Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 18
Mesenchymal Stem Cell Secretome Effectiveness in Healing Chronic Tendon Injury: Procollagen Analysis and Histopathology in Rat Tendons
Authors Sam ADP , Warsinggih W, Usman MA , Johan MP, Suroto H , Saleh MR, Sakti M, Zainuddin AA, Mubarak AF
Received 24 December 2024
Accepted for publication 1 April 2025
Published 9 April 2025 Volume 2025:18 Pages 35—43
DOI https://doi.org/10.2147/SCCAA.S512079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Andi Dhedie Prasatia Sam,1– 3 Warsinggih Warsinggih,4 Muhammad Andry Usman,5 Muhammad Phetrus Johan,5 Heri Suroto,6 M Ruksal Saleh,5 Muhammad Sakti,5 Andi Alfian Zainuddin,2,7 Andi Firman Mubarak1
1Faculty of Medicine, Universitas Muslim Indonesia, Makassar, Indonesia; 2Doctoral Study Program, Faculty of Medicine, Universitas Hasanuddin, Makassar, Indonesia; 3Stem Cell Research and Development Center, Universitas Airlangga, Surabaya, Indonesia; 4Department of Surgery, Faculty of Medicine, Universitas Hasanuddin, Makassar, Indonesia; 5Department of Orthopaedic and Traumatology, Faculty of Medicine, Universitas Hasanuddin, Makassar, Indonesia; 6Department of Orthopaedic and Traumatology, Faculty of Medicine, Dr. Soetomo General Academic Hospital, Universitas Airlangga, Surabaya, Indonesia; 7Faculty of Medicine, Universitas Hasanuddin, Makassar, Indonesia
Correspondence: Andi Dhedie Prasatia Sam, Faculty of Medicine, Universitas Muslim Indonesia, Jl. Urip Sumoharjo No. km.5, Makassar, South Sulawesi, 90231, Indonesia, Tel +62411-443280, Fax +62411– 432730, Email [email protected]
Background: Chronic tendon injuries often lead to diminished healing capacity, necessitating innovative treatments. Mesenchymal stem cells (MSCs) secretome has emerged as a promising option for enhancing tendon repair through paracrine signaling. This study evaluates the effectiveness of MSC secretome, derived from tendon-derived stem cells (TDSCs) and adipose-derived stem cells (ASCs) in healing chronic Achilles tendon injuries in a rat model. The focus is on Procollagen Type I N-Terminal Peptide (PINP) and Procollagen Type III N-Terminal Peptide (PIIINP) levels, and histopathological changes.
Methods: Fourteen adult male rats were divided into four groups: Group I (TDSC secretome), Group II (ASC secretome), Group III (combination of TDSC and ASC secretome), and Group IV (control). The healing response was assessed through PINP and PIIINP immunoserological markers, and histopathological changes were analyzed. The study adhered to ARRIVE and ICLAS guidelines and followed the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Results: The combination group showed significantly higher PINP levels compared to the control group (p = 0.004), suggesting enhanced Type I collagen synthesis. However, no significant differences in PIIINP levels were observed among the groups. Histopathological analysis showed no significant differences in collagen alignment or angiogenesis between treatment and control groups.
Conclusion: The MSC secretome, particularly the combination of TDSCs and ASCs, may accelerate collagen Type I synthesis and improve tendon microstructure. This suggests their potential for treating chronic tendon injuries. However, further research with longer observation periods and clinical trials is crucial to confirm these findings and advance our understanding of tendon healing.
