Back to Journals » International Journal of Nanomedicine » Volume 20
Mucoadhesive-to-Mucopenetrating Nanoparticles for Mucosal Drug Delivery: A Mini Review
Authors Zheng B, Liu D, Qin X, Zhang D, Zhang P
Received 8 November 2024
Accepted for publication 1 February 2025
Published 20 February 2025 Volume 2025:20 Pages 2241—2252
DOI https://doi.org/10.2147/IJN.S505427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Bin Zheng,1,2,* Dingyi Liu,1,* Xiaowen Qin,3,* Dahong Zhang,1 Pu Zhang1
1Urology & Nephrology Center, Department of Urology, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Urology, The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong, 510630, People’s Republic of China; 3Department of Nutrition and Food Hygiene, The National Key Discipline, School of Public Health, Harbin Medical University, Harbin, 15008, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dahong Zhang, Email [email protected]; Pu Zhang, Email [email protected]
Abstract: Mucosal tissue acts as a barrier between the human body’s internal environment and the external world. The mucosal tissue is shielded from injurious environmental chemicals, toxins, and pathogens by a mucus layer lining above the mucosal tissue, and meanwhile the periodic mucosal clearance accelerates the removal of mucoadhesive components. And therefore, transmucosal drug delivery is limited. Nanocarriers for mucosal drug delivery is recently developed to enhance either long retention of drugs within the mucus layer or rapid translocation of drugs across the mucus layer. Among all these types of drug delivery systems, mucoadhesive-to-mucopenetrating nanocarriers transport drugs most efficiently into targeted mucosal tissues. In this review, recent progress on the mucoadhesive-to-mucopenetrating drug delivery systems and their application are updated.
Keywords: nanoparticles, mucoadhesion, mucopenetration, drug delivery
Introduction
Mucous membrane is the main interface between the internal and external environment of the human body, which provides adequate containment of molecules and microorganisms while maintaining the ability to absorb nutrients.1 Mucous membranes cover the eye, nasopharynxes, the pulmonary airway, the gastrointestinal tract, the urogenital tract.2 Upon the mucosal surface there lines a layer of highly viscoelastic mucus secreted by epithelial cells.3 The main component of mucus is water (about 95%), with the remaining parts being glycoproteins, soluble proteins, enzymes, lipids, and immune factors.4 When foreign substances invade, they get trapped in the mucus layer due to their adsorptive interactions with mucinous proteins via van der Waals forces, hydrogen bonds and so forth,2,5 and meanwhile the mucus layer quickly removes out the trapped foreign substances in the following minutes to hours (Table 1).6–19
 |
Table 1 Characteristics of Mucus Lining Above the Different Organ Lumens |
Compared with systemic administration, mucosal local delivery of drugs has the advantages of fewer systemic side effects, targeted delivery to target tissues, and longer drug action time.6 However, mucosally-delivered drugs may get degraded by acid and enzymes, and limited into the superficial mucus layer due to its impermeability.20
Three types of systems open up new possibilities for more efficient mucosal drug delivery17,21 including mucoadhesive delivery systems, mucus-penetrating delivery systems,22 and mucoadhesive-to-mucopenetrating delivery systems.23 For the former two systems, they can either enhance the adhesion of drugs to the mucus layer to prolong their retention or weaken the interaction between drugs and the mucus layer to accelerate their translocation across the mucus layer. Only mucoadhesive-to-mucopenetrating strategy integrates both mucoadhesive delivery systems and mucus-penetrating delivery systems into one entity, which best enhance the concentration of drugs distributed underneath the mucus layer. Currently, mucosal drug delivery systems employing a mucoadhesive-to-mucopenetrating strategy has been explored for various mucosal tissues, including the ocular, nasal, respiratory, gastrointestinal, bladder, and vaginal mucosae. However, only a limited number of studies have successfully integrated both mucoadhesive and mucus-penetrating properties into a single entity. These delivery systems can be categorized into two main types. The first approach involves encapsulating mucus-penetrating drug delivery systems within mucoadhesive formulations, thereby constructing dual-functional drug delivery systems. The second approach entails functionalizing a delivery system with both adhesive and penetrating ligands, enabling it to interact effectively with the mucosal environment. This paper provides a comprehensive review of the mechanism underlying the mucoadhesive-to-mucopenetrating properties, design principles, and applications of such dual-functional delivery systems.
Mechanism of Mucoadhesion and Mucopenetration
In general, mucoadhesive-to-mucopenetrating drug delivery systems consist of mucoadhesive and mucopenetrating formulations. The mucus layer acts as a barrier that prevents nanoparticles from directly reaching the target epithelial tissue. Moreover, the mucus layer gets renewed regularly, leading to the removal of nanoparticles. Therefore, it is crucial to increase the residence time of the drugs in the mucus layer. One of the main strategies is to enhance the adsorptive interactions between nanoparticles and the mucus layer.
