Back to Journals » Infection and Drug Resistance » Volume 18
Mycobacterium tuberculosis Detection in Diverse Clinical Specimens by GeneXpert MTB/RIF: A Large-Scale Retrospective Study
Authors Li H, Li Z , Cui X, Liu X, Zhao J, Zhang Y, Wang C, Li B, Fan Y , Han J, Xia Y, Xiong Z, Zou X, Zhu Y, Li M, Lu B , Cao B
Received 22 January 2025
Accepted for publication 18 April 2025
Published 8 May 2025 Volume 2025:18 Pages 2401—2413
DOI https://doi.org/10.2147/IDR.S514220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Haibo Li,1,2,* Ziyao Li,1– 4,* Xiaojing Cui,1,2 Xinmeng Liu,1,2,5 Jiankang Zhao,1,2 Yulin Zhang,1,2 Chunlei Wang,1,2 Binbin Li,1,2 Yanyan Fan,1,2 Jiajing Han,1,2,5 Yudi Xia,1,2 Zhujia Xiong,1,2 Xiaohui Zou,1,2 Yue Zhu,1,2 Mengxue Li,1,2,5 Binghuai Lu,1– 3,5 Bin Cao1– 7
1Laboratory of Clinical Microbiology and Infectious Diseases, Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, National Center for Respiratory Medicine, New Cornerstone Science Laboratory, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 2National Center for Respiratory Medicine; State Key Laboratory of Respiratory Health and Multimorbidity; National Clinical Research Center for Respiratory Diseases; Institute of Respiratory Medicine, Chinese Academy of Medical Sciences; Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 3Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 4Changping Laboratory, Beijing, People’s Republic of China; 5China-Japan Friendship School of Clinical Medicine, Peking University, Beijing, People’s Republic of China; 6Tsinghua University-Peking University Joint Center for Life Sciences, Tsinghua University, Beijing, People’s Republic of China; 7Department of Respiratory Medicine, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Binghuai Lu; Bin Cao, Laboratory of Clinical Microbiology and Infectious Diseases, Department of Pulmonary and Critical Care Medicine, China-Japan Friendship Hospital, No. 2 East Yinghua Street, Chaoyang District, Beijing, 100029, People’s Republic of China, Tel/Fax +86-010-84206256, Email [email protected]; [email protected]
Purpose: Mycobacterium tuberculosis (MTB) infection poses a significant global health challenge, with conventional diagnostic methods like acid-fast smear and culture techniques exhibiting limitations in sensitivity and efficiency. This retrospective study examined 4098 clinical samples from China-Japan Friendship Hospital between March 2018 and March 2019, focusing on the diagnostic performance of GeneXpert MTB/RIF across different specimen types.
Methods: The study encompassed various sample types, including bronchoalveolar lavage fluid (BALF), sputum, lung tissues, hydrothorax, and others. All samples were performed via acid-fast-straining, GeneXpert MTB/RIF and culture.
Results: GeneXpert MTB/RIF demonstrated superior sensitivity (81.46%) and specificity (98.98%) in respiratory specimens compared to tissues (62.50%) and hydrothorax (46.15%). Notably, in acid-fast-straining-negative samples, GeneXpert MTB/RIF showed sensitivity and specificity of 73.43% and 98.8%, respectively, and reduced false negatives of acid-fast staining. Furthermore, the study explored Cycler Threshold (CT) values, revealing associations with bacterial load and sample types.
Conclusion: The findings highlight the importance of considering sample types in MTB diagnosis and underscore the potential of GeneXpert MTB/RIF as a valuable diagnostic tool, especially in respiratory specimens, contributing to improved tuberculosis management strategies. Additionally, the study recommended direct GeneXpert MTB/RIF testing of hydrothorax samples instead of acid-fast staining, further enhancing diagnostic accuracy and efficiency.
Keywords: acid-fast staining, threshold cycle, diagnosis, GeneXpert MTB/RIF, Mycobacterium tuberculosis, tuberculosis
Introduction
Mycobacterium tuberculosis (MTB) infection remains a significant global challenge.1 Approximately 1.2 million people succumb to MTB each year, with nearly 10 million new cases reported annually.2 China is also considered a high-burden region for MTB.3 TB is a major public health concern worldwide, ranking above HIV/AIDS and PTB/EPTB diagnosis exhibits serious challenges owing to paucibacillary nature of specimens and localization of disease at sites that are difficult to access.4,5 Until the COVID-19 pandemic, TB was the foremost cause of death from a single infectious agent and is now the second leading infectious killer after COVID-19.6,7
For decades, acid-fast staining and traditional culture techniques have been considered the mainstays of MTB diagnostics. While the sensitivity of acid-fast staining and its consistency was compromised by various factors,8 culture, the gold standard for detecting tuberculosis, was time-consuming.9 Both methods could effectively meet the clinical needs for MTB diagnosis. Since 2010, a rapid and accurate nucleic acid amplification test for diagnosing MTB emerged, marking a significant leap forward in tuberculosis diagnosis. GeneXpert MTB/RIF used for MTB detection, provided results within 2 hours with high sensitivity and specificity, representing a major improvement over traditional detection techniques.10 GeneXpert MTB/RIF is a cartridge-based NAAT (CBNAAT), using rpoB (Rv0664) that concomitantly identifies TB and rifamycin resistance.11 In 2010, GeneXpert MTB/RIF was co-developed by Cepheid and FIND, later the WHO endorsed the use of GeneXpert MTB/RIF (is automated, hemi-nested real-time PCR) for pulmonary tuberculosis diagnosis that has played a major role for high TB burden countries including India towards achieving the “End-TB Goal”.12 Afterward, the WHO endorsed GeneXpert MTB/RIF for rapid detection of pediatric-TB including other extra-pulmonary tuberculosis types and could be used as an alternative of traditional acid-fast staining for initial detection of adult TB patients.13
Due to its high sensitivity, GeneXpert MTB/RIF has been recommended by guidelines for the early and precise diagnosis and management of tuberculosis patients.14 It has been proven to effectively reduce clinical costs.15 Although GeneXpert MTB/RIF exhibits excellent diagnostic performance for pulmonary tuberculosis using sputum samples, its diagnostic efficacy in extrapulmonary tuberculosis remains suboptimal, and studies evaluating its performance in non-sputum specimens are limited.7,16–19 Several factors, including low bacterial load and sample processing methods, may contribute to diagnostic failures of GeneXpert MTB/RIF in certain clinical settings.20 Therefore, the influence of different specimen types on GeneXpert MTB/RIF results should not be overlooked and merits further investigation. The GeneXpert MTB/RIF Ultra, the advanced iteration of the GeneXpert MTB/RIF, exhibits enhanced sensitivity and demonstrates improved diagnostic performance across various specimen types.11,21 But WHO has not yet recommended it as the gold standard diagnostic method.21 Additionally, due to the low sensitivity of acid-fast staining and variations in disease progression, 40–50% of MTB patients might have negative acid-fast staining results, leading to potential missed diagnoses.22 Therefore, the evaluation of MTB diagnosis using GeneXpert MTB/RIF in conjunction with acid-fast staining as a preliminary screening test is worthy of exploration.
