Back to Journals » Drug Design, Development and Therapy » Volume 19
Mechanistic Insights into Flavonoid Subclasses as Cardioprotective Agents Against Doxorubicin-Induced Cardiotoxicity: A Comprehensive Review
Authors Shang W, Li XH, Zeng LH, Li Z , Hu Y, Wen HM, Cao FJ, Wan GX
Received 19 April 2025
Accepted for publication 19 June 2025
Published 1 July 2025 Volume 2025:19 Pages 5553—5596
DOI https://doi.org/10.2147/DDDT.S535517
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Wei Shang,1,* Xin-Hui Li,2,* Lang-Hong Zeng,2,* Zhi Li,3 Yu Hu,2 Hui-Min Wen,2 Feng-Jun Cao,2 Guo-Xing Wan2
1Department of Orthopedics, Renmin Hospital, Hubei University of Medicine, Shiyan, 442000, People’s Republic of China; 2Department of Oncology, Renmin Hospital, Hubei University of Medicine, Shiyan, 442000, People’s Republic of China; 3Department of Cardiology, The First Affiliated Hospital of Shantou University Medical College, Shantou, 515041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guo-Xing Wan; Feng-Jun Cao, Department of Oncology, Renmin Hospital, Hubei University of Medicine, 39# Chaoyang Road, Shiyan, Hubei, 442000, People’s Republic of China, Tel/Fax +86 719 8637385, Email [email protected]; [email protected]
Abstract: Doxorubicin (DOX) is an anthracycline chemotherapeutic agent widely used for treating various malignancies due to its remarkable efficacy. However, the dose-limiting cardiotoxicity induced by DOX remains a critical clinical concern with limited therapeutic strategy. Several molecular mechanisms underlying the pathogenesis of doxorubicin-induced cardiotoxicity (DIC) have been proposed, including oxidative stress, dysregulation of Top2β, mitochondrial damage, imbalance of calcium homeostasis, ferroptosis, and inflammatory responses. Increasing studies have posed the promise of the natural products flavonoids against DIC attributed to its advantages in antioxidant activity as well as anti-cancer properties. This paper reviews relevant publications to date and comprehensively summarizes the evidence from preclinical and clinical studies in support of the cardioprotective effect of seven flavonoids subclasses against DIC, including flavones with 18 compounds, flavonols with 11 compounds, isoflavones with 7 compounds, flavanones with 6 compounds, chalcones with 3 compounds, flavanols with 2 compounds and anthocyanins with 2 compounds. Specially, several lines of evidence have also demonstrated the anti-cancer property of flavonoids in addition to the cardioprotective property. This review synthesizes comprehensive mechanistic and translational insights to inform future preclinical and clinical investigations aiming at integrating flavonoid-based interventions into oncotherapeutic regimens. The accumulated evidence underscores flavonoids as promising candidates for DIC as well as adjuvant cancer therapy.
Keywords: doxorubicin, cardiotoxicity, flavonoids, mechanism, cardioprotection
Introduction
Doxorubicin (DOX), a member of the anthracycline family, is one of the most effective chemotherapeutic agents for treating various malignancies, including hematological cancers and solid tumors.1 Despite its therapeutic efficacy, DOX is associated with a dose-dependent cardiotoxicity, which remains a significant clinical challenge.2 Pharmacologically, intravenous DOX is quickly metabolized into the doxorubicinol (DOXol) by utilizing an enzyme NADPH-dependent aldo reductases.3 Although DOX the plasma concentration of DOX falls quickly after administration, DOXol exhibits higher hydrophilicity than DOX, resulting in slower clearance from cardiomyocytes and sustaining higher concentrations within myocardial tissue particularly after repeated injection.4 Both DOX and DOXol have the ability to inhibit DNA biosynthesis, form free radicals and disrupt the function of the ion pump in the sarcoplasmic reticulum of cardiac cells, inducing cell death.5 Consequently, the clinical concern with cardiotoxicity limits the cumulative dose of DOX to 400–700 mg/m² to minimize risks of acute and chronic cardiac damage.6 Acute toxicity manifests within days of administration as arrhythmias, myocarditis, or pericarditis, whereas chronic toxicity, occurring months or years later, may lead to irreversible heart failure.7 The underlying mechanisms of DOX-induced cardiotoxicity (DIC) are multifaceted, involving oxidative stress, mitochondrial dysfunction, calcium homeostasis dysregulation, intracellular iron overload, and DNA damage mediated through topoisomerase IIβ (Top2β). Of these, oxidative stress is a central driver, initiated by DOX’s quinone structure undergoing redox cycling, producing excessive reactive oxygen species (ROS) and reactive nitrogen species (RNS).8 These radicals damage cellular components, including lipids, proteins, and DNA, resulting in cardiomyocyte apoptosis and necrosis. Given these limitations, extensive research has focused on developing cardioprotective strategies to mitigate DIC without compromising its anti-tumor efficacy. Conventional approaches include the use of liposomal DOX, the restriction of cumulative doses, and the co-administration of the iron chelator dexrazoxane, an FDA-approved cardioprotective agent.6,9 However, these strategies have limitations, such as potential interference with DOX’s anti-tumor activity and insufficient protection against cardiac injury.10,11 Consequently, there is a growing interest in exploring compounds with anti-tumor and cytoprotective properties.
Flavonoids, a class of polyphenolic compounds widely distributed in fruits, vegetables, and medicinal plants, have emerged as promising candidates for cardioprotection.12,13 Chemically, flavonoids consist of a fifteen-carbon skeleton arranged as two benzene rings connected by a heterocyclic pyran ring.14 Based on structural differences, flavonoids are categorized into subgroups such as flavones, flavonols, flavanones, and anthocyanins.15 Several studies have demonstrated the pharmacological benefits of flavonoids, including antioxidant, anti-inflammatory, anti-apoptotic, anti-cancer, and iron-chelating activities, which make them particularly suitable for combating DIC.1 Mechanistically, flavonoids exert their cardioprotective effects by scavenging ROS, chelating free iron to prevent Fenton reactions, modulating apoptotic pathways, and attenuating mitochondrial dysfunction.11 For instance, quercetin, luteolin, and rutin have been shown to enhance the expression of Nrf2 and associated antioxidant enzymes, such as superoxide dismutase (SOD) and heme oxygenase 1 (Hmox1), while inhibiting pro-apoptotic proteins like Bax and Caspases.16,17 Additionally, flavonoids stabilize calcium homeostasis to mitigate calcium overload, which is pivotal in preventing cardiac contractile dysfunction.18,19
Despite the promising results from in vitro and in vivo studies, the clinical translation of flavonoids as cardioprotective agents against DOX toxicity remains in its infancy. Challenges such as bioavailability, pharmacokinetics, and potential interactions with chemotherapy necessitate further research. Previous reviews summarized relevant studies with limited data from particular perspective.1,11,16 This review aims to comprehensively evaluate the role of flavonoids in mitigating DIC, focusing on their mechanisms of action, preclinical evidence, and potential for clinical application. By integrating insights from molecular studies and translational research, we aim to highlight flavonoids as valuable candidates for enhancing the safety and efficacy of DOX-based chemotherapy regimens.
Overview of Mechanisms Underlying DIC
As shown in Figure 1, the cardiotoxicity mechanism of DOX is a complex and multifaceted process involving the interplay of various factors, including oxidative stress, dysregulation of Top2β, mitochondrial damage, imbalance of calcium homeostasis, ferroptosis, and inflammatory responses.6 Understanding these mechanisms is crucial for developing effective preventive and therapeutic strategies to mitigate DIC.
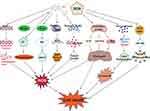 |
Figure 1 Core mechanisms involved in doxorubicin-induced cardiotoxicity. |
ROS Generated by Oxidative Stress
Oxidative stress plays a central role in DIC, encompassing several key aspects.6,8 First, DOX accumulates in mitochondrial compartments of cardiomyocytes, leading to excessive production of ROS, particularly through the redox cycling of complex I in the electron transport chain, which disrupts ATP synthesis.20 Second, DOX generates semiquinone radicals via its quinone moiety, which react with oxygen to produce superoxide anions (O2–), further converting into hydrogen peroxide (H2O2) and other ROS.21 Third, DOX induces the upregulation of nitric oxide synthase (Nos), increasing nitric oxide (NO) levels, which react with superoxide anions to form peroxynitrite (ONOO–), exacerbating oxidative damage.8 Simultaneously, NADPH oxidases (Noxs) are activated, catalyzing the oxidation of DOX’s quinone structure, and serve as a major source of ROS.8,22 Furthermore, DOX significantly depletes endogenous antioxidants such as glutathione (GSH) and catalase (CAT), leading to an imbalance between oxidative and antioxidative systems.23,24 The Nrf2 transcription factor plays a pivotal role in stabilization of DOX-induced oxidative stress by dissociating from kelch-like ECH-associated protein 1 (Keap1) and translocating into the nucleus to activate antioxidant genes, including NAD(P)H dehydrogenase, quinone 1 (Nqo1), Hmox1, and glutathione S-transferase (GST).25 Additionally, DOX induces mitochondrial and ER damage, disrupting calcium homeostasis, and interacts with iron metabolism-related proteins, resulting in iron overload, which further amplifies ROS generation.8,26,27 These interconnected mechanisms collectively lead to oxidative injury, dysfunction, and apoptosis in cardiomyocytes, ultimately contributing to DIC.
Top2β
DNA topoisomerase particularly Top2β has garnered significant attention for its involvement in cardiac damage in DIC. DOX interacts with Top2β to form a stable DNA-Top2β-DOX ternary complex, inhibiting the DNA helicase activity of Top2β, which results in DNA double-strand breaks and subsequent cell death.28 In cardiac tissue, the relatively high expression levels of Top2β render cardiomyocytes especially susceptible to DIC.29 Moreover, DOX-mediated Top2β inhibition may activate stress responses, induce transcriptional alterations, and disrupt signaling pathways involving P53, IGFBP, PDE10A, cAMP/PKA/FoxO3, cGMP/PKG/FoxO3, and PPARγ.4,30–32 These disruptions lead to mitochondrial dysfunction and oxidative stress.20 Notably, studies have demonstrated that cardiomyocyte-specific deletion of the Top2β gene protects mice from DOX-induced progressive heart failure, underscoring the mediating role of Top2β in DIC.33
Mitochondrial Damage
Previous studies have documented that mitochondrial damage is closely associated with DIC, involving several molecular mechanisms.3 First, DOX binds to mitochondrial DNA, inhibiting the activity of respiratory chain complexes I to IV and downregulating the expression of cytochrome c oxidase subunit 5A (Cox5a). These effects lead to mitochondrial dysfunction, increased production of ROS, and subsequent oxidative stress, ultimately resulting in myocardial injury.34,35 Second, DOX’s metabolite, doxorubicinol, accumulates in the heart, disrupting mitochondrial structure, impairing mitochondrial dynamics and autophagy, and preventing the effective clearance of damaged mitochondria, thereby exacerbating mitochondrial injury.4 Additionally, DOX interferes with mitochondrial calcium homeostasis, causing calcium overload, which triggers the opening of mitochondrial permeability transition pores (mPTPs). This results in mitochondrial membrane depolarization, matrix swelling, outer membrane rupture, and the activation of apoptotic signaling molecules such as cytochrome c (Cyt C) and Caspase-3, ultimately inducing cardiomyocyte apoptosis.36,37 Finally, DOX activates pro-apoptotic pathways, including P53 signaling, while suppressing the expression of key regulators of mitochondrial biogenesis and energy production, such as PGC1α and PGC1β. These disruptions affecting mitochondrial energy output further exacerbate cardiac injury.38,39
Calcium Signaling Dysregulation
The molecular mechanisms underlying calcium signaling dysregulation in DIC involve multiple levels of disruption. First, DOX disrupts intracellular calcium dynamics by inhibiting the expression of SERCA2 in cardiomyocytes, thereby impairing sarcoplasmic reticulum (SR)-mediated calcium regulation.40 Second, DOX directly interacts with cardiac RyR2 and SERCA2, altering SR calcium handling through thiol oxidation of these proteins.41,42 Furthermore, DOX activates PARP signal, exacerbating calcium-handling disruptions.43 DOX also influences calcium influx, inhibits SR calcium release, and suppresses Na+/Ca2+ exchange, which collectively affect the duration of action potential and impair diastolic function in cardiomyocytes.44,45 In diastolic dysfunction, DOX-treated cardiomyocytes exhibit impaired buffering of intracellular free Ca2+ ions, underscoring the importance of monitoring diastolic performance for early detection of DIC.46 Additionally, DOX damages intracellular calcium mobilization and buffering during the contraction-relaxation cycle under β-adrenergic receptor stimulation, as well as calcium transient responses.47–49 These calcium signaling abnormalities amplify ROS production, activate apoptotic pathways involving Caspase-3 (CASP3) and Caspase-9 (CASP9), and ultimately result in cardiomyocyte dysfunction.50–53
Ferroptosis
The molecular mechanisms of ferroptosis in DIC involve the interplay of multiple metabolic processes, primarily iron metabolism, GSH metabolism, and lipid metabolism.54 In terms of iron metabolism, DOX promotes iron accumulation in cardiomyocytes by inhibiting iron export proteins, such as ferroportin (FPN), and increasing iron uptake, leading to iron overload. This iron overload generates hydroxyl radicals through the Fenton reaction, triggering lipid peroxidation.55–57 Regarding GSH metabolism, DOX inhibits cysteine uptake and the activity of solute carrier family 7 member 11 (Slc7a11), which disrupts GSH synthesis and transport, thereby inducing ferroptosis.58 DOX also suppresses the activity of glutathione peroxidase 4 (Gpx4), a key enzyme that inhibits ferroptosis by detoxifying lipid peroxides.59 Impairment of Gpx4 activity results in the accumulation of lipid peroxidation products, further promoting ferroptosis.54 In lipid metabolism, DOX upregulates Acsl4 while downregulating Acot1, leading to an increase in polyunsaturated fatty acids (PUFAs), which serve as substrates for lipid peroxidation, thereby exacerbating ferroptosis.54,60 Additionally, DOX may regulate ferroptosis by affecting the iron-regulatory protein (IRP)-iron-responsive element (IRE) system, Nrf2 signaling pathway, and mitochondrial function.61,62 These interconnected molecular mechanisms collectively drive ferroptosis in cardiomyocytes, contributing to DIC.
Inflammation
Inflammatory responses are significantly involved in DIC. DOX treatment increases the production of ROS and RNS, which activate NF-κB signaling pathway and induce the release of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and interleukin 6 (IL-6).21 These cytokines directly contribute to programmed cell death processes in cardiomyocytes, including apoptosis, autophagy, and necrosis.63,64 The inflammatory responses also impair crosstalk between endothelial cells and cardiomyocytes, disrupting vascular endothelial function and promoting atherosclerosis and coronary artery damage.65 Furthermore, DOX-induced oxidative stress activates the Nlrp3 inflammasome, leading to pyroptosis, an inflammatory form of cell death, accompanied by the release of IL-1β and interleukin 18 (IL-18).21,66 This exacerbates inflammation and amplifies cardiac injury, further contributing to DIC.
Potentials of Flavonoids in the Treatment of DIC
Flavonoids are a class of polyphenolic plant secondary metabolites widely found in fruits, vegetables, tea, red wine, and various herbs.14,15 Chemically, flavonoids are based on a fifteen-carbon skeleton consisting of two benzene rings (A and B, as illustrated in Figure 2) connected via a heterocyclic pyran ring (C).12 Based on their chemical structures, flavonoids can be classified into several subgroups (Figure 3), including flavones, flavonols, flavanones, isoflavones, flavanols, chalcones, and anthocyanins.15 Pharmacologically, flavonoids are renowned for their diverse health-promoting and disease-preventing potential, including antioxidant, anti-inflammatory, anti-tumor, cardiovascular, neuroprotective, antibacterial, and antiviral effects.67 In recent years, flavonoids have attracted increasing attention for their role in mitigating DIC. The primary findings are summarized as Table 1.
 |
Table 1 Cardioprotective Effects of Flavonoids Against Doxorubicin-Induced Cardiotoxicity |
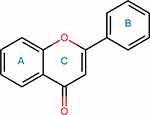 |
Figure 2 Common chemical structure of flavonoids. |
 |
Figure 3 Diagram summarization of flavonoids for the treatment of doxorubicin-induced cardiotoxicity. |
Isoflavones
Daidzein
Daidzein, 7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one is a naturally occurring isoflavone found prominently in soybeans and legumes, exhibits significant therapeutic potential due to its structural similarity to estrogen.161 Acting as a phytoestrogen, daidzein modulates estrogen receptor activity and displays a diverse range of pharmacological effects, including anti-tumor, antioxidant, anti-inflammatory, and cardioprotective properties.162 As documented, daidzein supports cardiovascular health by improving lipid metabolism, reducing oxidative stress, and enhancing endothelial function.163 Previous studies have demonstrated cardioprotective effects of Daidzein against DIC through various molecular mechanisms. Two key studies highlight its potential in mitigating cardiac dysfunction, oxidative stress, apoptosis, and autophagy, using both in vivo and in vitro models. In one study involving C57BL/6J mice and H9c2 cardiomyoblast cells, daidzein significantly improved cardiac function by preserving left ventricular ejection fraction (LVEF) and reducing inflammation, fibrosis, and oxidative damage caused by DOX. Mechanistically, daidzein enhanced mitochondrial function and energy metabolism by upregulating Sirt3 and its downstream target FoxO3.68 This pathway played a crucial role in alleviating oxidative stress and restoring metabolic homeostasis, including glucose, lipid, and ketone body metabolism. These findings suggested that daidzein exerted its cardioprotective effects through metabolic regulation and antioxidant activity. Another study using Sprague-Dawley rats and H9c2 cells further explored daidzein’s anti-apoptotic and anti-autophagic mechanisms. Rats treated with low-dose daidzein showed improved cardiac function and reduced myocardial damage. In vitro, daidzein reduced DOX-induced autophagy and apoptosis, as evidenced by lower levels of Bax, LC3 II, and cleaved Caspase-3, alongside increased Bcl-2 and cyclin D1 expression. These effects were mediated by the inhibition of the PI3K/Akt pathway, as activation of this pathway with an Akt agonist reversed the cardioprotective effects of daidzein.69
Calycosin
Calycosin is a typical phytoestrogen and the major bioactive isoflavonoids in the dry root extract of astragalus membranaceus which is widely used for the treatment of hypertension, nephritis, cancer, diabetes, cirrhosis, and many other disorders in traditional Chinese medicine (TCM).164 Calycosin and its derivatives have multiple biological effects, such as antioxidant, pro-angiogenesis, anti-tumour, antidiabetic, hepatoprotective, neuroprotective, and anti-inflammatory effects.165 For cancer, research has demonstrated its potential in modulating various signaling pathways, such as the inhibition of cell proliferation and the induction of apoptosis in cancer cells.166 Furthermore, calycosin has shown promising cardioprotective effects, including the prevention of myocardial injury and the improvement of heart function under conditions like ischemia/reperfusion injury and heart failure.167 Additionally, its role in regulating oxidative stress and inflammatory responses positions it as a valuable therapeutic agent for conditions associated with chronic inflammation and oxidative damage.166 Calycosin has been studied for its cardioprotective effects against DIC through multiple mechanisms. Two key studies highlighted its potential in mitigating oxidative stress, apoptosis, and autophagy dysregulation, employing both in vivo and in vitro models. In one study, calycosin significantly improved the viability of H9c2 cardiomyoblast cells and reduced DOX-induced apoptosis by modulating the PI3K/Akt signaling pathway and regulating the expression of Bcl-2 and Bax proteins. Additionally, calycosin alleviated oxidative stress in H9c2 cells and mouse models by enhancing the activities of key antioxidant enzymes such as glutathione peroxidase (GPx), CAT, and SOD. It also decreased oxidative damage markers, including malondialdehyde (MDA), lactate dehydrogenase (LDH), and aspartate aminotransferase (AST). Mechanistically, calycosin exerted its effects through the Sirt1-Nlrp3 pathway, as shown by the suppression of Nlrp3 inflammasome activation and Txnip expression. Inhibition of Sirt1 using Ex527 attenuated the protective effects of calycosin, confirming its role in this pathway.70 A second study utilized zebrafish embryo-adult models for rapid pharmacological screening and in vitro analyses to elucidate calycosin’s gene-specific mechanisms on chronic DIC. In adult zebrafish with late-onset DIC, calycosin treatment, initiated 28 days post-DOX injection, restored cardiac function and autophagic activity, which were severely impaired by DOX. Dysregulated autophagy, a key pathological feature of DIC, was ameliorated by calycosin through the activation of autophagy related 7 (Atg7), an E1-like activating enzyme essential for autophagy, suggesting the cardioprotective role of calycosin by modulating autophagy.71
Ononin
Ononin, an isoflavone glycoside found in sources like Ononis soybean, Glycyrrhiza uralensis, red clover, and other herbs, has shown promising medicinal properties.168 Recent studies highlight its anti-inflammatory, antitumor, antidiabetic, antioxidant, antiobesity, antiviral, cardioprotective, and neuroprotective effects.169 Ononin has shown promising cardioprotective effects against DIC by modulating apoptosis and endoplasmic reticulum stress through the Sirt3 pathway. This study employed both in vitro and in vivo models to investigate the underlying mechanisms. In vivo experiments involved DOX-induced cardiomyopathy in Wistar rats, where ononin was administered intragastrically two weeks prior to DOX treatment. Echocardiographic analysis demonstrated that ononin improved cardiac function by increasing LVEF and left ventricular systolic fractional shortening (LVFS). In vitro studies using DOX-treated H9c2 cardiomyoblast cells further corroborated these findings, showing that ononin significantly mitigated DOX-induced apoptosis and ER stress. Mechanistically, ononin reduced the Bax/Bcl-2 ratio and suppressed the expression of endoplasmic reticulum stress markers, including Grp78 and Chop. These effects were linked to the activation of Sirt3, as the use of a Sirt3 inhibitor (3-TYP) attenuated the protective effects of ononin, confirming its dependence on the Sirt3 pathway.72
8-Formylophiopogonanone B
8-Formylophiopogonanone B (8-FOB), a natural isoflavone derived from the root tubers of Ophiopogon japonicus, has demonstrated antitumor, hepatoprotective as well as cardioprotective effects against DIC.73,74,170 In the previous study, Qin et al employed a mouse model of acute cardiotoxicity and bioinformatics analyses to elucidate the mechanisms underlying DOX-induced cardiac damage and the protective role of 8-FOB. The in vivo experiments involved C57BL/6J mice revealed that 8-FOB administration effectively mitigated DOX-induced cardiac injury and dysfunction. These protective effects were closely associated with the downregulation of Hmox1, a hub gene identified through bioinformatics analysis and which acted as a key mediator of DIC. Mechanistically, Hmox1 expression was significantly upregulated in DOX-treated hearts, promoting myocardial inflammation and fibrosis. 8-FOB treatment inhibited Hmox1 expression, thereby reducing these pathological processes and preserving cardiac function.74
Genistein
Genistein (4′,5,7-trihydroxyisoflavone), a key nutraceutical molecule in soybeans, is a phytoestrogen with various pharmacological effects in animal cells. Since firstly isolated from the brooming plant Genista tinctoria, genistein is found widely distributed in the Fabaceae family and exerts estrogen-like functions.171 Preclinical studies have shown genistein’s antioxidant, anti-aging, anti-inflammatory, antitumor, antibacterial, antiviral, neuroprotective and potential benefits for angiogenesis, estrogenic activity, diabetes, and lipid metabolism.172 Genistein has been shown to mitigate DIC through multiple molecular mechanisms. Two studies utilizing both in vivo and in vitro models provide compelling evidence for genistein’s efficacy in reducing oxidative stress, inflammation, apoptosis, and autophagy in DOX-treated hearts. In the first study, male Sprague-Dawley rats pre-treated with genistein demonstrated improved cardiac function and reduced pathological remodeling following DOX administration. Genistein significantly inhibited Erk1/2 phosphorylation while upregulating Stat3 and c-Myc expression. This modulation of the Erk/Stat3/c-Myc pathway contributed to reduced cardiomyocyte apoptosis and autophagy, as evidenced by molecular docking analysis and the use of a Mek1/2 inhibitor (U0126), which mimicked genistein’s effects. Immunohistochemistry and electron microscopy revealed structural preservation in genistein-treated hearts, correlating with improved clinical cardiac function.75 The second study further elucidated genistein’s cardioprotective mechanisms, highlighting its ability to modulate redox and apoptotic pathways. In a mouse model, genistein treatment significantly reduced serum cardiac troponin levels and markers of oxidative stress, including ROS, lipid peroxidation (LPO), and 4-hydroxynonenal-protein adducts (HNE). Genistein also attenuated inflammatory responses by downregulating pro-inflammatory cytokines (TNF-α, IL-6, IL-8). Importantly, genistein activated the Nrf2/Hmox1 antioxidant signaling pathway, enhancing cellular defense mechanisms. Concurrently, it restored survival proteins such as p-Akt and Bcl-2 while suppressing pro-apoptotic markers, including Bax and cleaved Caspase-3.76
Puerarin
Puerarin (Pue) is a C-glucoside of the isoflavone daidzein extracted from Pueraria lobata (Willd). Ohwi, which is well known as Gegen (Chinese name) in TCM.173 Puerarin’s broad range of pharmacological properties, including vasodilation, cardioprotection, neuroprotection, antioxidant, anti-tumor, anti-inflammatory effects, pain relief, bone formation promotion, alcohol intake inhibition, and reduction of insulin resistance, may underlie its diverse medicinal benefits.174 Puerarin has shown significant cardioprotective effects against DIC through its modulation of oxidative stress, mitochondrial function, and autophagy pathways. This study investigated the mechanisms by which Pue pretreatment protects myocardial cells and tissue from DOX-induced damage using both in vivo and in vitro models. In adult mice and H9c2 cardiomyoblast cells, Pue pretreatment enhanced cell viability, reduced LDH activity, and decreased apoptosis levels. Pue also mitigated excessive oxidative stress, preserved mitochondrial function and energy metabolism, and improved overall myocardial function. These effects were associated with the upregulation of 14-3-3γ protein expression and its interaction with PKCε. This interaction facilitated the phosphorylation and translocation of PKCε to mitochondria, which subsequently activated adaptive autophagy, an essential mechanism for cardioprotection. However, the cardioprotective effects of Pue were weaken by the inhibition of 14-3-3γ expression, PKCε activity, or autophagy (using 3-methyladenine), demonstrating the critical role of the 14-3-3γ/PKCε pathway in mediating adaptive autophagy and myocardial protection.77
