Back to Journals » Drug Design, Development and Therapy » Volume 19
Model-Informed Precision Dosing of Remimazolam in General Anesthesia Patients
Authors Chen Y, Zhang ZJ, Zhang XF, Peng Y, Jiao Z, Wu JX
Received 10 September 2024
Accepted for publication 3 April 2025
Published 16 June 2025 Volume 2025:19 Pages 5099—5109
DOI https://doi.org/10.2147/DDDT.S495604
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Yueting Chen,1,* Zuo-Jing Zhang,2,* Xiao-Feng Zhang,2 Yuan Peng,2 Zheng Jiao,1,* Jing-Xiang Wu2,*
1Department of Pharmacy, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Anesthesiology, Shanghai Chest Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zheng Jiao, Department of Pharmacy, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200030, People’s Republic of China, Email [email protected] Jing-Xiang Wu, Department of Anesthesiology, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Xuhui District, Shanghai, 200030, People’s Republic of China, Tel +86 021-22200000-3302, Fax +86 021-32260806, Email [email protected]
Purpose: This study aimed to characterize the pharmacokinetics/pharmacodynamics (PK/PD) of remimazolam in patients under general anesthesia using a population analysis and to develop a web-based dashboard tool that directly displays the optimal dosing regimen for general anesthesia.
Patients and Methods: A total of 20 patients received remimazolam for general anesthesia, during which intensive arterial blood samples and bispectral index (BIS) values were collected. A population PK/PD model was established, and goodness-of-fit and visual predictive check plots were utilized to evaluate the model’s accuracy. Additionally, RxODE and Shiny in R were used to design a web-based dashboard tool to recommend optimal dosing regimens.
Results: The three-compartment model with first elimination best described the PK profiles of remimazolam. PK parameters were weight-adjusted via allometric scaling. The correlation between drug exposure and the BIS was optimally characterized through an effect compartment model employing an inhibitory sigmoid Emax model. In addition, a web-based dashboard tool was created to offer initial personalized dosing strategies for general anesthesia procedures, enhanced by graphical representations of the PK/PD profiles associated with the recommended dosing regimens.
Conclusion: The developed population PK/PD model effectively captured the dose-exposure-response relationship for remimazolam, allowing for the optimization of personalized dosing strategies.
Keywords: general anesthesia, monte carlo simulation, optimized dosing regimens, population pharmacokinetics/pharmacodynamics models, remimazolam, web-based dashboard
Introduction
Remimazolam, being a new type of benzodiazepine sedative, is frequently used for the induction and maintenance of anesthesia because of its rapid onset and elimination.1,2 It has a certain advantage in clinical anesthesia applications.3,4 A previous study5 has shown that during non-cardiac surgery, compared with propofol, remimazolam has less effect on hemodynamics during the induction phase of anesthesia, with fewer patients experiencing hypotension. Other studies6,7 have also indicated that during heart valve surgery and coronary artery bypass grafting, remimazolam provides more hemodynamically stable conditions during anesthesia induction compared to propofol, while also reducing the dosage of vasopressors required.
Administered intravenously, remimazolam has a plasma protein-binding ratio of approximately 90% and an apparent volume of distribution of 17.5 L. It is primarily metabolized in the liver by carboxylesterase-1 into inactive metabolites, with less than 1% of remimazolam eliminated through the kidneys.8 This process avoids the significant involvement of cytochrome P450 enzymes. The terminal half-life of remimazolam is less than one hour. Furthermore, remimazolam produces deep sedative and anesthetic effects that are usually assessed by electroencephalography (EEG) using the bispectral index (BIS) value.9 The BIS value can better monitor the changing state of the cerebral cortex and is considered the most sensitive and accurate objective indicator for evaluating sedative levels.10–12
The necessity for precision dosing of anesthetic drugs in surgery is increasingly recognized. Propofol has seen the development of multi-center population PK/PD models, leading to the construction of target-controlled infusion (TCI) systems.13,14 However, the scope of populations in the establishment of remimazolam’s PK/PD models is still narrow, with a notable absence of research involving Chinese surgery patients. This indicates that there are ongoing challenges in the development of a tailored dosing regimen for remimazolam.