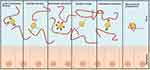
The mucus layer is characterized by a negative charge and hydrophobic properties.24,25 Drugs are surface-engineered with positively charged ligands to induce electrostatic interactions.17 Natali et al developed a delivery system that leverages the electrostatic interaction between cationic polymers and the anionic components on the surface of the nasal mucosa, thereby achieving adhesion to the nasal mucosa.26 Additionally, adhesion can be enhanced through van der Waals forces and hydrogen bonding interactions. In the study by Emily et al, the high flexibility of poly(vinyl alcohol) (PVA) and poly(vinyl pyrrolidone) (PVP) molecular chains allows them to conform to the irregular surface of the mucosa, increasing the number of contact points between the chains and the mucosal surface, thereby enhancing van der Waals forces and improving adhesion.27 The use of expandable foam formulations and the addition of pectin components also enhance adhesion through van der Waals forces.28,29 Furthermore, the hydroxyl groups (-OH) in PVA molecules and the amide groups (-CONH-) in PVP molecules can form hydrogen bonds with polar groups on the mucosal surface (such as carboxyl and hydroxyl groups), thereby strengthening the bond between the polymer and the mucosa. The formation of disulfide bonds is another mechanism that increases adhesion, achieved by forming covalent bonds with cysteine-rich subdomains in the mucus, thus adhering to the mucosa).23,30 Lectin-carbohydrate binding enhances adhesion based on the specific recognition and binding of lectins to glycosylated structures, which also increases binding to tumor cells with abnormal glycosylation.31 Physical entanglement of molecular chains with mucin increases the contact area, which is another way to enhance adhesion. Additionally, hydrophobic interactions enhance the binding force between nanoparticles and mucin by reducing the presence of water molecules between them (Figure 1).32,33
Once drugs become mucoadhesive, they are less mucus-penetrating and merely reach underneath the mucus layer. Mucus-penetrating drugs are generally coated with hydrophilic and net-neutral ligands.34 Coating the surface of delivery systems with densely packed Polyethylene glycol(PEG) chains creates a slippery effect, and the molecular weights of the PEG chains should be adjusted to prevent entanglement within mucus and collapse of the chains on the surface. PEG chains with a molecular weight of 5000–6000 Da are often preferred for this purpose.35,36 In addition, studies have shown that peptosomes self-assembly can also form nanoparticles with the function of penetrating the mucus layer.23,37 For example, peptides formed by hydrolysis of α-lactalbumin (α-lac) can self-assemble into nanotubes or nanoparticles that penetrate the mucous layer through rotational dynamics. Mucolytic enzymes represent other types of mucus-penetrating ligands because they degrade mucous glycoproteins and reduce the steric hindrance. Mucolytic enzymes include papain, bromelain, trypsin.38 The self-propulsion of nanomotors is also a means to enhance penetration through mucus. Zheng et al modified the surface of nanomotors with urease. When used for bladder treatment, the urease decomposed urea to generate propulsion, thereby facilitating the penetration of the nanomotors through the mucus layer.39
Design Principles of Mucoadhesive-to-Mucopenetrating Drug Delivery Systems
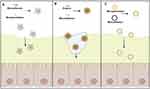
The application of mucoadhesive-to-mucopenetrating systems have been extended to mucosal drug delivery in various tissues (Table 2). 23,26–29,34–36,38,40–46This nano drug delivery system can be roughly divided into three systems. For the first type, a dual-function delivery system can be built by incorporating both mucoadhesive and mucus-penetrating ligands into a single delivery system. (Figure 2A). However, the surface ratio of mucoadhesive ligands and mucus-penetrating ligands should be well modulated. For example, in a delivery system coated with 2-hydroxypropyl methacrylamide(HPMA) as mucoadhesive ligands and polyethylene glycol (PEG) as mucus-penetrating ligands ligand,38 PEG is a hydrophilic substance, while the poly(propylene) backbone in methacrylamide provides sufficient hydrophobicity for the self-assembly of the polymer.47 The results showed that when the feed ratio of mPEG to HPMA was 1:70, the mucoadhesion of the polymer became strong with its mucopenetration weakening. When the feed ratio was adjusted to 1:150, the mucopenetration efficiency of the polymer got higher, but meanwhile its mucoadhesion got weaker.40 To achieve optimal mucoadhesive and mucus-penetrating properties of the polymer, more precise adjustments of the ligands responsible for these functions are required. Specifically, modifying the feed ratio to 1:100 or 1:120 might provide a more balanced and effective combination of mucoadhesion and mucus penetration compared to the previously discussed ratios. The second type of drug delivery system is surface-engineered with mucolytic enzymes and mucoadhesive ligands (Figure 2B).40 In this case, the depletion of the bioactivity of mucolytic enzymes by the fluctuation of pH and temperature may hamper the transmucosal efficiency of the drug delivery systems. And therefore, for example, bromelain or its conjugated delivery systems should be kept in the environment with the pH ranging from 5.5 to 8.0 and the temperature ranging from 50°C to 60°C.48 The optimal pH and temperature range for bromelain activity, as previously described, are crucial for achieving maximal efficacy. It is important to note that bromelain remains stable at temperatures below 40°C. Conversely, exposure to higher temperatures can lead to degradation of the enzyme. Furthermore, the influence of pH and temperature on adhesive ligands should not be overlooked. For instance, the stability of disulfide bonds is significantly reduced under neutral and elevated temperature conditions.23 Alternatively, a dual-functional delivery system could be generated by incorporating mucus-penetrating delivery systems into mucoadhesive formulations (Figure 2C). The release of the mucus-penetrating components from mucoadhesive formulations is a prerequisite for the next mucus-penetrating process and is generally accelerated by the degradation of mucoadhesive formulations. For instance, the integrity of orally administered thiolated microspheres was kept for 4 hours in the gastrointestinal tract, and approximately 40% of α-lac peptosome nanoparticles, encapsulated in the microspheres, got released. As a result, insufficient release of the mucus-penetrating ligands eventually leads to the retention of nanoparticles in the mucus layer and their removal.23 Improving the delivery efficiency of this system is contingent upon strategies to enhance the degradation of the mucoadhesive formulation. One approach could involve decreasing the quantity of mucoadhesive ligands, thereby reducing the stability of the nanoparticle shell. Hydrophilic-polymeric-materials-made nanofibers acted as mucoadhesive formulations to accommodate PEGylated PLGA nanoparticles and could release 85% of them within 30 minutes. The composite nanofibers also reduced the vaginal leakage of their loaded nanoparticles compared with free nanoparticles and therefore prolong the retention time of nanoparticles in vagina 30 times longer after 24 hours.27 Another concern is the steric hindrance exposed to passively diffused nanoparticles in Composite nanofibers, and the size of the liposome has a great influence on its diffusion. In addition, the crosslinking in the composite nanofibers is dynamic, so that the pore size can change to induce more strong release of the nanoparticles.
 |
Table 2 Mucoadhesive-to-Mucopenetrating Systems for Mucosal Drug Delivery |
Application of Mucoadhesive-to-Mucopenetrating Drug Delivery Systems
Ocular Mucosal Delivery
Local administration remains the most commonly used route for drug delivery to tackle ocular diseases. Unlike in other mucus-lined organs, mucins expressed at the ocular surface undergoes a post-secretion reduction of the molecular weight and does not aggregate into large multimers.49 And therefore, ocular mucins do not form a continuous viscous gel layer over the cornea but move with the tear film. An additional factor that limits drug bioavailability is the more frequent tear film turnover (between 0.5 and 2.2 µL/min under normal conditions in human) after topical administration, causing a rapid clearance (within 1–2 min) of the drug molecules via the nasolacrimal drainage.50 It has been estimated that 60% of the active ingredient can be eliminated two minutes after ocular administration, and only 1/1000 remained after 8 minutes.51 Moreover, many types of enzymes in the ocular mucus can degrade drugs, including esterase (acetyl, butyl and carboxy cholinesterase) and peptidase.52 Butyl cholinesterase can hydrolyze esters with more than four carbon chain lengths,53 and peptidase catalyzes the peptides from the N-terminal of peptide substrates.54
A variety of drug delivery systems have been investigated and marketed during the past decades including prodrugs, gels, ointments, permeation enhancers and liposomes nanocarriers.55 However, most of these drug delivery systems are not dual-functional. Sanjay et al utilized 2-Hydroxypropyl methacrylamide (HPMA) to interact with mucus polysaccharides via hydrogen bonding, electrostatic adsorption, and Van der Waals interactions, resulting in its strong adhesion to the mucus layer.38 Additionally, polyethylene glycol was linked with HPMA by free radical polymerization technique to induce mucus-penetration of mPEG-bHPMA nanoparticles. Their study demonstrated that the retention time of copolymer nanoparticles on the eye surface was up to 6 hours, and approximately four times higher transmucosal transport of drugs in the copolymer nanoparticles could be observed than that of free moxifloxacin (MOX). The antibacterial effect of copolymer nanoparticles was twofold stronger that of free MOX.
