Back to Journals » Infection and Drug Resistance » Volume 18
Metabolic Health Consequences of Switching to the Bictegravir/Emtricitabine/Tenofovir Alafenamide and Dolutegravir Plus Lamivudine Regimens in Virologically Suppressed People Living with HIV Aged > 40 Years: A Retrospective Real-World Study
Authors Shi J , Zhang W, Han J, Zhang Z, Yan D, Zheng R, Li F, Wang Y
Received 17 January 2025
Accepted for publication 21 May 2025
Published 29 May 2025 Volume 2025:18 Pages 2703—2716
DOI https://doi.org/10.2147/IDR.S516775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sandip Patil
Jinchuan Shi,1 Wenhui Zhang,1,2 Jie Han,1,2 Zhongdong Zhang,1 Dingyan Yan,1,2 Rongrong Zheng,1 Feng Li,1 Yi Wang1,3
1Department of Infection, Affiliated Hangzhou Xixi Hospital, Zhejiang Chinese Medical University, Hangzhou, 310023, People’s Republic of China; 2Department of Nursing, Affiliated Hangzhou Xixi Hospital, Zhejiang Chinese Medical University, Hangzhou, 310023, People’s Republic of China; 3Clinical Research Laboratory, Affiliated Hangzhou Xixi Hospital, Zhejiang Chinese Medical University, Hangzhou, 310023, People’s Republic of China
Correspondence: Yi Wang, Clinical Research Laboratory, Affiliated Hangzhou Xixi Hospital, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China, Email [email protected]
Objective: Both B/F/TAF and DTG/3TC are recommended in treatment guidelines for switch therapy in PLWH. This study aimed to evaluate the safety and metabolic health consequences of two switched regimens in a real-world setting among virologically suppressed PLWH previously treated with EFV/TDF/3TC.
Methods: This retrospective real-world study in Hangzhou included 220 virologically suppressed PLWH who switched from EFV/TDF/3TC to DTG/3TC or B/F/TAF between January 1, 2020 and October 30, 2023. All participants were examined the changes in weight, BMI, GLU levels, lipid parameters (TC, LDL-C, HDL-C, and TG), and eGFR at post-12-month.
Results: The mean age of included participants was 50.8 years (SD: 11.3). After 12 months of switching, the HIV RNA level was below the limit of detection (< 20 copies/mL) among all participants. The switch to DTG/3TC or B/F/TAF therapy was associated with significant improvement in LDL-C, GLU levels, and eGFR values (all P < 0.05), while other metabolic indexes did not change significantly. Furthermore, there was a significant difference in the incidence of hyperglycemia (5.7% vs 19.35%; P = 0.004) between the B/F/TAF and DTG/3TC groups, but not included the mean changes of weight, BMI, lipid profiles, GLU levels, and eGFR and incidence of high TC and high TG. For the aged 40– 59 years and aged ≥ 60 years PLWH, the differences in metabolic indicators were minimal between DTG/3TC and B/F/TAF groups post-12-month, with no significant differences between the arms in mean change from baseline in TC, TG, HDL-C, LDL-C, GLU, BMI, weight, and eGFR.
Conclusion: In this study, the B/F/TAF or DTG/3TC regimens are safe for virologically suppressed PLWH aged > 40 years. The transition to B/F/TAF demonstrated dual clinical benefits, significantly reducing hyperglycemia incidence while preserving renal function.
Keywords: people living with HIV, virological suppression, antiretroviral therapy, metabolic indices
Introduction
Acquired immunodeficiency syndrome (AIDS), caused by the human immunodeficiency virus (HIV), is a great threat to public health. Currently, antiretroviral therapy (ART) is the most effective treatment for HIV infection; ART efficiently blocks viral replication and achieves immune reconstitution, thus providing substantial benefits to people living with HIV (PLWH).1–3 Standard ART comprises two nucleoside reverse transcriptase inhibitors (NRTI) with a core drug that may include a non-nucleoside reverse transcriptase inhibitor (NNRTI), boosted protease inhibitor (PI), or integrase strand transfer inhibitor (INSTI).4 With extended lifespans, PLWH require drug treatment for decades and face chronic age-related diseases (such as incomplete immune reconstitution, metabolic abnormalities, reproductive dysfunction, and AIDS-related cancers) and damage from long-term drug treatment.4,5 Therefore, optimized ART regimens are needed to avoid drug side effects (including dyslipidaemia and poorly controlled glucose) and improve tolerance while maintaining antiviral efficacy.
