Back to Journals » International Journal of Nanomedicine » Volume 20
Nanomedicine-Driven Approaches for Kartogenin Delivery: Advancing Chondrogenic Differentiation and Cartilage Regeneration in Tissue Engineering
Authors Bhuyan S, Swain S, Misra RDK, Rautray TR
Received 28 February 2025
Accepted for publication 24 May 2025
Published 13 June 2025 Volume 2025:20 Pages 7443—7468
DOI https://doi.org/10.2147/IJN.S525580
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Samapika Bhuyan,1,* Subhasmita Swain,1,* RDK Misra,2 Tapash R Rautray1
1Biomaterials and Tissue Regeneration Laboratory, Centre of Excellence, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India; 2Metallurgical, Materials and Biomedical Engineering Department, The University of Texas at El Paso, El Paso, Texas, 79968, USA
*These authors contributed equally to this work
Correspondence: Tapash R Rautray, Email [email protected] RDK Misra, Email [email protected]
Abstract: Articular cartilage degradation and osteocartilage defects are the most prevalent concerns that vary from localized to more systemic forms of cartilage disease. However, regulating chondrogenic differentiation within the joints remains a significant challenge. Kartogenin, a small heterocyclic compound, has recently garnered considerable attention as a potential therapeutic agent, owing to both chondrogenic and chondroprotective properties for intra-articular therapy. Initially, it was created for osteoarthritis; it has also been used to address various diseased conditions, such as the regeneration of disc and bone-tendon junctions. On top of that, it preserves the equilibrium between cartilage catabolism and anabolism, while also mitigating inflammation and alleviating pain by preventing damage induced by cytokines. To modulate tissue function and cellular behaviour, it is crucial to have sustained release of ketogenic through an appropriate delivery system. A multitude of biomaterial-based carriers have been developed for the prolonged release of kartogenin. Moreover, many biological mechanisms of action of kartogenin have been identified. The most critical molecular mechanism among them is the dissociation of filamin A from core-binding factor (CBF)-β induced by kartogenin. Filamin A subsequently translocates to the nucleus, where it engages with RUNX-1 to transcribe genes implicated in the chondrogenesis of mesenchymal stem cells. This review focuses on the development of biomaterials functionalized with kartogenin, including their structure, design, physicochemical properties, biological roles, molecular mechanisms of action, and applications in tissue engineering and regenerative medicine. In conclusion, we discussed the future possibilities and challenges posed by recent advancements in kartogenin research and their potential applications in tissue regeneration.
Keywords: Kartogenin, cartilage regeneration, osteoarthritis, TGF-β, chondrogenesis
Introduction
Regenerative medicine is the branch of medicine that endeavours to restore normal functions by replacing or regenerating human cells, tissues, and organs.1 Osteoarthritis (OA) is a degenerative disease characterized by degeneration of articular cartilage, ligamentous injury, and loss of extracellular matrix (ECM), leading to pain, inflammation, and impaired joint functions.2 It has been stated as the most prevalent form of arthritis among adults, impacting approximately 32.5 million Americans, causing disability among the older population.3,4 It is challenging to successfully repeal the development of OA due to the avascular nature of cartilage and the low density of the dispersed chondrocytes, which are the limited resident cells in cartilage.5 The therapeutic strategies for OA are focused on alleviating pain and functional impairment, including non-pharmacological and pharmacological interventions.6 Non-pharmacological interventions might not be sufficient for patients developing symptomatic OA. The pharmacological interventions for OA include medications, such as oral non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen. Yet, serious concerns are raised by the fact that NSAIDs might cause adverse effects in the gastrointestinal, renal, and cardiovascular systems7 and are therefore restricted. Recently, there has been increasing interest in focused small-molecule therapies for the treatment of OA over palliative medications, using intra-articular (IA) injections. However, these methods always come with several problems, such as donor site morbidity, immunogenic rejection, limited availability, and disease transmission.8
In regenerative medicine, the most effective approach for addressing OA includes promoting chondrogenic differentiation of mesenchymal stem cells (MSCs) and the generation of new cartilage tissue. The ultimate objective of this strategy is to overcome the paucity of chondrocyte availability to repair injured cartilage.1 To solve this problem, in 2012, Kristen Johnson et al, after screening 22,000 drug-like compounds, discovered that Kartogenin (KGN) is a small non-protein molecule that promotes the regeneration of cartilage by stimulating the specific differentiation of mesenchymal stem cells (MSCs) into chondrocytes. It is a chondroprotective and chondrogenic agent.9 As compared to other growth factors, it has greater potential for cartilage regeneration.10 Growth factors, such as cytokines, which include bone morphogenetic protein (BMP), and protein growth factors like transforming growth factors-β (TGF-β), Insulin-like growth factor 1 (IGF-1), are crucial for inducing stem cells to differentiate into chondrogenic cells.11 Among them, TGF-β has been suggested as a possible therapeutic agent to improve MSC-based articular cartilage regeneration.
Furthermore, to achieve rapid cartilage regeneration, a substantial intra-articular (IA) dosage of TGF-β is required, which may result in osteophyte production, synovial fibrosis, joint swelling, synovitis, and deterioration of articular cartilage. However, the utilization of TGF-β and other growth factors might be restricted by their instability, short half-life, and low immunogenicity in vivo.12 The bone morphogenetic proteins (BMPs) were identified to promote chondrogenic differentiation of MSCs both in vitro and in vivo. However, BMPs might induce bone development after ectopic implantation, indicating that the management and modulation of BMPs are essential for optimum cartilage tissue engineering approaches. By regulating proteoglycan formation and breakdown in chondrocytes, IGF-1 may protect cartilage homeostasis and increase survival and proliferation. However, OA inflammation lowers IGF-1 levels and chondrocyte responsiveness to IGF-1, preventing functional and structural integrity.13–15 Meanwhile, KGN is a small heterocyclic stable molecule that can be stored and transported at room temperature. These advantages of KGN to peptide growth factors render it a promising candidate for cartilage regeneration. Therefore, it is attracting significant interest as an emerging chondrogenic candidate for IA treatment.
Even though KGN was initially established to cure OA, it has been utilized to treat other diseased conditions as well as promote disc and cartilage repair through bone-tendon regeneration.16 The cartilage nodules that develop in the presence of KGN comprise collagen II (COLII), aggrecan (ACAN), and proteoglycans, which are structurally fundamental elements of hyaline cartilage. While ACAN provides resistance to stress or compression in the cartilage region, COL II controls the metabolic balance.3 Furthermore, under pathophysiological conditions, KGN was able to drastically lower the release of pro-inflammatory nitric oxide (NO) and cytokine-induced glycosaminoglycans (GAGs) breakdown in cartilage tissues. The study revealed that IA injection of KGN can support cartilage regeneration as evidenced by a decrease in fibrillations in the superficial and mid-zone of articular cartilage using the collagenase VII–induced chronic joint injury model and the acute ligament ligation model.17 On the other hand, Patients with OA have defective chondrocytes that produce an excess of degrading enzymes such as matrix metalloproteinase 13 (MMP13) and a disintegrin metalloproteinase with thrombospondin motifs 5 (ADAMTS5), which exacerbates articular cartilage destruction.18 In this regard, KGN treatment has shown a dose-dependent rise in the production of COL II and ACAN in chondrocytes exposed to IL-1β.19 This impact is observed only in inflammatory conditions, whereas KGN seems to have little to no impact on ACAN and COL II levels in healthy chondrocytes. Furthermore, KGN functions to inhibit MMP13 and ADAMTS5, hence protecting the cartilage extracellular matrix and ameliorating inflammation.18
Johnson et al investigated the mechanism through which KGN functions by interacting with the actin-binding protein filamin A and disrupting its equilibrium with the transcription factor CBFβ. CBFβ enters the nucleus, where it interacts with RUNX-1 and forms a CBFβ-RUNX-1 complex that promotes the transcription of proteins associated with chondrogenesis and improves the production of ECM in cartilage.9 Additionally, a recent study revealed that KGN improved chondrogenesis by raising the levels of chondrogenic marker genes, including ACAN, COL II, and Sox9.20,21 It has been observed that RUNX-1 interacts with Sox5, Sox9, and Sox6, which are members of the SOX family, to stimulate chondrogenic differentiation and the development of cartilage matrix. It is well-established that they work by interacting with SOX DNA motifs that are located in enhancer regions. These regions are primarily associated with cartilage-specific genes, and as a result, they regulate the expression of a variety of genes that are involved in chondrogenesis, including those that encode ECM proteins like proteoglycans and collagens.22 In another studied mechanism, KGN primarily activates the JNK-RUNX-1 pathway, which causes it to have pro-chondrogenic effects, and it suppresses the β-catenin-RUNX-2 pathway, providing anti-osteogenic effects.23,24 It has been demonstrated that KGN stimulates the chondrogenic differentiation of stem cells, promotes tendon-bone junction regeneration in injured rabbits, and increases the proliferation of bone marrow-derived mesenchymal stem cells (BMSCs).25 However, KGN promotes the proliferation of BMCs and human adipose-derived stem cells by activating the AMPK-SIRT1 signalling pathway. In summary, KGN might be a potential molecule that can be implicated as an endogenous stem cell-based treatment for OA patients. KGN in vivo application was investigated in two OA mice models. Both models showed an enhanced new articular cartilage formation, a reduced level of cartilage breakdown products in the serum, and enhanced load-bearing capacity. KGN promotes cartilage nodule formation during mouse limb development by upregulating genes encoding hedgehog and TGF-β signalling.26
The chemical and physical properties of KGN include limited bioavailability, long-term efficacy, short retention time, and hydrophobicity, often constraining its effectiveness. The implementation of nanomedicine in bone and cartilage healing may enhance treatment outcomes by substituting damaged osteochondral tissue with healthy biological tissue, possibly reinstating joint function. Furthermore, advancements in nanomaterials have made it easier for the development of biomimetic scaffolds featuring nanostructures that improve cell proliferation and migration, thereby promoting the regeneration of damaged tissues.27 Likewise, nanoparticles (NPs) and extracellular vesicles serve as drug delivery systems that facilitate targeted treatment for different osteochondral lesions while mitigating drug adverse effects.28 Therefore, the advancement of nanomedicine has markedly improved the diagnostics and therapies for cartilage and bone disorders. Notably, the structure and properties of KGN, especially its hydrophobicity and carboxyl groups, make it facile to incorporate into a wide range of biomaterials. KGN used with other drugs may have synergistic effects. Tengfei et al synthesized two new conjugated KGN formulations and compared their drug release to free KGN to create a cartilage-targeting delivery strategy. However, KGN conjugated formulations showed that a single dosage of free KGN was ineffective. This conjugated charge-based cartilage-targeted delivery formulation reduces medication dosage with prolonged release.29
A study by Kang et al revealed that in vivo nano KGN conjugated chitosan IA injection resulted in much fewer degenerative defects in rats than in unconjugated KGN injected rats.30 IA injection of nano KGN conjugated materials has gained significant attention in drug delivery systems for cartilage repair, which was discussed later in this review.
Nano KGN-enriched biomaterials show potential for directing tissue regeneration, which is a practical approach. Regardless of the increasing understanding of KGN in cartilage regeneration, there are still several limitations to its clinical implementation. These include concerns about metabolic pathways, long-term safety, as well as other potential drug interactions. However, recently, an analogue of KGN called KA3475 has been developed after significant chemical modification, which substantially enhanced the chemical stability and potency of KGN. KA3475 finished a clinical trial (NCT03133676) in 60 OA patients. The results have not been released because of the safety and effectiveness of the drug.31,32 These potential improvements employ sustained-release formulations to avoid repeated injections, which create pain in local injection sites, inflammation, and issues. Biomaterial scientists, biologists, and clinicians must work together to solve these problems and progress KGN therapy. These teams may enhance KGN treatment by improving drug distribution and creating patient-specific solutions. This partnership speeds the development of new OA medicines and ensures their scientific efficacy and clinical viability, increasing patient outcomes. This cooperation will accelerate drug development and ensure scientific efficacy and clinical feasibility, improving OA patient outcomes.
