Back to Journals » Journal of Pain Research » Volume 18
Mechanistic and Clinical Evaluation of Lever-Positioning Manipulation in Lumbar Disc Herniation: A Study Protocol for a Randomized Controlled Trial Using Rs-fMRI
Authors Xie Y, Du H, Liang A, Yao J, Qu J, Zeng X
Received 19 February 2025
Accepted for publication 8 May 2025
Published 12 May 2025 Volume 2025:18 Pages 2379—2392
DOI https://doi.org/10.2147/JPR.S523613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Yunxing Xie,1 Honggen Du,1 An Liang,1 Juncheng Yao,1 Jianpeng Qu,2 Xiayang Zeng2
1Tui Na Department, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, People’s Republic of China; 2Tui Na Department, Zhejiang Hospital, Hangzhou, People’s Republic of China
Correspondence: Xiayang Zeng, Tui Na Department, Zhejiang Hospital, No. 1229 Gundun Road, Xihu District, Hangzhou City, Hangzhou, 310000, People’s Republic of China, Email [email protected]
Background: Lumbar disc herniation (LDH) is a prevalent clinical chronic pain disorder characterized by low back pain, lower limb pain, numbness, and claudication, among other symptoms. Lever positioning manipulation (LPM) has been demonstrated to alleviate pain by stimulating the paraspinal muscles and improving vertebral mechanical balance. Additionally, it has been shown to influence functional changes in the brain by acting on lumbar vertebral proprioceptors.
Patients and Methods: This was a randomized controlled study in which 60 eligible participants were randomly assigned to one of three groups: an observation group (LPM group), a control group (Inclined plate manipulation (IPM) group) and a normal control group (No Intervention Group). The ratio of participants in each group was 1:1:1. The LPM and IPM groups received treatment for five sessions per week over two weeks. The control group did not receive any form of intervention. The primary observation was rs-fMRI imaging of functional brain areas, whereas the secondary observations included surface electromyography, lumbar proprioceptive function, and VAS and JOA scores. All of these parameters were assessed before and after treatment. All analyses were conducted in accordance with the principles of treatment. Once data collection is complete, data will be analysed using SPSS 20.0 with ANOVA and rank-sum tests for comparisons. We will conduct follow-up monitoring at the first and third month after the end of treatment.
Conclusion: The objective of this study was to observe the changes in the relevant brain regions of LDH patients through LPM treatment of LDH patients, combined with rs-fMRI and lumbar proprioceptor detection, to elaborate the neural pathways of cortical changes, and to provide new ideas and methods for the treatment of LDH.
Registration for Trial: Chinese Clinical Trial Registry ChiCTR2400082255 on 25 March 2024.
Keywords: lumbar disc herniation, LDH, randomized controlled trial, lever positioning manipulation, LPM, rs-fMRI, lumbar vertebral proprioceptors
Introduction
Lumbar disc herniation (LDH) is a degenerative condition that primarily affects elderly individuals and is now more common in younger people because of changes in employment and lifestyle.1 LDH is characterized by rupture of the annulus fibrosus and protrusion of the nucleus pulposus, which results in local edema and inflammation. This leads to compression of adjacent tissues, including nerves, the spinal cord and blood vessels. The consequences of this are low back pain, neuralgia, numbness, and even symptoms that radiate to the lower limbs.2,4 LDH-induced low back pain and numerous other symptoms represent the third most common reason for surgical intervention and the fifth most common cause of hospitalization. These symptoms have a considerable negative impact on patients’ quality of life, with the potential to cause emotional distress and anxiety. This, in turn, may lead to a reduction in productivity and an associated burden on the health care system.5–8
Consequently, LDH treatment is becoming increasingly prevalent in the field of spine research. Currently, therapeutic approaches are predominantly classified as either conservative or surgical. It is estimated that only 15–20% of patients require surgical intervention because of the presence of severe neurological symptoms that significantly impair their quality of life.9 Furthermore, there is an ongoing debate regarding the comparative efficacy of surgical versus conservative treatment approaches. This is based on the observation that 2.7% of patients experience postoperative complications and that 11% of patients require reoperation within five years. Several studies have concluded that surgical intervention does not result in meaningful improvement.10–12 Consequently, the majority of patients elect to pursue conservative therapy, which is a secure and efficacious noninvasive treatment modality.13,14
Massage is recommended by the Royal College of Physiotherapists as a traditional conservative therapy for the treatment of pain conditions.15 Traditional Chinese massage (Tui Na) has been developed over millennia and is currently employed as a principal treatment for LDH. Tui Na manipulation effectively treats LDH by significantly improving the abnormal distribution of synovial stress and protruding nucleus pulposus caused by “musculoskeletal imbalance”, thereby promoting the reconstruction of the mechanical balance of the spinal column, relieving the pressure of the herniated discs on the nerve roots, and promoting the absorption of inflammatory substances.16–18 In the 2017 American College of Physicians (ACP) Clinical Practice Guidelines for the Noninvasive Treatment of Acute, Subacute, and Chronic Low Back Pain, Tui Na was identified as a highly recommended first-line treatment option.19 Lever position manipulation (LPM) has its roots in the development of traditional Tui Na manipulation, which is a form of chiropractic treatment for the spine that is widely used in clinical practice in China.20,21 The current research on the mechanism of action of the LPM on LDH has focused primarily on the impact of mechanical adjustment on the posterior lumbar joints and the biomechanical effects of muscle stabilization. Nevertheless, there is a paucity of research and randomized controlled trials in clinical practice on the mechanisms of brain function. Therefore, further randomized controlled trials are needed to elucidate the advantages of LPM and to investigate the neurotransmission mechanism of brain function under the influence of manipulation. These findings provide the latest experimental basis for the clinical application of lever manipulation in the treatment of LDH.
