Back to Journals » Cancer Management and Research » Volume 17
Nab-Paclitaxel Based Chemotherapy in the Treatment of Advanced Epithelioid Hemangioendothelioma: A Single-Institution Experience
Authors Liu X , Yang P, Liu L, Si S, Zhou R, Liu T , Tan H
Received 12 December 2024
Accepted for publication 18 February 2025
Published 21 February 2025 Volume 2025:17 Pages 373—381
DOI https://doi.org/10.2147/CMAR.S508673
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Xiaolei Liu,* Peijun Yang,* Liguo Liu, Shuang Si, Ruiquan Zhou, Tiantong Liu, Haidong Tan
Second Department of Hepatopancreatobiliary Surgery, China-Japan Friendship Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaolei Liu; Haidong Tan, Second Department of Hepatopancreatobiliary Surgery, China-Japan Friendship Hospital, 2 Yinghua Dongjie, Hepingli, Beijing, 100029, People’s Republic of China, Email [email protected]; [email protected]
Objective: To evaluate the clinical efficacy of nab-paclitaxel based chemotherapy in the treatment of advanced epithelioid hemangioendothelioma (EHE).
Methods: Since March 2022, chemotherapy has been recommended for patients with advanced EHE characterized by large tumors (liver tumors > 10 cm or tumors in other organs > 3 cm), rapid tumor progression, severe symptoms, serosal effusion, and treatment failure. Two chemotherapy regimens were administered: nab-paclitaxel plus bevacizumab and nab-paclitaxel plus sirolimus. Clinical data and outcomes were retrospectively analyzed.
Results: From March 2022 to August 2024, 21 patients with histologically confirmed EHE who received nab-paclitaxel based chemotherapy were included. At baseline, 18 patients (85.7%) presented with tumor-related symptoms, and serosal effusion was detected in 12 patients (57.1%). Among patients with hepatic EHE, six (28.6%) had tumors > 10 cm, while six (28.6%) with EHE at other sites had tumors > 3 cm. Partial response and stable disease were achieved in 5 (23.8%) and 12 (57.1%) patients, respectively, resulting in a disease control rate of 80.9%. Symptom relief was observed in 15 of 18 patients (83.3%), and decreased serosal effusion was noted in 6 of 12 patients (50.0%). The 1- and 2-year progression-free survival rates were 50.7% and 13.5%, respectively, while the 1- and 2-year overall survival rates were 70.6% and 51.5%, respectively.
Conclusion: Nab-paclitaxel based chemotherapy may offer an effective treatment option for patients with advanced EHE exhibiting adverse prognostic factors. However, further clinical trials are required to confirm its efficacy.
Keywords: epithelioid hemangioendothelioma, EHE, chemotherapy, sarcoma, survival
Introduction
Epithelioid hemangioendothelioma (EHE) is a rare, translocation-associated vascular malignancy that can occur in various organs, including the lung, liver, soft tissues, and bone.1–3 The biological behavior of EHE exhibits significant variability, with the tumor potentially behaving as an indolent growth in some patients, while developing into an aggressive sarcoma in others.4–6 The clinical symptoms of EHE are often dependent on the primary tumor site. In hepatic EHE, the tumor is typically asymptomatic in its early stages and is often discovered incidentally during radiological examination. However, large intrahepatic tumors may cause abdominal pain or ascites.7,8 Pulmonary EHE is similarly asymptomatic in many cases but can lead to symptoms such as chest pain, shortness of breath, or pleural effusion, particularly in patients with pleural metastases.9 Serosal effusion, large tumor size, and severe clinical symptoms have been identified as poor prognostic factors. However, predicting long-term outcomes based on the disease onset remains a challenge.7,10
Although standard guidelines for localized sarcomas are applied in clinical practice, systemic agents, such as anthracycline-based chemotherapy, have shown limited efficacy in treating EHE.11–13 Sirolimus has been utilized to control tumor growth in EHE; however, the objective response rate (ORR) is only 11.8% (2/17).14 Our previous study demonstrated that interferon-alpha 2b (IFN-α 2b) has clinical value in treating hepatic EHE, and the combination of sirolimus and IFN-α 2b yielded improved clinical outcomes.15,16 However, based on our experience, EHE patients with tumors at sites other than the liver tend to exhibit a weaker response to IFN-α 2b. For hepatic EHE patients with adverse prognostic factors, such as serosal effusion or severe pain, neither monotherapy with IFN-α 2b nor the combination of sirolimus and IFN-α 2b produces favorable outcomes. Therefore, at our center, nab-paclitaxel based chemotherapy has been recommended for patients with advanced EHE exhibiting rapid tumor progression, adverse prognostic factors, or failure of other systemic treatments. A retrospective study was conducted to assess the clinical outcomes of patients with advanced EHE who underwent nab-paclitaxel based chemotherapy.