Irigenin
Irigenin (IR), an isoflavonoid compound derived from the rhizome of Belamcanda chinensis, has shown antioxidative, anti-inflammatory and anti-tumor activity,175 but also has promising cardioprotective effects against DIC through the modulation of apoptosis, oxidative stress, and inflammation.78 This study utilized both in vivo and in vitro models to explore the underlying mechanisms, focusing on the regulatory role of miR-425 and its target, receptor interacting serine/threonine kinase 1 (Ripk1). In DIC models, IR significantly attenuated cardiac fibrosis, dysfunction, and injury. It reduced apoptosis, ROS accumulation, and inflammatory responses in heart tissue and HL-1 cells. Mechanistically, DOX treatment resulted in a substantial decrease in miR-425 levels, which was rescued by IR. miR-425 directly targeted Ripk1, a key mediator of cardiomyocyte injury, and IR effectively suppressed the DOX-induced overexpression of Ripk1 both in vivo and in vitro. Further experiments demonstrated that transfection with a miR-425 mimic inhibited Ripk1 expression, reducing apoptosis, oxidative stress, and inflammation in DOX-exposed cells. Conversely, miR-425 inhibition increased Ripk1 expression and exacerbated cardiomyocyte injury. Importantly, Ripk1 knockdown mirrored the protective effects of miR-425 overexpression, whereas Ripk1 overexpression negated these benefits, underscoring the critical role of the miR-425/Ripk1 axis in DIC.78
Flavones
Baicalin
Baicalin (7-D-glucuronic acid-5,6-dihydroxyflavone) belongs to the natural flavone extracted from the roots of Scutellaria baicalensis, exhibiting anti-inflammatory, antiviral, antitumor, antibacterial, anticonvulsant, antioxidant, hepatoprotective, and neuroprotective properties.79 Involving these pharmacological effects, the plant was widely used for treatment of various diseases including nervous system disorders (Alzheimer’s disease, Parkinson’s disease, and depression), metabolic disorders (obesity-related diseases), intestinal disorders (inflammatory bowel disease and dysbiosis) and cancers in TCM.176 Baicalin (BA) has demonstrated significant cardioprotective effects against DIC by targeting oxidative stress, inflammation, and ferroptosis pathways. Two studies provide insights into its mechanisms of action and therapeutic potential. In the first study, BA was delivered using angiotensin II receptor type 1 (AT1R)-targeted supramolecular nanofibers to selectively inhibit ferroptosis, an iron-dependent form of cell death implicated in DOX-induced cardiomyopathy. In vitro, BA delivery attenuated peroxide accumulation and suppressed ferroptosis in cardiomyocytes. In a murine model, targeted BA delivery achieved superior cardiac accumulation and therapeutic outcomes compared to systemic administration, effectively reducing cardiomyocyte death and preserving myocardial function.80 The second study focused on BA’s anti-inflammatory and antioxidant effects in a DIC model in Swiss albino mice. BA pretreatment significantly prevented DOX-induced elevations in serum cardiac biomarkers, such as cardiac Troponin-I (cTnI) and lactate dehydrogenase, and mitigated histopathological cardiac damage. Mechanistically, BA suppressed toll-like receptor 4 (TLR4) overexpression, subsequently inhibiting NF-κB and IL-1β pathways, which are critical mediators of DOX-induced inflammation. Additionally, BA reversed DOX-induced oxidative stress by reducing MDA and restoring GSH levels. BA also activated the Wnt/β-catenin pathway by suppressing dickkopf WNT signaling pathway inhibitor 1 (Dkk1), further contributing to its cardioprotective effects.81
Baicalein
Baicalein, a natural flavone extracted from the dried roots of Scutellaria baicalensis (S. baicalensis) Georgi (common name: Huangqin in China) which has been widely employed for many centuries in traditional Chinese herbal medicine as popular antibacterial and antiviral agents.177 Following years of research, the pharmacological activities of baicalein were further uncovered, including anti-tumor, antidiabetic, antimicrobial, antiaging, neuroprotective, respiratory protective, gastroprotective, hepatic protective, and renal protective effects.178 Of note, baicalein was found to be a strong free radical scavenger and xanthine oxidase inhibitor, enhancing endothelial function and providing cardiovascular protection against cell damage caused by oxidative stress.177 Baicalein has demonstrated significant cardioprotective effects against DIC by mitigating oxidative stress, apoptosis, and inflammation without compromising DOX’s anti-tumor efficacy. Two studies elucidate its mechanisms of action using both in vitro and in vivo models. In vitro, baicalein significantly reduced DOX-induced cardiomyocyte death in a chick cardiomyocyte model by attenuating ROS generation and preserving mitochondrial membrane potential. Baicalein decreased DNA fragmentation and inhibited the phosphorylation of the pro-apoptotic kinase JNK, a critical mediator of ROS-induced apoptosis. Co-treatment of cardiomyocytes with DOX and JNK inhibitor SP600125 also reduced JNK phosphorylation and enhanced cell survival, demonstrating that its protective effects are mediated via JNK signaling inhibition. Importantly, baicalein did not interfere with DOX’s antiproliferative effects against breast cancer MCF-7 cells, preserving its chemotherapeutic efficacy.82 In vivo, oral administration of baicalein significantly reduced serum markers of cardiac injury in BALB/c mice, including CK-MB, LDH, AST, and ALT, and ameliorated histopathological damage in the heart. Baicalein restored myocardial antioxidant defenses by upregulating Nrf2 and Hmox1 expression, thereby reducing oxidative stress. Additionally, it reversed the Bax/Bcl-2 ratio and suppressed the expression of p53, cleaved Caspase-3, and Parp, preventing apoptosis and DNA damage. Baicalein also inhibited DOX-induced NF-κB activation by suppressing IκBα phosphorylation and nuclear translocation of the p65 subunit, reducing inflammatory signaling. Elevated iNOS and NO levels in DOX-treated mice were significantly decreased by baicalein, further confirming its anti-inflammatory effects.83
Isoorientin
Isoorientin (ISO), a natural tetrahydroxyflavone and C-glycoside flavone found in herbs like Lophatherum gracile and Patrinia scabiosaefolia, exhibits various medicinal effects, including antibacterial, anti-inflammatory, and anti-tumor properties.179 Its strong antioxidant and anti-inflammatory activities have shown promise in addressing metabolic complications such as hyperglycemia, hyperlipidemia, and insulin resistance.180 ISO has also potential cardioprotective effects against DIC while enhancing the chemotherapeutic efficacy of DOX. The dual role of ISO in improving antiproliferation against tumor cell and protecting cardiomyocytes from DOX-induced damage was investigated using both in vitro and in vivo models. In vitro, ISO synergistically enhanced the antiproliferative effects of DOX on various tumor cell lines, including Hela, HepG2, HT-29, and A549 cells. Simultaneously, ISO significantly improved the survival rate of DOX-injured H9c2 cardiomyocytes by reducing ROS, maintaining mitochondrial integrity, and inhibiting apoptosis. These protective effects were further validated in a mouse model of DIC, where ISO improved survival, preserved cardiac function, and reduced myocardial injury, as demonstrated by improved electrocardiogram (ECG) profiles, myocardial enzyme levels, and histopathological analysis. Mechanistically, ISO exerted its dose-dependent cardioprotective effects through the inhibition of the MAPK and Caspase-dependent apoptosis pathways. Proteomics and pharmacological network analyses identified several key targets, including Caspase-3, EGFR, MAPK1, and Stat3. Further analysis revealed that ISO upregulated Nrf2 and TGF-β3 expression by downregulating the phosphorylation of JNK and p38 proteins in the MAPK pathway and suppressing Akt and Stat3 expression. Furthermore, ISO reduced cleaved Caspase-3 levels and increased Bcl-xL expression, confirming its inhibition of apoptosis in DIC.84
Vaccarin
Vaccarin is a kind of natural flavonoid glycoside which belongs to flavones and is found in the seeds of a Chinese herbal Vaccaria hispanica (Mill).181 Vaccarin possesses a multitude of pharmacological activities, including antioxidation, anti-inflammatory, antidiabetic and neuroprotective effects.182 Vaccarin has demonstrated significant cardioprotective effects against DIC by targeting oxidative stress and apoptosis pathways. This study explored vaccarin’s therapeutic potential in both in vivo and in vitro models, revealing its ability to mitigate cardiac dysfunction and cellular damage caused by DOX. In a mouse model, vaccarin effectively ameliorated DOX-induced cardiac dysfunction, reducing oxidative stress and preventing apoptosis. Mechanistically, vaccarin inhibited the activation of the p38 MAPK pathway, a key mediator of ROS-induced myocardial injury. In vitro studies using H9c2 cardiomyoblast cells further supported these findings, showing that vaccarin alleviated mitochondrial membrane depolarization and reduced ROS generation induced by DOX. However, the protective effects of vaccarin were reversed by anisomycin, a p38 MAPK agonist, confirming the role of this pathway in mediating its cardioprotective effects.85
Chrysin
Chrysin (5,7-dihydroxyflavone) which is categorized under the class of flavones occurs naturally in many plants, such as propolis, honey, passion fruit, and even in mushrooms and other plant sources.183 In general, chrysin exhibits many biological activities and pharmacological effects, including antioxidant, anti-inflammatory, anti-tumor, and antiviral activities.184 Chrysin has shown significant cardioprotective effects against chronic DIC in a rat model. The study elucidates the mechanisms underlying its protective effects, highlighting its role in mitigating oxidative stress, apoptosis, and inflammatory signaling while enhancing cardioprotective pathways. Male Sprague-Dawley rats were treated with DOX and/or chrysin for four weeks. Chrysin effectively prevented DOX-induced cardiomyopathy, evidenced by normalization of conduction abnormalities, reductions in serum CK-MB and LDH levels, and attenuation of histopathological cardiac damage. It also ameliorated oxidative stress by decreasing lipid peroxidation and upregulating antioxidant enzymes, restoring the redox balance disrupted by DOX. Mechanistically, chrysin inhibited DOX-induced activation of the p53-dependent apoptotic pathway by downregulating P53, Bax, Puma, Noxa, Cytochrome c, and Caspase-3, while upregulating the anti-apoptotic protein Bcl-2. Furthermore, chrysin suppressed the activation of MAPK, including p38 and JNK, and inhibited NF-κB signaling. These pathways are critical mediators of DOX-induced apoptosis and inflammation. Additionally, chrysin restored the VEGF/Akt pathway, which was suppressed by DOX. By decreasing PTEN expression and increasing VEGF and Akt levels, chrysin enhanced survival signaling in cardiomyocytes. This comprehensive modulation of apoptotic, inflammatory, and oxidative stress pathways underscores chrysin’s cardioprotective efficacy.86
Jaceosidin
Jaceosidin, a flavonoid compound found in several species of Artemisia, has garnered increasing attention for its potential therapeutic effects in cardiovascular diseases.185 Recent studies have highlighted its antioxidant, anti-inflammatory, antitumor and anti-apoptotic properties,186 which may offer protection against various forms of cardiac injury. One of the primary areas of focus has been its role in mitigating DIC. In an acute DIC model, jaceosidin orally administered was found to dose-dependently reduced oxidative stress, inflammation, and cardiomyocyte loss induced by DOX. Jaceosidin effectively inhibited myocardial oxidative damage and attenuated the inflammatory response, thereby preventing myocardial apoptotic death. These effects collectively improved cardiac function in mice exposed to DOX. Mechanistically, jaceosidin’s protective effects were mediated by the activation of Sirt1 signaling pathway. Jaceosidin enhanced Sirt1 activity, which played a crucial role in mitigating oxidative stress and apoptosis. However, in Sirt1-deficient cardiomyocytes and mice, the cardioprotective effects of jaceosidin were abrogated, confirming the essential role of Sirt1 activation in its mechanism of action.87
Chrysoeriol
Chrysoeriol is an active flavone compound derived from the Chinese medicinal herb Lonicerae japonicae flos in the dried flower bud or bloomed flower of Lonicera japonica Thunberg.187 The pharmacological properties including antitumor, anti-inflammatory, antibacterial, antifungal, anti-osteoporosis, anti-insecticide, and neuroprotective actions have been shown in a number of studies, showing its promising potential to prevent or treat diseases including cancer, diabetes, inflammation, osteoporosis, Parkinson’s disease, and cardiovascular diseases.188 Chrysoeriol has demonstrated cardioprotective potential against DIC by mitigating apoptosis and oxidative stress in H9c2 cells without compromising DOX’s antitumor efficacy. The study explored its effects and underlying mechanisms using a series of biochemical and cellular assays. Chrysoeriol significantly reduced DOX-induced apoptosis and cell death in H9c2 cells and LDH release measurements. At a dose of 20 µg/mL, chrysoeriol effectively decreased intracellular ROS levels and MDA concentrations while restoring the activities of critical antioxidant enzymes, such as SOD and GPx, to their normal levels. These findings suggest that chrysoeriol protects cardiomyocytes by neutralizing oxidative stress and enhancing cellular antioxidant defenses. Importantly, further analysis confirmed that the addition of chrysoeriol did not interfere with DOX’s antitumor activity, as demonstrated in HeLa cell models. This indicates that chrysoeriol selectively mitigates the cardiotoxic side effects of DOX without diminishing its chemotherapeutic efficacy.88
Pinocembrin
Pinocembrin (PCB, 5,7-dihydroxyflavone), a flavonoid compound derived from fungi and hive products, mainly honey and propolis, exhibits a wide range of biological activities, including anti-inflammatory, antioxidant, antimicrobial, neuroprotective, cardioprotective and anti-tumor activities.189 Recent studies have highlighted PCB’s cardioprotective effects against various forms of heart damage, including ischemia-reperfusion injury, heart failure and DIC.190,191 In vivo, PCB administration significantly improved cardiac function impaired by DOX, as evidenced by increased LVEF and LVFS, along with reductions in left ventricular internal diameters (LVIDd, LVIDs) and myocardial fibrotic area. PCB also attenuated cardiac injury markers, such as LDH and CK-MB levels, and decreased pro-inflammatory cytokines IL-1β and IL-18, highlighting its anti-inflammatory effects. Mechanistically, PCB was shown to inhibit Nlrp3-mediated pyroptosis and oxidative stress by activating the Nrf2/Sirt3 signaling pathway in DIC. However, inhibition of Nrf2 in H9c2 cells abolished the protective effects of PCB, confirming the critical role of Nrf2/Sirt3 pathway.89
7,8-Dihydroxyflavone
7,8-Dihydroxyflavone (7,8-DHF), a small-molecule agonist of the TrkB receptor, has attracted attention as a therapeutic candidate for diseases involving the BDNF pathway in recent years. While its potential in neurological disorders is well-documented, its role in cardiac diseases remains less understood. In the context of DIC, 7,8-DHF has demonstrated significant cardioprotective effects in both in vivo and in vitro models. Specifically, 7,8-DHF significantly improved cell viability, reduced cell death, and enhanced mitochondrial respiration, membrane potential, and the expression of OPA1 protein in H9c2 cells. In a DIC mouse model, 7,8-DHF improved cardiac function and reduced cardiac injury. Mechanistically, 7,8-DHF restored the expression of Ampk and Stat3 and modulated signaling pathways by activating Akt phosphorylation and reducing Erk activity. The protective effects were abolished by ANA-12, a TrkB antagonist, confirming the involvement of TrkB activation. Furthermore, the regulatory effects of 7,8-DHF on Stat3 and Ampk were dependent on Akt signaling, as they were reversed by an Akt inhibitor.90
Oroxylin A
Oroxylin A (5′7-dihydroxy-6-methoxy-2-phenyl-4H-1-benzopyran-4-one) is a monomethoxy and dihydroxy flavone, and is mainly found in the root-bark O. indicum, S. baicalensis (radix), S. lateriflora, Anchietea pyrifolia, and Aster himalaicus, which are used extensively in Ayurveda and TCM.192,193 A plethora of studies have reported that oroxylin A possesses a broad spectrum of pharmacological functions including anti-bacterial, anti-viral, anti-oxidant, antiinflammatory, antitumor, anti-invasive, neuroprotective, hepatoprotective, and pro-apoptotic properties, which buttresses its promising potential in the treatment of diseases.192,193 In a recent study, oroxylin A has demonstrated protective effects against DOX-induced acute cardiotoxicity, a critical limitation of DOX’s clinical use due to its adverse impact on cardiac function. Oroxylin A was administered to mice pre- and post-DOX exposure, effectively mitigating heart weight loss, cardiac functional decline, and elevations in myocardial injury markers. Mechanistically, oroxylin A alleviated DOX-induced oxidative stress, inflammation, and myocardial apoptosis, both in vivo and in vitro. These protective effects were mediated through activation of the Sirt1 signaling pathway via the cAMP/PKA axis and were abrogated in Sirt1-deficient models.91
Acacetin
Acacetin is a di-hydroxyl and mono-methoxide flavone (4′-methoxy-5,7-dihydroxyflavone), which is abundantly present in various herbs used in TCM, such as snow lotus (Saussurea).194 Literature indicates that acacetin demonstrates a wide range of pharmacological effects, including antitumor, anti-bacterial, anti-viral, antiinflammatory, neuroprotective, cardioprotective, antiobesity and hepatoprotective properties.195 A recent study has demonstrated a significant cardioprotective effect of acacetin against DOX-induced cardiomyopathy in a mouse model, with further mechanistic insights provided using cultured rat cardiomyocytes. In vivo, acacetin effectively mitigated cardiac dysfunction and myocardial fibrosis caused by DOX, largely through the restoration of impaired Nrf2/Hmox1 and Sirt1/Ampk signaling pathways. In vitro studies revealed that DOX-induced reductions in cell viability and increases in ROS production were counteracted by acacetin in a concentration-dependent manner. These effects were mediated by the activation of Sirt1/Ampk signaling and the enhancement of antioxidative (Nrf2/Hmox1, SOD1/SOD2) and anti-apoptotic defenses. Importantly, silencing Sirt1 abolished these protective effects, underscoring the centrality of Sirt1 in the cardioprotective mechanism.92
Dihydromyricetin
Dihydromyricetin (DHM), a 2,3-dihydroflavonol compound, represents the principal bioactive constituent extracted from the tender stems and leaves of the Chinese medicinal plant Ampelopsis grossedentata (A. grossedentata), which exhibits a wide range of biological activities, including anti-alcohol intoxication, anti-inflammatory, antibacterial, antioxidant, and anti-tumor properties, as well as regulatory effects on lipid metabolism and blood glucose levels.196 In recent years, DHM has garnered attention for its cardioprotective effects against DIC. Studies demonstrate that DHM mitigates DOX-induced cardiac injury through multiple mechanisms, offering a promising strategy for enhancing the therapeutic window of DOX without compromising its antitumor efficacy. In vivo experiments with C57BL/6 mice and in vitro studies using AC16 cardiomyocytes revealed that DHM preconditioning alleviated the inhibition of autophagy and excessive apoptosis triggered by DOX. These protective effects were mediated by the activation of the Ampk/mTOR signaling pathway, a crucial regulator of autophagy. DHM restored autophagic flux, reduced intracellular ROS levels, and inhibited oxidative stress, thereby preventing DOX-induced cardiac damage.93 DHM also exerts anti-inflammatory effects by targeting the Nlrp3 inflammasome, a key mediator of DOX-induced cardiac inflammation. In a rat model and H9c2 cell line, DHM inhibited Caspase-1 activity and suppressed the release of pro-inflammatory cytokines IL-1β and IL-18. These effects were closely associated with the upregulation of Sirt1, a protein known for its anti-inflammatory and antioxidative properties. The inhibition of Sirt1 abolished DHM’s cardioprotective effects, underscoring its pivotal role in mediating DHM’s actions.94 In addition to modulating autophagy and inflammation, DHM rescues the expression of anti-apoptotic proteins such as ARC, which are downregulated during DOX-induced myocardial injury. Restoration of ARC expression reduced myocardial cell apoptosis and prevented abnormal electrocardiographic changes. These effects were accompanied by decreases in serum markers of cardiac injury, such as ALT, LDH, and CK-MB, further highlighting DHM’s protective potential. Importantly, DHM preserves DOX’s anti-tumor efficacy while protecting against its cardiotoxicity. Studies on human leukemia U937 cells and xenograft models demonstrated that DHM enhanced DOX’s anti-tumor activity through a p53-dependent mechanism. This dual benefit of DHM protecting cardiac tissue while potentiating anti-tumor effects suggests that it could significantly expand the therapeutic window of DOX.95
Apigenin
Apigenin (4′,5,7,-trihydroxyflavone) is a natural phenolic flavone compound which is present principally as glycosylated in significant amount in vegetables (parsley, celery, onions), fruits (oranges), herbs (chamomile, thyme, oregano, basil), and plant-based beverages (tea, beer, and wine).197 Many studies have verified apigenin’s antiinflammatory, antioxidant, and anti-apoptotic activities, showcasing its therapeutic potential for diverse human diseases, such as cardiometabolic disorders, autoimmune and neurodegenerative diseases, skin inflammatory conditions and even several types of cancers.198,199 Numerous studies have highlighted the cardioprotective effects of apigenin against DIC, primarily through the enhancement of mitochondrial function via modulation of the mitochondrial unfolded protein response (UPRmt). In a murine model, co-administration of apigenin significantly improved cardiac function, attenuated myocardial edema, suppressed inflammatory responses, and upregulated the transcription of UPRmt-related genes, thereby promoting cardiomyocyte survival. In DOX-treated HL-1 cardiomyocytes, apigenin restored ATP production, enhanced mitochondrial antioxidant capacity, and reduced apoptotic cell death. Notably, these protective effects were abrogated upon inhibition of UPRmt, underscoring its critical role in apigenin’s mechanism of action. Mechanistically, apigenin prevented DOX-induced downregulation of Sirt1 and Atf5, key regulators of UPRmt, and its cardioprotective effects were abolished in Sirt1 knockout mice or following Sirt1 knockdown in vitro.96 Additionally, apigenin has been shown to protect against DIC by inhibiting cardiomyocyte pyroptosis through the modulation of GSK-3β signaling. In both a murine model of DIC and DOX-stimulated H9c2 cells, apigenin treatment significantly reduced the expression of pyroptosis-related factors. These effects were associated with increased phosphorylation of GSK-3β and decreased activation of NF-κB p65. The protective effects of apigenin were replicated by treatment with SB216763, a GSK-3β inhibitor, whereas siRNA-mediated knockdown of GSK-3β negated the benefits of apigenin in vitro. By inhibiting GSK-3β, apigenin reduced NF-κB p65 activation, thereby attenuating inflammation and pyroptosis in both cellular and animal models.97 Furthermore, apigenin exerts cardioprotection against DIC by improving cardiac function and mitigating cardiac injury through its anti-fibrotic, antioxidant, and anti-apoptotic properties. In a study involving male Wistar rats, apigenin administration significantly improved cardiac functional parameters, including EF, FS, LVIDs, and LVIDd. Apigenin treatment also markedly reduced serum levels of cardiac and hepatic injury markers, including LDH, CK-MB, cTnI, ALT, and AST. Additionally, apigenin attenuated cardiac fibrosis, decreased the expression of pro-apoptotic proteins (Caspase-3 and Bax), and increased the levels of the anti-apoptotic protein Bcl-2. Moreover, apigenin enhanced antioxidant defenses by significantly elevating SOD activity and reducing MDA levels, further supporting its multifaceted cardioprotective effects.98
Scutellarin
Scutellarin chemically named 4,5,6-trihydroxylflavone-7-O-glucuronoside is a polyphenolic monomer flavone compound widely found in a number of herbs including Scutellaria barbata and Erigeron breviscapus.200 Scutellarin exhibits a wide range of pharmacological properties, including antioxidant, anti-inflammatory, anti-apoptotic, antitumor and vasodilatory effects.201 These multifaceted protective effects render scutellarin a potentially valuable agent in addressing chronic conditions such as cerebrovascular diseases, cardiovascular disorders, neurodegenerative diseases, metabolic syndromes and several types of cancer.202 Previous studies have demonstrated that scutellarin exerts significant protective effects against DIC. In a rat model of DIC, co-administration of scutellarin significantly reduced LDH activity, MDA levels, and cTnT concentrations, while restoring LVEF and LVFS to near-normal levels compared to the DOX-treated group. Histopathological assessments further confirmed a marked reduction in cardiac tissue damage in scutellarin-treated animals. Pharmacokinetic analyses revealed that scutellarin decreased DOX accumulation in cardiac tissues without altering the plasma AUC, suggesting a cardioprotective mechanism mediated by reduced DOX exposure in the heart.99 Additionally, scutellarin has been shown to protect against DIC by targeting oxidative stress, DNA damage, apoptosis, and autophagy through modulation of the Akt/ mTOR signaling pathway. In vitro studies using H9c2 cardiomyocytes, cardiac fibroblasts (CFs), and human umbilical vein endothelial cells (HUVECs) demonstrated that scutellarin pretreatment significantly improved cell viability and attenuated DOX-induced mitochondrial dysfunction and apoptosis. Notably, H9c2 cells exhibited greater sensitivity to DOX compared to CFs and HUVECs. Scutellarin pretreatment dose-dependently reversed oxidative stress and mitochondrial dysfunction, while inhibiting DOX-induced Bax/Bcl-2-mediated apoptosis and autophagy activation. These findings underscore scutellarin’s potential as a cardioprotective agent against DIC, primarily through its antioxidant, anti-apoptotic, and autophagy-modulating properties.100 However, despite its multi-targeted therapeutic potential, scutellarin faces significant challenges, including low bioavailability and a paucity of robust clinical data, which currently limit its broader therapeutic application.
