Back to Journals » International Journal of Nanomedicine » Volume 19
Metal-Phenolic Networks: A Promising Frontier in Cancer Theranostics
Authors Li L, Pan J, Huang M, Sun J, Wang C , Xu H
Received 5 September 2024
Accepted for publication 23 October 2024
Published 6 November 2024 Volume 2024:19 Pages 11379—11395
DOI https://doi.org/10.2147/IJN.S491421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Jie Huang
Lingjun Li,1 Jiaoyang Pan,2 Mengwei Huang,3 Jiamin Sun,3 Cheng Wang,2 Hongbin Xu3
1Department of Reproductive Medicine Center, Changzhou Maternal and Child Health Care Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu Province, People’s Republic of China; 2School of Pharmacy, Changzhou University, Changzhou, Jiangsu Province, People’s Republic of China; 3Obstetrics and Gynecology Department, The Third Affiliated Hospital of Nanjing Medical University (Changzhou No. 2 People’s Hospital), Changzhou, Jiangsu Province, People’s Republic of China
Correspondence: Hongbin Xu, Email [email protected]
Abstract: The burgeoning field of cancer theranostics has been significantly advanced by the development of Metal-Phenolic Networks (MPNs), a new class of supramolecular architectures that integrate the advantages of metals and polyphenols. This review focuses on MPNs and their promising applications in cancer theranostics. Through a systematic literature search spanning from 2010 to 2023 in databases including PubMed, Scopus, and Web of Science. The period of search was justified by the rapid evolution of nanomaterials in cancer therapy, with MPNs emerging as a significant player in biomedical applications within the specified timeframe. This review discusses the classification and structure of polyphenolic compounds, as well as their mechanisms of action in cancer treatment. The applications of MPNs in chemotherapy drug delivery, photothermal therapy, chemodynamic therapy, biomedical imaging, and synergistic therapy are especially detailed. The authors emphasize the significance of MPNs in cancer nanomedicine and look forward to their future development directions.
Keywords: MPN, cancer, theranostics, polyphenolic compounds
Introduction
Carcinogenesis is a multifaceted condition that encompasses a diverse array of cellular entities, extracellular matrix components, immunological elements, signaling pathways, and physiological processes.1 The fundamental characteristics of malignancy encompass sustained proliferative signals, indefinite replication potential, neovascularization, invasive behavior, metastatic dissemination, defiance of apoptotic cues, escape from growth inhibitors, immunity subversion, and metabolic rewiring.2 Contemporary therapeutic strategies for cancer encompass surgical resection, pharmacological interventions, radiation therapy, and targeted modalities including hormonal, immunological, and genetic approaches; however, these are beset by drawbacks such as elevated likelihood of neoplastic resurgence, irreversible tissue alterations, operative hazards, and unwanted sequelae.3 Moreover, chemotherapeutics, predominantly small molecule agents, frequently grapple with issues like diminished bioavailability, polypharmacological resistance, inadequate specificity, and deleterious collateral effects. Radiation therapy, in addition, may inflict unwarranted injury upon healthy tissues, culminating in grave adverse reactions.4 The differences between the tumor microenvironment (TME) and normal physiological conditions in organs become crucial for the precise targeting of drug delivery platforms. Cancer is viewed as an evolutionary and ecological process,5 involving constant, dynamic, and static interactions between cancer cells and the TME. Due to the genomic instability of cancer cells, they readily develop drug resistance. In contrast, non-tumor cells within the TME have relatively stable genetic characteristics while being susceptible to external factors.6 The TME has a lower pH, reduced permeability, and considerable barrier effects through various mechanisms.7 Components of the TME, such as abnormal tumor vasculature, disordered extracellular matrix composition, and interstitial hypertension (elevated interstitial fluid pressure), which significantly determine the distribution of drug within the tumor tissue, are important parameters to be considered upon designing drugs for cancer treatment.8 Nanoplatforms, due to their small size, can preferentially accumulate at target sites, enhancing treatment efficacy while significantly reducing toxicity and side effects.9 With advancements in biomedicine and materials science, nanoplatforms that deliver drugs with good targeting, high drug loading capacity, excellent biocompatibility, and bioavailability have become a research hotspot for better cancer management.9–12
Polyphenolic compounds, endowed with potent antioxidant, antiplatelet aggregation, and anti-inflammatory activities, are recognized for their capacity to mitigate or avert the clinical manifestations of a spectrum of metabolic disorders. A wealth of scientific literature supports the notion that a diet replete with polyphenols or fortified with these phenolic substances can exert positive effects on the treatment and management of systemic disorders, neurodegenerative conditions, and amyloidopathies. Furthermore, polyphenols have established themselves as significant players in cancer prevention, with a plethora of evidence from animal studies demonstrating their efficacy in averting the onset of a diverse range of malignancies, including gastric cancer,13 ovarian cancer, breast cancer,14,15 colorectal cancer,16 prostate cancer,17 lymphoma,18 and lung cancer.19 Phenolic drugs act as cytotoxic anticancer agents, demonstrating significant promise in promoting apoptosis, reducing proliferation, and targeting various aspects in cancer progression (angiogenesis, growth, differentiation, and metastasis). However, stability and targeted delivery are critical factors for polyphenols to exert their biological activity while the bioavailability of polyphenolic compounds is a significant hurdle in their use as cancer therapeutic agents. Reports shown that polyphenols usually reach the desired targets at very low concentrations only while nano-formulated polyphenols represent a promising solution to this issue.20,21
Emerging research on nanodrugs composed of common metallic elements for tumor treatment and diagnostics has become a hotspot, with some metal-based nanodrugs successfully transitioning from laboratory to clinic and market due to their excellent biocompatibility and diverse application potentials, making them one of the most successful nanoplatforms.22 The catechol and gallol parts of polyphenols interact with other substances through hydrogen bonding, π-interactions, hydrophobic interactions, metal chelation, covalent bonds, and electrostatic interactions,23 with metal ion chelation being a significant characteristic of polyphenolic compounds.24–26 As common organic ligands, polyphenolic compounds can coordinate with metal ions to form self-assembled metal phenolic networks (MPNs)27 constitute a class of supramolecular amorphous structures, arising from the coordination bonding between polyvalent metal ions and polyphenolic constituents, attracting widespread attention for their remarkable physicochemical properties in various biomedical applications such as bioimaging, drug delivery, and surface coatings.28
The purpose of this review is to introduce the classification and structure of phenolic compounds, the potential actin mechanism of these compounds and summarize the main features of MPNs and their various applications in cancer treatments. Although many previous studies have summarized the recent advances of MPNs,29–31 we focus on the cutting-edge applications of MPNs in the integrated treatment of cancer. This not only serves as a beneficial supplement to the frontier research of MPNs but also points the way for their subsequent development.
Classification and Examples of Polyphenolic Compounds
Plant-derived polyphenols are inherently present in a diverse range of plant-based foods, including vegetables, fruits, cereals, tea, coffee, and other botanical sources. Given their ubiquity and relative abundance as chemical constituents within the plant kingdom, these polyphenols have garnered escalating interest among the scientific research community (Figure 1).32 Phenolic compounds play important roles in the clearance of hydrogen peroxide and the production of secondary metabolites in plant cells. Up to approximately 10,000 polyphenol structures have been identified and described in plants so far33 Polyphenols are a large class of compounds generally characterized by benzene rings with two or more phenolic hydroxy groups. Their distinctive chemical and biological properties arise from the presence of multiple phenol structural units. The biological activity of polyphenols is related to many oxidative stress-related diseases and the activation of cell death through various mechanisms.34 Based on structural features, they are generally categorized into phenolic acids, flavonoids, lignans, stilbenes, tannins, and coumarins.35 These compounds feature phenolic rings as basic monomers with complex structures (Table 1).
