Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Mechanisms of Vagus Nerve Stimulation in Improving Motor Dysfunction After Stroke
Authors Cai X, Jiang J, Zhou G, Zhang Y
Received 18 August 2024
Accepted for publication 16 December 2024
Published 21 December 2024 Volume 2024:20 Pages 2593—2601
DOI https://doi.org/10.2147/NDT.S492043
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rakesh Kumar
Xiaohu Cai,1,* Jiayao Jiang,2,* Guochao Zhou,3,* Yelei Zhang4
1Department of Rehabilitation Medicine, Xishan People’s Hospital of Wuxi City, Wuxi Branch of Zhongda Hospital Southeast University, Wuxi, People’s Republic of China; 2Department of Orthopedics, The 904th Hospital of the Joint Logistics Support Force of the PLA, Wuxi, Jiangsu, People’s Republic of China; 3Department of Orthopedics, The Army 947th Hospital, Kashgar, People’s Republic of China; 4Department of Neurosurgery, Xishan People’s Hospital of Wuxi City, Wuxi Branch of Zhongda Hospital Southeast University, Wuxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yelei Zhang, Department of Neurosurgery, Xishan People’s Hospital of Wuxi City, Wuxi Branch of Zhongda Hospital Southeast University, Wuxi, People’s Republic of China, Email [email protected]
Abstract: Patients with stroke would have persistent functional deficits despite undergoing physiotherapy and rehabilitation training. Recently, vagus nerve stimulation (VNS), a newly emerging neuroregulatory technique, has been shown to improve motor dysfunction after stroke. Evidence from clinical and preclinical studies has proven the safety, feasibility, and efficacy of invasive and noninvasive VNS. It has been reported that the positive effect may be related to anti-inflammatory effects, mediating neuroplasticity, increasing blood–brain barrier integrity, promoting angiogenesis and reducing spreading depolarization. However, the underlying mechanism remains poorly understood. In this review, we have summarized the potential molecular mechanisms by which VNS promotes stroke prognosis. We believe that VNS combined with upper-extremity rehabilitation can improve impairment and function among moderately to severely impaired stroke survivors. The applications and further exploration are discussed to provide new insights into this novel therapeutic technique.
Keywords: vagus nerve stimulation, stroke, neuroinflammatory response, brain plasticity
Introduction
Stroke is the leading cause of long-term disability worldwide, with 80% of patients suffering from motor dysfunction on one side of the body, which seriously affects their quality of life.1 According to the pathophysiology, stroke could be identified into two principal categories: ischemic and hemorrhagic stroke. Ischemic stroke occurred when blood flow is insufficient and unable to satisfy the brain tissue’s needs for oxygen and nutrients. In contrast, hemorrhagic stroke is due to the rupture of a blood vessel and represented by blood in the cerebral parenchyma, which can accumulate and press on the adjacent parenchyma.2 Similarly, both ischemic and hemorrhagic stroke would be associated with neurological dysfunction. Despite receiving physiotherapy and rehabilitation training, many patients still have persistent functional deficits, which would limit the quality of their lives and carry a huge economic burden on families and society.3 Therefore, novel therapeutic strategies are needed to better promote the improvement of damaged neurological function following stroke.
Vagus nerve stimulation (VNS) is a newly emerged neuroregulatory technique in recent years that has been widely used for the treatment of multiple neurological diseases such as refractory epilepsy, migraines, and refractory depression. An increasing number of studies have shown that VNS combined with rehabilitation can significantly reduce the size of post-stroke cerebral infarctions, reduce neurological symptoms, and improve limb motor function following stroke.4 VNS is commonly classified as invasive VNS (iVNS) or noninvasive VNS (nVNS). The nVNS containing auricular VNS in the ear (ta-VNS) and cervical VNS in the neck (tc-VNS) exhibited central nervous system (CNS) activation, similar to invasive VNS. However, the role of VNS in post-stroke motor function has not yet been elucidated and the mechanisms remain unclear. Given that the vagus nerve (VN) plays pivotal roles in the CNS regulating inflammatory responses and emerging evidence link inflammatory processes with VNS, the anti-inflammatory potential of VNS has been discovered.5 VNS has also been demonstrated to ameliorate gut microbiota dysbiosis and modulate microbiota-gut-brain axis to promote functional recovery after stroke.6,7 In this study, we summarized the anatomical, pre-clinical, and clinical evidence of VNS. We further emphasized the possible mechanism of VNS in improving motor dysfunction after stroke based current evidence and analyzed future challenges for its clinical application.