Resting-state functional magnetic resonance imaging (rs-fMRI), developed in the 1990s, represents one of the most recently applied and developed techniques for indirect visualization of neuronal activity through imaging of blood oxygen level-dependent (BOLD) effector mechanisms. This method is currently the only noninvasive in vivo technique for studying neural mechanisms, as it responds to the major functions of the brain and functional alterations in the presence of disease and is capable of high-resolution imaging.22 The primary clinical manifestations of LDH are low back and leg pain and numbness. In recent years, many domestic and international studies on brain function have demonstrated that chronic pain can result in the disruption of normal brain function. Additionally, human brain studies have indicated that pain transmission is associated with the anterior cingulate gyrus (ACG), cerebral conduction (INS), and thalamus, as well as the somatosensory cortex (SI), central gray matter, and other regions.23,24 Chronic pain is frequently accompanied by alterations in the volume of gray matter in the brain. The observation that chronic pain often involves specific areas of the brain indicates that these areas are regulated by the body during the experience of chronic pain.25 To ascertain the existence of motor-related brain regions in LDH patients, we conducted a previous study utilizing cranial resting-state fMRI scans of 10 LDH patients. The results demonstrated that, compared with those in healthy individuals, there were aberrant ReHo values in the motor functional regions of the brain in LDH patients, predominantly in the primary motor cortex (M1), premotor cortex (PMC) and posterior parietal cortex (PPC). The ReHo values exhibited variability in the primary motor cortex (M1), premotor cortex (PMC), and posterior parietal cortex (PPC) (as illustrated in Figure 1). Previous study also has found significant changes in ALFF and ReHO in the DMN, PFC, and S1 regions of the brain in LDH patients treated with LPM.20 The examination of rs-fMRI reveals that changes in the patient’s pain mood cause changes in the active areas of the cerebral cortex, and it is worthwhile to further explore whether such changes are neurally connected through the lumbar proprioceptors.
 |
Figure 1 Comparison of changes in ReHo values for resting-state brain function, (A) Healthy person; (B) LDH patient. The red points in the figure are anomalous ReHo values. |
In recent years, there has been a growing focus on the role of proprioceptive transmission pathways in the etiology of LDH. This process primarily involves the stimulation of muscles, tendons and joints, which in turn results in alterations in functional brain areas that are specific to the spinal joints.26,27 The lumbar joint capsule, intervertebral discs and ligamentous tissues are densely populated with proprioceptors, which convey sensory information regarding muscle contraction and joint position to the corresponding functional regions of the brain. This enables the brain to regulate motor function through the implementation of control and motor analysis and the subsequent generation of motor feedback via the central motor efferent system.28 When a specific level of stimulation is applied to an organism (for example, painful or pleasurable stimuli), the brain center will activate and excite the regions that form the basis of emotion in response to the stimulus. This results in functional regulation of the organism by the brain center. Researchers have found that stimulation induce activation of the “emotional brain” and to result in a reduction of grey matter in the prefrontal and somatosensory cortex.29–31 Kandel et al suggest that any external stimulus will leave traces in the brain and may trigger the switching of genes, the release of chemicals and hormones, and the growth and remodelling of the central nervous system itself.32 Therefore, we suspect that stimulation of lumbar acupoints or muscles or bone joints through Tui Na manipulation may induce changes in the brain area through lumbar proprioceptors, leaving traces in the brain and even inducing growth or remodelling of the central nervous system. In light of the aforementioned research, the preceding work on this project, and the latest findings from domestic and international research, we propose the following hypothesis: in the treatment of LDH by lever-positioning manipulation, the conduction of local lumbar proprioceptive sensation and the effector mechanism of the related brain functional area play important roles in regulating the functional activity of stabilizing muscles and remodeling the lumbar vertebrae to improve stability. Figure 2 illustrates the hypotheses.
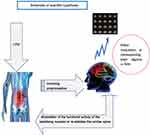 |
Figure 2 Schematic of scientific hypothesis. Abbreviations: LPM, Leveraged positioning manipulation; fMRI, functional magnetic resonance imaging. |
This project represents a further, more in-depth investigation into the mechanism of the lever positioning operation (LPM). According to articles published by Chinese scholars in Chinese journals, compared with the inclined plate manipulation (IPM), the finite element biomechanical model established by the LPM shows that, firstly, the LPM directly applies stress to the lumbar spine diseased segments, increases the lumbar spine posterior extensor force, so that the mechanical conduction of the lumbar spine is along the axis of equilibrium, and the intervertebral disc force is more balanced. Secondly, by improving the LPM, it can easily position and control the strength more stably, and can better adjust the strength, so that the adjustment is more targeted. Based on previous research, the present study focused on LDH patients. The efficacy of lever-positioning manipulation was compared with that of traditional manipulation, and a platform combining the efficacy of manipulation and experimental verification was established. By employing the most recent technological advancements, including rs-fMRI and proprioception, we elucidate the impact of lever-positioning manipulation on the proprioception of the lumbar spine and its influence on the corresponding brain functional areas. This will reveal the underlying mechanism through which the functional areas of the brain regulate the stabilization of muscular activities and remodel the lumbar spine through nerve conduction to improve stability. However, it is undeniable that this study suffers from a small sample size, a lack of multicentre clinical trials, as well as variability and uncertainty in manipulation, all of which will affect the accuracy of the results of the trial.
Methods
Objective
The objective of this study was to observe the changes in the relevant brain regions of LDH patients through LPM treatment of LDH patients, combined with rs-fMRI and lumbar proprioceptor detection, to elaborate the neural pathways of cortical changes, and to provide new ideas and methods for the treatment of LDH.
Hypotheses
- The utilization of contemporary techniques, including rs-fMRI and proprioception, revealed that lever-positioning manipulation elicited more pronounced alterations in ReHo values within the functional brain regions of patients with lumbar disc herniation than in those observed in the control group and those undergoing pharmacological intervention.
- The LPM notably enhanced patients’ lumbar spine functionality and quality of life, as evaluated through the use of the VAS and JOA.
