Back to Journals » International Journal of Nanomedicine » Volume 20
Nanodrug Delivery Systems for Direct Clearance or Neutralization of LPS
Authors Chen L , Pan R , Xu X , Guo J
Received 4 December 2024
Accepted for publication 28 April 2025
Published 3 July 2025 Volume 2025:20 Pages 8653—8673
DOI https://doi.org/10.2147/IJN.S510037
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Krishna Nune
Lu Chen,1 Ran Pan,1 Xiaoling Xu,2 Junping Guo1
1Rehabilitation and Nursing School, Hangzhou Vocational & Technical College, Hangzhou, Zhejiang, People’s Republic of China; 2Shulan International Medical College, Zhejiang Shuren University, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Xiaoling Xu, Shulan International Medical College, Zhejiang ShurenUniversity, Hangzhou, 310015, People’s Republic of China, Tel/Fax +86-571-88208435, Email [email protected] Junping Guo, Rehabilitation and Nursing School, Hangzhou Vocational & Technical College, Hangzhou, Zhejiang, 310018, People’s Republic of China, Tel/Fax +86-571-56700217, Email [email protected]
Abstract: Lipopolysaccharide (LPS) is a unique component of the outer wall of gram-negative bacteria and is a complex composed of lipids and polysaccharides. Normally, liver sinusoidal endothelial cells (LSECs) systemically clear LPS via unknown mechanisms. However, under pathological conditions, LPS causes lethal endotoxemia if it cannot be rapidly cleared from the blood circulation. Currently, an increasing number of drugs have demonstrated the ability to clear or neutralize LPS. However, due to their inherent characteristics, such as low biocompatibility and short half-life, their clinical applications are limited. Nanodrug delivery systems have the advantages of improving pharmacokinetics, increasing bioavailability, reducing the occurrence of side effects, and prolonging drug circulation time, which can compensate for the shortcomings of traditional drugs. This review summarizes nanodrug delivery systems that can clear or neutralize LPS, such as polymer nanodrug delivery systems, lipid-based nanodrug delivery systems, peptide-based nanodrug delivery systems, inorganic nanodrug delivery systems, nanosponges, and extracellular vesicles, which provides an outlook on the application and future prospects of nanodrug delivery systems for clearing and neutralizing LPS.
Keywords: nanodrug delivery system, LPS clearance, LPS neutralization, inflammation, nanomaterial
Graphical Abstract:

Introduction
Structure of LPS
LPS, also known as endotoxin, is a potent inflammatory molecule found in the outer membrane (OM) of gram-negative bacteria.1 LPS is an amphiphilic molecule anchored via its glycolipid component to the external leaflet of the OM of the gram-negative cell envelope, whereas the inner leaflet consists of phospholipids.2 It consists of three genetically, biologically, and chemically distinct domains: Lipid A, composed of two glucosamines, phosphates, and a certain amount of fatty acids, which are anchored in the bacterial OM and regarded as the bioactive center and main toxic component; the core oligosaccharide (OS) region, linked by 3-deoxy-d-manno-oct-ulosonic acid to lipid A; and the so-called O-antigen or O-specific polysaccharide chain, which points to the outside environment2 (Figure 1). Gram-negative colonies can display either smooth or rough morphology, depending on the type of LPS covering their OM. In bacteria with a smooth phenotype, LPS is called smooth LPS (S-LPS) and consists of lipid A, the core OS region and O-antigen, whereas rough LPS (R-LPS) lacks the O-antigen and consists of only lipid A with a core OS.3,4 Bacteria with a rough morphology, such as Neisseria or Haemophilus,5 express R-LPS, which is deprived of antigens and consists only of core OS and lipid A. Deviating from this general architecture, some core OS can also be substituted by other sugar polymers, such as capsules.6 Further research, the complexity and heterogeneity of the LPS structure revealed that LPS in the same strain is not a single molecule with a specific chemical structure but rather a series of molecular mixtures with inherent size and structural heterogeneity, and these properties may change in response to environmental signals.3
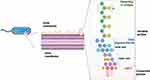 |
Figure 1 Structure of the LPS. LPS is an amphiphilic molecule anchored via its glycolipid component to the external leaflet of the OM of the gram-negative cell envelope, while the inner leaflet consists of phospholipids. Lipid A consists of two glucosamines, phosphates, and a certain number of fatty acids, which are highly conserved among bacteria. The core OS region has minor structural changes and can be further divided into the inner core and outer core. The O-antigen region is a polymer with different degrees of polymerization formed by the head-to-tail connection of oligosaccharide units of a certain length, and its structure is unstable and highly variable. Created in BioRender. Chen, L. (2025) https://BioRender.com/o4s9zqu. |
Biological Activity of LPS
In gram-negative bacteria, LPS constitutes 10%-15% of the total molecules in the OM; notably, 75% of LPS plays a crucial role in supporting the structural integrity of the bacterial OM.7 Furthermore, LPS is pivotal in safeguarding bacteria against stress and the host immune response. LPS is intrinsically linked to the natural resistance of gram-negative bacteria, enabling them to transform their OM into an efficient permeability barrier that obstructs the passage of small hydrophobic molecules through the phospholipid bilayer.8 Upon entry into the host body and subsequent induction of host immune responses, gram-negative bacteria assume a pivotal role in bacterial‒host interactions.9
In mammals, LPS is one of the most potent stimulators of the immune system and is responsible for inducing the expression of numerous cytokines, chemokines, and other immune mediators in human immune cells, such as monocytes, macrophages, and dendritic cells.10 Low doses of LPS may be beneficial for the activation of immune cells, and studies have demonstrated that the oral administration of LPS can rejuvenate small intestinal immunity and macrophage activity, thereby providing benefits in the treatment of malignant tumors.11 However, high concentrations of LPS cause a pathophysiological response, with a cascade of immune responses from the host that often results in severe inflammation and tissue damage.12 For example, high concentrations of lipopolysaccharides paired with certain cytokines (e.g., TNF, IL-18, IFN-γ or IL-1β) to regulate different cell types can produce changes similar to sepsis in a tissue state.12,13 Chronic exposure to LPS has been implicated in the pathogenesis of various diseases, including sepsis, inflammatory bowel disease, and certain types of cancer.14 An approximately twofold increase in LPS activity is considered “metabolic endotoxemia”.15 Chronic endotoxemia is involved in the pathogenesis of many inflammation-driven diseases, especially cardiometabolic diseases, including atherosclerotic cardiovascular disease, obesity, liver disease, diabetes and metabolic syndrome, and is therefore considered a risk factor.16
The Mechanism of LPS-Induced Inflammation and the Immune Response
Toll-like receptor 4 (TLR4) serves as the principal membrane receptor for LPS. LPS signals primarily through TLR4 via two distinct pathways: the TLR4/myeloid differentiation factor 88 (MyD88)/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway, which transduces plasma membrane signals; and the TLR4/β interferon TIR domain-containing adapter-inducing interferon-β (TRIF)/interferon regulatory factor 3 (IRF3) signaling pathway, which transduces CD14-dependent endocytic body signals.17–19 The former process culminates in the nuclear translocation of NF-κB, which triggers the production and release of proinflammatory cytokines such as IL-1β, TNF-α, and IL-6.20,21 Conversely, the latter process enables the translocation of phosphorylated IRF-3 to the nucleus, initiating the transcription of the IFN-β gene.22 In the MyD88-dependent pathway, activated MyD88 recruits IL-1 receptor-associated kinases (IRAK4, IRAK1, and IRAK2).23 These proteins are then phosphorylated and dissociated from the MyD88/IRAK complex, which binds to tumor necrosis factor (TNF) receptor-associated factor (TRAF6);24 the latter activates the TAK1-TAB1 complex, which in turn stimulates the IKKα/IKKβ complex. This causes the phosphorylation of the NF-κB inhibitor, which promotes its ubiquitination and degradation.23 Free NF-κB thus enters the nucleus to regulate transcription and activate the gene expression of proinflammatory cytokines.23 Moreover, TAK1-TAB1 can also activate mitogen-activated protein kinase (MAPK) family complexes. Phosphorylated MKK3/6 and MKK4/7 activate p38 and c-Jun N-terminal kinase (JNK), respectively.3 Thus, activator protein-1 (AP-1) and p38 are activated and enter the nucleus to initiate the gene expression of proinflammatory cytokines such as IL-1 and TNF-α.3,25 In the TRIF-dependent pathway, TLR4 recruits a TIR domain-containing adaptor in TRAM (TRIF-related adapter molecule) and TRIF. The latter recruits TRAF3 and receptor-interacting protein-1 (RIP1). Then, TRAF3 undergoes self-ubiquitination and forms a complex with TBK1 and IKKε.3 This complex then phosphorylates TBK1 or IKKε, thereby activating IRF, which forms homodimers and heterodimers with IRF7 and translocates to the nucleus to bind DNA target sequences, transcribing IFN and IFN-induced genes. In addition, TRIF can also activate NF-κB (late activation) by recruiting TRAF626 (Figure 2).
 |
Table 1 Removal/Adsorption Rate of LPS in Different Nanoparticles |
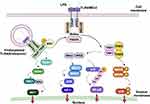 |
Figure 2 The mechanism of LPS-induced inflammation and the immune response. LPS stimulation of TLR4 induces the release of key proinflammatory cytokines required for the activation of a potent immune response. TLR4 signaling can be divided into MyD88-dependent and MyD88-independent (TRIF-dependent) pathways. MyD88 activates IRAKs/TRAF6 and the downstream transcription factors NF-κB, AP-1, and P38. These transcription factors induce the expression of proinflammatory cytokine genes. In the TRIF-dependent pathway, the TLR4-MD2-LPS complex is internalized and retained in endosomes, triggering signal transduction by recruiting TRAM and TRIF to activate IRF7 and late-phase NF-κB. Created in BioRender. Chen, L. (2025) https://BioRender.com/jpou0lm. |
In addition to the aforementioned classical signaling pathways, an increasing number of researchers have identified additional mechanisms underlying LPS-induced inflammatory and immune responses. Yeranddy A. Alpizar et al identified a signaling mechanism that operates independently of the traditional TLR4 immune pathway.27 They reported that LPS activates transient receptor potential vanilloid 4 (TRPV4), which in turn mediates an increase in intracellular calcium and the production of the crucial antimicrobial molecule nitric oxide. This leads to immediate protective responses, including direct antibacterial action, enhanced airway clearance, and the modulation of inflammation-intrinsic immune responses. Hashimoto et al discovered that MEK inhibitors amplified LPS-induced iNOS expression and NO production by stimulating interleukin-12 (IL-12) production in mouse bone marrow-derived macrophages, thereby increasing mortality rates in mice suffering from LPS-induced inflammation.28
Nanodrug Delivery Systems: A New Approach to Clear LPS
LPSs are bacterial toxins. Normally, LPSs are securely sequestered within the gut. However, when they breach this barrier and enter the bloodstream, they can instigate inflammation and contribute to various health issues. Numerous methods exist for the removal of endotoxins in biological preparations, as detailed in various studies.29,30 However, these techniques are largely unsuitable for in vivo applications. In the management of sepsis, polymyxins remain the primary therapeutic option for gram-negative bacteria. Despite their potent antibacterial activity against these bacteria, their clinical application is limited by their associated toxicity.31 Therefore, an increasing number of studies are currently designing nanodelivery systems on the basis of the production or mechanism of action of LPS, which can significantly increase drug activity and, more specifically, eliminate or neutralize LPS.