Anatomical Bases of Vagus Nerve
The vagus nerve is the longest and most widely distributed pair of mixed cranial nerves and can be divided into general somatosensory fibers, general visceral sensory fibers, general visceral motor fibers, and special visceral motor fibers. The vagus nerve runs from originates from the medulla oblongata and travels throughout the body to innervate various organs, including the heart, lung, liver, pancreas, stomach, intestines and spleen. The vagus nerve is composed of 80% afferent and 20% efferent fibers.8 When the vagus nerve is stimulated, excitation is transmitted to the nucleus tractus solitarii in the brainstem, which then transmits the excitation to the locus coeruleus and dorsal raphe nuclei, enhancing the release of norepinephrine, serotonin, and cholinergic neurotransmitters.9 Afferent projections of the vagus nerve to the dorsal raphe nucleus activate the serotonergic networks.10 The activation of these networks underpins the effect of VNS on neural plasticity.11 Thus, as a carrier of input and output information, the vagus nerve combines the central regions of the brain with the peripheral organs. It can also provide feedback on perceived information and build a bridge between the brain and peripheral crosstalk.12 For instance, the gut is a particularly important sensory organ. Enteric vagal afferents are transmitted via the nucleus tractus solitarius (NTS) to the vagal dorsal motor nucleus (DMV) and various forebrain structures such as the hypothalamus and limbic areas, partly in a lateralized manner, to modulate the reward system and control gut function. In the intestines, the VN regulates the contraction of smooth muscles and glandular secretions, and influences the peripheral immune system. The VN is also a major component of the parasympathetic nervous system. Its activation leads to the release of acetylcholine (ACh) at synaptic junctions with secretory cells, intrinsic nerve fibers and smooth muscles. The control of VN can be considered the ultimate effectors in the regulation of not only gut motility and secretion but also gut immunity.13 The spleen is considered as an important peripheral organ for the immune system and acts as a filter for blood within the immune system. Vagus nerves transmit bidirectional signals to communicate between the brain and peripheral organs, after which they control immune regulation and inflammatory reflexes, contributing to physiological homeostasis. Inflammatory reflex includes the afferent fibers of vagus nerve that senses pro-inflammatory inflammation from the spleen and other peripheral organs and the efferent fibers of vagus nerve that signals cholinergic anti-inflammatory integrated commands from the brain, such as brainstem dorsal motor nucleus (DMN), NTS, and higher forebrain regions. Finally, cholinergic anti-inflammatory signals act on the spleen via the splenic nerve, controlling immune activation and inhibiting proinflammatory cytokine release.14
In addition, the solitary nucleus emits fiber projections to the thalamus, hypothalamus, reticular region, limbic system, cerebellum, and cerebral cortex, which provides an anatomical basis for the VNS treatment of neurological diseases. The auricular region is the only area on the human body surface with a distribution of vagus nerve fibers. VNS stimulation in this area can activate the vagus nerve pathway. Therefore, it may be the best anatomical location for transcutaneous vagus nerve stimulation.
Neuromodulation Effects of the VNS for Stroke
Preclinical Evidence
Animal experiments have shown that VNS can be applied for the recovery of motor nerve function in rodent models of acute and chronic stroke, including hemorrhagic stroke and ischemic stroke.15 Sustained benefits were obtained from VNS stimulation in a post-stroke model. There was no correlation that has been found between the therapeutic effect and animal age, stroke type, or stroke time. For hemorrhagic stroke, VNS paired with rehabilitative training was found to significantly enhance the recovery of forelimb movement with 77% recovery of function, with only 29% recovery in a condition of rehabilitative training without VNS in a model of subcortical intracerebral hemorrhage (ICH).16 The results suggested that VNS paired with rehabilitative training significantly improved forelimb recovery after ICH compared to rehabilitative training without VNS.
For ischemic stroke, the therapeutic potential of VNS has also been excavated. To validate whether VNS paired with forelimb movements improves recovery after motor cortex ischemia, Khodaparast et al17 separated rats into 3 groups: vagus nerve stimulation during rehabilitation (rehabilitation), VNS after rehabilitation, and rehabilitation alone. The animals were allowed to recovery training 1-week post-operation. The VNS (500-ms train of 15 pulses at 30 hz) frequency was 5 days a week for 25 days. They found that forelimb function recovered completely to pre-lesion levels when VNS was performed during rehabilitation training. Khodaparast et al18 further tested the role of VNS in rat models six weeks after an ischemic lesion. The results showed that VNS paired with rehabilitative training significantly improved the recovery of forelimb function compared to the control group rats. The results showed that VNS also exhibits potential for the treatment of chronic stroke.
