Back to Journals » Journal of Pain Research » Volume 18
Multisite Chronic Pain and the Risk of Breast Cancer and Its Subtypes: A Mendelian Randomization Study
Authors Li W , Liu J, Wang T, Hou Y, Bao J, Song Y, Liu L, Ge S, Shang Y, Wang R, Zhang M, Xu M
Received 23 September 2024
Accepted for publication 7 April 2025
Published 7 May 2025 Volume 2025:18 Pages 2343—2357
DOI https://doi.org/10.2147/JPR.S489703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Amitabh Gulati
Wanyu Li,1,2 Jintao Liu,3 Teng Wang,2,3 Yudong Hou,3,4 Jianheng Bao,2,3 Yanyan Song,1,2 Longbi Liu,2,3 Shuke Ge,3 Yaohua Shang,5 Rongdi Wang,1 Min Zhang,1 Meng Xu1
1Department of Colorectal Surgery, Dalian University of Technology Affiliated Central Hospital (Dalian Central Hospital), Dalian, Liaoning Province, 116033, People’s Republic of China; 2Graduate School, Dalian Medical University, Dalian, Liaoning Province, 116041, People’s Republic of China; 3Department of Thyroid Surgery, Central Hospital Affiliated to Dalian University of Technology, Dalian, Liaoning Province, 116033, People’s Republic of China; 4Graduate School, China Medical University, Shenyang, Liaoning Province, 110122, People’s Republic of China; 5The First Department of Hand and Foot Surgery, Central Hospital Affiliated to Dalian University of Technology, Dalian, Liaoning Province, 116033, People’s Republic of China
Correspondence: Meng Xu, Email [email protected]
Background: Chronic pain (CP) is widespread and a major cause of disability. However, its genetic and environmental risk factors, as well as its relationship with breast cancer (BC), remain unclear. The study is the first to apply Mendelian randomization (MR) to explore the causal relationship between CP and BC.
Methods: Two-sample MR and multivariable MR (MVMR) were performed using genome-wide association study (GWAS) data. Univariable MR assessed the effect of CP on BC, while MVMR adjusted for body mass index (BMI). The inverse variance-weighted method was used as the primary method.
Results: Univariable MR found a strong genetic link between stomach/abdominal pain and overall BC risk (OR 3.411, 95% CI 1.029– 11.313, P=0.045). Neck/shoulder pain was associated with Luminal_A breast cancer risk (OR 1.999, 95% CI 1.263– 3.163, P=0.003). Multivariable MR, adjusting for BMI, confirmed these findings for stomach/abdominal pain to overall BC (OR 4.39, 95% CI 1.48– 13.06, P=0.008) and neck/shoulder pain to Luminal_A BC (OR 2.46, 95% CI 1.24– 4.87, P=0.010). No associations were found for other pain types (headache, hip pain, back pain, knee pain, facial pain) with BC subtypes.
Conclusion: Genetic evidence in this study suggests a causal link between stomach/abdominal pain and overall BC, and between neck/shoulder pain and Luminal-A BC risk in Europeans. Determining the cause of this discrepancy might shed light on the complicated link between breast cancer etiology and chronic pain genetics, emphasizing the need for further investigations and potential clinical applications to enhance breast cancer prevention and management.
Keywords: Mendelian randomization, chronic pain, breast cancer, BMI, confounder
Introduction
Chronic pain (CP) is widely defined as pain persisting beyond 3 months1,2 and can be a primary disorder or secondarily associated with injury, surgery, or a range of medical conditions. Around 30% of the global population suffers from chronic pain, with an average of three pain locations reported.3 The classification of chronic pain is a systematic categorization of multiple types of chronic pain conditions. The International Association for the Study of Pain (IASP) developed a classification of chronic pain for the International Classification of Diseases (ICD-11) Among 7 major diagnostic categories, chronic primary pain comprises chronic pain disorders that cannot be better explained by another specific chronic pain diagnosis.4 Headache, stomach or abdominal pain, neck or shoulder pain, back pain, facial pain, hip pain and knee pain are frequent causes of chronic pain in people. Previous work suggested that ∼50% variation in chronic pain development is heritable.5 Numerous aspects of chronic pain have been investigated from a genetic perspective. It has been demonstrated that several aspects are complex traits with moderate heritability.6 It is often associated with both specific and non-specific medical conditions such as cancers, HIV/AIDS, fibromyalgia and musculoskeletal conditions.7–9 Moreover, CP is associated with expensive health care costs, increasing disability and mortality.10–14
Breast cancer is a prominent cause of cancer incidence worldwide; 2.3 million people were newly diagnosed in 2020, accounting for 11.7% of all cancer cases. It is the sixth biggest cause of cancer-related mortality globally, with 685,000 deaths.15 Although the current treatment modalities, including surgical intervention, radiotherapy, chemotherapy, and targeted therapy, have significantly contributed to a reduction in breast cancer mortality rates, the etiology of this disease remains elusive. Furthermore, environmental factors such as radiation exposure and lifestyle choices alongside genetic predisposition play pivotal roles in breast cancer development.16 About 5–10% of cases of breast cancer are caused by genetic factors, such as single nucleotide polymorphisms (SNPs) in the genome and genetic abnormalities in susceptible genes.17 Defined by combinations of ER, PR, HER2 and grade, there are five subtypes of BC, including (1) luminal A-like (ER+ and/or PR+, HER2-, grade 1 and 2); (2) luminal B/HER2-negative-like (ER+ and/or PR+, HER2-, grade 3); (3) luminal B-like (ER+ and/or PR+, HER2+); (4) HER2-enriched-like (ER- and PR-, HER2+), and (5) triple-negative (ER-, PR-, HER2-).18 These definitions rely on immunohistochemical (IHC) markers and gene expression profiles, including: Estrogen receptor (ER) status, Progesterone receptor (PR) status, HER2/neu receptor status, Ki-67 proliferation index. Each subtype is characterized by distinct molecular drivers, responses to treatment, and progression rates, which may influence how chronic pain impacts breast cancer patients.
