Back to Journals » International Journal of Nanomedicine » Volume 19
Nanoparticle-Based Drug Delivery Systems Enhance Treatment of Cognitive Defects
Authors Wilar G , Suhandi C , Wathoni N , Fukunaga K, Kawahata I
Received 29 June 2024
Accepted for publication 24 October 2024
Published 6 November 2024 Volume 2024:19 Pages 11357—11378
DOI https://doi.org/10.2147/IJN.S484838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. RDK Misra
Gofarana Wilar,1,2,* Cecep Suhandi,2,* Nasrul Wathoni,2,* Kohji Fukunaga,3,4,* Ichiro Kawahata4,*
1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia; 2Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia; 3Department of Pharmacology, Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai, 980-8578, Japan; 4Department of CNS Drug Innovation, Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai, 980-8578, Japan
*These authors contributed equally to this work
Correspondence: Gofarana Wilar, Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia, Tel/Fax +62 812 97574112, Email [email protected]
Abstract: Nanoparticle-based drug delivery presents a promising solution in enhancing therapies for neurological diseases, particularly cognitive impairment. These nanoparticles address challenges related to the physicochemical profiles of drugs that hinder their delivery to the central nervous system (CNS). Benefits include improved solubility due to particle size reduction, enhanced drug penetration across the blood-brain barrier (BBB), and sustained release mechanisms suitable for long-term therapy. Successful application of nanoparticle delivery systems requires careful consideration of their characteristics tailored for CNS delivery, encompassing particle size and distribution, surface charge and morphology, loading capacity, and drug release kinetics. Literature review reveals three main types of nanoparticles developed for cognitive function enhancement: polymeric nanoparticles, lipid-based nanoparticles, and metallic or inorganic nanoparticles. Each type and its production methods possess distinct advantages and limitations. Further modifications such as coating agents or ligand conjugation have been explored to enhance their brain cell uptake. Evidence supporting their development shows improved efficacy outcomes, evidenced by enhanced cognitive function assessments, modulation of pro-oxidant markers, and anti-inflammatory activities. Despite these advancements, clinical trials validating the efficacy of nanoparticle systems in treating cognitive defects are lacking. Therefore, these findings underscore the need for researchers to expedite clinical testing to provide robust evidence of the potential of nanoparticle-based drug delivery systems.
Keywords: Nanoparticle, cognitive impairment, polymeric nanoparticle, inorganic nanoparticle, lipid-based nanoparticle
Introduction
Cognitive impairment, a disorder of the nervous system, results in difficulties in thinking, remembering, learning, or decision-making.1 This condition is particularly prevalent among older adults, with a global prevalence reaching up to 19%.2 It often arises as a secondary disorder linked to primary conditions such as Alzheimer’s disease, Parkinson’s disease, epilepsy, metabolic disorders like diabetes mellitus, and Huntington’s disease.3,4 The standard treatment for cognitive impairment involves the use of Donepezil, an acetylcholine esterase inhibitor, which remains the most effective option currently available.5
A recent meta-analysis demonstrated that Donepezil significantly improved cognitive performance, as evidenced by increased scores on cognitive assessments such as the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). Despite these benefits, Donepezil did not show a significant reduction in Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) scores, nor did it significantly delay the progression of cognitive decline. Moreover, the overall quality of evidence supporting these findings was low.6 Furthermore, long-term use of Donepezil is associated with several adverse effects, including nausea, vomiting, and diarrhea, which can significantly affect patient compliance and quality of life.7 Consequently, this has driven many researchers to seek alternative drugs for treating cognitive impairment.
One of the major challenges in treating brain disorders, including cognitive impairment, is overcoming the blood-brain barrier (BBB), which restricts the entry of drugs into the central nervous system (CNS).8 Generally, only drugs that are lipophilic, positively charged, and have a molecular weight below 400–600 Daltons can easily penetrate the BBB.9,10 The lipophilicity of a drug, indicated by a LogP value around 2, is typically adequate for this purpose.11 The more lipophilic a drug is (the higher the LogP), the easier it is for the drug to penetrate the BBB. However, while higher lipophilicity can enhance BBB permeability, it also compromises the drug’s solubility, leading to poor dissolution profiles and low bioavailability.12 Thus, while increased lipophilicity facilitates BBB penetration, it simultaneously poses challenges for the drug’s solubility and overall effectiveness.13 This highlights the challenge of balancing solubility and permeability in drug development for CNS diseases.
