Back to Journals » International Journal of Nanomedicine » Volume 20
Mitochondria-Targeted Biomaterials-Regulating Macrophage Polarization Opens New Perspectives for Disease Treatment
Authors Tian Z, Wang X, Chen S, Guo Z, Di J, Xiang C
Received 9 November 2024
Accepted for publication 18 January 2025
Published 4 February 2025 Volume 2025:20 Pages 1509—1528
DOI https://doi.org/10.2147/IJN.S505591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Zui Tian,* Xudong Wang,* Shuai Chen,* Zijian Guo, Jingkai Di, Chuan Xiang
Department of Orthopedics, Second Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chuan Xiang, Department of Orthopedics, Second Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China, Email [email protected]
Abstract: Macrophage immunotherapy is an emerging therapeutic approach designed for modulating the immune response to alleviate disease symptoms. The balance between pro-inflammatory and anti-inflammatory macrophages plays a pivotal role in the progression of inflammatory diseases. Mitochondria, often referred to as the “power plants” of the cell, are essential organelles responsible for critical functions such as energy metabolism, material synthesis, and signal transduction. The functional state of mitochondria is closely linked to macrophage polarization, prompting interest in therapeutic strategies that target mitochondria to regulate this process. To this end, biomaterials with excellent targeting capabilities and effective therapeutic properties have been developed to influence mitochondrial function and regulate macrophage polarization. However, a comprehensive summary of biomaterial-driven modulation of mitochondrial function to control macrophage phenotypes is still lacking. This review highlights the critical role of mitochondrial function in macrophage polarization and discusses therapeutic strategies mediated by biomaterials, including mitochondria-targeted biomaterials. Finally, the prospects and challenges of the use of these biomaterials in disease modulation have been explored, emphasizing their potential to be translated to the clinic. It is anticipated that this review will serve as a valuable resource for materials scientists and clinicians in the development of next-generation mitochondria-targeted biomaterials.
Keywords: macrophages, polarization, biomaterials, mitochondria
Introduction
Macrophages, originating from monocyte precursors in the bone marrow and bloodstream, serve as critical immune sentinels within the body. They play a pivotal role in bridging innate and adaptive immunity while contributing to host defense, resistance to invading pathogens, and the maintenance of tissue integrity. However, the macrophage response represents a double-edged sword, functioning both as a protective defender and, under certain conditions, as a potential contributor to disease pathogenesis.1–3 The classical model of macrophage polarization is characterized by two distinct phenotypic states: the pro-inflammatory M1 macrophage and the anti-inflammatory M2 macrophage.4 The equilibrium between M1 and M2 polarization plays a critical role in resolving inflammation.
During the inflammatory response, M1 macrophages promote T-helper lymphocyte type 1 (Th1) activation, driving pro-inflammatory responses and amplifying the inflammatory cascade through the secretion of cytokines such as IL-6 and IL-1.5–7 M1 macrophages produce nitric oxide (NO) and reactive oxygen species (ROS),8,9 which, while effective in eliminating infectious agents, can also cause tissue damage.M2 macrophages interact with type 2 T-helper lymphocytes (Th2) and secrete anti-inflammatory and tissue remodeling cytokines, including IL-10, TGF-β, and IL-13, thereby mitigating inflammatory damage.5,10 Disruptions in the balance between M1 and M2 polarization are implicated in the progression of various diseases, including osteoarthritis (OA),11 acute lung injury (ALI),12 myocardial infarction (MI),13 diabetic wounds,14 atherosclerosis,15 and inflammatory bowel disease (IBD)16 (Figure 1).
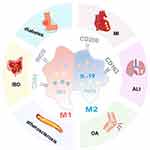 |
Figure 1 Disease caused by M1/M2 polarization imbalance of macrophages. |
Mitochondria serve as the primary energy source for cells and are critical regulators of cell fate. They play a dual role in cellular function: on one hand, they support cell viability by producing ATP to fuel various cellular processes; on the other, they regulate cell death by releasing pro-apoptotic factors that initiate apoptosis.17,18 Beyond ATP production,19,20 mitochondria are integral to numerous biological pathways, including apoptosis induction, ROS generation, and mitochondrial DNA (mtDNA) regulation. Mitochondrial dysfunction is implicated in the development of numerous diseases.21–23 As a result, mitochondria have become a central focus in therapeutic strategies, offering significant promise and far-reaching potential in disease treatment.23
Since the early 21st century, biomaterials have emerged as a cornerstone of modern medicine, driving significant advancements in therapeutic strategies. Among these, considerable effort has been dedicated to developing biomaterials for immunomodulation. These immunomodulatory biomaterials can directly interact with macrophages, influencing their behavior and function.24 While previous reviews have broadly addressed the properties of biomaterials, they have often overlooked macrophage polarization strategies based on precise targeting techniques, particularly those centered on mitochondrial targeting. This review addresses this gap by providing a comprehensive summary of the role of mitochondria in macrophage polarization (Figure 2) and detailing biomaterial-based strategies for mitochondrial targeting to modulate macrophage polarization. By offering insights into these innovative approaches, this review aims to equip materials scientists and clinicians with the knowledge needed to develop the next generation of advanced, stimulus-responsive immunomodulatory platforms. The discussion concludes with a synthesis of key findings and an exploration of future directions in the field.
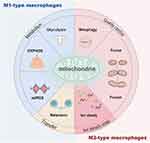 |
Figure 2 Overview of mitochondria and macrophage polarization. |
Effects of Mitochondrial Metabolism on Macrophages and Their Phenotype
Mitochondrial Glycolytic Response Promotes Macrophage Polarization Towards the M1 Phenotype
Classically activated M1 macrophages exhibit a predominantly aerobic glycolytic metabolic profile characterized by increased glucose consumption, lactate production, and a reduced rate of oxygen consumption.25,26 Glucose metabolism is pivotal in modulating M1 macrophage functions and immune responses, with enhanced glycolytic activity not only supplying energy and intermediate metabolites but also influencing the inflammatory response.26,27 Early studies reported elevated expression levels of hexokinase (HK) and glucose-6-phosphate dehydrogenase (G-6-PD) in inflammatory macrophages, indicating heightened glycolytic activity.28,29 The shift from a resting state to the M1 phenotype relies on glycolysis to meet the energy demands of anabolism. This metabolic adaptation supports the production of NO and ROS, which amplify the inflammatory response by activating nuclear factor-kappa B (NF-κB).30 M1 macrophage activation is closely regulated by NF-κB, which, in turn, induces the expression of hypoxia-inducible factor-1 alpha (HIF-1α).31 Stimulation of macrophages with IFN-γ or LPS promotes glycogen synthesis, a key regulator of M1-driven acute inflammatory responses.32–34 The molecular mechanism involves the UDPG/P2Y14/STAT1 signaling pathway, and blocking glycogen synthesis or this pathway may modulate inflammation.32 HIF-1α activation has emerged as a critical signaling mechanism for controlling aerobic glycolysis and M1 polarization. Inhibition of HIF-1α impacts glycolysis, M1 polarization, cell migration, and bactericidal functions.35,36 Activation of the AMP-activated protein kinase (AMPK) pathway can reprogram M1 glycolysis37 while inhibiting mTORC1 activity to regulate glucose metabolism and cell proliferation37 (Figure 3,4). These findings suggest that targeting glycolysis could provide novel therapeutic opportunities for treating diseases associated with inflammatory macrophage polarization.
 |
Figure 3 Overview of mitochondrial metabolism and macrophage polarization. |
 |
Figure 4 Molecular mechanisms of targeting mitochondria to regulate macrophage polarization. |
Biomaterial-based manipulation of mitochondrial glycolysis has centered on three key strategies:
(1) Encapsulation of glycolytic pathway regulators. 2-Deoxyglucose (2-DG), a common inhibitor of glycolysis, can modulate macrophage polarization by inhibiting the NF-κB signaling pathway. Xiao et al developed a novel chitosan/gelatin composite patch containing 2-DG, which significantly suppressed the expression of inflammatory cytokines and inhibited pro-inflammatory polarisation of macrophages, to attenuate the local inflammatory microenvironment in the ischaemic heart, improved cardiac function, reduced scar area and promotes post-MI angiogenesis. This suggests that a 2-DG composite patch may be a promising therapeutic strategy for cardiac repair following myocardial infarction.39,40 Silicon dioxide nanoparticles (SiNPs) are among the most commonly used nanomaterials due to their versatile applications. SiNPs therapy has been shown to promote macrophage M1 polarization by activating the NF-κB pathway and glycolytic mechanisms. This polarization plays a key role in inhibiting lung cancer progression and its associated processes.41
(2) Synthetic Nanomaterials Mimicking Natural Enzymes. Ling et al proposed a strategy to reprogram tumor-associated macrophages (TAMs) using SnSe nanosheets (SnSe NSs) that mimic the activity of lactate dehydrogenase (LDH) to enhance photothermal immunotherapy. Tumor cells undergoing rapid glycolysis produce excess lactate, which acidifies the tumor microenvironment (TME), creating an immunosuppressive environment and impairing TAM function. SnSe@ABS NSs were shown to shift the metabolic profile of TAMs from mitochondrial oxidative phosphorylation to glycolysis, promote TAM polarization from the M2 phenotype to the M1 phenotype, and restore macrophage tumor-killing activity. This metabolic reprogramming significantly enhanced the efficacy of TAM-based antitumor immunotherapy.42
(3) Encapsulation of Mitochondrial Metabolic Modulators. Ramesh et al synthesized supramolecular nanoparticles (MSNPs) loaded with a TLR7/8 agonist (R848) and a fatty acid oxidation (FAO) inhibitor (Etomoxir). This dual-drug delivery system targeted the mitochondrial metabolism of TAMs by inhibiting the tricarboxylic acid (TCA) cycle while upregulating glycolysis. This metabolic shift reprogrammed TAMs from the M2 phenotype to the M1 phenotype, significantly enhancing TAM phagocytic activity. MSNP therapy demonstrated superior efficacy compared to other approaches in slowing tumor growth by leveraging metabolic reprogramming to enhance antitumor activity43 (Table 1).