Obesity plays a significant role in breast cancer and pain. An excessive body mass index (BMI) may raise the risk of chronic pain.19 Obesity causes increased stress on joints, bones, and soft tissues, resulting in pain and inflammation. Obesity can also cause metabolic issues, chronic inflammation, and neurological abnormalities, all of which contribute to the development of chronic pain. An extremely low BMI may also be linked to chronic pain. Malnutrition and being underweight can induce muscle wasting and bone loss, both of which can be painful. It has been previously observed that BMI is both genetically correlated and epidemiologically associated with BC. The majority of prospective cohort studies found a negative correlation between BMI and the incidence of premenopausal breast cancer.20–25 There is a little positive correlation between BMI and the risk of postmenopausal breast cancer.20,21,26 Additionally, a significant MR Analysis revealed a consistent inverse relationship in all subgroups studied between premenopausal breast cancer risk and BMI predicted by GWAS-identified genetic variants.27 Thus, BMI can be considered the most important confounder to be controlled for.
While some research has explored the connection between chronic pain (CP) and breast cancer, the findings remain ambiguous. It is still unclear whether chronic pain plays a causal role in the initiation and progression of breast cancer, and the underlying mechanisms are not yet fully understood. Recent studies have suggested that chronic pain may influence cancer development through several biological pathways. Pro-inflammatory cytokines, such as IL-6 and TNF-α, are often elevated in chronic pain conditions and may contribute to a tumor-promoting microenvironment by enhancing angiogenesis, suppressing immune surveillance, and increasing metastatic potential.28 Furthermore, CP-induced chronic stress and inflammation may disrupt hormonal regulation, potentially altering estrogen metabolism and receptor signaling pathways in estrogen-sensitive breast cancer subtypes.29 Furthermore, chronic pain is frequently accompanied by oxidative stress, which can cause DNA damage and mutations, promoting cancer initiation.30 To better understand the causal relationship between chronic pain and breast cancer, we aimed to employ a genetic approach using Mendelian randomization (MR). Recent genome-wide association studies (GWAS) have identified multiple loci associated with CP, and in MR, a genetic score composed of CP-associated single nucleotide polymorphisms (SNPs) can serve as an instrumental variable. This approach allows us to evaluate the potential causal link between CP and breast cancer risk while minimizing selection bias and confounding factors typically encountered in traditional epidemiological studies.
Materials and Methods
Study Design
The publicly available studies that were approved in the corresponding studies by the institution itself provided us with the research summary dataset. As all exploited data was publicly accessible, no further approval is necessary. A two-sample Mendelian randomization was employed to evaluate the causal relationship among seven categories of chronic pain (including back pain, facial pain, headache, hip pain, knee pain, neck/shoulder pain, and stomach/abdominal pain) and breast cancer and its 5 subtypes (including Luminal A, Luminal B, Luminal B/HER2-negative, Her2-positive breast cancer, triple-negative breast cancer). The selection of these specific chronic pain types and breast cancer subtypes is based on their relevance to the study’s objectives, as detailed in the Introduction. Additionally, only these seven chronic pain types available in the database could be available for this analysis. Briefly, chronic pains served as the exposures, while all breast cancer and 5 subtypes of breast cancer served as the outcomes. Single-nucleotide polymorphisms (SNPs) that were highly correlated with seven different types of MCP were chosen to serve as instrumental variables (IVs). We first performed univariable MR analysis to identify the causal effect of chronic pain on breast cancer.
Given the association between obesity, chronic pain and breast cancer, we conducted a multivariable Mendelian randomization analysis to concurrently assess the direct impact of pain on breast cancer, accounting for obesity.
The MR method must adhere to three key assumptions: (i) the instrumental variables (IVs) should have a strong association with the exposures; (ii) the IVs are independent of any confounding factors in the relationship between exposures and outcomes and (iii) the instruments affect the outcomes only through the relevant exposures (Figure 1).