Keywords: mesenchymal stem cell secretome, chronic tendon injury, tendon healing, procollagen markers, rat model
Introduction
Tendons, composed of parallel collagen fibers within an extracellular matrix, are vital for musculoskeletal stability and movement but have limited regenerative capacity, making them prone to injury from repetitive motion and aging.1,2 The Achilles tendon, the body’s largest and strongest, withstands high tensile forces, connecting the gastrocnemius and soleus muscles to the calcaneus.3 Tendon and ligament injuries are among the most common musculoskeletal issues in sports, with Achilles tendon ruptures frequently affecting adults in their 30s to 50s.4,5 Though not life-threatening, these injuries often lead to prolonged pain, functional impairment, and significant healthcare costs, highlighting the need for better treatments to enhance healing and restore function.6,7
The healing of tendons is often poor, typically resulting in scar tissue structurally and functionally inferior to normal tendon tissue.8 Chronic tendon injuries are complicated to treat, requiring extensive intervention to restore pain-free function. Unlike acute injuries that trigger inflammation, chronic tendon injuries are characterized by collagen degeneration, a process where the collagen fibers in the tendon break down due to prolonged wear and tear and limited blood supply. This highlights the need for improved treatment strategies, especially for chronic cases.9
Stem cell-based therapies have emerged as promising approaches for tendon regeneration, primarily through paracrine signaling. Mesenchymal stem cells (MSCs) secrete bioactive molecules, collectively known as the secretome, which modulate inflammation, promote cell proliferation, and enhance extracellular matrix remodeling.10,11 The MSC secretome contains key factors such as transforming growth factor-beta (TGF-β), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), and extracellular vesicles carrying microRNAs (miRNAs), all of which contribute to tissue repair and angiogenesis.12–14
Compared to direct MSC transplantation, secretome-based therapy offers several advantages, including reduced immune rejection, lower tumorigenicity risk, and greater consistency in therapeutic effects. Secretome products can be standardized and stored for clinical applications, making them a more practical alternative to live-cell therapy. However, variations in secretome composition exist depending on the cell source and culture conditions.10,15,16 Tendon-derived stem cells (TDSC) secrete tenogenic factors such as scleraxis and tenomodulin, whereas adipose-derived stem cells (ASC) provide a broader spectrum of growth factors related to angiogenesis and anti-inflammatory activity. Combining TDSC and ASC secretomes may enhance collagen synthesis and tendon remodeling, offering a synergistic effect.17,18
Collagen synthesis, especially of Type I and III procollagen, is critical to tendon repair, with markers like procollagen type I N-terminal propeptide (PINP) and procollagen type III N-terminal propeptide (PIIINP) indicating active collagen production. While the stem cell secretome may significantly aid this process, particularly in chronic injuries, its specific impact on collagen metabolism and long-term tendon repair still needs to be explored.19,20
This study aims to evaluate the effects of MSC secretome from tedon- and adipose-derived stem cells on chronic Achilles tendon injuries in a rat model. By analyzing changes in PINP, PIIINP, and histological structure, this research seeks to clarify the regenerative potential of stem cell secretome in tendon repair and provide foundational insights for future clinical applications.
Materials and Methods
Study Design and Setting
This study was conducted in an experimental laboratory to evaluate the effects of MSCs-derived secretomes on tendon healing in a rat model of chronic tendinopathy. The reporting of this animal study is based on The Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.21 The research was carried out at the Stem Cell Research and Development Center, Universitas Airlangga, and the Laboratory of Rumah Sakit Pendidikan Tinggi Negeri, Universitas Hasanuddin, between July and December 2023. The study protocol has been approved by the Health Research Ethics Committee of the Faculty of Medicine, Universitas Hasanuddin (No.811/UN4.6.4.5.31/PP36/2023). All procedures involving animals were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and the guideline of the International Council for Laboratory Animal Science (ICLAS) for the welfare of laboratory animals.
Preparation of Animal Samples and Procedure
A total of 14 adult male Rattus norvegicus rats (8–12 weeks, 150–250 grams) were used in this study. The rats were housed under standard conditions. The Animal Ethics Committee of Universitas Airlangga approved all animal procedures. They were randomly divided into four groups (n=3-4 per group):
- Group I: Injection of MSC secretome from tendon tissues (TDSC)
- Group II: Injection of MSC secretome from Adipose Tissues (ASC)
- Group III: Injection of Mixed TDSC & ASC
- Group IV: Control
In two phases, chronic tendinopathy was induced in all rats using mechanical and enzymatic methods. In Phase 1, rats were anesthetized, and an aseptic longitudinal incision was made over the Achilles tendon, followed by a full-width transverse laceration. The injury was left unrepaired for four weeks to simulate chronic damage, with the wound closed and monitored.