Trial Design
The trial was a single-blind, randomized controlled trial in which researchers conducted a blinded comparison of the differences in patients’ functional brain areas and lumbar proprioceptive function between the lever-positioning manipulation group and the traditional technique (Inclined plate manipulation, IPM) group and the no intervention group. A total of 60 LDH patients were recruited and randomly assigned to one of three groups: an observation group (LPM group), a control group (IPM group) and a normal control group (No Intervention group). The trial has been registered with the Chinese Clinical Trial Registry (www.chictr.org.cn), and the registration number is ChiCTR2400082255. This protocol was prepared in accordance with the SPIRIT guidelines.33 The study process is illustrated in Figure 3, which presents a flow chart of the study process. Furthermore, Table 1 illustrates the process for the inclusion, treatment, and assessment of outcome indicators for patients participating in this trial.
 |
Table 1 Schedule of Enrollment, Treatment, and Assessments |
Participants, Enrollment, and Ethics
The participants with LDH were enrolled from the Tui Na Department of the First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), where they were either inpatients or outpatients. The participants were recruited primarily through the dissemination of information via posters and online sources. To enhance visibility, enrollment posters were displayed in both outpatient and inpatient departments. Furthermore, the recruitment information for this trial has been disseminated via online publicity. Individuals who met the inclusion criteria were informed of the potential benefits and risks associated with participation in the trial, signed an informed consent form, and were randomly assigned to groups. The study protocol will comply with the Declaration of Helsinki. The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine) Ethics Committee approved the study protocol (No. 2023-KLS-242-02).
Inclusion Criteria
Participants who met all of the following criteria were considered for inclusion in the study: (1) met the diagnostic criteria for lumbar disc herniation as proposed by the North American Spine Society and subsequently confirmed by lumbar MRI, MRI within 1 year; (2) Met the MSU Classification, lumbar disc herniation level 1a-2a; (3) were between the ages of 20 and 50 years old, with no sex restrictions; (4) cooperated with the research of this project and adhered to the treatment for two courses of treatment; (5) had a BMI of less than 24; (6) were willing to accept the treatment proposed by this project and signed the informed consent form.
Exclusion Criteria
The following criteria were used to exclude subjects from the study: (1) patients detected after DXA with a lumbar spine tumor, slip, tuberculosis, arch root fracture or severe osteoporosis; (2) according to the MSU classification, lumbar disc herniation at a level greater than 2a; (3) those with concomitant cardiovascular and cerebrovascular disease, liver, renal, and hematopoietic system disease, and other serious primary diseases; (4) those with infectious diseases, skin ulcers, skin breaks, or skin creases; (5) those with leukemia, thrombocytopenia, and other bleeding tendencies; (6) those with psychiatric disorders who were unable to cooperate with treatment; and (7) female patients during pregnancy or lactation.
Randomization and Allocation Concealment
An independent statistician, who was not involved in the trial, employed the Statistical Package for the Social Sciences (SPSS) version 20.0 software to generate 60 serial numbers, which were randomly divided into three groups at a 1:1:1 ratio. The first 20 numbers were assigned to the observation group, the next 20 to the control group, and the final 20 to the normal control group. The numbers were subsequently placed in opaque, sealed envelopes, with the outside of the envelopes displaying the patients’ screening order and the inside of the envelopes displaying the group assigned based on the order in which the patients were grouped. The envelopes were randomly sorted into opaque cardboard boxes, and the individual responsible for the procedure ensured that the confidentiality and security of the grouping information boxes were maintained. Patients who met the inclusion criteria were first required to complete the case report form (CRF). The envelopes were subsequently opened in accordance with the order of inclusion. The patient’s screening sequence number was recorded, and the resulting patient grouping was immediately recorded without further modification.
Blinding
This trial was conducted in a manner that ensured the blinding of both participants and assessors. Consequently, the participants were unaware of their subgroup and treatment allocation. Furthermore, they had an equal chance of being assigned to either the trial or control group. Similarly, the outcome assessors were unaware of the participants’ subgroupings. Although the therapists were not blinded and were aware of the grouping of the participants, they were not involved in the assessment of outcomes.
Intervention
Observation Group (Leveraged Positioning Manipulation Group)
Muscle Relaxation Techniques
The participants were positioned prone, and the practitioner employed massage techniques on the patient’s spinal lumbar region on both sides of the double lower limb bladder meridian alignment, focusing on the affected lumbar region, for approximately eight minutes. This was performed to relieve the meridians, activate the blood, and loosen the muscle spasms. Subsequently, the practitioner applied appropriate pressure and kneading to points such as the lumbar Jiaji of the lumbar (EX-B2, bilateral), Mingmen (DU4, bilateral), Shenshu (BL23, bilateral), Huantiao (GB30, bilateral), Zhibian (BL54, bilateral), Weizhong (BL40, bilateral) and Ashi points (ie, the point that feels most painful to localized pressure), requiring use of either the thumb or the tip of the elbow, for approximately seven minutes. The location of each point is shown in Table 2.
 |
Table 2 Location of Acupoints for Treating LDH |
Leveraged Positioning manipulation20,21
The participants assumed a prone position, with the doctor positioned on the right side of the participants, took the 0.5cm next to the segment of lumbar disc protrusion on the imaging as the positioning point. The participants were instructed to bend their knees and hips and to cross their two lower limbs. The doctor marked the leverage positioning point with the right-hand elbow eagle’s beak. This was followed by the doctor holding the patient’s two ankle joints with both hands from the left and right directions. The doctor then applied force to the patient’s upper limbs, using the leverage principle, to fix the lumbar spine in place. Finally, the doctor used the force of both arms to lift the lumbar spine, forced upward and backward lumbar vertebrae to 30°. When the lumbar vertebrae were subjected to a certain degree of resistance, the practitioner instructed the patient to perform rapid, limited backward extension of the trigger, and the practitioner made a rapid left and right control 5° trigger, and repeat the operation for 3 times with “clever force inch strength”. It is essential that patients cooperate with the leverage maneuver, maintain a calm and relaxed state, and finally assume a supine position on the bed for a 15-minute rest period. The participants received a total of five treatments per week over two weeks. We will conduct follow-up monitoring at the first and third month after the end of treatment.