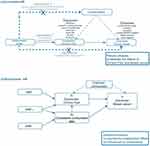 |
Figure 1 Diagrams illustrating associations examined in this study. (a) Univariable MR analysis; (b) Multivariable MR analysis. |
Data Sources
Single nucleotide variants (single-nucleotide polymorphisms [SNPs]) associated with CP were obtained from a comprehensive biomedical database of genome-wide association studies: the UK Biobank Consortium. This study identified facial pain (ukb-b-17107), headache (ukb-b-12181), hip pain (ukb-b-7289), knee pain (ukb-b-16254), stomach or abdominal pain (ukb-b-11413), neck or shoulder pain (ukb-b-18596), and back pain (ukb-b-9838) as prevalent contributors to chronic pain in individuals. SNPs associated with headache, knee pain, and back pain (P < 5×10−8), as well as SNPs associated with facial pain, hip pain, stomach/abdominal pain, and neck/shoulder pain (P < 5×10−6), were selected as instrumental variables (IVs). In assessing instrument strength and statistical power, we used a F statistic greater than 10 to signify a reasonably low probability of weak instrument bias for our Mendelian Randomization investigation.
GWAS summary data on breast cancer were obtained from the MRC-IEU. The SNPs for BRCA were sourced from a recent meta-analysis of GWAS datasets compiled by Zhang, Haoyu et al,18 encompassing 133,384 cases and 113,789 controls. They assessed variations with a minor allele frequency (MAF) over 0.01 (about 10 million) and a r² of at least 0.3, while excluding variants located within ±500 kb of, or in linkage disequilibrium (r² ≥ 0.1) with established susceptibility variants. A minimum allele frequency (MAF) greater than 0.01 was selected to guarantee a sufficient sample size. The five subtypes of breast cancer—luminal A, luminal B, luminal B/HER2-negative, HER2-enriched, and triple-negative—have been incorporated into research investigations. The SNPs for BMI were obtained from a meta-analysis of genome-wide association studies concerning body fat distribution in 694,649 individuals of European descent conducted by Pulit SL et al.31 They identified the most significantly linked SNPs (P < 5×10−9) and determined all SNPs in linkage disequilibrium (r2 > 0.05) with the top-associated SNP within a ±5Mb range. Table 1 contains the specifics of the data sources.
 |
Table 1 Details on the Characteristics of Each Included Dataset |
Statistical Analyses
Univariable MR analyses were conducted to systematically assess the total effect of each of the seven types of CPs on various subtypes of BCs. This study primarily employed the inverse-variance weighted (IVW) model as its main analytical method. The coherence of the causal estimates was assessed using the weighted median (WM) model and the MR-Egger regression model. The IVW approach assessed the causal consequences of each SNP as a valid natural experiment, evaluating the causal effects of each SNP on the outcome and employing the outcome as weights for meta-analysis to determine the combined causal effect. The IVW (fixed-effect) method yields an unbiased estimate when horizontal pleiotropy is absent or balanced. In the presence of heterogeneity, the random-effects IVW model was utilized, producing valid results contingent upon the assumption of balanced pleiotropy. The findings were reported as odds ratios (ORs) accompanied by their respective 95% confidence intervals (CIs), encompassing analyses of seven exposures and six outcomes. We define a positive result as having an odds ratio (OR) greater than 1 or less than 1, with both the IVW method and the weighted median method yielding P< 0.05. Given the exploratory nature of this study and the limited number of hypothesis tests, we did not apply multiple testing correction to the p-values to avoid over-adjustment, which could obscure meaningful associations. We then performed a multivariable MR (MVMR) analysis to estimate CPs and BMI on BCs. Multivariable MR entails collecting estimates for all instruments on each exposure under consideration, allowing each estimated effect to account for the impact of all other exposures in the model. We initially proceeded to handle instrumental variable (IV) data for exposure variables (CP) and confounding factors (BMI). Clumping of IV data was performed, followed by matching with GWAS data for both CP variables and BMI variables. Subsequently, outcome GWAS data from multiple files were read and formatted for the outcome variable (breast cancer). The MVMR process was then initiated, involving merging and formatting the data, as well as estimating the effect using the IVW (Inverse-Variance Weighted) method. The findings were reported as β coefficients and odds ratios (ORs), accompanied by their respective 95% confidence intervals (CIs). The statistical significance threshold was established at a P-value of less than 0.05. The conclusive positive outcomes are derived from the integration of the positive results from both univariate and multivariate MR analyses.
Pleiotropy was assessed using the MR-Egger intercept test to detect potential bias from SNP effects through alternative pathways. Heterogeneity testing helps determine whether the effect estimates across different SNPs are consistent. Cochran’s Q statistic was utilized to assess the heterogeneity among individual genetic variations. Leave-one-out analysis was employed to assess whether MR estimations were affected by a single SNP and to compute the meta-effect of the other SNPs.
All analyses were carried out using packages “TwoSampleMR”, and “MVMR” in R version 4.3.1.A flowchart detailing the statistical analyses is depicted in Figure 2.