Therefore, our study aims to develop a “fit for purpose” model that describes the PK/PD profiles of remimazolam to lay foundations for a TCI system in patients undergoing general anesthesia. In addition, to establish a convenient tool, we developed a web-based dashboard with the population PK/PD model to recommend optimal dosing regimens.
Materials and Methods
Study Design
Patients
All patients underwent thoracoscopic resection of lung cancer in the lateral decubitus position. The inclusion criteria were as follows: a) age between 18 and 75 years, with no limitations on gender; b) scheduled on an elective basis to undergo thoracic surgery under general anesthesia, with a minimum hospital stay of 3 days; c) expected operative time of 1 to 3 h; d) American Society of Anesthesiologists (ASA) physical status classification of grade I or II; e) body mass index between 18 kg/m2 and 30 kg/m2.
In contrast, the exclusion criteria were: a) allergy to benzodiazepines; b) severe respiratory disease, including acute respiratory infections; c) acute heart failure; d) moderate-to-severe heart valve disease; e) mental illness, long history of psychotropic drug use, or cognitive impairment; f) abnormal liver, kidney, or coagulation function; and g) history of benzodiazepine use, drug abuse, and alcohol abuse within 2 years before the start of the screening period. Detailed inclusion and exclusion criteria are provided in Table S1.
The study, conducted at Shanghai Chest Hospital, strictly followed the ethical principles of the 2013 Declaration of Helsinki. The Ethics Committee (Identifier: IS2152) granted approval for the research protocol. Written informed consent was secured from all participants before their enrollment in the study.
Study Design
This study enrolled a total of 20 patients undergoing general anesthesia. One group consisted of 10 patients who underwent thoracic surgery who were anesthetized with an intravenous injection of 0.2 mg/kg of remimazolam (Heng-rui Pharmaceuticals Co., Ltd., Jiangsu, China), and the depth of anesthesia was maintained with a continuous infusion of propofol (target-controlled at 2 μg/mL). The other group included 10 patients undergoing thoracic surgery, who received an initial dose of remimazolam at 0.25 mg/kg/min, subsequently maintained with a dosage of 1.2 mg/kg/h. During the surgery anesthesiologists adjusted the infusion rate of remifentanil in all 20 patients to within the range of 0.1–0.2 µg/kg/min, based on the patients’ vital signs, clinical experience, and the progress of the surgery. Intensive arterial blood sampling was performed, and the sampling time information is presented in Table 1. Additionally, to monitor the remimazolam response, the BIS values were assessed using EEG (BIS EEG VISTA) for up to 4 h post-dosing in both groups. The targeted BIS value range during surgery was set at 40–60. A flow chart representing the study is shown in Figure 1.
 |
Table 1 Study Design of Included Subjects in the PopPK/PD Analyses |
 |
Figure 1 Flow chart of study design. |
Bioassay
Remimazolam was detected using ultra high-performance liquid chromatography (ACQUITY UPLC system, Waters Corporation, Milford, MA) tandem mass spectrometry (QTRAP 5500, Applied Biosystems, Waltham, MA), a validated method with respect to selectivity, matrix effect, and stability in remimazolam analysis.15 Compounds were separated by a C18 column (Luna® 5 µm C18(2) 100 Å, LC Column 50×2 mm, Phenomenex Company, Tianjin, China) at a flow rate of 0.45 mL/min. The mobile phase included 10 mmol/L (mM) ammonium formate with 0.1% FA in water (A) and 10 mm ammonium formate with 0.1% formic acid (FA) in methanol: acetonitrile (B) in a gradient elution. The column and sampler temperatures were 30°C and 4°C, respectively.
Plasma (50 µL) was protein-precipitated with the above precipitate solution (150 µL) and centrifuged at 4°C and 12,000 rpm for 10 min. Supernatant (20 µL) was diluted by 40% acetonitrile containing 0.1% FA (180 µL); subsequently, 5 µL was injected into the UPLC system. The quantification range for remimazolam was 2.5–2000 ng/mL. The intra- and inter-batch accuracies were 85.0%–115.0%, with a precision of < 15%.
Population Pharmacokinetics and Pharmacodynamics Modeling
The population PK/PD model was constructed utilizing nonlinear mixed-effects modeling software, NONMEM (version 7.5, ICON plc, Ellicott City, MD). The first-order conditional estimation method, incorporating the η-ε interaction approach, was applied throughout the modeling process. For statistical analysis and visualization of data, R (version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria) was leveraged.