Currently, dolutegravir (DTG) and bictegravir (BIC) are the most commonly used integrase inhibitors for PLWH, with high resistance barrier, better efficacy and safety profiles, and fewer drug-drug interactions.6,7 Thus, DTG- or BIC-based regimens are recommended for initial and switch therapy in PLWH.8,9 In comparison to other 2-drug or 3-drug INSTI-based treatments, regimens of BIC/emtricitabine/tenofovir alafenamide (B/F/TAF) and DTG plus lamivudine (DTG/3TC) are currently more often prescribed clinically (including trials) for ART-naïve and virologically suppressed individuals.10–12 In Chinese cohort, Wei et al found that ART-naïve PLWH who adopted B/F/TAF regimens experienced a faster viral decline and fewer drug-related adverse effects (DRAEs) compared to those adopting DTG/3TC regimens.7 Gan et al proved that DTG/3TC and B/F/TAF regimens are well tolerated and effective for ART-naïve PLWH.13 Currently, research in China mainly focuses on comparing DTG/3TC and B/F/TAF as initial treatment regimens, with fewer comparative studies on switching to DTG/3TC and B/F/TAF after treatment. Although Gan et al reported favorable efficacy and tolerability when switching to these two regimens in PLWH with diverse ART histories,9 the heterogeneity in prior treatment exposure may introduce confounding factors. On the other hand, while multiple non-mainland clinical trials have demonstrated sustained virologic suppression after switching to either B/F/TAF or DTG/3TC in treatment-experienced individuals,14–17 the pharmacokinetic and pharmacodynamic profiles may differ across racial groups.18 Therefore, comparative studies evaluating switches to DTG/3TC versus B/F/TAF in Chinese patients with identical baseline ART regimens are warranted.
As outlined in the 2011 China National Guidelines for HIV/AIDS Diagnosis and Treatment, the efavirenz + lamivudine + tenofovir (EFV/TDF/3TC) regimen has been recommended as first-line therapy for Chinese populations and remains widely used in clinical practice.19 In 2019, dolutegravir (DTG) superseded EFV in first-line regimens due to its superior efficacy, higher genetic barrier to resistance, favorable safety profile, and cost-effectiveness.20 While tenofovir alafenamide (TAF) is the preferred alternative to tenofovir disoproxil fumarate (TDF) for patients with renal impairment, clinicians must carefully balance its renal protective effects against potential metabolic adverse effects, including dyslipidemia and weight gain.21 To date, robust clinical evidence remains insufficient to fully characterize the safety and metabolic profiles of switching ART-experienced PLWH to either bictegravir/emtricitabine/TAF (B/F/TAF) or dolutegravir/lamivudine (DTG/3TC) regimens, with limited data available from both controlled trials and real-world studies. Thus, differences in safety and metabolic health consequences among DTG/3TC and B/F/TAF groups must be investigated in the context of the same naïve regimen EFV/TDF/3TC.
Previous studies have highlighted the significant incidence of metabolic syndrome in ART-naïve PLWH in China, and showed that older age (40 years and over) had significant associations with metabolic diseases, including diabetes, hyperglycemia and others.22–24 In addition, there are significant associations between age (> 40 years) and metabolic diseases among PLWH.25 ART-naïve or ART-experienced PLWH of age ≥ 60 years have more frequent comorbidities, including metabolic diseases.26 Though some studies have focused on elderly PLWH switching to B/F/TAF or DTG/3TC, there have been limited data regarding changes of biochemical indicators of aged 40–59 years or aged ≥ 60 years groups after switching to B/F/TAF or DTG/3TC.
To address this knowledge gap, we conducted a single-center study to investigate and compare the safety and metabolic health consequences among virologically suppressed adult (> 40 years) PLWH who switching from EFV/TDF/3TC to DTG/3TC and B/F/TAF regimens. Notably, this study included the incidence of ART-related metabolic disorders at baseline and after switch 12-month for the Chinese populations.
Methods
Study Population and Design
This retrospective real-world study included 220 virologically suppressed PLWH who switched from EFV/TDF/3TC to DTG/3TC or B/F/TAF between January 1, 2020 and October 30, 2023 at Hangzhou Xixi Hospital, the largest dedicated AIDS hospital in Zhejiang Province, China. The inclusion criteria were: (1) receiving ART treatment with an EFV/TDF/3TC regimen for at least 6 months, (2) plasma HIV RNA < 50 copies/mL for at least 6 months, (3) age ≥ 40 years, (4) followed over the period 2020–2023. The exclusion criteria were: (1) pregnancy, (2) allergic history or high degree of sensitivity to any component or auxiliary material of the research drug, (3) with history of virologic failure (2 consecutive measurements ≥ 200 copies/mL), (4) receiving prior ART treatment with an DTG regimen. By these criteria, 48 PLWH were excluded because of prior DTG treatment. The remaining 220 PLWH received prior ART treatment with an EFV/TDF/3TC regimen for at least 6 months after HIV diagnosis. Among them, according to the different reasons and recommended usage guidelines for regimen switch, 62 and 158 PLWH switched to DTG/3TC or B/F/TAF regimens, respectively.
Baseline demographic data and clinical variables were collected from the Electronic Medical Records (EMR) management system; data included age, sex, weight, body mass index (BMI), smoking status, drinking status, hepatitis B virus (HBV) status, high blood pressure (HBP) status, diabetes status, and ART therapy time. A study flowchart is shown in Figure 1.
Outcomes of Interest
The primary outcome was the differences in weight, BMI, lipid profiles, glucose (GLU) levels, and eGFR between 12-month and baseline among virologically suppressed PLWH who switched from EFV/TDF/3TC to DTG/3TC or B/F/TAF. The secondary outcome were differences in the mean changes of the above metabolic indicators from baseline to post-12-month between DTG/3TC and B/F/TAF regimens.