Icariin
Icariin, a principal bioactive flavone constituent derived from Herba Epimedii, demonstrates a broad spectrum of pharmacological properties, including neuroprotective, cardioprotective, antitumor, antioxidative, immunomodulatory, lipid-lowering and reproductive-enhancing effects.203 Clinically, it has been extensively utilized for the management of various pathological conditions, such as osteoporosis, atherosclerosis, asthma, rheumatoid arthritis, diabetes mellitus, Alzheimer’s disease, Parkinson’s disease, and cerebral ischemia.203,204 Pharmacokinetic investigations in rodent models have elucidated the metabolic pathways of icariin, identifying its primary metabolites as icaritin, icariside I, icariside II, and desmethylicaritin.205 Evidence suggests that icariin and its metabolites confer significant cardioprotective benefits through multiple mechanisms, including the amelioration of inflammatory responses and oxidative stress, modulation of cellular proliferation and apoptosis, inhibition of vascular endothelial cell injury and senescence, and facilitation of stem cell differentiation and migration.203 According to the literatures, icariin exerts its pharmacological effects through multiple molecular mechanisms, including the activation of key signaling pathways such as Akt, Ppars, and Sirt1. Additionally, it inhibits NF-κB, MAPK signaling and the subsequent production of pro-inflammatory cytokines. Furthermore, icariin has been shown to suppress PDE5 activity and modulate the hypothalamic-pituitary-adrenal (HPA) axis.206 A previous study found that icariin exerts significant cardioprotective effects against DIC by targeting oxidative stress, mitochondrial dysfunction, and dysregulated autophagy. This is achieved through the modulation of Caveolin-1 expression and inhibition of PDE5a activity. In H9c2 cardiomyocytes, icariin treatment markedly enhanced cell viability, attenuated ROS generation, and inhibited the opening of the mitochondrial permeability transition pore (mPTP). Furthermore, icariin mitigated DOX-induced apoptotic cell death and restored autophagic flux, as evidenced by the downregulation of beclin-1 expression and reduced LC3-II lipidation. These protective effects were accompanied by improved mitochondrial function, decreased Caveolin-1 levels, and specific suppression of PDE5a activity. Collectively, these findings underscore the therapeutic potential of icariin in alleviating DIC, primarily through its antioxidative, mitochondrial-stabilizing, and autophagy-regulating mechanisms.101
Eupatilin
Eupatilin (5,7-dihydroxy-3,4,6-trimethoxyflavone, available as a commercial drug, Stillen®), a phenolic flavone isolated from Artemisia species, exerts anti-inflammatory, anti-tumor, antioxidant, antiallergic, cardioprotective, nephroprotective and neuroprotective activities.207 Eupatilin has been documented to exhibit significant therapeutic potential in the treatment of asthma, hyperlipidemia, hyperuricemia, renal injury, endometrial fibrosis, gastritis, periodontitis, hepatic fibrosis, pulmonary fibrosis, renal cell carcinoma and cervical cancer.208 In the field of cardiovascular disease, eupatilin has demonstrated protective effects against DIC by modulating oxidative stress, inflammation, and apoptosis. In a murine model of DIC, daily administration of eupatilin over a 7-day period significantly improved cardiac function, attenuated oxidative stress, and suppressed inflammatory and apoptotic responses. Mechanistic studies revealed that eupatilin exerts its cardioprotective effects primarily through the activation of the PI3K-Akt signaling pathway. These findings underscore the therapeutic potential of eupatilin as a novel agent for alleviating DIC, with its protective mechanisms centered on the regulation of oxidative stress, inflammation, and apoptosis.102
Luteolin
Luteolin (3′,4′,5,7-tetrahydroxyflavone), a member of the flavone subgroup within flavonoids, is a plant-derived secondary metabolite existing in aglycone or glycosidic forms across traditional herbs, vegetables, and fruits.209 Extensive research has demonstrated that luteolin (Lut) exhibits a wide spectrum of pharmacological activities, including antioxidative, antitumor, anti-inflammatory, antidiabetic, autophagic-regulatory, antimicrobial, cardioprotective, and neuroprotective effects. These activities are mechanistically linked to its modulation of key signaling pathways such as eNOS/Keap1/Nrf2, Ampk/PKC, p38 MAPK/NF-κB, JAK/STAT, Ras/Raf/MEK/Erk, PI3K/Akt, and Wnt/β-catenin.210 In TCM, Lut-rich plants have historically been employed to manage conditions including hypertension, inflammatory disorders, obesity, diabetes, and cancer.209 Lut demonstrates dual therapeutic potential by ameliorating DIC while enhancing its antitumor efficacy. In vitro studies in H9c2 and AC16 cardiomyocytes revealed that Lut attenuated DOX-induced oxidative stress, mitochondrial fission, and apoptosis. Mechanistically, Lut suppressed Drp1 upregulation and Ser616 phosphorylation, thereby preserving mitochondrial integrity. In vivo validation in zebrafish and murine models confirmed that Lut preserved ventricular function and prevented cardiac damage post-DOX exposure. Notably, Lut synergistically enhanced DOX’s antitumor activity in triple-negative breast cancer by inhibiting proliferation, metastasis, and promoting apoptosis, underscoring its role as both a cardioprotectant and chemotherapeutic adjuvant.211 Further studies in a rat model demonstrated that Lut alleviates DIC via activation of the Akt/Bcl-2 signaling pathway. Treatment with Lut restored cardiac function, normalized heart weight, and reduced serum biomarkers of cardiac injury, including brain natriuretic peptide, CK-MB, cTnT, and LDH. Lut mitigated oxidative stress by decreasing MDA levels and enhancing SOD activity. At the molecular level, Lut downregulated pro-apoptotic Bax and Caspase-3 while upregulating anti-apoptotic Bcl-2, of which effects mediated through inhibition of Phlpp1 and subsequent Akt/Bcl-2 pathway activation.103 Luteolin-7-O-glucoside (cynaroside), a glycosylated derivative predominantly found in honeysuckle, exhibits cardioprotective effects by targeting oxidative stress, pyroptosis, and mitochondrial dysfunction. In a murine DIC model, cynaroside improved cardiac function, reduced oxidative damage, and maintained apoptotic homeostasis. In vitro, it modulated pyroptosis-related genes (Nlrp3, Caspase-1, Gsdmd) and enhanced mitochondrial function via activation of the Ampk/Sirt3/Nrf2 axis.104 Additionally, cynaroside mitigates DIC by regulating the Pten/Akt and Erk pathways. In H9c2 cells, cynaroside pretreatment attenuated morphological damage, increased viability, and reduced ROS generation and mitochondrial depolarization. Molecular analyses revealed that cynaroside upregulated phosphorylated Pten while downregulating p-Akt, p-Erk, p-mTOR, and p-GSK-3β, counteracting DOX-induced pro-apoptotic signaling.105 Collectively, Lut and its derivatives exhibit multifaceted cardioprotective effects against DIC through modulation of oxidative stress, apoptosis, mitochondrial dynamics, and critical signaling pathways. These findings highlight their potential as adjuvant therapies to mitigate chemotherapy-associated cardiotoxicity while enhancing oncological efficacy.
Diosmin
Diosmin (3′,5,7-trihydroxy-4′-methoxyflavone-7-rutinoside, DS), a flavone glycoside chemically derived from the oxidation of hesperidin, is predominantly sourced from citrus fruits.212 First isolated from Scrophularia nodosa L. in 1925, DS was introduced as a therapeutic agent in 1969 for managing vascular disorders such as chronic venous insufficiency, hemorrhoids, and varicose veins.213 Extensive preclinical studies have established DS’s diverse pharmacological properties, including anti-inflammatory, antioxidant, anti-tumor, antidiabetic, antihyperlipidemic, cardioprotective, neuroprotective, hepatoprotective, antimicrobial, and antifibrotic effects across various disease models.214 Its therapeutic efficacy is largely attributed to its potent antioxidant activity, which mitigates oxidative stress-mediated cellular damage.215 The previous study has demonstrated the cardioprotective property of DS against DIC without compromising its antitumor efficacy. In vitro studies revealed that DS preserved DOX’s cytotoxic activity against MCF-7 breast cancer cells. In a female Wistar rat model, DS pretreatment significantly attenuated DOX-induced cardiac injury. DOX administration alone induced ECG abnormalities, elevated serum cardiac biomarkers (CK-MB, cTnT, and LDH), increased cardiac MDA and IL-1β levels, and reduced IL-10 and SOD activity. DOX also upregulated pro-apoptotic Bax, TNF-α, and HIF-1α, while downregulating anti-apoptotic Bcl-2 in cardiac tissues, accompanied by severe histopathological damage. In contrast, DS pretreatment normalized ECG parameters, suppressed IL-1β, enhanced IL-10 and SOD activity, and reduced MDA levels. DS also downregulated Bax, TNF-α, and HIF-1α while upregulating Bcl-2, effectively ameliorating DOX-induced histopathological alterations. These findings suggest that DS mitigates DIC through inhibition of inflammatory signaling pathways; however, the precise molecular mechanisms require further elucidation.106
Flavanones
Liquiritigenin
Liquiritigenin (LQG, 4′,7-dihydroxyflavanone) is a major bioactive flavanone ingredient extracted from Glycyrrhizae Radix et Rhizoma (Gan Cao), which is widely used in TCM.216 Holding various pharmacological and biochemical properties, such as neuroprotective, antibacterial, antioxidative, anti-inflammatory, anti-periodontitis, anti-asthmatic, anti-diabetic, anti-osteoporosis, hepatoprotective, nephroprotective, anti-mutagenic and anti-tumor activities, LQG-enriched medicinal plants were widely employed in the treatment of depression, anxiety, Parkinson’s disease, Alzheimer’s disease, stroke, nociception and brain glioma.217 While LQG is widely recognized for its neuropharmacological properties, recent studies have uncovered its cardioprotective potential against DIC. LQG was shown to ameliorate DOX-induced chronic heart failure (CHF) by targeting the Arhgap18/RhoA/Rock1 signaling axis. In both in vitro CHF cell models and in vivo rat models of DIC, LQG significantly improved cardiac function, reduced ROS accumulation, and suppressed cardiomyocyte apoptosis. Mechanistic investigations revealed that DOX treatment upregulated active RhoA expression while downregulating Arhgap18, thereby promoting ROS generation and apoptotic signaling. Overexpression of Arhgap18 attenuated these pathological effects, whereas Arhgap18 knockdown exacerbated them-a phenomenon reversible by RhoA inhibition. LQG mimicked the protective effects of Arhgap18 overexpression in CHF models and counteracted the detrimental consequences of Arhgap18 knockdown. In vivo, LQG administration enhanced left ventricular systolic pressure, reduced left ventricular end-diastolic pressure, and lowered serum levels of LDH and BNP, demonstrating its therapeutic efficacy in mitigating cardiac dysfunction.107 Nevertheless, LQG has low aqueous solubility and lipid solubility resulting the low bioavailability in vivo.218 To optimize its bioavailability and cardioprotective efficacy, a LQG-loaded submicron emulsion (Lq-SE) was developed using high-pressure homogenization and optimized through central composite design response surface methodology (CCD-RSM). Pharmacokinetic studies revealed a 59.5% increase in oral bioavailability compared to free LQG, highlighting the formulation’s enhanced delivery potential. In a murine model of DIC, Lq-SE treatment significantly reduced serum levels of cardiac injury biomarkers and ameliorated histopathological damage in cardiac tissues. Lq-SE attenuated oxidative stress by decreasing ROS levels, enhancing antioxidant enzyme activity, and downregulating NADPH oxidase isoforms Nox4 and Nox2. Furthermore, Lq-SE modulated inflammatory responses through inhibition of the MAPK/ NF-κB signaling pathway and suppressed cardiomyocyte apoptosis. These findings position Lq-SE as a promising therapeutic strategy to mitigate DIC, potentially enabling safer and more effective chemotherapy regimens.108
Naringin
Naringin (5,7-trihydroxyflavonone-7-rhamnoglucoside) commonly presented as naringenin-7-O-rhamnoglucoside comes under the category of flavanone glycoside isolated from grapes and citrus fruits.219 Naringin exhibits a broad spectrum of pharmacological and biological properties, demonstrating efficacy in modulating endogenous mediators to confer multiple physiological benefits. These include potent anti-oxidative, anti-inflammatory, and anti-apoptotic activities. The compound manifests therapeutic potential across diverse pathological conditions, notably neurodegeneration, asthma-induced tissue damage, chemical hepatotoxicity, tardive dyskinesia, and ligament regeneration. Of particular significance, preclinical investigations have consistently revealed naringin’s protective effects in organ-specific injuries, particularly within intestinal, cardiac, and pulmonary systems. Mechanistically, naringin modulates key signaling pathways to suppress the production of pro-inflammatory cytokines, such as Keap1/Nrf-2, RhoA/Rock, Ppar/Stat1, PI3K/Akt, and MAPK/Ampk.220 Naringin has demonstrated cardioprotective efficacy in both in vitro and in vivo models of DIC. Its protective mechanisms are primarily attributed to the mitigation of oxidative stress and preservation of mitochondrial function. In a rat model of DOX-induced cardiac injury, naringin significantly improved cardiac functional parameters by MDA levels, elevating GSH concentrations, and enhancing the activities of antioxidant enzymes, including SOD and CAT. Furthermore, naringin restored the impaired activities of mitochondrial electron transport chain complexes I–IV, which are critical for maintaining cellular energy homeostasis and redox balance.109 To elucidate the molecular basis of naringin’s cardioprotection, studies have focused on its interaction with the p38 MAPK signaling pathway. In H9c2 cardiomyocytes, pretreatment with naringin markedly increased cell viability and attenuated DOX-induced ROS accumulation. These effects were paralleled by the suppression of p38 MAPK phosphorylation, a key mediator of oxidative stress and apoptosis. Notably, the protective outcomes mirrored those observed with SB203580, a selective p38 MAPK inhibitor, confirming the pathway’s central role. These findings collectively establish that naringin alleviates DIC by inhibiting p38 MAPK activation, thereby reducing oxidative damage and preserving mitochondrial integrity.110
Hesperidin
Hesperidin (3′,5,7-trihydroxyflavanone-7-rhamnoglucoside, HES), a prominent member of the flavanone subclass within flavonoids, is predominantly found in citrus fruits of the Rutaceae family, such as oranges, grapefruits, tangerines, limes, and lemons.221 Recognized for its broad-spectrum health-promoting effects encompassing anti-inflammatory, antioxidant, anti-aging, anti-tumor, and antibacterial properties, HES has been extensively investigated for its therapeutic potential in managing type 2 diabetes, cardiovascular diseases, cancer, neurological disorders, and radiation-induced damage.222,223 Furthermore, it demonstrates notable benefits in modulating cutaneous functions under both physiological and pathological conditions.223 HES exhibits significant cardioprotective activity against DIC through its antioxidative, anti-inflammatory, and anti-apoptotic properties. In a Wistar rat model, HES administration attenuated DOX-induced cardiac injury by reducing serum levels of cardiac biomarkers (cTnI, CK-Total, CK-MB, LDH, and AST) and pro-inflammatory cytokines (IFN-γ, IL-1β, and TNF-α). Concurrently, HES enhanced antioxidant defenses by elevating GPx, SOD, and CAT activities. Histopathological analysis revealed that HES alleviated DOX-induced cardiomyocyte necrosis, sarcoplasmic vacuolization, inflammatory infiltration, and tissue disorganization.111 A complementary study in rats demonstrated that HES mitigates DIC by restoring redox homeostasis and NO balance, highlighting its dual regulatory role in oxidative stress and vascular function.112 Despite its therapeutic promise, hesperidin’s clinical utility is constrained by low aqueous solubility and limited bioavailability. To address this, hesperidin-loaded solid lipid nanoparticles (HES-SLNs) were developed, which significantly enhanced cardioprotective efficacy in a rat model of DIC. HES-SLNs improved cardiac biomarker profiles, ameliorated histopathological damage, reduced MDA levels, and upregulated CAT and SOD activities. Additionally, HES-SLNs suppressed Caspase-3 expression, underscoring their ability to attenuate oxidative stress and apoptosis more effectively than free HES.113 Hesperetin, the aglycone metabolite of HES, similarly protects against DIC by targeting oxidative stress and mitochondrial dysfunction. In vivo studies demonstrated that hesperetin reduced MDA levels, restored GSH content, and improved cardiac functional parameters in DOX-exposed rats. In vitro analyses revealed its capacity to mitigate DNA damage, apoptosis, and ROS generation. Mechanistically, hesperetin inhibits NF-κB and p38 MAPK signaling while suppressing Caspase-3 activation, thereby preserving mitochondrial integrity.114
Silibinin
Silibinin (SLB), a natural flavanone, derived from the milk thistle plant (Silybum marianum), was illustrated for several medicinal uses such as anti-tumor, antioxidant, anti-inflammatory, hypocholesterolemic, cardioprotective, neuroprotective, hepatoprotective, antimicrobial, and antidiabetic effects.224 Of note, this promising natural compound has been tested for its cardioprotective activities against doxorubicin DIC. In a DOX-injured human AC16 cardiomyocyte model, SLB attenuated cellular damage by restoring the activity of the IL6st/Jak2/ Stat3 signaling axis and enhancing autophagic flux. Network pharmacology and molecular docking analyses revealed strong binding affinities (≤ −7.0 kcal/mol) between SLB and key pathway components (IL6st, Jak2, and Stat3), suggesting direct molecular interactions. Experimental validation confirmed that SLB reduced mitochondrial ROS accumulation and promoted autophagy, of which effects were abolished upon IL6st, Jak2, or Stat3 knockdown or pharmacological inhibition of autophagy (via 3-methyladenine [3-MA] or beclin1 silencing). These findings indicate that SLB exerts its cardioprotection through dual modulation of the IL6st/Jak2/ Stat3 pathway and autophagy restoration, offering a novel mechanistic strategy to counteract DIC.115 Despite its therapeutic promise, SLB’s clinical translation is hindered by poor aqueous solubility and limited oral bioavailability. To address these limitations, a silibinin-phosphatidylcholine (SLB-PC) complex was developed to enhance solubility and pharmacokinetic profiles.225 While this formulation shows potential for improving drug delivery, its efficacy in mitigating DIC remains unexplored, warranting further preclinical and clinical investigation.