Chen et al synthesized multifunctional block copolymer vesicles via self-assembly of an amphiphilic copolymer, poly(polyethylene glycol methyl ether methacry-late)-co-Nbenzylacrylamide)-block-poly(2-methylthioethyl methacrylate) (P(PEGMA10-co-PBA2)-b-PMTEMA25), and ciprofloxacin hydrochloride (CIP).40 A broad-spectrum antibiotic was loaded into the hydrophilic lumen of the copolymer vesicles. Boric acid groups in phenylboronic acid (PBA) can strongly interact with 1, 2-cis-diol groups of sialic acid, whose abundance is high in the ocular mucus layer, to prolong the retention time. The study also examined mucous penetration of FICT-containing copolymer vesicles, and fluorescence signals from the cornea were observed four hours after topical application. The results showed that the penetration depth of the copolymer vesicles was about twice that of the free Fitc. In a mice model with bacterial keratitis, the antibacterial effect of CIP-loaded vesicles was about 4 times stronger than that of free CIP.
Nasal Mucosal Delivery
The application of intranasal administration dates back centuries and was only limited to treating respiratory infections or seasonal rhinitis. By the end of the 20th century, intranasal administration opens new was gaining traction as an alternative route to treat systemic conditions, such as cardiovascular indications and intranasal to brain (NTB) administration.56 In recent years, systemic nasal drug administration has become an important treatment option. Nasal administration offers several benefits such as bypassing first-pass metabolism, the high permeability of certain drugs in the nasal epithelium, rapid absorption, quick onset of action, improved patient compliance and comfort, and sustained and prolonged effects compared to oral delivery systems.57 Nasal administration is particularly beneficial for acid-sensitive drugs such as peptide hormones or proteins whose bioactivity can be diminished in the gastrointestinal tract, drugs with polar active substances that are not well absorbed orally, and drugs that require enhanced permeation to translocate physiological barriers. However, intranasal mucosal drug delivery also faces challenges. On the one hand, the ciliary clearance mechanism, which is unique to the nasal mucosal layer (clearance time: 20 min), can transport airborne microorganisms, dust and drugs to the throat and further to the gastrointestinal tract. On the other hand, there are active enzymes in the nasal mucosa that may decompose and eliminate the drug.58 To current knowledge, in the nasal mucosa there lie a diverse array of xenobiotic-metabolizing enzymes, including enzymes from the P450-dependent metabolism pathway (eg P450 monooxygenase), Phase I enzymes (such as flavin monooxygenases, aldehyde dehydrogenases, epoxide hydrolases, carboxylesterases, etc)., as well as Phase II enzymes (such as glucuronyl and sulphate transferases, glutathione transferase).59 It is plausible to suggest that these enzymes are also involved in the metabolism of intranasally administered small-molecule drugs, such as opioids, histamines, and corticosteroids.60
To achieve more efficient nasal mucosal drug delivery and to prevent foreign substances from being quickly cleared by the mucus layer, Natalia et al developed polyethylene glycolated(PEGylated) anionic copolymer nanoparticles (PEG-EPO-L100-55) for drug delivery into the nasal mucosa.26 EPO-L100-55 is a synthetic cationic terpolymer composed of N,N-dimethylaminoethyl methacrylate, methyl methacrylate and butyl methacrylate. This polymer interacts electrically with negatively charged mucin to act as a mucoadhesive component, and polyethylene glycol was also anchored to the particle surface to obtain “slippery” properties. Haloperidol was loaded into nanoparticles to compare the anesthetic effect of rats. After 10 minutes of nasal administration, PEG-EPO-L100-55 nanoparticles had about three times the paralyzing effect of EPO-L100-55 compared to unmodified nanoparticles.