This study thoroughly researched the physicochemical properties and biological functions of KGN. Furthermore, we provide a comprehensive analysis of cutting-edge functionalized biomaterials, emphasizing their design, structure, function, application, and mechanism of action. Moreover, we examine the essential characteristics for subsequent clinical applications in various biomaterials. Furthermore, Figure 1 illustrated the process of chondrocyte differentiation and cartilage formation. To achieve successful cartilage formation, it is important to understand the intricate molecular events and various pathways, which plays a significant role in cartilage formation.33
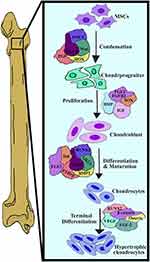 |
Figure 1 Mechanism of chondrocytes proliferation and differentiation. Data from Park et al.33 |
Structural Properties OF KGN
With a molecular weight of 317.3 g/mol, KGN is structurally composed of phthalic acid (PA) and 4-aminobiphenyl (4-ABP) linked together by an amide bond. The 4-ABP serves as a potent inducer of chondrogenic differentiation compared to KGN (Figure 2).34,35 Though the amide bond is stable, it is often hydrolysed by several enzymes, including alkaline ceramidases, allantiasis, metalloproteases, serine proteases, aldehyde oxidase, cysteine proteases, amidases, and peptidases, by releasing its hydrolysates, phthalic acid and 4-ABP.36 The structure of KGN can be confirmed by utilizing various analytical techniques, such as Fourier transform infrared spectroscopy (FTIR) and hydrogen nuclear magnetic resonance.(1HNMR)37 Other compounds, such as the new organic nitrate RS-7897, are shown to exhibit hydrolysis of an amide link that may be a more active molecule compared to the parent molecule.38 However, amide bonds are often utilized in drug design as a linker to bind the drugs to a specific peptide target. KGN has excellent biocompatibility, functions in a concentration-dependent manner, and possesses a prolonged half-life. Although their effects on various cell types vary, MSCs optimally respond to a concentration of 10 μM, whereas tendon stem cells (TSCs) respond best to a concentration of 100 μM.39 On the other hand, KGN hydrophobicity leads to poor solubility in water but is soluble in dimethyl sulfoxide, which affects its bioavailability and the efficacy of drug administration. Moreover, it demonstrates stable storage properties at room temperature.9
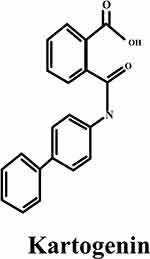 |
Figure 2 The chemical structure of KGN.40 |
In addition, KGN has two hydrogen bond donor sites along with three hydrogen bond acceptor sites. This property allows the implementation of polysaccharides to provide a reversible and defective attachment of KGN to the matrix structure by hydrogen bonding during the processing of the scaffold.35 Therefore, expensive or time-consuming binding techniques are not necessary. Moreover, the carboxyl group of KGN allows covalent bonding like esterification or amidation with hydroxyl and amine groups, respectively, which supports chemical cross-linking with other materials. Junqi Dai et al effectively linked KGN to HAMA by an esterification reaction, resulting in HAMA-KGN, which, when combined with GelMA and evaluated using a microfluidic system, yielded esterase-responsive GHKM. These microspheres demonstrate excellent biocompatibility, minimum invasiveness for injection, and sustained drug release properties.41 To let amidation reaction, Qing H et al prepared nanocarriers KGN conjugated to the surface of PAMAM and the end group of polyethylene glycol (PEG), using amide reaction between the carboxyl group of KGN amino group of PAMAM-PEG to obtain PEG-PAMAM-KGN (PPK) and KGN-PEG-PAMAM (KPP) conjugate. The resulting nanocarriers enhanced the chondrogenic differentiation, retention time in joints, and sustained drug release.42 Similarly, Wenshuai Fan et al synthesised PN-KGN conjugated nanospheres by reaction between the carboxyl group of KGN and the amine group of PN to form amide bonds, where PN-KGN facilitated the sustained release of KGN and provided prolonged cartilage preservation with minimal intra-articular injections.43 The chemical structure of KGN is depicted in Figure 2.40 In view of these chemical and physical properties, KGN may be used in biomedical engineering by chemical conjugation or physical encapsulation.
Biosynthesis and Mechanism of Action
The biosynthesis of KGN involves a mixture of 4-ABP and PA. This compound can be obtained by combining phenylboronic acid with either 4-iodoaniline or 4-bromoaniline. On the contrary, amide bond-breaking substances such as peptidases and amidases can hydrolyze KGN, resulting in the release of its hydrolysis products PA and 4-ABP. Interestingly, 4-ABP exhibited a greater ability to ameliorate the OA injury by promoting chondrogenic differentiation and MSC proliferation than KGN.4 Several different mechanisms of action have been proposed to provide evidence in favour of the chondrogenic effects of KGN.
Filamin A/CBFβ Signalling Pathway for Chondrogenesis
KGN promotes chondrogenic development in MSCs by interacting with an actin-binding protein, which is filamin A. KGN binds to the FC-1 segment of FLNA by displacing CBFβ from its binding site in the cytoplasm. Afterwards, CBFβ enters the nucleus and binds to the RUNX-1 factors that control the production of proteins and genes linked to chondrogenesis through a CBFβ-RUNX-1 transcription program (Figure 3). RUNX-1 induces osteoblast differentiation and chondrocyte proliferation, contributing to the repression of OA.34,44 Based on prior knowledge, the CBFβ-RUNX-1 transcription process may also preserve chondrogenesis function by keeping RUNX-2 at a low level.45 Despite this, KGN has been found to increase the levels of COLII, ACAN, and MMP inhibitors, indicating that it functions in both preserving the chondrocyte phenotype and safeguarding the cartilage matrix from degeneration. The schematic diagram representing the molecular mechanism of action of KGN by Filamin A/CBFβ/RUNX-1 Pathway has been demonstrated in Figure 3.46
 |
Figure 3 Schematic diagram of KGN molecular mechanism of action by the Filamin A/CBFβ/RUNX-1 Pathway. Data from Marini et al.46 |
Hippo/TAOK1 Signalling Pathway for Chondrogenesis
The Hippo signalling pathway is yet another well-known mechanism that controls cell differentiation and development. According to studies conducted by Jing et al small extracellular vesicles developed from KGN preconditioned hUCMSCs may transport miR-381-3p, target TAOK1, and stimulate chondrogenic differentiation of native MSCs. A possible target of miR-381-3p was selected to be TAOK1, an upstream regulator of the hippo signalling pathway (Figure 4). The dual luciferase reporter studies demonstrated that miR-381-3p enhanced chondrogenesis by directly targeting TAOK1 through limiting the 3′ untranslated region that blocked the Hippo signalling pathway.47,48
 |
Figure 4 Molecular mechanism of KGN induced Hippo/TAOK1 Signalling Pathway. Adapted from Fu M, Hu Y, Lan T, Guan KL, Luo T, Luo M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct Target Ther. 2022;7(1):376. Copyright © 2022, The Author(s). This article is licensed under a Creative Commons Attribution 4.0 International License.48 |
The AKT/P13K Signalling Pathway for Chondrogenesis
Besides the aforementioned pathways, a recent study revealed the role of KGN in the chondrogenic process regulated by the AKT/P13K pathway. It demonstrated that the lytic product of KGN, which is 4-ABP, promotes chondrogenic differentiation of MSC. 4-ABP can stimulate the PI3K-Akt pathway, leading to the proliferation of cartilage-derived stem/progenitor cells (CSPC) and MSCs in cartilage. PI3Ks include a family of lipid kinases that regulate a broad range of physiological activities, including cell proliferation, survival, trafficking, and migration.44 The PI3K-dependent signalling also promotes pluripotent embryonic stem cell self-renewal. The PI3K-Akt pathway plays an important role in governing chondrocyte proliferation and differentiation as well as terminal chondrocyte differentiation in both adult and embryonic chondrogenesis (Figure 5a). c-Jun N-terminal kinases are an essential enzyme in the PI3K-Akt pathway, involved in actin cytoskeleton reorganization, which is required for MSC chondrogenesis.49–52 The 4-ABP activates the PI3K-Akt pathway and increases the expression of a downstream molecule, which is the cell cycle protein CDK2. In addition, it suggested that the 4-ABP mechanism of action relies on RSK-3, which is a key regulator of cell differentiation and proliferation. RSK-3 promotes the proliferation of CSPC.44
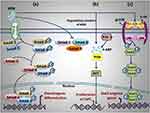 |
Figure 5 (a) AKT/P13K Signalling Pathway, (b) The molecular mechanism of KGN induced Smad/TGF-β Pathway, and (c) IL-6/Stat3 Pathway.51,52 |
The Smad/TGF-β Pathway for Chondrogenesis
Another significant regulatory system for skeletogenesis and joint formation is the TGF-β signalling pathway. Chondrogenesis and lubricin expression are both regulated by this mechanism. In contrast to TGF-β stimulation, which might increase Smad 1/5/8 phosphorylation in MSCs, Decker et al discovered that KGN had no impact on this process.53 The receptor-activated Smads are classified into two groups: Smad1/5/8 and Smad2/3. These Smads are again activated by distinct molecular compounds. Smad1/5/8 induces chondrocyte hypertrophy and OA pathogenesis, whereas Smad2/3 protects against chondrocyte hypertrophy.54 It has been shown that KGN influences the TGF-β/Smad pathway by enhancing phosphorylation and subsequently activating Smads (Figure 5b).51,52 The distinct function of KGN consists of its ability to stimulate Smad2/3, which leads to a chondroprotective effect. It has been shown that chondrogenic development of MSCs obtained from synovial fluid is more successfully stimulated by combining KGN and TGF-β3 than by using either factor separately. This effect is achieved by enhancing the Smad2/3 pathway, whereas KGN inhibits TGF-β3-induced hypertrophy via the Smad1/5/8 pathway.55,56 On the other hand, Wang et al discovered that KGN may stimulate type I collagen production in dermal fibroblasts via the TGF-β/Smad4/5 pathway. KGN enhances the p-Smad5 protein, which then entirely enters the nucleus.57,58 Likewise, the Smad4 protein is overexpressed and translocated to the nucleus. The p-Smad4 and p-Smad5 proteins consequently activate subsequent genes. This suggests that KGN may function via several routes, depending on the cell type.57
The IL-6/Stat3 Pathway for Chondrogenesis
Tao et al evidenced that KGN can stimulate mitosis in CSPCs, resulting in an increase in both the percentage and number of G2-M phase cells. The group treated with KGN resulted in a 10% higher proportion of G2-M phase cells, which was almost twice as high as in the control group. The transcriptomic analysis of rat CSPCs showed substantial differences between KGN-treated and control samples. Compared to the control group, the KGN-treated group had significantly greater levels of IL-6 as well as its co-receptor Gp130 gene expression. KGN stimulation improved the phosphorylation of Stat3, an IL-6 downstream protein (Figure 5c).51,52 Increased articular cartilage thickness was seen in the destabilization of the medial meniscus (DMM) animal model after IA KGN injection. Following IA KGN injection, IHC labelling also showed the increased distribution of CD44+/CD105+ cells in cartilage and elevation of Stat3 phosphorylation. The findings, therefore, indicated that KGN stimulated IL-6/Stat3-dependent proliferation, at least in part, to enhance cartilage regeneration.59 According to recent research, IL-6 has a role in maintaining MSCs in their undifferentiated form and is associated with MSC development via JAK/STAT3 signalling.60 Various factors, including periostin, TGF-β, mechanical stimulation, and platelet-rich plasma(PRP), have been shown to positively impact both of these cellular processes, hence promoting cartilage regeneration.59
The Indian Hedgehog (Ihh) Pathway for Chondrogenesis
The Indian hedgehog (Ihh) pathway is crucial for cartilage tissue formation and maintenance. As a crucial signalling molecule, it controls the differentiation, proliferation, and osteoblast differentiation of chondrocytes and is secreted by pre-hypertrophic chondrocytes in the growth plate. The absence of this protein inhibits the development of synovial joints in embryos.61 Ihh is produced in the growth plate by chondrocytes. When it binds to its trimeric receptor Patched-1 (Ptch1) in vertebrates, inhibition of Smoothened (Smo) would be relieved (Figure 6).62 Consequently, Smo activated and translocated to the cilium, where it recruited the suppressor of fused homologues, the Sufu-Gli complex. As discussed above, the active Smo dissociates the bonding between Sufu and Gli, enabling activated Gli2/3 to reach the nucleus and stimulate the production of Ihh target genes likeGli1 and Ptch1.63,64 Furthermore, it was also possible to inhibit KGN- Ihh signalling by treating KGN with cyclopamine (CPN), a drug that blocks hedgehogs.65 Another study by Chen et al suggests that Ihh and Sonic Hedgehog (Shh) regulate the activation of early cartilaginous differentiation in vivo and in vitro. It may have a synergistic impact on chondrogenic differentiation and chondrogenesis in the rotary cell culture system (RCCS) and in cartilage repair.66
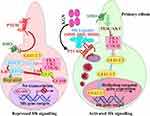 |
Figure 6 Molecular representation of repressed (left), in absence of hh ligands and activated (right) KGN induced hh signalling pathway, in presence of hh ligands. Adapted from Ok CY, Singh RR, Vega F. Aberrant activation of the hedgehog signaling pathway in malignant hematological neoplasms. Am J Med. 2012;180(1):2–11. Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.62 |
The AMPK-SIRT1/Antioxidant Signalling Pathway for Osteogenesis
It has been observed that KGN promoted the osteogenic differentiation of BMSCs, increased the level of production of antioxidant enzymes, and reduced intracellular reactive oxygen species (ROS).67 KGN increased BMSC levels of antioxidant enzymes and osteogenic differentiation in a dose-dependent manner. By suppressing the expression of p16INK4α, Silent Information Regulator Type 1 (SIRT1) prevented oxidative stress-induced premature senescence in MSCs and encouraged multi-lineage differentiation in MSCs.68 Furthermore, SIRT1 has been linked to the mediation of antioxidant activities via the reduction of intracellular ROS generation and the upregulation of the expression of Nuclear factor erythroid 2-related factor 2 (NRF2), a pivotal transcription factor that modulates over 200 cytoprotective genes. It has been shown to translocate to the nucleus in response to oxidative stress and transcribe many intracellular antioxidant enzymes, including superoxide dismutase 2 (SOD2) and catalase (CAT).69 Nonetheless, it has been shown that excessive ROS generation aids in cartilage damage and the advancement of OA. Apart from the AMPK-SIRT1 signalling pathway, it has been proposed that KGN therapy enhances the expression of TGF-β1 and other members of the transforming TGF-β superfamily genes. KGN has no impact on the mitogen-activated protein kinase (MAPK) signalling pathway, but it may potently trigger the phosphorylation of smad4/smad5 in the TGF-β signalling pathway (Figure 7).70 Therefore, KGN may hold promise for the therapeutic use of MSCs in bone tissue engineering in addition to articular cartilage repair.