Population Pharmacokinetics Modeling
We developed a remimazolam population PK model. Initially, we evaluated one-, two-, and three-compartment models with first-order elimination to ascertain the optimal PK structural model. The between-subject variability (BSV) in PK parameters was modeled using exponential functions, as depicted in equation (Eq. 1).
where  represents the BSV for population parameters of the
represents the BSV for population parameters of the  th individual and it is assumed to follow a normal distribution with be normally distributed with an average of 0 and a variance of ω2.
th individual and it is assumed to follow a normal distribution with be normally distributed with an average of 0 and a variance of ω2.
The residual unexplained variability (RUV) was characterized by examining models that incorporated only proportional errors, purely additive errors, and models that included a mix of both proportional and additive error models.
where  denotes the observed plasma concentration for the
denotes the observed plasma concentration for the  th individual in the
th individual in the  th individual’s dataset, while
th individual’s dataset, while  corresponds to the predicted plasma concentration for the same subject. The residual errors,
corresponds to the predicted plasma concentration for the same subject. The residual errors,  for proportional errors and
for proportional errors and  for additive errors, are each presumed to be normally distributed, both having a mean of 0 and a variance of
for additive errors, are each presumed to be normally distributed, both having a mean of 0 and a variance of  .
.
Continuous variables including weight (WT) and age were standardized to the median values of the population using a power transformation as shown in Eq. 5. WT was incorporated into the PK model using allometric scaling, as shown in Eq. 6:
where  denotes the parameter value of the
denotes the parameter value of the  th subject and
th subject and 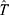 represents the typical parameter value within the population.
represents the typical parameter value within the population.  signifies the influence of covariates on the model parameters.
signifies the influence of covariates on the model parameters.  is the value of the covariate for
is the value of the covariate for 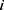 th individual, while
th individual, while  is the median value of the covariate.
is the median value of the covariate.  represents the WT of the
represents the WT of the 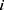 th subject, and
th subject, and  serves as the exponent, with values set to 0.75 for clearance (CL) and 1 for volume.
serves as the exponent, with values set to 0.75 for clearance (CL) and 1 for volume.
Additional variables such as age, gender, albumin, total protein, alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, glutamyl transferase, total bilirubin, direct bilirubin, and serum creatinine were analyzed utilizing a stepwise approach that included both forward selection and backward elimination. A covariate was considered significant if it resulted in a decrease in the objective function value (OFV) by more than 3.84 (χ2 test, P <0.05) during the forward selection phase, and an increase of more than 6.63 (χ2 test, P <0.05) during the backward elimination phase.
The selection of the model was based on an assessment of the OFV, Akaike information criterion (AIC),16 Bayesian information criterion (BIC),17 the accuracy of the parameter estimates, and the biological and pharmacological relevance of the model.
Population PK/PD Modeling
The population PK/PD model was sequentially developed.18,19 Once the population PK model was developed, individual PK parameters were determined using the empirical Bayes estimation approach. Subsequently, these individual parameters were integrated into the overall population PK/PD modeling framework.
The relationship between remimazolam exposure and the BIS value was investigated by examining an effect compartment model with an inhibitory effect, which could be either sigmoidal or non-sigmoidal. The sigmoid  model depicted in Eq. (7):
model depicted in Eq. (7):
where  refers to the BIS value prior to the administration of remimazolam.
refers to the BIS value prior to the administration of remimazolam.  denotes the maximum impact of remimazolam on the BIS for the
denotes the maximum impact of remimazolam on the BIS for the  th individual.
th individual.  signifies the concentration that elicits a half-maximum effect, and
signifies the concentration that elicits a half-maximum effect, and  is the concentration in the effect compartment for the
is the concentration in the effect compartment for the  subject at time
subject at time  . The
. The  coefficient characterizes the steepness of the dose-response curve. The concentrations in the effect compartment are governed by the rate constant for the effect compartment (
coefficient characterizes the steepness of the dose-response curve. The concentrations in the effect compartment are governed by the rate constant for the effect compartment ( ).
).
We evaluated both exponential and additive models to describe BSV. In the case of RUV, we utilized models that included proportional errors, additive errors, and a combination of both proportional and additive error components.
