Back to Journals » Infection and Drug Resistance » Volume 18
Multidrug-Resistant Staphylococcus aureus in Diabetic Foot Infections (DFI) from Beira, Mozambique: Prevalence and Virulence Profile
Authors Chaves CRS, Salamandane C, Maurício BDS, Machamba AAL, Salamandane A, Brito L
Received 10 February 2025
Accepted for publication 23 May 2025
Published 31 May 2025 Volume 2025:18 Pages 2779—2796
DOI https://doi.org/10.2147/IDR.S521876
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Celso Raul Silambo Chaves,1 Cátia Salamandane,2,3 Beatriz da Sorte Maurício,2 Almeida Abudo Leite Machamba,2 Acácio Salamandane,2,4 Luísa Brito4
1Laboratório Clínico do Centro de Saúde de Militar de Matacuane, Avenida Alfredo Lawley No. 42, Beira, Mozambique; 2Faculdade de Ciências de Saúde, Universidade Lúrio, Campus Universitário de Marrere, Nampula, 4250, Mozambique; 3Laboratório de Qualidade e Segurança Alimentar, Centro de Estudos Interdisciplinar Lúrio, Universidade Lúrio, Campus Universitário de Marrere, Nampula, 4250, Mozambique; 4LEAF—Linking Landscape, Environment, Agriculture and Food Research Center, Associate Laboratory TERRA, Instituto Superior de Agronomia, Universidade de Lisboa, Lisboa, 1349-017, Portugal
Correspondence: Cátia Salamandane, Faculdade de Ciências de Saúde, Universidade Lúrio, Campus Universitário de Marrere, Nampula, 4250, Mozambique, Email [email protected]
Introduction: Diabetic foot infection (DFI) represents a growing public health problem in Africa, caused by several microorganisms, with Staphylococcus aureus being one of the most prevalent pathogens associated with subsequent complications. This study aimed to characterize S. aureus isolated from the wounds of patients with type 2 diabetes, treated at a health center in Beira, Mozambique, in terms of antibiotic resistance and virulence genes.
Methods: Samples were collected by swab, after ulcer debridement, and cultivated onto mannitol salt Columbia agar supplemented with 5% sheep blood, for 24 to 48 h, at 37°C. The antibiotic resistance was assessed by disk diffusion on Mueller–Hinton agar, and Multiplex PCR was used to screen 32 virulence and seven antibiotic resistance genes.
Results: S. aureus isolates showed high phenotypic resistance to penicillin (100%), cefoxitin (53.3%), trimethoprim/sulfamethoxazole (40%) and vancomycin (22.2%), and a high percentage of multidrug resistance (68.9%). The most prevalent resistance genes were blaZ (penicillin, 100%), mecA (cefoxitin, 53.3%) and vancA (vancomycin, 28.9%). The most frequent virulence genes were TSST (toxic syndrome staphylococcal toxin, 57.8%), and the colonization factor clfB (37.8%), followed by the Panton-Valentine leukocidin (PLV) genes, lukPV (26.7%) and lukED (15.6%). The regulator factor coded by arcA (37.8%) and the adhesion factors coded by cap5 (20%) and by icaA (17.8%) were also found.
Conclusion: A high presence of virulence genes encoding exotoxins and colonization and adhesion factors, associated with a high rate of multidrug resistance, was found in S. aureus isolates. This anticipates increasing difficulty in treating DFI. The greatest resistance was to commonly used antibiotics, particularly penicillin, cefoxitin and vancomycin, with resistance genes, blaZ, mecA and vancA, frequently detected. This emphasizes the urgent need for improved antimicrobial stewardship, routine molecular surveillance, and improved management strategies for DFI in resource-limited settings to mitigate disease complications and reduce the burden of antimicrobial resistance.
Keywords: diabetic foot infection, DFI, Beira-Mozambique, multidrug-resistance, methicillin resistant Staphylococcus aureus, MRSA, staphylococcal exotoxin genes
Introduction
Diabetic foot infection (DFI) is a common complication resulting from soft tissue or bone infection, often associated with neuropathy or peripheral arterial disease in patients with type 2 diabetic mellitus.1 DFI is associated with considerable morbidity, an increased risk of lower limb amputation, and a high mortality.2 DFI is mainly polymicrobial, caused by different bacterial communities, and the presence of this wide variety of species is a factor that complicates diagnosis and treatment.3,4 Staphylococcus aureus is one of the most frequently isolated pathogens of DFI, often exhibiting worrying trends of high virulence and antibiotic resistance.5
Staphylococcus is one of the most common genera on the skin and nasal mucosa of humans and animals.5,6 The presence of Staphylococcus in wounds can cause increased inflammation and delay the healing process.6 This scenario is aggravated by the combination of the presence of different virulent genes and high antibiotic resistance, which may compromise the success of the treatment. The presence of virulence genes, such as those encoding the protein adhesin A (SpA), which facilitates the attachment of bacteria to host cells and tissues, fibronectin (FnBP), which binds to molecules on the surface of the host cells, and α-and-β-hemolysins (hlA and hlB) that can cause damage to host cells and induce cell death, contributes to the successful invasion and pathogenicity of S. aureus.7,8 Exfoliative toxins, expressed by the ET genes, are exotoxins produced by several staphylococcal species, capable of causing blistering in human and animal infections.9 Three of these toxins, encoded by the etA, etB, and etD genes, are directly linked to infections in humans.10 Toxic shock syndrome-associated toxin (TSST, or TSST-1), which encodes a protein considered a superantigen, and enterotoxins, such as those of the SE family (seA, seB, seD, seI and seH), are other examples of S. aureus virulence genes.11,12
The occurrence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA), is another factor that increases the severity of S. aureus infections in diabetic patients, making treatment of these infections challenging to say the least.13,14 MRSA strains thus play a significant role in DFI and have become a public health concern due to their high virulence and resistance to an increasingly broad spectrum of antibiotics.15 MRSA infections are associated with prolonged hospital stay, increased morbidity and mortality rates, and higher healthcare costs compared with infections caused by methicillin-sensitive strains.16 Furthermore, MRSA strains pose a significant threat to public health due to their propensity for nosocomial transmission and their ability to form antibiotic-resistant biofilms on medical devices.17 Biofilm formation involves several steps, such as adhesion, accumulation, maturation and biofilm dispersion, determined by several genes. In this process, the icaABCD operon, which encodes Polysaccharide Intercellular Adhesion (PIA), the main component of biofilms, plays a very relevant role.18,19 The regulation of the genes of this operon is complex and affected by several factors, including the presence of antimicrobials, the presence of cell wall-binding proteins and the presence of other bacterial virulence genes.20
S. aureus is a major opportunistic pathogen in DFIs and is frequently associated with persistent and severe infections in diabetic patients, often leading to poor healing outcomes, increased risk of amputation, and prolonged hospitalization.5,21 The evaluation of surveillance of this opportunistic pathogen is critically important for guiding effective clinical management and controlling the spread of antimicrobial resistance.21,22 Regular monitoring of its antibiotic resistance patterns and virulence profiles enables early detection of multidrug-resistant and highly virulent strains, informing targeted antimicrobial therapy and infection control measures.22 Moreover, surveillance data are essential for developing local and regional treatment guidelines, optimizing antibiotic stewardship programs, and shaping public health interventions aimed at reducing the clinical and economic burden of DFIs, particularly in resource-limited settings.23,24
In Africa, the increasing incidence and inadequate management of type 2 diabetes represent crucial public health challenges, requiring active surveillance.25 In Mozambique, there is a progressive increase in the prevalence of type 2 diabetes. The lack of awareness among the population about the disease and its control strategies is alarming.26 In view of this, there is an urgent need for immediate adaptation in health service delivery, especially in the diagnosis and characterization of opportunistic pathogens associated with type 2 diabetes, with a view to improving diabetes management. In this context, this study aimed at the molecular characterization of S. aureus isolates associated with DFI from patients assisted at the Matacuane military health center in the city of Beira, Mozambique.