Clinical Evidence
Based on the results obtained in animal models, VNS combined with rehabilitation training is gradually being used in clinical practice. Clinical trials have mainly focused on ischemic stroke, and research results have indicated the effectiveness of implantable VNS in improving post-stroke movement disorders. To avoid increasing the risk of cardiac complications, recent studies have chosen the left VNS with relatively fixed stimulation parameters and an intensity of mostly 0.8 mA. The Fugl-Meyer Assessment Upper Extremity (FMA-UE) score is commonly used to assess upper extremity motor function. In 2018, Kimberley et al19 conducted a randomized, multisite, double-blind, sham-controlled pilot study to examine the safety and effects of VNS combined with rehabilitation for improving upper limb function after chronic stroke. The results of the comparison between the active VNS group (n = 8) and the control VNS group (n = 9) suggest that the average FMA-UE scores significantly increased in the active VNS group at the 90-day follow-up. The investigation confirmed that VNS is a feasible way to treat upper limb motor deficits after chronic ischemic stroke although with a small sample size. To further determine whether VNS is a safe and effective treatment for improving arm function after stroke, a randomized, blinded, pivotal device trial was performed with 19 stroke rehabilitation services in 2021.20 Participants were selected at least 9 months after ischemic stroke. A total of 108 patients (two patients did not complete the trials) with moderate-to-severe arm weakness were randomly assigned (53:53) to either rehabilitation paired with active VNS (VNS group) or rehabilitation paired with sham stimulation (control group). The participants received 6 weeks of in-clinic therapy followed by a home exercise program. The results showed that on the first day after completion of the in-clinic therapy, the mean FMA-UE score increased by 5.0 points in the VNS group and by 2.4 points in the control group (p=0.0014). Ninety days after clinical therapy, a clinically meaningful response in the FMA-UE score was achieved in 23 (47%) of 53 patients in the VNS group versus 13 (24%) of 55 patients in the control group (p = 0.0098). This multicenter trial showed that VNS resulted in clinically meaningful improvements in upper limb function after ischemic stroke, which could be a safe and effective treatment strategy. It should be noted that most studies have a limited sample size, which is still in the preliminary stage of research. Patients with stroke usually experience upper and lower limb extremity deficits. However, whether VNS contributes to the improvement of lower limb motor dysfunction remains unclear.
However, current VNS-related clinical trials mainly focus on ischemic stroke, which lacks evaluation in patients with hemorrhagic stroke. Cummins et al21 reported an illustrative case of a 67-year-old male with a past 3 years medical history of intracerebral hemorrhage. The patient received implantable VNS therapy for over six weeks and showed an improvement in upper extremity function with an FMA-UE score of 30 (improvement of 14 points from 16 at baseline). The stimulus pattern of VNS was described as a frequency of 30 hz, amplitude up to 0.8 mA, pulse width of 100 msec, and lasting 0.5 seconds. Although further clinical trials are needed to confirm the efficacy of VNS for motor functional recovery after ICH, this case demonstrated a greater improvement in function than that after ischemic stroke. Rebeiz et al22 investigated the effects of nVNS on spontaneous subarachnoid hemorrhage (SAH). They found that nVNS was associated with a reduction in headache intensity and decreased opioid consumption in patients with SAH. However, the relationship between VNS and functional outcomes of SAH was not examined in this study. Overall, the relevant research conclusions regarding VNS and arm function after hemorrhagic stroke require further exploration.