Hence, this study aims to conduct a retrospective investigation of 4098 clinical samples collected from China-Japan Friendship Hospital (CJFH) between March 2018 and March 2019. The research intended to explore the influence of different sample types on the results of GeneXpert MTB/RIF in clinical MTB diagnosis.
Materials and Methods
Ethics Statement
Permission to use the information in the medical records of the patients for research purposes was granted by the Ethics Committee of the China-Japan Friendship Hospital (CJFH) (2022-KY-133). Our research is in line with the exemption type of informed consent and ethics approval that
Using identifiable human body materials or data for research, it is no longer possible to locate the subject, and the research project does not involve personal privacy disclosure or commercial interests.
As this is a retrospective cohort study based on previous clinical diagnosis and treatment results, the Ethics Committee of the China-Japan Friendship Hospital granted the study exemption status. In addition, we declare that this study is in line with the ethical guidelines of the Declaration of Helsinki, and the patient-related data is strictly confidential.
Sample Processing and Definitions
The clinical and laboratory data were collected from the medical record system and laboratory information system of China-Japan Friendship Hospital (CJFH) between March 2018 and March 2019, retrospectively. Patients who underwent acid-fast staining, GeneXpert MTB/RIF (Cepheid, Inc., Sunnyvale, CA, USA), and culture (Bactec Myco/F lytic culture and Bactec mycobacterial growth indicator tube 960 system) were included in the study. The sample data included the sample collection site, the results of acid-fast staining, culture system and GeneXpert MTB/RIF results including the Cycler Threshold (CT) values.
Fluid samples such as bronchoalveolar lavage fluid (BALF), hydrothorax, etc. were centrifuged at 3000 rpm for 3 min, and then subjected to acid-fast staining and culture. Long or large tissues were minced with sterile scissors or scalpel blades, and then the tissue samples were placed in a 10 mL glass container and grinder (Naitong Industrial Products Co., Ltd., Guangzhou, China) to grind and homogenize, then the acid-fast staining and GeneXpert MTB/RIF were employed considered as MTB positivity.
Statistical Analysis
To better describe diagnostic performance in detecting MTB, relevant indicators for methodological evaluation including sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), Likelihood Ratio (LR), and kappa value, were obtained via Microsoft Excel 16. The chi-square test and Fisher’s exact test were performed to compare the impact of different samples on GeneXpert MTB/RIF results using the GraphPad Prism 8, the two-side P value of <0.05 was considered statistically significant.
Data Availability
The original data presented in the current study are all included in the article. Further inquiries can be directed to the corresponding author.
Results
Clinical Characteristics
In the present study, a total of 4098 specimens were collected from CJFH between March 2018 and March 2019. After excluding individuals who met the exclusion criteria, there were 4098 samples included in the final analysis. Among these, 2405 (58.7%) were male. The age of the cohorts ranged from 14 to 97 years with the median age being 61 years. The types of clinical specimens from which used for MTB detection included BALF (1809, 44.14%), sputum (1633,39.85%), lung tissues (350, 8.54%), hydrothorax (283,6.91%), and others (23,0.56%), as shown in Figure 1 and Table 1.
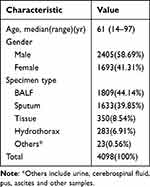 |
Table 1 Characteristics of All Patients and Samples Investigated in This Study |
 |
Figure 1 Diagnostic accuracy of GeneXpert MTB/RIF and acid-fast staining for detection of MTB from suspected tuberculosis patients based on retrospective data. |
Performance of the Acid-Fast Staining and the GeneXpert MTB/RIF in MTB Detection
In the study involving 4098 samples, sequential application of acid-fast staining and GeneXpert MTB/RIF revealed higher specificity for GeneXpert MTB/RIF (98.65%, 3814/3866) compared to acid-fast staining (99.48%, 3846/3866) as summarized in Table 2, Figures 1 and 2A. The sensitivity of GeneXpert MTB/RIF (78.88%, 183/232) surpassed that of acid-fast staining (27.59%, 64/232). GeneXpert MTB/RIF demonstrated slightly greater accuracy in MTB-positive diagnosis (PPV=77.87%, LR+=58.43) compared to acid-fast staining (PPV=76.19%, LR+=53.06). Notably, GeneXpert MTB/RIF exhibited superior negative predictive value (NPV=98.73%, LR-=0.21) compared to acid-fast staining (NPV=95.81%, LR-=0.73). The impact of different operators or experimental locations was more pronounced for acid-fast staining (kappa=0.387) than for GeneXpert MTB/RIF (kappa=0.771).
 |
Table 2 Detection Performance of GeneXpert MTB/RIF and Acid-Fast Staining in All Kinds of Samples, and the Diagnostic Efficiency of GeneXpert MTB/RIF Among Acid-Fast Staining Grouped Samples |
The MTB Diagnostic Efficacy on Different Specimen Types
The impact of specimen type on diagnostic efficacy was taken into account, as shown in Table 2 and Figure 2. The testing effectiveness of the GeneXpert MTB/RIF alone was more outstanding than the acid-fast staining only used among different specimen types as that on all samples.