Compared with free drugs, nanomedicines have advantages in terms of stability, solubility and pharmacokinetics in vivo, and approximately 250 nanomedicines are currently approved or at different stages of clinical evaluation (pre).32 The ultimate goal of nanomedicine is to improve patient outcomes by increasing the concentration of drugs in target tissues or cells while reducing exposure to healthy tissues to decrease toxicity. Currently, many nanodrug delivery systems have shown good clinical prospects.
Currently, several peptides,33,34 proteins,33,35,36 cell membranes,37–41 and cationic/amphipathic molecules and polymers42–45 have been reported to bind to LPS, and they can be effective methods for the clinical clearance or neutralization of LPS. Nanodrug delivery systems refer to technologies that utilize nanoscale particles as carriers to deliver organic small molecules or biological macromolecules to the cells and tissues under study. NPs are diverse in composition and possess advantages such as a high specific surface area, adjustable shape and size, and ease of modification for targeting,46 and they are widely used in in vivo delivery. On this basis, the nanodrug delivery system improves upon traditional drugs and has many advantages: (1) it enhances their pharmacokinetics, aiding drug molecules in crossing physiological and pathological barriers, thereby increasing bioavailability;47 (2) nanodrugs can achieve active and passive targeting, increasing the local concentration of nanodrugs within the lesion, enhancing efficacy while reducing side effects to achieve safer and more effective disease diagnosis and treatment;48,49 (3) it prolongs the circulation time of drugs, controls drug release, and improves patient compliance;50 (4) through clever “camouflage”, it reduces immune recognition and clearance by the reticuloendothelial system in organisms, protects active molecules from enzymatic degradation, increases the retention time of drugs in the body, extends the half-life of drugs, and enhances efficacy;37,38,40,41 and (5) it realizes diversity and intelligence. In addition to bioactive chemical molecules, nanocarriers can also carry biologically active substances such as peptides and nucleic acids.51
This review provides an overview of recent advancements in nanotechnology for the removal of LPS from biological systems. We discuss various nanodrug delivery strategies, including polymer nanodrug delivery systems, lipid-based nanodrug delivery systems, peptide-based nanodrug delivery systems, inorganic nanodrug delivery systems, nanosponges, and extracellular vesicles, and highlight their mechanisms of action and therapeutic potential. Additionally, we explore the challenges and opportunities associated with the development of nanodrug delivery systems for LPS detoxification, including biocompatibility, targeting efficiency, and clinical translation. This study helps elucidate the opportunities and challenges that current nanodrug delivery systems face in treating LPS-induced diseases.
Application of Different Types of Nanodrug Delivery Systems for the Clearance of LPS
In recent years, nanodrug delivery systems have emerged as promising tools for targeted drug delivery and therapy. These nanostructures typically range from 1 to 500 nanometers in size.52 They can compensate for the shortcomings of traditional drugs, serving as delivery systems to transport therapeutic agents or naturally active compounds to their target locations for treatment. In recent years, many studies have shown that nanodrug delivery systems can more effectively clear or neutralize LPS, and nanodrug delivery systems that facilitate advanced systems of drug delivery are currently being studied.
Polymer Nanodrug Delivery Systems for LPS scavenging
Polymers can be used in the fabrication of several nanostructures, such as polymeric micelles, dendrimers, nanoparticles, nanogels, nanocapsules and vesicles.53 Due to their good potential for surface modification via chemical transformations, excellent pharmacokinetic control, and suitability for the entrapment and delivery of a wide range of therapeutic agents, they are widely used as drug delivery systems.53
The utilization of polymeric nanomedicines in human subjects has a rich history. Toraymyxin, a blood endotoxin removal cartridge, was developed by Toray Industries Inc. in 1994 for direct hemoperfusion. This drug is based on a polystyrene-based fiber adsorbent composition that is covalently fixed with the ligand of the polymyxin B (PMB) antibiotic, serving as an effective adsorbent for LPS. Clinically, it has been employed to eliminate endotoxins in patients suffering from gram-negative septic shock.54 Since 1994, more than 30,000 patients have been treated with Toraymyxin in Japan. In the first multicenter clinical study, endotoxin was detected in 37 of 42 patients, and the mean blood endotoxin level decreased significantly from 83.7 ± 26.7 pg/mL before treatment to 56.4 ± 27.9 pg/mL after treatment.55 In another multicenter clinical study, the levels of interleukin-6 (IL-6), tumor necrosis factor α (TNF-α) and plasminogen activator inhibitor-1 (PAI-1) decreased significantly in 78 of 88 patients whose LPS levels decreased by more than 30%.56 These findings suggest that Toraymyxin treatment reduces the level of LPS and may also exert its effect by decreasing the levels of inflammatory mediators. This was a good result at the time, but PMX treatment still has some potentially serious side effects, such as a decrease in platelet count, and the optimal duration and frequency of DHP also need further study to determine. However, looking back, for a long time, one of the most promising treatments for sepsis was based on blood filtering.
An obvious shortcoming of the blood filtration approach is that proteins will rapidly adsorb onto the surface of the artificial membrane, leading to the activation of coagulation and compromising the effective clearance of endotoxin. To overcome this challenge, Mariia Vorobii et al engineered polymer brush-coated microparticles with antifouling properties.57 The modified material with specific functions, μP-PMB, was obtained by grafting poly(HPMA-co-CBMAA) polymer brushes onto high-porosity microparticles (μP-GMA) and subsequently immobilizing the antibacterial agent PMB onto μP-GMA, to specifically remove the most common endotoxin, LPS. Zhenqiang Shi et al reported a robust strategy for specific clearance of targeted LPS in circulating blood on the basis of phage display screening, and the design of a hemocompatible bottlebrush polymer with a peptide has been reported.58 The nanodrug delivery system contains a novel peptide, PEP-1 (HWKAVNWLKPWT), and the hemocompatible bottlebrush polymer [poly(PEGMEA-co-PEP-1)] bearing short peptides has high LPS selectivity, which can significantly reduce LPS levels from 2.63 ± 0.01 to 0.78 ± 0.05 EU mL−1 in septic rabbits via in vitro hemoperfusion and can reverse LPS-induced leukopenia and multiorgan damage. In this polymer, the short peptide shows high specificity for the LPS type, which enables the specific removal of LPS from circulating blood, improving the prognosis of sepsis patients.
Overall, polymer nanodrug delivery systems have the longest history among nanodrug delivery systems. Their core advantage lies in the flexibility and adjustability of their chemical structures: by selecting different monomers and polymerization methods, the physicochemical properties of nanoparticles (such as particle size, surface charge and hydrophobicity) can be precisely controlled, thus meeting the complex requirements of LPS clearance. These findings indicate that polymeric nanodrug delivery systems hold an important position in the field of LPS clearance.
Nanogel Delivery System for LPS Clearance
Gels are cross-connected polymer networks that swell in a fluid medium, and nanogels can be defined as highly cross-linked nanoscale hydrogel systems, which can be copolymers or monomers, ionic or nonionic, with a three-dimensional hydrophilic network that has a strong tendency to absorb water or physiological fluids without changing the internal network structure.59 Due to its unique properties, we classify it separately from the polymer nanodrug delivery system. Nanogels typically range in size from 20 to 200 nm, and they can escape renal clearance and prolong the serum half-life.60,61 By chemically modifying ligands and binding many ligands, they can be used for targeted drug delivery, stimuli-responsive drug release or the preparation of composite materials.62 Compared with other nanocarriers, such as liposomes, micelles, and nanoparticles, gels composed of nanoparticles result in greater drug accumulation.
Polymyxins, in addition to their antibacterial properties, act as positive ligands to bind and neutralize endotoxins.63 With this property, many strategies have been developed to target the neutralization of endotoxins in vivo. For example, Fei Wang et al prepared nanosponges by mechanically extruding purified mouse erythrocyte membranes with a poly(lactic-co-glycolic acid) (PLGA) polymer core and then mixing the preprepared nanosponges with acrylamide (as a monomer), poly(ethylene glycol) dimethacrylate (PEGDMA) (as a crosslinker), TEMED, and ammonium persulfate (both as initiators) for gelation to form nanosponge-hydrogel hybrids specifically for the topical treatment of MRSA infection.64 While these nanosponges can sequester toxins in vitro and in vivo, it is unclear which toxins are being removed. Xiaoyu Li et al used nanogels as carriers, prepared nanogels through inverse emulsion polymerization via biodegradable poly(acrylamide methacrylic acid) [P(AAm-co-MAA)] copolymers, and loaded three types of effector molecules—bactericidal colistin, SLAP-S25-neutralizing LPS, and acyloxyacyl hydrolase (AOAH)-degrading LPS—to form a type of nanodrug delivery system.63 This system is named GNGs and is a multifunctional drug delivery strategy that combines antibacterial, LPS neutralization, and detoxification effects. The principle of the system to clear LPS is very ingenious. First, colistin, an antibiotic with bactericidal activity, rapidly kills bacteria and subsequently releases the captured LPS. Then, AOAH removes the secondary fatty chain of lipid A in LPS and converts LPS into an inactive form, thus achieving in situ detoxification. In a mouse lung infection model infected with Pseudomonas aeruginosa PAO1, GNGs showed better anti-inflammatory activity than polymyxin (GC), and the production of TNF-α and IL-6 was approximately 4 times lower in the presence of GNGs than in the GC group. In a mouse acute peritonitis-sepsis model, compared with polymyxin alone, GNGs exhibited comparable antibacterial and anti-inflammatory activities against P. aeruginosa, and GNGs improved pathological damage, such as lung hemorrhage and congestion, in mice with severe sepsis. These findings demonstrate the excellent ability of GNGs to clear LPS and treat sepsis. This all-in-one nanoplatform combines antibacterial and anti-inflammatory effects to eradicate bacterial pathogens and inhibit LPS-induced inflammatory responses, which provides alternative strategies for the treatment of clinical inflammation associated with sepsis.
