Back to Journals » Journal of Pain Research » Volume 18
Microiontophoretic Application of Dynorphin in Dental Pain: Excitatory or Inhibitory Effects
Authors Choi SH, Kim YM, Son JY, Ahn DK
Received 2 October 2024
Accepted for publication 18 January 2025
Published 25 January 2025 Volume 2025:18 Pages 455—464
DOI https://doi.org/10.2147/JPR.S499040
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Keith
Seung-Ho Choi,* Yu-Mi Kim,* Jo-Young Son, Dong-Kuk Ahn
Department of Oral Physiology, School of Dentistry, Kyungpook National University, Daegu, Korea
*These authors contributed equally to this work
Correspondence: Dong-Kuk Ahn, Department of Oral Physiology, School of Dentistry, Kyungpook National University, 2177, Dalgubeol-Daero, Jung-Gu, Daegu, 41940, Korea, Tel +82-53-660-6840, Email [email protected]
Background: The tooth exhibits increased sensitivity to noxious stimuli due to the dense innervation of thin myelinated Aδ fibers and unmyelinated C fibers within the dental pulp. While prior research has identified dynorphin expression in layers I–II of the dorsal horn across the spinal cord in various pain models, its functional role in trigeminal nociception, including tooth pain, remains underexplored. This study examines the potential role of dynorphin in the nociceptive processing of dental stimuli.
Methods: Experiments were performed on adult male ferrets weighing 0.9– 1.4 kg. The effects of dynorphin on electrically evoked responses of tooth pulp neurons were recorded extracellularly.
Results: The results demonstrated that the microiontophoretic application of dynorphin A induced excitatory and inhibitory effects on N-methyl-D-aspartate (NMDA)-evoked responses in electrically stimulated tooth pulp neurons. Specifically, dynorphin A attenuated NMDA-evoked responses in 16 out of 32 neurons by 61 ± 6%, facilitated NMDA-evoked responses in 10 out of 32 neurons by 69 ± 17%, and elicited mixed inhibitory and facilitatory responses in six out of 32 neurons. The inhibitory effects of dynorphin were blocked by nor-binaltorphimine, a kappa receptor antagonist, whereas the facilitatory effects were inhibited by D,L-2-amino-5-phosphonovaleric acid, an NMDA receptor antagonist.
Conclusion: These findings suggest that dynorphin A-induced excitatory responses are mediated by NMDA receptors, whereas its inhibitory responses are mediated through kappa opioid receptors in dental pain. Thus, dynorphin exerts diverse effects, highlighting its role in the perception and modulation of dental pain.
Keywords: dynorphin, N-methyl-D-aspartate, kappa receptor, dental pulp, analgesics
Introduction
Dynorphin A, an endogenous opioid peptide, is a ligand for the kappa opioid receptor.1 Intrathecal injection of dynorphin A produces potent and long-lasting analgesic effects in behavioral tests.2,3 Similarly, intracerebroventricular administration elicits analgesic effects in models of formalin-induced nociceptive behavior and persistent inflammatory pain caused by complete Freund’s adjuvant in rats.4,5 Additionally, dynorphin A has been shown to attenuate itch sensations in the dorsal horn of the spinal cord and produce analgesic effects on neuropathic pain in rats.6,7 These findings suggest that dynorphin A has potential as an analgesic agent for various chronic pain conditions.8
Behavioral studies have produced conflicting findings regarding the role of dynorphin. Some studies suggest that kappa agonists induce analgesic effects in rats subjected to visceral chemical tests.9 Conversely, other studies report a lack of narcotic effects following intracerebroventricular injection and an absence of analgesic effects.10,11 Unlike other endogenous opioid peptides, dynorphin does not consistently produce antinociception, as it also interacts with non-opioid receptors.12 Emerging evidence indicates that endogenous spinal dynorphin plays a role in hyperalgesia associated with tissue or nerve injury. Peripheral inflammation has been shown to increase prodynorphin messenger ribonucleic acid levels and dynorphin immunoreactivity in the spinal cord.11,13–15 Furthermore, intrathecal administration of dynorphin antiserum inhibits thermal hyperalgesia in rats with spinal nerve ligation.16 These findings suggest that dynorphin might exert effects distinct from other enkephalin-containing peptides.17
The tooth exhibits heightened sensitivity to noxious stimuli, resulting in dental pain due to the extensive innervation of the dental pulp by thin myelinated Aδ fibers and unmyelinated C fibers, both of which mediate pain sensation.18,19 Previous studies have demonstrated that tooth movement increases dynorphin expression in the superficial layers of the trigeminal subnucleus caudalis, indicating a significant role for dynorphin in tooth movement-associated pain.20 Additionally, tooth inflammation has been shown to elevate dynorphin expression in regions such as the lateral solitary nucleus and the dorsal nucleus caudalis.21 While these studies suggest that dynorphin is involved in the transmission of noxious signals in the trigeminal nerve, research on its specific role in pain modulation remains limited.
