Back to Journals » International Journal of Nanomedicine » Volume 20
Nanoemulsions Based Therapeutic Strategies: Enhancing Targeted Drug Delivery against Breast Cancer Cells
Authors Izadiyan Z, Webster TJ , Kia P , Kalantari K, Misran M, Rasouli E, Maghareh Esfahan Z, Shameli K
Received 6 September 2024
Accepted for publication 28 April 2025
Published 14 May 2025 Volume 2025:20 Pages 6133—6162
DOI https://doi.org/10.2147/IJN.S488545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Farooq A. Shiekh
Zahra Izadiyan,1 Thomas J Webster,2– 4 Pooneh Kia,5 Katayoon Kalantari,6 Misni Misran,1 Elisa Rasouli,7 Zahra Maghareh Esfahan,8 Kamyar Shameli9
1Department of Chemistry, Universiti Malaya, Kuala Lumpur, Malaysia; 2School of Biomedical Engineering and Health Sciences, Hebei University of Technology, Tianjin, People’s Republic of China; 3School of Engineering, Saveetha University, Chennai, India; 4Division of Pre-College and Undergraduate Studies, Brown University, Providence, RI, USA; 5Institute of Bioscience, Universiti Putra Malaysia, Serdang, Selangor, Malaysia; 6Department of Chemical Engineering, Northeastern University, Boston, MA, USA; 7Department of Electrical and Electronics Engineering, Nanyang Technological University, Singapore; 8Department of Chemical and Environmental Engineering, Universiti Putra Malaysia, Serdang, Selangor, Malaysia; 9School of Medicine, Institute of Virology, Technical University of Munich, Munich, Germany
Correspondence: Zahra Izadiyan, Email [email protected] Kamyar Shameli, Email [email protected]
Abstract: Nanoemulsions (NEs), colloidal systems of nanoscale droplets (~100 nm), have emerged as transformative tools in oncology due to their high surface area-to-volume ratio, tunable physicochemical properties, and capacity for targeted drug delivery. While NEs find applications across diverse fields, their urgency in breast cancer therapy stems from critical limitations of conventional treatments, including systemic toxicity, poor bioavailability, and multidrug resistance. Unlike traditional chemotherapeutics, NEs enable precise tumor targeting via passive mechanisms (eg, enhanced permeability and retention effect) and active strategies (eg, ligand-functionalized surfaces), significantly reducing off-target effects. Their ability to encapsulate hydrophobic drugs, improve solubility, and sustain controlled release enhances therapeutic efficacy while overcoming resistance mechanisms prevalent in aggressive breast cancer subtypes, such as triple-negative and HER2-positive tumors. This review comprehensively analyzes NE formulation techniques (eg, ultrasonication, phase inversion temperature, bubble bursting), stability optimization through surfactant dynamics, and predictive modeling of droplet behavior. A focal point is their role in modulating tumor microenvironments, inducing apoptosis, and inhibiting angiogenesis in preclinical breast cancer models. By spotlighting NE-driven advancements in drug accumulation, reduced relapse rates, and adaptable combination therapies, this article underscores their potential to revolutionize oncology. Future research must prioritize clinical translation, scalability, and multifunctional NE designs to address unmet needs in precision breast cancer treatment.
Keywords: nanoemulsions, targeted drug delivery, breast cancer therapy, nanoscale droplets, tumor microenvironment
Graphical Abstract:

Introduction
Cancer is one of the most serious diseases threatening human health and remains a leading cause of death worldwide. It significantly reduces life expectancy, making it a major public health and economic burden across all nations.1 Currently, cancer accounts for nearly 16.8% of all global deaths and contributes to over 22.8% of all mortality cases.1 Among individuals aged 30–69, it is responsible for 30.3% of premature deaths and ranks as one of the top three causes of mortality in 177 out of 183 countries.2 Beyond its impact on longevity, cancer imposes substantial macroeconomic and societal costs, varying by type, region, and gender.3 A 2020 study revealed the significant impact of cancer mortality on women, reporting that nearly one million children lost their mothers to cancer, with breast and cervical cancers being responsible for nearly half of these deaths.4
As illustrated in Table 1, more than 60% of new cancer cases and fatalities stem from the ten most prevalent cancer types.2 Lung cancer ranks as the most diagnosed cancer globally, accounting for 12.4% of cases, followed by breast cancer in women (11.6%), colorectal cancer (9.6%), prostate cancer (7.3%), and stomach cancer (4.9%). In terms of mortality, lung cancer leads with 18.7% of all cancer-related deaths, followed by colorectal (9.3%), liver (7.8%), breast (6.9%), and stomach (6.8%) cancers. In women, breast cancer remains the most commonly diagnosed and the leading cause of death, whereas lung and colorectal cancers follow in both incidence and mortality. For men, lung cancer ranks highest in both cases and fatalities, with prostate and colorectal cancers being the next most frequently diagnosed, and liver and colorectal cancers ranking second and third in mortality.2
 |
Table 1 New Cases and Mortality Rates for the Top 10 Leading Cancers in 2022.2 |
Nanoemulsions (NEs) play a crucial role in cancer treatment by encapsulating drugs, enhancing solubility, and improving the stability and bioavailability of chemotherapeutic agents.5 Recent advancements in drug delivery systems have shown promising potential in overcoming limitations of conventional cancer treatments. These technologies enhance drug solubility, bioavailability, and therapeutic efficiency while minimizing toxicity and side effects. Additionally, they offer targeted drug release under tumor conditions, leading to high absorption and cytotoxic effects on cancer cells with minimal impact on healthy tissue.6
Breast cancer, the most frequently diagnosed malignancy in women, is a key focus of this discussion. Among its subtypes, Luminal A characterized by hormone receptor positivity and low proliferation rates is associated with favorable outcomes and high responsiveness to hormonal treatments.5 Another type is Luminal B. It is also receptor-positive but with higher proliferation rates compared to Luminal A; this subtype may require a combination of therapies, including chemotherapy and targeted agents. The next is HER2-positive, characterized by overexpression of the HER2 protein; this subtype may benefit from targeted therapies such as trastuzumab (Herceptin) but may also present aggressive behavior.2 The last one is triple-negative. This type of breast cancer, lacking hormone receptors (ER and PR) and HER2, is often more challenging to treat and is associated with poorer outcomes, requiring more research into targeted therapies.
To combat cancer, various treatment approaches such as surgery, radiotherapy, and chemotherapy have been implemented. Among these, chemotherapy is widely used due to its straightforward and accessible procedures.7 Over the past decade, nanotechnology has found applications in multiple fields, including cosmetics, food science, pharmaceuticals, and agricultural pesticides. Within the pharmaceutical sector, various nanocarriers such as nanoparticles, nanocapsules, solid lipid nanoparticles, and NEs have been developed to encapsulate bioactive compounds.8
NEs have emerged as a rapidly advancing class of colloidal carriers, facilitating effective and economical applications in diverse fields such as agriculture,9 food,10 cosmetics,11 and pharmaceuticals.12 Their classification is based on the chemical nature of the dispersed phase. Oil-in-water (O/W) emulsions occur when oil droplets are suspended in water, while water-in-oil (W/O) emulsions are formed when water is dispersed in oil. These are considered single emulsions. More complex systems, such as oil-in-water-in-oil (O/W/O) and water-in-oil-in-water (W/O/W) emulsions, involve a primary emulsion being dispersed in an additional liquid phase.13 This ability to incorporate different dispersed phases makes NEs ideal for encapsulating a wide range of bioactive compounds. The continual advancement of NEs, incorporating more intricate compositions and internal compartments,14 has positioned them as multifunctional drug delivery platforms. In medicine, NEs are valuable tools for addressing both existing and emerging health challenges. Their effectiveness in enhancing pharmaceutical compounds has been demonstrated across various therapeutic applications.15 Specifically, their high surface-area-to-volume ratio promotes efficient drug absorption, while their adjustable interfacial properties allow for targeted delivery of active agents.16 By modifying their surface to display biomolecules such as antibodies, NEs can achieve precise drug targeting, ensuring optimal doses reach intended sites while minimizing off-target effects.17 Additionally, encapsulating drugs within NE structures protects them from environmental fluctuations, including enzymatic degradation and pH changes.18 These attributes contribute to their growing adoption in pharmaceutical research, underlining their potential in healthcare advancements.
In nanocarrier-based drug delivery systems, targeted transport of therapeutic agents is further optimized through a combination of passive and active mechanisms, leveraging the unique properties of nanocarriers.19 Passive transport exploits the physicochemical traits of nanocarriers and the biological environment of the target tissue.20 For example, nanomedicines can accumulate at tumor sites through the enhanced permeability and retention (EPR) effect, which takes advantage of the leaky vasculature associated with tumors.19 Meanwhile, active transport provides precise drug delivery by interacting with specific cellular components, utilizing ligand-receptor binding, pH-sensitive release, or cell-penetrating peptides to ensure efficient drug uptake.21 Often, these mechanisms work together to maximize drug efficacy, specificity, and safety, particularly when integrated with strategic drug-loading methods.
The advancement of nanotechnology has led to increasing evidence supporting the role of nanocarriers in overcoming drug resistance in cancer chemotherapy.22 Traditional treatments, while widely used, remain suboptimal, as reflected in high mortality rates, particularly in women affected by certain cancers. Conventional therapies including surgery, radiotherapy, and chemotherapy face challenges such as limited bioavailability, poor cellular uptake, acquired drug resistance, and adverse side effects. In contrast, nanomedicine offers a promising alternative, particularly for breast cancer treatment. Recent breakthroughs in nanomedicine are fostering novel therapeutic applications and innovative healthcare strategies.23
This review article aimed to critically synthesize current advancements in NEs as versatile pharmacological carriers, emphasizing their formulation strategies, stability mechanisms, and transformative potential in breast cancer therapy. Key objectives included analyzing innovative synthesis techniques for precise droplet size control, elucidating the role of surfactants and predictive models in optimizing stability, and evaluating the efficacy of NEs in targeted drug delivery, tumor microenvironment modulation, and overcoming multidrug resistance. By consolidating recent research, the review underscores NEs’ capacity to enhance therapeutic precision through tumor-specific accumulation, minimized off-target effects, and adaptable delivery systems. The outcomes highlight NEs as promising tools for advancing oncology, bridging gaps between preclinical innovation and clinical translation. Future research should prioritize scalable production, biocompatibility assessments, and multifunctional NE designs to unlock their full potential in precision medicine and broader biomedical applications.
Nanoemulsions
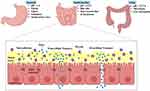
NEs are emulsions characterized by uniformly sized particles ranging from 20 to 200 nm. Prior research has demonstrated the effectiveness of NEs in drug encapsulation for distribution purposes.24 Due to their minuscule size, some NEs exhibit optical transparency or translucency. These systems comprise nanoscale droplets of one immiscible liquid uniformly distributed in another.25,26 Industrially, NEs are predominantly formulated as O/W or W/O mixtures (Figure 1). In O/W NEs, oil droplets are dispersed in an aqueous medium, whereas W/O systems feature water droplets suspended in an oil phase.27 O/W NEs are more widely adopted than W/O variants, with their droplets stabilized by hydrophilic surfactants, while lipophilic surfactants coat droplets in W/O systems.
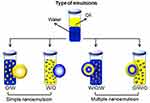 |
Figure 1 The structural variations of NEs depend on the relative positioning of the oil and aqueous phases. |
Engineers can design complex architectures like W/O/W or O/W/O NEs using these colloids as foundational units.28 Fabrication typically involves a dual-step process: for instance, creating W/O/W NEs requires first homogenizing an aqueous phase with an oil phase containing oil-soluble surfactants, followed by emulsification with a water phase containing hydrophilic surfactants. Building these systems can be challenging due to the requirement for extremely small droplet sizes in the internal and external droplets of parenteral double NEs, often less than 100 nm, driven by surfactant limitations in parenteral administration.29 Moreover, altering the internal droplet size significantly affects the oil phase’s characteristics.30 Different NEs offer advantages for specific applications, such as shielding hydrophilic substances from the external water phase, regulating the release of hydrophilic substances, reducing undesirable flavors, or minimizing overall fat content in the system.31–34 NE formulations, being non-toxic and non-irritating, are suitable for therapeutic use in humans and animals, without harmful effects on cells.35 These attributes find applications in drug delivery, biological agents, and diagnostic agents.
Nanocarriers can be differentiated by their unique physicochemical properties. A critical classification criterion lies in their biocompatibility and toxicity profiles, which serve as central factors defining their functional identity.17 The NEs for instance, are promising vehicles for drug delivery and pharmaceutical applications due to their tailored size, morphology, and biocompatibility.36 Standard preclinical evaluations of NEs often assess their interactions with red blood cells (RBCs) and platelets which are key components in blood clotting processes. The NP’s safety and biocompatibility are further validated through cytotoxicity testing across cell lines, histopathology, and serum biomarker analysis.17 The NE system preserves fragile drugs, enhances solubility, controls drug release, increases bioavailability, and reduces patient variability. For nearly four decades, NEs have been employed in the medical industry, particularly in the formulation of parenteral nutrition liquids.
The creation of NEs involves various manufacturing approaches, broadly categorized into low-energy approaches and high-energy approaches.25,37–39 In industrial applications, high-energy methods are predominantly employed, demanding a substantial energy input (∼108–1010 W/kg) to generate microscopic droplets. Common mechanical devices for NE production include high-pressure control device homogenization, micro fluidization, Rotor stator emulsification method and sonication. These techniques use specially designed mechanical equipment to break and blend the oil and water phases by creating significant shear, turbulent, and cavitation flow profiles.26,40 In contrast, low-energy approaches leverage specific system characteristics to create microscopic droplets while conserving energy (around 103 W/kg).
