Back to Journals » Drug Design, Development and Therapy » Volume 19
Model-Informed Precision Dosing of Levamlodipine Besylate in Smoking Patients
Authors Li G , Guan Y, Yang Y, Xiang Q, Chen S, Shao J , Chen Y, Yu X
Received 8 November 2024
Accepted for publication 11 April 2025
Published 24 April 2025 Volume 2025:19 Pages 3193—3207
DOI https://doi.org/10.2147/DDDT.S501762
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Guoxing Li,1,* Ying Guan,2,* YingYing Yang,3 Qiulin Xiang,1 Song Chen,3 Jiaqi Shao,3 Yue Chen,4 Xian Yu1
1Phase I Clinical Trial Center, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Joint Institute of Tobacco and Health, Kunming, Yunnan, People’s Republic of China; 3Chongqing Medical University, Chongqing, People’s Republic of China; 4Daxigou Street Health Service Center, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xian Yu, Email [email protected]
Object: To quantitatively investigate the influence of various factors, including nicotine, demographics, biochemical index, and genetic polymorphisms of PAHs and drug metabolising enzymes, on the steady-state trough concentrations of levamlodipine besylate and its therapeutic effects in smokers. Using models to promote rational and accurate medication dosing in smoking patients when administered as initial monotherapy.
Methods: A prospective study (NCT05126381) enrolled 43 patients receiving levamlodipine monotherapy. Pop PK/PD model of levamlodipine besylate was established to investigate the effects of nicotine concentration, demographics (age, sex, height, weight, BMI), biochemical index (ALT, AST, ALB, UA, eGFR), and the genetic polymorphisms (rs4646903, rs1048943, rs762551, rs12459249, rs776746, rs2740574) on the patients’ steady state trough concentrations and the antihypertensive efficacy (ΔSBP) of levamlodipine besylate after dosing.
Results: The Pop PK/PD model was conducted using the study data of 43 patients. One-compartment model was used to describe the PK characteristics, and the direct effect model was used to describe the antihypertensive effect of levamlodipine besylate. The final Pop PK/PD model showed that the typical value of V = 3521L, CL = 62.6L·h− 1, E0 = 168mmHg, Imax = 31mmHg, IC50 = 1.71ng·mL− 1; eGFR and UA were found in the model had significant effect on the CL of levamlodipine besylate.
Conclusion: Patients with lower eGFR and UA levels exhibited lower CL levels, higher dosages may be considered for initial monotherapy in such patients. The current study tentatively do not show that nicotine concentration and PAHs metabolizing enzymes have significant effect on PK and PD in patients taking the drug. More data may be needed in the future to refine the effects of the above covariates on the PK and PD parameters of the levamlodipine besylate.
Keywords: Levamlodipine besylate, model-informed precision dosing, pop PK/PD model, smoking patients
Graphical Abstract:

Introduction
Levamlodipine besylate is classified as the Calcium Channel Blockers (CCBs), as one of the first-line therapeutic agents for the initial treatment of hypertensive patients in China, which has a long-lasting and stable antihypertensive effect.1 It is well absorbed orally and is not significantly affected by food,2 and metabolized in the human body mainly by hepatic cytochrome P450 (CYP450) enzymes 3A4 / 3A5, and about 90% of the drug is metabolized in the liver to inactive products, and it is excreted in the body in the form of 10% of the prodrug and 60% of the metabolite via the kidneys.3
Polycyclic aromatic hydrocarbons (PAHs) and nicotine in tobacco compounds affect the metabolism and in vivo pharmacological effects of many drugs.4 This class of PAHs significantly induces the hepatic drug enzymes CYP1A1 and CYP1A2, thereby accelerating the metabolism of drugs such as propranolol, haloperidol, and olanzapine5,6 in vivo, and potentially affecting the metabolism of other drugs as well. Nicotine may have an effect on the pharmacological effects of the drug, especially its effects on the cardiovascular system,7 its ability to increase the level of catecholamine release in the body and thus stimulate the central and peripheral nervous system, this stimulation may reduce the original pharmacological effects of the drug.
