Back to Journals » International Journal of Nanomedicine » Volume 20
Nanoagent-Mediated Photothermal Therapy: From Delivery System Design to Synergistic Theranostic Applications
Authors Shang Y , Yi X , Xiang D, Zhou L
Received 14 February 2025
Accepted for publication 10 May 2025
Published 29 May 2025 Volume 2025:20 Pages 6891—6927
DOI https://doi.org/10.2147/IJN.S522736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Kamakhya Misra
Yating Shang,1 Xiangxiang Yi,1 Debiao Xiang,2,* Lili Zhou1,*
1School of Pharmacy, Hunan University of Chinese Medicine, Changsha, 410208, People’s Republic of China; 2Department of Pharmacy, The Third Hospital of Changsha, Changsha, 410035, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lili Zhou, Email [email protected] Debiao Xiang, Email [email protected]
Abstract: In contemporary medicine, cancer poses a perilous threat to human life, health, and quality of life, remaining a central focus of medical research and clinical practice. Although traditional cancer treatments, such as surgery, chemotherapy, and radiotherapy, have demonstrated some degree of success, they continue to encounter substantial challenges, including incomplete tumor eradication and the occurrence of severe adverse effects. Therefore, there is an urgent need to develop novel cancer treatment strategies characterized by high efficacy, low toxicity, and precise targeting. Photothermal therapy (PTT), as a newly emerging approach, holds considerable promise due to its unique mechanism of action. Upon laser irradiation, PTT utilizes a photothermal agent (PTA) to transform light energy into thermal energy, thereby inducing localized hyperthermia within tumor tissues. This process enables precise ablation of tumor cells while minimizing damage to surrounding healthy tissues. Recent advancements in photothermal agent research have yielded a diverse array of PTAs, and the synergistic integration of PTT with diagnostic and therapeutic techniques has further expanded its therapeutic efficacy and clinical applicability. This paper will provide an overview of the mechanisms underlying PTT, recent progress in photothermal agent development, synergistic theranostic strategies, and combined therapeutic approaches. The aim is to establish a foundation for optimizing PTAs design, elucidating PTT mechanisms, and advancing research on synergistic therapeutic protocols. Ultimately, these endeavors have the potential to result in more effective and safer disease treatments, thereby advancing the translation of photothermal therapy from foundational research to broad clinical application.
Keywords: photothermal therapy, photothermal agents, multimodal imaging theranostics, synergistic therapy, immunotherapy, photodynamic therapy
Introduction
Cancer is a leading global cause of mortality, characterized by the uncontrolled and disorganized proliferation and spread of malignant cells. Its impact substantially surpasses that of common diseases, including cardiovascular disease. In recent years, morbidity and mortality rates have been on the rise, presenting a significant challenge to public health. The complex process of tumor development and progression is influenced by a multitude of interacting factors,1 each contributing through distinct mechanisms. This complexity, coupled with the difficulty of early detection, poses significant challenges to effective cancer treatment. While the demonstrated importance of genomic variation and epigenetic modifications in cancer development represents a significant advancement with the potential to revolutionize treatment strategies. There are numerous obstacles remain,2 such as the development of drug resistance in tumor cells, strong resistance to radiotherapy (RT), and sophisticated mechanisms of immune evasion, which make effective treatment much more challenging. Furthermore, traditional therapies such as surgery, chemotherapy, and RT are limited in their clinical application due to drawbacks such as extensive surgical trauma, prolonged postoperative recovery, poor drug targeting, significant toxicity and side effects, high recurrence rates, and limited efficacy.3,4 Modern-era nanomedicines exhibit great potential in cancer diagnosis and therapy, attributed to their unique design, flexibility and modifiability, enabling targeted delivery and improved biocompatibility.5,6 These nanosystems can encapsulate drugs, facilitating combination therapies with chemotherapy, immunotherapy (IT), photothermal therapy (PTT), etc., to reduce systemic toxicity and enhance therapeutic efficacy. Moreover, the enhanced permeability and retention7 (EPR) effect in solid tumors promotes nanomedicine accumulation at tumor sites.8 Some nanomedicines possess signal-emitting properties (eg, fluorescence) that can be leveraged for both diagnostic and therapeutic purposes,9,10 aligning with the principles of precision medicine. Precision medicine aims to develop personalized treatment plans based on individual genes, environment and other differences. The photoacoustic imaging (PAI), magnetic resonance imaging (MRI) and fluorescence imaging (FI),11 as important imaging technologies, provide key support for precision medicine. In recent years, IT, PTT and other treatment modalities have been increasingly used. IT can accurately identify and attack tumor cells or pathogens. And compared with traditional RT and chemotherapy, IT yields less damage to normal tissues and organs of the body, although it may trigger autoimmune responses,12,13 and patient responses vary significantly,14,15 leading to diverse therapeutic outcomes. PTT is a novel tumor treatment that utilizing specific light wavelengths and photothermal conversion materials to achieve precise heating of specific lesion tissues, with less damage to surrounding normal tissues. This approach often employs fiber optics, lasers, or similar devices for localized irradiation, avoiding open surgery and thus reducing trauma, accelerating recovery, and improving patient quality of life.16,17 In comparison to traditional chemotherapy, photothermal therapy demonstrates a reduced likelihood of inducing drug resistance and is associated with fewer adverse effects. Furthermore, when contrasted with radiotherapy, photothermal therapy can be effectively combined with targeted nano-agents to facilitate localized hyperthermia at the tumor site, thereby minimizing the impact on surrounding healthy tissues. However, there are also shortcomings such as limited penetration depth, influence of tissue optical properties, and challenges in precisely controlling thermal damage. Integrating PTT with precision medicine and synergistic therapies has the potential to achieve more effective tumor ablation and significantly improve therapeutic efficacy and prognosis. This review centers on the research advancements of combined diagnostic techniques and therapeutic methods employing photothermal agents (PTAs) and PTT.
Mechanism of Photothermal Therapy
Photothermal therapy (PTT) represents a sophisticated therapeutic approach grounded in the principle of photothermal conversion. This method utilizes materials referred to as photothermal agents (PTAs), which inherently possess the ability to convert absorbed light energy into thermal energy. When exposed to light of specific wavelengths, these agents efficiently facilitate this conversion. Upon accumulation within pathological tissues, the resultant localized thermal energy elevates the tissue temperature, thereby inducing necrosis in tumor cells. PTT18 is characterized by minimal invasiveness tumor ablation, showing great potential in controlling drug-resistant bacteria19 and eliminating tumor cells.20 This method utilizes laser irradiation, mainly near-infrared (NIR) light, to deliver energy. NIR light offers greater potential for tumor diagnosis and treatment compared to other wavelengths due to its lower absorption and stronger tissue penetration within the biological window. The NIR spectrum encompasses two primary regions: NIR-I (750–900 nm)21 and NIR-II (1000–1700 nm).22 While 700–950 nm NIR light sources are more widely used in PTT treatment, the superior imaging quality and deeper tissue penetration of NIR-II23 make it a more suitable option for treating deeper-seated tumors.24–26 PTT leverages the unique property of PTAs to transform absorbed light energy into heat, thereby inducing cell death in tumors. Upon irradiation, PTAs absorb light energy, exciting electrons from the ground state to a higher energy singlet state.27 Subsequently, these electrons experience internal conversion to attain the lowest excited singlet state. The subsequent de-excitation of electrons to the ground state can occur through three primary pathways: (1) radiative decay via fluorescence emission, (2) non-radiative relaxation, and (3) intersystem crossing, leading to phosphorescence. The photothermal effect is primarily attributed to non-radiative relaxation,28 where excited electrons come back to the ground state while discharging energy as vibrational energy, causing local heating of the surrounding environment. Temperature significantly influences cellular function and viability. Elevated temperatures induce a range of cellular responses, including protein denaturation, membrane disruption, and DNA damage, ultimately leading to cell death. Mild hyperthermia (41°C) accelerates cellular metabolism and blood flow. In the range of 41–48°C,29 protein aggregation occurs, while cell sensitivity to RT and chemotherapy increases. Sustained exposure to temperatures within this range for more than 60 minutes can cause irreversible cell damage. In contrast, temperatures between 48–60°C induce rapid and severe cell damage within 4–6 minutes due to extensive protein denaturation and DNA damage. When the temperature of the organization exceeds 60°C, protein denaturation and membrane damage cause the cells to die almost instantaneously.30 This temperature-dependent cellular response forms the foundation for PTT in cancer treatment. Photothermal conversion efficiency (PCE) quantifies the capacity of a photothermal agent to convert absorbed light energy into thermal energy. This efficiency is determined by comparing the thermal energy output of the photothermal agent with the amount of light energy absorbed.31 The power conversion efficiency (PCE) is influenced by the material’s absorbance properties, the alignment of the laser wavelength, the structural design, and the energy scattering characteristics. To enhance the photothermal conversion efficiency,32 it is necessary to increase the photosensitivity of the PTAs, minimize light scattering, and optimize the attenuation of non-radiative relaxation pathways during energy conversion. Table 1 presents the factors influencing PCE and the methods for its improvement.
 |
Table 1 The Factors and Optimization Methods of Photothermal Conversion Efficiency |
Advantages of Photothermal Therapy
PTT, as a non-invasive treatment modality, exhibits highly effective and non-invasive properties.33,34 For example, compared with traditional antibiotic therapy, PTT demonstrates broad-spectrum antimicrobial activity against various pathogens,19 with a significantly shorter treatment time (only a few minutes)29 and negligible bacterial resistance. In tumor therapy, it enables precise control of the temperature within the tumor microenvironment by adjusting the dosage of the photothermal agent or the intensity of light, thereby controlling the degree of tumor ablation. In clinical applications, PTT is often employed synergistically with other therapeutic modalities, including IT, photodynamic therapy (PDT), chemotherapy, RT, gene therapy,35 ferroptosis therapy, and others. This synergistic approach enhances selectivity and improves therapeutic efficacy, demonstrating high clinical research value.
Limitations of Photothermal Therapy
Although photothermal therapy has numerous advantages, the following problems remain to be solved.36 Since photothermal therapy needs to be combined with laser irradiation, photothermal therapy may be less suitable for tumors that have spread, and is not conducive to the treatment of deep-seated tumors due to the limitation of light transmittance. Moreover, the optimal temperature of photothermal therapy is yet to be confirmed. Lower temperature is not suitable for killing cancer cells, but high temperature conditions may cause immune cells to gather like the tumor environment with changes such as accelerated blood flow, which in turn will kill normal immune cells, and high temperature may cause certain damage to normal cells or tissues. In the next studies, it is hoped that these problems can be remedied by combining appropriate agents or treatments.
Advances in Photothermal Agents
PTAs are required to have advantages such as high PCE, photostability, good biocompatibility, rapid clearance from the body37 after use, and ease of synthesis and modification. Nanopreparations38 can be directly used as PTAs in PTT, or as drug-delivery systems in combination with other therapies, or as contrast agents for imaging-guided applications. Nanopreparations, with their diverse applications, enhance the potential of photothermal therapy (PTT) by facilitating more precise treatment strategies. This review provides a comprehensive overview of the various types of photothermolysis agents, detailing their advantages, clinical potential, and potential challenges, as outlined in Table 2. Furthermore, Table 3 presents a summary of representative nano-agents, including their synergistic applications with other therapies, mechanisms of action, and therapeutic effects.
 |
Table 2 Types, Clinical Potential, Advantages and Potential Obstacles of Photothermal Agents |
 |
Table 3 Nanopreparations as Photothermal Agents |
Metal Photothermal Agents
Metal photothermal agents constitute a category of photothermal materials derived from metallic compositions.60 Metal photothermal agents are capable of absorbing specific wavelengths of light and converting them into thermal energy with high efficiency, thereby facilitating the treatment of various diseases. The fundamental mechanism underlying this process is the Localized Surface Plasmon Resonance (LSPR) effect exhibited by metal nanomaterials. In this section, a series of summary tables have been compiled and are presented in Table 4.
 |
Table 4 Representative Metal Photothermal Agents |
Precious Metal Nanoparticles
The first generation of precious metal materials employed as PTAs primarily includes gold (Au), platinum (Pt), and silver (Ag). These metals effectively capture NIR light, initiating a distinct LSPR effect. These precious metals can be fabricated into metal particles with sizes ranging from 1 to 100 nm, called metal nanoparticles (NPs), through reduction and other methods. When NPs encounter light matching an equally-distributed exciton band, the vibrating electromagnetic field of the light source triggers coordinated vibrations of the free-surface electrons. This leads to the separation of charges relative to the lattice and the creation of an oscillating dipole that aligns with the light’s electric field, a phenomenon called LSPR.65 LSPR facilitates efficient photothermal conversion in NPs, generating the heat necessary for ablating tumor cells.66–68 NPs have advantages including high photothermal conversion rates and imaging capabilities, which provide a basis for mild and effective non-invasive cancer diagnosis and treatment. Compared to traditional chemotherapeutic drugs, NPs demonstrate fewer adverse reactions and improved targeting. Furthermore, their absorption spectra are mainly dependent on size and adjustable factors such as the aspect ratio, which can be defined as the wavelength at which light energy is absorbed.69 Unique optical properties, coupled with chemical inertness, high biocompatibility, and low toxicity, make metallic nanoparticles suitable as raw materials for PTAs or biochemical sensors. These distinctive properties render metal nanoparticles ideal for use as raw materials in PTAs70 or biochemical sensors.71
The particle shape of NPs significantly influences their absorption wavelengths. Consequently, NPs exhibit diverse shapes, including nanospheres, nanorods, nanocages, nanocubes, and others, each suitable for specific applications. The influence of the shape and structure of NP on the optical absorption and conversion efficiency of materials was investigated. Utilizing identical surface modifications, three common gold nanoparticles (AuNPs)72 were developed: gold nanorods, gold nanorods and gold nanostars.73,74 The findings demonstrated that several types of AuNPs could effectively convert 808 nm NIR laser light energy into heat energy via LSPR. However, among these, gold nanostars exhibited the highest efficiency in converting light energy into thermal energy. Among precious metal photothermal materials, gold nanoparticles are some of the most widely utilized. Building upon these observations, for Plasmonic Nanoparticles (PNPs) capable of generating LSPR, researchers used simple harmonic resonance theory analysis incorporating defect-induced attenuation, and found that the attenuation arising from defects introduced into PNPs could effectively reduce optical scattering, thereby significantly enhancing energy conversion efficiency. The study further indicated that at larger scales (Au, Ag > 100 nm), the attenuation effect resulting from introduced defects in nanoparticles could greatly enhance the properties of light absorption and photothermal effect in the materials.
NPs have been shown to be useful in clinical applications, such as antimicrobial75 and anticancer therapies. They can kill cancer cells and reduce tumor mass upon irradiation with biocompatible NIR lasers. NPs can serve as PTAs, targeting tumor cells for ablation through PTT. Besides, they can function as contrast agents76 for improved diagnosis, sensitizers for RT,77,78 and tools to differentiate cancer cell populations by conjugating them with antibodies.79 Furthermore, combining NPs with IT and chemotherapy has shown promising synergistic treatment effects, which have undergone extensive research in recent years.80 For example, synthetic silver nanomaterials can significantly inhibit HIV-1 infection.81,82
In cancer treatment, Zhang et al developed a multifunctional metal nanoplatform, termed SFT-Au, that synergistically combined starvation therapy with physiological calcification.61 This platform comprised gold nanoparticles functionalized with salivary acid (SA), folic acid (FA),83 and triphenylphosphine (TPP) as targeting moieties. This system exhibited efficient light absorption and photosensitization, and demonstrated the effective integration of SA, FA, and TPP onto a single platform through PTT evaluation. By leveraging the ability of FA and TPP to target tumor cells and the ability of SFT-Au to target Ca2+ (abundant in tumor cell mitochondria), coupled with the excellent light-trapping and photothermal properties of SFT-Au, the platform establishes a precise tumor mitochondria-targeted calcification system, enhancing the efficacy of “starvation therapy”. The results are summarized in the be more specific. SFT-Au was found to promote Ca2+ chelation and trigger calcification, further amplified by a thermal agent at 808 nm optical radiation, resulting in persistent photodamage. A recent study has introduced a novel multifunctional nanomaterial, synthesized via glycol chitosan-assisted in situ formation of gold-coated PLGA nanoshells (AuPLGA-NS). This material integrates the biodegradability of PLGA with the near-infrared (NIR) photothermal properties of gold nanoshells and the imaging capabilities of X-ray/CT. Experimental results demonstrated that AuPLGA-NS exhibits substantial photothermal conversion efficiency when subjected to 808 nm laser irradiation, achieving an 80% mortality rate in MCF-7 breast cancer cells cultured in vitro after just 4 minutes of exposure. Additionally, its X-ray attenuation performance was found to be comparable to that of conventional iodine-based contrast agents, underscoring its potential utility as an imaging contrast medium. Biocompatibility assessments indicated that the material does not exhibit significant toxicity towards normal cells.84 This study substantiates the potential of this easily synthesized multifunctional nanosystem to concurrently facilitate photothermal therapy and image guidance, thereby offering a promising strategy for the integrated diagnosis and treatment of cancer. Similarly, Zou and Ma et al engineered a versatile Pt-based nanoplatform aimed at PTT with targeting capabilities, incorporating Arg-Gly-Asp peptide (RGD) combined polydopamine-altered Pt nanoparticles, designated as mPt@PDA-RGDNPs.39 Initially, K2PtCl4 and hexadecyltrimethylammonium bromide (CTAB) were fully reacted, followed by the addition of ascorbic acid to the reaction system. Upon reaction completion, anhydrous ethanol was utilized to remove excess CTAB, yielding an aqueous solution of mPtNPs. Subsequently, mPtNPs and PDA were reacted in a tris-HCl buffer to synthesize mPt@PDANPs. Finally, mPt@PDA-RGDNPs were obtained by reacting mPt@PDANPs with RGD in the tris-HCl buffer. The mPt@PDA-RGDNPs synthesized through this method exhibited high PCE under 808 nm NIR laser irradiation85 and demonstrated good biocompatibility. The specific interaction between RGD and the overexpressed αvβ3 integrins on SKOV-3 cells conferred targeting and migration inhibition capabilities. Cell scratch tests indicated that the photothermal impact of mPt@PDA-RGDNPs efficiently restricted the migration of SKOV-3 cells. Overall, mPt@PDA-RGDNPs exhibited significant potential in achieving targeted photothermal ablation of tumors and inhibiting the migration of cancer cells. On the platinum nanoplatform, Zhang, Zhao et al explored the utilization and possible mechanisms of Mesoporous Platinum Nanoparticles (MPNs)62 combined with the drug chlorosartan in PTT, utilizing neuroblastoma as a model system. Their findings demonstrated that chlorosartan-induced fibroblast ablation enhanced MPN penetration into the tumor, facilitated MPN deposition within tumor cells, thus boosting the effectiveness of PTT when it comes to eliminating tumor cells. Furthermore, this nanoplatform exhibited a synergistic effect in activating immune cells, thereby inducing immunogenic cell death. This strategic approach holds significant promise for the in vivo treatment of neuroblastoma.
Gold nanophotothermal agents are currently undergoing clinical trials. Notably, Ardeshir R. Rastinehad et al have reported preliminary findings from a trial involving gold-silica nanoshells (GSN). In this study, photothermal therapy was integrated with MRI-ultrasound imaging, and patients were monitored at various intervals post-procedure. The results demonstrated a 94% success rate for GSN in the photothermal ablation of tumors, with the feasibility and safety of the approach also being confirmed.86
Clearance of drugs from the body is an issue that requires special attention. This is closely related to the side effects or toxicity of the drug. Biodegradable drugs show big advantages. Aravind Kumar Rengan et al developed a biodegradable liposomal gold nanoparticles (Lipos Au NPs) for tumor photothermal therapy. In vitro and in vivo studies show that Lipos Au NPs significantly inhibit cancer cell growth. Biodistribution analysis showed that the nanoparticles were metabolized by the liver and kidneys and largely cleared within 14 days.87 The PDPC NPs developed by Tejaswini Appidi et al are also biodegradable nano-formulations for photothermal therapy. The therapeutic efficacy was verified.88 These systems combine efficient photothermal conversion, biodegradability and real-time bioluminescence monitoring, which provides a new idea for precision tumor therapy.