In Phase 2, four weeks post-injury, the rats were re-anesthetized, and an aseptic procedure was conducted. Using a single local injection technique, Group I received intratendinous injection of 3.75 × 10⁵ TDSC-secretome-treated cells in 100 µL phosphate-buffered saline (PBS), Group II received the same dose of 3.75 × 10⁵ ASC-secretome-treated cells in 100 µL PBS, and Group III received a combination of TDSC and ASC secretome in a 1:1 ratio, with each type contributing 1.875 × 10⁵ cells, making a total of 3.75 × 10⁵ cells in 100 µL PBS.22 Group IV received an injection of 100 µL PBS without any secretome treatment. All the cell therapies were allogeneic. Two weeks after the injections (week 6), the rats were sacrificed using an intraperitoneal injection of sodium pentobarbital.23 The blood samples were collected via cardiac puncture under anesthesia before euthanasia for PINP and PIIINP measurements. Serum PINP and PIIINP levels were measured using enzyme-linked immunosorbent assay (ELISA) kits. The histopathological assessment conducted via tendon biopsy.
Secretome Isolation and Preparation
TDSC were isolated from the Achilles tendons of donor rats. The cells were cultured up to passage 5 (P5) and induced to differentiate into a tenogenic lineage using connective tissue growth factor (CTGF) and ascorbic acid. TDSC were cultured in low-glucose Dulbecco’s modified Eagle medium (DMEM) supplemented with fetal bovine serum, penicillin, streptomycin, and neomycin. After five passages, TDSC were treated with CTGF (25 ng/mL) and ascorbic acid (25 μmol/L) to promote tenogenic differentiation. ASC were isolated from the adipose tissue of donor rats, and cells from the third passage (P3) were used for the experiments. ASC were cultured in α-minimum essential medium supplemented with fetal bovine serum, penicillin, streptomycin, and L-glutamine. The culture medium was replaced every three days, and the cells were harvested at the third passage for use in the experiments.
Secretomes were collected from MSC cultures after 48 hours of serum-free conditioning, filtered through a 0.22 µm membrane, and stored at −80°C until use. Processing controls included sterility testing and confirmation of exosome content using nanoparticle tracking analysis. Secretomes were applied directly into the tendon injury sites using a 30-gauge needle under sterile conditions.
Histopathological Analysis
The harvested tendons were fixed in 10% formalin, embedded in paraffin, and sectioned for histopathological evaluation. Hematoxylin and eosin (H&E) staining was performed to examine microscopic tissue changes at 2 and 4 weeks post-surgery to evaluate morphological alterations. The sections were examined under a light microscope, and histopathological scoring was based on the Grande Histological Biomechanical Correlation Score (GHBCS), which evaluates three components: collagen grade, degree of angiogenesis, and cartilage induction. The total score ranges from 0 to 8, with lower scores indicating optimal ultimate tensile strength.
Statistical Analysis
Data were analyzed using SPSS (version 25.0). The differences between groups were analyzed using one-way ANOVA followed by post hoc Tukey’s test for pairwise comparisons to see if the data is normally distributed and the Kruskal–Wallis test to see if the data is not normally distributed, with p <0.05 as significant.
Results
Procollagen I and III Levels in Tendon Samples
The levels of PINP and PIIINP were measured in rat tendon samples (Rattus Norvegicus) injected with MSC secretomes derived from various sources. Four groups were included: TDSCs, ASCs, a combination of TDSCs and ASCs, and a control group. The measured levels of PINP and PIIINP in the different groups are summarized in Table 1. The mean, standard deviation, median, minimum, and maximum values for PINP and PIIINP levels across the four groups are presented in Table 2. A Shapiro–Wilk test was performed to assess the normality of PINP and PIIINP data. The results indicate that the data for both PINP and PIIINP are not normally distributed (p < 0.05). Therefore, non-parametric tests were used for further analysis.