Several potential drug candidates have demonstrated promise in mitigating symptoms of cognitive impairment. Hesperidin, a flavonoid, has shown the ability to improve cognitive function in animal models, particularly in methotrexate-induced memory deficits in rats.14 This effect is attributed to hesperidin’s ability to promote neurogenesis through the activation of AMP-activated protein kinase (AMPK).14 Another promising compound is resveratrol, which improves cognitive function due to its potent antioxidant properties that protect neuronal cells from synaptic loss.15 However, both compounds face significant challenges: hesperidin, classified as a BCS Class IV drug, has low solubility and poor membrane permeability,16 while resveratrol, despite having good permeability, suffers from low solubility, limiting its bioavailability in brain tissues.17 These factors hinder their direct application as treatments for cognitive impairment.
Nanotechnology offers a promising solution to address various issues related to the pharmacokinetic profiles of drugs. Nanoparticle-based drug delivery systems can enhance solubility and improve drug permeability across lipophilic membranes.18,19 Commonly utilized nanoparticles for drug delivery include polymeric nanoparticles, metallic nanoparticles, and lipid-based nanoparticles.20,21 Among these, metallic nanoparticles, such as gold nanoparticles, are well-established for their good affinity towards brain neuronal cells.22 Additionally, nearly all lipid-based nanoparticles exhibit excellent capability to cross lipophilic membranes due to their lipid content.23 Polymeric nanoparticles, especially those with surfaces decorated with polydopamine, also show strong targeting properties towards brain cells.24 Comprehensive knowledge of nanoparticle utilization as drug carriers for cognitive impairment will undoubtedly drive significant advancements in the discovery and development of drugs for cognitive impairment. Therefore, this review aims to summarize the roles and characteristics of nanoparticles in improving treatments for neurodegenerative defects and to present evidence supporting nanoparticle-based drug delivery in enhancing cognitive function.
Method
This literature review is based on research studies obtained from the PubMed, Google Scholar, and Scopus databases. Keywords such as “nanoparticle”, “nano”, “nano-drug delivery”, “nanoformulation”, “cognitive impairment”, “cognitive defect”, and “memory deficit” were used to ensure a comprehensive search. The search was conducted without time restrictions to ensure the studies were comprehensive across a timeline. Selection criteria were established, detailing specific inclusion and exclusion parameters. The inclusion criteria consisted of literature focusing on drug development for cognitive improvement using nanoparticles as carriers and evaluating cognitive function improvement activities. The exclusion criteria included review articles, editorial letters, case series, case studies, non-English language literature, and inaccessible full-text articles.
Mechanisms of Blood-Brain Barrier (BBB) Penetration
The blood-brain barrier (BBB) is a semipermeable membrane that serves as a protective structure for the central nervous system (CNS), preventing harmful substances from entering the brain.9,25 Nearly 98% of small molecule drugs are hindered by the BBB from reaching the brain.26,27 This exclusion can occur due to the low lipophilicity of the drug, preventing it from merging with the lipid membrane of the BBB, or due to the drug being expelled through efflux mechanisms by membrane transporters such as P-glycoprotein (P-gp).28,29 P-gp, in particular, plays a vital role in protecting the brain by utilizing ATP-dependent mechanisms to expel potentially toxic substances.30 Despite these barriers, several mechanisms exist that enable drug molecules to cross the BBB, as shown in Figure 1.
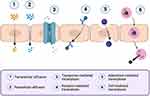 |
Figure 1 Mechanism of drug distribution to penetrate the blood-brain barrier. Created with BioRender.com. |
The routes of penetration through the BBB include paracellular and transcellular diffusion, receptor-mediated transcytosis, transporter-mediated transcytosis, cell-mediated transcytosis, and adsorptive-mediated transcytosis.9,25,31 In the paracellular diffusion mechanism, drug penetration occurs through the intercellular spaces between adjacent endothelial cells.10,32 This penetration is driven by the concentration gradient of the drug from the blood towards the brain cells.25 This pathway is limited to water-soluble drugs with small molecular sizes (MW < 400–600 Da). In contrast, transcellular diffusion occurs when the drug crosses the BBB by passing through the endothelial cells.33,34 This mechanism applies only to small molecules classified under BCS Class I, which possess sufficient lipophilicity to dissolve in the lipid membrane and high-water solubility.31,35 Like paracellular diffusion, transcellular diffusion is also driven by a concentration gradient from the blood to the brain.25 Both paracellular and transcellular diffusion mechanisms are non-specific.