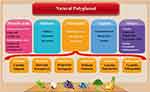 |
Figure 1 Classification of natural polyphenols. Reprinted from Zhang Z, Xie L, Ju Y, Dai Y. Recent advances in metal-phenolic networks for cancer theranostics. Small. 2021;17(43):2100314. © 2021 Wiley-VCH GmbH.32 |
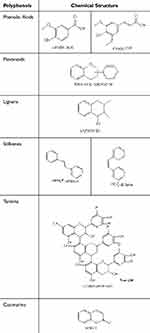 |
Table 1 Representative Polyphenols and Corresponding Chemical Structure |
Phenolic Acids
Phenolic acids represent a subset of phenolic compounds intrinsic to the plant kingdom, ubiquitously present in a vast array of plant-based consumables and forming a substantial component of the typical human diet. Their biosynthesis originates from the shikimate pathway, branching off from either L-phenylalanine or L-tyrosine.36 The classification of phenolic acids is further bifurcated into benzoic acid derivatives, characterized by a 6-carbon framework, and cinnamic acid derivatives, which possess a 9-carbon backbone. These molecules are distinguished by the presence of a carboxylic acid moiety linked to an aromatic ring, often accompanied by one or more hydroxyl or methoxy substituents.37 Notable representatives within this class encompass gallic acid, eugenol, cinnamic acid, ferulic acid, caffeic acid, and sinapic acid.
Flavonoids
In general, flavonoid compounds are characterized by a 15-carbon skeleton (C6-C3-C6), consisting of two aromatic rings (C6) connected by a three-carbon chain (C3), with both C6 units (rings A and B) exhibiting phenolic properties. The variation in hydroxylation patterns and the chromene ring (ring C) allows flavonoids to be further classified into distinct subclasses: flavonols, flavanones, flavan-3-ols, flavones, anthocyanidins, and isoflavones;38 (1) Flavonol Compounds: quercetin, kaempferol, myricetin, luteolin, etc.; (2) Flavanone Compounds: naringenin, hesperetin, eriodictyol, isosakuranetin; (3) Flavan-3-ol Compounds: Catechin, epicatechin, epigallocatechin, etc.; (4) Flavone Compounds: Luteolin, apigenin, chrysin, etc.; (5) Anthocyanidins: Cyanidin, pelargonidin; (6) Isoflavone Compounds: Genistein, daidzein, formononetin, biochanin A, prunetin, etc.35
Lignans
Lignans are a significant group of phenolic compounds that occur naturally and are extensively found throughout various species within the plant kingdom, originating from the shikimate biosynthetic pathway. These compounds display a dimeric structure characterized by a β,β′-bond between two phenylpropane units, with varying degrees of oxidation in the side chains and differing substitution patterns in the aromatic segments. According to the structural motifs of lignans (carbon skeleton, the mode of oxygen incorporation into the skeleton, and cyclization patterns), they are classified into eight categories: arylneolignans, aryltetrahydronaphthalenes, dibenzocyclooctadienes, dibenzylbutanes, dibenzylbutyrolactones, dibenzylbutyrolactols, and furans.39 Notable lignan compounds include magnolol, matairesinol, sesamin, silymarin, taxifolin A, bicyclicol, forsythoside B, and epicatechin, among others.
Stilbenes
Stilbenes are synthesized by plants as a defense mechanism against a variety of stressors, including pathogen attack, elevated temperatures, and oxidative challenges.40 Predominantly functioning as antimicrobial phytoalexins, these substances are specifically induced following plant injury or upon encountering pathogens. The core structure of stilbenes is a 14-carbon framework (C6-C2-C6), featuring two phenyl groups linked by an ethylene bridge. One phenyl group typically has two hydroxyl groups, while the other may have hydroxyl or methoxy groups at various positions. The unique architecture of stilbenes, which differs from that of flavonoids, arises from the polyketide segment’s susceptibility to cyclization and decarboxylation reactions, allowing them to exist in monomeric or oligomeric forms.41 Monomeric stilbenes can adopt two geometric isomers: the trans (E) configuration, which is sterically hindered, and the cis (Z) configuration, which is less hindered but less stable Resveratrol, pterostilbene, and piceatannol are notable examples of this class of polyphenols.
Tannins
Plant-derived tannins, often simply termed “tannins” or “tannic acids”, are ubiquitous components found in the natural flora. They are classified, according to their molecular structure and chemical behavior, into two primary groups: hydrolysable and condensed tannins. The former comprises polyphenolic cores with molecular weights varying from 500 to 3000 Da, encompassing gallotannins and ellagitannins that yield gallic acid and ellagic acid upon hydrolysis, respectively. Condensed tannins, alternatively named proanthocyanidins or leucoanthocyanidins, originate from flavan-3-ols and are oligomeric forms of flavan-3,4-diols. These are predominantly linked by carbon-carbon bonds, though carbon-oxygen-carbon linkages may also occur, contributing to their significant structural heterogeneity. Under acidic alcoholic conditions, condensed tannins break down, yielding a pigment known as phloroglucinol.42,43
Coumarins
Coumarins consist of a 1,2-benzopyranone skeleton (α-chromone), often existing in isoprenylated forms. Coumarin derivatives, as secondary metabolites, are naturally present in over 150 plant species across more than 30 plant families. Depending on their chemical composition and structural characteristics, natural coumarins can be classified into various categories, including but not limited to furanocoumarins, simple coumarins, biscoumarins, isocoumarins, pyranocoumarins, and phenylcoumarins.44 The coumarin core is often used as a template for synthesizing various pharmacologically important novel compounds, such as scopolamine, serpentine, phloroglucinol, etc.
Research on the Antitumor Activity of Polyphenolic Compounds
Essential architectural elements that contribute to the anticancer efficacy of phenolic compounds encompass the presence of aromatic ring systems, unsaturated side chains, and the quantity and spatial arrangement of unsubstituted hydroxyl moieties. In studies of structure-activity relationships, key functional groups that confer potent antitumor effects on phenolic compounds include aromatic rings and hydroxyl groups. Compounds with more hydroxyl groups exhibit superior antitumor activity compared to those without hydroxyl groups or those containing –OCH3 moieties.45
The anticancer mechanisms of polyphenols are multifaceted, encompassing the initiation of cell cycle halt, manifestation of antioxidant properties, enhancement of pro-oxidant actions, and suppression of specific kinases and enzymes that lead to the modulation of intracellular signaling pathways. Additionally, they impede angiogenesis, exert anti-estrogenic effects, curb cell proliferation, and possess immunomodulatory and anti-inflammatory capabilities.46 The strength of polyphenols as anti-neoplastic agents is predominantly due to their antioxidant potential. They also act as potent scavengers of free radicals, chelators of metal ions, and modulators of the body’s innate defenses, including enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), as well as regulators of the glutathione (GSH) redox cycle and various proteins and transcription factors like nuclear factor erythroid 2-related factor 2 (NRF2). Furthermore, their antitumor efficacy is characterized by the inhibition of cell proliferation markers (such as extracellular signal-regulated kinase, cyclin D, cyclin-dependent kinases, angiogenic factors, vascular endothelial growth factor, oncogenic signaling cascade proteins, and protein kinase B), the induction of programmed cell death (apoptosis), and the prevention of cell migration and metastatic processes.47
Antioxidant Activity
Within the context of cancer cells, reactive oxygen species (ROS) are integral to the modulation and initiation of apoptosis, exerting a pivotal influence on the regulation of cancer cell expansion, viability, and chemoresistance. Tumor hypoxia, a condition arising when the tumor vasculature fails to sufficiently nourish the proliferating mass, also leads to an escalation in ROS concentration. ROS, acting as intracellular signaling molecules, can inflict harm upon cellular macromolecules such as DNA, RNA, and proteins when in surplus. The consumption of polyphenolic compounds has demonstrated a significant protective role against ROS-induced damage and other reactive species within the human body, primarily due to their robust antioxidant capacity. Polyphenols mitigate ROS through a variety of pathways, which include 1) Engaging in direct radical scavenging, neutralizing both free radicals and diverse ROS species, including superoxide and peroxynitrite; 2) Exerting metal-chelating actions, thereby diminishing the catalytic potential of metal ions in ROS generation; 3) Augmenting the activity of intrinsic antioxidant defenses, such as the synthesis of superoxide dismutase (SOD) and glutathione (GSH); 4) Suppressing the activity of enzymes that generate ROS within the cell, including xanthine oxidase, myeloperoxidase, and NADPH oxidase. This multi-pronged approach by polyphenolic compounds contributes to their efficacy in countering oxidative stress and supports their potential utility in cancer therapy and prevention. Flavonoids exert fundamental antioxidant mechanisms by reacting with free radicals, which forms more stable, less reactive radicals. The antioxidant capacity of polyphenolic compounds stems from several structural features, such as the hydroxyl groups on the B-ring and the conjugated 2,3-double bond with the 4-oxo functional group in flavonoids, which can reduce ROS. Umbelliferone exhibits anticancer activity against human oral cancer (KB) cell lines, which was found to associated with the ROS mediated mitochondrial depolarization.48 Meanwhile, studies demonstrate the ability of flavonoids to prevent cardiomyocyte apoptosis, supporting their anti-apoptotic function under oxidative damage. Fruits and vegetables rich in anthocyanins and other flavonoids (such as quercetin, rutin, apigenin, etc.) significantly improve myocardial antioxidant status in models of drug-induced (doxorubicin), ischemia-induced heart dysfunction and alleviate the oxidative related damages in other organs.49–51 A plethora of research indicates that a diverse array of polyphenolic compounds and botanical extracts abundant in these constituents exert beneficial impacts on cardiovascular health. They achieve this by modulating oxidative stress at the cellular level, mitigating inflammatory responses, and influencing a spectrum of intracellular signaling cascades. Specifically, flavonoids have been observed to counteract oxidative stress in myocardial tissues. They accomplish this by suppressing the generation of reactive oxygen species (ROS) intrinsic to the cell, reducing the expression of inflammatory cytokines, and ameliorating the function of the mitochondrial respiratory chain. These actions collectively contribute to the cardioprotective effects of flavonoid-rich substances.
