Back to Journals » International Journal of Nanomedicine » Volume 20
Multifaceted Applications of Nanomaterials in Colorectal Cancer Management: Screening, Diagnostics, and Therapeutics
Received 18 February 2025
Accepted for publication 29 April 2025
Published 10 June 2025 Volume 2025:20 Pages 7271—7294
DOI https://doi.org/10.2147/IJN.S520616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
He Xin, Zhihui Chang, Meng Niu
Department of Radiology, Shengjing Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Meng Niu; Email [email protected] Zhihui Chang, Email [email protected]
Abstract: Colorectal cancer (CRC) is the third most common malignant tumor worldwide. Early detection and treatment of CRC can significantly improve patient survival and quality of life, while advanced-stage patients still face numerous challenges, such as drug resistance and adverse effects. Consequently, researchers are developing more efficient early screening and diagnostic strategies for CRC. Consequently, researchers are actively developing more efficient strategies for diagnosis and refined treatments. This review comprehensively examines the diverse applications of various nanomaterials in CRC management, including screening, diagnostic imaging, surgical guidance, drug delivery, radiotherapy, and modulation of the tumor microenvironment. Firstly, we explored how nanomaterials are revolutionizing CRC screening by enhancing the detection of early-stage tumors. In the realm of diagnostic imaging, nanomaterials are employed to improve the clarity and specificity of imaging modalities, thereby facilitating more accurate diagnoses. The review also examines the use of nanomaterials in surgical guidance, where they aid in the precise identification and removal of tumors, potentially improving surgical outcomes. Furthermore, the review underscores the significance of nanomaterials in drug delivery systems, which enable targeted therapy and reduce systemic side effects. We also discussed the role of nanomaterials in radio-sensitization, where they enhance the efficacy of radiotherapy by increasing the sensitivity of tumor cells to radiation. Additionally, the modulation of the tumor immune microenvironment using nanomaterials is highlighted as a promising strategy to induce immune response against cancer cells. Throughout the review, the mechanisms of action of these nanomaterials are meticulously examined, providing insights into how they interact with biological systems to achieve their therapeutic effects. The efficacy of these nanomaterials in overcoming drug resistance is also a focal point, as this is a critical factor in improving the long-term outcomes for CRC patients. In conclusion, while nanomaterials hold great promise for the management of CRC, addressing their biocompatibility and clinical translation challenges is crucial for their safe and effective application in clinical settings.
Keywords: Nanomaterials, Colorectal cancer, Clinical practice, Tumor microenvironment
Graphical Abstract:

Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors worldwide, significantly impacting global morbidity and mortality rates. According to the Global Cancer Statistics 2020, CRC ranks as the third most prevalent cancer globally, with over 1.9 million new cases and approximately 935,000 deaths annually.1 Despite advances in screening and treatment, CRC managing remains a significant clinical challenge. One of the primary challenges is the development of resistance to conventional chemotherapy drugs, which limits their effectiveness over time.2 Additionally, the side effects associated with chemotherapy and radiotherapy can be severe, impacting the patient’s quality of life and limiting the dosage that can be safely administered.3 Surgical options, while potentially curative in early-stage CRC, are often not viable for patients with advanced or metastatic disease. Furthermore, the heterogeneity of CRC tumors complicates treatment, as different genetic mutations and molecular profiles can influence the tumor’s response to therapy.1
Nanomaterials have gained significant attention in clinical applications, particularly in cancer treatment. Nanomaterials possess unique properties such as a high surface area to volume ratio, tunable physical and chemical characteristics, and the ability to interact with biological systems at the molecular level. Nanomaterials can be classified into several categories based on their composition and structure, including carbon-based nanomaterials (eg, carbon nanotubes, graphene), metal nanoparticles (eg, gold, silver), metal oxide nanoparticles (eg, iron oxide, zinc oxide), and mesoporous silica nanoparticles.4,5 Each class of nanomaterials has distinct properties that can be leveraged for specific medical applications. These nanomaterials can enhance drug delivery to tumor sites, improve imaging for more accurate diagnosis, and enhance drug efficacy and therapeutic effects compared to standard treatments.5
Some nanomaterials have used in the clinical management of CRC, offering enhanced therapeutic efficacy, targeted delivery, and reduced toxicity compared to traditional therapies (Table 1). Liposomal irinotecan (Onivyde®) is a significant example of a clinically approved nanomedicine for CRC treatment. It utilizes a liposomal delivery system to target tumor tissues via the Enhanced Permeability and Retention (EPR) effect, leading to DNA replication inhibition and apoptosis. Another FDA-approved nanoparticle is Ferumoxytol (Feraheme®), a superparamagnetic iron oxide nanoparticle used as an MRI contrast agent to detect lymph node metastasis.6 Additionally, Guardant360® has been approved for liquid biopsy to detect tumor gene mutations, utilizing nanomaterials for circulating tumor DNA (ctDNA) enrichment.7 A multitude of promising nanomaterials have already progressed to the clinical validation phase (Table 1). Some nanoparticles are under investigation to enhance chemotherapy efficacy and reduce toxicity. For instance, ASP3082 (NCT05382559) is being evaluated in a Phase 1 trial for its safety and tolerability in patients with advanced solid tumors, particularly those with KRAS G12D mutations. Nanoparticles are also being explored for lymph node mapping and staging in CRC. Carbon nanoparticles are being compared with indocyanine green to improve postoperative lymph node inspection (NCT04759820), and used for identifying metastatic nodes (NCT06783985). These ongoing clinical trials highlight the versatility and potential of nanoparticles in enhancing the precision and efficacy of CRC treatment. In this review, we will summarize the latest research progress on the application of nanotechnology in CRC, demonstrates significant potential in the early screening, diagnosis, treatment, and prognostic evaluation of CRC.
 |
Table 1 Summary of Nano Structures Used in Clinical Application or Trial for the Treatment and Diagnosis of CRC |
Advancements in Nanomaterial-Based Screening and Diagnosis of CRC
Advancements in Nanomaterial-Based Detection for CRC Screening
In the United States, CRC incidence and mortality have decreased by over 50% in the past 40 years, with screening estimated to account for more than half of this reduction.9 Colonoscopy has been particularly effective in reducing the risk of left-sided CRC; however, its efficacy for right-sided CRC is less pronounced. The quality of the colonoscopy procedure, including factors such as withdrawal time, plays a critical role in its effectiveness.10 While there is no universally ideal screening strategy, the best approach is influenced by factors such as economic feasibility and patient adherence. A range of nanomaterials and technologies have been explored for early CRC screening, including carbon nanotubes, dendrimers, gold nanoparticles, fluorescent nanospheres, nanotube arrays, and nanoparticle-based electrochemical sensing platforms (Table 2). These innovations have shown potential in distinguishing between healthy and tumor tissue samples.
 |
Table 2 Summary of Nano Structures Used for CRC Screening and Diagnosis |
Astolfi et al developed a sensor that utilizes gold nanoparticles decorated with tin and titanium oxides to detect volatile organic compounds (VOCs) in the blood of CRC patients. The sensor demonstrated a sensitivity of 80% and a specificity of 70% in differentiating healthy individuals from those with CRC.24 Similarly, Bhattacharyya employed functionalized titania nanotube arrays (TNAs) as a sensor to detect four VOCs associated with CRC, identifying these VOCs as a potential biomarker signature for CRC screening.20
The integration of nanoparticles with endoscopic procedures has further enhanced the efficiency of tumor screening. Zavaleta et al (2013) developed an optical fiber-based Raman spectroscopy device that uses surface-enhanced Raman scattering (SERS) nanoparticles as molecular imaging contrast agents. This technology provides real-time, multiplex functional information during conventional endoscopic procedures, helping endoscopists rapidly distinguish between normal and precancerous tissues, and identifying flat lesions that might otherwise go unnoticed.17 Additionally, Sakuma et al designed an innovative imaging agent for colonoscopy by immobilizing Peanut Agglutinin (PNA) on fluorescent nanospheres. In an animal model of human CRC in situ, PNA effectively indicated the invasion of implanted cancer cells on the mucosal side. The PNA-immobilized fluorescent nanospheres were able to recognize millimeter-sized tumors on the caecal mucosa with high specificity and affinity.16
The SEPT9 gene methylation (mSEPT9) assay is a blood-based test that offers moderate sensitivity (69%) and high specificity (92%) for CRC detection. However, it has limitations, including reduced effectiveness in detecting precancerous lesions and relatively high costs.26 In response to these challenges, Hanoglu et al developed an electrochemical sensing system based on magnetic nanoparticles, which is capable of selectively quantifying mSEPT9. The system has a limit of detection (LOD) as low as 0.37% and is suitable for point-of-care (POC) applications, enabling rapid, one-step detection.25
The identification of bacterial communities associated with CRC, such as Parvimonas micra and Fusobacterium nucleatum, has led to the development of highly specific aptamers. A colorimetric aptasensor using Au@Fe3O4 nanoparticles demonstrated high affinity towards P. micra, enabling CRC screening with a detection limit of 11 CFU/mL. Similarly, an aggregation-induced emission nanobioprobe for F. nucleatum showed a LOD of 82.97 CFU/mL, highlighting the potential of these bacteria as biomarkers for noninvasive CRC screening.14,15
Advancements in Nanomaterial-Based Detection of Circulating Tumor Biomarkers in CRC
CTC Detections
Circulating biomarkers, including circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and microRNAs (miRNAs), hold significant promise for the early detection, diagnosis, and prognosis of CRC.27 In early-stage CRC patients, the number of CTCs in the bloodstream is typically very low, making their detection a critical focus of current research. The development and application of various nanomaterials have greatly enhanced the capture efficiency and analytical accuracy of CTC detection.