In transporter-mediated transcytosis mechanisms at the BBB, two functional carrier proteins play crucial roles in facilitating drug penetration: large neutral amino acid transporter (LAT) and glucose transporter isoform GLUT-1.36,37 Unlike diffusion mechanisms, this process is highly specific, allowing only molecules with compatible conformations to penetrate.25,38 Penetration occurs when drug molecules fit the active site of the transporter protein facing the blood side, prompting a conformational change that permits drug molecules to enter.31 However, penetration via this mechanism is severely restricted due to the specificity that permits only drugs with structural modifications similar to glucose and amino acids to pass through.36,39
Another commonly utilized mechanism is receptor-mediated transcytosis, where drugs penetrate with the assistance of receptors on cell surfaces.40 Drug penetration through this mechanism occurs due to the interaction between targeting ligands and receptors on the endothelial cell surface.41,42 This interaction enables drugs to enter through endocytosis mechanisms. This mechanism is commonly employed in drugs modified into nanoparticle carriers, where the nanoparticle surface is decorated with receptor-targeting ligands.25
Similar to receptor-mediated transcytosis, adsorptive-mediated transcytosis is a mechanism through which drugs penetrate the BBB by utilizing interactions between the drug surface and the endothelial cell membrane.43 The distinction lies in the adsorptive mechanism, which exploits the positive charge on the drug surface interacting with the negative charge on the endothelial cell membrane surface.44 Like receptor-mediated transcytosis, this mechanism is commonly employed in nanoparticle-based drug delivery.25 For instance, polymeric nanoparticles based on chitosan can impart positive charges on their surfaces due to the presence of NH4+ ions, facilitating interaction with the negative charges (COO−) on the endothelial cell membrane surface.45 While more efficient than receptor-mediated transcytosis as it eliminates the need for ligand decoration, this technique has limitations due to its non-specific nature, where drugs may interact with negative charges on the membrane surface of other organs.46,47
Another mechanism utilized for drug delivery across the BBB is cell-mediated transcytosis. This mechanism is commonly employed for drugs encapsulated within liposomes.25 Penetration occurs with the assistance of immune cells that promptly engulf the liposomes and transport them across the endothelial cells into brain tissue.48 Once inside brain tissue, the drug contents within the liposomes act on affected cells through mechanisms such as chemotaxis and diapedesis.49 This method leverages the natural capabilities of immune cells to facilitate drug delivery to specific targets within the brain.25
Nanoparticles in Enhancing Drug Efficacy for Cognitive Impairment
Role and Mechanism of Brain Drug Delivery Utilizing Nanoparticles
Nanoparticles play an indirect yet significant role in enhancing the efficacy of cognitive impairment treatments through several functional mechanisms. These include improving solubility, stability, selectivity, and prolonging drug release, all of which ultimately contribute to better drug bioavailability in the brain, the target organ (Figure 2). One key mechanism is solubility enhancement, where drugs are encapsulated within nanoparticles, maintaining them in a nano-scaled or molecularly dispersed form within the nanoparticle matrix.50 This approach is particularly beneficial for lipophilic drugs, which can be encapsulated within lipid-based nanoparticle systems where the outer surface is hydrophilic.51 Such mechanisms are instrumental, especially in facilitating the distribution of drugs in the bloodstream, which typically favors hydrophilic environments.52 Consequently, nanoparticle-based strategy holds promise for overcoming challenges associated with the blood-brain barrier and enhancing therapeutic outcomes in cognitive impairment treatments.
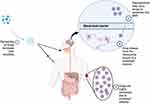 |
Figure 2 Mechanism by which nanoparticles serve as carriers for drugs to enhance the effectiveness of treatment for cognitive defects. Created with BioRender.com. |
In addition to enhancing solubility, nanoparticles also provide protection to loaded drugs during the delivery process.53 The encapsulated drugs are shielded from potential negative effects that may arise from degradative substances present in the bloodstream or target organs.54 Consequently, drugs can maintain their high concentration levels to reach the brain as the target organ. This high drug bioavailability indirectly reduces the need for effective dosage compared to unmodified drug administration.55 Moreover, enhanced bioavailability is supported by nanoparticles’ mechanisms to increase selectivity, both actively and passively.56 Passively, lipid-based nanoparticles exhibit high selectivity towards lipid components in the endothelial cell membrane, such as those found in the blood-brain barrier (BBB). Additionally, nanoparticles can actively target the brain by utilizing specific ligands targeting surface receptors on BBB endothelial cells, such as low-density lipoprotein receptor, insulin receptor, and transferrin receptor.57,58 These dual mechanisms—protection and enhanced targeting—make nanoparticles highly effective for drug delivery in cognitive impairment treatments.