Metal Sulfides and Oxides
Metal sulfides or oxides are less costly than precious metals and can also be used for PTT.89,90 For example, MoS2 is a representative two-dimensional transition metal dichalcogenide,91 exhibiting significant potential in the act of eradicating bacteria owing to its intrinsic antimicrobial properties, encompassing contact killing, PDT, and PTT.19 Furthermore, MoS2 is readily prepared with high PCE and low cost. However, metal sulfides or oxides typically exhibit negligible fluorescence and lack inherent tumor-targeting capabilities, hampering rapid real – time FI and exact localization of both primary tumors and lymph node metastases.92 To address these limitations, their combination with NIR organic dyes, such as Cy5.5, can be used for fluorescence dual-mode imaging therapy. For example, Shi et al developed RGD-CuS-Cy5.5,93 a CuS nanoparticle-based platform, for synergistic fluorescence/PTT applications.41 These nanoparticles effectively serve dual functions: FI and targeted PTT therapy for metastatic gastric tumor cells within lymph nodes. In vivo studies demonstrated that 808 nm laser irradiation, guided by RGD-CuS-Cy5.5-mediated FI, facilitated the targeted PTT-induced destruction of gastric cancer cells in lymph nodes while causing minimal systemic toxicity.
Complex nanostructures based on metal sulfides have been reported, such as ruthenium sulfide as core nanoclusters (NCs) constructed by Lu and Huang et al. Ruthenium sulfide nanodots (OA-RuS1.7NDs) encapsulated by oleic acid were initially prepared and subsequently coated with denatured bovine serum albumin (dBSA)94 and polyethylene glycol (PEG) to obtain PEG-dBSA-RuS1.7NCs.42 These nanostructures exhibited excellent light-to-heat conversion capabilities and possessed an optimal diameter and a near-neutral surface charge (approximately 0 mV). These characteristics contributed to prolonged in vivo circulation times and enhanced tumor targeting. Leveraging these advantages, PEG-dBSA-RuS1.7NCs could effectively target and ablate cancer cells under NIR irradiation. Metal oxides, such as manganese dioxide (MnO2), also offer potential for PTT. Wang, Wu et al developed a nanocomposite, BP@Mn-NC,63 comprising Black Phosphorus (BP) and Manganese Nanozymes (Mn-NZ) that exhibiting both photothermal and catalytic properties. The integration of Mn-NZ conferred glucose oxidase- and peroxidase-like activities to BP@Mn-NC, facilitating the generation of Reactive Oxygen Species (ROS).95 These ROS cause lipid peroxidation and the build-up of malondialdehyde in the bacterial cell membrane. This process disrupted the exposed and vulnerable respiratory chain complex of the bacterial cell membrane, leading to a decline in intracellular Adenosine Triphosphate (ATP) levels and a concomitant loss of normal function in ATP-dependent bacterial heat shock proteins. Consequently, mild PTT mediated by BP@Mn-NC effectively eradicated multidrug-resistant bacterial infections by specifically impairing bacterial heat resistance.
Biomimetic Metal Nanostructures
Metallic nanostructures, while exhibiting potential in cancer therapy, often lack active targeting capabilities and primarily rely on the passive EPR96 effect to accumulate at tumor sites. To address these limitations, researchers have drawn inspiration from biomimicry, employing biologically active cell membranes to encapsulate nanoparticles. This approach offers several advantages over traditional nanomedicines, including prolonged circulation time within the body, enhanced targeting and aggregation within the tumor region, and improved biosafety and biocompatibility.97 Common sources for these cell membranes include red blood cells, platelets, and tumor cells. Cell-derived membranes, when used to coat nanoparticles, effectively camouflage them from immune recognition, thereby extending their blood circulation time. This process involves the complete transfer of natural markers, typically found on the source cell’s membrane or extracellular vesicles, onto the surface of the chosen nanomaterial. The integration of gold nanoparticles (GNPs) with biofilms demonstrates significant potential within the field of drug delivery systems, especially when such integration can further optimize therapeutic effects.
Coating with erythrocyte membranes has been demonstrated to extend the drug’s half-life within the body, thereby enhancing its therapeutic efficacy.98 In a recent study, it was found that by delicately binding natural Red Blood Cell (RBC) membranes99 to the outer surface of Polyvinylpyrrolidone-noble metal nanocomposites (PVP-AuNCs),100 Piao and Wang et al successfully fabricated erythrocyte-derived loaded noble metal nanocomplexes (RBC-AuNCs).43 This innovative strategy not only preserved the biological activity and pharmacological properties of AuNCs but also enhanced their biocompatibility and improved their localization ability, thus showing more significant effects during the treatment of diverse diseases. The construction of RBC-AuNCs not only successfully facilitated the integration of nanomaterials with biological systems but also opened up innovative avenues for the development of efficient and safe biomedical applications. Notably, RBC-AuNCs could be constructed with tumor cell membranes possessing cell adhesion molecules homologous to the tumor cells,101 which promoted the aggregation of cancer cells and enables targeted delivery of the drug to tumor cells.
Liu, Wu, et al reported a novel nanostructure, MoOx@M-SN38, where molybdenum oxide (MoOx) nanoparticles were coated with egg yolk phospholipids and subsequently encapsulated in tumor cell membranes. This nanostructure was further loaded with 7-ethyl-10-hydroxycamptothecin (SN38). In vivo studies demonstrated that MoOx@M-SN3844 nanosheets effectively inhibited primary tumor growth and lung metastases compared to monotherapy. Notably, these novel nanosheets enabled the synergistic combination of PTT and chemotherapy,102 significantly enhancing anti-tumor immunity. Building upon this concept, researchers have explored the use of platelet membranes for drug delivery.103 Platelet membranes are now understood to possess unique properties, including the ability to reduce drug immune rejection, exhibit specific adsorption to tumors and damaged blood vessels, and facilitate drug accumulation at the lesion site. Leveraging these advantages, Zhao, Li, et al builted a biomimetic platform for tumour treatment. This platform comprised Mesoporous Platinum Nanoparticles (MPNPs) loaded with 17-Dimethylaminoethylamino-17-Demethoxygeldanamycin (17-DMAG),104 a heat shock protein (HSP) inhibitor,105 and subsequently encapsulated in a platelet membrane (PM) coating, resulting in the 17-DMAG/MPNPs@PM nanostructure.64 In vitro and in vivo studies demonstrated that 17-DMAG/MPNPs@PM exhibited efficient tumor accumulation and effectively inhibited tumor cell growth.
Carbon-Based Photothermal Agents
Carbon-based106 nanostructures, including graphene oxide, nanotubes, and carbon nanodots, have arisen as promising prospects107 for applications in the biomedical field, particularly in PTT and PDT.108,109 These nanostructures exhibit unique properties that enable precise heat generation within the treatment area and the generation of tunable ROS,110 facilitating highly efficient tumor cell elimination. Furthermore, their distinctive effects on bacteria and specific optical properties make them valuable tools in antibacterial applications, effectively mitigating the development of drug-resistant pathways.111,112
Carbon Nanotubes
Carbon Nanotubes (CNTs) are allotropes of carbon, formed by single or multiple layers of graphite flakes curled around a central axis at a certain helix angle.52,113 A single-walled carbon nanotube (SWNT) is characterized as a cylindrical structure composed of a single layer of carbon atoms organized in a hexagonal lattice.114 Multi-walled carbon nanotubes (MWCNTs) are characterized by their multi-layered nested structures.115 CNTs are characterized by high mechanical strength, and based on this property, there are now cases of animal experiments in which CNTs have been successfully utilized to achieve functional restoration of force-transmitting connective tissues (such as bone).116 Owing to the extensive surface area that they possess, strong chemical stability, and rich electronic polycyclic aromatic structure, CNTs exhibit excellent drug-carrying capacity, and a variety of drug molecules, antibodies, and nucleic acids can be attached or linked to carbon nanotubes.117,118 These molecular entities can be adhered to the surface of CNTs by means of non-covalent interactions (such as π-π stacking or van der Waals forces),118,119 or directly attached using covalent bonding methods, enhancing their stability and durability.120 CNTs can effectively transport these molecular payloads to target cells, safeguarding therapeutic molecules from metabolic alterations during transit. Consequently, CNTs emerge as promising carriers for the intracellular delivery of nucleic acids,121 proteins,122 and drug molecules,123 establishing a foundation for CNTs to synergize PTT with gene therapy. However, CNTs have smooth surfaces, and to load their surfaces with a certain composition, they need to undergo some treatment first to make their surfaces rough.124 Furthermore, CNTs exhibit the advantageous capability of readily crossing the blood-brain barrier.125 This characteristic renders them particularly suitable for the treatment of encephalopathy, offering enhanced efficacy compared to drugs with limited blood-brain barrier penetration. The high PCE of CNTs has driven their utilization in the development of PTAs. Carbon nanotubes are capable of efficiently combining chemotherapy and PTT to enhance anti-tumour effects. In addition to their therapeutic potential, CNTs serve as exceptional diagnostic tools, endowed with photoacoustic, fluorescence, and Raman imaging capabilities.126,127 As a result, CNTs have been thoroughly studied for numerous biomedical applications, serving as a potential treatment alternative for carcinoma. SWNTs128 are one of the particularly promising candidates for PTT, demonstrating strong absorption in the NIR region (700–1100 nm).129 Because biological systems display high translucency at this wavelength, SWNTs can be efficiently utilized for thermal excitation within living cells. This NIR wavelength can induce thermal stimulation by disrupting protein function and compromising the plasma membrane, leading to cell destruction. In this respect, SWNTs promote tumor ablation when the temperature reaches above 40 °C,52,118 while normal cells are typically resistant to this treatment. The local ablation effect based on CNTs overcomes the challenges of uneven tumor heating and the risk of tumor cell dissemination along the needle tract associated with invasive techniques like radiofrequency ablation. Zhou and Liu et al developed a SWNT-based thermosensitive hydrogel (SWNT-GEL) for treating xenografted gastric cancer in mice. Interestingly, SWNT-GEL could deliver the drug Doxorubicin (DOX) directly to the tumor site, enabling in situ anticancer effects. Concurrently, NIR light could penetrate the tissue to stimulate the SWNTs located at the tumor site, thereby providing thermal therapy. The DOX release profile demonstrated sustained drug delivery, with more than 20% released on the first day of injection and cumulative release reaching approximately 96% by day 28. In addition, after intratumoral injection of SWNT-GEL, NIR irradiation significantly reduced tumor growth rate in mice compared to the control group without NIR (156% vs 261%) after 28 days of treatment, suggesting sustained tumor remission under NIR irradiation.60,130 To further enhance PCE, MWCNTs characterized by nested columnar structures with diameters ranging from a few nanometers to a few micrometers, have been investigated. MWCNTs offer several advantages, including the capacity to load more drugs and absorb more NIR radiation than SWNTs, making them an ideal platform for combined chemo-thermal therapy.131 Oudjedi et al developed a photothermal agent utilizing gold nanorod (GNR)-modified MWCNTs (MWCNTs-GNRs).132 The LSPR property of GNRs enhanced light absorption133 in the NIR region,134 while the high PCE of MWCNTs facilitated efficient dispersion of heat into the surrounding environment. By synthesizing plasma MWCNTs-GNRs135 as PTAs, researchers achieved superior local absorption136 and heat conversion137 compared to bare carbon nanotubes, resulting in more effective thermal ablation of cancer cells than some carbon-metal-based PTAs.138 A similar MWCNT-based photothermal nanosystem is mTHPC/MWCNT, a complex of m-tetrahydroxyphenyl chlorin (mTHPC) and MWCNTs, developed by Marangon et al. The researchers confirmed its photothermal activity and demonstrated its low cytotoxicity and potency in promoting tumor cell apoptosis.139
Carbon Dots
Carbon Dots (CDs) correspond to carbon-based materials typically less than 10 nm in size and are usually composed of carbon, oxygen, and hydrogen. There are currently four main types of carbon quantum dots: graphene quantum dots, carbon nanodots, and carbonized polymer dots.140,141 CDs exhibit a number of outstanding properties, including good stability, high electrical conductivity, photo-induced photothermal conversion, tunable fluorescence, excellent photostability, very low cytotoxicity, and high biocompatibility.142,143 Intrinsic photosensitivity144 is also one of the core properties of CDs. The prominence of this trait stems from their unique chemical composition and surface modifications, enabling efficient and stable photoexcitation within the visible and NIR spectral intervals. Under photoexcitation, carbon dots not only facilitate a diverse array of photochemical reactions, such as electron and energy transfer, and photothermal conversion, but also exhibit a rich spectrum of photophysical properties through complex processes like inter-system crossing. These attributes have resulted in a large number of application potentials in the field of medical sciences.
Within the context of PDT and PTT, CDs demonstrate unique dual-functionality, with unprecedented potential and extensive applications in cancer treatment and microbial infections.145 In response to the simplified functionalized surface properties exhibited by CDs, their efficient combination with photosensitizers (PSs) has demonstrated significant synergistic effects in the combination therapy of PDT and PTT.146 The synthesis of Mannose-grafted Fe-doped Carbon Dots (M/Fe-CDs) was reported in a study by Ye and Zhang et al45 whereby M/Fe-CDs exhibited excellent photothermal properties, with a PCE significantly elevated to 30.1% compared to unmodified CDs. They also possessed good water solubility and biocompatibility. It has been demonstrated that M/Fe-CDs can achieve localized heating and killing of tumor cells under 808 nm NIR light irradiation. In addition to their excellent photothermal properties, M/Fe-CDs can be utilized in conjunction with IT. M/Fe-CDs are capable of binding CpG-oligodeoxynucleotides (CpG-ODN) via electrostatic interactions and transporting them into the cytoplasmic region of Dendritic Cells (DCs), significantly increasing CD80/86 expression on the surface of DCs. Therefore, M/Fe-CDs represent a multifunctional nanoplatform that synergizes PTT and IT. In addition to CDs synthesized from chemical materials, there are also CDs synthesized from natural materials. For instance, Liu and Cui et al synthesized fluorescent CDs from cinnamon fruit, exhibiting excellent photothermal properties and negligible toxicity. Their bright green fluorescence is conducive to bio-imaging. Experiments have demonstrated that these CDs can successfully and efficiently induce Hela cell death under 808 nm NIR irradiation.147 Zhang, Yang, et al have developed an advanced nanoplatform utilizing copper-nitrogen coordinated carbon dots (Cu-N-CDs) for targeted tumor therapy through hyaluronic acid modification. This material exhibits valence state switching in response to the glutathione-rich microenvironment, leading to dynamic modulation of its photophysical properties: the Cu2+ state predominantly facilitates photothermal therapy (PTT), while the Cu+ state enhances fluorescence imaging in conjunction with photodynamic therapy (PDT). The study demonstrated, through multimodal characterization, that a single laser irradiation can simultaneously achieve a combined PTT/PDT effect. Furthermore, residual tumors can be precisely targeted using Cu+-state fluorescence for complementary PDT-mediated eradication. This system is both biocompatible and responsive to the tumor microenvironment, offering a novel strategy for multimodal precision therapy of solid tumors.148
Graphene Oxide
Graphene is closely related to carbon nanotubes, and graphene and its derivatives, including Graphene oxide (GO) and reduced graphene oxide (rGO), have been extensively explored as PTT reagents. GO offers a potential new approach to treating cancer by assisting in the precise transfer of genetic components and therapeutic chemicals to the tumor region.149 GO possesses exceptional properties, being the thinnest and strongest material discovered to date,150 with excellent electrical and thermal conductivity. Its unique single-atom-thick, two-dimensional structure renders it an ideal scaffold for composite nanomaterials. Carbon-based materials, such as graphene oxide (GO), are considered promising substrates for metal ions due to their high surface area and uniform pore size, which provide a basis for further modification.151 The multi-layered and porous structure of GO endows it with a specific adsorption capacity, making it advantageous for addressing environmental pollution challenges.152 The advantages of GO nanostructures encompass encapsulating genes or drugs to protect them from degradation and enhancing cellular uptake, besides enhancing pharmacokinetics and bioavailability through concentrating therapeutic substances in a specific tumor region. Its excellent photothermal properties and ability to generate ROS can effectively convert NIR light into heat to selectively ablate cancer cells. Based on GO’s phototherapeutic capabilities, the combined application of GO nanoparticles with PDT and PTT has demonstrated significant potential in inhibiting tumor growth.153 The successful tumor eradication observed with GO nanoparticles is largely attributed to their internalization via the primary endocytosis pathway, ensuring precise delivery to the target site. Along with their potential medical utilizations, GO nanoparticles show promising prospects as vaccine candidates because of their capacity to trigger both cellular and innate immunity.149
These nanoparticles can also be used to detect, diagnose, and eradicate cancer. Gao and Pei et al synthesized GO-Austar@rHSA-FA@IR780 through a multi-step process: firstly, large-molecule GO was synthesized and then processed to obtain small-molecule GO. Subsequently, Au was modified to yield GO-Austar, which despite not improving targeting or PCE, was further modified with Reduced Human Serum Albumin-FA coupling (rHSA-FA) to enhance targeting and encapsulate IR780. In vitro experiments demonstrated excellent biocompatibility with MGC-803 cells and potent phototoxicity. In vivo studies revealed significant tumor growth inhibition and near-complete tumor elimination.46
In the field of antibacterial applications, Wu and Deng et al synthesized GO/Ag2S via chemical precipitation in a weak alkaline environment. GO/Ag2S exhibited excellent photothermal conversion properties and considerable antibacterial potency against Staphylococcus aureus and Escherichia coli. It disrupted bacterial membrane integrity and inhibits bacterial growth through various mechanisms.154,155 Ma and Qi et al prepared GO-PEG-NH2 for sterilization therapy. The positively charged amino group enabled GO-PEG-NH2 to selectively kill supracellular bacteria in the presence of cells, demonstrating superior efficacy in mammalian cells. Furthermore, it exhibited good photostability and effective photothermal ablation under 808 nm NIR light irradiation with low cytotoxicity.156
Organic Photothermal Agents
Semiconductor Polymer Nanoparticles
Semiconductor polymer nanoparticles (SPNs) are novel optical organic nanomaterials derived from Semiconductor Polymers (SPs),157 including polyaniline (PANI), polypyrrole (PPy) and quinoxaline. SPs offer several advantages for PTT, such as excellent optical properties, good photostability, large absorption coefficients, and facile surface functionalization. Importantly, the absence of metal ions in SPNs contributes to their good biocompatibility, low toxicity, and biodegradability.158,159 The optical properties of SPNs are primarily determined by the precursor SP and can be modulated by altering the molecular structure of the SP. Leveraging these properties, chemiluminescence and NIR fluorescence of SPNs have been explored for tumor visualization and detection of physiological indicators and biomarkers following photoacoustic imaging.160,161 Furthermore, SPNs can effectively respond to NIR light, generating localized heat and ROS for both PTT and PDT.162 By capitalizing on these superior PTT and PDT effects, SPNs can precisely modulate biological activities,163 such as neuronal activation and gene transfection.164 The integration of other therapeutic components into SPNs facilitates the development of combined phototherapy cancer treatments with significantly enhanced therapeutic efficacy.
PANI is a material renowned for its exceptional electrical properties and commendable stability, with a relatively straightforward synthesis process. It finds applications across various domains, including photothermal therapy and chemical sensing. However, a notable limitation of PANI is its poor solubility, which can lead to reduced bioavailability. Nevertheless, the properties of PANI can be substantially enhanced through chemical modifications, such as protonation doping165 or integration with other substances. Such modifications not only optimize its properties but also enable PANI to exhibit anticancer and antimicrobial effects.166 PANI is the first organic PTAs demonstrated to have pH-responsive properties,167 exhibits an extensive array of diagnostic and curative applications. For example, researchers have developed Polyaniline Nanoparticles (PANPs) modified by F127.168 These nanoparticles exhibited a high molar extinction coefficient (8.95*108M−1cm−1), achieving a PCE of 48.5%. In vivo tumor ablation studies under 808 nm NIR irradiation demonstrated efficacy comparable to other methods, while low toxicity was confirmed through tissue and hematology experiments. Research on polypyrrole has also been extensive, with innovative methods developed to synthesize Polypyrrole Nanosheets (PPy NSs). Xie and Xu et al utilized Manganese Dioxide Nanosheets (MnO2 NSs) as both the oxidizing agent and a self-sacrificial template, enabling the rapid synthesis of PPy NSs within minutes.169,170 PPy NSs exhibited strong NIR-II absorption with a PCE of 66.01% under 1064 nm NIR-II light irradiation and possessed PA imaging capabilities, allowing for synergistic PA imaging and PTT for both diagnosis and treatment. Furthermore, Zhu and Gao et al reported a multifunctional thiazole-fused quinoxaline imide semiconducting polymer (PQIA-BDTT).171 PQIA-BDTT demonstrated a high PCE of 72.6% under 1064 nm NIR light irradiation. In vivo and in vitro experiments demonstrated the significant anti-tumor effect of PQIA-BDTT in combination with PTT, while also confirming its biocompatibility.
