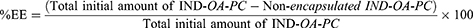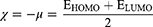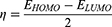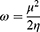Back to Journals » International Journal of Nanomedicine » Volume 19
Multifunctional Indomethacin Conjugates for the Development of Nanosystems Targeting Cancer Treatment
Authors Thiruchenthooran V , Świtalska M, Maciejewska G , Palko-Łabuz A, Bonilla-Vidal L, Wietrzyk J , Souto EB , Sánchez-López E , Gliszczyńska A
Received 10 July 2024
Accepted for publication 6 November 2024
Published 27 November 2024 Volume 2024:19 Pages 12695—12718
DOI https://doi.org/10.2147/IJN.S477512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sachin Mali
Vaikunthavasan Thiruchenthooran,1 Marta Świtalska,2 Gabriela Maciejewska,3,4 Anna Palko-Łabuz,5 Lorena Bonilla-Vidal,6,7 Joanna Wietrzyk,2 Eliana B Souto,8 Elena Sánchez-López,6,7,* Anna Gliszczyńska1,*
1Department of Food Chemistry and Biocatalysis, Wrocław University of Environmental and Life Sciences, Wrocław, Poland; 2Department of Experimental Oncology, Ludwik Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wrocław, Poland; 3Central Laboratory of the Instrumental Analysis, Wrocław University of Technology, Wrocław, Poland; 4Omics Research Center, Wrocław Medical University, Wrocław, Poland; 5Department of Biophysics and Neuroscience, Wrocław Medical University, Wrocław, Poland; 6Department of Pharmacy, Pharmaceutical Technology and Physical Chemistry, University of Barcelona, Barcelona, 08028, Spain; 7Institute of Nanoscience and Nanotechnology (IN2UB), University of Barcelona, Barcelona, 08028, Spain; 8UCD School of Chemical and Bioprocess Engineering, University College Dublin, Dublin, D04 V1W8, Ireland
*These authors contributed equally to this work
Correspondence: Anna Gliszczyńska; Elena Sánchez-López, Email [email protected]; [email protected]
Purpose: It is well known that the nonsteroidal anti-inflammatory drug (NSAID) indomethacin (IND) exhibits significant anticancer potential reported not only by in vitro and in vivo studies, but also in clinical trials. Despite promising results, IND is not widely used as an adjunctive agent in cancer therapy due to the occurrence of several gastrointestinal side effects, primarily after oral administration. Therefore, this study aimed to develop a nanosystem with reduced toxicity and risk of side effects for the delivery of IND for cancer treatment.
Methods: IND was encapsulated in nanostructured lipid carriers (NLC) in the form of a phospholipid conjugate, where a covalent bond exists between the drug and phosphatidylcholine skeleton. For this purpose, seven new hybrid molecules were synthesized, and subsequently evaluated as anticancer agents in an in vitro model against selected cancer cell lines.
Results: Biological studies demonstrated that the synthesized conjugates possessed excellent antiproliferative effects, exhibiting a 2.7-fold to even 100-fold higher activity against selected cancer cells, while remaining non-toxic to healthy cells. Based on biological studies and molecular calculations, heterosubstituted phosphatidylcholine containing IND and oleic acid (IND-OA-PC) in the sn-1 and sn-2 positions, respectively, was identified as the most potent molecule. Subsequently, IND-OA-PC was encapsulated in nanostructured lipid carriers (IND-OA-PC-NLC). The results revealed that IND-OA-PC-NLC has a spherical shape with an average diameter of 155 nm and a negatively charged surface (– 17.4 ± 0.49 mV). In this study, it was proven that the encapsulated conjugate of indomethacin with PC exhibits high activity against triple-negative (TNBC, Her2-, PR-, and ER-) breast cancer cells MDA-MB-468. While free IND was active at a concentration of 270.5 μM, in the form of the phospholipid conjugate (IND-OA-PC), it inhibited the growth of cancer cells at 67.5 μM and after conjugate encapsulation (IND-OA-PC-NLC) it was effective at only 10.3 μM.
Conclusion: Our study revealed that the conjugation of NSAID with phosphatidylcholine and its combination with nanotechnology techniques create opportunities to repurpose well-known drugs from this group for new therapeutic applications.
Keywords: indomethacin, phospholipid conjugates, long-chain fatty acids, nanostructured lipid carrier, nanoparticles, anticancer properties
Introduction
According to the report of the World Health Organization (WHO), cancer caused nearly 10 million deaths worldwide in 2020.1 This staggering toll not only reflects the magnitude of human suffering, but also underscores the urgency to pursue effective interventions. Commonly used conventional therapies, such as chemotherapy and radiation, are often insufficient for tumor treatment. Moreover, neoadjuvant chemotherapy does not improve the patient’s quality of life or guarantee overall survival compared to chemoradiotherapy.2 Even adjuvant chemotherapy combinations do not provide the expected magnitude of survival benefit and often lead to more serious side effects than chemoradiotherapy alone.3
Despite significant progress in understanding the molecular intricacies of various cancer types, translating this knowledge into effective therapies remains a complex endeavor.4 The ongoing search for new, targeted anticancer drugs stems from the urgent need to enhance the effectiveness of current therapies and minimize the side effects experienced during patient treatment. However, the development of new, more effective anticancer therapies is challenging due to the high costs of discovering de novo anticancer drugs (ranged from $157.3 to $1,950.8 million), the long duration of these studies (from 10 to 17 years) and the low success rate (less than 1% of molecules reach the clinical trials).5 In this aspect, drug repurposing takes advantage of the properties of active compounds approved for other diseases and relies on available data on their safety and pharmacology to develop new potential treatments. The use of known non-oncology drugs in cancer therapy offers significant benefits. Chief among them is the possibility of reducing the time required for developing new drugs by about 3–5 years and costs by about $0.3 billion.6,7
Nonsteroidal anti-inflammatory drugs (NSAID) have shown great antitumor therapeutic potential, because chronic or prolonged inflammation is often associated with cancer development. The high expression levels of cyclooxygenase-2 (COX-2) are associated with increased levels of cell proliferation and reduced levels of apoptosis, which may be due to the inhibitory effects of caspases.8–10 Inhibition of apoptosis and increased proliferation of tumor cells lead to invasive tumor growth and metastasis; therefore, COX-2 inhibition is one important tool to develop effective control of cancer progression.11 Consequently, the use of NSAID focused on one of the targets of cancer may turn out to be an effective and innovative strategy, especially since statistics reports that around 20% of all cancer types arise from chronic inflammatory diseases.12
According to the Biopharmaceutical Classification System (BCS), indomethacin (IND) (1) belongs to the Class II of drugs. IND exhibits poor water solubility (105.2 μg/mL at pH7), low absorption, and many side effects after oral administration. Therefore, in recent decades, extensive studies have been conducted to improve its solubility profile and, thus its oral bioavailability, and reduce its toxicity. Cyclodextrin complexes, microemulsions, ethosomes, liposomes, and lipid nanoparticles have been produced and evaluated for that purpose.13–16 Nanodrug delivery systems minimize systemic toxicity via the tumor-enhanced permeability and retention effect (EPR); thus, many nanoformulations such as Doxil®, Abraxane®, DepoCyt®, Genexol®-PM, and AmBisome® have been successfully transferred from preclinical research to clinical use.17 However, the technology of liposomes with encapsulated drugs hardly exhibits excellent long-term cure effects, and new nanotechnological systems are under development.18,19 In 2020, nanomedicine market was estimated to reach $142 billion, and economic forecast reports predict an additional 12.8% growth by 2025 driven by the evolution of vaccines against COVID-19.20
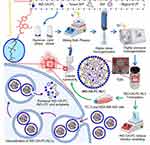
In previous studies, promising results were obtained for nanostructured lipid carriers (NLC) loaded with IND with the ability to achieve suitable physicochemical and pharmacological properties.21 Therefore, this strategy was combined with drug conjugation to enhance IND application in cancer therapy (Figure 1). Lipid nanocarriers can be formed from hydrophobic drug conjugates with phospholipids (PLs). These hybrid molecules can be assembled into nanoparticles with enhanced absorption, and improved pharmacokinetic and pharmacodynamic properties, even against multidrug-resistant (MDR) tumors. Moreover, nanoparticles produced from this type of bifunctional hybrids are characterized by high drug loading and avoid premature burst release compared to other nanocarriers. Over the last few decades, six lipid-drug conjugates (LDCs) have been approved by the American Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of depression, diabetes and schizophrenia.22
Taking these data into account, the main goal of our studies was to design a novel delivery system for IND to be used as an anticancer therapy. Therefore, we synthesized a new series of indomethacin-phospholipids conjugates (IND-PL) via the formation of ester bonds between IND and the phosphatidylcholine (PC) glycerol skeleton. Lysophosphatidylcholine (5) and six heterosubstituted phosphatidylcholines with IND in the sn-1 or sn-2 position and an acyl moiety were synthesized from the corresponding fatty acids (palmitic, stearic, and oleic acid) in the opposite position (6–8 and 18–20) were obtained. Molecular modelling was employed to estimate the features of the obtained hybrid molecules that are crucial for their anticancer properties and membrane permeation. After analyzing the physicochemical properties of the hybrids, their antiproliferative activities against selected cancer cell lines, including leukemia (MV-4-11), lung (A-549), breast (MDA-MB-468 and MCF-7), and prostate (PC-3), and the safety of their use against the non-tumorigenic human breast epithelial cell line MCF-10A were determined. Based on the obtained results, the molecular mechanisms of the hybrids with the highest potential, namely, indomethacinoyl-2-oleoyl-sn-glycero-3-phosphocholine (IND-OA-PC, 8) and 1-oleoyl-2-indomethacinoyl-sn-glycero-3-phosphocholine (OA-IND-PC, 20) were evaluated. Finally, nanotechnology techniques were applied to fabricate NLC with encapsulated phospholipids bearing IND in the position sn-1 and oleic acid in the sn-2 position IND-OA-PC (8) and the anticancer potential of this formulation IND-OA-PC-NLC was evaluated.