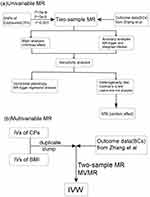 |
Figure 2 Flowchart of the statistical analyses, outlining the different analyses performed at each stage of the study. (a) Univariable MR flowchart, (b) Multivariable MR flowchart. |
Results
Mendelian Randomization Analysis
Overview of the results
Summary information on the SNPs used as genetic instruments are shown in Table S1. Independent SNPs linked with back pain, headache, and knee pain were set at a P-value < 5×10−8, and others were set at a P-value < 5×10−6. A clumping strategy with a threshold of r2 < 0.001 and kb = 10,000 was used to reduce linkage disequilibrium (LD). The F statistics of all identified SNPs were more than 10. Following a series of quality control measures, 23 SNPs with back pain, 19 SNPs with face pain, 58 SNPs with headache, 51 SNPs with hip pain, 11 SNPs with knee pain, 74 SNPs with neck/shoulder pain, and 38 SNPs with stomach/abdominal pain were classified as IVs. The causal relationships between 7 exposures and 6 outcomes were analyzed using Mendelian randomization. More specific results from the univariable MR analyses are summarized in Figures 3, 4 and Tables 2, 3; multivariable MR analysis is summarized in Figure 5. The results of sensitivity analyses are shown in Figures 6 and 7. All MR analysis results are shown in Table S1. Detailed information on each SNP is provided in Table S2.
 |
Table 2 Causal Effects of Pain on OVERALL Breast Cancer |
 |
Table 3 Causal Effects of Pain on Diabetic Luminal_A Breast Cancer |
 |
Figure 4 Forest plot. (A) MR effect size for ‘Stomach or Abdominal Pain’ on ‘OVERALL breast cancer’, (B) MR effect size for ‘Neck or Shoulder Pain’ on ‘Luminal A breast cancer’. |
 |
Figure 5 Independent effect of Pain on the risk of Breast cancer using multivariable Mendelian randomization analysis after adjusting BMI. |
 |
Figure 6 Funnel diagram of instrumental variable. (A) ‘Stomach or Abdominal Pain’ on ‘OVERALL breast cancer, (B) “Neck or Shoulder Pain’ on ‘Luminal A breast cancer. |
Causal Effects of CPs on Overall BC by Univariable MR Analysis
In univariable MR, as is apparent from Table 2, genetic liability to stomach/abdominal pain showed a strong association with a higher risk of overall BC (OR 3.411, 95% confidence interval [CI] 1.029–11.313, P<0.05) by the IVW method and (OR 3.525, 95% CI 1.021–12.176, P<0.05) by the weighted median method (WM). However, a slight causal effect of neck/shoulder pain on overall BC was observed with OR of 1.956 (95% CI 1.264–3.025, P<0.05) using the IVW, but an OR of 1.578 (95% CI 0.955–2.608, P=0.08) using the WM. The definition of a positive result, as mentioned earlier in the methodology section, requires a significant difference (P<0.05) between both the IVW and WM methods. Therefore, we do not believe that neck/shoulder pain could increase the risk of overall BC. The effect of stomach/abdominal pain on overall BC analysis is presented in a scatter plot and a forest plot (Figures 3 and 4). Besides, other chronic pains had no significant effect on overall BC: back pain (IVW: OR = 1.21, 95% CI = 0.72–2.02, P = 0.64), facial pain (IVW: OR = 1.40, 95% CI = 0.15–12.74, P = 0.76), headache (IVW: OR = 1.69, 95% CI = 0.83–3.44, P = 0.15), hip pain (IVW: OR = 0.89, 95% CI = 0.46–1.70, P = 0.72), knee pain (IVW: OR = 1.78, 95% CI = 0.74–4.27, P = 0.2) (Table S1).
Causal Effects of CPs on BC Subtypes by Univariable MR Analysis
Univariable MR revealed a connection between genetic susceptibility to neck/shoulder pain and an elevated risk of Luminal_A breast cancer (OR 1.999, 95% CI 1.263–3.163, P<0.05) by the IVW approach and (OR 1.917, 95% CI 1.029–3.572, P<0.05) by the WM method. In addition, three causal associations between CP and the subtypes of BC were shown by the IVW method, which is stomach/abdominal pain on Luminal-A BC breast cancer (OR=4.303, 95% CI =1.049–17.643, P=4.30E-02), headache on Luminal_B_HER2 Negative breast cancer (OR=2.914, 95% CI = 1.208–7.031, P=1.70E-02) and stomach/abdominal pain on HER2 (+) breast cancer (OR=86.30, 95% CI = 2.70–2760.51, P=1.20E-02), but there was no significant difference shown by the WM method. Therefore, we do not believe that those three results have a causal relationship with each other. The effect of neck/shoulder pain on Luminal_A BC analysis is presented in a scatter plot and a forest plot (Figures 3 and 4). Other 5 chronic pains had no significant effect on Luminal_A BC both by the IVW method and other MR analyses. (see more details in Table S1). Additionally, our IVW results showed that liability to the other 5 chronic pains (including back pain, facial pain, headache, hip pain, and knee pain) does not affect the other 4 subtypes of BCs (including luminal B, Luminal_B_HER2-negative, HER2-enriched, and triple-negative subtypes) which is consistent with results from the other MR methods including MR Egger and weighted median (see more details in Table S1).
Causal Effects of CPs on BCs by Multivariable MR Analysis
In the MVMR analysis of CPs-BMI-BCs, the direct effect of stomach/abdominal pain on overall breast cancer was OR 4.39 (95% CI = 1.48–13.06, P <0.05) after accounting for BMI, the direct effect of neck/shoulder pain on Luminal-A BC was OR 2.46 (95% CI = 1.24–4.87, P <0.05) after accounting for BMI. There were no statistically significant differences in other MVMR results by combining univariable results. All MVMR results are shown in Figure 5.