Gastrointestinal Mucosal Delivery
Orally treating gastrointestinal diseases has innate advantages, but the gastric environment, active intestinal enzymes and the impermeability of the gastrointestinal surface mucosal layer influence the effectiveness of oral drugs.61,62 Oral administration of drugs must first navigate the harsh acidic (pH 1–2) and enzymatically active environment of the stomach.63 These conditions can rapidly compromise the stability of drug formulations, particularly those containing proteins and peptide drugs. If the target site for drug delivery is the gastric epithelium, for either local or systemic therapy, nanoparticles must traverse a relatively thick (ranging from 40 to 450 μm) and complex gastric mucus layer.64 The gastric mucus forms a bilayer structure, transitioning from an outer loosely adherent, rapidly moving layer to a densely adherent, slowly moving layer near the epithelium, with a pH gradient from the acidic environment of the gastric epithelium to a neutral environment. If the target mucosa is located within the small intestine, nanoparticle formulations must also withstand a pH gradient, as the pH shifts from the acidic environment of the stomach to the alkaline conditions of the duodenum, jejunum, and ileum. In addition to proteins, lipids, and carbohydrates, the gastrointestinal tract harbors a microbiota within the loosely adherent outer mucus layer, which can interact with the surface of nanoparticles and thus affect the efficacy of the delivery system. Furthermore, the continuous digestion, expulsion, and replacement of the mucus layer with newly secreted mucus can also impact the adhesion and penetration of the drug delivery system. The thickness of the mucosal layer in the gastrointestinal tract varies; it is the thickest in the colon (approximately 830 µm thick) and thinner in the small intestine (100–500 µm).13,15,65,66 The clearance times of mucus vary across different regions of the gastrointestinal tract, which has significant implications for the retention time and bioavailability of drug delivery systems. Mucus in the stomach and small intestine may take approximately 6 hours to be completely renewed.67 In contrast, the clearance time for colonic mucus is relatively shorter, requiring about 1 hour.14 It has been reported that the pore size of gastrointestinal mucus ranges from approximately 100 nm to 200 nm.68,69
Liu et al developed bifunctional drug-carrying nanoparticles by utilizing Pluronic F127 (PF127) encapsulated in thiolated chitosan (CS)-modified liposomes.35 The positively charged thiolated CS shell interacted with the negatively charged mucin and sulfonic acid in the intestinal mucin via electrostatic adsorption. Moreover, thiolated CS-modified liposomes have the capability to generate covalent S-S bonds using the free thiol groups present on thiolated CS and the cysteine domain of mucus. This leads to the firm adhesion of liposomes to the mucus layer and an extended residence time on the mucus surface.18,70 To achieve mucus penetration, the hydrophilic, nonionic long-chain polymer PF127 enhances the ability of nanoparticles to penetrate intestinal mucus by approximately fivefold when compared to unmodified nanoparticles. Chen et al and Mohyeldin et al prepared nanoparticles with similar functions using chitosan derivatives and PEG modification and achieved satisfactory results in delivering gemcitabine for breast cancer and sulpiride depressive disorder therapy.41,42 PVMMA (poly (vinyl methyl ether/maleic anhydride)), which is a GRAS (generally recognized as safe) excipient, also has excellent biocompatibility.71 As well, PVMMA has been reported to show strong adhesion with the gastrointestinal tract due to the formation of carboxylic groups deriving from polyanhydride residues. J.M. Irache et al have previously reported nanoparticulate systems based on PVM/MA whether loaded with antigen or loaded with drug all had good bioadhesive property.72 Based on this, Wang et al prepared a PVMMA and mPEG-b-PLA-based nanoplatform (PVMMA/mPEG-b-PLA) through an emulsification method, to produce biodegradable and dual-functional polymeric nanoparticles. After oral administration in mice, the mucus-penetrating amount of PVMMA/mPEG-b-PLA was approximately 1.5 times higher than that of PVMMA-free nanoparticles, and the former was about six times more toxic to intestinal tumor cells than the latter.36
Compared with chemically modified mucoadhesive polysaccharides, natural adhesive polysaccharides are more environmentally friendly and biocompatible. Pectin is a mucoadhesive natural polysaccharides with abundant carboxyl groups,73 which can form adsorptive linking with the intestinal mucus layer by electrostatic attraction. In addition, carriers prepared with pectin could prolong the intestinal retention time of the payload.74 α-lac can be partially hydrolyzed and self-assembled into nanotubes (α-lac-NT). The noteworthy ability of α-lac-NT to penetrate mucus is attributed to their rotational dynamics, which are facilitated by the shear flow and the mesh structure of mucus.75 Yuan et al prepared mucoadhesive low methoxy pectin (LMP) as microgels into which mucus-penetrating α-lac NT was loaded (LMP-NT).28 The mucus-penetration of NT and LMP-NT across the murine intestinal mucus was evaluated, and the fluorescence intensity of LMP-NT- labeled with Cy5 across the mucus was much greater than that delivered by NT at 60 minutes, suggesting that the LMP microgel protected NT from gastric digestion and that the released NT was highly mucus-penetrating. Compared with free capsaicin, LMP-NT nanoparticle-delivered capsaicin can increase the damage caused by Salmonella by approximately twofold.
In a similar way, Zhao et al prepared mucoadhesive thiolated TEMPO-oxidized Konjac glucomannan (sOKGM) microspheres as microgels into which mucus-penetrating α-lac peptosomes was loaded, which successfully reached the target mucosal area after oral administration.75 The microspheres improved the adhesion of the nanoparticles to the mucus, resulting in a fourfold increase in gastrointestinal tract retention compared to that of systems without microspheres. Additionally, all α-lac peptidosomes penetrated the mucus layer within 60 minutes. Li et al also investigated drug delivery to the gastrointestinal mucosa using protein micelles.43 Lysine residues were partially hydrolyzed into amphiphiles, which then self-assembled into lysine nanoparticles. These nanoparticles were loaded into oxidized starch microgels to create a mucoadhesive-to-mucus-penetrating drug delivery system. One hour after oral administration, the drug delivery system covered 63.5% of the mucosal surface and remained there for 12 hours. The transmucosal amount of the lysine nanoparticles in the microgels was approximately twice that of the lysine nanoparticles alone. Sobia et al employed a different approach to achieve dual-functional drug delivery.44 Papain-functionalized thiolated redox micelles (PT-R-Ms) could form disulfide bonds with mucin glycoproteins and cysteine residues. Once the micelles adhered to the mucosal surface, papain gradually degraded the mucus structure by breaking the amide bonds presented in mucin glycoproteins to facilitate the transmucosal transport of drugs.76 However, only in vitro experiments were conducted in this study.
Bladder Mucosal Delivery
Drugs are directly instilled into the bladder cavity through a catheter. Intravesical therapy can be used as adjuvant chemotherapy or immunotherapy after transurethral resection of bladder tumors and is also commonly employed for tackling benign bladder diseases, such as interstitial cystitis, overactive bladder, and urinary incontinence.77 However, its clinical efficacy is limited, evidenced by the fact that it cannot prevent the recurrence and progression of high-risk bladder tumors after transurethral surgery.78 The primary reason is the impermeability presented by the mucus layer and other tissue barrier. The mucus layer serves as the first permeability barrier with a thickness ranging from 10 μm to 20 μm and the pore size larger than 200 nm.79,80 It is rich in negatively charged sulfated polysaccharides, which bind with core proteins.81 The bladder mucous layer differs from that found in other tissue sites, as it lacks a dynamic, continuously secreted mucous gel layer.79 The mucus layer reinforces the resistance against shear forces, acts as a buffer between irritants in urine and the urothelial cell membrane, and prevents the adsorption of bacteria, minerals, and carcinogens with the urothelium.81
Wu et al developed a dual-functional nano-based drug delivery system for intravesical use, in which wheat germ agglutinin (WGA) acted as a mucoadhesive component via linking with glycocalyx and polyethylene glycol was also anchored to the particle surface to obtain “slippery” properties.45 Remarkably, WGA could hydrolyze 2.3-dimethylmaleic anhydride (DMMA) from the nanoparticles under acidic environment to cationize the nanoparticle and afterwards enable them more adhesive to the urinary mucus. In murine models bearing with orthotopic bladder tumors, the nanoparticle-delivered ICG and αPD-L1 could inhibit the tumor growth twofold stronger than the ICG and αPD-L1 delivered by non-WGA-modified nanoparticles.