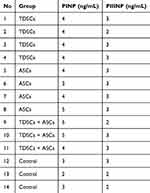 |
Table 1 Characteristics of PINP and PIIINP Levels in Rat Tendon Samples |
 |
Table 2 Descriptive Statistics of PINP and PIIINP Levels |
Comparative Analysis of PINP and PIIINP Levels
The non-parametric Kruskal–Wallis test compared the four groups’ PINP and PIIINP levels. The results showed a significant difference in PINP levels (p = 0.039), while no significant difference was found among PIIINP groups (p = 0.318). Post-hoc analysis using the Mann–Whitney U-test compared PINP levels between the experimental and control groups. The results indicate that combining TDSCs and ASCs significantly increased PINP levels compared to the control group (p= 0.004), indicating enhanced Type I collagen synthesis. The differences between the other groups and the control group were not statistically significant (p > 0.05) (Table 3).
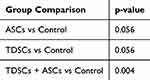 |
Table 3 Post-Hoc Analysis for PINP Levels Compared to Control |
Histopathological Findings and Analysis
Histopathological evaluation of the tendon samples was conducted to assess collagen formation, angiogenesis, and cartilage formation. The scoring of histopathological findings is presented in Table 4, and the histopathological appearance of the samples is shown in Figure 1. The results of the Kruskal–Wallis test for the variable Total Histopathological Scores show a p-value of 0.092, showing no significant difference in total histopathological scores among the four groups, indicating no differences in collagen fiber organization or angiogenesis.
 |
Table 4 Scoring and Histopathological Descriptions in Rat Tendon Samples |
Discussion
Our study results indicate that the group injected with the secretome of MSC, combining TDSCs and ASCs, showed a significant increase in PINP levels compared to the control group. However, the tested groups observed no significant differences between PIIINP levels. The findings align with previous research on MSC-derived therapies for tendon healing, reinforcing the role of secretome-based treatments in regenerative medicine. The observed increase in PINP levels in the TDSC + ASC secretome group suggests enhanced collagen synthesis, supporting the notion that combining tenogenic and angiogenic factors from different MSC sources may provide a more comprehensive regenerative response.24–26
Procollagen types I and III are crucial building blocks in all connective tissues. These two markers associated with collagen metabolism, specifically PINP and PIIINP, have been widely used in bone tissue as early predictors of the success of an intervention. PINP and PIIINP markers have recently been used to assess collagen metabolism in human Achilles tendons.19,27
The elevated PINP levels in the TDSCs and ASCs combination group suggest enhanced Type I collagen synthesis, a critical factor in tendon healing. Type I collagen is the primary structural component of the tendon extracellular matrix, providing strength and stability.28 This finding indicates that MSC secretome injections may accelerate new collagen formation in chronically injured tendons. Beyond PINP elevation, connective tissue healing relies on complex interactions between cell types, such as fibroblasts and macrophages, and the role of growth factors that MSC secretome releases. By modulating the microenvironment through enhancing fibroblast activity and reducing inflammation through paracrine signaling, the MSC secretome may further promote collagen synthesis and extracellular matrix remodeling, aiding tendon repair.29,30
On the other hand, the lack of significant differences in PIIINP levels suggests that, while Type I collagen synthesis increased, Type III collagen did not. Type III collagen, prominent in early wound healing, is typically replaced by the more resilient Type I collagen during tissue remodeling.31,32 Our measurements, taken at six weeks post-operation, likely reflect this remodeling phase, where Type I collagen production naturally rises, aligning with studies showing Type I collagen increases around 4–6 weeks post-injury. This suggests that the MSC secretome primarily supports structural repair through Type I rather than Type III collagen synthesis.33,34
Furthermore, although this study did not show significant differences in PIIINP levels, previous studies have suggested that combining cellular and molecular therapies, such as immunomodulated scaffolds, may yield more optimal outcomes in tendon healing, particularly regarding long-term structural and functional repair.35,36 Our findings align with previous research showing that applying MSC secretome, significantly when enriched with growth factors and cytokines, can enhance tissue injury healing by increasing Type I collagen synthesis without causing excessive fibrosis.37,38
Histopathological analysis of the rat tendons revealed no significant differences in the total histopathological scores between the groups injected with MSC secretome and the control group. The histopathological score includes assessments of collagen quantity, angiogenesis, and cartilage formation in the tested tendons. The increased PINP levels suggest enhanced Type I collagen synthesis, but significant histopathological changes may not appear short-term, possibly due to study duration, secretome dose, or the complexity of chronic tendon healing.39–41 To strengthen the interpretation of vascularization, future studies should incorporate additional immunohistochemical markers, such as VEGF or α-SMA, to better characterize neovascularization.