Pan et al23 developed ratiometric nanosensors capable of detecting CTCs by responding to changes in intracellular hydrogen peroxide levels, thus enabling the assessment of CTC activity. These sensors feature dual fluorescence emission peaks, which significantly improve the accuracy and reliability of detection. Additionally, Kuai et al21 utilized EGFR-targeted immunomagnetic liposomes (EILs) to capture CTCs, demonstrating superior capture efficiency and specificity compared to traditional EpCAM immunomagnetic beads. This approach offers a promising strategy for the diagnosis of CRC.
Antibody-loaded nanoplatforms have shown considerable potential in recognizing tumor cells and biomarkers, addressing the limitations of conventional screening methods.28 Xie et al19 found that nanomaterials dual-functionalized with antibodies significantly improved the capture specificity of CTCs. Moreover, the use of multi-Sialyl Lewis X antibodies (aSlex) conjugated to PAMAM dendrimers for the specific binding and capture of colon cancer cells (HT29) demonstrated a concentration-dependent enhancement in capture efficiency.18 These conjugates of multi-Sialyl Lewis X antibodies with PAMAM dendrimers specifically bind and capture HT29 colon cancer cells, showing a clear increase in capture efficiency with higher concentrations. Huang et al22 proposed a density gradient centrifugation method using gelatin nanoparticle-coated silica beads for the separation, release, and analysis of CTCs. This method not only improves the efficiency and purity of CTC capture but also preserves the viability of CTCs, thereby enabling subsequent molecular and functional analyses.
Other Biomarkers
In addition to circulating tumor cells (CTCs), other molecular markers have also demonstrated enhanced detection sensitivity through the application of nanomaterials. For instance, a study designed a nanozyme-based pump-free microfluidic chip for capturing and detecting circulating cancer stem cells (CCSCs) in peripheral blood and fecal samples. The use of CoPt3@HA probes for magnetic separation and colorimetric signal transduction enabled a detection limit of 3 cells per milliliter.12 MicroRNAs (miRNAs) also serve as effective markers for the early detection of CRC. A Surface-Enhanced Raman Scattering (SERS) biosensor was developed using Au nanocage@Au nanoparticles and Ag-coated Fe₃O₄ magnetic nanoparticles. This biosensor achieved detection limits of 3.46 aM for miR-21 and 6.49 aM for miR-31, while also demonstrating high specificity and anti-interference ability.13
In conclusion, nanomaterials have shown substantial potential in enhancing the diagnostics of CRC, ranging from the detection of genetic alterations to the identification of microbial biomarkers. However, challenges remain (Table 2). For instance, some technologies may require complex instrumentation or sample preparation procedures. Additionally, the cost-effectiveness and scalability of these methods need further investigation to ensure their practicality in diverse healthcare settings.
Nanomaterial-Enhanced Radiological Examination for CRC Diagnosis
Imaging detections provides detailed anatomical and functional information for tumor. However, it still faces challenges such as insufficient signal intensity and poor specificity. Nanomaterials, with their unique physicochemical properties, hold significant promise in enhancing the diagnostic accuracy of CRC by targeting tumors, boosting signal strength, and improving imaging contrast. Furthermore, they offer potential for real-time treatment monitoring (Table 3).
 |
Table 3 Advances in the Application of Nanomaterials for Imaging and Surgical Guidance in CRC |
Nanomaterials can serve as MRI contrast agents to improve tumor imaging quality. For instance, MnO2-coated nanoparticles, which undergo reduction reactions in the tumor microenvironment, have been successfully applied to achieve T1-weighted MRI imaging.38 Additionally, nanomaterials enable multimodal imaging techniques, combining MRI with other imaging modalities such as CT and fluorescence imaging. Fe3O4 nanoparticles, for example, have been employed to achieve dual-modal MRI and CT imaging of CRC.39 Additionally, nanomaterials facilitate molecular-level imaging by targeting tumor-specific molecules. The CEACAM5-targeted probe 2D5-IRDye800CW, which utilizes near-infrared-II (NIR-II) fluorescence, is crucial for real-time surgical guidance. This probe rapidly accumulates in tumors within 15 minutes and can detect tumors smaller than 2 mm29 Another example is [68Ga]Ga-HNI01, a CEA-targeted PET imaging radiotracer that can be synthesized within 10 minutes and achieves radiochemical purity exceeding 98%. It enables the detection of metastases in the liver, lung, and pancreas with high contrast.31 Furthermore, a novel nanobody Nb41 has been identified for PET and NIR fluorescence imaging, demonstrating significant accumulation in tumors and lymph node metastases.32
The use of nanomaterials in imaging diagnosis offers several advantages, including improved sensitivity and specificity for tumor detection. However, potential off-target effects and toxicity remain concerns, despite no adverse events reported in these studies. It is important to note that these studies are primarily preclinical, and further clinical trials are necessary to validate the safety and efficacy of these nanomaterials in humans.
Advancements in Nanomaterial-Based Therapy for CRC
Nanomaterial-Enhanced Precision Surgery in CRC
Surgery remains the cornerstone of CRC treatment, offering the potential for a cure in localized disease. Complete mesocolic excision and systematic lymph node dissection is critical for optimal outcomes. Recent advances include laparoscopic, robotic, and transanal total mesorectal excision techniques.40 With the progress of precision medicine and minimally invasive surgical approaches, the unique properties of nanomaterials are playing an increasingly pivotal role in improving surgical navigation, enhancing tumor identification, and optimizing therapeutic outcomes. This section explores the various applications of nanomaterials in CRC surgery (Table 3).
Several studies have shown that preoperative use of carbon nanoparticles (CNs) enhances lymph node detection. A meta-analysis of 1241 patients found that CNs led to an average of 5.21 additional lymph nodes detected per patient (mean difference = 5.21, 95% CI = 4.14–6.29, p < 0.001) and a 68% increase in the detection of micro lymph nodes (RR = 1.68, 95% CI = 1.38–2.04, p < 0.001). CNs also identified more metastatic lymph nodes (RR = 1.56, 95% CI = 1.40–1.75, p < 0.001), although the overall metastatic detection rate was similar between groups. In rectal cancer patients, the CN+ group had a higher number of lymph nodes retrieved at stations 251, 252, and 253, with 54.0% of patients retrieving ≥4 nodes at station 253 (vs 28.3%, p = 0.004). The retrieval of ≥4 nodes at station 253 was an independent risk factor for metastasis (odds ratio: 2.40, 95% CI: 1.22–4.74, p = 0.012).41 Another study analyzing 768 patients undergoing radical resection for rectal cancer found that the CNs group had a significantly higher total number of lymph nodes retrieved (p < 0.001), a significantly shorter total time for lymph node detection (p < 0.001), and a significantly increased percentage of lymph nodes <5mm in size (p < 0.001). Among patients with clinical stages I/II, there was a significant difference in positive lymph nodes (21.79% vs 11.95%, p = 0.029).35 These studies indicate that CNs are effective lymphatic tracers in CRC surgery, aiding in determining the extent of surgery and assessing prognosis.35,42
Wang et al employed preoperative endoscopic localization of CRC and used carbon nanoparticles for lymph node tracing during laparoscopic surgery. The experimental group demonstrated a higher average number of lymph nodes removed compared to the control group, with a greater proportion of patients in the experimental group achieving ≥12 lymph nodes resected.43 Additionally, Zinc-gallium-germanate (ZGC) nanoparticles doped with Cr³+ and modified with folic acid (ZGC-FA) exhibited strong near-infrared (NIR) luminescence with a signal-to-noise ratio of 23.9 in vivo. In a luciferase-expressing CRC model, 50 minutes after injection, the luminescence signal aligned closely with the tumor boundary (98% overlap), enabling complete tumor resection with minimal healthy tissue removal (2.3%). This improved surgical precision, reduced normal tissue damage, and lowered tumor recurrence, enhancing patient prognosis.44
Ma et al utilized a NIR-II organic donor-π-acceptor-π-donor probe, FE-2PEG, for high-resolution in vivo imaging of CRC. This probe exhibited bright fluorescence at 1100 nm and excellent photostability. Under fluorescence-guided surgery (FGS) using NIR-II fluorescence, the probe facilitated the resection of peritoneal micro-metastases with a sensitivity of 94.51%, specificity of 86.59%, a positive predictive value of 96.57%, and a negative predictive value of 79.78%. Notably, even for micro-metastases smaller than 3 mm, the positive predictive value remained as high as 96.07%.37
These advancements in CRC surgery underscore the critical role of nanomaterials in enhancing surgical precision. Preoperative use of carbon nanoparticles improves lymph node detection and metastatic identification, aiding in more accurate staging and prognosis. Nanoparticles such as ZGC-FA and NIR-II probes offer superior tumor localization, enabling complete resection with minimal damage to healthy tissue, ultimately improving surgical outcomes and reducing recurrence.