Sensitivity Analyses
Sensitivity analyses were performed to detect the presence of horizontal pleiotropy and heterogeneity. We assessed horizontal pleiotropy from the MR-Egger regression analysis and heterogeneity using Cochran’s Q test. The MR-Egger intercept provides evidence for balanced horizontal pleiotropy for all MR analyses (P>0.05) (Tables 2,3 and S1). Significant heterogeneity was apparent in stomach/abdominal pain IVs for overall BC (Q p-value= 2.16E−06), which is illustrated in the funnel plot (Figure 6). There was no significant heterogeneity revealed in neck/shoulder pain IVs for Luminal-A BC (Q p-value= 0.27), which is shown in the funnel plot (Figure 6). The leave-one-out analysis suggested that the risk estimates of stomach/abdominal pain for overall BC and neck/shoulder pain for Luminal-A BC generally remained consistent after eliminating each single SNP at a time, which are shown in the LOO plot (Figure 7).
Discussion
To our knowledge, this is the first large-scale univariable MR combining multivariable MR study to simultaneously elucidate the causal effect of each kind of pain on breast cancer with its subtypes. We found pieces of evidence that stomach/abdominal pain is a causative factor for overall BC and neck/shoulder pain is a causative factor for Luminal-A BC. MVMR was utilized to control for confounder (BMI) so that IVs of CPs are not subject to BMI. This helps minimize the potential for confounding bias in estimating causal effects. In this study, MR analysis provided an objective assessment of the causal relationship between specific risk factors and diseases, utilizing data from extensive published GWAS, thereby enhancing the validity and reliability of our findings. The use of data from multiple databases for exposure, confounders, and outcomes reduced potential bias in the estimation of the observational association. The advantages of MR analysis enhance the reliability of our findings.
A Mendelian randomization analysis examining the association between migraine and breast cancer indicates that migraine may serve as a risk factor for overall breast cancer and estrogen receptor-positive breast cancer, but not for estrogen receptor-negative breast cancer.32 Several studies and case reports have identified facial pain as a symptom associated with nonmetastatic lung cancer.33–35 A review indicated that the mean duration from the onset of facial pain to the pursuit of medical attention was 9 months, while the mean interval from seeking medical attention to cancer diagnosis was 8 months.36
The precise mechanistic relationship between chronic pain and breast cancer pathogenesis remains incompletely understood, with no conclusive evidence establishing either direct causality or shared risk factors. However, some evidence suggests potential pathophysiological interactions mediated through specific molecular pathways. The inflammatory hypothesis represents one plausible mechanistic link, as both chronic pain syndromes and breast carcinogenesis are associated with dysregulated inflammatory processes. Pro-inflammatory cytokines, particularly IL-1α and IL-6, have been implicated in both chronic pain maintenance and breast cancer progression. IL-1α demonstrates significant associations with resting low back pain 37 and breast cancer overall survival.38–42 Experimental evidence from Nature demonstrates that inflammatory cytokines (TNF-α, IL-1β, IL-6) enhance cellular invasiveness through chemokine receptor upregulation.28 Notably, Naugler et al identified sexual difference in tumor susceptibility mediated through estrogen-regulated macrophage IL-6 production, suggesting a complex interplay between inflammatory signaling, hormonal regulation, and carcinogenesis.29 Genetic polymorphisms in pain modulation pathways may further contribute to this association. Several Studies on OPRM1 genetic variants in cancer patients discovered that OPRM 118-G allele carriers were associated with high doses of opioids, which means that they were usually more sensitive to pain, compared to rs1799971-A carriers.43–46 A study by Cieślińska et al indicated a significant association between the G allele at position 118 of the μ-opioid receptor gene (OPRM1) and heightened breast cancer incidence (OR=3.3, 95% CI 2.2–5.0, p<0.0001).47,48 Zagon et al.49 Gach et al 50 and Hatzoglou et al 51 found that OPRM1 expression correlates with ER positivity, resulting in ERα activation via MOR activation, which facilitates its translocation to the plasma membrane and promotes the proliferation of breast cancer cells. Some genetic variations may be associated with breast cancer and chronic pain.Neuroimmune interactions represent another potential mechanistic link. Some research indicates that chronic pain-induced sensitization of the nervous system can alter immune responses in the tumor microenvironment. Ke Ren et al summarized that chronic pain leads to neuronal sensitization through complex interactions among immune cells (such as mast cells, macrophages, neutrophils, lymphocytes), glia (including satellite glial cells, microglia, astrocytes) and neurons, involving the release of inflammatory mediators, cytokines, chemokines and neurotransmitters, and the activation of multiple signaling pathways, which ultimately increase synaptic strength and alter pain sensitivity.52 This mechanism may indirectly influence the development of cancer by promoting inflammatory responses in the tumor microenvironment. This may also lead to tumor cell immune evasion, with pain affecting local immune responses through neuropeptide secretion.53 Chronic pain is often associated with increased stress hormones like cortisol, which have immune-suppressive effects. Elevated cortisol levels can impair immune cell function, reducing the body’s ability to respond to cancer cells. Prolonged stress and immune suppression may create a favorable environment for tumor growth and metastasis 54. The cumulative evidence suggests that chronic pain may influence breast cancer development and progression through interconnected mechanisms involving inflammatory signaling, hormonal regulation, genetic predisposition, neuroimmune modulation, and stress-mediated immunosuppression.