Subsequently, factors such as age, gender, albumin, total protein, alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, glutamyl transferase, total bilirubin, direct bilirubin, and serum creatinine were subjected to a two-step covariate selection process. Initially, they were included in the model using a forward selection method with a chi-squared (χ2) test at a significance level of P<0.05. Following this, a backward elimination approach was applied with the same chi-squared test, but at a more stringent significance level of P<0.01, to refine the model.
The selection of covariates for the population PK/PD model was guided by the OFV, AIC, BIC, the accuracy of the estimated parameters, and the biological relevance of the covariates. Following the screening process for covariates, PK and PD parameters were estimated together to reflect the correlation between drug exposure and therapeutic effect.
Model Evaluation
Diagnostic plots for goodness-of-fit (GOF) were utilized to evaluate the alignment of observed data with the PK/PD model’s predictive outcomes. Furthermore, visual predictive checks (VPCs) were implemented, encompassing 1000 simulations, to gauge the model’s predictive accuracy. The 2.5th, 50th, and 97.5th percentiles from the simulated concentration distributions were scrutinized and juxtaposed against actual observational data. A non-parametric bootstrap approach consisting of 1000 iterations was used to assess the model’s robustness. The 95% confidence intervals (CIs) of the bootstrap samples were determined and compared with the parameter estimates derived from the final model.
Hemodynamic Monitoring
No other sedative drugs were administered to any patient before entering the operating room. Peripheral venous access was established, and radial artery puncture was conducted under local anesthesia. During remimazolam administration, the systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure were continuously monitored using an arterial catheter. HR was continuously monitored using a standard five-lead electrocardiogram. Peripheral oxygen saturation (SpO2) was continually monitored using a pulse oximeter.
Anesthesia was induced using remimazolam, followed by sufentanil and rocuronium for muscle relaxation to facilitate endotracheal intubation. Intraoperative mean arterial pressure and HR were maintained within 20% of baseline values. To raise the blood pressure, ephedrine or phenylephrine was administered intravenously, whereas urapidil or perindopril was administered to lower the blood pressure. Atropine was used to treat bradycardia (HR <50 beats/min), and esmolol was used to treat tachycardia (HR >110 beats/min).
Adverse events were recorded during the operation, including, but not limited to, emergencies during the surgical procedure, surgical errors, and patient reactions. At the same time, a specific description of the adverse event, the cause of its occurrence, and countermeasures were provided. The occurrence of adverse events within 6 h postoperatively, including respiratory and circulatory depression, and postoperative agitation.
Dosing Regimen Design
Under surgical anesthesia, remimazolam was administered according to the measured body weight, a method that can yield varying drug exposures and influence the anesthetic effect in patients. By utilizing a Bayesian approach in conjunction with the established population PK/PD model, this study refined dosage predictions for both induction and maintenance of anesthesia, ensuring the prompt attainment of deep anesthesia.
For the convenience of anesthesiologists, we developed a user-friendly web-based dashboard tool. This tool leverages the R programming language packages RxODE (version 1.1.5) and Shiny (version 1.7.1) for its analytical capabilities. The recommended individual dosing regimens were directly displayed, and the associated concentration-surgery time profile and BIS-surgery time relationships were also visualized using the ggplot2 (version 3.2.1) package in R.
Results
Demographics
Twenty patients who underwent thoracic surgery received remimazolam according to the research protocol. A total of 190 PK observations and 531 BIS values for remimazolam were utilized to develop a population PK/PD model. The study cohort comprised 3 males and 17 females, aged between 27 and 69 years, and weighing between 53 and 71 kg. The demographic profiles of subjects are outlined in Table 2. No significant disparities were observed among patients across the various dosing regimens.
 |
Table 2 Demographic Characteristics of Subjects |
Population Pharmacokinetics and Pharmacodynamics Modeling
Population Pharmacokinetics Modeling
The three-compartment model with first-order elimination exhibited lower OFV, AIC, and BIC value than alternative models, and greater accuracy in the estimation of parameters and variability. Consequently, the structural model that best described PK profiles utilized a three-compartment model with first-order elimination, as illustrated in Figure 2. The RUV of remimazolam was described using a proportional model. Additionally, no covariates were found to affect remimazolam PK. The population PK parameters of remimazolam are summarized in Table 3.