Differences in diagnostic efficacy were observed among different specimen types when using GeneXpert MTB/RIF alone. The GeneXpert MTB/RIF performed best among the respiratory specimens, as shown in Table 2 and Figure 2B. The sensitivity was above 80% (Sen=81.46%), and the specificity was nearly 100% (Spe=98.98%). It also exhibited excellent performance in terms of accuracy and consistency (PPV=83.50%, NPV=98.83%, LR+=79.91, LR-=0.19, kappa=0.814). Among the respiratory specimens, the GeneXpert MTB/RIF was particularly effective in the sensitivity and excluding negative samples from BALF (Sen=82.4%, NPV=99.1%, LR-=0.18); meanwhile, the specificity and confirmation of positive samples were higher in sputum (Spe=99.1%, PPV=87.6%, LR+=89.67%) (shown in Table 2, Figure 2C and D). For the tissue and hydrothorax sample, the specificity and NPV were similar to those observed in respiratory specimens (Spetissue=96.71%, NPVtissue=98.18%; Spehydrothorax = 97.43%, NPVhydrothorax=98.15%). However, the sensitivity, PPV, LR, and consistency of GeneXpert MTB/RIF in tissue and hydrothorax samples significantly differed from those in respiratory samples (Sentissue=62.50%, PPVtissue=47.62%, LR+tissue=19.00, LR-tissue=0.39, kappatissue=0.515; Senhydrothorax = 54.55%, PPVhydrothorax=46.15%, LR+hydrothorax = 21.23%, LR-hydrothorax=0.47, kappahydrothorax = 0.478) (displayed in Table 2, Figure 2E and F). Only a single GeneXpert MTB/RIF-positive sample, considered negative by culture and acid-fast staining, was detected in pus.
Acid-fast staining also performed the best in respiratory specimens similarly to GeneXpert MTB/RIF. The most outstanding performance of the acid-fast staining was its nearly 100% specificity (Spe=99.44%), but the accuracy and the consistency were extremely poor (PPV=77.78%, NPV=95.78%, LR+=55.27, LR-=0.70, kappa=0.421). The diagnostic efficacy between sputum and BALF was similar, except for sensitivity which sputum had a higher sensitivity than BALF (Sensputum=34.2%; SenBALF=26.4%).
The Diagnostic Efficacy of GeneXpert MTB/RIF in Different Acid-Fast Staining Grouped Samples
The samples were then divided into two groups based on acid-fast staining. Both sensitivity and PPV were higher than those observed when using the GeneXpert MTB/RIF alone in smear-positive samples (Sen=98.44%, PPV=91.30%), yet the performance of GeneXpert MTB/RIF in smear-negative specimens was similar as the GeneXpert MTB/RIF employed individually. The consistency was also found to be relatively satisfactory (kappasmear-positive=0.749, kappasmear-negaitive=0.706).
In the respiratory specimens, GeneXpert MTB/RIF showed better efficiency after the samples were grouped by acid-fast staining. GeneXpert MTB/RIF did better in the sensitivity, PPV and LR- of the smear-positive sample (Sen=98.41%, PPV=93.94%, LR-=0.02). Furthermore, samples negative by both acid-fast staining and GeneXpert MTB/RIF can be confidently identified as negative with a high degree of certainty (Spe=99.1%, NPV=98.85%). The acid-fast-straining-negative patients with a positive GeneXpert MTB/RIF had a higher risk of suffering from MTB (LR+=82.08). However, compared to BALF samples, GeneXpert MTB/RIF exhibited superior diagnostic efficacy in detecting MTB in acid-fast-straining-positive sputum samples (SenBALF=95.8%, SpeBALF=62.5%, PPVBALF=88.5%, NPVBALF=83.3%, LR+BALF=2.55, LR-BALF=0.07, kappaBALF=0.636; Sensputum=100.0%, Spesputum=90.0%, PPVsputum=97.5%, NPVsputum=100.0%, LR+sputum=10, LR-sputum=0, kappaBALF=0.935). In acid-fast-straining-negative samples, the detection capabilities between the two types of specimens showed little difference (SpeBALF=99.0%, PPVBALF=75.4%, NPVBALF=99.1%, LR+BALF=77.60, LR-BALF=0.23, kappaBALF=0.755; Spesputum=99.2%, PPVsputum=81.5%, NPVsputum=98.6%, LR+sputum=88.38, LR-sputum=0.3, kappaBALF=0.746), except for in smear-negative patients, where GeneXpert MTB/RIF exhibited a stronger ability to detect MTB positivity in BALF (SenBALF=77.6%; Sensputum=70.7%). Therefore, in acid-fast-straining-positive respiratory specimens, when GeneXpert MTB/RIF testing was negative, there was a high likelihood of excluding MTB infection. However, in acid-fast-straining-negative cases, when GeneXpert MTB/RIF testing was positive, the possibility of infection cannot be ruled out. In contrast, when both acid-fast staining and GeneXpert MTB/RIF are negative, there was a strong basis for excluding MTB infection.