Cell Signal Regulation and Apoptosis
Cancer arises from imbalances and aberrant mechanisms in the apoptotic pathways. Apoptosis, a programmed cell death, responds to various signals of intracellular damage and is crucial for tissue homeostasis. The condition is distinguished by the presence of bleb-like protrusions on the cell membrane, a reduction in cellular volume, disintegration of the nucleus, and fragmentation of chromosomal DNA.34 The B lymphocyte leukemia/lymphoma-2 (Bcl-2) family regulates cell behavior through programmed cell death, primarily located on the outer mitochondrial membrane, chiefly regulating the release of cytochrome C. Apoptosis eliminates misplaced cells, effectively inhibiting the occurrence of metastatic dissemination. Thus, apoptosis plays an irreplaceably important role in preventing cell metastasis. Whether malignant cells evade the apoptotic process determines the success of metastasis. Resistance to apoptosis is essential for all steps of metastatic progression. Polyphenolic compounds modulate key components of the extracellular signal-regulated kinase (ERK), mitogen-activated protein kinase (MAPK), and protein kinase B (AKT) signaling pathways, demonstrating significant anticancer properties. Their efficacy is notable in aggressive breast malignancies, including the challenging triple-negative breast cancer subtypes.52 In human breast cancer cells MCF-7, quercetin induces apoptosis by increasing the expression of the pro-apoptotic gene Bax.53 Kaempferol induces DNA fragmentation and enhances the expression of the tumor suppressor gene p53, blocking the transmission of cell proliferation signals, thereby inducing cells to undergo self-apoptosis.54 Simultaneously, kaempferol effectively activates the MAPK cascade, a critical signaling pathway for normal cell proliferation, survival, and differentiation, and simultaneously activates the MEK1 and ELK1 pathways to reduce cell adhesion, migration, and invasion.55 Curcumin is capable of inhibiting the proliferation of cervical cancer cells, which is associated with the suppression of transcription factors such as nuclear factor kappa B (NF-κB).56
Transporter Regulation
Resistance to pharmaceuticals poses a significant impediment to the enduring efficacy of clinical cancer treatments. The phenomenon of multidrug resistance (MDR) arises when both microbial and cancerous cells develop an acquired imperviousness to a diverse array of chemotherapeutic agents that vary in chemical composition and functional mechanisms. The ATP-binding cassette (ABC) transporters represent a vast superfamily of proteins, comprising 49 distinct members, categorized according to genetic sequence and structural homology.57 Notably, a subset of at least 11 ABC transporters, such as P-glycoprotein (P-gp/ABCB1), multidrug resistance-associated proteins (MRPs/ABCCs including MRP1, MRP2, and others), and breast cancer resistance protein (BCRP/ABCG2), are implicated in the emergence of MDR. Specifically, ABC transporters like P-gp and MRP can exhibit heightened expression levels in cancerous cells, resulting in suboptimal intracellular drug concentrations that fall short of therapeutic requirements.58 P-gp stands out as the most extensively studied efflux pump associated with MDR in cancer. Its subcellular localization and expression levels are believed to significantly influence MDR across various cancer types. Resistance to chemotherapeutic agents, including taxanes like paclitaxel, vinca alkaloids such as vincristine, and anthracyclines like daunorubicin, is a leading cause of mortality in the majority of patients afflicted with advanced cancer stages, accounting for over 90% of deaths in this population.59 To overcome cancer resistance, alternative treatment strategies are necessary with combinations of drugs with natural molecules represent an excellent strategy. This strategy not only effectively overcomes cancer resistance but also contribute significantly to increased antitumor action and reduced systemic toxicity.60 The structural features of flavonoids give them strong affinity for P-gp, positively impacting the inhibition of P-gp, MRP1, MRP2, and BCRP.61 The anticancer and chemosensitizing activities of quercetin has been demonstrated in vitro and in vivo tests.62 Quercetin isolated from ginkgo leaves was shown to significantly inhibit ATPase activity, thus strongly inhibiting the transport of Hoechst-33342 (a fluorescent probe and P-gp substrate).63 Rutin, structurally similar to quercetin, significantly decreases the accumulation of P-gp substrates in the human colon adenocarcinoma cell line LS180 and reduces the bioavailability of cyclosporine by decreasing P-gp activation.64 Catecholamines, tannic acid, curcumin combined with doxorubicin (DOX) can enhance the sensitivity of MDR cells to DOX.
Influence on Microbiota
Studies indicate that the microbial metabolism of polyphenols is associated with cancer prevention, with differences in gut microbiota levels observed between patients with colorectal cancer and healthy individuals.65 In vitro and specific microbiota studies in rats suggest that lignans can transform the gut microbiome consortium. Furthermore, symbiosis with the lignan microbiota community protects germ-free rats from 7,12-dimethylbenz[a]anthracene-induced cancer, significantly reducing tumor numbers, sizes, and cellular proliferation while increasing tumor cell apoptosis.66 Certain polyphenols may also affect bacterial metabolic enzymes, influencing overall cancer risk. Resveratrol, a stilbene compound, is a treatment for colon cancer and exerts anti-inflammatory effects. In rat models, supplementation with resveratrol significantly reduced the activity of fecal and host colonic mucosal enzymes and decreased bacterial enzyme activity, resulting in a significant reduction in the incidence of colonic tumors.67
Multifunctional MPNs in Cancer Therapy
The variability of polyphenols and metals endows composite materials with unique characteristics and capabilities. A hallmark of most polyphenolic compounds is the presence of catechol groups within their molecular architecture, which offer specific binding sites for metal ions. The phenolic hydroxyl functionalities and aromatic ring structures of polyphenols facilitate their interaction with a range of metal ions, as well as other molecules and substrates, through both covalent (eg, Michael addition, Schiff base formation, and coordination chemistry) and non-covalent (eg, hydrogen bonding, π-π stacking, and electrostatic forces) interactions.25,68–72 The formation of metal-polyphenol coordination polymers arises from the synergistic combination of metal ions with phenolic ligands. The metal ions bring a spectrum of attributes to these polymers, including electronic, optical, radioactive, magnetic, and catalytic properties, which render MPNs as unique nanoplatforms suitable for applications in imaging and therapeutics.29
MPNs exhibit many superior properties in biomedicine due to their unique and novel structures, including adhesiveness, multifunctional drug-loading capabilities, stimulus-responsive dissociation, high photothermal conversion efficiency, and Fenton reaction activity.73 The variety of metal ions and polyphenols allows for the creation of nanomaterials with different shapes and functions, enabling flexible integration of various therapeutic modalities.