The rotor-stator technique is a predominant emulsification method in food industries and is increasingly adapted for NE synthesis.41 Its advantages include simple installation, cost-effectiveness, compatibility with viscous systems, and large-scale emulsion production. However, it is unsuitable for single-step NE generation due to challenges in achieving submicron droplet sizes (<1 µm). Common strategies to reduce droplet dimensions involve rotor redesign, gap minimization, increased rotational speed, and extended disruption zone residence time. High-pressure homogenization (HPH) is favored industrially for its scalability and flexibility.42 While efficient, most HPH systems struggle to produce droplets in the ~1 nm range. This method involves propelling liquid mixtures (oil, water, surfactants) through micro-orifices at pressures of 500–5,000.43 Though effective, HPH demands significant energy, risks thermal degradation, and is limited to O/W systems with <20% oil content.44 Excess surfactants mitigate coalescence, though post-dilution is often required. Microfluidization employs microchannels (50–300 µm) to fragment emulsions under pressure, differing from HPH in channel geometry.42 Ultrasonication, widely used in labs, reduces droplet size via acoustic cavitation by modulating sonication duration, power intensity, and surfactant concentration.
Ultrasonication serves as a homogenization technique for generating kinetically stable NEs,45 widely employed in research settings. This method generates acoustic pressure waves through electrical energy, which diminish droplet dimensions by adjusting sonication duration, power intensity, and emulsifier concentration. The process relies on high-frequency sound waves (≥20 kHz), where inertial cavitation induced by the ultrasonic probe provides the energy required to fragment droplets. A piezoelectric transducer converts electrical voltage into mechanical vibrations, generating these sound waves. Such low-energy approaches depend on the spontaneous generation of nanoscale droplets in surfactant-oil-water systems, driven by inherent structural properties or external stimuli like temperature shifts.46 Common low-energy strategies encompass spontaneous emulsification, phase inversion temperature (PIT), and emulsion inversion point techniques.
The spontaneous emulsification technique blends an organic phase (eg, oil and surfactants) with an aqueous phase containing co-surfactants and water, enabling the mobilization of water-soluble components like solvents and surfactants across phases.46 This diffusion of hydrophilic elements into the aqueous medium heightens interfacial instability at the oil-water boundary, enlarging the contact area. Disintegration of the transitional microemulsion structure triggers the autonomous generation of nanoscale oil droplets. Known as the Ouzo effect, this phenomenon can be expedited using solvents, regardless of surfactant presence.46 The method yields nano- or microemulsions irrespective of kinetic parameters. Although still in its early stages of industrial application, it demonstrates cost-efficiency, producing droplets as small as 10 nm.47
The phase inversion method refers to the reversible transition between O/W and W/O emulsions during emulsification and is a strategic process driven by surfactant behavior and phase dynamics.45 This transition arises from shifts in the surfactant’s spontaneous curvature, which can be modulated by temperature adjustments (for non-ionic surfactants) or compositional changes.45 The phase inversion composition (PIC) technique modifies the hydrophilic-lipophilic balance (HLB) of emulsifiers by altering ingredient ratios. For example, introducing salt to ionic-stabilized O/W NEs disrupts surfactant charge, inverting the system to W/O.48 Similarly, diluting W/O systems with water induces inversion to O/W. However, this method is less effective for hydrophobic compounds. Successful PIC implementation requires incremental water addition to the oil phase, gradually increasing aqueous content.
The PIT method involves a temperature-dependent transition between O/W and W/O emulsions, driven by molecular migration and temperature-modulated shifts in emulsifier hydrophilicity/lipophilicity at fixed composition.48 This approach generates NEs with minimal energy input, avoiding mechanical shear. PIT relies on transitional inversion in emulsions, governed by temperature-induced modifications to the HLB of surfactants.47 Membrane emulsification, often misclassified as high-energy, operates mechanically with low energy demands. The choice of the NE production method depends on component properties (eg, surfactant/oil phases) and target product features, including stability, optical/rheological behavior, release kinetics, and functionality. Table 2 summarizes the pros and cons of these techniques.
 |
Table 2 Preparation Method of Nanoemulsion Formulation With Advantages and Disadvantages |
Preparation of Nanoemulsions
Nanosized emulsions can be produced using various methods, each of which influences the properties of the resulting NE, making the choice of method a crucial factor in NE creation. The energy required for creating O/W NEs can vary, ranging from high-energy to low-energy techniques.38,49 High-energy methods, such as ultrasound and HPH, rely on mechanical forces to break down the dispersed phase into small oil droplets.26,50 Ultrasonication is commonly used in laboratory settings, while high-pressure homogenizers are favored for industrial applications. In contrast, low-energy techniques exploit the compositional properties of ENs (ie, combinations of oil, water, and emulsifiers) to reduce interfacial tension and promote spontaneous emulsification through temperature changes. The PIT and spontaneous or self-emulsification processes are common low-energy approaches.51,52
The creation of nano-sized emulsions by high-energy methods depends on the composition of the NEs and the energy input required. These techniques involve the application of substantial shear forces through great kinetic energy to generate small droplets, overcoming energy barriers between the designated oil and aqueous phases. The addition of surfactants and co-surfactants is employed to stabilize the NEs and reduce the interfacial tension. Lower interfacial tension reduces the external energy required to break up the NE droplets.53 For instance, by adjusting parameters such as ultrasonic wave frequency, energy input, plus duration, one can create NEs with specific characteristics such as size control, biodegradability, biocompatibility, the ability to overcome challenges related to drug loading and resistance, and controlled drug delivery. Consistent energy input and the oil phase’s residence time within the disruption zone (sonication waves) significantly affects droplet size and distribution.49,54 Increasing the sonication duration to a specific threshold, for example, leads to smaller NE droplets.55 Hence, when producing NEs for in vitro as well as in vivo applications, it’s essential to consider factors affecting physical stability and morphology. HPH is a scalable technique for industrial NE research, particularly in food production. However, it faces challenges related to high energy consumption as well as temperature increase.
For the duration of homogenization, NE mixtures flow under high pressure through a valve chamber, resulting in shearing energy that breaks the oil core into a nano-scale size.56 Parameters (for instance the type of homogenizer, sample composition, and operating settings (eg, duration, energy intensity, and temperature)) all influence droplet size during homogenization.57,58 The intensity of homogenization can influence droplet formation, however, the homogenization pressure levels (MPa) used should be appropriate for the NE composition, including the chemical nature of the oil core and the surfactant type. Recent research emphasizes the importance of investigating the effect of homogenization pressure on NE composition, stability, and encapsulation efficiency (EE), indicating that increasing homogenization pressure up to a certain level, such as 120 MPa, improves EE, but exceeding this threshold may have negative effects on the vehicle’s structure, such as soy protein isolate.59 Surfactant kind and concentration, oil proportion, and pressure homogenization conditions all have an impact on the physical stability of NEs.60
The selection of the NE production method has a significant impact on the physicochemical characteristics of NEs, including their rheology, stability, and payload release properties. Viscosity influences droplet shearing during emulsification, highlighting the importance of a thorough understanding of diverse NE formulation procedures, compositions, and intended pharmaceutical uses in order to produce NEs with desirable properties. In downstream applications like vaccines, it is critical to separate free and excess surfactants or polymer molecules from the NEs created. While centrifugation is commonly used to assess NE stability against phase separation, it may not effectively remove extraneous components from NE formulations. Instead, dialysis bags or chromatography-based separation techniques are employed to eliminate excess dyes, surfactants, and polymers like polyethylene glycol, with minimal impact on the physical characteristics of NEs.61
Characterization Methods for Nanoemulsions
Viscosity influences droplet shearing during emulsification, highlighting the importance of a thorough understanding of diverse NE formulation procedures, compositions, and intended pharmaceutical uses in order to produce NEs with desirable properties. In downstream applications like vaccines, it is critical to separate free and excess surfactants or polymer molecules from the created NEs. A widely used characterization method for NEs is dynamic light scattering (DLS), which relies on the principles of Brownian motion to determine droplet size. DLS exposes moving oil droplets to light, resulting in scattered light with varying intensities, which are quantified using the Stokes-Einstein equation. Key variables, such as the polydispersity index (PDI) and mean droplet diameter, are essential in describing the functional attributes of NEs, particularly their stability and dispersibility. Hence, it is imperative to continuously monitor these variables. Physically stable NEs exhibit properties that remain consistent over time.62,63
While DLS is a straight forward and rapid tool for the continuous monitoring of nanomaterial stability profiles, it may fall short in cases where test materials contain low proportions of droplet populations. In such cases, combining DLS data with other techniques, such as microscopic investigation, is advantageous. Electron microscopy allows for more detailed examinations into droplet structure and size.64 Transmission electron microscopy (TEM) is used to investigate the internal structure of the dispersed phase, whereas scanning electron microscopy (SEM) offers topographical information.65 Cryo electron microscopy, in addition to traditional TEM and SEM, is gaining popularity for several reasons. Cryo TEM, for example, allows for the direct examination of NEs as frozen hydrated droplets while preserving their original structural features.66 As a result, cryo-TEM is employed to study the internal structure and scattered phase of NEs in their native condition.67 While cryo TEM allows artifact-free inspection by distinguishing oil droplets from other structures,68 it may face difficulties when recognizing droplets with high oil content or viscosity. This constraint is overcome by studying the surface microstructure of NE droplet aggregates with cryo SEM, which can investigate larger sample regions and is the preferable method for determining overall NE structure and dispersity.69
Atomic force microscopy (AFM) serves as a tool for analyzing the surface structure of NEs. Although AFM offers critical insights into NE morphology, uniformity, and stability, its laborious and time-intensive workflow limits practicality. Consequently, DLS is frequently preferred for assessing long-term stability. Cryopreparation methods paired with electron energy loss spectroscopy enable chemical profiling of NEs. Additional characterization techniques encompass differential scanning calorimetry, isothermal titration calorimetry, small-angle X-ray scattering, and X-ray spectroscopy.69 The NEs provide a critical advantage in drug delivery by encapsulating diverse hydrophobic agents such as vitamins,70 essential oils (EOs),71 flavor compounds,72 fluorophores,73 and therapeutic drugs.74 This encapsulation safeguards cargo integrity against degradation, enhances bioavailability, and enables controlled release at target sites.75,76 The EE tests quantify retention within the NE’s hydrophobic core,77 with results influenced by formulation parameters, NE composition, physicochemical drug properties (eg, oil-phase solubility), and system stability. The EE of NEs is quantified using multiple analytical methods, including ultracentrifugation, dialysis, gel filtration, ultrafiltration, and microfiltration. Ultracentrifugation employs size-selective membranes to isolate unencapsulated molecules from NEs. The dialysis method relies on semipermeable membranes to differentiate NEs from unbound drugs through diffusion gradients. Gel filtration separates NEs by molecular mass using porous gel matrices.78 To enhance biocompatibility for in vivo applications, eukaryotic L-amino acid peptides are integrated into NPs design, leveraging their innate biodegradability, non-toxicity, and biocompatibility. The safety and biocompatibility of NPs can be evaluated using an MTT assay across diverse cell lines, where decreased cell viability post-NP exposure inversely correlates with the biocompatibility of the NP’s constituent materials. In vitro analyses include cell viability assays, cytotoxicity tests (eg, oxidative stress detection), and stress response evaluations (eg, gene expression profiling). These in vitro methodologies are widely adopted in cytotoxicity research, differing in their mechanisms for identifying cell death pathways. Commonly employed assays include the MTT test, trypan blue exclusion assay, Comet assay (for DNA damage), and 2ʹ,7ʹ-dichlorofluorescein diacetate assay.
