Back to Journals » International Journal of Nanomedicine » Volume 20
Nanomaterials Mediated Enhancement of CAR-T for HCC: Revolutionizing Immunotherapy Strategies
Authors Liu X, Liu Y, Zhao D, Shan D, Guo C, Jia L
Received 10 March 2025
Accepted for publication 3 June 2025
Published 13 June 2025 Volume 2025:20 Pages 7489—7500
DOI https://doi.org/10.2147/IJN.S527315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sachin Mali
Xudong Liu,1,* Ye Liu,2,* Danyu Zhao,1 Dehong Shan,1 Chenghao Guo,3,4 Lianqun Jia1
1College of Integrated Traditional Chinese and Western Medicine, Liaoning University of Traditional Chinese Medicine, Shenyang, Liaoning Province, 116600, People’s Republic of China; 2Department of Nephrology, Liaoning University of Traditional Chinese Medicine Affiliated Hospital, Shenyang, Liaoning Province, 110000, People’s Republic of China; 3Medical School of Yangzhou University, Yangzhou, Jiangsu Province, 225009, People’s Republic of China; 4Department of Urology, Ansteel Group General Hospital, Anshan, Liaoning Province, 617099, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lianqun Jia, Email [email protected]
Abstract: Hepatocellular carcinoma (HCC) presents significant challenges due to its aggressive nature and resistance to conventional treatments. While CAR-T therapy has shown promise in hematologic cancers, its application in HCC is limited by the tumor microenvironment (TME), insufficient T-cell infiltration, and antigenic heterogeneity. Nanomaterials offer a promising solution by enhancing CAR-T cell delivery, activation, persistence, and overcoming the immunosuppressive TME. This review focuses on the application of nanoparticles in CAR-T therapy, highlighting recent advancements in nanomaterials-based mRNA delivery, photothermal remodeling, and hydrogel platforms. Furthermore, nanomaterials-enhanced imaging tools enable real-time monitoring of CAR-T cell activity, improving therapeutic precision and safety. By addressing current limitations, nanomaterial-mediated CAR-T therapy holds the potential to transform HCC treatment, paving the way for more effective and personalized cancer immunotherapy.
Keywords: nanomaterials, CAR-T, HCC, tumor microenvironment, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and is a leading cause of cancer-related mortality worldwide.1,2 HCC is often diagnosed at an advanced stage, where treatment options are limited, and prognosis is poor.3 Conventional treatments, including surgical resection, chemotherapy, and radiation therapy, are often insufficient due to the aggressive nature of the disease, its high recurrence rates, and the resistance developed to systemic therapies.4,5 In recent years, immunotherapy, particularly Chimeric Antigen Receptor T-cell (CAR-T) therapy, has emerged as a promising approach for treating various cancers, including HCC.6,7
CAR-T therapy involves engineering T cells to express specific receptors that recognize and target tumor antigens. In hematologic cancers, CAR-T therapy has achieved remarkable success, leading to durable remissions.8 However, its application in solid tumors, such as HCC, remains challenging. Several factors contribute to the limited efficacy of CAR-T therapy in HCC, including the complex and immunosuppressive tumor microenvironment (TME), poor infiltration of CAR-T cells into solid tumors, and the heterogeneity of tumor antigen expression.9,10 Additionally, off-target toxicity and CAR-T cell exhaustion further limit the therapeutic potential of this approach in treating solid tumors.11,12 A critical factor in the development of CAR-T therapy for HCC is the identification of suitable tumor-associated antigens (TAAs). Among these, Glypican-3 (GPC3) is the most widely studied due to its high expression in HCC and limited presence in normal adult tissues.13 Other TAAs such as alpha-fetoprotein (AFP),14 particularly in its peptide–HLA complex form, Epithelial Cell Adhesion Molecule (EpCAM),15 and Mucin-1 (MUC1)16 have also been explored in preclinical and early clinical settings. However, the heterogeneous and often low expression of these antigens across patient populations presents an ongoing challenge for universal CAR-T targeting in HCC.
Nanotechnology has recently garnered attention as a potential strategy to overcome these challenges.17 By leveraging nanoparticles and nanoscale materials, researchers aim to enhance the delivery, targeting, and activation of CAR-T cells in the TME.18,19 Nanotechnology can facilitate the precise delivery of CAR-T cells to tumor sites, improve their persistence and activation, and modulate the immunosuppressive nature of the tumor microenvironment. Moreover, nanoparticles can be designed to reduce off-target effects and enhance the safety profile of CAR-T therapy.20,21
In this review, we explore how nanotechnology can be integrated into CAR-T therapy to address the current limitations in the treatment of HCC. We will discuss the key challenges faced in applying CAR-T therapy to solid tumors, the potential solutions offered by nanotechnology, and the preclinical and clinical advancements made in this field. By examining the role of nanotechnology in enhancing CAR-T therapy, this review aims to provide insights into how these innovative approaches can improve treatment outcomes for patients with HCC and potentially transform the therapeutic landscape.
CAR-T Therapy in HCC: Current Challenges
TME
Effective trafficking and infiltration into tumor tissues are essential for CAR-T cells to fulfill their anti-tumor functions. In contrast to hematological malignancies, where CAR-T cells can directly attack malignant cells, solid tumors require CAR-T cells to reach the tumor lesions to engage their targets, a process that is often impeded by the TME.