In this study, we adjusted for BMI as a potential confounder in the multivariable Mendelian randomization analysis to isolate the independent effect of chronic pain on breast cancer risk. BMI is a well-established risk factor for both chronic pain and breast cancer, with high BMI contributing to mechanical stress, systemic inflammation, and metabolic dysregulation, all of which may exacerbate chronic pain conditions as we mentioned before. Conversely, low BMI has been associated with malnutrition and musculoskeletal degradation, which can also lead to chronic pain. Additionally, BMI exhibits a complex relationship with breast cancer risk. A prior MR study conducted by Y G et al identified an inverse relationship between genetically predicted BMI and the risk of breast cancer.27 Previous studies agreed on whether a causal effect exists between BMI and BC. By accounting for BMI in our analysis, we aimed to minimize confounding and ensure that the observed associations between chronic pain and breast cancer are not driven by BMI-related pathways. However, further research is needed to explore the interplay between BMI, chronic pain, and breast cancer in greater detail.
This study has several limitations. This study’s focus on Europe may limit the generalizability of the findings to other populations and regions. While the use of large-scale GWAS datasets provides a robust foundation for the study, the reliance on historical data may not fully capture recent advances or population-specific variations. Secondly, certain MR analyses lacked sufficient power to identify effects, attributable to the limited number of IVs for each chronic pain condition explained by the SNP instruments or the restricted sample sizes of the outcome (BCs) GWAS. Increased quantities of instrumental variables and genome-wide association studies on biological contexts will enhance the capacity of future Mendelian randomization studies to identify associations. In the MVMR analysis, BMI was the sole confounder variable considered to estimate the direct effect of the CPs on BCs after controlling for it. The MVMR method may yield biased results if other genetic variants directly influence the outcome variables. To address this limitation, future studies should consider incorporating additional covariates, such as lifestyle and physiological factors (eg, hormonal levels, physical activity, smoking, and alcohol consumption), to improve the robustness of the analysis. This would provide a more comprehensive understanding of their potential influence on the relationship between chronic pain and breast cancer. As a result, our findings should be interpreted cautiously and substantiated by more studies.
In summary, our findings provide genetic evidence suggesting a potential causal link between stomach/abdominal pain and overall BC in the European population. In BC subtypes, our results supported that liability to neck/shoulder pain affects Luminal-A breast cancer risk in Europeans. Except for the above two, our findings did not support causal relationships between other types of pain and breast cancer and its subtypes. The MVMR method also validated the above two causal relationships by considering BMI as a confounder subsequently. Pain can significantly affect a patient’s health-related quality of life (HRQoL). It can cause increased anxiety, depression, and fatigue, adding to the disease’s overall burden. A study introduced pain neuroscience education (PNE) into the chronic pain management of breast cancer survivors, which helped improve the quality of life for breast cancer patients.55 People should therefore pay more attention to their pain. Raising awareness of the link between chronic pain and cancer may not delay a cancer diagnosis. Future research should utilize individual-level data and basic science methodologies to explore the mechanisms linking CPs with BMI and the development of BC. We hope that this study will inspire further investigations into the complex interactions between chronic pain and cancer, paving the way for a deeper understanding of how pain may influence cancer development and progression.
Data Sharing Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethic Statement
According to Article 24 of the Ethical Review Measures for Life Sciences and Medical Research Involving Humans issued in 2023, research projects meeting one of the following conditions may apply for exemption from ethical review: research conducted using legally obtained publicly available data, or research using anonymized data that cannot identify specific individuals and does not involve personal privacy or commercial interests.
Acknowledgments
The authors recognize the contributions of the consortia in delivering high-quality GWAS resources for researchers. Data and materials can be accessed from the relevant GWAS consortium. The authors express gratitude to the editors and reviewers for their constructive comments and suggestions that enhanced the quality of the paper. This paper has been uploaded to SSRN as a preprint: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4873289 - 2019.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work”.
Funding
There is no funding to report.
Disclosure
The authors affirm that the research was carried out without any commercial or financial relationships that might be interpreted as a potential conflict of interest.
References
1. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–2097. doi:10.1016/s0140-6736(21)00393-7
2. IASP. International Association for the Study of Pain. [Internet]. [cited 2020 Apr 14].
3. Kozak-Szkopek E, Broczek K, Slusarczyk P, et al. Prevalence of chronic pain in the elderly Polish population – results of the polsenior study. Arch Med Sci. 2017;13(5):1197–1206. doi:10.5114/aoms.2015.55270
4. Barke A, Korwisi B, Jakob R, Konstanjsek N, Rief W, Treede R-D. Classification of chronic pain for the international classification of diseases (ICD-11): results of the 2017 international world health organization field testing. Pain. 2022;163(2):e310–e318. doi:10.1097/j.pain.0000000000002287
5. Lj H, Ad M, Af D, Dj P, Bh S. Heritability of chronic pain in 2195 extended families. Eur J Pain. 2012;16(7). doi:10.1002/j.1532-2149.2011.00095.x
6. Johnston KJA, Adams MJ, Nicholl BI, et al. Genome-wide association study of multisite chronic pain in UK biobank. PLoS Genet. 2019;15(6):e1008164. doi:10.1371/journal.pgen.1008164
7. Greene SA. Chronic pain: pathophysiology and treatment implications. Top Companion Anim Med. 2010;25(1):5–9. [PMID: 20188333]. doi:10.1053/j.tcam.2009.10.009