Patients and Methods
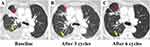
Since March 2022, chemotherapy has been recommended as an initial treatment for patients with advanced EHE with large tumors (liver tumor > 10 cm or tumors of other sites > 3 cm), rapid tumor progression, severe tumor-related symptoms (such as pain, fever, and shortness of breath), or serosal effusion. The patients should be pathologically diagnosed as EHE, while presence of WWTR1-CAMTA1 or YAP1-TFE3 translocation by fluorescence in situ hybridization (FISH) was performed for patients with uncertain pathological results (Figures 1 and 2). Chemotherapy has also been suggested for patients with EHE with tumor progression and failure of previous systematic treatment (sirolimus or sirolimus plus IFN-α 2b or other systematic agents). Two chemotherapy regimens were administered at our center. The protocol for regimen A consisted of one cycle every 21 days, included nab-paclitaxel (day 1, 130 mg/m2 and day 8, 130 mg/m2) and bevacizumab (day 1, 7.5 mg/kg). The protocol for regimen B included the administration of sirolimus (2 mg PO). qd), and nab-paclitaxel, administered every 21 days at the same dosage as the first regimen. For patients with treatment failure with sirolimus or sirolimus plus IFN-α 2b, the first protocol is usually suggested. However, no criteria have been established for the selection of appropriate chemotherapy regimens. Tolerability and safety were assessed before the start of treatment through interviewing and tests of blood cell counts, plasma sirolimus levels, and liver and renal function. Magnetic resonance imaging or computed tomography was performed every three cycles to assess the status of tumor, according to the Response Evaluation Criteria in Solid Tumors Committee (RECIST) criteria.17
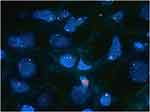 |
Figure 2 Positive result of fluorescence in situ hybridization on WWTR1 rearrangement, which showed the detachment of red and green signals. |
Chemotherapy was planned to be conducted 6 cycles, unless there were scenarios such as: occurrence of severe toxicity, tumor progression or treatment termination of patient’s decision. Safety assessments was conducted by monitoring and recording vital signs, laboratory results and adverse events (AE). AE was assessed according to the Common Terminology Criteria for Adverse Events, version 4.03. For patients with Eastern Cooperative Oncology Group (ECOG) performance status ≥ 3 or grade 3 AEs, chemotherapy would be temporarily withheld. If patients had both good treatment tolerability and tumor response, the treatment period of chemotherapy could be prolonged according to individual conditions. Until August 2024, the tumor and survival status were monitored and recorded.
ORR was calculated by adding the rates of complete response (CR) and partial response (PR). Disease control rate (DCR) was calculated by adding the rates of CR, PR and stable disease (SD) at six months. Before the start of treatment, all the patients needed to sign consent forms for chemotherapy and clinical data collection for medical research. All EHE patients who received nab-paclitaxel-based chemotherapy were retrospectively investigated. Patients who received chemotherapy less than three cycles and without tumor status evaluation were excluded. The research protocol was reviewed and approved by the ethics committee of the China-Japan Friendship Hospital (Number: 2023-KY-328) and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines.
Statistical Analysis
Categorical data were presented in the form of numbers and ratios. Continuous data were presented as mean ± standard deviation (SD). Progression-free survival (PFS) and overall survival (OS) were estimated using Kaplan-Meier method. PFS was calculated from the start of chemotherapy to the first evidence of progression disease (PD). OS was calculated from the start of chemotherapy to the time of death from any cause or last follow-up. Patients who remained alive or were lost to follow-up were censored at the last contact. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) (version 24.0, USA).