 |
Table 1 Effect of Biomaterial Compositions on Macrophage Polarization |
Mitochondrial Oxidative Phosphorylation Reactions Promote Macrophage Polarisation Towards the M2 Phenotype
Metabolic reprogramming has emerged as a critical hallmark of innate immunity. This process involves altering the predominant pathway of cellular energy production, shifting from glycolysis to oxidative phosphorylation (OXPHOS), a phenomenon often referred to as the “anti-Warburg effect”44 M1 macrophages primarily utilize glucose metabolism through glycolysis to meet their energy demands, whereas M2 macrophages rely on fatty acid oxidation (FAO) and OXPHOS to enhance their cellular functions.27,45 Recent studies have demonstrated that inhibiting OXPHOS in M2 macrophages can induce metabolic and phenotypic reprogramming toward the pro-inflammatory M1 phenotype.46 Recent studies have demonstrated that inhibiting OXPHOS in M2 macrophages triggers metabolic and phenotypic reprogramming, promoting their transition to the M1 phenotype.47,48 For example, IL-10 has been shown to suppress lipopolysaccharide (LPS)-induced glucose uptake and glycolysis while promoting the OXPHOS response, thereby inhibiting the transition of macrophages to the M1 phenotype.49 Similarly, IL-4 enhances mitochondrial oxidative phosphorylation via STAT6 activation, which interacts with PGC1β, facilitating the polarization of M2 macrophages.38 The AMPK pathway also plays a pivotal role in macrophage metabolism. Its activation reprograms glycolysis in M1 macrophages, inhibits mTORC1 activity, reduces protein synthesis, and regulates glucose metabolism and proliferation. Furthermore, AMPK activation enhances mitochondrial enzyme activity to support oxidative phosphorylation.37 In conclusion, M2 macrophages depend heavily on efficient OXPHOS to sustain their phenotypic and energetic demands, highlighting its significance in their anti-inflammatory functions (Figures 3 and 4).
Biomaterials designed to manipulate mitochondrial oxidative phosphorylation (OXPHOS) have been developed in three main approaches:
(1) Encapsulation of OXPHOS pathway regulators. Xie et al developed a highly adhesive polyethylene glycol (PEG) hydrogel incorporating Filgotinib (Fil@GEL), a Janus kinase (JAK) inhibitor. This hydrogel induces a metabolic shift in macrophages from glycolysis to oxidative phosphorylation by inhibiting the JAK-STAT signaling pathway, thereby suppressing M1 polarization. These properties highlight its potential as an effective wound dressing material.50
(2) Synthesis of immunometabolic biomaterials by adding appropriate concentrations of fluorine and selenium. Chen et al synthesized Fluorinated Hydroxyapatite (FPHA) and verified its compositional and structural integrity. Compared to unfluorinated hydroxyapatite (PHA), FPHA demonstrated enhanced biocompatibility and osteogenic potential. In a rat cranial bone defect model, FPHA improved mitochondrial function, shifted macrophage metabolism from glycolysis to OXPHOS, and promoted M2 macrophage polarization. FPHA also enhanced macrophage proliferation, fostering a favorable immune microenvironment for bone regeneration. These findings highlight the potential of fluorine-modified biomaterials in regulating macrophage mitochondrial function and immune metabolism, with significant implications for bone repair and clinical applications of fluorinated biomaterials.51 Chen et al developed Mesoporous Bioactive Glass (Se-MBG), a selenium-doped biomaterial that induces M2 macrophage polarization and enhances OXPHOS metabolism. The Se-MBG extracts were shown to promote mitochondrial function and metabolic reprogramming in macrophages, resulting in effective modulation of the immune response. This approach offers a promising strategy for preventing and managing peri-implantitis by targeting cellular metabolism to influence macrophage behavior. The findings provide a novel perspective on the design of multifunctional biomaterials for immune modulation and therapeutic applications.52
(3) Encapsulated microRNAs (miRNAs). MicroRNAs (miRNAs) are potent molecular regulators capable of modulating multiple endogenous processes simultaneously. Zhang et al developed a responsive and sustained miR-27a-releasing micro-and nanoimplant aimed at preventing and treating peri-implantitis. The design incorporated advanced techniques, such as using a flextronic laser to drill 100 μm osteogenic micropores on implant surfaces. This precise modification altered implant morphology without affecting its crystal phase. The micropore walls were coated with a miRNA-loaded metal-organic framework (MOF) membrane, a pH-responsive structure designed to release miR-27a in inflammatory environments. The implant induces repolarization of macrophage function by promoting the functional and metabolic reprogramming of macrophage mitochondria, shifting metabolism from glycolysis to oxidative phosphorylation (OXPHOS), while also restoring normal mitochondrial morphology and function. This approach regulates the macrophage immune response by targeting mitochondrial metabolism, resulting in a novel implant surface coating that can modulate macrophage polarization. The implant offers a promising strategy for preventing and treating peri-implantitis, providing new insights into the development of multifunctional biomaterials for clinical applications19 (Table 1).
Mitochondrial ROS and Macrophage Polarization
In mitochondria, ROS are considered a key indicator of changes in mitochondrial homeostasis and are frequently used as biomarkers of mitochondrial damage.53,54 Under normal physiological conditions, low levels of ROS produced by mitochondria can post-translationally and reversibly modify specific targets through oxidation,55 thereby regulating metabolic signaling pathways. Mitochondrial ROS (mtROS) can directly affect proteins within the mitochondrial matrix or membrane,56–58 ultimately influencing the oxidative phosphorylation (OXPHOS) process.59
mtROS also plays a critical role in regulating macrophage polarization.60 When cellular integrity is compromised, mtROS secretion increases, leading to mitochondrial dysfunction. mtROS has a dual role in macrophage polarization.61,62 At low levels, mtROS promotes M2 macrophage polarization, whereas at high levels, mtROS drives M1 polarization.63,64 NADPH oxidase 4 (NOX4), a key electron transfer mediator in the mitochondrial respiratory chain, is a major source of mtROS.65,66 Research on inflammatory bowel disease has shown that NOX4 enhances M1 polarization of intestinal macrophages by increasing mtROS production.67 Reduced mtROS levels inhibit M1 polarization and encourage M2 macrophage polarization.68 Porous Se@SiO2 nanosphere-coated catheters have been shown to promote macrophage polarisation to the M2 phenotype. This effect is achieved by inhibiting the ROS-NF-κB pathway, which helps reduce inflammatory responses and supports wound recovery following post-prostate urethroplasty.69 Accumulated ROS drives macrophage polarization toward the M1 phenotype by activating the NF-κB signaling or JAK/STAT pathways.60,70 (Figure 3,4). These findings highlight the significant relationship between macrophage polarization and mtROS levels, offering a potential starting point for developing more effective strategies to regulate macrophage polarization.
Biomaterials designed to regulate mitochondrial reactive oxygen species (mtROS) levels typically involve the following strategies.
(1) Incorporation of natural antioxidant enzymes such as cerium dioxide (CeO2) and superoxide dismutase (SOD). In a study by He et al, a novel biomaterial was developed by coating zeolite imidazoline skeleton-8 (ZIF-8) with cerium dioxide (CeO2), which encapsulated the Rho-associated protein kinase inhibitor Y-27632 (CeO2-Y@ZIF-8). This composite was further integrated into a photocrosslinked gelatin (GelMA) hydrogel containing cationic quaternary ammonium groups (CeO2-Y@ZIF-8@Gel) to impart antimicrobial properties. CeO2 exhibits dual enzymatic functions—superoxide dismutase (SOD) and catalase (CAT)—which help to neutralize excess mtROS, minimize mitochondrial damage, prevent leakage of oxidatively damaged mtDNA, and reduce cGAS-STING pathway activation. CeO2 also promotes the polarization of macrophages toward the M2 phenotype and enhances the secretion of anti-inflammatory cytokines. In vivo studies demonstrated that CeO2-Y@ZIF-8@Gel significantly accelerated wound healing in diabetic mice by stimulating angiogenesis and reducing inflammation. This multifunctional dressing effectively modulated macrophage and endothelial cell communication, supporting immunomodulation and facilitating rapid tissue repair in diabetic wounds.71
(2) Synthetic nanomaterials that emulate natural enzymes. Wei et al developed Pt-Se composite nanoenzymes through chemical reduction, harnessing the combined catalytic powers of platinum (Pt) and selenium (Se) for synergistic effects. These Pt-Se nanoenzymes exhibited strong mtROS scavenging abilities, which facilitated the repolarization of M1-type macrophages, decreased the expression of pro-inflammatory cytokines, and protected arthritic chondrocytes by restoring mitochondrial function in synovial macrophages. This study not only opens new possibilities for treating osteoarthritis (OA) and other chronic conditions linked to mtROS but also highlights the potential of nanoenzymes to enhance the microenvironment through their intrinsic bioactive properties.72
(3) Encapsulation of antioxidant substances such as EGCG,AST. Zhang et al developed an innovative nanocarrier for delivering astaxanthin (AST) and epigallocatechin-3-gallate (EGCG), both known for their antioxidant and anti-inflammatory properties. The nanocarriers were designed to target mitochondria through the Mannich reaction, utilizing EGCG as the wall material and incorporating glutathione (GSH) for responsive functionality. In vitro studies demonstrated that these nanocarriers exhibited enhanced mitochondrial accumulation, effectively scavenging mtROS and maintaining optimal mitochondrial membrane potential. The nanocarriers promoted M2-type macrophage polarization and significantly increased colon length in a mouse model of inflammatory bowel disease (IBD). This dual-nutrient nanocarrier offers promising potential for the treatment of IBD, particularly by enabling the oral delivery of hydrophobic bioactives.73 Ye et al developed an innovative wound dressing, E@C Gel, by combining catechin-3-gallate (EGCG) with cerium to form a complex (EGCG@Ce) that functions as a mtROS scavenger. This complex was then encapsulated in a poly(vinyl alcohol)-chitosan (PEG-CS) hydrogel, which also exhibits antimicrobial properties. In vitro experiments showed that EGCG@Ce demonstrated excellent cytocompatibility with macrophages and effectively scavenged mtROS, thereby protecting mitochondria. This also led to the reversal of M1-type macrophage polarization and a reduction in pro-inflammatory cytokine secretion. In vivo, the EGCG@Ce-loaded hydrogel significantly accelerated wound closure and skin tissue regeneration. It enhanced M2-type macrophage polarization and promoted angiogenesis by reducing mtROS accumulation. The study presents an effective antioxidant strategy for modulating the inflammatory microenvironment of wounds through mtROS scavenging and immunomodulation.70 Zhou et al developed an innovative therapeutic strategy that combines selenium nanoparticles (SeNPs) encapsulated in ZIF-8 (SeNPs@ZIF-8) and Ferrostatin-1 (FSZ NPs), an inhibitor of ferroptosis. This approach effectively enhances both antioxidant and anti-ferroptotic activities. In vitro studies demonstrated that this combination effectively scavenges mtROS, restores mitochondrial function, modulates inflammatory responses, and promotes macrophage polarization toward the M2 phenotype. In animal models, this therapeutic strategy significantly improved motor function, reduced glial scar formation and stimulated angiogenesis. This novel approach holds significant potential as an antioxidant-based treatment for spinal cord injury (SCI).74
(4) Synthetic nanomaterials for promoting ROS generation. Liet al conjugated BSA with fluorescent, lipophilic and cationic rhodamine molecules to prepare mitochondria-targeted RBSA, which was then attached to PSiNPs via hydrophobic interactions to obtain R-BSA@PSiNPs nanocomposites. Mechanistic studies demonstrated that the porous silica nanocarriers could efficiently deliver mitochondria-targeted BSA to macrophages by interfering with mitochondrial respiratory chain to generate mitochondrial ROS to effectively mediate macrophage pro-inflammatory transformation in vitro or in vivo.75 Such nanocomposites have important potential in combinatorial chemoimmunotherapy against cancer or virus/bacteria-associated infectious diseases (Table 1).
