Back to Journals » Drug Design, Development and Therapy » Volume 19
Minimum Effective Concentration of Ropivacaine for Ultrasound-Guided RISS Block in VATS: A Biased Coin Design Approach
Authors Li P, Yang Z, Zhang L, Wu R, Zhu M, Xie J
Received 19 February 2025
Accepted for publication 10 May 2025
Published 20 May 2025 Volume 2025:19 Pages 4151—4161
DOI https://doi.org/10.2147/DDDT.S520427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Tamer Ibrahim
Ropivacaine for rhomboid intercostal and subserratus plane blocks – Video abstract [520427]
Views: 55
Ping Li,1,2 Zhongsai Yang,1,3 Long Zhang,4 Ruilan Wu,2 Manhua Zhu,2 Junran Xie1
1Department of Anesthesiology, Zhejiang University School of Medicine Sir Run Run Shaw Hospital, Hangzhou, Zhejiang Province, 310016, People’s Republic of China; 2Department of Anesthesiology, Ningbo Medical Centre Lihuili Hospital, Ningbo, Zhejiang Province, 315040, People’s Republic of China; 3Department of Anesthesiology, Ningbo Women and Children’s Hospital, Ningbo, Zhejiang Province, 315012, People’s Republic of China; 4Department of Anesthesiology, Ningbo No.6 Hospital, Ningbo, Zhejiang Province, 315040, People’s Republic of China
Correspondence: Junran Xie, Department of Anesthesiology, Sir Run Run Shaw Hospital Affiliated Hospital of Zhejiang University School of Medicine, No. 3, Qingchun East Road, Shangcheng District, Hangzhou, Zhejiang Province, 310016, People’s Republic of China, Email [email protected]
Background: Postoperative pain management is essential after video-assisted thoracoscopic surgery (VATS). The rhomboid intercostal and sub-serratus (RISS) block is effective in providing analgesia. This study aims to determine the minimum effective concentration (MEC) of ropivacaine for ultrasound-guided RISS block in patients undergoing VATS, thereby optimizing the analgesia protocol and enhancing its clinical significance.
Methods: The biased coin design sequential method and isotonic regression were used. The ropivacaine initial concentration was 0.25% with a gradient of 0.025%. If the previous patient had a negative block, the concentration for the next patient would increase by 0.025%. If positive, the concentration would be adjusted based on biased coin randomization: an 11% chance of reducing it by 0.025% and an 89% chance of keeping it unchanged. MEC90 was calculated using isotonic regression with 95% confidence intervals (CI).
Results: A total of 49 patients were included in the analysis. The MEC90 of 40mL ropivacaine for RISS block in VATS was 0.220% (95% CI, 0.198 to 0.260%), the MEC95 was 0.248% (95% CI, 0.223 to 0.338%) and the MEC99 was 0.270% (95% CI, 0.261 to 0.351%). There was a negative correlation between ropivacaine concentration and VASpain at 0h, 0.5h, and 6h after surgery and the time of initial analgesia (r = – 0.396, – 0.594, – 0.309, 0.363; P = 0.005, 0.001, 0.031, 0.01). No significant correlation was observed between the VASpain at 12h and 24h after surgery and analgesia consumption (r = – 0.184, – 0.165; P = 0.205, 0.256).
Conclusion: The MEC90 of 40 mL ropivacaine for RISS block was 0.220%. While the MEC95 was 0.248% and the MEC99 was 0.270%.
Plain Language Summary: This study investigated the lowest effective dose of ropivacaine needed for pain relief after VATA using RISS block. Researchers used biased coin design sequential method to find the right dose of ropivacaine. They started with a certain concentration and adjusted it based on whether the previous patient’s block was successful or not. They found that a ropivacaine concentration of 0.220% was effective for 90% of the patients, while 0.248% was effective for 95% and 0.270% for 99% of the patients. Additionally, they observed that higher concentrations of ropivacaine were associated with less pain immediately after surgery and longer time before needing pain medication. No complications were reported related to this technique. In simple terms, this study helps doctors know the best dose of ropivacaine to use for pain relief after a certain type of lung surgery.