Nanoemulsion Stability
Stability in the context of NEs refers to their thermodynamic instability, where over time, these systems naturally tend to separate into two distinct phases. However, the introduction of surfactants can effectively prolong this process, rendering emulsions kinetically stable This extension of stability is crucial, particularly in applications like emulsion nanomedicine, where the alteration of a formulation over time can have significant implications for patient health and product quality, allowing NEs to maintain their original properties for extended periods, even months or years. To ensure the proper creation and preservation of NEs, it is essential to comprehend the mechanisms behind emulsion instability and stabilization. The DLVO theory, developed by Derjaguin, Landau, Verwey, and Overbeek, serves as a widely recognized model for predicting emulsion stability. This framework attributes colloidal stability to the balance between two opposing forces: van der Waals attraction and electrostatic repulsion from double-layer interactions. According to DLVO principles, these forces are assumed to act independently, permitting the total energy of contact (FT) to be calculated by summing the individual contributions of FA (attractive) and FR (repulsive) at varying distances. This approach offers a reliable approximation of colloidal stability, particularly at separation distances near 5 nm (Eq. 1).79
At larger inter-droplet distances, repulsive forces govern interaction energies, maintaining dispersion stability. However, as droplets approach, attractive forces prevail, destabilizing the system. Per the DLVO framework, colloidal instability arises when attraction dominates, manifesting through four primary instability mechanisms: coalescence, flocculation, creaming/sedimentation, and Ostwald ripening (Figure 2). 80 Coalescence involves the fusion of smaller droplets into a larger droplet. During collisions, interfacial distortion leads to the formation of a thin interfacial film. Film thickness is governed by the transport of the continuous phase and surfactants across the film. Critical thinning induces rupture due to thickness variations.81,82 The Marangoni effect, which drives mass transfer along interfaces due to tension gradients, contributes to film thinning.82 Droplet coalescence reduces the system’s total interfacial area, decreasing interfacial energy and Gibbs free energy. Flocculation involves the aggregation of dispersed droplets into clusters separated by thin continuous-phase films. While electrostatic repulsion delays coalescence, flocculation stability differs, as emulsions may rapidly aggregate before slowly coalescing.83 Flocculation reversibility depends on the strength of inter-droplet attraction forces.82 Proximity of droplets post-flocculation increases coalescence risk. Creaming/sedimentation occurs when buoyancy-driven separation positions droplets (based on density differences) at the top or bottom of the dispersion.84 Creaming and sedimentation elevate the risk of droplet coalescence by enhancing droplet proximity. Managing these processes is crucial for the food industry, which incurs significant annual costs, often mitigated by incorporating viscous polysaccharides to slow separation.85 Creaming can also result from droplet aggregation into expansive floc networks, either spontaneously or via polysaccharide bridging, which accelerates phase separation kinetics.85 Ostwald ripening describes the growth of larger droplets through the diffusion of the dispersed phase from smaller to larger droplets in the continuous medium.86 Ostwald ripening is driven by the Kelvin effect, which posits that smaller droplets exhibit higher solubility in the continuous phase. This reduces the total interfacial area of the dispersed phase, lowering the system’s Gibbs free energy.12
 |
Figure 2 Different mechanisms of emulsion instability. Reprinted with permission from Shao P, Feng J, Sun P, Xiang N, Lu B, Qiu D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res Int. 2020;137:109376. Copyright (2020), withpermission from Elsevier..80 |
To counteract this phenomenon, employing a dispersed phase with minimal solubility in the continuous medium and ensuring a narrow droplet size distribution are effective preventive measures.87 The DLVO framework proposes two mechanisms for stabilizing emulsions: preventing droplet aggregation to inhibit destabilization pathways. The first, electrostatic repulsion, arises when similarly charged droplets generate mutual repulsion upon approach. The second, steric stabilization, occurs when a dense interfacial coating physically blocks droplet contact, creating an energy barrier against coalescence. Ionic surfactants stabilize emulsions by drawing counterions from the medium, forming an electrical double layer that enhances electrostatic repulsion (Figure 3). 88,89 An electrical double layer comprises the charged droplet surface, a Stern layer of closely associated counterions, and a diffuse layer of mobile ions.90 The slip plane defines the boundary where the diffuse layer ceases to move with the droplets.91 Zeta potential, measured at this plane, reflects emulsion stability higher magnitudes (positive or negative) correlate with enhanced colloidal resistance to aggregation.92 Steric stabilization creates a thermodynamic barrier to droplet contact, a prerequisite for destabilization under DLVO theory.93 Nonionic surfactants with extended chains, Pickering particles, or surface-adsorbed polymers enhance stability.94 As droplets approach, interdigitating of these molecules elevates Gibbs free energy, counteracting van der Waals attraction.95
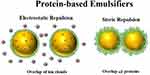 |
Figure 3 Protein-based emulsifiers prevent oil droplets from clumping together by using steric and electrostatic forces, which rely on the thickness, arrangement, and charge of the attached molecules. Reprinted with permission from McClements DJ, Lu J, Grossmann L. Proposed methods for testing and comparing the emulsifying properties of proteins from animal, plant, and alternative sources. Colloids Interfaces. 2022;6(2):19. © 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).89 |
Pharmaceutical Applications
Advances in technology have transformed pharmaceutical formulations, evolving from basic systems to highly complex platforms termed advanced drug delivery systems. In pharmaceutical applications, NEs utilize colloidal dispersions of oil and aqueous phases, with droplet diameters averaging 50–500 nm.31,32 The NEs primarily exist in two configurations: O/W or W/O, where droplets encapsulate either oil or aqueous components.96 To stabilize NEs, pharmaceutical-grade surfactants designated as Generally Recognized as Safe (GRAS) are employed as emulsifying agents.97 NEs offer unique advantages as drug carriers, efficiently solubilizing hydrophobic therapeutics and shielding active ingredients from degradation. Key benefits include enhanced drug payload capacity, solubility optimization, bioavailability enhancement, controlled release profiles, and protection against enzymatic/chemical breakdown. The nanoscale droplet size minimizes aggregation and gravitational separation compared to conventional emulsions, extending product shelf-life. Additionally, the high surface-area-to-volume ratio inherent to NEs may enhance targeted delivery of bioactive molecules.98 The nanoscale droplets in NEs demonstrate distinctive physicochemical properties, including exceptional optical clarity and novel viscoelastic characteristics. These systems display varied optical attributes, transitioning from opaque to translucent, alongside rheological behaviors that vary between fluid and gel-like states. It is essential to recognize that diverse colloidal systems comprising oil, water, and surfactants possess both shared and unique functional properties. Colloidal systems are categorized into three groups based on droplet size and thermodynamic stability: microemulsions (thermodynamically stable, optically isotropic dispersions with droplets 20–100 nm), NEs (kinetically stable systems with droplets <200 nm), and conventional emulsions (metastable systems with droplets ≥200 nm to micrometers).25 This review prioritizes NEs, which serve as versatile platforms for drug delivery via oral, transdermal, intranasal, or ocular routes. This analysis emphasizes recent advancements in NE-mediated therapeutic delivery.
Drug Delivery Applications
NEs have found extensive applications in the delivery of medications and various active compounds across multiple domains. They play a pivotal role in the pharmaceutical and medical sectors, offering a versatile solution for enhancing drug effectiveness and patient care. NEs are employed in diverse fields, including pharmaceuticals, cancer treatment, nutraceuticals, vaccine administration, ocular drug delivery, dermatological treatments, intranasal therapy, gene therapy, anti-inflammatory agents, antibiotics, central nervous system drug delivery, antiviral medications, gastrointestinal drug administration, and pulmonary drug delivery. Their ability to encapsulate and deliver a broad spectrum of drugs and bioactive substances makes them a valuable asset in the realm of healthcare and medicine (Figure 4). 99
 |
Figure 4 Routes of drug administration and associated dosage forms. Reprinted with permission from Khan MS, Roberts MS. Challenges and innovations of drug delivery in older age. Adv Drug Delivery Rev. 2018;135:3–38. Copyright (2018), withpermission from Elsevier.99 |
Oral Delivery
One of the primary methods for delivering drugs is orally, which is also one of the most widely used dose forms by patients. The oral delivery of proteins, vaccines, anti-cancer medications, and other medications has very poor bioavailability and is restricted, even with high patient compliance. For oral administration to be effective and sustained, it must overcome physiological hurdles such as limited solubility, permeability, and early breakdown. Through study, scientists attempt to address the physiological obstacles to oral drug distribution and explore technological solutions, including prodrugs, hydrogels, NPs, microemulsions, 3D printing, and other technical approaches. Oral medications have progressively transitioned from traditional to ultra-long-lasting drug administration because of these technological advancements (Figure 5). 100
 |
Figure 5 Challenges of oral drug delivery and technical design to overcome these challenges. Reprinted with permission from Majumdar. S. Oral drug delivery: conventional to long acting new-age designs. 2021. Copyright (2021), withpermission from Elsevier.100 |
Because the oral route is the most convenient, simplest, and cost-effective method for non-invasive medication administration, drug delivery systems based on this premise now dominate the pharmaceutical industry. Furthermore, enhanced patient compliance makes it the best method for attaining treatment goals. This method of administration, however, has limits in terms of elderly, pediatric, and perhaps trauma epileptic patients, where patient participation is a key constraint. Furthermore, several medications are intrinsically difficult to administer orally due to non-conducive physiochemical characteristics. Poorly soluble medications delivered orally have major issues with solubility, stability, and absorption in the gastrointestinal tract (GIT).101 Hydrolysis and enzymatic degradation of peptide medicines are known to impede their intestinal absorption and bioactivity. Additional downsides may include the restricted capacity of some medications to penetrate the epithelium cell wall. Micronization/nanonization,102 solid dispersions,103,104 complexation with cyclodextrins,105 amorphization,106 and use of particulate delivery systems that are dispersible in aqueous environments,107,108 are some of the methods that have been proposed to increase overall bioavailability of drugs.
NE technology has improved the oral bioavailability of poorly soluble and hydrophobic medicines significantly in the last decade, according to many research groups. When poorly soluble medicines were given via NEs, numerous researchers saw significant improvements in Cmax and the area under the curve (AUC). The most essential structural components of gut-associated lymphoid tissue are Peyer’s patches, which are specialized for endocytosis and transit into intraepithelial gaps. The M-cells ingest the NPs, which are quickly absorbed and “shuttled” to the lymphocytes.109 The advantage of lymphatic absorption over portal blood absorption is that it circumvents the liver’s presystemic processing of a medicine. Lymphatic targeting of NEs can facilitate the following: translocation of antineoplastic drugs for the treatment of lymphomas; sustained/controlled drug release; reduction of drug-related mucosal irritation; avoidance of the hepatic first-pass effect; oral delivery of vaccine antigens to gut-associated lymphoid tissue; and oral delivery of labile drugs protected by the carrier (Figure 6). 66
Lipids are successfully absorbed in the GIT by a variety of methods. As a result, one of the viable techniques for increasing medication absorption (particularly protein medicines) is to pack them inside lipids, resulting in a large increase in drug absorption as well as lipid absorption. The idea is to load medications with lipids, which are components of NEs, resulting in greater drug absorption in the GIT. It has been demonstrated that using NEs as oral drug delivery systems increases the medication’s efficacy at the target location.110,111 Increased penetration of flavonoids and iridoids from Vitex Agnus-castus extract-loaded NEs has been shown in in vitro transport tests (PAMPA and Caco-2 models) using triacetin as an oil phase and labrasol and cremophor EL as the surfactant and co-surfactant, respectively.112 Using a novel delivery method for NEs, Elkadi et al (2017) created self-nano emulsifying tablets to improve the solubility of simvastatin.113
Parenteral Delivery
Parenteral administration is a prevalent and effective method for delivering drugs characterized by a narrow therapeutic window and poor bioavailability.114,115 Parenteral drug delivery is a medical intervention that administers a drug directly into the bloodstream, bypassing the gastrointestinal tract.116 However, this route presents challenges for hydrophobic therapeutics. Current strategies outlined in research such as adjusting vehicular pH, incorporating co-solvents, or cyclodextrin complexation,101 face constraints such as adjuvant toxicity, injection-site discomfort, and post-administration drug crystallization. Nanostructured carriers have emerged as promising alternatives to address these limitations. Lipid-based emulsions have proven effective for decades in clinical settings requiring intravenous nutrition.117 Their viability stems from the biological compatibility of core components phospholipids and naturally derived or semi-synthetic oils enabling safe delivery of therapeutic and nutritional agents. Ensuring optimal parenteral delivery and system stability requires meticulous regulation of compositional design, structural integrity, and manufacturing parameters. Stability is critically influenced by factors such as formulation chemistry, production protocols, and storage conditions.118,119 The NEs are effective carriers for parenteral drug delivery due to their high solubilization capacity for hydrophobic bioactives, biocompatibility, and ability to protect therapeutics from chemical/enzymatic degradation.117,120 Stabilizing NEs for this route requires emulsifiers capable of forming monolayers or multilayers around oil droplets. Common emulsifiers include natural phospholipids (eg, lecithins) and semisynthetic derivatives like dioleoylphosphatidylethanolamine (DOPE) or distearoylphosphatidylcholine (DSPC). Lecithins, sourced from animal or plant origins, are inherently biocompatible and biodegradable. A significant proportion of lecithin phospholipids feature charged polar head groups, such as phosphatidylserine, phosphatidylglycerol, and phosphatidic acid, which confer a strong negative surface charge to droplets. This charge induces electrostatic repulsion, enhancing colloidal stability over time. While natural surfactants are favored for emulsification, formulations are often supplemented with auxiliary emulsifiers to improve stability. Nonionic surfactants, like poloxamers and polyoxyethylene sorbitan derivatives (eg, Tweens), demonstrate synergistic stabilization when combined with phospholipids, forming dense interfacial films that enhance formulation robustness. The hemolytic potential and post-autoclaving droplet size instability associated with Tween 80 limit its applicability in nanoemulsion (NE) formulations. Despite risks of toxicity and hemolysis linked to certain derivatives, polyoxyethylated castor oil variants remain under investigation for parenteral drug delivery.121,122 Lipophilic drugs delivered via NEs typically exhibit higher plasma levels post-parenteral administration compared to conventional solutions. This is attributed to enhanced distribution volume and reduced clearance rates, which collectively prolong elimination half-life.123 The sustained and controlled drug release from NEs minimizes injection frequency and dosage requirements throughout treatment regimens. Additionally, their reduced propensity for droplet coalescence or phase separation offers stability advantages over traditional emulsion systems.
Transdermal and Topical Delivery
For some clinical circumstances, systemic medication distribution via the skin is extremely convenient.124,125 Transdermal delivery methods are especially attractive because they offer the advantage of steady-state controlled drug delivery with self-administration, which may not be possible with the parenteral route.126 By taking off the transdermal patch, the patient can cease receiving medicine at any time. The skin’s ability to obstruct effective bioactive penetration is a significant limitation that now limits the application of this mode of administration for several uses. Drugs applied transdermally have the potential to enter the bloodstream through the stratum corneum, sweat ducts, or hair follicles. The stratum corneum, on the other hand, hinders their absorption and so reduces their bioavailability. The principal skin barriers must be addressed in order to optimize medication pharmacokinetics and targeting.86 NE droplets may readily penetrate the skin’s pores and enter the systemic circulation, where they are channeled for optimal distribution. The penetration of pharmaceuticals through the skin may be improved in a variety of ways by optimizing drug and vehicle characteristics. For example, the greatest penetration is frequently observed when the drug is at its highest thermodynamic activity, as in supersaturated solutions. Betamethasone,127 clobetasol,88 corticosterone,128 flufenamic acid,129 flurbiprofen,130 tocopheryl acetate,131 tolterodine tartrate,132 and amlodipine133 are only a few of the medicinal molecules that have been administered to or through the skin using nanoformulations.