Material and Methods
Study Area
The Matacuane Military Health Center is a military health facility integrated into the national health system of Mozambique, dedicated to providing healthcare services to military and paramilitary forces. However, as part of the national health system, this facility also provides primary healthcare services to civilians living in the vicinity of the health center. Located in the urban area of the municipality of Beira, on average, the center provides primary healthcare services to 1900 to 2500 patients per month, from various neighborhoods, with the majority of patients coming from the Matacuane neighborhood.27
The most important services offered include general medicine consultations, pediatric consultations, monitoring of patients on antiretroviral therapy and nursing services. Nursing services involve the administration of intramuscular or intravenous medications and the treatment of ulcers, wounds or surgical dressings, in patients referred by the Beira Central Hospital.
Inclusion and Exclusion Criteria
All civilian patients with a confirmed diagnosis of type 2 diabetes mellitus, who presented clinical signs of infected foot ulcers and provided informed consent, as stated under ethical considerations, were included in the study. On other way, military or paramilitary personnel, civilian patients who refused or were unable to provide informed consent, and those who had not initiated antibiotic treatment were excluded from the study.
Sampling and Characteristics of Patients and Lesions
Sampling was conducted between September 2023 and January 2024, in 45 civilian patients with type 2 diabetes and DFI ulcers. Ethical considerations were strictly observed. All patients were taking antibiotics to treat the infection. Demographic and lesion information, including age, sex, duration of DFI, occurrence of diabetes medication, lesion characteristics, and lesion location were collected.
Isolation and Identification of Staphylococcus aureus
A sample was collected from each patient by swab, after debridement of the ulcer. A total of 45 swabs were sampled and, respectively, inoculated into Brain Heart Infusion (BHI) broth (Oxoid Ltd., Basingstoke, UK) and incubated overnight at 37°C. From this culture broth, Mannitol Salt Agar (MSA) (Oxoid Ltd., Basingstoke, UK) and Columbia agar (supplemented with 5% sheep blood) plates were inoculated and incubated at 37°C for 24 to 48 h. The plates were inspected for characteristic staphylococcal morphology and presumptive Staphylococcus aureus colonies were identified using conventional methods (Gram staining, catalase and coagulase reactions) according to standard microbiological methods. Identification as S. aureus was performed by PCR targeting the 447-bp fragment of nuc gene, with the forward primer 5′-GCGATTGATGGTGATACGGTI-3′ and reverse primer 5′-AGCCAAGCCTTGACGAACTAAAGC-3′.28,29 The primers were commercially synthesized by STAB VIDA (STAB VIDA, Caparica, Portugal). S. aureus (ATCC 25923) and S. epidermidis (ATCC 12228) were used as positive and negative controls, respectively.
DNA extraction was performed using the QIAamp DNA Mini® Kit (Qiagen, Hilden, North Rhine-Westphalia, Germany) according to the manufacturer’s instructions. Briefly, bacterial pellets were resuspended in lysis buffer containing proteinase K and incubated at 56°C to ensure complete cell lysis. Ethanol was then added to the lysate, and the mixture was transferred to a QIAamp spin column. DNA was bound to the silica membrane, followed by successive washing steps to remove contaminants. Finally, purified DNA was eluted in the provided elution buffer and stored at –20°C until use.
The mix for PCR was prepared with 12.5 µL of Taq DNA Polymerase NZYTaq II2x Colourless Master Mix (MZYTech, Lisboa, Portugal), 1 µL of each forward and reverse primer (final concentration 0.3 µM), 2 µL of template DNA and sterile ultrapure water to fill 25 µL of total volume. The reaction mixtures were subjected to the following amplification conditions: initial denaturation, 94°C for 5 min, followed by 30 cycles of denaturation 94°C for 30 s, annealing 52°C for 30 s, elongation 72°C for 30 s, and a final elongation at 72°C for 5 min. PCR reactions were run in a thermocycler GeneAmp® PCR System 9700, Bio-Rad (Bio-Rad Laboratories, Segrate, Milan, Italy). The resulting PCR products were resolved on 2% (m/v) agarose gels in 1× TAE buffer, in an EC330 Thermo Fisher Scientific tank (Atlanta, GA, USA) at 8 V/cm for 60 min. The gels were stained with GelRed (Frilabo, Maia, Portugal) and analyzed using a Gel Doc™ EZ System (Bio-Rad Laboratories, Segrate, Milan, Italy). For calculating the size of the PCR products, the molecular marker 100 bp DNA Ladder (Invitrogen, CA, USA) was used.