Keywords: Ultrasound-guided, Ropivacaine, Rhomboid intercostal and subserratus, RISS, Minimum effective concentration, Dose finding
Introduction
Video-assisted thoracoscopic surgery (VATS) as a minimally invasive technique in thoracic surgery can partially mitigate patient trauma. However, it fails to reduce the risk of postoperative pain.1 Recovery from thoracic surgery is often complicated by an elevated risk of pain.2 A substantial proportion of patients who undergo VATS still exhibit moderate to severe postoperative pain and may even develop chronic pain. The impairment of respiratory function harms the immune, cardiovascular, gastrointestinal, hematological, and cognitive systems.3–5 Adequate postoperative analgesia enhances patient comfort, mitigates pain-related risks and complications, and facilitates prompt postoperative recovery.3–5
Regional nerve block as an analgesic method following thoracic surgery has been well established, facilitating opioid-sparing and opioid-free anesthesia and reducing the incidence of complications.6 Moreover, it has been widely adopted for postoperative pain management after VATS,7 aligning with the current enhanced recovery after surgery (ERAS) protocols.8 The rhomboid intercostal and subserratus (RISS) block represents an innovative nerve block technique that encompasses rhomboid intercostal and subserratus blocks. Additionally, rhomboid intercostal block (RIB) and serratus anterior plane block (SAPB) aim primarily to inhibit the giant lateral cutaneous branch of the intercostal nerve.9
The RISS block has developed rapidly and has demonstrated significant efficacy in postoperative analgesia across various surgical disciplines.10–12 Furthermore, the RISS block offers numerous advantages, including minimal impact on patients’ physiological function and reduced occurrence of side effects. However, limited reports exist regarding the correlation between drug concentration and dosage in RISS blocks, hindering their advancement in clinical applications.
This study employed a sequential method of biased coin design and isotonic regression calculations to investigate the minimal effective concentration (MEC) of ropivacaine for ultrasound-guided RISS block and analgesia in VATS patients to address this research gap and provide more comprehensive guidance for clinical practice.
Methods
This study was a prospective, double-masked, dose-finding trial. This study followed the Helsinki Declaration, and was approved by Lihuili Hospital, Ningbo Medical Center, with approval No. KY2022PJ030. Simultaneously, the study was registered in the clinical trial center (www.chictr.org.cn) on March 15th, 2022 (No. ChiCTR2200057584). Before each participant was selected for this research, they were comprehensively informed and retained the freedom to withdraw from the study at any time. The researchers ensured the comprehensive protection of participants’ personal privacy and data confidentiality throughout the research.
Patient Enrollment
Patients who underwent three-port VATS at Lihuili Hospital in Ningbo were prospectively enrolled in the study between March 24th, 2022, and December 31th, 2022. The inclusion criteria included age ranging from 18 to 65 years, American Society of Anesthesiologists (ASA) physical status I or II, and body mass index (BMI) between 18.5 and 30 kg/m2. The exclusion criteria included participation in concurrent clinical intervention trials, contraindications to nerve block or related medications, communication disorders, mental illness, and multiple surgeries within a short timeframe. Additional exclusion criteria included puncture failure, identification of severe adhesion of the thoracic cavity during postthoracoscopic exploration, voluntary withdrawal request by patients, and loss to follow-up or test interruptions for various reasons. The termination criterion consisted of the occurrence of severe complications requiring unplanned transfer to the intensive care unit after surgery and unscheduled secondary surgery within a short timeframe.
Biased Coin Design up and Down Sequential Method (BCD - UDM)
To achieve MEC90 based on statistical requirements, a minimum of 45 positive reactions (ie, greater than 40 and a multiple of 9) are necessary.13 The initial concentration of ropivacaine was 0.25%.14 The concentration of ropivacaine for each patient undergoing the RISS block is contingent on the block efficacy demonstrated by the previous patient. If the last patient exhibited a “negative”, the local anesthetic concentration administered to the subsequent patient was increased by 0.025%. Conversely, if the previous patient’s outcome was “positive”, the following patient’s local anesthetic concentration was subject to biased coin randomization, with an 11% probability of a 0.025% reduction and an 89% probability of no change. The study terminated when the number of successful block surgeries reached 45 (Figure 1). Should a patient discontinue treatment, the concentration of the agent in the patient who succeeded remains unaltered. Consequently, based on these considerations, the estimated sample size is determined to be 52 ± 2 cases.
 |
Figure 1 Biased coin design up-and-down sequential method (BCD-UDM). |
Ultrasound-Guided RISS Block and Anesthesia
Before the surgical procedure, a strict dietary regimen was enforced, with solid food being prohibited for eight hours and liquids for two hours in the absence of preoperative medications. Routine noninvasive blood pressure (NIBP), electrocardiogram (ECG), and oxygen saturation (SPO2) monitoring were implemented upon admission. The venous access site was established in the upper limb, with the contralateral radial artery being punctured under local anesthesia to facilitate the monitoring of invasive arterial blood pressure (ABP) and right internal jugular vein puncture.