Variables
To assess the study endpoint, we collected the data of the primary variables including weight, BMI, Tg, TC, LDL-C, HDL-C, GLU, creatinine, and estimated glomerular filtration rate (eGFR) at the time of switching (baseline measurement) and after 12 months. Changes in weight, BMI, and metabolic variables were calculated as the difference between post-12-month value with respect to the baseline measurement. The eGFR was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.27
Diagnostic Standards
The diagnosis of dyslipidaemia (high TG or High TC) is an abnormally elevation of TG (TG level > 1.7 mmol/L) or TC (TC level > 5.2 mmol/L); the diagnosis of dysglycemia is an abnormally elevation of GLU (fasting glucose level ≥ 5.6 mmol/L) in the blood.28
Statistical Analysis
All statistical analyses were performed using SPSS (v.25.0). Normally distributed continuous variables were described and compared using the means with standard deviations (SD) and t-test, respectively. Variables with non-normal distributions were described and compared using the median (M) and interquartile range (IQR, 1st quartile, 3rd quartile) and nonparametric Wilcoxon signed-rank tests (paired or unpaired), respectively. Categorical variables were reported with numbers and percentages. Proportions were compared using Pearson’s Chi-square test. All graphs were created using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA).29 All reported levels of statistical significance were two-sided, and p-value < 0.05 or lower were considered statistically significant.
Results
Baseline Characteristics of the Study Population
A total of 220 PLWH were enrolled in this study. As shown in Table 1, the primary reasons for regimen switch among 220 PLWH were the side effects (n = 57; 25.9%) and regimen simplification (n = 93; 42.3%). The mean participant age was 50.8 years (SD: 11.3). The cohort was 88.6% male and 11.4% female, with a mean BMI of 23.0 (SD: 2.7). The mean ART therapy time was 5.3 years (SD: 2.7). The CD4+ T cells count < 200 cells/μL, 200~499 cells/μL and ≥ 500 accounted for 6.4%, 43.2% and 50%, respectively. At baseline and post-12-month, the HIV RNA level was below the lower limit of detection (< 20 copies/mL) among all participants.
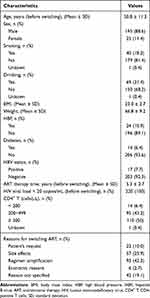 |
Table 1 Baseline Characteristics of All Participants (n = 220) |
Changes in the Levels of Clinical Indicators Among PLWH at 12-month After Switching to B/F/TAF or DTG/3TC Regimens
We compared the BMI, weight, and metabolic indices of PLWH after switching from EFV/TDF/3TC to DTG/3TC or B/F/TAF regimens between baseline and post-12-month. The metabolic changes are shown in Figure 2 and Table S1. Regarding blood lipid parameters, the levels of low-density lipoprotein cholesterol (LDL-C) at 12-month were significantly higher than at baseline (2.72 vs 2.43, P = 0.001, Figure 2D). There were significant decreases in glucose (GLU) level (5.58 vs 5.41, P = 0.005, Figure 2E) between two time points. Regarding renal function parameter, the change of estimated glomerular filtration rate (eGFR) was also markedly decreased at post-12-month (98.7 vs 89.2, P < 0.001, Figure 2H). However, another three metabolic parameters including total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) did not change markedly from baseline to post-12-month (all P > 0.05, Figure 2A–C and Table S1). In addition, the absolute mean BMI at baseline vs at 12-month was 23.02 vs 23.35 kg/m2 (Figure 2F), and the adjusted mean weight gain from baseline to 12-month (66.79 vs 67.71) was 0.92 kg among PLWH who switching to B/F/TAF or DTG/3TC regimens (Figure 2G), but there was no statistical significance (Both P > 0.05, Figure 2 and Table S1).
For PLWH cohort who switched to B/F/TAF, Table 2 summarizes the changes in metabolic indicators of PLWH at post-12-month. Comparing to the baseline, the LDL-C level (2.45 vs 2.73, P < 0.001) was clearly higher, and GLU level (5.59 vs 5.4, P = 0.009) and eGFR value (99.4 vs 90.47, P < 0.001) were significantly lower at post-12-month (Table 2). Nevertheless, other metabolic indices (including TC, TG, HDL-C levels, and BMI, and weight) and the incidence of hyperglycemia, high TC and high TG were comparable (All P > 0.05) (Table 2).
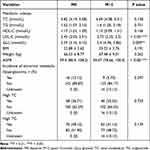 |
Table 2 Comparison of the Metabolic Indexes Between Baseline and Post-12-month Among B/F/TAF Group (n = 158) |
For PLWH cohort who switched to DTG/3TC, Table 3 summarizes the changes in metabolic indicators of PLWH at post-12-month. As shown in Table 3, comparing to the baseline, the eGFR value was dramatically reduced (92.7 vs 81.33, P = 0.014), but the incidence of hyperglycemia was higher at post-12-month (6.45% vs 19.35%, P = 0.030). However, eight metabolic indices (including TC, TG, HDL-C, LDL-C, GLU levels, and BMI, and weight, and eGFR) and the incidence of high TC and high TG were comparable (All P > 0.05) (Table 3).