Naringenin
Naringenin (4′,5,7-trihydroxyflavanone, NAR), a flavanone compound and the aglycone of naringin, is abundant in tomatoes, citrus fruits, and grapefruits.226 Despite its limited water solubility and subsequent bioavailability challenges,227 NAR exhibits diverse pharmacological effects, including antidiabetic, anti-tumor, antimicrobial, antiobesity, gastroprotective, immunomodulatory, cardioprotective, nephroprotective, and neuroprotective activities, primarily attributed to its antioxidative and anti-inflammatory properties.228 In a Dalton’s lymphoma ascites (DLA) tumor-bearing mouse model, NAR demonstrated dual functionality by alleviating DOX-induced systemic toxicity while enhancing chemotherapeutic efficacy. DOX treatment induced marked disruptions in hematological parameters, antioxidant enzyme levels (eg, SOD, CAT), and increased lipid peroxidation (MDA) across multiple organs, including the heart, kidney, liver, spleen, and tumor tissues. NAR supplementation restored tissue integrity, reduced oxidative damage, diminished tumor burden, and alleviated hypoxia within the tumor microenvironment, highlighting its potential to improve therapeutic outcomes while minimizing off-target toxicity.116 Additionally, in a rat model of DIC, NAR significantly improved cardiac function by restoring SOD, GPx, and CAT activities while reducing MDA levels. NAR attenuated the DOX-induced upregulation of inflammatory mediators, including TGF-β1, TNF-α, IL-6, and IL-10, and ameliorated histopathological damage such as myocardial necrosis and inflammatory infiltration.117 Moreover, NAR pretreatment in rats normalized DOX-induced alterations in serum LDH and CPK levels, reduced lipid peroxidation, and restored cardiac antioxidant enzyme activities (SOD, GST, CAT). Furthermore, NAR reversed DOX-mediated depletion of reduced GSH and total NO content in cardiac tissues, suggesting its role in balancing redox homeostasis and vascular function.118 Similar to NAR, Naringenin-7-O-glucoside (NARG), is a glycosylated derivative isolated from Dracocephalum rupestre Hance,119 which is capable of protecting against DIC by enhancing endogenous antioxidant defense and preventing apoptosis. In H9c2 cardiomyocytes, NARG pretreatment upregulated expression of Nqo1, Gclm and Gclc, key components of the cellular antioxidant system. Mechanistically, NARG promoted phosphorylation of Erk1/2, facilitating Nrf2 nuclear translocation to activate antioxidant gene expression.120 Furthermore, NARG was demonstrated to exert cardioprotective role by stabilizing membrane integrity and calcium signaling. In DOX-treated H9c2 cells, NARG alleviated morphological damage, enhanced viability, reduced LDH and CK leakage, and suppressed intracellular ROS and Ca²⁺ overload. These effects were associated with increased GPx activity, though the precise molecular mechanisms remain to be elucidated.19
7-Hydroxyflavanone
7-Hydroxyflavanone (7H), a member of the flavanone class, is a naturally occurring compound isolated from plants such as Flourensia oolepis, Virola surinamensis, Zuccagnia punctata, and Empetrum nigrum (black crowberry).229 It exhibits diverse pharmacological properties, including anti-tumor, anthelmintic, antioxidative, and antifungal activities, as well as inhibitory effects on the 20S proteasome and aromatase enzymes.121 Despite its broad bioactivity, no studies to date have explored its relevance to CVDs beyond DIC. A pioneering study revealed that 7H conferred protection against DIC by targeting oxidative stress and apoptosis. In an in vitro model using H9c2 cardiomyocytes exposed to DOX, 7H co-treatment significantly attenuated cardiac damage by enhancing total GSH content and SOD activity, while reducing ROS accumulation, MDA production, IL-6 secretion, and Caspase-3/7 activity. Furthermore, 7H restored mitochondrial bioenergetics, preserved mitochondrial membrane potential, and upregulated the expression of Pgc-1α, a master regulator of mitochondrial biogenesis. These effects were mechanistically linked to the activation of Ampk, a critical sensor of cellular energy status.121 These findings suggest that 7H may serve as a promising therapeutic candidate for mitigating DIC through its dual antioxidative and mitochondrial protective mechanisms. Future studies should investigate its broader applicability in CVDs and validate its efficacy in preclinical models to advance translational potential.
Chalcone
Licochalcone A
Licochalcone A (Lico A), a bioactive chalcone derivative (3-dimethylallyl-4,4′-dihydroxy-6-methoxychalcone), is isolated from the roots of Glycyrrhiza species (licorice), a cornerstone herb in TCM with historical applications in treating microbial infections, inflammatory disorders, and cancer.230,231 As a principal constituent of licorice, Lico A exhibits a broad pharmacological spectrum, including anti-tumor, anti-inflammatory, antioxidant, antimicrobial, antidiabetic, neuroprotective, and cardioprotective activities. These effects are mediated through modulation of critical signaling pathways such as PI3K/Akt/mTOR, p53, NF-κB, and p38 MAPK, alongside interactions with targets including TNF-α, VEGF, Fas/FasL, and Caspases.232 Lico A demonstrates protective efficacy against DIC by targeting oxidative stress and ferroptosis. In a murine DIC model, Lico A administration improved serum cardiac biomarkers, restored myocardial histoarchitecture, and normalized electrocardiographic abnormalities. In vitro, Lico A enhanced viability in DOX-injured H9c2 cardiomyocytes, reduced ROS, MDA, and ferrous iron levels, and elevated the GSH/glutathione disulfide (GSSG) ratio, indicative of restored redox homeostasis. Mechanistically, Lico A activated the PI3K/Akt/Mdm2 axis, suppressing p53 accumulation while upregulating ferroptosis-related proteins Slc7a11 and Gpx4. Crucially, PI3K/Akt pathway inhibition or p53 overexpression abolished these protective effects, confirming the pathway’s centrality.122 Complementary studies employing network pharmacology and ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS/MS) identified Lico A as a key licorice component mitigating DIC. In DOX-treated H9c2 cells, Lico A increased cell viability, SOD activity, and mitochondrial membrane potential while reducing MDA and ROS levels, further validating its antioxidative and mitochondrial protective roles.123
Aspalathin
Aspalathin (ASP), a dihydrochalcone C-glucoside exclusive to Aspalathus linearis (commonly known as rooibos), is a polyphenolic chalcone compound with well-documented biological activities, including antioxidant, anti-inflammatory, hypoglycemic, mitochondrial protective, and anti-apoptotic properties.233 Notably, ASP exhibits therapeutic potential for metabolic syndrome management, particularly type 2 diabetes (T2D) and its complications, through modulation of glucose/lipid metabolism and activation of critical signaling pathways such as p53, PI3K/Akt, Ampk, and mTOR.234 While substantial evidence supports its protective effects against hyperglycemia-induced oxidative damage, ischemia/reperfusion injury, and cardiac lipid toxicity,235–237 research on ASP’s cardioprotective efficacy against DIC remains limited. Recent studies, however, highlight ASP’s ability to mitigate DIC via dual regulation of oxidative stress and apoptosis. In H9c2 cardiomyoblasts, ASP counteracted DOX-induced oxidative damage by upregulating antioxidant enzymes (SOD, CAT, GSH) while suppressing ROS accumulation, lipid peroxidation (MDA), and apoptotic signaling.124 Mechanistically, ASP exerts cytoprotection in a p53-dependent manner by enhancing the Bcl-2/Bax ratio and attenuating apoptosis. Intriguingly, this effect coincides with Ampk/Foxo1-mediated activation of autophagy-related genes (Atgs) and subsequent p62 degradation, suggesting a synergistic interplay between apoptosis inhibition and autophagy induction.125 Importantly, co-administration of ASP with DOX preserved the latter’s antitumor efficacy in Caov-3 ovarian cancer cells, underscoring its clinical translatability.125 Taken together, these findings provide a credible evidence by which ASP co-treatment could protect against DIC without comprising its chemotherapeutic outcomes.
Cardamonin
Structurally identified as a 2′,4′-dihydroxy-6′-methoxychalcone, cardamonin (CAR), a member of the chalcone family, is a natural organic compound predominantly found in high concentrations within the seeds of Alpinia katsumadai.238 CAR exhibits a broad spectrum of pharmacological effects, such as antinociceptive, anti-inflammatory, antioxidant, cytotoxic, antiprotozoal, antiulcer, antihistaminic, and antitumor activities. Notably, CAR has shown protective efficacy against cisplatin-induced nephrotoxicity and has been implicated in modulating redox-sensitive pathways, including the inhibition of NF-κB and Wnt activation and cytokine production.126,238 Furthermore, due to its antioxidant, anti-inflammatory, and neuroprotective properties, CAR has been reported to mitigate the detrimental effects of oxidative stress and neuroinflammation, highlighting its potential therapeutic significance.238 Consistently, the cardioprotective effects of CAR against DIC has been revealed by a recent study. Through both in vitro and in vivo models, CAR has been demonstrated to effectively attenuate oxidative stress, apoptosis, and inflammatory responses, which are key pathological features of DOX-induced cardiomyopathy. In DOX-treated mouse cardiomyocytes, CAR was shown to significantly activate the Nrf2 signaling pathway while inhibiting its degradation, thereby bolstering the cellular antioxidant defense system. This activation led to the upregulation of critical antioxidant enzymes, including Hmox1, Nqo1, Gclm, SOD, GSH, and CAT. Concurrently, CAR suppressed the generation of ROS and MDA, both of which are established biomarkers of oxidative stress. Additionally, CAR was found to inhibit DOX-induced cardiomyocyte apoptosis by modulating the Caspase-3 pathway and to attenuate inflammatory responses through the downregulation of NF-κB signaling. In a murine model of DOX-induced cardiomyopathy, CAR administration significantly improved cardiac function by mitigating oxidative damage, apoptosis, and inflammation, of which effects were mechanistically linked to its potent activation of the Nrf2 signaling pathway. These findings underscore the therapeutic potential of CAR in addressing DIC.127
Flavonols
Galangin
Galangin (3,5,7-trihydroxyflavone, Gal), a bioactive flavonol derived from Alpinia officinarum Hance (Zingiberaceae),239 exhibits a broad spectrum of pharmacological properties, including antioxidant, anti-inflammatory, anti-tumor, antimicrobial, hepatoprotective, cardioprotective, neuroprotective, and metabolic regulatory activities.240–242 Preclinical studies demonstrate its therapeutic efficacy in diverse pathological conditions such as neurodegenerative disorders, cardiovascular/cerebrovascular diseases, diabetes, hepatic injury, asthma, and inflammatory arthritis.240–242 Mechanistically, Gal modulates key signaling pathways including p38 MAPK, NF-κB, PI3K/Akt, Sirt1, Trpv1, Nrf2, and Nlrp3 to counteract oxidative stress, inflammation, and cellular apoptosis.243 Emerging evidence highlights Gal’s potential in mitigating doxorubicin DIC through dual targeting of oxidative stress and ferroptosis. In murine models, Gal co-administration ameliorated DOX-induced cardiac dysfunction, attenuated myocardial histopathological damage, and normalized biomarkers of oxidative injury, including reduced ROS, MDA, and NADPH oxidase activity, while restoring SOD levels.128 Concurrently, Gal suppressed pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and activated the Nrf2/HO-1 axis, as evidenced by enhanced nuclear translocation of Nrf2 and upregulated Hmox1 expression. The critical role of Nrf2 was confirmed via ML385 (Nrf2 inhibitor), which abolished Gal’s cardioprotective effects.128 Furthermore, Gal mitigates ferroptosis, a lipid peroxidation-driven cell death, by rescuing Gpx4, Slc7a11, and FPN expression in H9c2 cardiomyocytes, while reducing iron accumulation and Ptgs2 levels.129 Mechanistic studies reveal that Gal upregulates Gstp1, facilitating its interaction with JNK to inhibit the JNK/c-Jun pathway, thereby attenuating ferroptotic cell death.129 Galangin represents a promising natural cardioprotectant against DIC, synergistically targeting oxidative stress, inflammation, and ferroptosis via Nrf2/Hmox1 and Gstp1/JNK pathways.
Morin
Morin (3,5,7,2′,4′-pentahydroxyflavone) is a natural flavonol predominantly extracted from the fruits, stems, and leaves of plants belonging to the Moraceae family, has been extensively studied for its therapeutic potential.244 Specifically, it demonstrates free radical scavenging, antioxidant, anti-inflammatory, anti-tumor, antimicrobial, antidiabetic, anti-arthritis, cardioprotective, neuroprotective, nephroprotective, and hepatoprotective properties. These multifaceted pharmacological activities are mediated through the modulation of key cellular signaling pathways, including NF-κB, MAPK, JAKs/STATs, Keap1/Nrf2, ER stress, mitochondrial-mediated apoptosis, Wnt/β-catenin, and the mTOR pathways. Accordingly, accumulating evidence indicates that morin exhibits a wide range of beneficial effects against numerous chronic and degenerative diseases.245 Recently, morin has been demonstrated to exert protective effects against DIC and neurotoxicity by attenuating oxidative stress, inflammation, and apoptosis. In a rat model, morin administration significantly improved cardiac function, as evidenced by reduced serum levels of cardiac biomarkers, including LDH, CK-MB, and cTnI, alongside amelioration of histopathological damage in cardiac tissues. Morin enhanced the endogenous antioxidant defense system by elevating GSH levels and increasing the activities of key antioxidant enzymes, such as SOD, CAT, and GPx, while concurrently reducing MDA levels, a marker of lipid peroxidation. Furthermore, morin suppressed DOX-induced inflammatory responses in both cardiac and brain tissues by downregulating the expression of pro-inflammatory mediators, including TNF-α, IL-1β, and NF-κB. The compound also exhibited anti-apoptotic effects by upregulating the expression of Bcl-2 and inhibiting Caspase-3 activation. In brain tissues, morin improved neural signaling through the modulation of AChE activity and reduced the levels of glial fibrillary acidic protein (GFAP), indicative of its neuroprotective potential. Collectively, these findings underscore morin’s dual cardioprotective and neuroprotective properties against DOX-induced toxicity, mediated via its antioxidant, anti-inflammatory, and anti-apoptotic mechanisms.130
Myricitrin
Myricitrin (myricetin-3-O-α-rhamnoside), a naturally occurring flavonol glycoside predominantly isolated from Myrica rubra and other dietary plants, has attracted substantial scientific interest due to its multifaceted pharmacological properties.246 Beyond its established application as a flavor modifier in food and beverages, this compound exhibits a remarkable spectrum of bioactivities encompassing antioxidant, anti-inflammatory, antinociceptive, anti-atherosclerotic, hepatoprotective, and anti-fibrotic effects.247 Emerging evidence from both in vitro and in vivo studies particularly highlights its therapeutic potential in cardiovascular pathologies through multimodal mechanisms. Experimental models have elucidated myricitrin’s cardioprotective efficacy against DOX-induced myocardial injury. In DOX-challenged rats, myricitrin administration significantly attenuated myocardial damage, as quantified by improved LVEF, reduced serum CK-MB, and histopathological amelioration of myocardial architecture. At the cellular level, myricitrin demonstrated cytoprotection against DOX-induced cardiomyocyte apoptosis through dual modulation of oxidative homeostasis and apoptotic signaling. These effects were associated with enhanced SOD activities, stabilized MMP, and regulation of apoptosis-related markers. Mechanistic studies reveal its regulation of stress-responsive pathways, particularly through inhibition of the Erk/p53 signaling cascade, thereby preventing mitochondrial dysfunction-mediated apoptosis.131 Given its recognized safety profile by international regulatory bodies and demonstrated efficacy across experimental models, myricitrin presents as a promising phytochemical candidate for developing nutraceutical interventions targeting oxidative stress-associated pathologies.
Quercetin
Quercetin (3,5,7,3′,4′-pentahydroxyflavone), a prominent phytochemical within the flavonol subclass of flavonoid polyphenols, is ubiquitously distributed in fruits, vegetables, beverages, flowers, leaves, and seeds, with onions representing its richest dietary source.248 As a multifunctional flavonoid, quercetin exhibits a broad spectrum of pharmacological properties, including antihypertensive, antihyperlipidemic, antihyperglycemic, antioxidant, antiviral, anti-tumor, anti-inflammatory, antimicrobial, neuroprotective, and cardioprotective effects.249 Extensive in vivo and in vitro studies have elucidated its therapeutic potential in addressing neurodegeneration, diabetes, cancer, and inflammation, solidifying its current utilization in diverse pharmaceutical formulations.250 Of particular significance is quercetin’s protective role against DIC, mediated through precise modulation of molecular and signaling pathways. Mechanistically, quercetin attenuates oxidative stress by upregulating critical antioxidant enzymes such as SOD, CAT, and GPx, while significantly reducing ROS and lipid peroxidation markers like MDA.132,133 Concurrently, it enhances mitochondrial function by restoring MMP, improving the GSH/GSSG ratio, and elevating expression of mitochondrial protective proteins, including Bmi-1 and 14-3-3γ.132,134 In apoptotic regulation, quercetin suppresses pro-apoptotic factors such as p53, Bid, and Nox1, while upregulating anti-apoptotic Bcl-2 and modulating Caspase-3 activation to mitigate myocardial apoptosis.135 It further orchestrates cellular energy homeostasis via activation of the Ampk pathway, enhancing downstream effectors Pparα and Pgc-1α, thereby promoting energy metabolism and reducing oxidative myocardial injury.136 The Akt kinase pathway, integral to anti-apoptotic signaling and ischemic tolerance, is also activated by quercetin.137 Quercetin’s anti-inflammatory action is achieved through inhibition of pro-inflammatory mediators, including TNF-α and iNOS, coupled with reduction of NO production.138 In DIC models, it attenuates oxidative damage and apoptosis by suppressing the SOD/p53 signaling axis.139 Synergistic strategies reveal that quercetin enhances the cardioprotective efficacy of agents such as losartan and resveratrol through cooperative pathway modulation, without compromising DOX’s anti-tumor activity.140,141 Advanced delivery systems, including liposomal formulations and polymeric micelles, have been engineered to improve its bioavailability and therapeutic precision via sustained release and targeted delivery.251 Collectively, these molecular insights underscore quercetin’s capacity to counteract the multifactorial mechanisms underlying chemotherapy-induced cardiotoxicity, positioning it as a promising adjuvant in clinical oncology. Further research is warranted to optimize its pharmacokinetic profile and validate its translational potential in combinatorial cancer therapies.
Fisetin
Fisetin (3,3′,4′,7-tetrahydroxyflavone), a flavonol subgroup member within the flavonoid class, occurs naturally in fruits and vegetables including apples, persimmons, grapes, cucumbers, and onions at concentrations ranging from 0.1 to 160 μg/g.252 Strawberries represent the richest dietary source.253 Fisetin demonstrates pleiotropic pharmacological activities with therapeutic potential across multiple disease domains, such as anti-tumor (PI3K/Akt/mTOR and Wnt/β-catenin, Trail and VEGF signal), anti-inflammatory (NF-κB and NO signal in hepatic ischemia/reperfusion), antioxidant (Nrf2 signal), neuroprotective, osteoprotective activity (Pten and mTORC2 signal).254 Of particular pharmacological significance, fisetin has been demonstrated to attenuate DIC through coordinated modulation of oxidative stress, inflammatory responses, and apoptotic pathways. In H9c2 cardiomyoblasts, fisetin was shown to reduce DOX-induced cell death in a dose-dependent manner, primarily through inhibition of the IGF-II receptor (IGF-IIR)-dependent apoptotic pathway via estrogen receptor (ER)-α/-β activation. In vivo studies using rat models revealed that fisetin administration improved cardiac functional parameters, decreased serum levels of cardiac injury markers (CK-MB, LDH, AST, ALT, ALP), and enhanced antioxidant defense mechanisms through elevated SOD activity and GSH levels, accompanied by reduced MDA and NO concentrations.142 Mechanistic investigations further demonstrated fisetin-mediated suppression of pro-inflammatory mediators (COX-II, TNF-α, IL-1β) and apoptotic markers (Caspase-3, cTn-I, iNOS), with these effects observed at both transcriptional and translational levels. Histopathological evaluations provided structural confirmation of these protective effects, demonstrating reduced myocardial tissue damage and oxidative injury markers in fisetin-treated specimens.143 These findings highlight fisetin’s ability to modulate key molecular pathways involved in oxidative stress, inflammation, and apoptosis, providing a promising therapeutic approach to mitigate DIC in cancer treatment.
Rutin
Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside), a flavonol glycoside ubiquitously present in crops such as apples, buckwheat, tea, and passion flower, is also known as rutoside, quercetin-3-O-rutinoside, or sophorin.255 The compound derives its name from Ruta graveolens L., a primary natural source.256 Recognized for its diverse biological activities including anti-inflammatory, antioxidant, anti-tumor, antimicrobial, antihyperglycemic, neuroprotective, nephroprotective, cardioprotective, and hepatoprotective effects, rutin has garnered significant interest as a therapeutic agent for managing cancer, neurodegenerative disorders, cardiovascular diseases, and diabetes.257,258 Of particular note are rutin’s cardioprotective properties against anthracycline-induced cardiotoxicity, such as that caused by DOX and pirarubicin (THP). Mechanistic studies reveal that rutin attenuates cardiomyocyte apoptosis and autophagy through Akt signaling pathway, leading to improved cardiac function and reduced myocardial damage in DOX-treated murine models.144 In THP-induced cardiotoxicity, rutin mitigated oxidative stress and apoptosis by upregulating miR-22-5p expression and downregulating Rap1/Erk pathway components, thereby.145 Furthermore, rutin suppresses miR-125b-1-3p expression, enhancing JunD signaling to reduce ROS accumulation and apoptotic activity.146 Rutin also activates PI3K/Akt/ mTOR pathway, bolstering antioxidative defenses and angiogenesis, which collectively enhance cell survival and cardiac function in THP-treated models.147 These findings underscore rutin’s ability to concurrently regulate oxidative stress, apoptosis, autophagy, and angiogenic pathways, offering a multifaceted therapeutic strategy to counteract DIC while preserving cardiac integrity.
Kaempferol
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), a naturally occurring flavonol glycoside, is widely distributed in fruits and vegetables such as onions, broccoli, strawberries, and grapes, as well as in medicinal plants like Ginkgo biloba.259 This compound and its glycosylated derivatives exhibit a broad spectrum of pharmacological activities, including cardioprotective, neuroprotective, anti-inflammatory, antidiabetic, antioxidant, antimicrobial, and antitumor effects.259,260 In CVD, kaempferol’s anti-inflammatory activity is mediated through suppression of pro-inflammatory cytokines (eg, IL-1β, TNF-α) and downregulation of adhesion molecules such as Vcam1, Icam1, and Mcp1, thereby attenuating monocyte infiltration.261 Its antioxidant properties involve activation of Nrf-2 pathway and upregulation of eNOS/dimethylarginine dimethylaminohydrolase II (DDAH II) expression, effectively reducing ROS accumulation.262 Preclinical studies in animal models demonstrate kaempferol’s ability to ameliorate high-fat diet-induced vascular dysfunction, dyslipidemia, and oxidative stress, while concurrently inhibiting inflammation and apoptosis via modulation of the GPER/PI3K/Akt signaling axis.263 In vitro investigations further reveal its endothelial protective effects, including reduced apoptosis in HUVECs, and inhibition of macrophage differentiation. Central to its molecular mechanisms are suppression of NF-κB and MAPK inflammatory pathways, activation of the Nrf-2-mediated antioxidant response, and regulation of vascular tone-related signaling.264 Kaempferol exhibits significant cardioprotective effects against DIC through dual modulation of p53-mediated apoptotic signaling and Erk pathway.148 In a rat model, prophylactic kaempferol administration attenuated DOX-induced oxidative stress, mitochondrial dysfunction, and cardiomyocyte apoptosis. DOX treatment impaired cardiac growth and disrupted Bcl-2 expression, which were counteracted by kaempferol through suppression of p53 expression and inhibition of its binding to the Bax promoter, thereby blunting mitochondrial apoptosis. Complementary in vitro studies corroborated these findings, demonstrating kaempferol’s inhibition of mitochondrion-dependent apoptotic pathways. Notably, kaempferol selectively inhibited Erk 1/2 phosphorylation without affecting p38 or JNK pathways, underscoring its specificity in modulating stress-responsive signaling. These dual mechanisms that attenuation of mitochondrial apoptosis and selective Erk pathway regulation highlight kaempferol’s potential as an adjunctive therapy to mitigate DOX-induced cardiac damage while preserving chemotherapeutic efficacy.