Each antioxidant strategy offers distinct advantages and limitations, making it essential to select the most appropriate approach based on the specific context. Naturally occurring antioxidant enzymes are often compromised by the disease microenvironment, leading to suboptimal mtROS scavenging efficiency.76 While antioxidant substances have the potential to scavenge mtROS, their effectiveness is limited by rapid metabolism and poor bioavailability.77 However, synthetic metal-containing nanoenzymes exhibit potent antioxidant properties but may pose cytotoxic risks due to the presence of non-degradable metal components, necessitating further exploration. To address these concerns, there is a need for the development of sustainable, low-toxicity, and highly biocompatible green mtROS scavenging platforms.78
Effect of Mitochondrial Ion Homeostasis on Macrophage Polarization
Metal ions play a critical role in maintaining homeostasis within the host and are involved in various physiological processes.79–81 However, imbalances in metal ion levels—whether through excess or deficiency—can disrupt cellular function.81 Mitochondria serve as a central site for the action of many metal ions, largely due to their role as storage organelles for these ions, which contributes to their involvement in these forms of cell function.82,83
Mitochondrial Iron Homeostasis Also Plays a Regulatory Role in Macrophage Polarization
Mitochondrial ferritin (FtMt) is a key mitochondrial protein responsible for iron storage, possessing ferrous oxidase activity that converts Fe2+into its ferric form. FtMt is stored within a spherical shell, capable of holding up to 4000 iron atoms.84–86 It plays an essential role in regulating intracellular iron distribution and ROS production. A deficiency in FtMt leads to an accumulation of free iron, which enhances lipid ROS production, thereby promoting macrophage polarization and inflammation.87 Iron, as a component of the mitochondrial iron-sulfur (Fe-S) cluster, is crucial for oxidative phosphorylation and the TCA cycle, both of which are vital mitochondrial processes that influence specific immune pathways.88 Therefore, Fe-S proteins and enzymes are integral in reconnecting metabolic pathways during macrophage polarization.89 Recent studies have shown significant Fe2+accumulation in M1-type macrophages, a phenomenon not observed in other polarized macrophage types, such as M2a, M2b, M2c, and M2d.90 This finding suggests that the dynamic regulation of mitochondrial iron may affect macrophage polarization, particularly during inflammatory activation. Thus, mitochondrial iron plays a complex role in macrophage polarization, influencing both its metabolic processes and redox reactions. It is also pivotal in regulating immune responses and maintaining intracellular homeostasis. Evidence suggests that iron accumulation in macrophages can activate the NF-κB signaling pathway, driving macrophage M1 polarization.91 Mitochondrial iron overload promotes ROS production and facilitates glycolysis, thereby driving macrophage M1 polarization92,93 (Figure 3,4).
Researchers have increasingly focused on modulating mitochondrial iron homeostasis to shape macrophage phenotypes for treating macrophage-driven diseases.
(1) Encapsulate metal ion chelating agent. Shi et al developed an injectable hydrogel composed of quaternized chitosan (QCS) and carboxymethyl cellulose (CMC), loaded with modified polydopamine nanoparticles (PDA NP), known as CQP hydrogel. This hydrogel helps prevent Fe2+accumulation, restores mitochondrial function and facilitates the polarization of microglia/macrophages from the M1 to M2 phenotype. This transition contributes to improved motor function in SCI rats. The CQP hydrogel serves as an iron-chelating system that supports recovery from SCI by targeting iron homeostasis and modulating macrophage polarization.94
(2) Synthetic nanomaterials for releasing Fe2+generation. Engineered macrophages have also shown promise in cancer therapy, particularly for drug delivery and immunotherapy. However, a significant challenge remains in achieving simultaneous targeted enrichment and controlled immune activation at the tumor site. To address this, researchers loaded macrophages with advanced nanoparticles (MNC-ICG-NIG@SiO2, or MINS), consisting of CpG-conjugated magnetic nanoclusters (MNC), indocyanine green (ICG), and Nigerian interferon (NIG). Upon release of Fe2+ from MINS@MΦ, macrophage polarization is strongly directed toward the M1 phenotype. This strategy enhances the expression of tumor-suppressor cytokines, offering a novel approach to treating bladder cancer.95 Biomaterials incorporating iron modulators release these agents to control mitochondrial iron levels in macrophages, thereby influencing macrophage polarization. However, uncontrolled release of iron modulators, whether in macrophages or other cells, can lead to dose-dependent toxicity due to nonspecific chelation of essential physiological iron. To address this, the development of stimulus-responsive biomaterials presents a promising solution, enabling precise control of cellular iron levels. These biomaterials would prevent regional over-enrichment and facilitate the sustainable, controlled release of iron modulators96,97 (Table 1).
Mitochondrial Copper Ion Homeostasis Plays a Regulatory Role in Macrophage Polarization
Copper ions play a crucial role in maintaining the inflammatory status of macrophages within the mitochondria.98 Studies have demonstrated that varying concentrations of Cu2+in macrophage cultures can influence the inflammatory response, with higher concentrations promoting inflammation due to their cytotoxic effects.99 In inflammatory macrophages, mitochondrial Cu2+levels are significantly elevated, contributing to key cellular processes such as metabolism and epigenetic programming.98 Mitochondrial Cu2+facilitates the redox cycling of NAD(H) by catalyzing the interaction between NADH and hydrogen peroxide (H2O2), a process essential for maintaining NAD+ levels, which are vital for the metabolism underlying the inflammatory response and the epigenetic regulation of macrophages100 (Figure 3,4).
(1) Synthetic nanomaterials for inactivating mitochondrial copper. Solier et al developed LCC-12, a metformin dimer that inactivates Cu2+within the mitochondria. LCC-12 counteracts macrophage activation by altering metabolic and epigenetic pathways, reducing the NAD(H) pool. In mouse models, LCC-12 effectively mitigated the acute inflammatory response and increased survival by targeting mitochondrial Cu2+, highlighting its potential to influence macrophage polarization.101
(2) Encapsulates copper ion carrier to translocate Cu(II) ions to mitochondriaI.In another innovative approach, Li et al designed a core-shell nanoscale coordination polymer (NCP) particle, featuring a Cu2+core and a triphenylphosphine (TPP) conjugate of IOX1 (TI) as the outer shell, to specifically target mitochondria. This design enables the simultaneous induction of cuprotosis (copper-induced cell death) and down-regulation of PD-L1 expression. The NCP particles efficiently deliver copper ions and therapeutic agents to the mitochondria of cancer cells, thereby increasing the proportion of anti-tumor M1 macrophages and reducing the tumor-promoting M2 macrophages in tumors. This approach demonstrates the potential of NCP nanoparticles as a versatile and biodegradable platform for targeted multidrug delivery to mitochondria102 (Table 1).
Mitochondrial Calcium Homeostasis Plays a Regulatory Role in Macrophage Polarization
Mitochondria play a critical role in regulating their homeostasis through the chelation and release of calcium ions (Ca2+).103–105 Calcium ions activate various enzymes that drive the tricarboxylic acid (TCA) cycle,106 regulate mitochondrial oxidative phosphorylation rates,107 and contribute to inflammation by controlling the opening of the mitochondrial permeability transition pore (mPTP).108 Transient increases in Ca2+levels promote normal mitochondrial functions, including oxidative respiration and ATP production. However, excessively elevated Ca2+concentrations can lead to several detrimental effects, such as disruption of the mitochondrial membrane potential, increased ROS production, and the release of mitochondrial DNA. These events activate inflammatory pathways and drive macrophage polarization towards the pro-inflammatory M1 phenotype.109–111 Recent studies have demonstrated that connexin 43 (Cx43) promotes macrophage polarization from M2 to M1 by promoting mitochondrial calcium overload, ROS generation and mitochondrial oxidative dysfunction.112 Gu et al demonstrated that mitochondrial calcium uptake, mediated by the mitochondrial calcium uniporter (MCU), regulates ROS and ATP production, influencing macrophage polarization. This process contributes to the development of a pro-fibrotic macrophage phenotype, ultimately driving the pathogenesis of pulmonary fibrosis113 (Figures 3 and 4).