Antibiotic Resistance Profile
Antimicrobial resistance profiling was performed by the disk diffusion method on Mueller–Hinton (MH) agar plates (Biokar Diagnostics, Beauvais, France) with antibiotic disks (Liofilchem, Roseto degli Abruzzi, Italy), according to the Clinical Laboratory Standards Institute (CLSI, 2021).30 Colonies grown on Trypto-Casein-Soy agar (Biokar Diagnostics, Beauvais, France) for 22 ± 2 h at 37°C were suspended in sterile saline until the turbidity was equivalent to the McFarland 0.5 standard (ca. 106 CFU/mL). Of the resulting bacterial suspensions, 100 µL was used to inoculate MH plates under the conditions described by CLSI.30 After deposition of the antibiotic disks, the plates were incubated for 18 ± 2 h at 37°C. Eight antibiotics were tested: cefoxitin (FOX) 30 μg; penicillin G 10U; vancomycin (VAN) 5 μg; chloramphenicol (CHL) 30 μg; tetracycline (TET) 30 μg; gentamicin (GEN) 10 μg; trimethoprim/sulfamethoxazole (SXT) 1.25/23.75 μg; erythromycin (ERY) 16 μg. On each 90 mm diameter plate, four different antibiotic disks were placed. For each isolate, two replicates were performed.
To assess the antimicrobial resistance profile of the S. aureus isolates, inhibition zones diameters were measured (millimeter) and compared to those described in the CLSI (2021).30 Isolates were considered non-susceptible to a given antibiotic when they showed intermediate or full resistance to that antibiotic, according to the CLSI clinical breakpoints. Multidrug resistance was considered as non-susceptibility to at least one agent, in three or more antimicrobial categories and/or resistance to methicillin, cefoxitin or oxacillin.31
 |
Table 1 Primers Used for the Detection of Virulent Genes and Respective Sizes of Amplified Product |
Identification of Virulence and Antibiotic Resistance Genes
Multiplex PCR (MPCR) investigated the presence of 32 virulence genes (Table 1). Namely: Genes encoding hemolysin (hlA, hlB and hlgCB); genes associated with colonization, such as fibronectins (fnbA and fnbB); genes encoding adhesins (clfA and clfB) and gene sasX related to nasal colonization; accessory gene regulators (agr I, Agr II, agr III, agr IV and arcA); biofilm regulator genes (icaA, icaB, icaC and icaD); exotoxins, such as exfoliative toxin genes (etA, etC and etD) and toxic shock syndrome (TSST); enterotoxins (sea, seb, sec, sed, see, seI and seH); capsular polysaccharides (cap5 and cap8); and genes encoding pore-forming leukotoxins (lukED and lukS/F-PV). Based on the annealing temperature and PCR product size of each primer, the reactions were grouped into different MPCRs. Namely: MPCR for seH, icaD, TSST, etD, hlgCB and fnbB genes; MPCR for fnbB, icaA and agr III genes; MPCR for hlB, Agr I, agr II and agr IV genes; MPCR for icaC, icaB, etB and clfA genes; MPCR for clfB, lukED, cap5 and cap8 genes; MPCR for seA, seB, seC and seE genes; MPCR for seD, ACME-arcA and lukS/F-PV genes; MPCR for etA, sasX and hlA genes. The gene seI was screened as a single PCR.
The MPCR master mixes were respectively prepared with 12.5 µL of Taq DNA Polymerase NZYTaq II2x Colourless Master Mix (MZYTech, Lisboa, Portugal), 1 µL of each forward and reverse primers (Table 2) (final concentration 0.3 µM), 2 µL of template DNA and sterile ultrapure water up to 25 µL of total volume. All primers commercially synthesized by STAB VIDA (STAB VIDA, Caparica, Portugal).
 |
Table 2 Primers Used for the Detection of Antimicrobial Resistance Genes and Respective Sizes of Amplified Products |
Genes encoding resistance to methicillin (mecA), penicillin (blaZ), erythromycin (ermA, ermB and ermC), and vancomycin (vancA and vancB) were screened by MPCR (Table 2). The MPCR master mix was prepared with 12.5 µL of Taq DNA Polymerase NZYTaq II2x Colourless Master Mix (MZTech, Lisboa, Portugal), 1 µL of each forward and reverse primers (Table 2) (final concentration 0.3 µM), 2 µL of template DNA, and sterile ultrapure water to complete 25 µL of total volume. MPCR conditions were performed as previous described.17,29,43 All primers commercially synthesized by STAB VIDA (STAB VIDA, Caparica, Portugal).
All PCR reactions were performed in a GeneAmp® PCR System 9700 thermocycler (Applied Biosystems, Bio-Rad Laboratories, Segrate, Milan, Italy). PCR products were resolved on 2% (m/v) agarose gels in 1×TAE buffer, in an EC330 Thermo Fisher Scientific tank (Georgia, USA) at 8 V/cm for 60 min. Gels were stained with GelRed (Frilabo, Maia, Portugal) and analysed using a Gel Doc™ EZ System (Bio-Rad Laboratories, Segrate, Milan, Italy). To calculate the size of PCR products, the molecular marker 100 bp DNA Ladder (InVitrogen, California, USA) was used.