In addition to immobilized PMB, some other nanogels can also be used to enrich and remove LPS through electrostatic adsorption. Changying Shi et al reported the rational synthesis of a telodendrimer (TD) nanotrap (NT) in size-exclusive hydrogel resins, e.g., PEGA resin with a pore size of ~50 kDa, to preferably capture inflammatory mediators with different charges, including LPS, for precise and effective immune modulation.45 The TD nanotrap was prepared via standard solid-phase peptide synthesis procedures, whereby the LPS-binding motifs were successively coupled to TentaGel (TG), PEGA or PVA–PEG resins, followed by Fmoc deprotection steps and Pbf deprotection steps. Finally, after the Fmoc removal step, the LPS-binding hydrophobic constructs were coupled to the α-amine of arginine via the insertion of a triethylene glycol linker. NT(+) resin exhibited excellent ability to remove LPS and treat sepsis. PEGA-(ArgC17)4 can remove approximately 96% of the LPS in whole blood in vitro. In vivo experiments using PEGA-(ArgC17)4 NT(+) resin by intraperitoneal injection for the treatment of septic mice revealed that PEGA-(ArgC17)4 NT(+) resin was able to effectively adsorb and remove the proinflammatory cytokines IL-1β and IL-6 in plasma with efficiencies ranging from 93.7% to 98.6%, respectively. Moreover, the resin could reduce the level of IL-10. When NT(+) resin was used in combination with antibiotics, all the mice survived until the end of the experiment (day 42), while the mortality rate of the mice treated with antibiotics alone was approximately 50% on day 7. The white blood cell count of the mice treated with NT(+) resin gradually decreased over time after treatment, and the white blood cell count remained stable during long-term observation. In contrast, the control mice presented a significantly low WBC count on day 2, followed by a sharp increase, indicating persistent inflammation and hematological instability. On the basis of the experimental data, the TD NT method does not selectively adsorb LPS. It also adsorbs other molecules that lead to increased inflammation, such as cytokines, free DNA/RNA, and other molecules, compromising the adsorption efficiency of LPS. Additionally, this method is currently used primarily in mice, and long-term transplantation into the abdominal cavity is unsuitable for clinical use in sepsis patients, nor is it applicable to sepsis patients in later stages of immunosuppression. Therefore, this method may not be sufficient to reverse multiorgan failure. Yupei Li et al described a green approach to prepare genipin crosslinked chitosan‒kappa‒carrageenan composite hydrogels (C‒K hydrogels) by modifying the traditional chitosan gel through phase transition and genipin crosslinking techniques to prepare chitosan hydrogels with the potential capacity for reducing endotoxin and bacterial loads, followed by the introduction of carrageenan onto the surface of the crosslinked chitosan hydrogels to improve their biocompatibility, especially their anticoagulant properties.65 The optimized C2‒K1 hydrogel could remove 63.3% of the endotoxins during a 3 h simulated hemoperfusion process with a maximum adsorption capacity of 95.0 EU/g. Bacteria cleansing experiments further demonstrated that the optimized C2-K1 hydrogel effectively reduced the loads of Escherichia coli and Staphylococcus aureus by 46.0% and 68.7%, respectively. Thus, the C-K hydrogel can simultaneously reduce the endotoxin level and bacterial load in septic blood and is expected to be a hemoperfusion adsorbent for patients with severe sepsis. In summary, the advantages of the C-K hydrogel, include that it can reduce both the endotoxin level and bacterial load in the blood during sepsis, and significantly inhibit adverse blood-material interactions, such as hemolysis, complement activation, platelet activation, and contact activation, resulting in better anticoagulant performance than other hydrogels. It is clear that C-K hydrogel, as a potential novel blood perfusion adsorbent, is likely to be applied in the treatment of severe sepsis in the near future.
In conclusion, nanogels are dispersions of hydrogel nanoparticles based on cross-linked polymeric networks and have been called next-generation drug delivery systems because of their relatively high drug encapsulation capacity, uniformity, tunable size, ease of preparation, minimal toxicity, etc., which show great potential in adsorbing and removing LPS.
Hydrogel nanodrug delivery systems, as emerging LPS clearance platforms, demonstrate significant advantages because of their unique physicochemical properties. The hydrogel network structure can not only efficiently load LPS capture agents (such as peptides, cationic polymers, antibiotics, etc.) but also achieve sustained and targeted drug delivery through pore size regulation. However, this system still faces many challenges: the insufficient mechanical strength of natural polymer hydrogels can lead to premature disintegration, the biocompatibility and degradation control of synthetic hydrogels need to be optimized, the delivery efficiency across biological barriers needs to be improved, and the potential foreign body reactions caused by long-term retention also need systematic evaluation. An increasing amount of research has focused on the innovative design of smart materials, such as the construction of biomimetic membrane-modified nanohydrogels. With the deep integration of material science and nanotechnology, hydrogel nanodelivery systems are expected to overcome the limitations of existing LPS clearance therapies.
Lipid-Based Nanodrug Delivery Systems
Lipid nanoparticles (LNPs) have a long history of development and are considered ideal carriers for drug delivery because of their excellent biocompatibility, biodegradability, and encapsulation efficiency. As early as 1995, the first lipid-based nanodrug, Doxil®, was approved by the FDA.66 Doxil was “passively targeted” to tumors, and the use of PEGylated nanoliposomes prolonged the drug circulation time, avoiding the occurrence of RES, and doxorubicin was released and acted on tumor cells.66 Unfortunately, no studies have shown that Doxil® clears LPS. Onpattro is also a lipid nanoparticle-based short interfering RNA drug and a nucleic acid-based nanodelivery system for the treatment of polyneuropathy caused by hereditary transthyretin amyloidosis, but no studies have shown its efficacy in LPS clearance.67 There is little development of lipid-based nanodrug delivery systems for the clearance of LPS.
Huiwen Liu et al prepared PLPs (polymyxins covalently conjugated to PEGylated liposomes) by covalently coupling PMB to the surface of PEGylated liposomes via a thin-film hydration method, which adsorbs circulating LPS through specific interactions between PMB and LPS.68 In the AS (atherosclerosis) mouse model, PLP treatment reduced the LPS concentration by approximately 24%, and the LPS concentration always remained significantly lower. The levels of the main cytokine TNF-α gradually decreased from 2.47 ± 0.32 to 1.71 ± 0.11 pg/mL after PLP treatment, and the levels of other inflammatory cytokines, such as IL-1β, IL-6 and IFN-γ, also tended to decrease. In the control groups, the serum TNF-α levels in the PBS and LPS groups continuously increased from 4.50±0.25 pg/mL and 3.60±0.18 pg/mL to 6.31±0.36 pg/mL and 4.90±0.52 pg/mL, respectively. Aorta root section staining revealed that the aortic plaque area in the PLP group was significantly reduced, and the proportion of lipid accumulation in the plaque area was also significantly decreased. The proportion of M1 macrophages in AS plaques was reduced, indicating that PLPs can stabilize AS plaques, reduce the plaque burden in the arteries, and ultimately slow the development of AS (Figure 3).
 |
Figure 3 PMB-conjugated PEGylated liposomes (PLPs) specifically adsorb LPS, alleviate the inflammatory response, and suppress atherosclerosis progression. (A) Schematic depiction of the preparation process of PLPs. (B) Representative images of oil red O (ORO)-stained atherosclerotic lesions of the aorta en face. (C) Corresponding quantitative analysis of plaque areas in the total intimal area of the aorta. (D) Serum levels of LPS. (E–H) Inflammatory cytokines, including TNF-α (E), IL-1β (F), IL-6 (G), and IFN-γ (H), in the 1st, 8th, and 16th weeks from AS model mice after different treatments (n = 4). All data were presented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001. ns, not significant. These figures were published in Liu H, Wang H, Li Q, et al. LPS adsorption and inflammation alleviation by polymyxin B-modified liposomes for atherosclerosis treatment. Acta Pharm Sin B. 2023;13(9):3817–3833. Copyright Elsevier.68 |
Lixian Jiang et al designed a bionic hybrid liposome (P-RL: PMB-modified, red blood cell-mimetic hybrid liposome) that covalently conjugated PMB with artificial phospholipids, and then, they fused the conjugate with red blood cell membranes, which anchored Escherichia coli and adsorbed LPS as a nanodecoy to achieve LPS clearance.41 In vitro, P-RL binds 73% of total LPS. In the mixed toxin-infected mouse model, PMB liposome or erythrocyte vesicle treatment did not significantly improve the survival of the mice, whereas P-RL treatment significantly prolonged the survival time of the mice in each group and reduced the mortality rate by 50% (p < 0.05). Morphological analysis revealed obvious damage to the liver in the toxin treatment group. Most hepatocytes were swollen and vacuolated, accompanied by obvious necrosis or nuclear dissolution, which may be due to inflammation-related injury. Compared with the other groups, P-RL significantly inhibited toxin-induced liver injury, and the cell morphology and arrangement were relatively normal. ELISA analysis of proinflammatory cytokines in the blood revealed that, compared with the other groups, the P-RL group presented significantly lower IL-1β, IL-6 and TNF-α levels, which is consistent with the in vitro results and indicates that P-RL can capture LPS and prohibit multitoxin-induced inflammation and tissue damage, thereby preventing death. Compared with existing RBC vesicles or PMB-modified liposomes, P-RL has excellent therapeutic effects against murine RBC hemolysis, macrophage activation and mixed toxin infection not only by neutralizing gram-positive bacterial toxins but also by anchoring gram-negative bacteria and their endotoxin and exotoxin. In addition, P-RL can be localized at the site of infection, which increases the probability of contact with toxins, indicating that P-RL may improve detoxification efficiency and significantly expand the detoxification range of the current antivirulence system, which may have a good potential application effect.
High-density lipoprotein (HDL) can naturally bind and detoxify LPS;69 however, acute inflammatory responses caused by LPS exposure lead to decreased circulating HDL levels, which in turn decrease the endotoxin neutralization capacity.70 HDL nanodrug delivery systems that possess the functions of natural HDL while overcoming the shortcomings of insufficient circulating levels of natural HDL have been designed. On the basis of the natural ability of human HDL to bind to LPS, Linda Foit et al, synthesized a set of HDL-like nanoparticles (HDL-like NPs),71 which utilize citrate-stabilized gold nanoparticles (AuNPs) as a scaffold to control the size and shape of conjugates. LPS exposure was found to induce the upregulation of multiple proinflammatory cytokines and agents involved in the recruitment of inflammatory cells, including IL-1a, IL-1b, IL-6, IL-8, CCL3 (MIP-1a), CCL4 (MIP-1b), CCL5 (RANTES) and CCL8 (MCP-2), to sites of infection by approximately 1000-fold. In contrast, LPS-induced inflammatory gene expression was significantly inhibited in the presence of NPs. These findings suggest that the NPs can effectively reduce the LPS-induced inflammatory response. HDL-like NPs can clear and neutralize LPS toxins by inhibiting TLR4-dependent inflammatory responses caused by LPS from multiple bacterial sources, which is a powerful endotoxin scavenger with significant potential for alleviating LPS-mediated inflammation.