Materials and Methods
Chemicals and Reagents
IND (3), stearic acid (10), oleic acid (11), triethylamine (TEA), dibutyltin (IV) oxide (DBTO), oxalyl chloride, 4-(N,N-dimethylamino)pyridine (DMAP), N,N’-dicyclohexylcarbodiimide (DCC), celite® 110, Dowex® 50WX8 H+ (an ion-exchange resin), refined beeswax (BW), and Tween® 80 were purchased from Sigma-Aldrich (St. Louis, MO, USA). sn-Glycero-3-phosphocholine (GPC) was ordered from Bachem AG (Bubendorf, Switzerland). Palmitic acid (9) is a product of MP Biomedicals GmbH (Eschwege, Germany). Miglyol® 812 was purchased from Roig Farma S.A. (Barcelona, Spain). Analytical grade chloroform and methanol were obtained from Stanlab (Lublin, Poland) for column chromatography and thin-layer chromatography (TLC). All other solvents used for high-performance liquid chromatography (HPLC) were of analytical grade. The deionized water was produced by a Millipore® Milli-Q® Q system (Merck KGaA, Darmstadt, Germany).
Analysis Conditions
Column and Thin-Layer Chromatography
Thin-layer chromatography (TLC) was performed on Merck Kieselgel 60 F254 plates (0.2 mm silica gel with fluorescent indicator UV254, NJ, USA). Visualization was performed using 0.05% primulin spray (acetone: water (80/20, v/v)) and checked under UV light (λ =365 nm). Purification of the synthesized products was performed in a column chromatography using silica gel (Kieselgel 60 with 0.1% Ca (230–400 mesh ASTM; Merck, NJ, USA) and a mixture of chloroform/ methanol/ water (65/25/4, v/v/v) as the eluent.
Reverse-Phase Higher Performance Liquid Chromatography (RP-HPLC)
The purity of all the synthesized phosphatidylcholines was analyzed using a reverse-phase higher performance liquid chromatography (RP-HPLC) Waters 2695 system (Waters Corporation, MA, USA) equipped with a photodiode array detector (Waters 2996, Waters Corporation, Milford, USA) at a wavelength of 220 nm. A C18 reverse-phase column (5 µm × 4.6×150 mm, Kromasil® C18, Nouryon, Amsterdam, Netherlands) was used. The mobile phase consisted of methanol: acetonitrile/ 0.05% orthophosphoric acid (80/15/5, v/v/v) and was pumped at a flow rate of 1mL/min at 25 °C. The applied injection volume was 20 µL. All chromatographs were processed using the Empower® 3 software (Waters Corporation, MA, USA).
NMR and Higher Resolution Mass Spectroscopy (HRMS)
Spectroscopic analysis was carried out on a Bruker Avance II 600 MHz spectrometer (Bruker, Billerica, MA, USA). All samples of the synthesized conjugates were dissolved in a mixture of CDCl3/CD3OD (2:1, v/v) and exposed to frequencies of 600 MHz for 1H, 150 MHz for 13C, and 243 MHz for 31P. The chemical shifts were calibrated using the methanol proton signals (δH = 3.31) in the 1H NMR and CDCl3 (δc = 77.0) in the 13C NMR spectra. For 31P NMR spectra, 85% phosphoric acid was used as an external standard. HRMS spectra were obtained for all samples of the synthesized compounds using an electron spray ionization (ESI) technique on a Waters ESI-Q-TOF Premier XE spectrometer (Waters Corporation, MA, USA).
Synthesis of Phospholipid – Indomethacin Conjugates
Synthesis of Chlorides (4, 12-14)
Indomethacin chloride (4), palmitic chloride (12), stearic chloride (13) and oleic chloric (14) were obtained according to procedure described by Bauer.23 Substrates (2.4 mmol) were suspended in 10 mL of anhydrous methyl chloride, followed by addition of oxalyl chloride (1230 µL, 14.4 mmol). A catalytic amount of dried dimethylformamide (DMF) was also used to efficiently exchange the OH groups by Cl. The reaction mixture was then stirred for 1 h at room temperature. Next, the solvent methyl chloride and excess of oxalyl chloride were evaporated under reduced pressure, and the obtained in situ chlorides were subjected without purification to the next step of the synthesis.
Synthesis of 1-Indomethacinoyl-2-Hydroxy-Sn-Glycero-3-Phosphocholine (IND-LPC) (5)
1-Indomethacinoyl-2-hydroxy-sn-glycero-3-phosphocholine (IND-LPC) (5) was obtained according to a slightly modified procedure.24 GPC (257 mg, 1 mmol) and DBTO (249 mg, 1 mmol) were suspended in 7 mL of anhydrous propan-2-ol and refluxed for 1h. The mixture was cooled to room temperature and TEA (334 µL, 2.4 mmol) and the corresponding chloride of IND (3) were added. The mixture was stirred for 1 h and, after this time, it was filtered using diatomaceous earth (Celite® 110, St. Louis, MO, USA) and washed with propan-2-ol and chloroform. The organic solvent was next evaporated under reduced pressure and the products were purified via silica gel column chromatography using chloroform: methanol: water (65:25:4, v/v/v). Spectroscopic data for IND-LPC are included in the Supplementary Material as Supplementary information 1.
Synthesis of Drug-Phospholipid Conjugates with Indomethacin in the Sn-1 Position (6-8)
IND-LPC (5) (250 mg, 0.42 mmol) was dissolved in anhydrous methyl chloride (4 mL). Next corresponding fatty acids: palmitic, stearic or oleic (0.84 mmol) dissolved in 2 mL of anhydrous methyl chloride were added, followed by DMAP (102.6 mg, 0.84 mmol in 2 mL of methyl chloride) and a solution of DCC (363.1 mg, 1.76 mmol, in 2 mL of methyl chloride).25 The mixture was stirred at room temperature for 72 h in the dark and after that the obtained products (6–8) were extracted and purified by silica gel column chromatography.26 The spectroscopic data of all synthesized conjugates are included in Supplementary Material as Supplementary information 2−4.
Synthesis of Drug-Phospholipid Conjugates with Indomethacin in the Sn-2 Position (18-20)
IND (3) (357.8 mg, 1 mmol) was added to an anhydrous methyl chloride solution (6 mL) containing 1-palmitoyl-2-hydroxy-sn-3-glycerophosphocholine (0.5 mmol) or 1-stearoyl-2-hydroxy-sn-3-glycerophosphocholine (0.5 mmol) or 1-oleoyl-2-hydroxy-sn-3-glycerophosphocholine (0.5 mmol) and DMAP (1 mmol). Next, DCC (2.1 mmol) dissolved in dry methyl chloride (4 mL) was added to the mixture.25 Reaction was performed for 72 h in a nitrogen atmosphere at room temperature and in the dark. The product was extracted and purified by silica gel column chromatography.26 The spectroscopic data of all the synthesized conjugates are included in the Supplementary Material as Supplementary information 5−7.
Molecular Modeling
The topological polar surface area (TPSA), octanol–water partition coefficient (logP), and number of hydrogen bond acceptors or donors were calculated using Molinspiration online property calculation toolkit software. Quantum calculations of the HOMO and LUMO energies (EHOMO and ELUMO, respectively), area, volume, ovality, and polarizability were performed using the SPARTAN’18 software (Wavefunction, Inc., USA). The molecular geometry of each compound in the aqueous phase was optimized utilizing the density functional theory (DFT) approach with the ωB97X-D exchange-correlation potential, incorporating empirical corrections for dispersive interactions, together with the 6–311+G** basic set. The optimized geometry of the specified molecule was validated as the true minimum using frequency analysis, which revealed no imaginary frequencies. The impact of the solvent on the geometry and quantum mechanical parameters was evaluated using the conductor-like polarizable continuum model (CPCM) technique.
Preparation of IND-OA-PC-NLC
The production of empty NLC and IND-OA-PC-NLC was performed using the hot high-pressure homogenization (HPH) technique (Homogenizer FPG 12800, Stansted, United Kingdom).27 In the first step, a primary emulsion was produced with a mixture of lipid phase containing Beeswax and Miglyol® 812 and IND-OA-PC and an aqueous phase containing Tween 80®, using an Ultra-Turrax® T10 basic (IKA-Werke GmbH & Co. KG, Staufen, Germany) at 8000 rpm for 30s. The emulsion was subsequently exposed to HPH at 800 bar and 85 °C (three cycles) using Stansted-pressure cell homogenizer FPG12800 (Stansted Fluid Power, Ltd., Essex, UK). Before further experiments, the final formulations were allowed to settle and cool overnight at room temperature. Labelling was carried out using Nile Red (NR) to prepare formulations following the same procedure, but with addition of a 0.04% (w/w) of NR to the formulation (IND-OA-PC-NLC-NR).28
Determination of Particle Size (Z-Ave), Polydispersity Index (PDI), Zeta Potential (ZP) and Entrapment Efficiency
NLC particle size (Z-Ave) and polydispersity index (PDI) were measured by dynamic light scattering (DLS) using a Malvern Nano Zetasizer (Malvern Instruments, Malvern, UK). In addition, the NLC surface charge was assessed via laser Doppler electrophoresis using zeta potential (ZP) measurements with the same apparatus. The formulations were diluted with ultrapure water for the measurement of Z-Ave, PDI (1/10, v/v) and ZP (1/20, v/v).29,30 All measurements were performed at 25 °C and reported as the mean ± standard deviation (n = 3).