(1) One approach involves encapsulating Ca2+modulators such as EGTA114 and BAPTA-AM.115,116 Kang et al115 developed a system where Ca2+modulators (DMNP-EDTA-Ca2+or BAPTA-AM) were loaded into mesoporous silica-modified upconversion nanoparticles (UCNP@mSiO2), which remotely regulate the immune function of M1- or M2-responsive RAW 264.7 cells. This system utilizes near-infrared (NIR) light to enable the on-demand release of Ca2+modulators, thereby enhancing or depleting [Ca2+] levels in a time-dependent manner. The nanoparticles facilitate targeted intracellular uptake and controlled release of the modulators.Similarly, Deng et al117 constructed calcium/manganese hybrid nanoparticles (CMS) that act as Ca2+ donors, inducing mitochondrial Ca2+overload. This system promotes the polarization of tumor-associated macrophages (TAMs) from M2 to M1, stimulates dendritic cell maturation, activates innate immunity, and enhances the infiltration of tumor-specific cytotoxic T lymphocytes (CTLs) into tumor tissues, thereby boosting anti-tumor immune responses. This strategy offers a novel approach to activate innate immunity, presenting new perspectives for effective tumor immunotherapy, particularly in triple-negative breast cancer (TNBC).
(2) Another strategy involves blocking aberrant Ca2+flux by targeting related channels or signaling pathways. Feng et al118 developed a macrophage-targeted release system using polyethyleneimine (PEI) and poly(β-aminoamine) (PBAA) to deliver siERN1, which regulated Ca2+levels in LPS-stimulated macrophages. This system facilitated the polarization of macrophages from M1 to M2 and helped maintain immune homeostasis. Their findings highlight ERN1 as a potent target for therapeutic intervention and provide valuable insights into Ca2+-regulated drug design and its mechanisms (Table 1).
Crosstalk between macrophage polarization and mitochondrial Ca2+levels plays a crucial role in various diseases. Manipulating Ca2+signaling can thus be explored as a potential therapeutic strategy. Nanocarriers containing Ca2+modulators offer a promising means to adjust intracellular oxidative stress by altering mitochondrial Ca2+levels, thereby influencing macrophage polarization. An in-depth understanding of Ca2+signaling mechanisms opens up new avenues for developing Ca2+-based therapeutics (Table 1).
Effect of Mitochondrial Quality Control on Macrophage Polarization
Mitophagy Regulates Macrophage Inflammatory Responses
Mitophagy is a selective process that removes damaged mitochondria from cells, contributing to cellular homeostasis.119 Recent research highlights its potential to influence macrophage phenotype and modulate immune-inflammatory responses by maintaining mitochondrial balance.120–123 Acrylamide has been shown to promote macrophage polarization toward the M2 phenotype through PINK1-induced mitophagy, enhancing anti-inflammatory responses.120 However, taurine inhibits PINK1-mediated mitophagy, preventing glycolytic shifts and reducing the expression of M1-associated markers, thus minimizing pro-inflammatory polarization while promoting M2 macrophage activity.124 Duan et al explored the effects of AIBP on mitochondrial regulation in atherosclerosis, demonstrating that AIBP induces PINK1/Parkin-mediated mitophagy. This process reduces mitochondrial ROS production, leading to the downregulation of M1 macrophage markers such as COX-2 and iNOS, while increasing the anti-inflammatory marker Arg1. These changes facilitate macrophage polarization toward an anti-inflammatory phenotype and improve atherosclerotic conditions. These findings suggest that enhancing mitophagy could be a promising strategy for preventing and treating diseases associated with macrophage-driven inflammation.125 Given the close relationship between macrophage polarization and mitophagy, targeting this process could offer new avenues for more effective regulation of macrophage polarization in therapeutic applications.
Methods to manipulate mitophagy levels using biomaterials are primarily divided into the following strategy:
Encapsulation of mitophagy-promoting agents. Yang et al developed a precision-targeted nanoscavenger, P@NB, featuring a ROS-responsive poly(l-propionate-ethanolate) core modified with Beclin1 and angiopoietin-2 peptides. Upon encountering high ROS levels in diseased tissues, P@NB rapidly releases nicotinamide adenine dinucleotide (NAD) and Beclin1, both of which stimulate mitophagy. This release restores mitochondrial homeostasis and induces microglial polarization toward the M2 phenotype, facilitating the phagocytosis of amyloid-beta (Aβ). The findings suggest that P@NB improves cognitive function in Alzheimer’s disease (AD) mice by restoring autophagic flux, accelerating Aβ degradation, and reducing excessive inflammatory responses. This multi-target approach utilizes synergistic effects to induce autophagy and mitophagy, thereby normalizing mitochondrial dysfunction, making it a promising therapeutic strategy for AD treatment.126
Mitochondrial Dynamics Regulates Macrophage Inflammatory Responses
Mitochondrial dynamics play a crucial role in maintaining cellular health,127 and alterations in mitochondrial fusion and fission have been implicated in various diseases.128–130 Research into these processes holds potential therapeutic value, particularly for inflammatory diseases. The relationship between macrophage polarization and mitochondrial dynamics is of significant interest, as it has been shown to influence disease progression.
Mitochondrial morphology has been linked to macrophage polarization. M1-type macrophages exhibit increased mitochondrial fragmentation, characterized by shortened network branching, low membrane potential, and elevated phosphorylation of DRP1. Reducing DRP1 activation can mitigate mitochondrial over-fragmentation and dysfunction.131,132 M2-type macrophages display enhanced mitochondrial fusion, forming larger networks with elongated branches. These macrophages also show higher efficiencies in electron transport chain (ETC) activity and oxidative phosphorylation (OXPHOS), which supports their anti-inflammatory properties.133 Gao et al demonstrated that the proteins MFN1, MFN2, and OPA1 promote mitochondrial fusion, and their knockdown leads to mitochondrial fission, which in turn impacts macrophage anti-tumor immunity. Their work on bone marrow-derived macrophages revealed distinct differences in mitochondrial morphology across various polarization states.134 Furthermore, research utilizing confocal microscopy, structured illumination microscopy, and transmission electron microscopy to examine the mitochondrial morphology of in vitro cultured bone marrow-derived macrophages showed that M1 and M2b polarized macrophages exhibited highly fragmented, discrete mitochondria, while M2a and M2c macrophages displayed elongated and interconnected mitochondrial networks.135 In sepsis models and patients with acute hypoxia syndrome, an imbalance in macrophage mitochondrial dynamics was observed, with modulation of mitochondrial dynamics shown to inhibit polarization towards pro-inflammatory phenotypes.136 PGAM5-Drp1 signaling has been identified as a key factor promoting macrophage polarization towards pro-inflammatory states and metabolic reprogramming. This suggests that PGAM5 signaling plays a critical role in linking altered mitochondrial dynamics to macrophage inflammatory responses, positioning it as a potential therapeutic target for inflammatory diseases.45
Wei et al found that the down-regulation of mitochondrial fusion protein 2 (Mfn2) expression in microglia leads to an imbalance between mitochondrial fusion and fission, causing aggravation of the cGas-Sting signaling pathway, which leads to microglial M1 polarization and exacerbation of inflammatory damage after spinal cord injury. MASM7 was found to promote Mfn2 expression. To deliver MASM7, the researchers developed a biomimetic microglial nanoparticle strategy, referred to as MSNs-MASM7@MI. In vitro experiments showed that MSNs-MASM7@MI was non-toxic and efficiently delivered MASM7. In vivo studies demonstrated that MSNs-MASM7@MI reduced the inflammatory response and improved neurological function after spinal cord injury. These findings provide new insights and potential therapeutic targets for spinal cord injury treatment.137
Effect of Mitochondrial Translocation on Macrophage Polarization
Mitochondrial transfer can be considered an extension of intracellular mitochondrial motility and intercellular communication, playing a critical role in restoring the respiratory function of recipient cells and enhancing their survival.138,139 This process has a regulatory effect on macrophage function.140 Upon receiving mitochondria, macrophages typically either reuse or degrade them. Mitochondrial reuse occurs through mitochondrial fusion, which also enhances oxidative phosphorylation (OXPHOS) levels in these macrophages. Alternatively, mitochondrial degradation is mediated by mitophagy.141–144 These findings suggest that targeting mitochondrial transfer could provide a potential therapeutic strategy for diseases associated with macrophage polarization.
Mesenchymal stem cells(MSCs) activate mitochondrial biosynthesis via PGC-1α and modulate lysosome-autophagy pathways through PGC-1α/TFEB signaling, facilitated by mitochondrial transfer. This process enhances mitochondrial function in macrophages, reducing ROS production, increasing ATP generation, and promoting polarization of M2-type macrophages. Simultaneously, it reduces the proportion of pro-inflammatory M1-type macrophages, thereby reducing inflammatory responses and alleviating kidney injury in mice.145 MSCs also promote macrophage polarization towards an anti-inflammatory phenotype and improve lung injury through extracellular vesicle (EV)-mediated mitochondrial transfer.146 These findings offer valuable insights for developing therapeutic strategies that harness mitochondrial transfer, potentially improving cell-based therapies or leading to cell-free approaches for treating inflammation-related diseases147 (Figure 5).
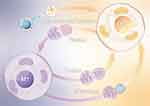 |
Figure 5 Overview of mitochondrial quality control, mitochondrial transfer and macrophage polarization. |
Methods for regulating mitochondrial transfer using biomaterials generally focus on the following approaches. Research has shown that iron oxide nanoparticles (IONPs) can selectively enhance mitochondrial transfer from human mesenchymal stem cells (hMSCs) to diseased cells. This enhancement occurs because ionized IONPs promote the formation of gap junction channels containing Cx43. In a mouse lung fibrosis model, IONP-engineered hMSCs significantly mitigated fibrosis progression by facilitating mitochondrial transfer. As a biocompatible material, IONPs increased the mitochondrial transfer capability of hMSCs by boosting the expression of Cx43, offering a novel approach for utilizing mitochondrial transfer in disease treatment.147 Utilizing photothermal nanoknife technology, the research team developed the “MitoPunch”, a pressure-driven device capable of simultaneously delivering isolated mitochondria to multiple target mammalian cells.148
The field of intracellular mitochondrial transfer is advancing rapidly, driven by significant improvements in biomaterials and technologies facilitating mitochondrial transfer. However, the application of biomaterials for treating macrophage-polarizing diseases remains underexplored, particularly from the perspective of intercellular mitochondrial transfer. Several critical questions warrant further investigation, including whether mitochondrial transfer occurs within human macrophages, under what physiological or pathophysiological conditions; what signaling mechanisms govern the delivery or receipt of mitochondria by macrophages; and how intercellular mitochondrial transfer influences the functions of both donor and recipient cells. While the answers to these questions may vary depending on cell type, tissue organization, and contextual factors, they hold the potential to transform our understanding of mitochondrial biology and may unveil novel biological materials for therapeutic and diagnostic applications.
