Back to Journals » International Journal of Nanomedicine » Volume 19
Nanomedicines Targeting Ferroptosis to Treat Stress-Related Diseases
Authors Kang H, Meng F, Liu F, Xie M, Lai H, Li P, Zhang X
Received 30 May 2024
Accepted for publication 3 August 2024
Published 12 August 2024 Volume 2024:19 Pages 8189—8210
DOI https://doi.org/10.2147/IJN.S476948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Farooq A. Shiekh
Hao Kang,1,2,* Fansu Meng,3,* Fengjie Liu,4 Mengjie Xie,4 Haibiao Lai,3 Pengfei Li,5 Xingwang Zhang4
1Department of Medicinal Chemistry and Pharmaceutical Analysis, Anhui College of Traditional Chinese Medicine, Wuhu, People’s Republic of China; 2Wuhu Modern Technology Research and Development Center of Chinese Medicine and Functional Food, Wuhu, People’s Republic of China; 3Zhongshan Hospital of Traditional Chinese Medicine Affiliated to Guangzhou University of Traditional Chinese Medicine, Zhongshan, People’s Republic of China; 4Department of Pharmaceutics, College of Pharmacy, Jinan University, Guangzhou, People’s Republic of China; 5Department of Oncology, Integrated Hospital of Traditional Chinese Medicine, Southern Medical University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haibiao Lai; Xingwang Zhang, Email [email protected]; [email protected]
Abstract: Ferroptosis, a unique form of regulated cell death driven by iron-dependent lethal lipid peroxidation, is implicated in various stress-related diseases like neurodegeneration, vasculopathy, and metabolic disturbance. Stress-related diseases encompass widespread medical disorders that are influenced or exacerbated by stress. These stressors can manifest in various organ or tissue systems and have significant implications for human overall health. Understanding ferroptosis in these diseases offers insights for therapeutic strategies targeting relevant pathways. This review explores ferroptosis mechanisms, its role in pathophysiology, its connection to stress-related diseases, and the potential of ferroptosis-targeted nanomedicines in treating conditions. This monograph also delves into the engineering of ferroptosis-targeted nanomedicines for tackling stress-related diseases, including cancer, cardia-cerebrovascular, neurodegenerative, metabolic and inflammatory diseases. Anyhow, nanotherapy targeting ferroptosis holds promise by both promoting and suppressing ferroptosis for managing stress-related diseases.
Keywords: ferroptosis, stress-related diseases, lipid peroxidation, nanoparticles, nanomedicine, targeted therapy
Introduction
Cell death is crucial for maintaining body development and metabolism balance. It occurs in two forms: accidental cell death (ACD) and regulatory cell death (RCD). ACD is a biologically uncontrolled process, whereas RCD involves strictly structured signaling cascades and molecularly definitive effector mechanisms.1 Ferroptosis, a newfound modality of RCD featured by iron-dependent lipid peroxidation, has emerged as a crucial player in the pathophysiology of various stress-related diseases. Its association with oxidative damage and cell death makes it a potential target for therapeutic interventions in conditions marked by cellular stress.2
Stress-related diseases are medical conditions that are either caused or exacerbated by chronic or excessive stress.3 Stress, particularly chronic and oxidative stresses, can have a profound impact on the body’s physiological processes, leading to a range of health problems. Many diseases are initially caused by stress and are aggravated with the haunting of stress, such as atherosclerosis, senile dementia, and diabetes. It has been shown that there exists a significant crosstalk between ferroptosis and stress. Ferroptosis can be induced or triggered by various stressors through multiple mechanisms.4 The dysregulation of ferroptosis pathways can exacerbate cellular damage and death, contributing to the progression of stressful disorders. In these conditions, the imbalance between oxidative stress and the body’s antioxidant defense mechanisms can trigger ferroptosis, putting ferroptosis at the crossroad of stress-related diseases. Targeting ferroptosis pathways may offer new therapeutic opportunities for managing stress-related diseases by modulating key regulators of ferroptosis, such as glutathione peroxidase 4 (GPX4) and iron metabolism.5–10 In addition, targeting ferroptosis in various diseases holds significant therapeutic implications due to its prominent role in cell death and disease onset. For instance, inducing ferroptosis in cancer can sensitize cancer cells to necrosis and inhibit tumor growth, especially for chemotherapy-resistant tumor cells.11 Suppression of ferroptosis can mitigate neuronal damage and slow the progression of neurodegenerative diseases.12 Otherwise, it may be possible to eliminate pathogens and reduce infection-associated stress by inducing ferroptosis in bacteria or viruses.13
Nanotechnology represents an emerging branch of science for designing nanomaterials with a nanoscale size and specific function at various microcosmic levels.14 The manipulation of enabling nanotechnology into biomedical applications is best known as nanomedicine. Nanomedicine can address big medical problems in small ways.15,16 Ferroptosis therapy is a relatively new remedial action, and there are several challenges such as insufficient pharmacologic activity and side effects caused by off-target that need to be overcome before access to the clinic.17 Ferroptosis-targeted nanomedicine can effectively dissolve this dilemma. Likewise, nanomedicines can be engineered to target specific cells or tissues affected by stress-related diseases. Functionalized nanoparticles (NPs) with ligands, antibodies or biomimetic membranes allow for targeted delivery of ferroptosis modulators by recognizing specific markers on diseased cells. They not only protect the therapeutic agents from elimination, but also enhance their stability and bioavailability.18 Nanomedicines can target ferroptosis pathways in stress-related diseases through rational engineering, leading to expectant and efficient therapeutic interventions for these diseases.
Nanomedicine and nanotechnology bring about an opportunity to fight against stress-related diseases through ferroptosis regulation. Exploring ferroptosis in stress-related diseases offers new perspectives on treatment approaches. This review examines the importance of ferroptosis in stress-related diseases, the spectrum of stress-related diseases, the engineering philosophy of ferroptosis-targeted nanomedicines, and the potential of nanotherapy in this context. It is supposed to bring inspiration for the treatment of stress-related diseases through ferroptosis.
What Do We Know About Ferroptosis and It Role in Diseases?
Iron is an essential trace element for the human body that is involved in the formation of many proteins, including hemoglobin, myoglobin, and ferritin, as well as various redox reactions in cells.19 Under normal physiological conditions, the complex and precise mechanisms of iron homeostasis ensure that intracellular iron concentrations remain stable and prevent intracellular iron overload from triggering iron toxicity. Ferroptosis, a newly defined form of cell death, is a non-apoptotic RCD driven by iron-dependent lethal lipid peroxidation.20 This unique programmed cell death is regulated by multiple cellular metabolic pathways (including redox homeostasis, iron metabolism, mitochondrial activity, and the metabolism of amino acids, lipids, and saccharides) as well as a variety of disease-related signaling pathways.21 The immanent cause of ferroptosis is the disequilibrium of iron metabolism that results in depletion of intracellular antioxidants, hence the accumulation of lipid peroxides, leading to cell death due to membrane damage. Mechanistically, ferroptosis is caused by various stresses on the cells that incurs iron overload (manifested by increased ferritinophagy) followed by lipid peroxidation with the involvement of reactive oxygen species (ROS), which causes alterations in the structure and function of the cell membrane and the onset of cell death.4,22
Unlike classical apoptosis, there is no cellular shrinkage and chromatin condensation during ferroptosis, but there is mitochondrial wrinkling (decreased cristae and membrane density) and increased lipid peroxides (elevated MDA and 4-hydroxynonenal).23 Common inhibitors for apoptosis, autophagy, and pyroptosis cannot affect ferroptosis, but ferric ion chelators can, indicating that ferroptosis is a ferric ion-dependent biological process. Initially, ferroptosis was believed to be a new mode of cell death, but recent studies reveal that ferroptosis exists in a shared pathway with other programmed cell deaths.24 Perhaps multiple modes of cell death occur simultaneously, or one of these modes is dominant in some diseases. Ferroptosis, as a fantastic mode of cell death, is closely related to cellular metabolism and stress, including disturbed metabolisms of iron, selenium, amino acids, and lipids as well as aberrant redox.
The occurrence of ferroptosis depends on the outcome of the confrontation between the execution system and defense system for ferroptosis (Figure 1).25 Ferroptosis execution refers to lipid peroxidation in the plasma membrane, mitochondria, endoplasmic reticulum, and lysosomes that results in Fe-dependent cell death, in which acyl-CoA synthetase long-chain family member 4 (ACSL4) and Fe2+-mediated Fenton reaction contribute to lipid peroxidation.26 Polyunsaturated fatty acid (PUFA)-containing phospholipids constitute the major source of lipid peroxidation. ACSL4 can facilitate the incorporation of PUFA into membrane phospholipids, thus playing a role in promoting ferroptosis. Fenton reaction is a process that Fe2+ catalyze the production of toxic hydroxyl radical (·OH) from hydrogen peroxide. The resultant -OH triggers lipid peroxidation of cellular membrane. The defense system of ferroptosis consists of GPX4 and ferroptosis suppressor protein 1 (FSP1).27 GPX4 is able to utilize glutathione (GSH) to protect cells from ferroptosis by reducing toxic lipid peroxides into non-toxic lipid alcohols. GPX4 system represents the most important defense mechanism against ferroptosis in cells. FSP1 is a NADPH-ubiquinone reductase that can abrogate lipid peroxidation by reducing COQ10 and Vitamin K whereby to inhibit ferroptosis.
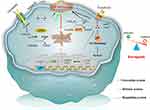 |
Figure 1 Schematic of ferroptosis. Ferroptosis is the result of a combined confrontation between execution and defense of the body’s redox systems, regulated by multiple signaling pathways. |
With the advances in ferroptosis research, it is beginning to be recognized that the regulatory network of ferroptosis plays a broad role in various physiopathologic processes.28 Ferroptosis can be activated under acute or chronic pathologic conditions. Accumulative findings suggest that ferroptosis is strongly associated with stress-related diseases, including metabolic diseases, neurodegenerative diseases, cardiovascular diseases, and carcinoma. Iron is the most abundant trace element in the human body, which plays a crucial role in various physiological functions, such as oxygen transport, energy production, and oxidative metabolism. Ferroptosis stands out from a variety of therapeutic targets in stress-related diseases because it involves fundamental regulation of iron metabolism and oxidative stress pathways. Conventional formulations are associated with a risk of off-target, though nanomedicine can circumvent this problem subtly by virtue of designable targetability. Actually, the progression of some diseases can be interfered with by activating or inhibiting ferroptosis.