Results
Demographic Data
Demographic and lesion information, including age, sex, duration of DFI, occurrence of diabetes medication, lesion characteristics, and lesion location are shown in Table 3. The age of the patients ranged from 36 to 75 years (mean 65.8 ± 13.76 years) and 64.4% were female (Table 3). Most patients (93%) were taking medications to control diabetes. Most patients (55.6%) had DIF for more than three months (Table 3). The types of lesions among the patients were mainly deep ulcers (86.7%) with chronic wounds (77.8%). Most lesions were located on the right toe (37.8%) and plantar region (28.8%) (Table 3).
 |
Table 3 Characterization of DFI Patients and Lesions |
The diabetic foot ulcers of the 45 patients evaluated in this study resulted in the identification of 45 isolates of S. aureus. These S. aureus isolates were subjected to analysis of the antibiotic resistance profile and the presence of virulence and antibiotic resistance genes.
 |
Table 4 Distribution of Virulent Genes by Function |
Virulent Genes
To facilitate the recognition of the most relevant virulence genes in the 45S. aureus isolates, the genes were grouped according to their respective functions (Table 4). Thus, the most frequent virulence genes were those encoding exotoxins (TSST, 57.8% and etA, 35.6%), followed by genes encoding colonization (clfB, 37.8%) and regulators factors (arcA, 37.8%) (Table 4). Regarding leukocidin genes, LukPV was found in 26.7% and LukED in 15.6 of the isolates. The cap5 (20%), icaA (17.8%) and icaD (11.1%) genes were the most representative adhesion genes. Among the seven genes investigated that encode staphylococcal enterotoxins, only the seI gene was found (13.3%) (Table 4). Among the 32 genes accessed, 15 (46.9%) were detected in at least one isolate (Table 5). Seventeen (clfA, etB, etD, fnbA, sasX, hlA, seA, seB, seC, seD, seE, seh, icaB, icaC, agr III, agr IV and cap8) of the 32 target genes were not found in any isolate.
 |
Table 5 Virulence Genes Detected in the 45 Staphylococcus aureus Isolates |
Most isolates that present virulent genes contain more than one gene, related or not (Tables 4 and 5). Regarding similar virulence genes, the most predominant co-occurrences were observed with the exotoxin gene TSST and etA (31.1%), and the adhesion factors icaA/icaD and cap5 (13.3%). The co-occurrence of multiples (three or more) virulence genes involved in different functions was found in 42.2% of the isolates. On the other hand, 31.1% of the isolates presented two genes encoding different types of virulence. In five of the 45 isolates, none of the 32 virulent genes under analysis were detected (Table 5).
 |
Table 6 Frequencies of Antibiotic Resistance Profile and Associated Genes |
Antibiotic Resistance
The most prevalent antimicrobial resistance profiles were penicillin (100%) and cefoxitin (53.3%) (Table 6). Among non-β-lactam antibiotics, the most frequent resistance profiles were to trimethoprim/sulfamethoxazole (40%), erythromycin (33.3%) and vancomycin (22.2%). Very few isolates showed resistance to chloramphenicol or gentamicin (8.9 and 4.4%, respectively) (Table 6). Among the 45 S. aureus isolates from DFI, 31 (68.9%) were multidrug-resistant as they showed resistance to cefoxitin and/or more than three groups of unrelated antibiotics (Table 7).
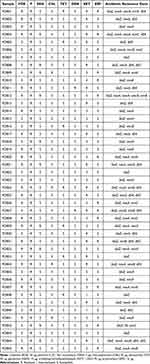 |
Table 7 Antibiotic Resistance Profile and Presence of Genes Encoding Antibiotic Resistance in the 45 Staphylococcus aureus Isolates from DFI |
Regard the frequency of antibiotic resistance genes, the blaZ gene, which encodes penicillin resistance, was found in all 45 isolates analyzed (Table 6). The mecA gene, encoding methicillin/penicillin resistance, was found in 53.3% of the isolates. Two types of genes encoding vancomycin resistance were found (vancA, in 28.9% and vancB, 15.6%) (Table 6). Among the genes encoding erythromycin resistance, the ermB gene (15.6%) was the most common gene, followed by ermC (11.1%). Gene encoding resistance to trimethoprim/sulfamethoxazole dfrA (31.1%) and dfrG (17.8%) was found among 40% of isolates that showed resistance profile.
Co-occurrence of resistance genes was observed in 84.4% of the isolates (Table 7). The most frequent co-occurrence was of blaZ and mecA (48.8%). In three of the 45 (6.7%) S. aureus isolates, all types of antibiotic resistance genes evaluated in this study were detected (blaZ, mecA, vanc, dfr and erm) (Table 7).
Beyond the high overall resistance to penicillin and cefoxitin, Table 7 shows a concerning trend of convergence between multidrug resistance and the presence of multiple virulence determinants in S. aureus isolates from DFI. Strains harboring both mecA and blaZ genes frequently co-expressed virulence genes such as TSST, etA, and leukocidin genes (lukPV, lukED), highlighting the potential for these strains to cause severe and hard-to-treat infections. Particularly notable were isolates like FDB33 and FDB34, which combined resistance to five or more antibiotics with genes associated with toxin production and biofilm formation (icaA, icaD, cap5), suggesting enhanced persistence and immune evasion capabilities.
Discussion
Methicillin-resistant S. aureus (MRSA) has been documented as the most dominant healthcare-associated Staphylococcus worldwide.49,50 However, in Mozambique, there is no information on the characterization of S. aureus isolated from DIF. Hopefully, this study will contribute to fill this gap. In general, low genetic diversity was found among the isolates, demonstrated by the similarity of the virulent genes found. The results of this study highlight high resistance to penicillin (100%), cefoxitin (53.3%) and trimethoprim/sulfamethoxazole (40%).
Among diabetic patients with foot ulcers, MRSA has emerged as an important and commonly opportunistic pathogen, often community- and hospital-associated. MRSA can infect ulcers, skin, and soft tissues, making treatment very difficult.51 Among the 53.3% (24/45) MRSA isolates identified in this study, 45.8% were resistant to trimethoprim/sulfamethoxazole, 41.7% to erythromycin and 29.2% to vancomycin. Similar results were found by other authors in Poland,10 Egypt16 and Brazil.52 S. aureus recovered from DFI in a Tunisian hospital showed 100% resistance to methicillin and 33.3% to trimethoprim/sulfamethoxazole.50 In Egypt, a high prevalence of MRSA strains (100%)16 exhibiting resistance to erythromycin and vancomycin was noted, possibly reflecting different prescription habits and infection control policies. In Brazil, high resistance rates (61%) of MRSA were also observed.52 However, the socioeconomic factors, such as better access to healthcare and structured diabetes care programs, might partially mitigate the burden compared to the Mozambican context. In Mozambique, co-trimoxazole (trimethoprim/sulfamethoxazole (SXT)) plays a very important role in the treatment of opportunistic diseases in immunocompromised patients and, together with amoxicillin and clavamox (another penicillin antibiotics), is the most prescribed and recommended antibiotic by health professionals.53
The mecA gene was found in all isolates that showed resistance to cefoxitin. These isolates also carried the blaZ gene encoding penicillin resistance and 29.2% had the vanc and or erm gene. Several studies have found a high prevalence of antibiotic resistance and genes associated with antibiotic resistance in S. aureus recovered from clinical and food samples in Mozambique.43,54,55 In food samples, Salamandane et al43 reported the occurrence of the blaZ gene in 42.1% of S. aureus isolates from ready-to-eat foods, as well as the occurrence of the mecA gene in 36.8% and vancA in 31.6%. On the other hand, in clinical isolates, blaZ was found in 79.2% of samples and mecA in 100%.55 Co-occurrence of multiple resistance genes further suggests potential for treatment failure and nosocomial spread.