Nanomaterial-Enhanced Radiotherapy in CRC
Radiotherapy plays a crucial role in the treatment of CRC, yet it is limited by challenges such as non-specific tissue damage and suboptimal efficacy. Recent advancements in nanotechnology have introduced innovative strategies to enhance the efficacy of radiotherapy by improving tumor targeting, radiosensitization, and immune modulation (Table 4).
 |
Table 4 Advances in the Application of Nanomaterials in Radiotherapy for CRC |
Radiosensitization
Gold nanoparticles (AuNPs) and carbon nanotubes (CNTs), among other nanomaterials, have demonstrated significant radiosensitizing effects in CRC treatment. AuNPs, owing to their unique optical properties, can enhance X-ray absorption, thereby increasing the radiation dose delivered to tumor tissues and improving the overall efficacy of radiotherapy. Additionally, AuNPs generate localized high temperatures upon laser irradiation, enabling a synergistic therapeutic effect in CRC treatment.66 AuNPs co-loaded with carboplatin in liposomes (LipoGold) have shown notable radiosensitizing effects in HCT116 cells, significantly enhancing tumor cell killing efficiency.67 Moreover, the combination of 17-AAG with AuNPs and radiation has been shown to significantly inhibit cell viability and induce apoptosis.68 In addition to AuNPs, CNTs, known for their excellent mechanical strength and thermal conductivity, have gained attention as effective radiosensitizers in radiotherapy. Studies indicate that CNTs can enhance the radiosensitivity of tumor cells, thereby improving the cytotoxic effects of radiotherapy. When combined with chemotherapy agents, CNTs further augment the radiosensitizing effects in CRC, significantly increasing tumor cell apoptosis.69 Additionally, bismuth disulfide nanoparticles (Bi2S₃@BSA-MTX NPs) combined with methotrexate have been shown to significantly reduce the survival rate of SW480 cells and exhibit enhanced radiosensitizing effects under X-ray irradiation.70 The use of X-ray-activated lanthanide-doped scintillators (LNS) combined with a photosensitive NO precursor to generate peroxynitrite in situ. This approach not only enhances radiosensitization but also promotes the polarization of tumor-associated neutrophils (TANs) from an immunosuppressive N2 phenotype to an anti-tumor N1 phenotype.56
Nanoparticles not only act as radiotherapy sensitizers but also inhibit tumor cell growth by modulating the tumor immune microenvironment. Wang L et al64 investigated the use of STING agonist cGAMP-loaded nanoparticles (DMPtNPS) in rectal cancer cells. As catalytic radiosensitizers, DMPtNPS facilitate X-ray energy transfer, generate reactive oxygen species, alleviate tumor hypoxia, and enhance radiosensitivity. The study demonstrated that DMPtNPS, when combined with radiotherapy, induced a dual regulatory effect on the immune response. Moreover, cGAMP reversed the negative impact of DMPtNPS and radiotherapy on the tumor immune microenvironment via a type I interferon-dependent pathway, thereby promoting cancer immunotherapy.
The integration of nanomaterials with antitumor drugs and radioactive isotopes has further enhanced tumor suppression and improved nuclear medicine treatments. In mouse models, metal-organic framework (MOF) nanoparticles, such as Hf-DBB-Ru developed by Ni et al,71 significantly reduced colorectal tumor size through combined radiotherapy and radiosensitization therapy (RDT). Additionally, DuRoss et al,72 developed targeted nanoparticles that enhanced chemotherapy and radiotherapy effects in CRC mouse models by targeting P-selectin. Similarly, Meng L et al73 developed manganese dioxide-modified albumin-bound paclitaxel nanoparticles, which improved the hypoxic tumor microenvironment and enhanced both chemotherapy and radiotherapy efficacy in CRC mouse models. The use of MIL-101(Cr)-NH2 MOFs as carriers for radioactive isotopes has also demonstrated efficient tumor-targeted delivery, improving nuclear medicine treatment.74 Furthermore, the development of a multifunctional nanoplatform (CCS) that integrates cetuximab for tumor targeting, Ni2+/Mn2+-doped carbon dots for NIR-II photothermal therapy (PTT), chemodynamic therapy (CDT), and photothermal/magnetic resonance/fluorescence imaging (PTI/MRI/FLI). In vitro and in vivo experiments demonstrated that CCS significantly inhibited tumor growth without detectable cytotoxicity.58
Overall, nanomaterials enhance the efficacy of radiotherapy in CRC by increasing tumor cell radiosensitivity, amplifying local radiation effects, and synergizing with chemotherapy, ultimately leading to cancer cell death via various mechanisms.
Nanomaterial-Enhanced Precision Chemotherapy in CRC
Chemotherapy plays a vital role in CRC treatment, significantly improving survival rates. In metastatic CRC, sequential use of FOLFOX and FOLFIRI regimens can extend median overall survival to approximately 20 months. The addition of targeted therapies, such as bevacizumab and cetuximab, has further improved outcomes, pushing median survival beyond two years in advanced CRC.75 However, drug resistance, driven by mechanisms such as reduced drug uptake and enhanced drug efflux, remains a major challenge. To address these issues, novel strategies are being explored to improve drug delivery and efficacy. Nanocarriers, including liposomes, solid lipid nanoparticles, and silica nanoparticles, have shown promise in enhancing the delivery and efficacy of oxaliplatin while minimizing side effects.76
Recent Advances in Nanomaterials for Drug Delivery and Chemotherapy Sensitization
Nanotechnology -based drug delivery systems have shown significant potential in improving the therapeutic efficacy of CRC treatments by enhancing drug solubility, stability, bioavailability, and reducing toxic side effects (Table 5).
 |
Table 5 Summary of Nanomaterial-Based Drug Delivery Systems and Mechanisms in Colorectal Cancer Research |
The nanoparticle-drug conjugate CRLX101, administered at 15 mg/m² weekly in neoadjuvant chemotherapy and radiotherapy for locally advanced rectal cancer, achieved a pathological complete response (pCR) rate of 19%, with a notable 33% pCR rate at the weekly dose.88 Ferritin nanoparticles (Ft NPs) and mucin 1 (MUC1) aptamer-targeted delivery of epirubicin (Epi) to CRC cells resulted in Apt-Epi Ft NPs, with an average size of 37.9 nm and an encapsulation efficiency of 67%. In acidic media, the drug release rate reached 90% within 4 hours, and targeted delivery significantly enhanced Epi’s anticancer effects both in vitro and in vivo. scFv biofunctionalized nanoparticles, created by conjugating an anti-CEA single-chain variable fragment (scFv), MFE-23, with PLGA-PEG polymers to deliver 5-fluorouracil (5-FU), achieved a threefold increase in cellular uptake by CEA-expressing CRC cells compared to non-targeted counterparts. The cytotoxicity of 5-FU-loaded nanoparticles was significantly higher in CEA-expressing cells after 24 h and 48 h of treatment, highlighting the specificity and efficacy of this targeted delivery system.78 Liposome-polymer hybrid nanoparticles (Cet-Iri-NPs), composed of PPG-PEG copolymer, lipid DSPE-PEG-Mal, and lecithin as carriers, were designed for targeted EGFR therapy in CRC. These nanoparticles, loaded with CPT-11 as the chemotherapy agent, indocyanine green (ICG) as the photothermal agent, and cetuximab as the targeting ligand, demonstrated significant photothermal effects. Upon near-infrared (NIR) laser irradiation, Cet-Iri-NPs facilitated faster CPT-11 release, inducing cell death in SW480 cells and inhibiting tumor growth in a xenograft mouse model.65 A layered double hydroxide (LDH) nanoparticle system, co-loaded with the anticancer drugs 5-fluorouracil (5FU) and albumin-bound paclitaxel (Abraxane, ABX), effectively delivered both drugs to colon cancer cells (HCT-116). This system synergistically induced apoptosis, accumulating efficiently at the tumor site and significantly inhibiting tumor growth after three intravenous injections, without detectable side effects.89
Calcium carbonate (CaCO3) nanoparticles loaded with curcumin (Cur) and the HDAC inhibitor QTX125, coated with hyaluronic acid (HA), demonstrated efficient cellular uptake and significant growth inhibition in CRC cells, including patient-derived organoid models. These nanoparticles exhibited a uniform spherical morphology with diameters around 450 nm and a Zeta potential of −8.11 mV.77 Meanwhile, diselenide-bridged nanovesicles encapsulating OSMI-1, an O-GlcNAc transferase inhibitor, demonstrated superior efficacy in reducing CRC cell proliferation, migration, and invasion both in vitro and in vivo compared to conventional nanovesicles.87 pH/cathepsin B sequential responsive nanoparticles (PSRNs) were designed for precise intracellular delivery of PROTACs targeting CDK4/6. Finally, genetically engineered ferritin nanoparticles (mHFn) represent another breakthrough, delivering mitoxantrone (MTO) specifically to tumor tissues for combined chemotherapy and photothermal therapy. Upon irradiation with a 660 nm laser, thermal-sensitive channels on mHFn facilitated efficient MTO release, generating reactive oxygen species and inducing significant tumor cell death.85
Collectively, these studies underscore the versatility and potential of nanomaterial-based strategies in overcoming the limitations of traditional chemotherapy. By enhancing cellular uptake, enabling targeted delivery, and modulating the tumor microenvironment, these innovations offer promising avenues for advancing CRC treatment.
Nanotechnology-Enhanced Therapies for CRC: Overcoming Resistance and Enhancing Efficacy
Chemotherapy’s lack of specificity for cancer cells often results in severe systemic toxicity, limiting its effectiveness. Targeted therapy, as a selective drug delivery system (SDDS), offers a promising strategy to reduce side effects. DNA nanocrosses (Holliday junctions, or HJ) have been used to target doxorubicin (Dox) delivery to colon cancer cells, significantly enhancing antitumor effects in vivo without increasing toxicity.90 Additionally, embedding superparamagnetic Fe3O4 nanoparticles (~10 nm) into chitosan polyelectrolyte complexes (PECs) enables efficient irinotecan (IRT) delivery to tumors under magnetic guidance, overcoming chemotherapy’s toxic side effects.91
Oxaliplatin resistance, a major obstacle in CRC treatment, contributes to recurrence and metastasis. Colorectal cancer cells secrete high levels of hyaluronic acid (HA), which mediates resistance to chemotherapy. The degradation product of HA, hyaluronic acid oligosaccharide (oHA), can reverse this resistance. OHA-loaded oxaliplatin liposome nanoparticles (oHA-Lipid-Oxa), synthesized via the thin-film hydration method, exhibit excellent tissue compatibility and targeting ability. These nanoparticles significantly suppress tumor growth, increase lymphocyte and macrophage infiltration, and do so without causing significant weight loss in mice. This suggests that oHA-Lipid-Oxa enhances oxaliplatin sensitivity while minimizing adverse effects.92 Furthermore, a nanodiamond platform enables oral chemotherapy combined with photothermal therapy, improving drug accumulation in tumor tissues and enhancing therapeutic efficacy through near-infrared laser-responsive drug release.93
In summary, the integration of nanotechnology into CRC treatment has led to significant advances in targeted drug delivery, overcoming chemotherapy resistance, and improving therapeutic outcomes with fewer side effects.