IND-OA-PC incorporated into the NLC was assessed by calculating the encapsulation efficiency (EE). EE was measured indirectly by quantifying the non-encapsulated conjugate in the NLC using the filtration-centrifugation technique. In brief, IND-OA-PC-NLC were diluted in water (1/10, v/v), filtered, and centrifuged using 0.5 mL Amicon® Ultracel 100 KDa filters (Merck kGaA, Darmstadt, Germany) at 14000 rpm for 15 min. The non-encapsulated IND-OA-PC in the supernatant was quantified by RP-HPLC using a Kromasil® C18 column and the program described in the Analysis Conditions section. The calibration curve was built using the IND-OA-PC concentration range of 0.25–500 µg/mL against the area of the corresponding detected peaks (n = 3). The percentage of EE was calculated using Equation 1:
Interaction Studies
Transmission Electron Microscopy (TEM)
Negative staining of IND-OA-PC-NLC was carried out using 2% of uranyl acetate placed on copper grids that were activated with UV light to visualize them. The morphology of IND-OA-PC-NLC was studied by transmission electron microscopy (TEM) using a JEOL 1010 microscope (JEOL, Akishima, Japan). The formulation was diluted to 1:5 (v/v) with ultrapure water.
Differential Scanning Calorimetry (DSC)
The thermodynamic profile was analyzed using a differential scanning calorimetry DSC 823e system (Mettler-Toledo 4000, Greifensee, Switzerland). Briefly, the samples were weighed in perforated aluminum pans using a Mettler M3 Microbalance (Mettler-Toledo, Greifensee, Switzerland). The Calorimetric curves were obtained in a nitrogen atmosphere by using a heating ramp from 25 to 200 °C at 10 °C/min. A pan with indium (purity ≥ 99.95%; Fluka, Switzerland) was used to calibrate of the calorimetric system, and an empty aluminum pan was used as a reference. The obtained data were evaluated using the Mettler STARe V 9.01 dB software (Mettler-Toledo, Barcelona, Spain).
X-Ray Diffraction (XRD)
X-ray diffraction (XRD) of air-dried samples were obtained by placing polyester films with a thickness of 3.6 µm and exposing them to Cu K” radiation (45 kV, 40 mA, λ = 1.5418 Å). The measurements were performed in the 2θ range of 2–60°. The step size and interval of the measurements were 0.026° and 200 s, respectively.
Fourier Transform Infrared Spectroscopy (ATR-FTIR)
Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectra of IND-OA-PC-NLC were obtained using a Thermo Scientific Nicolet iZ10 spectrometer equipped with an ATR crystal and DTGS detector (Thermo Scientific, Barcelona, Spain). Infrared spectra were analyzed within a frequency range of 500–4000 cm−1 regions with 1 cm−1 resolution.21
Stability of Synthesized Compounds and NLC
Hydrolytic Stability of Synthesized Compounds to pHs
The stability of synthesized compounds at pH 1.2 and pH 7.4 was assessed using Clark-Lubs and PBS buffers, respectively.31 IND-PL conjugates (4 mg/mL) in triplicates were prepared in each of the buffers and stirred (150 rpm) at 37 °C. At different time intervals (0.5, 1, 2, 4, 8, 12, and 24 h), a volume of 100 µL of each sample was withdrawn. The samples were subsequently dried under nitrogen flow and dissolved in 400 µL methanol for further analysis using RP-HPLC.
Storage Stability of NLC
IND-OA-PC-NLC were stored at 4, 25, and 38 °C monthly for 60 days. Changes in the particle size and electrical charge parameters (Z-Ave, PDI, and ZP) were assessed monthly.
Biopharmaceutical Behaviour
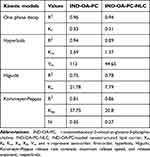
The in vitro release profile of IND-OA-PC from IND-OA-PC-NLC (n = 3)was carried out by comparison against the same concentration of free IND-OA-PC (n = 3), present in 50% of hydroalcoholic solution, using direct dialysis technique.32 In brief, dissolution medium was composed of ethanol (50/50, v/v) in PBS buffer (pH 7.4) solution. A volume of 0.5 mL was added to dialysis cassettes (MWCO 10KDa, Slide-A-LyzerTM, Thermo Scientific, Rockford, USA) and placed inside the dissolution medium, and incubated at 37 °C. At pre-estimated time intervals, a volume of 400 µL of the dissolution medium was collected and replaced with the same volume of fresh medium. The IND-OA-PC concentration was quantified using RP-HPLC and plotted as the cumulative drug release over time using Prism version 5.0 (GraphPad Software, MA, USA). The data were fitted to common kinetic equations (first-order, hyperbola, Higuchi, and Korsmeyer-Peppas equations), as explained elsewhere.33
Sterilization by Gamma Radiation
IND-OA-PC-NLC were sterilized using a dose of 25 KGy of 60Co equipped with a gamma irradiation source (Aragogamma, Barcelona, Spain). Modifications in Z-Ave, PDI, ZP and EE were estimated before and after gamma irradiation to ensure the safety of irradiation as a source of sterilization.
Biological Studies
Cell Lines and Cultured Mediums
The selected human biphenotypic B myelomonocytic leukemia MV4-11 cell line and normal breast epithelial MCF-10A cell line were obtained from the American Type Culture Collection (ATTC, Virginia, USA).Human non-small lung cancer A-549, prostate cancer PC-3, and breast cancer MCF-7 cells were purchased from the European Collection of Authenticated Cell Cultures (ECACC, Salisbury, UK). Human breast cancer MDA-MB-468 cells were obtained from the Leibniz Institute, DSMZ-German Collection of Microorganisms and Cell Cultures (Leibniz, Germany).
All cell lines were kept at the Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences (IIET PAS, Wroclaw, Poland). MV4-11, PC-3, and MDA-MB-468 cells (IIET PAS, Wroclaw, Poland) were cultured in RPMI 1640 medium (Gibco, London, UK) with 1.0 mm sodium pyruvate (only MV4-11), 10% (PC-3), or 20% (MDA-MB-468) fetal bovine serum (FBS) (Merck, Darmstadt, Germany). A549 cells (IIET PAS, Wroclaw, Poland) were cultured in RPMI 1640+Opti-MEM (1:1) (Gibco, London, UK) supplemented with 5% fetal bovine serum (Merck, Darmstadt, Germany). The MCF-7 cells were cultured in Eagle’s medium (IIET PAS, Wroclaw, Poland), supplemented with 8 microg/mL of insulin and 1% of MEM NON-essential amino acids (Merck, Darmstadt, Germany). Normal breast epithelial MCF-10A cells were cultured in the HAM’S F-12 medium (Corning, NY, USA), which was supplemented with 10% Horse Serum (Gibco, London, UK), 20 ng/mL EGFh, 10 µg/mL insulin, 0.5 µg/mL Hydrocortisone and 0.05 mg/mL Cholera Toxin from Vibrio cholerae (all from Merck, Darmstadt, Germany). All culture media were supplemented with 2 mm l-glutamine (Merck, Darmstadt, Germany), 100 units/mL penicillin (Polfa SA Tarchomin, Warsaw, Poland), and 100 µg/mL streptomycin (Merck, Darmstadt, Germany). All cell lines were cultured at 37 °C with 5% CO2 humidified environment.
Determination of Antiproliferative Activity
The antiproliferative activity of IND, its phospholipid conjugates, and the fabricated IND-OA-PC-NLC was tested towards five cancer cell lines (MV4-11, A549, PC-3, MDA-MB-468, and MCF-7). Cytotoxicity was also tested against the non-tumorigenic cell line MCF-10A. The test compounds (drug, conjugates and loaded NLC) were diluted in the culture medium to reach the final concentrations (1–625 μM). Before adding the test compounds (24 h prior), the cells were plated in 96-well plates (Sarstedt AG & Co., Sarstedt, Germany) at a density of 0.5×104 (A549) or 0.75×104 (MDA-MB-468, MCF-7), or 1×104 (PC-3, MV4-11, MCF-10A) cells. The assay was performed after 72 h of exposure to conjugate solutions at concentrations of 1–625 μM. The particles were diluted based on the total concentration of encapsulated IND-OA-PC concentration (1.5 mg/mL) in the IND-OA-PC-NLC. The in vitro cytotoxic effects of test compounds were examined using the MTT (MV4-11) or SRB assays (A549, PC-3, MDA-MB-468, MCF-7, MCF-10A) as described previously.25 The results were calculated as the IC50 (inhibitory concentration 50%), ie, the concentration of tested compounds that was cytotoxic to 50% of the cells. IC50 values were calculated for each experiment separately using the Prolab-3 system based on the Cheburator 0.4 software34 and the mean values ± SD are presented in Tables 1 and 2. IND, its phospholipid conjugates, and the fabricated IND-OA-PC-NLC, at each concentration, were tested in triplicate in a single experiment, which was repeated three to five times.
 |
Table 2 The Selectivity Index (SI) of Tested Compounds Against Selected Cancer Cell Lines |
Cell Cycle Analysis
MV4-11 and MDA-MB-468 cells were seeded at a density of 3×105 cells/well or 1.2×105 cells/well, respectively, in 6-well plates (Sarstedt AG & Co., Sarstedt, Germany) to a final volume of 4 mL. The cells were exposed for 72 h to the test compounds at concentrations about its 1.5 × IC50 (IND-LPC at 4.5 μM, IND-OA-PC and OA-IND-PC at 80 μM (MV4-11) or 90 μM (MDA-MB-468)). Following incubation, the cells were harvested and 1×106 of cells were washed twice in cold PBS and subsequently fixed for 24 h in 70% ethanol at −20 °C. Then, the cells were washed twice with PBS and incubated with RNAse (8 μg/mL; Fermentas, Germany) at 37 °C for 1 h. The cells were then stained for 30 min with propidium iodide (PI) (50 μg/mL, Merck, Darmstadt, Germany) at 4 °C, and the cellular DNA content was analyzed by flow cytometry using BD LSRFortessa cytometer (BD Biosciences, San Jose, CA, USA). The compounds at each concentration were tested independently at least thrice. The obtained results were analyzed using Flowing software 2.5.1 (Turku, Finland).