However, GeneXpert MTB/RIF in samples from other types yielded far fewer effective results compared to its usage in respiratory samples. As shown in Table 2. The sensitivity and the PPV in acid-fast-straining-negative tissue and acid-fast-straining-negative hydrothorax sample was below 60% (Sentissue=47.4%, PPVtissue=60.0%; Senhydrothorax =54.5%, PPVhydrothorax=46.15%), and the reliability and consistency are also not high (LR+tissue=26.33, LR-tissue=0.536, kappatissue=0.506; LR+hydrothorax =21.23, LR-hydrothorax=0.47, kappahydrothorax =0.478). Specificity and NPV were at an acceptable level (Spetissue=98.20%, NPVtissue=97.0%; Spehydrothorax =97.43%, NPVhydrothorax=98.15%). The diagnostic performance of the combined application in samples with acid-fast staining positivity had not been evaluated due to the limited number of samples with acid-fast staining positivity. Hence, in tissue and hydrothorax samples, when both acid-fast staining and GeneXpert MTB/RIF testing were negative, there was greater confidence in excluding MTB infection.
Impact of Specimen Types and Acid-Fast Staining on the CT of GeneXpert MTB/RIF
Different specimen types, owing to their varied processing methods, may have an impact on the results of GeneXpert MTB/RIF. The GeneXpert MTB/RIF cycle threshold value (CT) of all 235 GeneXpert MTB/RIF-positive samples was collected, and the values across different specimen types were compared. The median CT value of all culture-positive and all culture-negative samples was 23 and 26, respectively, showing a statistical difference between the two groups (P=0.0001) (Figure 3A). In the BALF samples, the median CT values for culture-negative and culture-positive samples were 23 and 27.5, respectively, with a statistically significant difference (P=0.0009) (Figure 3A). In sputum specimens, the median CT values for culture-negative and culture-positive samples were 22.5 and 26.5, and a statistically significant difference was also observed (P=0.423) (Figure 3A). However, there was no difference between the culture-negative and culture-positive tissue samples (P=0.5813), in which the median CT values were 23 and 26, respectively (Figure 3A). The median CT values for culture-negative and culture-positive samples from hydrothorax were 25 and 26, separately, with no statistical difference between the two groups (P>0.9999) (Figure 3A). There was no statistical difference between the GeneXpert MTB/RIF-negative and GeneXpert MTB/RIF-positive groups of different specimen types (Ppositive=0.7267, Pnegative=0.7405).
The acid-fast staining and culture results served as grouping criteria and divided samples of each specimen type into four groups: acid-fast-straining-positive and culture-positive (T+HL+), acid-fast-straining-positive and culture-negative (T+HL-), acid-fast-straining-negative and culture-positive (T-HL+), and acid-fast-straining-negative and culture-negative (T-HL-). The impact of different specimen types and acid-fast staining on GeneXpert MTB/RIF results was analyzed, with detailed results presented in Figure 3. Among all samples, the median CT values for T+HL+, T+HL-, T-HL+, and T-HL- groups, as displayed in Figure 3B, were 20, 23, 24, and 26, respectively, with statistically significant differences (P<0.0001). Statistical differences within groups were observed between T+HL+ vs T-HL-, T+HL+ vs T-HL+, as well as T-HL+ vs T-HL- (PALL T+HL+ vs ALL T-HL-<0.0001, PALL T+HL+ vs ALL T-HL+<0.0001, PALL T-HL+ vs ALL T-HL-=0.043), indicating that acid-fast smear results had an impact on the CT values. In BALF samples, as shown in Figure 3C, the median CT values for T+HL+, T+HL-, T-HL+, and T-HL- groups were 21, 26, 23, and 29, respectively, with significant intergroup differences (P=0.0003). Pairwise comparisons revealed statistical differences between BALF T+HL+ and BALF T-HL-, as well as BALF T-HL+ and BALF T-HL- within the groups (PBALF T+HL+ vs BALF T-HL-<0.0001, PBALF T-HL+ vs BALF T-HL-=0.0153). However, in sputum specimens, the median CT values for T+HL+, T+HL-, T-HL+, and T-HL- groups were 22.5, 31, 24, and 26, respectively, exhibiting significant intergroup differences (P=0.0348) (Figure 3D), though pairwise comparisons revealed no statistical differences (P>0.1). Due to the lack or minimal presence of acid-fast-straining-positive samples in hydrothorax and tissue samples, no comparisons were made between these types. The results indicate that specimen types had an impact on GeneXpert CT values after initial grouping by acid-fast staining. GeneXpert MTB/RIF had higher accuracy in detecting MTB in BALF.
Discussion
In this study, the effect of the specimen type on the GeneXpert MTB/RIF performance was investigated. The GeneXpert MTB/RIF demonstrated superior better on respiratory specimens than other samples. The diagnostic performance of GeneXpert MTB/RIF in hydrothorax and tissue samples was similar, yet not as robust as in respiratory samples across all metrics. The predominant representation of respiratory specimens reflects the importance of these samples in pulmonary MTB diagnosis, consistent with the report from WHO.3 Although all specimens analyzed with the GeneXpert MTB/RIF demonstrated high specificity (all Spe were above 98%), the sensitivity of different samples varied significantly. The sensitivity and the specificity of the GeneXpert MTB/RIF on respiratory samples were 81.46% and 98.8%, respectively, which was much higher than that on tissue (62.5%, 96.7%) and hydrothorax (54.6%, 98.2%). This finding aligns with that Akhter et al who reported the sensitivity and specificity were 52.2% and 100% on the biopsy samples, respectively.23 These results contrast significantly with findings from other researchers. They reported a sensitivity as high as 72.2%, and even up to 85.5%, in tissue samples.24–26 This variation may be closely linked to differences in sample collection and processing. The sensitivity of GeneXpert MTB/RIF in hydrothorax samples varied significantly, ranging from 16.6% to 63.6%. It may be attributed to differences in the choice of reference standards and the processing methods, such as centrifugation.25–31 However, the specificity across studies (98.6–100%) was similar to our findings. The accuracy and reliability of GeneXpert MTB/RIF in tissue and hydrothorax samples were significantly lower compared to its performance in respiratory samples, similar to other literature.25,26,30 In our study, the differences were observed in the specificity and sensitivity of GeneXpert MTB/RIF between the administration on sputum (Sen=80.7%, Spe=99.1%) and BALF (Sen=82.4%, Spe=98.8%) samples, but the variations were not substantial, as similar with others.32–34 Detecting BALF in patients with limited sputum is clinically more significant.34–36 Thus, GeneXpert MTB/RIF has significant advantages in the detection of respiratory samples.