In South Africa, MRSA prevalence among DFI patients has been reported at levels similar to or slightly higher than those observed in Mozambique, while studies from Nigeria and Kenya show even higher rates of multidrug resistance in S. aureus isolates.56 Several factors as limitations in healthcare infrastructure, with reduced laboratory capacity for culture and sensitivity testing, leading to widespread empirical antibiotic use without proper microbiological diagnosis in different African countries could explain these differences.57,58
Regarding the genes encoding virulence factors, the most frequent were exotoxins TSST (57.8%), colonization factor clfB (40%), regulator factor arcA (37.8%) and exfoliative toxin etA (33.3%). A total of 53.8% of the isolates that presented TSST also presented the etA gene. Several studies found a low prevalence of TSST in S. aureus isolates from DFI.50,59,60 In Costa Rica, Víquez-Molina et al detected the TSST gene in 5.2% of the samples,59 and in India, Shettigar et al60 found 13.9% of TSST in S. aureus recovered from individuals with foot ulcers. Combination of several factors, including high glucose, low oxygen levels resulting from the combination of neuropathy and poor circulation and indiscriminate use of a variety of antibiotics,61 may have contributed to the high frequency of TSST genes in this work. Exfoliative toxins cause scalded skin syndrome, most commonly in newborns, young children and immunosuppressed individuals. Scalded skin syndrome is a staphylococcal infection that causes redness, blisters and peeling, causing the top layer of skin to peel off.62
The gene encoding Panton-Valentine Leucocidin (PVL) was found in 40% of the isolates. Of these, 11 isolates were positive for lukPV, six isolates for lukED and one isolate showed both genes (lukPV and lukED). Detection of lukED in MRSA strains associated with DFI has also been reported in Iran.63 In Iran, lukED gene was found in 85% of S. aureus recovered from DIF. PVL is one of the main virulence factors associated with hospital-acquired pneumonia and skin infections. This toxin damages white blood cells, which are essential in fighting infection.64 Meeren et al found 37.5% and 90.5% of the gene encoding this toxin PVL in hospital- and community-acquired S. aureus in Beira city, Mozambique.54 Other relevant genes found in the present study are related to fibrinogen-binding adhesin of the host cell, such as clfB (37.8%) and fnbB (4.4%).
These genes encode proteins known as clumping factors, fibronectin-/fibrinogen-binding proteins, and adhesins,65 which play an important role in biofilm formation. A recent study reported high biofilm formation in MRSA isolated from street food in Mozambique.17 Regarding enterotoxins, only the seI gene was detected in 13.3% of the isolates. Although they are considered enterotoxins, seI and seH encode proteins that are also factors associated with the colonization of host tissues by S. aureus, due to their presumed participation in the infectious process.66,67
Some strains are of particular concern, as they are multidrug-resistant or multidrug-resistant MRSA, carry several virulent genes and isolated from high-risk patients. Namely, the MRSA isolates FDB03, that presented the clfB, TSST, etA, lukPV, lukED and agr I genes, and FDB04, with virulence genes clfB, TSST, etA and arcA, recovered from obese patients with ulcers for more than 90 days. Also, the MRSA isolates FDB11 and FDB39 recovered from ulcers older than 90 or 60 days, from elderly or obese patients, respectively, carried six virulence genes each. Although they were isolated from non-obese individuals with recent ulcers (less than 30 days), the multidrug-resistant isolate FDB33 carrying the hlB, TSST, etA, arcA, IcaA, icaD, cap5 and seI; and the MRSA isolate FDB34, carrying the genes TSST, etA, arcA, IcaA, icaD and cap5, may be considered high risk, as they present genes related to biofilm formation. In fact, in the biofilm state, bacteria are more resistant to antibiotics than their planktonic counterparts due to the multilevel protection conferred by the extracellular matrix (which hinders the penetration of antibiotics), altered metabolic states and lower growth rate.17,28,68
We did not find data on the prevalence of DFI in Mozambique. However, in South Africa, a country bordering Mozambique and sharing similarities in terms of prevalence of non-transmissible diseases, DIF is estimated to affect 28% of diabetic patients.69 The prevalence of DFI in Zimbabwe was estimated to be 53%. Longer duration of diabetes, absence of pedal pulses, and peripheral neuropathy were considered risk factors for foot ulceration in these populations.25,70–73
Conclusions
This study aimed to investigate antibiotic resistance and the presence of virulence genes in S. aureus isolated from DFI. To the best of our knowledge, this is the first study addressing antibiotic resistance and virulence genes in S. aureus isolated from DFI patients in Mozambique. The results revealed alarming levels of antibiotic resistance, particularly to penicillin, cefoxitin, co-trimoxazole (trimethoprim/sulfamethoxazole) and vancomycin, as well as high percentages of multidrug-resistant strains. Furthermore, the study identified a high presence of virulent genes. The presence of multiple virulence genes, including TSST, clfB, and lukPV, underscores the pathogenic potential of these strains and their capacity to cause severe infections. The presence of virulent genes in multidrug-resistant strains represents a worrying scenario, as it indicates the potential for greater severity of infections caused by these bacteria, making treatment difficult and increasing the risk of serious complications.
Abbreviations
BHI, Brain Heart Infusion; DFI, Diabetic foot infection; MSA, Mannitol Salt Agar; CLSI, Clinical Laboratory Standards Institute; MRSA, Methicillin-resistant S. aureus.