Intranasal Delivery
Intranasal drug delivery is another safe and effective way to provide some medications. Indeed, the nasal mucosa has emerged as a therapeutically feasible conduit for the delivery of systemic medications, as well as a promising means of circumventing the barriers to direct drug entrance to the target site. Since ancient times, this approach has been acknowledged in the Ayurvedic system of Indian medicine, and it has lately been recommended over oral administration of medications because it leads to improved systemic bioavailability, probably by bypassing drug metabolism in the gastrointestinal tract.134 Additionally, intranasal delivery is generally tolerated, painless, and noninvasive. Targeting the brain with medicines presents a number of challenges, especially with hydrophilic and high-molecular-weight molecules.135 This is because the endothelium, which divides the brain from the systemic circulation, is impermeable across the blood-brain barrier.136 The olfactory region of the nasal mucosa acts as a direct conduit between the nose and the brain.
The NEs encapsulating therapeutic agents have demonstrated clinical utility across diverse therapeutic areas, including osteoporosis (raloxifene), schizophrenia (olanzapine), motion sickness (metoclopramide), endometriosis (nafarelin), hormone replacement (estradiol), smoking cessation (nicotine), and enuresis (desmopressin).96
The potential of nasal administration for central nervous system (CNS) distribution of polar medications for the treatment of chronic CNS illnesses, like Parkinson’s or Alzheimer’s disease, has been investigated.137 Because the brain is thought to be one of HIV’s safe havens, saquinavir has been effectively used as an intranasal NE drug to target the brain to treat this problem.138 Vaccines can also be given as NEs via the intranasal route (described later in this review), which has several benefits over the oral and parenteral approaches. However, nasal medication delivery is limited by its capacity, the challenge of attaining exact dose measurements, and the consistency of delivery. The administration strategy, formulation, and delivery mechanism all affect permeability, duration of residence, and metabolism in the nasal cavity, which in turn affects drug distribution in the nasal cavity.
Ocular Delivery
Most ocular medications are administered as aqueous eye drops applied to the lower conjunctival.139 Key limitations of this approach include short ocular retention, inefficiency in delivering lipophilic/insoluble drugs, and susceptibility to rapid clearance from blinking and tear turnover. Less than 20% of the topical dose persists in the ocular cavity due to enzymatic degradation, absorption barriers, phagocytic activity, and systemic uptake via adjacent tissues.50 In topical ocular drug delivery, rapid clearance is a critical challenge, with <1% of the applied dose penetrating ocular tissues. Retinal delivery faces even greater inefficiency due to compounding barriers such as anatomical obstructions, aqueous humor dynamics, intraocular metabolism, pigment tissue binding, and non-targeted phagocytosis by resident cells.140 Developing effective ocular drug formulations presents major hurdles for the pharmaceutical industry, requiring prolonged ocular retention, sustained therapeutic concentrations, and regulatory compliance while accommodating diverse active ingredients. The NEs offer a potential solution, combining sustained release with enhanced permeation into ocular tissues and th aqueous humor.141 Cationic NEs, leveraging bioadhesive properties, outperform traditional eye drops by improving bioavailability and ensuring targeted delivery of therapeutics to ocular structures.142 The bioadhesive nature of NEs originates from electrostatic interactions between charged droplets and ocular surfaces, extending nanoscale oil droplet retention. Commercial systems like Novasorb utilize cationic droplets to electrostatically bind negatively charged ocular epithelia, enhancing therapeutic residence time.143 At physiological pH, the ocular surface including its glycosylaminoglycan-rich mucus layer, corneal/conjunctival cells carries a negative charge. A positively charged formulation applied topically induces electrostatic attraction, extending its ocular residence time. Additionally, nanoscale oil droplets may enhance absorption efficiency.
Nanoemulsions for Vaccine Delivery
In pharmaceutical applications, the surface properties of NEs play a crucial role in their physical stability and interactions within the body. These characteristics include the type, concentration, shape, and surface charge of the surfactant, coating material type, thickness, size, and architecture, as well as the nature of targeting ligands. Bioconjugation techniques are used to customize these surface characteristics in order to get the required pharmacological activity and physical stability (Figure 7). For instance, steric hindrance can be introduced at the O/W interface by the addition or presentation of polymers, such as polyethylene glycol (PEG), which will change the thickness and surface architecture of the NEs. This PEG shielding also mitigates surface charge, preventing unwanted electrostatic interactions with plasma proteins, which can lead to opsonization and rapid clearance. These positive surface modifications significantly influence the stability and biological interactions of NEs, enhancing their potential in drug delivery applications. However, surface functionalization must also be practical, cost-effective, and favorable for the encapsulated compounds during downstream processing (Figure 7).50
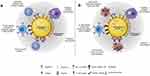 |
Figure 7 Surface features of O/W NEs play a crucial role in medicinal applications: (A) Biomolecules enhance O/W NEs for drug delivery, immune response, viral neutralization, and targeted infection treatment. (B) Customizable surfaces enable diverse strategies, including macrophage targeting, cancer cell recognition, immune activation, and T-cell receptor presentation. Reprinted with permission from Gassmann P, List M, Schweitzer A, Sucker H. Hydrosols: alternatives for the parenteral application of poorly water soluble drugs. Eur J Pharm Biopharm. 1994;40(2):64–72. © 2021 The Authors. Published by Elsevier B.V.114 |
Most of the current research on NEs vaccine delivery primarily focuses on intranasal mucosal techniques.144 In contrast to conventional vaccination methods, intranasal mucosal vaccination involves the introduction of an oil-based emulsion into the nostrils. This approach is preferred due to its ease of administration and the reduced amount of antigen required to trigger an immune response. Although the exact mechanism by which vaccination adjuvants enhance the immune response is not fully understood, it is believed that they promote enhanced antigen distribution and activate innate immune responses as potential pathways. NE-based vaccination adjuvants function by increasing the absorption of antigens by dendritic cells (DCs) and activating Toll-like receptors 2 and 4 (TLR 2 and 4). This, in turn, enhances both humoral and cell-mediated immune responses, particularly Th1 and Th17 responses.145 Moreover, NE-based mucosal adjuvants have demonstrated safety and tolerability in early-stage human clinical trials, with no harm to the mucosal epithelium.146 Given that HIV can target the mucosal immune system, there is a suggestion that bolstering mucosal immunity through the use of NEs could be a viable strategy for HIV prevention.143 Additionally, intranasal vaccinations have shown the ability to confer immunity to the vaginal mucosa.147 Adjuvants like NEs are currently being employed to deliver attenuated organisms to mucosal surfaces to provoke the appropriate immune response. For example, an intranasally administered NE-adjuvanted inactivated influenza vaccine, with a 1:6 ratio of cationic-to-nonionic surfactants, resulted in a robust mucosal influenza-specific IgA response, offering a notable advantage over responses generated by traditional parenteral immunization.148 Studies suggest that NEs are effective mucosal adjuvants for recombinant Bacillus anthracis protective antigen anthrax vaccines, as they induce long-lasting, robust, and specific humoral and cellular responses while maintaining antigen stability, all with no adverse effects.149
Nanoemulsions for Gene Delivery
Gene delivery is a fundamental concept aimed at introducing genes into a patient’s somatic cells to correct genetic defects or create new cellular functions. The excellent adhesion of liposomes to cell surfaces has led to their use as non-viral vectors for gene delivery.150,151 Although viral gene delivery techniques offer superior transfection capabilities, their higher toxicity limits their utility. This drawback has led researchers to explore non-viral methods, such as cationic NEs. These NEs, containing cationic surfactants, can interact electrostatically with negatively charged DNA, compacting it into nanocomplexes.152 Stearylamine, a cationic lipid, has been proposed as an effective surfactant for gene transport. Electrophoresis studies revealed that cationic NEs having a zeta potential greater than +30 mV and droplet diameters less than 200 nm, containing stearylamine, efficiently complexed with the model plasmid PIRES2-EGFP.153 Cationic NEs have also been used for intranasal delivery of TNF siRNA, demonstrating effectiveness in protecting against neuroinflammation in an LPS-induced model.154 Positive-strand RNA virus self-amplifying messenger RNA (mRNA) has emerged as a potential non-viral vector for creating vaccine antigens in situ.155 It has been demonstrated that cell-penetrating peptides improve the absorption of plasmid DNA (pDNA) by mucosal epithelial cells in self-nano emulsifying drug delivery systems (SNEDDS) for oral gene transfer.156 While liposomes are commonly used for gene transport, NEs offer an advantage in terms of physical stability. Unlike most liposomal preparations, which tend to aggregate upon contact with DNA, potentially causing an embolism when administered systemically,157 NE-based delivery systems are expected to mitigate this issue.
Nanoemulsions for Breast Cancer Treatment
Nanotechnology has emerged as a promising approach in cancer treatment, with significant research focus on addressing diverse malignancies. This discussion emphasizes prevalent cancer types, particularly breast cancer, which predominantly manifests as carcinomas originating in the milk-producing lobules (lobular carcinoma) or the epithelial lining of the milk ducts (ductal carcinoma). Metastatic progression often occurs via the bloodstream and lymphatic system, commonly affecting the brain, liver, lungs, and skeletal structures.158 Breast cancer pathogenesis is driven by disruptions in both genetic stability and epigenetic regulation. Mutations in high-penetrance genes such as p53, BRCA1, BRCA2, and PTEN (phosphatase and tensin homolog) frequently elevate susceptibility to hereditary breast cancer. Dysregulation in key signaling cascades including PI3K/Akt/mTOR, Ras/MAPK, NOTCH, and estrogen receptor (ER) pathways is also implicated in tumor cell proliferation and survival.159 Gene expression profiling revolutionized breast cancer biology and therapeutic strategies by uncovering its heterogeneous transcriptional landscape. These diverse molecular profiles correlate with variations in histological features, metastasis patterns, clinical outcomes, and treatment efficacy, underscoring the disease’s inherent complexity. Breast cancer subtypes are classified based on receptor expression: HER2-enriched (ER−, PR−, HER2+), luminal A (ER+/PR+, HER2−, Ki-67 <14%), normal-like (ER+/PR+, HER2−, low Ki-67), luminal B (ER+/PR+, HER2−, Ki-67 ≥14% or HER2+), and basal-like (triple-negative) (ER−, PR−, HER2−, CK5/6+ and/or EGFR+). Luminal and normal-like breast cancers generally exhibit favorable prognoses, whereas basal-like subtypes are associated with poorer outcomes compared to other variants.160 Although the exact causes of breast cancer remain unclear, certain modifiable risk factors elevate its likelihood. Breast cancer risk factors span multiple categories: hereditary (family history, genetic predisposition, etc)., demographic (age, female, sex, etc)., breast-specific (reduced lactation periods, benign breast conditions), environmental exposures, hormonal influences (hormone replacement therapy, fertility medications, oral contraceptives, etc)., and lifestyle-related (obesity, smoking, poor diet, vitamin D deficiency, sedentary behavior, alcohol use, and shift work).161 Breast cancer was the most frequently diagnosed cancer among women, accounting for 11.7% of all global cancer cases reported in 2018.162 In 2020, cancer led to 10 million deaths worldwide, with 19.3 million new cases (18.1 million excluding non-melanoma skin cancer and basal cell carcinoma, and 9.9 million when non-melanoma skin cancers excluding basal cell carcinoma were omitted). The accessibility of chemotherapeutic agents remains restricted due to their lower tumor concentrations compared to other organs, which contributes to heightened toxic effects. The effectiveness of chemotherapeutic drugs is hindered by their limited tumor-specific accumulation, resulting in unintended drug distribution and increased systemic toxicity. To enhance treatment outcomes, various strategies have been explored. Natural enantiomers, like the one formulated by Periasamy et al using Nigella sativa L. essential oil, have shown potential as a breast cancer therapy.163 This NE demonstrates anti-cancer effects by triggering apoptosis in MCF-7 breast cancer cells in vitro. Additionally, NE can encapsulate active breast cancer medications.164 Treatment approaches have also incorporated local drug delivery alongside advancements in C6 ceramide NEs. Scientists utilize NE-based drug delivery and local administration to target tumors and pre-tumor lesions while reducing systemic side effects. To enhance treatment, researchers have developed bioadhesive ceramide-loaded NEs coated with chitosan. Nanoencapsulation of C6 ceramide significantly improved its potency, lowering the concentration needed to reduce MCF-7 cell viability by 50% (EC50) by 4.5 times compared to its solution form. Additionally, incorporating tributyrin, a butyric acid pro-drug, into the NE’s oil phase further reduced the required concentration by 2.6 times. Compared to its solution form, intraductal administration of the NE prolonged drug retention in mammary tissue for over 120 hours.165 Natesan and colleagues also utilized chitosan to develop NEs, encapsulating camptothecin and evaluating its effectiveness both in vitro and in vivo, comparing the NE formulation with the free drug.166 Ongoing advancements in breast cancer treatment using NE-based drug delivery are outlined in Table 3.