However, it was recommended that direct GeneXpert MTB/RIF testing of hydrothorax samples rather than acid-fast staining according to our results. No positive result was detected in hydrothorax by acid-fast staining, while 54.6% (6/11) positive samples were detected via GeneXpert MTB/RIF. In previous studies, the sensitivity of the acid-fast staining was far lower than that of the GeneXpert MTB/RIF.24,37 So, we recommended direct GeneXpert MTB/RIF testing of hydrothorax samples instead of acid-fast staining based on our experiments and related literature.
However, despite its advantages, GeneXpert MTB/RIF demonstrated limited sensitivity in certain non-pulmonary specimens. In our study, approximately 22% of culture-positive tissue samples and 27.8% of culture-positive hydrothorax specimens were not detected by GeneXpert MTB/RIF. These findings suggest that the assay may not be suitable as a standalone diagnostic tool for extrapulmonary tuberculosis, particularly in resource-limited settings. Notably, a negative GeneXpert MTB/RIF result in non-pulmonary specimens does not definitively rule out TB infection, and supplementary diagnostic methods, such as culture, histopathology, or immunological assays, remain necessary to achieve accurate diagnosis.
In our study, the GeneXpert MTB/RIF in various samples, particularly respiratory samples, effectively excluded true-negative samples among the acid-fast-staining-negative samples. In our research, when GeneXpert MTB/RIF was applied to all acid-fast staining-negative samples, the sensitivity and specificity were 73.4% and 98.8%, respectively. Furthermore, the combination of acid-fast staining and GeneXpert MTB/RIF, with its high specificity, NPV, and low LR-, provides substantial confidence in excluding infection for samples negative in both tests. Among these, the results of respiratory samples were similar to the total samples, with only sputum samples having slightly lower sensitivity than BALF (Sensputum=70.7%; SenBALF=77.6%). Our results are consistent with previous studies, which reported sensitivities ranging from 72% to 80.3%.38–42 However, Dorman et al reported a sensitivity of only 46% in 137 sputum acid-fast-staining-negative but culture-positive samples when tested with GeneXpert MTB/RIF.43 Kumar et al found sensitivity, specificity, PPV, and NPV of 55.8%, 98.3%, 78.4%, and 95.1%, respectively, in acid-fast-straining-smear-negative cases,44 while Ngangue et al reported a sensitivity of approximately 53%.45 The reduced bacterial load in sputum samples may explain the lower sensitivity in acid-fast-staining-negative cases, emphasizing the impact of different sample types on GeneXpert MTB/RIF results. The application of GeneXpert MTB/RIF in non-respiratory samples that are acid-fast-staining negative but culture-positive significantly reduces sensitivity and PPV compared to respiratory samples, emphasizing the influence of sample type on sensitivity again. However, research on non-respiratory samples with acid-fast-straining -negative but culture-positive results was limited. Additionally, across all samples, GeneXpert MTB/RIF demonstrated a sensitivity and PPV of 98.44% and 91.3%, respectively, in acid-fast-straining-positive samples. In sputum and bronchoalveolar lavage fluid, the sensitivity was 100% and 98.41%, respectively. For tissue, hydrothorax, and other samples, there was insufficient data due to a low number of positive acid-fast staining. These results were consistent with previous studies, indicating that GeneXpert MTB/RIF can effectively confirm MTB infection and minimize false positives.42 Furthermore, 48 cases with negative acid-fast staining and cultures but positive GeneXpert results were observed. We speculated that it may be due to the presence of MTB DNA or intact MTB (viable or non-viable) in the samples or a combination of both. Some MTB patients may still test positive for MTB nucleic acid in sputum even after receiving tuberculosis treatment.46
The CT values of GeneXpert MTB/RIF appeared to be closely associated with bacterial load and sample types. We observed statistically significant differences in CT values between culture-positive and culture-negative samples (P<0.05). Within the culture-positive group, there were statistically significant differences between acid-fast-staining-positive and smear-negative samples (P<0.05), while within the culture-negative group, there were statistically significant differences between smear-positive and smear-negative samples (P<0.05). This correlation was only observed in the BALF sample group. Although there were differences in median CT values in the sputum sample group, there was no statistical difference between any two groups. It was reported that GeneXpert MTB/RIF could detect MTB with a bacterial load of 102.47 In 2019, Irene Najjingo et al confirmed that there was a moderate correlation between GeneXpert MTB/RIF CT values and acid-fast-staining grading. The correlation between GeneXpert MTB/RIF CT values and time to positive culture was relatively weak. It was found that an increase of one unit in GeneXpert MTB/RIF CT values corresponded to a 2.57-day increase in time to positive culture.48 Kui Li et al analyzed 980 sputum specimens and found differences when the bacterial load was within 1+ on microscopic examination (all P< 0.05). There was a strong negative correlation between CT values and acid-fast staining bacterial load (P< 0.0001), and when CT < 16, the diagnostic sensitivity and specificity were both 100.00%.49 Mary Mansfield also elaborated on the relationship between bacterial load and CT values of GeneXpert MTB/RIF.50 Acid-fast staining and culture reflect bacterial load. Our study also confirmed that the bacterial load carried by different sample types had an impact on the CT values of GeneXpert MTB/RIF. Above all, recent studies suggest that lower CT values may be associated with higher transmission risk, greater disease activity, and increased likelihood of smear positivity, although standardized clinical thresholds remain to be defined. We propose that CT values, when interpreted in combination with clinical and radiological findings, may aid clinicians in assessing disease severity and in triaging patients for isolation or treatment prioritization.