The patients were positioned in a lateral recumbent position, abducting the upper arm on the affected side and moving the scapula to the outside. Subsequently, the skin was disinfected, and a high-frequency linear array probe (4–15 hz, SonoSite, California, US) was inserted in an oblique sagittal position along the medial margin of the scapula at the T5-T6 level. The following ultrasound images were acquired in a shallow-to-deep sequence: trapezius muscle, rhomboid muscle, intercostal muscle, pleura, and lung (Figure 2A). The in-plane technique was employed for both intramuscular and extramuscular needle insertion. Position confirmation was achieved when the needle tip reached between the rhomboid muscle and the intercostal muscle. After being drawn back to ensure no blood or air, 20 mL of ropivacaine at varying concentrations was injected according to the experimental design (Figure 2B). The ultrasound probe was then shifted downward and laterally to the T7-T8 level, situated behind the posterior axillary line at the distal end of the subscapular angle. From shallow to deep ultrasound images revealed the latissimus dorsi, anterior serratus muscle, intercostal muscle, pleura, and lung (Figure 2C). The needle insertion process was identical to the previous step. Position confirmation was again established when the needle tip reached between the anterior serratus muscle and the intercostal muscle. After no blood or gas was withdrawn, 20 mL of ropivacaine at different concentrations was injected according to the experimental design (Figure 2D). After 30 minutes of RISS block, the range of head–tail pain at the axillary front was measured and mapped using acupuncture.
After completing the RISS block and evaluating its effects, midazolam (0.02 mg/kg), sufentanil (0.3 μg/kg), propofol (2.0 mg/kg), and rocuronium (1.0 mg/kg) were administered for anesthesia induction and endotracheal intubation. During the operation, the dosage of anesthetic agents and infusion rates were adjusted based on fluctuations in blood pressure and heart rate, and vasoactive medications, including norepinephrine, ephedrine, urapidil, atropine, and esmolol, were administered as needed. Upon completion of the procedure, patients were transferred to the postanesthesia care unit, and the tracheal tube was removed when the expected cough and swallowing reflexes were observed, allowing for the restoration of spontaneous breathing. The visual analog scale (VASpain) score postextubation was documented. None of the patients underwent patient-controlled intravenous analgesia.
If the static VASpain score was ≥ 4 or the cough VASpian score was ≥ 6, 2 mg/kg tramadol was administered. If the efficacy remains unsatisfactory with an intravenous sufentanil dose of 10 μg, 40 mg parecoxib sodium should be administered intravenously, and the patient should be discharged to the ward.
Outcomes
The primary outcome was the assessment of pain block efficacy 30 minutes post RISS block, defined as “negative” if the block did not extend beyond three consecutive spinal segments, and “positive” otherwise. The secondary outcomes were the VASpain scores, which were recorded at 0, 0.5, 6, 12, and 24 hours postoperation. Additionally, the duration of initial supplementary analgesia and the consumption of additional analgesics within the first 24 hours after surgery were documented. Furthermore, the incidence of complications related to RISS block (such as puncture injury, pneumothorax, or local anesthetic intoxication) and postoperative nausea and vomiting (PONV) were assessed.
Implementation and Blinding
Different concentrations of ropivacaine solutions were prepared by the same anesthesiologist into 20 mL each. The preparation results were kept confidential and all possible identifying marks were removed. All ultrasound-guided RISS blocks were performed by an experienced anesthesiologist who was blinded to the concentrations of ropivacaine used. The Blocking effect was evaluated by another anesthesiologist who was also blinded. Intraoperative anesthesia and the data recorders were blinded to the concentrations of ropivacaine utilized. Postoperative pain assessment and follow-up were conducted by the same nurse who was blinded to the study design, ensuring consistency in the evaluation. Statistical analysis was carried out by dedicated personnel not involved in the implementation of the experiment.