Results
Patients
Between March 2022 and August 2024, 22 patients with histologically confirmed EHE underwent chemotherapy at our center. One patient who chose to stop treatment after two cycles of chemotherapy without evaluation of the tumor status was excluded. Finally, 21 patients were included in this study and their clinical data and outcomes were retrospectively investigated. Twelve (57.1%) patients had FISH tests to confirm the diagnosis of EHE and all of them had WWTR1 rearrangement.
At baseline, tumor-related symptoms were recorded in 18 (85.7%) patients, and 8 (38.1%) patients had abnormalities in the hematopoietic system. The liver (57.1%) was the most common primary site, and 18 (85.7%) patients had metastases before the start of chemotherapy, including 14 (66.7%) patients with a single metastatic site and 4 (19.0%) patients with multiple metastatic sites. Serous effusion was detected in 12 (57.1%) patients, including pleural effusion in 7 (33.3%) patients and ascites in 5 (23.8%) patients. The median tumor size of the largest lesion was 6.3 cm, including 6 (28.6%) hepatic EHE patients with tumors larger than 10 cm and 6 (28.6%) EHE patients with tumors larger than 3 cm. The median time between diagnosis and the initiation of chemotherapy was 4 months (range, 1–103 months). Eleven patients (52.4%) were not treated prior to chemotherapy. The baseline demographic characteristics of the patients are summarized in Table 1.
 |
Table 1 Demographic and Clinical Characteristics of Patients |
Responses and AEs
Fourteen (66.7%) patients received regimen A and 7 (33.3%) patients received regimen B. The median number of chemotherapy cycles was six (range, 3–9 cycles). PR and SD were achieved in five (23.8%) and 12 (57.1%) patients, respectively, and the DCR was 80.9%. For patients with PR, 3 (3/14, 21.4%) patients received regimen A and 2 (2/7, 28.6%) patients received regimen B (Figure 3). One patient with PR received anlotinib before chemotherapy, which failed to control tumor growth. The other 4 patients with PR received chemotherapy as primary treatment. Among the patients with tumor-related symptoms, 15 (15/18, 83.3%) experienced symptom relief (Figure 4). In patients with serous effusion, a decrease in effusion was detected in 6 (6/12, 50.0%) patients (Figure 5). PD was detected in four (19.0%) patients, and no further treatment was provided. For patients with regimen B, the median plasma level of sirolimus was 6.8 ng/mL (range, 4.21–11.32 ng/mL).
AEs were recorded in 13 (61.9%) patients, and severe AEs (grade≥3) were recorded in three (14.3%) patients. The most common AEs was leukopenia (61.9%). Five patients (23.8%) experienced a delay in chemotherapy due to AEs. The detailed AE results of AEs were summarized in Table 2.
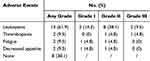 |
Table 2 Treatment-Related Adverse Events |
Survival Outcomes
Eight (8/17, 47.1%) patients with SD or PR experienced tumor progression after termination of chemotherapy. Four patients received anlotinib, and four patients received no other treatment. The 1-year and 2-years PFS rate were 50.7% and 13.5%, respectively (Figure 6A). The median PFS was 18 months (95% confidence intervals: 6.6–29.4 months). During the study period, eight patients died due to tumor progression. The 1-year and 2-years OS rate were 70.6% and 51.5%, respectively (Figure 6B). The median OS was 27 months (95% confidence intervals: 14.2–39.8 months). Detailed demographic and clinical information of each patient is provided in Supplementary File 1.