Spectrum of Stress-Related Diseases
Stress-related diseases are health conditions that are influenced or exacerbated by chronic or excessive stress.29 Stress refers to any physical or emotional strain or tension on the body, which can be caused by a variety of factors, including work, relationships, finances, and health problems. When the body is under stress, it responds by releasing hormones such as cortisol and adrenaline,30 which can have both positive and negative effects on the body. Short-term physiological responses help the body manage stress, but chronic or excessive stress can lead to health issues. In a variety of stresses, oxidative stress,31 an imbalance between the production of ROS and the body’s ability to neutralize them with antioxidants, has been linked to a wide range of diseases due to its ability to cause damage to cells, proteins, and DNA. As the body’s anti-oxidative defense mechanisms become overwhelmed and unable to neutralize the ROS produced by stress, our body will undergo pathophysiological changes, resulting in the genesis of stress-related diseases. These diseases can affect various systems of the body, including the cardiovascular, digestive, metabolic, immune, endocrine, nervous, muscular, and dermal systems (Figure 2).
There is a strong relationship between cardiovascular diseases and stress.32 In response to stress, our body releases a hormone called cortisol. This hormone can cause an increase in blood pressure and heart rate, putting additional strain on the heart. Over time, this can lead to the development of conditions such as hypertension, atherosclerosis, and coronary artery disease. In addition, chronic stress can trigger inflammation in the body,33 which is an additional risk factor for heart disease. Inflammatory reaction can damage the lining of blood vessels, resulting in the buildup of plaque and increasing the risk of heart attack and stroke. It exists a well-established relationship between inflammatory diseases and stress.34 Upon stress, the body releases stress hormones that impact the immune system and inflammatory response. Chronic stress inflicts our bodies in a state of low-grade inflammation.35 This persistent inflammation has been linked to a variety of inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, asthma, and even conditions like psoriasis. Likewise, the released hormones can affect the function of immune cells, breaking the balance in the immune response and increasing the susceptibility to inflammatory conditions. Besides, chronic stress can predispose to the aggravation of inflammatory conditions,36 leading to flare-ups and worsening symptoms. In turn, chronic inflammation amplifies the risk of developing other health problems, including diabetes and cancer. Some research suggests that stress conduces to the development and progression of some types of cancer.37 For example, stress hormones such as cortisol and adrenaline can promote the growth and spread of certain cancer cells.38 Chronic stress can also weaken or disrupt the immune system, which cause deficiency in immunological surveillance, prompting tumor progression and the occurrence of immune diseases.39,40 Chronic stress has also been proved to be associated with neurodegenerative diseases like Alzheimer’s and Parkinson’s diseases. Prolonged exposure to high levels of stress has detrimental effects on the brain health, including impairing memory and cognitive function, and even contributing to the loss of neurons in certain brain regions.41,42 The inflammation developing from chronic stress has been implicated in the genesis and progression of neurodegenerative diseases. Inflammatory responses increase the accumulation of abnormal proteins such as β-amyloid and α-synuclein in neurocytes.43 Otherwise, stress has been implicated in the onset of systemic lupus erythematosus (SLE) and aging.
Understanding the pathophysiological effects of stress on the body is important for developing strategies to manage and mitigate the negative impact of chronic stress on overall health and well-being. Stress can affect individuals differently, with disease severity influenced by factors like genetics, lifestyle, and environment. Many stress-related diseases may have overlapping symptoms with other disease conditions. Nevertheless, accumulative evidence manifests that most diseases originate from chronic stress, followed by inflammation, then lesion, and finally malignancy.
Crosstalk Between Ferroptosis and Stress-Related Diseases
Ferroptosis is defined as a programmed cell death with overladen accumulation of lipid peroxides, which leads to membrane oxidative damage and cell death. Ferroptosis is suggested to significantly impact stress-related diseases through lipid peroxidation in the cell membrane, a key factor in cellular stress.4,22,44 Among the factors of causing cellular stress, lipid oxidation modifications in the lipid bilayer, especially lipid peroxidation, have become a significant regulator in dictating the cell fate. Lipid peroxidation is subject to the modulation of redox homeostasis, which is ulteriorly modified by ROS and iron metabolism.45 Oxidative stress signifies high levels of ROS that likely result in the occurrence of ferroptosis. Oxidative stress and ferroptosis exist crosstalk in a great many of human diseases,46 including malignant tumors and vasculopathy. Ferroptosis is essentially involved in the regulation of oxidative stress and inflammatory responses. As known, these cellular pathophysiological processes are implicated in a variety of stress-related diseases.
Most organ damage and degenerative lesions are caused by ferroptosis.47 Drug-resistant tumor cells, especially those in a mesenchymal state and prone to metastasis, are very susceptible to ferroptosis.48,49 Iron, lipids, ROS, and cysteine in ferroptosis imply a significant connection between cell death, cellular metabolism, and stress. One study found that pro-ferroptotic signaling could accelerate arterial aging via vascular smooth muscle cell senescence, a condition that can be caused by oxidative stress.50 It was concluded that the activation of pro-ferroptotic signaling correlated with arterial stiffness in a human study. Another study revealed that ferroptosis was involved in the development of Alzheimer’s disease.51 Mechanistic investigation indicated that NOX4 promoted ferroptosis of astrocytes as a result of oxidative stress-induced lipid peroxidation via mitochondrial metabolic impairment. A parallel study also showed that the loss of ferroportin could worsen memory in Alzheimer’s by promoting ferroptosis.52 Activation of ferroptosis can contribute to diseases, while its inhibition can also trigger disease development. Tumorigenesis is a paradigm for the restriction of ferroptosis during cell proliferation. In some cases, cancer cells can develop resistance to ferroptosis, allowing them to evade cell death and promote tumor growth and survival.53 Tumor cells can also upregulate antioxidant pathways to counteract the effects of ferroptosis, enabling them to proliferate and resist ferroptotic cell death.54 As discussed previously, tumors can be deem as a chronic stressful disease in addition to genetic and chemical factors. Accordingly, it can be argued that ferroptosis is inextricably linked with the occurrence and development of tumor. Recent studies have shown that ferroptosis may be implicated in the development of other stress-related diseases, including diabetes,55 ischemia-reperfusion injury,56 fibrosis,57 and SLE.58 However, more research is required to fully understand the relationship between ferroptosis and these diseases, as well as the potential therapeutic implications of targeting ferroptosis.
Engineering of Ferroptosis-Targeted Nanomedicines
There is no doubt that ferroptosis is associated with the development of many diseases. However, ferroptosis plays a double-edged sword role in the progression of diseases.59 In terms of cancer, ferroptosis inhibition promotes unchecked growth of abnormal cells. In this case, activation of ferroptosis is beneficial for managing cancer, especially for metastasis-prone or highly-mutated cancerous cells that are sensitive to ferroptosis. On the other hand, most stress-related diseases highlight inhibition of ferroptosis to halt the disease progression, since ferroptosis mediates or participates in the evolution of these diseases. Thus, whether ferroptosis is induced or inhibited depends on the type of disease and the role of ferroptosis plays in the disease. Anyway, the medication intended for targeting ferroptosis has shown great promise in addressing refractory diseases.
Physiologically, ferroptosis can be modulated by the use of various chemical agents, natural active ingredients, nanoparticles, or signaling molecules.60 The implementation of ferroptotic therapy hinges on the intervention to the execution system, defense system, and regulating system of ferroptosis. Yet, the challenge lies in the ineffective delivery of many regulators to their targets due to undesirable biopharmaceutical properties. Thus, nanomedicine design becomes crucial for precise ferroptotic therapy, leveraging various pathways and compounds. Ferroptosis-targeting nanomedicines can be designed by utilizing various pathways with different medications or molecules. Ferroptosis can be upregulated by inducers (eg, erastin, RSL5 and sorafenib) that act on the execution system or inhibitors (eg, ferrostatin, deferiprone and liproxstatin) that target the defense system through promoting or blocking lipid peroxidation. Likewise, ferroptosis suppression can be implemented to target both the execution and defense systems. The regulating system of ferroptosis consisting of various signaling pathways is also the therapeutic target. Tables 1 and 2 generalizes the commonly used ferroptosis inducers and inhibitors as well as their action mechanisms.
 |
Table 1 Representative Inducers of Ferroptosis |
 |
Table 2 Representative Inhibitors of Ferroptosis |
Despite the therapeutic activities, the daunting challenge faced by ferroptotic therapy is the targeting efficiency of ferroptotic medicaments. Many agents demonstrate good regulatory effects on ferroptosis in vitro, but ideal therapeutic outcomes are not frequently gained in vivo, resulting in requirements for the design of targeted delivery systems. Generally, there are three design philosophies available to optimize ferroptotic therapy: i) developing antibody conjugated NPs (ACNPs) to deliver ferroptotic agents; ii) employing nanocarriers to deliver ferroptotic agents; and iii) biomimicking ferroptotic NPs with biomembrane (Figure 3). For small molecule compounds, targeting modification can be achieved by encapsulating them into NPs followed by conjugation with an antibody of ligand.18,99 For pure NPs with a ferroptotic activity, they can be camouflaged with autologous biomembrane to improve their targetability.100 Rational targeting design can capacitate the synergy and attenuation of therapeutic agents, insuring a success of ferroptotic therapy.
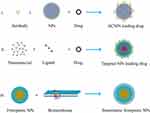 |
Figure 3 Design strategies of ferroptosis-targeted nanomedicines. |
Nanotherapy via Promoting Ferroptosis
Cancer
The approach to sensitizing ferroptosis is most frequently utilized to treat cancer. Ferroptosis often dysregulates in human cancer. Cancer cells have developed mechanisms to evade ferroptosis,101 contributing to treatment resistance and tumor survival. Meanwhile, chemotherapy-resistant and metastasis-susceptible cancer cells show increased sensitivity to ferroptosis.102 Hence, targeting the regulatory network of ferroptosis in cancer can bring about new therapeutic opportunities. However, ferroptosis induction, while inhibiting tumor cells, can also affect the function and activity of immune cells situated in the tumor microenvironment, including T cells, macrophages, neutrophils, and B cells.103 The presence of multiple enzymes that regulate ferroptosis also enables the development of ferroptosis-targeted therapies. Cancer treatment based on ferroptosis induction are being actively sought. There have been attempts to use non-targeted NPs to deliver iron, peroxides, and other cytotoxic substances to kill tumor cells.104
The most prominent target of ferroptosis is GPX4, which is expressed in most cancer cells and is essential for their survival. Due to GPX4’s atypical structure without conventional binding sites, current inhibitors can alter selenocysteine residues in GPX4 and other selenoproteins, raising concerns about their specificity and toxicity.21 GPX4 is vital for a variety of peripheral tissues (renal tubular cells), so targeting GPX4 may have side effects unless therapies are intended for the tumor target. Unlike targeting GPX4, the knockout of SLC7A11 gene does not cause major pathological changes and affect the expression of SLC3A2 and/or SLC7A11, so the strategy to block cysteine uptake by inhibiting the system Xc− keeps great promise. Tumor is more sensitive to the inhibition of system Xc− than normal tissues because of its metabolic reprogramming and evolutional survival mechanism.105 To prevent excessive peroxidation, tumor cells become more dependent on the detoxification function of system Xc− to withstand oxidative stress. Similar to SLC7A11 subduction, the knockout of FSP1 does not impact the embryo development and cause any overt phenotype in terms of animal experiments,106 presenting a broad therapeutic window for targeting FSP1. FSP1, highly expressed in many cancer cell lines, is the top gene linked with GPX4 inhibitor resistance.106 Therefore, FSP1 inhibitors are ideal for treating drug-resistant or differentiated tumors. Other targets toward the execution, defense and regulating systems of ferroptosis can also be opted for the design of ferroptosis-targeted therapy.