8. Merskey H, Bogduk N. Classification of chronic pain. IASP pain terminology. 1994;240.
9. Vellucci R. Heterogeneity of chronic pain. Clin Drug Invest. 2012;32:3–10. doi:10.2165/11630030-000000000-00000
10. Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302(20):2214–2221. doi:10.1001/jama.2009.1738
11. Stubbs B, Eggermont L, Patchay S, Schofield P. Older adults with chronic musculoskeletal pain are at increased risk of recurrent falls and the brief pain inventory could help identify those most at risk. Geriatrics Gerontol Int. 2015;15(7):881–888. doi:10.1111/ggi.12357
12. Williams A, Kamper SJ, Wiggers JH, et al. Musculoskeletal conditions may increase the risk of chronic disease: a systematic review and meta-analysis of cohort studies. BMC Med. 2018;16(1):167. doi:10.1186/s12916-018-1151-2
13. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi:10.1016/j.jpain.2012.03.009
14. Parsons B, Schaefer C, Mann R, et al. Economic and humanistic burden of post-trauma and post-surgical neuropathic pain among adults in the United States. J Pain Res. 2013;6:459–469. doi:10.2147/jpr.S44939
15. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
16. Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the global burden of disease study 2017. J Hematol Oncol. 2019;12(1):140. DOI:10.1186/s13045-019-0828-0
17. Tan DS, Marchio C, Reis-Filho JS. Hereditary breast cancer: from molecular pathology to tailored therapies. J Clin Pathol. 2008;61(10):1073–1082. doi:10.1136/jcp.2008.057950
18. Zhang H, Ahearn TU, Lecarpentier J. Haoyu et al.Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat Genet. 2020;52(6):572–581. doi:10.1038/s41588-020-0609-2 Epub 2020 May 18. PMID: 32424353; PMCID: PMC7808397.
19. Stokes AC, Xie W, Lundberg DJ, et al. Increases in BMI and chronic pain for US adults in midlife, 1992 to 2016. SSM Popul Health. 2020;12:100644. doi:10.1016/j.ssmph.2020.100644 PMID: 33134473; PMCID: PMC7585155.
20. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi:10.1016/S0140-6736(08)60269-X PMID: 18280327.
21. van den Brandt PA, van den Brandt PA, Spiegelman D, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. [Epub 2000/09/21. PMID: 10997541]. doi:10.1093/aje/152.6.514
22. Vatten LJ, Kvinnsland S. Prospective study of height, body mass index and risk of breast cancer. Acta Oncol. 1992;31(2):195–200. [Epub 1992/01/01. PMID: 1622634]. doi:10.3109/02841869209088902
23. Ursin G, Longnecker MP, Haile RW, Greenland S. A meta-analysis of body mass index and risk of pre-menopausal breast cancer. Epidemiology. 1995;6(2):137–141. [Epub 1995/03/01. PMID: 7742399]. doi:10.1097/00001648-199503000-00009
24. Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166(21):2395–2402. doi:10.1001/archinte.166.21.2395 PMID: 17130395 Epub 2006/11/30. 166/21/2395 [pii].
25. Dowsett M, Folkerd E. Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: a new hypothesis. Breast Cancer Res Treat. 2015;149(1):1–4. doi:10.1007/s10549-014-3211-4 PMID: 25414027 Epub 2014/11/22.
26. Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–765. doi:10.1016/S0140-6736(14)60892-8 PMID: 25129328; PubMed Central PMCID: PMC4151483.
27. Guo Y, Warren Andersen S, Shu X-O, et al. Genetically predicted body mass index and breast cancer risk: Mendelian randomization analyses of data from 145,000 women of European descent. PLoS Med. 2016;13(8):e1002105. doi:10.1371/journal.pmed.1002105 PMID: 27551723; PMCID: PMC4995025.
28. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205.
29. Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi:10.1126/science.1140485
30. Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. doi:10.1038/nri3621 Epub 2014 Feb 28. PMID: 24577438; PMCID: PMC5525062.
31. Pulit SL, Stoneman C, Morris AP, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28(1):166–174. PMID: 30239722; PMCID: PMC6298238. doi:10.1093/hmg/ddy327
32. Fang T, Zhang Z, Zhou H, Wu W, Ji F, Zou L. Effect of genetic liability to migraine and its subtypes on breast cancer: a mendelian randomization study. BMC Cancer. 2023;23(1):887. doi:10.1186/s12885-023-11337-9 PMID: 37730543; PMCID: PMC10510189.
33. Palmieri A. Lung cancer presenting with unilateral facial pain: remission after laryngeal nerve palsy. Headache. 2006;46(5):813–815. doi:10.1111/j.1526-4610.2006.00458.x PMID: 16643587.