Vaginal Mucosal Delivery
The vaginal mucosa forms multiple elevations known as rugae, the number of which varies with hormonal changes. The turnover rate of the mucus layer on the vaginal surface is rapid (only a few hours), and its viscosity is also influenced by hormonal levels. In healthy premenopausal women, the vaginal pH is acidic, ranging from 3.8 to 4.2, while in postmenopausal women, the vaginal pH is higher, ranging from 6 to 7.5.82 The maintenance of an acidic vaginal environment depends on the production of lactic acid by lactobacilli, a common bacterium found in the vagina, and this acidic environment provides conditions for environment-responsive drug delivery systems. The vagina has a dense vascular network and a large surface area, and it is subject to the first-pass effect of the uterus. Vaginal mucosal drug delivery can be used to deliver drugs locally to female reproductive organs, such as the vagina itself, cervix, uterus, and even the fallopian tubes.83–85 Li et al developed an expansive hydrogel delivery system,29 composed of propylene glycol liposome nanoparticles (PGLNs), hydroxyethyl cellulose and sodium dodecyl sulfate, and the latter two components could be released under compression induction to form post-expansile hydrogel foam aerosol (PEHFE). Its superior bioadhesion properties lie in the fact that the cellulose skeleton shows the ability to form hydrogen bonds with oligosaccharide side chains of mucin. Additionally, the subsequent expansion of the hydrogel improved mucus permeability by creating a foam-like structure, significantly enhancing the penetration efficiency of the propylene glycol liposome nanoparticles into the mucus. The penetration efficiency of PEHFL was approximately three times greater than that of PGLN.
Conclusions and Future Perspectives
The mucosa covers the largest surface area of the human body that is exposed to the external environment, making it the main gateway for drug absorption via topical administration. The mucus layer acts as the first line of defense against mucosal drug delivery. Numerous nanosized drug delivery systems have been developed to enhance either the adhesion of drugs to the mucus layer or their transmucosal efficiency. Many studies have demonstrated the effectiveness of mucoadhesion-to-mucopenetration strategies for mucosal administration. At the same time, the efficiency of mucosal drug delivery varies among different strategies. For example, two types of ligands are distributed on the surface of the nanoparticles, and their functions are interdependent; therefore, dual-functional delivery systems may be less efficient than integrating a mucus-penetrating delivery system into a mucus adhesion preparation. The development of dual-functional drug delivery systems presents both opportunities and challenges. Achieving optimal adhesion and penetration requires more refined adjustments in the ratio of ligands. This necessitates not only the optimization of experimental protocols but also more rigorous validation. Additionally, the integration of penetrating ligands into adhesive ligands must address the issue of ligand release. The efficiency of penetrating ligand release in current studies remains suboptimal, which could significantly impact the efficacy of the drug delivery system. The regular clearance of the mucus layer limits the penetration time of drug delivery systems. For instance, ocular mucus is cleared within minutes, posing a significant challenge for the design of drug delivery systems employing this strategy. Furthermore, the pH gradient present in the mucus layer of some organs requires nanoparticles to possess robust stability to withstand these conditions. Future research should focus on enhancing the stability and release efficiency of penetrating ligands to improve the overall performance of dual-functional drug delivery systems. Furthermore, it is important to consider the compatibility between different formulations, as well as the impact of factors such as mucoadhesive segment viscosity, loaded transporter softness and size, and interactions between these components on release kinetics. In addition, the biological safety of the composite system also needs investigation to avoid normal cavity blockage. Finally, for clinical translation, it is necessary to strictly control the preparation of the composite system.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (82300868), Medical Technology Plan of Zhejiang Province (grant number: 2022497314), the Natural Science Foundation of Zhejiang Province (grant number: LQ21H160041), the Natural Science Foundation of Zhejiang Province (grant number: LBQ20H050001).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Dan Y, Zhicheng N, Wenyu Z, et al. Mind the particle rigidity: blooms the bioavailability via rapidly crossing the mucus layer and alters the intracellular fate of curcumin. ACS Nano. 2024;18(39):27026–27041. doi:10.1021/acsnano.4c09838
2. Bianca H, Daniel JR, Oliver GH, et al. Mucoadhesion across scales: towards the design of protein-based adhesives. Adv Colloid Interface Sci. 2024;334. doi:10.1016/j.cis.2024.103322
3. Anton NB, Natallia VD, Yury AS. Chemical modification of hyaluronic acid as a strategy for the development of advanced drug delivery systems. Carbohydr Polym. 2024;337. doi:10.1016/j.carbpol.2024.122145
4. Radha K, Suraj F, Diane JB. Mucoadhesive drug delivery systems: a promising non-invasive approach to bioavailability enhancement. Part i: biophysical considerations. Expert Opin Drug Deliv. 2023;20(3). doi:10.1080/17425247.2023.2181331
5. Varsha VN, Pablo C, Constanza R, et al. Buccal delivery of small molecules and biologics: of mucoadhesive polymers, films, and nanoparticles - an update. Int J Pharm. 2023;636. doi:10.1016/j.ijpharm.2023.122789