Delivery of Chemotherapy Drugs
Compared to free drugs, nanodrug offer numerous advantages.74 They protect drugs from degradation during in vivo transport, control drug release in specific areas or cells in response to specific signals, increase specificity for tumor targeting, reduce cytotoxic side effects on normal cells, decrease systemic side effects, increase drug solubility, and augment maximum tolerated doses.75 Nanodrugs with covalent bonds are stable in the circulatory system, but excessive stability may hinder drug release within the body. Additionally, covalently-based nanodrugs involves numerous chemical reactions and toxic reagents, with low reproducibility due to complex preparation procedures, potentially affecting drug activity.76 An alternative strategy uses non-covalent interactions to construct nanomaterials through supramolecular self-assembly, mainly involving non-covalent interactions such as hydrophobic, electrostatic, and hydrogen bonding.77 However, non-covalent bonds can be somewhat unstable, leading to the disruption and leakage of nanodrugs after entering the complex circulatory system, potentially harming normal tissues and failing to achieve desired therapeutic outcomes.78 Recently, strategies utilizing metal-ion coordination to guide the self-assembly of different organic molecules have attracted considerable attention. Since coordination bonds exhibit stability intermediate between strong covalent bonds and weak non-covalent interactions, they maintain stability in complex in vivo environments while possessing dynamic behavior. The porous structure of MPNs offers favorable conditions for loading chemotherapy drugs.28,79–81
Caruso et al82 developed MPN-based capsules featuring a responsive gating system, modulated by adjusting intermolecular forces. These capsules are sensitive to pH fluctuations between 4 and 9, enabling reversible “closed” to “open” state changes, which in turn allow for the precise management of drug encapsulation and release kinetics, as depicted in Figure 2. In another study, Gang et al83 fabricated a delivery system for cabazitaxel (Cab), integrating MPNs with chitosan (CS) to enhance Cab delivery efficacy in melanoma therapy. The application of a CS layer resulted in an elevated drug payload, rising from 7.56% to 9.28%. The Cab@MPN/CS system was shown to release Cab in a sustained manner within the acidic TME, effectively inhibiting melanoma growth in both cellular and animal models.
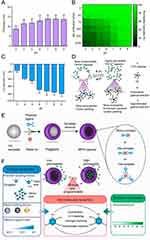 |
Figure 2 (A) Graphical representation of the wall thickness for MPN MPN capsules fabricated across a pH range of 3 to 9. (B) A heatmap depicting the permeability of capsules to FITC-dextran across a molecular weight spectrum of 4 to 2000 kDa at varying pH levels; the assessment was based on 100 capsules per pH condition. (C) The ζ-Potential measurements of MPN capsules dispersed under a range of pH environments. (D) A conceptual model illustrating the influence of pH on the permeability characteristics of the capsules. (E) A stepwise schematic outlining the fabrication of an MPN film on a polystyrene template particle, resulting in the formation of a PS@MPN construct, and the subsequent generation of an MPN capsule post-template elimination. (F) A diagrammatic representation of the process to programmatically regulate the permeability of MPN capsules by manipulating intermolecular interactions through a combination of intrinsic properties and extrinsic factors. Reprinted with permission from Chen J, Pan S, Zhou J, et al. Programmable permeability of metal–phenolic network microcapsules. Chem Mater. 2020;32(16):6975–6982. Copyright 2020, ACS Publications.82 |
Photothermal Therapy
Photothermal therapy (PTT) is an emerging, widely applied treatment modality in oncology, offering advantages such as high selectivity, low invasiveness, and minimal side effects. PTT mechanisms primarily involve the selective enrichment of photothermal agents (PTAs) in tumor tissues, converting absorbed near-infrared light into localized heat to kill cancer cells.84,85 However, traditional PTAs have limitations due to poor biocompatibility, low photothermal conversion efficiency, and complex synthesis processes.86 In contrast, MPNs with exceptional photothermal conversion efficiency, good photothermal stability, and biocompatibility hold greater promise and warrant further investigation. Combining PTT with other treatments, such as chemotherapy, can produce synergistic effects, significantly enhancing the efficacy of chemotherapeutics. Moreover, PTT can facilitate the release of chemotherapeutics upon near-infrared light stimulation, amplifying their antitumor effects.87,88 MPNs are excellent photothermal materials due to their versatile and superior physical-chemical properties. The mechanism may be attributed to the coordination interaction between metal ions and polyphenols in MPNs, leading to the splitting of metal ions’ d-orbitals and d-d electron transitions, resulting in new absorption peaks. Given that the d-d electron transitions of metal ions are largely determined by the nature of their ligands, the near-infrared (NIR) absorption capability can be controlled through judicious design of coordinating ligands.89 Feng et al90 systematically explored the photothermal capabilities of MPNs induced by various metal ions, including Gd3+, Ru3+, Fe3+, Cu2+, Ni2+, Mn2+, and V3+. Under near-infrared 808 nm laser irradiation, the temperature of 200 μg/mL Fe-TA nanoparticles increased by approximately 44.5 °C above water’s 3.8 °C. Further measurements showed that V-TA and Ru-TA nanoparticles exhibited broad absorption in the near-infrared window and near-infrared-activated photothermal conversion. Gd3+, Cu2+, Ni2+, and Mn2+-based MPNs also showed broad absorption. Chen’s team91 developed a nano-complex FeAP-NPs using natural cyanidin (CAN), Fe3+, and PLG-g-mPEG. Photothermal conversion assays demonstrated that FeAP-NPs exhibited photothermal heating efficacy that was contingent upon their concentration upon irradiation with an 808 nm laser. Following five sequential photothermal cycles, the temperature elevation profiles were found to be nearly indistinguishable from one another, thereby validating the superior photothermal stability of FeAP-NPs. These nanoparticles also displayed exceptional dual-modal photoacoustic (PA) and magnetic resonance (MR) imaging properties coupled with high photothermal conversion efficiency. In vivo studies conducted on mice bearing MCF-7 tumors resulted in the complete eradication of the tumors upon PTT treatment.
Chemodynamic Therapy
Chemodynamic therapy (CDT) refers to an emerging in situ treatment strategy utilizing Fenton or Fenton-like reactions to generate hydroxyl radicals (·OH) at the tumor site. Compared to chemotherapy, CDT offers significant advantages, which includes high locality and selectivity, and initiation by endogenous stimuli.92–97 Ferroptosis is a non-apoptotic form of cell death induced by iron-mediated lipid peroxidation accumulation, causing lipid peroxidation and membrane damage.98 Ferrous ion (Fe2+) can directly catalyze H2O2 decomposition into·OH through the Fenton reaction, promoting intracellular ROS accumulation and lipid peroxidation.99 Excessive free Fe2+ can directly or indirectly promote lipid peroxidation, making iron overload and triggers ferroptosis. Ferric ions (Fe3+) are stable and can react with many chemical drugs to form complexes with small particle sizes, exhibiting good stability in the body. However, the catalytic activity of Fe3+ is much lower than that of Fe2+. To address this bottleneck, in many studies, Fe3+ serve as precursors to chelate with chemical drugs upon in vitro assembly while transferring into Fe2+ in vivo for ferroptosis therapy. Liu et al100 combined ferroptosis inducers, iron ions, and an excess of acid-activated reductant for concurrent drug delivery to tumors with continuously supplying exogenous Fe2+. Specifically, Fe3+ was self-deposited onto sorafenib (SRF) along with tannic acid (TA), forming a core-shell structured nanocrystal SRF@FeIII. TA, acting as an activated reductant, reduced Fe3+ to Fe2+ within the lysosomes of tumor cells, initiating the Fenton reaction, while SRF inhibited glutathione peroxidase GPX4 (Figure 3). However, due to limited H2O2 availability and low catalytic efficiency, the Fenton reaction often falls short of expectations. Numerous MPNs designed to catalyze Fenton reactions have emerged. Zhang et al101 synthesized an ATP-responsive self-catalytic Fenton nanosystem (GOx@ZIF@MPN) for tumor ablation. Overexpression of ATP in tumor cells led to the degradation of the MPN-coated GOx@ZIF@MPN complex, releasing Fe3+ and TA, exposing the internal GOx complex. Subsequently, GOx reacted with endogenous glucose to produce abundant H2O2, followed by TA reducing Fe3+ to Fe2+, generating toxic·OH via H2O2 catalyzation through Fe2+. The Fe3+ was rapidly reduced to reactive Fe2+ under TA mediation, forming an accelerated Fe3+/Fe2+ conversion, ensuring effective CDT mediated by the Fenton reaction. Zheng et al102 formed MPNs on the surface of PEI/p53 complexes through chemical bonding between TA and Fe3+, successfully preparing MON-p53 complexes. The prepared complexes could effectively enhance the ferroptosis-inducing effect of p53 through MON coating, holding great potential for application.