Nanomedicines Target Cancer Stem Cells to Control Tumor Growth
Cancer stem cells (CSCs) are a subpopulation of tumor cells that contribute to chemotherapy resistance, tumor recurrence, and metastasis, making them key targets for effective therapeutic interventions. Nanomaterials, through precise targeting and controlled drug release, can enhance chemotherapeutic efficacy while minimizing damage to normal cells. Magnetothermal therapy (MHT) combined with local chemotherapy effectively suppresses colorectal CSCs. Iron oxide nanocubes, used as heat mediators for MHT, are coated with a thermoresponsive polymer (TR-Cubes) and loaded with doxorubicin (TR-DOXO) or oxaliplatin. Cells exposed to both chemotherapy agents and MHT show reduced colony formation, and tumor growth is undetectable in exposed mouse models.94
Nanomaterials Targeting the Tumor Microenvironment for CRC Treatment
Tumor immune microenvironment (TIME) plays a crucial role in tumor progression and therapeutic response, with immunosuppressive elements often hindering effective treatment. Nanomaterials offer significant advantages by specifically targeting and modulating the TIME to enhance antitumor immunity, overcoming limitations of traditional therapies. Some nanomedicines induce reactive oxygen species (ROS) production, kill tumor cells, stimulate the release of immunogenic cells, enhance T-cell infiltration, and reverse immunosuppression (Table 6).
 |
Table 6 Advances in the Application of Nanomaterials for Modulating Tumor Microenvironments in CRC |
Targeting Immune Checkpoints and Signaling Pathways
In a liver metastasis mouse model, the combination of nanomedicine (NCG) and anti-PD-L1 inhibited the growth of colon cancer liver metastasis. NCG contains the transforming growth factor-β receptor inhibitor galunisertib (Gal) and the photosensitizer chlorin e6 (Ce6) generates ROS under ultrasound irradiation, leading to tumor immunogenic cell death and the release of immunostimulatory signals. This also induces M1-like polarization of tumor-associated macrophages and disrupts the immunosuppressive barrier of tumor-associated fibroblasts, increasing effector T cell infiltration, reversing tumor immunosuppression, and enhancing the efficacy of anti-PD-L1 antibodies.63 Additionally, a highly stable cerasomal nano-modulator (DMC@P-Cs) has been developed, effectively accumulating in tumor tissues. It carries the immunotherapeutic adjuvant demethylcantharidin (DMC) in its hydrophilic core. Under ultrasound induction, DMC@P-Cs generates ROS to kill tumor cells and induce immunogenic cell death (ICD), while releasing DMC to downregulate regulatory T cells (Tregs) and enhance antitumor immune responses. The tumor inhibition rate reached 94.73%, with no significant toxic side effects observed.121 A dual-targeted nano-delivery system (GOx@FeNPs) uses photothermal Fe3O4 nanoparticles to release tumor-specific antigens from tumor tissues, stimulating dendritic cell (DC) maturation in the lymph nodes and enhancing CD8+ T cell infiltration into the tumor. Combined with PD-L1, this system achieves a synergistic therapeutic effect, with a tumor suppression rate exceeding 90%.122
Ionizable STING-activating nanoadjuvants have been engineered to activate the STING pathway, inducing innate immunity and reshaping the TME. These nanoadjuvants demonstrated robust antitumor effects in mouse models of CRC, shifting the tumor immune landscape from immunosuppressed to tumoricidal.30 Similarly, STING agonist-loaded nanoparticles (DMPtNPS@cGAMP) have shown the ability to enhance type I interferon-dependent radioimmunotherapy, leading to durable complete responses at the primary site and enhanced abscopal effects at distant sites.64
Nanomaterials Modulating the Tumor Microenvironment by Polarizing Immune Cells
CRC tissues that are microsatellite stable/proficient in mismatch repair (MSS/pMMR) are typically considered immunologically “cold”, with poor immunogenicity and limited CD8+ T cell infiltration. Nanomaterials that can induce the polarization of immune cells towards a pro-inflammatory phenotype have shown potential in reshaping the TME. One approach utilizes nano-drug delivery of cyclin-dependent kinase (CDK) inhibitors to downregulate phosphorylated retinoblastoma and RNA polymerase II, arresting the G2/M cell cycle. This promotes the release of immunogenic signals, stimulates DC maturation, and enhances CD8+ T cell infiltration. This strategy has shown significant efficacy in activating immune responses in patient-derived xenograft and organoid models of CRC liver metastasis.123 In another study, inulin-based nanoparticles (UIRN) encapsulating the chemotherapeutic drug regorafenib have demonstrated the ability to increase the intratumoral concentration and accumulation time of the drug, leading to the polarization of tumor-associated macrophages (TAMs) towards the M1-type, which enhances antitumor immunity.117
In summary, nanomaterials are emerging as powerful agents to enhance CRC immunotherapy by modulating the tumor immune microenvironment, inducing immunogenic cell death, and synergizing with immune checkpoint inhibitors to achieve high tumor suppression rates. These approaches have demonstrated substantial efficacy in preclinical models, including immunologically cold tumors, offering promising strategies for overcoming treatment resistance and improving patient outcomes.
Nanomaterials Modulating the Tumor Microenvironment by Regulating the Microbiota
Nanomedicines enhance the efficacy of chemotherapeutic drugs by modulating tumor-associated bacteria. Fusobacterium nucleatum (F. nucleatum), prevalent in CRC, is linked to cancer cell proliferation, metastasis, and poor treatment outcomes. Incorporating highly immunostimulatory cholesterol-modified CpG oligonucleotides into autologous F. nucleatum membranes using nanovaccines significantly improves chemotherapy responses in F. nucleatum-infected CRC and reduces metastasis.124 Additionally, phage-guided nanotechnology markedly enhances chemotherapy efficacy in mouse models of CRC by inhibiting tumor-promoting F. nucleatum and boosting the effectiveness of first-line chemotherapeutic agents.125 Nano-selenium probiotic complexes (SeNVs@NE-IL32-EcN) have been developed to enhance CD8+ T cell-mediated immune responses and overcome immunotherapy resistance in CRC. This complex significantly improved the proliferation and activity of CD8+ T cells and reduced tumor progression in humanized mouse models.118
Nanomaterials Modulating the TME by Inducing Oxidative Stress and Immunogenic Cell Death
Nanomedicines can eliminate tumor cells by inducing the production of reactive oxygen species (ROS). In photodynamic therapy, photosensitizers are commonly used to generate ROS that target cancer cells. Nanoparticles were constructed via self-assembly of an amphiphilic hyperbranched polyphosphoester containing thioketal units and photosensitizers, synthesized through self-condensing ring-opening polymerization of a novel cyclic phosphate monomer and end-capped with Chlorin e6. These nanoparticles serve as drug carriers, loading camptothecin and maintaining stability in blood circulation. The Chlorin e6 in the nanoparticles effectively generates ROS to kill cancer cells.126 In another study, nanoparticles delivered to tumor tissues gradually released chemotherapeutic drugs and Mn2+ through glutathione (GSH)-triggered biodegradation. The released chemotherapeutic drugs not only exhibited potent anticancer effects but also enhanced H2O2 generation.38 Moreover, H2O2/ultrasound (US)-driven mesoporous manganese oxide (MnOx)-based nanomotors, upon entering the tumor microenvironment, decompose excess H2O2 into singlet oxygen via Mn2+ mediated Fenton-like reactions, inducing ferroptosis in tumor cells and releasing tumor antigens. This strategy directly kills cancer cells, reverses the immunosuppressive microenvironment, and boosts systemic antitumor immunity, thereby controlling both primary and distant tumors.127 These nanoformulations induce ROS production and ferroptosis, leading to direct tumor cytotoxicity and stimulating systemic antitumor immunity, thus offering a multifaceted approach to improving chemotherapy outcomes in CRC.
The use of nanomaterials to target the TME in CRC offers several advantages, including enhanced drug delivery, specific targeting of tumor cells, and the ability to modulate the immune response. However, these approaches also face limitations, such as potential toxicity, the need for precise targeting to avoid systemic side effects, and the challenge of translating preclinical success to clinical settings. Future research should focus on optimizing nanomaterial design to minimize toxicity, improve biocompatibility, and enhance therapeutic efficacy.
Conclusions
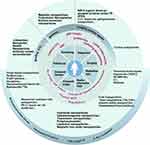
Nanomaterials, including polymeric nanoparticles, liposomes, micelles, and exosomes, have shown great potential in medicine due to their unique physicochemical properties. The applications of nanomaterials in CRC were summarized in Figure 1. These nanoscale formulations enhance drug solubility, bioavailability, and tumor specificity, potentially overcoming multidrug resistance and reducing toxic side effects.128 These materials have shown significant potential in enhancing the precision of drug delivery systems, enabling targeted therapies that minimize damage to healthy tissues while maximizing therapeutic efficacy. Given their ability to directly target tumor cells, nanomaterials have also shown considerable promise in tumor radiotherapy, enabling the selective destruction of cancer cells, activation of the immune microenvironment, and enhancement of radiotherapy efficacy. By leveraging tumor organoid technology to identify nanodrugs to which patients are most responsive and to evaluate potential toxicities, we can facilitate the selection of personalized therapeutic agents.