This study investigated the functional significance of dynorphin in trigeminal nociception associated with dental structures. Specifically, it explored the potential role of dynorphin in nociceptive processing originating from the tooth by assessing its effects on thermally or electrically evoked tooth pulp neurons (ETPNs).
Methods
Animals and Surgery
All animal experiments were approved by the Animal Experiment Management Committee of the School of Dentistry at Kyungpook National University (approval number: KNU2018-0021) and the Institutional Care and Use Committee of the University of North Carolina at Chapel Hill (approval number: DE 11661). The experiments were conducted in accordance with the ethical guidelines established by the International Association for the Study of Pain. The study involved 25 male ferrets (Mustela putorius furo), each weighing between 0.9 and 1.4 kg. All animals were housed individually in a controlled environment with consistent temperature and lighting conditions, following a 12-h light and 12-h dark cycle. Water and food were provided ad libitum at all times.
Twenty-five ferrets were anesthetized using 3% isoflurane in oxygen for induction, followed by a combination of chloral hydrate (120 mg/kg; intraperitoneal, [i.p.]) and pentobarbital sodium (20 mg/kg; i.p.). Anesthesia was maintained throughout the experiment with a mixture of chloral hydrate (42.5 mg/kg; intravenous; [i.v.]) and pentobarbital sodium (8.8 mg/kg; i.v.). Arterial blood pressure and body temperature were continuously monitored and maintained within the ranges of 90–120 mmHg and 37–38°C, respectively. The ferrets were secured in a stereotaxic apparatus, their neck muscles were dissected to remove the dura mater overlying the medullary surface exposing the obex and medulla. The exposed medullary surface was covered with warmed saline. While recording from brainstem neurons, the ferrets were immobilized with vecuronium bromide (0.4 mg/kg) and artificially ventilated via a tracheal cannula. The end-tidal carbon dioxide level was maintained at 3.5–4.5%.
Extracellular Recording of ETPNs
The upper canine was prepared for electrical stimulation. The central barrel of a seven-barrel micropipette (tip diameter: 10 µm; impedance: 2–10 MΩ) was utilized for extracellular recordings. Typically, the initial penetration was performed at the level of the obex, approximately 2–2.5 mm lateral to the midline. This region exhibited c-Fos activity following the application of noxious thermal stimulation to the ferret canines.18
A dental rubber dam was placed to encircle the tooth and minimize inadvertent stimulation of surrounding intraoral tissues. The cathode electrode was positioned on the canines, while the anode electrode was attached to the neck muscle. Each canine was secured with a stimulating probe containing colloidal silver liquid (number: 16031; TED PELLA Inc. Redding, CA, USA). Recording depths and their locations in relation to the brainstem surface and obex were carefully documented. For search stimulation, constant current square wave pulses (2 ms duration) were delivered to the canine at 1-s intervals. Current intensity was monitored by measuring the voltage drop across a 100 Ω resistor connected in series with the experimental setup. The applied current was maintained at a maximum of 150–200 mA to prevent stimulation of adjacent tissues.19–21
The single-unit activity of ETPNs was amplified, input into a window discriminator and an audio monitor, and displayed on an oscilloscope. Discriminated spikes were transmitted to a digital interface (number: 1401; Cambridge Electronic Design Ltd. Cambridge, UK) and stored on a computer for online and offline analysis. ETPNs were further characterized by evaluating cutaneous receptive fields using non-noxious tactile stimuli, such as von Frey filaments and soft brushes, as well as noxious stimuli, including pinching with toothed forceps and exposure to harmful heat and cold. Based on cutaneous receptive field responses, neurons were categorized into three groups: low-threshold mechanoreceptive (LTM) neurons, wide dynamic range (WDR) neurons, or nociceptive specific (NS) neurons.22,23
Thermal Stimulation of the Tooth
The maxillary canine was prepared for thermal stimulation. Sensitivity to quantitative heat stimuli was assessed using a computer-controlled contact thermode applied to the maxillary canine as described previously.21 Heat stimuli were delivered as a single 15-s pulse at a temperature of 50°C.