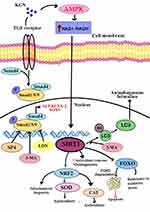 |
Figure 7 Molecular mechanism of KGN induced AMPK-SIRT1/Antioxidant signalling pathway.71,72 |
The Autophagy-Induced Response for Osteogenesis
KGN enhances BMSCs osteogenesis by increasing the Smad1/5/9 pathway and autophagy activity. Autophagy is a conserved biological mechanism which promotes cell survival under stress and maintains homeostasis.73,74 Previous studies have shown that autophagy holds an indispensable mechanism for regulating bone regeneration. It has also been established that autophagy can improve osteogenesis by supporting osteoblast mineralization and differentiation, which affects autophagosomes in calcium extracellular transportation. Autophagic activity plays a significant role in determining the potential for MSC differentiation through its regulation of osteoblastic or adipogenic lineages.75,76 Furthermore, autophagic levels may affect the survival rate of bone-related cells in adverse conditions. Several autophagy marker genes, including (LC3), Atg7 and Beclin1, have been proposed for regulating autophagy. LC3 is available in two forms: LC3-I, which is unconjugated to lipids and distributed in the cytoplasm. Whereas LC3-II is conjugated to phosphatidyl ethanolamine and utilised as a specific autophagosome marker to access autophagic flux.77 In addition, the involvement of autophagy during KGN treatment was confirmed by (3-MA), an inhibitor of autophagy. The action of 3-MA to inhibit autophagy resulted in a notable decrease in the mRNA and protein expression levels of alkaline phosphatase (ALP), as well as Runt related transcription factor-2 (RUNX-2), in BMSCs treated with KGN.78 As a result, the activity of osteogenic differentiation was significantly reduced. According to Decker et al investigation, they suggested that KGN might stimulate the phosphorylation of Smad2/ 3 of E11.5 limb bud mesenchymal cells, which would generally stimulate the development of mice embryonic limbs.53 Furthermore, bone marrow-MSCs (BM-MSC) differentiation potential may be suppressed by LDN, a specific inhibitor of the Smad signalling pathway, whereas 3-MA’s inhibitory effects on osteogenic differentiation in BMSCs can be reduced by the activator SB4.79 The findings suggested that KGN stimulated BMMSCs autophagic activity and improved their potential for osteogenic differentiation via increasing Smad1/5/9 phosphorylation. Thus, autophagy and Smad1/5/9 play an important role in osteogenic differentiation in BMSCs as well as fibroblasts and endothelial cells (Figure 7).70–72,80
The Anti-Osteogenic Mechanism of KGN
A recent study has revealed the anti-osteogenic effect of KGN. KGN preconditioning may have two advantageous effects on chondrogenic differentiation triggered by TGF-β3. It committed MSCs to a pre-cartilaginous stage accompanied by reduced β-catenin expression enhanced JNK phosphorylation (Figure 8).3,81 These anti-ossific and pro-chondrogenic effects may be linked with the inhibition of β-catenin/RUNX-2 pathways and the activation of JNK/RUNX-1 signalling pathways. JNK functions through the remodelling of the actin cytoskeleton, requisite for chondrogenic differentiation.50 On the other hand, β-catenin related signalling pathways, including the PI3K/Akt/GSK-3b/β-catenin pathways and canonical Wnt/β-catenin, play a major role in MSC osteogenic development.50 The in vivo findings revealed that tracheal defects in a rabbit model might be restored using tissue-engineered patches made of KGN-preconditioned hUC-MSCs along with electrospun nanofilms encapsulating TGF-β3. Re-epithelization was also observed, demonstrating a promising strategy for developing tissue-engineered trachea.50
 |
Figure 8 The molecular mechanism of KGN induced JNK/RUNX-1 signalling pathway. Reproduced from Jing H, Zhang X, Gao M, et al. Kartogenin preconditioning commits mesenchymal stem cells to a precartilaginous stage with enhanced chondrogenic potential by modulating JNK and β‐catenin–related pathways. FASEB J. 2019;33(4):5641–5653. © FASEB.3 |
The mechanism of action of KGN has been increasingly elucidated by an increasing number of investigations. The mechanism behind the actions of KGN, initially discovered by Johnson et al is filamin A/CBFβ/RUNX1, which is the most predominant and generally established mechanism involved in KGN activity. Apart from this, the Ihh and TGFβ/Smad pathways are quite widespread. However, research fails to define the number of mechanisms by which KGN addresses cartilage defects. Consequently, comprehensive study is required to investigate the complex interactions between these pathways and their wider significance in cartilage regeneration and MSC development.
The Application of Biomaterial-Mediated Drug Delivery of KGN in The Biomedical Field
KGN has been employed in many tissue engineering fields as well as in regenerative fields, including cartilage protection and regeneration, tendon-bone healing, wound healing, scaffold engineering, and limb development. Regenerative medicine is the process of replacing old or damaged cells with genetically identical new and functioning cells to repair or establish normal function of cells. KGN could be helpful in cartilage repair, stimulate collagen synthesis for wound healing, encourage the establishment of a cartilage-like transition zone in tendon-bone junctions, and govern limb growth in a coordinated manner. Owing to its bioactivity, KGN may be incorporated into nanoparticles, nanofibers, hydrogel, liposomes, nanovesicles, and dendrimers - as nanodrug delivery systems (Figure 9). In order to efficiently target bone or cartilage regeneration, some essential applications of KGN are as discussed below:
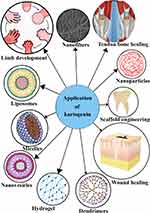 |
Figure 9 Schematic diagram of KGN delivery system and application as biomaterials. |
Scaffold Engineering
A scaffold is a three-dimensional (3D) matrix structure that promotes cell survival and holds significant promise for tissue regeneration. An ideal scaffold should have several properties, such as a porous structure, an effective mode of action, a low immunogenicity, excellent biocompatibility, an appropriate degradation rate, and excellent mechanical strength.82 Grafted scaffolds need to be effectively rigid to withstand multidirectional compressive loads.83 Shi et al employed a quick cross-linkable, ultraviolet-reactive scaffold loaded with KGN NPs to treat full-thickness cartilage lesions in an in vivo model. The NPs-incorporated scaffolds were loaded into the defect sites and then exposed to ultraviolet (UV) light. The scaffold that was combined with KGN had a major beneficial effect on cartilage regeneration.84,85 Similarly, Hong et al developed a unique multi-dimensional combination treatment that promoted cartilage regeneration. The scaffold was composed of soluble poly-L lysine/KGN (L−K) NPs and poly (lactic-co-glycolic acid) PLGA/methacrylate hyaluronic acid (PLHA). The porous PLHA scaffold was developed by combining UV and co-precipitation crosslinking. Through in vitro cytotoxicity tests, the synthesized scaffold was revealed to be biocompatible with adipose-derived stem cells (ADSCs) and to enhance the secretion of COL II by ADSCs in a dosage-dependent manner, as shown by immune fluorescence assay. It demonstrated that the PLHA scaffold degraded and finally differentiated into cartilage in vivo, while the nascent tissue subsequently regenerated.86,87
Furthermore, Xuan et al created scaffolds made up of poly (1,3-propylene sebacate) (PPS), poly (glycerol sebacate) (PGS), and bioactive KGN. The scaffolds supported enhanced chondrogenic differentiation and cartilage repair in full-thickness lesions of rat femoro-patellar groove after 12 weeks and suppressed osteogenic differentiation of BMSCs in a dosage-dependent manner.88 Similarly, Li et al designed a KGN-filled PLGA-PEG-PLGA thermo gel to fix cartilage damage. Based on the in vitro results, 42.4% of KGN was released after 196 hours. BMSCs and KGN both were combined in the thermo gel. The in vivo result reported that the scaffold-encapsulated drug can help cartilage tissue regeneration. The findings showed that the newly developed cartilage had high content of COLII and GAGs, a fully smooth surface, along with decreased cartilage matrix degradation.89 In another study, Liu et al created a layer-specific biomimetic biphasic osteochondral scaffold (BBOS) with stem cell differentiation inducer. Additionally, the BBOS cartilage regeneration layer (cartilage scaffold, CS) had a hyaluronic acid that looks like cartilage that is mechanically enhanced by host-guest supramolecular units, regulating the release of KGN. Furthermore, the bone regeneration layer (bone scaffold, BS) is a 3D-printed hydroxyapatite (HA) scaffold which releases alendronate (ALN).90 The two semi-immersion-bound layers may control the stem cell hierarchical targeted differentiation activity. In comparison to the drug-free scaffold, it was possible to encourage the MSCs in the BBOS to develop into osteoblasts and chondrocytes. The in vivo findings showed that the regeneration of bone or cartilage is strongly promoted in the corresponding layers. This BBOS with layered specific inducer release is predicted to be developed into a novel osteochondral regeneration technique.91,92 Likewise, Zhao et al developed a biocompatible scaffold containing poly(lactic-co-glycolic acid) (PLGA) and chondrocyte extracellular matrix (CECM) microspheres. This scaffold can mimic the regenerative microenvironment and promote chondrogenic differentiation of BMSCs both in vivo and in vitro.93 In contrast, Sepahdar et al reported an effective composite composed of polyvinyl alcohol- polyurethane (PVA-PU) hydrogel with KGN-incorporated PLGA NPs for cartilage tissue regeneration isolated from MSC. Increasing the concentration of KGN increased the cytotoxicity of the examined NP scaffold. Their findings revealed that the KGN-loaded NP Scaffold assisted MSC differentiation into chondrocytes.78
These investigations explained how porous scaffolds can be used as drug delivery systems for KGN in tissue regeneration applications. Utilizing a variety of methods, including 3D printing, conjugation with microspheres, nanoparticles, or hydrogels and reversible attachment, offers scaffold designing sustained drug delivery strategies. However, their method of preparation and implantation are more intricate, elevating both costs, invasiveness for the patients, and they may be more appropriate for long-term repair conditions, particularly in cartilage regeneration that demands robust mechanical support in weight-bearing joints. There is potential for improving cartilage regeneration and the management of diseases related to cartilage through more research and development in this area, as scaffold can mimic the extracellular matrix to promote cell attachment and tissue formation. Drug-containing scaffolds greatly improved MSC chondrogenic and osteogenic development in comparison to drug-free scaffolds.
Nanoscale Applications of KGN
NPs have been broadly used in various fields, including gene and drug delivery, bioimaging, anti-microbial and antitumor targeting because of their wide range of functions and diverse forms.94 These particles can preserve bioactive substances, reduce their adverse effects, control their release profiles, maximizing their therapeutic benefits, and effectively distribute them to target cells.95 It is worth mentioning that NPs can be produced with exceptional precision in terms of their shape, surface characteristics, and size, which can substantially improve their solubility, cellular uptake, and immuno-compatibility.96 In addition, the utilization of NPs to encapsulate active molecules provides several benefits, such as improved solubility, protection against degradation in biological fluids, as well as facilitating controlled, sustained release across space and time.97 Particle size is a key aspect of the therapeutic effects because it influences penetration as well as retention time in the cartilage matrix.98 However, the exact size of the drug formulation for IA delivery is still under debate.99 Rothenfluh et al proposed that NPs that self-assemble with a size of less than 60 nm could easily pass through the heavy collagen network found in the cartilage extracellular matrix. This would transform the matrix of the cartilage from a barrier into a reservoir for the NPs. To improve bioavailability in cartilage, they engineered NPs with a regular and spherical shape with an average size of 25 nm that should be able to efficiently penetrate the matrix of cartilage under the dynamic compression of normal transportation. This would allow an intra-tissue release of nano KGN rather than just an IA release with enhanced bioavailability.100,101
Almeida et al examined how surface chemistry affects the properties of KGN-loaded nanoparticles and how they interact with hematopoietic MSCs by investigating the KGN-loaded PLGA, PLGA–PEG–HA, and PLGA–PEGNPs. The PLGA–PEG–HA and PLGA–PEG NPs released more KGN than the PLGA NPs. This suggested that KGN interacts more with the hydrophobic PLGA than with the hydrophilic HA or PEG at the surface of the NPs. The larger PLGA–PEG and PLGA–PEG–HA particles may have larger pores, as they released the most KGN. Over short periods of time (approximately 7 days), MSCs that were formulated with nano KGN displayed a high rise in sulphated GAGs, which was an indication of chondrogenic differentiation in comparison to non-KGN formulations.101 Similarly, Kang et al utilized chitosan, a chitin derivative distinguished by its amino group and polycationic properties, conjugated with KGN through an amide bond involving 1-ethyl-3-(3-dimethyl aminopropyl) carbamide along with N-hydroxy succinimide (EDC/NHS), to produce NPs using ionic gelation method. This involved the tripolyphosphate anion interaction with the cationic chitosan through electrostatic forces. The result showed that the chitosan NPs conjugated with nano KGN enhanced the biocompatibility and aqueous solubility of hydrophobic KGN. Furthermore, these NPs exhibited sustained in vitro release for a period of seven weeks. These polymer-drug conjugates showed a promising technique for delivering drugs to treat OA.102 Additionally, they created thermo-responsive polymeric NPs based on chitosan. KGN was covalently cross-linked with dual drug-release NP outside and revealed that changes in temperature can control the release of each drug separately.103 According to a study by Fan et al nano KGN-conjugated polyurethane NPs (PN-KGN) were successfully developed by synthesizing amphiphilic PN with pendant amino groups. The amine group of PN and the carboxyl group of KGN then reacted to form amide bonds. Additionally, an IA injection of PN neither caused any joint swelling nor accelerated the progression of OA compared to the control group. This deduced that PN was biocompatible in vivo. Their findings revealed that PN-KGNNPs can protect joint cartilage better than KGN and stop the progression of OA.43 Recently there was a study stated that injecting NPs into mice joints caused severe inflammation.104
In addition to the size of particle, the charge of particles also determines their retention and penetration into the cartilage matrix. The ECM inside cartilage is negatively charged, it offers an exceptional opportunity of employing electrostatic interactions to improve drug carriers’ movement, uptake, and binding. Drug-carriers that were positively charged exhibited greater ingestion as well as faster penetration than their neutral counterparts of the same size.105,106 Furthermore, studies have documented that cationic polymeric NPs formed electrostatic clusters with endogenous hyaluronic acid following IA injection, which resulted in an extended-release profile and increased retention time in joints.107,108 A prior study indicated that pendant amino groups (–NH2) can enhance the cationic properties of PU.109 Hence, it suggested that the amino groups could extend retention time in the cartilage matrix and facilitate electrostatic interactions with anionic proteoglycans.