Additionally, the application of nanomaterials in tumor diagnosis and imaging has been steadily increasing. For instance, nanomaterials have demonstrated superior performance in the efficient capture of tumor biomarkers and the detection of minimal metastatic lesions. Nanomaterials offer enhanced efficiency in capturing circulating tumor cells and cell-free tumor DNA (ctDNA), thereby improving the sensitivity of early screening and diagnosis for CRC. Moreover, when combined with colonoscopy and intraoperative imaging, these materials can effectively identify micrometastases and delineate tumor margins from normal tissue, thus optimizing therapeutic strategies.
In addition to improving drug delivery efficiency, researchers are exploring alternative therapeutic approaches. Over the past few years, an increasing number of studies have reported the use of nanomaterials to target and modulate the tumor microenvironment. One strategy involves regulating the internal homeostasis of tumors, such as by altering levels of reactive oxygen species or modulating intratumoral microbiota, to control tumor progression. With the advent of immunotherapy, the tumor immune microenvironment has emerged as a novel therapeutic target. In the past two years, there has been a notable increase in studies utilizing nanomaterials to modulate immune cells within tumors or to activate tumor immune cells by regulating molecular pathways, thereby enhancing antitumor immune responses.
Moreover, researchers are developing nanomaterials to improve drug administration methods. For instance, oral nanotherapeutics have been designed to overcome gastrointestinal barriers, selectively target tumor cells, and exert therapeutic effects, potentially transforming the chemotherapy experience for patients. The development of these innovative technologies expands the scope of nanomaterial applications and holds promise for advancing cancer treatment strategies.
Challenges in the Clinical Translation of Nanomaterials
However, significant challenges remain in translating nanomedicines from the laboratory to clinical practice. Biocompatibility and potential toxicity are primary concerns for their clinical application. Studies have shown that the size, shape, and surface properties of nanomaterials can influence their distribution, metabolism, and excretion in the body, affecting their safety profiles. Additionally, many nanomaterials are fabricated from heavy metals such as copper (Cu) and gold (Au), and their potential toxic effects on the human body also need to be validated. Therefore, a comprehensive evaluation of the biocompatibility and toxicological properties of nanomaterials is crucial for their safe clinical use.
Another critical challenge is the large-scale production and quality control of nanomaterials. To ensure consistency and efficacy in clinical applications, standardized manufacturing processes and stringent quality control standards must be established. This includes rigorous monitoring of the physicochemical properties of nanomaterials, batch-to-batch consistency, and long-term stability. Additionally, the development of efficient synthesis methods and scalable production technologies is essential to reduce costs and improve yield.
In future studies, establishing standardized manufacturing processes and stringent quality control standards is crucial for ensuring consistency and efficacy in clinical applications. This includes rigorous monitoring of the physicochemical properties of nanomaterials, batch-to-batch consistency, and long-term stability. Additionally, further investigation into the biocompatibility and toxicity profiles of these nanomaterials is necessary to ensure their safety and effectiveness in patients. As nanotechnology continues to advance, it is expected that more nanomaterials will successfully transition into clinical applications, providing patients with safer and more effective therapeutic options.
Highlights
- Nanomaterials Revolutionize CRC Management: Enhancing Screening, Diagnostics, and Therapeutics.
- Advanced Nanotechnologies Improve Early Detection and Precision Surgery Outcomes.
- Nanomaterials enable precise targeting of tumor cells, thereby enhancing the efficacy of radiotherapy and chemotherapy;
- Nanomaterials specifically target the tumor microenvironment, modulating reactive oxygen species and activating tumor-infiltrating immune cells.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the National Natural Science Foundation of China (General Program, Award No. 62371474); National Key Research and Development Program of China (Award No. 2023YFC2308405); Natural Science Foundation of Liaoning Province (Award No. 2023JH2/101600006).
Disclosure
The authors declare that they have no competing interests.
References
1. Morgan E, Arnold M, Gini A, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–344. doi:10.1136/gutjnl-2022-327736
2. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233–254. doi:10.3322/caac.21772
3. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi:10.3322/caac.21834
4. Yan L, Zhao F, Wang J, Zu Y, Gu Z, Zhao Y. A safe-by-design strategy towards safer nanomaterials in nanomedicines. Adv Mater. 2019;31:e1805391. doi:10.1002/adma.201805391
5. Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci. 2020;8:4653–4664. doi:10.1039/d0bm00558d
6. Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. New Engl J Med. 2003;348:2491–2499. doi:10.1056/NEJMoa022749
7. Lam SW, Cleton-Jansen A-M, Cleven AHG, et al. Molecular analysis of gene fusions in bone and soft tissue tumors by anchored multiplex PCR-based targeted next-generation sequencing. J Mol Diagnostics. 2018;20:653–663. doi:10.1016/j.jmoldx.2018.05.007
8. Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as drug delivery systems: a review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers. 2023;15. doi:10.3390/polym15071596.
9. Mendelsohn RB, Winawer SJ, Ahnen DJ. Incidence of colorectal cancer matters. Gastroenterology. 2020;158:1191–1195. doi:10.1053/j.gastro.2019.11.304
10. Li D. Recent advances in colorectal cancer screening. Chronic Dis Transl Med. 2018;4:139–147. doi:10.1016/j.cdtm.2018.08.004
11. Ishwar D, Venkatakrishnan K, Tan B, Haldavnekar R. DNA methylation signatures of tumor-associated natural killer cells with self-functionalized nanosensor enable colorectal cancer diagnosis. Nano Lett. 2023;23:4142–4151. WOS:000985506300001. doi:10.1021/acs.nanolett.2c04914.
12. Liu X, Fang Y, Liu J, Chen X, Teng F, Caolong L. Nanozyme-based pump-free microfluidic chip for colorectal cancer diagnosis via circulating cancer stem cell detection. ACS Sens. 2024;9:5090–5098. WOS:001330668200001. doi:10.1021/acssensors.4c00774.
13. Jiamin W, Shengrong L, Yiling M, et al. 3D hierarchic interfacial assembly of Au nanocage@Au along with IS-AgMNPs for simultaneous, ultrasensitive, reliable, and quantitative SERS detection of colorectal cancer related miRNAs. Biosens Bioelectron. 2024. WOS:001154194700001. doi:10.1016/j.bios.2023.115993 248
14. Feng S, Zhang P, Chen H, et al. Au@Fe3O4 nanoparticle-based colorimetric aptasensor for noninvasive screening of colorectal cancer via detection of parvimonas micra. ACS Sens. 2025;10:1053–1062. WOS:001435863000001. doi:10.1021/acssensors.4c02885.
15. Liu H, Tengling W, Yunjian Y, et al. A peptide-derived aggregation-induced emission nanobioprobe: unlocking selective detection of Fusobacterium nucleatum and noninvasive screening of colorectal cancer. Aggregate. 2025:e740. WOS:001396113000001. doi:10.1002/agt2.740
16. Sakuma S, Yano T, Masaoka Y, et al. Detection of early colorectal cancer imaged with peanut agglutinin-immobilized fluorescent nanospheres having surface poly(N-vinylacetamide) chains. Eur J Pharm Biopharm. 2010;74:451–460. doi:10.1016/j.ejpb.2010.01.001
17. Zavaleta CL, Garai E, Liu JT, et al. A Raman-based endoscopic strategy for multiplexed molecular imaging. Proc Natl Acad Sci U S A. 2013;110:E2288–97. doi:10.1073/pnas.1211309110
18. Xie J, Wang J, Chen H, et al. Multivalent conjugation of antibody to dendrimers for the enhanced capture and regulation on colon cancer cells. Sci Rep. 2015;5:9445. doi:10.1038/srep09445
19. Xie J, Gao Y, Zhao R, et al. Ex vivo and in vivo capture and deactivation of circulating tumor cells by dual-antibody-coated nanomaterials. J Control Release. 2015;209:159–169. doi:10.1016/j.jconrel.2015.04.036
20. Bhattacharyya D, Kumar P, Mohanty SK, Smith YR, Misra M. Detection of four distinct volatile indicators of colorectal cancer using functionalized titania nanotubular arrays. Sensors. 2017;17. doi:10.3390/s17081795.
21. Kuai JH, Wang Q, Zhang AJ, et al. Epidermal growth factor receptor-targeted immune magnetic liposomes capture circulating colorectal tumor cells efficiently. World J Gastroenterol. 2018;24:351–359. doi:10.3748/wjg.v24.i3.351
22. Huang Q, Wang FB, Yuan CH, et al. Gelatin nanoparticle-coated silicon beads for density-selective capture and release of heterogeneous circulating tumor cells with high purity. Theranostics. 2018;8:1624–1635. doi:10.7150/thno.23531
23. Pan R, Lu X, Ju J, et al. Ratiometric nanoprobe for circulating tumor cell detection and intracellular hydrogen peroxide evaluation in colorectal cancer patients. Bioorg Med Chem. 2021;30:115930. doi:10.1016/j.bmc.2020.115930
24. Astolfi M, Rispoli G, Anania G, et al. Tin, Titanium, Tantalum, Vanadium and Niobium Oxide based sensors to detect colorectal cancer exhalations in blood samples. Molecules. 2021;26. doi:10.3390/molecules26020466.