Parts of literature8–10 had reported that with regular use of medication following medical advice, some patients still had poor control of their blood pressure to the extent that they needed to be treated with other antihypertensive medications for better control. Therefore, it is important to further explore the factors that differentiate the efficacy of patients with different group characteristics after the same initial monotherapy in order to develop an individualised initial treatment regimen. In patients who smoke, it may be necessary to adjust the dosage administered according to the smoking behaviour of the smoker in order to achieve better therapeutic effects and improve the safety of the medication. The genetic polymorphisms of PAHs and nicotine metabolising enzymes may be significant contributors to the considerable inter-individual variability observed in PK and PD parameters11 following drug administration in patients with chronic diseases who smoke. However, there is a paucity of quantitative studies investigating the relationship between these genetic polymorphisms and the PK and PD parameters. This study aims to establish a population PK/PD model that incorporates the genetic polymorphisms of PAHs and nicotine-related metabolising enzymes, in conjunction with demographic and pathological factors. The objective of this study is to develop a population pharmacokinetic/pharmacodynamic model to quantitatively investigate the effects of the aforementioned factors on steady-state blood concentrations and efficacy in smoking patients with chronic diseases. This will be achieved through simulation, which will enable the identification of the optimal dosage to be administered to patients in order to achieve individualised dosing, thus ensuring safe, effective and cost-effective treatment.
Methods
Study Design
A prospective study was conduct in which patients were selected from hospitals and community healthcare organizations. This study was performed in accordance with the principles stated in the Declaration of Helsinki, and it has received approval from the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University, with the approval number: 2021LCYJ094. It has also been registered on ClinicalTrails.gov, with the ClinicalTrials.gov ID: NCT05126. All patients provided written informed consent before participating in this study. All subjects were screened through the inclusion and exclusion criteria of the study protocol, and demographic information, medical history information, personal history, and medication information of the enrolled subjects were recorded. During the study period, a medication information record card was given to the subjects to record the time and dosage, and the number of cigarettes smoked (cigarettes were uniformly provided by investigator).
Subjects were enrolled in the group and underwent the necessary examinations and blood collection in D1 ~ D3. D1: 0 ~ 30 min before drug administration on the same day, PK blood samples of 4mL, blood biochemistry samples of 4mL, genetic blood samples of 2mL were collected. Blood pressure was measured using a standardized mercury sphygmomanometer, with participants seated and after a 5-minute rest period. Measurements were taken in triplicate, and the average of three readings was recorded to ensure accuracy. And a new drug administration information card was collected and distributed. D2 ~ D3: 0 ~ 30 min before drug administration on the same day, PK blood samples of 4mL were collected and blood pressure was measured, and a new drug administration information card was recovered. D2 ~ D3: 0 ~ 30 min before drug administration on the same day, 4mL of PK blood samples were collected and blood pressure values were measured.
Subjects were discharged from the group after completing the last blood sample collection and safety assessment by the investigator.
Inclusion and Exclusion Criteria
Inclusion
(1) Age: 18 ~ 70 years old (including borderline values), gender is not limited; (2) Patients with a previous diagnosis of hypertension, who are on antihypertensive treatment with levamlodipine besylate tablets alone and adhere to the long-term regular medication. (3) Use of a fixed antihypertensive drug regimen within 1 month prior to enrollment, and can continue that dosing regimen after enrollment. (4) Subjects who understand the risks and regulations of the trial and are able to comply with the study protocol, participate voluntarily and sign the informed consent form.
Exclusion
(1) Subjects have combined other drugs with antihypertensive effects or Chinese herbal or proprietary medicines containing antihypertensive components during the study period. (2) Subject has combined drugs with strong inducing or inhibiting effects on CYP3A4 or CYP3A5 enzymes within the first 2 weeks of the study period or during the study period. (3) History of alcohol abuse (drinking more than 14 units of alcohol per week, 1 unit = 350 mL of beer or 44 mL of spirits with 40% alcohol or 150 mL of wine) or prior history of alcohol abuse with current abstinence less than 3 months. (4) Subject has a pathophysiological condition that interferes with drug absorption or a history of surgery that interferes with drug absorption. (5) Subjects who have previously tested positive for HBsAg, HCV or syphilis antibodies. (6) Pregnant and lactating women. (7) Subjects who, in the opinion of the investigator, are not suitable for participation in this trial due to safety or compliance factors.