 |
Figure 6 (A) Kaplan–Meier curve showing progression-free survival of all EHE patients. (B) Kaplan–Meier curve showing overall survival of all EHE patients. |
Discussion
EHE is a rare vascular malignant tumor that originates from endothelial cells and can develop in various organs, including the liver, lung, bone, and soft tissues.18 Its reported incidence is less than 1 per million, with a slightly higher prevalence in women.19 Radiological imaging plays a crucial role in diagnosing EHE, which typically presents as a solid nodule with occasional calcifications.20 However, a definitive diagnosis requires histological confirmation. Pathologically, the tumor is usually composed of epithelioid, dendritic, and intermediate cells embedded in a fibromyxoid stroma.21 The detection of WWTR1-CAMTA1 or YAP1-TFE3 gene fusions may serve as valuable molecular diagnostic markers.22 According to the World Health Organization (WHO) classification, EHE is categorized as a malignant vascular tumor, resembling angiosarcoma but generally with a more favorable prognosis.23
The biological behavior of EHE is typically indolent in most patients, with a reported 5-year survival rate of 70–80%.24,25 However, long-term survival is closely linked to the tumor’s primary site. EHE in the lungs, particularly when associated with pleural metastases, is known to have a poor prognosis.26 Several adverse prognostic factors for EHE have been identified, including older age, larger tumor size, tumor-related symptoms, serosal effusion, and anemia.7,22,27 Tumors greater than 10 cm in size in hepatic EHE and those greater than 3 cm in size in EHE at other sites are associated with poorer outcomes.7,22,28 Our recent report on the long-term prognosis of hepatic EHE identified both age ≥60 years and tumor size >10 cm as significant poor prognostic factors.29 These adverse factors were considered as reference indices for chemotherapy selection in the present study.
For patients with localized EHE, radical resection should be considered, provided it is technically feasible. However, our previous studies have shown that surgical resection is often not possible for most hepatic EHE patients due to the presence of multiple metastases, and recurrence is commonly observed post-surgery.20,30 Liver transplantation has been reported as an effective treatment for hepatic EHE, with favorable long-term survival outcomes.31 However, metastases in the early stages of the disease often hinder the feasibility of liver transplantation for most patients with hepatic EHE. As a result, systemic therapy is typically recommended for patients with tumor progression or tumor-related symptoms. Unfortunately, none of the currently used agents for sarcoma treatment demonstrate significant clinical efficacy in EHE.
Sirolimus, an mTOR inhibitor, has been reported to be effective in treating EHE. Stacchiotti et al documented the results of 18 patients with progressive EHE treated with sirolimus, reporting one PR and 12 instances of SD, with a median PFS of 12 months.14 A separate retrospective study involving pediatric EHE patients also demonstrated the activity of sirolimus, with four PRs observed among six patients.32 Since 2021, sirolimus has been used in our center to treat EHE, both as monotherapy and in combination with IFN-α 2b. One of our previous studies indicated that hepatic EHE patients treated with IFN-α 2b monotherapy achieved favorable long-term survival.15 It was speculated that IFN-α exerts immunomodulatory effects through the activation of both innate and adaptive immunity, although the precise mechanism remains unclear.33 Furthermore, the combination of sirolimus and IFN-α 2b exhibited synergistic effects in the treatment of hepatic EHE.16 However, based on our clinical experience, neither monotherapy with sirolimus nor the combination with IFN-α 2b effectively controls tumor progression in advanced EHE patients with adverse prognostic factors, such as large tumor size, tumor-related symptoms, and serosal effusion. As a result, chemotherapy is recommended for this subgroup of patients with EHE.
Various cytotoxic treatments have been explored for metastatic EHE. Tumor regression was observed with doxorubicin alone, while the combination of epirubicin and dacarbazine showed no significant effect.34,35 Recent studies indicate that paclitaxel induces cell death through chromosome missegregation on multipolar spindles, making it a key chemotherapy option for vasogenic malignancies.36 Additionally, the combination of paclitaxel and targeted drugs has demonstrated favorable efficacy in the treatment of sarcoma.37 Compared to docetaxel and paclitaxel, nab-paclitaxel—a newer taxane chemotherapeutic agent—has several clinical advantages and promising efficacy in treating subtypes of sarcoma, including epithelioid sarcoma and angiosarcoma.37
Several other agents have also been explored for treating EHE, such as sorafenib, bevacizumab, and trametinib.38–40 A Phase 2 study of sorafenib found that 13.3% (2/15) of EHE patients achieved PR.38 Another phase 2 study of bevacizumab in patients with advanced angiosarcoma and EHE reported a 28.6% (2/7) PR in EHE patients, though the small sample size and lack of disease severity data limit the interpretation of these findings.39 Nevertheless, this study provided valuable evidence for further investigation of bevacizumab in EHE treatment. Trametinib, a MEK inhibitor, was also evaluated in a phase 2 trial for EHE, but its ORR was only 3.7%, suggesting that monotherapy with these agents may have limited effectiveness for EHE.40
Survival outcomes for EHE patients with adverse prognostic factors are typically poor, and no effective therapy has been reported for this subgroup.10 In our previous report on long-term outcomes in hepatic EHE, including 26 patients receiving chemotherapy, only a few were treated with nab-paclitaxel as the primary therapy.29 This study aimed to assess the effectiveness of nab-paclitaxel based chemotherapy in patients with advanced EHE and adverse prognostic factors. At baseline, 85.7% of patients presented with tumor-related symptoms, 57.1% had serous effusion, and 57.1% had large tumor sizes, all indicating disease severity. After nab-paclitaxel based chemotherapy, 23.8% of patients achieved a PR, and the DCR was 80.9%. The median PFS was 18 months, which is an improvement compared to previous studies.13,14,38–40 In patients treated with regimen B, the plasma levels of sirolimus were similar to those in our prior report, suggesting that nab-paclitaxel does not significantly impact the metabolism of sirolimus.16 Thus, nab-paclitaxel-based chemotherapy is a promising treatment for advanced EHE patients with adverse prognostic factors.