Apoptosis Determination by Annexin V Staining
MV4-11 and MDA-MB-468 cells were cultured at a density of 1 × × 105 cells/well or 0.4×105 cells/well, respectively, in culture medium on 24-well plates (Sarstedt AG & Co., Sarstedt, Germany) to a final volume of 2 mL. The cells were exposed to the test compounds for 72 h at concentrations of its 1.5 × IC50 (IND-LPC at 4.5 μM, IND-OA-PC, and OA-IND-PC at 80 μM (MV4-11) or 90 μM (MDA-MB-468)). After incubation, the cells were harvested, and 2×105 cells were washed twice with PBS and suspended in 100 µL of binding buffer (HEPES buffer: 10 mm HEPES/NaOH, pH 7.4, 150 mm NaCl, 5 mm KCl, 1 mm MgCl2, 1.8 mm CaCl2, (IIET, Wrocław, Poland) with 4 µL of APC-Annexin V (BD Pharmingen, USA, 2 µg/mL). Following a 15-minute incubation in the dark at ambient temperature, PI solution was introduced before analysis to achieve a final concentration of 4 µg/mL. Data acquisition was conducted via flow cytometry utilizing a BD LSRFortessa cytometer (BD Biosciences, San Jose, USA). The test compounds at each concentration were analyzed independently at least thrice. The results were analyzed using Flowing software 2.5.1 (Turku, Finland). The data are displayed as a two-color dot plot with APC-annexin V vs PI., PI−/Annexin V+ were early apoptotic cells, PI+/Annexin V+ were late apoptotic or necrotic cells and double-negative cells were live cells.
Caspase-3/7 Activity Determination
MV4-11 and MDA-MB-468 cells were seeded at a density of 0.5×105 cells/well or 0.2×105 cells/well, respectively, of culture medium in 48-well plates (Sarstedt AG & Co., Sarstedt, Germany) to a final volume of 1 mL. Cells were exposed to the test compounds for 24h or 72h at concentrations of its 1.5 × IC50 (IND-LPC at 4.5 μM, IND-OA-PC, and OA-IND-PC at 80 μM (MV4-11), or 90 μM (MDA-MB-468). After incubation cells were suspended in 50 µL of ice-cold lysis buffer (50 mm HEPES, 10% (w/v) sucrose, 150 mm NaCl, 2 mm EDTA, 10 mm DTT, 1% (v/v) Triton X-100, and pH 7.3, IIET PAS, Warszawa, Poland) and incubated for 30 min at 4 °C. After the incubation, 40 µL of each sample was transferred to a white 96-well plate (Corning, NY, USA) containing 160 µL of the reaction buffer (20 mm HEPES, 10% sucrose, 100 mm NaCl, 1 mm EDTA, 10 mm DTT, and 0.02% Triton X-100, pH 7.3) (IIET PAS, Warszawa, Poland) with Ac-DEVD-AMC (final concentration 10 µM) fluorogenic substrate (λex = 360 nm, λem = 460 nm). The fluorescence increase correlated with the caspase-3/7 levels was continuously recorded at 37 °C for 90 min using a Biotek Synergy H4 (Biokom, Warsaw, Poland). The test compounds at each concentration were analysed in duplicate in a single experiment, and each experiment was independently repeated at least thrice. The results were normalized to the cells count in each well and are presented as the mean relative caspase-3/7 activity compared to the untreated control sample ± standard deviation.
Determination of Compounds Accumulation in Cells by Flow Cytometry
Both IND-OA-PC-NLC and empty NLC were fluorescently labelled with NR to determine their accumulation in the cancer cells. MDA-MB-468 and prostate cancer PC-3 cells (2 × 105 cells) were incubated for 5, 15, and 30 min and 1, 2, and 4 h with IND-OA-PC-NLC-NR (20 µM). After incubation, cells were washed with PBS and analyzed by flow cytometry using a BD LSRFortessa cytometer (BD Biosciences, San Jose, USA). Untreated cells served as the unlabeled controls. The The findings were checked utilizing the Flowing software 2 (Cell Imaging Core, Turku Centre for Biotechnology, University of Turku Åbo Akademi University).
Statistical Analysis
Student’s t-tests followed by Tukey’s post-hoc tests were performed for two-group comparisons to indicate significant differences between treatments using StatGraphics 18.
Results and Discussion
Synthesis of Indomethacin-Phospholipid Prodrugs
PLs are used in the pharmaceutical industry as components of clinically approved drug formulations.35,36 Their biocompatible nature makes PLs safe materials to be administered via oral, and parenteral routes. The conjugation of lipids with drugs changes the physicochemical properties of these latter and their absorption, distribution, metabolism and elimination (ADME) properties, which are capable of overcoming restrictive barriers.37,38 This strategy makes possible the conjugates achieve increased cell penetration, bioavailability and accumulation within the tumor, as well as prolonged release time, leading to increased chances of achieving the desired therapeutic effects with reduced toxicity. Moreover, phospholipid-drug conjugates are biomaterials that can assemble into nanoparticles with even higher permeation with improved pharmacokinetics.39
In the present work, we proposed and developed a biodegradable lipid-based nanoparticulated delivery system for IND in cancer therapeutics (based on lipid-drug conjugation). For the synthesis of IND-PL conjugates, optically pure GPC with the natural R-configuration of a chiral center was used as the substrate. Two series of phosphatidylcholines containing IND at the sn-1 or sn-2 position of GPC were synthesized, as shown in Schemes 1 and 2, respectively.
The first GPC was transformed into cyclic styrene ketal (2) by treatment with DBTO and then selectively acylated with previously synthesized in situ indomethacin chloride (IND-Cl) (4). IND-Cl was obtained by the reaction of IND with oxalyl chloride in the presence of catalytic amounts of anhydrous DMF. 1-Indomethacinoyl-2-hydroxy-sn-glycero-3-phosphocholine (IND-LPC) (5) was obtained next, according to known procedures24 in 31% yield and its formation was confirmed by spectroscopic data (Figures S1–S4). Next, IND-LPC (5) was esterified using palmitic, stearic, and oleic acids. The heterosubstituted phosphatidylcholines (6–8) were obtained in good 25–50% yields under the Steglich conditions (Figures S5–S16).
The second group of phospholipids, phosphatidylcholines with IND at the sn-2 position (18–20), was synthesized in good yields (26–65%) (Figures S17–S28) by reacting IND with corresponding 1-acyl-2-hydroxy-sn-glycero-3-phosphocholines bearing as the acyl palmitic, stearic, or oleic acids (15–17) (Scheme 2).
The compounds were purified, and their structures were established by nuclear magnetic resonance (NMR) (1H, 13C, 31P NMR), correlation spectroscopy (COSY), heteronuclear quantum correlation spectroscopy (HSQC), and mass spectrometry (ESI-MS). Spectroscopic data for all synthesized products are reported in Supplementary Material.
Determination of Antiproliferative Activity of Synthesized Conjugates
The antiproliferative activities of IND and its hybrids were evaluated in selected cancer cell lines: leukemia (MV4-11), lung (A549), prostate (PC-3), and breast (MDA-MB-468 and MCF-7), whereas their safety was evaluated in the non-tumorigenic human breast epithelial cell line MCF-10A. Based on these results, conjugation of IND with PLs significantly increased the anticancer potential of this drug. Among all the tested compounds, IND-LPC (5) was the most active molecule in all cancer cell lines (Table 1). This compound inhibited effectively and selectively leukemia (MV4-11) and breast (MDA-MB-468) cancer cells at concentrations of 3.1 μM and 2.7 μM, respectively. The determined values were 66- and 100-fold lower than those observed for free IND, and were not toxic at these doses towards the non-tumorigenic human breast epithelial cell line MCF-10A. IND-LPC was 3-times more active against triple negative (TNBC, HER-, PR-, ER-) breast cancer cells MDA-MB-468 than estrogenic positive (ER+) breast cancer MCF-7 cells.
Among studied heterosubstituted phosphatidylcholines (6–8 and 18–20) the best results were observed for hybrids containing oleic acid in the structures of phosphatidylcholines IND-OA-PC (8) and OA-IND-PC (20). These hybrids exhibited strong antiproliferative activity towards all studied cancer cell lines, with IC50 values in the range 33.5–67.5 μM. Hybrids with oleoyl acyl residues were 2–4 times more active than free IND, and 3–10 times more active than hybrids with acyl from stearic acid and palmitic acid. The hybrids had similar antiproliferative activity against triple-negative (MDA-MB-468) and ER-positive (MCF-7) breast cancer cells.