Data Collection
The following data were collected and recorded using the chart report form (CRF) and medication information diary card: (1) demographic information: including age, gender, height, weight, and race. (2) Medical history data: including current medical history, past history, surgical history; (3) Other data: including smoking history, alcohol consumption history. (4) Medication-related information: name of current medication, dosage, time of medication. (5) Sample collection time. (6) Blood biochemistry results: liver function (ALT, AST, ALB, TBIL), kidney function (Scr, UA, UREA). (7) Metabolic enzyme gene polymorphism results: cigarette PAHs: CYP1A1, CYP1A2; nicotine: CYP2A6; levamlodipine besylate tablets: CYP3A4, CYP3A5; (8) PK: plasma concentrations of the target drug were collected for each subject (3 portions), and plasma concentrations of nicotine (3 portions, only for the smoking group). (9) PD: baseline blood pressure values, follow-up blood pressure values. (10) Information on medication taken during the trial, number of cigarettes smoked per day in the smoking group.
Plasma Concentration Detection
Levamlodipine Besylate Plasma Concentration
After collecting whole blood from the study patients using anticoagulation blood collection tubes containing sodium citrate, the samples were processed under the conditions of sodium lamp, temperature: 4°C, rotational speed: 3000 r/min, centrifugation time: 10 min, and the drug concentration was detected by the Research Center for Innovative Drugs and Excipients Analysis Technology of the School of Pharmacy, Chongqing Medical University, using the HPLC-MS / MS method. The chromatographic conditions were as follows: the chromatographic column was ACE Excel 2C18-PFP (2.1*100 mm); the flow rate was 0.4 mL-min-1; the column temperature was 35.0 °C; the mobile phases were: 0.1% formic acid solution for mobile phase A, 0.1% formic acid acetonitrile solution for mobile phase B, and gradient elution. Mass spectrometry conditions: multi-reaction ion monitoring was used, and ESI positive ionization was used for detection. 100 uL of plasma sample and 400 uL of internal standard working solution (0.1% formic acid acetonitrile solution containing fluoxetine hydrochloride 8.5 ng/mL), mixing and vortexing for 3 min, centrifuged at low temperature for 15 min, and the supernatant was injected into the sample for detection at 2 uL.
Nicotine Plasma Concentration
The detection was performed by LC-MS under the following chromatographic conditions: the column was an ACE Excel 2 C18-PFP column (100×2.1 mm, 1.7 μmm; Waters); the mobile phase A was acetonitrile; and the mobile phase B was 0.1% formic acid in water. Gradient elution was performed. Mass spectrometry was performed in positive ion mode (MRM mode) with capillary voltage of +0.35 kV, ion source temperature of 350 °C, desolventised gas flow rate of 10 L/min, and conical pore gas flow rate of 11 L/min. 100 μL of plasma was transferred to a 1.5 mL centrifuge tube, and 10 μL of the internal standard working solution and 300 μL of ethyl acetate were added to the tube, and then the plasma was vortexed and mixed for 5 min, and centrifuged for 15 min at 13000 rpm. The sample was centrifuged at 13000 rpm for 15 min, and the supernatant was injected into the sample for detection at 2 uL.
Gene Polymorphism Detection
The study samples were collected from 2 mL of whole blood of the study patients using anticoagulation blood collection tubes containing sodium citrate, frozen in a refrigerator at −80°C, and handed over to Shanghai Tianhao Biotechnology Co. The target SNP loci were detected and typed using imLDR typing technology, and the priority of SNP loci selection for genotyping was as follows: loci reported in the literature > functional loci > high mutation rate loci > other loci. The SNP sites for polymorphisms in the metabolizing enzyme genes related to PAHs and nicotine were selected as CYP1A1: rs1048943, rs4646903;12 CYP1A2: rs762551;13 CYP2A6: rs12459249;14 and for drug metabolizing enzyme genes, the following were selected: CYP3A4: rs2740574; CYP3A5: rs776746.15
Pop PK / PD Model Construction
The Pop PK/PD model of levamlodipine besylate was constructed by using the “NLME” in the Phoenix 64 (V 8.3) software of Certara, and the FOCE-ELS was used to estimate the model parameters. The model was constructed by constructing a Pop PK model of levamlodipine besylate, followed by the Pop PK model based on Pop PK model. Since the study was designed for trough concentration sampling, the absorption phase could not be characterized, and the absorption rate constant Ka had little effect on the clearance rate, so it was fixed at the literature value of 0.79.16 Basic model equation with fixed and random effects was as follows:
Note: exp(nV, nCL, nE0, nImax, nIC50): inter-individual variation in V, CL, E0, Imax, IC50 corresponding to random effects in the model; which conforms to a normal distribution with a mean of 0 and variance of ω2.