 |
Table 3 Breast Cancer Treatment Summary Using NE Drug Delivery |
Clinically Approved Nanomedicines for the Treatment of Breast Cancer
Nanodrug formulations loaded with NEs are increasingly prominent in global cancer therapy markets. Notably, Abraxane, utilizing albumin-bound paclitaxel, commands a significant share due to its proven clinical efficacy and safety. Doxil and Myocet, leveraging liposomal NEs for doxorubicin delivery, also feature prominently in breast cancer treatment. These drugs are globally accessible and favored for their enhanced therapeutic outcomes. Ongoing research is geared towards refining these drugs and broadening their applications in different cancer types. Clinical trials and studies aim to optimize drug delivery, minimize side effects, and enhance treatment efficacy. Nano drug formulations, which reduce toxicity and improve drug solubility, are set to play an expanding role in personalized cancer therapy, catering to individual patient profiles. While Abraxane, Doxil, and Myocet are established leaders, newer entrants like Onivyde, Nanoxel, NANOXEL-E, and Paclitaxel are making significant inroads. These formulations offer innovative features such as sustained drug release kinetics and reduced cytotoxicity, adding diversity to NEs-based cancer therapy. As research progresses, market shares may shift, reflecting the dynamic landscape of cancer treatment and personalized approaches. The Table 4 provides a comprehensive overview of various nano-drug formulations loaded with NEs for breast cancer therapy, highlighting their nanomedicine characteristics, therapeutic and nanotheranostic applications, years of FDA approval, clinical efficacy, safety profiles, and mechanisms of action on cancer cells.
 |
Table 4 Clinically Approved Nanomedicine Drugs |
These NEs offer enhanced drug delivery, reducing toxicity while improving treatment outcomes. In a study, researchers developed a nanocomposite by crosslinking chitosan and agarose to form a polymeric hydrogel, incorporating γ-alumina NPs within the hydrogel for 5-FU delivery. This nanocomposite was encapsulated in a W/O/W NE system. Experiments with MCF-7 breast cancer cells revealed that the 5-FU-loaded NE was more effective in eliminating cancer cells than crude 5-FU.192 Another study focused on the design and characterization of a pequi oil-based NE with anticancer properties. The NE, made with lecithin as a surfactant, showed excellent physicochemical stability under stress and during long-term storage. It demonstrated significant dose- and time-dependent antitumoral effects against 4T1 breast cancer cells and exhibited lower cytotoxicity toward non-cancerous NIH/3T3 cells. Pequi oil served as both a structural element and a cytotoxic agent. Overall, the pequi oil-based NE shows great potential as a therapeutic platform for breast cancer treatment.179
Therapeutic Potential and Hurdles
NEs offer several key benefits as drug delivery systems, particularly for breast cancer treatment. Their ultra-small droplet sizes (usually between 20 and 200 nm) provide a high surface area-to-volume ratio, improving the solubility and bioavailability of poorly soluble drugs.193 Additionally, NEs can encapsulate both hydrophobic and hydrophilic compounds, enabling the co-delivery of various therapeutic agents, including chemotherapeutics and immunomodulatory drugs. This bimodal approach can facilitate enhanced therapeutic effectiveness while potentially mitigating systemic toxicity. Additionally, the stability of NEs under various environmental stressors (eg, temperature fluctuations, pH changes, etc) allows for prolonged shelf life and ease of formulation, which are crucial for clinical applications. The capacity for surface modification using various surfactants and targeting ligands affords NEs the ability to achieve site-specific delivery, thereby minimizing off-target effects and enhancing localized therapeutic efficacy against breast cancer cells.194 Despite these advantages, several challenges hinder the widespread clinical adoption of NEs. One prominent issue is the formulation scalability and reproducibility, which can pose significant hurdles when transitioning from laboratory to industrial production. The production processes often involve complex methodologies (eg, HPH or ultrasonication), which can require specialized equipment and may lead to variations in droplet size distribution that impact therapeutic outcomes. Moreover, NEs could exhibit potential immunogenicity or toxicity due to their surfactants or any active pharmaceutical ingredients they carry. The long-term safety of repeated administration of NEs remains an area of concern and requires thorough clinical evaluation, especially considering the particularly sensitive population of breast cancer patients. Furthermore, understanding the pharmacokinetics and dynamics of NEs within the biological system is still in its infancy. Factors such as biological barriers, enzyme interactions, and patient variability can significantly influence the performance of NEs, leading to unpredictable therapeutic responses. To overcome these hurdles, future strategies must focus on several key areas: Developing more streamlined and reproducible production methods will be vital. This could include the integration of continuous flow methods or microfluidics to ensure uniformity in droplet size and improve manufacturability while minimizing energy consumption. Research efforts should investigate novel surfactants and co-surfactants that are more biocompatible and possess lower toxicity profiles.195
Exploring natural surfactants could offer safer alternatives while preserving the effective drug delivery properties of NEs. To improve the targeting of NEs to tumor sites, research should focus on attaching ligands to NEs that specifically recognize tumor markers or developing stimulus-responsive systems that release drugs in response to specific triggers like pH or temperature changes in the tumor microenvironment. Future research might also utilize advancements in AI and machine learning to create predictive models that customize NE formulations and treatment protocols based on individual patient profiles, optimizing therapeutic outcomes while minimizing side effects. Long-term, comprehensive studies are crucial to assess the safety of NEs with repeated systemic administration. This includes assessing potential immune responses, cytotoxic effects on non-tumoral tissues, and any implications from cumulative exposure to formulation components. By addressing these current disadvantages and leveraging their unique advantages, NEs can transform into a robust platform for breast cancer therapy, paving the way for more effective, targeted, and patient-centric treatment modalities. There is a lack of comprehensive research detailing the in vivo mechanisms governing drug release and bio-distribution of NEs. Understanding how NEs interact within the biological environment, including their absorption, distribution, metabolism, and excretion, is crucial for predicting therapeutic outcomes and potential side effects. Current studies often focus more on formulation and preliminary efficacy rather than these essential pharmacokinetic properties. Many of the formulation approaches remain empirical and may vary significantly between studies. This unpredictability can result in inconsistencies in droplet size, zeta potential, and stability, affecting the overall therapeutic effectiveness of NEs. Additionally, the lack of standardized protocols for production and characterization further complicates the comparative assessment of different NE formulation. The long-term safety of repeated NE administration has not been adequately addressed.196 Potential cytotoxicity and immunogenic responses to NEs and their components need thorough investigation, particularly given the recurring nature of breast cancer treatments. Current studies often do not account for inter-patient variability in terms of genetics, disease stage, and prior treatments. This variability can significantly impact the effectiveness and safety of NEs, highlighting the need for personalized therapy approaches that have yet to be significantly explored in the context of NEs. Many research labs may lack the necessary funding, infrastructure, or technical expertise to perform extensive pharmacokinetic studies or to develop large-scale production methods that ensure uniformity and quality in NE formulation. The intricate nature of biological systems makes it challenging to predict how NEs will behave within different patient populations. This complexity may discourage researchers from pursuing these avenues due to the uncertain outcomes and high costs associated with advanced studies. A significant emphasis in current research is placed on the initial efficacy of NEs against specific cancer cell lines.197 While this is critical for establishing the potential of NEs, it often overshadows the longer-term considerations such as safety, pharmacokinetics, and individualized treatment approaches.
There are a few possible ways to overcome these issues. Future studies should adopt an integrative approach that encompasses formulation, pharmacokinetics, and safety assessments from the outset.197 This includes collaboration between formulation scientists, pharmacologists, and clinicians to create a more holistic view of how NEs will perform in clinical settings. Establishing standardized protocols for both NE production and characterization can improve reproducibility and comparability across studies, thus fostering a more systematic evaluation of their potential clinical applications. Prioritizing long-term safety studies that could utilize in vivo models to assess potential immune responses and cytotoxicity associated with repeated NE administration is crucial. Such studies would provide critical data necessary for regulatory approvals and clinical trials. Emphasizing personalized approaches could enhance treatment effectiveness. This may involve high-throughput screening of patient-derived models to understand how individual tumor profiles respond to various NE formulations, leading to tailored treatment regimens that consider the unique characteristics of each patient’s cancer. By rigorously addressing these caveats, the translation of NE technology into effective clinical therapies for breast cancer could be significantly enhanced, ultimately leading to improved patient outcomes.
Future Research and Development
In the realm of utilizing NEs as carriers for the pharmacological treatment of breast cancer, there are notable challenges that forthcoming research must confront. These challenges encompass the development of multifunctional NEs capable of concurrently delivering chemotherapeutic agents, gene therapy vectors, and immunomodulatory agents. Special attention should be given to the design of NEs that exhibit superior targeting precision, heightened bioavailability, and reduced toxicity. It is also imperative to delve into the synergistic effects of combination therapies delivered through NEs and comprehend the in vivo mechanisms governing drug release for optimal therapeutic outcomes. A crucial aspect of this research is the investigation of the long-term safety and immunological implications associated with repeated NEs-based treatments for breast cancer patients. Furthermore, the integration of artificial intelligence and machine learning for predictive modeling of patient responses holds substantial promise in tailoring treatment regimens to individual patients. Subsequent studies may extend their focus to the application of multifunctional NEs in the context of breast cancer treatment, leveraging advanced analytical techniques and patient-derived models to enable highly personalized therapeutic approaches.
Conclusion
This review highlights the considerable potential of NEs as versatile drug carriers in breast cancer treatment. The unique physicochemical properties of NEs, such as their high surface area, stability, and customizable characteristics, make them strong candidates for improving drug delivery systems. By encapsulating a variety of therapeutic agents, NEs can enhance bioavailability and treatment efficacy while reducing systemic toxicity, a major issue in traditional breast cancer treatments. However, despite promising results, significant challenges remain in optimizing the clinical use of NEs. Future research should focus on developing multifunctional NEs that can deliver a combination of chemotherapeutics, gene therapies, and immunomodulatory agents, while improving targeting accuracy and minimizing off-target effects. Investigating the long-term safety and immunological consequences of repeated NE administration is also critical to ensure that treatments remain both effective and safe for patients. Additionally, the integration of artificial intelligence and machine learning could play a crucial role in personalizing treatment regimens based on individual patient characteristics and tumor profiles. As research in this field advances, standardizing NE formulation and characterization methods will be essential to ensure reproducibility and comparability across studies. Ultimately, the progress of NE technology has the potential to transform breast cancer therapy. With ongoing research and a focus on personalized medicine, NEs could lead to more effective and targeted treatments, improving both patient outcomes and quality of life.
Highlights
- Nanoemulsions (NEs) were synthesized using various homogenization techniques to achieve sub-100 nm droplet sizes.
- Surfactants and predictive models enhanced NE stability, preventing coalescence and extending shelf-life.
- NEs demonstrated targeted drug delivery to breast cancer tumors, reducing systemic toxicity and improving therapeutic efficacy.
- Dual O/W and W/O nanoemulsions systems disrupted tumor microenvironments by inducing apoptosis and inhibiting angiogenesis in preclinical models.
Acknowledgments
The authors would like to sincerely thank the Technical University of Munich for their essential support in enabling the publication of this research. They also express their gratitude to the University of Malaya for their generous provision of resources and access to library databases.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author(s) report no conflicts of interest in this work.
References
1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi:10.1002/cncr.33587
2. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
3. Chen S, Cao Z, Prettner K, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023;9(4):465–472. doi:10.1001/jamaoncol.2022.7826
4. Guida F, Kidman R, Ferlay J, et al. Global and regional estimates of orphans attributed to maternal cancer mortality in 2020. Nature Med. 2022;28(12):2563–2572. doi:10.1038/s41591-022-02109-2
5. Dhiman R, Bazad N, Mukherjee R, et al. Enhanced drug delivery with nanocarriers: a comprehensive review of recent advances in breast cancer detection and treatment. Discover Nano. 2024;19(1):143. doi:10.1186/s11671-024-04086-6
6. Alven S, Aderibigbe BA. The therapeutic efficacy of dendrimer and micelle formulations for breast cancer treatment. Pharmaceutics. 2020;12(12):1212. doi:10.3390/pharmaceutics12121212
7. Jardim GA, da Cruz EH, Valença WO, et al. Synthesis of selenium-quinone hybrid compounds with potential antitumor activity via Rh-catalyzed CH bond activation and click reactions. Molecules. 2017;23(1):83. doi:10.3390/molecules23010083
8. Izadiyan Z, Basri M, Masoumi HRF, Karjiban RA, Salim N, Kalantari K. Improvement of physicochemical properties of nanocolloidal carrier loaded with low water solubility drug for parenteral cancer treatment by response surface methodology. Mater Sci Eng C. 2019;94:841–849. doi:10.1016/j.msec.2018.10.015
9. Kaur P, Gupta S, Kaur K, Kaur N, Kumar R, Bhullar MS. Nanoemulsion of Foeniculum vulgare essential oil: a propitious striver against weeds of Triticum aestivum. Ind Crops Prod. 2021;168:113601. doi:10.1016/j.indcrop.2021.113601
10. Jamali SN, Assadpour E, Feng J, Jafari SM. Natural antimicrobial-loaded nanoemulsions for the control of food spoilage/pathogenic microorganisms. Adv Colloid Interface Sci. 2021;295:102504. doi:10.1016/j.cis.2021.102504
11. Imran M, Iqubal MK, Imtiyaz K, et al. Topical nanostructured lipid carrier gel of quercetin and resveratrol: formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int J Pharm. 2020;587:119705. doi:10.1016/j.ijpharm.2020.119705
12. Wilson RJ, Li Y, Yang G, Zhao C-X. Nanoemulsions for drug delivery. Particuology. 2022;64:85–97. doi:10.1016/j.partic.2021.05.009
13. Chatzidaki MD, Mitsou E. Advancements in nanoemulsion-based drug delivery across different administration routes. Pharmaceutics. 2025;17(3):337. doi:10.3390/pharmaceutics17030337
14. Croissant JG, Butler KS, Zink JI, Brinker CJ. Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nature Rev Mater. 2020;5(12):886–909.