Control Group (Inclined Plate Manipulation Group)
Muscle Relaxation Techniques: Muscle Relaxation, as in the Observation Group
Inclined Plate Manipulation (IPM)34
The patient was positioned laterally, with the healthy and affected sides positioned in the upper and lower extremities, respectively. The affected side was subsequently flexed at the hip while the doctor stood in front of the patient, and pressure was applied to the root of the palm of one hand against the affected shoulder and the root of the palm of the other hand against the lumbosacral area. The doctor’s two elbows were directed in opposite directions, applying force to the lumbar vertebrae until they reached the limit of their range of motion. The operator subsequently applied the requisite degree of force to the lumbar vertebrae with the appropriate oblique plate. Once the procedure was complete, the patient was advised to rest in the supine position for a period of fifteen minutes. The treatment regimen for the patient cohort was identical to that of the control group. We will conduct follow-up monitoring at the first and third month after the end of treatment.
Normal Control Group (No Intervention Group)
The 20 participants were not subjected to any intervention. It is worth emphasising that we had considered using a placebo group (sham manipulation group) as a control group, but to consider the uncertainty of the manipulation of the placebo group, we therefore had to use the no-intervention group as a control group in this study. Those who were taking medication or using other treatments during the trial period were excluded from the study, as they were deemed to have discontinued their participation. We will conduct follow-up monitoring at the first and third month after the end of treatment.
Outcome Measures
Prior to and following the administration of the treatment, all three groups underwent a series of assessments and analyses, including brain functional area testing and analysis, lumbar proprioceptive function assessment, surface electromyography assessment, and evaluation of the JOA and VAS scores. These assessments were conducted to assess the efficacy of the two groups, namely, the LPM and IPM groups, and to observe the changes in the brain functional areas, surface electromyography, and lumbar proprioceptive function of the participants in the LPM and IPM groups compared with those in the normal control group.
Primary Outcome
Detection of Functional Brain Areas (Rs-fMRI)
A functional MRI was conducted via a GEDiscovery MR750 3.0T functional magnetic resonance system. The T1 structural image and resting-state functional image of the patients were acquired for the purpose of evaluating and analyzing the brain-related parameters of the test patients. This allows for the accurate localization of changes in the relevant brain functional areas of LDH patients and the clarification of the different response patterns of the relevant brain functional areas after treatment. The rs-fMRI raw images were imported into the Statistical Parametric Mapping (SPM) software, and the SPM package on the MATLAB platform was used to analyse the Dicom-formatted fMRI raw images, which mainly included time-layer correction, head-motion correction, de-baseline drifting, and filtering (0.01 < f < 0.1 hz), to obtain the raw data. Rs-fMRI raw data we usually use ALFF and ReHo analyses to obtain the frequency wave signals, and then calculate the ALFF and ReHo values, which will have a statistically significant brain images are superimposed on the standard brain so that the corresponding brain structures can be displayed and the 3D spatial location of activated regions can be calibrated. The reading of the coordinates, anatomical position and anatomical location of the activated brain regions, as well as the T-values in the MNI template, is achieved by utilising graphical plug-ins such as Slice Viewer and Xjview 95.
Secondary Outcome
Surface Electromyography Assessment
The objective quantitative index for evaluating the efficacy of lumbar disc herniation, the median frequency decline rate (MFs) of the paraspinal muscles in the isometric contraction state, was the primary focus of the study. The specific test method employs the use of Mega-T8 surface electromyography to measure the surface electromyographic frequency index of the paraspinal muscles in the lumbar 4–5 plane bilaterally under the isometric contraction state. The specific method is the Biering‒Sorensen test. The subject was positioned prone, with the end of the body or leg below the upper edge of the iliac crest fixed. The hands were placed on both sides of the body or crossed in front of the chest, and the contralateral shoulder was grasped. The change in surface electromyographic frequency was recorded by lifting the head of the subject by 15 cm and maintaining it in this position for 30 seconds. The test was conducted three times, with a five-minute interval between each trial to minimize the influence of systematic errors. Upon completion of the test, the raw data file was obtained, the median frequency change was analyzed, and the average value of the three test indices was obtained, which represented the value of the index.
Assessment of Lumbar Proprioceptive Function
Lumbar proprioceptive function was evaluated through tracking of the lumbar spine and measuring neuromuscular control of the lumbar spine, employing the Italian Pro-kin Rehabilitation Training and Evaluation System (PK254). The pre- and posttreatment effects on local lumbar proprioception in patients with LDH were analyzed in terms of average trajectory error (ATE) and time. ATE is the mean error rate between the trajectory and the ideal trajectory when the affected limb is placed on an electronic tilt plate and a circular continuous movement is made in all directions. Time is the time consumed by the affected limb to complete the trajectory.
JOA and VAS Score Measurements
The Japanese Orthopedic Association (JOA) score and visual analog scale (VAS) score index are currently internationally accepted objective indicators for evaluating the status of lumbar spine dysfunction and the degree of pain. A lower JOA score indicates a greater degree of lumbar spine dysfunction, whereas a higher VAS score indicates a more pronounced experience of low back pain.
The VAS is a numerical scale comprising 11 digits ranging from 0 to 10, where 0 represents no pain and 10 represents the most severe pain. Patients are instructed to select one of the 11 digits to represent their pain level. A higher score indicates a greater degree of low back pain.
The JOA lower back pain score comprises eight domains: main lower back pain, leg pain and/or tingling sensation, gait, the straight leg raising test (including hamstrings), sensory disturbances, decreased muscle strength (MRC classification), the degree of limitation of daily activities, and bladder function. The score ranges from 0 to 29 points, with lower scores indicating more pronounced lumbar spine function symptoms.