Physical barriers such as abnormal neovascularization, wide gaps in blood vessel walls, excessive vascular leakage, and dense extracellular matrix (ECM) create significant challenges for CAR-T cells attempting to reach tumor sites.22,23 HCC, which frequently develops from liver fibrosis and cirrhosis, presents additional difficulties due to its pronounced fibrotic nature, further limiting CAR-T cell migration and infiltration. Moreover, HCC often produce chemokines like CXCL1, CXCL2, and CXCL5, which hinder T cell migration and penetration.24–26 Interestingly, a nanoprodrug was developed, self-assembled from polyethylene glycol-poly-4-borono-L-phenylalanine (mPEG-PBPA) conjugated with quercetin (QUE) via boronic ester bonds. Additionally, the immune adjuvant imiquimod (R837) was incorporated. This nanodrug effectively remodels the TME, promoting high infiltration of cytotoxic T lymphocytes, thus enhancing immunotherapy for HCC.27 This suggests that nanotechnology could potentially improve the penetration and infiltration of CAR-T cells in HCC, thereby boosting their anti-tumor efficacy.
Additionally, in HCC, the TME is dominated by an array of immunosuppressive cells, including regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs). These cells release immunosuppressive molecules that hinder CAR-T cell efficacy and accelerate their exhaustion.28 Importantly, nanotechnology provides a promising approach to tackling the challenges posed by the TME by employing nanocarriers to deliver targeted immunomodulatory therapies, significantly reducing adverse effects29 (Figure 1). In addition, immune pathways involving PD-1 on T cells and PD-L1 within the TME of HCC are key factors that contribute to CAR-T cell dysfunction. The application of PD-1/PD-L1 inhibitors can enhance the immunosuppressive environment and prolong CAR-T cell activity.30,31 Notably, recent studies have found that a multifunctional colloidosomal microreactor was constructed by encapsulating catalase within calcium carbonate nanoparticle-assembled colloidosomes, with anti-PD-1 co-loaded inside. This nanodrug significantly enhances the efficacy of CAR-T cells against solid tumors by promoting tumor infiltration and the secretion of effector cytokines.32
CAR-T Cell Persistence and Exhaustion
When CAR-T cells are infused into the human body, they often undergo exhaustion, which diminishes their antitumor capabilities. This exhaustion is primarily driven by continuous antigenic stimulation, leading to a weakened immune response. As a result, the cells lose their ability to proliferate, produce cytokines, and effectively eliminate tumor cells.33 Additionally, they begin to express elevated levels of inhibitory receptors such as PD-1, TIM-3, and LAG-3, further hampering their function.34 Notably, ganglioside-functionalized nanoparticles can bind to CD169-expressing APCs and localize to the contact sites between APCs and CAR T cells during cell conjugation initiation, thereby promoting a strong and durable antigen-specific T cell-mediated immune response.35
Currently, mRNA-based CAR-T cells represent a promising engineering strategy. In all clinical studies, the delivery of CAR mRNA has primarily been achieved through electroporation (EP).36–38 However, this method can lead to irreversible membrane damage and the loss of cellular contents, which in turn decreases cell viability and increases the risk of alterations in gene and protein expression.39 To overcome the challenges associated with electroporation-induced cellular manipulation, researchers have utilized lipid nanoparticles (LNPs) to encapsulate mRNA within fully synthetic lipid shells for the in vivo production of mRNA-based CAR T cells. Compared to traditional EP-CAR T cells, LNP-CAR T cells exhibit significantly prolonged persistence.40
Future research should go beyond current CAR T cell strategies and explore the application of nanotechnology in broader immune cell engineering, such as modifying natural killer (NK) cells and macrophages. Additionally, the potential of nanoparticles in modulating the tumor microenvironment should not be overlooked, as they can deliver drugs in a targeted manner, alter immunosuppressive environments, and thereby enhance immune responses. When combined with gene editing technologies like CRISPR/Cas9, nanoparticles can precisely modify immune or tumor cells in vivo, improving treatment specificity (Figure 2). Future studies should also focus on developing multifunctional nanoparticles capable of delivering not only mRNA but also immunomodulatory molecules, providing more sophisticated and precise therapies. Moreover, the design of personalized nanoparticles tailored to a patient’s genetic profile will drive the advancement of precision medicine.
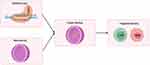 |
Figure 2 Flowchart for CRISPR/Cas9 integration with nanotechnology. |
Targeting and Off-Target Toxicity
One of the key challenges in the success of CAR T cell therapy for HCC is the difficulty in identifying optimal tumor-associated antigens (TAAs). In many solid tumors, only a subset of cells displays the target antigen. Even when TAAs are consistently expressed in HCC, there is still a risk of antigen loss or escape, leading to significant antigen heterogeneity.41 Furthermore, tumor antigens are often expressed to varying degrees in normal tissues, making it more challenging to target them specifically. For instance, in neuroblastoma treatment, high-affinity anti-GD2 CAR not only targets GD2 on neuroblastoma cells but also affects low levels of GD2 in the brain, resulting in fatal encephalitis. These severe side effects indicate that even low-level expression of target antigens in normal tissues can be a major source of toxicity.42 Therefore, selecting the appropriate antigen is crucial in CAR design to ensure therapeutic effectiveness and avoid “on-target off-tumor” toxicity.