Robinin
Robinin (kaempferol-3-O-robinoside-7-O-rhamnoside), a naturally occurring flavonol glycoside, was initially isolated from the aerial parts of Astragalus falcatus Lam.265 This bioflavonoid has been demonstrated to exhibit multifunctional pharmacological activities, including anti-inflammatory, anti-osteoarthritis, anti-tumor, neuroprotective, nephroprotective, cardioprotective, and antioxidant effects, primarily through modulation of critical signaling pathways such as TLR2/PI3K/Akt, TLR/NF-κB, and Hmgb1/Rage signaling.266 Notably, experimental evidence from Sprague Dawley rat model indicated that robinin attenuated DIC via regulation of the TGF-β1 signaling pathway.149 In this study, DOX administration induced significant elevations in cardiac injury biomarkers (LDH and CPK) and hepatic toxicity markers (serum glutamate oxaloacetate transaminase [SGOT] and serum glutamate pyruvate transaminase [SGPT]). Concurrently, DOX treatment increased lipid peroxidation levels and pro-inflammatory mediators (Cox2, Lox15), while markedly suppressing antioxidant enzyme activities. Molecular analyses revealed DOX-induced dysregulation of TGF-β1 signaling components, including altered expression of Smad2, Smad3, Mdm2, Smad7, Cdkn2a, and Smad4. Apoptotic protein expression profiles were similarly affected, with increased p53 and Bax levels accompanied by decreased Bcl-2 expression. Co-administration of robinin effectively normalized these pathological alterations, restoring antioxidant capacity and attenuating oxidative stress. Complementary in vitro investigations demonstrated that robinin pretreatment significantly reduced apoptotic rates through enhancement of endogenous antioxidant activity with concomitant reduction in MDA and LDH levels and inhibition of ROS generation. Notably, comparative analyses with the clinical cardioprotectant dexrazoxane (DEX) revealed differential efficacy profiles, while DEX showed superior protection against DIC, robinin exhibited significant protective effects against both H2O2 and DOX induced stress.150 These findings collectively highlight robinin’s potential as a multifactorial therapeutic agent for mitigating chemotherapy-associated cardiac damage.
Isorhamnetin
Isorhamnetin (3-methylquercetin), a naturally occurring flavonol chemically defined as 3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-chromen-4-one, is predominantly isolated from Hippophae rhamnoides L. (sea buckthorn) fruits and Ginkgo biloba L. leaves.267 Accumulating preclinical evidence underscores its therapeutic potential in various disease, attributed to its multifaceted pharmacological properties, including anti-atherosclerotic, lipid-lowering, anti-inflammatory, antioxidant, antithrombotic, antiplatelet, antihypertensive, and cardioprotective effects.268 Mechanistically, isorhamnetin modulates critical signaling pathways such as PI3K/Akt/Pkb, NF-κB, and MAPK cascades, thereby regulating downstream inflammatory cytokines and cellular kinases.269 In the context of DIC, isorhamnetin demonstrates robust cardioprotection through dual modulation of oxidative stress and apoptotic pathways.151 In vivo studies in rat models revealed that isorhamnetin pretreatment significantly attenuated DOX-induced myocardial injury, evidenced by improved cardiac functional parameters, reduced serum levels of cardiac enzymes (eg, CK-MB, LDH), and alleviated histopathological alterations such as myocardial vacuolation. Complementary in vitro experiments using H9c2 cardiomyocytes further confirmed its protective role, showing that isorhamnetin reduced intracellular ROS accumulation and suppressed mitochondrial apoptosis via inhibition of Caspase activation. Additionally, isorhamnetin attenuated DOX-triggered MAPK pathway activation, further mitigating cardiomyocyte damage. Notably, isorhamnetin exhibits dual therapeutic efficacy while protecting cardiac tissue from DOX toxicity, it synergistically enhances DOX’s anti-tumor activity in MCF-7, HepG2, and Hep2 cancer cell lines. This dual action highlights its potential as an adjunctive therapy to mitigate chemotherapy-induced cardiotoxicity without compromising antitumor efficacy.
Anthocyanins
Anthocyanins, a subclass of flavonoids widely distributed as natural pigments in fruits and vegetables, exist predominantly in their glycosylated forms, which are chemically derived from their aglycone counterparts, anthocyanidins.270 Among identified anthocyanidins, six are most prevalent: cyanidin (Cy), delphinidin (Dp), malvidin (Mv), pelargonidin (Pg), peonidin (Pn), and petunidin (Pt).271 These compounds have garnered significant scientific interest due to their high dietary bioavailability and diverse health-promoting properties, including antineoplastic, radioprotective, vasoprotective, anti-inflammatory, and chemoprotective effects, largely attributed to their potent antioxidant capacity in mitigating lipid peroxidation and LDL oxidation.152 Of note, cyanidin-3-glucoside (C3G), a prominent anthocyanin in purple corn, demonstrates cardioprotective efficacy against DIC. In vitro studies using murine HL-1 cardiomyocytes revealed that pretreatment with purified C3G or purple corn extract significantly enhanced cell viability under DOX exposure, without compromising DOX’s cytotoxic effects on human cancer cell lines. Corroborating these findings, in vivo experiments showed that mice fed a C3G-enriched diet exhibited improved survival rates and reduced histopathological cardiac damage following DOX administration, underscoring its selective cardioprotection without interfering with DOX’s antitumor activity.153 Cyanidin chloride (CyCl), identified via a deep-learning-assisted zebrafish phenotypic screening platform combined with cardiac functional analysis, emerged as a potent inhibitor of DIC. Subsequent validation in vitro and in vivo DIC models demonstrated that CyCl attenuates cardiomyocyte death, restores cardiac function, and mitigates lipid peroxidation and mitochondrial dysfunction by suppressing ferroptosis and apoptosis. Mechanistic investigations revealed that CyCl directly binds to Keap1, disrupting its interaction with Nrf2, thereby promoting Nrf2 nuclear translocation and upregulating antioxidant defenses, including Gpx4. A Keap1 R415A mutation abolished CyCl’s protective effects, confirming the critical role of Keap1-Nrf2 axis modulation.154 These findings highlight anthocyanins’ dual role in cardioprotection and chemotherapeutic synergy, offering a strategic avenue to enhance the safety profile of DOX-based regimens. Further research is warranted to translate these preclinical insights into clinical applications, optimizing bioavailability and therapeutic efficacy.
Flavanols
Flavanols, a prominent class of plant-derived polyphenolic compounds, are recognized for their diverse health-promoting properties, including antioxidant, cardioprotective, anti-microbial, anti-viral, and neuroprotective, anti-inflammatory, and chemopreventive activities.272 Structurally classified into flavan-3-ols, flavan-4-ols, isoflavan-4-ols, and flavan-3,4-ols, these metabolites have garnered significant pharmacological interest.273 Among them, catechins, a subclass of flavan-3-ols abundant in Camellia sinensis (green tea), are particularly notable for their therapeutic potential.274 Key catechin derivatives include (-)-epigallocatechin gallate (EGCG), (-)-epicatechin gallate (ECG), (-)-epigallocatechin (EGC), (-)-epicatechin (EC), and (+)-catechin (CAT), all of which exhibit robust antioxidant and cytoprotective effects.275 Catechins demonstrate significant cardioprotection against DIC via modulation of oxidative stress and apoptotic pathways. In a rat model of DIC, CAT pretreatment markedly improved cardiac function by reducing intracellular ROS levels and enhancing the activity of antioxidant enzymes, including CAT, SOD, and GST. Additionally, CAT attenuated DOX-induced apoptosis by downregulating pro-apoptotic markers (eg, Bax, Caspase-3) and restoring reduced GSH homeostasis in cardiac tissues. Histopathological assessments revealed that CAT mitigated DOX-induced myofibrillar loss, hemorrhage, and vascular congestion, while ultrastructural analysis confirmed its protective effects against mitochondrial degeneration and preservation of intercalated disc integrity.155 Mechanistically, CAT suppressed the expression of pro-inflammatory mediators such as NF-κB, TNF-α, and iNOS, highlighting its anti-inflammatory role in cardioprotection.156 EGCG, the most abundant and bioactive catechin in green tea, exerts multifaceted protection against DIC. In vivo studies demonstrated that EGCG administration alleviated cardiac injury by reducing LDH release, attenuating apoptosis, and restoring mitochondrial membrane potential (ΔΨm) via upregulation of MnSOD. EGCG also ameliorated myocardial ROS generation and calcium overload, key contributors to DOX-induced cardiomyocyte dysfunction.157 Functional assessments in isolated cardiomyocytes revealed that EGCG restored impaired contraction-relaxation dynamics, including cell shortening and the maximum velocity of contraction (+dL/dt), by enhancing both electrically- and caffeine-induced Ca²⁺ transients.158 This suggests EGCG’s ability to replenish sarcoplasmic reticulum Ca²⁺ stores, thereby improving calcium handling. Further mechanistic studies in murine models and cardiomyocytes showed that EGCG mitigated DOX-induced ECG abnormalities, leakage of cardiac enzymes (CK-MB and LDH), lipid peroxidation, and histopathological damage.159,160 These effects were linked to the restoration of ErbB2 and Hsp70 expression, alongside suppression of NF-κB, p53, calpain-2, and Caspases-3/12.160 Notably, co-administration of EGCG with DOX synergistically enhanced tumor growth inhibition and apoptosis induction in cancer cells, without compromising DOX’s chemotherapeutic efficacy.157 The dual cardioprotective and chemosensitizing properties of catechins, particularly EGCG, underscore their potential as adjunctive therapies in DOX-based chemotherapy. By targeting oxidative stress, calcium dysregulation, and apoptotic/inflammatory pathways, these compounds offer a strategic approach to mitigate cardiotoxicity while preserving anti-tumor activity. Future research should prioritize clinical validation of these preclinical findings, focusing on bioavailability optimization and dose-response studies to facilitate translational applications.
Flavonoids From Basic Research to Clinical Trial
Although a plenty of flavonoids have been extensively investigated for the potential application in DIC, the therapeutic value of most flavonoids is not tested by clinical trials except for 7-Monohydroxyethylrutoside and silymarin. The primary findings from basic research to clinical trial regarding 7-Monohydroxyethylrutoside and silymarin are summarized in Table 2.
 |
Table 2 Flavonoids from Basic Research to Clinical Trial |
7-Monohydroxyethylrutoside
7-Monohydroxyethylrutoside (monoHER), a semisynthetic flavonoid derived from hydroxyethylrutosides, has emerged as a promising cardioprotective agent against doxorubicin DIC. MonoHER’s antioxidant properties have been extensively studied across various preclinical models to mitigate these deleterious effects while preserving DOX’s anti-tumor efficacy. MonoHER has demonstrated high tissue uptake and stability under specific conditions, with bioavailability varying across administration routes. Intravenous and intraperitoneal injections yielded significant plasma and heart tissue concentrations, while oral bioavailability was negligible.276,294 Pharmacokinetic studies confirmed that monoHER’s protective effects are not mediated by interactions with DOX metabolism but rather by its antioxidant activity.295 MonoHER exhibited a peak plasma concentration of approximately 130 µM (IP) and 230 µM (subcutaneous), with sustained cardioprotective levels in preclinical studies.276 MonoHER’s cardioprotective effects are primarily attributed to its antioxidant capacity, neutralizing ROS and attenuating oxidative stress pathways. Studies on neonatal rat cardiac myocytes (NeRCaMs) revealed that monoHER significantly reduced DOX-induced cytotoxicity, apoptosis, and lipid peroxidation.277–279 MonoHER suppressed Caspase-dependent and independent apoptotic pathways, particularly by inhibiting mitochondrial damage and p53 activation. These effects were consistent across other cell types, including endothelial and ovarian cancer cells, indicating monoHER’s selective cytoprotection in non-cancerous tissues.279 MonoHER was compared with clinically established cardioprotective agents like dexrazoxane (ICRF-187). Preclinical studies in mice demonstrated that monoHER provided comparable or superior protection against DIC, as evidenced by reduced ECG changes (eg, ST interval prolongation), cardiomyocyte damage, and histological markers of cardiac injury.280 Importantly, monoHER did not compromise DOX’s antitumor efficacy, distinguishing it from some alternative agents. Optimal dosing schedules were critical for monoHER’s efficacy. A single dose administered one hour prior to DOX injection was sufficient to confer protection, aligning with its pharmacokinetic profile.281 Frequent dosing regimens, however, raised concerns about potential pro-oxidant effects, especially over extended periods. Long-term studies revealed that monoHER’s initial cardioprotective effects diminished when dosing frequency increased, highlighting the importance of tailored administration schedules.282 MonoHER also demonstrated anti-inflammatory properties by reducing the accumulation of Nepsilon-(carboxymethyl) lysine (CML), a marker of oxidative stress-induced inflammation. In murine models, monoHER pre-treatment significantly decreased the incidence of CML-positive cardiomyocytes and intramyocardial vessels.283 These findings underline monoHER’s role in modulating inflammation in DIC. Despite its efficacy, monoHER’s cardioprotective potential is constrained by administration challenges, such as the need for parenteral delivery due to poor oral bioavailability. Additionally, while monoHER outperformed adenoviral CuZn-superoxide dismutase (CuZn-SOD) gene therapy, its pro-oxidant effects under certain conditions warrant further investigation to refine dosing strategies.277
Given the excellent anti-tumor and cardioprotection potential, monoHER was tested in clinical trials. In a phase I study to develop a safe and feasible dose in cancer patients treated with doxorubicin, the possible side effects and the pharmacokinetics of monoHER were evaluated in healthy volunteers. The results showed that The mean values of C (max) and AUC(infinity) were 360±69.3 microM and 6.8±2.1 micromol min/mL, which were comparable to the C (max) and AUC(infinity) observed under the protecting conditions in mice. Although the dose was escalated up to 1,500 mg/m2, no serious side effects occurred during the entire study, indicating a feasible and safe dose to be evaluated in a phase I study.284 Subsequently, the safety and efficacy was further tested on patients with metastatic cancer treated with DOX in a phase II study. Surprisingly, monoHER did not alleviate but enhance DIC with an intravenous infusion of 1,500 mg/m2. However, the antitumour activity of DOX seemed better than expected. The investigators explained that the relatively high dose of monoHER may account for the observed lack of cardioprotection and the high response rate in patients with soft-tissue sarcoma, potentially through depletion of the GSH defense system in both cardiac and tumor tissues.285
Silymarin
Silymarin, a flavonolignan complex derived from Silybum marianum, has been widely investigated for its cardioprotective properties against DIC. These studies utilized diverse experimental approaches, including various animal models and analytical methods, to uncover the mechanisms underlying silymarin’s protective effects. Notably, silymarin, particularly its active component silibinin, mitigates DIC through antioxidant, anti-apoptotic, and anti-inflammatory mechanisms. Evidence from both acute and chronic models highlights silymarin’s efficacy. For instance, in male Wistar rats, oral administration of silymarin (60 mg/kg) over 12 days significantly alleviated oxidative stress, myocardial apoptosis, and functional impairments caused by DOX. The benefits were observed in improved ECG profiles, reduced lipid peroxidation markers such as MDA, and enhanced activities of antioxidant enzymes like SOD and GPx.286,287 Histological analysis further corroborated these findings by revealing reduced myocardial necrosis and fibrosis.287 A critical component of silymarin’s protective action lies in its ability to maintain mitochondrial integrity. Histopathological and electron microscopic analyses revealed that silymarin prevented mitochondrial swelling and cytochrome c leakage, which are pivotal steps in apoptosis.288 At the molecular level, silymarin exerts its cardioprotective effects by modulating key apoptotic and oxidative stress pathways. For instance, studies on BALB/c mice demonstrated that silymarin reduced DOX-induced cardiomyopathy by upregulating anti-apoptotic proteins (eg, Bcl-xL) and downregulating pro-apoptotic markers (eg, p53).288 Additionally, silymarin inhibited DOX-induced Top2β-mediated DNA damage and decreased expression of γH2AX, improving mitochondrial function and preserving cardiac contractility.289 Comparative studies with other flavonoids, such as quercetin, emphasized silymarin’s unique protective mechanisms. While quercetin exhibited superior iron-chelating properties, silymarin’s primary effects were attributed to its potent free radical scavenging and lipid peroxidation inhibition.290 Moreover, combination therapy experiments, such as those involving verapamil, demonstrated that silymarin enhanced the cardioprotective effects of co-administered agents. For example, silymarin increased the verapamil dose required to induce toxic responses in DOX-treated models, showcasing its synergistic potential.291 Beyond cardioprotection, silymarin demonstrated systemic benefits, as seen in studies involving albino rats. Pre-treatment with silymarin significantly reduced serum markers of cardiac and kidney injury, including NO, CPK, LDH and CK, creatinine and urea, while also normalizing renal MDA and GSH levels. These findings suggest that silymarin provides comprehensive protection, encompassing both the heart and other organs like the kidneys and liver.286,287,292
Based on the findings from preclinical studies, a perspective study examined the protective role of silymarin in early doxorubicin-induced cardiac dysfunction in children with acute lymphoblastic leukemia. As expected, silymarin was shown to alleviate early doxorubicin-induced left ventricular systolic function disturbances (LVEF, LVFS and S wave). Moreover, silymarin significantly decreased the level of troponin induced by doxorubicin. These findings supported the recommendation of silymarin as an adjuvant drug in early and late DIC.293
Challenges and Future Perspectives
While emerging evidence highlights the cardioprotective potential of flavonoids against DIC, critical challenges and knowledge gaps persist in translating preclinical findings to clinical applications. First, numerous studies remain descriptive, focusing on phenotypic observations (eg, reduced ROS, antioxidant enzymes and cardiac markers) without elucidating the underlying regulatory mechanisms of key signaling pathways such as ferroptosis and immunomodulation of the cardiac microenvironment. Current literature disproportionately emphasizes common subclasses such as flavones and flavonols, with limited exploration of other flavonoids. Beyond monoHER and silymarin, most compounds remain confined to cellular and animal studies, lacking comprehensive pharmacokinetic profiles and long-term safety assessments. Furthermore, the synergistic mechanisms between flavonoids and other natural products (eg, phenolic compounds) remain poorly characterized. Pharmacologically, many flavonoids (eg, quercetin, luteolin) face inherent limitations, including poor aqueous solubility and pronounced first-pass metabolism, which compromise bioavailability. Advanced strategies such as nanocarriers (eg, liposomes, polymeric micelles) and chemical modifications (eg, glycosylation) show promise in enhancing stability and myocardial targeting. Although nanotechnology has been developed to address the low bioavailability of flavonoids, current flavonoid delivery systems face significant challenges: (1) physical instability and low drug loading capacity, primarily owing to the requisite abundance of pharmaceutical excipients, multi-step synthesis, and complex preparation methods; and (2) off-target toxicity resulting from the nonspecific biodistribution of oral formulations, byproduct generation, and incomplete carrier degradation.296 Natural flavonoid extracts often exhibit compositional complexity, necessitating stringent quality control protocols to ensure batch-to-batch consistency in therapeutic efficacy. The combinatorial effects of flavonoids with conventional cardioprotective agents (eg, dexrazoxane) warrant rigorous validation to assess additive or synergistic benefits. Addressing these challenges will advance flavonoids as viable adjuvants in oncological regimens, balancing cardioprotection with chemotherapeutic efficacy. Additionally, the publication bias is unavoidable although articles concerning the potential of flavonoids against DIC are comprehensively reviewed. Most of the current evidence is derived from model organism such as H9c2 cells and mice which are not fully representative of adult human cardiomyocytes, and therefore is suggested to be further validated in human induced pluripotent stem cell-derived cardiomyocytes (hi-PSCMs).
Conclusion
Cardiotoxicity is a major limitation when considering dose escalation of DOX to enhance therapeutic efficacy. This review highlights 7 subclasses of flavonoids encompassing over 50 compounds that target oxidative stress, mitochondrial dysfunction, calcium imbalance, ferroptosis, inflammation and apoptotic pathways to mitigate doxorubicin-induced cardiotoxicity. Despite their promising preclinical efficacy, clinical translation remains hindered by challenges including suboptimal bioavailability, undefined drug-drug interactions, and insufficient clinical data. Future efforts which prioritize nanotechnology-driven delivery systems and structural optimization to improve bioavailability may enhance clinical applicability. Additionally, heterogeneity in study designs (eg, dosing, timing, treatment duration) complicates cross-study comparisons and hinders robust flavonoid prioritization. Crucially, most existing data lack justification for clinical applicability. To bridge this gap, critical next steps include: (1) Formulation optimization and PK/PD studies to define bioavailability and dosing regimens; (2) well-designed pre-clinical studies to determine the cardioprotection and antitumor efficacy of flavonoids based on tumor-cardiac dual models. Remarkably, quercetin emerges as the most promising candidate based on extensive preclinical evidence of multi-organ protection without compromising DOX efficacy, which deserves further clinical studies to confirm its clinical significance. Collectively, advancing flavonoids-based therapies from bench to bedside will provide safer adjunctive chemotherapeutic agents for cancer patients.
Data Sharing Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgment
We appreciate the contributions of all the research teams mentioned in the article. The GPT-4o and deepseek aid partially in the translation and embellishment of the language without model-generated text, which was followed by a manual check for accuracy. All co-authors declare that they take full responsibility for all of its contents.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82204540), Hubei Provincial Natural Science Foundation and Shiyan of China (No. 2025AFD209), the Discipline Construction Foundation of Hubei University of Medicine (No. X1204310), and the College Student Innovation and Entrepreneurship Training Program (No. 202413249002).
Disclosure
The authors declare no competing interests in this work.