This study has several notable limitations. First, the sample size was small. However, EHE is a rare and typically indolent tumor, making it difficult to recruit patients with advanced EHE and adverse prognostic factors. Second, the use of two nab-paclitaxel based chemotherapy regimens in this study may have introduced ambiguity in the results, and the absence of a comparison between nab-paclitaxel monotherapy and its combination with bevacizumab or sirolimus is another limitation. Given the retrospective nature of the analysis, it is unclear whether combination therapy offers any advantage over single-agent nab-paclitaxel. Additionally, many pathologists confirm EHE diagnosis via detection of WWTR1-CAMTA1 or YAP1-TFE3 translocations through FISH. Not all patients in this study underwent FISH testing, which may impact the interpretation and broader applicability of the results.
In conclusion, nab-paclitaxel based chemotherapy shows promise as an effective treatment for advanced EHE. Although this was a single-arm retrospective study, the findings provide valuable clinical evidence for treating patients with advanced EHE and adverse prognostic factors. The encouraging results of nab-paclitaxel based chemotherapy position it as a promising option. However, further studies are needed to determine whether the observed treatment effects are primarily due to nab-paclitaxel or the result of its combination with sirolimus or bevacizumab.
Data Sharing Statement
The original contributions of this study are included in the article and Supplementary File 1. Further inquiries can be directed to the corresponding author.
Consent to Participate
Written informed consent was obtained from all individual participants included in the study. Consent to publish identifiable information was obtained from study participants. All authors have provided their consent for the publication of this manuscript.
Acknowledgments
The authors are grateful to all patients included in this study for their support.
Funding
This research was supported by the National High Level Hospital Clinical Research Funding and Elite Medical Professionals Project of the China-Japan Friendship Hospital (Grant Number: ZRJY2023-GG12).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stacchiotti S, Miah AB, Frezza AM, et al. Epithelioid hemangioendothelioma, an ultra-rare cancer: a consensus paper from the community of experts. ESMO Open. 2021;6(3):100170. doi:10.1016/j.esmoop.2021
2. Stacchiotti S, Frezza AM, Blay JY, et al. Ultra-rare sarcomas: a consensus paper from the connective tissue oncology society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127(16):2934–2942. doi:10.1002/cncr.33618
3. Rosenberg A, Agulnik M. Epithelioid hemangioendothelioma: update on diagnosis and treatment. Curr Treat Options Oncol. 2018;19(4):19. doi:10.1007/s11864-018-0536-y
4. Errani C, Zhang L, Sung YS, et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50(8):644–653. doi:10.1002/gcc.20886
5. Antonescu CR, Le Loarer F, Mosquera JM, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. 2013;52(8):775–784. doi:10.1002/gcc.22073
6. Witte S, Weidema M, Kaal S, et al. The heterogeneity of epithelioid hemangioendothelioma (EHE): a case series and review of the literature with emphasis on treatment options. Semin Oncol. 2021;48(2):111–118. doi:10.1053/j.seminoncol.2021.04.002