Enhancing CAR-T Therapy for HCC Using Nanotechnology
Various nanotechnologies are being applied to overcome the challenges associated with CAR T cell therapy for HCC. These include approaches such as transient CAR expression through RNA delivery, hydrogels, and nanoparticle conjugation, among others. These nano-integrated therapies have shown great potential in inducing robust antitumor immunity, effectively eradicating both primary tumors and metastatic lesions (Table 1).
 |
Table 1 Key Nanotechnology Strategies for Enhancing CAR-T Therapy in HCC |
Nano-Based mRNA CAR Delivery in T Cells
mRNA therapeutics offer transformative potential for modern medicine by providing a gene therapy platform that allows rapid development. The stabilization of therapeutic mRNA can be enhanced through the incorporation of modified nucleosides, synthetic caps, and extended poly-A tails, along with codon optimization to further improve stability.54 LNPs-based mRNA therapies have emerged as promising approaches in CAR T cell immunotherapy. LNP-mRNA CAR can be produced in cell-free systems, with its expression easily regulated, and it offers a safer alternative as it eliminates the risk of genetic integration. The delivery efficiency of LNP systems hinges on the use of ionizable cationic lipids.43 As mRNA is negatively charged, it binds within the LNP through electrostatic interactions, enhancing its stability in vivo and protecting it from lysosomal degradation.55 Once LNPs are internalized by cells, the acidic environment of the endosomes promotes fusion with LNPs, facilitating the release of mRNA into the cytoplasm. Studies have shown that LNP-based mRNA systems achieve high transfection efficiency and lower cytotoxicity across various cell types.56 Interestingly, in a study focused on HCC, mRNA encoding CAR was encapsulated in LNPs. Importantly, researchers developed a specific lipid formulation for the LNPs, enabling them to selectively bind to certain plasma proteins and target macrophages associated with HCC.44 Therefore, future research should focus on identifying lipid formulations for LNPs that can specifically target HCC-associated T cells. Notably, Billingsley identified seven formulations capable of enhancing mRNA delivery compared to lipofectamine. Among them, the top-performing LNP formulation, C14-4, was selected for delivering CAR mRNA to primary human T cells.43 Additionally, by modifying LNPs with antibody conjugation (Ab-LNPs), the LNPs were covalently bound to anti-human CD3 mAb, which enhanced T cell targeting45 and could potentially improve the transfection efficiency of CAR-encoding mRNA in T cells. Since LNPs often accumulate in the liver, the aforementioned strategy could help enhance nano-based mRNA CAR delivery in T cells.
In efforts to stimulate or enhance CAR T therapy, cytokine and vaccine mRNA LNPs have also been developed. For instance, Lu et al engineered GPC3-CAR-T cells to secrete human IL-7 and CCL19. These secreted factors enhance CAR-T cell infiltration and persistence within the body, while also recruiting more mature dendritic cells (DCs). As a result, the IL-7 and CCL19 nanochaperones significantly boosted CAR-T cells’ antitumor activity, leading to the effective eradication of HCC46 (Figure 3).
 |
Figure 3 Nano-based mRNA CAR delivery in T cells. The LNP loaded with CAR mRNA induces T cells to express CAR, which results in tumor targeting and cell death. |
Hydrogel for CAR T Cells Delivery
Over the past few years, scientists have introduced an innovative injectable hydrogel delivery system aimed at enhancing the effectiveness of CAR-T cells in targeting solid tumors.57,58 Grosskopf et al, for instance, engineered a practical hydrogel formulation that facilitates the controlled release of CAR-T cells alongside stimulatory cytokines to improve therapeutic outcomes in solid tumor treatment. This hydrogel’s distinct design effectively limits the passive spread of embedded cytokines while enabling the active movement of the contained cells. This feature supports prolonged retention, improved viability, and activation of CAR-T cells. Following administration, a temporary inflammatory microenvironment is established, which continuously exposes CAR-T cells, promotes the development of tumor-reactive phenotypes, and boosts treatment efficacy.47 Moreover, it is essential for the material used in this platform to exhibit signs of breakdown during the treatment of solid tumors. The previously discussed covalently cross-linked hydrogel systems may remain intact for extended periods post-treatment, potentially heightening the risk of systemic toxicity due to their inability to degrade within a clinically relevant timeframe.48 These observations indicate that this hydrogel-based CAR T cell delivery approach holds promise for treating hard-to-reach solid tumors and metastatic cancers (Figure 4). In addition, a recent study developed a multifunctional hydrogel system composed of γ-polyglutamic acid (γ-PGA), fifth-generation polyamide-amine dendrimers (G5), and polydopamine (PDA) nanoparticles, enabling a synergistic approach that combines photothermal therapy and chemotherapy. This hydrogel integrates PDA nanoparticles with the chemotherapeutic agent doxorubicin (DOX), exhibiting excellent photothermal conversion efficiency and temperature-responsive drug release. In vivo experiments demonstrated significant tumor growth inhibition with minimal systemic toxicity, alongside favorable biocompatibility, photothermal stability, and biodegradability. Unlike conventional hydrogels, the γ-PGA-based platform offers both therapeutic versatility and precise stimulus-responsiveness, representing a promising strategy to enhance the local efficacy of CAR-T cell therapy through combined photothermal and chemotherapeutic mechanisms.59
 |
Figure 4 Hydrogel for CAR T cells delivery. |
Photothermal-Remodeling CAR-T Cell Therapy
Regional hyperthermia has been demonstrated to partially degrade the tumor extracellular matrix, promote blood vessel dilation to enhance circulation, and directly increase CAR T-cell penetration into solid tumors. The heat-induced destruction of cancer cells triggers an inflammatory response that boosts chemokine secretion, further supporting CAR-T cell activation and recruitment. In their study, Chen et al showed that nanoparticles made from poly (lactic-co-glycolic) acid (PLGA) and loaded with indocyanine green (ICG), a near-infrared photothermal (NIR) dye, enhanced the trafficking and accumulation of CAR. CSPG4+ T cells at tumor sites.49 Additionally, Miller et al engineered heat-responsive constructs to modify T cells for the regulation of various immunostimulatory genes, including CARs, BiTEs, and a cytokine superagonist designed to enhance T cell proliferation.50 Gold nanoparticles, which naturally accumulate in tumors, were administered intravenously to mice with hind limb tumors that had received these modified CAR-T cells. The combined treatment of heat therapy and engineered CAR-T cells resulted in significant tumor regression compared to mice treated with either the modified CAR-T cells or heat therapy alone.50 It is worth noting that photothermal ablation enhances T cell infiltration within HCC tumor tissues,60 with gold nanoparticles playing a critical role in the photothermal treatment of HCC61 (Figure 5). Future research should focus on investigating whether gold nanoparticle-based photothermal therapy can further promote the infiltration of CAR-T cells into HCC tumor tissues.