This study has several limitations. First, the direct injury to healthy rat tendons differs from the natural degenerative process in chronic injuries, limiting direct clinical applicability. While PINP increase with secretome injection suggests a potential for enhanced tendon repair, further research is needed to validate these benefits. Despite this, our results support secretome injections as a promising approach for tendon repair, offering advantages in scalability, storage, and reduced risks compared to cell transplantation, such as immune reactions, cell survival issues, and infection.42
The significant increase in PINP levels in the combination group of TDSCs and ASCs compared to the control group suggests that this secretome combination could accelerate the healing of chronic tendon injuries. However, the histopathological results highlight that molecular-level improvements do not always correlate with observable microscopic changes in the short term. Therefore, long-term studies with continuous observation are needed to confirm the effectiveness of this therapy in repairing the structure and function of injured tendons.
Conclusion
The injection of MSC secretome, combining TDSCs and ASCs, increases Type I collagen synthesis, improves tendon microstructure, and reduces inflammation, making it effective in accelerating the healing of chronic tendon injuries in rats. Further research on larger animal models, long-term evaluations, and human clinical trials is recommended. Developing standard protocols and considering cost factors are essential for the broad application of this therapy.
Acknowledgments
We thank the Stem Cell Research and Development Center and Universitas Airlangga’s laboratory staff for their technical support.
Funding
The Indonesian Education Scholarship (BPI), Higher Education Financing Agency (BPPT), Ministry of Education, Culture, Research and Technology of the Republic of Indonesia and the Indonesia Endowment Fund for Education (LPDP), Ministry of Finance, Republic of Indonesia, fully funded this study.
Disclosure
The authors declare that they have no competing interests.
This paper has been uploaded to ResearchSquare as a preprint: [https://doi.org/10.21203/rs.3.rs-5257458/v1].
References
1. Stauber T, Blache U, Snedeker JG. Tendon tissue microdamage and the limits of intrinsic repair. Matrix Biol. 2020;85–86:68–79. doi:10.1016/j.matbio.2019.07.008
2. Kwan KYC, Ng KWK, Rao Y, et al. Effect of aging on tendon biology, biomechanics and implications for treatment approaches. Int J Mol Sci. 2023;24(20):15183. doi:10.3390/ijms242015183
3. Naňka O, Sedmera D, Rammelt S, Bartoníček J. Anatomy of the Achilles tendon-A pictorial review. Orthopadie. 2024;53(10):721–730. doi:10.1007/s00132-024-04555-x
4. Gimigliano F, Resmini G, Moretti A, et al. Epidemiology of Musculoskeletal injuries in adult athletes: a scoping review. Medicina. 2021;57(10):1118.
5. Clark ST, Zhu M, Gamble GD, et al. Epidemiology of tendon and ligament injuries in Aotearoa/New Zealand between 2010 and 2016. Inj Epidemiol. 2020;7(5). doi:10.1186/s40621-020-0231-x.
6. Aldanyowi SN, AlOraini LI. Personalizing injury management and recovery: a cross-sectional investigation of Musculoskeletal injuries and quality of life in athletes. Orthop Res Rev. 2024;16:137–151. doi:10.2147/ORR.S460748
7. Leong NL, Kator JL, Clemens TL, et al. Tendon and ligament healing and current approaches to tendon and ligament regeneration. J Orthop Res Off Publ Orthop Res Soc. 2020;38(1):7–12.