Potential Mechanisms of VNS on Motor Function
Inhibiting Neuroinflammatory Response
The anti-inflammatory role of vagus nerve has been widely recognized, especially the cholinergic anti-inflammatory pathway, which is mediated through vagal efferent fibers that synapse onto enteric neurons that release acetylcholine (ACh) at the synaptic junction with macrophages. ACh binds to α-7-nicotinic ACh receptors (α7nAchR) of those macrophages to inhibit the release of tumour necrosis (TNF)α, a pro-inflammatory cytokine. Another pathway is the splenic sympathetic anti-inflammatory pathway, where the VN stimulates the splenic sympathetic nerve. Norepinephrine (noradrenaline) released at the distal end of the splenic nerve links to the β2 adrenergic receptor of splenic lymphocytes that release ACh. Finally, ACh inhibits the release of TNFα by spleen macrophages through α-7-nicotinic ACh receptors.23 Understanding of these pathways is important to explain the mechanism of VNS regulating the immune response of the CNS through various pathways and exerting a protective effect on the cells. The cholinergic anti-inflammatory pathway is closely related to the neuroinflammatory processes during cerebral ischemia/reperfusion (I/R) injury. Tang et al24 identified that VNS may be involved in different neuroprotective and neuroplastic pathways via theα7nAchR in rat models of focal cerebral ischemic stroke. α7nAchR is a key component of acetylcholine, and VNS can modulate inflammatory responses and metabolism through the action of acetylcholine.25 Similarly, in a transient middle cerebral artery occlusion (tMCAO) model of mice, VNS was found to preserve α7nAChR expression in the penumbra regions, inhibit NLRP3 inflammasome activation and ensuing neuroinflammation, and rescue cerebral neurons. The role of the α7nAChR in microglial NLRP3 inflammasome activation in ischemic stroke was further validated.26 In stroke, activation of the NF-κB signaling upregulates the expression of NLRP3 and pro-IL-1β, followed by assembly and activation of the NLRP3 inflammasome, which promotes the maturation and release of IL-1β, resulting in neuroinflammation and neuronal death. However, VNS treatment activates microglial α7nAChR, which negatively regulates the NF-κB signaling, thereby inhibiting the NLRP3 inflammasome-induced neuroinflammation and neuronal death.26
Thus, VNS may have a neuroprotective effect against ischemic stroke via the α7nAchR-dependent inactivation of the microglial NLRP3 inflammasome. Ubiquitin-specific protease 10 (USP10) belongs to a member of the ubiquitin-specific protease family. Previous studies have reported that USP10 could inhibit the activation of NF-kB and the release of inflammatory cytokines, while USP10 silencing can markedly increase NF-kB activation.27,28 In mice with tMCAO, the relationship between USP10 and VNS stimulation was reported by Xie et al.29 They found that USP10 expression in the brain was decreased after ischemic stroke, and VNS treatment could counteract it by increasing the expression of USP10 and ameliorating neurological deficits. However, this protective effect was inhibited by silencing of USP10. These results suggest that neuroinflammation in VNS alleviating neurological deficits.29 Neuroinflammatory responses and neuronal apoptosis are closely related to the pathological processes in ischemic stroke. The effects of VNS on suppressing neuronal apoptosis were also demonstrated by the decreased levels of Bax and cleaved caspase-3, as well as increased levels of Bcl-2,30 which reduced infarct volume and improved neurological deficit scores.
Microglia participate widely in ischemia/reperfusion (I/R)-related inflammatory processes following an ischemic stroke. Zhang et al have reported that VNS suppresses p-NF-κB in microglial.31 The M2 microglial phenotype plays a critical role in anti-inflammatory activity and is a promising target. In a study by Zhao et al,32 non-invasive VNS (nVNS) alleviated cerebral I/R in mice by promoting microglial M2 polarization via interleukin-17A inhibition. The M1-to-M2 phenotype conversion is accelerated by VNS to alleviate the inflammatory response. Brain injury was subsequently reduced through the inhibition of microglial neuroinflammation following ischemia-reperfusion. Using a rat model of MCAO, Ay et al33 found that nVNS inhibited ischemia-induced immune activation, including microglial activation and cytokine levels. The extent of tissue injury and functional deficits was reduced without adverse cardiac or hemodynamic effects. In addition to microglial activation, VNS inhibited MCAO-induced immune response in the brain by reducing the number of Iba-1-, CD68-, and TNF-α-positive cells and increasing the number of HMGB1 positive cells.33 In view of the close relationship between neuroinflammatory response and stroke outcome, VNS might promote neurological function prognosis by exerting anti-inflammatory effects.
Enhancing Brain Plasticity
Brain plasticity is an inherent characteristic of the CNS and its ability to modify the structure and function of the brain in response to external stimuli or injuries. Injured neural circuits require substantial rewiring after neurological insult from a stroke. VNS may play a critical role in restoring corrective feedback patterns and promoting cortical reorganization.34,35 However, the mechanisms of how VNS exerts the beneficial effects of VNS on cortical plasticity are not completely understood. The VNS fosters a neurochemical milieu that facilitates synaptic plasticity and supports reinforcement. Specifically, it promotes the expression of neurotrophic factors, increases neurotransmitter release, and alters the synaptic and intrinsic neuronal properties. In a rat model of unilateral cortical and subcortical ischemic stroke,36 VNS paired with rehabilitative training improved forelimb function 12 weeks after stroke. Four rats in the rehab group and four rats in the Rehab + VNS group successfully received retrograde transneuronal tracer injections of pseudorabies virus (PRV-152) into the forelimb muscles. The results showed that VNS paired with rehabilitative training increased cortical PRV labeling in both the left and right sensorimotor cortices, suggesting that VNS enhanced plasticity in corticospinal motor networks to increase synaptic connectivity to the musculature of the rehabilitated forelimbs. Thus, the role of VNS in promoting motor recovery may be associated with enhanced structural plasticity in motor networks.