Conjugated Polymer Nanoparticles
Conjugated Polymer Nanoparticles (CPNs) are a subtype of SPNs. CPNs constructed upon π-extended conjugated systems exhibit significant advantages in cell-penetrating nanoparticles, including very high fluorescence brightness, extremely low cytotoxicity, excellent photostability, efficient ROS generation capability, and high PCE.172 These combined properties endow π-extended CPNs with the potential to serve as efficient therapeutic and diagnostic tools. CPNs can be utilized as drug-carrying systems,173 contrast agents,174 and PTAs concurrently, demonstrating numerous applications within the biomedical domain. In addition, the distinct light-gathering and energy-effective transport properties of CPNs enable their conversion into intelligent functional nanocomposites with significantly enhanced properties. Given these numerous features and the availability of simple and efficient preparation and isolation processes, the rapid development of CPNs and their hybrids within the medical community has been propelled to become a key player in leading cutting-edge research, with notable achievements observed in several specialized fields. As efficient photothermal conversion materials, CPN-based organic photothermal materials hold significant promise for NIR-II applications with good deep tissue penetration and low scattering properties.175,176 The D-π-A-π-D type conjugated small molecules synthesized with177 fluorene as the donor unit can achieve NIR-II absorption by simply replacing one atom within the molecule.178 Moreover, the assembly of these molecules into nanoparticles exhibits good photothermal stability and high PCE, which has been confirmed to demonstrate high efficiency for tumor treatment in both in vitro and in vivo experiments. CPNs are also suitable for NIR-I applications. Some researchers successfully synthesized Diketopyrrolopyrrole-Borondipyrromethene (DPP-BDP), a conjugated polymer with NIR-I absorption properties, through an innovative cross-coupling reaction. Utilizing Pluronic F127 as a stabilizer and functionalizing the polymer with folic acid enabled the creation of a targeted photothermolysis agent. Subsequent formation into conjugated polymer nanoparticles with uniform particle size resulted in a photothermolysis agent capable of achieving a PCE of 38.9% and a tumor reduction of 99.2%.179
Organic Small Molecule Dyes
Organic Small Molecule Dyes (OSMDs) are organic photothermal materials180,181 that can be used as PTAs,182 with the advantages of low toxicity along with excellent biocompatibility.183 When irradiated with NIR light, OSMDs produce heat via the PTT effect and also generate ROS181 via the PDT effect, acting as PS.184–186 The combined synergy of PTT and ROS treatment boosts cancer cell destruction and improves therapeutic outcomes.187 Notably, a novel organic small-molecule photothermal material demonstrated a PCE exceeding 72.7% under 808 nm laser irradiation.188 OSMDs include anthocyanine-based dyes (such as Indocyanine Green, ICG), IR series, porphyrins, boron dipyrrole dyes, rhodamine dyes, and others. These small molecules hold significant promise for clinical antimicrobial PTT applications. For instance, Diketopyrrolopyrrole (DPP) exhibits excellent photophysical properties, photostability, and structural tunability. Consequently, PTT agents based on DPP derivatives have found widespread use in PTT and have garnered considerable attention for FI and PAI diagnostics.189–191 Furthermore, ICG has been approved by the Food and Drug Administration (FDA) for over fifty years for medical imaging and the treatment of patients with internal contamination from cesium or thallium exposure. In a clinical trial, ICG was used in the treatment of periodontitis with PTT, which induced minimal bacterial resistance and less cytotoxicity to oral cells than chlorhexidine gel.192 IR780 is an appropriate candidate for utilization as a photothermal agent. Upon loading nanosystems with IR780, they demonstrate fluorescence properties. This characteristic facilitates the integration with fluorescence imaging, thereby enhancing the efficacy of the therapeutic system.88,193 However, these PTAs possess certain intrinsic limitations. For instance, they may have a low PCE, exhibit poor stability, show hydrophobic characteristics and fast removal rate. It has been reported that although the PCE of OSMDs was lower than that of metallic agents, they could be immobilized on metallic or nonmetallic materials. This approach not only enhanced OSMD stability but also improved the overall PCE, enabling synergistic exploitation of OSMDs’ PCE and ROS generation.194 For instance, Biomimetic cell membrane-coated polymer vesicles were created by binding cetyltrimethylammonium bromide, ICG, and polycaprolactone onto the surface of hydrophobic gold nanorods, achieving self-assembly in aqueous solution. The increased stability of vesicles facilitated synergistic roles for PTT and PDT in prostate cancer treatment. This method enhanced ICG loading while maintaining ICG fluorescence and activity. In comparison with free ICG or Au nanorods, this material exhibited superior stability, PCE (up to 47.7 °C), and effects of photodynamic action, significantly inhibiting tumor growth. For the IR series of organic dyes, Yang and Xu et al synthesized PPy(Fe)-IR820-BSA195 by polymerizing PPy nanoparticles using Fe3+ as an oxidant, followed by Bovine Serum Albumin (BSA) coating and IR820 loading. Fe3+ acted as a Chemodynamic Therapy (CDT) agent, PPy and IR820 as photothermite, and IR820 produced toxic ROS under NIR irradiation, acting as PDT. These nanoparticles were demonstrated to promote immunogenic cell death. A class of anthocyanin dyes, similar to IR820 but with higher PCE, namely the naphthylimide-modified anthocyanin dye ML880, featured a stronger electron-leaving domain due to the naphthamide modification. Jin and Cheng et al constructed a PDT/PTT/CDT multimodal synergistic nanoplatform by loading both ML880 and desired chemotherapeutic agents into ROS-responsive Mesoporous Organosilicon (RMON), significantly inhibiting tumor growth.196 For another organic small molecule dye, porphyrin, researchers prepared a combined anthocyanin-porphyrin nanoparticle demonstrating good efficacy in PTT treatment of prostate cancer. Rhodamine and semicarbocyanine derivatives (RC) also exhibited strong NIR absorption. The nanoparticles RC-BSANPs, prepared by combining RC with BSA, effectively eliminated tumors via the PTT mechanism under 915 nm NIR irradiation.197
Metal-Organic Frameworks
Metal-organic Frameworks (MOFs) belong to a novel class of crystalline materials characterized by periodic and internally porous structures. They are primarily constructed from metal atoms, metal clusters, and organic ligands (such as azoles, porphyrins, and carboxylates)198 containing oxygen or nitrogen donor atoms, which coordinate with a variety of metals including Fe, Ca, Mg, and Zn. As crystalline materials assembled from metal nodes and organic ligands, MOFs display a multitude of distinctive benefits. These include a large specific surface area, high porosity, an adjustable pore size, a high drug-loading capacity, good biocompatibility, and the potential for surface modification. MOFs are porous nanoreactors. It also has good adsorption capacity to adsorb pollutants.199 MOFs also have excellent thermal stability and can be used as photocatalysts.200,201 Furthermore, their porosity can be harnessed to encapsulate a diverse range of materials, such as pharmaceuticals, targeting agents, or imaging probes. This porous structure enables the controlled release of therapeutic agents and the design of stimulus-responsive systems that can be activated by light, pH, and other external stimuli.202–204 Therefore, MOFs serve as versatile multifunctional therapeutic platforms, finding widespread applications in catalysis, gas adsorption, energy storage, and drug delivery. They represent ideal platforms for the design of drug delivery systems, demonstrating significant potential for the development of cancer therapies.
MOFs with azoles as organic ligands include Zeolitic Imidazolate Frameworks (ZIFs), which consist of a transition metal coordinated to an imidazole-based linker (eg, 2-methylimidazole). ZIF-8, for example, comprises Zn as a metal node coordinated to 2-methylimidazole via two nitrogen atoms, exhibiting a sodalite topology. ZIF-8 and its biocomposites have shown promise in various biomedical applications, including biosensing, cancer and bacterial therapy, wound healing, and biocatalysis.205 Cancer therapy utilizing ZIF-8-based materials can be achieved through diverse strategies such as drug delivery, PTT, PDT, CDT, starvation therapy, and gene therapy. Notably, the encapsulation of indocyanine green within ZIF-8 significantly enhanced photostability, thereby improving PTT efficacy. Similarly, ZIF-8 nanostructures encapsulating Hollow Silver Sulfide (HAg2S), forming HAg2S/ZIF-8 structures, might be additionally modified using hyaluronic acid to target the CD44 receptor overexpressed in cancer cells. In this study, this nanoplatform was further functionalized to encapsulate azithromycin hydrochloride, enabling the synergistic combination of PTT and CDT for enhanced therapeutic outcomes.206
Zhang and Yuan designed and synthesized a porphyrin-cored, Cu2+-doped MOF, namely PCN-224(Cu), and subsequently modified its surface with Polydopamine (PDA) to obtain PCN-224(Cu)@PDA (PCP).207 This porphyrin-based organic ligand was then incorporated into a multifunctional nanocomposite hydrogel through dynamic Schiff base bonding between oxidized sodium alginate and methyl chitosan. This resulted in injectable and self-repairing hydrogels. Within this system, Cu2+ within the MOF was converted to Cu+ by reacting with glutathione, thereby reducing the antioxidant activity of tumors and enhancing CDT effects. The Cu2+/Cu+ redox couple induced a Fenton-like reaction within tumor cells, generating highly toxic hydroxyl radicals. Simultaneously, PDA enabled efficient photothermal conversion for PTT under NIR irradiation. Moreover, the porphyrin core acted as a photosensitizer, generating ROS for effective PDT. This injectable, self-healing hydrogel served as a versatile platform for in situ delivery of PCP to the tumor site, followed by cellular endocytosis. This approach facilitated precise and synergistic CDT-PDT-PTT therapy.
Unlike the aforementioned ligands, carboxylic acids have also been employed in MOF synthesis. For instance, Hao and Liu et al synthesized MOF nanoparticles using amino-terephthalic acid as a ligand and Zr44 as a metal ion.208 Subsequently, the NH2 group was modified to N3 using an azide-transferring reagent. Through a click chemistry reaction without copper, DBCO disulfide bond-functionalized PD-1/PD-L1209 encapsulating AUNP-12 was covalently incorporated into the MOF nanoparticles. The nanoparticles were then loaded with the PTAs ICG to successfully obtain ICG-MOF-SS-AUNP12210 nanoparticles with uniform and stable size.50 These nanoparticles displayed the release of PD −1/PD -L1 blockers triggered by GSH and demonstrated a powerful photothermal effect be able to effectively killing cancer cells. By 808 nm NIR irradiation, ICG-MOF-SS-AUNP12 successfully facilitated the maturation of DCs and initiated the immune response.
Multimodal Imaging Theranostics
Numerous photothermal agents possess bio-imaging capabilities, and the comprehensive utilization of this property could significantly enhance the optimization and application of photothermal therapy. Therapeutic diagnostic probes, which integrate diagnostic functions with therapeutic efficacy, represent a promising class of tools for cancer treatment. A close and indispensable relationship exists between diagnosis and treatment. Integrated diagnostics, achieved through the fusion of multidisciplinary technologies, enable comprehensive and accurate diagnosis, characterization, and monitoring of disease efficacy. This integrated approach is crucial for enhancing the quality of medical care and facilitating patient recovery.16,211,212 The synergistic application of PTT and imaging techniques is shown in Figure 1.
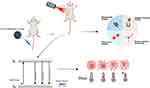 |
Figure 1 Schematic diagram of PTT-imaging theranostics (Created in BioRender. shang, y. (2025) https://BioRender.com/dbx88p9). |
Photoacoustic Imaging
Photoacoustic Imaging (PAI) represents a new-fangled biomedical imaging technique. It capitalizes on the photoacoustic effect, integrating the high-contrast feature of optical imaging with the deep-tissue-penetrating ability of ultrasound imaging. In PAI, a brief laser pulse irradiates biological tissue, inducing light absorption and subsequent thermoelastic expansion. This expansion generates ultrasound waves (ie, photoacoustic signals).213,214 These signals are detected by ultrasound transducers, and subsequent signal processing and image reconstruction algorithms generate images that reflect the optical absorption properties of the tissue. PAI exhibits high resolution,215 exceptional contrast, and non-invasive nature, with applications in diverse fields such as tumor detection, vascular imaging, and brain function mapping. As a non-ionizing imaging technique, PAI surpasses traditional optical imaging by mitigating the significant light scattering that limits deep tissue penetration, as acoustic waves experience far less scattering.216 PAI represents an innovative imaging technique achieving deep tissue penetration while maintaining high spatial resolution,217 demonstrating significant potential for real-time, in vivo monitoring of biological processes. To enhance therapeutic efficacy, minimize side effects, and reduce the risk of overdose, accurately setting the therapeutic window and monitoring therapeutic efficacy through imaging and diagnostic tools is crucial. Consequently, the design of PTT photothermolysis agents should adhere to PAI principles,218 aiming to improve overall treatment effectiveness, safety, and precision.211 In the context of physically assisted interferometry, PNP sare widely utilized. Their unique properties trigger substantial light-matter interactions, ensuring that nanoparticle absorption efficiency significantly surpasses that of dye molecules, facilitating efficient photoacoustic signal generation. These signals originate from the activation of endogenous molecules within living cells or the release of exogenous contrast agents.219 The optimal excitation light wavelength220 can be selected based on nanoparticle size, geometry, and constituent components. For instance, Antimony (Sb) semimetallic nanomaterials exhibit high efficiency as PAI and PTT agents.221 Sb Nanorods (Sb NPs) can be synthesized using a mixture of 1-octadecane and oleylamine as a solvent.222 Aqueous dispersions of polyethylene glycolized Sb NPs demonstrate high-intensity light absorption across UV to NIR wavelengths, enabling their function as PTAs for 808 nm NIR light, achieving a PCE of 41% without significant toxicity. In vivo experiments revealed that injecting this reagent into tumors produced a strong photoacoustic signal upon NIR irradiation, confirming its suitability as a NIR PAI reagent. Similar multimodal nanoplatforms based on metallic nanomaterials include AuNCs/Se, comprising gold nanocages (AuNCs) coated with selenium-modified Chitosan Shells (CS) and modified with iRGD for targeted delivery. Fang et al further developed AuNCs/DOX@Se-iRGD by loading the platform with the chemotherapeutic drug DOX, creating a CDT/PTT/PAI multimodal antitumor nanoplatform.223,224
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI), as a non-invasive imaging technique,225 is favored for its ability to perform precise imaging and track various pathological changes, especially in diseases such as cancer. Its principle of operation relies on the phenomenon of relaxation of protons within tissues,226,227 a process significantly influenced by the unique properties of the chemical environment of matter.228 MRI probes are divided into two main types: MRI contrast agents based on T2-weighting and MRI contrast agents based on T1-weighting. In T2-weighted MRI imaging, the contrast agent achieves this effect by modulating the transverse relaxation time of the tissue. Specifically, the contrast agent increases the transverse relaxivity (r2) of the tissue, which results in the region appearing darker on T2-weighted images. This technique facilitates clearer identification and contrast of different tissue characteristics, particularly crucial for the diagnosis and evaluation of certain pathological states. In contrast, T1-weighted MRI contrast agents substantially enhance image brightness by adjusting the longitudinal relaxivity (r1) of the tissue.76 Both contrast agents possess distinct advantages in clinical use and are suitable for different imaging needs and diagnostic goals. Achieving contrast in MRI applications with nanosystems following intravenous injection hinges on their ability to significantly enhance the plasmonic relaxation properties of specific tissues relative to surrounding tissues. This concept also applies to the design of photothermal media within PTT. By integrating MRI contrast agent functionality, PTT achieves highly effective thermal therapy and enables precise image monitoring.229,230 For example, Mo and Qiu et al synthesized polydopamine-manganese dioxide-IR780 iodide, integrating the tumor therapeutic diagnostic function of IR780, the photothermal conversion ability of IR780, and the property of Mn to promote T1-weighting in MRI, and the preparation reduced the biotoxicity of IR780 and enhanced the PTT effect. And the component MnO2 can reverse the hypoxic environment in the tumor environment and facilitate the occurrence of PDT.231
A research team implemented an innovative strategy to load manganese carbonyls at the core of hollow mesoporous copper sulfide nanoparticles, aiming to develop a composite material with both MRI contrast enhancement and photothermal effects. This design not only significantly improved the efficiency of the PTT process but also enabled real-time MRI images to monitor the treatment process, thus supporting more precise and customized treatment strategies.232 He et al reported the preparation of a novel core-shell hollow copper sulfate-metal-organic framework HCuS@MIL-100.233,234 HCuSNPs were first synthesized, and then MIL-100 was coated on the outer layer of HCuSNPs to form HCuS@MIL-100, which showed good photothermal effect under 1064 nm NIR-II light irradiation and excellent biocompatibility, showing strong T2MRI capability. The biomimetic photothermite mentioned above is also widely used in PTT/MRI dual-mode synergy. Zhang et al combined a photothermite gold nanobipyramid (AuNBP) with MRI-capable MnO2 to prepare a core/shell structure (Au@MnO2), and then modified the surface of Au@MnO2 with plasma membranes (PMs) of homologous cancer cells that have targeting capabilities. Au@MnO2@PM (AMP)235 was obtained to achieve tumor targeting. It was experimentally demonstrated that AMP possessed multimodal imaging functions, such as computed tomography, photothermography, and MRI, and showed significant PTT efficacy in both in vivo and in vitro experiments.