Covariates of the study were assigned with the following rules: continuous covariate=original value; continuous covariate: male = 0, female = 1; non-smoking patient = 0, smoking patient = 1; rs2740574 phenotype for TT = 0, TC = 1; rs776746 phenotype for CC = 0, TC = 1, TT = 2. The inclusion criteria for covariates were “forward inclusion” and “backward exclusion” to investigate the effects of covariates on PK/PD parameters, with P < 0.05 for forward inclusion and P < 0.01 for backward exclusion. The value of the objective function (OFV) is used as a measure of the overall fit of the model, and the degree of influence of the covariates was assessed by comparing the decreasing values of the model OFV values. Similarly, covariates were screened stepwise to investigate the effect of covariates on PD parameters.
Results
General Information of Patients
The recruitment information for the study participants is presented in Figure 1. A total of 43 patients were included in this study, including 9 in the smoking group and 34 in the non-smoking group. Among them, 16 were males (smoker: 9, non-smoker: 7) and 27 were females (non-smoker). The general data information of the patients is shown in Table 1.
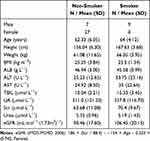 |
Table 1 The General Information of Study Patients |
 |
Figure 1 Recruitment flow of study. The figure illustrates the recruitment process and the number of patients included/excluded in the study and the number of research samples collected of the study. |
Concomitant Diseases and Combined Medications
In addition to the underlying disease of hypertension, some of the patients included in the study had other concomitant diseases including: hyperlipidaemia, hyperuricaemia, cerebral infarction, type 2 diabetes mellitus, and carotid plaque. The patients’ combined medications were mainly glucose-lowering and lipid-lowering medications; the glucose-lowering medications: acarbose, gliclazide, glimepiride, miglitol, selegiline, and metformin; and lipid-lowering medications: atorvastatin calcium. The other combined medications included colchicine and aspirin. Overall the patients’ combined medications had no significant antihypertensive effect and did not significantly induce or inhibit CYP3A4 and CYP3A5 enzymes.
PK/PD Samples
During the study period, each patient collected 3 steady-state blood drug concentration data. A total of 125 steady-state trough concentration data are available for the Pop PK model, with 2 data points at the lower limit of detection were excluded. And 3 nicotine concentrations were collected from each of the smoking patients, and 27 nicotine concentrations were collected from nine smoking patients. Meanwhile, a total of 129 blood pressure values in 43 patients, with SBP as the primary indicator for the evaluation of PD. The results of the patients’ PK/PD Sample of the study period are shown in Table 2.
 |
Table 2 Results of PK/PD Sample of the Study |
Gene Polymorphisms
2 mL of whole blood was collected from each patient for gene polymorphism testing, for a total of 43 study samples collected. The imLDR typing technique was used to detect and type each blood sample. The phenotypic results of the patients’ genes at the SNP loci are shown in Table 3.
 |
Table 3 SNP Locus Genotypes of Patients |
Pop PK/PD Analysis
Base Model
The study data were import the model for analysis, and select the “exponential” model for the random effects model to describe the degree of variation. The population typical values of the base model are shown in Table 4. A one-compartment model is used to describe the pharmacokinetic characteristics of the drug. A direct effect model with baseline values and suppression effects was selected for the PD model of the cohort, and a “proportionality” model was selected to characterize the residual error of the model. The population typical value of the V is 3521 L, the typical value of the CL is 62.59 L·h−1, the typical value of E0 is 168 mmHg, the typical value of Imax = 31.1 mmHg, and the typical value of IC50 = 1.71ng·mL−1, with the OFV was −2604.25 and proportional residual value of the model is 0.22.