TME Targeted CAR-T Therapy
The TME in HCC is characterized by a complex network of cellular and molecular components that hinder CAR-T cell infiltration, persistence, and functionality. Key immunosuppressive factors include Tregs, MDSCs, and various cytokines like TGF-β and IL-10, which collectively contribute to the suppression of T cell activity and promote tumor progression.
To overcome these barriers, nanotechnology-based approaches have been developed to enhance the efficacy of CAR-T cell therapy by targeting and modulating the TME.62 One strategy involves engineering CAR-T cells to carry nanoparticles that can specifically deliver therapeutic agents to the TME. For instance, nanoparticle delivery systems can encapsulate small molecule inhibitors that block immunosuppressive pathways within the TME. A prominent example is the use of CAR-T cells loaded with cross-linked multilamellar liposomal vesicles (cMLVs) carrying A2aR-specific small molecule antagonists, such as SCH-58261, which can mitigate the effects of adenosine—an immunosuppressive metabolite—and improve CAR-T cell function.51 Recent advancements have also included the synthesis of tumor-specific nanozyme systems, such as HA@Cu2−xS-PEG (PHCN) nanozymes, that catalyze the production of reactive oxygen species (ROS) within the TME. The increase in ROS levels induces a synergistic photothermal and nanocatalytic effect that not only promotes direct tumor cell killing but also enhances the release of tumor antigens, thereby facilitating the activation and effector function of CAR-T cells.52
Furthermore, the inhibitory microenvironment and the inherent heterogeneity of individuals contribute to the downregulation of tumor-associated antigens in HCC cells.63,64 Notably, nanobodies (Nbs) have the ability to specifically recognize tumor-associated antigens on the surface of cancer cells, allowing for the direct delivery of therapeutic molecules or drugs to the tumor site. Due to their small size, nanobodies can effectively penetrate the tumor microenvironment, enhancing both targeting specificity and therapeutic outcomes. In a recent study, Lin et al injected FGFR4-ferritin (FGFR4-HPF) nanoparticles into alpacas and created a phage display library of nanobodies derived from alpaca antibodies, successfully obtaining nanobodies targeting FGFR4. Notably, CAR-T cells engineered with high-affinity and high-specificity nanobodies targeting FGFR4 demonstrated significantly improved antitumor effects both in vitro and in vivo.53
These nanotechnology-enhanced strategies represent a promising frontier in overcoming the limitations imposed by the TME in HCC and improving the therapeutic outcomes of CAR-T cell therapy. By leveraging targeted delivery, responsive release mechanisms, and advanced imaging techniques, researchers are paving the way for more effective and precise treatment modalities for HCC patients.
Advancing CAR-T Cell Therapy Monitoring in HCC Through Nanotechnology
Nanotechnology-based imaging agents have transformed the landscape of monitoring and diagnosing CAR-T therapies, addressing many of the shortcomings of conventional approaches.18,65 Traditional techniques such as enzyme-linked immunosorbent assays (ELISA) are constrained by their long processing times, large sample requirements, limited sensitivity, and inability to provide real-time detection.66 In comparison, bioimmunosensors, a multidisciplinary innovation, leverage methods such as double-antibody sandwich or competition detection, supported by optical and electrical systems, to achieve superior detection sensitivity and lower limits of detection.67 Nanomaterials play a pivotal role in enhancing the performance of these sensors, acting as carriers or catalysts to amplify detection signals, thus improving overall accuracy and efficiency.68,69 For example, interleukin-6 (IL-6), a key biomarker in cytokine release syndrome (CRS), has been detected using an innovative biosensor that combines a gold nanoparticle (AuNP)-based surface-enhanced Raman spectroscopy (SERS) substrate with a DNA aptamer. This approach demonstrated exceptional sensitivity and selectivity for IL-6 detection.70
Beyond biosensors, nanotechnology-based imaging agents provide critical insights into CAR-T cell distribution and activity, allowing for early detection and intervention to mitigate off-tumor effects.71 One approach involves ex vivo loading of nanoparticles onto CAR-T cells. For instance, in preclinical mouse models, the biodistribution of CAR-T cells was effectively tracked by incorporating radiolabeled or contrast-enhanced nanoparticles into the cells before infusion.72 Furthermore, light-inducible systems, such as the light-inducible nuclear translocation and dimerization platform, enable precise control over CAR-T cell activation. By leveraging light to regulate nuclear translocation and dimerization, this system facilitates the fine-tuning of gene expression in CAR-T cells with high specificity.73 These advancements have paved the way for more precise tracking and improved therapeutic efficacy of CAR-T cells while reducing potential risks.74
Conclusion and Future Perspectives
Nanotechnology has emerged as a transformative tool for overcoming the challenges associated with CAR-T cell therapy in HCC. By leveraging nanoparticles and nanoscale materials, researchers have achieved significant advancements in improving CAR-T cell delivery, activation, and persistence within the immunosuppressive TME. These innovations have paved the way for enhanced therapeutic outcomes, offering hope for a more effective treatment of solid tumors like HCC.