Statistical Analysis
The data processing and statistical analysis were conducted using SPSS software (ver. 26.0, IBM Corporation, NY, US). The MEC90 for sequential series was calculated using R for Windows software (ver.4.3.2, New Zealand), and graphs were generated using GraphPad Prism software (ver.8.0.1, California, US). The normality of continuous variables was assessed using the Shapiro‒Wilk test. Normally distributed measurement data are presented as the mean ± standard deviation (SD), while nonnormally distributed measurement data are presented as the median and upper and lower quartiles. Count data are reported as frequencies and percentages (%). MEC90 was computed using a preprogrammed R package script to accurately determine its value within the dataset’s context.15 Utilizing centered isotonic regression (CIR), we further extrapolated MEC95 and MEC99.
Results
Forty-nine patients, with a mean age of 51.0 (38.5, 59.5) years, were enrolled in this study. The average BMI was 22.8 ± 2.6 kg/m2. Among them were nine males (18.4%) and 40 females (81.6%). ASA grade I was observed in 26 patients (53.1%), while grade II was observed in 23 patients (46.9%). The surgical sites included the left upper lung in 11 patients (22.4%), the left lower lung in 12 patients (24.5%), the right upper lung in 13 patients (26.5%), the right middle lung in 1 patient (2.0%), and the right lower lung in 12 patients (24.5%) (Table 1). All patients received RISS block and the range of the block plane was determined (Supplementary Table 1).
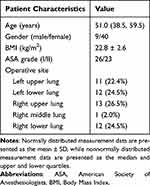 |
Table 1 Demographic Characteristics |
The MEC90 of ropivacaine for ultrasound-guided RISS block in VATS was determined to be 0.220% (95% CI 0.198 to 0.260%) according to the findings presented in Figure 3. Furthermore, we calculated the MEC95 to be 0.248% (95% CI 0.223 to 0.338%) and the MEC99 to be 0.270% (95% CI 0.261 to 0.351%). The cephalic and caudal boundaries of successful patients’ block planes are presented in Table 2, extending from the lateral boundary to the posterior axillary line and from the medial boundary of the flat papilla to the anterior axillary line. This study revealed no complications related to ultrasound-guided RISS block in any of the 49 patients. PONV was observed in 16 patients (32.7%).
 |
Table 2 Clinical Outcomes |
The ropivacaine concentration, postoperative VASpain score, time to first relief of analgesia, and duration of relief of analgesia were all found to be nonnormally distributed. Spearman correlation analysis revealed a positive correlation between ropivacaine concentration and intraoperative sufentanil consumption (r = 0.283, P = 0.049) and a negative correlation between ropivacaine concentration and VASpain score at 0 h, 0.5 h, and 6 h after surgery (r = −0.396, −0.594, and −0.309; P = 0.005, 0.001, and 0.031, respectively). However, no significant correlation was observed between the VASpain scores at 12 h and 24 h (r = −0.184; P = 0.205). Furthermore, the concentration of ropivacaine was positively correlated with the time of initial analgesia (r = 0.363; P = 0.01) but was not significantly correlated with the duration of analgesia (r = −0.165; P = 0.256), as depicted in Figure 4.
Discussion
The MEC90 of 40 mL ropivacaine for ultrasound-guided RISS block in VATS patients was determined to be 0.220%. Then, this study employed the CIR to calculate MEC95 and MEC99 as 0.248% and 0.270%, respectively. Furthermore, probit regression yielded comparable results (0.241% and 0.268%), thereby corroborating the reliability of these findings. Considering the practicality of drug administration in clinical settings, we recommend utilizing either a 0.25% or 0.3% ropivacaine solution at a volume of 40 mL for RISS block procedures.
This study adopts a more rigorous and scientific approach by considering the hypoalgesia plane measured through acupuncture as a positive indicator, so these outcomes can also be applied to other thoracic surgery. The VASpain score was commonly employed as a reliable metric in other studies to assess the efficacy of block procedures.16 However, this method was significantly influenced by the patient’s subjective emotions and surgical trauma. Furthermore, postoperative pain assessment revealed that patients classified as having successful blocks in this study exhibited a VASpain score of fewer than 3 points within 24 hours after surgery, with minimal need for additional analgesia within 6 hours postsurgery; thus, these findings were consistent with previous studies.