 |
Table 4 Parameters of the Pop PK/PD Base Model |
Covariates and Final Model
By screening covariates with stepwise method, it was initially found that ALT and eGFR were significant covariates of CL. None smoking covariates, including nicotine concentration, cigarette metabolic enzyme polymorphism, drug metabolic enzyme polymorphism and other covariates, were found to have significant effects on the PK/PD of levamlodipine besylate.
The final Pop PK/PD model parameters including covariates are shown in Table 5. The text of the equations for the group PK model with covariates included is shown below:
 |
Table 5 Parameters of the Pop PK/PD Final Model |
In the formula, the mean value of UA was 311.0 μmol·L−1; and the mean value of eGFR was 90.5 mL·min−1·(1.73 m2)−1. Typical values of the model with the inclusion of covariates were altered compared to the base model, with a tvV of 3383 L and a tvCL of 58.76 L·h−1. The final model had an OFV of −2617.95, which decreased compared to the base model.
Goodness-of-Fit
The DV vs IPRED, ICWRES vs IPRED and CWRES-QQ plot are used to describe the prediction performance and stability of the model by the degree of fitting. Figures 2–4 shows the final Pop PK model; Figures 5–7 shows the Pop PD model of the final model. The final DV vs IPRED has a good degree of fit, indicating that the model has good enough prediction performance; IWRES vs IPRED has a better zero-line shrinkage trend and a more even distribution; The CWRES-QQ diagram deviates slightly from the reference line at the tail, but overall falls within On the reference line, it is suggested that the conditional weighted residuals of the model mostly conform to the normal distribution.
 |
Figure 2 Pop DV vs IPRED of the final Pop PK model. The figure illustrates the comparison of population observed values and individual predicted values of the final Pop PK model. |
 |
Figure 3 Pop IWRES vs IPRED of the final Pop PK model. The figure illustrates the comparison of individual weighted residuals values and individual predicted values of the final Pop PK model. |
 |
Figure 4 Pop CWERS - QQ of the final Pop PK model. The figure illustrates the quantiles of conditional weighted residuals to the quantiles of a standard normal distribution of the final Pop PK model. |
 |
Figure 5 Pop DV vs IPRED of the final Pop PD model. The figure illustrates the comparison of population observed values and individual predicted values of the final Pop PD model. |
 |
Figure 6 Pop IWRES vs IPRED of the final Pop PD model. The figure illustrates the comparison of individual weighted residuals values and individual predicted values of the final Pop PD model. |
 |
Figure 7 Pop CWERS - QQ of the final Pop PD model. The figure illustrates the quantiles of conditional weighted residuals to the quantiles of a standard normal distribution of the final Pop PD model. |
Bootstrap and VPC
Bootstrap with 1000 resampling times was used to evaluate the stability of the final model. The fitting parameters and Bootstrap results of the final model are shown in Table 6. In the output results, the estimated values of the final model are included in the 95% CI (2.5% ~ 97.5%) range of the calculated parameters, indicating that the final model has good stability. We assume that the patient takes levamlodipine besylate continuously once a day starting from 0h, and uses the VPC graph (Figures 8 and 9) to predict the three trough concentrations and systolic blood pressure valuesafter 7 days (168h, 192h, 216h). Most of the observed values fall within the corresponding centimeters. Within the 90% prediction interval of the quantiles, this indicates that the final model has better prediction performance.