Data Sharing Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Restrictions are applied for confidential personal data.
Ethics Approval and Informed Consent
The study was conducted according with the Declaration of Helsinki, and the protocol was approved by the National Committee for Bioethics in Health of Ministry of Health of Mozambique, protocol reference number 039/2022/DFI/BS/MSM. All participants provided written informed consent to take part in the study and confirmed their consent before participation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Khan MS, Azam M, Khan MN. et al. Identification of contributing factors, microorganisms and antimicrobial resistance involved in the complication of diabetic foot ulcer treatment. Microb Pathog. 2023;184:106363. doi:10.1016/j.micpath.2023.106363
2. Raspovic KM, Wukich DK. Self-reported quality of life and diabetic foot infections. J Foot Ankle Surg. 2014;53(6):716–719. doi:10.1053/j.jfas.2014.06.011
3. Gemechu FW, Curley CA, Curley CA. Diabetic Foot Infections. Am Fam Physician. 2013;88(3):177–184.
4. Wang X, Yuan C-X, Xu B, Yu Z. Diabetic foot ulcers: classification, risk factors and management. World J Diabetes. 2022;13(12):1049–1065. doi:10.4239/wjd.v13.i12.1049
5. Dunyach-Remy C, Essebe CN, Sotto A, Lavigne JP. Staphylococcus aureus toxins and diabetic foot ulcers: role in pathogenesis and interest in diagnosis. Toxins. 2016;8(7):1–20. doi:10.3390/toxins8070209
6. Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. 2015;13(9):529–543. doi:10.1038/nrmicro3521
7. Foster TJ, Fischetti VA, Novick RP. Surface Proteins of Staphylococcus aureus. Microbiol Spectr. 2019;7(4). doi:10.1128/microbiolspec.gpp3-0046-2018
8. Otto M. Staphylococcus aureus toxins. Curr Opin Microbiol. 2014;17:32–37. doi:10.1016/j.mib.2013.11.004
9. Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, Microbial, and Biochemical Aspects of the Exfoliative Toxins Causing Staphylococcal Scalded-Skin Syndrome. Clin Microbiol Rev. 1999;12(2):224–242. doi:10.1128/CMR.12.2.224
10. Kot B, Piechota M, Jakubczak A, et al. The prevalence of virulence determinants in methicillin-resistant Staphylococcus aureus isolated from different infections in hospitalized patients in Poland. Sci Rep. 2022;12(1). doi:10.1038/s41598-022-09517-x
11. Chen Q, Xie S. Genotypes, Enterotoxin Gene Profiles, and Antimicrobial Resistance of Staphylococcus aureus Associated with Foodborne Outbreaks in Hangzhou, China. Toxins. 2019;11(6):307. doi:10.3390/toxins11060307
12. Nasaj M, Saeidi Z, Tahmasebi H, Dehbashi S, Arabestani MR. Prevalence and distribution of resistance and enterotoxins/enterotoxin-like genes in different clinical isolates of coagulase-negative Staphylococcus. Eur J Med Res. 2020;25(1). doi:10.1186/s40001-020-00447-w
13. Boyanova L, Mitov I. Antibiotic resistance rates in causative agents of infections in diabetic patients: rising concerns. Expert Rev Anti Infect Ther. 2013;11(4):411–420. doi:10.1586/ERI.13.19
14. Abbas M, Uçkay I, Lipsky BA. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin Pharmacother. 2015;16(6):821–832. doi:10.1517/14656566.2015.1021780
15. Feng S-H, Chu Y-J, Wang P-H, Jun X, Min D, Li X-M. Risk Factors and Gene Type for Infections of MRSA in Diabetic Foot Patients in Tianjin, China. Int J Low Extrem Wounds. 2013;12(2):106–112. doi:10.1177/1534734613489991
16. Abalkhail A, Elbehiry A. Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infections: protein Profiling, Virulence Determinants, and Antimicrobial Resistance. Appl Sci. 2022;12(21):10803. doi:10.3390/app122110803
17. Salamandane A, Correia J, Muetanene BA, Dos Santos M, Malfeito-Ferreira M, Brito L. Methicillin Resistance of Food-Borne Biofilm-Forming Staphylococci. Appl Sci. 2023;13(13):7725. doi:10.3390/app13137725
18. Arciola CR, Campoccia D, Ravaioli S, Montanaro L, Vasil ML, Otto M. Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Front Cell Infect Microbiol. 2015;5(7). doi:10.3389/fcimb.2015.00007
19. Gajewska J, Chajecka-Wierzchowska W. Biofilm Formation Ability and Presence of Adhesion Genes among Coagulase-Negative and Coagulase-Positive Staphylococci Isolates from Raw Cow’s Milk. Pathogens. 2020;9(8):654. doi:10.3390/pathogens9080654
20. Cue D, Lei MG, Lee CY. Genetic regulation of the intercellular adhesion locus in staphylococci. Front Cell Infect Microbiol. 2012;2. doi:10.3389/fcimb.2012.00038.
21. Sohail MU, Mashood F, Oberbach A, Chennakkandathil S, Schmidt F. The role of pathogens in diabetes pathogenesis and the potential of immunoproteomics as a diagnostic and prognostic tool. Front Microbiol. 2022;13. doi:10.3389/fmicb.2022.1042362.
22. Ranjalkar J, Chandy S. India’s National Action Plan for antimicrobial resistance – an overview of the context, status, and way ahead. J Family Med Prim Care. 2019;8(6):1828. doi:10.4103/jfmpc.jfmpc_275_19
23. Pezzani MD, Mazzaferri F, Compri M, et al. Linking antimicrobial resistance surveillance to antibiotic policy in healthcare settings: the COMBACTE-Magnet EPI-Net COACH project. J Antimicrob Chemother. 2020;75(Supplement_2):ii2–ii19. doi:10.1093/jac/dkaa425
24. Trivedi KK, Pollack LA. The Role of Public Health in Antimicrobial Stewardship in Healthcare. Clinl Infect Dis. 2014;59(suppl_3):S101–S103. doi:10.1093/cid/ciu544
25. Silva-Matos C, Gomes A, Azevedo A, Damasceno A, Prista A, Lunet N. Diabetes in Mozambique: prevalence, management and healthcare challenges. Diabetes Metab. 2011;37(3):237–244. doi:10.1016/j.diabet.2010.10.006
26. Li M, Du X, Villaruz AE, et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med. 2012;18(5):816–819. doi:10.1038/nm.2692