Given the chronic nature of cognitive impairment conditions, maintaining drug availability in the brain over extended periods is essential.59 Through sustained release mechanisms, nanoparticles offer optimal advantages to support this requirement. The selection of nanoparticle types based on appropriate bases is critical in determining the drug release characteristics.60 The use of polymeric nanoparticles such as chitosan and polylactic-co-glycolic acid (PLGA) typically provides sustained drug release profiles.61–63 Similarly, lipid-based nanoparticles often achieve sustained drug release tailored to the needs, where drug retention occurs due to chemotactic effects between the drug and target receptors within brain neuronal cells.64 These sustained release capabilities make nanoparticles particularly suitable for treating cognitive impairment, where consistent drug levels in the brain are critical for effective management of the condition.
Nanoparticle Characteristics Affecting Their Effectiveness as Carriers
The use of nanoparticles as carriers in cognitive impairment therapy necessitates nanoparticle systems with optimal physicochemical properties. Developing an effective nanoparticle system requires careful consideration of the characteristics of the nanoparticles to support the optimization of the delivered drug (Figure 3). Several crucial physicochemical properties that influence the efficacy of these nanoparticle systems are discussed in detail below.
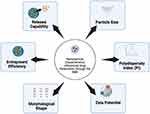 |
Figure 3 Characteristics of nanoparticles influencing the effectiveness of nanoparticle systems in enhancing efficacy in cognitive defect treatment. Created with BioRender.com. |
Particle Size
Particle size is a primary physical parameter that determines whether a formulation is within the nanoparticulate scale. Generally, materials are considered nanoscale if they have particle sizes in the range of 1–100 nm.65 Functionally, nanoparticle systems with sizes between 10–1000 nm are typically effective as drug carriers.66 An optimal particle size is crucial to meet the needs of a nano-drug delivery system, particularly to ensure good solubility in water.67 Smaller particle sizes are generally associated with increased solubility, as they provide a larger surface area in contact with water.46
Moreover, nanoparticles with smaller particle sizes are more likely to cross the blood-brain barrier (BBB), a critical factor in cognitive impairment treatments.68 However, excessively small particle sizes can also have negative consequences. Smaller particle sizes increase the probability of interparticle contact due to the higher Gibbs free energy, leading to particulate aggregation.69 This aggregation can be detrimental during storage, causing the formulation to form larger particle conglomerates, and can result in clotting if aggregation occurs during drug delivery.
Polydispersity Index
A well-defined particle size distribution is another critical characteristic of an effective nanoparticle system. The polydispersity index (PI) is commonly used to assess the uniformity of particle size distribution.70 Typically, a nanoparticle system with a PI value below 0.7 indicates a narrow size distribution, while a PI value above 0.7 suggests a broad size distribution.70 More specifically, the ideal PI value for nano-drug delivery systems is below 0.3.71,72
A uniform particle size distribution plays a crucial role in maintaining consistent drug delivery to the target site. High variability in particle size can result in differences in solubility profiles, drug adsorption, and release profiles.73 Furthermore, varying particle sizes can lead to inconsistent drug penetration capabilities across the BBB.74 This variability can cause non-uniform drug action. For instance, smaller nanoparticles typically interact more efficiently with body fluids, leading to faster drug release, whereas larger particles tend to release the drug more slowly.73 Achieving a uniform particle size distribution (monodisperse particles) requires optimal production conditions, including controlled stirring speed and precise melting temperature.75
Zeta Potential
The surface charge of nanoparticles plays a crucial role in ensuring the optimal state of nanoparticle formulations. This can be characterized through the evaluation of the zeta potential of the preparation.73 For a nanoparticle system to be considered stable, it should exhibit a zeta potential greater than +30 mV or less than −30 mV, which provides sufficient electrostatic repulsion between particles to prevent aggregation.76 On the other hand, a zeta potential between +10 mV and −10 mV is considered less stable, as the particles possess neutral surface charges, making them prone to aggregation.77
Additionally, the zeta potential can be adjusted according to the specific requirements of the nanoparticle system. For instance, when the nanocarrier is intended to cross the blood-brain barrier (BBB) via adsorptive-mediated transcytosis, a positive surface charge is required.78 This positive charge is achieved when the zeta potential of the formulation exceeds +10 mV.77 This can be achieved by utilizing appropriate nanoparticle bases, such as chitosan, which imparts a positive surface charge due to the presence of NH4+ side groups.79 Additionally, a positive charge can be obtained by surface modification with positively charged compounds like poly(β-amino ester), poly(N-isopropylacrylamide), poly(amidoamine) (PANAM), and poly(L-lysine) (PLL).80
Particle Shape
While spherical particles are generally favored due to their optimal surface area for interaction, other shapes have shown distinct advantages depending on the type of nanoparticle used.81 For example, gold nanoparticles exhibit variable cellular uptake depending on their shape. Studies have found that triangular gold nanoparticles have the highest cellular uptake due to their ability to penetrate cells not only through clathrin-mediated endocytosis but also via cytoskeletal rearrangement and dynamin pathways.82,83 In contrast, star-shaped particles demonstrate the lowest cellular uptake, likely due to significant steric hindrance.83 Therefore, selecting the appropriate particle shape is a crucial parameter to consider in the process of nanoparticle-based drug delivery.