The selectivity of the synthesized conjugates was assessed by comparing the cytotoxic activity (IC50) of each compound tested against each human cancer cell line with their activity against healthy MCF-10A cells (Table 2). Heterosubstituted phosphatidylcholine (8) bearing IND in the sn-1 position and oleic acid in the sn-2 position possessed the highest selectivity indices (much higher than free IND). This was especially visible against prostate (SI=5.43) and lung (SI=4.31) cancers and confirmed that hybrids are selective antitumor agents. The comparison of IC50s values for breast cancer cells (MCF-7 and MDA-MB-468) and non-tumorigenic breast MCF-10A cells showed statistically significant higher antiproliferative activity of these two compounds against cancer cells.
The Effect of Compounds 5, 8 and 20 on the Cell Cycle of MV4-11 and MDA-MB-468
The effects of the three most active compounds (IND-LPC, IND-OA-PC and OA-IND-PC) were analyzed on the cell cycle progression of the most sensitive among studied lines of leukemia (MV4-11) and breast cancer (MDA-MB-468) cells. Flow cytometry analysis was performed after staining cellular DNA with propidium iodide (PI). As this dye binds stoichiometrically to DNA, it is possible to determine its content in an individual cell and thus determine the size of the cell population in a given phase of the life cycle (the amount of PI bound to a cell is proportional to the amount of DNA).
Using this technique, the percentage of cells in each phase of the cell cycle (G0/G1, S, and G2/M) was estimated (Figure 2A and B). After 72 h exposure of MV4-11 cells to the test compounds at their predetermined active doses (1.5 × IC50), the test compounds caused only a slight decrease in the percentage of cells in the G0/G1 phase (comprising non-dividing cells and just after mitotic division with a single amount of 2N genetic material) and a decrease in the number of cells in the S phase of the cell cycle (comprising cells in the process of synthesizing genetic material, 4N). This indicates that they are weak inhibitors of the cell cycle and may act via other independent mechanisms. IND-LPC and OA-IND-PC strongly induced cell death at these concentrations. The opposite situation was observed in breast cancer cells (MDA-MB-468), in which heterosubstituted PC caused an increase in the percentage of cells in the G0/G1 phase. Compound OA-IND-PC stopped the cell cycle in the G0/G1 phase, but did not induce cell death, and compound IND-OA-PC stopped the cell cycle in the G0/G1 and S phases. IND-LPC strongly induces breast cancer cell death.
Apoptosis Determination by Annexin-V Staining
To determine the type of death induced, a further study was carried out using an Annexin-V antibody because it binds to phosphatidylserine, which is present on the outer side of the cell membrane during early and late apoptosis. Using PI, which penetrates only through damaged cell membranes, we confirmed that only IND-LPC and OA-IND-PC (at a concentration of 1.5 × IC50) slightly increased the number of apoptotic (AnV+/PI+) MV4-11 cells (Figure 2C and D). No effect was observed on MDA-MB-468 cells.
Caspase-3/7 Activity Determination
Activation of caspase cascades is one of the mechanisms involved in apoptosis, with caspase-3 and −7 serving as the primary effectors. The peptide substrate for caspase-3/7: Ac-DEVD-AMC was used to evaluate the expression of active caspase-3/7. Although caspase-3 and −7 share very similar primary sequence specificity, it was found that they cleave a particular set of native substrates at different rates based on factors other than the primary sequence. The results from caspase-3 and/or caspase-7 activation together allowed to check whether “executors of apoptosis” caspases are activated and whether the observed cell death occurs via the caspase pathway. The leukemia MV4-11 and breast cancer MDA-MB-468 cells were incubated for 24 h or 72 h with the test compounds (at a concentration of 1.5 × IC50), and using camptothecin as the positive control (at a concentration of 0.025 µg/mL) (Figure 2E and F). Compound IND-LPC induced activation of caspase-3 in higher level after 24 h than 72 h and in higher level in breast cancer cells than in leukemia cells (the same concentration, 4.5 µM, was applied). Heterosubstituted PC induced caspase-3 activity in MV4-11 and MDA-MB-468 cells at similar level after 24 h and 72 h except for OA-IND-PC, which strongly increased caspase activity in MV4-11 cells after 72 h. This result was in accordance with those obtained for MV4-11 cell death induction (increase in phosphatidylserine expression, AnV+).
Molecular Calculations
Molecular modeling was carried out to determine the molecular characteristics of the conjugates responsible for their anticancer properties. The obtained results are summarized in Table 3. The energy difference ΔE is presented as the “energy gap” which is the difference between the highest occupied molecular orbital (EHOMO) and lowest unoccupied molecular orbital (ELUMO). The values of absolute electronegativity (χ), chemical potential (μ), absolute hardness (ɳ) and electrophilicity index (ω) were calculated on the basis of the calculated EHOMO and ELUMO using equations 2–4.40–42
 |
Table 3 Parameters Describing the Electronic Properties of Studied Molecules Calculated Using SPARTAN’18 Software |
Any significant differences between ΔE, χ and ƞ calculated for IND and its conjugates were observed. However, the highest values of ΔE and ƞ were observed for IND-OA-PC which indicates that this conjugate is the most stable among studied. In parallel, the lowest values of ΔE and ƞ were noticed for OA-IND-PC. These results suggest that the double bond in the acyl chain and the position of this unsaturated chain in the conjugate structure may determine the reactivity of the molecule. The higher reactivity of OA-IND-PC was also indicated by the highest value of ω which expresses the propensity of the electron acceptors to acquire additional electronic charges from the environment. On the other hand, all studied conjugates were characterized by higher polarizability and dipole moment compared to the values of these parameters calculated for IND. This suggests that the differences in the biological activities of these compounds may result from changes in their trend toward charge distribution following conjugation.
Table 4 presents the descriptor values for the thermodynamic (logP) and structural (TPSA, area, volume, and ovality) properties of these molecules. IND and its conjugates containing long-chain fatty acids are lipophilic compounds. Lipophilicity is characterized by the octanol/water partition coefficient (logP), which has been used to predict the biological activity of compounds and describe their ability to reach specific molecular targets. Lipophilic molecules possess logP higher than 0.43 Among studied compounds, only IND-LPC has a higher affinity for the aqueous phase (logP lower than 0). Conjugation of IND with LPC or PC and palmitic, stearic, and oleic acid increased the molecular mass and, thus, all structural parameters of the synthesized compounds. Chemical modifications allow IND to acquire new biological properties in vitro. The Lipinski’s rule of five, published in 1997 by Christopher A. Lipinski, states that molecules with the best solubility and permeability should exhibit a molecular mass below 500, logP < 5, no more than 5 hydrogen bond donors (HBD) and no more than 10 hydrogen bond acceptors (HBA).44 Molecules violating more than one of these principles may have problems with bioavailability. For the studied conjugates, both their HBA and molecular weights were high. Therefore, in the next step, we applied nanotechnology techniques to target the active molecules and increase their bioavailability. Topological polar surface area (TPSA) is a good indicator of intestinal drug absorption and penetration through the blood-brain barrier. Generally, the TPSA of orally administered drugs should be lower than 140 Å2.45 For the synthesized hybrids, the values for this parameter ranged from 136.37 to 142.44.
 |
Table 4 Parameters Describing Thermodynamic and Structural Properties of Studied Molecules Calculated Using SPARTAN’18 (Volume, Area, Ovality) or Molinspiration (logP, TPSA, HBA, HBD) Software |
Preparation of Nanostructured Lipid Carriers Loading Selected Hybrid IND-OA-PC and Characterization of IND-OA-PC-NLC
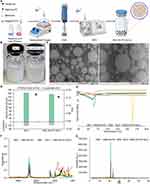
After characterizing the conjugates, IND-OA-PC was encapsulated into an NLC formulation prepared using the combined methodology of high shear homogenization (HSH) and HPH techniques, as illustrated in Figure 3A. Empty NLC were fabricated following the same procedure but without the addition of IND-OA-PC.
To fabricate empty NLC, an aqueous phase containing Tween 80® and a lipid phase consisting of BW and Miglyol® 812 were used. IND-OA-PC was dissolved in the lipid phase. Tween 80®, a sorbitan oleate ester, was selected as a non-toxic and non-ionic surfactant that prevents particle aggregation and degradation.46 Miglyol® 812 is a glycerol triester of caprylic and capric acids, known for its high chemical stability and low viscosity47 whereas BW is a natural wax that consists mainly of esters of fatty acids and various long-chain alcohols.48 Selected excipients are on the list of products with Generally Recognized As Safe (GRAS) status and are dedicated to the production of formulations administrated by the oral route.49 Moreover, all the above products are also on the list of safe substances in the inactive ingredients database of the FDA.50
According to the procedure presented in Materials and Methods, the aqueous and lipid phases were heated at 85 °C in a water bath and their mixture was homogenized using HSH at 8000 rpm for 30s. The obtained pre-emulsion was then subjected to hot HPH technique (900 bar, 85 °C) (Figure 3A). The final formulations of NLC and IND-OA-PC-NLC were milky and of white color (Figure 3B).