25. Hanoglu SB, Man E, Harmanci D, et al. Magnetic nanoparticle-based electrochemical sensing platform using ferrocene-labelled peptide nucleic acid for the early diagnosis of colorectal cancer. Biosensors. 2022;12. doi:10.3390/bios12090736
26. Hariharan R, Jenkins M. Utility of the methylated SEPT9 test for the early detection of colorectal cancer: a systematic review and meta-analysis of diagnostic test accuracy. BMJ Open Gastroenterol. 2020;7:e000355. doi:10.1136/bmjgast-2019-000355
27. Marcuello M, Vymetalkova V, Neves RPL, et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Aspects Med. 2019;69:107–122. doi:10.1016/j.mam.2019.06.002
28. Djermane R, Nieto C, Vega MA, Del Valle EMM. Antibody-loaded nanoplatforms for colorectal cancer diagnosis and treatment: an update. Pharmaceutics. 2023;15. doi:10.3390/pharmaceutics15051514.
29. Guo X, Changjian L, Jia X, et al. NIR-II fluorescence imaging-guided colorectal cancer surgery targeting CEACAM5 by a nanobody. Ebiomedicine. 2023. WOS:000953875300001. doi:10.1016/j.ebiom.2023.104476 89
30. Xian S, Chen X, Ren S, Chen X, Wang H. Ionizable STING-activating nanoadjuvants enhance tumor immunogenicity and potentiate immunotherapy efficacy in solid tumors. Cancer Res. 2024;84:3044–3057. WOS:001313818100007. doi:10.1158/0008-5472.CAN-23-3511.
31. Liqiang L, Lin X, Wang L, et al. Immuno-PET of colorectal cancer with a CEA-targeted [68 Ga]Ga-nanobody: from bench to bedside. Eur J Nuclear Med Mol Imaging. 2023;50:3735–3749. WOS:001022074500003. doi:10.1007/s00259-023-06313-1.
32. Xiao Y, Mei C, Duo X, et al. Identification of a CEACAM5 targeted nanobody for positron emission tomography imaging and near-infrared fluorescence imaging of colorectal cancer. Eur J Nuclear Med Mol Imaging. 2023;50:2305–2318. WOS:000948428500001. doi:10.1007/s00259-023-06183-7.
33. Zhang B, Jialiang L, Lin X, et al. Injectable and sprayable fluorescent nanoprobe for rapid real-time detection of human colorectal tumors. Adv Mater. 2024. WOS:001279841500001. doi:10.1002/adma.202405275 36
34. Ding Y, Zhou R, Shi G, et al. Cadherin 17 nanobody-mediated near-infrared-II fluorescence imaging-guided surgery and immunotoxin delivery for colorectal cancer. Biomater Res. 2024. WOS:001252732700001. doi:10.34133/bmr.0041 28
35. Ji H, Hu Y, Cheng J, et al. Use of carbon nanoparticles to improve the efficiency of harvesting lymph nodes in rectal cancer. Surg Laparosc Endosc Percutan Tech. 2023;33:382–390. doi:10.1097/sle.0000000000001194
36. Li J, Lin C, Zhu Y, Shao C, Wang T, Chen B. Colorectal cancer cell membrane biomimetic ferroferric oxide nanomaterials for homologous bio-imaging and chemotherapy application. Med Oncol. 2023;40:322. doi:10.1007/s12032-023-02175-7
37. Ma S, Sun B, Li M, et al. High-precision detection and navigation surgery of colorectal cancer micrometastases. J Nanobiotechnol. 2023;21:403. doi:10.1186/s12951-023-02171-z
38. Wang L, Xia J, Fan H, et al. A tumor microenvironment responsive nanosystem for chemodynamic/chemical synergistic theranostics of colorectal cancer. Theranostics. 2021;11:8909–8925. doi:10.7150/thno.61651
39. Dong X, Luo J, Lan P, et al. Magnetic resonance colonography with intestine-absorbable nanoparticle contrast agents in evaluation of colorectal inflammation. Eur Radiol. 2021;31:4615–4624. doi:10.1007/s00330-020-07609-8
40. Matsuda T, Yamashita K, Hasegawa H, et al. Recent updates in the surgical treatment of colorectal cancer. Ann Gastroenterol Surg. 2018;2:129–136. doi:10.1002/ags3.12061
41. Li K, Li Z, Yan B, et al. Preoperative carbon nanoparticle injection improves inferior mesenteric artery lymph node retrieval in patients with rectal cancer. Surgery. 2022;171:1177–1184. doi:10.1016/j.surg.2021.08.023
42. Li J, Deng X, Wang L, Liu J, Xu K. Clinical application of carbon nanoparticles in lymphatic mapping during colorectal cancer surgeries: a systematic review and meta-analysis. Dig Liver Dis. 2020;52:1445–1454. doi:10.1016/j.dld.2020.08.020
43. Wang Q, Chen E, Cai Y, et al. Preoperative endoscopic localization of colorectal cancer and tracing lymph nodes by using carbon nanoparticles in laparoscopy. World J Surg Oncol. 2016;14:231. doi:10.1186/s12957-016-0987-1
44. Yan Z, Wang Y, Qiu M, et al. Persistent luminescence nanoparticles with high intensity for colorectal cancer surgery navigation and precision resection. J Mater Chem B. 2024;12:8655–8661. doi:10.1039/d4tb01062k
45. Wang S, Zhou L, Tian H, et al. Site-specific nanomodulator capable of modulation apoptosis for enhanced colorectal cancer chemo-photothermal therapy. J Nanobiotechnol. 2023;21. WOS:000920479700002. doi:10.1186/s12951-023-01779-5
46. Liang Q, Chen J, Hou S, et al. Activatable Mn2+-Armed nanoagonist augments antitumor immunity in colorectal cancer: a NIR-II Photonic neoadjuvant paradigm. Biomaterials. 2023. WOS:001033626600001. doi:10.1016/j.biomaterials.2023.122206 300
47. Zhang Z-J, Liu Z-T, Huang Y-P, et al. Magnetic resonance and fluorescence imaging superparamagnetic nanoparticles induce apoptosis and ferroptosis through photodynamic therapy to treat colorectal cancer. Mat Today Physics. 2023;36. WOS:001035130500001. doi:10.1016/j.mtphys.2023.101150
48. Lee Y-H, Nu Thu Pham U. Engineered indocyanine green and PD-L1 inhibitors co-loaded perfluorochemical double-layered nanodroplets offer effective photoimmunotherapy against colorectal cancer. Chem Eng J. 2023;460. WOS:000948429500001. doi:10.1016/j.cej.2023.141819
49. Xiao Y, Zhu T, Zeng Q, Tan QN, Jiang G, Huang X. Functionalized biomimetic nanoparticles combining programmed death-1/programmed death-ligand 1 blockade with photothermal ablation for enhanced colorectal cancer immunotherapy. Acta Biomater. 2023;157:451–466. WOS:000926678000001. doi:10.1016/j.actbio.2022.11.043.
50. Deng K, Tian H, Zhang T, et al. Chemo-photothermal nanoplatform with diselenide as the key for ferroptosis in colorectal cancer. J Control Release. 2024;366:684–693. WOS:001168340300001. doi:10.1016/j.jconrel.2024.01.024.
51. Zhuang Z-N, Yong-Dan Q, Huang Q-X, et al. Storable polydopamine nanoparticles combined with bacillus Calmette-Guerin for photothermal-immunotherapy of colorectal cancer. Adv Funct Mater. 2024;34. WOS:001281891300001. doi:10.1002/adfm.202404381
52. de Almeida, Alexandre M, Andree SF, et al. Enhancing phototoxicity in human colorectal tumor cells through nanoarchitectonics for synergistic photothermal and photodynamic therapies. ACS App Mater Interfaces. 2024;16:23742–23751. WOS:001227731600001. doi:10.1021/acsami.4c02247.
53. Xie J, Li D, Niu S, et al. Nano-titanium oxide-coated carbon nanotubes for photothermal therapy in the treatment of colorectal cancer. Adv Healthcare Mater. 2024;13. WOS:001251667000001. doi:10.1002/adhm.202401009
54. Niu S, Qiu P, Meng J, et al. Light/glutathione-ignited nanobombs integrating azo and tetrasulfide bonds for multimodal therapy of colorectal cancer. J Colloid Interface Sci. 2024;659:474–485. WOS:001154809100001. doi:10.1016/j.jcis.2024.01.002.
55. Axin H, Xia F, Han D, Yang Q, Tan W. Self-assembled amphiphilic NIR-II emissive nano-micelles for imaging-guided photothermal therapy of colorectal cancer. Sci China-Chem. 2024;67:2767–2774. WOS:001251530200003. doi:10.1007/s11426-023-1920-0.
56. Hui L, Zeng J, You Q, et al. X-ray-activated nanoscintillators integrated with tumor-associated neutrophils polarization for improved radiotherapy in metastatic colorectal cancer. Biomaterials. 2025. WOS:001393193100001. doi:10.1016/j.biomaterials.2024.123031 316
57. Yan F, Ruiyuan L, Liu J, et al. Hybrid near-infrared-activated luminescent gold nanoparticle platform for efficient cancer therapy. Adv Composites Hybrid Mat. 2025. WOS:001423644000001. doi:10.1007/s42114-024-01141-9 8
58. Ren G, Wang X, Cao J, et al. NIR II laser-triggered photothermal nanoplatform for multimodal imaging-guided synergistic therapy toward colon cancer. ACS App Mater Interfaces. 2025;17:5984–5994. WOS:001389973800001. doi:10.1021/acsami.4c18748
59. Xiong Y, Jinhong L, Jiang X, Zhen W, Xin M, Lin W. Nitric oxide-releasing nanoscale metal-organic layer overcomes hypoxia and reactive oxygen species diffusion barriers to enhance cancer radiotherapy. Adv Sci. 2025;12. WOS:001386294700001. doi:10.1002/advs.202413518
60. Long X, Wang J, Wang H, et al. Injectable 2D-MoS2-integrated bioadhesive hydrogel as photothermal-derived and drug-delivery implant for colorectal cancer therapy. Adv Healthcare Mater. 2025. WOS:001446132700001. doi:10.1002/adhm.202404842.