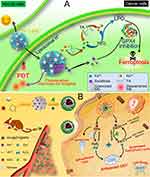 |
Figure 3 (A) Schematic Illustration of SFT-Mediated Combination of Ferroptosis and Image-Guided PDTa. Reprinted with permission from Liu T, Liu W, Zhang M, et al. Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano. 2018;12(12):12181–12192. Copyright 2018, ACS Publications.100 (B) Schematic Illustration of GOx@ZIF@MPN as an ATP-Responsive Autocatalytic Fenton Nanoparticle for Tumor Ablation. Reprinted with permission from Zhang L, Wan -S-S, Li C-X, Xu L, Cheng H, Zhang X-Z. An adenosine triphosphate-responsive autocatalytic Fenton nanoparticle for tumor ablation with self-supplied H2O2 and acceleration of Fe(III)/Fe(II) conversion. Nano Lett. 2018;18(12):7609–7618. Copyright 2018, ACS Publications.101 |
Biomedical Imaging
In the recent past, the utilization of nanomaterials for imaging purposes has garnered significant interest in the medical field for diagnosing and treating a spectrum of conditions. Standard imaging techniques encompass photoacoustic imaging (PAI), positron emission tomography (PET), computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US).103–105 The realm of MRI contrast agents extends from paramagnetic molecular complexes to nanoscale materials embedded with such complexes, as well as inorganic magnetic nanomaterials. The synergistic integration of the distinctive attributes of metal ions with the adaptable network structures of nanomaterials facilitates the convergence of diagnostic and therapeutic applications. Frequently employed magnetic metals ions such as Mn2+, Gd3+, and Fe3+ can engage in coordination with polyphenols, yielding MPNs suitable for biomedical imaging. These imaging modalities are favored for their minimally invasive nature, heightened sensitivity, precise spatial resolution, and the ability to penetrate deeply into tissues, attracting considerable attention from researchers in the field. They overcome drawbacks of conventional small molecule contrast agents, such as high cytotoxicity, poor photostability, and inadequate biological stability. PET is a molecular imaging technique based on radioactive nuclides. Traditional PET contrast agents require additional chelators, whereas the polyphenols in MPNs have strong chelating abilities, allowing for rapid and easy binding with radioactive nuclides. Wang’s research team106 reported the formation of supramolecular nanoparticles (PPNPs) through self-assembly of TA and Poloxamer. PPNPs were synthesized through the interaction of TA with F-127. The phenolic hydroxyl groups present in TA engaged in hydrogen bonding with the ether oxygen atoms in the Polyoxyethylene segments of F-127. The hydrophobic nature of the Polyoxypropylene chains in F-127 facilitated the encapsulation of the near-infrared fluorescent dye IR780 within the PPNPs, allowing for near-infrared fluorescence (NIRF) imaging. Furthermore, the presence of numerous free -OH groups in the PPNPs enabled the chelation of the positron-emitting radioisotope 89ZrIV, forming stable O-Zr linkages. NIRF and PET imaging effectively detected tumors in mice bearing human breast cancer cell line tumors (SKBR3) overexpressing human epidermal growth factor receptor 2 (Her2). Additionally, nanoporous metal-phenolic granules (npFeIII-TA RPs) and nanoporous CaCO3 granules were prepared through self-assembly coordination of Fe3+ and TA. Fe3+ chelated in the npFeIII-TA RP network catalyzed the conversion of H2O2 to O2, serving as a reagent for US imaging.107 Xie et al79 developed a novel phototherapeutic MPN using phenolic semiconductor polymers and PEG-modified semiconductor polymers. Within this nanoscale construct, the Fe3+, an efficacious inducer of ferroptosis, was coordinated with the phenolic moieties present on the phenolic polymer backbone. To enhance the nanoparticles’ surface properties, hydrophilic PEG polymers were integrated, endowing the nanoparticles with amphiphilic characteristics and resulting in the formation of PEGylated, polymer-encapsulated Fe3+ Metal-Polyphenolate Nanoparticles (PF MPN). The hydrophobic exosome inhibitor GW4869 was embedded within the core of the PF MPN, serving the dual purpose of phototherapy and diagnostic imaging. Upon accumulation at the tumor site, the PF MPNs demonstrated enhanced NIRF and PA imaging capabilities under the influence of near-infrared laser irradiation.
Synergistic Therapy
From the aforementioned summary, MPNs can be flexibly designed into multifunctional, multi-pathway nanosystems for cancer diagnosis and therapy. For instance, Yao’s team108 developed a combinatorial therapy using DOX as a chemotherapeutic agent, ferric chloride (FeCl3) as a ferroptosis inducer, and TA as an activator for the SOD-like reaction in intracellular cascades, for breast cancer treatment. DFTA nanoparticles, with dimensions of 106.4 ± 0.7 nm and an irregular nanomesh morphology, were observed to generate a pronounced photothermal response upon aggregation. The release of the encapsulated drug from DFTA was demonstrated to be efficiently activated by photothermal stimulation. ELISA-based assays revealed that the combination of DFTA with laser irradiation significantly depleted intracellular GSH levels, an effect attributed to the intensified oxidative stress induced by ROS and the ROS-mediated PTT. Yu and colleagues109 reported the self-assembly synthesis of Fe-GA@BSA nanoparticles, incorporating the chemotherapeutic agent SRF to create the Fe-GA@BSA-SRF nanomedicine. Experimental data suggested that this nanomedicine could trigger ferroptosis in cancer cells by enhancing the Fenton reaction. The inherent photothermal properties of Fe-GA@BSA-SRF were harnessed for mild thermotherapy to augment the ferrous therapy. The nanoformulation exhibited potent anticancer efficacy by inducing ROS production and suppressing the expression of glutathione peroxidase 4 (GPX4) in both in vitro and in vivo settings. Xu et al110 described the development of a synergistic theranostic nanoplatform, which involved the in situ coordination of TA with Fe3+ to form DOX-loaded TAF nanocomplexes. These were further functionalized with a fibronectin (FN) coating on the DOX-TAF surface through hydrogen bonding interactions, yielding DOX-TAF@FN nanocomplexes. This nanosystem integrated chemotherapy and CDT to activate immune responses, with the aim of enhancing tumor treatment efficacy. The therapeutic approach was further complemented by the use of an anti-PD-L1 strategy to target immune checkpoint blockade (ICB), thereby eliciting a robust immune response against the tumor.
Conclusion and Perspective
The preceding sections reviewed the structure and classification of polyphenolic compounds, elucidated their mechanisms against tumors, introduced the application of MPNs formed by the chelation of polyphenols and metal ions in cancer therapy, and cited various studies on multifunctional MPNs. Generally, the construction of MPNs encompasses multiple antitumor pathways. These multifunctional MPNs exhibit potent antitumor capabilities, and depending on the type of polyphenolic compound, metal ion, and surface coating, various tumor treatment strategies can be flexibly developed. As a critical component of the field of anticancer nanomedicines, the reticular porous structure of MPNs not only facilitates the construction of drug delivery systems suitable for conventional chemotherapeutics but also promotes the release of chemotherapy drugs at specific target sites, mitigating the various side effects associated with chemotherapy. With the advancement of nanomedicine, the role of MPNs in antitumor treatment has become increasingly prominent, encompassing light-mediated PTT, multimodal bioimaging, among others. Compared to traditional nanomaterial fabrication strategies, the coordination between metal-polyphenol compounds is relatively facile, requiring inexpensive and readily available raw materials, employing relatively simple equipment during the reaction process, and permitting adjustments. MPNs possess green, nontoxic, and pH-sensitive characteristics, displaying excellent photothermal performance, good tunability, and good biocompatibility.