 |
Table 6 Bootstrap Parameters of the Final PK/PD Model |
 |
Figure 8 VPC of the final Pop PK model. The figure illustrates the visual predictive check of the final Pop PK model. |
 |
Figure 9 VPC of the final Pop PD model. The figure illustrates the visual predictive check of the final Pop PD model. |
Discussion
Levamlodipine is the active structural component of amlodipine, which exhibits the advantages of a prolonged antihypertensive effect and a low incidence of adverse effects.17 In the initial antihypertensive treatment of patients with hypertension, a reasonable dose is significant to achieve the standard of blood pressure control. This study employed a “non-linear mixed-effects” model to quantitatively analyse the influence of smoking-related factors, including nicotine concentration, and cigarette metabolising enzyme gene polymorphisms on the PK/PD of levamlodipine besylate. Given that levamlodipine besylate is a long-acting antihypertensive drug with a peak drug concentration of 6~12 h and a half-life of approximately 30~50 h,18 an intensive sampling design would introduce complications and present a significant challenge to the study’s implementation. Consequently, a trough concentration sampling design19 was employed in this investigation. In this study, the Ka values reported in the literature were fixed,16 and the steady-state trough concentration after multiple doses of the drug in patients was employed as the exposure parameter for the purpose of performing a quantitative and quantitative effect relationship analysis. The utilisation of a PK blood sampling design with three steady-state trough concentrations not only reduces the number of blood sampling points, but also allows for a more comprehensive assessment of intra-individual.20 Patients on monotherapy with antihypertensive agents were selected for this study to avoid the confounding effect of combining other antihypertensive drugs on PD outcomes. Furthermore, the distribution of patient medication diary cards during the study period may have effectively improved patient medication adherence and reduced the influence of medication irregularity or missed doses on therapeutic efficacy.
The final PK/PD model showed that the typical value of the V group was 3383 L and the typical value of the CL group was 58.76 L·h−1, which was analogous to the ranges of V and CL in the results of Courlet21 and Ngo.22 SBP was selected as the primary PD indicator in our study because levamlodipine besylate demonstrated a more pronounced effect in reducing SBP in patients. Preliminary findings revealed that the reduction in DBP after administration of levamlodipine besylate was not strongly correlated with the covariates. The Pop PK model exhibited good stability and predictive performance, as indicated by VPC. However, the Pop PD model’s VPC results were less satisfactory than PK, likely due to physiological fluctuations in blood pressure.
Nicotine’s cardiovascular effects may influence the PD of cardiovascular drugs.23–25 In the Pop PK/PD model, nicotine concentration was used as a covariate instead of the number of cigarettes smoked, allowing patients to smoke without restriction. Since polycyclic aromatic hydrocarbons (PAHs) could not be measured directly, polymorphisms in PAH-metabolizing enzymes were included as covariates. However, the model results suggested no significant differences in PK/PD parameters based on nicotine concentration or PAH-related genetic polymorphisms.
Regarding genetic polymorphisms, the National Center for Biotechnology Information (NCBI) database indicates that the reference allele for rs2740574 is C, with substitution alleles C > A, C > G, and C > T.26 The study conducted by Coto27 reported that patients with the GG genotype metabolized drugs faster than those with the CC wild type. However, the limited variability in rs2740574 genotypes in this study precluded a robust assessment of its influence on PK/PD. Similarly, rs776746 polymorphisms in the Chinese population did not significantly affect levamlodipine metabolism.
Levamlodipine and its metabolites are primarily renally excreted,28 making estimated glomerular filtration rate (eGFR) a key determinant of drug clearance. Given the correlation between uric acid levels and eGFR, dose adjustments based on eGFR are more clinically relevant than those based on uric acid levels.29 The established model indicated that a patient with an eGFR of 60 mL·min−1·(1.73 m2)−1 would have a CL approximately 46.94% of the tvCL. Lower eGFR levels result in prolonged drug retention, higher trough concentrations, and sustained antihypertensive effects. For smokers on levamlodipine besylate monotherapy, pretreatment dosing should consider baseline blood pressure and eGFR. Model simulations suggest that for patients with eGFR levels of 90 ~ 120 mL·min−1·(1.73 m2)−1, a daily dose of 2.5 mg is appropriate for those with baseline SBP ≤ 165 mmHg, maintaining SBP below 140 mmHg until the next dose. For baseline SBP > 165 mmHg, a daily dose of 5.0 mg or 2.5 mg combined with another antihypertensive agent may be necessary. Patients with eGFR > 120 mL·min−1·(1.73 m2)−1 may require additional medications due to increased drug clearance, while those with eGFR < 90 mL·min−1·(1.73 m2)−1 may experience prolonged drug exposure without requiring dose reductions, given levamlodipine’s favorable safety profile. However, adverse effects should be monitored during treatment.