Fluorescence Imaging
Fluorescence Imaging (FI) refers to the phenomenon where a fluorescent substance emits light with different colors or intensities when irradiated with light, and this emission ceases quickly upon cessation of irradiation. Artificial design can be carried out for exogenous fluorescent probes to exhibit unique fluorescence in the UV, visible, or NIR spectrum. Furthermore, their fluorescent properties, including excitation and emission wavelengths, are highly sensitive to their local environment (polarity, viscosity, etc.). FI offers several advantages, such as high sensitivity and temporal resolution, low cost, and excellent safety, making it widely used in clinical settings. Some nanoprobes employed in FI exhibit good photothermal conductivity, enabling their use as PTAs in PTT. Through targeted processing and modification, these PTAs can be specifically directed towards certain tumor cells.236 The integration of FI with PTT/PDT can reveal tumor location in real time, which has great potential in early diagnosis and precision treatment of cancer. Moreover, combining FI with drug delivery facilitates the development of dual-function systems for both diagnosis and therapy.237,238 However, conventional FI in the visible and NIR-I regions can be susceptible to interference from tissue scattering and autofluorescence, leading to reduced imaging resolution and high false-positive rates.239 Deeper tissue penetration, lower autofluorescence background, and reduced light absorption and scattering in biological tissues make NIR-II FI a promising solution to these challenges. Integrating NIR-II FI with PTT and PDT can notably enhance the precision of cancer therapeutic diagnostics. Simultaneously, it reduces the harm inflicted on healthy tissues to the greatest extent possible. However, due to the principle of energy conservation, the two energy dissipation modes of PTT (non-radiative decay) and FI (radiative decay) are inherently in conflict.240 Therefore, a primary objective in FI-guided PTT is to develop methods that effectively balance the photothermal effect with fluorescence. IR-780, an anthocyanin dye, exemplifies an agent possessing both fluorescence emission and photothermal conversion properties, facilitating real-time monitoring of tumor sites.241 Although IR-780 offers improved therapeutic efficacy, its high toxicity at elevated doses restricts its further application as a PTT agent. IR-825 exhibits two absorption peaks at 600 nm and 820 nm, a characteristic that is favorable for mitigating this issue. Upon excitation with a 600 nm laser, fluorescence emission occurred at approximately 650 nm with a fluorescence quantum yield (Φ) of 42%.242 Conversely, when stimulated by the 820 nm laser, Φ decreased markedly, while the PCE increased significantly. Dual excitation wavelengths enabled energy dispersion, thereby reducing the degree of energy concentration at a specific wavelength. Furthermore, this approach could partially compensate for the limitations inherent in single-wavelength excitation, leading to a more comprehensive and homogeneous excitation effect. This, in turn, diminished the risk of localized high-energy accumulation and associated toxicity. Leveraging this unique property of IR-825, researchers developed a dual-excitation light-induced therapeutic and diagnostic agent capable of both FI-guided and simultaneous PTT. This innovative agent showed efficient thermal ablation within a mouse breast cancer model.243 Wang, Xu, and their research team have engineered a biomimetic nanodelivery system, designated as M-HMnO2@ICG NP, tailored for multimodal imaging-guided therapy targeting cervical cancer. Within the tumor microenvironment, the degradation of the carrier particles releases indocyanine green (ICG), which facilitates the self-generation of oxygen for PDT and reduces glutathione levels, thereby enhancing therapeutic efficacy through the combined effects of CDT and PDT. Furthermore, ICG contributes to synergistic PDT/PTT. Manganese ions (Mn2+) function as contrast agents for magnetic resonance imaging, while ICG is employed for photothermal and fluorescence imaging. The M-HMnO2@ICG system demonstrates efficient tumor site accumulation, exhibiting promising therapeutic efficacy and biocompatibility. This approach represents an innovative strategy for the synergistic treatment of cervical cancer.244
Raman Spectroscopy
Raman spectroscopy is an imaging technique that relies on the inelastic scattering of incident light caused by molecular vibrations, rotations, and other energy level transitions, enabling “fingerprint” identification.245 The frequency difference between the scattered and incident light corresponds to the energy level differences in molecular vibrations or rotations. By analyzing the frequency and intensity of Raman scattered light, valuable insights into molecular structure and chemical bonding can be obtained. Raman scattering can be utilized for diagnosing and monitoring diseases, such as employing serum Raman spectroscopy to detect the presence of cancer cells in a patient’s body, thereby providing a preliminary diagnosis. Surface-enhanced Raman scattering, which is based on Localized Surface Plasmon Resonance (LSPR), occurs near metal ions and further amplifies the Raman spectra.246 Wang, Sun, et al developed a hyaluronic acid-modified, doxorubicin-loaded gold nanoframework (AuNF). This AuNF exhibits strong absorbance in the near-infrared II region, facilitates photothermal therapy (PTT), and generates a robust photoacoustic signal. The LSPR effect of gold enhances the capabilities of AuNF.247
Synergistic Therapy
When PTT technology is applied within a tissue, its effectiveness may be affected by a decrease in light intensity.248 PTT-treated tumor tissues exhibit inhomogeneous heat distribution and significant hypoxia, hindering complete tumor cell eradication through a single therapeutic approach. Consequently, synergistic therapies112 have emerged as an effective strategy to address these challenges.249 Secondly, a significant advantage of combinatorial therapies lies in their ability to effectively reduce the occurrence of side effects. By combining multiple therapies, the dosage of individual therapies can be reduced without significantly affecting the overall therapeutic efficacy. For example, the heat energy produced by the photothermal effect is capable of augmenting the therapeutic effectiveness of some anti-cancer medications. Furthermore, heat generation induced by NIR radiation can not only cause damage to malignant cells but also significantly enhance the efficacy of other therapeutic modalities, serving as the foundation for the realization of photothermal synergistic therapies. Photothermal synergistic therapy operates through a variety of mechanisms, including but not limited to: photo-controlled drug release, photothermal-enhanced enzyme activation, photothermal-regulated gene expression, photothermal-stimulated immune response, and photothermal-promoted chemical reactions. These mechanisms work synergistically with the aim of optimizing therapeutic effects and enhancing the precision and efficiency of biomedical interventions.250 The synergistic effects of different therapies and PTT are shown in Figure 2. A statistical study was conducted regarding the efficacy of synergistic therapies. Several cases are summarized in Figure 3. This highlights the fact that the efficacy of synergistic therapies is significantly better than that of monotherapies.
 |
Figure 2 Synergy of different therapies with PTT (Created in BioRender. shang, y. (2025) https://BioRender.com/9ze5nbo). |
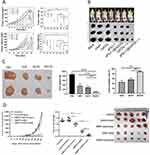 |
Figure 3 Continued. |
 |
Figure 3 Synergistic therapy statistics. (A) The Integrated Impact of Synergistic PTT, IT and CDT Employing PMo@CCM. Reprinted with permission from Advanced Functional Materials. 2024; 34(41):2402692. Ma S, Li D, Jia X, Xu W, Ding G, He J et al. Homologous tumor targeting molybdenum‐doped prussian blue for enhancing immunotherapy via PTT/CDT and remodeled tumor immune microenvironment. Copyright © 2024 John Wiley and Sons.49; (B) The Integrated Impact of Synergistic PTT, PDT, and ICB Employing aPD-L1@GdCDs. Reprinted with permission from Journal of Colloid and Interface Science. 2025; 678:1088–1103. Fan Y, Zhang R, Shi J, Tian F, Zhang Y, Zhang L et al. Mild near-infrared laser-triggered photo-immunotherapy potentiates immune checkpoint blockade via an all-in-one theranostic nanoplatform. Copyright © 2025 with permission from Elsevier.251; (C) The Integrated Impact of Synergistic PTT, Chemotherapy and Ferroptosis Employing MnO2-SOR-Ce6@PDA-PEG-FA. Reprinted with permission from Nanoscale Research Letters. 2022; 17(1). Wang CG, Cheng XL, Peng H, Zhang YW. NIR-Triggered and ROS-Boosted Nanoplatform for Enhanced Chemo/PDT/PTT Synergistic Therapy of Sorafenib in Hepatocellular Carcinoma. Copyright © 2022 Springer Nature. Creative Commons.53; (D) The Integrated Impact of Synergistic PTT, GT and IT Employing GMPF-siIDO. Reprinted with permission from Frontiers in Immunology. 2020; 11:968. Zhang Y, Feng Y, Huang Y, Wang Y, Qiu L, Liu Y et al. Tumor-targeted gene silencing IDO synergizes PTT-induced apoptosis and enhances anti-tumor immunity. Copyright © 2020 Zhang, Feng, Huang, Wang, Qiu, Liu, Peng, Li, Kuang, Shi, Shi, Chen, Joshi, Wang, Yuan and Min. Creative Commons.252; (E) The Integrated Impact of Synergistic PTT, Ferroptosis and IT Employing CMCTNPs@OVA. Reprinted with permission from Materials Today Chemistry. 2023; 27:101308. Fang T, Ma S, Wei Y, Yang J, Zhang J, Shen Q. Catalytic immunotherapy-photothermal therapy combination for melanoma by ferroptosis-activating vaccine based on artificial nanoenzyme. Copyright © 2023 Elsevier.54 Based on the research data presented, it can be concluded that the therapeutic efficacy of the synergistic treatment group is significantly superior to that of the single treatment group. |
Immunotherapy
Immunotherapy (IT)253 is an approach that depends on the patient’s native immune system for combating cancer, demonstrating a higher level of safety and efficacy compared to traditional treatments.254 Immunotherapy operates through four primary mechanisms: enhancing antigen recognition, initiating an immune response, diminishing immune escape, and alleviating immunosuppression. These mechanisms are illustrated in Figure 4. To activate and enhance the effects of IT, a range of strategies have been widely adopted, including but not limited to checkpoint blockade therapy (ICB),255,256 adoptive T-cell transfer, as well as the application of emerging technologies such as chimeric antigen receptor T-cell (CAR-T)257 therapy and cancer vaccines.258–261 The combined application of PTT and IT has demonstrated remarkable potential and effectiveness in the field of cancer therapy.262 Through this combination, PTT not only directly destroys tumor cells. It also releases specific tumor antigens in situ within them, thereby activating the body’s specific anti-tumor immune response against these antigens.263,264 This process enables patients to rely on their own immune system to eliminate diffuse lesions that are not covered by laser irradiation, effectively preventing tumor recurrence. Meanwhile, as an immune adjuvant, PTAs effectively promotes the activation of the immune system and further enhances the ability to recognize and attack tumors. This synergistic effect not only improves treatment efficiency but also significantly enhances the safety of the treatment and the long-term survival rate of patients. A team of researchers designed an innovative microneedle delivery system that incorporated sulfhydryl-modified Au nanorods with the immune checkpoint inhibitor anti-PD-1 peptide, aiming to achieve precise drug release and enhance immune response.265,266 With its superior mechanical properties, the microneedle system enabled accurate transdermal drug delivery. Upon insertion into human skin, it released anti-PD-1 peptides and gold nanorods. These components enhanced the cytotoxic T-lymphocyte-mediated destruction of tumor cells. When exposed to NIR radiation, the system generated heat, facilitating PTT for tumor elimination. Notably, this microneedle delivery system significantly enhanced the immune response and prolonged the survival of mice. Bionic-type nano-delivery systems have also demonstrated significant potential in IT synergistic with PTT. Ma and Li et al49 constructed a Prussian blue variant of PMo@CCM doped with Molybdenum (Mo) and coated with cancer cell membranes (CCMs). This system could activate the immune system through a combination of PTT and CDT, inducing immunogenic cell death and promoting antigen presentation.267 Modification with anti-programmed cell death Protein-1 (PD-1) could effectively modulate the tumor immunosuppressive microenvironment, further enhancing the efficacy of IT. Building upon ICB strategies, Fan, Zhang, et al designed and synthesized anti-PD-L1-modified gadolinium-doped carbon dots (aPD-L1@GdCDs), which integrate PTT, PDT, and ICB. This composite material exhibits potential for multimodal imaging applications. In vivo experimental results demonstrated that aPD-L1@GdCDs significantly enhance immune cell activity and exhibit pronounced cytotoxic effects against tumor cells, while causing no discernible damage to normal tissues. The biosafety of this system has been successfully validated.251
 |
Figure 4 The main process of immunotherapy. Reprinted with permission from Signal Transduction and Targeted Therapy. 2023; 8(1):306. Tang, L, Huang, Z, Mei, H. et al. Immunotherapy in hematologic malignancies: achievements, challenges and future prospects. Copyright © 2023 Springer Nature. Creative Commons.253 |
Photodynamic Therapy
Photodynamic therapy (PDT) is a treatment approach that makes use of photosensitizers and lasers to selectively destroy cancer cells.268 It is a localized therapy where ROS production is induced by the photosensitizer. The Photosensitizer (PS), enriched at the tumor site, is excited to an excited state by laser irradiation. PDT comprises three core elements: light, PS, and O2.269 When activated at specific NIR wavelengths, the presence of PS leads to oxygen depletion, which in turn triggers a large amount of ROS. These highly oxidative species effectively destroy tumor cells and inhibit their further proliferation. During the PDT process, the photosensitizer is first incorporated into the target area, followed by exposuring to light of a particular wavelength that matches the photosensitizer’s absorption spectrum. After irradiation, two types of redox reactions, type I and type II, are initiated.270 Type I ROS generation mainly involves electron transfer within the PS, producing superoxide anion radicals (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•−).271 Type II ROS primarily consists of singlet oxygen (1O2) generated via energy transfer from oxygen.268 Several studies have demonstrated that OH•− exhibit the highest cytotoxicity against cancer among free radical oxides. The core process involves photons exciting PS from its ground state to a singlet excited state (1PS·). This singlet state undergoes intersystem crossing to a triplet state (3PS·), which then either interacts with an oxygen molecule to generate 1O2 or reacts with substances in the body to generate free radicals, including H2O2 and O2−, which triggers the generation of ROS. The basic mechanism of Type I and Type II photodynamic therapy is shown in the following Figure 5. ROS have a vital part in the redox control of ion channels, transcriptional regulators, and protein phosphorylation processes. When maintaining steady levels, ROS are able to function as messengers for the regulation of physiological activities.272,273 However, excess ROS can have toxic effects,274 causing to cell death and tissue dysfunction.275 Scientists have been inspired by this effect to utilize ROS for cancer cell destruction. Many compounds, such as arsenic oxides, platinum drugs, and paclitaxel, exert their anticancer effects by promoting cellular ROS production.276 Both PTT and PDT are advanced non-invasive medical treatments that play an important role in the treatment of cancer and other diseases.16,277 Combining these technologies not only significantly enhanced the photothermal enhancement effect of PDT but also triggered synergistic mechanisms. This strategy promoted blood circulation in the tumor region, increasing the local oxygen supply and significantly enhancing PDT’s therapeutic efficacy. This integrated application not only expands the range of therapeutic tools but also provides a new way to improve the success rate of cancer treatment.
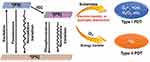 |
Figure 5 Schematic illustration for the basic process of type I and type II PDT. Reprinted with permission from Small. 2021; 17(31):2006742. Dapeng Chen, Qian Xu, Wenjun Wang et al. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Copyright © 2021 John Wiley and Sons.278 |
Zhang and Ma et al developed multifunctional metal-organic nanocomposite nanoparticles—Fe-TCPP@CS NPs—whose excellent properties arise from the utilization of chitosan (CS) as the outer layer. This successful integration of chitosan facilitated a triple therapeutic approach: targeted delivery, PDT, and PTT, demonstrating significant potential for biomedical applications. A stable Fe-TCPP core structure was achieved through the formation of a coordination complex between Tetrakis (4-carboxyphenyl) porphyrin (TCPP) and Fe3+ ions. This core not only provided the basis for low-temperature photothermal therapeutic technology but also enhanced efficient 1O2 generation capacity by reducing spontaneous aggregation of porphyrin molecules. Upon light irradiation, these nanoparticles exhibited excellent ROS generation capacity and PCE, synergistically achieving highly efficient inhibition and eradication of a wide range of bacteria, such as Staphylococcus aureus and Escherichia coli. This groundbreaking achievement not only validated the remarkable potential of Fe-TCPP@CS NPs as a highly effective and comprehensive antimicrobial agent but also established a novel and innovative approach to antimicrobial therapy. And the researchers evaluated their biosafety by cytocompatibility and hemolytic activity, which proved that Fe-TCPP@CS NPs have low toxicity to normal cells without laser irradiation, and the percentage of cytolysis is negligible.279
There are also many cases in the fight against cancer, such as Au@MOF, have been developed. Cai and Zhao et al took advantage of the carboxylic acid groups present on the surface of gold nanorods (AuNRs) to fabricate Au@MOF core-shell hybrids via a step-by-step metal-porphyrin metal self-assembly process.280 In Au@MOF, AuNRs facilitate photothermal conversion, while metal nodes, such as Fe3O(OAc)6(H2O)3+ clusters within the MOF, catalyze the decomposition of H2O2 into O2 to modulate the hypoxic tumor environment. Furthermore, porphyrin ligands convert O2− into 1O2 under light conditions, synergistically promoting PDT.280 Experimental data confirmed that Au@MOF exhibited negligible biotoxicity and excellent biocompatibility, effectively killing cancer cells and significantly inhibiting tumor growth. Moreover, the surface of Au@MOF could be further modified, such as with folic acid, to enhance tumor targeting, thereby reducing toxicity and side effects. PDT/PTT bimodal nanoparticles were also synthesized without the use of metal nodes, relying solely on organic components. Sha and Deng et al synthesized BDP-BTNPs by modifying the benzene ring of Boron-dipyrrolidinylmethylene (BODIPY) with poly ethylene glycol (PEG) chains to improve biocompatibility and hydrophilicity. Subsequently, a Donor-acceptor-donor (D-A-D) structured molecule, BDP-BT, was synthesized employing BODIPY as the donor and benzothiadiazole as the acceptor. Amphiphilic BDP-BT spontaneously formed corresponding nanoparticles (BDP-BTNPs) through π-π stacking and hydrophilic interactions. The results demonstrated that BDP-BTNPs effectively generated ROS and ablated tumor cells through local thermotherapy.281
Chemotherapy
Chemotherapy282 usually combines one or more adjuvant drugs to eradicate cancer cells, with these drugs distributed across various tissues and organs to achieve systemic therapy. However, chemotherapy faces several limitations,283 including the accelerated development of drug resistance, non-selective systemic distribution of antitumor drugs, inadequate drug concentration at the target site, and significant toxic side effects, which together result in serious therapeutic challenges including interference with normal cell proliferation and damage to healthy tissues and organs.
The integration of PTT and chemotherapy has shown a synergistic impact,284 significantly enhancing the control of tumor metastasis, leading to more precisely targeted therapeutic strategies while reducing the harm inflicted on healthy tissues. Recent research has focused on the design of innovative nanosystems aimed at improving the productivity and meticulousness of therapeutic agent delivery. For instance, researchers have developed a photoresponsive drug carrier composed of amorphous carbon-coated rGO sheets with mesoporous silica, exhibiting a unique nanocookie structure.285 This design endowed the carrier with a photothermal effect, enabling it to effectively release the drug upon NIR light irradiation while generating significant heat, thereby introducing an efficient therapeutic strategy for drug delivery. With its superior biocompatibility and efficient cellular uptake, the material achieved effective clearance of subcutaneous tumors within fourteen days after five minutes of NIR light irradiation without causing distant tissue damage, demonstrating its excellent potential as a platform for chemotherapy and PTT co-administration. For chemotherapeutic agents such as Sorafenib (SOR), a first-line therapeutic agent for advanced hepatocellular carcinoma (HCC), the effectiveness is frequently restricted by drug resistance and adverse effects. To address this, Zeng and Zhang et al constructed a multifunctional nano-platform, MnO2-SOR-Ce6@PDA-PEG-FA (MSCPF), which improved the targeting and efficacy of SOR.53 The synthesis involved loading SOR into MnO2 NPs, followed by the sequential addition of chlorine 6 (Ce6), PDA, and HS-PEG-FA to form MSCPF. This platform utilized MnO2 as a photothermal agent and Ce6 as a photosensitizer, enabling it to generate excess ROS to alleviate tumor hypoxia, enhance SOR accumulation in tumor cells, and induce ferroptosis through the inactivation of Glutathione Peroxidase 4 (GPX4) and Solute Carrier Family 7 Member 11 (SLC7A11), as well as the inhibition of P-glycoprotein expression. These synergistic pathways significantly enhanced the antitumor efficacy of SOR. The experimental results (shown in Figure 5) indicated that the MSCPF group of the synergistic treatment strategy was significantly better than the SOR group. Similarly, Yang and Zhang et al developed a calcium carbonate-based pH and NIR dual-responsive nanoparticle, DOX-CuS@CaCO3@PL-PEG (DCCP), which co-loaded DOX and CuS nanoparticles into CaCO3 and modified the surface with PEG. DCCP enabled the combined application of PTT, CDT, chemotherapy, and Ca2+ overloading, offering controlled drug release and improved chemotherapy efficiency.102
Radiotherapy
Radiotherapy (RT)286 plays a pivotal role in clinical oncology, its mechanism relying on the generation of localized ionizing radiation287 to eradicate cancer cells.288 This modality is indispensable for patients with surgically inoperable tumors, those with incomplete initial resection, and those experiencing disease recurrence.289 Notably, RT trigger immunogenic cell death under specific circumstances, thereby stimulating systemic immunotherapeutic responses. In addition, local irradiation not only eradicates the treated tumor but also exerts significant therapeutic effects beyond the irradiated site.290 However, the efficacy of RT is significantly hindered by several factors: inherent tumor radioresistance, hypoxic tumor microenvironments, and substantial systemic and local toxicities. Consequently, there is a pressing necessity to create novel therapeutic strategies that reduce harm to normal tissues to the greatest extent possible while optimizing antitumor impacts. Analogous to the working principle of PDT, the efficacy of radiation therapy is significantly dependent on the oxygen concentration within the tumor. Fortunately, scientists have innovatively researched Oxygen-Independent Radiodynamic Therapy, which combining radiation therapy with novel sensitizers.291 Oxygen-Independent Radiodynamic Therapy overcomes the limitations of conventional RT and PDT.