Overall, liposome nanodrug delivery systems that can clear or neutralize LPS have many advantages: they possess inherent membrane affinity, reducing immune rejection; they have multimechanism drug loading capabilities, where liposomes can simultaneously encapsulate hydrophobic drugs to clear LPS, and can also directly neutralize negatively charged LPS through electrostatic adsorption, or deliver drugs to block the binding of LPS with host receptors. However, the clinical application of pure liposomes still faces key challenges: the physical stability of the phospholipid bilayer is poor, and it is easily affected by lipoproteins in the blood or enzymatic action, leading to leakage and other issues. To address these problems, increasing research has focused on the development of multifunctional hybrid liposomes that balance stability and functional diversity; or the design of biomimetic modification strategies, such as the use of macrophage membrane-coated liposomes to enhance immune evasion and lesion-targeting capabilities. In the future, further exploration of cascading response systems, integrating multistep collaborative mechanisms of LPS recognition, neutralization, and anti-inflammatory signal regulation, is possible. Perhaps with the advancement of computational simulation technology, liposome nanodelivery systems are expected to achieve breakthroughs in the entire chain, from efficient clearance to immune regulation in the treatment of LPS-related diseases.
Peptide-Based Nanodrug Delivery Systems
Peptides are relatively common types of natural polymers that are typically composed of multiple amino acids that can form secondary structures (α-helices, β-sheets) through hydrogen bonding. Peptides can be prepared via solid-phase synthesis, which is a simple method. Peptides possess biological activity and have the advantages of being easily metabolized, having low immunogenicity, and causing fewer toxic side effects. Peptides, as emerging building blocks for drug delivery systems, possess high biological activity. Their side chains can carry a variety of active functional groups (carboxylic acids, hydroxyl groups, amino groups, and thiol groups), allowing various chemical modifications to achieve the functions of the drug delivery system.35 Peptides have attracted widespread research interest as drug delivery systems, demonstrating tremendous application potential.
Tripti Kumari et al identified a synthetic MyD88 fragment, KRCRRMVVVV (M3), which is rich in 10 arginine‒valine residues, from the crystal structure of the innate immune protein MyD88.35 This fragment has a β-sheet structure when it binds with the OM component LPS of gram-negative bacteria. The isothermal titration calorimetry (ITC) experimental data indicate that M3 has a high affinity for LPS and that the interaction is hydrophobic. Computational studies suggest that the multiple consecutive valine residues of M3 strongly interact with the acyl chains of LPS. In vivo experiments revealed that a single dose of 5 mg/kg M3 inhibited the proinflammatory response (TNF-α and IL-6) induced by LPS (10 mg/kg) by approximately 60%. When the mice were given a single intraperitoneal injection of 5 mg/kg or 10 mg/kg M3, the survival rates of the mice injected with a lethal dose of LPS (12 mg/kg) were 60% and 80%, respectively, after 7 days. On the basis of the methods described in the article, peptides with specific secondary structures can be designed, which not only have significant effects on the clearance of LPS, but also provide new valuable active peptides for further research.
The peptide nanonet designed by Nhan Dai Thien Tram et al selectively captures negatively charged LPS and the proinflammatory cytokines TNF-α and IL-6, inhibits the production of cytokines (TNF-α and IL-6) from a mouse macrophage line induced by LPS and rescues the antibacterial activity of antibiotics by binding to LPS.36 These nanonets are assumed to specifically bind and capture endotoxin and proinflammatory cytokines by leveraging the large negative surface charge on these inflammation mediators, and minimizing the undesirable inactivation of beneficial anti-inflammatory mediators. Peng Tan et al constructed self-assembled chimeric peptide nanoparticles (NPs) 1 and 2 that bind through charge interactions and potentially disrupt bacterial membranes to clear LPS, with broad-spectrum antibacterial activity and desirable biocompatibility, and maintain their antibacterial ability in physiological saline environments.33 Although this study demonstrated only the affinity of nanopeptides for LPS and did not directly prove the ability of nanopeptides to clear LPS, a significant decrease in the serum levels of the proinflammatory cytokines tumor necrosis factor α (TNF-α), interleukin 6 (IL-6) and interleukin 1β (IL-1β) was observed in mice treated with peptide nanoparticles 1 and 2 compared with those in the saline treatment group in a mouse model of acute sepsis. These findings suggest that peptide nanoparticles have not only direct antibacterial effects but also immunomodulatory properties, which complement each other to help prevent systemic bacterial infection. In conclusion, peptide-based nanodrug delivery systems can effectively remove LPS both in vitro and in vivo, showing promising anti-inflammatory activity.
Due to their unique molecular programmability and biocompatibility, peptide-based nanodrug delivery systems have great potential in the field of LPS clearance. From the above discussion, nanostructures formed by the self-assembly of peptide molecules can efficiently capture and neutralize LPS through cationic groups and hydrophobic groups. Their small molecular weight and precisely designable structure allow for the customization of high-affinity LPS-binding domains. Notably, peptide-based nanodrug delivery systems also share the shortcomings of traditional nanomedicines, especially the high risk of premature drug release/leakage and low stability.72 In the complex human body environment, peptides are easily degraded by proteases, leading to loss of function, and some hydrophobic peptides may cause hemolytic toxicity. Moreover, the in vivo metabolic pathways and long-term safety of nanostructures still need systematic verification. With the advancement of biotechnology, the above challenges will be gradually overcome. Therefore, functional peptides have broad application prospects in biologically related fields, and peptide-based nanodrug delivery systems may be applied in clinical practice in the near future.
Inorganic Nanodrug Delivery Systems
The efficient and safe delivery of drugs, proteins, or genes to the therapeutic site has always been a research hotspot. Currently, LPS-binding peptides have been reported to react with LPS or LPS-related receptors, exhibiting antibacterial or anti-inflammatory effects;73 however, the proteolytic stability of these peptides limits their application. On the other hand, inorganic nanodrug delivery systems do not have these shortcomings. Inorganic nanoparticles are promising gene vectors with several advantages over nature-derived/organic materials, including easily tunable physicochemical properties, relatively high stability, and generally low fabrication costs.74 Currently, many types of inorganic nanodrug delivery systems, including metals, oxides, semiconductors, and carbon-based structures, are available.75 However, not all materials can directly remove or neutralize LPS. We classify inorganic nanodrug delivery systems that can directly remove or neutralize LPS into metal nanodrug delivery systems, nonmetal nanodrug delivery systems, and magnetic nanodrug delivery systems on the basis of their composition and characteristics.
Some metal nanodrug delivery systems can directly capture LPS, reducing the extent of endotoxemia. Fang-Hsuean Liao et al designed a supramolecular trap fabricated from subnanometer gold nanosheets for the capture and inactivation of free LPS, which can not only reduce the degree of endotoxemia but also enhance the low-dose antibacterial activity of polymyxins.76 The anti-LPS activity of the supramolecular trap is achieved by blocking lipid A of free LPS to provide effective steric hindrance, thereby preventing the interaction between polymyxins and free LPS. Without interference from free LPS, polymyxins can maintain their maximum antibacterial activity at low doses, minimizing endotoxemia and bacteremia.76 Injection of LPS in mice increased the survival rate from 0% to 90% after treatment with SAuM. It follows that supramolecular traps made of gold nanoplates can not only minimize endotoxemia to the greatest extent, possible but also maximize the antibacterial efficacy of colistin, so that colistin can be used at a much lower dose. Thus, potential colistin resistance can be avoided.
Nonmetallic nanodrug delivery systems are also important types of inorganic nanodrug delivery systems. Yun Meng et al proposed a boron-trapping strategy to prevent infection and excessive inflammation by synthesizing a class of reactive metal boride nanoparticles (MgB2 NPs).77 The MB nanoparticles gradually hydrolyze to generate boron dihydrides and metal cations while generating a local alkaline microenvironment, which greatly enhances the capture of LPS or PGN (peptidoglycan) by boron dihydrides through an esterification reaction, not only increasing metal cation-induced bacterial death but also inhibiting the dead bacterium-induced excessive inflammatory response in vitro and in vivo, ultimately accelerating wound healing.77 In the Pseudomonas aeruginosa skin infection model, Nano-MgB2 treatment significantly inhibited the lesion area induced by P. aeruginosa infection, reduced the number of bacteria in the lesion area, and significantly reduced the number of neutrophils and macrophages induced by P. aeruginosa, as well as the inflammatory factors IL-6, TNF-α, and MCP-1. These data once again show that Nano-MgB2 can inhibit bacterial growth and reduce bacterium-induced inflammation in vivo (Figure 4). This boron-trapping strategy is quite novel or inhibits the viability and pathogenicity of pathogens by trapping their key component, LPS/PGN. Not only did it inhibit bacterial survival, but it also reduced the excessive inflammation induced by dead bacteria. These effects ultimately promoted the healing of infected wounds. Similarly, Yuk et al developed a nanoparticle system called D-TZP, which consists of iron-complexed tannic acid nanocapsules containing vitamin D cores encapsulated with PMB and chitosan derivatives, where the chitosan derivative controls the interaction of PMB with LPS, bacteria, and host cells to both inactivate endotoxins and kill gram-negative bacteria.78 The in vivo experimental results revealed that when LPS and D-TZP were simultaneously injected intraperitoneally, the survival rate of the mice increased from 20% to 100%. When D-TZP was administered immediately after intraperitoneal injection of LPS, the animals treated with D-TZP (PMB [40 mg/kg]) presented a 100% survival rate, whereas none of the mice in the control group survived (0% survival) after treatment with D5W. After LPS was fully absorbed systemically (2 hours post-injection), all the control animals died, while the survival rate of the animals treated with. In addition, the levels of TNF-α and IL-10 in mouse plasma were reduced nearly 10-fold, indicating that D-TZP could effectively protect mice from the LPS-induced lethal inflammatory response in a model of endotoxemia induced by LPS. D-TZP attenuates the membrane toxicity associated with PMB, is safer, and retains the ability of PMB to inactivate endotoxins and kill gram-negative bacteria. The entire design concept is very novel. Higher biocompatibility also means that it has more applications. However, its large particle size, short half-life, and limited renal distribution may mean that it cannot address the issue of acute kidney injury caused by LPS. In the future, if the challenges of reducing its particle size and extending its half-life can be overcome, D-TZP will be a promising systemic therapy for gram-negative sepsis.