Microiontophoretic Application of Drugs
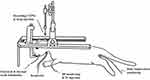
The outer barrels of the micropipette were filled with the following pharmacological agents: dynorphin A (1–17) at concentrations of 0.5, 1, and 2.5 mm in 150 mm sodium chloride (NaCl) (pH 5.5); N-methyl-D-aspartate (NMDA) at 50 mm in 150 mm NaCl (pH 8.0); D,L-2-amino-5-phosphonovaleric acid (AP-5) at 50 mm in 150 mm NaCl (pH 8.0); and nor-binaltorphimine (Nor-BIN) at 4 mm in 150 mm NaCl (pH 6.5). Additionally, 1% pontamine sky blue in 0.5 M sodium acetate was included for current balancing purposes. Nor-BIN, a kappa-opioid receptor antagonist, and AP-5, an NMDA receptor antagonist, were administered to investigate the receptor mechanisms underlying the dual effects of dynorphin A. Iontophoretic ejection currents were monitored and regulated using a BH2 system (Harvard Apparatus, Holliston, MA, USA). All pharmacological agents were applied iontophoretically using a cationic current, except for NMDA and AP-5, which were delivered using an anionic current. Retaining currents were maintained within the range of 5–10 nA. A balancing barrel was used throughout all chemical applications to neutralize currents. All experimental procedures followed previously established methodologies.24 Figure 1 presents a diagram illustrating the monitoring of experimental animals, the recording of ETPNs, and drug injections.
Histological Procedure
Upon completion of the experiments, the animals were deeply anesthetized with an overdose of pentobarbital sodium for euthanasia. Following this, they were perfused with 0.01 M phosphate-buffered saline (PBS), followed by 10% formaldehyde fixative via the cardiac route. The brainstem was then excised, post-fixed in 10% formaldehyde for approximately 24 h, and immersed in a 30% sucrose solution in 0.01 M PBS for several days. The tissue was sectioned into 60 µm slices, which were mounted on gelatin-coated slides and stained to verify the locations of the recording sites.
Statistical Analysis
Single-cell activity variations were analyzed using a Student’s t-test. The results are presented as the mean ± standard error of the mean. A P-value of < 0.05 was considered statistically significant.
Results
Recording of ETPNs
A total of 53 ETPNs were evaluated for their responses to dynorphin A. These neurons were located in the superficial and deep regions of the trigeminal subnucleus caudalis (Vc) and the transition zone between the subnucleus caudalis and interpolaris (Vc/Vi). Of the 53 ETPNs, 46 exhibited cutaneous receptive fields, which were categorized based on their responses to noxious and non-noxious stimuli. Among these, 13 neurons were classified as NS neurons, responding exclusively to noxious stimulation; 15 were classified as WDR neurons, responding to noxious and non-noxious stimuli; and 18 were classified as LTM neurons, responding solely to non-noxious stimuli. Seven ETPNs did not exhibit identifiable cutaneous receptive fields.
Dual Response of ETPNs to Dynorphin A
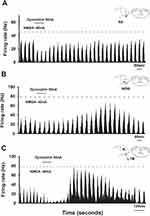
The microiontophoretic application of dynorphin A produced diverse effects on NMDA-evoked responses, as illustrated in Figure 1. A representative example of an inhibitory response from an ETPN is illustrated in Figure 2A. The NS neuron, situated in the deep lamina, exhibited a cutaneous receptive field on the lower lip. The cyclic NMDA-evoked neuronal activity was inhibited by the iontophoretic application of dynorphin A in 16 out of 32 neurons, resulting in a significant reduction of 61 ± 6% (Figure 2A, P <0.05). Conversely, dynorphin A enhanced the cyclic NMDA-evoked neuronal activity in 10 out of 32 neurons, with an increase of 69 ± 17% (Figure 2B, P <0.05). Furthermore, a WDR neuron with a cutaneous receptive field in the mouth angle region was located in the deep lamina. Dynorphin A elicited inhibitory (61 ± 5%, P <0.05) and facilitatory responses (57 ± 11%) in six out of 32 neurons (Figure 2C). Although the iontophoretic application of dynorphin A exhibited inhibitory and facilitatory effects, these responses were independent of the specific cell type (NS, WDR, or LTM) or their location within the lamina.