Ropivacaine was a frequently employed local anesthetic for nerve blockers. Previous studies on RISS block have reported satisfactory outcomes using ropivacaine of 0.375%12,17,18 and 0.5%,9,14 which were higher than our recommended concentration. However, some studies have also achieved successful results utilizing 0.2% ropivacaine for continuous RISS block.9,10,14 Studies investigating ropivacaine for nerve blocks in other areas have revealed varying minimum effective concentrations. For adductor canal block during total knee arthroplasty, the MEC90 was found to be 0.247% with a volume of 20 mL.19 Ultrasound-guided supraclavicular brachial plexus block requires an MEC90 of 0.257% with a volume of 40 mL ropivacaine.20 Following cesarean section, a quadratus lumborum block necessitated an MEC90 of 0.335% with a volume of 25 mL of ropivacaine.16 Quadratus lumborum block during total hip arthroplasty had an MEC90 of 0.352%, requiring 30 mL of ropivacaine.21 Finally, an adductor canal block during knee arthroscopic surgery requires an MEC90 of 0.477%, utilizing only 10 mL of ropivacaine.22 Variations in examining different anatomical regions may be attributed to variations in nerve block intensity, neural blood supply distribution, and drug diffusion mechanisms. Most studies yielded results similar to those of this study, ensuring consistent clinical significance.
This study also utilized correlation analysis to obtain results. An increase in the ropivacaine concentration was found to enhance the analgesic effect, but this improvement did not exceed 12 hours postsurgery. Deng et al used 0.2%, 0.3%, and 0.4% ropivacaine for RIB and reported that the blocking effect of 0.3% was superior to that of 0.2% but essentially equivalent to that of 0.4%. Increasing the anesthetic concentration does not significantly improve analgesia once a certain block level has been achieved.23 Increasing the concentration of ropivacaine may only extend the duration of the block and is limited, which aligns with previous research.23 Additionally, the increase in ropivacaine concentration did not have any beneficial impact on intraoperative or postoperative opioid utilization. An increased concentration of local anesthetics has the potential to exacerbate patient anxiety, heighten the likelihood of rebound pain occurrence, and diminish overall patient satisfaction.24 These findings underscore the significance of our study and suggest the use of low concentrations of ropivacaine for RISS blocks. Although the correlation analysis indicated a positive association between the intraoperative dosage of sufentanil and the concentration of ropivacaine, the strength of this correlation was very weak. Based on clinical experience, we are inclined to conclude that no meaningful correlation exists between the two variables.
The MEC50 calculated by the Dixon and Mood up-and-down sequential method (DM-UDM) holds less clinical significance. Notably, there is a considerable margin of error when extrapolating MEC90 from MEC50 obtained through DM-UDM. Thus, statisticians recommend employing BCD-UDM instead. To accurately estimate the MEC90, a minimum of 45 positive reactions are needed; however, increasing the response rate from 90% to 95% or 99% will significantly augment the sample size.25 The measurement of MEC90 is, therefore, more feasible, making it a commonly used indicator for determining the lowest effective concentration in numerous studies.16 The present study employed ordinal regression to account for interindividual variations among patients, while the pooled adjacent violators algorithm algorithm was utilized to estimate the target value. Order-preserving regression is a widely adopted statistical approach in drug clinical trials, premised on the assumption that the probability of drug efficacy remains nondecreasing with increasing dosage. Extensive evidence has demonstrated the accuracy of this method.15,26,27 The 95% CI was estimated using 2000 bootstrap replicates.28,29
This study once again demonstrated the effectiveness of the RISS block for postoperative analgesia in patients who underwent VATS, and these patients experienced fewer postoperative adverse reactions. Patients were more satisfied with the analgesic effect of the RISS block, which was consistent with previous studies.12,17 The RISS block was a two-point injection technique that involves the diffusion of local anesthesia to both the deep surface of the serratus anterior muscle through the rhomboid muscle plane and to the posterior branch of the thoracic spinal nerve and lateral cutaneous branches of the T3-T8 intercostal nerves. Additionally, by combining it with an anterior serratus plane block, this approach extends the blockade range medially to include the deep surface of erector spinae muscles and laterally to encompass the deep surface of anterior serratus muscles. Consequently, this combined technique covers a larger area, including lateral cutaneous branches from the T3 to T9 intercostal nerves.9 The RISS block can be effectively employed for postoperative analgesia in upper abdominal and thoracic surgeries, exhibiting a favorable analgesic effect that significantly alleviates patient pain.10–12,30 In certain instances, the utilization of the RISS block can also be extended to encompass anesthesia management for breast and axillary surgery.31
In this study, patients did not experience any significant complications, which aligns with the findings of numerous scholars. The RISS block minimally impacts patients’ physiological function and is associated with a low incidence of complications such as pneumothorax and vascular nerve injury.9,12,17,30 The clinical advantage lies in the distant injection point from the surgical incision, ensuring minimal interference with the surgical area.9 Moreover, compared to the erector spinae plane block, implementing the RISS block is technically more straightforward because it involves patient localization and identification of the scapula’s boundary marker. The local anesthetic is then distributed between one side of the rhomboid muscle or front tooth and the other side of the intercostal muscle. Additionally, the RISS block was less invasive than epidural and paravertebral blocks, theoretically reducing hemodynamic instability and minimizing complications and side effects such as hypotension. It reduces the cardiovascular burden caused by anesthesia and the occurrence of complications such as myocardial damage.32 Consequently, RISS block has gained significant attention as a highly regarded analgesic method within multimodal analgesia protocols. However, accurate identification of ultrasonic anatomical structures, tip positioning, and precise execution remain crucial.