References
1. Navarro-Hortal MD, Varela-Lopez A, Romero-Marquez JM, et al. Role of flavonoids against Adriamycin toxicity. Food Chem Toxicol. 2020;146:111820. doi:10.1016/j.fct.2020.111820
2. Bhagat A, Kleinerman ES. Anthracycline-induced cardiotoxicity: causes, mechanisms, and prevention. Adv Exp Med Biol. 2020;1257:181–192. doi:10.1007/978-3-030-43032-0_15
3. Sheibani M, Azizi Y, Shayan M, et al. Doxorubicin-induced cardiotoxicity: an overview on pre-clinical therapeutic approaches. Cardiovasc Toxicol. 2022;22(4):292–310. doi:10.1007/s12012-022-09721-1
4. Jones IC, Dass CR. Doxorubicin-induced cardiotoxicity: causative factors and possible interventions. J Pharm Pharmacol. 2022;74(12):1677–1688. doi:10.1093/jpp/rgac063
5. Harahap Y, Ardiningsih P, Corintias Winarti A, et al. Analysis of the Doxorubicin and Doxorubicinol in the plasma of breast cancer patients for monitoring the toxicity of Doxorubicin. Drug Des Devel Ther. 2020;14:3469–3475. doi:10.2147/DDDT.S251144
6. Rawat PS, Jaiswal A, Khurana A, et al. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139:111708. doi:10.1016/j.biopha.2021.111708
7. Wan G, Chen P, Sun X, et al. Weighted gene co-expression network-based approach to identify key genes associated with anthracycline-induced cardiotoxicity and construction of miRNA-transcription factor-gene regulatory network. Mol Med. 2021;27(1):142. doi:10.1186/s10020-021-00399-9
8. Songbo M, Lang H, Xinyong C, et al. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019;307:41–48. doi:10.1016/j.toxlet.2019.02.013
9. O’Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a Phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi:10.1093/annonc/mdh097
10. Schlitt A, Jordan K, Vordermark D, et al. Cardiotoxicity and oncological treatments. Dtsch Arztebl Int. 2014;111(10):161–168. doi:10.3238/arztebl.2014.0161
11. Bast A, Haenen GR, Bruynzeel AM, et al. Protection by flavonoids against anthracycline cardiotoxicity: from chemistry to clinical trials. Cardiovasc Toxicol. 2007;7(2):154–159. doi:10.1007/s12012-007-0018-0
12. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013;2013:162750. doi:10.1155/2013/162750
13. Xu H, Yu S, Lin C, et al. Roles of flavonoids in ischemic heart disease: cardioprotective effects and mechanisms against myocardial ischemia and reperfusion injury. Phytomedicine. 2024;126:155409. doi:10.1016/j.phymed.2024.155409
14. Billowria K, Ali R, Rangra NK, et al. Bioactive flavonoids: a comprehensive review on pharmacokinetics and analytical aspects. Crit Rev Anal Chem. 2024;54(5):1002–1016. doi:10.1080/10408347.2022.2105641
15. Chen S, Wang X, Cheng Y, et al. A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules. 2023;28(13):4982. doi:10.3390/molecules28134982
16. Syahputra RA, Harahap U, Dalimunthe A, et al. The role of flavonoids as a cardioprotective strategy against Doxorubicin-induced cardiotoxicity: a review. Molecules. 2022;27(4):1320. doi:10.3390/molecules27041320
17. Razavi-Azarkhiavi K, Iranshahy M, Sahebkar A, et al. The protective role of phenolic compounds against Doxorubicin-induced cardiotoxicity: a comprehensive review. Nutr Cancer. 2016;68(6):892–917. doi:10.1080/01635581.2016.1187280
18. Wang SQ, Han XZ, Li X, et al. Flavonoids from Dracocephalum tanguticum and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Bioorg Med Chem Lett. 2010;20(22):6411–6415. doi:10.1016/j.bmcl.2010.09.086
19. Han XZ, Gao S, Cheng YN, et al. Protective effect of naringenin-7-O-glucoside against oxidative stress induced by doxorubicin in H9c2 cardiomyocytes. Biosci Trends. 2012;6(1):19–25. doi:10.5582/bst.2012.v6.1.19
20. Wenningmann N, Knapp M, Ande A, et al. Insights into Doxorubicin-induced cardiotoxicity: molecular mechanisms, preventive strategies, and early monitoring. Mol Pharmacol. 2019;96(2):219–232. doi:10.1124/mol.119.115725
21. Vitale R, Marzocco S, Popolo A. Role of oxidative stress and inflammation in Doxorubicin-induced cardiotoxicity: a brief account. Int J Mol Sci. 2024;25(13):7477. doi:10.3390/ijms25137477
22. Gilleron M, Marechal X, Montaigne D, et al. NADPH oxidases participate to doxorubicin-induced cardiac myocyte apoptosis. Biochem Biophys Res Commun. 2009;388(4):727–731. doi:10.1016/j.bbrc.2009.08.085
23. Kondru SK, Potnuri AG, Allakonda L, et al. Histamine 2 receptor antagonism elicits protection against doxorubicin-induced cardiotoxicity in rodent model. Mol Cell Biochem. 2018;441(1–2):77–88. doi:10.1007/s11010-017-3175-x
24. Krause MS, Oliveira LJ, Silveira EM, et al. MRP1/GS-X pump ATPase expression: is this the explanation for the cytoprotection of the heart against oxidative stress-induced redox imbalance in comparison to skeletal muscle cells? Cell Biochem Funct. 2007;25(1):23–32. doi:10.1002/cbf.1343
25. Zhao L, Qi Y, Xu L, et al. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018;15:284–296. doi:10.1016/j.redox.2017.12.013
26. Halili-Rutman I, Hershko C, Link G, et al. Inhibition of calcium accumulation by the sarcoplasmic reticulum: a putative mechanism for the cardiotoxicity of Adriamycin. Biochem Pharmacol. 1997;54(1):211–214. doi:10.1016/s0006-2952(97)00108-1
27. Kim E, Giri SN, Pessah IN. Iron(II) is a modulator of ryanodine-sensitive calcium channels of cardiac muscle sarcoplasmic reticulum. Toxicol Appl Pharmacol. 1995;130(1):57–66. doi:10.1006/taap.1995.1008
28. Lyu YL, Kerrigan JE, Lin CP, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67(18):8839–8846. doi:10.1158/0008-5472.CAN-07-1649
29. Mordente A, Meucci E, Martorana GE, et al. Topoisomerases and Anthracyclines: recent advances and perspectives in anticancer therapy and prevention of cardiotoxicity. Curr Med Chem. 2017;24(15):1607–1626. doi:10.2174/0929867323666161214120355
30. Zhu H, Sarkar S, Scott L, et al. Doxorubicin redox biology: redox cycling, Topoisomerase inhibition, and oxidative stress. React Oxyg Species. 2016;1(3):189–198. doi:10.20455/ros.2016.835
31. Chen J, Chapski DJ, Jong J, et al. Integrative transcriptomics and cell systems analyses reveal protective pathways controlled by Igfbp-3 in anthracycline-induced cardiotoxicity. FASEB J. 2023;37(6):e22977. doi:10.1096/fj.202201885RR
32. Chen S, Chen J, Du W, et al. PDE10A inactivation prevents Doxorubicin-induced cardiotoxicity and tumor growth. Circ Res. 2023;133(2):138–157. doi:10.1161/CIRCRESAHA.122.322264
33. Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–1642. doi:10.1038/nm.2919
34. Zhang P, Chen Z, Lu D, et al. Overexpression of COX5A protects H9c2 cells against doxorubicin-induced cardiotoxicity. Biochem Biophys Res Commun. 2020;524(1):43–49. doi:10.1016/j.bbrc.2020.01.013
35. Zhang P, Lu H, Wu Y, et al. COX5A alleviates Doxorubicin-induced cardiotoxicity by suppressing oxidative stress, mitochondrial dysfunction and cardiomyocyte apoptosis. Int J Mol Sci. 2023;24(12):10400. doi:10.3390/ijms241210400
36. Wu BB, Leung KT, Poon EN. Mitochondrial-targeted therapy for Doxorubicin-induced cardiotoxicity. Int J Mol Sci. 2022;23(3):1912. doi:10.3390/ijms23031912
37. Gao F, Xu T, Zang F, et al. Cardiotoxicity of anticancer drugs: molecular mechanisms, clinical management and innovative treatment. Drug Des Devel Ther. 2024;18:4089–4116. doi:10.2147/DDDT.S469331
38. Wu L, Wang L, Du Y, et al. Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol Sci. 2023;44(1):34–49. doi:10.1016/j.tips.2022.10.003
39. Wang T, Xing G, Fu T, et al. Role of mitochondria in doxorubicin-mediated cardiotoxicity: from molecular mechanisms to therapeutic strategies. Int J Med Sci. 2024;21(5):809–816. doi:10.7150/ijms.94485
40. Arai M, Yoguchi A, Takizawa T, et al. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca(2+)-ATPase gene transcription. Circ Res. 2000;86(1):8–14. doi:10.1161/01.res.86.1.8
41. Hanna AD, Lam A, Tham S, et al. Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol Pharmacol. 2014;86(4):438–449. doi:10.1124/mol.114.093849
42. Zhang Y, Chen Y, Zhang M, et al. Doxorubicin induces sarcoplasmic reticulum calcium regulation dysfunction via the decrease of SERCA2 and phospholamban expressions in rats. Cell Biochem Biophys. 2014;70(3):1791–1798. doi:10.1007/s12013-014-0130-2
43. Szenczi O, Kemecsei P, Holthuijsen MF, et al. Poly(ADP-ribose) polymerase regulates myocardial calcium handling in doxorubicin-induced heart failure. Biochem Pharmacol. 2005;69(5):725–732. doi:10.1016/j.bcp.2004.11.023
44. Matsushita T, Okamato M, Toyama J, et al. Adriamycin causes dual inotropic effects through complex modulation of myocardial Ca2+ handling. Jpn Circ J. 2000;64(1):65–71. doi:10.1253/jcj.64.65
45. Maeda A, Honda M, Kuramochi T, et al. Doxorubicin cardiotoxicity: diastolic cardiac myocyte dysfunction as a result of impaired calcium handling in isolated cardiac myocytes. Jpn Circ J. 1998;62(7):505–511. doi:10.1253/jcj.62.505
46. Wang TH, Ma Y, Gao S, et al. recent advances in the mechanisms of cell death and dysfunction in Doxorubicin cardiotoxicity. Rev Cardiovasc Med. 2023;24(11):336. doi:10.31083/j.rcm2411336
47. Fajardo G, Zhao M, Berry G, et al. beta2-adrenergic receptors mediate cardioprotection through crosstalk with mitochondrial cell death pathways. J Mol Cell Cardiol. 2011;51(5):781–789. doi:10.1016/j.yjmcc.2011.06.019
48. Freiwan M, Kovacs MG, Kovacs Z, et al. Investigation of the antiremodeling effects of Losartan, Mirabegron and their combination on the development of doxorubicin-induced chronic cardiotoxicity in a rat model. Int J Mol Sci. 2022;23(4):2201. doi:10.3390/ijms23042201
49. Maeda A, Honda M, Kuramochi T, et al. A calcium antagonist protects against doxorubicin-induced impairment of calcium handling in neonatal rat cardiac myocytes. Jpn Circ J. 1999;63(2):123–129. doi:10.1253/jcj.63.123
50. Maghraby N, El-Baz M, Hassan A, et al. Metformin alleviates Doxorubicin-induced cardiotoxicity via preserving mitochondrial dynamics balance and calcium homeostasis. Appl Biochem Biotechnol. 2025;197(4):2713–2733. doi:10.1007/s12010-024-05141-9
51. Agustini FD, Arozal W, Louisa M, et al. Cardioprotection mechanism of mangiferin on doxorubicin-induced rats: focus on intracellular calcium regulation. Pharm Biol. 2016;54(7):1289–1297. doi:10.3109/13880209.2015.1073750
52. Ikeda S, Matsushima S, Okabe K, et al. Blockade of L-type Ca(2+) channel attenuates doxorubicin-induced cardiomyopathy via suppression of CaMKII-NF-kappaB pathway. Sci Rep. 2019;9(1):9850. doi:10.1038/s41598-019-46367-6
53. Lin R, Peng X, Li Y, et al. Empagliflozin attenuates doxorubicin-impaired cardiac contractility by suppressing reactive oxygen species in isolated myocytes. Mol Cell Biochem. 2024;479(8):2105–2118. doi:10.1007/s11010-023-04830-z
54. Wu L, Zhang Y, Wang G, et al. Molecular mechanisms and therapeutic targeting of ferroptosis in Doxorubicin-induced cardiotoxicity. JACC Basic Transl Sci. 2024;9(6):811–826. doi:10.1016/j.jacbts.2023.10.009
55. Ajoolabady A, Aslkhodapasandhokmabad H, Libby P, et al. Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol Metab. 2021;32(7):444–462. doi:10.1016/j.tem.2021.04.010
56. Wu L, Wang LT, Du YX, et al. Asiatic acid ameliorates doxorubicin-induced cardiotoxicity by promoting FPN-mediated iron export and inhibiting ferroptosis. Acta Pharmacol Sin. 2025;46(1):81–95. doi:10.1038/s41401-024-01367-9
57. Ye H, Wu L, Liu Y. Iron metabolism in doxorubicin-induced cardiotoxicity: from mechanisms to therapies. Int J Biochem Cell Biol. 2024;174:106632. doi:10.1016/j.biocel.2024.106632
58. Zhang H, Pan J, Huang S, et al. Hydrogen sulfide protects cardiomyocytes from doxorubicin-induced ferroptosis through the SLC7A11/GSH/GPx4 pathway by Keap1 S-sulfhydration and Nrf2 activation. Redox Biol. 2024;70:103066. doi:10.1016/j.redox.2024.103066
59. Tadokoro T, Ikeda M, Ide T, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5(9):e132747. doi:10.1172/jci.insight.132747
60. Cui J, Chen Y, Yang Q, et al. Protosappanin A protects DOX-induced myocardial injury and cardiac dysfunction by targeting ACSL4/FTH1 axis-dependent ferroptosis. Adv Sci. 2024;11(34):e2310227. doi:10.1002/advs.202310227
61. Wang B, Jin Y, Liu J, et al. EP1 activation inhibits doxorubicin-cardiomyocyte ferroptosis via Nrf2. Redox Biol. 2023;65:102825. doi:10.1016/j.redox.2023.102825
62. Yi X, Wang Q, Zhang M, et al. Ferroptosis: a novel therapeutic target of natural products against doxorubicin-induced cardiotoxicity. Biomed Pharmacother. 2024;178:117217. doi:10.1016/j.biopha.2024.117217
63. Syukri A, Budu, Hatta M, et al. Doxorubicin induced immune abnormalities and inflammatory responses via HMGB1, HIF1-alpha and VEGF pathway in progressive of cardiovascular damage. Ann Med Surg. 2022;76:103501. doi:10.1016/j.amsu.2022.103501
64. Li Y, Yan J, Yang P. The mechanism and therapeutic strategies in doxorubicin-induced cardiotoxicity: role of programmed cell death. Cell Stress Chaperones. 2024;29(5):666–680. doi:10.1016/j.cstres.2024.09.001
65. Avagimyan A, Pogosova N, Kakturskiy L, et al. Doxorubicin-related cardiotoxicity: review of fundamental pathways of cardiovascular system injury. Cardiovasc Pathol. 2024;73:107683. doi:10.1016/j.carpath.2024.107683
66. Maayah ZH, Takahara S, Dyck J. The beneficial effects of reducing NLRP3 inflammasome activation in the cardiotoxicity and the anti-cancer effects of doxorubicin. Arch Toxicol. 2021;95(1):1–9. doi:10.1007/s00204-020-02876-2
67. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;
68. Li H, Zhang M, Wang Y, et al. Daidzein alleviates doxorubicin-induced heart failure via the SIRT3/FOXO3a signaling pathway. Food Funct. 2022;13(18):9576–9588. doi:10.1039/d2fo00772j
69. Wu J, Li K, Liu Y, et al. Daidzein ameliorates doxorubicin-induced cardiac injury by inhibiting autophagy and apoptosis in rats. Food Funct. 2023;14(2):934–945. doi:10.1039/d2fo03416f
70. Zhai J, Tao L, Zhang S, et al. Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1-NOD-like receptor protein 3 pathway. Phytother Res. 2020;34(3):649–659. doi:10.1002/ptr.6557
71. Lu X, Lu L, Gao L, et al. Calycosin attenuates doxorubicin-induced cardiotoxicity via autophagy regulation in zebrafish models. Biomed Pharmacother. 2021;137:111375. doi:10.1016/j.biopha.2021.111375
72. Zhang H, Weng J, Sun S, et al. Ononin alleviates endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity by activating SIRT3. Toxicol Appl Pharmacol. 2022;452:116179. doi:10.1016/j.taap.2022.116179
73. Qian JY, Deng P, Liang YD, et al. 8-Formylophiopogonanone B Antagonizes Paraquat-induced hepatotoxicity by suppressing oxidative stress. Front Pharmacol. 2019;10:1283. doi:10.3389/fphar.2019.01283
74. Qin D, Yue R, Deng P, et al. 8-Formylophiopogonanone B antagonizes doxorubicin-induced cardiotoxicity by suppressing heme oxygenase-1-dependent myocardial inflammation and fibrosis. Biomed Pharmacother. 2021;140:111779. doi:10.1016/j.biopha.2021.111779
75. Wu J, Feng A, Liu C, et al. Genistein alleviates doxorubicin-induced cardiomyocyte autophagy and apoptosis via ERK/STAT3/c-Myc signaling pathway in rat model. Phytother Res. 2024;38(8):3921–3934. doi:10.1002/ptr.8236
76. Bai Z, Wang Z. Genistein protects against doxorubicin-induced cardiotoxicity through Nrf-2/HO-1 signaling in mice model. Environ Toxicol: Int J. 2019;34(5):645–651. doi:10.1002/tox.22730
77. Peng Y, Wang L, Zhang Z, et al. Puerarin activates adaptive autophagy and protects the myocardium against doxorubicin-induced cardiotoxicity via the 14-3-3gamma/PKCepsilon pathway. Biomed Pharmacother. 2022;153:113403. doi:10.1016/j.biopha.2022.113403
78. Guo L, Zheng X, Wang E, et al. Irigenin treatment alleviates doxorubicin (DOX)-induced cardiotoxicity by suppressing apoptosis, inflammation and oxidative stress via the increase of miR-425. Biomed Pharmacother. 2020;125:109784. doi:10.1016/j.biopha.2019.109784
79. Wen Y, Wang Y, Zhao C, et al. The pharmacological efficacy of Baicalin in inflammatory diseases. Int J Mol Sci. 2023;24(11):9317. doi:10.3390/ijms24119317
80. Zeng Y, Liao X, Guo Y, et al. Baicalin-peptide supramolecular self-assembled nanofibers effectively inhibit ferroptosis and attenuate doxorubicin-induced cardiotoxicity. J Control Release. 2024;366:838–848. doi:10.1016/j.jconrel.2023.12.034
81. El-Ela S, Zaghloul RA, Eissa LA. Promising cardioprotective effect of baicalin in doxorubicin-induced cardiotoxicity through targeting toll-like receptor 4/nuclear factor-kappaB and Wnt/beta-catenin pathways. Nutrition. 2022;102:111732. doi:10.1016/j.nut.2022.111732
82. Chang W-T, Li J, Haung -H-H, et al. Baicalein protects against doxorubicin-induced cardiotoxicity by attenuation of mitochondrial oxidant injury and JNK activation. J Cell Biochem. 2011;112(10):2873–2881. doi:10.1002/jcb.23201
83. Sahu BD, Kumar JM, Kuncha M, et al. Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sci. 2016;144:8–18. doi:10.1016/j.lfs.2015.11.018
84. Li S, Liu H, Lin Z, et al. Isoorientin attenuates doxorubicin-induced cardiac injury via the activation of MAPK, Akt, and Caspase-dependent signaling pathways. Phytomedicine. 2022;101:154105. doi:10.1016/j.phymed.2022.154105
85. Shi X, Cao Y, Wang H, et al. Vaccarin ameliorates Doxorubicin-induced cardiotoxicity via inhibition of p38 MAPK mediated mitochondrial dysfunction. J Cardiovasc Transl Res. 2024;17(5):1155–1171. doi:10.1007/s12265-024-10525-7
86. Mantawy EM, Esmat A, El-Bakly WM, et al. Mechanistic clues to the protective effect of chrysin against doxorubicin-induced cardiomyopathy: Plausible roles of p53, MAPK and AKT pathways. Sci Rep. 2017;7(1):4795. doi:10.1038/s41598-017-05005-9
87. Liu Y, Zhou L, Du B, et al. Protection against Doxorubicin-related cardiotoxicity by Jaceosidin involves the Sirt1 signaling pathway. Oxid Med Cell Longev. 2021;2021:9984330. doi:10.1155/2021/9984330
88. Liu Z, Song XD, Xin Y, et al. Protective effect of chrysoeriol against doxorubicin-induced cardiotoxicity in vitro. Chin Med J. 2009;122(21):2652–2656. doi:10.3760/cma.j.issn.0366-6999.2009.21.024
89. Gu J, Huang H, Liu C, et al. Pinocembrin inhibited cardiomyocyte pyroptosis against doxorubicin-induced cardiac dysfunction via regulating Nrf2/Sirt3 signaling pathway. Int Immunopharmacol. 2021;95:107533. doi:10.1016/j.intimp.2021.107533
90. Zhao J, Du J, Pan Y, et al. Activation of cardiac TrkB receptor by its small molecular agonist 7, 8-dihydroxyflavone inhibits doxorubicin-induced cardiotoxicity via enhancing mitochondrial oxidative phosphorylation. Free Radic Biol Med. 2019;130:557–567. doi:10.1016/j.freeradbiomed.2018.11.024
91. Zhang WB, Zheng YF, Wu YG. Protective effects of Oroxylin A against Doxorubicin-induced cardiotoxicity via the activation of Sirt1 in mice. Oxid Med Cell Longev. 2021;2021:6610543. doi:10.1155/2021/6610543
92. Wu WY, Cui YK, Hong YX, et al. Doxorubicin cardiomyopathy is ameliorated by acacetin via Sirt1-mediated activation of AMPK/Nrf2 signal molecules. J Cell Mol Med. 2020;24(20):12141–12153. doi:10.1111/jcmm.15859
93. Li X, Wang X, Wang B, et al. Dihydromyricetin protects against Doxorubicin-induced cardiotoxicity through activation of AMPK/mTOR pathway. Phytomedicine. 2022;99:154027. doi:10.1016/j.phymed.2022.154027
94. Sun Z, Lu W, Lin N, et al. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem Pharmacol. 2020;175:113888. doi:10.1016/j.bcp.2020.113888
95. Zhu H, Luo P, Fu Y, et al. Dihydromyricetin prevents cardiotoxicity and enhances anticancer activity induced by Adriamycin. Oncotarget. 2015;6(5):3254–3267. doi:10.18632/oncotarget.2410
96. Li H, Chen D, Zhang X, et al. Screening of an FDA-approved compound library identifies apigenin for the treatment of myocardial injury. Int J Biol Sci. 2023;19(16):5233–5244. doi:10.7150/ijbs.85204
97. Wang F, Yan X, Yue A, et al. Apigenin alleviates doxorubicin-induced myocardial pyroptosis by inhibiting glycogen synthase kinase-3beta in vitro and in vivo. Drug Dev Res. 2024;85(4):e22196. doi:10.1002/ddr.22196
98. Zare M, Rakhshan K, Aboutaleb N, et al. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci. 2019;232:116623. doi:10.1016/j.lfs.2019.116623
99. Sun XP, Wan LL, Yang QJ, et al. Scutellarin protects against doxorubicin-induced acute cardiotoxicity and regulates its accumulation in the heart. Arch Pharm Res. 2017;40(7):875–883. doi:10.1007/s12272-017-0907-0
100. Zhou L, Han Y, Yang Q, et al. Scutellarin attenuates doxorubicin-induced oxidative stress, DNA damage, mitochondrial dysfunction, apoptosis and autophagy in H9c2 cells, cardiac fibroblasts and HUVECs. Toxicol In Vitro. 2022;82:105366. doi:10.1016/j.tiv.2022.105366
101. Scicchitano M, Carresi C, Nucera S, et al. Icariin protects H9c2 rat cardiomyoblasts from Doxorubicin-induced cardiotoxicity: role of Caveolin-1 upregulation and enhanced autophagic response. Nutrients. 2021;13(11):4070. doi:10.3390/nu13114070
102. Lu Y, Min Q, Zhao X, et al. Eupatilin attenuates doxorubicin-induced cardiotoxicity by activating the PI3K-AKT signaling pathway in mice. Mol Cell Biochem. 2024;479(4):869–880. doi:10.1007/s11010-023-04769-1
103. Zhang Y, Ma C, Liu C, et al. Luteolin attenuates doxorubicin-induced cardiotoxicity by modulating the PHLPP1/AKT/Bcl-2 signalling pathway. PeerJ. 2020;8:e8845. doi:10.7717/peerj.8845
104. Zou H, Zhang M, Yang X, et al. Cynaroside regulates the AMPK/SIRT3/Nrf2 pathway to inhibit doxorubicin-induced cardiomyocyte pyroptosis. J Zhejiang Univ Sci B. 2024;25(9):756–772. doi:10.1631/jzus.B2300691
105. Yao H, Shang Z, Wang P, et al. Protection of Luteolin-7-O-Glucoside against Doxorubicin-induced injury through PTEN/Akt and ERK pathway in H9c2 cells. Cardiovasc Toxicol. 2016;16(2):101–110. doi:10.1007/s12012-015-9317-z
106. Abohashem RS, Ahmed HH, Sayed AH, et al. Primary protection of Diosmin against Doxorubicin cardiotoxicity via inhibiting oxido-inflammatory stress and apoptosis in rats. Cell Biochem Biophys. 2024;82(2):1353–1366. doi:10.1007/s12013-024-01289-7
107. Xu Z, Hu Z, Xu H, et al. Liquiritigenin alleviates doxorubicin-induced chronic heart failure via promoting ARHGAP18 and suppressing RhoA/ROCK1 pathway. Exp Cell Res. 2022;411(2):113008. doi:10.1016/j.yexcr.2022.113008
108. Shi C, Wu H, Xu K, et al. Liquiritigenin-loaded submicron emulsion protects against Doxorubicin-induced cardiotoxicity via antioxidant, anti-inflammatory, and anti-apoptotic activity. Int J Nanomed. 2020;15:1101–1115. doi:10.2147/IJN.S235832
109. Kwatra M, Kumar V, Jangra A, et al. Ameliorative effect of naringin against doxorubicin-induced acute cardiac toxicity in rats. Pharm Biol. 2016;54(4):637–647. doi:10.3109/13880209.2015.1070879
110. Jian CY, Ouyang HB, Xiang XH, et al. Naringin protects myocardial cells from doxorubicin‑induced apoptosis partially by inhibiting the p38MAPK pathway. Mol Med Rep. 2017;16(6):9457–9463. doi:10.3892/mmr.2017.7823
111. Alharbi FK, Alshehri ZS, Alshehri FF, et al. The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: the antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials. Open Vet J. 2023;13(12):1718–1728. doi:10.5455/OVJ.2023.v13.i12.20
112. Abdel-Raheem IT, Abdel-Ghany AA. Hesperidin alleviates doxorubicin-induced cardiotoxicity in rats. J Egypt Natl Canc Inst. 2009;21(2):175–184.