On the other hand, Silva et al prepared nano KGN-incorporated coaxial poly (glycerol sebacate)/PCL aligned nanofibers using the chemical cross-linking method. These fibres released KGN over time and caused MSCs to differentiate into chondrogenic cells.110 A flexible nanofiber structure was created to heal wounds at the interface tissue.111 In another study, nano KGN was linked to the polydopamine layer on the silk fibroin nanofiber surface. Using the core-shell nanofiber structure helped to interconnect tendons with bones.112
It is possible to manage the loading and release of KGN, the stability of suspension, as well as the cellular uptake of nanoparticles, by adjusting their composition, structure, and biological activity. This will ultimately affect the functional efficacy of the particles. Combining nanoparticles with hydrogels to create composite biomaterial systems for controlled drug delivery is becoming more common to further improve therapeutic efficacy, particularly for localized applications.
KGN-Loaded Exosomes
Exosomes are attracting significant interest because of their distinctive biological properties, multidisciplinary functions and potential therapeutic applications. According to reports, MSCs have a therapeutic impact that involves stimulating tissue receptor cell activity via paracrine mechanisms, as opposed to directly replacing cells.113 When MSCs are in an active or resting state, they release a membrane secretory system called exosomes into the extracellular matrix. They have several advantages, including targeted distribution, stable chemical properties, simple preservation, selective assembly, efficient tissue regeneration, and the ability to retain the active components of MSCs.114 When compared to direct cell transplantation, exosome therapy shows an improved homing effect, increased flexibility, and increased immune modulatory activity.115 The paracrine system secretes extracellular vesicles that attach to target cells and release a range of cytokines. Following that, these cytokines control tissue regeneration. They have a diameter of 40–120 nm and are packed with proteins, lipids, nucleic acids, and other substances released by cells.116,117 According to Xu et al exosomes containing the surface-displayed E7 peptide (E7-Exo) may target synovial fluid-derived MSCs (SF-MSCs), which can be produced by fusing the peptide E7 with Lamp 2b (the exosomal membrane protein). In an OA rat model, IA injection of SF-MSCs along with E7-Exo/KGN co-administration at the knee joints exhibited more marked therapeutic advantages compared to KGN alone and KGN injected by exosomes without E7.118 As per Wang et al secretory elements derived from UC-MSCs possessed the ability to control MSC differentiation.57 Following this, Zhang et al observed that exosomes derived from embryonic stem cells (ESCs) could stimulate osteochondral regeneration.119 Similarly, Cosenza et al observed that exosomes extracted from BM-MSCs could protect the bone and cartilage from deterioration in OA patients.120 In a recent study, Huang et al postulated that MSC-derived exosomes might serve as an alternative therapy for cartilage repair in an approach called cell-based tissue engineering.121,122 In a later investigation, Tao et al discovered that exosomes derived from synovial mesenchymal stem cells (SMSCs) had a noteworthy capacity to prevent OA and that the effectiveness of this method might be markedly augmented by the upregulation of miR-140-5p in SMSCs.123 Wu et al proposed that IPFP-MSC-EXOs rich in miR-100-5p might suppress mTOR in OA and so preserve articular cartilage.124 Qi et al found that BMSC exosomes may block the p38, ERK, and Akt pathways that cause mitochondrial dysfunction-induced apoptosis in chondrocytes.125 As a result, several studies have shown the benefits of MSC-EXOs for OA prevention and cartilage regeneration. Exosome components and functions, however, are very diverse and may change depending on whether they are taken from other cell types or else the same cell types under various circumstances.126
In their study, Kato et al demonstrated that exosome function can be controlled by pre-treatment. They employed to activate synovial fibroblasts and reported that exosomes isolated from IL-1 cells induced a greater number of osteoarthritic alterations in articular chondrocytes than those without IL-1 stimulation.127,128 Similarly, Shao et al observed that exosomes produced by IPFP-MSCs might increase the chondrocytes production by increasing its anabolic effects, decrease the catabolic effects by lowering MMP expression, and raise the COL-II, ACAN, and SOX9 expression. In addition, compared to exosomes produced from IPFP-MSCs without pre-treatment, exosomes extracted from IPFP-MSCs treated with KGN may considerably increase anabolic effects and decrease catabolic effects.2 In another report, Liu et al, found that exosomes obtained from nano KGN preconditioned BMSCs (KGN-BMSC-Exos) outperformed exosomes derived from BMSCs. The chondral matrix development and degradation of BMSC-Exos were both enhanced by nano KGN preconditioning.129 However, there are numerous challenges for using MSCs clinically to treat cartilage damages, such as ethical clearance and policy issues. Because of these, we need to keep looking for a cell-free method to support cartilage repair that might be helpful to put into practice. Thus, exosomes provide a potential cell-free approach to stimulate cartilage regeneration based on MSCs.
The use of exosomes increased the effective concentration of KGN within cells and considerably facilitated the chondrogenesis of MSCs both in vitro and in vivo. Despite the increasing number of studies on the impacts of exosomes, there are still some shortcomings in the area of drug delivery systems. Native exosomes, for example, are not naturally made to deliver specific cargo. Instead, cargo is delivered to different types of cells in vivo without any preference. However, with an enhanced comprehension of exosomes, it is anticipated that superior techniques would be produced to maximize their design and functionality.
KGN for Wound Healing
Wound healing is a complicated process that involves several cell lineages and tissues. The preliminary goals of wound healing are to ensure fast wound closure while leaving a biologically similar and functionally appealing scar. The collagen-rich granulation tissue created by fibroblasts is thought to be crucial for wound healing.130,131 KGN promotes lubricin accumulation through the ADAMTS5 and c-Myc pathways as well as reducing intervertebral disk degeneration and alleviating pain. Wang et al discovered that KGN promotes collagen I production by activating Smad4/Smad5/TGF-β pathway, indicating its potential application in wound healing.132 Furthermore, KGN had no obvious adverse impacts on fibroblast viability, morphology, or survival. In vivo, experiments have shown that KGN may significantly enhance collagen production in the skin and improve wound healing in mice. The group treated with KGN had a significantly faster wound healing time than the control group (12.8±2.3 days vs 16.4±3.4 days).133
Chondrogenic Potential of KGN: Tendon-Bone Healing
The rising risk of tendon-bone junction (TBJ) injuries is high, particularly in the field of sports. TBJ injuries heal slowly and often result in the formation of scar tissue that impairs normal function. The unique structure of TBJ consists of three components, including a tendon, a zone of transitional fibrocartilage (both calcified and uncalcified), and bone.134,135 The fibrocartilage structure acts like a shock absorber and minimizes the level of stress that is transferred to tendons. Since tendons allow complicated movements in many aspects, TBJs are subjected to high-stress levels, which increase their risk of acute or chronic injuries, especially in athletes who engage in intensely competitive sports. KGN chondrogenic potential was used to promote the fibro-cartilaginous zone during TBJ repair.136 Zhang et al studied the in vitro nano KGN effect on chondrogenic differentiation and proliferation of rabbit BMSCs as well as patellar tendon stem/progenitor cells (PTSCs). Nano KGN stimulated chondrogenic differentiation of stem cells and concentration-dependently increased cell proliferation in both cell type, as indicated by the upregulation of chondrogenic markers Sox9, COL II, and ACAN expression. In vivo, nano KGN stimulated the development of cartilage- tissues in rat patellar tendons and improved wound healing at damaged rat Achilles TBJs.137
KGN Mediated Limb Development
Besides the KGN chondrogenic effect, the biological effects on other different tissues have been investigated. Limb development requires the coordinated development of several tissue types, including cartilage, bones, ligaments, tendons, and joints. The joint formation is critical in vertebrate limb development, allowing animal limbs to adapt successfully to an array of ecological niches.138 The preliminary sign of joint development is the formation of an interzone, which occurs when extremely flat and condensed mesenchymal cells arise. The interzone is composed of three layers: a dense middle cell layer and two outer cell layers. The middle interzone layer is directly linked with the formation of articular chondrocytes, while the outer layers support the expansion of long bone anlagen through appositional growth.139 Decker et al investigated whether KGN controls limb developmental processes by examining its effects on complete limb explants while committing pre-skeletal mesenchymal cells from limb buds in mouse embryos. KGN not only increased cartilage nodule production but also increased tendon maturation, digit cartilaginous anlage elongation, synovial joint creation, and interzone compaction.52 KGN worked through comprehensive, central processes that ordinarily encourage, direct, and coordinate total limb development. KGN increased gene expression for TGF-β (particularly TFG-β1) and hedgehog superfamily members. Exogenous TGF-β1 induced cartilage nodule development like KGN, where both TGF-β1 and KGN significantly increased the expression of SZP/lubricin/PRG4 in articular superficial zone cells. Overall, their findings indicated that KGN might be an effective tool for tissue repair and limb regeneration strategies.52,140
KGN-Loaded Liposomes
Liposomes are lipid bi-layered membrane structures that effectively encapsulate hydrophilic and hydrophobic drugs, respectively, inside the core and in the lipid bilayers. Besides this, liposomes have significant benefits in drug delivery systems (for example, high biocompatibility, effectively controlled drug release, and passive targeting capabilities).141,142 Yang et al presented a simple and successful technique for producing mono-disperse liposomes employing microfluidics technology, a photo-cross-linkable gelatine methacryloyl (GelMA) matrix combined with KGN-loaded liposomes. Further, to enhance liposome stability and alleviate drug release, GelMA micro-gels were used to immobilize liposomes in the matrix structure by non-covalent interaction and physical network obstruction. They deduced that KGN-loaded GelMA@Lipomicrogels (GelMA@Lipo@KGN) may release prolonged KGN for more than three weeks and significantly increase chondrocyte differentiation of BMSCs in vitro when compared to KGN-loaded liposomes (Lipo@KGN). Furthermore, an in vivo study showed that injecting GelMA@Lipo@KGN into the joints of rats with surgically induced OA prevented cartilage degeneration, osteophyte burden, and subchondral bone changes while improving joint residence over five weeks.143
The Liposome-based delivery system acts as dynamic carriers that improved the biocompatibility and controlled release of KGN. By integrating liposomes within scaffolds, this hybrid system can enhance the mechanical support without changing its therapeutic efficacy.
KGN-Loaded Dendrimers
Dendrimers are molecules that are about nanometers in size and have a radially symmetrical shape that is uniform and monodisperse. Their structure appears to be a large, hyper-branched molecules deliberately engineered such that their end groups, or those extending to the outside, might be functionalized to alter their biological or physicochemical properties. They have various applications in supra molecular chemistry, especially in host-guest interactions and self-assembly. Their distinctive characteristics make them potential candidates for many different purposes.144 Qing et al utilized a partially PEGylated polyamidoamine (PAMAM) dendrimer as a nanocarrier to transport nano KGN into the cytoplasm of MSCs and stimulate chondrogenic differentiation. In that case, PEG-PAMAM-KGN (PPK) and KGN-PEG-PAMAM (KPP) conjugates were created by linking nano KGN to the surface of PAMAM and the end group of polyethylene glycol, respectively. In comparison to PPK and free KGN, the KPP increased the expression of chondrogenic markers by increasing the level of CBFβ nuclear localization. Both OA and healthy rats possessed fluorescein-labeled PPK that could persist in the joint space for a long time. To treat OA, PPK might be a good nanocarrier for IA medication delivery system.42
The branching architecture of dendrimers enables targeted administration and high drug loading capacity, which may enhance KGN localization at damaged sites.