27. EUROSIS. Relatório da Avaliação Anual de Desempenho do Município da Beira. Beira: EUROSIS; 2020.
28. Salamandane A, Cahango G, Muetanene BA, Malfeito-Ferreira M, Brito L. Multidrug Resistance in Enterococci Isolated from Cheese and Capable of Producing Benzalkonium Chloride-Resistant Biofilms. Biology. 2023;12(10):1353. doi:10.3390/biology12101353
29. Salamandane A, Leech J, Almeida R, et al. Metagenomic analysis of the bacterial microbiome, resistome and virulome distinguishes Portuguese Serra da Estrela PDO cheeses from similar non-PDO cheeses: an exploratory approach. Food Res Int. 2024;189:114556. doi:10.1016/j.foodres.2024.114556
30. CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing an Informational Supplement for Global Application Developed Through the Clinical and Laboratory Standards Institute Consensus Process. 2021.
31. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
32. Peacock SJ, Moore CE, Justice A, et al. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun. 2002;70(9):4987–4996. doi:10.1128/IAI.70.9.4987-4996.2002
33. Tristan A, Ying L, Bes M, Etienne J, Vandenesch F, Lina G. Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. J Clin Microbiol. 2003;41(9):4465–4467. doi:10.1128/JCM.41.9.4465-4467.2003
34. Campbell SJ, Deshmukh HS, Nelson CL, et al. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol. 2008;46(2):678–684. doi:10.1128/JCM.01822-07
35. Lin T, Li Q, Jin D, Liu W, Tang C, Zhang X. Investigation of Virulence Genes of Staphylococcus aureus Isolated from Sterile Body Fluid Samples and Their Correlation with Clinical Symptoms and Outcomes. Can J Infect Dis Med Microbiol. 2021;2021:1–8. doi:10.1155/2021/5354747
36. Jarraud S, Mougel C, Thioulouse J, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002;70(2):631–641. doi:10.1128/IAI.70.2.631-641.2002
37. Kumar JD, Negi YK, Gaur A, Khanna D. Detection of virulence genes in Staphylococcus aureus isolated from paper currency. Inter J Infect Dis. 2009;13(6):e450–e455. doi:10.1016/j.ijid.2009.02.020
38. Růžičková V, Voller J, Pantůček R, Petráš P, Doškař J. Multiplex PCR for detection of three exfoliative toxin serotype genes inStaphylococcus aureus. Folia Microbiol. 2005;50(6):499–502. doi:10.1007/BF02931437
39. Li X, Fang F, Zhao J, et al. Molecular characteristics and virulence gene profiles of Staphylococcus aureus causing bloodstream infection. Braz J Infect Dis. 2018;22(6):487–494. doi:10.1016/j.bjid.2018.12.001
40. Strommenger B, Braulke C, Pasemann B, Schmidt C, Witte W. Multiplex PCR for rapid detection of Staphylococcus aureus isolates suspected to represent community-acquired strains. J Clin Microbiol. 2008;46(2):582–587. doi:10.1128/JCM.01600-07
41. Becker K, Roth R, Peters G. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J Clin Microbiol. 1998;36(9):2548–2553. doi:10.1128/JCM.36.9.2548-2553.1998
42. Solati SM, Tajbakhsh E, Khamesipour F, Gugnani HC. Prevalence of virulence genes of biofilm producing strains of Staphylococcus epidermidis isolated from clinical samples in Iran. AMB Express. 2015;5(1):47. doi:10.1186/s13568-015-0134-3
43. Salamandane A, Oliveira J, Coelho M, et al. Enterotoxin- and Antibiotic-Resistance-Encoding Genes Are Present in Both Coagulase-Positive and Coagulase-Negative Foodborne Staphylococcus Strains. Appl Microbiol. 2022;2(2):367–380. doi:10.3390/applmicrobiol2020028
44. Moura GS, Mota RA, Marques MFS, et al. Gangrenous mastitis in sheep caused by multidrug-resistant Staphylococcus haemolyticus. Pesquisa Veterinária Brasileira. 2020;40(12):947–954. doi:10.1590/1678-5150-pvb-6658
45. Acosta AC, Oliveira PRF, Albuquerque L, et al. Frequency of Staphylococcus aureus virulence genes in milk of cows and goats with mastitis. Pesquisa Veterinária Brasileira. 2018;38(11):2029–2036. doi:10.1590/1678-5150-pvb-5786
46. Al-Amery K, Elhariri M, Elsayed A, et al. Vancomycin-resistant Staphylococcus aureus isolated from camel meat and slaughterhouse workers in Egypt. Antimicrob Resist Infect Control. 2019;8(129). doi:10.1186/s13756-019-0585-4
47. Ho YM, Sun Y-J, Wong S-Y, Lee ASG. Contribution of dfrA and inhA Mutations to the Detection of Isoniazid-Resistant Mycobacterium tuberculosis Isolates. Antimicrob Agents Chemother. 2009;53(9):4010–4012. doi:10.1128/AAC.00433-09
48. Burgold-Voigt S, Monecke S, Simbeck A, et al. Characterisation and Molecular Analysis of an Unusual Chimeric Methicillin Resistant Staphylococcus Aureus Strain and its Bacteriophages. Front Genet. 2021;12:723958. doi:10.3389/fgene.2021.723958
49. Zhou S, Hu X, Wang Y, Fei W, Sheng Y, Que H. The Global Prevalence of Methicillin-Resistant Staphylococcus Aureus in Patients with Diabetic Foot Ulcers: a Systematic Review and Meta-Analysis. Diabetes Metabolic Syndrome Obesity. 2024;17:563–574. doi:10.2147/DMSO.S446911
50. Arfaoui A, Sallem R, Fernández-Fernández R. Methicillin-Resistant Staphylococcus aureus from Diabetic Foot Infections in a Tunisian Hospital with the First Detection of MSSA CC398-t571. Antibiotics. 2022;11(12):1755. doi:10.3390/antibiotics11121755
51. Chen Y, Yang J, Wang Y, et al. Community-associated methicillin-resistant Staphylococcus aureus infection of diabetic foot ulcers in an eastern diabetic foot center in a tertiary hospital in China: a retrospective study. BMC Infect Dis. 2023;23(1):652. doi:10.1186/s12879-023-08631-z
52. Perim MC, Celeste SRC, Orsolin EF, et al. Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev Soc Bras Med Trop. 2015;48(5):546–554. doi:10.1590/0037-8682-0146-2015
53. Rodrigues CF. Self-medication with antibiotics in Maputo, Mozambique: practices, rationales and relationships. Palgrave Commun. 2020;6(1):1–12. doi:10.1057/s41599-019-0385-8
54. Van der Meeren BT, Millard PS, Scacchetti M, et al. Emergence of methicillin resistance and Panton-Valentine leukocidin positivity in hospital- and community-acquired Staphylococcus aureus infections in Beira, Mozambique. Tropical Medicine and International Health. 2014;19(2):169–176. doi:10.1111/tmi.12221
55. Vubil D, Garrine M, Ruffing U, et al. Molecular Characterization of Community Acquired Staphylococcus aureus Bacteremia in Young Children in Southern Mozambique, 2001–2009. Front Microbiol. 2017;8. doi:10.3389/fmicb.2017.00730.