As a neuromodulatory therapy, VNS has recently been shown to enhance neuroplasticity by promoting myelin repair.37 With the application of VNS, demyelinating injury was assessed using longitudinal myelin dynamics and functional recovery. The effect of VNS was further confirmed by an increase in the generation of myelinating oligodendrocytes. The magnitude of the sheath pattern restoration correlates with long-term motor functional improvement. Considering that demyelination of corticospinal tract neurons leads to long-term disability after cortical stroke,38 VNS has the potential to treat stroke-related demyelinating diseases as a remyelination therapy.
Protecting the Blood-Brain Barrier (BBB)
Disruption of the BBB structure and function is related to infarct size and secondary brain damage following stroke. The impact of VNS on BBB permeability following I/R injury was assessed according to a study by Wang et al.6 Evaluation of BBB structure by EB extravasation assay, FITC-dextran permeability assay, and transmission electron microscopy analysis showed that VNS could improve BBB integrity and attenuate BBB damage. Another study verified the neuroprotective role of nVNS in BBB integrity in rat MCAO models.39 Compared with the control group, MRI images showed significantly reduced infarct sizes in the nVNS group. Dynamic contrast-enhanced (DCE)-MRI showed an obviously decreased BBB transfer rate in the lesion area in the nVNS group, which was spatially associated with attenuation of infarct size. In addition, nVNS protected vascular tight junction proteins from disruption in microvessels and reduced the expression of matrix metalloproteinases-2/9 in reactive astrocytes surrounding compromised vessels in ischemic hemispheres. This suggests that VNS may reduce BBB disruption in ischemic stroke. Aquaporin-4 (AQP-4) is a unique bidirectional water channel protein which is present on astrocytes and lines the endothelial cells fortifying the BBB. Investigating the effect of VNS on AQP-4 expression is important to clarify its relation to BBB permeability and brain edema. In mice models of traumatic brain injury (TBI), it has been found that VNS could inhibit TBI-induced upregulation of perivascular AQP-4, suggesting that modulation of AQP-4 may be another neuroprotective effect of VNS.40 However, current evidence is poor in stroke models, which may need to be validated in the future.
Promoting Angiogenesis
Angiogenesis after ischemic brain injury is positively related to reduced cerebral infarction and the recovery of neurological function. A surge in angiogenesis represents increased cerebral blood volume, and cerebral vascular remodeling benefits the functional recovery of stroke.41 VNS has been shown to induce angiogenesis in a rat tMCAO model of stroke. Histopathologically, increased microvessel density and endothelial cell proliferation surrounding the infarct area were observed using ta-VNS.42 Upregulated BDNF and VEGF protein and mRNA levels at the border of the ischemic zone were validated using immunofluorescence staining and Western blotting after 21 days of reperfusion. Li et al43 also found that ta-VNS induced angiogenesis and improved neurological function in a rat model of cerebral I/R injury. PPAR-γ is a potential mediator of ta-VNS-induced angiogenesis.
Reducing Spreading Depolarization
Cortical spreading depolarization (SD) is an intense depolarization wave that originates in the ischemic penumbra and slowly propagates across the gray matter, constricting the arteries in the ischemic brain region and imposing a tremendous metabolic demand, thus increasing the supply–demand mismatch.44,45 It reflects cytotoxic edema and negatively affects neuronal survival after an ischemic stroke.46 Novel therapies targeting SDs are effective intervention tools for stroke treatment. Jan et al47 investigated the effects of VNS on SDs in a rat model of focal ischemia and found that VNS significantly reduced the frequency of SDs in the cortical peri-infarct area, contributing to improved outcomes. Similarly, Lindemann et al explored the alteration in SD during a VNS intervention.47 They found that VNS significantly reduced the frequency of SDs in the cortical peri-infarct area in a rat model of MCAO. Moreover, elevated glutamate levels are proposed triggers of SDs following stroke. VNS has also been reported to reduce ischemia-induced glutamate release and alleviate excitotoxicity, leading to better outcomes in stroke patients.15,48
Safety of VNS
The main adverse events of VNS include pain, sleep disturbance, flu-like symptoms, and local skin discomfort,49 which could be mild. However, a few cases with transient vocal cord palsy and dysphagia after implantation, atrial fibrillation, reduced oxygen saturation, and chest pain have also been reported, which would seem to be serious.50 A meta-analysis confirmed previous findings that instantaneous heart rate and systolic, diastolic, and mean blood pressures are not significantly modified by ta-VNS.51 Another meta-analysis also showed that there was no significant difference in adverse events between invasive VNS and control group.52 Therefore, VNS is safe and tolerable.