KGN in Hydrogels
For many years, hydrogel has been utilized in 3D cell culture research applications. The capacity to retain high water and elasticity makes them mimic native tissues. The absorption of water causes hydrogels to swell.145 Water gets absorbed by the functional groups attached to the polymeric backbone and prevents it from dissolving.146 Since the formation of inter-fibrillar or intermolecular cross-link structures between the network chains of fibrillar proteins or polymer molecules, respectively.147,148 Hydrogels comprise desirable and controlled properties that are essential for tissue engineering. These include tunable mechanical strength and stiffness, intrinsic adaptability and biocompatibility, swellability, as well as biodegradability. It has been utilized to repair damaged tissue since they have a complex structure and function that are reminiscent of the ECM.149 In 2017, Zhu et al prepared an injectable hydrogel comprising HA and chitosan in conjugation with KGN, which released KGN continuously. This hydrogel successfully stimulated cell differentiation in the nucleus pulposus of an intervertebral disk. In view of its excellent mechanical characteristics, KGN-conjugated hydrogel was selected as the best gel for enhancing cell proliferation and differentiation.150 To achieve thermo-sensitivity and KGN, Dorsa Dehghan-Baniani chemically modified chitosan using β-Glycerophosphate (β-GP), N-(β-maleimidopropyloxy) succinimide ester BMPs and KGN to create in situ formation of chitosan hydrogels with an improved shear modulus, in order to achieve thermo-sensitivity. A large range of shear moduli, from 50 to 250 kPa, was covered by the hydrogen with a shear modulus of 78 ± 5 kPa.151 It takes just a few minutes for it to gel at 37 °C after being injected non-invasively into the defect site. The thermo-sensitive hydrogel that was produced had several intriguing properties for cartilage tissue engineering, including an improved shear modulus, gelation behaviour, long-term drug release and injectability.152 In a later investigation, Yuan worked on a thermos-sensitive dual drug-loaded hydroxypropyl chitin hydrogel (HPCH) system that could release stromal-derived factor-1α-like polypeptides (SDFP) and KGN for stem cell recruitment and chondrogenic differentiation. The network structure of the hydrogel allowed for the exchange of nutrients and enhanced cell growth. However, it worked effectively by fixing defects with temperature-sensitive properties. The system demonstrated superior biocompatibility in vitro as well as promoted chondrogenic differentiation and stem-cell recruitment. Additionally, it downregulated chondrocyte catabolism in inflammatory conditions. Experimental evidence suggests that the dual-drug hydrogel systems may stimulate articular cartilage regeneration in rats. This research provided strong evidence that the HPCH system, including KGN along with SDFP, is a promising approach for treating articular cartilage defects.153
The incorporation of nanomaterials within hydrogels has improved drug delivery.154 For instance, Diaet al studied nano KGN-loaded PLGA MPs in a collagen-based hydrogel using the emulsification evaporation method.155 Fan et al also used the EDC/NHS condensation process to attach KGN to polyurethane nanoparticles, which led to a 14% loading efficiency.156 Houreh et al developed a multi-layered scaffold using a polycaprolactone (PCL) mat and CS hydrogel to improve the stability, integrity, and mechanical properties of CS, specifically for developing in vivo transplantation. The conjugated nano KGN in the matrix structure stimulated MSC chondrogenesis with its slow release. Furthermore, the gene expression study revealed that in the presence of KGN, the expression levels of COLL2, SOX9, and ACAN were higher. However, the expression of COLLX was downregulated, suggesting a synergistic impact of TGF-β and hypertrophy. The study confirmed that the multilayer structure system of KGN-loaded SDFP and HPCH not only effectively repaired articular cartilage defects but also demonstrated a successful treatment strategy.157,158 Zhang et al synthesized a metal-ion-collagen hydrogel that was supra-molecularly wrapped with the chondro-inductive factor KGN using cyclodextrin (CD). Furthermore, the hydrogel was able to greatly increase the production of cartilage matrix components (ACAN as well as type II collagen) in articular chondrocytes without having any effect on cell proliferation or migration in vitro. The protective effects were based on improving intracellular antioxidant properties and mitochondrial activity in chondrocytes. In vivo, hydrogel injection enhanced cartilage regenerating capability and subchondral bone restoration after full-thickness lesions. This work highlighted the potential of a dynamic chondrogenic hydrogel as a new approach to treat refractory cartilage injury by facilitating healing processes via mitochondrial reinforcement.159 An additional investigation by Mohsenifard et al designed an interpenetrating polymer network (IPN) hydrogel containing beta-cyclodextrin (β-CD)-modified alginate/cartilage ECM, KGN, and 3D-printed poly (ε-caprolactone) (PCL)/starch microfiber network. This prototype demonstrated promise as a treatment for articular cartilage defects.160 Massaro et al created a new halloysite nanotube (HNT)-based carrier system for the possible intra-articular administration of KGN using laponite (Lap) hydrogel (HNT/KGN/Lap), which displayed no harmful effects.161
Yuan et al employed Schiff base cross-linking reaction to develop an injectable CMC-OCS hydrogel incorporating MPs, and the PME method was utilized to fabricate PLGA MPs@KGN. When compared to control group hydrogels, the procured CMC-OCS@MPs@KGN resulted in a reduced gelation time along with a slower rate of weight loss. It was helpful for the cartilage healing process since the hydrogel@MPs had a significantly increased compressive elastic modulus.162,163 Fan et al prepared a cell-free hydrogel containing TGF-β3 and polyurethane NPs (PN-KGN) to promote cartilage repair by simultaneously captivating endogenous MSCs and stimulating chondrogenesis in the recruited cells. The results revealed that the chondrogenesis of MSCs was improved by a combination of nano KGN and TGF-β3. It was also found that KGN promoted MSC chondrogenesis by reducing the degradation of RUNX-1, a protein that physically interacts with p-Smad3 in MSC nuclei.156 Furthermore, Zere et al created two distinct configurations of laminated composite scaffolds using PCL: Gelatin electrospun mat, alginate sulphate hydrogel, and nano KGN. The prepared composite scaffold exhibited several advantages, including enhanced mechanical properties, reduced damage potential (less energy wasted), interconnected pores of fibre and hydrogel, a good swelling ratio, and properly controlled biodegradability.164,165 In another study, Mungal et al reported the successful preparation of self-assembled PEGylated KGN (PEG/KGN) micelles through covalent crosslinking of OH-PEG-NH2 along with 3-hydroxypropanoic acid-grafted KGN using carbodiimide chemistry. Over a period of five days in vitro, the HA/PEG/KGN hydrogels showed continuous KGN release. In vivo studies demonstrated that, in comparison to free HA hydrogel, HA/PEG/KGN hydrogel considerably reduced OA development in rats.166 Chen et al designed a biocompatible hydrogel scaffold system for cartilage regeneration loaded nano KGN and with synthetic melanin nanoparticles (SMNP). The matrix was composed of cellulose nanocrystals, HAMA (hyaluronic acid-methacrylate anhydride) and GelMA (gelatin-methacrylate anhydride). The SMNP-KGN/Gel exhibited a suitable degradation rate, favourable mechanical properties, adequate MRI contrast enhancement and prolonged KGN release. The multi-parametric MRI could be utilized to evaluate hydrogel scaffold functionalization and degradation rate longitudinally and non-invasively in vivo. The SMNP-KGN/Gel demonstrated better thermal stability, excellent mechanical properties, and detectable MRI contrast enhancement. At the same time, both in vitro as well as in vivo studies revealed that nano KGN prolonged release encouraged BMSCs chondrocyte differentiation and proliferation, which in turn facilitated cartilage repair.167,168 Liu et al developed a functional and convenient hydrogel-based CM-KGN@GelMA, which consists of TGF-β1, imitating CM-10 peptide and KGN encapsulation in liposomes. Since CM-10 was covalently crosslinked with GelMA, it had preserved its bioactivity to control BMSC chondrogenesis persistently to avoid the drawbacks of TGF-β1. A combination of KGN@Lipo and CM-10 stimulated chondrogenic differentiation of BMSCs by upregulating the expression of SOX9 and RUNX-1 in vitro.169,170
Hydrogel exhibits a biocompatible environment that is extremely hydrophilic, facilitating cell growth and differentiation, which can also release KGN in a controlled manner, thus boosting its therapeutic efficiency. Furthermore, the amalgamation of microspheres and hydrogel can simultaneously leverage the benefits of both. Numerous natural hydrogel materials degrade too quickly, hindering the process of prolonged drug release. Moreover, hydrogels generally exhibit low mechanical strength, rendering them inadequate for bearing the stresses of heavily loaded joint sites. Consequently, hydrogels may have more potent drug for early intervention in cartilage injuries or conditions necessitating injectable therapies, particularly treatments that do not demand long-term mechanical support but require sustained drug release. Future advancements in this area should result in joint repair options that are more personalized, efficient, and long-lasting.
Summary
In summary, KGN is unequivocally a pathway for cartilage regeneration. It has shown potential as a small chemical for stem cell-mediated cartilage repair. The biosafety, chondroprotective properties, and capacity to induce chondrogenesis have all been established. Irrespective of its prospective applications, there are scientific challenges and difficulties that need to be resolved. Particular aspects include the exact mechanism of action that needs more investigation. The results of KGN need the enhancement of its long-term stability in water environments. Nonetheless, the brief retention and rapid elimination of KGN are notable limitations for clinical use, limiting its potential for long-term therapeutic advantages. The intra-articular injection of KGN has been found to enhance cartilage regeneration. However, oral administration presents a more straightforward and non-invasive application method that requires further studies, especially when combined with other drugs. Moreover, there is a paucity of research about the actual in vivo drug dose or its metabolites, despite KGN’s use in the development and functionalization of various materials and the investigation of in vitro release profiles. Before proceeding with clinical applications, it is crucial to evaluate the safety and efficacy of KGN and its degradation products. When combined with biomaterials, it has enhanced synergistic actions that modify the physicochemical and surface properties. Over the last decade, the biofunctions and applications of KGN have significantly advanced, enhancing our understanding and augmenting its therapeutic potential. Hydrogels were used for drug delivery and regulated release of medications to enhance retention duration in joints. Conversely, there are contradictory reports on the efficacy of KGN works. It is stated that the chondrogenic induction of KGN is much inferior to that of TGF, indicating its constraints for drug delivery in cartilage regeneration. Despite the potential of biomaterial-based KGN delivery, issues persist in maintaining its stability and bioactivity. In order to enhance the targeting and efficacy of delivery systems, investigation on innovative biomaterials, such as, nanoscale 3D composites and stimuli responsive materials (eg, pH, light, temperature) have become mandatory. The ultimate goal of advance fabrications strategies is to create functionalized carriers that overcome the challenges of conventional treatments. There are various advantages of using different delivery systems for long term therapy which are supported by polymeric microspheres and for short term therapy exosomes provide rapid release for efficient delivery of drugs. Additionally, novel biomaterials must be investigated for the incorporation, transport, and release of KGN.