Despite the comprehensive evaluation presented in this study, some limitations should be acknowledged. The impact of varying operator skills or experimental locations on acid-fast staining results suggested a potential source of variability that should be addressed in future studies. Additionally, the limited number of acid-fast-straining-positive samples in tissue and hydrothorax samples constrained the evaluation of combined diagnostic performance in these specimen types.
Due to the clinical context, we were unable to apply traditional molecular tools like PCR or loop-mediated isothermal amplification (LAMP) in our study, both of which have certain limitations. PCR, for example, can yield false results and may not detect a broad range of pathogens PCR can produce false results and may not detect a broad range of pathogens.51 LAMP, while rapid, can suffer from non-specific primer interactions, false positives, and contamination risks.52,53 These challenges underscore the need for alternative methods like the one we developed.
This study provides a comprehensive evaluation of GeneXpert MTB/RIF performance across diverse clinical specimen types, offering practical insights into its application in real-world settings. The inclusion of CT value analysis adds semi-quantitative depth that may assist clinical decision-making. However, the study has limitations, including a limited number of positive cases in certain specimen types and its single-center retrospective design, which may affect the generalizability of the findings.
Conclusion
In summary, a retrospective evaluation of 4098 clinical samples collected from CJFH between March 2018 and March 2019, assessing the application of GeneXpert MTB/RIF in different types of samples was conducted. Our study provides valuable insights into the clinical characteristics and diagnostic performance of GeneXpert MTB/RIF in different specimen types. The research findings support the practicality of GeneXpert MTB/RIF as a highly sensitive and specific diagnostic tool, especially in respiratory specimens. It was recommended that direct GeneXpert MTB/RIF testing of hydrothorax samples rather than acid-fast staining. The GeneXpert MTB/RIF showed promise, particularly in reducing the false-negative rate of acid-fast staining, optimizing the sensitivity of acid-fast-straining-positive samples, and assessing the exclusion accuracy of samples negative for both acid-fast staining and GeneXpert MTB/RIF. The impact of observed sample types on the CT values of GeneXpert MTB/RIF was also observed.
Abbreviation
AFS, Acid-Fast Stain; BALF, Broncho Alveolar Lavage Fluid; CT, Threshold Cycle; Mtb, Mycobacterium tuberculosis.
Ethics Statement
Permission to use the information in the medical records of the patient for research purposes was granted by the Ethics Committee of the China-Japan Friendship Hospital (2022-KY-133). Our research is in line with the exemption type of informed consent and ethics approval that “Using identifiable human body materials or data for research, it is no longer possible to locate the subject, and the research project does not involve personal privacy disclosure or commercial interests”. As this is a retrospective Cohort study based on previous clinical diagnosis and treatment results, the Ethics Committee of the China-Japan Friendship Hospital granted the study exemption status. In addition, we declare that this study is in line with the ethical guidelines of the Declaration of Helsinki, and the patient-related data is strictly confidential.
Acknowledgments
We wish to thank the support of Changping Laboratory.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by National High Level Hospital Clinical Research Funding (grant no. 2024-NHLHCRF-LX-01-0101, grant no. 022-NHLHCRF-YS-02, and grant no. 2022-NHLHCRF-LX-01-0208). Elite Medical Professionals Project of China-Japan Friendship Hospital (grant no. ZRJY2023-QM08), the Natural Science Foundation of China (grant no. 82470007), Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (grant no. CIFMS 2021-I2M-1-048 and grant no. 2021-I2M-1-030), Beijing Research Ward Excellence Program BRWEP2024W114060103, Noncommunicable Chronic Diseases-National Science and Technology Major Project (grant no. 2023ZD0506200 and grant no. 2023ZD0506203) and this work has been supported by the New Cornerstone Science Foundation.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Walzl G, McNerney R, du Plessis N, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. 2018;18(7):e199–e210. doi:10.1016/S1473-3099(18)30111-7
2. Springer YP, Kammerer JS, Silk BJ, Langer AJ. Tuberculosis in indigenous persons - United States, 2009–2019. J Racial Ethn Health Disparities. 2022;9(5):1750–1764. doi:10.1007/s40615-021-01112-6
3. World Health Organization. Global tuberculosis report 2023. 2023. Available from: https://www.who.int/tb/publications/global_report/en/.