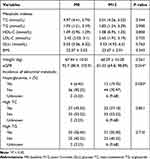 |
Table 3 Comparison of the Metabolic Indexes Between Baseline and Post-12-month Among DTG/3TC Group (n = 62) |
Comparison of Metabolic Indicators in DTG/3TC and B/F/TAF Groups
Among 220 PLWH, there were 62 PLWH in the DTG/3TC cohort and 158 PLWH in the B/F/TAF cohort. With the exceptions of HBP and TG (P < 0.05), other baseline characteristics were largely similar between the DTG/3TC and B/F/TAF groups (All P > 0.05), including age, sex, BMI, smoking status, drinking status, HBV status, diabetes status, ART therapy time, CD4, CD8, CD4/CD8 ratio, GLU, TC, HDL, LDL, ALT, AST, AST/ALT ratio, eGFR (Table S2). First, no adverse reactions and discontinuation were observed among all participants after switching to DTG/3TC or B/F/TAF regimens (Table S3). In addition, Figure 3 and Table S3 demonstrated the differences in metabolic indicators were minimal between DTG/3TC and B/F/TAF groups at post-12-month, with no significant differences between the arms in mean change from baseline in TC, TG, HDL-C, LDL-C and GLU (All P > 0.05, Figure 3A–E).
Mean BMI change from baseline to 12-month was +0.332 kg/m2 in the B/F/TAF group and +0.231 kg/m2 in the DTG/3TC group (P = 0.688, Figure 3F and Table S3). Similarly, mean weight change from baseline to 12-month was +0.968 kg in the B/F/TAF group and +0.759 kg in the DTG/3TC group (P = 0.767, Figure 3G and Table S3). And so beyond that, mean change from baseline to 12-month in eGFR were comparable between the B/F/TAF and DTG/3TC groups (−6.26 vs −6.99 mL/min/1.73m2, P = 0.721, Figure 3H and Table S3).
In addition, we compared the differences in the occurrence of abnormal metabolic indexes between B/F/TAF and DTG/3TC groups. At the baseline, there were no significant statistical differences in the occurrence of hyperglycemia (10.12% vs 6.45%), high TC (36.71% vs 43.55%) and high TG (48.1% vs 56.45%) between two groups (All P > 0.05) (Table 4). However, at post-12-month, the occurrence of hyperglycemia in DTG/3TC group was significantly higher than those in B/F/TAF group (19.35% vs 5.70%, P = 0.004, Table 4). But beyond that, there was no significant difference in the occurrence of high TC (37.1% vs 25.32%) and high TG (50% vs 41.14%) between B/F/TAF and DTG/3TC groups (Both P > 0.05, Table 4).
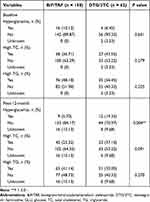 |
Table 4 Comparison of the Abnormal Metabolic Indexes Between B/F/TAF and DTG/3TC Groups |
Changes of Clinical Indicators from Baseline to 12-month for the Middle-Aged (40–59 Years) PLWH or Old-Aged (≥ 60 Years) PLWH
Among middle-aged (40–59 years) PLWH, there were 118 PLWH in B/F/TAF group and 41 PLWH in DTG/3TC group. With the exceptions of ART therapy time, TC, TG and BMI (P < 0.05), other baseline characteristics were largely similar between the DTG/3TC and B/F/TAF groups (All P > 0.05), including sex, smoking status, drinking status, HBP status, diabetes status, HBV status, CD4, CD8, CD4/CD8 ratio, GLU, HDL, LDL, ALT, AST, AST/ALT ratio, weight, and eGFR (Table S4). We further compared the mean changes in metabolic indicators at post-12-month between B/F/TAF and DTG/3TC groups among middle-aged PLWH. As shown in Table 5, there were no significant differences between B/F/TAF and DTG/3TC groups in mean change from baseline in TC, TG, HDL-C, LDL-C, GLU, BMI, weight, and eGFR (All P > 0.05).
 |
Table 5 Comparison of the Levels of Metabolic Indicators Between B/F/TAF and DTG/3TC Groups Among Aged 40–59 years PLWH |
For old-aged (≥ 60 years) PLWH, there were 40 B/F/TAF group and 21 DTG/3TC group. With the exceptions of drinking status, HBP status, diabetes status, and ART therapy time (P < 0.05), other baseline characteristics were largely similar between the DTG/3TC and B/F/TAF groups (All P > 0.05), including sex, smoking status, drinking status, HBP status, diabetes status, HBV status, CD4, CD8, CD4/CD8 ratio, GLU, TC, TG, HDL-C, LDL-C, ALT, AST, AST/ALT ratio, BMI, weight, and eGFR (Table S5). In addition, Table 6 summarizes the differences in metabolic indicators. The minimal differences between DTG/3TC and B/F/TAF groups were observed at post-12-month, with no significant differences between the arms in mean change from baseline in TC, TG, HDL-C, LDL-C, GLU BMI, weight, and eGFR (All P > 0.05).