Conclusion and Perspective
In general, VNS could be considered an effective and feasible treatment for improving upper-limb motor dysfunction after stroke with obvious cardiac complications. These positive effects may be related to anti-inflammatory effects, mediating neuroplasticity, increasing blood–brain barrier integrity, promoting angiogenesis, and reducing spreading depolarization (Figure 1). However, the mechanisms are not completely clear.
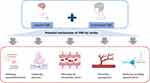 |
Figure 1 Potential mechanisms of vagus nerve stimulation (VNS) for stroke recovery. The figure is created with BioRender. Zhang, Y (2024) BioRender.com/q60u863. |
For example, the role of neurogenesis in VNS enhancing motor function is poorly reported. The vagus nerve has been identified to modulate BDNF expression and neurogenesis in the hippocampus.53 BDNF is critical for maintaining adult neurogenesis. Similarly, could VNS improve BDNF expression in the motor cortex of the brain, thereby enhancing motor function? More hypotheses need to be verified.
There is still uncertainty regarding the application plan of VNS for treating upper limb motor dysfunction after stroke, including the optimal stimulation parameters, stimulation duration, and optimal intervention treatment time for VNS to achieve successful results. With an implanted closed-loop system trend, the physiological feedback and spatiotemporal precision of VNS must be considered. To identify the optimal stimulation parameters and intervention times in future research, VNS should be combined with neuroimaging to study the pathological and physiological mechanisms of disease occurrence and development. Currently, clinical research focuses more on ischemic stroke and less on hemorrhagic stroke. Further exploration of the effectiveness of VNS in hemorrhagic stroke is required. More importantly, current evidence is insufficient to reveal the mechanism of VNS. With the development of optogenetics, chemogenetics, and neural tracing techniques, the enhanced brain plasticity must be accurately confirmed. Clarification of the reorganized neural circuit is essential for more precise treatment. A better understanding of the pathological mechanisms underlying VNS could improve its clinical efficacy.
Funding
This work was supported by grants from the Scientific Research Program of the Wuxi Health Commission (no. Q202209) to Yelei Zhang.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–754. doi:10.1016/s1474-4422(09)70150-4
2. Maida CD, Norrito RL, Rizzica S, et al. Molecular pathogenesis of ischemic and hemorrhagic strokes: background and therapeutic approaches. Int J Mol Sci. 2024;25:6297. doi:10.3390/ijms25126297
3. Rajsic S, Gothe H, Borba HH, et al. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ. 2019;20:107–134. doi:10.1007/s10198-018-0984-0
4. Du L, He X, Xiong X, et al. Vagus nerve stimulation in cerebral stroke: biological mechanisms, therapeutic modalities, clinical applications, and future directions. Neural Regen Res. 2024;19:1707–1717. doi:10.4103/1673-5374.389365
5. Schiweck C, Sausmekat S, Zhao T, et al. No consistent evidence for the anti-inflammatory effect of vagus nerve stimulation in humans: a systematic review and meta-analysis. Brain Behav Immun. 2024;116:237–258. doi:10.1016/j.bbi.2023.12.008
6. Wang Y, Tan Q, Pan M, et al. Minimally invasive vagus nerve stimulation modulates mast cell degranulation via the microbiota-gut-brain axis to ameliorate blood-brain barrier and intestinal barrier damage following ischemic stroke. Int Immunopharmacol. 2024;132:112030. doi:10.1016/j.intimp.2024.112030
7. Hesampour F, Bernstein CN, Ghia JE. Brain-gut axis: invasive and noninvasive vagus nerve stimulation, limitations, and potential therapeutic approaches. Inflamm Bowel Dis. 2024;30:482–495. doi:10.1093/ibd/izad211
8. Bonaz B. Is-there a place for vagus nerve stimulation in inflammatory bowel diseases? Bioelectron Med. 2018;4:4. doi:10.1186/s42234-018-0004-9
9. Mravec B. The role of the vagus nerve in stroke. Auton Neurosci. 2010;158:8–12. doi:10.1016/j.autneu.2010.08.009
10. Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci. 2009;34:272–280.