Elements such as gold and Bismuth (Bi) exhibit potential as radiosensitizers.292,293 Yu and Li et al synthesized ultramicrogranular Bismuth Nanoparticles (BiNPs) for combined PTT and radiation therapy. These BiNPs were prepared by reducing Bi ions to BiNPs through heating oleylamine in a nitrogen atmosphere. These nanoparticles were then transferred to aqueous media by encapsulating them with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)] (DSPE-PEG) and further functionalized with a specific peptide chain (CGNKRTRGC, ie, LyP-1) to enable targeted delivery to tumor sites. Upon irradiation with a 1064 nm laser, the BiNPs exhibited strong light absorption, leading to efficient photothermal conversion and generating heat. In addition, BiNPs acted as radiosensitizers, enhancing the efficacy of RT. The study demonstrated that while individual therapeutic strategies only temporarily delayed tumor growth, the combination of PTT and RT effectively halted tumor progression.294 In another study, Hu and Tian et al synthesized AuNCs embedded within a PDA matrix and modified with Arg-Gly-Asp (RGD) peptides for targeted PTT/RT of non-small cell lung cancer (NSCLC). AuNCs were synthesized by reducing HAuCl4 with dopamine, followed by PDA coating to form a protective shell. Subsequent modification with RGD peptides imparted tumor-targeting capabilities to the AuNC/PDA-RGD nanostructures, enhancing their cellular uptake. These nanostructures exhibited excellent colloidal stability, low cytotoxicity, and synergistic therapeutic efficacy when combined with PTT and RT compared to monotherapy.295
Gene Therapy
Gene Therapy (GT) has demonstrated significant promise in the treatment of numerous disorders through the down-regulation of the expression of particular genes.296 The advancement of gene vectors has substantially promoted the extensive clinical utilization of nucleic acid substances (mRNA, siRNA, etc.).297,298 Both GT and PTT, as effective complements to conventional chemotherapy and RT, have shown great potential with their non-invasive nature and reduced side effects.299,300 However, the therapeutic efficacy of monotherapies is limited. GT often exhibits slow response rates and is constrained by rapid enzymatic degradation and low intracellular uptake. In contrast, PTT is mainly limited to the ablative treatment of localized tumors. Among the approaches integrating photothermal and physical tumor therapy, the fusion of photothermal gene nanocarriers shows significant advantages. These nanocarriers act as effective gene delivery vehicles, forming stable bonds with negatively charged gene fragments, thereby enhancing the stable accumulation of genes in serum and tumor sites. GT promotes the efficacy of PTT by enhancing the cellular sensitivity to heat, which in turn provides GT performance enhancement by optimizing the process of gene transfer, deconvolution, and expression. This integrated approach presents a unique opportunity for cancer therapy, characterized by rapid response and high efficacy.
Electrostatic attraction was employed to integrate heat shock protein 70 (HSP70)-regulated genes into Ferrocene-based dendritic polymers (DSPs), resulting in the formation of stable nanocomplexes.297 Upon NIR light irradiation, DSPs induced localized hyperthermia, promoting the conversion of heat shock factors from their monomeric to trimeric state. Subsequently, these trimers made their way to the nucleus, attached themselves to heat shock elements, and initiated the transcription of target genes.301 The transgene expression capacity under NIR activation was evaluated in HeLa cell lines by employing enhanced green fluorescent protein with luciferase as a biomarker. NIR laser irradiation significantly enhanced gene transcription and expression, as evidenced by increased fluorescence signal intensity. Notably, while Zhao et al reported nanoplatforms utilizing RNA-based regulation, other studies have explored the use of Peptide Lipid (PL) and Sucrose Laurate (SL)-coated SCNTs and MCNTs as bifunctional transport systems (SCNT-PS and MCNT-PS) with excellent temperature sensitivity and photothermal properties.52 These CNT/siRNA systems could inhibit tumor growth by silencing survivin expression and exerting a photothermal effect upon NIR irradiation, with SCNT-PS/siRNA demonstrating high anti-tumor efficacy. Similarly, Zhang and Feng et al developed GMPF-siIDO,252 a gold nanorod (GNR)-based platform modified with tumor-targeting FA and Indoleamine-2,3-dioxygenase (IDO)-specific siRNA. This system combined PTT with GT and IT, enabling tumor cell ablation, silencing of IDO gene expression, and enhancement of the body’s immune response, thereby significantly improving therapeutic efficacy.
Ferroptosis Therapy
Iron, an essential element for human life, plays a crucial role in numerous physiological processes. However, abnormal iron metabolism can lead to disturbances in these activities. Ferroptosis is a regulated cell death process characterized by Fe2+-catalyzed lipid peroxidation, resulting in elevated levels of lipid peroxides302 and iron overload.303 This process leads to increased ROS production and oxidative stress, ultimately causing cell damage and death.304 Genes involved in iron and lipid metabolism can regulate ferroptosis by regulating iron overload and lipid peroxidation through direct or indirect pathways.305 Glutathione (GSH), a key antioxidant, plays a crucial role in combating lipid peroxidation.306,307 As a cofactor for GPX4, GSH facilitates the conversion of toxic lipid peroxides to less harmful alcohols.308,309 While not directly involved in lipid peroxide reduction, GPX4 plays a vital role in inhibiting lipid peroxidation by maintaining the reduced state of selenocysteine residues within its active site.310,311 Cysteine, a rate-limiting precursor for GSH synthesis, requires extracellular cystine uptake for intracellular cysteine production. SLC7A11, a cystine transporter protein, thus plays a crucial role in ferroptosis.312,313 Studies have shown that multiple genes and drugs can regulate ferroptosis by targeting GPX4, GSH, or SLC7A11.314 Morphological features of ferroptotic cells include aberrant mitochondria with reduced or lost cristae and membrane condensation. Mechanistically, ferroptosis differs from classical cell death pathways, often relying on oxidized phospholipids containing polyunsaturated fatty acids. Notably, mesenchymal and dedifferentiated cancer cells, which are frequently resistant to apoptosis and traditional therapies, display a high level of susceptibility to ferroptosis. This highlights the potential of ferroptosis as a promising therapeutic approach, especially for cancers that are difficult to treat. Researchers fabricated multifunctional PDA-coated Manganese Sulfide (MnS) nanoclusters. The polyhydroxyl structure of PDA facilitated enhanced hydration of pH-responsive MnS nanoclusters through hydrogen bonding, significantly increasing the spin-lattice relaxation rate from 5.76 to 19.33 mM−1·s−1 at pH 5.5, thereby improving their efficacy as MRI contrast agents for tumor visualization.169 In addition, PDA conferred excellent biocompatibility and PCE to the MnS nanoclusters for PTT. Notably, the acidic tumor microenvironment triggered the release of H2S from MnS nanoclusters, efficiently suppressing mitochondrial respiration and the generation of ATP. This consequently downregulated the expression of heat shock proteins, mitigating tumor cell resistance to photothermal stimulation and enhancing PTT efficacy. Concurrently, the liberated Mn2+ ions demonstrated strong peroxidase-like and glutathione oxidase-like activities, synergistically provoking ferroptosis and apoptosis in tumor cells. These findings demonstrate the potential of this multifunctional nano-platform for synergistic MRI-guided ferroptosis-mediated PTT in cancer therapy. These findings demonstrate the potential of combining ferroptosis therapy with PTT as a novel approach for addressing refractory cancers. Deng and Zhang et al315 developed a nanoplatform (MP@PI) that exemplified this synergistic strategy. MP@PI, composed of MOFs modified with PDA and IR820 and loaded with Piperonyl Longumine (PL), leveraged MOFs as an iron source and PL as a source of H₂O₂ to induce ferroptosis. Concurrently, the iron source contributed to pyroptosis. PDA, responding to pH changes and depleting GSH, reduced GPX4 levels, further promoting ferroptosis. The photosensitizer IR820 generated heat upon NIR light irradiation, enhancing both ferroptosis and pyroptosis. In addition, MP@PI stimulated immune responses, augmenting anti-tumor efficacy. Similarly, Fang and Ma et al54 synthesized CMCTNPs@OVA, a catalytic immunotherapeutic ferroptosis-activated vaccine, which combined ferroptosis, IT, and PTT. This platform utilized copper telluride nanoparticles, mimicking peroxidase to deplete GPX4, and incorporates Ovalbumin (OVA) as an exogenous antigen. The photothermal effect of copper telluride enhanced PTT efficacy, while Cu2+ released initiates ferroptosis. Furthermore, ferroptosis, in conjunction with PTT, induced ICD, emanating damage-related molecular patterns to facilitate dendritic cell maturation and the recruitment of T-cells. The results demonstrated the efficacy of CM CTNPs@OVA in treating melanoma, highlighting the potential of this combined strategy for combating cancer. The researchers verified that CM CTNPs@OVA did not induce any abnormal damage to normal tissues, and its biosafety was confirmed through the examination of pathological tissue sections.
Summary and Outlook
This review comprehensively examines PTT, encompassing its underlying mechanism, the developmental landscape of PTAs, and the advancements in PTT-based diagnostic and therapeutic strategies. Research on PTAs, including metal-based, carbon-based, and organic-based materials, alongside their diverse nanoformulations, are introduced. Recognizing PTAs as the cornerstone of PTT, the rational design of these agents leverages the inherent advantages of the nanomedicine,316 facilitating enhanced diagnosis and treatment. This includes advancements in FI, PAI, and MRI-guided PTT, enabling real-time tumor visualization during surgery and paving the way for complete tumor eradication. Furthermore, synergistic approaches combining PTT with chemotherapy, RT, PDT, gene therapy, and ferroptosis therapy are explored. These combinatorial strategies aim to capitalize on the complementary strengths of each modality, thereby improving therapeutic efficacy, enhancing patient quality of life, and demonstrating promising clinical translation potential. While preclinical and clinical studies have shown encouraging results in treating breast, lung, and prostate cancers, further optimization of PTAs remains crucial. Photothermal therapy still faces difficulties in clinical translation and is not conducive to deeper levels of treatment. And developing nanoformulations with superior PCE, facile synthesis and application, and compatibility with synergistic therapies is essential. Moreover, innovative combined treatment strategies are necessary to address current clinical limitations and ultimately achieve the goal of effective tumor eradication. Future advancements in photothermal therapy research should establish systematic integration with existing scientific foundations, with prioritized investigation focusing on three pivotal aspects: (1) innovative development of photothermal conversion agents, (2) synergistic therapeutic modalities combining multiple treatment approaches, and (3) real-time imaging-guided intervention systems. Crucially, greater emphasis must be placed on accelerating clinical translation through standardized preclinical evaluation protocols and multidisciplinary collaboration, thereby expediting practical applications to advance cancer treatment paradigms.
Author Contributions
All authors have made significant contributions to the reported work, whether in conceptualization, execution, literature review, statistical analysis, data interpretation, writing, or any combination of these elements. All authors participated in drafting, revising, or critically reviewing the article and gave final approval of the version to be published. All authors have agreed on the journal to which the article will be submitted and agreed to accept responsibility for all aspects of the work.
Funding
This study was supported by Natural Science Foundation of Hunan Province (No. 2024JJ6079, 2025JJ80119), the Excellent Youth Project of Hunan Provincial Department of Education (No. 23B0362), the General Subjects of Hunan Provincial Administration of Traditional Chinese Medicine (No. B2024015), the Key Discipline Project on Chinese Pharmacology of Hunan University of Chinese Medicine [202302], the Hunan Innovation and Entrepreneurship Training Program for College Students(S202410541076).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Filella X, Rodríguez-Garcia M, Fernández-Galán E. Clinical usefulness of circulating tumor markers. Clin Chem Lab Med. 2023;61(5):895–905. doi:10.1515/cclm-2022-1090
2. Yang P, Yang W, Wei Z, Li Y, Yang Y, Wang J. Novel targets for gastric cancer: the tumor microenvironment (TME), N6-methyladenosine (m6A), pyroptosis, autophagy, ferroptosis and cuproptosis. Biomed Pharmacother. 2023;163:114883. doi:10.1016/j.biopha.2023.114883
3. Zhuang Y, Zhao YY, Wang BY, Wang Q, Cai TG, Cai Y. Strategies for preparing different types of lipid polymer hybrid nanoparticles in targeted tumor therapy. Current Pharmaceutical Design. 2021;27(19):2274–2288. doi:10.2174/1381612826666201120155558
4. Yin XJ, He ZQ, Ge WY, Zhao ZH. Application of aptamer functionalized nanomaterials in targeting therapeutics of typical tumors. Front Bioeng Biotechnol. 2023;11:1092901.
5. Kumar V, Rahman M, Gahtori P, Al-Abbasi F, Anwar F, Kim HS. Current status and future directions of hepatocellular carcinoma-targeted nanoparticles and nanomedicine. Expert Opinion Drug Deliv. 2021;18(6):673–694. doi:10.1080/17425247.2021.1860939
6. Han MM, Fan YK, Zhang Y, Dong ZQ. Advances in herbal polysaccharides-based nano-drug delivery systems for cancer immunotherapy. J Drug Target. 2024;32(3):311–324. doi:10.1080/1061186X.2024.2309661
7. Yuan Y, Sun W, Xie J, et al. RNA nanotherapeutics for hepatocellular carcinoma treatment. Theranostics. 2025;15(3):965. doi:10.7150/thno.102964
8. Xu LN, Xu M, Sun X, et al. Quantitative comparison of gold nanoparticle delivery via the enhanced permeation and retention (EPR) effect and mesenchymal stem cell (MSC)-based targeting. Acs Nano. 2023;17(3):2039–2052. doi:10.1021/acsnano.2c07295
9. Hasannia M, Tabasi H, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M. Theranostic gold nanoparticles mediated drug delivery. Theranostics Nanomat Drug Deliv. 2025;2025:211–229.
10. Zhang HY, Wang XY, Zhang YD, et al. Hyaluronic acid modified indocyanine green nanoparticles: a novel targeted strategy for NIR-II fluorescence lymphatic imaging. Front Chem. 2024;12:1435627.
11. Yuen R, West FG, Wuest F. Dual probes for positron emission tomography (PET) and fluorescence imaging (FI) of cancer. Pharmaceutics. 2022;14(3):645. doi:10.3390/pharmaceutics14030645
12. Li QY, Liu YL, Huang ZH, Guo YJ, Li QJ. Triggering immune system with nanomaterials for cancer immunotherapy. Front Bioeng Biotechnol. 2022;10:878524.
13. Jiang N, Yu Y, Wu DW, et al. HLA and tumour immunology: immune escape, immunotherapy and immune-related adverse events. J Cancer Res Clin Oncol. 2023;149(2):737–747. doi:10.1007/s00432-022-04493-1
14. Taefehshokr S, Parhizkar A, Hayati S, et al. Cancer immunotherapy: challenges and limitations. Pathol Res Pract. 2022;229:153723.
15. Li ZT, Jiang LL, Zhao R, et al. MiRNA-based model for predicting the TMB level in colon adenocarcinoma based on a LASSO logistic regression method. Medicine. 2021;100(21):e26068.
16. Cuadrado CF, Lagos KJ, Stringasci MD, Bagnato VS, Romero MP. Clinical and pre-clinical advances in the PDT/PTT strategy for diagnosis and treatment of cancer. Photodiag Photodyn Ther. 2024;50:104387.
17. Huang SD, Ye YN, Jiang CY, et al. Current and promising applications of MOFs loaded with PTAs on photothermal therapy. Reactive Funct Polymers. 2023;193:10743.
18. Gadeval A, Chaudhari S, Bollampally SP, et al. Integrated nanomaterials for non-invasive photothermal therapy of rheumatoid arthritis. Drug Discov Today. 2021;26(10):2315–2328. doi:10.1016/j.drudis.2021.04.026
19. Chen Y, Gao Y, Chen Y, Liu L, Mo A, Peng Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J Control Release. 2020;328:251–262. doi:10.1016/j.jconrel.2020.08.055
20. Xu P, Liang F. Nanomaterial-based tumor photothermal immunotherapy. Int J Nanomed. 2020;15:9159–9180. doi:10.2147/IJN.S249252
21. Zhang Y, Zhang S, Zhang Z, et al. Recent progress on NIR-II photothermal therapy. Front Chem. 2021;9:728066. doi:10.3389/fchem.2021.728066
22. Liu S, Li Y, Kwok RT, Lam JW, Tang BZ. Structural and process controls of AIEgens for NIR-II theranostics. Chem Sci. 2021;12(10):3427–3436. doi:10.1039/D0SC02911D
23. Li J, Zhang Q, Chen Z, Guo S, Guo J, Yan F. Postsynthetic modification of thermo-treated metal–organic framework for combined photothermal/photodynamic antibacterial therapy. ACS Appl Mat Interfaces. 2024;16(7):8459–8473. doi:10.1021/acsami.3c17955
24. Chu Y, Xu XQ, Wang Y. Ultradeep photothermal therapy strategies. J Phys Chem Lett. 2022;13(41):9564–9572. doi:10.1021/acs.jpclett.2c02642
25. Shinn J, Lee S, Lee HK, et al. Recent progress in development and applications of second near-infrared (NIR-II) nanoprobes. Arch Pharm Res. 2021;44(2):165–181. doi:10.1007/s12272-021-01313-x
26. Yu H, Wang Y, Chen Y, et al. Transmissible H-aggregated NIR-II fluorophore to the tumor cell membrane for enhanced PTT and synergistic therapy of cancer. Nano Converg. 2023;10(1):3. doi:10.1186/s40580-022-00352-4
27. Xu L, Yu Y, Li M, et al. Pyrene‐based emitter with a small energy gap between high‐energy triplet and singlet state for high‐performance blue organic light‐emitting diodes. Adv Opt Mat. 2024;12(29):2401275. doi:10.1002/adom.202401275
28. Fang L, Chen Z, Dai J, et al. Recent advances in strategies to enhance photodynamic and photothermal therapy performance of single-component organic phototherapeutic agents. Adv Sci. 2025;12(7):e2409157. doi:10.1002/advs.202409157
29. D’Acunto M, Cioni P, Gabellieri E, Presciuttini G. Exploiting gold nanoparticles for diagnosis and cancer treatments. Nanotechnology. 2021;32(19):192001. doi:10.1088/1361-6528/abe1ed
30. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi:10.1038/s41571-020-0410-2
31. Weng X-L, Liu J-Y. Strategies for maximizing photothermal conversion efficiency based on organic dyes. Drug Discovery Today. 2021;26(8):2045–2052. doi:10.1016/j.drudis.2021.03.009
32. Li J, Wang J, Zhang J, et al. A facile strategy of boosting photothermal conversion efficiency through state transformation for cancer therapy. Adv Mat. 2021;33(51):2105999. doi:10.1002/adma.202105999
33. Fan F, Hou Y, Zhang Y, et al. Tumor imaging and photothermal therapy in second near infrared window: a systematic review and meta-analysis. Front Oncol. 2022;12:987491. doi:10.3389/fonc.2022.987491
34. Oudjedi F, Kirk AG. Near‐infrared nanoparticle‐mediated photothermal cancer therapy: a comprehensive review of advances in monitoring and controlling thermal effects for effective cancer treatment. Nano Select. 2024;6:e202400107.
35. Zhang L, Liu Y, Huang H, et al. Multifunctional nanotheranostics for near infrared optical imaging-guided treatment of brain tumors. Adv Drug Deliv Rev. 2022;190:114536. doi:10.1016/j.addr.2022.114536
36. Huang X, Lu Y, Guo M, Du S, Han N. Recent strategies for nano-based PTT combined with immunotherapy: from a biomaterial point of view. Theranostics. 2021;11(15):7546. doi:10.7150/thno.56482
37. Ota Y, Inagaki R, Takanashi Y, et al. Targeting tumor-associated macrophages with the immune-activating nanomedicine for achieving strong antitumor activity with rapid clearance from the body. ACS Nano. 2024;18(34):23757–23772. doi:10.1021/acsnano.4c08811
38. Biswas S, Torchilin VP. Nanopreparations for organelle-specific delivery in cancer. Adv Drug Deliv Rev. 2014;66:26–41. doi:10.1016/j.addr.2013.11.004
39. Zou XW, Ma GQ, Zhu PY, et al. A polydopamine-coated platinum nanoplatform for tumor-targeted photothermal ablation and migration inhibition. Front Oncol. 2022;12:860718.
40. Becker A, Leskau M, Schlingmann-Molina BL, et al. Functionalization of gold-nanoparticles by the Clostridium perfringens enterotoxin C-terminus for tumor cell ablation using the gold nanoparticle-mediated laser perforation technique. Sci Rep. 2018;8(1):14963. doi:10.1038/s41598-018-33392-0
41. Shi H, Yan RQ, Wu LY, et al. Tumor-targeting CuS nanoparticles for multimodal imaging and guided photothermal therapy of lymph node metastasis. Acta Biomaterialia. 2018;72:256–265. doi:10.1016/j.actbio.2018.03.035
42. Lu Z, Huang FY, Cao R, et al. Long blood residence and large tumor uptake of ruthenium sulfide nanoclusters for highly efficient cancer photothermal therapy. Sci Rep. 2017;7:41571. doi:10.1038/srep41571
43. Piao JG, Wang LM, Gao F, You YZ, Xiong YJ, Yang LH. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. Acs Nano. 2014;8(10):10414–10425. doi:10.1021/nn503779d
44. Liu D, Wu H, Kong X, et al. Biomimetic layered molybdenum oxide nanosheets with excellent photothermal property combined with chemotherapy for enhancing anti-tumor immunity. Appl Mat Today. 2024;36:102027. doi:10.1016/j.apmt.2023.102027
45. Ye HY, Zhang L, Qiu XN, et al. Construction of mannose-grafting Fe-doped carbon dots for CpG delivery and photothermal ablation of tumor. J Nanopart Res. 2024;26(2):25.