4. Khan A, Kamra E, Singh R, et al. Diagnosis of osteoarticular tuberculosis: multi-targeted loop-mediated isothermal amplification assay versus multiplex PCR. Future Microbiol. 2021;16:935–948. doi:10.2217/fmb-2021-0030
5. Khan A, Singh R, Sharma S, et al. Diagnosis of osteoarticular tuberculosis by immuno-PCR assay based on mycobacterial antigen 85 complex detection. Lett Appl Microbiol. 2022;74(1):17–26. doi:10.1111/lam.13567
6. Dahiya B, Mehta N, Soni A, Mehta PK. Diagnosis of extrapulmonary tuberculosis by GeneXpert MTB/RIF Ultra assay. Expert Review of Molecular Diagnostics. 2023;23(7):561–582. doi:10.1080/14737159.2023.2223980
7. Kumar N, Khan A, Boora S, et al. Diagnosis of tuberculous lymphadenitis by molecular and immunological tools. Med Microecol. 2024;22:100116. doi:10.1016/j.medmic.2024.100116
8. Steingart KR, Ng V, Henry M, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6(10):664–674. doi:10.1016/S1473-3099(06)70602-8
9. Moore DF, Guzman JA, Mikhail LT. Reduction in turnaround time for laboratory diagnosis of pulmonary tuberculosis by routine use of a nucleic acid amplification test. Diagn Microbiol Infect Dis. 2005;52(3):247–254. doi:10.1016/j.diagmicrobio.2005.02.014
10. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–1015. doi:10.1056/NEJMoa0907847
11. World Health Organization. Use of Xpert MTB/RIF and Xpert MTB/RIF Ultra on GeneXpert 10-Colour Instruments: WHO Policy Statement. World Health Organization; 2021.
12. Khan A, Khan N, Singh R. Tuberculosis diagnosis versus GeneXpert(®)MTB/RIF formats. Bioanalysis. 2024;16(16):843–848. doi:10.1080/17576180.2024.2349423
13. World Health Organization. Xpert MTB/RIF Implementation Manual: Technical and Operational ‘How-To’; Practical Considerations. World Health Organization; 2014.
14. WHO Guidelines Approved by the Guidelines Review Committee. Policy Statement: Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System. World Health Organization; 2011.
15. Hickey AJ, Cummings MJ, Zafari Z, Louh IK, Li J, O’Donnell MR. Evaluation of screening strategies for pulmonary tuberculosis among hospitalized patients in a low-burden setting: cost-effectiveness of GeneXpert MTB/RIF compared to smear microscopy. Infect Control Hosp Epidemiol. 2022;43(7):892–897. doi:10.1017/ice.2021.247
16. Tadesse M, Abebe G, Bekele A, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a diagnostic evaluation study. Clin Microbiol Infect. 2019;25(8):1000–1005. doi:10.1016/j.cmi.2018.12.018
17. Maynard-Smith L, Larke N, Peters JA, Lawn SD. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis. 2014;14:709. doi:10.1186/s12879-014-0709-7
18. Mezgebe H, Gebrecherkos T, Hagos DG, Muthupandian S. Prevalence of smear-positive, rifampicin-resistant mycobacterium tuberculosis and related factors among residents with cough in northern Ethiopian refugee health facilities. Infect Drug Resist. 2024;17:1135–1145. doi:10.2147/idr.S453306
19. Mechal Y, Benaissa E, El Mrimar N, et al. Evaluation of GeneXpert MTB/RIF system performances in the diagnosis of extrapulmonary tuberculosis. BMC Infect Dis. 2019;19(1):1069. doi:10.1186/s12879-019-4687-7
20. Wei JH, Qian XQ, Wan YM, et al. Analysis of unsuccessful tests and the effect of prolonged clinical sample preprocessing in the GeneXpert MTB/RIF assay. BMC Infect Dis. 2024;24(1):770. doi:10.1186/s12879-024-09684-4
21. World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis. World Health Organization; 2025.
22. Deshwal H, Avasarala SK, Ghosh S, Mehta AC. Forbearance with bronchoscopy: a review of gratuitous indications. Chest. 2019;155(4):834–847. doi:10.1016/j.chest.2018.08.1035
23. Akhter N, Sumalani KK, Chawla D, Ahmed Rizvi N. Comparison between the diagnostic accuracy of Xpert MTB/Rif assay and culture for pleural tuberculosis using tissue biopsy. ERJ Open Res. 2019;5(3). doi:10.1183/23120541.00065-2019
24. Du J, Huang Z, Luo Q, et al. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay using pleural biopsy and pleural fluid specimens. J Res Med Sci. 2015;20(1):26–31.
25. Allahyartorkaman M, Mirsaeidi M, Hamzehloo G, Amini S, Zakiloo M, Nasiri MJ. Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: a multicenter surveillance. Sci Rep. 2019;9(1):18515. doi:10.1038/s41598-019-55112-y
26. Tortoli E, Russo C, Piersimoni C, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J. 2012;40(2):442–447. doi:10.1183/09031936.00176311
27. Lokhande L, Malhotra AG, Vishwakarma SP, et al. Diagnosis of tuberculous pleural effusion in a tertiary care hospital of central India: the role of xpert Mycobacterium tuberculosis/rifampicin. Int J Mycobacteriol. 2023;12(2):162–167. doi:10.4103/ijmy.ijmy_96_23
28. Aggarwal AN, Agarwal R, Dhooria S, Prasad KT, Sehgal IS, Muthu V. Xpert MTB/RIF Ultra versus Xpert MTB/RIF for diagnosis of tuberculous pleural effusion: a systematic review and comparative meta-analysis. PLoS One. 2022;17(7):e0268483. doi:10.1371/journal.pone.0268483
29. Sehgal IS, Dhooria S, Aggarwal AN, Behera D, Agarwal R. Diagnostic performance of Xpert MTB/RIF in tuberculous pleural effusion: systematic review and meta-analysis. J Clin Microbiol. 2016;54(4):1133–1136. doi:10.1128/JCM.03205-15
30. Jain J, Jadhao P, Banait S, Salunkhe P. Diagnostic accuracy of GeneXpert MTB/RIF assay for detection of tubercular pleural effusion. PLoS One. 2021;16(6):e0251618. doi:10.1371/journal.pone.0251618
31. Friedrich SO, von Groote-Bidlingmaier F, Diacon AH. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J Clin Microbiol. 2011;49(12):4341–4342. doi:10.1128/JCM.05454-11
32. Lu Y, Zhu Y, Shen N, Tian L, Sun Z. Evaluating the diagnostic accuracy of the Xpert MTB/RIF assay on bronchoalveolar lavage fluid: a retrospective study. Int J Infect Dis. 2018;71:14–19. doi:10.1016/j.ijid.2018.01.030
33. Sharma SK, Kohli M, Yadav RN, et al. Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosis. PLoS One. 2015;10(10):e0141011. doi:10.1371/journal.pone.0141011
34. Kilaru SC, Chenimilla NP, Syed U, et al. Role of Xpert MTB/RIF in Bronchoalveolar lavage fluid of sputum-scarce, suspected Pulmonary TB patients. J Clin Tuberc Other Mycobact Dis. 2019;14. doi:10.1016/j.jctube.2018.11.003
35. Khalil KF, Butt T. Diagnostic yield of Bronchoalveolar Lavage gene Xpert in smear-negative and sputum-scarce pulmonary tuberculosis. J Coll Physicians Surg Pak. 2015;25(2):115–118.