11. Dawson J, Abdul-Rahim AH, Kimberley TJ. Neurostimulation for treatment of post-stroke impairments. Nat Rev Neurol. 2024;20:259–268. doi:10.1038/s41582-024-00953-z
12. Fonseca RC, Bassi GS, Brito CC, et al. Vagus nerve regulates the phagocytic and secretory activity of resident macrophages in the liver. Brain Behav Immun. 2019;81:444–454. doi:10.1016/j.bbi.2019.06.041
13. Mikami Y, Tsunoda J, Kiyohara H, et al. Vagus nerve-mediated intestinal immune regulation: therapeutic implications of inflammatory bowel diseases. Int Immunol. 2022;34:97–106. doi:10.1093/intimm/dxab039
14. Wei Y, Wang T, Liao L, et al. Brain-spleen axis in health and diseases: a review and future perspective. Brain Res Bull. 2022;182:130–140. doi:10.1016/j.brainresbull.2022.02.008
15. Baig SS, Kamarova M, Ali A, et al. Transcutaneous vagus nerve stimulation (tVNS) in stroke: the evidence, challenges and future directions. Auton Neurosci. 2022;237:102909. doi:10.1016/j.autneu.2021.102909
16. Hays SA, Khodaparast N, Hulsey DR, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. 2014;45:3097–3100. doi:10.1161/strokeaha.114.006654
17. Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil Neural Repair. 2014;28:698–706. doi:10.1177/1545968314521006
18. Khodaparast N, Kilgard MP, Casavant R, et al. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats. Neurorehabil Neural Repair. 2016;30:676–684. doi:10.1177/1545968315616494
19. Kimberley TJ, Pierce D, Prudente CN, et al. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke. Stroke. 2018;49:2789–2792. doi:10.1161/strokeaha.118.022279
20. Dawson J, Liu CY, Francisco GE, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 2021;397:1545–1553. doi:10.1016/s0140-6736(21)00475-x
21. Cummins DD, Kalagara R, Downes MH, et al. Vagus nerve stimulation for enhanced stroke recovery after intracerebral hemorrhage: illustrative case. J Neurosurg Case Lessons. 2024;7. doi:10.3171/case23676
22. Rebeiz T, Sabirov T, White TG, et al. Noninvasive vagus nerve stimulation in spontaneous subarachnoid hemorrhage (VANQUISH): a randomized safety and feasibility study. Brain Stimul. 2024;17:543–549. doi:10.1016/j.brs.2024.04.004
23. Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016;594:5781–5790. doi:10.1113/jp271539
24. Tang H, Li J, Zhou Q, et al. Vagus nerve stimulation alleviated cerebral ischemia and reperfusion injury in rats by inhibiting pyroptosis via α7 nicotinic acetylcholine receptor. Cell Death Discov. 2022;8:54. doi:10.1038/s41420-022-00852-6
25. Wang YY, Lin S-Y, Chang C-Y, et al. α7 nicotinic acetylcholine receptor agonist improved brain injury and impaired glucose metabolism in a rat model of ischemic stroke. Metab Brain Dis. 2023;38:1249–1259. doi:10.1007/s11011-023-01167-w
26. Xia XM, Duan Y, Wang Y-P, et al. Vagus nerve stimulation as a promising neuroprotection for ischemic stroke via α7nAchR-dependent inactivation of microglial NLRP3 inflammasome. Acta Pharmacol Sin. 2024;45:1349–1365. doi:10.1038/s41401-024-01245-4
27. Niu J, Shi Y, Xue J, et al. USP10 inhibits genotoxic NF-κB activation by MCPIP1-facilitated deubiquitination of NEMO. EMBO j. 2013;32:3206–3219. doi:10.1038/emboj.2013.247
28. Luo P, Qin C, Zhu L, et al. Ubiquitin-specific peptidase 10 (USP10) inhibits hepatic steatosis, insulin resistance, and inflammation through Sirt6. Hepatology. 2018;68:1786–1803. doi:10.1002/hep.30062
29. Xie C, Gao X, Liu G, Tang H, Li C. USP10 is a potential mediator for vagus nerve stimulation to alleviate neuroinflammation in ischaemic stroke by inhibiting NF-κB signalling pathway. Front Immunol. 2023;14:1130697. doi:10.3389/fimmu.2023.1130697
30. Zhang L, Ma J, Jin X, et al. L-PGDS mediates vagus nerve stimulation-induced neuroprotection in a rat model of ischemic stroke by suppressing the apoptotic response. Neurochem Res. 2017;42:644–655. doi:10.1007/s11064-016-2121-8
31. Zhang L, Zhang X, Liu Y, Wang S, Jia G. Vagus nerve stimulation promotes the M1-to-M2 transition via inhibition of TLR4/NF-κB in microglial to rescue the reperfusion injury. J Stroke Cerebrovasc Dis. 2022;31:106596. doi:10.1016/j.jstrokecerebrovasdis.2022.106596