6. Wang L, Zhou Y, Wu M, et al. Functional nanocarrier for drug and gene delivery via local administration in mucosal tissues. Nanomedicine. 2018;13(1):69–88. doi:10.2217/nnm-2017-0143
7. Leal J, Smyth HDC, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 2017;532(1):555–572. doi:10.1016/j.ijpharm.2017.09.018
8. Mahmoud SA, Jeffrey PP. Upper airway mucin gene expression: a review. Laryngoscope. 2007;117(5). doi:10.1097/MLG.0b013e3180383651
9. Mainardes RM, Urban MCC, Cinto PO, et al. Liposomes and micro/nanoparticles as colloidal carriers for nasal drug delivery. Curr Drug Deliv. 2006;3(3):275. doi:10.2174/156720106777731019
10. Bajka BH, Rigby NM, Cross KL, et al. The influence of small intestinal mucus structure on particle transport ex vivo. Colloids Surf B. 2015:13573–13580. doi:10.1016/j.colsurfb.2015.07.038
11. Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. 2019;157(1):44–53. doi:10.1053/j.gastro.2019.04.011
12. Ngwuluka NC, Choonara YE, Modi G, et al. Design of an interpolyelectrolyte gastroretentive matrix for the site-specific zero-order delivery of levodopa in Parkinson’s disease. AAPS Pharm Sci Tech. 2013;14(2):605–619. doi:10.1208/s12249-013-9945-1
13. Hock N, Racaniello GF, Aspinall S, et al. Thiolated nanoparticles for biomedical applications: mimicking the workhorses of our body. Adv Sci. 2022;9(1). doi:10.1002/advs.202102451
14. Subramanian DA, Langer R, Traverso G. Mucus interaction to improve gastrointestinal retention and pharmacokinetics of orally administered nano-drug delivery systems. J Nanobiotechnology. 2022;20(1). doi:10.1186/s12951-022-01539-x
15. Leichner C, Jelkmann M, Bernkop-Schnürch A. Thiolated polymers: bioinspired polymers utilizing one of the most important bridging structures in nature. Adv Drug Deliv Rev. 2019;151–152191–221. doi:10.1016/j.addr.2019.04.007
16. Ways TMM, Lau WM, Ng KW, et al. Synthesis of thiolated, pegylated and pozylated silica nanoparticles and evaluation of their retention on rat intestinal mucosa in vitro. Eur J Pharm Sci. 2018:122230–122238. doi:10.1016/j.ejps.2018.06.032
17. Zhang P, Wu G, Zhang D, et al. Mechanisms and strategies to enhance penetration during intravesical drug therapy for bladder cancer. J Control Release. 2023:35469–35479. doi:10.1016/j.jconrel.2023.01.001
18. Olmsted SS, Padgett JL, Yudin AI, et al. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81(4):1930–1937. doi:10.1016/S0006-3495(01)75844-4
19. Shapiro RL, Delong K, Zulfiqar F, et al. In vitro and ex vivo models for evaluating vaginal drug delivery systems. Adv Drug Deliv Rev. 2022:191114543. doi:10.1016/j.addr.2022.114543
20. Yumei W, Wei L, Zhenwei H, et al. Multichiral mesoporous silica screws with chiral differential mucus penetration and mucosal adhesion for oral drug delivery. ACS Nano. 2024;18(25). doi:10.1021/acsnano.4c01245
21. Dünnhaupt S, Barthelmes J, Hombach J, et al. Distribution of thiolated mucoadhesive nanoparticles on intestinal mucosa. Int J Pharm. 2011;408(1–2):191–199. doi:10.1016/j.ijpharm.2011.01.060
22. Chen D, Liu J, Wu J, et al. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin Drug Deliv. 2021;18(5):595–606. doi:10.1080/17425247.2021.1854222
23. Zhao R, Du S, Liu Y, et al. Mucoadhesive-to-penetrating controllable peptosomes-in-microspheres co-loaded with anti-mir-31 oligonucleotide and curcumin for targeted colorectal cancer therapy. Theranostics. 2020;10(8):3594–3611. doi:10.7150/thno.40318
24. Amina A, Shumaila A, Alamgeer M, et al. Zeta potential changing self-nanoemulsifying drug delivery systems: a newfangled approach for enhancing oral bioavailability of poorly soluble drugs. Int J Pharm. 2024;655. doi:10.1016/j.ijpharm.2024.123998
25. Lenard ML. Development of the PC-NSAID technology: from contact angle to vazalore®. Drug Discov Today. 2022;28(1). doi:10.1016/j.drudis.2022.103411
26. Porfiryeva NN, Semina II, Salakhov IA, et al. Mucoadhesive and mucus-penetrating interpolyelectrolyte complexes for nose-to-brain drug delivery. Nanomedicine. 2021:37102432. doi:10.1016/j.nano.2021.102432
27. Krogstad EA, Ramanathan R, Nhan C, et al. Nanoparticle-releasing nanofiber composites for enhanced in vivo vaginal retention. Biomaterials. 2017:1441–1516. doi:10.1016/j.biomaterials.2017.07.034
28. Yuan Y, Liu Y, He Y, et al. Intestinal-targeted nanotubes-in-microgels composite carriers for capsaicin delivery and their effect for alleviation of salmonella induced enteritis. Biomaterials. 2022:287121613. doi:10.1016/j.biomaterials.2022.121613
29. Li W, Zhao N, Zhou Y, et al. Post-expansile hydrogel foam aerosol of pg-liposomes: a novel delivery system for vaginal drug delivery applications. Eur J Pharm Sci. 2012;47(1):162–169. doi:10.1016/j.ejps.2012.06.001
30. Köllner S, Dünnhaupt S, Waldner C, et al. Mucus permeating thiomer nanoparticles. Eur J Pharm Biopharm. 2015:97265–97272. doi:10.1016/j.ejpb.2015.01.004
31. Netsomboon K, Bernkop-Schnürch A. Mucoadhesive vs. Mucopenetrating particulate drug delivery. Eur J Pharm Biopharm. 2016;9876–9889. doi:10.1016/j.ejpb.2015.11.003
32. Rossi S, Vigani B, Sandri G, et al. Recent advances in the mucus-interacting approach for vaginal drug delivery: from mucoadhesive to mucus-penetrating nanoparticles. Expert Opin Drug Deliv. 2019;16(8):777–781. doi:10.1080/17425247.2019.1645117
33. Ensign LM, Lai SK, Wang Y, et al. Pretreatment of human cervicovaginal mucus with pluronic f127 enhances nanoparticle penetration without compromising mucus barrier properties to herpes simplex virus. Biomacromolecules. 2014;15(12):4403–4409. doi:10.1021/bm501419z
34. Amidi M, Krudys KM, Snel CJ, et al. Efficacy of pulmonary insulin delivery in diabetic rats: use of a model-based approach in the evaluation of insulin powder formulations. J Control Release. 2008;127(3):257–266. doi:10.1016/j.jconrel.2008.01.019