Despite these advancements, several hurdles remain. The heterogeneity of TAAs, the physical barriers of the TME, and the risk of off-target toxicity continue to limit the broad application of CAR-T therapy in HCC. Future strategies should focus on developing personalized nanotechnologies capable of addressing these limitations. Tailoring nanoparticles to individual genetic and molecular profiles can enhance the precision and safety of CAR-T cell therapies, particularly in targeting antigens that are selectively expressed in tumor cells.
Given the unique histological features of HCC, such as a highly fibrotic TME, the development of nanocarriers that specifically target hepatic stellate cells (HSCs) may offer a promising solution. These cells are central to ECM production and stromal stiffening, which impair T cell infiltration. Nanoparticles delivering matrix-modifying enzymes or TGF-β inhibitors to activated HSCs could help reverse fibrosis, remodel the TME, and facilitate CAR-T cell penetration and expansion within tumor lesions.
To address the high degree of antigen heterogeneity, multi-targeted CAR constructs may be integrated with nanoparticle-based co-delivery systems that either present multiple tumor-associated antigens (eg, GPC3, AFP, EpCAM) or deliver mRNA sequences for poly-specific CAR expression. This would increase recognition breadth while reducing immune escape. Combining these strategies with stimuli-responsive nanoparticles—capable of releasing immune modulators in response to pH, ROS, or enzymatic signals—may further refine CAR-T activation kinetics within the tumor.
Nanomedicine-based CAR-T cell therapies can also be categorized into two principal delivery strategies: ex vivo and in vivo. Ex vivo approaches involve nanoparticle-mediated T cell engineering outside the body before reinfusion, offering high transfection efficiency and control, but requiring labor-intensive processes and specialized manufacturing infrastructure. In contrast, in vivo approaches use systemically administered nanoparticles to directly generate or modulate CAR-T cells within the patient, potentially reducing production cost and improving scalability. However, challenges such as targeting specificity, immune clearance, and safety concerns remain. Future research should aim to optimize and integrate both strategies based on HCC-specific TME characteristics and clinical feasibility.
Moreover, combining nanotechnology with cutting-edge genetic tools, such as CRISPR/Cas9, holds promise for the precise modification of immune cells or tumor cells, thereby increasing the specificity of therapeutic interventions. These approaches could further broaden the scope of immune-based therapies by integrating nanoparticles into the engineering of other immune cells, such as NK cells and macrophages.
Another critical research direction is the optimization of real-time monitoring systems for CAR-T therapy using nanotechnology-enhanced imaging modalities. Advanced imaging tools, including nanoparticle-based MRI and PAT, provide critical insights into CAR-T cell distribution, activity, and therapeutic efficacy. These tools enable clinicians to track CAR-T cells more accurately, intervene earlier to mitigate off-target effects, and optimize treatment protocols in a timely manner.
Notably, despite the increasing preclinical interest in nano-assisted CAR-T therapy for HCC, no clinical trials to date have specifically investigated such approaches. Current trials remain focused on conventional CAR-T modalities targeting GPC3 or AFP, without integrating nanomaterial-based delivery or modulation systems. This highlights a major unmet need in translational development. Future clinical efforts should prioritize early-phase trials to evaluate the safety and feasibility of nanotechnology-enhanced CAR-T systems, while addressing scalability, targeting efficiency, and regulatory challenges.
In conclusion, nanotechnology offers an unprecedented opportunity to enhance CAR-T therapy for HCC by addressing its current challenges and expanding its therapeutic potential. Through interdisciplinary collaboration between oncologists, immunologists, and nanotechnology experts, these innovations can be translated from the laboratory to clinical practice, ultimately improving patient outcomes and redefining the future of cancer immunotherapy.
Disclosure
The authors declare that they have no competing interests.