Statistical Methods
Sample Size
This was a prospective study comprising one observation group and two control groups, each with 20 participants. The study was designed according to a randomized controlled protocol.
Data Collection and Missing Data Management
All data from this study will be recorded on a case report form, which will be entered into the computer by two independent researchers at the end of the trial, and all case report forms and data will be placed in a locked tin cupboard, so that uninvolved persons will not have the right to view them, to ensure the security of the data.
During data collection we followed the intention-to-treat (ITT) principle, and retained all subjects after randomisation grouping for analysis, regardless of whether they had completed treatment or had missing data, and analysed them in the original grouping to maintain the randomisation effect, and used the last observation carry-over method (LOCF) for missing data. Detailed records were also kept of the number of missing, temporal distribution, and reasons for shedding, and differences in baseline characteristics were compared between the populations with/without missing.
Statistical Analysis
The results were collated and entered into the computer by an independent statistical analyst. The data were subjected to statistical analysis via the SPSS 20.0 software package. The statistical analyses of the baseline data were conducted via either analysis of variance (ANOVA) with a group design (assuming a normal distribution with homogeneous variance) or a rank-sum test with a group design (in cases where the data did not conform to a normal distribution or homogeneous variance). The statistical analyses of the test outcome indicators were conducted in accordance with the methodology employed for the baseline data. The statistical analysis of within-group count data was conducted via the t test for the league table A rank-sum test for multiple comparisons was applied to the rank data in the between-group analysis, and the results were analyzed via the following methods: mean ± standard deviation (M±SD), with a significance level ofp < 0.05. A p value of 0.05 indicates that the results are statistically significant.
Patient Safety and Quality Control
Prior to the commencement of the trial, all massage therapists and outcome assessors underwent specialized training to ensure consistency. This training encompassed diagnostic criteria for LDH, inclusion and exclusion criteria, manipulation criteria, and evaluation criteria for the CRF form. Any instances of patient dislodgement or rejection were meticulously documented throughout the course of the trial.
The occurrence of adverse events and adverse reactions was systematically documented throughout the treatment course. A statistical comparison was conducted to evaluate the safety of the various treatment regimens in this study, with particular attention given to adverse reactions. Throughout the course of the trial, all the data were recorded and entered into the computer system by independent researchers.
The trial was monitored throughout by the Department of Science and Education of the First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), which is responsible for monitoring the data and has the right to disclose the blinded data and to verify the authenticity of the raw and recorded data to ensure the accuracy and authenticity of the trial.
Discussion
In the case of lumbar disc herniation, the clinical manifestations that are most commonly observed include lower back pain or radicular pain, which may or may not be accompanied by numbness or tingling in the lower extremities.35 An increasing number of individuals are concerned that LDH not only induces pain but may also cause damage to the central nervous system, which could impede recovery and rehabilitation from the condition.36–39 rs-fMRI can be used to assess any functional network of the brain, including those associated with motor, language, visual, and other processes.40–42 Several studies in the literature have demonstrated that structural and functional changes in the brain that are induced by LDH have been shown to occur via rs-fMRI. These alterations include abnormalities in neuronal metabolites,43 cerebral gray matter changes with localized atrophy of the pain matrix and prefrontal cortex (PFC),44,45 damage to the microstructure of the white matter of the corpus callosum and the internal capsule,46,47 and increased activity in relevant pain areas such as the thalamus and cingulate cortex.48 One study revealed that the activity of nerve cells in the prefrontal cortex and in the motor‒sensory cortex was greater in individuals with LDH.49 A significant finding of another study was that the default mode network (DMN) and the pain matrix processing system were diminished in patients with LDH.36 Furthermore, patients with LDH also exhibit impaired excitability in the somatosensory and motor cortex, reduced homogeneity of the default mode network, and dysfunctional connectivity (FC).50 The collective findings of these studies indicate that low back pain is associated with alterations in brain structure.
Nevertheless, it remains unclear how the observed correlation between low back pain in LDH patients and alterations in brain structure is established. This topic warrants further investigation. An increasing number of studies have identified a significant correlation between lumbar proprioceptive deficiencies and functional alterations in the brain in patients with low back pain.51–53 A reduction in tactile and proprioceptive acuity has been observed in the lumbar spine of patients with LDH compared with healthy individuals, which may contribute to the altered motor cortex areas of the brain.54–56 Recent studies have demonstrated that the lumbar cortex undergoes reorganization in patients with LDH, resulting in diminished proprioceptive function and sensory deficits within the lumbar spine. In the lower back of LDH patients, the primary motor cortex (M1) and the secondary somatosensory cortex (S2) exhibited overlapping patterns within the superficial and deep muscles, with a notable anterior shift,57–60 and the primary somatosensory cortex (S1) exhibited an expansion of representations in the lower lumbar region.61,62 The findings revealed that S1 underwent reorganization in the lumbar region of the cortex in response to painful or physical stimuli applied to the muscles.61,62 In contrast, S2 reorganizes following the application of nonpainful pressure, which stimulates different receptors in the musculoskeletal structures of the spine and in the skin.60 The involvement of these sensorimotor brain regions in proprioception was evidenced by stimulation of the muscular spindle, either through muscle movement or passive vibration.63–65 These findings provide a physiological rationale for the efficacy of massage in the treatment of low back pain. This technique induces structural changes in the brain by stimulating the muscles.