 |
Figure 4 Bacterial infection and accompanying inflammation can be treated via a boron-trapping strategy. (A) Schematic diagram of Nano-MgB2 capturing LPS: Reactive metal borides (such as nano-MgB2) gradually hydrolyze to generate boron dihydroxy groups (HO-B-OH) and metal cations (Mg2+) while generating a local alkaline microenvironment. The alkaline microenvironment promotes the capture of key components (LPS/PGN) of bacteria by HO-B-OH. (B) Photos of the skin inflammation caused by 10 μg or 50 μg of Nano-MgB2-treated or untreated dead bacteria (HIB, heat-inactivated bacteria, Pseudomonas aeruginosa) in mice (n = 5). H&E staining of the HIB-induced mouse skin. Scale bar = 100 μm. (C) Protein expression level. IL-6(C), TNF-α(D) and MCP-1(E) protein expression was measured with a CBA mouse inflammation kit from the HIB-induced mouse skin described in (B) (n = 5 biologically independent mice). Data are representative of at least three independent experiments. Values are the mean ± SEM. One-way ANOVA with Bonferroni post test was used to analyze multiple groups. Reprinted from Meng Y, Chen L, Chen Y, et al. Reactive metal boride nanoparticles trap lipopolysaccharide and peptidoglycan for bacteria-infected wound healing. Nat Commun. 2022;13(1):7353. http://creativecommons.org/licenses/by/4.0/).77 Under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). |
Magnetic drug delivery systems, as the member of the inorganic nanodrug delivery systems, directly adsorb LPS and pathogens through their unique electrostatic adsorption action. Zhenqiang Shi et al designed and synthesized a novel imidazole-based ionic liquid with good antibacterial activity.79 They used a polydopamine coating as a hemocompatibility platform to fix the ionic liquid onto Fe3O4 nanoparticles, forming hemocompatible magnetic particles (Fe3O4@PDA-IL). Magnetic particles have good hemocompatibility and effectively remove various pathogens of clinical significance in human whole blood, with broad-spectrum bacterial capture ability. The removal efficiencies for Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and even MRSA were 80.5 ± 0.4%, 61.3 ± 0.8%, 27.3 ± 1.5% and 32.5 ± 1.4%, respectively. In addition, they can eliminate endotoxins from bacteria in the blood and inhibit further deterioration caused by sepsis. The experimental results revealed that the LPS endotoxins released by pathogenic bacteria (24.8 ± 1.2 EU/mg in total 30 EU/mg) can be removed simultaneously by electrostatic action. Fe3O4@PDA-IL possesses both hemocompatibility and broad-spectrum bacterial scavenging ability, but the residual issue of magnetic particles remains unaddressed. The in vivo distribution, accumulation, and degradation of residual magnetic particles still need further confirmation. Donald E. Ingber et al developed a hemodiafiltration filter with hollow fibres, the FcMBL-HF (mannose binding lectin linked to Fc domain hemoadsorption filter) device, which uses magnetic nanoparticles coated with genetically engineered human mannose-binding lectin, a lectin that lacks complement-fixing and coagulation domains and is linked to an antibody Fc domain. MBL binds to LPS but not to mammalian cells so that LPS clearance can be achieved.43,44 The results of the experiment showed that FcMBL-HF had as good an ability to remove endotoxins as to remove live E. coli, and it did so more quickly, with LPS levels reduced by over 80% within 30 min and over 95% within 5 h after initiating FcMBL-HF hemoperfusion treatment.
Inorganic nanodrug delivery systems, due to their high stability, tunable physicochemical properties, and unique magnetic responsiveness, demonstrate irreplaceable advantages in the field of LPS clearance. Inorganic nanocarriers, which include metal oxides, precious metals, and other nonmetals, can be efficiently loaded with LPS-binding ligands through surface functionalization modifications. However, as seen from the previous examples, the limitations of this system must also be taken into account: some metal nanoparticles carry a risk of biological accumulation, and the long-term toxicity evaluation system is not yet perfected; the mechanisms of how surface charge and particle size distribution affect the phagocytosis of immune cells are complex, and in the inflammatory microenvironment, they might exacerbate tissue damage due to oxidative stress. Future research could explore degradable inorganic-organic hybrid nanosystems to balance functionality with safety, and also integrate CRISPR delivery technology to endow carriers with the ability to actively regulate inflammatory signaling pathways.
Nanosponges as Nanodecinators That Neutralize LPS
Currently, in clinical practice, the main approach for treating severe infections caused by LPS is through controlling the infection, maintaining hemodynamics, and providing major organ support.80 Commonly used methods include PMB or activated carbon to clear blood LPS, but their selectivity and removal rates are not ideal. Once LPS enters the circulation, it is recognized by toll-like receptor 4 (TLR-4) of innate immune cells with the assistance of LPS binding protein (LBP) and CD14 receptors, activating intracellular signals and triggering the release of effector cytokines such as TNF-α, IL-1, and IL-6.81 In this process, innate immune cells, including monocytes and macrophages, play a vital role in recognizing LPS and mediating inflammatory responses. The specific ability of immune cells, such as macrophages and neutrophils, to recognize LPS can be used to design nanodecoys to capture LPS. Cell membrane-coated nanoparticles have emerged as nanodecoys to absorb bacterial toxins, thus diverting them from their intended cellular targets. Nanosponges have the potential to develop broad-spectrum neutralization strategies for pathogenic factors, irrespective of their diversity, by emulating host cells.82
Macrophage membrane-coated nanosponges inherit the ability of macrophages to neutralize LPS and can significantly improve the survival rate of endotoxemic mice. Song Shen et al developed nanoparticles, called Fe3O4@MM, by wrapping iron oxide nanoclusters with macrophage membranes.37 Fe3O4@MMs had a significant affinity for LPS, and the results of the in vitro experiments revealed that its removal rate was as high as 81.1%, whereas the removal rate of PMB as a control was only 7.8%. The Fe3O4@MMs could clear different types of proinflammatory cytokines, and 0.5 mg of the Fe3O4@MMs could neutralize 13.7 pg of TNF-α and 24.3 pg of IL-1β, with corresponding removal rates of 13.7% and 2.9%, respectively. When the amount of Fe3O4@MMs increased to 2 mg, the removal rates increased to 24.9% and 5.5%, respectively. It also significantly improved the survival rate of endotoxemic mice. In the in vivo experiments, all the mice challenged with 15 mg/kg LPS died within 48 h after injection. However, in the group of mice treated with Fe3O4@MMs, the mortality rate was significantly reduced to 30%. In contrast, PMB did not improve the survival rate of endotoxemic mice.37 Soracha Thamphiwatana et al also used the membrane of macrophages, which have the same antigenic outer surface as that of macrophages and thus inherit their ability to bind and neutralize endotoxins, to design MΦ-NPs.38 These nanoparticles bind LPS with high affinity through the macrophage membrane on their surface, especially through the binding of the lipid A moiety of LPS to the pattern recognition receptor CD14. This binding prevents LPS from effectively activating pattern recognition receptors on the host cell surface, thereby preventing the initiation of subsequent immune responses and inflammatory cascades. In vitro, the removal capacity was 62.5 ng of LPS per mg of MΦ-NPs, and the clearance efficiency was approximately 92%. Mice treated with MΦ-NPs (300 mg/kg) in the E. coli lethal infection model presented a survival rate of 60%, whereas no significant improvement was observed in mice treated with RBC-NPs or PEG-NPs. Compared with those in the control group, the bacterial counts in key organs, such as the blood, spleen, kidney and liver, were significantly lower in the MΦ-NP-treated group, and the levels of proinflammatory cytokines in these organs were lower. Fangyu Zhang et al made biohybrid motors, called MΦ‒Mg motors, by incubating titanium dioxide-coated magnesium microparticles and poly(L-lysine) (PLL) layers with living MΦs at low temperature.39 MΦ–Mg motors removed 66.82 ± 6.31% of the LPS from the system, which was approximately 13% greater than that of free macrophages (as a positive control) (53.34 ± 4.48%).
Neutrophil membrane-coated nanosponges can adsorb LPS both in vivo and in vitro. Neutrophil membrane nanodecoys fabricated via a simple extrusion method by Yao Xiao et al can effectively remove LPS, and their ability to bind to LPS, IL-1β, TNF-α, and SAA is dose-dependently enhanced. They can also significantly alleviate LPS-induced hepatocyte injury, such as by reducing the levels of inflammatory cytokines and liver injury biomarkers in plasma, including aspartate aminotransferase, alanine aminotransferase, and direct bilirubin, ultimately improving the survival rate of septic mice.40
Red blood cell (RBC) membrane-coated nanosponges, which mimic erythrocytes in vivo, can not only neutralize LPS but also endow nanosponges with superior drug transport capabilities. Yijie Chen et al engineered a nanosponge that adeptly employs natural protein receptors on RBC membranes to mimic host cells. This design enables the neutralization of various harmful molecules without specifically targeting the pathogen’s structure.83 Furthermore, this nanosponge can emulate the extended circulation properties of natural red blood cells, which can be achieved by applying a coating of the RBC membrane onto the polymer core, thereby endowing the nanosponge with superior drug transport capabilities.83 Lixian Jiang et al designed a nanodecoy that covalently conjugated PMB with artificial phospholipids, and then, we fused the conjugate with RBC membranes to prepare a bionic hybrid liposome (P-RL), which could anchor Escherichia coli and adsorb LPS as a nanodecoy to achieve LPS clearance.41 P-RL is essentially a liposome nanodrug delivery system. It does not utilize the antigen on the surface of RBC membranes to adsorb LPS but rather connects PMB to the membrane surface, thereby endowing the nanoparticles with the ability to adsorb LPS. Moreover, it significantly reduced the levels of IL-1β, IL-6, and TNF-α, prolonged the survival time of the mice in each group and reduced the mortality rate by 50% (p < 0.05).
In addition to the use of natural cell membrane-coated nanoparticles, some polymers can also be used to make nanosponges for LPS adsorption. Yao Huang et al utilized the good stability of PAN (polyacrylonitrile) and the good biocompatibility of SiO2 to select PAN nanofibers and SiO2 nanofibers to prepare a nanofiber sponge.84 The nanofiber sponge prepared by electrospinning and freeze-drying technology has the advantages of good blood compatibility and high porosity, which results in high throughput; thus, most substances in the blood can pass through, and then, PMB is fixed on the nanofiber sponge to successfully prepare an endotoxin adsorbent. These nanosponges achieved a 90% endotoxin removal rate in human plasma, and adsorption reached equilibrium within 60 min, demonstrating that the PMB-immobilized nanofibrous sponge has great potential for clinical blood purification.