References
1. Huang M, Huang X, Huang N. Exosomal circGSE1 promotes immune escape of hepatocellular carcinoma by inducing the expansion of regulatory T cells. Cancer Sci. 2022;113:1968–1983. doi:10.1111/cas.15365
2. Zheng H, Peng X, Yang S, et al. Targeting tumor-associated macrophages in hepatocellular carcinoma: biology, strategy, and immunotherapy. Cell Death Discov. 2023;9:65. doi:10.1038/s41420-023-01356-7
3. Xia X, Huang M, Hu Y, et al. Rational design of a tandem activatable fluorescent probe for differential diagnosis and therapeutic assessment of hepatocellular carcinoma. Anal Chem. 2024. doi:10.1021/acs.analchem.4c05202
4. Chan YT, Zhang C, Wu J, et al. Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol Cancer. 2024;23:189. doi:10.1186/s12943-024-02101-z
5. Shen S, Qiu X, Yang C, et al. Prognostic importance of the Scottish inflammatory prognostic score in patients with hepatocellular carcinoma after hepatectomy: a retrospective cohort study. BMC Cancer. 2024;24:1393. doi:10.1186/s12885-024-13174-w
6. Ren T, Huang Y. Recent advancements in improving the efficacy and safety of chimeric antigen receptor (CAR)-T cell therapy for hepatocellular carcinoma. Naunyn Schmiedebergs Arch Pharmacol. 2024;2024:1–4.
7. Zhou L, Li Y, Zheng D, et al. Bispecific CAR-T cells targeting FAP and GPC3 have the potential to treat hepatocellular carcinoma. Mol Ther Oncol. 2024;32:200817. doi:10.1016/j.omton.2024.200817
8. Su M, Zhang Z, Jiang P, Wang X, Tong X, Wu G. CAR-T-cell therapy based on immune checkpoint modulation in the treatment of hematologic malignancies. Cell Transplant. 2024;33:9636897241293964. doi:10.1177/09636897241293964
9. Zhu X, Xue J, Jiang H, Xue D. CAR-NK cells for gastrointestinal cancer immunotherapy: from bench to bedside. Mol Cancer. 2024;23:237. doi:10.1186/s12943-024-02151-3
10. Wang Y, Barrett A, Hu Q. Nanotechnology-assisted CAR-T-cell therapy for tumor treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024;16:e2005. doi:10.1002/wnan.2005
11. Chen X, Gao Y, Zhang Y. Allogeneic CAR-T cells for cancer immunotherapy. Immunotherapy. 2024;16:1079–1090. doi:10.1080/1750743X.2024.2408048
12. Sirini C, De Rossi L, Moresco MA, Casucci M. CAR T cells in solid tumors and metastasis: paving the way forward. Cancer Metastasis Rev. 2024;43:1279–1296. doi:10.1007/s10555-024-10213-7
13. Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20:6418–6428. doi:10.1158/1078-0432.CCR-14-1170
14. Liu H, Xu Y, Xiang J, et al. Targeting Alpha-Fetoprotein (AFP)-MHC complex with CAR T-cell therapy for liver cancer. Clin Cancer Res. 2017;23:478–488. doi:10.1158/1078-0432.CCR-16-1203
15. Chen Y, E CY, Gong ZW, et al. Chimeric antigen receptor-engineered T-cell therapy for liver cancer. Hepatobiliary Pancreat Dis Int. 2018;17:301–309. doi:10.1016/j.hbpd.2018.05.005
16. Supimon K, Sangsuwannukul T, Sujjitjoon J, et al. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci Rep. 2021;11:6276. doi:10.1038/s41598-021-85747-9
17. Dhas N, Kudarha R, Kulkarni S, et al. Nanoengineered platform-based microenvironment-triggered immunotherapy in cancer treatment. Front Biosci. 2024;29:349. doi:10.31083/j.fbl2910349
18. Ku KS, Tang J, Chen Y, Shi Y. Current advancements in anti-cancer chimeric antigen receptor t cell immunotherapy and how nanotechnology may change the game. Int J Mol Sci. 2024;25:5361. doi:10.3390/ijms25105361
19. Bockamp E, Rosigkeit S, Siegl D, Schuppan D. Nano-enhanced cancer immunotherapy: immunology encounters nanotechnology. Cells. 2020;9:2102. doi:10.3390/cells9092102
20. Nawaz W, Xu S, Li Y, Huang B, Wu X, Wu Z. Nanotechnology and immunoengineering: how nanotechnology can boost CAR-T therapy. Acta Biomater. 2020;109:21–36. doi:10.1016/j.actbio.2020.04.015
21. Chada NC, Wilson JT. Jump-starting chimeric antigen receptor-T cells to go the extra mile with nanotechnology. Curr Opin Biotechnol. 2024;89:103179. doi:10.1016/j.copbio.2024.103179
22. Zhang GZ, Li TF, Han SY. Mesothelin-targeted CAR-T cells for adoptive cell therapy of solid tumors. Arch Med Sci. 2021;17:1213–1220. doi:10.5114/aoms.2019.84888
23. Katayama Y, Uchino J, Chihara Y, et al. Tumor neovascularization and developments in therapeutics. Cancers. 2019;11:316. doi:10.3390/cancers11030316
24. Xie P, Yu M, Zhang B, et al. CRKL dictates anti-PD-1 resistance by mediating tumor-associated neutrophil infiltration in hepatocellular carcinoma. J Hepatol. 2024;81:93–107. doi:10.1016/j.jhep.2024.02.009
25. Akasu M, Shimada S, Kabashima A, et al. Intrinsic activation of beta-catenin signaling by CRISPR/Cas9-mediated exon skipping contributes to immune evasion in hepatocellular carcinoma. Sci Rep. 2021;11:16732. doi:10.1038/s41598-021-96167-0