Future Perspectives
Mitochondria, as key organelles of cells, play a central role in cell fate and function. Targeting mitochondria is a promising strategy for the development of novel regulatory materials. As such, an in-depth investigation of their intrinsic connection with the mechanism of macrophage polarisation is of great significance for the development of novel biomaterials. The study provides a systematic and comprehensive overview of the close relationship between various mitochondrial functions and macrophage polarization. As research in this area progresses, it is expected to lead to breakthroughs in understanding the mitochondrial pathways involved in macrophage polarization and the development of novel biomaterials. These advancements could have significant implications for the treatment of inflammation-related diseases.
However, the current study has several limitations. On one hand, most of the preclinical results are based on cell lines and animal models, which differ from human metabolic profiles. Factors such as poor evaluation models and limited understanding of nano-biological interactions further hinder the clinical translation of macrophage nanomedicines. Moreover, there is an inadequate understanding of the potential safety concerns associated with nanomedicines. For nanomedicines targeting mitochondria or cellular energy sources, critical aspects such as long-term safe delivery, biodegradability, pharmacokinetics, and long-term toxicity require thorough investigation. A more systematic and comprehensive approach is needed to bridge the gap from in vitro studies to clinical applications. Furthermore, the unique characteristics of nanomedicines must be clearly distinguished from other emerging medical technologies to ensure their effective development and integration into therapeutic strategies.
Although immunotherapy has emerged as a new strategy in cancer therapy, cancer cells are prone to developing resistance to immune checkpoint inhibitors. Combining mitochondria-targeted drugs with immune checkpoint inhibitors offers a promising strategy to overcome this challenge. This combination regulates macrophage energy metabolism, suppresses the survival and proliferation of cancer cells, and establishes a supportive microenvironment for optimal immune cell function.149,150
Traditional techniques for macrophage identification are time-consuming. Digital or optical microscopy, in contrast, offers simpler and more effective morphological identification, which can present a comprehensive picture of macrophage polarisation. Computer-based morphometric tools, including artificial intelligence and machine learning, offer high accuracy and cost-effectiveness for predictive analyses. These technologies are anticipated to become a focal point in biological research, particularly when integrated with single-cell cytomics, enabling a more detailed understanding of immune cell heterogeneity.
Advancements in modern biotechnology have brought genome editing tools, such as CRISPR/Cas9, to the forefront of research. These tools enable precise macrophage engineering to regulate specific polarization and enhance tissue-specific immunity. The development of innovative biomaterial nanocarriers for the controlled release of CRISPR/Cas9 is essential for achieving dynamic macrophage immunomodulation. Future research should focus on designing novel platforms while integrating these advanced tools to support tissue healing and regeneration effectively.
With continued global investment in research on mitochondria and macrophage polarization, significant breakthroughs in the mitochondrial pathways of macrophage polarization are anticipated. These advancements, coupled with the development of innovative biomaterials, are expected to drive transformative progress. It is anticipated that they will contribute substantially to the treatment of inflammation-related diseases, offering more effective therapeutic options for patients.
Funding
This study was supported by Central Guidance of Local Science and Technology Development Funds [NO. YDZJSX20231A062] and Shanxi Provincial Scientific and Technological Achievement Transformation Guidance Special Program[NO. 202204021301067].
Disclosure
Zui Tian, Xudong Wang, and Shuai Chen contributed equally to this work and should be considered co-first authors. The authors report no conflicts of interest in this work.
References
1. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi:10.1016/j.immuni.2010.05.007
2. Xu W, Zhao X, Daha MR, van Kooten C. Reversible differentiation of pro- and anti-inflammatory macrophages. Mol Immunol. 2013;53(3):179–186. doi:10.1016/j.molimm.2012.07.005
3. Parisi L, Gini E, Baci D, et al. Macrophage polarization in chronic inflammatory diseases: killers or builders. J Immunol Res. 2018;2018:8917804. doi:10.1155/2018/8917804
4. Kolliniati O, Ieronymaki E, Vergadi E, Tsatsanis C. Metabolic regulation of macrophage activation. J Innate Immun. 2022;14(1):51–68. doi:10.1159/000516780
5. Tan N, Jian G, Peng J, Tian X, Chen B. Chishao - Fuzi herbal pair restore the macrophage M1/M2 balance in acute-on-chronic liver failure. J Ethnopharmacol. 2024;328:118010. doi:10.1016/j.jep.2024.118010
6. Chen Q, Zhao Y, Xie C, et al. Therapeutic effect of a novel M1 macrophage-targeted nanodrug in chronic periodontitis mice. Mol Pharm. 2024;21(4):1677–1690. doi:10.1021/acs.molpharmaceut.3c00954
7. Hrčková G, Mačak Kubašková T, Mudroňová D, Jurčacková Z, Ciglanová D. Co-treatment with human leukocyte extract and albendazole stimulates drug’s efficacy and th1 biased immune response in mesocestoides vogae (Cestoda) infection via modulation of transcription factors, macrophage polarization, and cytokine profiles. Pharmaceutics. 2023;15(2):541. doi:10.3390/pharmaceutics15020541
8. Chen Z, Tang L, Luo L, et al. Enhancing the treatment of uncontrolled inflammation through the targeted delivery of TPCA-1-loaded nanoparticles. Pharmaceutics. 2023;15(10):2435. doi:10.3390/pharmaceutics15102435
9. Song J, Peng D, Peng Y, et al. The new pattern for dual NOTCH pathway involving nuclear transcription and mitochondrial regulation supports therapeutic mechanism of 4-butyl benzophenone derivatives against SIRS. Free Radic Biol Med. 2024;223:306–324. doi:10.1016/j.freeradbiomed.2024.07.036
10. Liu C, Liu D, Zhang X, Hui L, Zhao L. Nanofibrous polycaprolactone/amniotic membrane facilitates peripheral nerve regeneration by promoting macrophage polarization and regulating inflammatory microenvironment. Int Immunopharmacol. 2023;121:110507. doi:10.1016/j.intimp.2023.110507
11. Yang X, Wang Q, Shao F, et al. Cell volume regulation modulates macrophage-related inflammatory responses via JAK/STAT signaling pathways. Acta Biomater. 2024;186:286–299. doi:10.1016/j.actbio.2024.07.046
12. Bao-Yuan H, Shu-Ru L, Le-Xin C, et al. Shikonin ameliorated LPS-induced acute lung injury in mice via modulating MCU-mediated mitochondrial Ca(2+) and macrophage polarization. Phytomedicine. 2024;135:156043. doi:10.1016/j.phymed.2024.156043
13. Paccalet A, Badawi S, Pillot B, et al. Deleterious anti-inflammatory macrophage recruitment in early post-infarction phase: unraveling the IL-6/MCP-1/STAT3 axis. JACC Basic Transl Sci. 2024;9(5):593–604. doi:10.1016/j.jacbts.2024.01.019
14. Ma Y, Wang X, Huang X, et al. Radial egg white hydrogel releasing extracellular vesicles for cell fate guidance and accelerated diabetic skin regeneration. Adv Healthc Mater. 2024;13(31):e2400016. doi:10.1002/adhm.202400016
15. Liang M, Wang Q, Zhang S, et al. Polypyridiniums with inherent autophagy-inducing activity for atherosclerosis treatment by intracellularly co-delivering two antioxidant enzymes. Adv Mater. 2024;36(46):e2409015. doi:10.1002/adma.202409015
16. Li T, Li Q, Liu S, et al. Targeted V-type peptide-decorated nanoparticles prevent colitis by inhibiting endosomal TLR signaling and modulating intestinal macrophage polarization. Biomaterials. 2024;314:122843. doi:10.1016/j.biomaterials.2024.122843
17. Bock FJ, Tait S. Mitochondria as multifaceted regulators of cell death. Nat Rev mol Cell Biol. 2020;21(2):85–100. doi:10.1038/s41580-019-0173-8
18. Lotz C, Herrmann J, Notz Q, Meybohm P, Kehl F. Mitochondria and pharmacologic cardiac conditioning-at the heart of ischemic injury. Int J mol Sci. 2021;22(6):3224. doi:10.3390/ijms22063224
19. Zhang H, Yuan Y, Xue H, et al. Reprogramming mitochondrial metabolism of macrophages by miRNA-released microporous coatings to prevent peri-implantitis. J Nanobiotechnol. 2023;21(1):485. doi:10.1186/s12951-023-02244-z
20. Sprent N, Cheung C, Shameer S, Ratcliffe RG, Sweetlove JL, Töpfer N. Metabolic modelling reveals distinct roles of sugars and carboxylic acids in stomatal opening and uncovers unexpected carbon fluxes. Plant Cell. 2024;37:
21. Clayton SA, MacDonald L, Kurowska-Stolarska M, Clark AR. Mitochondria as key players in the pathogenesis and treatment of rheumatoid arthritis. Front Immunol. 2021;12:673916. doi:10.3389/fimmu.2021.673916