8. Gross G, Hoffmann A. Therapeutic strategies for tendon healing based on novel biomaterials, factors and cells. Pathobiology. 2013;80(4):203–210. doi:10.1159/000347059
9. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87(7):486–490.
10. González-González A, García-Sánchez D, Dotta M, Rodríguez-Rey JC, Pérez-Campo FM. Mesenchymal stem cells secretome: the cornerstone of cell-free regenerative medicine. World Stem Cells. 2020;12(12):1529–1552. doi:10.4252/wjsc.v12.i12.1529
11. Pokrovskaya LA, Zubareva EV, Nadezhdin SV, Lysenko AS, Litovkina TL. Biological activity of mesenchymal stem cells secretome as a basis for cell-free therapeutic approach. Res Results Pharmacol. 2020;6(1):57–68. doi:10.3897/rrpharmacology.6.49413
12. Ulpiano C, da Silva CL, Monteiro GA. Mesenchymal stromal cells (MSCs): a promising tool for cell-based angiogenic therapy. Curr Gene Ther. 2021;21(5):382–405. doi:10.2174/1566523221666210917114353
13. Maacha S, Sidahmed H, Jacob S, et al. paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020;2020:4356359. doi:10.1155/2020/4356359
14. Chinnici CM, Iannolo G, Cittadini E, et al. Extracellular vesicle-derived microRNAs of human wharton’s jelly mesenchymal stromal cells may activate endogenous VEGF-A to promote angiogenesis. Int J Mol Sci. 2021;22(4):2045.
15. Shin S, Lee J, Kwon Y, et al. Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and Wharton’s Jelly. Int J Mol Sci. 2021;22(2):845. doi:10.3390/ijms22020845
16. Chouaib B, Haack-Sørensen M, Chaubron F, Cuisinier F, Collart-Dutilleul P-Y. Towards the standardization of mesenchymal stem cell secretome-derived product manufacturing for tissue regeneration. Int J Mol Sci. 2023;24(16):12594. doi:10.3390/ijms241612594
17. Xue Z, Chen Z, Wu T, et al. VEGFA-enriched exosomes from tendon-derived stem cells facilitate tenocyte differentiation, migration, and transition to a fibroblastic phenotype. Biomed Res Int. 2022;2022:8537959. doi:10.1155/2022/8537959
18. Kokubu S, Inaki R, Hoshi K, Hikita A. Adipose-derived stem cells improve tendon repair and prevent ectopic ossification in tendinopathy by inhibiting inflammation and inducing neovascularization in the early stage of tendon healing. Regen Ther. 2020;14:103–110. doi:10.1016/j.reth.2019.12.003
19. Vestergaard P, Jørgensen JOL, Olesen JL, et al. Local administration of growth hormone stimulates tendon collagen synthesis in elderly men. J Appl Physiol. 2012;113(9):1432–1438. doi:10.1152/japplphysiol.00816.2012
20. Flodin J, Juthberg R, Edman G, Flodin J. Patient-reported outcome and healing biomarkers in patients treated by female versus male surgeons– a cohort study on achilles tendon ruptures. Muscle Ligaments Tendons J. 2019;09:531.
21. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. 2020;40(9):1769–1777. doi:10.1177/0271678X20943823
22. Sevivas N, Teixeira FG, Portugal R, et al. Mesenchymal stem cell secretome improves tendon cell viability in vitro and tendon-bone healing in vivo when a tissue engineering strategy is used in a rat model of chronic massive rotator cuff tear. Am J Sports Med. 2018;46(2):449–459. doi:10.1177/0363546517735850
23. Sevivas N, Teixeira FG, Portugal R, et al. Mesenchymal stem cell secretome: a potential tool for the prevention of muscle degenerative changes associated with chronic rotator cuff tears. Am J Sports Med. 2017;45(1):179–188. doi:10.1177/0363546516657827
24. Fredianto M, Herman H, Ismiarto YD, et al. Secretome of hypoxia-preconditioned mesenchymal stem cells enhance the expression of HIF-1a and bFGF in a rotator cuff tear model. Med Glas Off Publ Med Assoc. 2023;20.