7. Chahrour MA, Khachfe HH, Habib JR, El-Asmar R, Saifi O, Jamali FR. Treatment and prognosis of hepatic epithelioid hemangioendothelioma: a SEER DATABASE ANAlysis. World J Surg. 2021;45(9):2886–2894. doi:10.1007/s00268-021-06165-6
8. Kaltenmeier C, Stacchiotti S, Gronchi A, et al. Treatment modalities and long-term outcomes of hepatic hemangioendothelioma in the United States. HPB. 2022;24(10):1688–1696. doi:10.1016/j.hpb.2022.03.013
9. Jung H, Kim HN, Jang Y, Park CK, Ha SY. CAMTA-1 expression in 24 cases of hepatic epithelioid hemangioendothelioma in a single institute: diagnostic utility for differential diagnosis from hepatic angiosarcoma. Vivo. 2019;33(6):2293–2297. doi:10.21873/invivo.11736
10. Frezza AM, Napolitano A, Miceli R, et al. Clinical prognostic factors in advanced epithelioid haemangioendothelioma: a retrospective case series analysis within the Italian rare cancers network. ESMO Open. 2021;6(2):100083. doi:10.1016/j.esmoop.2021
11. Strauss SJ, Frezza AM, Abecassis N, et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(12):1520–1536. doi:10.1016/j.annonc.2021.08.1995
12. Gronchi A, Miah AB, Dei Tos AP, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021;32(11):1348–1365. doi:10.1016/j.annonc.2021.07.006
13. Frezza AM, Ravi V, Lo Vullo S, et al. Systemic therapies in advanced epithelioid haemangioendothelioma: a retrospective international case series from the world sarcoma network and a review of literature. Cancer Med. 2021;10(8):2645–2659. doi:10.1002/cam4.3807
14. Stacchiotti S, Provenzano S, Dagrada G, et al. Sirolimus in advanced epithelioid hemangioendothelioma: a retrospective case-series analysis from the Italian Rare Cancer Network Database. Ann Surg Oncol. 2016;23(9):2735–2744. doi:10.1245/s10434-016-5331-z
15. Liu X, Zhang Z, Huang J, Tan H, Yang Z. Efficacy and safety of interferon-alpha 2b for patients with hepatic epithelioid hemangioendothelioma: outcomes of a case-series analysis. Cancer Manag Res. 2021;13:8273–8279. doi:10.2147/CMAR.S334171
16. Liu X, Zhou R, Liu L, et al. Short-term outcomes of combined therapy with sirolimus and interferon-alpha 2b for advanced hepatic epithelioid hemangioendothelioma. Ther Adv Med Oncol. 2024;16:17588359231220509. doi:10.1177/17588359231220509
17. Therasse P, Arbuck SG, Eisenhauer EA, et al.; European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi:10.1093/jnci/92.3.205
18. Orsini G, Fioroni M, Rubini C, Piattelli A. Epithelioid hemangioendothelioma of the oral cavity: report of case. J Oral Maxillofac Surg. 2001;59(3):334–337. doi:10.1053/joms.2001.21007
19. Liu Z, He S. Epithelioid hemangioendothelioma: incidence, mortality, prognostic factors, and survival analysis using the surveillance, epidemiology, and end results database. J Oncol. 2022;2022:2349991. doi:10.1155/2022/2349991
20. Tan H, Zhou R, Yu H, et al. CT appearances and classification of hepatic epithelioid hemangioendothelioma. Insights Imaging. 2023;14(1):56. doi:10.1186/s13244-023-01410-z
21. Thin LW, Wong DD, De Boer BW, et al. Hepatic epithelioid haemangioendothelioma: challenges in diagnosis and management. Intern Med J. 2010;40(10):710–715. doi:10.1111/j.1445-5994.2009.02043.x
22. Stacchiotti S, Tap W, Leonard H, Zaffaroni N, Baldi GG. New molecular insights, and the role of systemic therapies and collaboration for treatment of epithelioid hemangioendothelioma (EHE). Curr Treat Options Oncol. 2023;24(6):667–679.