35. Yanhua L, Tong Y, Shijie W, et al. Mucus adhesion- and penetration-enhanced liposomes for paclitaxel oral delivery. Int J Pharm. 2017;537. doi:10.1016/j.ijpharm.2017.12.044
36. Wang Q, Li C, Ren T, et al. Poly(vinyl methyl ether/maleic anhydride)-doped peg–pla nanoparticles for oral paclitaxel delivery to improve bioadhesive efficiency. Mol Pharm. 2017;14(10):3598–3608. doi:10.1021/acs.molpharmaceut.7b00612
37. Inchaurraga L, Martín-Arbella N, Zabaleta V, et al. In vivo study of the mucus-permeating properties of peg-coated nanoparticles following oral administration. Eur J Pharm Biopharm. 2015:97280–97289. doi:10.1016/j.ejpb.2014.12.021
38. Ch S, Mishra P, Bhatt H, et al. Hydroxypropyl methacrylamide-based copolymeric nanoparticles loaded with moxifloxacin as a mucoadhesive, cornea-penetrating nanomedicine eye drop with enhanced therapeutic benefits in bacterial keratitis. Colloids Surf B. 2021:208112113. doi:10.1016/j.colsurfb.2021.112113
39. Zheng B, Zhang H, Wang J, et al. A mucoadhesive-to-penetrating nanomotors-in-hydrogel system for urothelium-oriented intravesical drug delivery. J Nanobiotechnology. 2024;22(1). doi:10.1186/s12951-024-02816-7
40. Qiumeng C, Xiaopeng H, Lu L, et al. Multifunctional polymer vesicles for synergistic antibiotic-antioxidant treatment of bacterial keratitis. Biomacromolecules. 2023;24(11). doi:10.1021/acs.biomac.3c00754
41. Chen G, Svirskis D, Lu W, et al. N-trimethyl chitosan coated nano-complexes enhance the oral bioavailability and chemotherapeutic effects of gemcitabine. Carbohydr Polym. 2021:273118592. doi:10.1016/j.carbpol.2021.118592
42. Mohyeldin SM, Samy WM, Ragab D, et al. Hybrid lipid core chitosan-tpgs shell nanocomposites as a promising integrated nanoplatform for enhanced oral delivery of sulpiride in depressive disorder therapy. Int J Biol Macromol. 2021:188432–188449. doi:10.1016/j.ijbiomac.2021.08.035
43. Li X, Guo W, Xu R, et al. The interaction mechanism between gold nanoparticles and proteins: lysozyme, trypsin, pepsin, γ-globulin, and hemoglobin. Spectrochimica Acta Part A. 2022:272120983. doi:10.1016/j.saa.2022.120983
44. Razzaq S, Rauf A, Raza A, et al. A multifunctional polymeric micelle for targeted delivery of paclitaxel by the inhibition of the p-glycoprotein transporters. Nanomaterials. 2021;11(11):2858. doi:10.3390/nano11112858
45. Wu X, Wei Y, Lin R, et al. Multi‐responsive mesoporous polydopamine composite nanorods cooperate with nano‐enzyme and photosensitiser for intensive immunotherapy of bladder cancer. Immunology. 2022;167(2):247–262. doi:10.1111/imm.13534
46. Fida S, Jalil A, Habib R, et al. Development of mucus-penetrating iodine loaded self-emulsifying system for local vaginal delivery. PLoS One. 2022;17(3):e0266296. doi:10.1371/journal.pone.0266296
47. Han F, Gao J, Lv G, et al. Magnetic resonance imaging with upconversion nanoprobes capable of crossing the blood-cerebrospinal fluid barrier. J Nanobiotechnology. 2024;22(1). doi:10.1186/s12951-024-02301-1
48. Kumar V, Mangla B, Javed S, et al. Bromelain: a review of its mechanisms, pharmacological effects and potential applications. Food Funct. 2023;14(18):8101–8128. doi:10.1039/D3FO01060K
49. Moonjung C, Anna Ablamowicz T. Regional conjunctival differences in glycocalyx mucin expression in dry eye and normal subjects. Invest Ophthalmol Vis Sci. 2024;65(2). doi:10.1167/iovs.65.2.20
50. Bo T, Evan B, Sean D, et al. Ocular drug delivery: advancements and innovations. Pharmaceutics. 2022;14(9). doi:10.3390/pharmaceutics14091931
51. Yaru W, Changhong W. Novel eye drop delivery systems: advance on formulation design strategies targeting anterior and posterior segments of the eye. Pharmaceutics. 2022;14(6). doi:10.3390/pharmaceutics14061150
52. Lorenzo G, Maria Grazia C, Elisabetta R. Light-responsive polymeric nanoparticles for retinal drug delivery: design cues, challenges and future perspectives. Heliyon. 2024;10(5). doi:10.1016/j.heliyon.2024.e26616
53. Lee VHL, Chang S, Oshiro CM, et al. Ocular esterase composition in albino and pigmented rabbits: possible implications in ocular prodrug design and evaluation. Curr Eye Res. 1985;4(11):1117–1125. doi:10.3109/02713688509003358
54. Stratford RE, Lee VHL. Ocular aminopeptidase activity and distribution in the albino rabbit. Curr Eye Res. 1985;4(9):995–1000. doi:10.3109/02713689509000007
55. Yang Y, Lockwood A. Topical ocular drug delivery systems: innovations for an unmet need. Exp Eye Res. 2022;218109006. doi:10.1016/j.exer.2022.109006
56. Tekade AR, Mittha PS, Pisal CS. Nanostructured lipid carriers for nose to brain delivery targeting cns: diversified role of liquid lipids for synergistic action. Adv Pharm Bull. 2021. doi:10.34172/apb.2022.078
57. Laffleur F, Bauer B. Progress in nasal drug delivery systems. Int J Pharm. 2021;607120994. doi:10.1016/j.ijpharm.2021.120994
58. Akel H, Ismail R, Csóka I. Progress and perspectives of brain-targeting lipid-based nanosystems via the nasal route in Alzheimer’s disease. Eur J Pharm Biopharm. 2020;14838–14853. doi:10.1016/j.ejpb.2019.12.014
59. Keller L, Merkel O, Popp A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv Transl Res. 2022;12(4):735–757. doi:10.1007/s13346-020-00891-5
60. Brewster PR, Mohammad Ishraq Bari S, Walker GM, et al. Current and future directions of drug delivery for the treatment of mental illnesses. Adv Drug Deliv Rev. 2023:197114824. doi:10.1016/j.addr.2023.114824
61. Chia-Ming W, Matthew TF, Benjamin MW, et al. Native gastrointestinal mucus: critical features and techniques for studying interactions with drugs, drug carriers, and bacteria. Adv Drug Deliv Rev. 2023;200. doi:10.1016/j.addr.2023.114966
62. John D, Nada A, Michael C. Gut goo: physiology, diet, and therapy of intestinal mucus and biofilms in gastrointestinal health and disease. Clin Gastroenterol Hepatol. 2024. doi:10.1016/j.cgh.2024.09.007