The HSH method, performed using Ultra-Turrax® T10 basic, was used to obtain emulsions by mechanically mixing all components. Subsequently, the HPH method was applied. This technique is based on the application of high-pressure and mechanical forces in the production of NLC, and offers many advantages. The process is easy to scale up and fast in the production of uniform and stable NLC with a small size. Moreover, this method avoids toxic chemicals, which is important for maintaining good manufacturing standards for pharmaceutical products.51
DLS technique was applied to determine the main parameters (Z-Ave and PDI) of the fabricated nanoformulations. Size of the empty NLC and IND-OA-PC-NLC were 163 ± 1.39 and 155.5 ± 1.35 nm, respectively. They were monomodal populations with PDI from 0.17 ± 0.01 to 0.15 ± 0.01 and negative surface charge (ZP) values of –21 ± 0.27 mV and –17.4 ± 0.49 mV (Figure 3D). According to the TEM images (Figure 3C), the IND-OA-PC-NLC showed no particle aggregation, attributed to the negative electric repulsion of the particles. Furthermore, TEM images confirmed that the IND-OA-PC-NLC were spherical, and the Z-Ave was very similar to that obtained using DLS. The shape of NLC plays a vital role in cellular internalization of the particles. In this regard, spherical particles are internalized by cells quicker than any other particle shapes.52
The physicochemical parameters of NLC play a crucial role in their anticancer applications. NLC require a higher half-life and circulation time.53 Considering particle targets; particles within a certain size range can be withdrawn by organs, particles < 100 nm can be expelled through the liver, and those between 10 and 20 nm can be expelled through the kidney. The favored particle size range for a higher circulation time range is 150–200 nm, which is in accordance with the size of the obtained IND-OA-PC-NLC.54 It is also worth noting that the circulation time is often compromised by the mononuclear phagocyte system (MPS), and possible bypassing of the MPS is determined by the particle surface. Cationic surface charge makes particles identified by MPS faster than anionic and neutral particles but neutral surface charge may cause particle aggregation.52 In an attempt to avoid these obstacles, we prepared anionic IND-OA-PC-NLC for anticancer application. Notably, anionic nanoparticles reduce adverse toxic effects during blood circulation by preventing their binding to blood proteins. Cationic and neutral nanoparticles bind to blood proteins.52
The encapsulation efficacy (EE) of IND-OA-PC in NLC was estimated from quantification of non-encapsulated amount of IND-OA-PC, and the value was 99.9 ± 0.2%. Its excellent EE and reduction in particle size in DLS analysis were associated with the interaction of IND-OA-PC with other lipids. This was further confirmed by the interaction between IND-OA-PC and the lipid matrix characterized by different techniques.
Analysis of Interactions
DSC was performed to identify the compatibility between the selected lipids and IND-OA-PC (Figure 3E). IND and IND-OA-PC showed melting endothermic peaks at 164 °C and 91 °C, respectively. The incorporation of IND into phosphatidylcholine formed a hybrid molecule in an amorphous state and prevented the recrystallization of IND. A change in the heating enthalpy (ΔH) of IND from 111.01 to 22.34 J/g was observed after the conjugation of the drug with PC. Tonset 162.6 °C was observed for IND, whereas for IND-OA-PC was detected at 80.7 °C. BW during the fabrication of NLC resulted in good compatibility with other excipients with similar Tonset at 61.3 °C and ΔH change reduction from 134.14 to 56.72 J/g. The addition of liquid lipid Miglyol® 812 reduced the crystallinity of BW. In IND-OA-PC-NLC, Tonset and ΔH were further reduced to 47.7 °C and 53.64 J/g, respectively. The above evidence indicates that loading IND-OA-PC further distorted the lipid matrix. The thermograms obtained in our study produced a well-distorted lipid matrix that was suitable for IND-OA-PC encapsulation. IND-OA-PC was well homogenized and compatible with the lipid matrix.55 Tonset of NLC and IND-OA-PC-NLCs were slightly different at 64 °C and 62 °C, respectively.
In addition, the interactions between IND-OA-PC, the lipid matrix, and the surfactant were analyzed using FTIR spectroscopy. Figure 3F presents the FTIR spectra of IND, IND-OA-PC, solid lipid BW, empty nanoparticles (NLC), and IND-OA-PC-NLC. The IND spectrum presented characteristic bands at 1716 and 1690 cm−1 assigned to the vibration of the carbonyl (C=O) group, and bands from the aromatic ring (C=C) at 1479 cm−1. Additional bands between 1262 and 1221 cm−1 originate from the ether group (=C-O), whereas the band below 1012 cm−1 and the band at 737 cm−1 correspond to the C–Cl stretching and C–H deformation bonds, respectively.56
The structure of IND-OA-PC was confirmed by the spectrum band at 1729 cm−1 from the carbonyl group (C=O) and at 1235 cm−1 from the ester bonds. For BW in the FTIR spectrum in the fingerprint region, the bands are in accordance with those presented in the literature48 whereas in the spectrum of empty NLC, bands at 1157 and 1104 cm−1 correspond to the presence of Miglyol® 812 in the lipid matrix.57
The XRD profiles presented in Figure 3G allow the assessment of the crystallinity of IND-OA-PC in NLC. IND presents sharp 2-theta peaks at 10.2°, 11.8°, 17.0°, 19.9°, and 21.9°, whereas IND-OA-PC showed minor intense peaks at 3.9° and 20.8°. These peaks were not detected in IND-OA-PC-NLC, indicating that they were present in a dissolved state in the NLC. BW presents 2-theta peaks at 21.4° and 23.8° (2Ɵ) characteristic of the β’ form of triacylglycerols,58 which is reduced in the spectra, attributed to the interaction with Miglyol® 812. This may contribute to BW recrystallization, prevent IND-OA-PC expulsion, and boost the loading capacity.55
FTIR showed no additional changes in functional groups during IND-OA-PC loading due to hydrophobic interactions, hydrogen bonds, and van der Waals interactions.59
Stability of IND-OA-PC and IND-OA-PC-NLC
Ester bonds between hydroxyl group of glycerol skeleton and IND/OA have been reported to be pH-sensitive.39 Therefore, stability of IND-OA-PC was assessed at pH 1.2 and 7.2 to resemble gastric and blood pH, respectively. Hydrolysis of IND-OA-PC was not observed during the initial 12 h at any pH value (Figure 4A). After this time, at pH 1.2, hydrolysis of IND-OA-PC begin to, and after 24 h only 13.67 ± 2.32% remained unaltered. IND-OA-PC was stable at pH 7.2 throughout the study period.
Moreover, long-term storage stability of IND-OA-PC-NLC formulation was carried out at 4, 25, and 38 °C. The particle size, PDI, and ZP were monitored for up to 60 days, and the results are presented in Figure 4B and C. The Z-Ave did not show significant variations at any of the studied temperatures, and the PDI values decreased to below 0.2. In addition, no significant modifications in surface charge were observed for 60 days at 4 and 25 °C, but they occurred at 38 °C (p<0.0001).
Conjugation of biologically active molecules with lipids improves encapsulation efficacy and prevents particle denaturation, as a result of enhancing interactions within the matrix.60,61 The level of stability of the formulation is also influenced by the manufacturing technique, and HPH favors the production of small-sized nanoparticles.51 We observed the short-term stability of the produced formulation up to 30 days. After this time, the encapsulation efficacy (EE) was reduced at 25 and 38 °C to 98.45 ± 0.87% and 97.58 ± 0.11%, respectively. However, at 4 °C, no modification of EE was observed. During storage, Miglyol® 812 incorporation prevented IND-OA-PC leakage from the BW matrix because of its ability to reduce BW crystallinity.55,58,62
Furthermore, NLC and IND-OA-PC-NLC were sterilized using gamma radiation at 25 KGy of 60Co.63 There was no significant influence (p>0.05) of gamma radiation on the physicochemical properties of the NLC and IND-OA-PC-NLC (Figure 4D). EE remained the constant at 99.9%. Furthermore, gamma-irradiated NLC and IND-OA-PC-NLC were assessed as potential antitumor agents.
Release, in vitro Antiproliferative Activity and Bioavailability of IND-OA-PC-NLC
The release profiles of IND-OA-PC and IND-OA-PC-NLC were measured for 24 h. The rapid release kinetics of free IND-OA-PC is shown in Figure 5. IND-OA-PC diffused up to 91.75 ± 4.33% during the first 10 h, whereas IND-OA-PC-NLC released 40.53 ± 2.79% of IND-OA-PC after the same time period.
 |
Figure 5 Release profile of IND-OA-PC from free IND-OA-PC and IND-OA-PC-NLC after 24 h of incubation, data are adjusted by first-order and hyperbola models, respectively. |
The obtained IND-OA-PC release from the NLC indicated prolonged drug release kinetics due to the conjugation of IND with OA and PC. Therefore, a much faster release profile of IND-OA-PC was observed in the initial phase due to its presence at the surface of the nanoparticles. Later, the release was prolonged, probably due to diffusion of IND-OA-PC from the inner nanoparticle core.64 This type of release model is commonly observed in lipid nanoparticles.65 Further, release data were fitted to several biopharmaceutical equations being the best fit the one-phase decay model for both free and IND-OA-PC-NLC (Table 5).