The nerve block effect of ropivacaine was influenced by its concentration and the injection dose.33,34 Previous studies have demonstrated that 40 mL of 0.375% ropivacaine in ultrasound-guided anterior serratus block results in a broader range of skin sensory blocks than 20 mL.34 Furthermore, different doses of ropivacaine lead to varying MEC90s under the same block mode.19–22 This study’s findings are based on the premise of administering 40 mL of ropivacaine for the RISS blocks, which is a commonly used clinical practice dosage. However, it should be noted that the diffusion of the fascia layer affects the efficacy of the RISS block, implying that altering the injection dose may yield different outcomes. Future research will focus on determining the optimal injection dose while considering other factors, such as puncture point selection, which can influence plane diffusion during practical application.14
The limitations of this study were that it failed to take into account the influence of individual patient differences and surgical factors on the results. Previous studies have indicated that women may require higher drug concentrations than men when using ropivacaine for sacral block,35 although its overall usage remains relatively low.36 Nevertheless, current research still lacks fully elucidating the potential impact of sex on the analgesic efficacy of nerve blocks, highlighting an imperative avenue for future investigations. In addition, if possible, increasing the sample size can further enhance the value of the research.25
Conclusion
The minimum effective concentration (MEC90) of ropivacaine for ultrasound-guided RISS block was determined to be 0.220% (95% CI 0.198 to 0.260%) when it was administered in a volume of 40 mL. Based on these findings, utilizing a clinical concentration of either 0.25% or 0.3% is recommended. Exploring the optimal dose and volume of local anesthetics for different surgical procedures and patient characteristics could refine the application of the RISS block, enhancing its clinical utility.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. The datasets used and/or analyzed during the current study are also available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study underwent rigorous ethical review, duly completed trial registration in accordance with the established guidelines, and fully informed consent was obtained from all participating patients. The research was approved by Lihuili Hospital, Ningbo Medical Center, Ningbo City, Zhejiang Province, China, with approval no. KY2022PJ030. Simultaneously, the study was registered in the clinical trial center on March 15th, 2022 (the registration number is ChiCTR2200057584).
Consent for Publication
Written informed consent was obtained from all patients, their legally authorized representatives, or their guardians for the publication of this report.
Acknowledgments
We express our gratitude to Dr. Bingwei Hu for generously providing the R software code and offering valuable assistance in the statistical analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by the Key projects in the domains of agriculture and social development in Hangzhou (20231203A06).
Disclosure
All authors declare that the implementation of this study, data analysis, or interpretation of the results have no economic or non-economic associations that could be construed as conflicts of interest.