22. Park SY, Kim KY, Gwak DS, Shin SY, Jun DY, Kim YH. L-Cysteine mitigates ROS-induced apoptosis and neurocognitive deficits by protecting against endoplasmic reticulum stress and mitochondrial dysfunction in mouse neuronal cells. Biomed Pharmacother. 2024;180:117538. doi:10.1016/j.biopha.2024.117538
23. Lyu Y, Wang T, Huang S, Zhang Z. Mitochondrial damage-associated molecular patterns and metabolism in the regulation of innate immunity. J Innate Immun. 2023;15(1):665–679. doi:10.1159/000533602
24. Yin X, Lin S, Xiong Y, Zhang P, Mei X. Biomimetic nanoplatform with anti-inflammation and neuroprotective effects for repairing spinal cord injury in mice. Mater Today Bio. 2023;23:100836. doi:10.1016/j.mtbio.2023.100836
25. Wang F, Liao W, Li C, Zhu L. Silencing BMAL1 promotes M1/M2 polarization through the LDHA/lactate axis to promote GBM sensitivity to bevacizumab. Int Immunopharmacol. 2024;134:112187. doi:10.1016/j.intimp.2024.112187
26. Ye L, Jiang Y, Zhang M. Crosstalk between glucose metabolism, lactate production and immune response modulation. Cytokine Growth Factor Rev. 2022;68:81–92. doi:10.1016/j.cytogfr.2022.11.001
27. Dong T, Chen X, Xu H, et al. Mitochondrial metabolism mediated macrophage polarization in chronic lung diseases. Pharmacol Ther. 2022;239:108208. doi:10.1016/j.pharmthera.2022.108208
28. Rabbani N, Xue M, Thornalley PJ. Hexokinase-2-linked glycolytic overload and unscheduled glycolysis-driver of insulin resistance and development of vascular complications of diabetes. Int J mol Sci. 2022;23(4):2165. doi:10.3390/ijms23042165
29. De Azevedo RB, Rosa LF, Lacava ZG, Curi R. Gonadectomy impairs lymphocyte proliferation and macrophage function in male and female rats. Correlation with key enzyme activities of glucose and glutamine metabolism. Cell Biochem Funct. 1997;15(4):293–298. doi:10.1002/(SICI)1099-0844(199712)15:4<293::AID-CBF755>3.0.CO;2-1
30. Zhang CJ, Li JM, Xu D, et al. Surface molecularly engineered mitochondria conduct immunophenotype repolarization of tumor-associated macrophages to potentiate cancer immunotherapy. Adv Sci. 2024;11(38):e2403044. doi:10.1002/advs.202403044
31. Zhang J, Li N, Hu X and Passero L Felipe. (2024). Metabolic Reprograming of Macrophages: A New Direction in Traditional Chinese Medicine for Treating Liver Failure. Journal of Immunology Research, 2024(1), 10.1155/jimr/5891381
32. Yan Z, Ji F, Yan R, et al. Reyanning mixture inhibits M1 macrophage polarization through the glycogen synthesis pathway to improve lipopolysaccharide-induced acute lung injury. J Ethnopharmacol. 2024;328:118005. doi:10.1016/j.jep.2024.118005
33. Feng X, Li M, Lin Z, et al. Tetramethylpyrazine promotes axonal remodeling and modulates microglial polarization via JAK2-STAT1/3 and GSK3-NFκB pathways in ischemic stroke. Neurochem Int. 2023;170:105607. doi:10.1016/j.neuint.2023.105607
34. Ma J, Wei K, Liu J, et al. Glycogen metabolism regulates macrophage-mediated acute inflammatory responses. Nat Commun. 2020;11(1):1769. doi:10.1038/s41467-020-15636-8
35. Osada-Oka M, Goda N, Saiga H, et al. Metabolic adaptation to glycolysis is a basic defense mechanism of macrophages for Mycobacterium tuberculosis infection. Int Immunol. 2019;31(12):781–793. doi:10.1093/intimm/dxz048
36. Semba H, Takeda N, Isagawa T, et al. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun. 2016;7:11635. doi:10.1038/ncomms11635
37. Cheng JW, Yu Y, Zong SY, et al. Berberine ameliorates collagen-induced arthritis in mice by restoring macrophage polarization via AMPK/mTORC1 pathway switching glycolytic reprogramming. Int Immunopharmacol. 2023;124(Pt B):111024. doi:10.1016/j.intimp.2023.111024
38. Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. doi:10.1016/j.cmet.2006.05.011
39. Xiao W, Zhu Z, Yu Z, et al. A composite patch loaded with 2-Deoxy Glucose facilitates cardiac recovery after myocardial infarction via attenuating local inflammatory response. Sci Rep. 2024;14(1):20368. doi:10.1038/s41598-024-71473-5
40. Xiao W, Chen M, Zhou W, et al. An immunometabolic patch facilitates mesenchymal stromal/stem cell therapy for myocardial infarction through a macrophage-dependent mechanism. Bioeng Transl Med. 2023;8(3):e10471. doi:10.1002/btm2.10471
41. Xiang M, Chen C, Chen Y, et al. Unexpected inhibitory role of silica nanoparticles on lung cancer development by promoting M1 polarization of macrophages. Int J Nanomed. 2024;19:11087–11104. doi:10.2147/IJN.S472796
42. Ling J, Chang Y, Yuan Z, Chen Q, He L, Chen T. Designing lactate dehydrogenase-mimicking SnSe nanosheets to reprogram tumor-associated macrophages for potentiation of photothermal immunotherapy. ACS Appl Mater Interfaces. 2022;14(24):27651–27665. doi:10.1021/acsami.2c05533
43. Ramesh A, Malik V, Brouillard A, Kulkarni A. Supramolecular nanotherapeutics enable metabolic reprogramming of tumor-associated macrophages to inhibit tumor growth. J Biomed Mater Res A. 2022;110(8):1448–1459. doi:10.1002/jbm.a.37391
44. Yong J, Bischof H, Burgstaller S, et al. Mitochondria supply ATP to the ER through a mechanism antagonized by cytosolic Ca(2). Elife. 2019;8:e49682. doi:10.7554/eLife.49682
45. Bang BR, Miki H, Kang YJ. Mitochondrial PGAM5-Drp1 signaling regulates the metabolic reprogramming of macrophages and regulates the induction of inflammatory responses. Front Immunol. 2023;14:1243548. doi:10.3389/fimmu.2023.1243548
46. Wu X, Xia Y, Dai H, et al. Metabolic control during macrophage polarization by a citrate-functionalized scaffold for maintaining bone homeostasis. Adv Healthc Mater. 2024;13(22):e2400770. doi:10.1002/adhm.202400770
47. Wang F, Zhang S, Vuckovic I, et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab. 2018;28(3):463–475.e4. doi:10.1016/j.cmet.2018.08.012
48. Zheng X, Liu Y, Liu Y, et al. Arginine-assembly as NO nano-donor prevents the negative feedback of macrophage repolarization by mitochondrial dysfunction for cancer immunotherapy. Biomaterials. 2024;306:122474. doi:10.1016/j.biomaterials.2024.122474
49. Zhu Y, Zhang X, Xie S, et al. Oxidative phosphorylation regulates interleukin-10 production in regulatory B cells via the extracellular signal-related kinase pathway. Immunology. 2022;167(4):576–589. doi:10.1111/imm.13554
50. Xie J, Huang Y, Hu X, et al. A constant filgotinib delivery adhesive platform based on polyethylene glycol (PEG) hydrogel for accelerating wound healing via restoring macrophage mitochondrial homeostasis. Small;2024. e2408791. doi:10.1002/smll.202408791
51. Chen K, Ha S, Xu L, et al. Fluorinated hydroxyapatite conditions a favorable osteo-immune microenvironment via triggering metabolic shift from glycolysis to oxidative phosphorylation. J Transl Med. 2024;22(1):437. doi:10.1186/s12967-024-05261-0
52. Chen D, Liang Z, Su Z, et al. Selenium-doped mesoporous bioactive glass regulates macrophage metabolism and polarization by scavenging ROS and promotes bone regeneration in vivo. ACS Appl Mater Interfaces. 2023;15(29):34378–34396. doi:10.1021/acsami.3c03446
53. Song Y, Zhu M, Islam MA, et al. Glutathione peroxidase 3 is essential for countering senescence in adipose remodelling by maintaining mitochondrial homeostasis. Redox Biol. 2024;77:103365. doi:10.1016/j.redox.2024.103365
54. Qiao J, Zhong C, Zhang Q, Yang G, Li S, Jin J. ASA VI controls osteoarthritis in mice by maintaining mitochondrial homeostasis through Sirtuin 3. Int Immunopharmacol. 2024;140:112858. doi:10.1016/j.intimp.2024.112858
55. Teyani RL, Moghaddam F, Moniri NH. ROS-mediated regulation of β2AR function: does oxidation play a meaningful role towards β2-agonist tachyphylaxis in airway obstructive diseases. Biochem Pharmacol. 2024;226:116403. doi:10.1016/j.bcp.2024.116403
56. Tiwari S, Dewry RK, Srivastava R, Nath S, Mohanty TK. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology. 2022;179:22–31. doi:10.1016/j.theriogenology.2021.11.013
57. Lv C, Zeng Q, Qi L, et al. Sodium selenite induces autophagy and apoptosis in cervical cancer cells via mitochondrial ROS-activated AMPK/mTOR/FOXO3a pathway. Antioxidants. 2024;13(8):1004. doi:10.3390/antiox13081004
58. Shi TF, Zhou Z, Jiang WJ, Huang TL, Si JQ, Li L. Hyperglycemia-induced oxidative stress exacerbates mitochondrial apoptosis damage to cochlear stria vascularis pericytes via the ROS-mediated Bcl-2/CytC/AIF pathway. Redox Rep. 2024;29(1):2382943. doi:10.1080/13510002.2024.2382943
59. Teixeira RB, Pfeiffer M, Zhang P, et al. Reduction in mitochondrial ROS improves oxidative phosphorylation and provides resilience to coronary endothelium in non-reperfused myocardial infarction. Basic Res Cardiol. 2023;118(1):3. doi:10.1007/s00395-022-00976-x
60. Gao Y, Huang D, Huang S, Li H, Xia B. Rational design of ROS generation nanosystems to regulate innate immunity of macrophages, dendrtical and natural killing cells for immunotherapy. Int Immunopharmacol. 2024;139:112695. doi:10.1016/j.intimp.2024.112695
61. Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224(2):242–253. doi:10.1016/j.imbio.2018.11.010