Antiproliferative Activity of IND-OA-PC-NLC Against Prostate PC-3 and Breast Cancer MDA-MB-468 Cell Lines
Anticancer potential of empty NLC and IND-OA-PC-NLC was studied after 24 h, 48 h and 72 h of treatment with human prostate PC-3 and human breast MDA-MB-468 cancer cells (Table 6). These cancer lines were chosen for the study because the non-encapsulated conjugate showed the highest activity by effectively reducing the proliferation of cancer cells. IND-OA-PC-NLC possessed 2–3 times higher antiproliferative activity after a longer time (48 h and 72 h) of incubation than after 24 h. The IND-OA-PC incorporated into NLC exhibited greater activity against prostate cancer cells PC3 (IC50 = 15.2 μM) and breast cancer cells MDA-MB-468 (IC50 = 10.3 μM) (Table 6) compared to the free hybrid solution with IC50 33.5 μM and 67.5 μM, respectively (Table 1).
 |
Table 6 The Half Maximal Inhibitory Concentrations (IC50) of Empty-NLC and IND-OA-PC-NLC Against Selected Cancer Cell Lines PC-3 and MDA-MB-468 |
Determination of Compounds Accumulation in Cells by Flow Cytometry
Cellular uptake of IND-OA-PC-NLC was evaluated using the fluorescent dye NR. Prostate and breast cancer cells were incubated with the tested formulation at 20 µM for 5, 15, 30 min., 1 h, 2 h and 4 h (Figure 6). NR fluorescence intensity was measured by flow cytometry (untreated cells were used as unlabeled controls). Flow cytometry confirmed the rapid cellular uptake during the first 30 min in both cancer cell types. During the study, the fluorescence intensity increased over time, with the highest signals observed after 1h for PC-3 cells and after 4 h for breast cancer cells MDA-MB-468. A higher accumulation (higher fluorescence intensity) of IND-OA-PC-NLC was observed in the culture of breast cancer cells than in prostate cancer cells. These results confirmed that lipid nanoparticles effectively penetrated lipid membranes, primarily owing to the presence of liquid lipids in the composition of NLC.66
 |
Figure 6 Accumulation of IND-OA-PC-NLC-NR in MDA-MB-468 and PC-3 cells. (A) Mean fluorescent intensity after 5, 15, 30 min and 1, 2 and 4 h; (B) Flow cytometry histograms of IND-OA-PC-NLC-NR. |
Conclusion
In this study, seven novel indomethacin-phospholipid conjugates molecules were synthesized. Cytotoxicity studies revealed that among the obtained molecules, the most active were lysophosphatidylcholines substituted with indomethacin (IND-LPC) and phosphatidylcholines structured with drugs at the sn-2 position (IND-OA-PC and OA-IND-PC). These molecules exhibited higher activity and selectivity towards cancer cells than toward healthy cells. Molecular analysis predicted that PC with indomethacin attached to sn-1 the position (IND-OA-PC) was the most stable among the synthesized compounds. From the perspective of the tumor environment, conjugates with drug attached to the sn-1 position are of therapeutic significance. Phospholipase A2 (PLA2) is overexpressed in inflammatory and tumor tissues; therefore, heterosubstituted phospholipids with the drug in the sn-1 position may act as prodrugs, and after selective hydrolysis, yield a more active anticancer hybrid IND-LPC. Moreover, the encapsulation of IND-OA-PC into NLC is a suitable strategy with enhanced antitumor properties. This promising approach could also serve as a strategy for developing an effective drug delivery system for hydrophilic IND-LPC hybrids, which will be a subject of future research. The results offer a new solution for delivery of NSAID and confirm that their use in the form of nanocarriers could be a promising alternative for cancer treatment.
Acknowledgments
This work was supported by the Wrocław University of Environmental and Life Sciences (Poland) under the Ph.D. research programme “Innovative Doctorate” no. N070/0005/22. This manuscript is part of a doctoral dissertation entitled “Development of lipid nanosystems of nonsteroidal anti-inflammatory drugs (NSAIDs) and their multifunctional bioconjugates for targeting cancer treatment”. The APC was financed by the Wrocław University of Environmental and Life Sciences. This work was supported by the Spanish Ministry of Science and Innovation under the project PID2021-122187NB-C32.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Kenter G, Greggi S, Vergote I, et al. Results from neoadjuvant chemotherapy followed by surgery compared to chemoradiation for stage Ib2-IIb cervical cancer, EORTC 55994. Am Soc Clin Oncol. 2016;37(15_suppl):5503. doi:10.1200/JCO.2019.37.15_SUPPL.5503
3. Horeweg N, Mittal P, Gradowska PL, Boere I, Chopra S, Nout RA. Adjuvant systemic therapy after chemoradiation and brachytherapy for locally advanced cervical cancer: a systematic review and meta-analysis. Cancers. 2021;13(8):1880. doi:10.3390/CANCERS13081880/S1
4. Xu X, Xu S, Wan J, et al. Disturbing cytoskeleton by engineered nanomaterials for enhanced cancer therapeutics. Bioact Mater. 2023;29:50–71. doi:10.1016/J.BIOACTMAT.2023.06.016
5. Prasad V, Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med. 2017;177(11):1569. doi:10.1001/JAMAINTERNMED.2017.3601
6. Aggarwal S, Chandra Gupta S, Singh Verma S, Aggarwal S, Chandra Gupta S. Drug repurposing for breast cancer therapy: old weapon for new battle. Semin Cancer Biol. 2021;82:68. doi:10.1016/j.semcancer.2019.09.012
7. Basso J, Miranda A, Sousa J, Pais A, Vitorino C. Repurposing drugs for glioblastoma: from bench to bedside. Cancer Lett. 2018;428:173–183. doi:10.1016/J.CANLET.2018.04.039
8. Qin S, Xu C, Li S, et al. Indomethacin induces apoptosis in the EC109 esophageal cancer cell line by releasing second mitochondria-derived activator of caspase and activating caspase-3. Mol Med Rep. 2015;11(6):4694–4700. doi:10.3892/mmr.2015.3331
9. Deb J, Majumder J, Bhattacharyya S, Jana SS. A novel naproxen derivative capable of displaying anti-cancer and anti-migratory properties against human breast cancer cells. BMC Cancer. 2014;14(1):1–8. doi:10.1186/1471-2407-14-567
10. Wang G, Li J, Zhang L, Huang S, Zhao X, Zhao X. Celecoxib induced apoptosis against different breast cancer cell lines by down-regulated NF-κB pathway. Biochem Biophys Res Commun. 2017;490(3):969–976. doi:10.1016/j.bbrc.2017.06.148
11. Sun L, Chen K, Jiang Z, et al. Indometacin inhibits the proliferation and activation of human pancreatic stellate cells through the downregulation of COX-2. Oncol Rep. 2018;39(5):2243–2251. doi:10.3892/OR.2018.6321/HTML
12. Ko Y. Computational drug repositioning: current progress and challenges. Appl Sci. 2020;Vol 10(15):5076. doi:10.3390/APP10155076
13. Lin SZ, Wouessidjewe D, Poelman MC, Duchêne D. Indomethacin and cyclodextrin complexes. Int J Pharm. 1991;69(3):211–219. doi:10.1016/0378-5173(91)90363-S
14. Srinath P, Vyas SP, Diwan PV. Preparation and pharmacodynamic evaluation of liposomes of indomethacin. Drug Dev Ind Pharm. 2000;26(3):313–321. doi:10.1081/DDC-100100359
15. Froelich A, Osmałek T, Snela A, et al. Novel microemulsion-based gels for topical delivery of indomethacin: formulation, physicochemical properties and in vitro drug release studies. J Colloid Interface Sci. 2017;507:323–336. doi:10.1016/J.JCIS.2017.08.011
16. Balguri SP, Adelli GR, Majumdar S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. Eur J Pharm Biopharm. 2016;109:224–235. doi:10.1016/J.EJPB.2016.10.015
17. Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int J Nanomed. 2014;9(1):4357–4373. doi:10.2147/IJN.S46900
18. Li M, Xia W, Khoong YM, et al. Smart and versatile biomaterials for cutaneous wound healing. Biomater Res. 2023;27(1):1–32. doi:10.1186/S40824-023-00426-2/FIGURES/13
19. Ma G, Zhang X, Zhao K, et al. Polydopamine nanostructure-enhanced water interaction with pH-responsive manganese sulfide nanoclusters for tumor magnetic resonance contrast enhancement and synergistic ferroptosis-photothermal therapy. ACS Nano. 2024;18(4):3369–3381. doi:10.1021/ACSNANO.3C10249/SUPPL_FILE/NN3C10249_SI_001.PDF
20. Market Data Forecast. Global nanomedicine market size, share, trends and growth analysis report - segmented by products, disease type, nanomolecule type and region - industry forecast (2023 to 2028). Availabel from: https://www.marketdataforecast.com/market-reports/nanomedicine-market.