23. Fletcher CD. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology. 2014;64(1):2–11. doi:10.1111/his.12267
24. Lau K, Massad M, Pollak C, et al. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140(5):1312–1318. doi:10.1378/chest.11-0039
25. Bagan P, Hassan M, Le Pimpec Barthes F, et al. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann Thorac Surg. 2006;82(6):2010–2013. doi:10.1016/j.athoracsur.2006.06.068
26. Yurkiewicz IR, Zhou M, Ganjoo KN, et al. Management strategies for patients with epithelioid hemangioendothelioma: charting an indolent disease course. Am J Clin Oncol. 2021;44(8):419–422. doi:10.1097/COC.0000000000000827
27. Amin RM, Hiroshima K, Kokubo T, et al. Risk factors and independent predictors of survival in patients with pulmonary epithelioid haemangioendothelioma. Review of the literature and a case report. Respirology. 2006;11(6):818–825. doi:10.1111/j.1440-1843.2006.00923.x
28. Deyrup AT, Tighiouart M, Montag AG, Weiss SW. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol. 2008;32(6):924–927. doi:10.1097/pas.0b013e31815bf8e6
29. Liu X, Yang P, Liu L, et al. Long-term prognosis and treatment modalities of hepatic epithelioid hemangioendothelioma: a retrospective study of 228 patients. BMC Cancer. 2024;24(1):1285. doi:10.1186/s12885-024-13053-4
30. Liu XL, Yang ZY. Outcomes of hepatic epithelioid hemangioendothelioma with different managements: a retrospective investigation. Eur Rev Med Pharmacol Sci. 2021;25(12):4274–4282. doi:10.26355/eurrev_202106_26133
31. Agrawal N, Parajuli S, Zhao P, et al. Liver transplantation in the management of hepatic epithelioid hemangioendothelioma: a single-center experience and review of the literature. Transplant Proc. 2011;43(7):2647–2650. doi:10.1016/j.transproceed.2011.06.035
32. Engel ER, Cournoyer E, Adams DM, Stapleton S. A retrospective review of the use of sirolimus for pediatric patients with epithelioid hemangioendothelioma. J Pediatr Hematol Oncol. 2020;42(8):e826–e829. doi:10.1097/MPH.0000000000001643
33. Hirata A, Hashimoto H, Shibasaki C, Narumi K, Aoki K. Intratumoral IFN-α gene delivery reduces tumor-infiltrating regulatory T cells through the downregulation of tumor CCL17 expression. Cancer Gene Ther. 2019;26(9–10):334–343. doi:10.1038/s41417-018-0059-5
34. Kelly H, O’Neil BH. Response of epithelioid haemangioendothelioma to liposomal doxorubicin. Lancet Oncol. 2005;6(10):813–815. doi:10.1016/S1470-2045(05)70392-2
35. Idilman R, Dokmeci A, Beyler AR, et al. Successful medical treatment of an epithelioid hemangioendothelioma of liver. Oncology. 1997;54(2):171–175. doi:10.1159/000227683
36. Weaver BA. How Taxol/paclitaxel kills cancer cells. mol Biol Cell. 2014;25(18):2677–2681. doi:10.1091/mbc.E14-04-0916
37. Tian Z, Yao W. Albumin-bound paclitaxel: worthy of further study in sarcomas. Front Oncol. 2022;12:815900. doi:10.3389/fonc.2022.815900
38. Chevreau C, Le Cesne A, Ray-Coquard I, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French sarcoma group (GSF/GETO). Cancer. 2013;119(14):2639–2644. doi:10.1002/cncr.28109
39. Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, Phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24(1):257–263. doi:10.1093/annonc/mds237
40. Schuetze SM, Ballman KV, Heise R, et al. A single-arm phase 2 trial of trametinib in patients with locally advanced or metastatic epithelioid hemangioendothelioma. Clin Cancer Res. 2024;30(20):4584–4592. doi:10.1158/1078-0432.CCR-23-3817
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Overweight and Obesity are Associated with Poorer Survival Among Patients with Advanced Non-Small Cell Lung Cancer Receiving Platinum-Based Chemotherapy
Sutandyo N, Hanafi AR, Jayusman AM, Kurniawati SA, Hanif MA
International Journal of General Medicine 2023, 16:85-93
Published Date: 6 January 2023
Neoadjuvant Radiation Therapy with Interdigitated High-Dose LRT for Voluminous High-Grade Soft-Tissue Sarcoma
Hatoum GF, Temple HT, Garcia SA, Zheng Y, Kfoury F, Kinley J, Wu X
Cancer Management and Research 2023, 15:113-122
Published Date: 5 February 2023