15. Kah M, Johnston LJ, Kookana RS, et al. Comprehensive framework for human health risk assessment of nanopesticides. Nature Nanotechnol. 2021;16(9):955–964. doi:10.1038/s41565-021-00964-7
16. Demokritou P, Gass S, Pyrgiotakis G, et al. An in vivo and in vitro toxicological characterisation of realistic nanoscale CeO2 inhalation exposures. Nanotoxicology. 2013;7(8):1338–1350. doi:10.3109/17435390.2012.739665
17. Ahmad A, Imran M, Sharma N. Precision nanotoxicology in drug development: current trends and challenges in safety and toxicity implications of customized multifunctional nanocarriers for drug-delivery applications. Pharmaceutics. 2022;14(11):2463. doi:10.3390/pharmaceutics14112463
18. Pal AK, Aalaei I, Gadde S, et al. High resolution characterization of engineered nanomaterial dispersions in complex media using tunable resistive pulse sensing technology. ACS Nano. 2014;8(9):9003–9015. doi:10.1021/nn502219q
19. Li S, Wang H, Shan Y. The mechanism of nano-drug delivery. Current Pharmacol Rep. 2019;5(6):410–420. doi:10.1007/s40495-019-00205-5
20. Rabanel JM, Aoun V, Elkin I, Mokhtar M, Hildgen P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr Med Chem. 2012;19(19):3070–3102. doi:10.2174/092986712800784702
21. Ma X, Tian Y, Yang R, et al. Nanotechnology in healthcare, and its safety and environmental risks. J Nanobiotechnol. 2024;22(1):715. doi:10.1186/s12951-024-02901-x
22. Gowda R, Jones NR, Banerjee S, Robertson GP. Use of nanotechnology to develop multi-drug inhibitors for cancer therapy. J Nanomed Nanotechnol. 2013;4(6):184. doi:10.4172/2157-7439.1000184
23. Tagde P, Najda A, Nagpal K, et al. Nanomedicine-based delivery strategies for breast cancer treatment and management. Int J mol Sci. 2022;23(5):2856. doi:10.3390/ijms23052856
24. Musa SH, Basri M, Masoumi HRF, et al. Formulation optimization of palm kernel oil esters nanoemulsion-loaded with chloramphenicol suitable for meningitis treatment. Colloids Surf B. 2013;112:113–119. doi:10.1016/j.colsurfb.2013.07.043
25. McClements DJ. Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter. 2011;7(6):2297–2316. doi:10.1039/C0SM00549E
26. McClements DJ, Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr. 2011;51(4):285–330. doi:10.1080/10408398.2011.559558
27. Jafari SM, McClements DJ. Nanotechnology approaches for increasing nutrient bioavailability. Adv Food Nutr Res. 2017;81:1–30.
28. McClements DJ. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr Opin Colloid Interface Sci. 2012;17(5):235–245. doi:10.1016/j.cocis.2012.06.002
29. Shakeel F, Haq N, Al-Dhfyan A, Alanazi FK, Alsarra IA. Double w/o/w nanoemulsion of 5-fluorouracil for self-nanoemulsifying drug delivery system. J mol Liq. 2014;200:183–190. doi:10.1016/j.molliq.2014.10.013
30. Garti N. Double emulsions—scope, limitations and new achievements. Colloids Surf A. 1997;123:233–246. doi:10.1016/S0927-7757(96)03809-5
31. Assadpour E, Maghsoudlou Y, Jafari S-M, Ghorbani M, Aalami M. Optimization of folic acid nano-emulsification and encapsulation by maltodextrin-whey protein double emulsions. Int J Biol Macromol. 2016;86:197–207. doi:10.1016/j.ijbiomac.2016.01.064
32. Assadpour E, Jafari S-M, Maghsoudlou Y. Evaluation of folic acid release from spray dried powder particles of pectin-whey protein nano-capsules. Int J Biol Macromol. 2017;95:238–247. doi:10.1016/j.ijbiomac.2016.11.023
33. Esfanjani AF, Jafari SM, Assadpoor E, Mohammadi A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J Food Eng. 2015;165:149–155. doi:10.1016/j.jfoodeng.2015.06.022
34. McClements DJ. Food Emulsions: Principles, Practices, and Techniques. CRC press; 2015.
35. Aboofazeli R. Nanometric-scaled emulsions (nanoemulsions). Iran J Pharm Res. 2010;9(4):325.
36. Gorain B, Choudhury H, Nair AB, Dubey SK, Kesharwani P. Theranostic application of nanoemulsions in chemotherapy. Drug Discovery Today. 2020;25(7):1174–1188. doi:10.1016/j.drudis.2020.04.013
37. Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–2841. doi:10.1039/C5SM02958A
38. Shams N, Sahari MA. Nanoemulsions: preparation, structure, functional properties and their antimicrobial effects. Appl Food Biotechnol. 2016;3(3):138–149.
39. Sampieri-Morán JM, Bravo-Alfaro DA, Uribe-Lam E, et al. Delivery of Magnolia bark extract in nanoemulsions formed by high and low energy methods improves the bioavailability of Honokiol and Magnolol. Eur J Pharm Biopharm. 2025;208:114627. doi:10.1016/j.ejpb.2025.114627
40. Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108:303–318. doi:10.1016/j.cis.2003.10.023
41. van der Schaaf US, Karbstein HP. Fabrication of nanoemulsions by rotor-stator emulsification. In: Nanoemulsions. Elsevier; 2018:141–174.
42. Salvia-Trujillo L, Soliva-Fortuny R, Rojas-Graü MA, McClements DJ, Martín-Belloso O. Edible nanoemulsions as carriers of active ingredients: a review. Annu Rev Food Sci Technol. 2017;8(1):439–466. doi:10.1146/annurev-food-030216-025908
43. Setya S, Talegaonkar S, Razdan B. Nanoemulsions: formulation methods and stability aspects. World J Pharm Pharm Sci. 2014;3(2):2214–2228.
44. Safaya M, Rotliwala YC. Nanoemulsions: a review on low energy formulation methods, characterization, applications and optimization technique. Mater Today. 2020;27:454–459.
45. Jasmina H, Džana O, Alisa E, Edina V, Ognjenka R. Preparation of Nanoemulsions by High-Energy and Lowenergy Emulsification Methods. Springer; 2017:317–322.
46. Komaiko JS, McClements DJ. Formation of food‐grade nanoemulsions using low-energy preparation methods: a review of available methods. Compr Rev Food Sci Food Saf. 2016;15(2):331–352. doi:10.1111/1541-4337.12189
47. Mushtaq A, Wani SM, Malik A, et al. Recent insights into Nanoemulsions: their preparation, properties and applications. Food Chem X. 2023;18:100684. doi:10.1016/j.fochx.2023.100684
48. Aswathanarayan JB, Vittal RR. Nanoemulsions and their potential applications in food industry. Front Sustain Food Syst. 2019;3:95. doi:10.3389/fsufs.2019.00095
49. Sigurdson GT, Tang P, Giusti MM. Natural colorants: food colorants from natural sources. Annu Rev Food Sci Technol. 2017;8:261–280. doi:10.1146/annurev-food-030216-025923
50. Tayeb HH, Felimban R, Almaghrabi S, Hasaballah N. Nanoemulsions: formulation, characterization, biological fate, and potential role against COVID-19 and other viral outbreaks. Colloid Interface Sci Commun. 2021;45:100533. doi:10.1016/j.colcom.2021.100533
51. Kumar M, Bishnoi RS, Shukla AK, Jain CP. Techniques for formulation of nanoemulsion drug delivery system: a review. Prev Nutr Food Sci. 2019;24(3):225. doi:10.3746/pnf.2019.24.3.225
52. Kotta S, Khan AW, Ansari S, Sharma R, Ali J. Formulation of nanoemulsion: a comparison between phase inversion composition method and high-pressure homogenization method. Drug Delivery. 2015;22(4):455–466. doi:10.3109/10717544.2013.866992
53. Yukuyama M, Ghisleni D, Pinto T, Bou‐Chacra N. Nanoemulsion: process selection and application in cosmetics–a review. Int J Cosmet Sci. 2016;38(1):13–24. doi:10.1111/ics.12260
54. Singh Y, Meher JG, Raval K, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. doi:10.1016/j.jconrel.2017.03.008
55. Kentish S, Wooster T, Ashokkumar M, Balachandran S, Mawson R, Simons L. Innovative Food Sci. Emerg Technol. 2008;9(2):170–175.
56. Saffarionpour S. Preparation of food flavor nanoemulsions by high-and low-energy emulsification approaches. Food Eng Rev. 2019;11(4):259–289. doi:10.1007/s12393-019-09201-3
57. Håkansson A, Rayner M. General principles of nanoemulsion formation by high-energy mechanical methods. In: Nanoemulsions. Elsevier; 2018:103–139.
58. Akbas E, Soyler B, Oztop MH. Formation of capsaicin loaded nanoemulsions with high pressure homogenization and ultrasonication. Lwt. 2018;96:266–273. doi:10.1016/j.lwt.2018.05.043
59. Espitia P, Fuenmayor C, Otoni C. Nanoemulsions: synthesis, characterization, and application in bio-based active food packaging. Compr Rev Food Sci Food Saf. 2018;18:264–285. doi:10.1111/1541-4337.12405
60. Silva HD, Cerqueira MA, Vicente AA. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J Food Eng. 2015;167:89–98. doi:10.1016/j.jfoodeng.2015.07.037
61. Chuacharoen T, Prasongsuk S, Sabliov CM. Effect of surfactant concentrations on physicochemical properties and functionality of curcumin nanoemulsions under conditions relevant to commercial utilization. Molecules. 2019;24(15):2744. doi:10.3390/molecules24152744
62. Faria M, Björnmalm M, Thurecht KJ, et al. Minimum information reporting in bio–nano experimental literature. Nature Nanotechnol. 2018;13(9):777–785. doi:10.1038/s41565-018-0246-4
63. Jaiswal M, Dudhe R, Sharma P. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–127. doi:10.1007/s13205-014-0214-0
64. Ganta S, Amiji M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. mol Pharmaceut. 2009;6(3):928–939. doi:10.1021/mp800240j
65. Klang V, Matsko NB, Valenta C, Hofer F. Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron. 2012;43(2–3):85–103. doi:10.1016/j.micron.2011.07.014
66. Klang V, Matsko N, Raupach K, El-Hagin N, Valenta C. Development of sucrose stearate-based nanoemulsions and optimisation through γ-cyclodextrin. Eur J Pharm Biopharm. 2011;79(1):58–67. doi:10.1016/j.ejpb.2011.01.010
67. Kuntsche J, Horst JC, Bunjes H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int J Pharm. 2011;417(1–2):120–137. doi:10.1016/j.ijpharm.2011.02.001
68. Fox CB. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;14(9):3286–3312. doi:10.3390/molecules14093286
69. Severs NJ, Robenek H. Freeze-fracture cytochemistry in cell biology. Methods Cell Biol. 2008;88:181–204.
70. Vickers NJ. Animal communication: when I’m calling you, will you answer too? Curr Biol. 2017;27(14):R713–R715. doi:10.1016/j.cub.2017.05.064
71. Pavoni L, Perinelli DR, Bonacucina G, Cespi M, Palmieri GF. An overview of micro-and nanoemulsions as vehicles for essential oils: formulation, preparation and stability. Nanomaterials. 2020;10(1):135. doi:10.3390/nano10010135
72. Liu Q, Huang H, Chen H, Lin J, Wang Q. Food-grade nanoemulsions: preparation, stability and application in encapsulation of bioactive compounds. Molecules. 2019;24(23):4242. doi:10.3390/molecules24234242
73. Patel SK, Beaino W, Anderson CJ, Janjic JM. Theranostic nanoemulsions for macrophage COX-2 inhibition in a murine inflammation model. Clin Immunol. 2015;160(1):59–70. doi:10.1016/j.clim.2015.04.019
74. Meng L, Xia X, Yang Y, et al. Co-encapsulation of paclitaxel and baicalein in nanoemulsions to overcome multidrug resistance via oxidative stress augmentation and P-glycoprotein inhibition. Int J Pharm. 2016;513(1–2):8–16. doi:10.1016/j.ijpharm.2016.09.001
75. Streck L, Sarmento VH, de Menezes RP, Fernandes-Pedrosa MF, Martins AM, Da silva-júnior AA. Tailoring microstructural, drug release properties, and antichagasic efficacy of biocompatible oil-in-water benznidazol-loaded nanoemulsions. Int J Pharm. 2019;555:36–48. doi:10.1016/j.ijpharm.2018.11.041
76. Salvia-Trujillo L, Martín-Belloso O, McClements DJ. Excipient nanoemulsions for improving oral bioavailability of bioactives. Nanomaterials. 2016;6(1):17. doi:10.3390/nano6010017
77. da Rocha Neto AC, da Rocha ABDO, Maraschin M, Di Piero RM, Almenar E. Factors affecting the entrapment efficiency of β-cyclodextrins and their effects on the formation of inclusion complexes containing essential oils. Food Hydrocoll. 2018;77:509–523. doi:10.1016/j.foodhyd.2017.10.029
78. Che Marzuki NH, Wahab RA, Abdul Hamid M. An overview of nanoemulsion: concepts of development and cosmeceutical applications. Biotechnol Biotechnol Equip. 2019;33(1):779–797. doi:10.1080/13102818.2019.1620124
79. Buschow KJ. Encyclopedia of materials: science and technology. 2001.
80. Shao P, Feng J, Sun P, Xiang N, Lu B, Qiu D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res Int. 2020;137:109376. doi:10.1016/j.foodres.2020.109376
81. Dickinson E, Miller R. Food Colloids: Fundamentals of Formulation. Vol. 258. Royal Society of Chemistry; 2001.
82. Ivanov IB, Danov KD, Kralchevsky PA. Flocculation and coalescence of micron-size emulsion droplets. Colloids Surf A. 1999;152(1–2):161–182. doi:10.1016/S0927-7757(98)00620-7
83. Hubbard AT. Encyclopedia of Surface and Colloid Science. Vol. 1. CRC press; 2002.
84. Grumezescu A, Oprea AE. Nanotechnology Applications in Food: Flavor, Stability, Nutrition and Safety. Academic Press; 2017.