36. Hong J, Lee SH, Ryu B-H, et al. Diagnostic usefulness of bronchoalveolar lavage fluid xpert MTB/RIF in pauci-bacillary pulmonary tuberculosis. Infect Dis. 2018;50(9):725–727. doi:10.1080/23744235.2018.1467037
37. Yang J, Ye W, Zhang C, et al. Accuracy of nanopore sequencing as a diagnostic assay for pulmonary tuberculosis versus smear, culture and Xpert MTB/RIF: a head-to-head comparison. Trop Med Infect Dis. 2023;8(9). doi:10.3390/tropicalmed8090441
38. Piersimoni C, Gherardi G, Gracciotti N, Pocognoli A. Comparative evaluation of Xpert MTB/RIF and the new Xpert MTB/RIF ultra with respiratory and extra-pulmonary specimens for tuberculosis case detection in a low incidence setting. J Clin Tuberc Other Mycobact Dis. 2019;15:100094. doi:10.1016/j.jctube.2019.100094
39. Lombardi G, Di Gregori V, Girometti N, Tadolini M, Bisognin F, Dal Monte P. Diagnosis of smear-negative tuberculosis is greatly improved by Xpert MTB/RIF. PLoS One. 2017;12(4):e0176186. doi:10.1371/journal.pone.0176186
40. Li M, Qiu Y, Guo M, et al. Comparison of Xpert MTB/RIF Ultra with Xpert MTB/RIF for the detection of Mycobacterium tuberculosis and rifampicin resistance in a primary-level clinic in rural China. Tuberculosis. 2023;142:102397. doi:10.1016/j.tube.2023.102397
41. Agrawal M, Bajaj A, Bhatia V, Dutt S. Comparative study of GeneXpert with ZN stain and culture in samples of suspected pulmonary tuberculosis. J Clin Diagn Res. 2016;10(5):DC09–DC12. doi:10.7860/JCDR/2016/18837.7755
42. Zifodya JS, Kreniske JS, Schiller I, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021;2:CD009593. doi:10.1002/14651858.CD009593.pub5
43. Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76–84. doi:10.1016/S1473-3099(17)30691-6
44. Singh A, Karmakar D, Jha R. Comparative evaluation of cbnaat with smear microscopy, symptom screen and chest X-ray for diagnosis of pulmonary tuberculosis. Inter J Scient Res. 2019;8:1–5.
45. Ngangue YR, Mbuli C, Neh A, et al. Diagnostic accuracy of the Truenat MTB plus assay and comparison with the Xpert MTB/RIF assay to detect tuberculosis among hospital outpatients in Cameroon. J Clin Microbiol. 2022;60(8):e0015522. doi:10.1128/jcm.00155-22
46. Malherbe ST, Shenai S, Ronacher K, et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med. 2016;22(10):1094–1100. doi:10.1038/nm.4177
47. Pang Y, Lu J, Su B, Zheng H, Zhao Y. Misdiagnosis of tuberculosis associated with some species of nontuberculous mycobacteria by GeneXpert MTB/RIF assay. Infection. 2017;45(5):677–681. doi:10.1007/s15010-017-1044-x
48. Najjingo I, Muttamba W, Kirenga BJ, et al. Comparison of GeneXpert cycle threshold values with smear microscopy and culture as a measure of mycobacterial burden in five regional referral hospitals of Uganda- A cross-sectional study. PLoS One. 2019;14(5):e0216901. doi:10.1371/journal.pone.0216901
49. Li K, Hu Q, Liu J, Liu S, He Y. Effects of sputum bacillary load and age on GeneXpert and traditional methods in pulmonary tuberculosis: a 4-year retrospective comparative study. BMC Infect Dis. 2023;23(1):831. doi:10.1186/s12879-023-08832-6
50. Mansfield M, McLaughlin AM, Roycroft E, et al. Diagnostic performance of Xpert MTB/RIF ultra compared with predecessor test, Xpert MTB/RIF, in a low TB incidence setting: a retrospective service evaluation. Microbiol Spectr. 2022;10(3):e0234521. doi:10.1128/spectrum.02345-21
51. Vaneechoutte M, Van Eldere J. The possibilities and limitations of nucleic acid amplification technology in diagnostic microbiology. J Med Microbiol. 1997;46(3):188–194. doi:10.1099/00222615-46-3-188
52. Hardinge P, Murray JAH. Lack of specificity associated with using molecular beacons in loop mediated amplification assays. BMC Biotech. 2019;19(1):55. doi:10.1186/s12896-019-0549-z
53. Wong Y-P, Othman S, Lau Y-L, Radu S, Chee H-Y. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. J Appl Microbiol. 2018;124(3):626–643. doi:10.1111/jam.13647
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