References
1. Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi:10.1016/S1470-2045(16)00173-X
2. Marshall K, McLaughlin K. Pain management in thoracic surgery. Thorac Surg Clin. 2020;30(3):339–346. doi:10.1016/j.thorsurg.2020.03.001
3. Mehran RJ, Martin LW, Baker CM, Mena GE, Rice DC. Pain management in an enhanced recovery pathway after thoracic surgical procedures. Ann Thorac Surg. 2016;102(6):e595–e596. doi:10.1016/j.athoracsur.2016.05.050
4. Thompson C, French DG, Costache I. Pain management within an enhanced recovery program after thoracic surgery. J Thorac Dis. 2018;10(Suppl 32):S3773–S3780. doi:10.21037/jtd.2018.09.112
5. Batchelor T, Ljungqvist O. A surgical perspective of ERAS guidelines in thoracic surgery. Curr Opin Anaesthesiol. 2019;32(1):17–22. doi:10.1097/ACO.0000000000000685
6. D’Amico F, Barucco G, Licheri M, et al. Opioid free anesthesia in thoracic surgery: a systematic review and meta analysis. J Clin Med. 2022;11(23):6955. doi:10.3390/jcm11236955
7. Almeida CR. Parascapular sub-iliocostalis plane block: comparative description of a novel technique for posterior rib fractures. Pain Pract. 2021;21(6):708–714. doi:10.1111/papr.13003
8. Powers BK, Ponder HL, Findley R, et al. Enhanced recovery after surgery (ERAS ®) Society abdominal and thoracic surgery recommendations: a systematic review and comparison of guidelines for perioperative and pharmacotherapy core items. World J Surg. 2024;48(3):509–523. doi:10.1002/wjs.12101
9. Elsharkawy H, Maniker R, Bolash R, et al. Rhomboid intercostal and subserratus plane block: a cadaveric and clinical evaluation. Reg Anesth Pain Med. 2018;43(7):745–751. doi:10.1097/AAP.0000000000000824
10. Elsharkawy H, Ince I, Pawa A. Rhomboid intercostal and sub-serratus (RISS) plane block for analgesia after lung transplant. J Clin Anesth. 2019;56:85–87. doi:10.1016/j.jclinane.2019.01.042
11. Yayik AM, Aydin ME, Tekin E, et al. An alternative plane block for multiple rib fractures: rhomboid Intercostal and Sub-Serratus block (RISS). Am J Emerg Med. 2019;37(12):2263.e5–2263.e7. doi:10.1016/j.ajem.2019.158429
12. Deng W, Hou XM, Zhou XY, et al. Rhomboid intercostal block combined with sub-serratus plane block versus rhomboid intercostal block for postoperative analgesia after video-assisted thoracoscopic surgery: a prospective randomized-controlled trial. BMC Pulm Med. 2021;21(1):68. doi:10.1186/s12890-021-01432-7
13. Sotthisopha T, Elgueta MF, Samerchua A, et al. Minimum effective volume of lidocaine for ultrasound-guided costoclavicular block. Reg Anesth Pain Med. 2017;42(5):571–574. doi:10.1097/AAP.0000000000000629
14. Elsharkawy H, Hamadnalla H, Altinpulluk EY, Gabriel RA. Rhomboid intercostal and subserratus plane block -a case series. Korean J Anesthesiol. 2020;73(6):550–556. doi:10.4097/kja.19479
15. Hu B, Li L, Wang H, et al. Determining the minimum effective concentration of ropivacaine in epidural anesthesia for tolerable pain in transforaminal percutaneous endoscopic lumbar discectomy to avoid nerve injury: a double-blind study using a biased-coin design. Drug Des Devel Ther. 2022;16:315–323. doi:10.2147/DDDT.S334605
16. Cao R, Li X, Yang J, Deng L, Cui Y. The minimum effective concentration (MEC90) of ropivacaine for ultrasound-guided quadratus lumborum block for analgesia after cesarean delivery: a dose finding study. BMC Anesthesiol. 2022;22(1):410. doi:10.1186/s12871-022-01954-5
17. Longo F, Piliego C. Rhomboid intercostal and subserratus plane block for non-intubated video-assisted thoracoscopic surgery. J Clin Anesth. 2020;61:109612. doi:10.1016/j.jclinane.2019.09.006
18. Longo F, Piliego C, Martuscelli M, et al. Rhomboid intercostal and subserratus plane block for intubated uniportal video-assisted thoracoscopic surgery lobectomy. J Clin Anesth. 2020;65:109881. doi:10.1016/j.jclinane.2020.109881