Efforts to enhance cancer treatment by boosting ferroptosis have led to the ingenious design of vehicles for targeted delivery of ferroptosis inducers to tumors,107 even enabling combination therapy. For instance, living bacteria and cancer cell membranes-coated Fe3O4 NPs were developed for improving targeted delivery of NPs and the payload sulfasalazine (a ferroptosis inducer) into the tumor tissue in order for synergistic ferroptosis and immunotherapy.108 Enhanced ferroptosis induced by both Fe3O4 NPs and the loaded sulfasalazine effectively suppressed tumor growth and produced immune response through immunogenic cell death (ICD). A nanomedicine composed of pure sorafenib, Ce6 and Fe3+ significantly improved the photodynamic therapy of oral cancer via promoting ferroptosis.109 The carrier-free NPs loading sorafenib, a ferroptosis inducer, and Ce6, a photosensitizer, markedly elevated intracellular ROS and O2 that induced phototoxicity through the ferroptotic pathway. Ding et al110 constructed pyropheophorbide-a (ppa)-loaded mesoporous Fe3O4 NPs with PDA coating and biotin decoration for jointly targeting ferroptosis and apoptosis. The NPs increased ferroptosis sensitivity by depleting GSH and generating hydroxyl radical (OH). In addition, the nanomedicine overcame hypoxia of SDT and promoted ·OH generation through a SOD-like action, thus synergistically improving ferroptosis and apoptosis of tumor cells. Likewise, a diselenide-containing polymer was synthesized to promote tumor ferroptosis by inactivating GPX4 via GSH depletion in the form of self-assembled micelles with a targeting peptide.111 The nanoscale micelles can pertinently bind to the active site cysteine of GSH, resulting in a thorough depletion of GSH and inducing ferroptosis. The antitumor effects of NPs were confirmed in vitro and in vivo in the BxPC-3 tumor model. In another study, targeting GPX4 to induce ferroptosis was engineered to actualize an immunogenic translation from “cold” to “hot” of prostate cancer.112 The excellent work developed a transformable self-assembled peptide TEP-FFG-CRApY with alkaline phosphatase (ALP) responsiveness and GPX4) protein targeting. The self-assembled NPs from bioactive peptide were transformed into nanofibers in response to ALP in the endosome and lysosomes after uptake. The in situ formed nanofibers targeted GPX4 and selectively degrade its protein under the light irradiation, thereby inducing cascade ferroptosis. The leaked Fe2+ and intracellular Fenton reaction enhanced the ferroptotic effect. Nanoparticles induced immunogenic ferroptosis, leading to increased maturation of dendritic cells and T-cell infiltration in tumors, which stimulated IFN-γ secretion to promote ferroptosis (Figure 4).
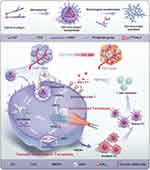 |
Figure 4 Schematic diagram of self-assembly, morphological transformation, and induction of cascade ferroptosis with TEP-FFG-CRApY in prostate cancer. Reproduced from Wang H, Jiao D, Feng D, et al. Transformable supramolecular self-assembled peptides for cascade self-enhanced ferroptosis primed cancer immunotherapy. Adv Mater. 2024;36(21):e2311733, © 2024 Wiley-VCH GmbH.112 |
Ferroptosis induction based on iron overload has also been explored to fight against cancer. Feng et al113 developed iron-based NPs as a nanocatalytic medicine to target the niches of cancer stem cells (CSC) and trigger ferroptosis. The NPs effectively accumulated in the microlesions of lung cancer through dextran-mediated TAM targeting via nebulization administration and resulted in a rise in iron level surrounding CSC niches and damage of redox homeostasis, thus enhancing the sensitivity of CSCs to ferroptosis. Carrier-free nanomedicines have also been developed to treat cancer through the ferroptotic pathway. Zhu et al114 engineered ferroptotic nanomedicines with aurantiamide acetate (Aa), scutebarbatine A (SA), and palmitin (P) as self-assembly materials and evaluated their promoting effects on apoptosis and ferroptosis in breast cancer. Intriguingly, the components Aa and P in nanomedicine induced significant mitochondrial apoptosis of cancer cells, while SA and P inhibited triple-negative breast cancer (TNBC) by ferroptosis and upregulating p53. Recently, nanomedicines or NPs pinpointing other targets of ferroptosis have also been reported to ameliorate cancer therapy. For example, Cao et al115 constructed small activating RNA (saRNA) tailored to ALOX15 and loaded it into mesoporous PDA NPs followed by biomimetic modification with macrophage membranes. The resulting hybrid NPs induced ferroptosis in glioblastoma by promoting mitochondrial damage through mediating the expression of ALOX15. Additionally, Tang’s group designed a brain-targeting NIR-II ferroptosis nanosystem to enable visualization and oncotherapy for orthotopic glioblastoma.116 Au(I)-based NIR-II ferroptotic nanoparticles (termed TBTP-Au NPs) released TBTP-Au in the brain that specifically activated HMOX1-regulated ferroptosis of glioma cells, hence greatly prolonging the survival period of glioma-bearing mice. In Fan et al’s study,117 MMP-2-sensitive NPs loaded with bispecific antibodies (biABs) were developed for IFN-induced ferroptosis using a dimer of epigallocatechin-3-gallate (EGCG) with hyaluronic acid as a ligand. The self-assembled NPs loaded with B7-H3×CD3 biAbs displayed uplifted intracranial accumulation and GBM elimination by boosting ferroptosis and enhancing immune checkpoint blockade. The most prevailing paradigm of ferroptosis induction is programmed for various cancers, and these excellent examples have shed light on negotiating the bottleneck of tumor therapy.
Senescence
The resistance of senescent cells to ferroptosis has been found to be linked with poor healing of chronic wounds.118 Senescent cells are cells that have stopped dividing and entered a state of irreversible cell cycle arrest in response to various stressors, such as DNA damage or oxidative stress.119 Targeting senescent cells with senolytic drugs, which selectively eliminate senescent cells, is proposed as a potential therapeutic strategy for chronic wound healing. As an example, Wei et al120 developed a Fe3O4 nanosystem (F@GP) with galactose-modified poly (lactic-co-glycolic acid) (PLGA) to induce ferroptosis via the Fenton reaction in the presence of elevated intracellular H2O2 levels. After administration, F@GP worked as a chemodynamic therapy that eliminated senescent cells by increasing their sensitivity to ferroptosis, thus deadening cellular senescence, stimulating cell proliferation, promoting re-epithelialization, and accelerating the healing of diabetic wounds in mice. Further, ferroptosis induction can be explored for treating other diseases featured with ferroptotic resistance, such as aging and various age-related diseases.
Infectious Diseases
Infectious diseases are caused by pathogenic microorganisms such as bacteria, viruses, fungi, or parasites. These microorganisms can invade the body and disrupt normal cellular functions, leading to a variety of symptoms and potentially life-threatening complications, including infection-associated stress. Studies have shown that pathogens like bacteria and viruses can manipulate cellular iron metabolism to promote their own survival and replication, including through resistance to ferroptosis.121 Developing nanomedicines that trigger ferroptosis in pathogens could be a promising strategy to combat infections. A ferroptosis inducer comprising hybrid biomimetic membrane coated NPs made of Fe3O4 and cinnamaldehyde (CA) has been developed to mediate bacterial ferroptosis for acute Staphylococcus aureus (MRSA) pneumonia treatment.122 In this system, Fe3O4 can induce ferroptosis, while CA can advance ferroptosis by consuming GSH. To render a lung-targetability, the particles are further camouflaged with erythrocyte membrane and platelet membrane. Under ultrasonic stimulation, the NPs can efficiently release Fe3O4 and CA, thus synergistically inducing MRSA death with the characteristics of ferroptosis. Furthermore, the nanomedicine exhibits a capacity of suppressing the quorum sensing, removing biofilms, and reducing strain virulence. The anti-pneumonic effect of NPs by inducing ferroptosis of bacteria has been confirmed in a mouse model of MRSA pneumonia. This study provides pioneering insight into the feasibility of treating infectious diseases via promoting ferroptosis of pathogens.
Nanotherapy via Suppressing Ferroptosis
Stress, whether physical, psychological, or environmental, can trigger cellular responses that lead to ferroptosis activation, contributing to disease development and progression. One key player in the crosstalk between ferroptosis activation and stress-related diseases is oxidative stress.22 Stressors can induce the generation of ROS and disrupt cellular redox balance, resulting in lipid peroxidation and ferroptosis. Chronic stress has been associated with increased oxidative stress and lipid peroxidation,123 which can promote ferroptosis in various tissues and organs. Furthermore, stress-related diseases such as cardiovascular diseases, metabolic disorders and neurodegenerative disorders are characterized by dysregulated iron metabolism, conversely exacerbating ferroptosis. Excessive iron accumulation increases the susceptibility of cells to ferroptosis by providing a source of iron for lipid peroxidation.124 In addition, inflammation, another hallmark of stress-related diseases, can also promote ferroptosis.125 Inflammatory cytokines/mediators trigger the expression of pro-oxidant enzymes and disrupt cellular antioxidant defenses, leading to ferroptosis activation. Inhibition of ferroptosis has emerged as a potential therapeutic strategy for treating these diseases. One approach to suppress ferroptosis is to target the key regulators of ferroptotic process, such as GPX4 enzyme. Another approach is to modulate iron metabolism by chelation or iron transport inhibition.
Atherosclerosis
Atherosclerosis is a chronic inflammatory disease characterized by the deposition of lipids, immune cells, and fibrous elements in the arterial walls, leading to the formation of plaques. Studies have shown that the interplay between ferroptosis and oxidative stress/inflammation plays a critical role in atherosclerosis.126 Oxidized lipids accumulating in atherosclerotic plaques can cause ferroptotic cell death in vascular cells, as an initiating factor, contributing to plaque instability and progression. In addition, iron overload in these lesions further enhances ferroptosis by boosting lipid peroxidation.127 With the growing importance of ferroptosis in atherosclerosis, there is a rising expectation for enhanced arteriosclerosis treatment by inhibiting ferroptosis. Targeting key molecular components in the ferroptotic pathway offers potential to improve therapeutic results. Utilizing iron chelators, antioxidants, lipid peroxidation inhibitors, genetic manipulation, and tailored ferroptosis inhibitors to suppress ferroptosis provide many preferred treatment options for atherosclerosis.