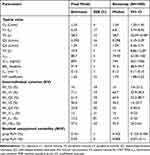 |
Table 3 Final popPK/PD Model Parameters Estimation of Remimazolam |
Population PK/PD Modeling
The population PK/PD model of remimazolam was described using a sigmoid  model. The proportional model was determined to be optimal for capturing the characteristics of RUV. No significant covariates were identified within the PD model. Table 3 also presents the final PD parameters of remimazolam.
model. The proportional model was determined to be optimal for capturing the characteristics of RUV. No significant covariates were identified within the PD model. Table 3 also presents the final PD parameters of remimazolam.
Model Evaluation
GOF plots were utilized to assess the population PK/PD models of remimazolam, as illustrated in Figure 3. The VPC plots, displayed in Figure 4, demonstrated the developed population PK/PD model accurately captured the PK and PD profiles of remimazolam. Figures 3A and 4A are utilized to evaluate the population PK model, while Figures 3B and 4B are employed to assess the population PD model. Table 3 shows the results of the bootstrapping analysis. Parameter estimates of the final model are contained within the 95% CIs obtained from the bootstrap process. The bootstrap’s successful estimation rate of 94.5% suggests that the final model exhibits stability. These assessments confirm that the population PK/PD model is robustly constructed, indicating that the recommended dose is effective in helping anesthesiologists rapidly achieve the target BIS value.
 |
Figure 3 Goodness-of-fit plots of the population PK/PD model. (A) population PK model; (B) population PD model. |
Hemodynamic Monitoring
Ten patients were administered remimazolam for both induction and maintenance of anesthesia, during which blood pressure (Figure 5A) and HR decreased (Figure 5B). Compared to baseline, the maximum decrease in mean arterial pressure was 26±11%, and the maximum decrease in HR was 17±11%. During the surgical procedure, the SpO2 of nine patients was maintained at >95%. For one patient, SpO2 was maintained between 90–95% for 5 min during the one-lung ventilation process and above 95% at all other times (Figure 5C).
No adverse events occurred during the surgery, and no respiratory or circulatory depression or postoperative agitation was observed during the 6-hour follow-up period.
Dosing Regimen Design
The web-based dashboard tool is divided into two parts, including the input and the output sides. On the input side, users must enter the target BIS values, actual surgery time, and patient weight. Population PK and PD parameters can be customized based on different clinical settings, with the initial population PK and PD parameters set as default. On the output side, the tool displays the recommended bolus dose and infusion dose of remimazolam, along with direct visualizations of concentration-surgery time profiles and BIS-surgery time correlations. Furthermore, the online web-based dashboard facilitates access to personalized dosing regimens (https://chen12yue-ting.shinyapps.io/remimazolam-XK/).
Figure S1 presents screenshots of the dashboard, illustrating a scenario for a 60 kg, 40-year-old patient undergoing a 2-hour surgical procedure. On the input side, the target BIS values, actual surgery time, and patient weight had been entered (Figure S1a). To meet surgical anesthesia needs, the BIS was set at 40–60. On the output side, the recommended dosing regimen consisted of an initial bolus dose of 1.1 mg/kg, followed by a continuous infusion at a rate of 0.4 mg/kg/h (Figure S1b). It also shows that the BIS rapidly decreased to levels of 40 and 60, maintaining a consistent level throughout the surgery (Figure S1c).
Discussion
The study characterized the remimazolam PK and PD profiles in patients undergoing surgery and developed a convenient web-based tool for remimazolam to recommend optimal dosage regimen. Furthermore, we also determined that remimazolam administration induced a stable hemodynamic response and with no adverse events observed in patients undergoing general anesthesia.
The PK profiles of remimazolam were similar to those reported in previous studies. Sheng et al20 reported that the remimazolam CL was 1.24 L/min/70 kg in healthy Chinese subjects. Additionally, a previous study21 encompassing 11 Phase I–III trials demonstrated that the remimazolam CL was 1.18 L/min/70 kg, which suggests that ethnicity is not a significant clinical factor affecting PK. Furthermore, the results of these previous studies were similar to those of our study, with a CL of 1.33 L/min/70 kg, showing that remimazolam has stable PK profiles in different populations.