Despite the demonstrated superior and broad biological properties of MPNs, they are still in the nascent stage, with many issues yet to be addressed. Further emphasis should be placed on integrating the carrier function of MPNs with the characteristics of the loaded drugs to achieve synergistic therapeutic effects, which goes beyond merely stacking several effects together. On another front, the stability of MPNs as carriers deserves significant attention. A sufficiently stable carrier can prevent damage to normal tissues and reduction in drug efficacy during drug delivery, but overly stable carriers may lead to difficulties in drug release. Therefore, the underlying mechanisms responsible for the stability adjustment of MPNs should be explored in future works. Moreover, regarding preparation strategies, in the design process of MPNs, more importance should be given to exploring the possibility of multifunctional platforms, including the integration of tumor targetability and TME responsiveness. Additionally, the biosafety of MPNs is also worthy of exploration for they further application into clinic.
Declaration of Generative AI in Scientific Writing
In the composition of this manuscript, the authors have employed artificial intelligence tools, specifically Kimi and Tongyi QianWen, to refine the manuscript’s prose. Following the application of these AI-based technologies, the authors have conducted a thorough review and revision of the manuscript content. The authors assume complete accountability for all aspects of the published work, ensuring that the final submission aligns with the highest standards of academic integrity and scholarly communication.
Data Sharing Statement
Data will be made available on request from Hongbin Xu ([email protected]).
Acknowledgment
The authors acknowledge the funding from The Changzhou Sci & Tech Program (No. CJ20220137); The Third Affiliated Hospital of Nanjing Medical University (Changzhou No. 2 People’s Hospital), Changzhou Medical Center, Nanjing Medical University (No. CMCC202215).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discovery. 2022;12(1):31–46. doi:10.1158/2159-8290.CD-21-1059
3. Bidram E, Esmaeili Y, Ranji-Burachaloo H, et al. A concise review on cancer treatment methods and delivery systems. J Drug Delivery Sci Technol. 2019;54:101350.
4. Tynga IM, Abrahamse H. Nano-mediated photodynamic therapy for cancer: enhancement of cancer specificity and therapeutic effects. Nanomaterials. 2018;8(11):923–930. doi:10.3390/nano8110923
5. Maley CC, Aktipis A, Graham TA, et al. Classifying the evolutionary and ecological features of neoplasms. Nat Rev Cancer. 2017;17(10):605–619. doi:10.1038/nrc.2017.69
6. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. doi:10.1016/j.pharmthera.2020.107753
7. Khawar IA, Kim JH, Kuh H-J. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release. 2015;201:78–89. doi:10.1016/j.jconrel.2014.12.018
8. Hu J, Yuan X, Wang F, Gao H, Liu X, Zhang W. The progress and perspective of strategies to improve tumor penetration of nanomedicines. Chin Chem Lett. 2021;32(4):1341–1347. doi:10.1016/j.cclet.2020.11.006
9. Rosic G, Selakovic D, Omarova S. CANCER SIGNALING, CELL/GENE THERAPY, DIAGNOSIS AND ROLE OF NANOBIOMATERIALS. Adv Biol Earth Sci. 2024;9(Special Issue):11–34. doi:10.62476/abes9s11
10. Al-Thani AN, Jan AG, Abbas M, Geetha M, Sadasivuni KK. Nanoparticles in cancer theragnostic and drug delivery: a comprehensive review. Life Sci. 2024;352:122899. doi:10.1016/j.lfs.2024.122899
11. Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Delivery Rev. 2012;64:24–36. doi:10.1016/j.addr.2012.09.006
12. Wang C. Reconstituted lipid nanoparticles from cells/tissues for drug delivery in cancer. Mol Pharmaceut. 2023;20(6):2891–2898. doi:10.1021/acs.molpharmaceut.2c01033
13. Deng Y-T, Lin J-K. EGCG Inhibits the Invasion of Highly Invasive CL1-5 lung cancer cells through suppressing mmp-2 expression via jnk signaling and induces g2/M arrest. J Agricultural Food Chem. 2011;59(24):13318–13327. doi:10.1021/jf204149c
14. Hung J-Y, Hsu Y-L, Li C-T, et al. 6-shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells. J Agricultural Food Chem. 2009;57(20):9809–9816. doi:10.1021/jf902315e
15. Wang K, Zhu X, Zhang K, Zhu L, Zhou F. Investigation of gallic acid induced anticancer effect in human breast carcinoma MCF-7 cells. J Biochem Molecular Toxicol. 2014;28(9):387–393. doi:10.1002/jbt.21575
16. Zhao Y, Jiang Q. Roles of the polyphenol–gut microbiota interaction in alleviating colitis and preventing colitis-associated colorectal cancer. Adv Nutr. 2021;12(2):546–565. doi:10.1093/advances/nmaa104
17. Dasari S, Samy ALPA, Kajdacsy-Balla A, Bosland MC, Munirathinam G. Vitamin K2, a menaquinone present in dairy products targets castration-resistant prostate cancer cell-line by activating apoptosis signaling. Food Chem Toxicol. 2018;115:218–227. doi:10.1016/j.fct.2018.02.018
18. Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 2019;20(13):3177–3182. doi:10.3390/ijms20133177
19. Wang S-F, Wu M-Y, Cai C-Z, Li M, Lu J-H. Autophagy modulators from traditional Chinese medicine: mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J Ethnopharmacol. 2016;194:861–876. doi:10.1016/j.jep.2016.10.069
20. Jakobek L, Matić P. Non-covalent dietary fiber - Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci Technol. 2019;83:235–247. doi:10.1016/j.tifs.2018.11.024
21. Li Y, He D, Li B, et al. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci Technol. 2021;110:470–482. doi:10.1016/j.tifs.2021.02.009
22. Dadfar SM, Roemhild K, Drude NI, et al. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev. 2019;138:302–325. doi:10.1016/j.addr.2019.01.005
23. Zhou J, Lin Z, Ju Y, Rahim MA, Richardson JJ, Caruso F. Polyphenol-mediated assembly for particle engineering. Acc Chem Res. 2020;53(7):1269–1278. doi:10.1021/acs.accounts.0c00150
24. Perron NR, Wang HC, DeGuire SN, Jenkins M, Lawson M, Brumaghim JL. Kinetics of iron oxidation upon polyphenol binding. Dalton Trans. 2010;39(41):9982–9987. doi:10.1039/c0dt00752h
25. Hider RC, Liu ZD, Khodr HH. Metal chelation of polyphenols. Flavonoids Other Polyphenols. 2001;335:190–203.
26. McGee EJT, Diosady LL. Development of spectrophotometric quantification method of iron-polyphenol complex in iron-fortified black tea at relevant pH levels. Food Anal Methods. 2018;11(6):1645–1655. doi:10.1007/s12161-018-1147-8
27. Chang Y, Cui P, Zhou S, et al. Metal-phenolic network for cancer therapy. J Drug Delivery Sci Technol. 2023;81:104194. doi:10.1016/j.jddst.2023.104194
28. Ejima H, Richardson JJ, Caruso F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today. 2017;12:136–148. doi:10.1016/j.nantod.2016.12.012
29. Guo Y, Sun Q, Wu FG, Dai Y, Chen X. Polyphenol‐containing nanoparticles: synthesis, properties, and therapeutic delivery. Adv Mater. 2021;33(22):2007356. doi:10.1002/adma.202007356
30. Andersen A, Chen Y, Birkedal H. Bioinspired metal–polyphenol materials: self-healing and beyond. Biomimetics. 2019;4(2):30. doi:10.3390/biomimetics4020030
31. Qin J, Guo N, Yang J, Chen Y. Recent advances of metal–polyphenol coordination polymers for biomedical applications. Biosensors. 2023;13(8):776. doi:10.3390/bios13080776
32. Zhang Z, Xie L, Ju Y, Dai Y. Recent advances in metal-phenolic networks for cancer theranostics. Small. 2021;17(43):2100314. doi:10.1002/smll.202100314
33. Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–1246. doi:10.3390/nu2121231
34. Braicu C, Zanoaga O, Zimta -A-A, et al. Natural compounds modulate the crosstalk between apoptosis- and autophagy-regulated signaling pathways: controlling the uncontrolled expansion of tumor cells. Semi Cancer Biol. 2022;80:218–236. doi:10.1016/j.semcancer.2020.05.015
35. Abotaleb M, Samuel SM, Varghese E, et al. Flavonoids in cancer and apoptosis. Cancers. 2019;11(1):28. doi:10.3390/cancers11010028
36. Heleno SA, Martins A, Queiroz MJRP, Ferreira ICFR. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chem. 2015;173:501–513. doi:10.1016/j.foodchem.2014.10.057
37. Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21(1):381–406. doi:10.1146/annurev.nutr.21.1.381
38. Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A. Dietary (Poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signaling. 2012;18(14):1818–1892. doi:10.1089/ars.2012.4581
39. Teponno RB, Kusari S, Spiteller M. Recent advances in research on lignans and neolignans. Nat Product Reports. 2016;33(9):1044–1092. doi:10.1039/C6NP00021E
40. Hapeshi A, Benarroch JM, Clarke DJ, Waterfield NR. Iso-propyl stilbene: a life cycle signal? Microbiology. 2019;165(5):516–526. doi:10.1099/mic.0.000790
41. Teka T, Zhang L, Ge X, Li Y, Han L, Yan X. Stilbenes: source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry. 2022;197:113128. doi:10.1016/j.phytochem.2022.113128