Effects of Blocking a Kappa-Opioid Receptor on Dual Response to Dynorphin A
Figure 3 illustrates the effects of dynorphin A on ETPNs following the blockade of kappa-opioid receptors. The iontophoretic application of dynorphin A suppressed cyclic NMDA-evoked neuronal activity in ETPNs. The administration of Nor-BIN, a kappa-opioid receptor antagonist, effectively inhibited the suppression of NMDA-evoked neuronal activity induced by dynorphin A in seven identified ETPNs (Figure 3A). Additionally, the study investigated the excitatory effects of dynorphin A following kappa-opioid receptor blockade. Notably, Nor-BIN did not influence the excitatory effects elicited by dynorphin A in seven identified ETPNs (Figure 3B).
Effects of Blocking an NMDA Receptor on Thermal Stimulation to the Maxillary Canine
Figure 4 illustrates the effects of dynorphin A on ETPNs following the blockade of NMDA receptors. Thermal stimuli were applied to the maxillary canine, and noxious heat stimuli at 50°C for 15s elicited responses in ETPNs. The iontophoretic application of dynorphin A enhanced thermal-evoked responses. Notably, five out of the seven ETPNs exhibited facilitated heat-evoked responses, with an average increase of 99 ± 31% (P <0.05). These responses returned to baseline levels 20 min after dynorphin A application (Figure 4A). Furthermore, the study explored the excitatory effects of dynorphin A following NMDA receptor blockade. The microiontophoretic application of AP-5, an NMDA receptor antagonist, effectively inhibited the facilitation responses induced by dynorphin A in ETPNs (Figure 4B).
Discussion
This study demonstrated that the microiontophoretic application of dynorphin A elicited excitatory and inhibitory effects on the evoked responses of ETPNs. The excitatory effects were inhibited by an NMDA receptor antagonist, while the inhibitory effects were blocked by a kappa opioid receptor antagonist. These findings suggest that although pulpal inflammation likely elevates dynorphin levels, dynorphin induces NMDA receptor-mediated excitatory effects and kappa opioid receptor-mediated inhibitory effects.
This study demonstrates that dynorphin directly modulates excitatory transmission in the medullary dorsal horn. The microiontophoretic application of dynorphin A elicited inhibitory and excitatory responses in NMDA-evoked neuronal activity. This dual response aligns with previous research that highlights the bifunctional role of dynorphin in NMDA receptor-mediated synaptic currents, as observed in guinea pig CA3 pyramidal neurons.25 Additionally, the dynorphin-induced inhibition of evoked neuronal activity is substantiated by prior behavioral studies that demonstrate significant and prolonged analgesic effects following intrathecal administration.2,3 Furthermore, the inhibitory effects of dynorphin on NMDA receptor-mediated synaptic currents are sensitive to naloxone, an opioid receptor antagonist.25 This supports the current findings, as the iontophoretic application of Nor-BIN effectively blocked the inhibitory effects of dynorphin. These observations suggest that the inhibitory action of dynorphin is mediated through the kappa opioid receptor.