Entrapment Efficiency
The effectiveness of nanoparticles as drug delivery systems is also determined by their success in loading drugs into the particle cavities. A higher amount of drug loaded into the nanoparticle system indicates a superior drug loading capacity. This is evaluated by measuring the percentage of entrapment efficiency (%EE) of the drug.73 Nanoparticle systems with an %EE greater than 70% are considered to have a high loading capacity.84 However, for optimal drug delivery via nanoparticles, an ideal %EE value is greater than 90%.85 This high level of entrapment efficiency ensures that the nanoparticle system is capable of effectively carrying and delivering the therapeutic agents to the target site.
Achieving high %EE depends on the compatibility between the nanoparticle system and the drug being delivered. For lipophilic drugs, lipid-based nanoparticle systems are ideal, as their lipid content facilitates better dissolution and entrapment of lipophilic compounds.86 Additionally, the choice of method significantly impacts achieving optimal %EE. In some cases, melting the components that form the nanoparticle system is necessary, as it enhances the mobility of the materials in the liquid phase, thereby improving drug entrapment within the system.87
Drug Release Capability
The drug release capability of nanoparticles plays a crucial role in enhancing drug efficacy in cognitive impairment therapy. Selecting the appropriate drug release profile must align with the loaded drug’s characteristics and the therapeutic requirements. Particularly for drugs with narrow therapeutic windows, it is essential to ensure that the drug release maintains therapeutic concentrations without exceeding potentially toxic doses.88 This consideration is critical given that at the nano scale, drugs generally exhibit higher bioavailability. Therefore, optimizing the drug release kinetics from nanoparticles is pivotal in achieving effective therapeutic outcomes in cognitive impairment treatments.89
In the case of cognitive impairment, which is a chronic condition requiring long-term management, sustained drug release is especially important.90 To achieve this objective, the formed nanoparticle systems must be capable of releasing drugs in a sustained manner. Sustained-release systems are commonly achieved through the utilization of chitosan-based nanoparticles and various types of lipid-based nanoparticles.62,91 These systems enable controlled drug release, thereby enhancing therapeutic efficacy by ensuring prolonged drug availability within the therapeutic range.88 Such approaches are pivotal in addressing the complexities associated with chronic conditions like cognitive impairment.
Progress and Developments in Nanoparticle-Based Drug Delivery for Cognitive Defects
There are various types of nanoparticles that can be employed in drug delivery applications, offering a range of options to suit different therapeutic needs. Based on their constituent materials, at least five major categories of nanoparticles have been widely recognized: polymeric nanoparticles (such as polymeric micelles), lipid-based nanoparticles (such as liposomes, solid lipid nanoparticles (SLN), and nanostructured lipid carriers (NLC)), metallic nanoparticles (such as gold nanoparticles and silver nanoparticles), ceramic nanoparticles (such as mesoporous silica nanoparticles (MSNs)), and carbon nanoparticles (such as carbon nanotubes and graphene).92 In addition to material-based classifications, nanoparticles can also be distinguished by their shapes. At least five forms have been identified, including nanospheres, nanocapsules, nanofibers, nanorods, and nanotubes.65,93 However, the nomenclature of nanoparticles is more commonly based on their material composition. According to the literature reviewed, nanoparticle-based drug delivery systems for the treatment of cognitive defects have predominantly been developed using polymeric nanoparticles, lipid-based nanoparticles, and metallic nanoparticles, as summarized in Figure 4. A detailed discussion of the developments in each nanoparticle type will be presented as follows.