61. Xin Y, Yunjian Y, Mengdi W, et al. Tumor and intratumoral pathogen cascade-targeting photothermal nanotherapeutics for boosted immunotherapy of colorectal cancer. J Control Release. 2025;379:574–591. WOS:001411058000001. doi:10.1016/j.jconrel.2025.01.048.
62. Zhang Y, Zhao J, Zhang L, et al. A cascade nanoreactor for enhancing sonodynamic therapy on colorectal cancer via synergistic ROS augment and autophagy blockage. Nano Today. 2023. WOS:000949891100001. doi:10.1016/j.nantod.2023.101798 49
63. Huang S, Ding D, Lan T, et al. Multifunctional nanodrug performs sonodynamic therapy and inhibits TGF-β to boost immune response against colorectal cancer and liver metastasis. Acta Biomater. 2023;164:538–552. doi:10.1016/j.actbio.2023.04.001
64. Wang L, Zhou H, Chen Q, et al. STING agonist-loaded nanoparticles promotes positive regulation of type I interferon-dependent radioimmunotherapy in rectal cancer. Adv Sci. 2024;11:e2307858. doi:10.1002/advs.202307858
65. Fang F, Chen YY, Zhang XM, et al. EGFR-targeted and NIR-triggered lipid-polymer hybrid nanoparticles for chemo-photothermal colorectal tumor therapy. Int J Nanomed. 2024;19:9689–9705. doi:10.2147/ijn.S473473
66. Liu J, Hsieh CL, Gelincik O, et al. Proteomic characterization of outer membrane vesicles from gut mucosa-derived fusobacterium nucleatum. J Proteomics. 2019;195:125–137. doi:10.1016/j.jprot.2018.12.029
67. Charest G, Tippayamontri T, Shi M, et al. Concomitant chemoradiation therapy with gold nanoparticles and platinum drugs co-encapsulated in liposomes. Int J Mol Sci. 2020;21. doi:10.3390/ijms21144848.
68. Moradi Z, Mohammadian M, Saberi H, et al. Anti-cancer effects of chemotherapeutic agent; 17-AAG, in combined with gold nanoparticles and irradiation in human colorectal cancer cells. Daru. 2019;27:111–119. doi:10.1007/s40199-019-00251-w
69. Gabrielaitis D, Zitkute V, Saveikyte L, et al. Nanotubes from bacteriophage tail sheath proteins: internalisation by cancer cells and macrophages. Nanoscale Adv. 2023;5:3705–3716. doi:10.1039/d3na00166k
70. Faghfoori MH, Nosrati H, Rezaeejam H, et al. Anticancer effect of X-Ray triggered methotrexate conjugated albumin coated bismuth sulfide nanoparticles on SW480 colon cancer cell line. Int J Pharm. 2020;582:119320. doi:10.1016/j.ijpharm.2020.119320
71. Ni K, Lan G, Veroneau SS, Duan X, Song Y, Lin W. Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nat Commun. 2018;9:4321. doi:10.1038/s41467-018-06655-7
72. DuRoss AN, Landry MR, Thomas CR, Neufeld MJ, Sun C. Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Lett. 2021;500:208–219. doi:10.1016/j.canlet.2020.11.021
73. Meng L, Cheng Y, Gan S, et al. Facile deposition of manganese dioxide to albumin-bound paclitaxel nanoparticles for modulation of hypoxic tumor microenvironment to improve chemoradiation therapy. Mol Pharm. 2018;15:447–457. doi:10.1021/acs.molpharmaceut.7b00808
74. Belyaev IB, Zelepukin IV, Tishchenko VK, et al. Nanoparticles based on MIL-101 metal-organic frameworks as efficient carriers of therapeutic(188)Re radionuclide for nuclear medicine. Nanotechnology. 2023;35. doi:10.1088/1361-6528/ad0c74
75. Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther. 2020;206:107447. doi:10.1016/j.pharmthera.2019.107447
76. Mahaki H, Mansourian M, Meshkat Z, et al. Nanoparticles containing oxaliplatin and the treatment of colorectal cancer. Curr Pharm Des. 2023;29:3018–3039. doi:10.2174/0113816128274742231103063738
77. Shengyun H, Xia K, Huang X, et al. Multifunctional CaCO3@Cur@QTX125@HA nanoparticles for effectively inhibiting growth of colorectal cancer cells. J Nanobiotechnol. 2023;21. WOS:001075239200001. doi:10.1186/s12951-023-02104-w
78. Silveira MJ, Martins C, Cruz T, et al. scFv biofunctionalized nanoparticles to effective and safe targeting of CEA-expressing colorectal cancer cells. J Nanobiotechnol. 2023;21. WOS:001081991800001. doi:10.1186/s12951-023-02126-4
79. Zhang Y, Zhao Z, Jingli L, et al. Treatment of colorectal cancer by anticancer and antibacterial effects of hemiprotonic phenanthroline-phenanthroline+ with nanomicelle delivery. Asian J Pharm Sci. 2023;18. WOS:001007110000001. doi:10.1016/j.ajps.2023.100801
80. Yuan M, Chen T, Jin L, et al. A carrier-free supramolecular nano-twin-drug for overcoming irinotecan-resistance and enhancing efficacy against colorectal cancer. J Nanobiotechnol. 2023. WOS:001092173200002. doi:10.1186/s12951-023-02157-x 21
81. Jiaxue W, Shixiong Y, Cao Y, et al. Dual-driven nanomotors enable tumor penetration and hypoxia alleviation for calcium overload-photo-immunotherapy against colorectal cancer. Biomaterials. 2023. WOS:001088684700001. doi:10.1016/j.biomaterials.2023.122332 302
82. Shenshen W, Yun J, Tang W, et al. Therapeutic m6A eraser ALKBH5 mRNA-loaded exosome-liposome hybrid nanoparticles inhibit progression of colorectal cancer in preclinical tumor models. ACS Nano. 2023;17:11838–11854. WOS:001008550000001. doi:10.1021/acsnano.3c03050.
83. Wang Z, Wenpan L, Jiang Y, et al. Camptothesome-based combination nanotherapeutic regimen for improved colorectal cancer immunochemotherapy. Biomaterials. 2024;306. WOS:001181669600001. doi:10.1016/j.biomaterials.2024.122477
84. Cui R, Zhou J, Yang W, et al. Ultrasound-triggered nanogel boosts chemotherapy and immunomodulation in colorectal cancer. ACS App Mater Interfaces. 2024;17:211–221. WOS:001375019200001. doi:10.1021/acsami.4c13358.
85. Cheng J, Jiaxin L, Qilin Y, et al. Laser-activable murine ferritin nanocage for chemo-photothermal therapy of colorectal cancer. J Nanobiotechnol. 2024;22. WOS:001234795600001. doi:10.1186/s12951-024-02566-6
86. Liu X, Chen F, Saeed M, et al. In-situ vaccination immunotherapy of colorectal cancer with STING agonist-integrated supramolecular nanovectors. Nano Today. 2024;56. WOS:001234968100001. doi:10.1016/j.nantod.2024.102273
87. Wang H, Zhuang H, Chunyan W, et al. Colorectal cancer treatment strategy: targeting O-GlcNAcylation of Yes-associated protein utilizing diselenide-bridged nanovesicles. Chem Eng J. 2024. WOS:001301123900001. doi:10.1016/j.cej.2024.154750 497
88. Sanoff HK, Moon DH, Moore DT, et al. Phase I/II trial of nano-camptothecin CRLX101 with capecitabine and radiotherapy as neoadjuvant treatment for locally advanced rectal cancer. Nanomedicine. 2019;18:189–195. doi:10.1016/j.nano.2019.02.021
89. Li L, Qian Y, Sun L, et al. Albumin-stabilized layered double hydroxide nanoparticles synergized combination chemotherapy for colorectal cancer treatment. Nanomedicine. 2021;34:102369. doi:10.1016/j.nano.2021.102369
90. Yao F, An Y, Li X, Li Z, Duan J, Yang XD. Targeted therapy of colon cancer by aptamer-guided holliday junctions loaded with doxorubicin. Int J Nanomed. 2020;15:2119–2129. doi:10.2147/ijn.S240083
91. Wu D, Zhu L, Li Y, et al. Superparamagnetic chitosan nanocomplexes for colorectal tumor-targeted delivery of irinotecan. Int J Pharm. 2020;584:119394. doi:10.1016/j.ijpharm.2020.119394
92. Du W, Yang X, He S, et al. Novel hyaluronic acid oligosaccharide-loaded and CD44v6-targeting oxaliplatin nanoparticles for the treatment of colorectal cancer. Drug Deliv. 2021;28:920–929. doi:10.1080/10717544.2021.1914777
93. Li Y, Su Y, Pan H, et al. Nanodiamond-based multifunctional platform for oral chemo-photothermal combinational therapy of orthotopic colon cancer. Pharmacol Res. 2022;176:106080. doi:10.1016/j.phrs.2022.106080
94. Fernandes S, Fernandez T, Metze S, et al. Magnetic nanoparticle-based hyperthermia mediates drug delivery and impairs the tumorigenic capacity of quiescent colorectal cancer stem cells. ACS Appl Mater Interfaces. 2021;13:15959–15972. doi:10.1021/acsami.0c21349
95. Wang W, Zhu Q, Jin Y, et al. Self-immolated nanoadjuvant for in situ vaccination immunotherapy of colorectal cancer. Adv Healthcare Mater. 2023;12. WOS:001008371500001. doi:10.1002/adhm.202300524
96. Meng Q, Jie X, Wang J, et al. Investigation of the enhanced antitumour potency of CD46-specific chimeric antigen receptor-T cells in human colorectal cancer liver metastases after combination with nanotherapeutics. Nano Today. 2023. WOS:001106576600001. doi:10.1016/j.nantod.2023.101985 52
97. Haiting X, Wang Y, Liu G, et al. Nano-armed limosilactobacillus reuteri for enhanced photo-immunotherapy and microbiota tryptophan metabolism against colorectal cancer. Adv Sci. 2025;12. WOS:001388271100001. doi:10.1002/advs.202410011
98. Lijun H, Tan L, Deng S, et al. Tertiary lymphoid structure formation induced by LIGHT-engineered and photosensitive nanoparticles-decorated bacteria enhances immune response against colorectal cancer. Biomaterials. 2025;314. WOS:001324329700001. doi:10.1016/j.biomaterials.2024.122846
99. Caiying L, Chen G, Tan L, et al. Multifunctional nanodrug-mediated immunotherapy in microsatellite stable colorectal cancer via promoting m6A modification and M1-like tumor-associated macrophages polarization. Small Struct. 2024;5. WOS:001249854700001. doi:10.1002/sstr.202400100
100. Yoonhee S, Yim D, Keun Kim H, et al. Functional nanosheet immunoswitches reprogramming innate macrophages for immunotherapy of colorectal cancer and sepsis. ACS Nano. 2025;19:5165–5177. WOS:001413735200001. doi:10.1021/acsnano.4c08828
101. Chen Y, Cao H, Jiang C, Youbin L. Tumor-microenvironment-mediated second near-infrared light activation multifunctional cascade nanoenzyme for self-replenishing O2/H2O2 multimodal tumor therapy. J Colloid Interface Sci. 2025;683:930–943. WOS:001403139000001. doi:10.1016/j.jcis.2024.12.228.