113. Saad S, Ahmad I, Kawish SM, et al. Improved cardioprotective effects of hesperidin solid lipid nanoparticles prepared by supercritical antisolvent technology. Colloids Surf B Biointerfaces. 2020;187:110628. doi:10.1016/j.colsurfb.2019.110628
114. Trivedi PP, Kushwaha S, Tripathi DN, et al. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovasc Toxicol. 2011;11(3):215–225. doi:10.1007/s12012-011-9114-2
115. Li W, Qu X, Kang X, et al. Silibinin eliminates mitochondrial ROS and restores autophagy through IL6ST/JAK2/STAT3 signaling pathway to protect cardiomyocytes from doxorubicin-induced injury. Eur J Pharmacol. 2022;929:175153. doi:10.1016/j.ejphar.2022.175153
116. Kathiresan V, Subburaman S, Krishna AV, et al. Naringenin ameliorates Doxorubicin toxicity and hypoxic condition in Dalton’s Lymphoma Ascites tumor mouse model: evidence from electron paramagnetic resonance imaging. J Environ Pathol Toxicol Oncol. 2016;35(3):249–262. doi:10.1615/JEnvironPatholToxicolOncol.2016013997
117. Subburaman S, Ganesan K, Ramachandran M. Protective role of naringenin against doxorubicin-induced cardiotoxicity in a rat model: histopathology and mRNA expression profile studies. J Environ Pathol Toxicol Oncol. 2014;33(4):363–376. doi:10.1615/jenvironpatholtoxicoloncol.2014010625
118. Arafa HM, Abd-Ellah MF, Hafez HF. Abatement by naringenin of doxorubicin-induced cardiac toxicity in rats. J Egypt Natl Canc Inst. 2005;17(4):291–300.
119. Han X, Ren D, Fan P, et al. Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9C2 cells. Eur J Pharmacol. 2008;581(1–2):47–53. doi:10.1016/j.ejphar.2007.11.048
120. Han X, Pan J, Ren D, et al. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food Chem Toxicol. 2008;46(9):3140–3146. doi:10.1016/j.fct.2008.06.086
121. Sangweni NF, Gabuza K, van Aarde R, et al. Doxorubicin-induced cardiomyopathy: a preliminary study on the cardioprotective benefits of 7-Hydroxyflavanone. Int J Mol Sci. 2023;24(20):15395. doi:10.3390/ijms242015395
122. Chen G, Luo S, Guo H, et al. Licochalcone A alleviates ferroptosis in doxorubicin-induced cardiotoxicity via the PI3K/AKT/MDM2/p53 pathway. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(6):4247–4262. doi:10.1007/s00210-023-02863-1
123. Sun P, Chen H, Fan X, et al. Exploring the effective components of honey-processed licorice (Glycyrrhiza uralensis Fisch.) in attenuating Doxorubicin-induced myocardial cytotoxicity by combining network pharmacology and in vitro experiments. J Ethnopharmacol. 2024;329:118178. doi:10.1016/j.jep.2024.118178
124. Shabalala SC, Dludla PV, Muller C, et al. Aspalathin ameliorates doxorubicin-induced oxidative stress in H9c2 cardiomyoblasts. Toxicol In Vitro. 2019;55:134–139. doi:10.1016/j.tiv.2018.12.012
125. Johnson R, Shabalala S, Louw J, et al. Aspalathin reverts doxorubicin-induced cardiotoxicity through increased autophagy and decreased expression of p53/mTOR/p62 signaling. Molecules. 2017;22(10):1589. doi:10.3390/molecules22101589
126. Daimary UD, Parama D, Rana V, et al. Emerging roles of cardamonin, a multitargeted nutraceutical in the prevention and treatment of chronic diseases. Curr Res Pharmacol Drug Discov. 2021;2:100008. doi:10.1016/j.crphar.2020.100008
127. Qi W, Boliang W, Xiaoxi T, et al. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed Pharmacother. 2020;122(109547). doi:10.1016/j.biopha.2019.109547
128. Fang G, Li X, Yang F, et al. Galangin attenuates doxorubicin-induced cardiotoxicity via activating nuclear factor erythroid 2-related factor 2/heme oxygenase 1 signaling pathway to suppress oxidative stress and inflammation. Phytother Res. 2023;37(12):5854–5870. doi:10.1002/ptr.7991
129. Shu G, Chen K, Li J, et al. Galangin alleviated Doxorubicin-induced cardiotoxicity by inhibiting ferroptosis through GSTP1/JNK pathway. Phytomedicine. 2024;134:155989. doi:10.1016/j.phymed.2024.155989
130. Kuzu M, Kandemir FM, Yildirim S, et al. Morin attenuates doxorubicin-induced heart and brain damage by reducing oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2018;106:443–453. doi:10.1016/j.biopha.2018.06.161
131. Sun J, Sun G, Cui X, et al. Myricitrin protects against Doxorubicin-induced cardiotoxicity by counteracting oxidative stress and inhibiting mitochondrial apoptosis via ERK/P53 pathway. Evid Based Complement Alternat Med. 2016;2016:6093783. doi:10.1155/2016/6093783
132. Han Y, Yu H, Wang J, et al. Quercetin alleviates myocyte toxic and sensitizes anti-leukemic effect of Adriamycin. Hematology. 2015;20(5):276–283. doi:10.1179/1607845414Y.0000000198
133. Chen JY, Hu RY, Chou HC. Quercetin-induced cardioprotection against doxorubicin cytotoxicity. J Biomed Sci. 2013;20(1):95. doi:10.1186/1423-0127-20-95
134. Chen X, Peng X, Luo Y, et al. Quercetin protects cardiomyocytes against doxorubicin-induced toxicity by suppressing oxidative stress and improving mitochondrial function via 14-3-3gamma. Toxicol Mech Methods. 2019;29(5):344–354. doi:10.1080/15376516.2018.1564948
135. Dong Q, Chen L, Lu Q, et al. Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br J Pharmacol. 2014;171(19):4440–4454. doi:10.1111/bph.12795
136. Zakaria N, Khalil SR, Awad A, et al. quercetin reverses altered energy metabolism in the heart of rats receiving Adriamycin chemotherapy. Cardiovasc Toxicol. 2018;18(2):109–119. doi:10.1007/s12012-017-9420-4
137. Bartekova M, Simoncikova P, Fogarassyova M, et al. Quercetin improves postischemic recovery of heart function in doxorubicin-treated rats and prevents doxorubicin-induced matrix metalloproteinase-2 activation and apoptosis induction. Int J Mol Sci. 2015;16(4):8168–8185. doi:10.3390/ijms16048168
138. Matouk AI, Taye A, Heeba GH, et al. Quercetin augments the protective effect of losartan against chronic doxorubicin cardiotoxicity in rats. Environ Toxicol Pharmacol. 2013;36(2):443–450. doi:10.1016/j.etap.2013.05.006
139. Pei TX, Xu CQ, Li B, et al. Protective effect of quercetin against Adriamycin-induced cardiotoxicity and its mechanism in mice. Yao Xue Xue Bao. 2007;42(10):1029–1033.
140. Majhi S, Singh L, Yasir M. Evaluation of Ameliorative Effect of Quercetin and Candesartan in Doxorubicin-induced cardiotoxicity. Vasc Health Risk Manag. 2022;18:857–866. doi:10.2147/VHRM.S381485
141. Cote B, Carlson LJ, Rao DA, et al. Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J Control Release. 2015;213:128–133. doi:10.1016/j.jconrel.2015.06.040
142. Lin KH, Ramesh S, Agarwal S, et al. Fisetin attenuates doxorubicin-induced cardiotoxicity by inhibiting the insulin-like growth factor II receptor apoptotic pathway through estrogen receptor-alpha/-beta activation. Phytother Res. 2023;37(9):3964–3981. doi:10.1002/ptr.7855
143. Ma T, Kandhare AD, Mukherjee-Kandhare AA, et al. Fisetin, a plant flavonoid ameliorates doxorubicin-induced cardiotoxicity in experimental rats: the decisive role of caspase-3, COX-II, cTn-I, iNOs and TNF-alpha. Mol Biol Rep. 2019;46(1):105–118. doi:10.1007/s11033-018-4450-y
144. Ma Y, Yang L, Ma J, et al. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):1904–1911. doi:10.1016/j.bbadis.2016.12.021
145. Qin M, Li Q, Wang Y, et al. Rutin treats myocardial damage caused by pirarubicin via regulating miR-22-5p-regulated RAP1/ERK signaling pathway. J Biochem Mol Toxicol. 2021;35(1):e22615. doi:10.1002/jbt.22615
146. Li Q, Qin M, Li T, et al. Rutin protects against pirarubicin-induced cardiotoxicity by adjusting microRNA-125b-1-3p-mediated JunD signaling pathway. Mol Cell Biochem. 2020;466(1–2):139–148. doi:10.1007/s11010-020-03696-9
147. Fei J, Sun Y, Duan Y, et al. Low concentration of rutin treatment might alleviate the cardiotoxicity effect of pirarubicin on cardiomyocytes via activation of PI3K/AKT/mTOR signaling pathway. Biosci Rep. 2019;39(6):BSR20190546. doi:10.1042/BSR20190546
148. Xiao J, Sun GB, Sun B, et al. Kaempferol protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. Toxicology. 2012;292(1):53–62. doi:10.1016/j.tox.2011.11.018
149. Janeesh PA, Abraham A. Robinin modulates doxorubicin-induced cardiac apoptosis by TGF-beta1 signaling pathway in Sprague Dawley rats. Biomed Pharmacother. 2014;68(8):989–998. doi:10.1016/j.biopha.2014.09.010
150. Abhirami N, Ayyappan JP. Cardioprotective effect of Robinin ameliorates endoplasmic reticulum stress and apoptosis in H9c2 cells. Cell Biochem Biophys. 2024;82(4):3681–3694. doi:10.1007/s12013-024-01456-w
151. Sun J, Sun G, Meng X, et al. Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS One. 2013;8(5):e64526. doi:10.1371/journal.pone.0064526
152. Choi EH, Chang HJ, Cho JY, et al. Cytoprotective effect of anthocyanins against doxorubicin-induced toxicity in H9c2 cardiomyocytes in relation to their antioxidant activities. Food Chem Toxicol. 2007;45(10):1873–1881. doi:10.1016/j.fct.2007.04.003
153. Petroni K, Trinei M, Fornari M, et al. Dietary cyanidin 3-glucoside from purple corn ameliorates doxorubicin-induced cardiotoxicity in mice. Nutr Metab Cardiovasc Dis. 2017;27(5):462–469. doi:10.1016/j.numecd.2017.02.002
154. Liu C, Wang Y, Zeng Y, et al. Use of deep-learning assisted assessment of cardiac parameters in Zebrafish to Discover Cyanidin Chloride as a Novel Keap1 inhibitor against Doxorubicin-induced cardiotoxicity. Adv Sci. 2023;10(30):e2301136. doi:10.1002/advs.202301136
155. Saleh AA. Potential protective effect of catechin on doxorubicin-induced cardiotoxicity in adult male albino rats. Toxicol Mech Methods. 2022;32(2):97–105. doi:10.1080/15376516.2021.1972375
156. Abd ET, Mohamed RH, Pasha HF, et al. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in Adriamycin-treated rats. Clin Exp Med. 2012;12(4):233–240. doi:10.1007/s10238-011-0165-2
157. Yao YF, Liu X, Li WJ, et al. (-)-Epigallocatechin-3-gallate alleviates doxorubicin-induced cardiotoxicity in sarcoma 180 tumor-bearing mice. Life Sci. 2017;180:151–159. doi:10.1016/j.lfs.2016.12.004
158. Zheng J, Lee HC, Bin SM, et al. Cardioprotective effects of epigallocatechin-3-gallate against doxorubicin-induced cardiomyocyte injury. Eur J Pharmacol. 2011;652(1–3):82–88. doi:10.1016/j.ejphar.2010.10.082
159. Li W, Nie S, Xie M, et al. A major green tea component, (-)-epigallocatechin-3-gallate, ameliorates doxorubicin-mediated cardiotoxicity in cardiomyocytes of neonatal rats. J Agric Food Chem. 2010;58(16):8977–8982. doi:10.1021/jf101277t
160. Saeed NM, El-Naga RN, El-Bakly WM, et al. Epigallocatechin-3-gallate pretreatment attenuates doxorubicin-induced cardiotoxicity in rats: a mechanistic study. Biochem Pharmacol. 2015;95(3):145–155. doi:10.1016/j.bcp.2015.02.006
161. Ahmad S, Ahsan F, Ansari JA, et al. Bioflavonoid Daidzein: therapeutic insights, formulation advances, and future directions. Drug Res. 2024;74(9):433–455. doi:10.1055/a-2379-6849
162. Goleij P, Sanaye PM, Alam W, et al. Unlocking daidzein’s healing power: present applications and future possibilities in phytomedicine. Phytomedicine. 2024;134:155949. doi:10.1016/j.phymed.2024.155949
163. Laddha AP, Kulkarni YA. Pharmacokinetics, pharmacodynamics, toxicity, and formulations of daidzein: an important isoflavone. Phytother Res. 2023;37(6):2578–2604. doi:10.1002/ptr.7852
164. Gao J, Liu ZJ, Chen T, et al. Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of Radix astragali. Pharm Biol. 2014;52(9):1217–1222. doi:10.3109/13880209.2013.879188
165. Li M, Han B, Zhao H, et al. Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine. 2022;98:153918. doi:10.1016/j.phymed.2021.153918
166. Deng M, Chen H, Long J, et al. Calycosin: a review of its pharmacological effects and application prospects. Expert Rev Anti Infect Ther. 2021;19(7):911–925. doi:10.1080/14787210.2021.1863145
167. Pan L, Zhang XF, Wei WS, et al. The cardiovascular protective effect and mechanism of calycosin and its derivatives. Chin J Nat Med. 2020;18(12):907–915. doi:10.1016/S1875-5364(20)60034-6
168. Sharma U, Sharma B, Mishra A, et al. Ononin: a comprehensive review of anticancer potential of natural isoflavone glycoside. J Biochem Mol Toxicol. 2024;38(6):e23735. doi:10.1002/jbt.23735
169. Ganesan K, Xu C, Wu J, et al. Ononin inhibits triple-negative breast cancer lung metastasis by targeting the EGFR-mediated PI3K/Akt/mTOR pathway. Sci China Life Sci. 2024;67(9):1849–1866. doi:10.1007/s11427-023-2499-2
170. Zhang YJ, Mu ZL, Deng P, et al. 8-Formylophiopogonanone B induces ROS-mediated apoptosis in nasopharyngeal carcinoma CNE-1 cells. Toxicol Res. 2021;10(5):1052–1063. doi:10.1093/toxres/tfab087
171. Dixon RA, Ferreira D. Genistein. Phytochemistry. 2002;60(3):205–211. doi:10.1016/s0031-9422(02)00116-4
172. Sharifi-Rad J, Quispe C, Imran M, et al. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev. 2021;2021:3268136. doi:10.1155/2021/3268136
173. Meng F, Guo B, Ma YQ, et al. Puerarin: a review of its mechanisms of action and clinical studies in ophthalmology. Phytomedicine. 2022;107:154465. doi:10.1016/j.phymed.2022.154465
174. Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28(7):961–975. doi:10.1002/ptr.5083
175. Xu J, Sun S, Zhang W, et al. Irigenin inhibits glioblastoma progression through suppressing YAP/beta-catenin signaling. Front Pharmacol. 2022;13:1027577. doi:10.3389/fphar.2022.1027577
176. Hu Q, Hou S, Xiong B, et al. Therapeutic effects of Baicalin on diseases related to gut-brain axis dysfunctions. Molecules. 2023;28(18):6501. doi:10.3390/molecules28186501
177. Huang Y, Tsang SY, Yao X, et al. Biological properties of baicalein in cardiovascular system. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5(2):177–184. doi:10.2174/1568006043586206
178. Munjal K, Goel Y, Gauttam VK, et al. Molecular targets and therapeutic potential of baicalein: a review. Drug Target Insights. 2024;18:30–46. doi:10.33393/dti.2024.2707
179. Wei Z, Chen J, Zuo F, et al. Traditional Chinese medicine has great potential as candidate drugs for lung cancer: a review. J Ethnopharmacol. 2023;300:115748. doi:10.1016/j.jep.2022.115748
180. Ziqubu K, Dludla PV, Joubert E, et al. Isoorientin: a dietary flavone with the potential to ameliorate diverse metabolic complications. Pharmacol Res. 2020;158:104867. doi:10.1016/j.phrs.2020.104867
181. Wu T, Ma W, Lu W, et al. Vaccarin alleviates cisplatin-induced acute kidney injury via decreasing NOX4-derived ROS. Heliyon. 2023;9(11):e21231. doi:10.1016/j.heliyon.2023.e21231
182. Zhu X, Meng X, Du X, et al. Vaccarin suppresses diabetic nephropathy through inhibiting the EGFR/ERK1/2 signaling pathway. Acta Biochim Biophys Sin. 2024;56(12):1860–1874. doi:10.3724/abbs.2024141
183. Naz S, Imran M, Rauf A, et al. Chrysin: pharmacological and therapeutic properties. Life Sci. 2019;235:116797. doi:10.1016/j.lfs.2019.116797
184. Mani R, Natesan V. Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry. 2018;145:187–196. doi:10.1016/j.phytochem.2017.09.016
185. Nageen B, Rasul A, Hussain G, et al. Jaceosidin: a natural flavone with versatile pharmacological and biological activities. Curr Pharm Des. 2021;27(4):456–466. doi:10.2174/1381612826666200429095101
186. Liu J, Li SM, Tang YJ, et al. Jaceosidin induces apoptosis and inhibits migration in AGS gastric cancer cells by regulating ROS-mediated signaling pathways. Redox Rep. 2024;29(1):2313366. doi:10.1080/13510002.2024.2313366
187. Law SK, Wu XX, Jiang Z, et al. Pharmacological activities of Lonicerae japonicae flos and its derivative-“Chrysoeriol” in skin diseases. Molecules. 2024;29(9):1972. doi:10.3390/molecules29091972
188. Aboulaghras S, Sahib N, Bakrim S, et al. Health benefits and pharmacological aspects of chrysoeriol. Pharmaceuticals. 2022;15(8):973. doi:10.3390/ph15080973
189. Shen X, Liu Y, Luo X, et al. Advances in biosynthesis, pharmacology, and pharmacokinetics of pinocembrin, a promising natural small-molecule drug. Molecules. 2019;24(12):2323. doi:10.3390/molecules24122323
190. Chen X, Wan W, Guo Y, et al. Pinocembrin ameliorates post-infarct heart failure through activation of Nrf2/HO-1 signaling pathway. Mol Med. 2021;27(1):100. doi:10.1186/s10020-021-00363-7
191. Elbatreek MH, Mahdi I, Ouchari W, et al. Current advances on the therapeutic potential of pinocembrin: an updated review. Biomed Pharmacother. 2023;157:114032. doi:10.1016/j.biopha.2022.114032
192. Lu L, Guo Q, Zhao L. Overview of Oroxylin A: a promising flavonoid compound. Phytother Res. 2016;30(11):1765–1774. doi:10.1002/ptr.5694
193. Sajeev A, Hegde M, Girisa S, et al. Oroxylin A: a promising flavonoid for prevention and treatment of chronic diseases. Biomolecules. 2022;12(9):1185. doi:10.3390/biom12091185
194. Wang SY, Wang YJ, Dong MQ, et al. Acacetin is a promising drug candidate for cardiovascular diseases. Am J Chin Med. 2024;52(6):1661–1692. doi:10.1142/S0192415X24500654
195. Singh S, Gupta P, Meena A, et al. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem Toxicol. 2020;145:111708. doi:10.1016/j.fct.2020.111708
196. Wang Z, Cao Z, Yue Z, et al. Research progress of dihydromyricetin in the treatment of diabetes mellitus. Front Endocrinol. 2023;14:1216907. doi:10.3389/fendo.2023.1216907
197. Salehi B, Venditti A, Sharifi-Rad M, et al. The Therapeutic Potential of Apigenin. Int J Mol Sci. 2019;20(6). doi:10.3390/ijms20061305
198. Charriere K, Schneider V, Perrignon-Sommet M, et al. Exploring the role of Apigenin in neuroinflammation: insights and implications. Int J Mol Sci. 2024;25(9):5041. doi:10.3390/ijms25095041
199. Lee IG, Lee J, Hong SH, et al. Apigenin’s therapeutic potential against viral infection. Front Biosci. 2023;28(10):237. doi:10.31083/j.fbl2810237
200. Ge HC, Zhong XH. Research progress on anti-tumor mechanisms of scutellarin. J Asian Nat Prod Res. 2024;26(11):1261–1275. doi:10.1080/10286020.2024.2362375
201. Xie Y, Sun G, Tao Y, et al. Current advances on the therapeutic potential of scutellarin: an updated review. Nat Prod Bioprospect. 2024;14(1):20. doi:10.1007/s13659-024-00441-3
202. Nie S, Zhang S, Wu R, et al. Scutellarin: pharmacological effects and therapeutic mechanisms in chronic diseases. Front Pharmacol. 2024;15:1470879. doi:10.3389/fphar.2024.1470879
203. Zeng Y, Xiong Y, Yang T, et al. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: from effects to molecular mechanisms. Biomed Pharmacother. 2022;147:112642. doi:10.1016/j.biopha.2022.112642
204. Luo Z, Dong J, Wu J. Impact of Icariin and its derivatives on inflammatory diseases and relevant signaling pathways. Int Immunopharmacol. 2022;108:108861. doi:10.1016/j.intimp.2022.108861
205. Wang M, Gao H, Li W, et al. Icariin and its metabolites regulate lipid metabolism: from effects to molecular mechanisms. Biomed Pharmacother. 2020;131:110675. doi:10.1016/j.biopha.2020.110675
206. Jin J, Wang H, Hua X, et al. An outline for the pharmacological effect of icariin in the nervous system. Eur J Pharmacol. 2019;842:20–32. doi:10.1016/j.ejphar.2018.10.006