Abbreviations
4-ABP, 4-aminobiphenyl; ACAN, Aggrecan; ADAMTS5, ADAM metallopeptidase with thrombospondin type 1 motif 5; ALP, Alkaline phosphatase; BMP, Bone morphogenetic protein; BMSC, Bone marrow-derived mesenchymal stem cell; CBFβ, Core binding factorβ; CECM, Chondrocyte extracellular matrix; COLII, collagen II; CPN, Cyclopamine; CSPC, Cartilage derived stem/progenitor cells; DMM, Destabilization of the medial meniscus; ECM, extracellular matrix; EVs, Extracellular vesicles; FTIR, Fourier transform infrared spectroscopy; GAG, Glycosaminoglycans; HA, Hydroxyapatite; HNMR, Hydrogen nuclear magnetic resonance; IA, Intra articular; IGF-1, Insulin-like Growth Factor-1; IHH, Indian hedgehog; KGN, Kartogenin; LC3, Light chain-3; MAPK, Mitogen activated protein kinase; MMP13, Matrix metalloproteinase-13; MSC, Mesenchymal stem cells; NO, Nitric oxide; NP, Nanoparticle; NRF2, Nuclear factor erythroid 2-related factor 2; NSAIDS, Nonsteroidal anti-inflammatory drugs; OA, Osteoarthritis; PA, Phthalic acid; PRP, Platelet-rich plasma; Ptch1, Patched-1; PTSCSs, Patellar tendon stem/progenitor cells; RCCS, Rotary cell culture system; ROS, Reactive oxygen species; RUNX-1, Runt-related transcription factor 1; RUNX-2, Runt-related transcription factor 2; SHH, Sonic Hedgehog; SIRT1, Silent information regulator Type 1; SMNP, Synthetic melanin nanoparticles; Smo, Smoothened; SOX9, SRY-box transcription factor 9; TBJ, Tendon bone junction; TGF-β, Transforming growth factor- β; TSC, Tendon stem cells.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Im GI, Kim TK. Regenerative therapy for osteoarthritis: a perspective. Int J Stem Cells. 2020;13(2):177–181. doi:10.15283/ijsc20069
2. Shao J, Zhu J, Chen Y, et al. Exosomes from Kartogenin‐pretreated infrapatellar fat pad mesenchymal stem cells enhance chondrocyte anabolism and articular cartilage regeneration. Stem Cells Int. 2021;2021(1):6624874. doi:10.1155/2021/6624874
3. Jing H, Zhang X, Gao M, et al. Kartogenin preconditioning commits mesenchymal stem cells to a precartilaginous stage with enhanced chondrogenic potential by modulating JNK and β‐catenin–related pathways. FASEB J. 2019;33(4):5641–5653. doi:10.1096/fj.201802137RRR
4. Swain S, Priyadarshini I, Rautray TR, et al. Processing and Characterization of Materials. In:
5. Clouet J, Vinatier C, Merceron C, et al. From osteoarthritis treatments to future regenerative therapies for cartilage. Drug Discov Today. 2009;14(19–20):913–925. doi:10.1016/j.drudis.2009.07.012
6. Deng J, Zong Z, Su Z, et al. Recent advances in pharmacological intervention of osteoarthritis: a biological aspect. Front Pharmacol. 2021;12:772678. doi:10.3389/fphar.2021.772678
7. Weng C, Xu J, Wang Q, Lu W, Liu Z. Efficacy and safety of duloxetine in osteoarthritis or chronic low back pain: a systematic review and meta-analysis. Osteoarthr Cartil. 2020;28(6):721–734. doi:10.1016/j.joca.2020.03.001
8. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the US bone and joint initiative. In: Seminars in Arthritis and Rheumatism. WB Saunders; 2014. doi:10.1016/j.semarthrit.2013.11.012
9. Johnson K, Zhu S, Tremblay MS, et al. A stem cell–based approach to cartilage repair. Science. 2012;336(6082):717–721. doi:10.1126/science.1215157
10. Wang D, Tan H, Lebaschi AH, et al. Kartogenin enhances collagen organization and mechanical strength of the repaired enthesis in a murine model of rotator cuff repair. Arthrosc J Arthrosc Relat Surg. 2018;34(9):2579–2587. doi:10.1016/j.arthro.2018.04.022
11. Im GI. Application of kartogenin for musculoskeletal regeneration. J Biomed Mater Res A. 2018;106(4):1141–1148. doi:10.1002/jbm.a.36300
12. Jiao D, Wang J, Yu W, Zhang N, Zhang K, Bai Y. Gelatin reduced graphene oxide nanosheets as kartogeninnanocarrier induces rat ADSCs chondrogenic differentiation combining with autophagy modification. Materials. 2021;14(5):1053. doi:10.3390/ma14051053
13. Peng XB, Zhang Y, Wang YQ, He Q, Yu Q. IGF‐1 and BMP‐7 synergistically stimulate articular cartilage repairing in the rabbit knees by improving chondrogenic differentiation of bone‐marrow mesenchymal stem cells. J Cell Biochem. 2019;120(4):5570–5582. doi:10.1002/jcb.27841
14. Józefiak A, Larska M, Pomorska-Mól M, Ruszkowski JJ. The IGF-1 signaling pathway in viral infections. Viruses. 2021;13(8):1488. doi:10.3390/v13081488
15. Qiao K, Xu L, Tang J, et al. The advances in nanomedicine for bone and cartilage repair. J Nanobiotechnology. 2022;20(1):141. doi:10.1186/s12951-022-01342-8
16. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi:10.1016/S0140-6736(11)60243-2
17. Kwon JY, Lee SH, Na HS, et al. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci Rep. 2018;8(1):13832. doi:10.1038/s41598-018-32206-7
18. Wang M, Sampson ER, Jin H, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15:1–11. doi:10.1186/ar4133
19. Sekino Y, Takemoto K, Murata D, et al. CD44 is involved in sunitinib resistance and poor progression-free survival after sunitinib treatment of renal cell carcinoma. Anticancer Res. 2021;41(10):4875–4883. doi:10.21873/anticanres.15301
20. Mishra S, Swain P, Swain S, Kennedy VJ, Rautray TR. Smart Biomaterial Surface. In: Behera A, Nayak D, Swain BK, editors. Surface Engineering of Biomaterials. United States: CRC Press; 2024:284–305.
21. Yuan T, Zhang J, Zhao G, Zhou Y, Zhang CQ, Wang JH. Creating an animal model of tendinopathy by inducing chondrogenic differentiation with kartogenin. PLoS One. 2016;11(2):148557. doi:10.1371/journal.pone.0148557
22. Lefebvre V, Behringer RR, De Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. OARSI. 2001;9(1):S69–75. doi:10.1053/joca.2001.0447
23. Liu H, Liu P. Kartogenin promotes the BMSCs chondrogenic differentiation in osteoarthritis by down-regulation of miR-145-5p targeting Smad4 pathway. Tissue Eng Regen Med. 2021;18:989–1000. doi:10.1007/s13770-021-00390-9
24. Mangaraj S, Swain S, Rautray TR. Smart biomaterials and their applications. In: Behera A, Nayak D, Swain BK, editors. Surface Engineering of Biomaterial. United States: CRC Press; 2024:59–84.
25. Swain S, Mishra S, Patra A, Praharaj R, Rautray T. Dual action of polarised zinc hydroxyapatite-guar gum composite as a next generation bone filler material. Mater Today Proc. 2022;62:6125–6130. doi:10.1016/j.matpr.2022.05.022
26. Liu F, Xu H, Huang H. A novel kartogenin-platelet-rich plasma gel enhances chondrogenesis of bone marrow mesenchymal stem cells in vitro and promotes wounded meniscus healing in vivo. Stem Cell Res Ther. 2019;10:1–2. doi:10.1186/s13287-019-1314-x
27. Lei Y, Xu Z, Ke Q, et al. Strontium hydroxyapatite/chitosan nanohybrid scaffolds with enhanced osteoinductivity for bone tissue engineering. Mater Sci Eng C. 2017;72:134–142. doi:10.1016/j.msec.2016.11.063
28. Li X, Dai B, Guo J, et al. Nanoparticle–cartilage interaction: pathology-based intra-articular drug delivery for osteoarthritis therapy. Nanomicro Lett. 2021;13(1):149. doi:10.1007/s40820-021-00670-y
29. He T, Shaw I, Vedadghavami A, Bajpayee AG. Single-dose intra-cartilage delivery of kartogenin using a cationic multi-arm avidinnanocarrier suppresses cytokine-induced osteoarthritis-related catabolism. Cartilage. 2022;13(2):19476035221093072. doi:10.1177/19476035221093072
30. Kang ML, Kim JE, Ko JY, Im GI. THU0462 intra-articular delivery of Kartogenin-conjugated Chitosan nano/microparticles for cartilage regeneration. Ann Rheum Dis. 2015;74(Suppl 2):367. doi:10.1136/annrheumdis-2015-eular.1119
31. Mangaraj S, Priyadarshini I, Swain S, Rautray TR. ZnO-doped β-TCP ceramic-based scaffold promotes osteogenic and antibacterial activity. Bioinspired Biomimetic Nanobiomater. 2024;13(2):37–44. doi:10.1186/s13287-019-1314-x
32. Cho Y, Jeong S, Kim H, et al. Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp Mol Med. 2021;53(11):1689–1696. doi:10.1038/s12276-021-00710-y
33. Park IK, Cho CS. Stem cell-assisted approaches for cartilage tissue engineering. Int J Stem Cells. 2010;3(2):96–102. doi:10.15283/ijsc.2010.3.2.96
34. Swain S, Ong JL, Narayanan R, Rautray TR. Ti‐9Mn β‐type alloy exhibits better osteogenicity than Ti‐15Mn alloy in vitro. J Biomed Mater Res B Appl Biomater. 2021;109(12):2154–2161. doi:10.1002/jbm.b.34863
35. Assaf Z, Faber K, Hall M. Scope, limitations and classification of lactamases. J Biotech. 2016;235:11–23. doi:10.1016/j.jbiotec.2016.03.050
36. Pei J, Millay DP, Olson EN, Grishin NV. CREST-a large and diverse superfamily of putative transmembrane hydrolases. Biol Direct. 2011;6:1–7. doi:10.1186/1745-6150-6-37
37. Yuan Z, Li H, He S, et al. Kartogenin releasing decellularized umbilical cord Wharton’s jelly scaffold promotes rotator cuff fibrocartilaginous interface regeneration. Mater Des. 2022;218:110710. doi:10.1016/j.matdes.2022.110710
38. Abe K, Yamada M, Terao T, et al. Novel organic nitrate prodrug 4 (R)-N-(2-Nitroxyethyl)-2-oxothiazolidine-4-carboxamide (RS-7897) serves as a xenobiotic substrate for pyroglutamyl aminopeptidase I in dogs. Drug Metab Pharmacokinet. 2003;18(6):373–380. doi:10.2133/dmpk.18.373
39. Huang H, Xu H, Zhao J. A novel approach for meniscal regeneration using kartogenin-treated autologous tendon graft. AJSM. 2017;45(14):3289–3297. doi:10.1177/0363546517721192
40. Westin CB, Trinca RB, Zuliani C, Coimbra IB, Moraes ÂM. Differentiation of dental pulp stem cells into chondrocytes upon culture on porous chitosan-xanthan scaffolds in the presence of kartogenin. Mater Sci Eng C. 2017;80:594–602. doi:10.1016/j.msec.2017.07.005
41. Dai J, Ni L, Jin C, et al. Esterase-responsive Kartogenin composite hydrogel microspheres boost nucleus pulposus regeneration in intervertebral disc degeneration. ActaBiomater. 2025. doi:10.1016/j.actbio.2025.04.001
42. Hu Q, Ding B, Yan X, et al. Polyethylene glycol modified PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomed Nanotechnol Biol Med. 2017;13(7):2189–2198. doi:10.1016/j.nano.2017.05.011
43. Fan W, Li J, Yuan L, et al. Intra-articular injection of kartogenin-conjugated polyurethane nanoparticles attenuates the progression of osteoarthritis. Drug Deliv. 2018;25(1):1004–1012. doi:10.1080/10717544.2018.1461279
44. Zhang S, Hu P, Liu T, et al. Kartogenin hydrolysis product 4-aminobiphenyl distributes to cartilage and mediates cartilage regeneration. Theranostics. 2019;9(24):7108. doi:10.7150/2Fthno.38182
45. Ye L, Cao Z, Tan X, Zhao C, Cao Y, Pan J. Kartogenin potentially protects temporomandibular joints from collagenase-induced osteoarthritis via core binding factor β and runt-related transcription factor 1 binding-A rat model study. J Dent Sci. 2023;18(4):1553–1560. doi:10.1016/j.jds.2023.03.002
46. Marini JC, Forlino A. Replenishing cartilage from endogenous stem cells. N Engl J Med. 2012;366(26):2522–2524. doi:10.1056/NEJMcibr1204283
47. Jing H, Zhang X, Luo K, et al. miR-381-abundant small extracellular vesicles derived from kartogenin-preconditioned mesenchymal stem cells promote chondrogenesis of MSCs by targeting TAOK1. Biomaterials. 2020;231:119682. doi:10.1016/j.biomaterials.2019.119682
48. Fu M, Hu Y, Lan T, Guan KL, Luo T, Luo M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct Target Ther. 2022;7(1):376. doi:10.1038/s41392-022-01191-9
49. Zhang L, Zhang Q, Cui L, Wu L, Gao S. Kartogenin combined platelet-rich plasma (PRP) promoted tendon-bone healing for anterior cruciate ligament (ACL) reconstruction by suppressing inflammatory response via targeting AKT/PI3K/NF-κB. Appl Biochem Biotechnol. 2023;195(2):1284–1296. doi:10.1007/s12010-022-04178-y
50. Kim D, Song J, Kim S, et al. MicroRNA-34a modulates cytoskeletal dynamics through regulating RhoA/Rac1 cross-talk in chondroblasts. J Biol Chem. 2012;287(15):12501–12509. doi:10.1074/jbc.M111.264382
51. Chen H, Tan XN, Hu S, et al. Molecular mechanisms of chondrocyte proliferation and differentiation. Front Cell Dev Biol. 2021;9:664168. doi:10.3389/fcell.2021.664168
52. Manore SG, Doheny DL, Wong GL, Lo HW. IL-6/JAK/STAT3 signaling in breast cancer metastasis: biology and treatment. Front Oncol. 2022;12:866014. doi:10.3389/fcell.2021.664168
53. Decker RS, Koyama E, Enomoto-Iwamoto M, et al. Mouse limb skeletal growth and synovial joint development are coordinately enhanced by Kartogenin. Dev Biol. 2014;395(2):255–267. doi:10.1016/j.ydbio.2014.09.011
54. Matsumoto Y, Otsuka F, Hino J, et al. Bone morphogenetic protein-3b (BMP-3b) inhibits osteoblast differentiation via Smad2/3 pathway by counteracting Smad1/5/8 signaling. Mol Cell Endocrinol. 2012;350(1):78–86. doi:10.1016/j.mce.2011.11.023
55. Jia Z, Wang S, Liang Y, Liu Q. Combination of kartogenin and transforming growth factor-β3 supports synovial fluid-derived mesenchymal stem cell-based cartilage regeneration. Am J Transl Res. 2019;11(4):2056.