42. Khanbabaee K, van Ree T. Tannins: classification and definition. Nat Product Reports. 2001;18(6):641–649.
43. Tong Z, He W, Fan X, Guo A. Biological function of plant tannin and its application in animal health. Frontiers Veterinary Sci. 2022;8:803657. doi:10.3389/fvets.2021.803657
44. Zhu -J-J, Jiang J-G. Pharmacological and nutritional effects of natural coumarins and their structure–activity relationships. Mol Nutr Food Res. 2018;62(14):1701073. doi:10.1002/mnfr.201701073
45. Roleira FMF, Tavares-da-silva EJ, Varela CL, et al. Plant derived and dietary phenolic antioxidants: anticancer properties. Food Chem. 2015;183:235–258. doi:10.1016/j.foodchem.2015.03.039
46. Abotaleb M, Liskova A, Kubatka P, Büsselberg D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. 2020;10(2):221–233. doi:10.3390/biom10020221
47. Lee S-H, Lee J, Herald T, et al. Anticancer activity of a novel high phenolic sorghum bran in human colon cancer cells. Oxid Med Cell Longev. 2020;2020:2890536. doi:10.1155/2020/2890536
48. Vijayalakshmi A, Sindhu G. Umbelliferone arrest cell cycle at G0/G1 phase and induces apoptosis in human oral carcinoma (KB) cells possibly via oxidative DNA damage. Biomed Pharmacother. 2017;92:661–671. doi:10.1016/j.biopha.2017.05.128
49. Sadzuka Y, Sugiyama T, Shimoi K, Kinae N, Hirota S. Protective effect of flavonoids on doxorubicin-induced cardiotoxicity. Toxicol Lett. 1997;92(1):1–7. doi:10.1016/S0378-4274(97)00028-3
50. Ma Y, Yang L, Ma J, et al. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochimica et Biophysica Acta (BBA)-Molecular Basis Dis. 2017;1863(8):1904–1911. doi:10.1016/j.bbadis.2016.12.021
51. Eftekhari A, Ahmadian E, Panahi-Azar V, Hosseini H, Tabibiazar M, Maleki Dizaj S. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: in vitro/in vivo studies. Artif Cells Nanomed Biotechnol. 2018;46(2):411–420. doi:10.1080/21691401.2017.1315427
52. Collard M, Gallagher PE, Tallant EA. A polyphenol-rich extract from muscadine grapes inhibits triple-negative breast tumor growth. Integr Cancer Ther. 2020;19:1534735420917444. doi:10.1177/1534735420917444
53. Chou -C-C, Yang J-S, Lu H-F, et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharmacal Res. 2010;33(8):1181–1191. doi:10.1007/s12272-010-0808-y
54. Luo H, Rankin GO, Li Z, DePriest L, Chen YC. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011;128(2):513–519. doi:10.1016/j.foodchem.2011.03.073
55. Kim B-W, Lee E-R, Min H-M, et al. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol Ther. 2008;7(7):1080–1089. doi:10.4161/cbt.7.7.6164
56. Ghasemi F, Shafiee M, Banikazemi Z, et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol Res Pract. 2019;215(10):152556. doi:10.1016/j.prp.2019.152556
57. Amawi H, Sim HM, Tiwari AK, Ambudkar SV, Shukla S. ABC transporter-mediated multidrug-resistant cancer. In: Liu X, Pan G, editors. Drug Transporters in Drug Disposition, Effects and Toxicity. Cham: Springer International Publishing Ag; 2019:549–580.
58. Dong J, Qin Z, Zhang WD, et al. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: an update. Drug Resistance Updates. 2020;49:100681. doi:10.1016/j.drup.2020.100681
59. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance. A Brief Rev, Adv Pharmaceutical Bulletin. 2017;7(3):339–348. doi:10.15171/apb.2017.041
60. Maleki Dana P, Sadoughi F, Asemi Z, Yousefi B. The role of polyphenols in overcoming cancer drug resistance: a comprehensive review. Cell Mol Biol Lett. 2022;27(1):1–26. doi:10.1186/s11658-021-00301-9
61. Falcone Ferreyra ML, Rius S, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012;3:34352. doi:10.3389/fpls.2012.00222
62. Chen FY, Cao LF, Wan HX, et al. Quercetin enhances Adriamycin cytotoxicity through induction of apoptosis and regulation of mitogen‑activated protein kinase/extracellular signal‑regulated kinase/c‑Jun N‑terminal kinase signaling in multidrug‑resistant leukemia K562 cells. Molecular Med Reports. 2015;11(1):341–348. doi:10.3892/mmr.2014.2734
63. Yu J, Zhou P, Asenso J, Yang XD, Wang C, Wei W. Advances in plant-based inhibitors of P-glycoprotein. J Enzyme Inhibition Med Chem. 2016;31(6):867–881. doi:10.3109/14756366.2016.1149476
64. Zhang X, Yu A, Zhang G. M-matrix-based delay-range-dependent global asymptotical stability criterion for genetic regulatory networks with time-varying delays. Neurocomputing. 2013;113:8–15. doi:10.1016/j.neucom.2012.12.046
65. Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013;24(8):1415–1422. doi:10.1016/j.jnutbio.2013.05.001
66. Mabrok HB, Klopfleisch R, Ghanem KZ, Clavel T, Blaut M, Loh G. Lignan transformation by gut bacteria lowers tumor burden in a gnotobiotic rat model of breast cancer. Carcinogenesis. 2012;33(1):203–208. doi:10.1093/carcin/bgr256
67. Sengottuvelan M, Nalini N. Dietary supplementation of resveratrol suppresses colonic tumour incidence in 1, 2-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Br J Nutr. 2006;96(1):145–153. doi:10.1079/BJN20061789
68. Scalbert A, Mila I, Expert D, et al. Polyphenols, metal ion complexation and biological consequences. Basic Life Sci. 1999;66:545–554. doi:10.1007/978-1-4615-4139-4_30
69. Mucchino C, Musci M. Extraction of Al, Cu, Fe, Mn, Ni and Zn - polyphenol complexes from black tea infusions by Amberlite resins. J Sci Food Agric. 2014;94(11):2234–2238. doi:10.1002/jsfa.6547
70. Kim J, Lee K, Nam YS. Metal-polyphenol complexes as versatile building blocks for functional biomaterials. Biotechnol Bioprocess Eng. 2021;26(5):689–707. doi:10.1007/s12257-021-0022-4
71. Halake K, Cho S, Kim J, et al. Applications using the metal affinity of polyphenols with mussel-inspired chemistry. Macromol Res. 2018;26(2):93–99. doi:10.1007/s13233-018-6051-x
72. Borowska S, Brzoska MM, Tomczyk M. Complexation of bioelements and toxic metals by polyphenolic compounds - implications for health. Curr Drug Targets. 2018;19(14):1612–1638. doi:10.2174/1389450119666180403101555
73. Liu P, Shi X, Zhong S, et al. Metal-phenolic networks for cancer theranostics. Biomater Sci. 2021;9(8):2825–2849. doi:10.1039/D0BM02064H
74. Zhang JF, Wang LY, You XR, Xian TZ, Wu J, Pang J. Nanoparticle therapy for prostate cancer: overview and perspectives. Curr Top Med Chem. 2019;19(1):57–73. doi:10.2174/1568026619666190125145836
75. Dang Y, Guan J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater Med. 2020;1:10–19. doi:10.1016/j.smaim.2020.04.001
76. Li Y, Zou Q, Yuan C, Li S, Xing R, Yan X. Amino acid coordination driven self-assembly for enhancing both the biological stability and tumor accumulation of curcumin. Angew Chem-Int Ed. 2018;57(52):17084–17088. doi:10.1002/anie.201810087
77. Fan X, Lv S, Lv F, et al. Type-I photodynamic therapy induced by Pt-coordination of type-II photosensitizers into supramolecular complexes. Chemistry. 2024;30(17):e202304113. doi:10.1002/chem.202304113
78. Zhou C-H, Zhang -Y-Y, Yan C-Y, Wan K, Gan -L-L, Shi Y. Recent researches in metal supramolecular complexes as anticancer agents. Anti-Cancer Agents Med Chem. 2010;10(5):371–395. doi:10.2174/1871520611009050371
79. Xie L, Li J, Wang G, et al. Phototheranostic metal-phenolic networks with antiexosomal PD-L1 enhanced ferroptosis for synergistic immunotherapy. J Am Chem Soc. 2022;144(2):787–797. doi:10.1021/jacs.1c09753
80. Luo W, Xiao G, Tian F, et al. Engineering robust metal-phenolic network membranes for uranium extraction from seawater. Energy Environ Sci. 2019;12(2):607–614. doi:10.1039/C8EE01438H
81. Li Y, Miao Y, Yang L, et al. Recent advances in the development and antimicrobial applications of metal-phenolic networks. Adv Sci. 2022;9(27):2202684. doi:10.1002/advs.202202684
82. Chen J, Pan S, Zhou J, et al. Programmable permeability of metal–phenolic network microcapsules. Chem Mater. 2020;32(16):6975–6982. doi:10.1021/acs.chemmater.0c02279
83. Mu M, Liang XY, Chuan D, et al. Chitosan coated pH-responsive metal-polyphenol delivery platform for melanoma chemotherapy. Carbohydr Polym. 2021;264:1569–1577. doi:10.1016/j.carbpol.2021.118000
84. Overchuk M, Weersink RA, Wilson BC, Zheng G. Photodynamic and photothermal therapies: synergy opportunities for nanomedicine. Acs Nano. 2023;17(9):7979–8003.