207. Lee BE, Park SJ, Kim GH, et al. Anti-inflammatory effects of eupatilin on Helicobacter pylori CagA-induced gastric inflammation. PLoS One. 2024;19(11):e0313251. doi:10.1371/journal.pone.0313251
208. Zhao B, Chen Z, Li T, et al. Eupatilin suppresses osteoclastogenesis and periodontal bone loss by inhibiting the MAPKs/Siglec-15 pathway. Int Immunopharmacol. 2024;139:112720. doi:10.1016/j.intimp.2024.112720
209. Mahwish, Imran M, Naeem H, et al. Antioxidative and anticancer potential of Luteolin: a comprehensive approach against wide range of human malignancies. Food Sci Nutr. 2025;13(1):e4682. doi:10.1002/fsn3.4682
210. Ren F, Li Y, Luo H, et al. Extraction, detection, bioactivity, and product development of luteolin: a review. Heliyon. 2024;10(24):e41068. doi:10.1016/j.heliyon.2024.e41068
211. Shi Y, Li F, Shen M, et al. Luteolin prevents cardiac dysfunction and improves the chemotherapeutic efficacy of Doxorubicin in breast cancer. Front Cardiovasc Med. 2021;8:750186. doi:10.3389/fcvm.2021.750186
212. Huwait E, Mobashir M. Potential and therapeutic roles of Diosmin in human diseases. Biomedicines. 2022;10(5):1076. doi:10.3390/biomedicines10051076
213. Hassanein E, Althagafy HS, Baraka MA, et al. Hepatoprotective effects of diosmin: a narrative review. Naunyn Schmiedebergs Arch Pharmacol. 2025;398(1):279–295. doi:10.1007/s00210-024-03297-z
214. Gerges SH, Wahdan SA, Elsherbiny DA, et al. Pharmacology of Diosmin, a Citrus Flavone Glycoside: an updated review. Eur J Drug Metab Pharmacokinet. 2022;47(1):1–18. doi:10.1007/s13318-021-00731-y
215. Mustafa S, Akbar M, Khan MA, et al. Plant metabolite diosmin as the therapeutic agent in human diseases. Curr Res Pharmacol Drug Discov. 2022;3:100122. doi:10.1016/j.crphar.2022.100122
216. Shi M, Zhang J, Li M, et al. Liquiritigenin confers liver protection by enhancing NRF2 signaling through both canonical and non-canonical signaling pathways. J Med Chem. 2023;66(16):11324–11334. doi:10.1021/acs.jmedchem.3c00815
217. Ramalingam M, Kim H, Lee Y, et al. Phytochemical and pharmacological role of Liquiritigenin and Isoliquiritigenin from Radix Glycyrrhizae in human health and disease models. Front Aging Neurosci. 2018;10:348. doi:10.3389/fnagi.2018.00348
218. Shi CC, Qin KM, Xu K, et al. Development of liquiritigenin-phospholipid complex with the enhanced oral bioavailability. Chin J Nat Med. 2020;18(12):916–921. doi:10.1016/S1875-5364(20)60035-8
219. Shilpa VS, Shams R, Dash KK, et al. Phytochemical properties, extraction, and pharmacological benefits of Naringin: a review. Molecules. 2023;28(15):5623. doi:10.3390/molecules28155623
220. Bajgai B, Suri M, Singh H, et al. Naringin: a flavanone with a multifaceted target against sepsis-associated organ injuries. Phytomedicine. 2024;130(155707). doi:10.1016/j.phymed.2024.155707
221. Hu HY, Zhang ZZ, Jiang XY, et al. Hesperidin anti-osteoporosis by regulating estrogen signaling pathways. Molecules. 2023;28(19):6987. doi:10.3390/molecules28196987
222. Hajialyani M, Hosein FM, Echeverria J, et al. Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules. 2019;24(3):648. doi:10.3390/molecules24030648
223. Pyrzynska K. Hesperidin: a review on extraction methods, stability and biological activities. Nutrients. 2022;14(12):2387. doi:10.3390/nu14122387
224. Zare MP, Asadi S, Ehsani E, et al. Silibinin as a major component of milk thistle seed provides promising influences against diabetes and its complications: a systematic review. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(10):7531–7549. doi:10.1007/s00210-024-03172-x
225. Polachi N, Bai G, Li T, et al. Modulatory effects of silibinin in various cell signaling pathways against liver disorders and cancer - A comprehensive review. Eur J Med Chem. 2016;123:577–595. doi:10.1016/j.ejmech.2016.07.070
226. Goyal A, Verma A, Dubey N, et al. Naringenin: a prospective therapeutic agent for Alzheimer’s and Parkinson’s disease. J Food Biochem. 2022;46(12):e14415. doi:10.1111/jfbc.14415
227. Alam MA, Subhan N, Rahman MM, et al. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5(4):404–417. doi:10.3945/an.113.005603
228. Ucar K, Goktas Z. Biological activities of naringenin: a narrative review based on in vitro and in vivo studies. Nutr Res. 2023;119:43–55. doi:10.1016/j.nutres.2023.08.006
229. Wroblewski T, Ushakou DV. Photophysical properties of 7-hydroxyflavanone: quantum chemical calculations and experimental studies. Spectrochim Acta A Mol Biomol Spectrosc. 2019;215:81–87. doi:10.1016/j.saa.2019.02.092
230. Olloquequi J, Ettcheto M, Cano A, et al. Licochalcone A: a potential multitarget drug for Alzheimer’s disease treatment. Int J Mol Sci. 2023;24(18):14177. doi:10.3390/ijms241814177
231. Kwon YJ, Son DH, Chung TH, et al. A review of the pharmacological efficacy and safety of Licorice Root from Corroborative clinical trial findings. J Med Food. 2020;23(1):12–20. doi:10.1089/jmf.2019.4459
232. Li MT, Xie L, Jiang HM, et al. Role of Licochalcone A in potential pharmacological therapy: a review. Front Pharmacol. 2022;13:878776. doi:10.3389/fphar.2022.878776
233. Mazibuko-Mbeje SE, Dludla PV, Johnson R, et al. Aspalathin, a natural product with the potential to reverse hepatic insulin resistance by improving energy metabolism and mitochondrial respiration. PLoS One. 2019;14(5):e0216172. doi:10.1371/journal.pone.0216172
234. Muller C, Joubert E, Chellan N, et al. New insights into the efficacy of aspalathin and other related phytochemicals in Type 2 Diabetes-A review. Int J Mol Sci. 2021;23(1):356. doi:10.3390/ijms23010356
235. Dludla PV, Muller CJ, Joubert E, et al. Aspalathin protects the heart against hyperglycemia-induced oxidative damage by up-regulating Nrf2 expression. Molecules. 2017;22(1):129. doi:10.3390/molecules22010129
236. Smit SE, Manirafasha C, Marais E, et al. Cardioprotective function of Green Rooibos (Aspalathus linearis) extract supplementation in ex vivo ischemic prediabetic rat hearts. Planta Med. 2022;88(1):62–78. doi:10.1055/a-1239-9236
237. Johnson R, Dludla PV, Muller CJ, et al. The transcription profile unveils the cardioprotective effect of aspalathin against lipid toxicity in an in vitro H9c2 model. Molecules. 2017;22(2):219. doi:10.3390/molecules22020219
238. Barber K, Mendonca P, Soliman K. The neuroprotective effects and therapeutic potential of the Chalcone Cardamonin for Alzheimer’s Disease. Brain Sci. 2023;13(1):145. doi:10.3390/brainsci13010145
239. Thapa R, Afzal O, Alfawaz AA, et al. Galangin as an inflammatory response modulator: an updated overview and therapeutic potential. Chem Biol Interact. 2023;378:110482. doi:10.1016/j.cbi.2023.110482
240. Wang D, Chen J, Pu L, et al. Galangin: a food-derived flavonoid with therapeutic potential against a wide spectrum of diseases. Phytother Res. 2023;37(12):5700–5723. doi:10.1002/ptr.8013
241. Zhang F, Yan Y, Zhang LM, et al. Pharmacological activities and therapeutic potential of galangin, a promising natural flavone, in age-related diseases. Phytomedicine. 2023;120:155061. doi:10.1016/j.phymed.2023.155061
242. Hassanein E, Abd EM, Ibrahim IM, et al. The molecular mechanisms underlying anti-inflammatory effects of galangin in different diseases. Phytother Res. 2023;37(7):3161–3181. doi:10.1002/ptr.7874
243. Khawaja G, El-Orfali Y, Shoujaa A, et al. Galangin: a promising flavonoid for the treatment of rheumatoid arthritis-mechanisms, evidence, and therapeutic potential. Pharmaceuticals. 2024;17(7):963. doi:10.3390/ph17070963
244. Caselli A, Cirri P, Santi A, et al. Morin: a Promising Natural Drug. Curr Med Chem. 2016;23(8):774–791. doi:10.2174/0929867323666160106150821
245. Rajput SA, Wang XQ, Yan HC. Morin hydrate: a comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed Pharmacother. 2021;138:111511. doi:10.1016/j.biopha.2021.111511
246. Geng Y, Xie Y, Li W, et al. Toward the bioactive potential of myricitrin in food production: state-of-the-art green extraction and trends in biosynthesis. Crit Rev Food Sci Nutr. 2024;64(29):10668–10694. doi:10.1080/10408398.2023.2227262
247. Zhang X, Zhang K, Wang Y, et al. Effects of myricitrin and relevant molecular mechanisms. Curr Stem Cell Res Ther. 2020;15(1):11–17. doi:10.2174/1574888X14666181126103338
248. Nguyen T, Bhattacharya D. Antimicrobial activity of quercetin: an approach to its mechanistic principle. Molecules. 2022;27(8):2494. doi:10.3390/molecules27082494
249. Hosseini A, Razavi BM, Banach M, et al. Quercetin and metabolic syndrome: a review. Phytother Res. 2021;35(10):5352–5364. doi:10.1002/ptr.7144
250. Deepika, Maurya PK. Health benefits of quercetin in age-related diseases. Molecules. 2022;27(8):2498. doi:10.3390/molecules27082498
251. Dorostkar H, Haghiralsadat BF, Hemati M, et al. Reduction of doxorubicin-induced cardiotoxicity by co-administration of smart liposomal doxorubicin and free quercetin: in vitro and in vivo studies. Pharmaceutics. 2023;15(7):1920. doi:10.3390/pharmaceutics15071920
252. Szymczak J, Cielecka-Piontek J. Fisetin-in search of better bioavailability-from macro to nano modifications: a review. Int J Mol Sci. 2023;24(18):14158. doi:10.3390/ijms241814158
253. Markowska A, Antoszczak M, Kacprzak K, et al. Role of fisetin in selected malignant neoplasms in women. Nutrients. 2023;15(21):4686. doi:10.3390/nu15214686
254. Tavenier J, Nehlin JO, Houlind MB, et al. Fisetin as a senotherapeutic agent: evidence and perspectives for age-related diseases. Mech Ageing Dev. 2024;222:111995. doi:10.1016/j.mad.2024.111995
255. Forouzanfar F, Sahranavard T, Tsatsakis A, et al. Rutin: a pain-relieving flavonoid. Inflammopharmacology. 2025;33(3):1289–1301. doi:10.1007/s10787-025-01671-8
256. Ganeshpurkar A, Saluja AK. The pharmacological potential of rutin. Saudi Pharm J. 2017;25(2):149–164. doi:10.1016/j.jsps.2016.04.025
257. Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed Pharmacother. 2017;96:305–312. doi:10.1016/j.biopha.2017.10.001
258. Pandey P, Khan F, Qari HA, et al. Rutin (Bioflavonoid) as cell signaling pathway modulator: prospects in treatment and chemoprevention. Pharmaceuticals. 2021;14(11):1069. doi:10.3390/ph14111069
259. Chen M, Xiao J, El-Seedi HR, et al. Kaempferol and atherosclerosis: from mechanism to medicine. Crit Rev Food Sci Nutr. 2024;64(8):2157–2175. doi:10.1080/10408398.2022.2121261
260. Imran M, Salehi B, Sharifi-Rad J, et al. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24(12):2277. doi:10.3390/molecules24122277
261. Kong L, Luo C, Li X, et al. The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis. 2013;12:115. doi:10.1186/1476-511X-12-115
262. Xiao HB, Jun-Fang, Lu XY, et al. Protective effects of kaempferol against endothelial damage by an improvement in nitric oxide production and a decrease in asymmetric dimethylarginine level. Eur J Pharmacol. 2009;616(1–3):213–222. doi:10.1016/j.ejphar.2009.06.022
263. Feng Z, Wang C, Yue, et al. Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm Biol. 2021;59(1):1106–1116. doi:10.1080/13880209.2021.1961823
264. Devi KP, Malar DS, Nabavi SF, et al. Kaempferol and inflammation: from chemistry to medicine. Pharmacol Res. 2015;99:1–10. doi:10.1016/j.phrs.2015.05.002
265. Tsiklauri L, Svik K, Chrastina M, et al. Bioflavonoid Robinin from Astragalus falcatus Lam. Mildly improves the effect of metothrexate in rats with adjuvant arthritis. Nutrients. 2021;13(4):1268. doi:10.3390/nu13041268
266. Li N, Alzahrani FM, El SM, et al. Nephroprotective potential of robinin to counteract aldicarb induced renal dysfunction via modulating TLR4/MyD88, HMGB1/RAGE, NF-kappaB pathway: a biochemical and pharmacodynamic approach. Food Chem Toxicol. 2025;197:115298. doi:10.1016/j.fct.2025.115298
267. Wq L, Li J, Liu WX, et al. Isorhamnetin: a novel natural product beneficial for cardiovascular disease. Curr Pharm Des. 2022;28(31):2569–2582. doi:10.2174/1381612828666220829113132
268. Gong G, Guan YY, Zhang ZL, et al. Isorhamnetin: a review of pharmacological effects. Biomed Pharmacother. 2020;128:110301. doi:10.1016/j.biopha.2020.110301
269. Rodriguez L, Badimon L, Mendez D, et al. Antiplatelet activity of isorhamnetin via mitochondrial regulation. Antioxidants. 2021;10(5):666. doi:10.3390/antiox10050666
270. Alappat B, Alappat J. Anthocyanin pigments: beyond aesthetics. Molecules. 2020;25(23):5500. doi:10.3390/molecules25235500
271. Zhao CL, Chen ZJ, Bai XS, et al. Structure-activity relationships of anthocyanidin glycosylation. Mol Divers. 2014;18(3):687–700. doi:10.1007/s11030-014-9520-z
272. Al-Dashti YA, Holt RR, Stebbins CL, et al. Dietary flavanols: a review of select effects on vascular function, blood pressure, and exercise performance. J Am Coll Nutr. 2018;37(7):553–567. doi:10.1080/07315724.2018.1451788
273. Luo Y, Jian Y, Liu Y, et al. Flavanols from nature: a phytochemistry and biological activity review. Molecules. 2022;27(3):719. doi:10.3390/molecules27030719
274. Farhan M. Green tea catechins: nature’s way of preventing and treating cancer. Int J Mol Sci. 2022;23(18):10713. doi:10.3390/ijms231810713
275. Bernatoniene J, Kopustinskiene DM. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23(4):965. doi:10.3390/molecules23040965
276. Abou EHM, Kedde MA, Zwiers UT, et al. Bioavailability and pharmacokinetics of the cardioprotecting flavonoid 7-monohydroxyethylrutoside in mice. Cancer Chemother Pharmacol. 2003;52(5):371–376. doi:10.1007/s00280-003-0667-z
277. Abou EHM, Heijn M, Rabelink MJ, et al. The protective effect of cardiac gene transfer of CuZn-sod in comparison with the cardioprotector monohydroxyethylrutoside against doxorubicin-induced cardiotoxicity in cultured cells. Cancer Gene Ther. 2003;10(4):270–277. doi:10.1038/sj.cgt.7700564
278. Abou-El-Hassan MA, Rabelink MJ, van der Vijgh WJ, et al. A comparative study between catalase gene therapy and the cardioprotector monohydroxyethylrutoside (MonoHER) in protecting against doxorubicin-induced cardiotoxicity in vitro. Br J Cancer. 2003;89(11):2140–2146. doi:10.1038/sj.bjc.6601430
279. Bruynzeel AM, Abou EHM, Torun E, et al. Caspase-dependent and -independent suppression of apoptosis by monoHER in doxorubicin treated cells. Br J Cancer. 2007;96(3):450–456. doi:10.1038/sj.bjc.6603598
280. van Acker SA, Kramer K, Grimbergen JA, et al. Monohydroxyethylrutoside as protector against chronic doxorubicin-induced cardiotoxicity. Br J Pharmacol. 1995;115(7):1260–1264. doi:10.1111/j.1476-5381.1995.tb15034.x
281. van Acker FA, van Acker SA, Kramer K, et al. 7-monohydroxyethylrutoside protects against chronic doxorubicin-induced cardiotoxicity when administered only once per week. Clin Cancer Res. 2000;6(4):1337–1341.
282. Bruynzeel AM, Vormer-Bonne S, Bast A, et al. Long-term effects of 7-monohydroxyethylrutoside (monoHER) on DOX-induced cardiotoxicity in mice. Cancer Chemother Pharmacol. 2007;60(4):509–514. doi:10.1007/s00280-006-0395-2
283. Bruynzeel AM, Abou EHM, Schalkwijk C, et al. Anti-inflammatory agents and monoHER protect against DOX-induced cardiotoxicity and accumulation of CML in mice. Br J Cancer. 2007;96(6):937–943. doi:10.1038/sj.bjc.6603640
284. Willems AM, Bruynzeel AM, Kedde MA, et al. A phase I study of monohydroxyethylrutoside in healthy volunteers. Cancer Chemother Pharmacol. 2006;57(5):678–684. doi:10.1007/s00280-005-0083-7
285. Bruynzeel AM, Niessen HW, Bronzwaer JG, et al. The effect of monohydroxyethylrutoside on doxorubicin-induced cardiotoxicity in patients treated for metastatic cancer in a phase II study. Br J Cancer. 2007;97(8):1084–1089. doi:10.1038/sj.bjc.6603994
286. Cecen E, Dost T, Culhaci N, et al. Protective effects of silymarin against doxorubicin-induced toxicity. Asian Pac J Cancer Prev. 2011;12(10):2697–2704.
287. Raskovic A, Stilinovic N, Kolarovic J, et al. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules. 2011;16(10):8601–8613. doi:10.3390/molecules16108601
288. Patel N, Joseph C, Corcoran GB, et al. Silymarin modulates doxorubicin-induced oxidative stress, Bcl-xL and p53 expression while preventing apoptotic and necrotic cell death in the liver. Toxicol Appl Pharmacol. 2010;245(2):143–152. doi:10.1016/j.taap.2010.02.002
289. Ipek E, Tunca R. Silymarin protects against doxorubicin induced cardiotoxicity by down-regulating topoisomerase IIbeta expression in mice. Biotech Histochem. 2023;98(6):412–423. doi:10.1080/10520295.2023.2218648
290. Psotova J, Chlopcikova S, Grambal F, et al. Influence of silymarin and its flavonolignans on doxorubicin-iron induced lipid peroxidation in rat heart microsomes and mitochondria in comparison with quercetin. Phytother Res. 2002;16(Suppl 1):S63–S67. doi:10.1002/ptr.811
291. Stilinovic N, Raskovic A. [Influence of silymarin and doxorubicin on the myocardial function in rats]. Med Pregl. 2008;61(1–2):95–98.
292. El-Shitany NA, El-Haggar S, El-desoky K. Silymarin prevents Adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food Chem Toxicol. 2008;46(7):2422–2428. doi:10.1016/j.fct.2008.03.033
293. Hagag AA, El SW, El-Abasy AI, et al. Protective role of silymarin in early doxorubicin-induced cardiac dysfunction in children with acute lymphoblastic leukemia. Infect Disord Drug Targets. 2019;19(2):133–140. doi:10.2174/1871526518666180803141827
294. Abou EHM, Kedde MA, Bast A, et al. Determination of monohydroxyethylrutoside in heart tissue by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl. 2001;757(1):191–196. doi:10.1016/s0378-4347(01)00050-0
295. Abou EHM, Kedde MA, Zwiers UT, et al. The cardioprotector monoHER does not interfere with the pharmacokinetics or the metabolism of the cardiotoxic agent doxorubicin in mice. Cancer Chemother Pharmacol. 2003;51(4):306–310. doi:10.1007/s00280-003-0582-3
296. Teng H, Zheng Y, Cao H, et al. Enhancement of bioavailability and bioactivity of diet-derived flavonoids by application of nanotechnology: a review. Crit Rev Food Sci Nutr. 2023;63(3):378–393. doi:10.1080/10408398.2021.1947772
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Evaluation of Ameliorative Effect of Quercetin and Candesartan in Doxorubicin-Induced Cardiotoxicity
Majhi S, Singh L, Yasir M
Vascular Health and Risk Management 2022, 18:857-866
Published Date: 13 December 2022
Analysis and Validation of Critical Signatures and Immune Cell Infiltration Characteristics in Doxorubicin-Induced Cardiotoxicity by Integrating Bioinformatics and Machine Learning
Huang C, Pei J, Li D, Liu T, Li Z, Zhang G, Chen R, Xu X, Li B, Lian Z, Chu XM
Journal of Inflammation Research 2024, 17:669-685
Published Date: 2 February 2024
Irisin Mitigates Doxorubicin-Induced Cardiotoxicity by Reducing Oxidative Stress and Inflammation via Modulation of the PERK-eIF2α-ATF4 Pathway
Zhang Z, Yu X, Li J, Shen X, Fu W, Liu Y, Dong X, Wang Z
Drug Design, Development and Therapy 2025, 19:1067-1081
Published Date: 15 February 2025