56. Swain S, Koduru JR, Rautray TR. Mangiferin-enriched Mn–hydroxyapatite coupled with β-TCP scaffolds simultaneously exhibit osteogenicity and anti-bacterial efficacy. Materials. 2023;16(6):2206. doi:10.3390/ma16062206
57. Wang KX, Xu LL, Rui YF, et al. The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS One. 2015;10(3):e0120593. doi:10.1371/journal.pone.0120593
58. Fazal-Ur-Rehman B, Karen AH, Hongsik C. Kartogenin induced chondrogenesis of stem cells and cartilage repair. Int J Stem Cell Res Ther. 2016;3:36. doi:10.1007/2Fs13770-021-00390-9
59. Liu T, Li X, Wang T, et al. Kartogenin mediates cartilage regeneration by stimulating the IL-6/Stat3-dependent proliferation of cartilage stem/progenitor cells. Biochem Biophys Res Commun. 2020;532(3):385–392. doi:10.1016/j.bbrc.2020.08.059
60. Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–248. doi:10.1038/nrclinonc.2018.8
61. Minina E, Wenzel HM, Kreschel C, et al. BMP and Ihh/PTHrPsignaling interact to coordinate chondrocyte proliferation and differentiation. Development. 2001;22:4523–4534. doi:10.1242/dev.128.22.4523
62. Ok CY, Singh RR, Vega F. Aberrant activation of the hedgehog signaling pathway in malignant hematological neoplasms. Am J Med. 2012;180(1):2–11. doi:10.1016/j.ajpath.2011.09.009
63. Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu–Gli protein complexes. J Cell Biol. 2010;191(2):415–428. doi:10.1083/jcb.201004108
64. Swain S, Padhy RN, Rautray TR. Electrically stimulated hydroxyapatite–barium titanate composites demonstrate immunocompatibility in vitro. J Korean Ceram Soc. 2020;57(5):495–502. doi:10.1007/s43207-020-00048-7
65. Mohan G, Magnitsky S, Melkus G, et al. Kartogenin treatment prevented joint degeneration in a rodent model of osteoarthritis: a pilot study. J Orthop Res. 2016;34(10):1780–1789. doi:10.1002/jor.23197
66. Chen L, Liu G, Li W, Wu X. Synergistic effects of Indian hedgehog and sonic hedgehog on chondrogenesis during cartilage repair. J Mol Histol. 2021;52:407–418. doi:10.1007/s10735-021-09964-2
67. Zhou L, Chen X, Liu T, et al. Melatonin reverses H2O2‐induced premature senescence in mesenchymal stem cells via the SIRT 1‐dependent pathway. J Pineal Res. 2015;59(2):190–205. doi:10.1111/jpi.12250
68. Wang Y, Chen G, Yan J, et al. Upregulation of SIRT1 by kartogenin enhances antioxidant functions and promotes osteogenesis in human mesenchymal stem cells. OxidMed Cell Longevity. 2018;2018(1):1368142. doi:10.1155/2018/1368142
69. Swain S, Rautray TR. Estimation of trace elements, antioxidants, and antibacterial agents of regularly consumed Indian medicinal plants. Biol Trace Elem Res. 2021;199(3):1185–1193. doi:10.1007/s12011-020-02228-2
70. Wang J, Zhou J, Zhang N, Zhang X, Li Q. A heterocyclic molecule kartogenin induces collagen synthesis of human dermal fibroblasts by activating the smad4/smad5 pathway. Biochem Biophys Res Commun. 2014;450(1):568–1574. doi:10.1016/j.bbrc.2014.06.016
71. Shi Y, Shen HM, Gopalakrishnan V, Gordon N. Epigenetic regulation of autophagy beyond the cytoplasm: a review. Front Cell Dev Biol. 2021;9:675599. doi:10.3389/fcell.2021.675599
72. Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J. The role of different SIRT1-mediated signaling pathways in toxic injury. CMBL. 2019;24:1–10. doi:10.1186/s11658-019-0158-9
73. An X, Cao H, Liu S, Cao B. Effects of TG interaction factor 1 on synthesis of estradiol and progesterone in granulosa cells of goats through SMAD2/3-SP1 signaling pathway. Anim Reprod Sci. 2021;229:106750. doi:10.1016/j.anireprosci.2021.106750
74. Swain S, Rautray TR. Silver doped hydroxyapatite coatings by sacrificial anode deposition under magnetic field. Mater Sci Mater Med. 2017;28:1–5. doi:10.1007/s10856-017-5970-z
75. Jakovljevic J, Harrell CR, Fellabaum C, Arsenijevic A, Jovicic N, Volarevic V. Modulation of autophagy as new approach in mesenchymal stem cell-based therapy. Biomed Pharmacother. 2018;104:404–410. doi:10.1016/j.biopha.2018.05.061
76. Swain S, Bowen C, Rautray T. Dual response of osteoblast activity and antibacterial properties of polarized strontium substituted hydroxyapatite—Barium strontium titanate composites with controlled strontium substitution. J Biomed Mater Res A. 2021;109(10):2027–2035. doi:10.1002/jbm.a.37195
77. Nuschke A, Rodrigues M, Stolz DB, Chu CT, Griffith L, Wells A. Human mesenchymal stem cells/multipotent stromal cells consume accumulated autophagosomes early in differentiation. Stem Cell Res Ther. 2014;5:1–4. doi:10.1186/scrt530
78. Xiao L, Xiao Y. The autophagy in osteoimmonology: self-eating, maintenance, and beyond. Front Endocrinol. 2019;10:490. doi:10.3389/fendo.2019.00490
79. Swain S, Pradhan M, Bhuyan S, Misra RD, Rautray TR. On the ion implantation synthesis of Ag-embedded over Sr-substituted hydroxyapatite on a nano-topography patterned Ti for application in acetabular fracture sites. Int J Nanomed. 2024;Volume 19:4515–4531. doi:10.2147/IJN.S464905
80. Yan H, Yu T, Li J, et al. Kartogenin improves osteogenesis of bone marrow mesenchymal stem cells via autophagy. Stem Cells Int. 2022;2022(1):1278921. doi:10.1155/2022/1278921
81. Wang Y, Chen J, Fan W, et al. Stromal cell-derived factor-1α and transforming growth factor-β1 synergistically facilitate migration and chondrogenesis of synovium-derived stem cells through MAPK pathways. Am J Transl Res. 2017;9(5):2656. doi:10.4252/wjsc.v15.i5.490
82. Gao X, Ge J, Li W, Zhou W, Xu L. LncRNA KCNQ1OT1 promotes osteogenic differentiation to relieve osteolysis via Wnt/β-catenin activation. Cell Biosci. 2018;8:1–2. doi:10.1186/s13578-018-0216-4
83. Bhuyan S, Swain S, Rautray TR. Criteria for biomaterial surface selection. In: Behera A, Nayak D, Swain BK, editors. Surface Engineering of Biomaterial. United States: CRC Press; 2024:17–43.
84. Shi D, Xu X, Ye Y, et al. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS nano. 2016;10(1):1292–1299. doi:10.1021/acsnano.5b06663
85. Swain S, Patra A, Kumari S, Praharaj R, Mishra S, Rautray T. Corona poled gelatin-magnesium hydroxyapatite composite demonstrates osteogenicity. Mater Today Proc. 2022;62:6131–6435. doi:10.1016/j.matpr.2022.05.024
86. Hong Y, Liu N, Zhou R, et al. Combination therapy using kartogenin-based chondrogenesis and complex polymer scaffold for cartilage defect regeneration. ACS Appl Bio Mater. 2020;6(11):6276–6284. doi:10.1021/acsbiomaterials.0c00724
87. Pradhan M, Swain S, Rautray TR. Electrically polarized hydroxyapatite with Sr containing barium titanate incorporated with mangiferin for enhanced osteogenic and antibacterial activity. Ferroelectrics. 2024;618(4):1157–1169. doi:10.1080/00150193.2024.2324630
88. Xuan H, Hu H, Geng C, et al. Biofunctionalizedchondrogenic shape-memory ternary scaffolds for efficient cell-free cartilage regeneration. ActaBiomater. 2020;105:97–110. doi:10.1016/j.actbio.2020.01.015
89. Li X, Ding J, Zhang Z, et al. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl Mater Interfaces. 2016;8(8):5148–5159. doi:10.1021/acsami.5b12212
90. Swain S, Misra S, Rautray TR. Osteogenic trace element doped ceramic coating for bioimplant applications. In: Gupta R, Motallebzadeh A, Kakooei S, Nguyen TA, Behera A, editors. Advanced Ceramic Coatings for Biomedical Applications. United States: Elsevier; 2023:293–322.
91. Liu X, Wei Y, Xuan C, et al. A biomimetic biphasic osteochondral scaffold with layer‐specific release of stem cell differentiation inducers for the reconstruction of osteochondral defects. Adv Healthc Mater. 2020;9(23):2000076. doi:10.1002/adhm.202000076
92. Gupta R, Motallebzadeh A, Kakooei S, Nguyen TA, Behera A, editors.. Advanced Ceramic Coatings for Biomedical Applications. United States: Elsevier; 2023:347–368.
93. Zhao Y, Zhao X, Zhang R, et al. Cartilage extracellular matrix scaffold with kartogenin-encapsulated PLGA microspheres for cartilage regeneration. Front Bioeng Biotechnol. 2020;8:600103. doi:10.3389/fbioe.2020.600103
94. Sepahdar A, Nazbar A, Bahadorikhalili S, et al. Cartilage tissue regeneration using kartogenin loaded hybrid scaffold for the chondrogenic of adipose mesenchymal stem cells. J Drug Deliv Sci Technol. 2022;73:103384. doi:10.1016/j.jddst.2022.103384
95. Das S, Swain S, Rautray TR. Incorporation of hydroxyapatite and cerium oxide nanoparticle scaffold as an antibacterial filler matrix for biomedical applications. Int J Artif Organs. 2024;47(5):356–361. doi:10.1177/03913988241234548
96. Somiya M, Kuroda SI. Development of a virus-mimicking nanocarrier for drug delivery systems: the bio-nanocapsule. Adv Drug Deliv Rev. 2015;95:77–89. doi:10.1016/j.addr.2015.10.003
97. Parodi A, Molinaro R, Sushnitha M, et al. Bio-inspired engineering of cell-and virus-like nanoparticles for drug delivery. Biomaterials. 2017;147:155–168. doi:10.1016/j.biomaterials.2017.09.020
98. Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf B Biointerfaces. 2005;44(2 Pt 3):65–73. doi:10.1016/j.colsurfb.2005.06.001
99. Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7(3):248–254. doi:10.1038/nmat2116
100. Swain S, Rautray TR, Narayanan R. Sr, Mg, and Co substituted hydroxyapatite coating on TiO2 nanotubes formed by electrochemical methods. Adv Sci Lett. 2016;22(2):482–487. doi:10.1166/asl.2016.6888
101. Almeida B, Wang Y, Shukla A. Effects of nanoparticle properties on kartogenin delivery and interactions with mesenchymal stem cells. Ann Biomed Eng. 2020;48(7):2090–2102. doi:10.1007/s10439-019-02430-x
102. Kang ML, Ko JY, Kim JE, Im GI. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35(37):9984–9994. doi:10.1016/j.biomaterials.2014.08.042
103. Swain S, Mishra S, Das S, Rautray TR. Magnesium-substituted hydroxyapatite/spider silk fibroin/N-carboxymethyl chitosan based microsphere: a potential template for bone regeneration; 2024. Available from: 10.21203/rs.3.rs-4198253/v1.
104. Vermeij EA, Koenders MI, Bennink MB, et al. The in-vivo use of superparamagnetic iron oxide nanoparticles to detect inflammation elicits a cytokine response but does not aggravate experimental arthritis. PLoS One. 2015;10(5):126687. doi:10.1371/journal.pone.0126687
105. Bajpayee AG, Grodzinsky AJ. Cartilage-targeting drug delivery: can electrostatic interactions help. Nat Rev Rheumatol. 2017;13(3):183–193. doi:10.1038/nrrheum.2016.210
106. Priyadarshini I, Swain S, Rautray TR. Mechanism of ceramic coatings degradation. In: Gupta R, Motallebzadeh A, Kakooei S, Nguyen TA, Behera A, editors. Advanced Ceramic Coatings for Biomedical Applications. United States: Elsevier; 2023:33–45.
107. Kim SR, Ho MJ, Kim SH, et al. Increased localized delivery of piroxicam by cationic nanoparticles after intra-articular injection. Drug Des Devel Ther. 2016;10:3779–3787. doi:10.2147/DDDT.S118145
108. Tripathy N, Swain S, Rautray TR, Karri RR. Material release and toxicity at micro-and nanoscales. In: Behera A, Nayak, Mohapatra RK, Rabaan AA, editors. Smart Micro-and Nanomaterials for Pharmaceutical Applications. United States: CRC Press; 2024:179–205.
109. Fang J, Ye SH, Shankarraman V, Huang Y, Mo X, Wagner WR. Biodegradable poly (ester urethane) urea elastomers with variable amino content for subsequent functionalization with phosphorylcholine. Acta Biomater. 2014;10(11):4639–4649. doi:10.1016/j.actbio.2014.08.008
110. Silva JC, Udangawa RN, Chen J, et al. Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Mater Sci Eng C. 2020;107:110291. doi:10.1016/j.msec.2019.110291
111. Swain S, Rautray TR. Ceramic coatings for dental implant applications. In: Gupta R, Motallebzadeh A, Kakooei S, Nguyen TA, Behera A, editors. Advanced Ceramic Coatings for Biomedical Applications. United States: Elsevier; 2023:249–268.