62. Formentini L, Santacatterina F, Núñez de Arenas C, et al. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep. 2017;19(6):1202–1213. doi:10.1016/j.celrep.2017.04.036
63. West AP, Brodsky IE, Rahner C, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476–480. doi:10.1038/nature09973
64. Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81(3):457–464. doi:10.1093/cvr/cvn335
65. Dilly S, Romero M, Solier S, Feron O, Dessy C, Slama Schwok A. Targeting M2 macrophages with a novel NADPH oxidase inhibitor. Antioxidants. 2023;12(2):440. doi:10.3390/antiox12020440
66. Halimu G, Zhang Q, Liu L, et al. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J Hazard Mater. 2022;430:128485. doi:10.1016/j.jhazmat.2022.128485
67. Han C, Sheng Y, Wang J, et al. NOX4 promotes mucosal barrier injury in inflammatory bowel disease by mediating macrophages M1 polarization through ROS. Int Immunopharmacol. 2022;104:108361. doi:10.1016/j.intimp.2021.108361
68. Qu R, Peng Y, Xu S, et al. RBPJ knockdown promotes M2 macrophage polarization through mitochondrial ROS-mediated Notch1-Jagged1-Hes1 signaling pathway in uveitis. Inflammation. 2024. doi:10.1007/s10753-024-02053-y
69. Yang BY, Deng GY, Zhao RZ, et al. Porous Se@SiO(2) nanosphere-coated catheter accelerates prostatic urethra wound healing by modulating macrophage polarization through reactive oxygen species-NF-κB pathway inhibition. Acta Biomater. 2019;88:392–405. doi:10.1016/j.actbio.2019.02.006
70. Ye J, Li Q, Zhang Y, et al. ROS scavenging and immunoregulative EGCG@Cerium complex loaded in antibacterial polyethylene glycol-chitosan hydrogel dressing for skin wound healing. Acta Biomater. 2023;166:155–166. doi:10.1016/j.actbio.2023.05.027
71. He S, Li Z, Wang L, et al. A nanoenzyme-modified hydrogel targets macrophage reprogramming-angiogenesis crosstalk to boost diabetic wound repair. Bioact Mater. 2024;35:17–30. doi:10.1016/j.bioactmat.2024.01.005
72. Wei H, Huang H, He H, et al. Pt-Se hybrid nanozymes with potent catalytic activities to scavenge ROS/RONS and regulate macrophage polarization for osteoarthritis therapy. Research. 2024;7:0310. doi:10.34133/research.0310
73. Zhang X, Su W, Chen Y, et al. Bi-functional astaxanthin macromolecular nanocarriers to alleviate dextran sodium sulfate-induced inflammatory bowel disease. Int J Biol Macromol. 2024;256(Pt 2):128494. doi:10.1016/j.ijbiomac.2023.128494
74. Zhou H, Li Z, Jing S, et al. Repair spinal cord injury with a versatile anti-oxidant and neural regenerative nanoplatform. J Nanobiotechnology. 2024;22(1):351. doi:10.1186/s12951-024-02610-5
75. Li J, Fan J, Gao Y, et al. Porous silicon nanocarriers boost the immunomodulation of mitochondria-targeted bovine serum albumins on macrophage polarization. ACS Nano. 2023;17(2):1036–1053. doi:10.1021/acsnano.2c07439
76. Liang M, Yan X. Nanozymes: from new concepts, mechanisms, and standards to applications. Acc Chem Res. 2019;52(8):2190–2200. doi:10.1021/acs.accounts.9b00140
77. Sridhar K, Inbaraj BS, Chen BH. Recent advances on nanoparticle based strategies for improving carotenoid stability and biological activity. Antioxidants. 2021;10(5):713. doi:10.3390/antiox10050713
78. Chen H, Guo Y, Zhang Z, et al. Symbiotic algae-bacteria dressing for producing hydrogen to accelerate diabetic wound healing. Nano Lett. 2022;22(1):229–237. doi:10.1021/acs.nanolett.1c03693
79. Senpradit Y, Wacharasindhu S, Sukwattanasinitt M. Novel highly selective quinoline-based fluorescent chemosensors for quantitative analysis of Cu(II) ion in water and food. Spectrochim Acta A Mol Biomol Spectrosc. 2024;326:125128. doi:10.1016/j.saa.2024.125128
80. Wang S, Qin M, Fan X, et al. The role of metal ions in stroke: current evidence and future perspectives. Ageing Res Rev. 2024;101:102498. doi:10.1016/j.arr.2024.102498
81. Lai Y, Gao FF, Ge RT, Liu R, Ma S, Liu X. Metal ions overloading and cell death. Cell Biol Toxicol. 2024;40(1):72. doi:10.1007/s10565-024-09910-4
82. Wang X, An P, Gu Z, Luo Y, Luo J. Mitochondrial metal ion transport in cell metabolism and disease. Int J mol Sci. 2021;22(14):7525. doi:10.3390/ijms22147525
83. Bravo-Sagua R, Parra V, López-Crisosto C, Díaz P, Quest AF, Lavandero S. Calcium Transport and Signaling in Mitochondria. Compr Physiol. 2017;7(2):623–634.
84. Guan H, Yang H, Yang M, et al. Mitochondrial ferritin protects SH-SY5Y cells against H(2)O(2)-induced oxidative stress and modulates α-synuclein expression. Exp Neurol. 2017;291:51–61. doi:10.1016/j.expneurol.2017.02.001
85. Wang P, Cui Y, Liu Y, et al. Mitochondrial ferritin alleviates apoptosis by enhancing mitochondrial bioenergetics and stimulating glucose metabolism in cerebral ischemia reperfusion. Redox Biol. 2022;57:102475. doi:10.1016/j.redox.2022.102475
86. Abu Bakar ZH, Bellier JP, Yanagisawa D, Kato T, Mukaisho KI, Tooyama I. LC3/FtMt colocalization patterns reveal the progression of FtMt accumulation in nigral neurons of patients with progressive supranuclear palsy. Int J mol Sci. 2022;23(1):537. doi:10.3390/ijms23010537
87. Wang P, Cui Y, Ren Q, et al. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021;12(5):447. doi:10.1038/s41419-021-03725-5
88. Song X, Yang X, Ying Z, Wu K, Liu J, Liu Q. Regulation of mitochondrial energy metabolism by glutaredoxin 5 in the apicomplexan parasite neospora caninum. Microbiol Spectr. 2023;11(1):e0309122. doi:10.1128/spectrum.03091-22
89. Behmoaras J. The versatile biochemistry of iron in macrophage effector functions. FEBS J. 2021;288(24):6972–6989. doi:10.1111/febs.15682
90. Ma H, Shu Q, Li D, et al. Accumulation of intracellular ferrous iron in inflammatory-activated macrophages. Biol Trace Elem Res. 2023;201(5):2303–2310. doi:10.1007/s12011-022-03362-9
91. Zhang J, Jiang J, Wang B, et al. SAP130 released by ferroptosis tubular epithelial cells promotes macrophage polarization via Mincle signaling in sepsis acute kidney injury. Int Immunopharmacol. 2024;129:111564. doi:10.1016/j.intimp.2024.111564
92. Pereira M, Chen TD, Buang N, et al. Acute iron deprivation reprograms human macrophage metabolism and reduces inflammation in vivo. Cell Rep. 2019;28(2):498–511.e5. doi:10.1016/j.celrep.2019.06.039
93. Zhou Y, Que KT, Zhang Z, et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7(8):4012–4022. doi:10.1002/cam4.1670
94. Shi T, Chen Y, Zhou L, et al. Carboxymethyl cellulose/quaternized chitosan hydrogel loaded with polydopamine nanoparticles promotes spinal cord injury recovery by anti-ferroptosis and M1/M2 polarization modulation. Int J Biol Macromol. 2024;275(Pt 1):133484. doi:10.1016/j.ijbiomac.2024.133484
95. Guo P, Dai P, Yang S, et al. Engineered macrophages tune intratumoral cytokines through precisely controlled self-pyroptosis to enhance bladder cancer immunotherapy. Small. 2024;20(13):e2306699. doi:10.1002/smll.202306699
96. Liu Z, Qiao J, Nagy T, Xiong MP. ROS-triggered degradable iron-chelating nanogels: safely improving iron elimination in vivo. J Control Release. 2018;283:84–93. doi:10.1016/j.jconrel.2018.05.025
97. Yang Y, Tian Q, Wu S, et al. Blue light-triggered Fe(2+)-release from monodispersed ferrihydrite nanoparticles for cancer iron therapy. Biomaterials. 2021;271:120739. doi:10.1016/j.biomaterials.2021.120739
98. Lu Y, Fan X, Pan Q, He B, Pu Y. A mitochondria-targeted anticancer copper dithiocarbamate amplifies immunogenic cuproptosis and macrophage polarization. J Mater Chem B. 2024;12(8):2006–2014. doi:10.1039/D3TB02886K
99. Díez-Tercero L, Delgado LM, Bosch-Rué E, Perez RA. Evaluation of the immunomodulatory effects of cobalt, copper and magnesium ions in a pro inflammatory environment. Sci Rep. 2021;11(1):11707. doi:10.1038/s41598-021-91070-0
100. Wang S, Cazelles R, Liao WC, et al. Mimicking horseradish peroxidase and NADH peroxidase by heterogeneous Cu(2+)-modified graphene oxide nanoparticles. Nano Lett. 2017;17(3):2043–2048. doi:10.1021/acs.nanolett.7b00093
101. Solier S, Müller S, Cañeque T, et al. A druggable copper-signalling pathway that drives inflammation. Nature. 2023;617(7960):386–394. doi:10.1038/s41586-023-06017-4
102. Li Y, Liu J, Weichselbaum RR, Lin W. Mitochondria-targeted multifunctional nanoparticles combine cuproptosis and programmed cell death-1 downregulation for cancer immunotherapy. Adv Sci. 2024;e2403520. doi:10.1002/advs.202403520
103. He Y, Ge J, Tombran-Tink J. Mitochondrial defects and dysfunction in calcium regulation in glaucomatous trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2008;49(11):4912–4922. doi:10.1167/iovs.08-2192
104. Zhang L, Qi J, Zhang X, et al. The regulatory roles of mitochondrial calcium and the mitochondrial calcium uniporter in tumor cells. Int J mol Sci. 2022;23(12):6667. doi:10.3390/ijms23126667
105. Zhang D, Wang F, Li P, Gao Y. Mitochondrial Ca(2+) homeostasis: emerging roles and clinical significance in cardiac remodeling. Int J mol Sci. 2022;23(6):3025. doi:10.3390/ijms23063025