56. Wada FW, Mekonnen MF, Sawiso ED, et al. Bacterial profile and antimicrobial resistance patterns of infected diabetic foot ulcers in sub-Saharan Africa: a systematic review and meta-analysis. Sci Rep. 2023;13(1):14655. doi:10.1038/s41598-023-41882-z
57. Ehsan H. Antibiotic Resistance in Developing Countries: emerging Threats and Policy Responses. Public Health Challenges. 2025;4(1). doi:10.1002/puh2.70034
58. Iskandar K, Molinier L, Hallit S, et al. Surveillance of antimicrobial resistance in low- and middle-income countries: a scattered picture. Antimicrob Resist Infect Control. 2021;10(1):63. doi:10.1186/s13756-021-00931-w
59. Víquez-Molina G, Aragón-Sánchez J, Pérez-Corrales C, Murillo-Vargas C, López-Valverde ME, Lipsky BA. Virulence Factor Genes in Staphylococcus aureus Isolated From Diabetic Foot Soft Tissue and Bone Infections. Int J Low Extrem Wounds. 2018;17(1):36–41. doi:10.1177/1534734618764237
60. Shettigar K, Jain S, Bhat DV, et al. Virulence determinants in clinical Staphylococcus aureus from monomicrobial and polymicrobial infections of diabetic foot ulcers. J Med Microbiol. 2016;65(12):1392–1404. doi:10.1099/jmm.0.000370
61. Zhao H, Xu S, Yang H, et al. Molecular Typing and Variations in Amount of tst Gene Expression of TSST-1-Producing Clinical Staphylococcus aureus Isolates. Front Microbiol. 2019;10. doi:10.3389/fmicb.2019.01388.
62. Mishra AK, Yadav P, Mishra A. A Systemic Review on Staphylococcal Scalded Skin Syndrome (SSSS): a Rare and Critical Disease of Neonates. Open Microbiol J. 2016;10(1):150–159. doi:10.2174/1874285801610010150
63. Kananizadeh P, Moghadam SO, Sadeghi Y, Foroushani AR, Adibi H, Pourmand MR. Molecular characteristics of methicillin-resistant staphylococcus aureus (MRSA) isolated from diabetic foot infection. Iran J Pathol. 2019;14(4):329–337. doi:10.30699/IJP.2019.101092.2035
64. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9(8):978–984. doi:10.3201/eid0908.030089
65. Abraham NM, Jefferson KK. Staphylococcus aureus clumping factor B mediates biofilm formation in the absence of calcium. Microbiology. 2012;158(6):1504–1512. doi:10.1099/mic.0.057018-0
66. Ortega E, Abriouel H, Lucas R, Gálvez A. Multiple Roles of Staphylococcus aureus Enterotoxins: pathogenicity, Superantigenic Activity, and Correlation to Antibiotic Resistance. Toxins. 2010;2(8):2117–2131. doi:10.3390/toxins2082117
67. Roetzer A, Gruener C, Haller G, Beyerly J, Model N, Eibl M. Enterotoxin Gene Cluster-Encoded SEI and SElN from Staphylococcus aureus Isolates are Crucial for the Induction of Human Blood Cell Proliferation and Pathogenicity in Rabbits. Toxins. 2016;8(11):314. doi:10.3390/toxins8110314
68. Silva V, Almeida L, Gaio V, et al. Biofilm formation of multidrug-resistant mrsa strains isolated from different types of human infections. Pathogens. 2021;10(8):970. doi:10.3390/pathogens10080970
69. Turner MJ, van Vuuren S, Leigh-De Rapper S. Analysing patient factors and treatment impact on diabetic foot ulcers in South Africa. S Afr J Sci. 2024;120(3–4). doi:10.17159/sajs.2024/16301
70. Kuguyo O, Mukona DM, Chikwasha V, Gwanzura L, Chirenda J, Matimba A. Prevalence and risk factors for diabetic foot complications among people living with diabetes in Harare, Zimbabwe: a cross-sectional study. BMC Public Health. 2024;24(1):677. doi:10.1186/s12889-023-17610-7
71. Madede T, Damasceno A, Lunet N, et al. Changes in prevalence and the cascade of care for type 2 diabetes over ten years (2005-2015): results of two nationally representative surveys in Mozambique. BMC Public Health. 2022;22(1). doi:10.1186/s12889-022-14595-7
72. Fontes F, Damasceno A, Jessen N, et al. Prevalence of overweight and obesity in Mozambique in 2005 and 2015. Public Health Nutr. 2019;22(17):3118–3126. doi:10.1017/S1368980019002325
73. Nutor JJ, Duodu PA, Agbadi P, Duah HO, Oladimeji KE, Gondwe KW. Predictors of high HIV+ prevalence in Mozambique: a complex samples logistic regression modeling and spatial mapping approaches. PLoS One. 2020;15(6). doi:10.1371/journal.pone.0234034
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