112. Chen P, Wang S, Huang Z, et al. Multi-functionalized nanofibers with reactive oxygen species scavenging capability and fibrocartilage inductivity for tendon-bone integration. J Mater Sci Technol. 2021;70:91–104. doi:10.1016/j.jmst.2020.09.006
113. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. doi:10.1016/j.scr.2009.12.003
114. Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233. doi:10.1089/scd.2013.0479
115. Bari E, Perteghella S, Catenacci L, et al. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine. 2019;14(6):753–765. doi:10.2217/nnm-2018-0240
116. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteom. 2010;73(10):1907–1920. doi:10.1016/j.jprot.2010.06.006
117. Mishra S, Swain S, Rautray TR. Preparation of eggshell derived HA-BT composite for biomedical applications. Integr Ferroelectr. 2024;240(1):163–174. doi:10.1080/10584587.2023.2296316
118. Xu X, Liang Y, Li X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. doi:10.1016/j.biomaterials.2020.120539
119. Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartil. 2016;24(12):2135–2140. doi:10.1016/j.joca.2016.06.022
120. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. doi:10.1038/s41598-017-15376-8
121. Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi:10.1016/j.biomaterials.2016.04.018
122. Swain S, Patra A, Dwibedy P, Guru BM, Rautray TR. Electrochemical synthesis of ceramics for biomedical applications.In. In: Gupta R, Motallebzadeh A, Kakooei S, Nguyen TA, Behera A, editors. Advanced Ceramic Coatings for Biomedical Applications. United States: Elsevier; 2023:87–110.
123. Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180. doi:10.7150/2Fthno.17133
124. Wu J, Kuang L, Chen C, et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. doi:10.1016/j.biomaterials.2019.03.022
125. Qi H, Liu DP, Xiao DW, Tian DC, Su YW, Jin SF. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. In Vitro Cell Dev Biol Anim. 2019;55:203–210. doi:10.1007/s11626-019-00330-x
126. Chang YH, Wu KC, Harn HJ, Lin SZ, Ding DC. Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transplant. 2018;27(3):349–363. doi:10.1177/0963689717723636
127. Kato T, Miyaki S, Ishitobi H, et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16:1–11. doi:10.1186/ar4679
128. Swain S, Kumari S, Swain P, Rautray T. Polarised strontium hydroxyapatite–xanthan gum composite exhibits osteogenicity in vitro. Mater Today Proc. 2022;62:6143–6147. doi:10.1016/j.matpr.2022.05.026
129. Liu C, Li Y, Yang Z, Zhou Z, Lou Z, Zhang Q. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomedicine. 2020;15(3):273–288. doi:10.2217/nnm-2019-0208
130. Liu C, Ma X, Li T, Zhang Q. Kartogenin, transforming growth factor-β1 and bone morphogenetic protein‐7 coordinately enhance lubricin accumulation in bone‐derived mesenchymal stem cells. Cell Biol Int. 2015;39(9):1026–1035. doi:10.1002/cbin.10476
131. Swain S, Misra RD, Rautray TR. Ceragenin-CSA13 loaded high strength coatings of nano-and micro-SrHA implanted N-Carboxymethyl chitosan–Polyetheretherketone by low temperature high speed collision approach: in vitro pro-osteogenicity and bactericidal activity against MRSA. Mater Chem Phys. 2023;309:128367. doi:10.1016/j.matchemphys.2023.128367
132. Wang J, Langhe D, Ponting M, Wnek GE, Korley LT, Baer E. Manufacturing of polymer continuous nanofibers using a novel co-extrusion and multiplication technique. Polymer. 2014;55(2):673–685. doi:10.1016/j.polymer.2013.12.025
133. Cai JY, Zhang L, Chen J, Chen SY. Kartogenin and its application in regenerative medicine. Curr Med Sci. 2019;39(1):16–20. doi:10.1007/s11596-019-1994-6
134. Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471–490. doi:10.1111/j.1469-7580.2006.00540.x
135. Swain SU, Rautray TR. Cu and Zn substituted hydroxyapatite coatings on titanium oxie anotubes formed by electrochemical methods. Innovare J Eng Technol. 2016;4:7–10.
136. Zhou Y, Zhang J, Yang J, et al. Kartogenin with PRP promotes the formation of fibrocartilage zone in the tendon–bone interface. J Tissue Eng Regen Med. 2017;11(12):3445–3456. doi:10.1002/term.2258
137. Zhang J, Wang JH. Kartogenin induces cartilage-like tissue formation in tendon–bone junction. Bone Res. 2014;2(1):1–10. doi:10.1038/boneres.2014.8
138. Storm EE, Kingsley DM. GDF5 coordinates bone and joint formation during digit development. Dev Biol. 1999;209(1):11–27. doi:10.1006/dbio.1999.9241
139. Holder N. An experimental investigation into the early development of the chick elbow joint. Development. 1977;39(1):115–127. doi:10.1242/dev.39.1.115
140. Swain S, Swain P, Parida SK, Rautray TR. Ceramic scaffolds for biomaterials applications. In: Gupta R, Motallebzadeh A, Kakooei S, Nguyen TA, Behera A, editors. Advanced Ceramic Coatings for Biomedical Applications. United States: Elsevier; 2023:223–248.
141. Cheng R, Liu L, Xiang Y, et al. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials. 2020;232:119706. doi:10.1016/j.biomaterials.2019.119706
142. Swain S, Sahoo S, Rautray TR, You CK. Current Approaches to Smart Drug Delivery. In: Behera A, Nayak D, Swain BK, editors. Smart Micro-and Nanomaterials for Drug Delivery. United States: CRC Press; 2024:18–46.
143. Yang J, Zhu Y, Wang F, Deng L, Xu X, Cui W. Microfluidic liposomes-anchored microgels as extended delivery platform for treatment of osteoarthritis. J Chem Eng. 2020;400:126004. doi:10.1016/j.cej.2020.126004
144. Abbasi E, Aval SF, Akbarzadeh A, et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9:1–10. doi:10.1186/2F1556-276X-9-247
145. Swain S, Kwon TY, Rautray TR. Fabrication of silver doped nano hydroxyapatite-carrageenan hydrogels for articular cartilage applications. bioRxiv. 2021;202. doi:10.1101/2020.12.31.424664
146. Swain S, Lenka R, Rautray T. Synthetic strategy for the production of electrically polarized polyvinylidene fluoride‐trifluoroethylene—co‐polymer osseo‐functionalized with hydroxyapatite scaffold. J Biomed Mater Res A. 2024;112(10):1675–1687. doi:10.1002/jbm.a.37720
147. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6(2):105–121. doi:10.1016/j.jare.2013.07.006
148. Priyadarshini I, Swain S, Koduru JR, Rautray TR. Electrically polarized withaferin and alginate-incorporated biphasic calcium phosphate microspheres exhibit osteogenicity and antibacterial activity in vitro. Molecules. 2022;28(1):86. doi:10.3390/molecules28010086
149. Worthington P, Pochan DJ, Langhans SA. Peptide hydrogels–versatile matrices for 3D cell culture in cancer medicine. Front Oncol. 2015;5:92. doi:10.3389/fonc.2015.00092
150. Zhu Y, Tan J, Zhu H, et al. Development of kartogenin-conjugated chitosan–hyaluronic acid hydrogel for nucleus pulposus regeneration. Biomater Sci. 2017;5(4):784–791. doi:10.1039/C7BM00001D
151. Swain S, Swain P, Mishra S, Rautray T. Processing and characterization of materials. In:
152. Dehghan-Baniani D, Chen Y, Wang D, Bagheri R, Solouk A, Wu H. Injectable in situ forming kartogenin-loaded chitosan hydrogel with tunable rheological properties for cartilage tissue engineering. Colloids Surf B Biointerfaces. 2020;192:111059. doi:10.1016/j.colsurfb.2020.111059
153. Yuan X, Wan J, Yang Y, et al. Thermosensitive hydrogel for cartilage regeneration via synergistic delivery of SDF-1α like polypeptides and kartogenin. Carbohydr Polym. 2023;304:120492. doi:10.1016/j.carbpol.2022.120492
154. Das S, Swain S, Rautray TR, Kennedy JV. Metals for smart drug delivery. In: Behera A, Nayak, Mohapatra RK, Rabaan AA, editors. Smart Micro-and Nanomaterials for Pharmaceutical Applications. United States: CRC Press; 2024:74–95.
155. Dai W, Liu Q, Li S, et al. Functional injectable hydrogel with spatiotemporal sequential release for recruitment of endogenous stem cells and in situ cartilage regeneration. J Mater Chem B. 2023;11(18):4050–4064. doi:10.1039/D3TB00105A
156. Fan W, Yuan L, Li J, et al. Injectable double-crosslinked hydrogels with kartogenin-conjugated polyurethane nano-particles and transforming growth factor β3 for in-situ cartilage regeneration. Mater Sci Eng C. 2020;110:110705. doi:10.1016/j.msec.2020.110705
157. Houreh AB, Masaeli E, Nasr-Esfahani MH. Chitosan/polycaprolactone multilayer hydrogel: a sustained Kartogenin delivery model for cartilage regeneration. Int J Biol Macromol. 2021;177:589–600. doi:10.1016/j.ijbiomac.2021.02.122
158. Satpathy A, Mohanty R, Rautray TR. Bio‐mimicked g uided tissue regeneration/guided bone regeneration membranes with hierarchical structured surfaces replicated from teak leaf exhibits enhanced bioactivity. J Biomed Mater Res B Appl Biomater. 2022;110(1):144–356. doi:10.1002/jbm.b.34898
159. Zhang Y, Yin W, Liu Y, et al. Dynamic protein hydrogel with supramolecularly enveloped kartogenin promotes cartilage regeneration through mitochondrial activation. Compos B Eng. 2022;246:110257. doi:10.1016/j.compositesb.2022.110257
160. Mohsenifard S, Mashayekhan S, Safari H. A hybrid cartilage extracellular matrix-based hydrogel/poly (ε-caprolactone) scaffold incorporated with Kartogenin for cartilage tissue engineering. J Biomater Appl. 2023;37(7):1243–1258. doi:10.1177/08853282221132987
161. Massaro M, Buscemi G, Arista L, et al. Multifunctional carrier based on halloysite/laponite hybrid hydrogel for kartogenin delivery. ACS Med Chem Lett. 2018;10(4):419–424. doi:10.1021/acsmedchemlett.8b00465
162. Yuan FZ, Wang HF, Guan J, et al. Fabrication of injectable chitosan-chondroitin sulfate hydrogel embedding kartogenin-loaded microspheres as an ultrasound-triggered drug delivery system for cartilage tissue engineering. Pharmaceutics. 2021;13(9):1487. doi:10.3390/pharmaceutics13091487
163. Swain S, Rautray TR, You CK. Swellable Polymers for Drug Delivery Applications.In: behera. In:: Nayak A, Mohapatra RK, Rabaan AA, editors. Smart Micro-and Nanomaterials for Pharmaceutical Applications. United States: CRC Press; 2024:115–131.
164. Zare P, Pezeshki-Modaress M, Davachi SM, et al. Alginate sulfate-based hydrogel/nanofiber composite scaffold with controlled Kartogenin delivery for tissue engineering. Carbohydr Polym. 2021;266:118123. doi:10.1016/j.carbpol.2021.118123
165. Swain S, Misra RD, You CK, Rautray TR. TiO2 nanotubes synthesised on Ti-6Al-4V ELI exhibits enhanced osteogenic activity: a potential next-generation material to be used as medical implants. Mater Tech. 2021;36(7):393–399. doi:10.1080/10667857.2020.1760510
166. Kang ML, Jeong SY, Im GI. Hyaluronic acid hydrogel functionalized with self-assembled micelles of amphiphilic PEGylated kartogenin for the treatment of osteoarthritis. Tissue Eng Part A. 2017;23(13 Pt 14):630–699. doi:10.1089/ten.tea.2016.0524
167. Chen C, Huang S, Chen Z, et al. Kartogenin (KGN)/synthetic melanin nanoparticles (SMNP) loaded theranostic hydrogel scaffold system for multiparametric magnetic resonance imaging guided cartilage regeneration. Bioeng Transl Med. 2023;8(1):10364. doi:10.1002/btm2.10364
168. Behera DR, Nayak P, Rautray TR. Phosphatidylethanolamine impregnated Zn-HA coated on titanium for enhanced bone growth with antibacterial properties. J King Saud Univ, Sci. 2020;32(1):848–852. doi:10.1016/j.jksus.2019.03.004
169. Liu G, Guo Q, Liu C, et al. Cytomodulin-10 modified GelMA hydrogel with kartogenin for in-situ osteochondral regeneration. Acta Biomater. 2023;169:317–333. doi:10.1016/j.actbio.2023.08.013
170. Lee KW, Bae CM, Jung JY, et al. Surface characteristics and biological studies of hydroxyapatite coating by a new method. J Biomed Mater Res B Appl Biomater. 2011;98(2):395–407. doi:10.1002/jbm.b.31864
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Current and Novel Therapeutics for Articular Cartilage Repair and Regeneration
Cong B, Sun T, Zhao Y, Chen M
Therapeutics and Clinical Risk Management 2023, 19:485-502
Published Date: 20 June 2023
Network Analysis of Osteoarthritis Progression Using a Steiner Minimal Tree Algorithm
Xie Y, Shao F, Ji Y, Feng D, Wang L, Huang Z, Wu S, Sun F, Jiang H, Miyamoto A, Wang H, Zhang C
Journal of Inflammation Research 2024, 17:3201-3209
Published Date: 18 May 2024