26. Cao M, Huang W, Chen Y, et al. Chronic restraint stress promotes the mobilization and recruitment of myeloid-derived suppressor cells through beta-adrenergic-activated CXCL5-CXCR2-Erk signaling cascades. Int, J, Cancer. 2021;149:460–472. doi:10.1002/ijc.33552
27. Hong K, Cao J, Jiang W, et al. A nanodrug provokes antitumor immune responses via synchronous multicellular regulation for enhanced cancer immunotherapy. J Colloid Interface Sci. 2025;678:750–762. doi:10.1016/j.jcis.2024.09.016
28. Fu Y, Guo X, Sun L, et al. Exploring the role of the immune microenvironment in hepatocellular carcinoma: implications for immunotherapy and drug resistance. Elife. 2024;13. doi:10.7554/eLife.95009
29. Zhang F, Stephan SB, Ene CI, Smith TT, Holland EC, Stephan MT. Nanoparticles that reshape the tumor milieu create a therapeutic window for effective T-cell therapy in solid malignancies. Cancer Res. 2018;78:3718–3730. doi:10.1158/0008-5472.CAN-18-0306
30. Guo X, Jiang H, Shi B, et al. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol. 2018;9:1118. doi:10.3389/fphar.2018.01118
31. Li D, Qin J, Zhou T, et al. Bispecific GPC3/PD‑1 CAR‑T cells for the treatment of HCC. Int J Oncol. 2023;62. doi:10.3892/ijo.2023.5501
32. Dong Z, Liu Y, Wang C, et al. Tumor microenvironment modulating CaCO(3) -based colloidosomal microreactors can generally reinforce cancer immunotherapy. Adv Mater. 2024;36:e2308254. doi:10.1002/adma.202308254
33. Bhagwat AS, Torres L, Shestova O, et al. Cytokine-mediated CAR T therapy resistance in AML. Nat Med. 2024;30:3697–3708. doi:10.1038/s41591-024-03271-5
34. Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi:10.1038/ni.2035
35. Zang H, Siddiqui M, Gummuluru S, Wong WW, Reinhard BM. Ganglioside-functionalized nanoparticles for chimeric antigen receptor T-Cell activation at the immunological synapse. ACS Nano. 2022;16:18408–18420. doi:10.1021/acsnano.2c06516
36. Beatty GL, O’Hara MH, Lacey SF, et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a Phase 1 trial. Gastroenterology. 2018;155:29–32. doi:10.1053/j.gastro.2018.03.029
37. Tchou J, Zhao Y, Levine BL, et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol Res. 2017;5:1152–1161. doi:10.1158/2326-6066.CIR-17-0189
38. Svoboda J, Rheingold SR, Gill SI, et al. Nonviral RNA chimeric antigen receptor-modified T cells in patients with Hodgkin lymphoma. Blood. 2018;132:1022–1026. doi:10.1182/blood-2018-03-837609
39. DiTommaso T, Cole JM, Cassereau L, et al. Cell engineering with microfluidic squeezing preserves functionality of primary immune cells in vivo. Proc Natl Acad Sci U S A. 2018;115:E10907–E10914. doi:10.1073/pnas.1809671115
40. Kitte R, Rabel M, Geczy R, Park S, Fricke S, Koehl U. U.S. Tretbar, Lipid nanoparticles outperform electroporation in mRNA-based CAR T cell engineering. Mol Ther Methods Clin Dev. 2023;31:101139. doi:10.1016/j.omtm.2023.101139
41. Aggeletopoulou I, Kalafateli M, Triantos C. Chimeric antigen receptor T cell therapy for hepatocellular carcinoma: where do we stand? Int J Mol Sci. 2024;25:2631. doi:10.3390/ijms25052631
42. Richman SA, Nunez-Cruz S, Moghimi B, et al. High-Affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol Res. 2018;6:36–46. doi:10.1158/2326-6066.CIR-17-0211
43. Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–1589. doi:10.1021/acs.nanolett.9b04246
44. Yang Z, Liu Y, Zhao K, et al. Dual mRNA co-delivery for in situ generation of phagocytosis-enhanced CAR macrophages augments hepatocellular carcinoma immunotherapy. J Control Release. 2023;360:718–733. doi:10.1016/j.jconrel.2023.07.021
45. Kheirolomoom A, Kare AJ, Ingham ES, et al. In situ T-cell transfection by anti-CD3-conjugated lipid nanoparticles leads to T-cell activation, migration, and phenotypic shift. Biomaterials. 2022;281:121339. doi:10.1016/j.biomaterials.2021.121339
46. Pang N, Shi J, Qin L, et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14:118. doi:10.1186/s13045-021-01128-9
47. Grosskopf AK, Labanieh L, Klysz DD, et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci Adv. 2022;8:eabn8264. doi:10.1126/sciadv.abn8264
48. Hu Q, Li H, Archibong E, et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat Biomed Eng. 2021;5:1038–1047. doi:10.1038/s41551-021-00712-1
49. Chen Q, Hu Q, Dukhovlinova E, et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019;31:e1900192. doi:10.1002/adma.201900192
50. Miller IC, Zamat A, Sun LK, et al. Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control. Nat Biomed Eng. 2021;5:1348–1359. doi:10.1038/s41551-021-00781-2