85. An D, Fu J, Zhang B, et al. NIR-II responsive inorganic 2D nanomaterials for cancer photothermal therapy: recent advances and future challenges. Adv Funct Mater. 2021;31(32):2101625.
86. Feng S, Lu J, Wang K, et al. Advances in smart mesoporous carbon nanoplatforms for photothermal-enhanced synergistic cancer therapy. Chem Eng J. 2022;435:134886. doi:10.1016/j.cej.2022.134886
87. Hu -J-J, Cheng Y-J, Zhang X-Z. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale. 2018;10(48):22657–22672. doi:10.1039/C8NR07627H
88. Chen Y, Gao Y, Chen Y, Liu L, Mo A, Peng Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J Control Release. 2020;328:251–262. doi:10.1016/j.jconrel.2020.08.055
89. Liu Y, Jing J, Jia F, et al. Tumor microenvironment-responsive theranostic nanoplatform for in situ self-boosting combined phototherapy through intracellular reassembly. ACS Appl Mater Interfaces. 2020;12(6):6966–6977. doi:10.1021/acsami.9b22097
90. Liu T, Zhang M, Liu W, et al. Metal ion/tannic acid assembly as a versatile photothermal platform in engineering multimodal nanotheranostics for advanced applications. Acs Nano. 2018;12(4):3917–3927. doi:10.1021/acsnano.8b01456
91. Xu C, Wang Y, Yu H, Tian H, Chen X. Multifunctional theranostic nanoparticles derived from fruit-extracted anthocyanins with dynamic disassembly and elimination abilities. ACS Nano. 2018;12(8):8255–8265. doi:10.1021/acsnano.8b03525
92. Cao C, Wang X, Yang N, Song X, Dong X. Recent advances of cancer chemodynamic therapy based on Fenton/Fenton-like chemistry. Chem Sci. 2022;13(4):863–889. doi:10.1039/D1SC05482A
93. Huang Y, Wu S, Zhang L, Deng Q, Ren J, Qu X. A metabolic multistage glutathione depletion used for tumor-specific chemodynamic therapy. Acs Nano. 2022;16(3):4228–4238. doi:10.1021/acsnano.1c10231
94. Tian H, Zhang M, Jin G, Jiang Y, Luan Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J Colloid Interface Sci. 2021;587:358–366. doi:10.1016/j.jcis.2020.12.028
95. Xuan W, Xia Y, Li T, Wang L, Liu Y, Tan W. Molecular self-assembly of bioorthogonal aptamer-prodrug conjugate micelles for hydrogen peroxide and pH-independent cancer chemodynamic therapy. J Am Chem Soc. 2020;142(2):937–944. doi:10.1021/jacs.9b10755
96. Wang X, Zhong X, Liu Z, Cheng LJNT. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today. 2020;35:100946. doi:10.1016/j.nantod.2020.100946
97. Jia C, Guo Y, Wu FGJS. Chemodynamic therapy via Fenton and Fenton‐like nanomaterials: strategies and recent advances. Small. 2022;18(6):2103868. doi:10.1002/smll.202103868
98. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi:10.1016/j.cell.2012.03.042
99. Qian X, Zhang J, Gu Z, Chen Y. Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials. 2019;211:1–13. doi:10.1016/j.biomaterials.2019.04.023
100. Liu T, Liu W, Zhang M, et al. Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano. 2018;12(12):12181–12192. doi:10.1021/acsnano.8b05860
101. Zhang L, Wan -S-S, Li C-X, Xu L, Cheng H, Zhang X-Z. An adenosine triphosphate-responsive autocatalytic Fenton nanoparticle for tumor ablation with self-supplied H2O2 and acceleration of Fe(III)/Fe(II) conversion. Nano Lett. 2018;18(12):7609–7618. doi:10.1021/acs.nanolett.8b03178
102. Zheng D-W, Lei Q, Zhu J-Y, et al. Switching apoptosis to ferroptosis: metal–organic network for high-efficiency anticancer therapy. Nano Lett. 2017;17(1):284–291. doi:10.1021/acs.nanolett.6b04060
103. Peng Z, Han X, Li S, et al. Carbon dots: biomacromolecule interaction, bioimaging and nanomedicine. Coord Chem Rev. 2017;343:256–277.
104. Yang M, Huang J, Fan J, Du J, Pu K, Peng X. Chemiluminescence for bioimaging and therapeutics: recent advances and challenges. Chem Soc Rev. 2020;49(19):6800–6815. doi:10.1039/D0CS00348D
105. Qian J, Tang BZ. AIE luminogens for bioimaging and theranostics: from organelles to animals. Chem. 2017;3(1):56–91. doi:10.1016/j.chempr.2017.05.010
106. Wang X, Yan J, Pan D, et al. Polyphenol–poloxamer self-assembled supramolecular nanoparticles for tumor NIRF/PET imaging. Adv Healthcare Mater. 2018;7(15):1701505. doi:10.1002/adhm.201701505
107. Guo J, Wang X, Henstridge DC, et al. Nanoporous metal–phenolic particles as ultrasound imaging probes for hydrogen peroxide. Adv Healthcare Mater. 2015;4(14):2170–2175. doi:10.1002/adhm.201500528
108. Xiong H, Wang C, Wang Z, Jiang Z, Zhou J, Yao J. Intracellular cascade activated nanosystem for improving ER+ breast cancer therapy through attacking GSH-mediated metabolic vulnerability. J Control Release. 2019;309:145–157. doi:10.1016/j.jconrel.2019.07.029
109. Yu X, Shang T, Zheng G, et al. Metal-polyphenol-coordinated nanomedicines for Fe(II) catalyzed photoacoustic-imaging guided mild hyperthermia-assisted ferroustherapy against breast cancer. Chin Chem Lett. 2022;33(4):1895–1900. doi:10.1016/j.cclet.2021.10.021
110. Xu Y, Guo Y, Zhang C, et al. Fibronectin-coated metal–phenolic networks for cooperative tumor chemo-/chemodynamic/immune therapy via enhanced ferroptosis-mediated immunogenic cell death. ACS Nano. 2022;16(1):984–996. doi:10.1021/acsnano.1c08585
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