46. Gao A, Pei L, Liu G, Chen Y, Zhang A, Cui D. UV-assisted synthesis of ultra-small GO–Austar for efficient PTT therapeutic architectonic construction. RSC Adv. 2024;14(15):10714–10725. doi:10.1039/D4RA00742E
47. Kim TE, Jang HJ, Park SW, et al. Folic acid functionalized carbon dot/polypyrrole nanoparticles for specific bioimaging and photothermal therapy. ACS Appl Bio Mat. 2021;4(4):3453–3461. doi:10.1021/acsabm.1c00018
48. Chen X, Zhang M, Li S, et al. Facile synthesis of polypyrrole@ metal–organic framework core–shell nanocomposites for dual-mode imaging and synergistic chemo-photothermal therapy of cancer cells. J Mat Chem B. 2017;5(9):1772–1778. doi:10.1039/C6TB03218D
49. Ma S, Li D, Jia X, et al. Homologous tumor targeting molybdenum‐doped prussian blue for enhancing immunotherapy via PTT/CDT and remodeled tumor immune microenvironment. Adv Funct Mat. 2024;34(41):2402692. doi:10.1002/adfm.202402692
50. Hao Y, Liu TL, Zhou H, Peng JR, Li K, Chen YW. The GSH responsive indocyanine green loaded PD-1 inhibitory polypeptide AUNP12 modified MOF nanoparticles for photothermal and immunotherapy of melanoma. Front Bioeng Biotechnol. 2023;11:1294074.
51. Luo LH, Yang J, Zhu CQ, et al. Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J Controlled Release. 2018;278:87–99. doi:10.1016/j.jconrel.2018.04.002
52. Zhao YN, Zhao TY, Cao YN, et al. Temperature-sensitive lipid-coated carbon nanotubes for synergistic photothermal therapy and gene therapy. Acs Nano. 2021;15(4):6517–6529. doi:10.1021/acsnano.0c08790
53. Wang CG, Cheng XL, Peng H, Zhang YW. NIR-triggered and ROS-boosted nanoplatform for enhanced Chemo/PDT/PTT synergistic therapy of sorafenib in hepatocellular carcinoma. Nanoscale Res Lett. 2022;17(1). doi:10.1186/s11671-022-03729-w
54. Fang T, Ma S, Wei Y, Yang J, Zhang J, Shen Q. Catalytic immunotherapy-photothermal therapy combination for melanoma by ferroptosis-activating vaccine based on artificial nanoenzyme. Mat Today Chem. 2023;27:101308. doi:10.1016/j.mtchem.2022.101308
55. Cano-Mejia J, Burga RA, Sweeney EE, et al. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomed –Nanotechnol Biol Med. 2017;13(2):771–781. doi:10.1016/j.nano.2016.10.015
56. Zhou B, Wu Q, Wang M, et al. Immunologically modified MnFe2O4 nanoparticles to synergize photothermal therapy and immunotherapy for cancer treatment. Chem Eng J. 2020;396:125239. doi:10.1016/j.cej.2020.125239
57. Zhou R, Liu X, Wu Y, et al. Suppressing the radiation-induced corrosion of bismuth nanoparticles for enhanced synergistic cancer radiophototherapy. ACS Nano. 2020;14(10):13016–13029. doi:10.1021/acsnano.0c04375
58. Zheng XH, Wang L, Guan YY, Pei Q, Jiang J, Xie ZG. Integration of metal-organic framework with a photoactive porous-organic polymer for interface enhanced phototherapy. Biomaterials. 2020;235:119792.
59. Cetin Ersen B, Goncu B, Dag A, Birlik Demirel G. GLUT-targeting phototherapeutic nanoparticles for synergistic triple combination cancer therapy. ACS Appl Mat Interfaces. 2023;15(7):9080–9098. doi:10.1021/acsami.2c21180
60. Li C, Cheng Y, Li D, et al. Antitumor applications of photothermal agents and photothermal synergistic therapies. Int J Mol Sci. 2022;23(14):7909. doi:10.3390/ijms23147909
61. Chang X, Tang X, Liu J, et al. Precise starving therapy via physiologically dependent photothermal conversion promoted mitochondrial calcification based on multi‐functional gold nanoparticles for effective tumor treatment. Adv Funct Mat. 2023;33(35):2303596. doi:10.1002/adfm.202303596
62. Zhang X, Zhao Y, Teng Z, et al. Combination of losartan and platinum nanoparticles with photothermal therapy induces immunogenic cell death effective against neuroblastoma. Int J Nanomed;2024:10213–10226. doi:10.2147/IJN.S467968
63. Wang F, Wu Q, Jia G, et al. Black phosphorus/MnO2 nanocomposite disrupting bacterial thermotolerance for efficient mild‐temperature photothermal therapy. Adv Sci. 2023;10(30):2303911. doi:10.1002/advs.202303911
64. Zhao G, Li J, Fangfang L, et al. Biomimetic platform based on mesoporous platinum for multisynergistic cancer therapy. ACS Biomater Sci Eng. 2021;7(11):5154–5164. doi:10.1021/acsbiomaterials.1c00912
65. Mauro N, Utzeri MA, Varvarà P, Cavallaro G. Functionalization of metal and carbon nanoparticles with potential in cancer theranostics. Molecules. 2021;26(11):3085. doi:10.3390/molecules26113085
66. Chang Y, Bai Q, Wang M, et al. Plasmonic Bi nanoparticles encapsulated by N-Carbon for dual-imaging and photothermal/photodynamic/chemo-therapy. Biomater Adv. 2022;134:112546. doi:10.1016/j.msec.2021.112546
67. Kong X, Li M, Xiao W, et al. Ω-Shaped fiber optic LSPR coated with hybridized nanolayers for tumor cell sensing and photothermal treatment. Talanta. 2024;278:126381. doi:10.1016/j.talanta.2024.126381
68. Nejabat M, Samie A, Ramezani M, Alibolandi M, Abnous K, Taghdisi SM. An overview on gold nanorods as versatile nanoparticles in cancer therapy. J Control Release. 2023;354:221–242. doi:10.1016/j.jconrel.2023.01.009
69. Yu S, Xia G, Yang N, et al. Noble Metal Nanoparticle-Based Photothermal Therapy: development and Application in Effective Cancer Therapy. Int J Mol Sci. 2024;25(11):5632.
70. Turkmen Koc SN, Rezaei Benam S, Aral IP, Shahbazi R, Ulubayram K. Gold nanoparticles-mediated photothermal and photodynamic therapies for cancer. Int J Pharm. 2024;655:124057. doi:10.1016/j.ijpharm.2024.124057
71. Li J, Wang H, Li Z, Su Z, Zhu Y. Preparation and application of metal nanoparticals elaborated fiber sensors. Sensors. 2020;20(18):5155.
72. Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114(21):10869–10939. doi:10.1021/cr400532z
73. Dahan KA, Li Y, Xu J, Kan C. Recent progress of gold nanostructures and their applications. Phys Chem Chem Phys. 2023;25(28):18545–18576. doi:10.1039/D3CP01549A
74. Khlebtsov BN, Burov AM, Zarkov SV, Khlebtsov NG. Surface-enhanced Raman scattering from Au nanorods, nanotriangles, and nanostars with tuned plasmon resonances. Phys Chem Chem Phys. 2023;25(45):30903–30913. doi:10.1039/D3CP04541B
75. Magdy G, Aboelkassim E, Abd Elhaleem SM, Belal F. A comprehensive review on silver nanoparticles: synthesis approaches, characterization techniques, and recent pharmaceutical, environmental, and antimicrobial applications. Microchem J. 2024;196:109615. doi:10.1016/j.microc.2023.109615
76. Caschera L, Lazzara A, Piergallini L, Ricci D, Tuscano B, Vanzulli A. Contrast agents in diagnostic imaging: present and future. Pharmacol Res. 2016;110:65–75.
77. Wang H, Mu X, He H, Zhang X-D. Cancer radiosensitizers. Trends Pharmacolog Sci. 2018;39(1):24–48. doi:10.1016/j.tips.2017.11.003
78. Zhang Y, Han X, Liu Y, Wang S, Han X, Cheng C. Research progress on nano-sensitizers for enhancing the effects of radiotherapy. Mat Adv. 2022;3(9):3709–3725. doi:10.1039/D2MA00094F
79. Carter P, Smith L, Ryan M. Identification and validation of cell surface antigens for antibody targeting in oncology. Endocrine Rel Cancer. 2004;11(4):659–687. doi:10.1677/erc.1.00766
80. Raheem MA, Rahim MA, Gul I, et al. Advances in nanoparticles-based approaches in cancer theranostics. OpenNano. 2023;12:100152. doi:10.1016/j.onano.2023.100152
81. Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol. 2011;9:1–8. doi:10.1186/1477-3155-9-30
82. Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. 2010;8(1):1–10. doi:10.1186/1477-3155-8-1
83. Sijilmassi O. Folic acid deficiency and vision: a review. Graefes Arch Clin Exp Ophthalmol. 2019;257(8):1573–1580. doi:10.1007/s00417-019-04304-3
84. Shanavas A, Rengan AK, Chauhan D, et al. Glycol chitosan assisted in situ reduction of gold on polymeric template for anti-cancer theranostics. Int J Biol Macromol. 2018;110:392–398. doi:10.1016/j.ijbiomac.2017.11.127
85. Liang A, Yang J, Zhu X, Zhou X. The glowing frontier: carbon dots’ journey for customized cancer treatments. J Drug Deliv Sci Technol. 2025;2024:106658.
86. Rastinehad AR, Anastos H, Wajswol E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Nat Acad Sci. 2019;116(37):18590–18596. doi:10.1073/pnas.1906929116
87. Rengan AK, Bukhari AB, Pradhan A, et al. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Letters. 2015;15(2):842–848. doi:10.1021/nl5045378
88. Appidi T, Rajalakshmi P, Chinchulkar SA, et al. A plasmon-enhanced fluorescent gold coated novel lipo-polymeric hybrid nanosystem: synthesis, characterization and application for imaging and photothermal therapy of breast cancer. Nanoscale. 2022;14(25):9112–9123. doi:10.1039/D2NR01378A
89. Pachaiappan R, Manavalan K. Role of metals, metal oxides, and metal sulfides in the diagnosis and treatment of cancer. Metal Metal Oxides Metal Sulphides Biomed Applic. 2021;2021:165–207.
90. Li J, Zhang W, Ji W, et al. Near infrared photothermal conversion materials: mechanism, preparation, and photothermal cancer therapy applications. J Mat Chem B. 2021;9(38):7909–7926. doi:10.1039/D1TB01310F
91. Domask A, Gurunathan R, Mohney S. Transition metal–MoS 2 reactions: review and thermodynamic predictions. J Electron Mat. 2015;44:4065–4079.
92. Y-j H, X-x Y, R-q L, et al. Pathological mechanism of photodynamic therapy and photothermal therapy based on nanoparticles. Int J Nanomed. 2020;15:6827–6838. doi:10.2147/IJN.S269321
93. Molkenova A, Atabaev TS, Hong SW, Mao C, Han D-W, Kim KS. Designing inorganic nanoparticles into computed tomography and magnetic resonance (CT/MR) imaging-guidable photomedicines. Mat Today Nano. 2022;18:100187.
94. Lu Z, Huang F-Y, Cao R, et al. Intrinsic, cancer cell-selective toxicity of organic photothermal nanoagent: a simple formulation for combined photothermal chemotherapy of cancer. ACS Appl Mat Interfaces. 2018;10(31):26028–26038. doi:10.1021/acsami.8b07801
95. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiolog Rev. 2014;94(3):909–950. doi:10.1152/physrev.00026.2013
96. Onzi G, Guterres SS, Pohlmann AR, Frank LA. Passive targeting and the enhanced permeability and retention (EPR) effect. ADME Encyclo. 2021;2021:1–13.
97. Johnson AP, Sabu C, Nivitha K, et al. Bioinspired and biomimetic micro-and nanostructures in biomedicine. J Controlled Release. 2022;343:724–754. doi:10.1016/j.jconrel.2022.02.013
98. Li ST, Meng XR, Peng B, et al. Cell membrane-based biomimetic technology for cancer phototherapy: mechanisms, recent advances and perspectives. Acta Biomaterialia. 2024;174:26–48. doi:10.1016/j.actbio.2023.11.029
99. Hu H, Hua SY, Lin X, et al. Hybrid biomimetic membrane coated particles-mediated bacterial ferroptosis for acute MRSA pneumonia. ACS Nano. 2023;17(12):11692–11712. doi:10.1021/acsnano.3c02365
100. Peng YW, Gao L, Pidamaimaiti G, et al. Facile construction of highly luminescent and biocompatible gold nanoclusters by shell rigidification for two-photon pH-edited cytoplasmic and in vivo imaging. Nanoscale. 2022;14(23):8342–8348. doi:10.1039/D2NR01078J
101. Jana S, Jana S. Particulate Technology for Delivery of Therapeutics. Springer; 2017.
102. Yang X, Zhang H, Wu Z, et al. Tumor therapy utilizing dual-responsive nanoparticles: a multifaceted approach integrating calcium-overload and PTT/CDT/chemotherapy. J Controlled Release. 2024;376:646–658. doi:10.1016/j.jconrel.2024.10.029
103. Rajaram J, Kuthati Y. Metal peroxide nanoparticles for modulating the tumor microenvironment: current status and recent prospects. Cancers. 2024;16(21):3581. doi:10.3390/cancers16213581
104. Safdar A, Wang P, Muhaymin A, Nie G, Li S. From bench to bedside: platelet biomimetic nanoparticles as a promising carriers for personalized drug delivery. J Controlled Release. 2024;373:128–144. doi:10.1016/j.jconrel.2024.07.013
105. Isambert N, Delord J-P, Soria J-C, et al. Debio0932, a second-generation oral heat shock protein (HSP) inhibitor, in patients with advanced cancer—results of a first-in-man dose-escalation study with a fixed-dose extension phase. Annals Oncol. 2015;26(5):1005–1011. doi:10.1093/annonc/mdv031
106. Liu X, Dai L. Carbon-based metal-free catalysts. Nat Rev Mat. 2016;1(11):1–12.
107. Egbedina AO, Bolade OP, Ewuzie U, Lima EC. Emerging trends in the application of carbon-based materials: a review. J Environ Chem Eng. 2022;10(2):107260. doi:10.1016/j.jece.2022.107260
108. Han B, Zhang YL, Chen QD, Sun HB. Carbon‐based photothermal actuators. Adv Funct Mat. 2018;28(40):1802235. doi:10.1002/adfm.201802235
109. Miao W, Shim G, Lee S, Oh Y-K. Structure-dependent photothermal anticancer effects of carbon-based photoresponsive nanomaterials. Biomaterials. 2014;35(13):4058–4065. doi:10.1016/j.biomaterials.2014.01.043
110. Lagos KJ, Buzzá HH, Bagnato VS, Romero MP. Carbon-based materials in photodynamic and photothermal therapies applied to tumor destruction. Int J Mol Sci. 2021;23(1):22. doi:10.3390/ijms23010022
111. Kaur K, Reddy S, Barathe P, et al. Combating drug-resistant bacteria using photothermally active nanomaterials: a perspective review. Front Microbiol. 2021;12:747019. doi:10.3389/fmicb.2021.747019
112. Huo J, Jia Q, Huang H, et al. Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections. Chem Soc Rev. 2021;50(15):8762–8789. doi:10.1039/D1CS00074H
113. Nasir A, Khan A, Li JY, et al. Nanotechnology, A tool for diagnostics and treatment of cancer. Current Topics Med Chem. 2021;21(15):1360–1376. doi:10.2174/1568026621666210701144124
114. Hirsch A. Functionalization of single‐walled carbon nanotubes. Angewandte Chem Int Edition. 2002;41(11):1853–1859. doi:10.1002/1521-3773(20020603)41:11<1853::AID-ANIE1853>3.0.CO;2-N
115. Saito T, Matsushige K, Tanaka K. Chemical treatment and modification of multi-walled carbon nanotubes. Physica B. 2002;323(1–4):280–283. doi:10.1016/S0921-4526(02)00999-7
116. Li C, Jia R, Yang Y, Liao G. A Hierarchical helical Carbon Nanotube Fiber Artificial Ligament. Springer; 2023.
117. Mahor A, Singh PP, Bharadwaj P, et al. Carbon-based nanomaterials for delivery of biologicals and therapeutics: a cutting-edge technology. C. 2021;7(1):19.
118. Sharma M, Alessandro P, Cheriyamundath S, Lopus M. Therapeutic and diagnostic applications of carbon nanotubes in cancer: recent advances and challenges. J Drug Targeting. 2024;32(3):287–299. doi:10.1080/1061186X.2024.2309575
119. Tuncel D. Non-covalent interactions between carbon nanotubes and conjugated polymers. Nanoscale. 2011;3(9):3545–3554. doi:10.1039/c1nr10338e
120. Jogi H, Maheshwari R, Raval N, et al. Carbon nanotubes in the delivery of anticancer herbal drugs. Nanomedicine. 2018;13(10):1187–1220. doi:10.2217/nnm-2017-0397
121. Chargaff E. The Nucleic Acids. Elsevier; 2012.
122. Whitford D. Proteins: Structure and Function. John Wiley & Sons; 2013.
123. Romao CC, Blättler WA, Seixas JD, Bernardes GJ. Developing drug molecules for therapy with carbon monoxide. Chem Soc Rev. 2012;41(9):3571–3583. doi:10.1039/c2cs15317c
124. Wu Q, Yang X, Yang J, et al. Size effect of ruthenium nanoparticles on water cracking properties with different crystal planes for boosting electrocatalytic hydrogen evolution. J Colloid Interface Sci. 2023;644:238–245. doi:10.1016/j.jcis.2023.04.076
125. Guo Q, Shen XT, Li YY, Xu SQ. Carbon nanotubes-based drug delivery to cancer and brain. J Huazhong Univ Sci Technol-Med Sci. 2017;37(5):635–641.
126. Gong H, Peng R, Liu Z. Carbon nanotubes for biomedical imaging: the recent advances. Adv Drug Delivery Rev. 2013;65(15):1951–1963. doi:10.1016/j.addr.2013.10.002
127. Bartelmess J, Quinn S, Giordani S. Carbon nanomaterials: multi-functional agents for biomedical fluorescence and Raman imaging. Chem Soc Rev. 2015;44(14):4672–4698. doi:10.1039/C4CS00306C
128. Movia D, Prina-Mello A, Bazou D, Volkov Y, Giordani S. Screening the cytotoxicity of single-walled carbon nanotubes using novel 3D tissue-mimetic models. ACS Nano. 2011;5(11):9278–9290. doi:10.1021/nn203659m
129. Khalid A, Yi W, Yoo S, et al. Single-chirality of single-walled carbon nanotubes (SWCNTs) through chromatography and its potential biological applications. New J Chem. 2023;47(3):992–1022.
130. Zhou M, Liu S, Jiang Y, et al. Doxorubicin‐loaded single wall nanotube thermo‐sensitive hydrogel for gastric cancer chemo‐photothermal therapy. Adv Funct Mat. 2015;25(29):4730–4739. doi:10.1002/adfm.201501434
131. Wang X, Li B, Jing H, Dong X, Leng X. MWCNT-mediated combinatorial photothermal ablation and chemo-immunotherapy strategy for the treatment of melanoma. J Mat Chem B. 2020;8(19):4245–4258. doi:10.1039/C9TB02238D
132. Oudjedi F, Lee SS, Paliouras M, Trifiro M, Kirk AG. Enhancing in vitro photothermal therapy using plasmonic gold nanorod decorated multiwalled carbon nanotubes. Biomed Optics Express. 2023;14(12):6629–6643. doi:10.1364/BOE.504746
133. Nakayama K, Tanabe K, Atwater HA. Plasmonic nanoparticle enhanced light absorption in GaAs solar cells. Appl Physics Lett. 2008;93(12). doi:10.1063/1.2988288
134. Chen Y, Xue L, Zhu Q, Feng Y, Wu M. Recent advances in second near-infrared region (NIR-II) fluorophores and biomedical applications. Front Chem. 2021;9:750404. doi:10.3389/fchem.2021.750404
135. Oudjedi F, Lee SS, Paliouras M, Trifiro M, Wachsmann-Hogiu S, Kirk AG. In vitro raman thermometry using gold nanorod-decorated carbon nanotubes. ACS Appl Nano Mat. 2024;7(17):20942–20953. doi:10.1021/acsanm.4c03865
136. Stout A, Friedly J, Standaert CJ. Systemic absorption and side effects of locally injected glucocorticoids. Pm&r. 2019;11(4):409–419. doi:10.1002/pmrj.12042
137. Alefeld G, Radermacher R. Heat Conversion Systems. CRC press; 2023.
138. Oudjedi F. Exploring the therapeutic potential of plasmonic hybrid multiwalled carbon nanotubes in cancer treatment and beyond. 2024.