21. Thiruchenthooran V, Espina M, Świtalska M, et al. Combination of indomethacin with nanostructured lipid carriers for effective anticancer therapy. Int J Nanomed. 2024;19:7033–7048. doi:10.2147/IJN.S464239
22. Liu H, Bolleddula J, Nichols A, Tang L, Zhao Z, Prakash C. Metabolism of bioconjugate therapeutics: why, when, and how? Drug Metab Rev. 2020;52(1):66–124. doi:10.1080/03602532.2020.1716784
23. Bauer ST. The preparation of fatty acid chlorides. Oil Soap. 1946;23(1):1–5. doi:10.1007/BF02593227
24. Gliszczyńska A, Niezgoda N, Gladkowski W, Świtalska M, Wietrzyk J. Isoprenoid-phospholipid conjugates as potential therapeutic agents: synthesis, characterization and antiproliferative studies. PLoS One. 2017;12(2):e0172238. doi:10.1371/JOURNAL.PONE.0172238
25. Czarnecka M, Świtalska M, Wietrzyk J, Maciejewska G, Gliszczyńska A. Synthesis and biological evaluation of phosphatidylcholines with cinnamic and 3-methoxycinnamic acids with potent antiproliferative activity. RSC Adv. 2018;8(62):35744–35752. doi:10.1039/C8RA07002D
26. Czarnecka M, Świtalska M, Wietrzyk J, Maciejewska G, Gliszczyńska A. Synthesis characterization, and in vitro cancer cell growth inhibition evaluation of novel phosphatidylcholines with anisic and veratric acids. Molecules. 2018;23(8):2022. doi:10.3390/MOLECULES23082022
27. Thiruchenthooran V, Świtalska M, Bonilla L, et al. Novel strategies against cancer: dexibuprofen-loaded nanostructured lipid carriers. Int J Mol Sci. 2022;23(19):11310. doi:10.3390/ijms231911310
28. Lacerda SP, Cerize NNP, Ré MI. Preparation and characterization of carnauba wax nanostructured lipid carriers containing benzophenone-3. Int J Cosmet Sci. 2011;33(4):312–321. doi:10.1111/J.1468-2494.2010.00626.X
29. Anaraki NI, Sadeghpour A, Iranshahi K, et al. New approach for time-resolved and dynamic investigations on nanoparticles agglomeration. Nano Res. 2020;13(10):2847–2856. doi:10.1007/s12274-020-2940-4
30. Sánchez-López E, Egea MA, Cano A, et al. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen—in vitro, ex vivo and in vivo characterization. Colloids Surf B Biointerfaces. 2016;145:241–250. doi:10.1016/j.colsurfb.2016.04.054
31. Yang J, Zhong W, Xue K, Wang Z. Design, synthesis and pharmacological evaluation of CVIB, a codrug of carvacrol and ibuprofen as a novel anti-inflammatory agent. Int Immunopharmacol. 2019;71:76. doi:10.1016/J.INTIMP.2019.105856
32. Abla MJ, Banga AK. Formulation of tocopherol nanocarriers and in vitro delivery into human skin. Int J Cosmet Sci. 2014;36(3):239–246. doi:10.1111/ICS.12119
33. Carvajal-Vidal P, González-Pizarro R, Araya C, et al. Nanostructured lipid carriers loaded with Halobetasol propionate for topical treatment of inflammation: development, characterization, biopharmaceutical behavior and therapeutic efficacy of gel dosage forms. Int J Pharm. 2020;585:119480. doi:10.1016/J.IJPHARM.2020.119480
34. Nevozhay D. Cheburator software for automatically calculating drug inhibitory concentrations from in vitro screening assays. PLoS One. 2014;9(9):e106186. doi:10.1371/JOURNAL.PONE.0106186
35. Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115(19):10938–10966. doi:10.1021/ACS.CHEMREV.5B00046/ASSET/ACS.CHEMREV.5B00046.FP.PNG_V03
36. Singh RP, Gangadharappa HV, Mruthunjaya K. Phospholipids: unique carriers for drug delivery systems. J Drug Deliv Sci Technol. 2017;39:166–179. doi:10.1016/J.JDDST.2017.03.027
37. Dvir E, Elman A, Simmons D, et al. DP-155, a lecithin derivative of indomethacin, is a novel nonsteroidal antiinflammatory drug for analgesia and Alzheimer’s disease therapy. CNS Drug Rev. 2007;13(2):260–277. doi:10.1111/J.1527-3458.2007.00014.X
38. Dahan A, Duvdevani R, Shapiro I, Elmann A, Finkelstein E, Hoffman A. The oral absorption of phospholipid prodrugs: in vivo and in vitro mechanistic investigation of trafficking of a lecithin-valproic acid conjugate following oral administration. J Control Release. 2008;126(1):1–9. doi:10.1016/J.JCONREL.2007.10.025
39. Irby D, Du C, Li F. Lipid–drug conjugate for enhancing drug delivery. Mol Pharm. 2017;14(5):1325. doi:10.1021/ACS.MOLPHARMACEUT.6B01027
40. Pearson RG. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci U S A. 1986;83(22):8440. doi:10.1073/PNAS.83.22.8440
41. Kohn W, Becke AD, Parr RG. Density functional theory of electronic structure. J Phys Chem. 1996;100(31):12974–12980. doi:10.1021/JP960669L
42. Parr RG, Szentpály LV, Liu S. Electrophilicity index. J Am Chem Soc. 1999;121(9):1922–1924. doi:10.1021/JA983494X
43. Chmiel T, Mieszkowska A, Kempińska-Kupczyk D, Kot-Wasik A, Namieśnik J, Mazerska Z. The impact of lipophilicity on environmental processes, drug delivery and bioavailability of food components. Microchem J. 2019;146:393–406. doi:10.1016/J.MICROC.2019.01.030
44. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1–3):3–25. doi:10.1016/S0169-409X(96)00423-1
45. Da Silva MM, Comin M, Duarte TS, et al. Synthesis, antiproliferative activity and molecular properties predictions of Galloyl derivatives. Moleclus. 2015;20(4):5360–5373. doi:10.3390/MOLECULES20045360
46. Zukancic D, Suys EJA, Pilkington EH, Algarni A, Al-Wassiti H, Truong NP. The importance of poly(ethylene glycol) and lipid structure in targeted gene delivery to lymph nodes by lipid nanoparticles. Pharm. 2020;12(11):1068. doi:10.3390/PHARMACEUTICS12111068
47. Souto EB, Doktorovova S, Gonzalez-Mira E, Egea MA, Garcia ML. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr Eye Res. 2010;35(7):537–552. doi:10.3109/02713681003760168
48. Svečnjak L, Baranović G, Vinceković M, Prđun S, Bubalo D, Gajger IT. An approach for routine analytical detection of beeswax adulteration using ftir-atr spectroscopy. J Apic Sci. 2015;59(2):37–49. doi:10.1515/JAS-2015-0018
49. Chauhan I, Yasir M, Verma M, Singh AP. Nanostructured lipid carriers: a groundbreaking approach for transdermal drug delivery. Adv Pharm Bull. 2020;10(2):150–165. doi:10.34172/APB.2020.021
50. US Food & Drug Administration. Inactive ingredient search for approved drug products. Available from: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm.
51. Amasya G, Aksu B, Badilli U, Onay-Besikci A, Tarimci N. QbD guided early pharmaceutical development study: production of lipid nanoparticles by high pressure homogenization for skin cancer treatment. Int J Pharm. 2019;563:110–121. doi:10.1016/j.ijpharm.2019.03.056
52. Di J, Gao X, Du Y, Zhang H, Gao J, Zheng A. Size, shape, charge and “stealthy” surface: carrier properties affect the drug circulation time in vivo. Asian J Pharm Sci. 2021;16(4):444–458. doi:10.1016/J.AJPS.2020.07.005
53. Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27(1):27–36. doi:10.1016/J.EJPS.2005.08.002
54. Jasinski DL, Li H, Guo P. The effect of size and shape of RNA nanoparticles on biodistribution. Mol Ther. 2018;26(3):784–792. doi:10.1016/J.YMTHE.2017.12.018
55. Muller RH, Ranjita S, Cornelia MK. 20 years of lipid nanoparticles (SLN & NLC): present state of development & industrial applications. Curr Drug Discov Technol. 2011;8(3):207–227. doi:10.2174/157016311796799062
56. Jamrógiewicz M, Józefowicz M. Preparation and characterization of indomethacin supramolecular systems with β-Cyclodextrin in order to estimate photostability improvement. Molecules. 2021;26(24):7436. doi:10.3390/MOLECULES26247436
57. Ajiboye AL, Nandi U, Galli M, Trivedi V. Olanzapine loaded nanostructured lipid carriers via high shear homogenization and ultrasonication. Sci Pharm 2021. 2021;89(2):25. doi:10.3390/SCIPHARM89020025
58. Jenning V, Gohla S. Comparison of wax and glyceride solid lipid nanoparticles (SLN®). Int J Pharm. 2000;196(2):219–222. doi:10.1016/S0378-5173(99)00426-3
59. Hu Q, Zhou F, Ly NK, et al. Development of multifunctional nanoencapsulated trans-resveratrol/Chitosan nutraceutical edible coating for Strawberry preservation. ACS Nano. 2023;17(9):8586–8597. doi:10.1021/ACSNANO.3C01094/SUPPL_FILE/NN3C01094_SI_001.PDF
60. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2020;20(2):101–124. doi:10.1038/s41573-020-0090-8
61. Sun B, Luo C, Li L, et al. Core-matched encapsulation of an oleate prodrug into nanostructured lipid carriers with high drug loading capability to facilitate the oral delivery of docetaxel. Colloids Surf B Biointerfaces. 2016;143:47–55. doi:10.1016/J.COLSURFB.2016.02.065
62. Ban C, Lim S, Chang PS, Choi YJ. Enhancing the stability of lipid nanoparticle systems by sonication during the cooling step and controlling the liquid oil content. J Agric Food Chem. 2014;62(47):11557–11567. doi:10.1021/JF503489V/SUPPL_FILE/JF503489V_SI_001.PDF
63. Carvajal-Vidal P, Fábrega MJ, Espina M, Calpena AC, García ML. Development of Halobetasol-loaded nanostructured lipid carrier for dermal administration: optimization, physicochemical and biopharmaceutical behavior, and therapeutic efficacy. Nanomed Nanotechnol Biol Med. 2019;20:102026. doi:10.1016/J.NANO.2019.102026
64. Son GH, Lee BJ, Cho CW. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J Pharm Invest. 2017;47(4):287–296. doi:10.1007/S40005-017-0320-1/FIGURES/7
65. Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288. doi:10.4103/1735-5362.235156
66. Neves AR, Queiroz JF, Costa lima SA, Figueiredo F, Fernandes R, Reis S. Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: relevance for oral drug delivery. J Colloid Interface Sci. 2016;463:258–265. doi:10.1016/j.jcis.2015.10.057
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.