 |
Figure 4 Overview of nanoparticle types used in cognitive impairment treatment. |
Polymeric Nanoparticles and Their Role in Cognitive Impairment
Polymeric nanoparticles offer several advantages as drug carriers, particularly in the treatment of cognitive impairment. One of their primary benefits is the ability to significantly enhance drug solubility.94 Furthermore, these nanoparticles provide a sustained release effect, which is particularly beneficial for drug delivery in the treatment of cognitive defects.95 In some cases, the release mechanism can also be controlled, especially when the polymer used exhibits sensitivity to specific environmental conditions. For instance, chitosan-based polymeric nanoparticles tend to swell in acidic pH conditions, thereby demonstrating a specific release profile under such conditions.96 Despite these advantages, polymeric nanoparticles face limitations, particularly in terms of lower entrapment efficiency and physical stability compared to lipid-based nanoparticles.97
The evidence supporting the use of polymeric nanoparticles for drug delivery in optimizing cognitive function therapy is summarized in Table 1. Three main types of polymeric nanoparticles—chitosan-based, PLGA-based, and collagen-based nanoparticles—have demonstrated efficacy in enhancing treatments for cognitive defects.98–107 Chitosan-based nanoparticles have been effectively used for delivering empagliflozin, nicotinamide, and ascorbic acid.98,99 These nanoparticles are consistently fabricated using the ionic gelation method. In this process, chitosan acts as the base and is ionically crosslinked by adding sodium tripolyphosphate (Na-TPP), where the negative ions of TPP serve as crosslinkers binding to NH4+ ions on the chitosan polymer chains.108
 |
Table 1 Evidence of Polymeric Nanoparticle Utilization to Elevate Drug Efficacy in Treating Cognitive Defects |
The mechanism of action of chitosan-based nanoparticles in drug delivery for cognitive defects primarily involves their ability to act as sustained release agents.98,99 Additionally, in a study by Herrera et al, polysorbate 80 (PS80) was used as a coating agent for chitosan-based nanoparticles to deliver α-melanocyte-stimulating hormone analog (NDP-MSH).105 This coating improved nanoparticle uptake, as PS80 interacts with components of the blood-brain barrier (BBB) by binding to apolipoprotein and low-density lipoprotein (LDL), which facilitates endocytosis into neuronal cells.109
PLGA-based nanoparticles offers similar advantages to chitosan-based nanoparticles, particularly in providing sustained release mechanisms.110 These nanoparticles have been used to deliver therapeutic agents such as Brain-Derived Neurotrophic Factor (BDNF), curcumin, and phytol, which are known to support cognitive function.102–104 PLGA nanoparticles are relatively simple to produce through methods like emulsification and nanoprecipitation, but they have limitations in crossing the BBB, as their primary mechanism of transport is passive diffusion, which depends on particle size.111 To address this limitation, a study by Valenza et al involved the modification of PLGA-based nanoparticles through conjugation with glycopeptide (g-7) for the delivery of cholesterol.101 This modification significantly enhanced the uptake of nanoparticles via receptor-mediated transcytosis, thereby improving their effectiveness in crossing the BBB and delivering therapeutic agents to target sites in the brain.
Another alternative approach involves the use of collagen-based nanoparticles, which offer superior safety due to their biodegradable and biocompatible properties with the body’s components.112 These properties make them particularly safe for use in drug delivery systems targeting the central nervous system. This system has been successfully developed for delivering resveratrol to the central nervous system to improve cognitive function.100 Loading drugs into collagen-based nanoparticles results in high bioavailability due to increased solubility and stability during delivery. However, collagen-based nanoparticles exhibit weak specificity and penetration capabilities for crossing the BBB.113 To overcome this, glutathione is used as a coating agent to enhance selectivity towards brain cells.100
Lipid-Based Nanoparticles and Their Role in Cognitive Impairment
Lipid-based nanoparticles present several advantages over polymeric nanoparticles. These systems demonstrate high physical stability under extreme environmental conditions.114 Additionally, they offer substantial versatility, being capable of encapsulating both hydrophilic and lipophilic active compounds.115 In their application as carriers for cognitive function therapy drugs, lipid-based nanoparticles also provide significant benefits in terms of ease of penetration through the blood-brain barrier (BBB).116 The lipid components within these nanoparticles readily merge with the lipid components of endothelial cells, facilitating entry via endocytosis.116 Several types of lipid-based nanoparticles—liposomes, nanoemulsions, and solid lipid nanoparticles (SLNs)—have been developed to address cognitive defects, as summarized in Table 2.