106. Perry RJ. Regulation of hepatic lipid and glucose metabolism by INSP3R1. Diabetes. 2022;71(9):1834–1841. doi:10.2337/dbi22-0003
107. Wang CH, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca(2+) homeostasis in the pathophysiology of insulin resistance and type 2 diabetes. J Biomed Sci. 2017;24(1):70. doi:10.1186/s12929-017-0375-3
108. D’Angelo D, Vecellio Reane D, Raffaello A. Neither too much nor too little: mitochondrial calcium concentration as a balance between physiological and pathological conditions. Front Mol Biosci. 2023;10:1336416. doi:10.3389/fmolb.2023.1336416
109. Giorgi C, Danese A, Missiroli S, Patergnani S, Pinton P. Calcium dynamics as a machine for decoding signals. Trends Cell Biol. 2018;28(4):258–273. doi:10.1016/j.tcb.2018.01.002
110. Ferreira JJ, Cassina A, Irigoyen P, et al. Increased mitochondrial activity upon CatSper channel activation is required for mouse sperm capacitation. Redox Biol. 2021;48:102176. doi:10.1016/j.redox.2021.102176
111. Popoiu TA, Maack C, Bertero E. Mitochondrial calcium signaling and redox homeostasis in cardiac health and disease. Front Mol Med. 2023;3:1235188. doi:10.3389/fmmed.2023.1235188
112. Zhou Q, Wang Y, Lu Z, et al. Cx43 acts as a mitochondrial calcium regulator that promotes obesity by inducing the polarization of macrophages in adipose tissue. Cell Signal. 2023;105:110606. doi:10.1016/j.cellsig.2023.110606
113. Gu L, Larson-Casey JL, Carter AB. Macrophages utilize the mitochondrial calcium uniporter for profibrotic polarization. FASEB J. 2017;31(7):3072–3083. doi:10.1096/fj.201601371R
114. Lei X, Tan G, Wang Y, et al. Mitochondrial calcium nanoregulators reverse the macrophage proinflammatory phenotype through restoring mitochondrial calcium homeostasis for the treatment of osteoarthritis. Int J Nanomed. 2023;18:1469–1489. doi:10.2147/IJN.S402170
115. Kang H, Zhang K, Wong D, Han F, Li B, Bian L. Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization. Biomaterials. 2018;178:681–696. doi:10.1016/j.biomaterials.2018.03.007
116. Wang Y, Qian D, Wang X, et al. Biomimetic trypsin-responsive structure-bridged mesoporous organosilica nanomedicine for precise treatment of acute pancreatitis. ACS Nano. 2024;18(29):19283–19302. doi:10.1021/acsnano.4c05369
117. Deng X, Liu T, Zhu Y, et al. Ca & Mn dual-ion hybrid nanostimulator boosting anti-tumor immunity via ferroptosis and innate immunity awakening. Bioact Mater. 2024;33:483–496. doi:10.1016/j.bioactmat.2023.11.017
118. Feng N, Liang L, Fan M, et al. Treating autoimmune inflammatory diseases with an siERN1-nanoprodrug that mediates macrophage polarization and blocks Toll-like receptor signaling. ACS Nano. 2021;15(10):15874–15891. doi:10.1021/acsnano.1c03726
119. Yi J, Wang HL, Lu G, et al. Spautin-1 promotes PINK1-PRKN-dependent mitophagy and improves associative learning capability in an Alzheimer disease animal model. Autophagy. 2024;20:2655–2676. doi:10.1080/15548627.2024.2383145
120. Hung CH, Lin YC, Tsai YG, et al. Acrylamide induces mitophagy and alters macrophage phenotype via reactive oxygen species generation. Int J mol Sci. 2021;22(4):1683. doi:10.3390/ijms22041683
121. Huang J, Zhu T, Rong R, You M, Ji D, Li H. FUN14 domain-containing 1-mediated mitophagy suppresses interleukin-1β production in macrophages. Int Immunopharmacol. 2020;88:106964. doi:10.1016/j.intimp.2020.106964
122. Esteban-Martínez L, Boya P. BNIP3L/NIX-dependent mitophagy regulates cell differentiation via metabolic reprogramming. Autophagy. 2018;14(5):915–917. doi:10.1080/15548627.2017.1332567
123. Zhu W, Wang C, Xue L, et al. The SMYD3-MTHFD1L-formate metabolic regulatory axis mediates mitophagy to inhibit M1 polarization in macrophages. Int Immunopharmacol. 2022;113(Pt A):109352. doi:10.1016/j.intimp.2022.109352
124. Meng L, Lu C, Wu B, et al. Taurine antagonizes macrophages M1 polarization by mitophagy-glycolysis switch blockage via dragging SAM-PP2Ac transmethylation. Front Immunol. 2021;12:648913. doi:10.3389/fimmu.2021.648913
125. Duan M, Chen H, Yin L, et al. Mitochondrial apolipoprotein A-I binding protein alleviates atherosclerosis by regulating mitophagy and macrophage polarization. Cell Commun Signal. 2022;20(1):60. doi:10.1186/s12964-022-00858-8
126. Yang Z, Shi H, Cai G, Jiang S, Hu Z, Wang Z. A reactive oxygen species-responsive targeted nanoscavenger to promote mitophagy for the treatment of alzheimer’s disease. Small. 2023;19(42):e2302284. doi:10.1002/smll.202302284
127. Yuan G, Luo Y, Qian P, He N. Mitochondrial labeling with mulberrin-Cy3: a new fluorescent probe for live cell visualization. Biosensors. 2024;14(9):428. doi:10.3390/bios14090428
128. Jin Y, Wu O, Chen Q, et al. Hypoxia-preconditioned BMSC-derived exosomes induce mitophagy via the BNIP3-ANAX2 axis to alleviate intervertebral disc degeneration. Adv Sci;2024. e2404275. doi:10.1002/advs.202404275
129. Sun M, Li Y, Xu G, et al. Sirt3-mediated Opa1 deacetylation protects against sepsis-induced acute lung injury by inhibiting alveolar macrophage pro-inflammatory polarization. Antioxid Redox Signal. 2024;41(16–18):1014–1030. doi:10.1089/ars.2023.0322
130. Yang L, Wu C, Parker E, et al. Non-invasive photobiomodulation treatment in an Alzheimer disease-like transgenic rat model. Theranostics. 2022;12(5):2205–2231. doi:10.7150/thno.70756
131. Yu W, Wang X, Zhao J, et al. Stat2-Drp1 mediated mitochondrial mass increase is necessary for pro-inflammatory differentiation of macrophages. Redox Biol. 2020;37:101761. doi:10.1016/j.redox.2020.101761
132. Lin AJ, Joshi AU, Mukherjee R, et al. δPKC-mediated DRP1 phosphorylation impacts macrophage mitochondrial function and inflammatory response to endotoxin. Shock. 2022;57(3):435–443. doi:10.1097/SHK.0000000000001885
133. Ning Y, Cai Y, Dai Y, et al. Mitochondrial fusion mediated by mitofusin 1 regulates macrophage mycobactericidal activity by enhancing autophagy. Infect Immun. 2021;89(11):e0030621. doi:10.1128/IAI.00306-21
134. Gao Z, Li Y, Wang F, et al. Mitochondrial dynamics controls anti-tumour innate immunity by regulating CHIP-IRF1 axis stability. Nat Commun. 2017;8(1):1805. doi:10.1038/s41467-017-01919-0
135. Li Y, He Y, Miao K, Zheng Y, Deng C, Liu TM. Imaging of macrophage mitochondria dynamics in vivo reveals cellular activation phenotype for diagnosis. Theranostics. 2020;10(7):2897–2917. doi:10.7150/thno.40495
136. Sun M, Zeng Z, Xu G, et al. Promoting mitochondrial dynamic equilibrium attenuates sepsis-induced acute lung injury by inhibiting proinflammatory polarization of alveolar macrophages. Shock. 2023;60(4):603–612. doi:10.1097/SHK.0000000000002206
137. Wei FL, Wang TF, Wang CL, et al. Cytoplasmic escape of mitochondrial DNA mediated by Mfn2 downregulation promotes microglial activation via cGas-Sting axis in spinal cord injury. Adv Sci. 2024;11(4):e2305442. doi:10.1002/advs.202305442
138. Zampieri LX, Silva-Almeida C, Rondeau JD, Sonveaux P. Mitochondrial Transfer in Cancer: a Comprehensive Review. Int J mol Sci. 2021;22(6):3245. doi:10.3390/ijms22063245
139. Liu D, Gao Y, Liu J, et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct Target Ther. 2021;6(1):65. doi:10.1038/s41392-020-00440-z
140. Chi A, Yang B, Dai H, et al. Stem Leydig cells support macrophage immunological homeostasis through mitochondrial transfer in mice. Nat Commun. 2024;15(1):2120. doi:10.1038/s41467-024-46190-2
141. Zhang TG, Miao CY. Mitochondrial transplantation as a promising therapy for mitochondrial diseases. Acta Pharm Sin B. 2023;13(3):1028–1035. doi:10.1016/j.apsb.2022.10.008
142. Cowan DB, Yao R, Thedsanamoorthy JK, Zurakowski D, Del Nido PJ, McCully JD. Transit and integration of extracellular mitochondria in human heart cells. Sci Rep. 2017;7(1):17450. doi:10.1038/s41598-017-17813-0
143. Pacak CA, Preble JM, Kondo H, et al. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol Open. 2015;4(5):622–626. doi:10.1242/bio.201511478
144. Liu Z, Sun Y, Qi Z, Cao L, Ding S. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci. 2022;12(1):66. doi:10.1186/s13578-022-00805-7
145. Yuan Y, Yuan L, Li L, et al. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1α activation. Stem Cells. 2021;39(7):913–928. doi:10.1002/stem.3375
146. Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–1286. doi:10.1164/rccm.201701-0170OC
147. Huang T, Zhang T, Jiang X, et al. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Sci Adv. 2021;7(40):eabj0534. doi:10.1126/sciadv.abj0534
148. Sercel AJ, Patananan AN, Man T, et al. Stable transplantation of human mitochondrial DNA by high-throughput, pressurized isolated mitochondrial delivery. Elife. 2021;10:e63102. doi:10.7554/eLife.63102
149. Gao Y, Tong H, Li J, et al. Mitochondria-targeted nanomedicine for enhanced efficacy of cancer therapy. Front Bioeng Biotechnol. 2021;9:720508. doi:10.3389/fbioe.2021.720508
150. Gao Y, Xia B. Editorial: mitochondria-targeted nanocarriers for enhanced efficacy of cancer therapy. Front Bioeng Biotechnol. 2022;10:905999. doi:10.3389/fbioe.2022.905999
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