Due to the different membrane sources used, the abovementioned “macrophage membrane-coated nanosponges”, “neutrophil membrane-coated nanosponges”, and “RBC membrane-coated nanosponges” have different characteristics. Macrophage membrane-coated nanosponges often contain macrophage surface molecules (eg, CD14), which can specifically capture LPS and can also be widely used to treat various diseases related to LPS. However, the manufacturing process is relatively complicated, and the production cost may be high, which may limit its promotion in a wide range of clinical applications. Neutrophil membrane nanosponges play a key role in the pathogenesis of sepsis-related liver injury and reduce hepatocyte damage caused by inflammatory mediators. The neutrophil membrane can also directly regulate the chemotaxis and adhesion of neutrophils, thereby reducing neutrophil infiltration and apoptosis in the liver and improving therapeutic effects. Compared with RBC membrane biomimetic nanosponges, neutrophil membranes have shown stronger therapeutic advantages, especially in neutralizing endotoxin, TNF-α and CXCL6. However, the optimal effective dose range of the neutrophil membrane and its dose-response relationship need to be further determined, and this research has focused mainly on the effects of the neutrophil membrane in the acute phase. The long-term effects and possible long-term side effects are still unclear. RBC membrane-coated nanosponges have superior drug transport capabilities and can be combined with a variety of delivery systems. Although it has good biocompatibility, the degradation process inside the human body and potential immune responses need further study.
Extracellular Vesicles as a Natural Nanodrug Delivery System for LPS Clearance
Extracellular vesicles (EVs) are classified on the basis of their cellular origin, biological function or biogenesis. As determined by their biogenesis, the three main classes of extracellular vesicles are exosomes, microvesicles and apoptotic bodies. They are all cell-derived vesicles that are enclosed by a lipid bilayer ranging from 30 nm to 2,000 nm in diameter, depending on their origin.52 EVs can transfer contents containing complex biomolecules from one cell to another, even over long distances; therefore, they are considered natural carrier systems.85 They have recently received much attention because of their instrumental role in physiological and pathological processes.
Artificially synthesized drug nanocarriers have long been developed to improve the therapeutic efficacy, pharmacokinetics and pharmacodynamics of treatment while reducing toxic side effects.86 However, synthetic drug delivery systems still face many setbacks, such as nonspecific drug targeting, carrier toxicity, immunogenicity, and unsatisfactory efficacy.85 Compared with artificially synthesized nanocarriers, EVs have many advantages as delivery carriers. First, EVs are derived from cells and are more easily metabolized after drug delivery. Second, exosomes exhibit lower immunogenicity and avoid unwanted immune responses. In addition, EVs can cross other in vivo barriers that are difficult for other nanocarriers to overcome, such as the blood‒brain barrier.87 Cell-released bionanoparticles, including EVs, are emerging as a new class of highly sophisticated drug carriers (Figure 5). EVs are natural nanoparticles released by prokaryotic and eukaryotic cells, they are similar in size, shape, and structure to liposomes but have a more complex bilayer structure containing hundreds of different lipid, protein, and carbohydrate types, as well as internal cargo and surface-associated molecules.87 Witwer KW et al analyzed and compared the characteristics of free drugs, clinically approved synthetic nanoparticles, and EVs.87 They reported that EVs have greater complexity and variability and more complicated manufacturing, and although the effective time in vivo is not high enough, they have more functions. The high production cost and relatively difficult extraction of EVs mean that the separation and characterization of EVs are challenging, which might be very regrettable if they do not have good therapeutic effects. Therefore, we cannot conclude that EVs nanodrug delivery systems are superior to other nanodrug delivery systems in many aspects. However, it is undeniable that EVs, as drug carriers, have shown great promise in multiple fields and are gradually overcoming the aforementioned challenges. The use of natural carrier system EVs in therapy has also become one of the many methods used to overcome the limitations of liposomes.88
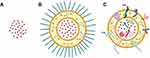 |
Figure 5 Structural comparison of free drugs, clinically approved synthetic nanoparticles, and EVs. (A) Free drug; (B) synthetic nanoparticles, with lipid-based nanoparticles used as an example. (C) Extracellular vesicles, a natural nanodrug delivery system similar to liposomes in terms of size, shape and structure, but with a more complex bilayer. Created in BioRender. Chen, L. (2025) https://BioRender.com/lhr63v9. |
EVs inherently possess the ability to absorb LPS. Puja Kumari et al reported that extracellular vesicles capture and chaperone systemic LPS to the cytosol, thereby activating an atypical inflammasome response.89 These EVs were isolated from mouse plasma by ultracentrifugation, and researchers injected FITC–LPS EVs or PBS EVs into Casp11−/− mice. Five hours after injection, the cytoplasm of the splenic myeloid cells was extracted. Through LAL analysis, LPS was detected in the cytoplasm of sorted splenic myeloid cells from mice injected with FITC–LPS-EVs, and the survival period of the mice was significantly prolonged, with a survival rate greater than 70%. There have been reports on the use of extracellular vesicles as nanodelivery systems for clearing LPS. Dongmei Sun et al reported that the levels of IL-6 and TNF-α in the serum of mice treated with exosome-curcumin were significantly reduced and that the survival of these mice was prolonged, indicating that exosome-curcumin can effectively reduce inflammatory responses and may help to clear LPS from the body.90
Although EVs naturally capture and bind to LPS through their lipid bilayer, such binding does not depend on proteins on the surface of EVs. EVs may also be heterogeneous in cargo and function because of their source cell type and pathological state. The loading capacity of EVs is relatively limited and less easily improved than that of other nanodrug delivery systems, which might limit their application for massive LPS delivery. The clinical application of EVs still requires time, and more breakthroughs are needed.
Others
In addition to the above classical nanodrug delivery systems, in recent years, due to the development and contribution of various interdisciplinary fields, innovative means for achieving the clearance or neutralization of inflammatory mediators have emerged continuously, and clever carrier design has provided new ideas for the clearance and neutralization of LPS.
Marta Pacheco et al designed a micromotor, which was assembled by using the biocompatible polymer polycaprolactone for the encapsulation of CdTe or CdSe@ZnS quantum dots (QDs) as photoactive materials and an asymmetric Fe3O4 patch for propulsion.91 The micromotors can be activated with visible light (470–490 nm) to propel them in peroxide or glucose media via a diffusiophoretic mechanism that clears LPS in human blood serum.91 For 20 μg/mL endotoxin, the highest removal percentage of 90% was achieved after 30 min of navigation in glucose and peroxide media. However, efficient micromotor propulsion was observed in the blood, and the toxin removal efficiency of this medium was low (less than 30%), which could be due to micromotor biofouling, with red blood cells and other blood components attaching to the micromotors, preventing endotoxin diffusion into the pores and attachment to the micromotor surface. Therefore, future work should aim at incorporating appropriate antibiofouling coatings in micromotors. While the design of biomedical technologies to overcome the limitations of light penetration beyond the dermis is still needed, the combination of biocompatible polymers with adsorptive capacity for toxins and photoactive quantum dots with exchange capacity has provided a powerful platform for future biomedical or environmental applications of light-driven nanodrug delivery systems.
Conclusion
This review summarizes the current nanodrug delivery systems for the clearance or neutralization of LPS, including their types, clearance mechanisms and clearing effects (Table 1). As a common endotoxin, LPS can activate monocytes, endothelial cells, epithelial cells, etc., in the body through cellular signal transduction systems; these cells synthesize and release various cytokines and inflammatory mediators, which in turn trigger a series of responses in the organism, and it can even be life-threatening if it is not removed in time.10 To address inflammation and adverse immune responses caused by LPS, clinical treatments often involve eliminating pathogenic bacteria or inhibiting the activation of downstream immune cells by LPS. However, specific clearance of LPS from circulating blood is highly challenging because of its structural complexity and its variation between/within bacterial species.58
Currently, nanodrug delivery systems that can directly clear or neutralize LPS mainly include polymer nanodrug delivery systems, inorganic nanodrug delivery systems, peptide nanodrug delivery systems, exosomal nanodrug delivery systems, lipid nanodrug delivery systems, nanodecoys, and nanosponges. They overcome many problems existing in traditional drug administration and can change properties, such as solubility, drug release, diffusion, bioavailability, and immunogenicity, not only providing convenient routes of administration but also reducing toxicity, minimizing side effects, improving biodistribution, and extending the life cycle of drugs. However, several key issues remain. For example, the safety and toxicity profiles of these nanomedicines lack effective regulation. Despite the considerable number of approved nanomedicines, the absence of distinct regulatory guidelines for the development and characterization of these nanomaterials has hindered their clinical potential.92 In addition, when these nanomedicines are introduced into biological systems, various nanomaterials have structural and functional relationships and their characteristics, and components and surface coatings interact with the biological system. This has the potential to produce aggregates and agglomerates that may result in unexpected toxic effects.92 However, many scientific researchers and clinical workers are still trying to overcome these obstacles.
From this review, we have gained a clear understanding of the advantages of nanodrug delivery systems for direct LPS clearance, as well as the existing deficiencies, which may be addressed by other functional nanodrug platforms. Among nanodrug platforms in other fields, stimulus-responsive nanodelivery platforms have emerged.93 We boldly predict that future nanodrug delivery systems for clearing and neutralizing LPS may place more emphasis on their ability to respond dynamically to the inflammatory microenvironment. For example, by designing multiresponsive carriers such as those sensitive to pH, reactive oxygen species (ROS), and others, precise release of LPS neutralizers at the lesion site can be achieved. Photothermal or photocontrolled drug release systems can also be utilized to achieve more controllable spatiotemporal LPS clearance through external stimuli, reducing off-target effects. Moreover, the singular function of LPS clearance no longer meets complex pathological needs. Multifunctional integration and synergistic therapy might lead to more breakthroughs. In the future, we can focus on integrating “capture-neutralize-anti-inflammation” multifunctional modules within nanodrug delivery systems. We might even utilize gene editing tools (such as CRISPR) to endow systems with the ability to regulate inflammatory signaling pathways, achieving an integrated “treatment-repair” effect. With the rapid development of artificial intelligence, its combination with nanotechnology may accelerate the optimization of nanodrug delivery systems. For example, molecular dynamics simulations assisted by machine learning can efficiently screen for high-affinity LPS-binding ligands. Biomimetic technology can, to a certain extent, prolong circulation time and reduce toxicity, but the risk of long-term accumulation remains undeniable. In the future, perhaps more degradable hybrid materials can be explored to balance functionality with metabolic safety.