With a deeper understanding of the role of ferroptosis in atherosclerosis, nanomedicines that target ferroptosis may dominate in the future development of medicaments. By leveraging the unique properties, such as targeted delivery, responsiveness, and versatility, practitioners have developed innovative nanomedicines to intervene atherosclerosis. For instance, a broad-spectrum ROS scavenging NPs with intrinsic anti-inflammatory activity was purposed for anti-atherosclerotic treatment.128 The NPs were efficiently internalized by macrophages and vascular smooth muscle cells and attenuated ROS-induced inflammation and cell apoptosis in vitro. Increased accumulation in atherosclerotic lesions and inhibited development of atherosclerosis were presented by anti-oxidative NPs in vivo after i.v. injection. More importantly, therapy with NPs afforded stabilized plaques except for fewer arterial abnormalities compared with the control. Although ferroptosis has not been related in this report, the broad-spectrum ROS scavenging capability of fabricative NPs lends a notion that the anti-atherosclerotic mechanism of NPs is likely associated with ferroptosis. In another report, Wang et al129 contrived macrophage membrane-camouflaged biomimetic NPs for targeting atherosclerosis through a macrophage “homing” effect. The biomimetic NPs loading rapamycin, a mTOR inhibitor with certain anti-inflammatory activity, effectively targeted and accumulated in the atherosclerotic lesions in vivo and significantly retarded the progression of atherosclerosis. Unfortunately, the model drug is not a ferroptotic inhibitor, otherwise the anti-atherosclerosis effect may be more encouraging. Ferroptosis is a promising target for atherosclerosis treatment, and nanomedical research should pay enough attention to this area in the future.
Diabetes and Its Complications
Numerous studies have indicated that ferroptosis plays a pivotal role in the pathophysiology and pathogenesis of diabetes mellitus and its associated complications, including diabetic nephropathy, retinopathy, and neuropathy.130 Factors such as oxidative stress, inflammation, and mitochondrial dysfunction, which are common in diabetes, can promote the induction of ferroptosis. Ferroptosis and diabetes are interconnected through mechanisms involving iron metabolism, lipid peroxidation, and cellular stress responses. Ferroptosis as a novel therapeutic target has been widely investigated for diabetes and its complications.131 Ferroptosis inhibitors show a promise to rescue pancreatic β-cell function and alleviate diabetes and complications thereof.132 However, due to the limitations of off-target effects or unsatisfactory pharmacokinetics with ferroptosis inhibitors, nanoformulations are required to be developed.
While there are no direct reports on treating diabetes by inhibiting ferroptosis with nanomedicines, some artificial NPs loaded with antioxidants or therapeutic molecules have demonstrated strong anti-diabetic effects linked to ferroptosis inhibition. In our group, we synthesized selenium nanoparticles (SeNPs) loading herbal extracts (Folium Mori and Puerariae Radix) for antidiabetic application.133 After treatment for two weeks, the relevant oxidative stress indices were all positively regulated. More importantly, the pancreatic function of model rates was restored to a large extent as manifested by raised islet β cell quantity (Figure 5). Additionally, our latest findings reveal that the antidiabetic effect of selenium-enriched NPs is associated with suppressed ferroptosis (data not shown). Coincidentally, green-synthesized SeNPs incorporating luteolin and diosmin showed significant antidiabetic effect.134 The flavonoids-containing SeNPs exhibited good antioxidant activity as examined by catalase (CAT), SOD, and GPX. Nanotherapy for six weeks restored the blood glucose, lipid profile, glycogen, glycosylated hemoglobin, and insulin level in STZ induced-diabetic mice. Antioxidant therapy for diabetes has shown potential, but direct evidence of ferroptosis inhibition in antidiabetic studies is lacking. Yet, research on using nanomedicines to address diabetic complications is actively ongoing.
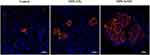 |
Figure 5 MPE-SeNPs animate β cells in the pancreas islet of diabetic rats after treatment for two weeks compared with MPE-NPs and control groups. Insulin-secreted cells are specifically labeled by Cy3-conjugated antibody. MPE-SeNPs, Folium Mori and Puerariae Radix extracts-loaded selenium NPs; MPE-NPs, selenium-free NPs. Reprinted from Deng W, Wang H, Wu B, Zhang X. Selenium-layered nanoparticles serving for oral delivery of phytomedicines with hypoglycemic activity to synergistically potentiate the antidiabetic effect. Acta Pharm Sin B. 2019;9(1):74–86.133 |
Hypertensive nephropathy is secondary to diabetes as a causation of chronic kidney disease. Hao et al135 communicated a report on inhibition of ubiquitin specific peptidase 1 (USP1) that ameliorated the hypertensive nephropathy through regulating oxidative stress and ferroptosis via nanodelivery. The expression of USP1 in human kidney cells was found to be modulated by angiotensin II. They formulated USP1 inhibitor SJB3-019A into NPs with MIL-100 (Fe-based metal–organic frameworks) and PEGTK (polyethylene glycol-thioketal) as nanomaterials. The nanomedicine exhibited excellent alleviating effects on hypertension-induced oxidative stress and ferroptosis in renal cells both in vitro and in vivo. Diabetic osteoporosis (DOP) betokens a serious complication that continues to threaten the bone health of people with diabetes. As a way out, Hao et al136 synthesized ferroptosis-suppressing NPs to deliver curcumin, a natural phytomedicine, to the bone marrow using tetrahedral framework nucleic acid (tFNA). The nanoformulation could enhance the mitochondrial function by activating the NRF2/GPX4 pathway, thereby inhibiting ferroptosis, promoting osteogenic differentiation of BMSCs in the diabetic context, reducing trabecular loss, and increasing bone formation. In addition to artificial NPs, authigenous nanovesicles secreted by living cells have also been utilized to treat diabetic complications. For example, Cui et al137 isolated secretory autophagosomes (SAPs) from human umbilical vein endothelial cells and loaded them into gelatin-methacryloyl (GelMA) hydrogels to fabricate functional wound dressings. SAPs successfully inhibited ferroptosis in high glucose-induced ferroptosis in human dermal fibroblasts, thus improving their proliferation and migration. SAPs inhibited ferroptosis by reducing Fe2+ generation through ER stress and increasing Fe2+ release via exosomes, showcasing the therapeutic potential of nanomedicines in addressing diabetic complications by targeting ferroptosis.
Ischemia-Reperfusion Injury
Ischemia-reperfusion (IR) injury is a type of tissue damage that occurs when blood flow is restored to a tissue or organ after a period of reduced blood supply (ischemia). This can lead to oxidative stress and inflammation, hence cell death and tissue damage.138 In the context of ischemia-reperfusion injury, ferroptosis plays a role in the cell death and tissue damage that occurs during the reperfusion phase.139 The accumulation of iron and lipid peroxidation lead to oxidative stress and cell death, exacerbating the damage caused by the initial ischemic insult. In the absence of effective medicines, nanotechnology offers significant therapeutic improvements in IR.
Nanomedicines that can be used for IR therapy involve pure NPs, RNA/enzyme-loaded NPs, anti-ferroptotic agent-loaded NPs, and exosome-like NPs. Polydopamine (PDA) NPs and Cu-based NPs have been reported for anti-ferroptotic applications against IR injury.91,140 Drug-free PDA NPs efficiently reduced Fe2+ deposition and lipid peroxidation in a myocardial IR injury model, while neutrophil membrane-inspired Cu-based NPs (N-Cu5.4O@DFO NPs) ameliorated IR-induced acute kidney injury through an antioxidant mechanism. Potent antioxidants are preferably chosen to treat IR injury by virtue of suitable formulations. To this end, Wang et al141 developed nano-integrated cascade antioxidases opsonized by albumin to bypass the blood brain barrier (BBB) for treatment of IR injury in the brain. Albumin NPs co-loading CAT and SOD1, integrated with a Se crosslinker, targeted neutrophil and effectively penetrated BBB in vitro and in vivo and alleviated IR injury in the brain via inhibiting neuronal ferroptosis. Besides antioxidative enzyme-loaded NPs, NPs with antioxidative enzyme activity, NPs with antioxidative enzyme activity, such as recombinant human heavy chain ferritin NPs, were discovered meritorious for treating IR diseases like ischemic stroke.142 Pleiotropic microenvironment remodeling micelles were constructed for targeted delivery of idebenone to the brain to rescue the ischemic penumbra.143 CREKA target peptide-modified micelles accumulated in the damaged brain via binding to microthrombi in the ipsilateral microvessels accompanied with transformation of diselenide bond into hydrophilic seleninic acid upon ROS stimulation. Allosteric transition of NPs synchronously consumed ROS and released drug that prevented oxidative stress-induced neuronal ferroptosis and protected the brain from IR injury. The nose-to-brain route was also utilized to deliver anti-ferroptotic miRNA for cerebral IR injury remedy. Zhao’s team prepared anti-CHAC1 exosomes from adipose-derived mesenchymal stem cells (ADSC-Exo) for nose-to-brain delivery of miR-760-3p to treat cerebral IR injury.144 ADSC-Exo given intranasally improved mouse neurobehavior IR and ferroptosis-related outcomes by targeting CHAC1 in neurons. In addition to cardio-cerebrovascular IR injury, nanomedicines have also been designed for hepatic and intestinal IR injury repair. Qiu et al145 engineered neutrophil membrane-coated taurine NPs to protect against hepatic IR injury. Nanosized taurine decreased inflammatory cytokines and various oxides, showing good anti-inflammatory and antioxidant properties. The upregulated expression of both SLC7A11 and GPX4 suggested that ferroptosis inhibition may be involved in the mechanism against the hepatic IR injury. Notably, it was reported that a phytomedicine-loaded nanoparticle significantly alleviated intestinal IR injury by ATF3/SLC7A11-mediated ferroptosis.146 In this study, the researchers synthesized two amphiphilic molecules, DTPA-N10-10 and mPEG-TK-DA, capable of scavenging free radicals and ROS, and then fabricated them into NPs with apigenin-7-O-glucoside (AGL) by self-assembly. The nanomedicine (PDN@AGL) protected intestinal tissues by reducing lipid peroxidation, ROS levels, and suppressing ferroptosis during intestinal IR (Figure 6). Additionally, the ATF3/SLC7A11 pathway was identified as key in mitigating intestinal IR injury.