Previous research has demonstrated that massage can facilitate the remodeling of endogenous and exogenous stability imbalances in the lumbar spine, thereby correcting spinal joint and paravertebral muscle disorders.66,67 Tui Na, as a traditional Chinese therapy, now combines the traditional Chinese meridian and acupoint theories with modern anatomical theories, which has been found to be efficacious in the reduction of pain, as well as in the alleviation of tension and anxiety in patients with LDH.68 Nevertheless, more recent studies have demonstrated that massage also influences spontaneous brain activity in patients with low back pain. For example, studies have demonstrated that massage enhances functional brain connectivity between the cognitive, emotional and sensorimotor areas of the brain that are involved in the experience of pain.69 A further study revealed that the amplitude of low-frequency fluctuations (ALFF) and the fractional ALFF (fALFF) in the brains of LDH patients exhibited a notable decline following manipulative therapy, as identified through rs-fMRI.70
Leveraged positioning manipulation (LPM) represents a modified iteration of the traditional Chinese Tui Na technique, which has demonstrated notable clinical efficacy in China. Nevertheless, the majority of current clinical reports are based on comparisons between lever-positioning manipulation and healthy population groups, with a paucity of exploration into the differences in efficacy between different therapies and different changes in functional brain areas. The present study sought to elucidate the efficacy of lever-positioning manipulation in the treatment of LDH by comparing it with traditional acupressure techniques. Furthermore, this study aimed to elucidate the mechanism through which the LPM modifies the functional structure of the brain by acting on lumbar proprioceptors and to provide technical guidance for clinical practice. Notably, the present study is subject to several limitations, including a relatively small sample size, a lack of multicenter trials with a large sample size, and the potential for bias in the data. In addition, various aspects such as patients’ expectations, psychological factors, and psychiatric factors may have an impact on the results of fMRI, with potential bias in outcomes. However, we hope that this research project will yield new therapeutic methods and ideas for the treatment of LDH, provide patients with pain relief and improve their quality of life.
Data Sharing Statement
This trial has been audited by the China Clinical Trials Registry, and the experimental data will be made available on the Public Management Platform for Clinical Trials. The latter can be accessed at http://www.medresman.org.cn.
Ethics Approval and Consent to Participate
The studies involving human participants were subject to review and approval by The First Affiliated Hospital of Zhejiang University of Traditional Chinese Medicine Ethics Committee, which approved the study protocol (No. 2023-KLS-242-02). Prior to their participation in this study, written informed consent was obtained from all patients/participants. All methods were conducted in accordance with the relevant guidelines and regulations.
Acknowledgments
The authors of this study would like to express their gratitude to all those who participated in this project, including the patients and researchers.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The research was conducted with the support of the Chinese Medicine Science and Technology Programme Fund of the Chinese Medicine Administration of Zhejiang Province (2023ZF022).
Disclosure
The authors confirm that they have no commercial or financial interests that may constitute a potential conflict of interest.
References
1. Yu P, Mao F, Chen J. et al. Characteristics and mechanisms of resorption in lumbar disc herniation. Arthritis Res Therapy. 2022;24(1):205. doi:10.1186/s13075-022-02894-8
2. Kreiner DS, Hwang SW, Easa JE, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014;14(1):180–191.
3. Fardon DF, Williams AL, Dohring EJ, et al. Lumbar Disc Nomenclature: version 2.0. Spine. 2014;39(24):E1448–E1465. doi:10.1097/BRS.0b013e3182a8866d
4. Deyo RA, Mirza SK. Herniated lumbar intervertebral disk. N Engl J Med. 2016;374(18):1763–1772. doi:10.1056/NEJMcp1512658
5. Taylor VM, Deyo RA, Cherkin DC, et al. Low back pain hospitalization: recent United States trends and regional variations. Spine. 1994;19(11):1207–1212. doi:10.1097/00007632-199405310-00002
6. Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–585. doi:10.1016/S0140-6736(99)01312-4
7. Kuai S, Liu W, Ji R, et al. The effect of lumbar disc herniation on spine loading characteristics during trunk flexion and two types of picking up activities. J Healthcare Engine. 2017;2017:1–10. doi:10.1155/2017/6294503
8. Arts MP, Kuršumović A, Miller LE, et al. Comparison of treatments for lumbar disc herniation: systematic review with network meta-analysis. Medicine. 2019;98(7):e14410. doi:10.1097/MD.0000000000014410
9. Zhang B, Xu H, Wang J, Liu B, Sun G. A narrative review of non-operative treatment, especially traditional Chinese medicine therapy, for lumbar intervertebral disc herniation. Biosci Trends. 2017;11(4):406–417. doi:10.5582/bst.2017.01199
10. Fjeld OR, Grøvle L, Helgeland J, et al. Complications, reoperations, readmissions, and length of hospital stay in 34 639 surgical cases of lumbar disc herniation. Bone Joint J. 2019;101-B(4):470–477. doi:10.1302/0301-620X.101B4.BJJ-2018-1184.R1
11. Kim CH, Chung CK, Choi Y, et al. The long-term reoperation rate following surgery for lumbar herniated intervertebral disc disease: a nationwide sample cohort study with a 10-year follow-up. Spine. 2019;44(19):1382–1389. doi:10.1097/BRS.0000000000003065
12. Gugliotta M, Da Costa BR, Dabis E, et al. Surgical versus conservative treatment for lumbar disc herniation: a prospective cohort study. BMJ Open. 2016;6(12):e012938. doi:10.1136/bmjopen-2016-012938
13. Gadjradj PS, Arts MP, Van Tulder MW, et al. Management of symptomatic lumbar disk herniation: an international perspective. Spine. 2017;42(23):1826–1834. doi:10.1097/BRS.0000000000002294
14. Wan ZY, Shan H, Liu TF, et al. Emerging issues questioning the current treatment strategies for lumbar disc herniation. Front Surg. 2022;9:814531. doi:10.3389/fsurg.2022.814531
15. UK BEAM Trial Team. United Kingdom back pain exercise and manipulation (UK BEAM) randomised trial: effectiveness of physical treatments for back pain in primary care. BMJ. 2004;329(7479):1377. doi:10.1136/bmj.38282.669225.AE
16. Wang CA, Zhao HF, Ju J, et al. Reabsorption of intervertebral disc prolapse after conservative treatment with traditional Chinese medicine: a case report. World J Clin Cases. 2023;11(10):2308–2314. doi:10.12998/wjcc.v11.i10.2308
17. Zhou X, Kong L, Ren J, et al. Effect of traditional Chinese exercise combined with massage on pain and disability in patients with lumbar disc herniation: a multi-center, randomized, controlled, assessor-blinded clinical trial. Front Neurol. 2022;13:952346.