139. Marangon I, Ménard-Moyon C, Silva AK, Bianco A, Luciani N, Gazeau F. Synergic mechanisms of photothermal and photodynamic therapies mediated by photosensitizer/carbon nanotube complexes. Carbon. 2016;97:110–123. doi:10.1016/j.carbon.2015.08.023
140. Xia C, Zhu S, Feng T, Yang M, Yang B. Evolution and synthesis of carbon dots: from carbon dots to carbonized polymer dots. Adv Sci. 2019;6(23):1901316. doi:10.1002/advs.201901316
141. Lagos KJ, García D, Cuadrado CF, et al. Carbon dots: types, preparation, and their boosted antibacterial activity by photoactivation. Current status and future perspectives. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15(4):e1887. doi:10.1002/wnan.1887
142. Mansuriya BD, Altintas Z. Carbon Dots: classification, properties, synthesis, characterization, and applications in health care—An updated review (2018–2021). Nanomaterials. 2021;11(10):2525. doi:10.3390/nano11102525
143. Li X, Fu Y, Zhao S, et al. Metal ions-doped carbon dots: synthesis, properties, and applications. Chem Eng J. 2022;430:133101. doi:10.1016/j.cej.2021.133101
144. Chen H, Luo K, Xie C, Zhou L. Nanotechnology of carbon dots with their hybrids for biomedical applications: a review. Chem Eng J. 2024;496:153915. doi:10.1016/j.cej.2024.153915
145. Wang S, McCoy CP, Li P, et al. Carbon dots in photodynamic/photothermal antimicrobial therapy. Nanomaterials. 2024;14(15):1250.
146. Yi J, Liu L, Gao W, et al. Advances and perspectives in phototherapy-based combination therapy for cancer treatment. J Mat Chem B. 2024;12(26):6285–6304. doi:10.1039/D4TB00483C
147. Liu S, Cui H, Huang J, Tian B, Bao J. Osmanthus-derived carbon dots for cell imaging and NIR photothermal therapy. Mat Lett. 2024;377:137347. doi:10.1016/j.matlet.2024.137347
148. Zhang L, Yang A, Ruan C, et al. Copper-nitrogen-coordinated carbon dots: transformable phototheranostics from precise PTT/PDT to post-treatment imaging-guided PDT for residual tumor cells. ACS Appl Mat Interfaces. 2023;15(2):3253–3265. doi:10.1021/acsami.2c17525
149. Bhattacharya S, Belemkar S, Prajapati BG. The application of graphene oxide nanoarchitectures in the treatment of cancer: phototherapy, immunotherapy, and the development of vaccines. Curr Med Chem. 2024;31(27):4320–4339. doi:10.2174/0109298673288750240117115141
150. Ghuge A D, R Shirode A, Kadam V J. Graphene: a comprehensive review. Current Drug Targets. 2017;18(6):724–733. doi:10.2174/1389450117666160709023425
151. Wu Q, Wang J, Wang Z, et al. High-loaded single Cu atoms decorated on N-doped graphene for boosting Fenton-like catalysis under neutral pH. J Mat Chem A. 2020;8(27):13685–13693. doi:10.1039/D0TA04943C
152. Yan Z, Wu T, Fang G, Ran M, Shen K, Liao G. Self-assembly preparation of lignin–graphene oxide composite nanospheres for highly efficient Cr (vi) removal. RSC Adv. 2021;11(8):4713–4722. doi:10.1039/D0RA09190A
153. Guo W, Chen Z, Feng X, et al. Graphene oxide (GO)-based nanosheets with combined chemo/photothermal/photodynamic therapy to overcome gastric cancer (GC) paclitaxel resistance by reducing mitochondria-derived adenosine-triphosphate (ATP). J Nanobiotechnol. 2021;19(1):146. doi:10.1186/s12951-021-00874-9
154. Wu J, Deng T, Wu Q, et al. NIR-responsive GO/Ag2S heterostructure for rapid bacteria-killing and wound healing. Appl Mat Today. 2023;32:101826. doi:10.1016/j.apmt.2023.101826
155. Chen YK, Wang RZ, Wang D, Fang JJ, Dong RL, Dai BY. Harnessing near-infrared light for highly efficient photocatalysis. Chemsuschem. 2024;18(3):e202401786
156. Ma G, Qi JJ, Cui QF, Bao XY, Gao D, Xing CF. Graphene oxide composite for selective recognition, capturing, photothermal killing of bacteria over mammalian cells. Polymers. 2020;12(5):1116. doi:10.3390/polym12051116
157. Li M, Zhao M, Li JC. Near-infrared absorbing semiconducting polymer nanomedicines for cancer therapy. Wiley Interdisciplinary Rev –Nanomed Nanobiotechnol. 2023;15(3). doi:10.1002/wnan.1865
158. Zhen X, Pu KY, Jiang XQ. Photoacoustic imaging and photothermal therapy of semiconducting polymer nanoparticles: signal amplification and second near-infrared construction. Small. 2021;17(6):2004723.
159. Rejinold NS, Choi G, Choy JH. Recent developments on semiconducting polymer nanoparticles as smart photo-therapeutic agents for cancer treatments-a review. Polymers. 2021;13(6):981.
160. Zhu J, Zhu R, Miao Q. Polymeric agents for activatable fluorescence, self-luminescence and photoacoustic imaging. Biosensors Bioelectron. 2022;210:114330.
161. Ding F, Feng J, Zhang X, Sun J, Fan C, Ge Z. Responsive optical probes for deep-tissue imaging: photoacoustics and second near-infrared fluorescence. Adv Drug Delivery Rev. 2021;173:141–163.
162. Xu C, Pu K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem Soc Rev. 2021;50(2):1111–1137.
163. Li J, Duan H, Pu K. Nanotransducers for near‐infrared photoregulation in biomedicine. Adv Mat. 2019;31(33):1901607. doi:10.1002/adma.201901607
164. Chen G, Cao Y, Tang Y, et al. Advanced near‐infrared light for monitoring and modulating the spatiotemporal dynamics of cell functions in living systems. Adv Sci. 2020;7(8):1903783. doi:10.1002/advs.201903783
165. Liao G, Li Q, Xu Z. The chemical modification of polyaniline with enhanced properties: a review. Progress Organic Coat. 2019;126:35–43. doi:10.1016/j.porgcoat.2018.10.018
166. Liao G, Gong Y, Yi C, Xu Z. Soluble, antibacterial, and anticorrosion studies of sulfonated polystyrene/polyaniline/silver nanocomposites prepared with the sulfonated polystyrene template. Chin J Chem. 2017;35(7):1157–1164. doi:10.1002/cjoc.201600816
167. Korupalli C, Kalluru P, Nuthalapati K, Kuthala N, Thangudu S, Vankayala R. Recent advances of polyaniline-based biomaterials for phototherapeutic treatments of tumors and bacterial infections. Bioengineering. 2020;7(3):94. doi:10.3390/bioengineering7030094
168. Zhou J, Lu Z, Zhu X, et al. NIR photothermal therapy using polyaniline nanoparticles. Biomaterials. 2013;34(37):9584–9592. doi:10.1016/j.biomaterials.2013.08.075
169. Ma G, Zhang X, Zhao K, et al. Polydopamine nanostructure-enhanced water interaction with ph-responsive manganese sulfide nanoclusters for tumor magnetic resonance contrast enhancement and synergistic ferroptosis–photothermal therapy. ACS Nano. 2024;18(4):3369–3381. doi:10.1021/acsnano.3c10249
170. Xie YX, Xu J, Jin H, et al. Polypyrrole nanosheets prepared by rapid in situ polymerization for nir-ii photoacoustic-guided photothermal tumor therapy. Coatings. 2023;13(6):1037.
171. Zhu CG, Gao Q, Wang CX, et al. Quinoxalineimide-based semiconducting polymer nanoparticles as an effective phototheranostic for the second near-infrared fluorescence imaging and photothermal therapy. Acs Appl Mat Interfaces. 2023;15(24):29396–29405. doi:10.1021/acsami.3c06853
172. Li T, Wu M, Wei Q, et al. Conjugated polymer nanoparticles for tumor theranostics. Biomacromolecules. 2023;24(5):1943–1979. doi:10.1021/acs.biomac.2c01446
173. Zheng Q, Duan Z, Zhang Y, et al. Conjugated polymeric materials in biological imaging and cancer therapy. Molecules. 2023;28(13):5091.
174. Bag A, Maheshwari R. Advances in polymer-centric nanomedicines for theranostic cancer treatment. J Drug Deliv Sci Technol. 2024;100:106105.
175. Pham -T-TD, Phan LMT, Cho S, Park J. Enhancement approaches for photothermal conversion of donor–acceptor conjugated polymer for photothermal therapy: a review. Sci Technol Adv Mat. 2022;23(1):707–734. doi:10.1080/14686996.2022.2134976
176. Lin H, Lin Z, Zheng K, et al. Near‐infrared‐II nanomaterials for fluorescence imaging and photodynamic therapy. Adv Opt Mat. 2021;9(9):2002177. doi:10.1002/adom.202002177
177. He K, Chen S, Chen Y, et al. Water-soluble donor–acceptor–donor-based fluorophore for high-resolution nir-ii fluorescence imaging applications. ACS Appl Polymer Mat. 2021;3(6):3238–3246. doi:10.1021/acsapm.1c00456
178. Wei J, Liu Y, Yu J, et al. Conjugated polymers: optical toolbox for bioimaging and cancer therapy. Small. 2021;17(43):2103127. doi:10.1002/smll.202103127
179. Wei ZW, Zhu BL, Cai Y, et al. Near-infrared-absorbing diketopyrrolopyrrole-based semiconducting polymer nanoparticles for photothermal therapy. Particle Particle Syst Characterization. 2020;37(3). doi:10.1002/ppsc.201900433
180. Chen G, Sun J, Peng Q, et al. Biradical‐featured stable organic‐small‐molecule Photothermal materials for highly efficient solar‐driven water evaporation. Adv Mat. 2020;32(29):1908537. doi:10.1002/adma.201908537
181. Guo S, Gu D, Yang Y, Tian J, Chen X. Near-infrared photodynamic and photothermal co-therapy based on organic small molecular dyes. J Nanobiotechnol. 2023;21(1):348. doi:10.1186/s12951-023-02111-x
182. Huang CC, Shi TY, Zhang JJ, et al. An NIR-II-absorbing photothermal agent containing multiple rotors with enhanced photothermal conversion capacity for multimodal-imaging-guided photothermal therapy. Dyes Pigments. 2023;210:110932.
183. Li L, Dong XG, Li JR, Wei J. A short review on NIR-II organic small molecule dyes. Dyes Pigments. 2020;183:108756.
184. Li L, Chen YS, Chen WJ, Tan Y, Chen HY, Yin J. Photodynamic therapy based on organic small molecular fluorescent dyes. Chin Chem Lett. 2019;30(10):1689–1703. doi:10.1016/j.cclet.2019.04.017
185. Shao W, Lee J, Li FY, Ling DS. Organic small molecule nanoparticles for phototheranostics. Chem J Chin Univ -Chin. 2020;41(11):2356–2382.
186. Zhou W, Yin LK, Zhang XH, et al. Recent advances in small molecule dye-based nanotheranostics for NIR-II photoacoustic imaging-guided cancer therapy. Front Bioeng Biotechnol. 2022;10:1002006.
187. Ali MF, Muday GK. Reactive oxygen species are signaling molecules that modulate plant reproduction. Plant Cell Environ. 2024;47(5):1592–1605. doi:10.1111/pce.14837
188. Chen GY, Sun JM, Peng Q, et al. Biradical-featured stable organic-small-molecule photothermal materials for highly efficient solar-driven water evaporation. Adv Mat. 2020;32(29):121290.
189. Feng L, Li C, Liu L, et al. Acceptor planarization and donor rotation: a facile strategy for realizing synergistic cancer phototherapy via type I PDT and PTT. ACS Nano. 2022;16(3):4162–4174. doi:10.1021/acsnano.1c10019
190. Sun W, Wang X, Cheng Z, et al. Phototheranostics for NIR fluorescence image guided PDT/PTT with extended conjugation and enhanced TICT. Biomed Pharmacother. 2023;158:114071. doi:10.1016/j.biopha.2022.114071
191. Wang L, Lai B, Ran X, Tang H, Cao D. Recent advances of diketopyrrolopyrrole derivatives in cancer therapy and imaging applications. Molecules. 2023;28(10):4097.
192. Chiang C-P, Hsieh O, Tai W-C, Chen Y-J, Chang P-C. Clinical outcomes of adjunctive indocyanine green-diode lasers therapy for treating refractory periodontitis: a randomized controlled trial with in vitro assessment. J Formosan Med Assoc. 2020;119(2):652–659. doi:10.1016/j.jfma.2019.08.021
193. Appidi T, Sivasankaran RP, Chinchulkar SA, et al. A lipo-polymeric hybrid nanosystem with metal enhanced fluorescence for targeted imaging of metastatic breast cancer. Nanotheranostics. 2024;8(2):239. doi:10.7150/ntno.92410
194. Kang Y, Li Z, Yang Y, Su Z, Ji X, Zhang S. Antimonene nanosheets‐based Z‐scheme heterostructure with enhanced reactive oxygen species generation and photothermal conversion efficiency for photonic therapy of cancer. Adv Healthcare Mat. 2021;10(3):2001835. doi:10.1002/adhm.202001835
195. Yang Q, Xu L, Wang J, et al. Iron-decorated, IR820-loaded polypyrrole nanocomposites for synergistic tumor photothermal, photodynamic, and chemodynamic therapy. J Nanopart Res. 2021;24:1–10.
196. Jin T, Cheng D, Jiang G, et al. Engineering naphthalimide-cyanine integrated near-infrared dye into ROS-responsive nanohybrids for tumor PDT/PTT/chemotherapy. Bioact Mat. 2022;14:42–51. doi:10.1016/j.bioactmat.2021.12.009
197. Zhou B, Li Y, Niu G, Lan M, Jia Q, Liang Q. Near-infrared organic dye-based nanoagent for the photothermal therapy of cancer. ACS Appl Mater Interfaces. 2016;8(44):29899–29905. doi:10.1021/acsami.6b07838
198. Duan H, Wang F, Xu W, Sheng G, Sun Z, Chu H. Recent advances in the nanoarchitectonics of metal-organic frameworks for light-activated tumor therapy. Dalton Trans. 2023;52(44):16085–16102. doi:10.1039/D3DT02725B
199. Qiao Y, Sun C, Jian J, et al. Efficient removal of organic pollution via photocatalytic degradation over a TiO2@ HKUST-1 yolk-shell nanoreactor. J Mol Liquids. 2023;385:122383. doi:10.1016/j.molliq.2023.122383
200. Li C, Huang N-Y, Yang Y, Xu Q, Liao G. Emerging metal-organic framework-based photocatalysts for solar-driven fuel production. Coord Chem Rev. 2025;524:216292.
201. Qiao Y, Sun C, Jian J, et al. Multifunctional luminescent 3D Ln-MOFs with high sensitivity for trace detection of micronutrients. Inorganic Chem. 2024;63(4):2060–2071. doi:10.1021/acs.inorgchem.3c03838
202. Luo Z, Sheng Y, Jiang C, et al. Recent advances and prospects of metal-organic frameworks in cancer therapies. Dalton Trans. 2023;52(47):17601–17622. doi:10.1039/D3DT02543H
203. Feng Y, Wu W, Li M. Metal-organic frameworks for hepatocellular carcinoma therapy and mechanism. Front Pharmacol. 2022;13:1025780. doi:10.3389/fphar.2022.1025780
204. Huang X, Sun X, Wang W, et al. Nanoscale metal-organic frameworks for tumor phototherapy. J Mater Chem B. 2021;9(18):3756–3777.
205. Abdelhamid HN. Zeolitic imidazolate frameworks (ZIF-8) for biomedical applications: a review. Curr Med Chem. 2021;28(34):7023–7075. doi:10.2174/0929867328666210608143703
206. Lou Y, Wang Z, Wang Y. Construction of pH/NIR responsive silver sulfide metal-organic frameworks for photothermal/chemotherapy synergistic treatment of tumor. Colloids Surfaces A. 2024;703:135217. doi:10.1016/j.colsurfa.2024.135217
207. Zhang H, Yuan W. Self-healable oxide sodium alginate/carboxymethyl chitosan nanocomposite hydrogel loading Cu2+-doped MOF for enhanced synergistic and precise cancer therapy. Int J Biol Macromol. 2024;262:129996. doi:10.1016/j.ijbiomac.2024.129996
208. Ліннік О, Смірнова Н, Г Є, Wilson WB, Campiglia AD. Особливості золь-гель синтезу напівпровідникових азотвмісних плівок TiO₂, модифікованих іонами металів (Zn²⁺, Zr⁴⁺, Pt²⁺). Доповіді НАН України. 2020;1100:163–173. doi:10.1016/j.aca.2019.10.067
209. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. doi:10.1186/s12943-021-01489-2
210. Hao Y, Liu T, Zhou H, Peng J, Li K, Chen Y. The GSH responsive indocyanine green loaded PD-1 inhibitory polypeptide AUNP12 modified MOF nanoparticles for photothermal and immunotherapy of melanoma. Front Bioeng Biotechnol. 2023;11:1294074. doi:10.3389/fbioe.2023.1294074
211. Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–2108. doi:10.1039/c8cs00618k
212. Zhi D, Yang T, O’Hagan J, Zhang S, Donnelly RF. Photothermal therapy. J Control Release. 2020;325:52–71. doi:10.1016/j.jconrel.2020.06.032
213. Attia ABE, Balasundaram G, Moothanchery M, et al. A review of clinical photoacoustic imaging: current and future trends. Photoacoustics. 2019;16:100144. doi:10.1016/j.pacs.2019.100144
214. Steinberg I, Huland DM, Vermesh O, Frostig HE, Tummers WS, Gambhir SS. Photoacoustic clinical imaging. Photoacoustics. 2019;14:77–98. doi:10.1016/j.pacs.2019.05.001
215. Choi W, Park B, Choi S, Oh D, Kim J, Kim C. Recent advances in contrast-enhanced photoacoustic imaging: overcoming the physical and practical challenges. Chem Rev. 2023;123(11):7379–7419. doi:10.1021/acs.chemrev.2c00627
216. Jiang D, Zhu L, Tong S, Shen Y, Gao F, Gao F. Photoacoustic imaging plus X: a review. J Biomed Opt. 2024;29(S1):S11513. doi:10.1117/1.JBO.29.S1.S11513
217. Liu Y, Nie L, Chen X. Photoacoustic molecular imaging: from multiscale biomedical applications towards early-stage theranostics. Trends Biotechnol. 2016;34(5):420–433. doi:10.1016/j.tibtech.2016.02.001
218. Pu Y, Zhu Y, Qiao Z, et al. A Gd-doped polydopamine (PDA)-based theranostic nanoplatform as a strong MR/PA dual-modal imaging agent for PTT/PDT synergistic therapy. J Mat Chem B. 2021;9(7):1846–1857. doi:10.1039/D0TB02725A
219. Luke GP, Yeager D, Emelianov SY. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Annals Biomed Eng. 2012;40:422–437. doi:10.1007/s10439-011-0449-4
220. Mito D, Eda H, S-i O, et al. Optimizing excitation light for accurate rapid bacterial species identification with autofluorescence. J Fluorescence. 2024;34(4):1737–1745. doi:10.1007/s10895-023-03383-0
221. Yu X, Liu X, Yang K, Chen X, Li W. Pnictogen semimetal (Sb, Bi)-based nanomaterials for cancer imaging and therapy: a materials perspective. ACS Nano. 2021;15(2):2038–2067. doi:10.1021/acsnano.0c07899
222. Li W, Rong P, Yang K, Huang P, Sun K, Chen X. Semimetal nanomaterials of antimony as highly efficient agent for photoacoustic imaging and photothermal therapy. Biomaterials. 2015;45:18–26. doi:10.1016/j.biomaterials.2014.12.037
223. Fang X, Lui K-H, Li S, et al. Multifunctional nanotheranostic gold nanocage/selenium core-shell for PAI-guided chemo-photothermal synergistic therapy in vivo. Int J Nanomed. 2020;15:10271–10284. doi:10.2147/IJN.S275846
224. Fang X. Novel and multifunctional nanoplatforms for efficient synergistic cancer therapy and multimodal imaging. 2022.