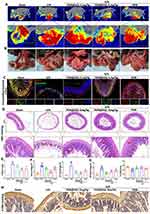 |
Figure 6 Effects of PDN@AGL on protecting intestinal blood flow, alleviating IR injury and inhibiting ferroptosis in vivo: (A) Perfusions of the microcirculation of intestine via laser speckle blood perfusion imaging; (B) Representative images of intestine morphologic changes; (C) Double fluorescence staining of ZO-1 and occluding of intestine tissues under IR with PDN@AGL; (D) H&E staining of intestine tissues under IR with PDN@AGL; (E) Chiu’s score of small intestine injury; (F) Total iron and Fe2+ levels in serum under IR injury with PDN@AGL; (G) SOD and MDA levels in serum under IR injury with PDN@AGL; and (H) 4-HNE immunohistochemical staining of intestine tissues under IR injury with PDN@AGL. Unpaired t-test, *P < 0.05 and **P < 0.01 compared with Sham group, and ##P < 0.01 compared with II/R group. Reproduced from Journal of Controlled Release, Volume 366, Zhao X, Wang Z, Wu G, et al, Apigenin-7-glucoside-loaded nanoparticle alleviates intestinal ischemia-reperfusion by ATF3/SLC7A11-mediated ferroptosis, pages 182–193, Copyright 2024, with permission from Elsevier.146 |
Alzheimer’s Disease
Alzheimer’s disease (AD) is a neurodegenerative disorder that is characterized by the progressive loss of memory and cognitive function. It is believed to be caused by the accumulation of abnormal protein deposits in the brain, leading to the death of nerve cells.147 Studies have shown that the brains of Alzheimer’s patients have higher levels of iron and lipid peroxides, which are key triggers of ferroptosis.148 This has led researchers to speculate that ferroptosis should be a promising target for management of Alzheimer’s disease. In this regard, nanomedicines are also demonstrating a bright therapeutic landscape.
Nawar et al149 examined the neuroprotective and anti-ferroptotic effects of nanocurcumin and donepezil against AD in AlCl3 and D-galactose-induced models. Treatment with nanocurcumin alone or in combination with donepezil ameliorated oxidative stress, liver and kidney functions, iron profile and decreased plasma fibrinogen. These indicators forecast a remission of AD possibly related to ferroptosis relief. In another brilliant study, Liu et al150 artistically engineered an in situ self-assembled phytopolyphenol-coordinated intelligent nanotherapeutic system for multipronged management of ferroptosis-driven AD. Utilizing brain-targeting and mitochondria-targeting features, the triphenylphosphonium-modified quercetin-derived smart nanomedicine efficiently chelated iron through phytopolyphenol-mediated spontaneous coordination. This led to self-assembly into metal-phenolic nanocomplexes, boosting defensive actions against excess iron and free radicals. In addition, the Nrf2 signaling-mediated endogenous defensive system was also reconstituted that restored the iron metabolism homeostasis and enhanced the cytoprotective antioxidant cascades. Nanotherapy improved neurodegenerative symptoms related to brain iron buildup and reversed cognitive decline in AD mice (Figure 7). Although AD intervention via ferroptosis inhibition is in the ascendant phase, these findings suggest that targeting ferroptosis pathways may hold promise as a potential therapeutic approach for treating AD.
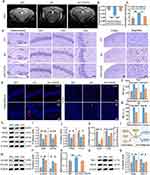 |
Figure 7 Multipronged anti-AD efficacy of TQCN against ferroptosis: (a) representative SWI images; (b) phase signal value analysis in the hippocampus (n = 5); (c) iron content detection in mouse brain after various treatments; (d) representative images of Perl’s Prussian blue staining on brain slices (Red arrows indicate iron-positive plaques); (e) representative confocal fluorescence images; (f and g) quantitative analysis of DHE and Aβ staining; (h−j) representative immunoblots and quantitative analysis for Nrf2, GPX4, FPN1 and FTH1; (k) SOD and CAT activity detection; (l) schematic of Nrf2-mediated defense pathways activated by TQCN; (m and n) representative immunoblots and quantitative analysis for 15-LOX and 4-HNE; (o) MDA content analysis; and (p and q) representative immunoblots and quantitative analysis for Bax and Bcl-2. Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, statistically significant between two groups. Adapted from Liu Y, Zhao D, Yang F, et al. In situ self-assembled phytopolyphenol-coordinated intelligent nanotherapeutics for multipronged management of ferroptosis-driven Alzheimer’s disease. ACS Nano. 2024;18(11):7890–7906. Copyright American Chemical Society.150 |
Inflammatory Conditions
In recent years, there has been growing interest in targeting ferroptosis as a potential therapeutic strategy for inflammatory conditions or diseases.151 Excessive ferroptosis has been implicated in various inflammatory conditions, including acute lung injury, sepsis, inflammatory bowel disease (IBD), and rheumatoid arthritis. It has been shown that modulating the key regulators of ferroptosis, such as GPX4, iron metabolism, and lipid peroxidation,125 can have a protective effect against inflammation. Targeting iron metabolism or lipid peroxidation pathways with nanomedicines shows promise in reducing inflammation linked to ferroptosis.
Different nanotherapeutic agents for IBD have been designed based upon the principle of ferroptosis inhibition. Yang et al152 fabricated pH-sensitive molybdenum-based polyoxometalate (POM) nanoclusters for IBD treatment by counteracting ferroptosis. POM nanoclusters displayed potent ROS scavenging ability and significantly alleviated the enteric symptoms and inflammatory indicators in DSS-induced ulcerative colitis (UC) mouse model by attenuating ferroptosis. In the work of Zhu et al153 they produced a zero-valence selenium-enriched nanozyme by a hard template method used to reconstruct intestinal barrier against IBD via suppressing ferroptosis and T cells differentiation. In addition, exosomes have also been shown to be useful nanocarriers to tackle IBD via inhibiting ferroptosis.154 Li et al introduced a groundbreaking study on treating IBD by combining ferroptosis inhibition with macrophage polarization.155 They developed a nanosystem that targets IBD by polarizing M2 macrophages and inhibiting ferroptosis. CaCO3-mineralized liposomes encapsulating ferroptosis inhibitor Fer-1 (CLF) prevent premature release of Fer-1 from liposomes in circulation, while Ca2+ is released in the acidic-inflammatory environment that promotes M2 polarization through the CaSR/AKT/β-catenin pathway. The subsequently released Fer-1 upregulates GSH and GPX4, scavenges ROS, and inhibits ferroptosis in M2 macrophages (Figure 8). CLF improved the targeting efficiency of ferroptotic antagonist in IBD lesions in vivo due to EPR effect and reinforced IBD therapy by elevating the M2/M1 macrophage ratio and suppressing ferroptosis.
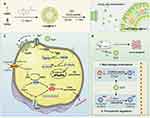 |
Figure 8 Schematic of the preparation of CaCO3@Lipo@Fer-1 (CLF) and therapeutic mechanisms in IBD. (A) Preparation of CLF mineralized liposome. (B) CLF treats IBD by regulating macrophage polarization and inhibiting ferroptosis. (C) Mechanisms of CLF promoting polarization and inhibiting ferroptosis of M2 macrophages. Reproduced from Journal of Controlled Release, Volume 367, Zhao Y, Yin W, Yang Z, et al, Nanotechnology-enabled M2 macrophage polarization and ferroptosis inhibition for targeted inflammatory bowel disease treatment, pages 339–353, Copyright 2024, with permission from Elsevier.155 |
Apart from IBD, nanomedicines against ferroptosis have been employed for the treatment of other inflammatory diseases. For instance, Yu et al156 created supramolecular self-assembly of EGCG-selenomethionine nanodrug for treating osteoarthritis (OA). Through a Mannich condensation reaction between EGCG and selenomethionine, they synthesized polyphenol-based nanodrug in aqueous medium. Molecular biology experiments disclosed that the EGCG-based nanomedicine effectively reduced GPX4 inactivation, abnormal Fe2+ accumulation, and lipid peroxidation induced by oxidative stress. It also improved chondrocyte metabolism and addressed various pathological processes related to ferroptosis, highlighting its therapeutic potential for OA. Overall, the development of therapies that specifically target ferroptosis pathways holds great potential for the treatment of inflammatory diseases.
The Future Prospective
Ferroptosis, a burgeoning concept of RCD in the biomedical realm, plays a pleiotropic role in stress-related diseases by contributing to oxidative damage and cell death. Targeting ferroptosis in various diseases presents new therapeutic avenues by regulating key factors like GPX4 and iron levels. Currently, there are 11 completed or ongoing clinical trials on using ferroptosis for treatment, including one focusing on nanomedicine for advanced solid tumors (Carbon nanoparticle-loaded iron, NCT06048367, Clinicaltrials). The growing recognition of ferroptosis in disease pathogenesis is leading to the emergence of specific agonists and antagonists. Ferroptosis is seen as a promising target for future treatment strategies, despite being in the early stages of research. Developing ferroptosis-related nanomedicines hinges on confirming its role in a disease and ensuring targeted delivery to the affected site, thus minimizing side effects. Overcoming these hurdles paves the way for the clinical use of such nanomedicines. Customizable solutions can tackle these challenges effectively.
Engineered nanomedicines targeting ferroptosis in stress-related diseases offer a promising approach for disease management. Utilizing responsive nanocarriers or biomimetic NPs for targeted delivery of ferroptotic agents and combining therapies with other mechanisms allow for efficient interventions against stress-related diseases. Due to the double-edged sword role of ferroptosis in diseases, we can both activate ferroptosis and inhibit ferroptosis upon designing nanomedicines in order to achieve satisfactory treatment outcomes. Targeted nanomedicines allow precise modulation of ferroptotic pathways, mitigating off-target toxicity and side effects. With the advance in nanotechnology, new therapeutic avenues are opened for managing complex stress-related diseases, including but not limited to cancer, neurodegenerative diseases, and inflammatory conditions.
Funding
This work was jointly supported by the Major Projects Supported by Anhui Provincial Department of Education for Outstanding Young Talents of Universities (gxyqZD2022104), Domestic Visiting Scholar Program for Young Backbone Teachers of Higher Education Institutions of the Ministry of Education in Central and Western China, International Programs & Strategic Innovative Programs of National Key Research and Development Program of China (2023YFE0112200), International Joint Research Center for Anti-tumor Nanomedicine Innovation and Application (2023A0505090002), Natural Science Foundation of Guangdong Province (2023A151522022; 2023A1515012326), and Foundation of Zhongshan Hospital of Traditional Chinese Medicine (YN2024A001, YN2024A004).
Disclosure
The authors declare no conflicts of interest.