18. De Zoete A, Rubinstein SM, De Boer MR, et al. The effect of spinal manipulative therapy on pain relief and function in patients with chronic low back pain: an individual participant data meta-analysis. Physiotherapy. 2021;112:121–134. doi:10.1016/j.physio.2021.03.006
19. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American college of physicians. Ann Internal Med. 2017;166(7):514. doi:10.7326/M16-2367
20. Zhou X, Huang Y, Wu S, et al. Lever positioning manipulation alters real-time brain activity in patients with lumbar disc herniation: an amplitude of low-frequency fluctuation and regional homogeneity study. Psychiatry Res Neuroim. 2023;334:111674. doi:10.1016/j.pscychresns.2023.111674
21. Lyu LJ, Mao LY, Li JH. Lever positioning manipulation combined with pulsed electric field on the analgesic effect of patients with lumbar disc herniation and its influence on IL-1β and TNF-α. Zhongguo Gu Shang. 2021.
22. Smith SM, Beckmann CF, Andersson J, et al. Resting-state fMRI in the Human Connectome Project. NeuroImage. 2013;80:144–168. doi:10.1016/j.neuroimage.2013.05.039
23. Borsook D, Edwards R, Elman I, et al. Pain and analgesia: the value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi:10.1016/j.pneurobio.2013.02.003
24. Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain. 2013;154(Supplement 1):S29–S43. doi:10.1016/j.pain.2013.09.001
25. Liao X, Mao C, Wang Y, et al. Brain gray matter alterations in Chinese patients with chronic knee osteoarthritis pain based on voxel-based morphometry. Medicine. 2018;97(12):e0145. doi:10.1097/MD.0000000000010145
26. Kristjansson E, Treleaven J. Sensorimotor function and dizziness in neck pain: implications for assessment and management. J Orthop Sports Phys Ther. 2009;39(5):364–377. doi:10.2519/jospt.2009.2834
27. Gao Z, Yang Y, Feng Z, et al. Chemogenetic stimulation of proprioceptors remodels lumbar interneuron excitability and promotes motor recovery after SCI. Mol Ther. 2021;29(8):2483–2498. doi:10.1016/j.ymthe.2021.04.023
28. Ianuzzi A, Little JS, Chiu JB, et al. Human lumbar facet joint capsule strains: i. During physiological motions. Spine J. 2004;4(2):141–152. doi:10.1016/j.spinee.2003.07.008
29. Apkarian AV, Sosa Y, Sonty S. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. doi:10.1523/JNEUROSCI.2541-04.2004
30. Schmidt-Wilcke T, Leinisch E, Ganßbauer S, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125(1):89–97. doi:10.1016/j.pain.2006.05.004
31. Baliki M N, Chialvo D R, Geha P Y, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26(47):12165–12173. doi:10.1523/JNEUROSCI.3576-06.2006
32. Kandel ER. Biology and the future of psychoanalysis: a new intellectual framework for psychiatry revisited. Am J Psych. 1999;4(4):505–524. doi:10.1176/ajp.156.4.505
33. Chan A-W, Tetzlaff J M, Altman D G, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Internal Med. 2013;158(3):200. doi:10.7326/0003-4819-158-3-201302050-00583
34. Zhang R, Mo Z, Li D, et al. Biomechanical comparison of lumbar fixed-point oblique pulling manipulation and traditional oblique pulling manipulation in treating lumbar intervertebral disk protrusion. J Manipul Physiolog Therape. 2020;43(5):446–456. doi:10.1016/j.jmpt.2019.10.004
35. Zhang Y, Guo T, Guo X, et al. Clinical diagnosis for discogenic low back pain[J]. Int J Bio Sci;2009. 647–658. doi:10.7150/ijbs.5.647
36. Zhou F, Gu L, Hong S, et al. Altered low-frequency oscillation amplitude of resting state-fMRI in patients with discogenic low-back and leg pain. J Pain Res. 2018;11:165–176. doi:10.2147/JPR.S151562
37. Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014;17(2):192–200. doi:10.1038/nn.3628
38. Butler S. Important new insight in pain and pain treatment induced changes in functional connectivity between the pain matrix and the salience, central executive, and sensorimotor networks. Scandinavian J Pai. 2017;16(1):64–65. doi:10.1016/j.sjpain.2017.02.005
39. Chan AYP, Ford JJ, Surkitt LD, et al. Individualised functional restoration plus guideline-based advice vs advice alone for non-reducible discogenic low back pain: a randomised controlled trial. Physiotherapy. 2017;103(2):121–130. doi:10.1016/j.physio.2016.08.001
40. Sbardella E, Petsas N, Tona F, et al. Resting-state fMRI in MS: general concepts and brief overview of its application. Biomed Res Int. 2015;2015:1–8. doi:10.1155/2015/212693
41. Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. Am J Neuroradiol. 2013;34(10):1866–1872. doi:10.3174/ajnr.A3263
42. Smitha K, Akhil Raja K, Arun K, et al. Resting state fMRI: a review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017;30(4):305–317. doi:10.1177/1971400917697342
43. Cirstea CM. Primary somatosensory cortex in chronic low back pain – a 1H-MRS study. J Pain Res. 2011;143. doi:10.2147/JPR.S19297
44. Fritz H-C, McAuley JH, Wittfeld K, et al. Chronic back pain is associated with decreased prefrontal and anterior insular gray matter: results from a population-based cohort study. J Pain. 2016;17(1):111–118. doi:10.1016/j.jpain.2015.10.003
45. Cauda F, Palermo S, Costa T, et al. Gray Matter Alterations in Chronic Pain: A Network-Oriented Meta-Analytic Approach. Vol. 4. NeuroImage: Clinical; 2014:676–686.