In addition, the PD profiles were found to be consistent with other reported studies. In our study, 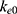 was found to be comparable to that of previous studies [18, 19] (0.13 min−1 vs 0.141 min−1 and 0.13 min−1). However, for
was found to be comparable to that of previous studies [18, 19] (0.13 min−1 vs 0.141 min−1 and 0.13 min−1). However, for 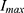 , significant variations were observed among studies. Previous studies22,23 established an
, significant variations were observed among studies. Previous studies22,23 established an  of 39.3 at doses ranging from 0.01 to 0.3 mg/kg, whereas our study observed an
of 39.3 at doses ranging from 0.01 to 0.3 mg/kg, whereas our study observed an 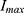 of 80.7 across doses ranging from 0.025 to 0.4 mg/kg at an infusion rate of 1 mg/kg/h. Additionally, Zhou et al22 reported an
of 80.7 across doses ranging from 0.025 to 0.4 mg/kg at an infusion rate of 1 mg/kg/h. Additionally, Zhou et al22 reported an  of 73.3 across infusion rates ranging from 0.5 to 3 mg/kg/h, which is consistent with our study. This difference may be attributed to the different doses used.
of 73.3 across infusion rates ranging from 0.5 to 3 mg/kg/h, which is consistent with our study. This difference may be attributed to the different doses used.
No covariates exerted a significant influence on the remimazolam PK and PD profiles. Previous studies21,22 have identified gender, ASA status, and extracorporeal circulation as factors influencing the CL and central volume of remimazolam. However, our study only included patients in a relatively good physical condition and did not identify significant covariates. This is consistent with the findings of a previous study24 that included only healthy subjects and also did not identify significant covariates. In addition, gender was not a clinically significant factor associated with remimazolam PK/PD.21 Hence, future research should encompass a broader spectrum of the population, such as obese individuals, pediatric and geriatric patients, or those with unique medical conditions, to uncover the factors that may influence remimazolam PK and PD profiles.
The population PK/PD-based dashboard tool is highly convenient for clinical use. Anesthesiologists can enter patient information into the tool to obtain predicted initial dosing regimens prior to surgery. However, initial feedback on its practicality is still being gathered in clinical settings.
During remimazolam administration, hemodynamics remained relatively stable Compared with baseline, the maximum decrease in mean arterial pressure was approximately 26%, which is similar to the maximum decrease of approximately 24% in mean arterial pressure observed in a previous study24 of healthy adults receiving continuous infusion of remimazolam. Previous studies have shown that in healthy adults, continuous infusion of remimazolam alone results in an increase in HR of approximately 28%, which is in contrast to the 17% decrease in HR observed in this study.24 This discrepancy may be attributed to the combined use of opioids, which had a HR-lowering effect, during the induction and maintenance phases of our study.
This study has several limitations. First, we did not investigate the synergistic effect of combined therapy with opioids in the study patients who received remimazolam along with synergistic drugs. Additionally, remimazolam metabolism is influenced by liver function, which may affect the patient’s anesthetic depth and awakening time. Therefore, investigating variability in liver function among patients is also necessary. Lastly, the developed web-based dashboard tool only provides the initial dosing regimen for patients undergoing general anesthesia, and adjustment of remimazolam dosage during the surgical procedure has not yet been achieved.