References
1. Tang D, Kang R, Berghe TV, et al. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi:10.1038/s41422-019-0164-5
2. Tang D, Kang R. From oxytosis to ferroptosis: 10 years of research on oxidative cell death. Antioxid Redox Signal. 2023;39(1–3):162–165. doi:10.1089/ars.2023.0356
3. Xavier CH, de Menezes RCA, Chianca DA, et al. Editorial: stress-related diseases and dysfunctions. Front Physiol. 2022;13:896842. doi:10.3389/fphys.2022.896842
4. Zhang C, J-j Y, Yang C, et al. Crosstalk between ferroptosis and stress—implications in cancer therapeutic responses. Cancer Innovation. 2022;1(1):92–113. doi:10.1002/cai2.7
5. Wang S, Liao H, Li F, Ling D. A mini-review and perspective on ferroptosis-inducing strategies in cancer therapy. Chin Chem Lett. 2019;30(4):847–852. doi:10.1016/j.cclet.2019.03.025
6. Gu Y, Li Y, Wang J, et al. Targeting ferroptosis: paving new roads for drug design and discovery. Eur J Med Chem. 2023;247:115015. doi:10.1016/j.ejmech.2022.115015
7. Pan Y, Wang X, Liu X, et al. Targeting ferroptosis as a promising therapeutic strategy for ischemia-reperfusion injury. Antioxidants. 2022;11(11):2196. doi:10.3390/antiox11112196
8. Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830–849. doi:10.1016/j.ccell.2019.04.002
9. Ni L, Yuan C, Wu X. Targeting ferroptosis in acute kidney injury. Cell Death Dis. 2022;13(2):182. doi:10.1038/s41419-022-04628-9
10. Li J, Jia B, Cheng Y, et al. Targeting molecular mediators of ferroptosis and oxidative stress for neurological disorders. Oxid Med Cell Longev. 2022;2022:3999083. doi:10.1155/2022/3999083
11. Li L, Sun B, Sun J, et al. Binary prodrug nanoassemblies combining chemotherapy and ferroptosis activation for efficient triple-negative breast cancer therapy. Chin Chem Lett. 2024;35(10):109538. doi:10.1016/j.cclet.2024.109538
12. Abdalkader M, Lampinen R, Kanninen KM, et al. Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front Neurosci. 2018;12:466. doi:10.3389/fnins.2018.00466
13. Huang M, Wang Z, Yao L, et al. Ferric chloride induces ferroptosis in Pseudomonas aeruginosa and heals wound infection in a mouse model. Int J Antimicrob Agents. 2023;61(5):106794. doi:10.1016/j.ijantimicag.2023.106794
14. Zhang Y, Ai L, Gong Y, Jin Y. Preparation and usage of nanomaterials in biomedicine. Biotechnol Bioeng. 2023;120(10):2777–2792. doi:10.1002/bit.28472
15. Zhu MJ, Zhu SP, Liu QB, et al. Selenized liposomes with ameliorative stability that achieve sustained release of emodin but fail in bioavailability. Chin Chem Lett. 2023;34(1):107482. doi:10.1016/j.cclet.2022.04.080
16. Zhang C, Yan L, Wang X, et al. Progress, challenges, and future of nanomedicine. Nano Today. 2020;35:101008. doi:10.1016/j.nantod.2020.101008
17. Liu Q, Zhao Y, Zhou H, Chen C. Ferroptosis: challenges and opportunities for nanomaterials in cancer therapy. Regen Biomater. 2023;10:rbad004. doi:10.1093/rb/rbad004
18. Yu W, Shevtsov M, Chen X, Gao H. Advances in aggregatable nanoparticles for tumor-targeted drug delivery. Chin Chem Lett. 2020;31(6):1366–1374. doi:10.1016/j.cclet.2020.02.036
19. Roemhild K, von Maltzahn F, Weiskirchen R, et al. Iron metabolism: pathophysiology and pharmacology. Trends Pharmacol Sci. 2021;42(8):640–656. doi:10.1016/j.tips.2021.05.001
20. Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi:10.1038/s41419-020-2298-2
21. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–282. doi:10.1038/s41580-020-00324-8
22. Yu Y, Yan Y, Niu F, et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discovery. 2021;7(1):193. doi:10.1038/s41420-021-00579-w
23. Chen X, Comish PB, Tang D, Kang R. Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol. 2021;9:637162. doi:10.3389/fcell.2021.637162
24. Zhang Q, Fan X, Zhang X, Ju S. Ferroptosis in tumors and its relationship to other programmed cell death: role of non-coding RNAs. J Transl Med. 2023;21(1):514. doi:10.1186/s12967-023-04370-6
25. Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022;289(22):7038–7050. doi:10.1111/febs.16059
26. Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861(8):1893–1900. doi:10.1016/j.bbagen.2017.05.019
27. Lin X, Zhang Q, Li Q, et al. Upregulation of CoQ shifts ferroptosis dependence from GPX4 to FSP1 in acquired radioresistance. Drug Resist Updat. 2024;73:101032. doi:10.1016/j.drup.2023.101032
28. Han C, Liu Y, Dai R, et al. Ferroptosis and its potential role in human diseases. Front Pharmacol. 2020;11:239. doi:10.3389/fphar.2020.00239
29. Song H, Fang F, Tomasson G, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA. 2018;319(23):2388–2400. doi:10.1001/jama.2018.7028
30. Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15(9):525–534. doi:10.1038/s41574-019-0228-0
31. Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi:10.1146/annurev-biochem-061516-045037
32. Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9(6):360–370. doi:10.1038/nrcardio.2012.45
33. Wirtz PH, von Känel R. Psychological stress, inflammation, and coronary heart disease. Curr Cardiol Rep. 2017;19(11):111. doi:10.1007/s11886-017-0919-x
34. Liu YZ, Wang YX, Jiang CL. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. doi:10.3389/fnhum.2017.00316
35. Le Bras A. Chronic stress and inflammation. Lab Anim. 2021;50(11):309. doi:10.1038/s41684-021-00884-y
36. Chaudhary R, Prasad A, Agarwal V, et al. Chronic stress predisposes to the aggravation of inflammation in autoimmune diseases with focus on rheumatoid arthritis and psoriasis. Int Immunopharmacol. 2023;125:111046. doi:10.1016/j.intimp.2023.111046
37. Liu Y, Tian S, Ning B, et al. Stress and cancer: the mechanisms of immune dysregulation and management. Front Immunol. 2022;13:1032294. doi:10.3389/fimmu.2022.1032294
38. Aquino-Acevedo AN, Knochenhauer H, Castillo-Ocampo Y, et al. Stress hormones are associated with inflammatory cytokines and attenuation of T-cell function in the ascites from patients with high grade serous ovarian cancer. Brain Behav Immun Health. 2022;26:100558. doi:10.1016/j.bbih.2022.100558
39. Lempesis IG, Georgakopoulou VE, Papalexis P, et al. Role of stress in the pathogenesis of cancer (Review). Int J Oncol. 2023;63(5):124. doi:10.3892/ijo.2023.5572
40. Stojanovich L, Marisavljevich D. Stress as a trigger of autoimmune disease. Autoimmun Rev. 2008;7(3):209–213. doi:10.1016/j.autrev.2007.11.007
41. Guo H, Zheng L, Xu H, et al. Neurobiological links between stress, brain injury, and disease. Oxid Med Cell Longev. 2022;2022:8111022. doi:10.1155/2022/8111022
42. Dolotov OV, Inozemtseva LS, Myasoedov NF, Grivennikov IA. Stress-induced depression and Alzheimer’s disease: focus on astrocytes. Int J Mol Sci. 2022;23(9):4999. doi:10.3390/ijms23094999
43. Keskin E, Gezen-Ak D, Dursun E. Amyloid β,α-synuclein and amyloid β-α-synuclein combination exert significant but different alterations in inflammatory response profile in differentiated human SH-SY5Y cells. ACS Omega. 2023;8(48):45519–45534. doi:10.1021/acsomega.3c05585
44. Li J, Zhou Y, Wang H, et al. Oxidative stress-induced ferroptosis in cardiovascular diseases and epigenetic mechanisms. Front Cell Dev Biol. 2021;9:685775. doi:10.3389/fcell.2021.685775
45. Schieber M, Chandel Navdeep S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi:10.1016/j.cub.2014.03.034
46. Wang X, Xu Y, Dai L, et al. A novel oxidative stress- and ferroptosis-related gene prognostic signature for distinguishing cold and hot tumors in colorectal cancer. Front Immunol. 2022;13:1043738. doi:10.3389/fimmu.2022.1043738
47. Du X, Dong R, Wu Y, Ni B. Physiological effects of ferroptosis on organ fibrosis. Oxid Med Cell Longev. 2022;2022:5295434. doi:10.1155/2022/5295434
48. Wang Y, Zhao G, Condello S, et al. Frizzled-7 identifies platinum-tolerant ovarian cancer cells susceptible to ferroptosis. Cancer Res. 2021;81(2):384–399. doi:10.1158/0008-5472.Can-20-1488
49. Lee J, You JH, Kim MS, Roh JL. Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020;37:101697. doi:10.1016/j.redox.2020.101697
50. Sun D-Y, Wu W-B, Wu -J-J, et al. Pro-ferroptotic signaling promotes arterial aging via vascular smooth muscle cell senescence. Nat Commun. 2024;15(1):1429. doi:10.1038/s41467-024-45823-w
51. Park MW, Cha HW, Kim J, et al. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021;41:101947. doi:10.1016/j.redox.2021.101947
52. Bao WD, Pang P, Zhou XT, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28(5):1548–1562. doi:10.1038/s41418-020-00685-9
53. Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–296. doi:10.1038/s41571-020-00462-0
54. Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43(2):143–181. doi:10.1080/10408360500523878
55. Sha W, Hu F, Xi Y, et al. Mechanism of ferroptosis and its role in type 2 diabetes mellitus. J Diabetes Res. 2021;2021:9999612. doi:10.1155/2021/9999612
56. Cai W, Liu L, Shi X, et al. Alox15/15-HpETE aggravates myocardial ischemia-reperfusion injury by promoting cardiomyocyte ferroptosis. Circulation. 2023;147(19):1444–1460. doi:10.1161/circulationaha.122.060257
57. Zhu Q, Yao S, Ye Z, et al. Ferroptosis contributes to endometrial fibrosis in intrauterine adhesions. Free Radic Biol Med. 2023;205:151–162. doi:10.1016/j.freeradbiomed.2023.06.001
58. Chen Q, Xiang M, Gao Z, et al. The role of B-cell ferroptosis in the pathogenesis of systemic lupus erythematosus. Clin Immunol. 2023;256:109778. doi:10.1016/j.clim.2023.109778
59. Dang Q, Sun Z, Wang Y, et al. Ferroptosis: a double-edged sword mediating immune tolerance of cancer. Cell Death Dis. 2022;13(11):925. doi:10.1038/s41419-022-05384-6