225. van Oostveen WM, de Lange EC. Imaging techniques in Alzheimer’s disease: a review of applications in early diagnosis and longitudinal monitoring. Int J Mol Sci. 2021;22(4):2110. doi:10.3390/ijms22042110
226. Koenig SH, Brown RD III. Field-cycling relaxometry of protein solutions and tissue: implications for MRI. Prog Nuclear Magnet Resonance Spectros. 1990;22(6):487–567. doi:10.1016/0079-6565(90)80008-6
227. Brown MA, Semelka RC. MRI: Basic Principles and Applications. John Wiley & Sons; 2011.
228. Yousaf T, Dervenoulas G, Politis M. Advances in MRI methodology. Int Rev Neurobiol. 2018;141:31–76.
229. Wu Q, Chen G, Gong K, et al. MnO2-laden black phosphorus for MRI-guided synergistic PDT, PTT, and chemotherapy. Matter. 2019;1(2):496–512. doi:10.1016/j.matt.2019.03.007
230. Chen L, Chen J, Qiu S, et al. Biodegradable nanoagents with short biological half‐life for SPECT/PAI/MRI multimodality imaging and PTT therapy of tumors. Small. 2018;14(4):1702700. doi:10.1002/smll.201702700
231. Mo Z, Qiu M, Zhao K, et al. Multifunctional phototheranostic nanoplatform based on polydopamine-manganese dioxide-IR780 iodide for effective magnetic resonance imaging-guided synergistic photodynamic/photothermal therapy. J Colloid Interface Sci. 2022;611:193–204. doi:10.1016/j.jcis.2021.12.071
232. Zhang L, Oudeng G, Wen F, Liao G. Recent advances in near-infrared-II hollow nanoplatforms for photothermal-based cancer treatment. Biomat Res. 2022;26(1):61. doi:10.1186/s40824-022-00308-z
233. He J, Ramachandraiah K, Huang T, et al. Core− shell structured hollow copper sulfide@ metal− organic framework for magnetic resonance imaging guided photothermal therapy in second near-infrared biological window. Biochem Biophys Res Commun. 2023;638:51–57. doi:10.1016/j.bbrc.2022.11.036
234. Huang S, Ye Y, Jiang C, et al. Current and promising applications of MOFs loaded with PTAs on photothermal therapy. Reactive Funct Polymers. 2023;193:105743. doi:10.1016/j.reactfunctpolym.2023.105743
235. Zhang M, Zhang Y, Hang L, et al. Bionic nanotheranostic for multimodal imaging-guided NIR-II-photothermal cancer therapy. Nanoscale. 2024;16(12):6095–6108. doi:10.1039/D4NR00230J
236. Wallyn J, Anton N, Akram S, Vandamme TF. Biomedical imaging: principles, technologies, clinical aspects, contrast agents, limitations and future trends in nanomedicines. Pharmaceut Res. 2019;36:1–31. doi:10.1007/s11095-019-2608-5
237. Liu XY, Yang Y, Wang XY, et al. Self-assembled Au4Cu4/Au25 NCs@liposome tumor nanotheranostics with PT/fluorescence imaging-guided synergetic PTT/PDT. J Mat Chem B. 2021;9(32):6396–6405. doi:10.1039/D1TB01092A
238. Yu HL, Wang YS, Chen Y, et al. Transmissible H-aggregated NIR-II fluorophore to the tumor cell membrane for enhanced PTT and synergistic therapy of cancer. Nano Convergence. 2023;10(1):3.
239. Chen Y, Wang S, Zhang F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat Rev Bioeng. 2023;1(1):60–78. doi:10.1038/s44222-022-00002-8
240. Shao C, Li Z, Zhang C, et al. Optical diagnostic imaging and therapy for thyroid cancer. Mat Today Bio. 2022;17:100441. doi:10.1016/j.mtbio.2022.100441
241. Chen X, Li J, Roy S, et al. Development of polymethine dyes for NIR‐II fluorescence imaging and therapy. Adv Healthcare Mat. 2024;13:2304506. doi:10.1002/adhm.202304506
242. Rurack K, Spieles M. Fluorescence quantum yields of a series of red and near-infrared dyes emitting at 600− 1000 nm. Anal Chem. 2011;83(4):1232–1242. doi:10.1021/ac101329h
243. Sogomonyan AS, Deyev SM, Shipunova VO. Internalization-responsive poly (lactic-co-glycolic acid) nanoparticles for image-guided photodynamic therapy against HER2-positive breast cancer. ACS Appl Nano Mat. 2023;6(13):11402–11415. doi:10.1021/acsanm.3c01446
244. Wang Y, Xu Y, Song J, et al. Tumor cell-targeting and tumor microenvironment–responsive nanoplatforms for the multimodal imaging-guided photodynamic/photothermal/chemodynamic treatment of cervical cancer. International journal of nanomedicine. 2024:5837–5858.
245. Ma X, Cheng H, Hou J, et al. Detection of breast cancer based on novel porous silicon Bragg reflector surface-enhanced Raman spectroscopy-active structure. Chin Opt Lett. 2020;18(5):051701. doi:10.3788/COL202018.051701
246. Das A, Tsai H-C, Sen T, Moirangthem RS. Plasmonic nanoparticle-based surface-enhanced Raman spectroscopy-guided photothermal therapy: emerging cancer theranostics. Nanomedicine. 2023;18(6):555–576. doi:10.2217/nnm-2023-0010
247. Wang J, Sun J, Wang Y, et al. Gold nanoframeworks with mesopores for Raman–photoacoustic imaging and photo‐chemo tumor therapy in the second near‐infrared biowindow. Adv Functi Mat. 2020;30(9):1908825. doi:10.1002/adfm.201908825
248. Wilson BC, Weersink RA. The Yin and Yang of PDT and PTT. Photochem Photobiol. 2020;96(2):219–231. doi:10.1111/php.13184
249. Ailioaie LM, Ailioaie C, Litscher G. Synergistic nanomedicine: photodynamic, photothermal and photoimmune therapy in hepatocellular carcinoma: fulfilling the myth of prometheus? Int J Mol Sci. 2023;24(9):8308. doi:10.3390/ijms24098308
250. Lyu X, Yu J, Zhang L, et al. AIEgens for synergistic anticancer therapy. J Mater Chem B. 2023;11(26):5953–5975. doi:10.1039/D3TB00219E
251. Fan Y, Zhang R, Shi J, et al. Mild near-infrared laser-triggered photo-immunotherapy potentiates immune checkpoint blockade via an all-in-one theranostic nanoplatform. J Colloid Interface Sci. 2025;678:1088–1103. doi:10.1016/j.jcis.2024.09.020
252. Zhang Y, Feng Y, Huang Y, et al. Tumor-targeted gene silencing IDO synergizes PTT-induced apoptosis and enhances anti-tumor immunity. Front Immunol. 2020;11:968. doi:10.3389/fimmu.2020.00968
253. Tang L, Huang Z, Mei H, Hu Y. Immunotherapy in hematologic malignancies: achievements, challenges and future prospects. Signal Transduction Targeted Ther. 2023;8(1):306. doi:10.1038/s41392-023-01521-5
254. Koury J, Lucero M, Cato C, et al. Immunotherapies: exploiting the immune system for cancer treatment. J Immunol Res. 2018;2018(1):9585614. doi:10.1155/2018/9585614
255. Cramer GM, Moon EK, Cengel KA, Busch TM. Photodynamic therapy and immune checkpoint blockade(†). Photochem Photobiol. 2020;96(5):954–961. doi:10.1111/php.13300
256. Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–5337. doi:10.1016/j.cell.2021.09.020
257. Chung JB, Brudno JN, Borie D, Kochenderfer JN. Chimeric antigen receptor T cell therapy for autoimmune disease. Nat Rev Immunol. 2024;24(11):830–845. doi:10.1038/s41577-024-01035-3
258. Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19(12):775–790. doi:10.1038/s41571-022-00689-z
259. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. doi:10.3322/caac.21596
260. Oliveira G, Wu CJ. Dynamics and specificities of T cells in cancer immunotherapy. Nat Rev Cancer. 2023;23(5):295–316. doi:10.1038/s41568-023-00560-y
261. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi:10.1038/s41423-020-0488-6
262. Chang M, Hou Z, Wang M, Li C, Lin J. Recent advances in hyperthermia therapy-based synergistic immunotherapy. Adv Mater. 2021;33(4):e2004788. doi:10.1002/adma.202004788
263. Hou X, Tao Y, Pang Y, Li X, Jiang G, Liu Y. Nanoparticle‐based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143(12):3050–3060.
264. Kong C, Xu B, Qiu G, et al. Multifunctional nanoparticles-mediated PTT/PDT synergistic immune activation and antitumor activity combined with anti-PD-L1 immunotherapy for breast cancer treatment. Int J Nanomed. 2022;17:5391. doi:10.2147/IJN.S373282
265. Feng ZH, Li ZT, Zhang S, et al. A combination strategy based on an Au nanorod/doxorubicin gel via mild photothermal therapy combined with antigen-capturing liposomes and anti-PD-L1 agent promote a positive shift in the cancer-immunity cycle. Acta Biomater. 2021;136:495–507. doi:10.1016/j.actbio.2021.09.052
266. Weng J, Zheng G, Wen J, et al. Construction and application of microneedle-mediated photothermal therapy and immunotherapy combined anti-tumor drug delivery system. Drug Deliv. 2023;30(1):2232950. doi:10.1080/10717544.2023.2232950
267. Jiang J, Huang Y, Zeng Z, Zhao C. Harnessing engineered immune cells and bacteria as drug carriers for cancer immunotherapy. ACS Nano. 2023;17(2):843–884.
268. Correia JH, Rodrigues JA, Pimenta S, Dong T, Yang Z. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13(9):1332. doi:10.3390/pharmaceutics13091332
269. Li M, Xu Y, Peng X, Kim JS. From low to no O2-dependent hypoxia photodynamic therapy (hPDT): a new perspective. Accounts Chem Res. 2022;55(22):3253–3264.
270. Sun H, Guo R, Guo Y, Song J, Li Z, Song F. Boosting type-I and type-II ROS production of water-soluble porphyrin for efficient hypoxic tumor therapy. Mol Pharm. 2023;20(1):606–615. doi:10.1021/acs.molpharmaceut.2c00822
271. Ozougwu JC. The role of reactive oxygen species and antioxidants in oxidative stress. Int J Res. 2016;1(8):1–8.
272. Moris D, Spartalis M, Tzatzaki E, et al. The role of reactive oxygen species in myocardial redox signaling and regulation. Annals Transl Med. 2017;5(16):324.
273. Checa J, Aran JM. Reactive oxygen species: drivers of physiological and pathological processes. J Inflam Res. 2020;2020:1057–1073.
274. Gammella E, Recalcati S, Cairo G. Dual role of ROS as signal and stress agents: iron tips the balance in favor of toxic effects. Oxidative Med Cell Longevity. 2016;2016(1):8629024. doi:10.1155/2016/8629024
275. Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. 2020;467:1–12. doi:10.1007/s11010-019-03667-9
276. Aggarwal V, Tuli HS, Varol A, et al. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;9(11):735.
277. Chen H, Wu L, Wang T, et al. PTT/ PDT-induced microbial apoptosis and wound healing depend on immune activation and macrophage phenotype transformation. Acta Biomater. 2023;167:489–505. doi:10.1016/j.actbio.2023.06.025
278. Chen D, Xu Q, Wang W, Shao J, Huang W, Dong X. Type I photosensitizers revitalizing photodynamic oncotherapy. Small. 2021;17(31):2006742. doi:10.1002/smll.202006742
279. Zhang Y, Ma J, Wang D, et al. Fe-TCPP@ CS nanoparticles as photodynamic and photothermal agents for efficient antimicrobial therapy. Biomat Sci. 2020;8(23):6526–6532. doi:10.1039/D0BM01427C
280. Cai X, Zhao Y, Wang L, et al. Synthesis of Au@ MOF core–shell hybrids for enhanced photodynamic/photothermal therapy. J Mat Chem B. 2021;9(33):6646–6657. doi:10.1039/D1TB00800E
281. Sha Q, Deng J, Zhang H, Luo X, Wu F. Organic nanomaterials from self-assembly of BODIPY-benzothiadiazole conjugate for PDT/PTT synergistic therapy. J Porphyrins Phthalocyanines. 2023;27(06):852–860. doi:10.1142/S1088424623500621
282. Mitchison DA. Basic mechanisms of chemotherapy. Chest. 1979;76(6):771–780. doi:10.1378/chest.76.6.771
283. Fang X, Xu J, Jin K, Qian J. Combining of immunotherapeutic approaches with chemotherapy for treatment of gastric cancer: achievements and limitations. Int Immunopharmacol. 2023;118:110062. doi:10.1016/j.intimp.2023.110062
284. Zhang X, He Q, Sun J, et al. Near-infrared-enpowered nanomotor-mediated targeted chemotherapy and mitochondrial phototherapy to boost systematic antitumor immunity. Adv Healthc Mater. 2022;11(14):e2200255. doi:10.1002/adhm.202200255
285. Chen YW, Chen PJ, Hu SH, Chen IW, Chen SY. NIR‐triggered synergic photo‐chemothermal therapy delivered by reduced graphene oxide/carbon/mesoporous silica nanocookies. Adv Funct Mat. 2014;24(4):451–459. doi:10.1002/adfm.201301763
286. Chandra RA, Keane FK, Voncken FE, Thomas CR. Contemporary radiotherapy: present and future. Lancet. 2021;398(10295):171–184. doi:10.1016/S0140-6736(21)00233-6
287. Gong L, Zhang Y, Liu C, Zhang M, Han S. Application of radiosensitizers in cancer radiotherapy. Int J Nanomed. 2021;16:1083–1102. doi:10.2147/IJN.S290438
288. Srinivasan D, Subbarayan R, Srivastava N, et al. A comprehensive overview of radiation therapy impacts of various cancer treatments and pivotal role in the immune system. Cell Biochem Funct. 2024;42(6):e4103. doi:10.1002/cbf.4103
289. Busato F, Khouzai BE, Mognato M. Biological mechanisms to reduce radioresistance and increase the efficacy of radiotherapy: state of the art. Int J Mol Sci. 2022;23(18):10211. doi:10.3390/ijms231810211
290. Cui J, Wang T-J, Zhang Y-X, She L-Z, Zhao Y-C. Molecular biological mechanisms of radiotherapy-induced skin injury occurrence and treatment. Biomed Pharmacother. 2024;180:117470. doi:10.1016/j.biopha.2024.117470
291. Chen Y, Deng Y, Li Y, et al. Oxygen-independent radiodynamic therapy: radiation-boosted chemodynamics for reprogramming the tumor immune environment and enhancing antitumor immune response. ACS Appl Mat Interfaces. 2024;16(17):21546–21556. doi:10.1021/acsami.4c00793
292. Shahbazi‐Gahrouei D, Choghazardi Y, Kazemzadeh A, Naseri P, Shahbazi‐Gahrouei S. A review of bismuth‐based nanoparticles and their applications in radiosensitising and dose enhancement for cancer radiation therapy. IET nanobiotechnol. 2023;17(4):302–311. doi:10.1049/nbt2.12134
293. Wen S, Ovais M, Li X, et al. Tailoring bismuth-based nanoparticles for enhanced radiosensitivity in cancer therapy. Nanoscale. 2022;14(23):8245–8254. doi:10.1039/D2NR01500E
294. Yu N, Li X, Wen M, et al. Doxorubicin-loaded Bi-PEG nanoparticles as novel chemo-photothermal nanoagents for efficiently killing cancer cells. J Nanosci Nanotechnol. 2020;20(4):2032–2039. doi:10.1166/jnn.2020.17212
295. Hu P, Tian X, Sun Z, et al. Au nanoclusters embedded in polydopamine for photothermal radiotherapy on non-small-cell lung cancer. ACS Appl Nano Ma. 2024;7(16):19724–19736. doi:10.1021/acsanm.4c03967
296. Abas MDM, Asri MFM, Yusafawi NAS, et al. Advancements of gene therapy in cancer treatment: a comprehensive review. Pathol-Res Pract. 2024;261:155509. doi:10.1016/j.prp.2024.155509
297. Jiang Y, Fan M, Yang Z, et al. Recent advances in nanotechnology approaches for non-viral gene therapy. Biomat Sci. 2022;10(24):6862–6892. doi:10.1039/D2BM01001A
298. Han X, Mitchell MJ, Nie G. Nanomaterials for therapeutic RNA delivery. Matter. 2020;3(6):1948–1975. doi:10.1016/j.matt.2020.09.020
299. Huang H, Yuan G, Xu Y, et al. Photoacoustic and magnetic resonance imaging-based gene and photothermal therapy using mesoporous nanoagents. Bioact Mater. 2022;9:157–167. doi:10.1016/j.bioactmat.2021.07.025
300. Zhi D, Yang T, Zhang T, Yang M, Zhang S, Donnelly RF. Microneedles for gene and drug delivery in skin cancer therapy. J Control Release. 2021;335:158–177. doi:10.1016/j.jconrel.2021.05.009
301. Li J, Pu K. Semiconducting polymer nanomaterials as near-infrared photoactivatable protherapeutics for cancer. Accounts Chem Res. 2020;53(4):752–762. doi:10.1021/acs.accounts.9b00569
302. Wang D, Fang Y, Lin L, et al. Upregulating miR-181b promotes ferroptosis in osteoarthritic chondrocytes by inhibiting SLC7A11. BMC Musculoskeletal Disorders. 2023;24(1):862. doi:10.1186/s12891-023-07003-7
303. González PM, Piloni NE, Puntarulo S. Iron overload and lipid peroxidation in biological systems. Lipid Peroxidation. 2012;4:89–108.
304. Liu T, Sun L, Zhang Y, Wang Y, Zheng J. Imbalanced GSH/ROS and sequential cell death. J Biochem Mol Toxicol. 2022;36(1):e22942. doi:10.1002/jbt.22942
305. Rochette L, Dogon G, Rigal E, Zeller M, Cottin Y, Vergely C. Lipid peroxidation and iron metabolism: two corner stones in the homeostasis control of ferroptosis. Int J Mol Sci. 2022;24(1):449. doi:10.3390/ijms24010449
306. Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018;63(1):68–78. doi:10.1016/j.advms.2017.05.005
307. Irato P, Santovito G. Enzymatic and non-enzymatic molecules with antioxidant function. MDPI. 2021;10:579.
308. Weaver K, Skouta R. The selenoprotein glutathione peroxidase 4: from molecular mechanisms to novel therapeutic opportunities. Biomedicines. 2022;10(4):891. doi:10.3390/biomedicines10040891
309. Pei J, Pan X, Wei G, Hua Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front Pharmacol. 2023;14:1147414. doi:10.3389/fphar.2023.1147414
310. Forcina GC, Dixon SJ. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics. 2019;19(18):1800311. doi:10.1002/pmic.201800311
311. Gong Y, Wang N, Liu N, Dong H. Lipid peroxidation and GPX4 inhibition are common causes for myofibroblast differentiation and ferroptosis. DNA Cell Biol. 2019;38(7):725–733. doi:10.1089/dna.2018.4541
312. Sun -L-L, He H-Y, Li W, Jin W-L, Wei Y-J. The solute carrier transporters (SLCs) family in nutrient metabolism and ferroptosis. Biomarker Res. 2024;12(1):94. doi:10.1186/s40364-024-00645-2
313. Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599–620. doi:10.1007/s13238-020-00789-5
314. Huang Q, Ru Y, Luo YL, et al. Identification of a targeted ACSL4 inhibitor to treat ferroptosis-related diseases. Sci Adv. 2024;10(13):eadk1200.
315. Deng H, Zhang J, Yang Y, et al. Chemodynamic and photothermal combination therapy based on dual-modified metal-organic framework for inducing tumor ferroptosis/pyroptosis. ACS Appl Mater Interfaces. 2022;14(21):24089–24101. doi:10.1021/acsami.2c00574
316. Naletova I, Tomasello B, Attanasio F, Pleshkan VV. Prospects for the use of metal-based nanoparticles as adjuvants for local cancer immunotherapy. Pharmaceutics. 2023;15(5):1346. doi:10.3390/pharmaceutics15051346
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