 |
Table 6 Comparison of the Levels of Metabolic Indicators Between B/F/TAF and DTG/3TC Groups Among Aged ≥ 60 years PLWH |
Discussion
Both B/F/TAF and DTG/3TC are recommended in worldwide treatment guidelines for switch therapy in PLWH.30–32 In this study, the switch to B/F/TAF or DTG/3TC regimens was safe and conducive to the decrease of GLU at post-12-month among virologically suppressed adult PLWH who aged > 40 years. While no significant differences in metabolic indices were observed between B/F/TAF and DTG/3TC groups over 12 months. Our data demonstrate that switching to B/F/TAF was associated with a statistically significant reduction in hyperglycemia incidence compared to DTG/3TC.
Prior work has evaluated the safety and efficacy of B/F/TAF and DTG/3TC switch therapy regimens for virologically suppressed adult PLWH (≥ 18 years).30 The current study focused on the switching to B/F/TAF or DTG/3TC in virologically suppressed middle-aged (40–59 years) and old-aged (≥ 60 years) PLWH. We found no significant changes in the majority of biochemical indices among PLWH who switching to B/F/TAF or DTG/3TC regimens; only the levels of LDL-C, GLU and eGFR value varied significantly at post-12-month. The alterations in certain lipid profiles are a typical occurrence following therapeutic regimen changes, a phenomenon that has also been noted in prior clinical research.33–35 For example, increases in LDL-C were also observed in Hsu’ study.36 One interesting study revealed that higher LDL-C levels in PLWH were associated with a reduced risk of poor immune reconstitution,37 which supports a switch to B/F/TAF or DTG/3TC in virologically suppressed PLWH. These evidence underpins the switch to B/F/TAF or DTG/3TC regimens was safe for middle-aged and elderly PLWH.
Growing evidence supports the use of B/F/TAF as the preferred ART regimens because of their high barrier to resistance.11,36,38–40 However, several studies have demonstrated the efficacy of the two-drug DTG/3TC regimen, which is not inferior to the three-drug regimens.13,41–43 Thus, we further compared the metabolic health consequences of B/F/TAF and DTG/3TC in real-world settings. Given that the mean age in the DTG/3TC group was slightly higher than in the B/F/TAF group, this age disparity may account for the observed baseline differences in HBP and TG between the two treatment groups. Previous studies showed that HBP was associated with older age among PLWH,44 TG levels were higher in the older PLWH.45 Except for HBP and TG, other baseline characteristics were largely similar between the DTG/3TC and B/F/TAF groups. We did not detect significant differences between B/F/TAF and DTG/3TC group in the adjusted mean change of weight, BMI, lipid parameters (TC, TG, HDL-C, and LDL-C), and fasting GLU. Furthermore, our findings are similar to others.9,43 A long-term study favored that the changes in weight, fasting GLU and lipid parameters through Week 144 are similar between B/F/TAF and DTG-containing regimens.46 Our data showed that there was not significant differences in eGFR value between two groups. Van Wyk J et al’ data also favored that the changes in renal biomarkers were similar between DTG/3TC and TAF-based regimens.47 On the other hand, although both regimens showed statistically significant eGFR declines after 12 months, these changes may reflect the limited observation period rather than clinically meaningful renal impairment. Importantly, the B/F/TAF group maintained mean eGFR levels within the normal range (> 90 mL/min/1.73m²), suggesting preserved renal function. This result may imply that the impact of B/F/TAF regimen on renal function was relative minimal. Longer-term data from our cohort are planned to determine the significance of changes in these parameters.
Interestingly, at post-12-month, the incidence of dysglycemia in the DTG/3TC group was significantly increased, and higher than those in the B/F/TAF group. In addition, we also found that the switch to B/F/TAF treatment lowered GLU level and incidence of hyperglycemia among the virologically suppressed PLWH at post-12-month. A newest findings provide important information regarding the effect of B/F/TAF on the low incidence of treatment-emergent diabetes (dysglycemia) in PLWH.46 These data illustrated that the switched B/F/TAF regimens could bring more benefits for virologically suppressed PLWH with hyperglycemia problem.
Another shining point of the article is the comparison of the safety and metabolic health consequences of switching to B/F/TAF or DTG/3TC in old-aged (≥ 60 years) PLWH. Generally, elderly patients with weakened immune systems, especially those over age 60, were more likely to experience comorbidities.26 Previous studies primarily revealed the efficacy and safety of switching to B/F/TAF in older PLWH,35,48 which was also observed in our study. In addition, our data also reveals that there were no significant differences between B/F/TAF and DTG/3TC groups in eight metabolic indices among old-aged (≥ 60 years) PLWH. These results highlights either DTG/3TC or B/F/TAF regimens could be considered in virologically suppressed older PLWH.