60. Du Y, Guo Z. Recent progress in ferroptosis: inducers and inhibitors. Cell Death Dis. 2022;8(1):501. doi:10.1038/s41420-022-01297-7
61. Shi Z, Li Z, Jin B, et al. Loss of LncRNA DUXAP8 synergistically enhanced sorafenib induced ferroptosis in hepatocellular carcinoma via SLC7A11 de-palmitoylation. Clin Transl Med. 2023;13(6):e1300. doi:10.1002/ctm2.1300
62. Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245. doi:10.1016/j.chembiol.2008.02.010
63. Wang L, Liu Y, Du T, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc(). Cell Death Differ. 2020;27(2):662–675. doi:10.1038/s41418-019-0380-z
64. Li S, He Y, Chen K, et al. RSL3 Drives ferroptosis through NF-κB pathway activation and GPX4 depletion in glioblastoma. Oxid Med Cell Longev. 2021;2021:2915019. doi:10.1155/2021/2915019
65. Xiong D, Geng C, Zeng L, et al. Artesunate induces ferroptosis by regulating MT1G and has an additive effect with doxorubicin in diffuse large B-cell lymphoma cells. Heliyon. 2024;10(7):e28584. doi:10.1016/j.heliyon.2024.e28584
66. Hendricks JM, Doubravsky CE, Wehri E, et al. Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis. Cell Chem Biol. 2023;30(9):1090–1103.e1097. doi:10.1016/j.chembiol.2023.04.007
67. Qin X, Zhang J, Wang B, et al. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy. 2021;17(12):4266–4285. doi:10.1080/15548627.2021.1911016
68. Jiang X, Gao H, Cao Y, et al. SiNPs induce ferroptosis in HUVECs through p38 inhibiting NrF2 pathway. Front Public Health. 2023;11:1024130. doi:10.3389/fpubh.2023.1024130
69. Yuan H, Xia P, Sun X, et al. Photothermal nanozymatic nanoparticles induce ferroptosis and apoptosis through tumor microenvironment manipulation for cancer therapy. Small. 2022;18(41):e2202161. doi:10.1002/smll.202202161
70. Zheng H, Jiang J, Xu S, et al. Nanoparticle-induced ferroptosis: detection methods, mechanisms and applications. Nanoscale. 2021;13(4):2266–2285. doi:10.1039/d0nr08478f
71. Fernández-Acosta R, Iriarte-Mesa C, Alvarez-Alminaque D, et al. Novel iron oxide nanoparticles induce ferroptosis in a panel of cancer cell lines. Molecules. 2022;27(13):3970. doi:10.3390/molecules27133970
72. Zhang Y, Luo M, Cui X, et al. Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ. 2022;29(9):1850–1863. doi:10.1038/s41418-022-00970-9
73. Wang Z, Chen X, Liu N, et al. A Nuclear Long Non-Coding RNA LINC00618 Accelerates ferroptosis in a manner dependent upon apoptosis. Mol Ther. 2021;29(1):263–274. doi:10.1016/j.ymthe.2020.09.024
74. Zhang B, Bao W, Zhang S, et al. LncRNA HEPFAL accelerates ferroptosis in hepatocellular carcinoma by regulating SLC7A11 ubiquitination. Cell Death Dis. 2022;13(8):734. doi:10.1038/s41419-022-05173-1
75. Ta N, Qu C, Wu H, et al. Mitochondrial outer membrane protein FUNDC2 promotes ferroptosis and contributes to doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA. 2022;119(36):e2117396119. doi:10.1073/pnas.2117396119
76. Zhang HL, Hu BX, Li ZL, et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24(1):88–98. doi:10.1038/s41556-021-00818-3
77. Miotto G, Rossetto M, Di Paolo ML, et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020;28:101328. doi:10.1016/j.redox.2019.101328
78. Zhang B, Chen X, Ru F, et al. Liproxstatin-1 attenuates unilateral ureteral obstruction-induced renal fibrosis by inhibiting renal tubular epithelial cells ferroptosis. Cell Death Dis. 2021;12(9):843. doi:10.1038/s41419-021-04137-1
79. Guo Z, Lin J, Sun K, et al. Deferoxamine alleviates osteoarthritis by inhibiting chondrocyte ferroptosis and activating the Nrf2 pathway. Front Pharmacol. 2022;13:791376. doi:10.3389/fphar.2022.791376
80. Dixon SJ, Patel DN, Welsch M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi:10.7554/eLife.02523
81. Poon JF, Zilka O, Pratt DA. Potent ferroptosis inhibitors can catalyze the cross-dismutation of phospholipid-derived peroxyl radicals and hydroperoxyl radicals. J Am Chem Soc. 2020;142(33):14331–14342. doi:10.1021/jacs.0c06379
82. Dai Y, Chen Y, Mo D, et al. Inhibition of ACSL4 ameliorates tubular ferroptotic cell death and protects against fibrotic kidney disease. Commun Biol. 2023;6(1):907. doi:10.1038/s42003-023-05272-5
83. Du R, Cheng X, Ji J, et al. Mechanism of ferroptosis in a rat model of premature ovarian insufficiency induced by cisplatin. Sci Rep. 2023;13(1):4463. doi:10.1038/s41598-023-31712-7
84. Lee N, Park SJ, Lange M, et al. Selenium reduction of ubiquinone via SQOR suppresses ferroptosis. Nat Metab. 2024;6(2):343–358. doi:10.1038/s42255-024-00974-4
85. Lin Z, Wang J. Taxifolin protects against doxorubicin-induced cardiotoxicity and ferroptosis by adjusting microRNA-200a-mediated Nrf2 signaling pathway. Heliyon. 2023;9(11):e22011. doi:10.1016/j.heliyon.2023.e22011
86. Li Q, Li QQ, Jia JN, et al. Baicalein exerts neuroprotective effects in FeCl(3)-induced posttraumatic epileptic seizures via suppressing ferroptosis. Front Pharmacol. 2019;10:638. doi:10.3389/fphar.2019.00638
87. Li M, Meng Z, Yu S, et al. Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem Biol Interact. 2022;366:110137. doi:10.1016/j.cbi.2022.110137
88. Yu M, Li H, Wang B, et al. Baicalein ameliorates polymyxin B-induced acute renal injury by inhibiting ferroptosis via regulation of SIRT1/p53 acetylation. Chem Biol Interact. 2023;382:110607. doi:10.1016/j.cbi.2023.110607
89. Wang J, Wang Z, Li Y, et al. Blood brain barrier-targeted delivery of double selenium nanospheres ameliorates neural ferroptosis in Alzheimer’s disease. Biomaterials. 2023;302:122359. doi:10.1016/j.biomaterials.2023.122359
90. Ye XQ, Zhu YR, Yang YY, et al. Biogenic selenium nanoparticles synthesized with alginate oligosaccharides alleviate heat stress-induced oxidative damage to organs in broilers through activating Nrf2-mediated anti-oxidation and anti-ferroptosis pathways. Antioxidants. 2023;12(11):1973. doi:10.3390/antiox12111973
91. Zhang Y, Ren X, Wang Y, et al. Targeting ferroptosis by polydopamine nanoparticles protects heart against ischemia/reperfusion injury. ACS Appl Mater Interfaces. 2021;13(45):53671–53682. doi:10.1021/acsami.1c18061
92. Yang X, Chen Y, Guo J, et al. Polydopamine nanoparticles targeting ferroptosis mitigate intervertebral disc degeneration via reactive oxygen species depletion, iron ions chelation, and GPX4 ubiquitination suppression. Adv Sci. 2023;10(13):e2207216. doi:10.1002/advs.202207216
93. Shan X, Li J, Liu J, et al. Targeting ferroptosis by poly(acrylic) acid coated Mn(3)O(4) nanoparticles alleviates acute liver injury. Nat Commun. 2023;14(1):7598. doi:10.1038/s41467-023-43308-w
94. Shen L, Zhang J, Zheng Z, et al. PHGDH inhibits ferroptosis and promotes malignant progression by upregulating SLC7A11 in bladder cancer. Int J Biol Sci. 2022;18(14):5459–5474. doi:10.7150/ijbs.74546
95. Li D, Wang Y, Dong C, et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene. 2023;42(2):83–98. doi:10.1038/s41388-022-02537-x
96. Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi:10.1038/s41586-019-1705-2
97. Zhang T, Sun L, Hao Y, et al. ENO1 suppresses cancer cell ferroptosis by degrading the mRNA of iron regulatory protein 1. Nat Cancer. 2022;3(1):75–89. doi:10.1038/s43018-021-00299-1
98. Lin Z, Song J, Gao Y, et al. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022;52:102312. doi:10.1016/j.redox.2022.102312
99. Johnston MC, Scott CJ. Antibody conjugated nanoparticles as a novel form of antibody drug conjugate chemotherapy. Drug Discov Today Technol. 2018;3063–3069. doi:10.1016/j.ddtec.2018.10.003
100. Hasan N, Imran M, Jain D, et al. Advanced targeted drug delivery by bioengineered white blood cell-membrane camouflaged nanoparticulate delivery nanostructures. Environ Res. 2023;238(Pt 1):117007. doi:10.1016/j.envres.2023.117007
101. Zhou Q, Meng Y, Li D, et al. Ferroptosis in cancer: from molecular mechanisms to therapeutic strategies. Signal Transduct Target Ther. 2024;9(1):55. doi:10.1038/s41392-024-01769-5
102. Liu X, Zhang Y, Wu X, et al. Targeting ferroptosis pathway to combat therapy resistance and metastasis of cancer. Front Pharmacol. 2022;13:909821. doi:10.3389/fphar.2022.909821
103. Kim R, Taylor D, Vonderheide RH, Gabrilovich DI. Ferroptosis of immune cells in the tumor microenvironment. Trends Pharmacol Sci. 2023;44(8):542–552. doi:10.1016/j.tips.2023.06.005
104. Li Y, Wei C, Yan J, et al. The application of nanoparticles based on ferroptosis in cancer therapy. J Mater Chem B. 2024;12(2):413–435. doi:10.1039/d3tb02308g
105. Liu MR, Zhu WT, Pei DS. System Xc(-): a key regulatory target of ferroptosis in cancer. Invest New Drugs. 2021;39(4):1123–1131. doi:10.1007/s10637-021-01070-0
106. Nakamura T, Mishima E, Yamada N, et al. Integrated chemical and genetic screens unveil FSP1 mechanisms of ferroptosis regulation. Nat Struct Mol Biol. 2023;30(11):1806–1815. doi:10.1038/s41594-023-01136-y
107. Kang N, Son S, Min S, et al. Stimuli-responsive ferroptosis for cancer therapy. Chem Soc Rev. 2023;52(12):3955–3972. doi:10.1039/d3cs00001j
108. Xie B, Zhao H, Ding YF, et al. Supramolecularly engineered conjugate of bacteria and cell membrane-coated magnetic nanoparticles for enhanced ferroptosis and immunotherapy of tumors. Adv Sci. 2023;10(34):e2304407. doi:10.1002/advs.202304407
109. Xu Y, Yang L, Wang C, et al. Ferroptosis boosted oral cancer photodynamic therapy by carrier-free Sorafenib-Ce6 self-assembly nanoparticles. J Control Release. 2024;366:798–811. doi:10.1016/j.jconrel.2023.12.056