85. Robins MM. Emulsions—creaming phenomena. Curr Opin Colloid Interface Sci. 2000;5(5–6):265–272. doi:10.1016/S1359-0294(00)00065-0
86. Behrens MA, Franzén A, Carlert S, Skantze U, Lindfors L, Olsson U. On the Ostwald ripening of crystalline and amorphous nanoparticles. Soft Matter. 2025;21(12):2349–2354. doi:10.1039/D4SM01544D
87. Mitra AK, Cholkar K, Mandal A. Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices. William Andrew; 2017.
88. Verwey E. Electrical double layer and stability of emulsions. Trans Faraday Soc. 1940;35:192–203. doi:10.1039/tf9403500192
89. McClements DJ, Lu J, Grossmann L. Proposed methods for testing and comparing the emulsifying properties of proteins from animal, plant, and alternative sources. Colloids Interfaces. 2022;6(2):19. doi:10.3390/colloids6020019
90. Park S-J, Seo M-K. Types of composites. In: Interface Science and Technology. Elsevier; 2011:501–629.
91. Delgado ÁV, González-Caballero F, Hunter R, Koopal L, Lyklema J. Measurement and interpretation of electrokinetic phenomena. J Colloid Interface Sci. 2007;309(2):194–224. doi:10.1016/j.jcis.2006.12.075
92. Wooster TJ, Augustin MA. β-Lactoglobulin–dextran Maillard conjugates: their effect on interfacial thickness and emulsion stability. J Colloid Interface Sci. 2006;303(2):564–572. doi:10.1016/j.jcis.2006.07.081
93. Tambe DE, Sharma MM. Factors controlling the stability of colloid-stabilized emulsions: II. A model for the rheological properties of colloid-laden interfaces. J Colloid Interface Sci. 1994;162(1):1–10. doi:10.1006/jcis.1994.1001
94. Claesson PM, Kjellin M, Rojas OJ, Stubenrauch C. Short-range interactions between non-ionic surfactant layers. Phys Chem Chem Phys. 2006;8(47):5501–5514. doi:10.1039/b610295f
95. Napper D, Netschey A. Studies of the steric stabilization of colloidal particles. J Colloid Interface Sci. 1971;37(3):528–535. doi:10.1016/0021-9797(71)90330-4
96. McClements DJ. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8(6):1719–1729. doi:10.1039/C2SM06903B
97. Mehrnia M-A, Jafari S-M, Makhmal-Zadeh BS, Maghsoudlou Y. Rheological and release properties of double nano-emulsions containing crocin prepared with Angum gum, Arabic gum and whey protein. Food Hydrocoll. 2017;66:259–267. doi:10.1016/j.foodhyd.2016.11.033
98. Jafari SM, Fathi M, Mandala I. Emerging product formation. Food Waste Recovery. 2021;257–275.
99. Khan MS, Roberts MS. Challenges and innovations of drug delivery in older age. Adv Drug Delivery Rev. 2018;135:3–38. doi:10.1016/j.addr.2018.09.003
100. Majumdar. S. Oral drug delivery: conventional to long acting new-age designs. 2021.
101. Mallick S, Pattnaik S, Swain K, De PK. Current perspectives of solubilization: potential for improved bioavailability. Drug Dev Ind Pharm. 2007;33(8):865–873. doi:10.1080/03639040701429333
102. Pattnaik S, Swain K, Manaswini P, et al. Fabrication of aceclofenac nanocrystals for improved dissolution: process optimization and physicochemical characterization. J Drug Delivery Sci Technol. 2015;29:199–209. doi:10.1016/j.jddst.2015.07.021
103. Senta-Loys Z, Bourgeois S, Valour J-P, Briançon S, Fessi H. Orodispersible films based on amorphous solid dispersions of tetrabenazine. Int J Pharm. 2017;518(1–2):242–252. doi:10.1016/j.ijpharm.2016.12.036
104. Huang Y, Dai W-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharmaceutica Sinica B. 2014;4(1):18–25. doi:10.1016/j.apsb.2013.11.001
105. Kumar Mahapatra A, Narsimha Murthy P, Kumari Patra R, Pattnaik S. Solubility enhancement of modafinil by complexation with β-cyclodextrin and hydroxypropyl β-cyclodextrin: a response surface modeling approach. Drug Deliv Lett. 2013;3(3):210–219. doi:10.2174/22103031113039990005
106. Mallick S, Pattnaik S, Swain K, et al. Formation of physically stable amorphous phase of ibuprofen by solid state milling with kaolin. Eur J Pharm Biopharm. 2008;68(2):346–351. doi:10.1016/j.ejpb.2007.06.003
107. Pattnaik S, Pathak K. Mesoporous silica molecular sieve based nanocarriers: transpiring drug dissolution research. Curr Pharm Des. 2017;23(3):467–480. doi:10.2174/1381612822666161026162005
108. Pattnaik S, Swain K, Rao JV, Talla V, Prusty KB, Subudhi SK. Polymer co-processing of ibuprofen through compaction for improved oral absorption. RSC Adv. 2015;5(91):74720–74725. doi:10.1039/C5RA13038G
109. Yoo M-K, Kang S-K, Choi J-H, et al. Targeted delivery of chitosan nanoparticles to Peyer’s patch using M cell-homing peptide selected by phage display technique. Biomaterials. 2010;31(30):7738–7747. doi:10.1016/j.biomaterials.2010.06.059
110. Khatri P, Shao J. Transport of lipid nano-droplets through MDCK epithelial cell monolayer. Colloids Surf B. 2017;153:237–243. doi:10.1016/j.colsurfb.2017.02.024
111. Mamadou G, Charrueau C, Dairou J, Nzouzi NL, Eto B, Ponchel G. Increased intestinal permeation and modulation of presystemic metabolism of resveratrol formulated into self-emulsifying drug delivery systems. Int J Pharm. 2017;521(1–2):150–155. doi:10.1016/j.ijpharm.2017.02.036
112. Piazzini V, Monteforte E, Luceri C, Bigagli E, Bilia AR, Bergonzi MC. Nanoemulsion for improving solubility and permeability of Vitex agnus-castus extract: formulation and in vitro evaluation using PAMPA and Caco-2 approaches. Drug Delivery. 2017;24(1):380–390. doi:10.1080/10717544.2016.1256002
113. Elkadi S, Elsamaligy S, Al-Suwayeh S, Mahmoud H. The development of self-nanoemulsifying liquisolid tablets to improve the dissolution of simvastatin. AAPS Pharm Sci Tech. 2017;18:2586–2597. doi:10.1208/s12249-017-0743-z
114. Gassmann P, List M, Schweitzer A, Sucker H. Hydrosols: alternatives for the parenteral application of poorly water soluble drugs. Eur J Pharm Biopharm. 1994;40(2):64–72.
115. Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharmaceutica Sinica B. 2015;5(5):442–453. doi:10.1016/j.apsb.2015.07.003
116. Czerniel J, Gostyńska-Stawna A, Sommerfeld-Klatta K, Przybylski T, Krajka-Kuźniak V, Stawny M. Development and validation of in vitro assessment protocol of novel intravenous nanoemulsions for parenteral nutrition. Pharmaceutics. 2025;17(4):493. doi:10.3390/pharmaceutics17040493
117. Hörmann K, Zimmer A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J Control Release. 2016;223:85–98. doi:10.1016/j.jconrel.2015.12.016
118. Cheng Y, Liu M, Hu H, Liu D, Zhou S. Development, optimization, and characterization of PEGylated nanoemulsion of prostaglandin E1 for long circulation. AAPS Pharm Sci Tech. 2016;17:409–417. doi:10.1208/s12249-015-0366-1
119. Tan SF, Masoumi HRF, Karjiban RA, et al. Ultrasonic emulsification of parenteral valproic acid-loaded nanoemulsion with response surface methodology and evaluation of its stability. Ultrason Sonochem. 2016;29:299–308. doi:10.1016/j.ultsonch.2015.09.015
120. Gué E, Since M, Ropars S, Herbinet R, Le Pluart L, Malzert-Fréon A. Evaluation of the versatile character of a nanoemulsion formulation. Int J Pharm. 2016;498(1–2):49–65. doi:10.1016/j.ijpharm.2015.12.010
121. Söderlind E, Wollbratt M, von Corswant C. The usefulness of sugar surfactants as solubilizing agents in parenteral formulations. Int J Pharm. 2003;252(1–2):61–71. doi:10.1016/S0378-5173(02)00599-9
122. Lu Y, Wang Y, Tang X. Formulation and thermal sterile stability of a less painful intravenous clarithromycin emulsion containing vitamin E. Int J Pharm. 2008;346(1–2):47–56. doi:10.1016/j.ijpharm.2007.06.003
123. Hwang SR, Lim S-J, Park J-S, Kim C-K. Phospholipid-based microemulsion formulation of all-trans-retinoic acid for parenteral administration. Int J Pharm. 2004;276(1–2):175–183. doi:10.1016/j.ijpharm.2004.02.025
124. Pattnaik S, Swain K, Rao JV, Varun T, Mallick S. Temperature influencing permeation pattern of alfuzosin: an investigation using DoE. Medicina. 2015;51(4):253–261. doi:10.1016/j.medici.2015.07.002
125. Swain K, Pattnaik S, Yeasmin N, Mallick S. Preclinical evaluation of drug in adhesive type ondansetron loaded transdermal therapeutic systems. Eur J Drug Metab Pharmacokinet. 2011;36(4):237–241. doi:10.1007/s13318-011-0053-x
126. Swain K, Pattnaik S, Sahu SC, Mallick S. Feasibility assessment of ondansetron hydrochloride transdermal systems: physicochemical characterization and in vitro permeation studies. Lat Am J Pharm. 2009;28(5):706–714.
127. Sivaramakrishnan R, Nakamura C, Mehnert W, Korting H, Kramer K, Schäfer-Korting M. Glucocorticoid entrapment into lipid carriers—characterisation by parelectric spectroscopy and influence on dermal uptake. J Control Release. 2004;97(3):493–502. doi:10.1016/S0168-3659(04)00169-5
128. Jensen LB, Magnussson E, Gunnarsson L, Vermehren C, Nielsen HM, Petersson K. Corticosteroid solubility and lipid polarity control release from solid lipid nanoparticles. Int J Pharm. 2010;390(1):53–60. doi:10.1016/j.ijpharm.2009.10.022
129. Luengo J, Weiss B, Schneider M, et al. Influence of nanoencapsulation on human skin transport of flufenamic acid. Skin Pharmacol Physiol. 2006;19(4):190–197. doi:10.1159/000093114
130. Jain S, Chourasia M, Masuriha R, et al. Solid lipid nanoparticles bearing flurbiprofen for transdermal delivery. Drug Delivery. 2005;12(4):207–215. doi:10.1080/10717540590952591
131. Zhao Y, Moddaresi M, Jones SA, Brown MB. A dynamic topical hydrofluoroalkane foam to induce nanoparticle modification and drug release in situ. Eur J Pharm Biopharm. 2009;72(3):521–528. doi:10.1016/j.ejpb.2009.03.002
132. Elshafeey AH, Kamel AO, Fathallah MM. Utility of nanosized microemulsion for transdermal delivery of tolterodine tartrate: ex-vivo permeation and in-vivo pharmacokinetic studies. Pharm Res. 2009;26(11):2446–2453. doi:10.1007/s11095-009-9956-5
133. Kumar D, Aqil M, Rizwan M, Sultana Y, Ali M. Investigation of a nanoemulsion as vehicle for transdermal delivery of amlodipine. Die Pharmazie. 2009;64(2):80–85.
134. Rahisuddin SP, Garg G, Salim M. Review on nasal drug delivery system with recent advancement. Int J Pharm Pharm Sci. 2011;3(2):1–5.