32. Zhao XP, Zhao Y, Qin XY, Wan LY, Fan XX. Non-invasive vagus nerve stimulation protects against cerebral ischemia/reperfusion injury and promotes microglial M2 polarization via Interleukin-17A inhibition. J Mol Neurosci. 2019;67:217–226. doi:10.1007/s12031-018-1227-7
33. Ay I, Nasser R, Simon B, Ay H. Transcutaneous cervical vagus nerve stimulation ameliorates acute ischemic injury in rats. Brain Stimul. 2016;9:166–173. doi:10.1016/j.brs.2015.11.008
34. Ganzer PD, Darrow MJ, Meyers EC, et al. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife. 2018;7. doi:10.7554/eLife.32058
35. Ching-Tzu T, Jackson B, Solomon J,G, Bilaal SH, Catherine AT. Vagus nerve stimulation promotes cortical reorganization and reduces task-dependent calorie intake in male and female rats. Brain Res. 2020;1748. doi:10.1016/j.brainres.2020.147099
36. Meyers EC, Solorzano BR, James J, et al. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke. 2018;49:710–717. doi:10.1161/strokeaha.117.019202
37. Huang R, Carter ER, Hughes EG, Welle CG. Paired vagus nerve stimulation drives precise remyelination and motor recovery after myelin loss. bioRxiv. 2024. doi:10.1101/2024.05.10.593609
38. Strecker JK, Schmidt‐Pogoda A, Diederich K, et al. Anti-LINGO-1 treatment restores myelination of corticospinal tract neurons and improves functional recovery after stroke. Brain Pathol. 2024:e13280. doi:10.1111/bpa.13280
39. Yang Y, Yang LY, Orban L, et al. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain Stimul. 2018;11:689–698. doi:10.1016/j.brs.2018.01.034
40. Lopez NE, Krzyzaniak MJ, Costantini TW, et al. Vagal nerve stimulation decreases blood-brain barrier disruption after traumatic brain injury. J Trauma Acute Care Surg. 2012;72:1562–1566. doi:10.1097/TA.0b013e3182569875
41. Liu J, Wang Y, Akamatsu Y, et al. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–156. doi:10.1016/j.pneurobio.2013.11.004
42. Jiang Y, Li L, Ma J, et al. Auricular vagus nerve stimulation promotes functional recovery and enhances the post-ischemic angiogenic response in an ischemia/reperfusion rat model. Neurochem Int. 2016;97:73–82. doi:10.1016/j.neuint.2016.02.009
43. Li J, Zhang K, Zhang Q, et al. PPAR-γ mediates Ta-VNS-induced angiogenesis and subsequent functional recovery after experimental stroke in rats. Biomed Res Int. 2020;2020:8163789. doi:10.1155/2020/8163789
44. Ayata C, Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev. 2015;95:953–993. doi:10.1152/physrev.00027.2014
45. Andalib S, Divani AA, Ayata C, et al. Vagus nerve stimulation in ischemic stroke. Curr Neurol Neurosci Rep. 2023;23:947–962. doi:10.1007/s11910-023-01323-w
46. Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron. 2015;86:902–922. doi:10.1016/j.neuron.2015.04.004
47. Lindemann, J., Rakers, C., Matuskova, H. et al. Vagus nerve stimulation reduces spreading depolarization burden and cortical infarct volume in a rat model of stroke. PLoS One. 2020;15:e0236444. doi:10.1371/journal.pone.0236444
48. Miyamoto O, Pang J, Sumitani K, et al. Mechanisms of the anti-ischemic effect of vagus nerve stimulation in the gerbil hippocampus. Neuroreport. 2003;14:1971–1974. doi:10.1097/00001756-200310270-00018
49. Yang H, Shi W, Fan J, et al. Transcutaneous auricular vagus nerve stimulation (ta-VNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial. Neurotherapeutics. 2023;20:870–880. doi:10.1007/s13311-023-01353-9
50. Dawson J, Pierce D, Dixit A, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke. 2016;47:143–150. doi:10.1161/strokeaha.115.010477
51. Kim AY, Marduy A, de Melo PS, et al. Safety of transcutaneous auricular vagus nerve stimulation (taVNS): a systematic review and meta-analysis. Sci Rep. 2022;12:22055. doi:10.1038/s41598-022-25864-1
52. Abdullahi A, Wong TWL, Ng SSM. Effects and safety of vagus nerve stimulation on upper limb function in patients with stroke: a systematic review and meta-analysis. Sci Rep. 2023;13:15415. doi:10.1038/s41598-023-42077-2
53. O’Leary OF, Ogbonnaya ES, Felice D, et al. The vagus nerve modulates BDNF expression and neurogenesis in the hippocampus. Eur Neuropsychopharmacol. 2018;28:307–316. doi:10.1016/j.euroneuro.2017.12.004
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