Our study is not without limitations. First, all safety incidents were self-reported and may be under-reported. Another study limitation is the smaller size of the old-aged (≥ 60 years) group. However, the two groups were well balanced for all variables, thus potentially limiting the confounders. Additionally, the external validity of these results may be constrained when applied to patients with uncontrolled comorbid non-communicable conditions. Finally, this was a single-center study that lasted for only 12 months, limiting the ability to extrapolate.
Conclusions
This study confirmed the safety of both regimens while revealing B/F/TAF’s metabolic advantages over DTG/3TC, most notably in glucose metabolism where it significantly lowered dysglycemia incidence among the virologically suppressed PLWH aged > 40 years at post-12-month. This study provides theoretical support for PLWH who are considering changing their treatment regimens. Future studies would focus on the B/F/TAF- and DTG/3TC-associated long-term metabolic health consequences of weight gain, lipid and glucose parameter changes.
Ethical Approval
This retrospective study was reviewed and approved by the Clinical Research Ethics Committee of the Hangzhou Xixi Hospital (No. 2023-Research ethics-97) in accordance with the tenets of the Declaration of Helsinki. This study met requirements for consent waived by the Ethics Committee. This study was a retrospective study, where researchers only conducted retrospective analysis on the medical records and data from laboratory examinations, not involving personal privacy or commercial interests. Meanwhile, strict confidentiality was maintained for all patient information, and no any intervention was performed on patients. Hence, the patient consent was waived by the Ethics Committee.
Acknowledgments
We would like to appreciate all patients who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, including its conception, study design, execution, acquisition of data, analysis, and/or interpretation. The authors took part in drafting, revising, or critically reviewing the article, gave final approval of the version to be published, agreed on the journal to which the article has been submitted, and agreed to be accountable for all aspects of the work.
Funding
This research was supported by grants from the Science and Technology Project of Disease Control and Prevention Administration of Zhejiang Province (2025JK065), Medical and Health Science and Technology Project of Hangzhou (A20251320 and A20251296).
Disclosure
Regarding the publication of this paper, the authors declare that they have no conflicts of interest.
References
1. Lundgren JD, Babiker AG.; INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi:10.1056/NEJMoa1506816
2. Mendoza I, Lázaro A, Torralba M. Effectiveness, durability, and safety of dolutegravir and lamivudine versus dolutegravir, lamivudine, and abacavir in a real-life cohort of HIV-infected adults. Ann Pharmacother. 2022;56:412–421. doi:10.1177/10600280211034176
3. Chen LY, Sun HY, Chuang YC, et al. Patient-reported outcomes among virally suppressed people living with HIV after switching to Co-formulated bictegravir, emtricitabine and tenofovir alafenamide. J Microbiol Immunol Infect. 2023;56:575–585. doi:10.1016/j.jmii.2023.01.015
4. Patel R, Evitt L, Mariolis I, et al. HIV treatment with the two-drug regimen dolutegravir plus lamivudine in real-world clinical practice: a systematic literature review. Infect Dis Ther. 2021;10:2051–2070. doi:10.1007/s40121-021-00522-7
5. Kumar S, Samaras K. The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front Endocrinol. 2018;9:705. doi:10.3389/fendo.2018.00705
6. Zhao AV, Crutchley RD, Guduru RC, et al. A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection. Retrovirology. 2022;19:22. doi:10.1186/s12977-022-00608-1
7. Wei Y, Li J, Xu R, et al. Efficacy and safety profiles of dolutegravir plus lamivudine vs. bictegravir/emtricitabine/tenofovir alafenamide in therapy-naïve adults with HIV-1. Chin Med J. 2023;136:2677–2685. doi:10.1097/CM9.0000000000002907
8. Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the international antiviral society-USA panel. JAMA. 2023;329:63–84. doi:10.1001/jama.2022.22246
9. Gan L, Xie X, Fu Y, et al. Bictegravir/Emtricitabine/Tenofovir alafenamide versus dolutegravir plus lamivudine for switch therapy in patients with HIV-1 infection: a real-world cohort study. Infect Dis Ther. 2023;12:2581–2593. doi:10.1007/s40121-023-00879-x
10. Cahn P, Sierra Madero J, Arribas JR, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy - naive adults with HIV-1 infection. AIDS. 2022;36:39–48. doi:10.1097/QAD.0000000000003070