51. Siriwon N, Kim YJ, Siegler E, et al. CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunol Res. 2018;6:812–824. doi:10.1158/2326-6066.CIR-17-0502
52. Zhu L, Liu J, Zhou G, et al. Remodeling of tumor microenvironment by tumor-targeting nanozymes enhances immune activation of CAR T cells for combination therapy. Small. 2021;17:e2102624. doi:10.1002/smll.202102624
53. Lin K, Xia B, Wang X, et al. Development of nanobodies targeting hepatocellular carcinoma and application of nanobody-based CAR-T technology. J Transl Med. 2024;22:349. doi:10.1186/s12967-024-05159-x
54. Liu C, Shi Q, Huang X, Koo S, Kong N, Tao W. mRNA-based cancer therapeutics. Nat Rev Cancer. 2023;23:526–543. doi:10.1038/s41568-023-00586-2
55. Billingsley MM, Hamilton AG, Mai D, et al. Orthogonal design of experiments for optimization of lipid nanoparticles for mRNA engineering of CAR T cells. Nano Lett. 2022;22:533–542. doi:10.1021/acs.nanolett.1c02503
56. Weissman D. mRNA transcript therapy. Expert Rev Vaccines. 2015;14:265–281. doi:10.1586/14760584.2015.973859
57. Burgos JM, Vega E, Garcia ML, Pujol M, Sanchez-Lopez E, Souto EB. Biodegradable nanoplatforms for antigen delivery: part II - nanoparticles, hydrogels, and microneedles for cancer immunotherapy. Expert Opin Drug Deliv. 2024;21:1385–1394. doi:10.1080/17425247.2024.2400291
58. Lin Y, Chen Y, Luo Z, Wu YL. Recent advances in biomaterial designs for assisting CAR-T cell therapy towards potential solid tumor treatment. Nanoscale. 2024;16:3226–3242. doi:10.1039/D3NR05768B
59. Jia X, Wang S. A multifunctional gamma-polyglutamic acid hydrogel for combined tumor photothermal and chemotherapy. Gels. 2025;11:217. doi:10.3390/gels11030217
60. Munoz NM, Dupuis C, Williams M, et al. Molecularly targeted photothermal ablation improves tumor specificity and immune modulation in a rat model of hepatocellular carcinoma. Commun Biol. 2020;3:783. doi:10.1038/s42003-020-01522-y
61. Taghizadeh S, Alimardani V, Roudbali PL, Ghasemi Y, Kaviani E. Gold nanoparticles application in liver cancer. Photodiagnosis Photodyn Ther. 2019;25:389–400. doi:10.1016/j.pdpdt.2019.01.027
62. Alexandru I, Davidescu L, Motofelea AC, et al. Emerging nanomedicine approaches in targeted lung cancer treatment. Int J Mol Sci. 2024;25:11235. doi:10.3390/ijms252011235
63. Slingluff CL. Targeting unique tumor antigens and modulating the cytokine environment may improve immunotherapy for tumors with immune escape mechanisms. Cancer Immunol Immunother. 1999;48(7):371–373. doi:10.1007/s002620050588
64. Ferrone S, Whiteside TL. Tumor microenvironment and immune escape. Surg Oncol Clin N Am. 2007;16:755–774,viii. doi:10.1016/j.soc.2007.08.004
65. Yan H, Zhai B, Yang F, et al. Nanotechnology-based diagnostic and therapeutic strategies for neuroblastoma. Front Pharmacol. 2022;13:908713. doi:10.3389/fphar.2022.908713
66. Gabaldon JA, Maquieira A, Puchades R. Current trends in immunoassay-based kits for pesticide analysis. Crit Rev Food Sci Nutr. 1999;39:519–538. doi:10.1080/10408699991279277
67. Zheng Y, Li Y, Li M, et al. COVID-19 cooling: nanostrategies targeting cytokine storm for controlling severe and critical symptoms. Med Res Rev. 2024;44:738–811. doi:10.1002/med.21997
68. Lei J, Ju H. Signal amplification using functional nanomaterials for biosensing. Chem Soc Rev. 2012;41:2122–2134. doi:10.1039/c1cs15274b
69. Liu X, Wu W, Cui D, Chen X, Li W. Functional micro-/nanomaterials for multiplexed biodetection. Adv Mater. 2021;33:e2004734. doi:10.1002/adma.202004734
70. Kang X, Mita N, Zhou L, et al. Nanotechnology in advancing chimeric antigen receptor T cell therapy for cancer treatment. Pharmaceutics. 2024;16:1228. doi:10.3390/pharmaceutics16091228
71. Balakrishnan PB, Sweeney EE. Nanoparticles for enhanced adoptive T cell therapies and future perspectives for CNS tumors. Front Immunol. 2021;12:600659. doi:10.3389/fimmu.2021.600659
72. Jakobczyk H, Sciortino F, Chevance S, Gauffre F, Troadec MB. Promises and limitations of nanoparticles in the era of cell therapy: example with CD19-targeting chimeric antigen receptor (CAR)-modified T cells. Int J Pharm. 2017;532:813–824. doi:10.1016/j.ijpharm.2017.07.075
73. Huang Z, Wu Y, Allen ME, et al. Engineering light-controllable CAR T cells for cancer immunotherapy. Sci Adv. 2020;6:eaay9209. doi:10.1126/sciadv.aay9209
74. Bhatnagar P, Li Z, Choi Y, et al. Imaging of genetically engineered T cells by PET using gold nanoparticles complexed to Copper-64. Integr Biol. 2013;5:231–238. doi:10.1039/c2ib20093g
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.