46. Ung H, Brown JE, Johnson KA, et al. Multivariate classification of structural MRI data detects chronic low back pain. Cereb Cortex. 2014;24(4):1037–1044. doi:10.1093/cercor/bhs378
47. Čeko M, Shir Y, Ouellet JA, et al. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Human Brain Mapp. 2015;36(6):2075–2092. doi:10.1002/hbm.22757
48. Giesecke T, Gracely RH, Grant MAB, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50(2):613–623. doi:10.1002/art.20063
49. Huang S, Wakaizumi K, Wu B, et al. Whole-brain functional network disruption in chronic pain with disk herniation. Pain. 2019;160(12):2829–2840. doi:10.1097/j.pain.0000000000001674
50. Chen XM, Wen Y, Chen S. 等 Traditional Chinese Manual Therapy (Tuina) reshape the function of default mode network in patients with lumbar disc herniation. Front Neurosci. 2023;17:1125677. doi:10.3389/fnins.2023.1125677
51. Goossens N, Rummens S, Janssens L, et al. Association between sensorimotor impairments and functional brain changes in patients with low back pain: a critical review. Am J Phys Med Rehab. 2018;97(3):200–211.
52. Wand B M, James M, Abbaszadeh S, et al. Assessing self-perception in patients with chronic low back pain: development of a back-specific body-perception questionnaire. J Back Musculoskeletal Rehab. 2014;27(4):463–473. doi:10.3233/BMR-140467
53. Wand BM, Catley MJ, Rabey MI, et al. Disrupted self-perception in people with chronic low back pain. further evaluation of the Fremantle Back Awareness Questionnaire. J Pain. 2016;17(9):1001–1012. doi:10.1016/j.jpain.2016.06.003
54. Wand BM, Di Pietro F, George P, et al. Tactile thresholds are preserved yet complex sensory function is impaired over the lumbar spine of chronic non-specific low back pain patients: a preliminary investigation. Physiotherapy. 2010;96(4):317–323. doi:10.1016/j.physio.2010.02.005
55. Luomajoki H, Moseley GL. Tactile acuity and lumbopelvic motor control in patients with back pain and healthy controls. Br J Sports Med. 2011;45(5):437–440. doi:10.1136/bjsm.2009.060731
56. Tong MH, Mousavi SJ, Kiers H, et al. Is there a relationship between lumbar proprioception and low back pain? A systematic review with meta-analysis. Arch Phys Med Rehabil. 2017;98(1):120–136.e2. doi:10.1016/j.apmr.2016.05.016
57. Tsao H, Danneels LA, Hodges PW. ISSLS prize winner: smudging the motor brain in young adults with recurrent low back pain. Spine. 2011;36(21):1721–1727. doi:10.1097/BRS.0b013e31821c4267
58. Elgueta-Cancino E, Schabrun S, Hodges P. Is the organization of the primary motor cortex in low back pain related to pain, movement, and/or sensation? Clin J Pain. 2018;34(3):207–216. doi:10.1097/AJP.0000000000000535
59. Schabrun SM, Elgueta-Cancino EL, Hodges PW. Smudging of the motor cortex is related to the severity of low back pain. Spine. 2017;42(15):1172–1178. doi:10.1097/BRS.0000000000000938
60. Hotz-Boendermaker S, Marcar VL, Meier ML, et al. Reorganization in secondary somatosensory cortex in chronic low back pain patients. Spine. 2016;41(11):E667–E673. doi:10.1097/BRS.0000000000001348
61. Flor H, Braun C, Elbert T, et al. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett. 1997;224(1):5–8. doi:10.1016/S0304-3940(97)13441-3
62. Lloyd D, Findlay G, Roberts N, et al. Differences in low back pain behavior are reflected in the cerebral response to tactile stimulation of the lower back. Spine. 2008;33(12):1372–1377.
63. Radovanovic S, Korotkov A, Ljubisavljevic M, et al. Comparison of brain activity during different types of proprioceptive inputs: a positron emission tomography study. Exp Brain Res. 2002;143(3):276–285. doi:10.1007/s00221-001-0994-4
64. Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92(4):1651–1697. doi:10.1152/physrev.00048.2011
65. Naito E, Nakashima T, Kito T, et al. Human limb‐specific and non‐limb‐specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur J Neurosci. 2007;25(11):3476–3487. doi:10.1111/j.1460-9568.2007.05587.x
66. Wu L, Wan B, Xu M, et al. Massage for protrasion of the lumbar intervertebral disci: a systematic review protocol. Medicine. 2020;99(31):e20614. doi:10.1097/MD.0000000000020614
67. Du H, Liao S, Jiang Z, et al. Biomechanical analysis of press-extension technique on degenerative lumbar with disc herniation and staggered facet joint. Saudi Pharm J. 2016;24(3):305–311. doi:10.1016/j.jsps.2016.04.002
68. Yin Z, Shuaipan Z, He P, Zhang Q, Fang M, Lu P. 等Efficacy of Tuina in chronic low back pain with anxiety: study protocol for a randomised controlled trial. BMJ Open. 2023;13(10):e073671. doi:10.1136/bmjopen-2023-073671
69. Isenburg K, Mawla I, Loggia ML, et al. Increased salience network connectivity following manual therapy is associated with reduced pain in chronic low back pain patients. J Pain. 2021;22(5):545–555. doi:10.1016/j.jpain.2020.11.007
70. Wen Y, Chen X-M, Jin X, et al. A spinal manipulative therapy altered brain activity in patients with lumbar disc herniation: a resting-state functional magnetic resonance imaging study. Front Neurosci. 2022;16(1):974792. doi:10.3389/fnins.2022.974792
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