102. Liu J, Chen Z, Deng L, et al. Metal-phenolic networks specifically eliminate hypoxic tumors by instigating oxidative and proteotoxic stresses. Bioact Mater. 2025;47:361–377. WOS:001426920000001. doi:10.1016/j.bioactmat.2025.01.022
103. Xiaoling L, Duan Z, Chen X, et al. Impairing tumor metabolic plasticity via a stable metal-phenolic-based polymeric nanomedicine to suppress colorectal cancer. Adv Mater. 2023. WOS:000974645100001. doi:10.1002/adma.202300548 35
104. Yan Z, Wang B, Shen Y, et al. Bisphosphonate-mineralized nano-IFNγ suppresses residual tumor growth caused by incomplete radiofrequency ablation through metabolically remodeling tumor-associated macrophages. Theranostics. 2025;15:1057–1076. WOS:001414730200015. doi:10.7150/thno.100998
105. Lei D, Wang W, Zhao J, et al. An injectable gambogic acid loaded nanocomposite hydrogel enhances antitumor effect by reshaping immunosuppressive tumor microenvironment. Mater Today Bio. 2025. WOS:001437589500001. doi:10.1016/j.mtbio.2025.101611 31
106. Wang H, Zhou R, Chengchao X, et al. GRP78 nanobody-directed immunotoxin activates innate immunity through STING pathway to synergize tumor immunotherapy. Adv Sci. 2025:e2408086–e2408086. MEDLINE:40135833. doi:10.1002/advs.202408086
107. Xinchen L, Jin J, Wu Y, et al. Self-assembled PROTACs enable protein degradation to reprogram the tumor microenvironment for synergistically enhanced colorectal cancer immunotherapy. Bioact Mater. 2025;43:255–272. WOS:001332550300001. doi:10.1016/j.bioactmat.2024.09.022
108. Peng J, Yang Q, Lei R, Wang Y, Liu G, Qian Z. Preferential activation of type I interferon-mediated antitumor inflammatory signaling by CuS/MnO2/diAMP nanoparticles enhances anti-PD-1 therapy for sporadic colorectal cancer. J Nanobiotechnol. 2024;22. WOS:001353326800001. doi:10.1186/s12951-024-02970-y
109. Jiang H, Tian H, Wang Z, et al. Laser-activatable oxygen self-supplying nanoplatform for efficiently overcoming colorectal cancer resistance by enhanced ferroptosis and alleviated hypoxic microenvironment. Biomater Res. 2023. WOS:001073747500001. doi:10.1186/s40824-023-00427-1 27
110. Baoyi L, Menghang Z, Jiang A, et al. Magnetic natural lipid nanoparticles for oral treatment of colorectal cancer through potentiated antitumor immunity and microbiota metabolite regulation. Biomaterials. 2024;307. WOS:001219132400001. doi:10.1016/j.biomaterials.2024.122530
111. Jin X-K, Liang J-L, Zhang S-M, et al. Orchestrated copper-based nanoreactor for remodeling tumor microenvironment to amplify cuproptosis-mediated anti- tumor immunity in colorectal cancer. Mater Today. 2023;68:108–124. WOS:001082267000001. doi:10.1016/j.mattod.2023.06.024.
112. Wang X, Chen Q, Zhu Y, et al. Destroying pathogen-tumor symbionts synergizing with catalytic therapy of colorectal cancer by biomimetic protein-supported single-atom nanozyme. Signal Transduction Targeted Ther. 2023. WOS:001032891800001. doi:10.1038/s41392-023-01491-8 8
113. Cun J-E, Ziyun H, Fan X, et al. Copper-Based bio-coordination nanoparticle for enhanced pyroptosis-cuproptosis cancer immunotherapy through redox modulation and glycolysis inhibition. Small. 2025;21. WOS:001389864500001. doi:10.1002/smll.202409875
114. Lin X, Chen H, Deng T, et al. Improved immune response for colorectal cancer therapy triggered by multifunctional nanocomposites with self-amplifying antitumor ferroptosis. ACS App Mater Interfaces. 2024;16:13481–13495. WOS:001181880500001. doi:10.1021/acsami.3c16813.
115. Zhilong Y, Wang C, Yingjiang Y, Wang S, Jiang K. Therapeutic potentials of FexMoyS-PEG nanoparticles in colorectal cancer: a multimodal approach via ROS-ferroptosis-glycolysis regulation. J Nanobiotechnol. 2024;22. WOS:001227239700005. doi:10.1186/s12951-024-02515-3
116. Xiaohui L, Yanmei M, Xin Y, Feihe M, Gao H. Tumor-targeting nanoassembly for enhanced colorectal cancer therapy by eliminating intratumoral Fusobacterium nucleatum. ACS App Mater Interfaces. 2023;15:14164–14172. WOS:001009920700001. doi:10.1021/acsami.3c01210.
117. Zhu R, Yuan W, Xia A, et al. Inulin-based nanoparticle modulates gut microbiota and immune microenvironment for improving colorectal cancer therapy. Adv Funct Mater. 2024;34. WOS:001301950000001. doi:10.1002/adfm.202407685
118. Shiquan L, Liu T, Chenyao L, Zhang Z, Zhang J, Sun D. Overcoming immunotherapy resistance in colorectal cancer through nano-selenium probiotic complexes and IL-32 modulation. Biomaterials. 2025;320. WOS:001446354700001. doi:10.1016/j.biomaterials.2025.123233
119. Lei L, Shouhua H, Liao B, et al. Orally administrated hydrogel harnessing intratumoral microbiome and microbiota-related immune responses for potentiated colorectal cancer treatment. Research. 2024. WOS:001235073800001. doi:10.34133/research.0364 7
120. Fan Y, Jiamin Y, Kang Y, et al. Biomimetic piezoelectric nanomaterial-modified oral microrobots for targeted catalytic and immunotherapy of colorectal cancer. Sci Adv. 2024;10:
121. Zhang J, Sun L, Jiang L, et al. Regulation of CTLs/Tregs via highly stable and ultrasound-responsive cerasomal nano-modulators for enhanced colorectal cancer immunotherapy. Adv Sci. 2024;11:e2400485. doi:10.1002/advs.202400485
122. Li Y, Chen J, Xia Q, et al. Photothermal Fe(3)O(4) nanoparticles induced immunogenic ferroptosis for synergistic colorectal cancer therapy. J Nanobiotechnol. 2024;22:630. doi:10.1186/s12951-024-02909-3
123. Ding D, Liang R, Li T, et al. Nanodrug modified with engineered cell membrane targets CDKs to activate aPD-L1 immunotherapy against liver metastasis of immune-desert colon cancer. J Control Release. 2024;369:309–324. doi:10.1016/j.jconrel.2024.03.052
124. Chen L, Kang Z, Shen J, et al. An emerging antibacterial nanovaccine for enhanced chemotherapy by selectively eliminating tumor-colonizing bacteria. Sci Bull. 2024;69:2565–2579. doi:10.1016/j.scib.2024.06.016
125. Zheng DW, Dong X, Pan P, et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat Biomed Eng. 2019;3:717–728. doi:10.1038/s41551-019-0423-2
126. Jin H, Zhu T, Huang X, et al. ROS-responsive nanoparticles based on amphiphilic hyperbranched polyphosphoester for drug delivery: light-triggered size-reducing and enhanced tumor penetration. Biomaterials. 2019;211:68–80. doi:10.1016/j.biomaterials.2019.04.029
127. Cao Y, Liu S, Ma Y, et al. Oral nanomotor-enabled mucus traverse and tumor penetration for targeted chemo-sono-immunotherapy against colon cancer. Small. 2022;18:e2203466. doi:10.1002/smll.202203466
128. Cabeza L, Perazzoli G, Mesas C, et al. Nanoparticles in colorectal cancer therapy: latest in vivo assays, clinical trials, and patents. AAPS Pharm Sci Tech. 2020;21:178. doi:10.1208/s12249-020-01731-y
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.