This study has several limitations. First, the trough concentration sampling design and fixed Ka values limited the characterization of the drug’s absorption phase and compartmental structure, as well as inter-individual variability in absorption rates. Second, the accuracy of initial parameter estimates is constrained by the small sample size (n=43), which may affect population-typical values and covariate effects as the sample size increases. Additionally, the inclusion of only nine smokers limited the ability to quantitatively assess smoking’s influence on PK/PD parameters. The preliminary model did not reveal significant effects of genetic polymorphisms on PK/PD, suggesting the need for larger studies to better evaluate covariate influences.
Data Sharing Statement
The authors do not intend to share de-identified individual participant data due to concerns regarding participant privacy and confidentiality, as well as restrictions imposed by the ethical review board that approved the study. Sharing this data could compromise the privacy of participants and is not permitted under the current ethical guidelines governing this research.
Funding
Open funding from the Joint Institute of Tobacco and Health; Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu Z, Zheng X, Yang X, Wang E, Wang J. Affinity and specificity of levamlodipine-human serum albumin interactions: insights into its carrier function. Biophys J. 2009;96(10):3917–3925. doi:10.1016/j.bpj.2008.12.3965
2. FDA. FDA approved drug products: conjupri Levamlodipine oral tablets. 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212895s000lbl.pdf.
3. Li X, Wang C, Li T, et al. Bioequivalence of levamlodipine besylate tablets in healthy Chinese subjects: a single-dose and two-period crossover randomized study. BMC Pharmacol Toxicol. 2020;21(1):80. doi:10.1186/s40360-020-00459-6
4. Münzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J. 2020;41(41):4057–4070. doi:10.1093/eurheartj/ehaa460
5. Konstandi M, Johnson EO, Marselos M, Kostakis D, Fotopoulos A, Lang MA. Stress-mediated modulation of B(alpha)P-induced hepatic CYP1A1: role of catecholamines. Chem Biol Interact. 2004;147(1):65–77. doi:10.1016/j.cbi.2003.10.007
6. Desai HD, Seabolt J, Jann MW. Smoking in patients receiving psychotropic medications: a pharmacokinetic perspective. CNS Drugs. 2001;15(6):469–494. doi:10.2165/00023210-200115060-00005
7. Neczypor EW, Mears MJ, Ghosh A, et al. E-cigarettes and cardiopulmonary health. Rev Clinicians Circulation. 2022;145(3):219–232. doi:10.1161/circulationaha.121.056777
8. Bahiru E, de Cates AN, Farr MR, et al. Fixed-dose combination therapy for the prevention of atherosclerotic cardiovascular diseases. Cochrane Database Syst Rev. 2017;3(3):Cd009868. doi:10.1002/14651858.CD009868.pub3
9. Al Dhabyi O, Bakris GL. Initial single-pill blood pressure-lowering therapy: should it be for most people? J Am Heart Assoc. 2017;6(11). doi:10.1161/jaha.117.007760
10. Volpe M, de la Sierra A, Kreutz R, Laurent S, Manolis AJ. ARB-based single-pill platform to guide a practical therapeutic approach to hypertensive patients. High Blood Press Cardiovasc Prev. 2014;21(2):137–147. doi:10.1007/s40292-014-0043-6
11. Duan X, Yang Y, Zhang D, et al. Genetic polymorphisms, mRNA expression levels of telomere-binding proteins, and associates with telomere damage in PAHs-Exposure workers. Chemosphere. 2019;231:442–449. doi:10.1016/j.chemosphere.2019.05.134
12. Sánchez-Siles M, Pelegrín-Hernández JP, Hellin-Meseguer D, et al. Genotype of null polymorphisms in genes GSTM1, GSTT1, CYP1A1, and CYP1A1*2A (rs4646903 T>C)/CYP1A1*2C (rs1048943 A>G) in patients with larynx cancer in Southeast Spain. Cancers. 2020;12(9). doi:10.3390/cancers12092478