25. Migliorini F, Tingart M, Maffulli N. Progress with stem cell therapies for tendon tissue regeneration. Expert Opin Biol Ther. 2020;20(11):1373–1379. doi:10.1080/14712598.2020.1786532
26. Rhatomy S, Sumarwoto T, Prijosedjati A, Romaniyanto, Prasetyo TE. Role of mesenchymal stem cell-conditioned medium (MSC-CM) in ligament or tendon healing: a systematic review from 1998-2018. Orthop J Sport Med. 2020;8.
27. Alim MA, Peterson M, Pejler G. Do mast cells have a role in tendon healing and inflammation? Cells. 2020;9(5):1134. doi:10.3390/cells9051134
28. Buckley MR, Evans EB, Matuszewski PE, et al. Distributions of types I, II and III collagen by region in the human supraspinatus tendon. Connect Tissue Res. 2013;54(6):374–379. doi:10.3109/03008207.2013.847096
29. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–1219. doi:10.1161/CIRCRESAHA.108.176826
30. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi:10.1002/jcb.20886
31. Boyer MI, Strickland JW, Engles DR, Sachar K, Leversedge FJ. Flexor tendon repair and Rehabilitation. JBJS. 2002;84(9):1684–1706. doi:10.2106/00004623-200209000-00025
32. Müller SA, Todorov A, Heisterbach PE, Martin I, Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surgery, Sport Traumatol Arthrosc. 2013;23(7):2097–2105. doi:10.1007/s00167-013-2680-z
33. Sandrey MA. Acute and chronic tendon injuries: factors affecting the healing response and treatment. J Sport Rehabil. 2003;12(1):70–91. doi:10.1123/jsr.12.1.70
34. Valkering K, Aufwerber S, Ranuccio F, et al. Functional weight-bearing mobilization after Achilles tendon rupture enhances early healing response: a single-blinded randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(6):1807–1816. doi:10.1007/s00167-016-4270-3
35. Oryan A, Moshiri A, Meimandi-Parizi A. Role of embedded pure xenogenous bovine platelet gel on experimental tendon healing, modelling and remodelling. BioDrugs. 2014;28(6):537–556. doi:10.1007/s40259-014-0107-0
36. Lohan P, Murphy N, Treacy O, et al. Third-party allogeneic mesenchymal stromal cells prevent rejection in a pre-sensitized high-risk model of corneal transplantation. Front Immunol. 2018;9:2666. doi:10.3389/fimmu.2018.02666
37. Chen T, You Y, Jiang H, Wang ZZ. Epithelial-mesenchymal transition (EMT): a biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol. 2017;232(12):3261–3272. doi:10.1002/jcp.25797
38. Tamama K, Kerpedjieva SS. Acceleration of wound healing by multiple growth factors and cytokines secreted from multipotential stromal cells/mesenchymal stem cells. Adv Wound Care. 2012;1(4):177–182. doi:10.1089/wound.2011.0296
39. Pajala A, Melkko J, Leppilahti J, et al. Tenascin-C and type I and III collagen expression in total Achilles tendon rupture. An immunohistochemical study. Histol Histopathol. 2009;24(10):1207. doi:10.14670/HH-24.1207
40. Kjær M, Langberg H, Heinemeier K, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009;19(4):500–510. doi:10.1111/j.1600-0838.2009.00986.x
41. Christensen B, Dyrberg E, Aagaard P, Kjaer M, Langberg H. Short-term immobilization and recovery affect skeletal muscle but not collagen tissue turnover in humans. J Appl Physiol. 2008;105(6):1845–1851. doi:10.1152/japplphysiol.90445.2008
42. Lange-Consiglio A, Rossi D, Tassan S, et al. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22(22):3015–3024. doi:10.1089/scd.2013.0214
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