110. Ding X, Wang Z, Yu Q, et al. Superoxide dismutase-like regulated Fe/Ppa@PDA/B for synergistically targeting ferroptosis/apoptosis to enhance anti-tumor efficacy. Adv Healthc Mater. 2023;12(29):e2301824. doi:10.1002/adhm.202301824
111. Zhou C, Zhao Y, Yang M, et al. Diselenide-containing polymer based on new antitumor mechanism as efficient GSH depletion agent for ferroptosis therapy. Adv Healthc Mater. 2024;13(17):e2303896. doi:10.1002/adhm.202303896
112. Wang H, Jiao D, Feng D, et al. Transformable supramolecular self-assembled peptides for cascade self-enhanced ferroptosis primed cancer immunotherapy. Adv Mater. 2024;36(21):e2311733. doi:10.1002/adma.202311733
113. Feng Q, Fang W, Guo Y, et al. Nebulized therapy of early orthotopic lung cancer by iron-based nanoparticles: macrophage-regulated ferroptosis of cancer stem cells. J Am Chem Soc. 2023;145(44):24153–24165. doi:10.1021/jacs.3c08032
114. Zhu J, Zhang K, Zhou Y, et al. A Carrier-free nanomedicine enables apoptosis-ferroptosis synergistic breast cancer therapy by targeting subcellular organelles. ACS Appl Mater Interfaces. 2023;15(18):22403–22414. doi:10.1021/acsami.3c01350
115. Cao Z, Liu X, Zhang W, et al. Biomimetic macrophage membrane-camouflaged nanoparticles induce ferroptosis by promoting mitochondrial damage in glioblastoma. ACS Nano. 2023;17(23):23746–23760. doi:10.1021/acsnano.3c07555
116. Zhang J, Han L, Wu H, et al. A Brain‐targeting NIR‐II ferroptosis system: effective visualization and oncotherapy for orthotopic glioblastoma. Adv Sci. 2023;10(13):e2206333. doi:10.1002/advs.202206333
117. Fan R, Chen C, Mu M, et al. Engineering MMP-2 activated nanoparticles carrying B7-H3 bispecific antibodies for ferroptosis-enhanced glioblastoma immunotherapy. ACS Nano. 2023;17(10):9126–9139. doi:10.1021/acsnano.2c12217
118. Wei X, Liu M, Zheng Z, et al. Defective NCOA4-dependent ferroptosis in senescent fibroblasts retards diabetic wound healing. Cell Death Discov. 2023;9(1):138. doi:10.1038/s41420-023-01437-7
119. Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16(10):718–735. doi:10.1038/nrd.2017.116
120. Wei X, Zheng Z, Liu M, et al. Enzyme-responsive nanospheres target senescent cells for diabetic wound healing by employing chemodynamic therapy. Acta Biomater. 2023;172:407–422. doi:10.1016/j.actbio.2023.10.015
121. Gao J, Wang Q, Tang YD, et al. When ferroptosis meets pathogenic infections. Trends Microbiol. 2023;31(5):468–479. doi:10.1016/j.tim.2022.11.006
122. Hu H, Hua SY, Lin X, et al. Hybrid biomimetic membrane coated particles-mediated bacterial ferroptosis for acute mrsa pneumonia. ACS Nano. 2023;17(12):11692–11712. doi:10.1021/acsnano.3c02365
123. Chen Y, Guo X, Zeng Y, et al. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci Rep. 2023;13(1):15515. doi:10.1038/s41598-023-42760-4
124. Zhang DD. Ironing out the details of ferroptosis. Nat Cell Biol. 2024. doi:10.1038/s41556-024-01361-7
125. Chen Y, Fang Z-M, Yi X, et al. The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis. 2023;14(3):205. doi:10.1038/s41419-023-05716-0
126. Ouyang S, You J, Zhi C, et al. Ferroptosis: the potential value target in atherosclerosis. Cell Death Dis. 2021;12(8):782. doi:10.1038/s41419-021-04054-3
127. Vinchi F, Porto G, Simmelbauer A, et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J. 2020;41(28):2681–2695. doi:10.1093/eurheartj/ehz112
128. Wang Y, Li L, Zhao W, et al. Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano. 2018;12(9):8943–8960. doi:10.1021/acsnano.8b02037
129. Wang Y, Zhang K, Li T, et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics. 2021;11(1):164–180. doi:10.7150/thno.47841
130. Liu P, Zhang Z, Cai Y, et al. Ferroptosis: mechanisms and role in diabetes mellitus and its complications. Ageing Res Rev. 2024;94:102201. doi:10.1016/j.arr.2024.102201
131. Yang XD, Yang YY. Ferroptosis as a Novel Therapeutic Target for Diabetes and Its Complications. Front Endocrinol. 2022;13:853822. doi:10.3389/fendo.2022.853822
132. Prasad MK, Mohandas S, Kunka Mohanram R. Role of ferroptosis inhibitors in the management of diabetes. BioFactors. 2023;49(2):270–296. doi:10.1002/biof.1920
133. Deng W, Wang H, Wu B, Zhang X. Selenium-layered nanoparticles serving for oral delivery of phytomedicines with hypoglycemic activity to synergistically potentiate the antidiabetic effect. Acta Pharm Sin B. 2019;9(1):74–86. doi:10.1016/j.apsb.2018.09.009
134. Gutiérrez R, Gómez J, Urby R, et al. Evaluation of diabetes effects of selenium nanoparticles synthesized from a mixture of luteolin and diosmin on streptozotocin-induced type 2 diabetes in mice. Molecules. 2022;27(17):5642. doi:10.3390/molecules27175642
135. Hao F, Li Y, Zhang Y, et al. Inhibition of USP1 ameliorates hypertensive nephropathy through regulating oxidative stress and ferroptosis: a precise treatment via SJB3-019A nanodelivery. Eur J Pharm Biopharm. 2023;193:187–197. doi:10.1016/j.ejpb.2023.11.009
136. Li Y, Cai Z, Ma W, et al. A DNA tetrahedron-based ferroptosis-suppressing nanoparticle: superior delivery of curcumin and alleviation of diabetic osteoporosis. Bone Res. 2024;12(1):14. doi:10.1038/s41413-024-00319-7
137. Cui S, Liu X, Liu Y, et al. autophagosomes defeat ferroptosis by decreasing generation and increasing discharge of free Fe(2+) in skin repair cells to accelerate diabetic wound healing. Adv Sci. 2023;10(25):e2300414. doi:10.1002/advs.202300414
138. Sánchez-Hernández CD, Torres-Alarcón LA, González-Cortés A, Peón AN. Ischemia/reperfusion injury: pathophysiology, current clinical management, and potential preventive approaches. Mediators Inflamm. 2020;2020:8405370. doi:10.1155/2020/8405370
139. Yan HF, Tuo QZ, Yin QZ, Lei P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool Res. 2020;41(3):220–230. doi:10.24272/j.issn.2095-8137.2020.042
140. Ding C, Wang B, Zheng J, et al. Neutrophil membrane-inspired nanorobots act as antioxidants ameliorate ischemia reperfusion-induced acute kidney injury. ACS Appl Mater Interfaces. 2023;15(34):40292–40303. doi:10.1021/acsami.3c08573
141. Wang W, Zhang Z, Liu Y, et al. Nano-integrated cascade antioxidases opsonized by albumin bypass the blood-brain barrier for treatment of ischemia-reperfusion injury. Biomater Sci. 2022;10(24):7103–7116. doi:10.1039/d2bm01401g
142. Qi M, Cheng Y, Liu K, et al. Recombinant human heavy chain ferritin nanoparticles serve as ros scavengers for the treatment of ischemic stroke. Int J Nanomed. 2024;19:2285–2299. doi:10.2147/ijn.S449606
143. Li C, Wu Y, Chen Q, et al. Pleiotropic microenvironment remodeling micelles for cerebral ischemia-reperfusion injury therapy by inhibiting neuronal ferroptosis and glial overactivation. ACS Nano. 2023;17(18):18164–18177. doi:10.1021/acsnano.3c05038
144. Wang Y, Niu H, Li L, et al. Anti-CHAC1 exosomes for nose-to-brain delivery of miR-760-3p in cerebral ischemia/reperfusion injury mice inhibiting neuron ferroptosis. J Nanobiotechnol. 2023;21(1):109. doi:10.1186/s12951-023-01862-x
145. Qiu S, Li X, Zhang J, et al. Neutrophil membrane-coated taurine nanoparticles protect against hepatic ischemia-reperfusion injury. Eur J Pharmacol. 2023;949:175712. doi:10.1016/j.ejphar.2023.175712
146. Zhao X, Wang Z, Wu G, et al. Apigenin-7-glucoside-loaded nanoparticle alleviates intestinal ischemia-reperfusion by ATF3/SLC7A11-mediated ferroptosis. J Control Release. 2024;366:182–193. doi:10.1016/j.jconrel.2023.12.038
147. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590. doi:10.1016/s0140-6736(20)32205-4
148. Jakaria M, Belaidi AA, Bush AI, Ayton S. Ferroptosis as a mechanism of neurodegeneration in Alzheimer’s disease. J Neurochem. 2021;159(5):804–825. doi:10.1111/jnc.15519
149. Nawar NF, Beltagy DM, Mohamed TM, et al. Ameliorative anti-coagulant, anti-oxidative and anti-ferroptotic activities of nanocurcumin and donepezil on coagulation, oxidation and ferroptosis in Alzheimer’s disease. Toxicol Res. 2024;13(2):tfae054. doi:10.1093/toxres/tfae054
150. Liu Y, Zhao D, Yang F, et al. In situ self-assembled phytopolyphenol-coordinated intelligent nanotherapeutics for multipronged management of ferroptosis-driven Alzheimer’s disease. ACS Nano. 2024;18(11):7890–7906. doi:10.1021/acsnano.3c09286
151. Mao H, Zhao Y, Li H, Lei L. Ferroptosis as an emerging target in inflammatory diseases. Prog Biophys Mol Biol. 2020;155:20–28. doi:10.1016/j.pbiomolbio.2020.04.001
152. Yang F, Chen Y, Xiao Y, et al. pH-sensitive molybdenum (Mo)-based polyoxometalate nanoclusters have therapeutic efficacy in inflammatory bowel disease by counteracting ferroptosis. Pharmacol Res. 2023;188:106645. doi:10.1016/j.phrs.2023.106645
153. Zhu D, Wu H, Jiang K, et al. Zero-valence selenium-enriched Prussian blue nanozymes reconstruct intestinal barrier against inflammatory bowel disease via inhibiting ferroptosis and T cells differentiation. Adv Healthc Mater. 2023;12(12):e2203160. doi:10.1002/adhm.202203160
154. Wei Z, Hang S, Wiredu Ocansey DK, et al. Human umbilical cord mesenchymal stem cells derived exosome shuttling mir-129-5p attenuates inflammatory bowel disease by inhibiting ferroptosis. J Nanobiotechnology. 2023;21(1):188. doi:10.1186/s12951-023-01951-x
155. Zhao Y, Yin W, Yang Z, et al. Nanotechnology-enabled M2 macrophage polarization and ferroptosis inhibition for targeted inflammatory bowel disease treatment. J Control Release. 2024;367:339–353. doi:10.1016/j.jconrel.2024.01.051
156. Yu H, Song Z, Yu J, et al. Supramolecular self-assembly of EGCG-selenomethionine nanodrug for treating osteoarthritis. Bioact Mater. 2024;32:164–176. doi:10.1016/j.bioactmat.2023.09.020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.