11. Sax PE, Arribas JR, Orkin C, et al. Bictegravir/emtricitabine/tenofovir alafenamide as initial treatment for HIV-1: five-year follow-up from two randomized trials. EClinicalMedicine. 2023;59:101991. doi:10.1016/j.eclinm.2023.101991
12. Daar ES, DeJesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, Phase 3, non-inferiority trial. Lancet HIV. 2018;5:e347–e356. doi:10.1016/S2352-3018(18)30091-2
13. Gan L, Xie X, Fu Y, et al. Comparison of dolutegravir+Lamivudine and bictegravir/emtricitabine/tenofovir alafenamide in antiretroviral therapy-naïve patients infected with HIV: preliminary results from clinical practice. Expert Rev Anti Infect Ther. 2023:1–8. doi:10.1080/14787210.2023.2279719
14. Cossarizza A, Cozzi-Lepri A, Mattioli M, et al. Evaluating immunological and inflammatory changes of treatment-experienced people living with HIV switching from first-line triple cART regimens to DTG/3TC vs. B/F/TAF: the DEBATE trial. Front Immunol. 2023;14:1279390. doi:10.3389/fimmu.2023.1279390
15. Llibre JM, Brites C, Cheng CY, et al. Efficacy and safety of switching to the drug regimen dolutegravir/lamivudine versus continuing a 3- or 4-drug 2-regimen for maintaining virologic suppression in adults living with human immunodeficiency virus 1 (HIV-1): week 48 results from the phase 3, noninferiority SALSA randomized trial. Clin Infect Dis. 2023;76:720–729. doi:10.1093/cid/ciac130
16. Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the phase 3, noninferiority TANGO randomized trial. Clin Infect Dis. 2022;75:975–986. doi:10.1093/cid/ciac036
17. Rojas J, de Lazzari E, Negredo E, et al. Efficacy and safety of switching to dolutegravir plus lamivudine versus continuing triple antiretroviral therapy in virologically suppressed adults with HIV at 48 weeks (DOLAM): a randomised non-inferiority trial. Lancet HIV. 2021;8:e463–e473. doi:10.1016/S2352-3018(21)00100-4
18. Du X, Peng W, Fu Q, et al. A review of the clinical pharmacokinetic and pharmacodynamic profiles of select antiretrovirals: focus on differences among Chinese patients. Pharmacotherapy. 2019;39:1179–1189. doi:10.1002/phar.2333
19. Cao J, Tang W, Wang N, et al. Treatment persistence of bictegravir/emtricitabine/tenofovir alafenamide and efavirenz + lamivudine + tenofovir disoproxil among HIV-1 patients newly starting treatment in Hunan Province in China. BMC Infect Dis. 2023;23:396. doi:10.1186/s12879-023-08359-w
20. Bronwyn B, Godspower A, Nomathemba C, et al. Weight and metabolic changes after switching from tenofovir Alafenamide/Emtricitabine (FTC)+Dolutegravir (DTG), Tenofovir Disoproxil Fumarate (TDF)/FTC + DTG, and TDF/FTC/Efavirenz to TDF/Lamivudine/DTG. Clinl Infect Dis. 2023;76:1492–1495. doi:10.1093/cid/ciac949
21. Patrick W, Laurence B, Jennifer S, et al. Lipid changes after switch from TDF to TAF in the OPERA cohort: LDL cholesterol and triglycerides. Open Forum Infect Diseases. 2022;9:ofab621. doi:10.1093/ofid/ofab621
22. He N, Ding Y, Li J, et al. HIV and aging in Mainland China: implications for control and prevention research. Curr HIV/AIDS Rep. 2019;16:439–447. doi:10.1007/s11904-019-00473-2
23. Shen Y, Wang Z, Liu L, et al. Prevalence of hyperglycemia among adults with newly diagnosed HIV/AIDS in China. BMC Infect Dis. 2013;13:79. doi:10.1186/1471-2334-13-79
24. Tang J, Chen L, Pan W, et al. Prevalence of metabolic syndrome in people living with HIV and its multi-organ damage: a prospective cohort study. BMC Infect Dis. 2025;25:351. doi:10.1186/s12879-025-10735-7
25. Ye RH, Li J, Yao ST, et al. Prevalence and related factors on diabetes among HIV/AIDS receiving antiretroviral therapy in Dehong Dai and Jingpo Autonomous Prefecture. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40(6):654–659. doi:10.3760/cma.j.issn.0254-6450.2019.06.010
26. Yang X, Fu Y, Xie X, et al. Real-world implementation of dolutegravir plus lamivudine in people living with HIV in Southwest China. Expert Rev Anti Infect Ther. 2022;20:1501–1508. doi:10.1080/14787210.2022.2128766
27. Wang L, Xu X, Zhang M, et al. Prevalence of chronic kidney disease in China: results from the sixth china chronic disease and risk factor surveillance. JAMA Intern Med. 2023;183(4):298–310. doi:10.1001/jamainternmed.2022.6817
28. Yang K, Liu J, Fu S, et al. Vitamin D status and correlation with glucose and lipid metabolism in Gansu Province, China. Diabetes Metab Syndr Obes. 2020;13:1555–1563. doi:10.2147/DMSO.S249049
29. Wang Y, Li J, Zhang W, et al. Extending the dosing interval of COVID-19 vaccination leads to higher rates of seroconversion in people living with HIV. Front Immunol. 2023;14:1152695. doi:10.3389/fimmu.2023.1152695