63. Kaori M, Akira S, Hiroshi M. Advances in the evaluation of gastrointestinal absorption considering the mucus layer. Pharmaceutics. 2023;15(12). doi:10.3390/pharmaceutics15122714
64. Watchorn J, Clasky AJ, Prakash G, et al. Untangling mucosal drug delivery: engineering, designing, and testing nanoparticles to overcome the mucus barrier. ACS Biomater Sci Eng. 2022;8(4):1396–1426. doi:10.1021/acsbiomaterials.2c00047
65. Caffarel-Salvador E, Abramson A, Langer R, et al. Oral delivery of biologics using drug-device combinations. Curr Opin Pharmacol. 2017:368–413. doi:10.1016/j.coph.2017.07.003
66. Han H, Shin H, Ha DH. Improved oral bioavailability of alendronate via the mucoadhesive liposomal delivery system. Eur J Pharm Sci. 2012;46(5):500–507. doi:10.1016/j.ejps.2012.04.002
67. Bandi SP, Bhatnagar S, Venuganti VVK. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021;11913–11929. doi:10.1016/j.actbio.2020.10.031
68. Krupa L, Bajka B, Staroń R, et al. Comparing the permeability of human and porcine small intestinal mucus for particle transport studies. Sci Rep. 2020;10(1). doi:10.1038/s41598-020-77129-4
69. Wang C, Fernez MT, Woolston BM, et al. Native gastrointestinal mucus: models and techniques for studying interactions with drugs, drug carriers, and bacteria. Adv Drug Deliv Rev. 2023:200114966. doi:10.1016/j.addr.2023.114966
70. Jain S, Patil SR, Swarnakar NK, et al. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes). Mol Pharm. 2012;9(9):2626–2635. doi:10.1021/mp300202c
71. Zhang D, Pan X, Wang S, et al. Multifunctional poly(methyl vinyl ether-co -maleic anhydride)-graft -hydroxypropyl-β-cyclodextrin amphiphilic copolymer as an oral high-performance delivery carrier of tacrolimus. Mol Pharm. 2015;12(7):2337–2351. doi:10.1021/acs.molpharmaceut.5b00010
72. Agüeros M, Zabaleta V, Espuelas S, et al. Increased oral bioavailability of paclitaxel by its encapsulation through complex formation with cyclodextrins in poly(anhydride) nanoparticles. J Control Release. 2010;145(1):2–8. doi:10.1016/j.jconrel.2010.03.012
73. Serra L, Doménech J, Peppas NA. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur J Pharm Biopharm. 2009;71(3):519–528. doi:10.1016/j.ejpb.2008.09.022
74. Luo R, Lin M, Fu C, et al. Calcium pectinate and hyaluronic acid modified lactoferrin nanoparticles loaded rhein with dual-targeting for ulcerative colitis treatment. Carbohydr Polym. 2021:263117998. doi:10.1016/j.carbpol.2021.117998
75. Bao C, Liu B, Li B, et al. Enhanced transport of shape and rigidity-tuned α-lactalbumin nanotubes across intestinal mucus and cellular barriers. Nano Lett. 2020;20(2):1352–1361. doi:10.1021/acs.nanolett.9b04841
76. Kali G, Knoll P, Bernkop-Schnürch A. Emerging technologies to increase gastrointestinal transit times of drug delivery systems. J Control Release. 2022;346289–346299. doi:10.1016/j.jconrel.2022.04.016
77. Joice GA, Bivalacqua TJ, Kates M. Optimizing pharmacokinetics of intravesical chemotherapy for bladder cancer. Nat Rev Urol. 2019;16(10):599–612. doi:10.1038/s41585-019-0220-4
78. Ensign LM, Cone R, Hanes J. Nanoparticle-based drug delivery to the vagina: a review. J Control Release. 2014;190500–190514. doi:10.1016/j.jconrel.2014.04.033
79. Dow J N, Jordan N, Robson CN, et al. The bladder does not appear to have a dynamic secreted continuous mucous gel layer. J Urol. 2005;173(6):2025–2031. doi:10.1097/01.ju.0000158454.47299.ae
80. Poinard B, Lam SAE, Neoh KG, et al. Mucopenetration and biocompatibility of polydopamine surfaces for delivery in an ex vivo porcine bladder. J Control Release. 2019:300161–300173. doi:10.1016/j.jconrel.2019.02.041
81. Yoon HY, Yang HM, Kim CH, et al. Current status of the development of intravesical drug delivery systems for the treatment of bladder cancer. Expert Opin Drug Deliv. 2020;17(11):1555–1572. doi:10.1080/17425247.2020.1810016
82. Taurin S, Almomen AA, Pollak T, et al. Thermosensitive hydrogels a versatile concept adapted to vaginal drug delivery. J Drug Target. 2018;26(7):533–550. doi:10.1080/1061186X.2017.1400551
83. Suk JS, Xu Q, Kim N, et al. Pegylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016:9928–9951. doi:10.1016/j.addr.2015.09.012
84. Zierden HC, Josyula A, Shapiro RL, et al. Avoiding a sticky situation: bypassing the mucus barrier for improved local drug delivery. Trends Mol Med. 2021;27(5):436–450. doi:10.1016/j.molmed.2020.12.001
85. Buya AB, Beloqui A, Memvanga PB, et al. Self-nano-emulsifying drug-delivery systems: from the development to the current applications and challenges in oral drug delivery. Pharmaceutics. 2020;12(12):1194. doi:10.3390/pharmaceutics12121194
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
pH/Redox Dual-Responsive Drug Delivery System with on-Demand RGD Exposure for Photochemotherapy of Tumors

Li Y, Nie J, Dai J, Yin J, Huang B, Liu J, Chen G, Ren L
International Journal of Nanomedicine 2022, 17:5621-5639
Published Date: 23 November 2022
Multifunctional Nanoparticles Codelivering Doxorubicin and Amorphous Calcium Carbonate Preloaded with Indocyanine Green for Enhanced Chemo-Photothermal Cancer Therapy

Yu J, Wang L, Xie X, Zhu W, Lei Z, Lv L, Yu H, Xu J, Ren J
International Journal of Nanomedicine 2023, 18:323-337
Published Date: 18 January 2023
From Pioneering Discoveries to Innovative Therapies: A Journey Through the History and Advancements of Nanoparticles in Breast Cancer Treatment
Basingab FS, Alshahrani OA, Alansari IH, Almarghalani NA, Alshelali NH, Alsaiary AH, Alharbi N, Zaher KA
Breast Cancer: Targets and Therapy 2025, 17:27-51
Published Date: 21 January 2025
Progress in the Application of Novel Nanomaterials in Targeted Therapy for Liver Cancer

Wei X, Cao W, Wang S, Zhang Y, Gao Z, Wang S, Yao L, Zhang Z, Li X, Deng W, Xie Y, Li M
International Journal of Nanomedicine 2025, 20:2623-2643
Published Date: 3 March 2025