Conclusion
Our study characterized the population PK/PD profiles of remimazolam in patients undergoing general anesthesia. Furthermore, we developed a web-based dashboard tool based on the established remimazolam population PK/PD model to recommend an individualized initial dosing regimen for general anesthesia. In the future, studies using remimazolam should include a more diverse population and a variety of clinical settings, such as those involving combination drugs, to investigate its population PK/PD profiles.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Shanghai Jiao Tong University School of Medicine Education Development Foundation (HXKT202006) and Shanghai Chest Hospital Basic Cultivation Program for Young Professionals (2023YNTCQ14); Shanghai Jiao Tong University’s “Star of SJTU” Program Fund for Medical-Engineering Interdisciplinary Research (No. YG2024LC10).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang H, Li H, Zhao S, Bao F. Remimazolam in general anesthesia: a comprehensive review of its applications and clinical efficacy. Drug Des Devel Ther. 2024;18:3487–3498. doi:10.2147/dddt.S474854
2. Wegner BM, Wegner GM, Spagnol LW, Costa LA, Spagnol VW, Paiva DF. Comparison between hemodynamic effects of remimazolam and propofol during general anesthesia: a systematic review and meta-analysis. Minerva Anestesiol. 2024;90(10). doi:10.23736/s0375-9393.24.18041-8
3. Sekiguchi R, Kinoshita M, Kawanishi R, Kakuta N, Sakai Y, Tanaka K. Comparison of hemodynamics during induction of general anesthesia with remimazolam and target-controlled propofol in middle-aged and elderly patients: a single-center, randomized, controlled trial. BMC Anesthesiol. 2023;23(1):14. doi:10.1186/s12871-023-01974-9
4. Tang F, Yi JM, Gong HY, et al. Remimazolam benzenesulfonate anesthesia effectiveness in cardiac surgery patients under general anesthesia. World J Clin Cases. 2021;9(34):10595–10603. doi:10.12998/wjcc.v9.i34.10595
5. Choi JY, Lee HS, Kim JY, et al. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: a randomized non-inferiority trial. J Clin Anesth. 2022;82:110955. doi:10.1016/j.jclinane.2022.110955
6. Liu T, Lai T, Chen J, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021;9(5):e00851. doi:10.1002/prp2.851
7. Ju JW, Lee DJ, Chung J, et al. Effect of remimazolam versus propofol on hypotension after anesthetic induction in patients undergoing coronary artery bypass grafting: a randomized controlled trial. J Clin Anesth. 2024;98:111580. doi:10.1016/j.jclinane.2024.111580
8. Freyer N, Knöspel F, Damm G, et al. Metabolism of remimazolam in primary human hepatocytes during continuous long-term infusion in a 3-D bioreactor system. Drug Des Devel Ther. 2019;13:1033–1047. doi:10.2147/dddt.S186759
9. Xin Y, Ma L, Xie T, et al. Comparative analysis of the effect of electromyogram to bispectral index and 95% spectral edge frequency under remimazolam and propofol anesthesia: a prospective, randomized, controlled clinical trial. Front Med. 2023;10:1128030. doi:10.3389/fmed.2023.1128030
10. Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet. 2004;363(9423):1757–1763. doi:10.1016/s0140-6736(04)16300-9
11. Al-Kadi MI, Reaz MB, Ali MA. Evolution of electroencephalogram signal analysis techniques during anesthesia. Sensors. 2013;13(5):6605–6635. doi:10.3390/s130506605
12. Musizza B, Ribaric S. Monitoring the depth of anaesthesia. Sensors. 2010;10(12):10896–10935. doi:10.3390/s101210896
13. Vuyk J, Schnider T, Engbers F. Population pharmacokinetics of propofol for target-controlled infusion (TCI) in the elderly. Anesthesiology. 2000;93(6):1557–1560. doi:10.1097/00000542-200012000-00049
14. Schüttler J, Ihmsen H. Population pharmacokinetics of propofol: a multicenter study. Anesthesiology. 2000;92(3):727–738. doi:10.1097/00000542-200003000-00017
15. Zhou Y, Wang H, Jiang J, Hu P. Simultaneous determination of remimazolam and its carboxylic acid metabolite in human plasma using ultra-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2015;976–977:78–83. doi:10.1016/j.jchromb.2014.11.022
16. Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6(2):165–175. doi:10.1007/bf01117450
17. Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods. 2012;17(2):228–243. doi:10.1037/a0027127
18. Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn. 2003;30(6):387–404. doi:10.1023/b:jopa.0000012998.04442.1f
19. Zhang L, Beal SL, Sheinerz LB. Simultaneous vs. sequential analysis for population PK/PD data II: robustness of methods. J Pharmacokinet Pharmacodyn. 2003;30(6):405–416. doi:10.1023/b:jopa.0000012999.36063.4e
20. Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–391. doi:10.1007/s00228-019-02800-3
21. Zhou J, Curd L, Lohmer LL, et al. Population pharmacokinetics of remimazolam in procedural sedation with nonhomogeneously mixed arterial and venous concentrations. Clin Transl Sci. 2021;14(1):326–334. doi:10.1111/cts.12875
22. Zhou J, Leonowens C, Ivaturi VD, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899. doi:10.1016/j.jclinane.2020.109899
23. Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled Phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115(2):284–296. doi:10.1213/ANE.0b013e318241f68a
24. Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–651. doi:10.1097/aln.0000000000003103
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.