19. Wang Q, Hu J, Cai L, Bahete A, Yang J, Kang P. Minimum effective concentration of ropivacaine for ultrasound-guided adductor canal + IPACK block in total knee arthroplasty. J Orthop Surg. 2022;30(2):10225536221122339. doi:10.1177/10225536221122339
20. Fang G, Wan L, Mei W, Yu HH, Luo AL. The minimum effective concentration (MEC90) of ropivacaine for ultrasound-guided supraclavicular brachial plexus block. Anaesthesia. 2016;71(6):700–705. doi:10.1111/anae.13445
21. Hu J, Li X, Wang Q, Yang J. Minimum effective concentration of ropivacaine for ultrasound-guided transmuscular quadratus lumborum block in total Hip arthroplasty: a randomized clinical trial. Braz J Anesthesiol. 2023;74(2):S0104–0014(23)00095–7[pii]. doi:10.1016/j.bjane.2023.08.005
22. Rey Moura EC, de Oliveira C, da Cunha Leal P, Kimiko Sakata R. Minimum effective analgesic concentration of ropivacaine in saphenous block guided by ultrasound for knee arthroscopic meniscectomy: randomized, double-blind study. J Pain Res. 2021;14:53–59. doi:10.2147/JPR.S282286
23. Deng W, Jiang CW, Qian KJ, et al. Evaluation of rhomboid intercostal block in video-assisted thoracic surgery: comparing three concentrations of ropivacaine. Front Pharmacol. 2021;12:774859. doi:10.3389/fphar.2021.774859
24. Sun G, Atary J, Raju AV, Pozek JJ, Schwenk ES. Sometimes less is more when it comes to peripheral nerve blocks. J Clin Anesth. 2024;94:111376. doi:10.1016/j.jclinane.2024.111376
25. Sharma N, Piazza M, Marcotte PJ, et al. Implications of anesthetic approach, spinal versus general, for the treatment of spinal disc herniation. J Neurosurg Spine. 2018;30(1):78–82. doi:10.3171/2018.7.SPINE18460
26. Saranteas T, Finlayson RJ, Tran DQ. Dose-finding methodology for peripheral nerve blocks. Reg Anesth Pain Med. 2014;39(6):550–555. doi:10.1097/AAP.0000000000000157
27. Grelet T, Besch G, Puyraveau M, et al. Minimum effective concentration of ropivacaine for 90% ultrasound-guided axillary brachial plexus block, with or without intravenous dexamethasone. J Clin Anesth. 2021;75:110468. doi:10.1016/j.jclinane.2021.110468
28. Drew T, Balki M, Farine D, Ye XY, Downey K, Carvalho J. Carbetocin at elective caesarean section: a sequential allocation trial to determine the minimum effective dose in obese women. Anaesthesia. 2020;75(3):331–337. doi:10.1111/anae.14944
29. Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometrics. 2002;58(1):171–177. doi:10.1111/j.0006-341X.2002.00171.x
30. Ökmen K, Gürbüz H, Özkan H. Application of unilateral rhomboid intercostal and subserratus plane block for analgesia after laparoscopic cholecystectomy: a quasi-experimental study. Korean J Anesthesiol. 2022;75(1):79–85. doi:10.4097/kja.21229
31. Kozanhan B, Aksoy N, Yildiz M, et al. Rhomboid Intercostal and Subserratus Plane block for modified radical mastectomy and axillary curettage in a patient with severe obstructive sleep apnea and morbid obesity. J Clin Anesth. 2019;57:93–94. doi:10.1016/j.jclinane.2019.03.026
32. Duchnowski P, Śmigielski W. Usefulness of myocardial damage biomarkers in predicting cardiogenic shock in patients undergoing heart valve surgery. Kardiol Pol. 2024;82(4):423–426. doi:10.33963/v.phj.99553
33. Biswas A, Castanov V, Li Z, et al. Serratus plane block: a cadaveric study to evaluate optimal injectate spread. Reg Anesth Pain Med. 2018;43(8):854–858. doi:10.1097/AAP.0000000000000848
34. Kunigo T, Murouchi T, Yamamoto S, et al. Injection volume and anesthetic effect in serratus plane block. Reg Anesth Pain Med. 2017;42(6):737–740. doi:10.1097/AAP.0000000000000649
35. Li Y, Zhou Y, Chen H, Feng Z. The effect of sex on the minimum local analgesic concentration of ropivacaine for caudal anesthesia in anorectal surgery. Anesth Analg. 2010;110(5):1490–1493. doi:10.1213/ANE.0b013e3181d6bade
36. Li X, Li J, Zhang P, et al. The minimum effective concentration (MEC90) of ropivacaine for ultrasound-guided caudal block in anorectal surgery. A dose finding study. PLoS One. 2021;16:e0257283. doi:10.1371/journal.pone.0257283
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