 |
Table 2 Evidence of Lipid-Based Nanoparticle Utilization to Elevate Drug Efficacy in Treating Cognitive Defects |
Among these, SLNs are the most frequently chosen for developing drugs to treat cognitive defects. SLNs have been successfully utilized for delivering quercetin and sesamol.119–121 Their production can be achieved using two methods: the solvent evaporation method and the oil-in-water emulsification method. The latter is preferred as it avoids the use of organic solvents, reducing the risk of toxicity from solvent residues.122 However, the use of SLNs is limited by stability issues due to the crystallization tendency of the solid lipid components within the nanoparticles.123
Nanoemulsions offer a simpler alternative to SLNs and are easier to manufacture. Nanoemulsions can be easily obtained by dispersing lipid droplets into an aqueous phase containing surfactants.124 This system has been successfully developed for the delivery of L-tryptophan, aiming to enhance the uptake of this amino acid into neuronal brain cells.118 Despite their effectiveness in increasing drug solubility, nanoemulsions are generally less stable than SLNs, making them more prone to phase separation over time.125
Liposomes, known for their biocompatibility, are another valuable lipid-based option, particularly for central nervous system (CNS) drug delivery. Their phospholipid shell mimics the composition of cell membranes, making them highly compatible with biological systems.126 Liposomes also excel in loading capacity, as demonstrated by their successful encapsulation of gold nanoparticles and plasmid DNA (pDNA) in a study by Liu et al.117 This study utilized the thin-film evaporation method to form the liposomes. The primary penetration mechanism of liposomes is endocytosis, facilitated by the fusion of the phospholipid components of the liposome with the cell membrane.127
In more advanced applications, lipid-based nanoparticle systems can be hybridized with polymeric systems to enhance their properties. For instance, Darwish et al demonstrated the use of a hybrid nanoparticle system to optimize the delivery of piperine for cognitive enhancement.107 In this system, nanoparticles were produced via the oil-in-water homogenization method, with a chitosan polymer core coated with lipid components. This hybrid approach improves the physical stability of the nanoparticles, making them particularly well-suited for encapsulating highly hydrophobic drugs.128 However, the hybrid system shows limitations in encapsulating hydrophilic drugs, which restricts its versatility.129
Metallic Nanoparticles and Their Role in Cognitive Impairment
Metallic nanoparticles, also known as inorganic nanoparticles, offer several advantages over organic nanoparticles, primarily in terms of superior physical stability.130 Despite being metal-based, their small size and unique properties make them biocompatible for medical applications in humans. Their nanoscale dimensions also confer hydrophilic characteristics, enhancing solubility in aqueous environments. This increased solubility is primarily due to the large surface area of the nanoparticles, which promotes better interaction with water molecules.131 Metallic nanoparticles are emerging as highly effective drug delivery systems for brain applications, as their ability to penetrate the blood-brain barrier (BBB) has become a key focus in cognitive impairment treatments.132 These nanoparticles not only act as carriers but, in some cases, also provide therapeutic effects due to their metal composition.131 To date, at least seven types of metals have been successfully developed into metallic nanoparticles for drug delivery aimed at improving cognitive function (Table 3).
 |
Table 3 Evidence of Metallic Nanoparticle Utilization to Elevate Drug Efficacy in Treating Cognitive Defects |
Evidence indicates that magnesium and zinc at the nanoscale can effectively serve as drug carriers.133 Using green synthesis or biogenic methods, these metals can be administered to brain cells while simultaneously delivering Datura alba leaf extract as a therapeutic agent. This combination exhibits a mutualistic relationship: magnesium and zinc nanoparticles enhance the solubility and act as carriers for Datura alba leaves, while the extract provides physical protection to the nanoparticles during delivery, ensuring high bioavailability in the brain and supplying essential minerals for brain cell activity. Similar approaches have been successfully employed by Qiao et al and Baraka et al134,135 Qiao et al utilized the biogenic method to deliver Lactobacillus casei with selenium nanoparticles,134 while Baraka et al used cerium oxide nanoparticles for the delivery of Carissa carandas extract.135
Besides biogenic synthesis, metallic nanoparticles can also be produced through wet chemistry methods for phytoconstituent delivery. This approach typically follows a bottom-up strategy, where nanoparticle components are first synthesized at the molecular scale.143 After all components interact at this level, physical treatments are applied to form nanoparticles composed of these molecular structures. This method was employed by Ninsiima et al, who successfully created green tea silver nanoparticles by combining green tea leaf extract with silver nanoparticles.140 This combination offers similar benefits to the mutualistic symbiosis observed in the production of metallic nanoparticles using biogenic methods, enhancing both solubility and stability during delivery.