34. Sarlani E, Schwartz AH, Greenspan JD, Grace EG. Facial pain as first manifestation of lung cancer: a case of lung cancer-related cluster headache and a review of the literature. J Orofac Pain. 2003;17(3):262–267. PMID: 14558496.
35. Abraham PJ, Capobianco DJ, Cheshire WP. Facial pain as the presenting symptom of lung carcinoma with normal chest radiograph. Headache. 2003;43(5):499–504. doi:10.1046/j.1526-4610.2003.03097.x PMID: 12752757.
36. Katchky L, Gilbert M, Grossman A, Eskander A, Klieb H. Referred orofacial pain as an initial symptom of distant, nonmetastatic cancer: report of a case and review of the literature. J Endod. 2021;47(11):1801–1807. doi:10.1016/j.joen.2021.08.002 Epub 2021 Aug 13. PMID: 34400198.
37. Alghamdi A, Alyami AH, Althaqafi RMM 2nd, et al. Cytokines’ role in the pathogenesis and their targeting for the prevention of frozen shoulder: a narrative review. Cureus. 2023;15(3):e36070. doi:10.7759/cureus.36070 PMID: 37056530; PMCID: PMC10092900.
38. Xia H, Chen Y, Meng J, Liang C. Effect of polymorphisms on IL1A to cancer susceptibility: evidence based on 34,016 subjects. Artif Cells Nanomed Biotechnol. 2019;47:3138–3152. doi:10.1080/21691401.2019.1646750
39. Grimm C, Kantelhardt E, Heinze G, et al. The prognostic value of four interleukin-1 gene polymorphisms in Caucasian women with breast cancer: a multicenter study. BMC Cancer. 2009;9:78. doi:10.1186/1471-2407-9-78
40. Dethlefsen C, Højfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat. 2013;138:657–664. doi:10.1007/s10549-013-2488-z
41. Masjedi A, Hashemi V, Hojjat-Farsangi M, et al. The significant role of IL-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed Pharmacother. 2018;108:1415–1424. doi:10.1016/j.biopha.2018.09.177
42. Korobeinikova E, Ugenskiene R, Insodaite R, et al. Association of angiogenesis and inflammation-related gene functional polymorphisms with early-stage breast cancer prognosis. Oncol Lett. 2020. doi:10.3892/ol.2020.11521
43. Sia AT, Lim Y, Lim ECP, et al. Influence of Mu-opioid receptor variant on morphine use and self-rated pain following abdominal hysterectomy. J Pain. 2013;14:1045–1052. doi:10.1016/j.jpain.2013.03.008
44. Somogyi AA, Coller JK, Barratt DT. Pharmacogenetics of opioid response. Clin Pharmacol Ther. 2015;97:125–127. doi:10.1002/cpt.23
45. Gong XD, Wang JY, Liu F, et al. Gene polymorphisms of OPRM1 A118G and ABCB1 C3435T may influence opioid requirements in Chinese patients with cancer pain. Asian Pac J Cancer Prev. 2013;14(5):2937–2943. doi:10.7314/APJCP.2013.14.5.2937
46. Agulló L, Muriel J, Margarit C, et al. Sex differences in opioid response linked to OPRM1 and COMT genes DNA methylation/genotypes changes in patients with chronic pain. J Clin Med. 2023;12(10):3449. doi:10.3390/jcm12103449 PMID: 37240556; PMCID: PMC10219447.
47. Cieślińska A, Sienkiewicz-Szłapka E, Kostyra E, et al. μ-opioid receptor gene (OPRM1) polymorphism in patients with breast cancer. Tumor Biol. 2015;36(6):4655–4660. doi:10.1007/s13277-015-3113-z
48. Li L, Li S, Qin S, et al. Sports, and psychological stress as modulators of breast cancer risk: focus on OPRM1 methylation. Front Nutr. 2021;8:747964. doi:10.3389/fnut.2021.747964
49. Zagon IS, McLaughlin PJ, Goodman SR, Rhodes RE. Opioid receptors and endogenous opioids in diverse human and animal cancers. J Natl Cancer Inst. 1987;79:1059–1065.
50. Gach K, Piestrzeniewicz M, Fichna J, Stefanska B, Szemraj J, Janecka A. Opioid-induced regulation of mu-opioid receptor gene expression in the MCF-7 breast cancer cell line. Biochem Cell Biol. 2008;86:217–226. doi:10.1139/O08-001
51. Hatzoglou A, Bakogeorgou E, Castanas E. The antiproliferative effect of opioid receptor agonists on the T47D human breast cancer cell line, is partially mediated through opioid receptors. Eur J Pharmacol. 1996;296:199–207. doi:10.1016/0014-2999(95)00703-2
52. Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. doi:10.1038/nm.2234 Epub 2010 Oct 14. PMID: 20948535; PMCID: PMC3077564.
53. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi:10.1038/ni.1863 Epub 2010 Apr 20. PMID: 20404851.
54. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi:10.1038/nri.2015.5 PMID: 26711676; PMCID: PMC5542678.
55. Balordi M, Tiberio P, Castaldo M, et al. Empowering beyond pain: pain neuroscience education interventions in breast cancer survivorship care. Cancers. 2024;16(16):2806. doi:10.3390/cancers16162806 PMID: 39199580; PMCID: PMC11353171.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