13. Mao Y, Yang L, Chen Q, et al. The influence of CYP1A1 and CYP1A2 polymorphisms on stroke risk in the Chinese population. Lipids Health Dis. 2020;19(1):221. doi:10.1186/s12944-020-01370-z
14. Chenoweth MJ, Ware JJ, Zhu AZX, et al. Genome-wide association study of a nicotine metabolism biomarker in African American smokers: impact of chromosome 19 genetic influences. Addiction. 2018;113(3):509–523. doi:10.1111/add.14032
15. Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part II. Clin Pharmacokinet. 2010;49(4):207–221. doi:10.2165/11317550-000000000-00000
16. Kang D, Verotta D, Schwartz JB. Population analyses of amlodipine in patients living in the community and patients living in nursing homes. Clin Pharmacol Ther. 2006;79(1):114–124. doi:10.1016/j.clpt.2005.09.007
17. Kojima S, Shida M, Yokoyama H. Comparison between cilnidipine and amlodipine besilate with respect to proteinuria in hypertensive patients with renal diseases. Hypertens Res. 2004;27(6):379–385. doi:10.1291/hypres.27.379
18. Elliott HL, Meredith PA. The clinical consequences of the absorption, distribution, metabolism and excretion of amlodipine. Postgrad Med J. 1991;67(Suppl 3):S20–3.
19. Punyawudho B, Thammajaruk N, Ruxrungtham K, Avihingsanon A. Population pharmacokinetics and dose optimisation of ritonavir-boosted atazanavir in Thai HIV-infected patients. Int J Antimicrob Agents. 2017;49(3):327–332. doi:10.1016/j.ijantimicag.2016.11.019
20. Cojutti PG, Rinaldi M, Zamparini E, et al. Population pharmacokinetics of dalbavancin and dosing consideration for optimal treatment of adult patients with staphylococcal osteoarticular infections. Antimicrob Agents Chemother. 2023;65(5):110–128. doi:10.1128/aac.02260-20
21. Courlet P, Guidi M, Alves Saldanha S, et al. Population pharmacokinetic modelling to quantify the magnitude of drug-drug interactions between amlodipine and antiretroviral drugs. Eur J Clin Pharmacol. 2021;77(7):979–987. doi:10.1007/s00228-020-03060-2
22. Ngo L, Cho HY, Lee YB. Effects of hydrochlorothiazide and amlodipine on single oral dose pharmacokinetics of valsartan in healthy Korean subjects: population model-based approach. Eur J Pharm Sci. 2018;118:154–164. doi:10.1016/j.ejps.2018.03.031
23. Kaplan NM. Strategies to reduce risk factors in hypertensive patients who smoke. Am Heart J. 1988;115(1 Pt 2):288–294. doi:10.1016/0002-8703(88)90652-7
24. Mallah MA, Changxing L, Mallah MA, et al. Polycyclic aromatic hydrocarbon and its effects on human health: an overeview. Chemosphere. 2022;296:133948. doi:10.1016/j.chemosphere.2022.133948
25. Saino A, Pomidossi G, Perondi R, et al. Effects of amlodipine on coronary hemodynamics and vascular responses to sympathetic stimulation in patients with coronary heart disease. J Cardiovasc Pharmacol. 1994;24(6):875–882.
26. NCBI. Reference SNP (rs) Report Available from: https://www.ncbi.nlm.nih.gov/snp/rs2740574.
27. Coto E, Tavira B, Marín R, et al. Functional polymorphisms in the CYP3A4, CYP3A5, and CYP21A2 genes in the risk for hypertension in pregnancy. Biochem Biophys Res Commun. 2010;397(3):576–579. doi:10.1016/j.bbrc.2010.06.003
28. Jain R, Sinha A, Khan AL. Polyaniline-graphene oxide nanocomposite sensor for quantification of calcium channel blocker levamlodipine. Mater Sci Eng C Mater Biol Appl. 2016;65:205–214. doi:10.1016/j.msec.2016.03.115
29. Srivastava A, Kaze AD, McMullan CJ, Isakova T, Waikar SS. Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis. 2018;71(3):362–370. doi:10.1053/j.ajkd.2017.08.017
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.