Dynorphin A has been shown to interact with non-opioid receptors, leading to effects that are not mediated by opioid pathways.12 This phenomenon is attributed to the absence of the critical N-terminal tyrosine residue necessary for binding to opioid receptors.26 Previous research has documented interactions involving non-opioid receptors that result in hyperalgesia. Specifically, low intrathecal doses of dynorphin have been found to induce prolonged tactile allodynia, an effect that is not mitigated by opioid receptor antagonists.27,28 Furthermore, dynorphin has been observed to antagonize the analgesic effects of intrathecal morphine administration.29,30 In line with these previous findings, the current study demonstrates that dynorphin enhances heat-evoked neuronal activity in ETPNs, an effect that is inhibited by NMDA receptor antagonists. These results suggest that the enhanced effects of dynorphin are mediated through non-opioid receptors. Supporting this assertion, earlier studies have shown that dynorphin can induce hind limb paralysis and tactile allodynia via NMDA receptor mechanisms.27,28,31,32 Notably, a recent investigation revealed that dynorphin triggers non-opioid receptor-mediated neural excitation through bradykinin receptors.33 Additionally, the blockade of dynorphin-activated spinal bradykinin receptors has been shown to reverse persistent neuropathic pain in rat models.34 The upregulation of spinal dynorphin A interacts with bradykinin receptors to facilitate hyperalgesia through a neuroexcitatory (pronociceptive) mechanism under chronic pain conditions.35 Collectively, these findings provide evidence of the mechanisms by which dynorphin contributes to pain through interactions with bradykinin receptors.
This study highlights the dual inhibitory and excitatory effects of dynorphin, prompting the question of why it exhibits such duality in animals. This phenomenon might reflect a protective mechanism inherent to individuals against noxious stimuli. Acute injury and nociceptive input trigger the immediate release of dynorphin, which can attenuate nociception through opioid receptor activation. Simultaneously, the hypersensitivity induced by dynorphin might function to protect the injured area from further damage.36,37 Therefore, this dual action might elucidate why dynorphin produces analgesic and hyperalgesic effects.
The tooth is a unique structure comprising densely innervated and vascularized soft tissue surrounded by mineralized hard tissue. It is extensively innervated by thin, myelinated Aδ fibers and unmyelinated C fibers, both of which are thought to mediate pain perception.18,19 Previous studies have suggested that spinal dynorphin plays a significant role in pain sensation. Tissue injury has been shown to increase dynorphin levels in spinal neurons across various animal models, including rats with painful peripheral neuropathy,38–40 hind paw inflammation,13 and polyarthritis.41 Dynorphin also plays a crucial role in modulating pain in the orofacial region. For example, tooth movement increases dynorphin expression in the superficial layers of the trigeminal subnucleus caudalis,20 while tooth inflammation enhances dynorphin expression in the lateral solitary nucleus and the dorsal nucleus caudalis.21 While these studies demonstrate the involvement of dynorphin in transmitting noxious signals within the trigeminal nerve, limited research has specifically explored its role in toothache. The present study reveals that dynorphin A modulates the evoked responses of ETPNs. These findings could inform the development of improved clinical strategies for managing toothache.
Conclusion
Dynorphin A exerts dual effects on the evoked responses of ETPNs. The microiontophoretic application of Nor-BIN selectively inhibits the inhibitory responses without affecting the excitatory responses, while AP-5 effectively blocks the excitatory responses induced by dynorphin. These findings suggest that dynorphin plays a crucial role in the initial perception of pain and the persistence of dental pain associated with pulpal inflammation. Although dynorphin is implicated in dental pain, it exerts diverse effects, including excitatory responses mediated by NMDA receptors and inhibitory responses mediated by kappa opioid receptors.
Acknowledgment
One of the contributors of this paper, Professor Maxiner W, has unfortunately passed away.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (2022R1A2C2092262).
Disclosure
The authors have no conflicts of interests to declare related to this study.
References
1. Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–415. doi:10.1126/science.6120570
2. Han JS, Xie CW. Dynorphin: potent analgesic effect in spinal cord of the rat. Life Sci. 1982;31(16–17):1781–1784. doi:10.1016/0024-3205(82)90209-0
3. Herman BH, Goldstein A. Antinociception and paralysis induced by intrathecal dynorphin A. J Pharmacol Exp Ther. 1985;232(1):27–32.