Therefore, we strongly believe that the emerging science of “nanodrug delivery systems that directly clear or neutralize LPS”, a field only over 20 years old, is an area of great potential and is key to future breakthroughs in the treatment of diseases caused by LPS. Its precise delivery, efficient neutralization, and synergistic therapeutic characteristics are unparalleled by those of other treatment methods.
Abbreviations
LPS, Lipopolysaccharide; LSECs, Liver sinusoidal endothelial cells; OM, Outer membrane; OS, Oligosaccharide; S-LPS, Smooth LPS; R-LPS, Rough LPS; MAMPs, Microbe-associated molecular patterns; DAMPs, Damage-associated molecular patterns; TLRs, Toll-like receptors; MyD88, Myeloid differentiation factor 88; NF-κb, Nuclear factor kappa-light-chain-enhancer of activated B cells; TRIF, β interferon TIR domain-containing adapter-inducing interferon-β; TRAM, TRIF-related adapter molecule; IRF3, Interferon regulatory factor 3; TNF, Tumor necrosis factor; MAPK, Mitogen-activated protein kinase; JNK, C-Jun N-terminal kinase; AP-1, Activator protein-1; RIP1, Receptor-interacting protein-1; TRPV4, Transient receptor potential vanilloid 4; PAI-1, Plasminogen activator inhibitor-1; PMB, Polymyxin B; PLGA, Poly (lactic-co-glycolic acid); AOAH, Acyloxyacyl hydrolase; TD, Telodendrimer; NT,Nanotrap; TG, TentaGel; LNPs, Lipid nanoparticles; PLPs, Polymyxin covalently conjugated to PEGylated liposomes; AS, Atherosclerosis; HDL, High-density lipoprotein; AuNPs, Gold nanoparticles; ITC, Isothermal titration calorimetry; PGN, Peptidoglycan; FcMBL-HF, Mannose Binding Lectin linked to Fc domain hemoadsorption filter; PAN, Polyacrylonitrile; EVs, Extracellular vesicles.
Acknowledgments
This work was supported by the High-Level Talent Research Project of Hangzhou Vocational & Technical College [Grant No. RCXY202309]. We would like to thank Guanghui Yan from HIKVISION for his contribution to polishing the figures of this article. We would like to thank the BioRender team again for their support of our work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Campbell NA, Reece JB. Biology. Pearson Education India; 2005.
2. Pussinen PJ, Kopra E, Pietiäinen M, et al. Periodontitis and cardiometabolic disorders: the role of lipopolysaccharide and endotoxemia. Periodontol 2000. 2022;89(1):19–40. doi:10.1111/prd.12433
3. Di Lorenzo F, Duda KA, Lanzetta R, Silipo A, De Castro C, Molinaro A. A journey from structure to function of bacterial lipopolysaccharides. Chem Rev. 2022;122(20):15767–15821. doi:10.1021/acs.chemrev.0c01321
4. Di Lorenzo F, De Castro C, Lanzetta R, Parrilli M, Silipo A, Molinaro A In: Jimenez-Barbero J, Canada FJ, Martin-Santamaria S, eds. Lipopolysaccharides as Microbe-Associated Molecular Patterns: A Structural Perspective. Lipopolysaccharides as Microbe-Associated Molecular Patterns: A Structural Perspective.The Royal Society of Chemistry; 2015pp. 38–63.
5. John CM, Phillips NJ, Stein DC, Jarvis GA. Innate immune response to lipooligosaccharide: pivotal regulator of the pathobiology of invasive Neisseria meningitidis infections. Pathog Dis. 2017;75(3). doi:10.1093/femspd/ftx030
6. Amor PA, Whitfield C. Molecular and functional analysis of genes required for expression of group IB K antigens in Escherichia coli: characterization of the his-region containing gene clusters for multiple cell-surface polysaccharides. Mol Microbiol. 1997;26(1):145–161. doi:10.1046/j.1365-2958.1997.5631930.x
7. Erridge C, Bennett-Guerrero E, Poxton IR. Structure and function of lipopolysaccharides. Microbes Infect. 2002;4(8):837–851. doi:10.1016/S1286-4579(02)01604-0
8. Alexander MK, Miu A, Oh A, et al. Disrupting gram-negative bacterial outer membrane biosynthesis through inhibition of the lipopolysaccharide transporter MsbA. Antimicrob Agents Chemother. 2018;62(11). doi:10.1128/AAC.01142-18.
9. Simpson BW, Trent MS. Pushing the envelope: LPS modifications and their consequences. Nat Rev Microbiol. 2019;17(7):403–416. doi:10.1038/s41579-019-0201-x
10. Davydova VN, Yermak IM, Gorbach VI, Krasikova IN, Solov’eva TF. Interaction of bacterial endotoxins with chitosan. Effect of endotoxin structure, chitosan molecular mass, and ionic strength of the solution on the formation of the complex. Biochemistry. 2000;65(9):1082–1090.
11. Morishima A, Inagawa H. Clinical effects of orally administered lipopolysaccharide derived from pantoea agglomerans on malignant tumors. Anticancer Res. 2016;36(7):3747–3751.
12. Bone RC. The sepsis syndrome. Definition and general approach to management. Clin Chest Med. 1996;17(2):175–181. doi:10.1016/S0272-5231(05)70307-5
13. Takahama M, Patil A, Richey G, et al. A pairwise cytokine code explains the organism-wide response to sepsis. Nat Immunol. 2024;25(2):226–239. doi:10.1038/s41590-023-01722-8
14. Lundin JI, Checkoway H. Endotoxin and cancer. Environ Health Perspect. 2009;117(9):1344–1350. doi:10.1289/ehp.0800439
15. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi:10.2337/db06-1491
16. Kallio E, Begon M, Birtles RJ. Lipopolysaccharide: a link between periodontitis and cardiometabolic disorders. Vector Borne Zoonotic Dis 2014;14:389–393. doi:10.1089/vbz.2013.1383
17. Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi:10.1016/j.cyto.2008.01.006
18. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi:10.1038/ni.1863
19. Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–1066.
20. Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews Immunology. 2004;4(7):499–511.
21. Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874.
22. Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25(3):349–360. doi:10.1016/j.immuni.2006.08.009
23. Haque MA, Jantan I, Harikrishnan H. Zerumbone suppresses the activation of inflammatory mediators in LPS-stimulated U937 macrophages through MyD88-dependent NF-κB/MAPK/PI3K-Akt signaling pathways. Int Immunopharmacol. 2018;55:312–322. doi:10.1016/j.intimp.2018.01.001
24. Srikanth N, Deliz-Aguirre R, Gola DK, Bilay M, Ziska E, Taylor MJ. IRAK4 autophosphorylation controls inflammatory signaling by activating IRAK oligomerization. bioRxiv. 2024;
25. Baig MS, Liu D, Muthu K, et al. Heterotrimeric complex of p38 MAPK, PKCδ, and TIRAP is required for AP1 mediated inflammatory response. Int Immunopharmacol. 2017;48:211–218. doi:10.1016/j.intimp.2017.04.028
26. Weiss J, Barker J. Diverse pro-inflammatory endotoxin recognition systems of mammalian innate immunity. F1000Res. 2018;7:516. doi:10.12688/f1000research.13977.1
27. Alpizar YA, Boonen B, Sanchez A, et al. TRPV4 activation triggers protective responses to bacterial lipopolysaccharides in airway epithelial cells. Nat Commun. 2017;8(1):1059. doi:10.1038/s41467-017-01201-3
28. Hashimoto R, Koide H, Katoh Y. MEK inhibitors increase the mortality rate in mice with LPS-induced inflammation through IL-12-NO signaling. Cell Death Discov. 2023;9(1):374. doi:10.1038/s41420-023-01674-w
29. Sulc R, Szekely G, Shinde S, et al. Phospholipid imprinted polymers as selective endotoxin scavengers. Sci Rep. 2017;7:44299. doi:10.1038/srep44299
30. Szermer-Olearnik B, Boratyński J. Removal of endotoxins from bacteriophage preparations by extraction with organic solvents. PLoS One. 2015;10(3):e0122672. doi:10.1371/journal.pone.0122672
31. Davies B, Cohen J. Endotoxin removal devices for the treatment of sepsis and septic shock. Lancet Infect Dis. 2011;11(1):65–71. doi:10.1016/S1473-3099(10)70220-6
32. Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine. 2013;9(1). doi:10.1016/j.nano.2012.05.013
33. Tan P, Tang Q, Xu S, Zhang Y, Fu H, Ma X. Designing self-assembling chimeric peptide nanoparticles with high stability for combating piglet bacterial infections. Adv Sci. 2022;9(14):e2105955. doi:10.1002/advs.202105955
34. Kasetty G, Kalle M, Mörgelin M, Brune JC, Schmidtchen A. Anti-endotoxic and antibacterial effects of a dermal substitute coated with host defense Peptides. Biomaterials. 2015;53:415–425. doi:10.1016/j.biomaterials.2015.02.111
35. Kumari T, Verma DP, Kuldeep J, et al. 10-residue MYD88-peptide adopts β-sheet structure, self-assembles, binds to lipopolysaccharides, and rescues mice from endotoxin-mediated lung-infection and death. ACS Chem Biol. 2022;17(12):3420–3434. doi:10.1021/acschembio.2c00569
36. Tram NDT, Tran QTN, Xu J, et al. Multifunctional antibacterial nanonets attenuate inflammatory responses through selective trapping of endotoxins and pro-inflammatory cytokines. Adv Healthc Mater. 2023;12(20):e2203232. doi:10.1002/adhm.202203232
37. Shen S, Han F, Yuan A, et al. Engineered nanoparticles disguised as macrophages for trapping lipopolysaccharide and preventing endotoxemia. Biomaterials. 2019;189:60–68. doi:10.1016/j.biomaterials.2018.10.029
38. Thamphiwatana S, Angsantikul P, Escajadillo T, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci U S A. 2017;114(43):11488–11493. doi:10.1073/pnas.1714267114
39. Zhang F, Mundaca-Uribe R, Gong H, et al. A macrophage-magnesium hybrid biomotor: fabrication and characterization. Adv Mater. 2019;31(27):e1901828. doi:10.1002/adma.201901828
40. Xiao Y, Ren C, Chen G, et al. Neutrophil membrane-mimicking nanodecoys with intrinsic anti-inflammatory properties alleviate sepsis-induced acute liver injury and lethality in a mouse endotoxemia model. Mater Today Bio. 2022;14:100244. doi:10.1016/j.mtbio.2022.100244
41. Jiang L, Zhu Y, Luan P, et al. Bacteria-anchoring hybrid liposome capable of absorbing multiple toxins for antivirulence therapy of Escherichia coli infection. ACS Nano. 2021;15(3):4173–4185. doi:10.1021/acsnano.0c04800
42. Zhao H, Zeng Z, Liu L, et al. Polydopamine nanoparticles for the treatment of acute inflammation-induced injury. Nanoscale. 2018;10(15):6981–6991. doi:10.1039/C8NR00838H
43. Didar TF, Cartwright MJ, Rottman M, et al. Improved treatment of systemic blood infections using antibiotics with extracorporeal opsonin hemoadsorption. Biomaterials. 2015;67:382–392. doi:10.1016/j.biomaterials.2015.07.046
44. Kang JH, Super M, Yung CW, et al. An extracorporeal blood-cleansing device for sepsis therapy. Nat Med. 2014;20(10):1211–1216. doi:10.1038/nm.3640
45. Shi C, Wang X, Wang L, et al. A nanotrap improves survival in severe sepsis by attenuating hyperinflammation. Nat Commun. 2020;11(1):3384. doi:10.1038/s41467-020-17153-0
46. Xia Y, Fu S, Ma Q, Liu Y, Zhang N. Application of nano-delivery systems in lymph nodes for tumor immunotherapy. Nanomicro Lett. 2023;15(1):145. doi:10.1007/s40820-023-01125-2
47. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi:10.1038/nrc.2016.108
48. Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi:10.1038/nrd2591
49. Wang K, Shang J, Tao C, et al. Advancements in betulinic acid-loaded nanoformulations for enhanced anti-tumor therapy. Int J Nanomedicine. 2024;19:14075–14103. doi:10.2147/IJN.S493489
50. Zhou L, Zou M, Xu Y, Lin P, Lei C, Xia X. Nano drug delivery system for tumor immunotherapy: next-generation therapeutics. Front Oncol. 2022;12:864301. doi:10.3389/fonc.2022.864301
51. Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nature Reviews Materials. 2016;2(1):16075.