30. Sax PE, Rockstroh JK, Luetkemeyer AF, et al. Switching to Bictegravir, Emtricitabine, and Tenofovir Alafenamide in virologically suppressed adults with human immunodeficiency virus. Clin Infect Dis. 2021;73:e485–e493. doi:10.1093/cid/ciaa988
31. Basso M, Battagin G, Nicolè S, et al. Predicting factors of plasma HIV RNA undetectability after switching to co-formulated bictegravir, emtricitabine, and tenofovir alafenamide in experienced HIV-1 patients: a multicenter study. Viruses. 2023;15:1727. doi:10.3390/v15081727
32. Baldin G, Ciccullo A, Lombardi F, et al. Short Communication: comparing Lamivudine+Dolutegravir and Bictegravir/Emtricitabine/Tenofovir Alafenamide as Switch Strategies: preliminary Results from Clinical Practice. AIDS Res Hum Retroviruses. 2021;37:429–432. doi:10.1089/AID.2020.0219
33. Heseltine T, Hughes E, Mathew J, et al. The effect of changing to Bictegravir on lipids using real world data: a brief report. J Clin Pharm Ther. 2022;47:2182–2187. doi:10.1111/jcpt.13789
34. Bendala-Estrada AD, Diaz-Almiron M, Busca C, et al. Change in metabolic parameters after switching from triple regimens with tenofovir alafenamide to dolutegravir-based dual therapy. Bi-lipid study. HIV Med. 2023;24:558–567. doi:10.1111/hiv.13432
35. Rolle CP, Nguyen V, Patel K, et al. Real-world efficacy and safety of switching to bictegravir/emtricitabine/tenofovir alafenamide in older people living with HIV. Medicine. 2021;100:e27330. doi:10.1097/MD.0000000000027330
36. Hsu JY, Sun HY, Chen LY, et al. Weight and metabolic changes among virally-suppressed people living with HIV who switched to co-formulated bictegravir/emtricitabine/tenofovir alafenamide. J Glob Antimicrob Resist. 2023:S2213–7165(23)00181–9. doi:10.1016/j.jgar.2023.10.012
37. Wang Y, Liu S, Zhang W, et al. Development and evaluation of a nomogram for predicting the outcome of immune reconstitution among HIV/AIDS patients receiving antiretroviral therapy in China. Adv Biol. 2023;8:e2300378. doi:10.1002/adbi.202300378
38. Mounzer K, Brunet L, Fusco JS, et al. Advanced HIV infection in treatment-naïve individuals: effectiveness and persistence of recommended 3-Drug regimens. Open Forum Infect Dis. 2022;9:ofac018. doi:10.1093/ofid/ofac018
39. Kong L, Xie X, Fu Y, et al. Clinical efficacy, safety, and subjective experience based on ePRO in HIV-infected individuals administered Bictegravir/Emtricitabine/Tenofovir Alafenamide in southwest China. Immun Inflamm Dis. 2023;11:e974. doi:10.1002/iid3.974
40. Corona D, Pérez-Valero I, Camacho A, et al. Effectiveness and safety of bictegravir/emtricitabine/tenofovir alafenamide in HIV late presenters. Int J Antimicrob Agents. 2023;63:107016. doi:10.1016/j.ijantimicag.2023.107016
41. Priest J, Germain G, Laliberté F, et al. Comparing real-world healthcare costs associated with single-tablet regimens for HIV-1: the 2-drug regimen dolutegravir/lamivudine vs. standard 3- or 4-drug regimens. Infect Dis Ther. 2023;12:2117–2133. doi:10.1007/s40121-023-00848-4
42. Rocabert A, Borjabad B, Berrocal L, et al. Tolerability of bictegravir/tenofovir alafenamide/emtricitabine versus dolutegravir/lamivudine as maintenance therapy in a real-life setting. J Antimicrob Chemother. 2023;78:2961–2967. doi:10.1093/jac/dkad338
43. Rolle CP, Castano J, Nguyen V, Hinestrosa F, DeJesus E. Efficacy, safety, and tolerability of switching from bictegravir/emtricitabine/tenofovir alafenamide to dolutegravir/lamivudine among adults with virologically suppressed HIV: the DYAD Study. Open Forum Infect Dis. 2024;11(10):ofae560. doi:10.1093/ofid/ofae560
44. Migisha R, Ario AR, Kadobera D, et al. High blood pressure and associated factors among HIV-infected young persons aged 13 to 25 years at selected health facilities in Rwenzori region, western Uganda, September–October 2021. Clin Hypertens. 2023;29(6). doi:10.1186/s40885-022-00230-5
45. Sun L, Liu J, He Y, et al. Evolution of blood lipids and risk factors of dyslipidemia among people living with human immunodeficiency virus who had received first-line antiretroviral regimens for 3 years in Shenzhen. Chinese Med J. 2020;133(23):2808–2815. doi:10.1097/CM9.0000000000001245
46. Daar ES, Orkin C, Sax PE, et al. Long-term metabolic changes with bictegravir/emtricitabine/tenofovir alafenamide or dolutegravir-containing regimens for HIV. AIDS Res Ther. 2025;22(45). doi:10.1186/s12981-025-00732-w
47. Van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO Study. Clin Infect Dis. 2020;71:1920–1929. doi:10.1093/cid/ciz1243
48. Maggiolo F, Rizzardini G, Molina JM, et al. Bictegravir/emtricitabine/tenofovir alafenamide in virologically suppressed people with HIV aged ≥ 65 years: week 48 results of a phase 3b, open-label trial. Infect Dis Ther. 2021;10:775–788. doi:10.1007/s40121-021-00419-5
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