4. Tan-No K, Ohshima K, Taira A, et al. Antinociceptive effect produced by intracerebroventricularly administered dynorphin A is potentiated by p-hydroxymercuribenzoate or phosphoramidon in the mouse formalin test. Brain Res. 2001;891(1–2):274–280. doi:10.1016/s0006-8993(00)03225-x
5. Jiang YL, He XF, Shen YF, et al. Analgesic roles of peripheral intrinsic met-enkephalin and dynorphin A in long-lasting inflammatory pain induced by complete Freund’s adjuvant in rats. Exp Ther Med. 2015;9(6):2344–2348. doi:10.3892/etm.2015.2407
6. Kardon AP, Polgár E, Hachisuka J, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82(3):573–586. doi:10.1016/j.neuron.2014.02.046
7. Hall SM, Lee YS, Hruby VJ. Dynorphin A analogs for the treatment of chronic neuropathic pain. Future Med Chem. 2016;8(2):165–177. doi:10.4155/fmc.15.164
8. Brust A, Croker DE, Colless B, et al. Conopeptide-derived κ-Opioid Agonists (Conorphins): potent, selective, and metabolic stable Dynorphin A mimetics with antinociceptive properties. J Med Chem. 2016;59(6):2381–2395. doi:10.1021/acs.jmedchem.5b00911
9. Schmauss C, Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral, chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984;228:1–12.
10. Walker JM, Moises HC, Coy DH, Baldrighi G, Akil H. Nonopiate effects of dynorphin and des-Tyr-dynorphin. Science. 1982;218(457):1136–1138. doi:10.1126/science.6128791
11. Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15(3):96–103. doi:10.1016/0166-2236(92)90019-5
12. Shukla VK, Lemaire S. Non-opioid effects of dynorphins: possible role of the NMDA receptor. Trends Pharmacol Sci. 1994;15(11):420–424. doi:10.1016/0165-6147(94)90091-4
13. Ruda MA, Iadarola MJ, Cohen LV, Young WS. In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci U S A. 1988;85(2):622–626. doi:10.1073/pnas.85.2.622
14. Sapio MR, Iadarola MJ, Loydpierson AJ, et al. Dynorphin and enkephalin opioid peptides and transcripts in spinal cord and dorsal root ganglion during peripheral inflammatory hyperalgesia and allodynia. J Pain. 2020;21(9–10):988–1004. doi:10.1016/j.jpain.2020.01.001
15. Riley RC, Zhao ZQ, Duggan AW. Spinal release of immunoreactive dynorphin A (1-8) with the development of peripheral inflammation in the rat. Brain Res. 1996;710(1–2):131–142. doi:10.1016/0006-8993(95)01394-6
16. Bian D, Ossipov MH, Ibrahim M, et al. Loss of antiallodynic and antinociceptive spinal/supraspinal morphine synergy in nerve-injured rats: restoration by MK-801 or dynorphin antiserum. Brain Res. 1999;831(1–2):55–63. doi:10.1016/s0006-8993(99)01393-1
17. Dickenson AH, Sullivan AF. Electrophysiological studies on the effects of intrathecal morphine on nociceptive neurons in the rat dorsal horn. Pain. 1986;24(2):211–222. doi:10.1016/0304-3959(86)90044-8
18. Närhi M. The neurophysiology of the teeth. Dent Clin North Am. 1990;34(3):439–448. doi:10.1016/S0011-8532(22)01127-2
19. Närhi M, Jyväsjärvi E, Virtanen A, Huopaniemi T, Ngassapa D, Hirvonen T. Role of intradental A- and C-type nerve fibres in dental pain mechanisms. Proc Finn Dent Soc. 1992;88(Suppl 1):507–516.
20. Kato J, Wakisaka S, Tabata MJ, Itotagawa T, Kurisu K. Appearance of dynorphin in the spinal trigeminal nucleus complex following experimental tooth movement in the rat. Arch Oral Biol. 1995;40(1):79–81. doi:10.1016/0003-9969(94)00134-w
21. Byers MR, Chudler EH, Iadarola MJ. Chronic tooth pulp inflammation causes transient and persistent expression of Fos in dynorphin-rich regions of rat brainstem. Brain Res. 2000;861(2):191–207. doi:10.1016/s0006-8993(00)01936-3
22. Chattipakorn SC, Light AR, Willcockson HH, Narhi M, Maixner W. The effect of fentanyl on c-fos expression in the trigeminal brainstem complex produced by pulpal heat stimulation in the ferret. Pain. 1999;82(2):207–215. doi:10.1016/S0304-3959(99)00046-9
23. Matthews B, Searle BN. Electrical stimulation of teeth. Pain. 1976;2(3):245–251. doi:10.1016/0304-3959(76)90003-8
24. Ahn DK, Doutova EA, McNaughton K, Light AR, Närhi M, Maixner W. Functional properties of tooth pulp neurons responding to thermal stimulation. J Dent Res. 2012;91(4):401–406. doi:10.1177/0022034511435703
25. Caudle RM, Chavkin C, Dubner R. Kappa 2 opioid receptors inhibit NMDA receptor-mediated synaptic currents in Guinea pig CA3 pyramidal cells. J Neurosci. 1994;14(9):5580–5589. doi:10.1523/JNEUROSCI.14-09-05580.1994
26. Lai J, Ossipov MH, Vanderah TW, Malan TP, Porreca F. Neuropathic pain: the paradox of dynorphin. mol Intervent. 2001;1(3):160–167.