135. Marianecci C, Rinaldi F, Hanieh PN, Paolino D, Di Marzio L, Carafa M. Nose to brain delivery: new trends in amphiphile-based “soft” nanocarriers. Curr Pharm Des. 2015;21(36):5225–5232. doi:10.2174/1381612821666150923095958
136. Pardridge WM. Blood-brain barrier biology and methodology. J Neurovirol. 1999;5(6):556–569. doi:10.3109/13550289909021285
137. Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379(1):146–157. doi:10.1016/j.ijpharm.2009.06.019
138. Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Delivery. 2014;21(2):148–154. doi:10.3109/10717544.2013.838014
139. Tatham A, Sarodia U, Gatrad F, Awan A. Eye drop instillation technique in patients with glaucoma. Eye. 2013;27(11):1293–1298. doi:10.1038/eye.2013.187
140. Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharm. 2013;2(2):47. doi:10.5497/wjp.v2.i2.47
141. Daull P, Lallemand F, Garrigue J-S. Benefits of cetalkonium chloride cationic oil-in-water nanoemulsions for topical ophthalmic drug delivery. J Pharm Pharmacol. 2014;66(4):531–541. doi:10.1111/jphp.12075
142. du Toit LC, Pillay V, Choonara YE, Govender T, Carmichael T. Ocular drug delivery–a look towards nanobioadhesives. Expert Opin Drug Delivery. 2011;8(1):71–94. doi:10.1517/17425247.2011.542142
143. Demberg T, Robert-Guroff M. Mucosal immunity and protection against HIV/SIV infection: strategies and challenges for vaccine design. Int Rev Immunol. 2009;28(1–2):20–48. doi:10.1080/08830180802684331
144. Sun H, Wei C, Liu B, et al. Induction of systemic and mucosal immunity against methicillin-resistant Staphylococcus aureus infection by a novel nanoemulsion adjuvant vaccine. Int j Nanomed. 2015;10:7275. doi:10.2147/IJN.S91529
145. Bielinska AU, Gerber M, Blanco LP, et al. Induction of Th17 cellular immunity with a novel nanoemulsion adjuvant. Crit Rev Immunol. 2010;30(2):189–199. doi:10.1615/CritRevImmunol.v30.i2.60
146. Makidon PE, Bielinska AU, Nigavekar SS, et al. Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS One. 2008;3(8):e2954. doi:10.1371/journal.pone.0002954
147. Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi:10.1038/nri3251
148. Das SC, Hatta M, Wilker PR, et al. Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine. Vaccine. 2012;30(48):6871–6877. doi:10.1016/j.vaccine.2012.09.007
149. Bielinska AU, Janczak KW, Landers JJ, et al. Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect Immun. 2007;75(8):4020–4029. doi:10.1128/IAI.00070-07
150. Kaufmann KB, Büning H, Galy A, Schambach A, Grez M. Gene therapy on the move. EMBO mol Med. 2013;5(11):1642–1661. doi:10.1002/emmm.201202287
151. Ropert C. Liposomes as a gene delivery system. Braz J Med Biol Res. 1999;32:163–169. doi:10.1590/S0100-879X1999000200004
152. Dai Z, Gjetting T, Mattebjerg MA, Wu C, Andresen TL. Elucidating the interplay between DNA-condensing and free polycations in gene transfection through a mechanistic study of linear and branched PEI. Biomaterials. 2011;32(33):8626–8634. doi:10.1016/j.biomaterials.2011.07.044
153. Silva AL, Marcelino HR, Veríssimo LM, Araujo IB, Agnez-Lima LF, Do Egito ES. Stearylamine-containing cationic nanoemulsion as a promising carrier for gene delivery. J Nanosci Nanotechnol. 2016;16(2):1339–1345. doi:10.1166/jnn.2016.11671
154. Yadav S, Gandham SK, Panicucci R, Amiji MM. Intranasal brain delivery of cationic nanoemulsion-encapsulated TNFα siRNA in prevention of experimental neuroinflammation. Nanomed Nanotechnol Biol Med. 2016;12(4):987–1002. doi:10.1016/j.nano.2015.12.374
155. Bogers WM, Oostermeijer H, Mooij P, et al. Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J Infect Dis. 2015;211(6):947–955. doi:10.1093/infdis/jiu522
156. Mahmood A, Prüfert F, Efiana NA, et al. Cell-penetrating self-nanoemulsifying drug delivery systems (SNEDDS) for oral gene delivery. Expert Opin Drug Delivery. 2016;13(11):1503–1512. doi:10.1080/17425247.2016.1213236
157. Yi SW, Yune TY, Kim TW, et al. A cationic lipid emulsion/DNA complex as a physically stable and serum-resistant gene delivery system. Pharm Res. 2000;17(3):314–320. doi:10.1023/A:1007553106681
158. Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–428. doi:10.1007/s10549-008-0086-2
159. Wang J, Wang GG. No easy way out for EZH2: its pleiotropic, noncanonical effects on gene regulation and cellular function. Int J mol Sci. 2020;21(24):9501. doi:10.3390/ijms21249501
160. Zhang L, Huang Y, Feng Z, et al. Comparison of breast cancer risk factors among molecular subtypes: a case‐only study. Cancer Med. 2019;8(4):1882–1892. doi:10.1002/cam4.2012
161. Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer. 2019;151–164.
162. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
163. Sánchez-López E, Guerra M, Dias-Ferreira J, et al. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials. 2019;9(6):821. doi:10.3390/nano9060821
164. Moura JA, Valduga CJ, Tavares ER, Kretzer IF, Maria DA, Maranhão RC. Novel formulation of a methotrexate derivative with a lipid nanoemulsion. Int j Nanomed. 2011;2285–2295. doi:10.2147/IJN.S18039
165. Winter E, Dal Pizzol C, Locatelli C, et al. In vitro and in vivo effects of free and chalcones-loaded nanoemulsions: insights and challenges in targeted cancer chemotherapies. Int J Environ Res Public Health. 2014;11(10):10016–10035. doi:10.3390/ijerph111010016
166. Periasamy VS, Athinarayanan J, Alshatwi AA. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason Sonochem. 2016;31:449–455. doi:10.1016/j.ultsonch.2016.01.035
167. Alkhatib MH, Bawadud RS, Gashlan HM. Incorporation of docetaxel and thymoquinone in borage nanoemulsion potentiates their antineoplastic activity in breast cancer cells. Sci Rep. 2020;10(1):18124. doi:10.1038/s41598-020-75017-5
168. Nayila I, Sharif S, Lodhi MS, et al. Formulation, characterization and evaluation of anti-breast cancer activity of 2-carene nanoemulsion; in silico, in vitro and in vivo study. Arabian J Chem. 2024;17(9):105937. doi:10.1016/j.arabjc.2024.105937
169. Miranda SEM, de Alcântara Lemos J, Fernandes RS, et al. Enhanced antitumor efficacy of lapachol-loaded nanoemulsion in breast cancer tumor model. Biomed Pharmacother. 2021;133:110936. doi:10.1016/j.biopha.2020.110936
170. Kaur H, Kaur K, Singh A, et al. Frankincense oil-loaded nanoemulsion formulation of paclitaxel and erucin: a synergistic combination for ameliorating drug resistance in breast cancer: in vitro and in vivo study. Front Pharmacol. 2022;13:1020602. doi:10.3389/fphar.2022.1020602
171. Sharma P, Chaturvedi S, Khan M, et al. Nanoemulsion Myricetin preparation increases the anticancer efficacy against triple-negative breast cancer cells. Authorea Preprints. 2023.
172. Miranda SEM, de Alcantara Lemos J, Ottoni FM, et al. Preclinical evaluation of L-fucoside from lapachol-loaded nanoemulsion as a strategy to breast cancer treatment. Biomed Pharmacother. 2024;170:116054. doi:10.1016/j.biopha.2023.116054
173. Kumar R, Uppal S, Mansi K, et al. Ultrasonication induced synthesis of TPGS stabilized clove oil nanoemulsions and their synergistic effect against breast cancer cells and harmful bacteria. J mol Liq. 2022;349:118130. doi:10.1016/j.molliq.2021.118130
174. Cabral ÁS, Leonel ECR, Candido NM, et al. Combined photodynamic therapy with chloroaluminum phthalocyanine and doxorubicin nanoemulsions in breast cancer model. J Photochem Photobiol B Biol. 2021;218:112181. doi:10.1016/j.jphotobiol.2021.112181
175. Uppal S, Sharma P, Kumar R, Kaur K, Bhatia A, Mehta S. Effect of benzyl isothiocyanate encapsulated biocompatible nanoemulsion prepared via ultrasonication on microbial strains and breast cancer cell line MDA MB 231. Colloids Surf A. 2020;596:124732. doi:10.1016/j.colsurfa.2020.124732
176. Pawar VK, Panchal SB, Singh Y, et al. Immunotherapeutic vitamin E nanoemulsion synergies the antiproliferative activity of paclitaxel in breast cancer cells via modulating Th1 and Th2 immune response. J Control Release. 2014;196:295–306. doi:10.1016/j.jconrel.2014.10.010
177. Machado FC, de Matos RPA, Primo FL, Tedesco AC, Rahal P, Calmon MF. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Bioorg Med Chem. 2019;27(9):1882–1890. doi:10.1016/j.bmc.2019.03.044
178. Salehi F, Behboudi H, Kavoosi G, Ardestani SK. Incorporation of Zataria multiflora essential oil into chitosan biopolymer nanoparticles: a nanoemulsion based delivery system to improve the in-vitro efficacy, stability and anticancer activity of ZEO against breast cancer cells. Int J Biol Macromol. 2020;143:382–392. doi:10.1016/j.ijbiomac.2019.12.058
179. Ombredane AS, Araujo VH, Borges CO, et al. Nanoemulsion-based systems as a promising approach for enhancing the antitumoral activity of pequi oil (Caryocar brasilense Cambess.) in breast cancer cells. J Drug Delivery Sci Technol. 2020;58:101819. doi:10.1016/j.jddst.2020.101819
180. Mojeiko G, Apolinário AC, Salata GC, Chorilli M, Lopes LB. Optimization of nanoemulsified systems containing lamellar phases for co-delivery of celecoxib and endoxifen to the skin aiming for breast cancer chemoprevention and treatment. Colloids Surf A. 2022;646:128901. doi:10.1016/j.colsurfa.2022.128901
181. Chaudhuri A, Shrivastava N, Kumar S, Singh AK, Ali J, Baboota S. Designing and development of omega-3 fatty acid based self-nanoemulsifying drug delivery system (SNEDDS) of docetaxel with enhanced biopharmaceutical attributes for management of breast cancer. J Drug Delivery Sci Technol. 2022;68:103117. doi:10.1016/j.jddst.2022.103117
182. Pourmadadi M, Ahmadi M, Abdouss M, et al. The synthesis and characterization of double nanoemulsion for targeted co-delivery of 5-fluorouracil and curcumin using pH-sensitive agarose/chitosan nanocarrier. J Drug Delivery Sci Technol. 2022;70:102849. doi:10.1016/j.jddst.2021.102849
183. Alkhatib MH, AlBishi HM. In vitro evaluation of antitumor activity of doxorubicin-loaded nanoemulsion in MCF-7 human breast cancer cells. J Nanopart Res. 2013;15(3):1–15. doi:10.1007/s11051-013-1489-5
184. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi:10.1200/JCO.2005.04.937
185. Tang S-C. Strategies to decrease taxanes toxicities in the adjuvant treatment of early breast cancer. Cancer Invest. 2009;27(2):206–214. doi:10.1080/07357900802178520
186. O’Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi:10.1093/annonc/mdh097
187. Batist G, Ramakrishnan G, Rao CS, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19(5):1444–1454. doi:10.1200/JCO.2001.19.5.1444
188. Yoo C, Im H-S, Kim K-P, et al. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/leucovorin in patients with metastatic pancreatic adenocarcinoma: a study by the Korean Cancer Study Group. Ther Adv Med Oncol. 2019;11:1758835919871126. doi:10.1177/1758835919871126
189. Cai W, Gao H, Chu C, et al. Engineering phototheranostic nanoscale metal–organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl Mater Interfaces. 2017;9(3):2040–2051. doi:10.1021/acsami.6b11579
190. Rege MD, Ghadi R, Katiyar SS, Kushwah V, Jain S. Exploring an interesting dual functionality of anacardic acid for efficient paclitaxel delivery in breast cancer therapy. Nanomedicine. 2019;14(1):57–75. doi:10.2217/nnm-2018-0138
191. Fan Y, Zhang Q. Development of liposomal formulations: from concept to clinical investigations. Asian J Pharm Sci. 2013;8(2):81–87. doi:10.1016/j.ajps.2013.07.010
192. Anjum S, Naseer F, Ahmad T, et al. Enhancing therapeutic efficacy: sustained delivery of 5-fluorouracil (5-FU) via thiolated chitosan nanoparticles targeting CD44 in triple-negative breast cancer. Sci Rep. 2024;14(1):11431. doi:10.1038/s41598-024-55900-1
193. Prajapati A, Kumar R. A comprehensive review of the use of nanoparticles in cosmeceutical science. J Pharma Insight Res. 2024;2(3):030–037. doi:10.69613/rckvna64
194. Yingngam B. Advances in nanomaterials for drug delivery. Cutting-Edge Applications of Nanomaterials in Biomedical Sciences. 2024;22–85.
195. Kothapalli P, Vasanthan M. Lipid-based nanocarriers for enhanced delivery of plant-derived bioactive molecules: a comprehensive review. Ther Deliv. 2024;15(2):135–155. doi:10.4155/tde-2023-0116
196. Rana R, Kuche K, Jain S, Chourasia MK. Addressing overlooked design considerations for nanoemulsions. Nanomedicine. 2024;19(30):2727–2745. doi:10.1080/17435889.2024.2429947
197. Kumar A, Singh AK, Chaudhary RP, et al. Unraveling the multifaceted role of nanoemulsions as drug delivery system for the management of cancer. J Drug Delivery Sci Technol. 2024;100:106056. doi:10.1016/j.jddst.2024.106056
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.