52. El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi:10.1038/nrd3978
53. Venditti I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: a review. Journal of King Saud University - Science. 2019;31(3):398–411. doi:10.1016/j.jksus.2017.10.004
54. Shoji H. Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin). Ther Apher Dial. 2003;7(1):108–114. doi:10.1046/j.1526-0968.2003.00005.x
55. Aoki H, Kodama M, Tani T, Hanasawa K. Treatment of sepsis by extracorporeal elimination of endotoxin using polymyxin B-immobilized fiber. Am J Surg. 1994;167(4):412–417. doi:10.1016/0002-9610(94)90126-0
56. Tani T, Hanasawa K, Endo Y, et al. Therapeutic apheresis for septic patients with organ dysfunction: hemoperfusion using a polymyxin B immobilized column. Artif Organs. 1998;22(12):1038–1044. doi:10.1046/j.1525-1594.1998.06086.x
57. Vorobii M, Kostina NY, Rahimi K, et al. Antifouling microparticles to scavenge lipopolysaccharide from human blood plasma. Biomacromolecules. 2019;20(2):959–968. doi:10.1021/acs.biomac.8b01583
58. Shi Z, Zhang X, Yang X, et al. Specific clearance of lipopolysaccharide from blood based on peptide bottlebrush polymer for sepsis therapy. Adv Mater. 2023;35(33):e2302560. doi:10.1002/adma.202302560
59. Karg M, Pich A, Hellweg T, et al. Nanogels and microgels: from model colloids to applications, recent developments, and future trends. Langmuir. 2019;35(19):6231–6255. doi:10.1021/acs.langmuir.8b04304
60. Ansari S, Karimi M. Novel developments and trends of analytical methods for drug analysis in biological and environmental samples by molecularly imprinted polymers. TrAC Trends in Analytical Chemistry. 2017;89:146–162. doi:10.1016/j.trac.2017.02.002
61. Anooj ES, Charumathy M, Sharma V, et al. Nanogels: an overview of properties, biomedical applications, future research trends and developments. Journal of Molecular Structure. 2021;1239:130446. doi:10.1016/j.molstruc.2021.130446
62. Sivaram AJ, Rajitha P, Maya S, Jayakumar R, Sabitha M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(4):509–533. doi:10.1002/wnan.1328
63. Li X, Qu S, Song X, Wu C, Shen J, Zhu K. In situ neutralization and detoxification of lps to attenuate hyperinflammation. Adv Sci. 2023;10(26):e2302950. doi:10.1002/advs.202302950
64. Wang F, Gao W, Thamphiwatana S, et al. Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant staphylococcus aureus infection. Adv Mater. 2015;27(22):3437–3443. doi:10.1002/adma.201501071
65. Li Y, Li J, Shi Z, et al. Anticoagulant chitosan-kappa-carrageenan composite hydrogel sorbent for simultaneous endotoxin and bacteria cleansing in septic blood. Carbohydr Polym. 2020;243:116470. doi:10.1016/j.carbpol.2020.116470
66. Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi:10.1016/j.jconrel.2012.03.020
67. Akinc A, Maier MA, Manoharan M, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14(12):1084–1087. doi:10.1038/s41565-019-0591-y
68. Liu H, Wang H, Li Q, et al. LPS adsorption and inflammation alleviation by polymyxin B-modified liposomes for atherosclerosis treatment. Acta Pharm Sin B. 2023;13(9):3817–3833. doi:10.1016/j.apsb.2023.06.005
69. Oh I. Muslim governance and the duty to protect. The Journal of Religious Ethics. 2013;41(1):15–19. doi:10.1111/jore.12001
70. Murch O, Collin M, Hinds CJ, Thiemermann C. Lipoproteins in inflammation and sepsis. I. Basic Science. Intensive Care Med. 2007;33(1):13–24. doi:10.1007/s00134-006-0432-y
71. Foit L, Thaxton CS. Synthetic high-density lipoprotein-like nanoparticles potently inhibit cell signaling and production of inflammatory mediators induced by lipopolysaccharide binding Toll-like receptor 4. Biomaterials. 2016;100:67–75. doi:10.1016/j.biomaterials.2016.05.021
72. Wang Q, Jiang N, Fu B, Huang F, Liu J. Self-assembling peptide-based nanodrug delivery systems. Biomater Sci. 2019;7(12):4888–4911. doi:10.1039/C9BM01212E
73. Saravanan R, Holdbrook DA, Petrlova J, et al. Structural basis for endotoxin neutralisation and anti-inflammatory activity of thrombin-derived C-terminal peptides. Nat Commun. 2018;9(1):2762. doi:10.1038/s41467-018-05242-0
74. Sun B, Wu W, Narasipura EA, et al. Engineering nanoparticle toolkits for mRNA delivery. Adv Drug Deliv Rev. 2023;200:115042. doi:10.1016/j.addr.2023.115042
75. Yang J, Shang J, Yang L, et al. Nanotechnology-based drug delivery systems for honokiol: enhancing therapeutic potential and overcoming limitations. Int J Nanomedicine. 2023;18:6639–6665. doi:10.2147/IJN.S431409
76. Liao F-H, Wu T-H, Yao C-N, et al. A supramolecular trap to increase the antibacterial activity of colistin. Angew Chem Int Ed Engl. 2020;59(4):1430–1434. doi:10.1002/anie.201912137
77. Meng Y, Chen L, Chen Y, et al. Reactive metal boride nanoparticles trap lipopolysaccharide and peptidoglycan for bacteria-infected wound healing. Nat Commun. 2022;13(1):7353. doi:10.1038/s41467-022-35050-6
78. Yuk SA, Kim H, Abutaleb NS, et al. Nanocapsules modify membrane interaction of polymyxin B to enable safe systemic therapy of Gram-negative sepsis. Sci Adv. 2021;7(32). doi:10.1126/sciadv.abj1577.
79. Shi Z, Jin L, He C, et al. Hemocompatible magnetic particles with broad-spectrum bacteria capture capability for blood purification. J Colloid Interface Sci. 2020;576:1–9. doi:10.1016/j.jcis.2020.04.115
80. Weil BR, Manukyan MC, Herrmann JL, et al. Mesenchymal stem cells attenuate myocardial functional depression and reduce systemic and myocardial inflammation during endotoxemia. Surgery. 2010;148(2):444–452. doi:10.1016/j.surg.2010.03.010
81. Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78(4):1233–1261.
82. Zhou Y, Gao C, Vong CT, et al. Rhein regulates redox-mediated activation of NLRP3 inflammasomes in intestinal inflammation through macrophage-activated crosstalk. Br J Pharmacol. 2022;179(9):1978–1997. doi:10.1111/bph.15773
83. Chen Y, Chen M, Zhang Y, et al. Broad-spectrum neutralization of pore-forming toxins with human erythrocyte membrane-coated nanosponges. Adv Healthc Mater. 2018;7(13):e1701366. doi:10.1002/adhm.201701366
84. Huang Y, Yuan Z, Zhao D, et al. Polymyxin B immobilized nanofiber sponge for endotoxin adsorption. European Polymer Journal. 2019;110:69–75. doi:10.1016/j.eurpolymj.2018.11.008
85. van der Meel R, Mham F, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72–85. doi:10.1016/j.jconrel.2014.07.049
86. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi:10.1021/acsnano.1c04996
87. Witwer KW, Wolfram J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 2021;6(2):103–106. doi:10.1038/s41578-020-00277-6
88. Elsharkasy OM, Nordin JZ, Hagey DW, et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–343. doi:10.1016/j.addr.2020.04.004
89. Kumari P, Vasudevan SO, Russo AJ, et al. Host extracellular vesicles confer cytosolic access to systemic LPS licensing non-canonical inflammasome sensing and pyroptosis. Nat Cell Biol. 2023;25(12):1860–1872. doi:10.1038/s41556-023-01269-8
90. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
91. Pacheco M, Jurado-Sánchez B, Escarpa A. Visible-light-driven janus microvehicles in biological media. Angew Chem Int Ed Engl. 2019;58(50):18017–18024. doi:10.1002/anie.201910053
92. Wacker MG, Proykova A, Santos GML. Dealing with nanosafety around the globe-Regulation vs. innovation. Int J Pharm. 2016;509(1–2):95–106. doi:10.1016/j.ijpharm.2016.05.015
93. Long J, Liang X, Ao Z, et al. Stimulus-responsive drug delivery nanoplatforms for inflammatory bowel disease therapy. Acta Biomater. 2024;188:27–47. doi:10.1016/j.actbio.2024.09.007
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