27. Vanderah TW, Laughlin T, Lashbrook JM, et al. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;68(2–3):275–281. doi:10.1016/s0304-3959(96)03225-3
28. Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, Porreca F, Wilcox GL. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72(1–2):253–260. doi:10.1016/s0304-3959(97)00046-8
29. Schmauss C, Herz A. Intrathecally administered dynorphin-(1-17) modulates morphine-induced antinociception differently in morphine-naive and morphine-tolerant rats. Eur J Pharmacol. 1987;135(3):429–431. doi:10.1016/0014-2999(87)90695-9
30. Fujimoto JM, Holmes B. Systemic single dose morphine pretreatment desensitizes mice to the spinal antianalgesic action of dynorphin A (1-17). J Pharmacol Exp Ther. 1990;254(1):1–7.
31. Caudle RM, Isaac L. A novel interaction between dynorphin (1-13) and an N-methyl-D-aspartate site. Brain Res. 1988;443(1–2):329–332. doi:10.1016/0006-8993(88)91628-9
32. Bakshi R, Faden AI. Blockade of the glycine modulatory site of NMDA receptors modifies dynorphin-induced behavioral effects. Neurosci Lett. 1990;110(1–2):113–117. doi:10.1016/0304-3940(90)90797-d
33. Lee YS, Rankin D, Hall SM, et al. Structure-activity relationships of non-opioid [des-Arg(7)]-dynorphin A analogues for bradykinin receptors. Bioorg Med Chem Lett. 2014;24(21):4976–4979. doi:10.1016/j.bmcl.2014.09.033
34. Lai J, Luo MC, Chen Q, et al. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci. 2006;9(12):1534–1540. doi:10.1038/nn1804
35. Lee YS, Hall SM, Ramos-Colon C, et al. Blockade of non-opioid excitatory effects of spinal dynorphin A at bradykinin receptors. Receptors Clin Investig. 2015;2(1):517. doi:10.14800/rci.517
36. Caudle RM, Mannes AJ. Dynorphin: friend or foe? Pain. 2000;87(3):235–239. doi:10.1016/S0304-3959(00)00360-2
37. Podvin S, Yaksh T, Hook V. The emerging role of spinal dynorphin in chronic pain: a therapeutic perspective. Annu Rev Pharmacol Toxicol. 2016;56:511–533. doi:10.1146/annurev-pharmtox-010715-103042
38. Cho HJ, Basbaum AI. Increased staining of immunoreactive dynorphin cell bodies in the deafferented spinal cord of the rat. Neurosci Lett. 1988;84(2):125–130. doi:10.1016/0304-3940(88)90395-3
39. Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ. Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 1990;11(4):719–728. doi:10.1016/0196-9781(90)90187-a
40. Nishimori T, Moskowitz MA, Uhl GR. Opioid peptide gene expression in rat trigeminal nucleus caudalis neurons: normal distribution and effects of trigeminal deafferentation. J Comp Neurol. 1988;274(1):142–150. doi:10.1002/cne.902740113
41. Weihe E, Millan MJ, Hrllt V, Nohr D, Herz A. Induction of the gene encoding pro-dynorphin by experimentally induced arthritis enhances staining for dynorphin in the spinal cord of rats. Neuroscience. 1989;31(1):77–95. doi:10.1016/0306-4522(89)90031-6
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.