Among metallic nanoparticles, gold nanoparticles stand out in the treatment of cognitive defects. Besides their exceptional capability to deliver drugs with high uptake rates across the blood-brain barrier (BBB), gold itself exhibits potent pharmacological activity against numerous neurological issues.144 While research into the molecular mechanisms underlying gold’s pharmacological effects is ongoing, it is already known that gold nanoparticles improve cognitive function in Alzheimer’s patients by inhibiting β-amyloid aggregation and α-synuclein activity in Parkinson’s disease.145 Evidence has shown that gold nanoparticles have successfully delivered vildagliptin, vitamin E, and hesperidin.138,139 Most of these nanoparticles are produced using the Turkevich method, a widely-used technique due to its simplicity, despite sometimes yielding less spherical nanoparticles.146
Recent advancements in drug delivery systems have expanded significantly to include the utilization of superparamagnetic metals such as iron oxide (Fe3O4).147 Superparamagnetic iron oxide nanoparticles (SPIONs) offer compelling advantages by providing three distinct benefits. Firstly, they serve effectively as carriers for therapeutic agents.148 Secondly, their paramagnetic properties enable their use in diagnostic magnetic resonance imaging (MRI).149 Thirdly, they exhibit promising pharmacological effects on the neuronal system, potentially aiding in neuronal function improvement through axon differentiation, although the exact mechanisms are yet to be fully elucidated.150 Mechanistically, SPIONs are favored as drug carriers due to their low tendency for aggregation, ensuring excellent colloidal dispersibility and stability.147 Their exceptional stability has facilitated the successful delivery of biological agents like human umbilical cord mesenchymal stem cells (hUC-MSCs) using methods combining thermal reduction and emulsification techniques.142 Moreover, research by Chamgordani et al has demonstrated the effective delivery of secondary metabolites such as quercetin to brain cells to enhance cognitive function.141 To improve targeting specificity, SPIONs have been conjugated with polydopamine, effectively enhancing their uptake selectivity into brain cells.
Challenges and Future Directions
The utilization of nanoparticle systems for drug delivery in treating neurodegenerative diseases, such as cognitive impairment, remains significantly limited. To date, no nanoparticle-modified drugs for cognitive impairment treatment have received FDA approval. This is partly due to ongoing development and several challenges encountered in the process. One major challenge is technology transfer, as the equipment required for producing nanoparticle-based drugs is relatively advanced.151 This limits the number of industries capable of production and negatively impacts costs, making these products relatively expensive.152 Additionally, the lack of standardized regulations for overseeing the quality production of nanoparticle-based drugs presents a significant hurdle.153 The modifications applied to these drugs necessitate adjustments in both the instruments used and the established acceptance criteria for quality evaluation.
Nevertheless, the development of nanoparticle-based drugs for the treatment of cognitive defects is achievable. Various pieces of evidence demonstrate positive trends in the benefits of modifying drugs into nanoparticle systems to enhance cognitive function. Strategic steps include conducting cost-effectiveness studies of drugs modified into nanoparticle systems. The results of these evaluations can serve as guidelines for improvement and provide substantial evidence to gain confidence from investors during the development process. In addition, clinical studies on the use of nanoparticles for central nervous system (CNS) disorders are still quite limited. One promising application is in diagnostics, where gold nanoparticles are utilized as diagnostic agents. These nanoparticles have demonstrated the ability to detect soluble amyloid-β protein oligomers (AβO), which are crucial biomarkers for Alzheimer’s disease (AD), in just five minutes.154 Moreover, the therapeutic application of nanoparticle systems for drug delivery to the CNS has proven successful in treating various cancers, including brain cancer. Notable examples include Abraxane, which consists of paclitaxel bound to albumin nanoparticles, and Doxil, a pegylated liposomal formulation of doxorubicin.155,156 Both of these formulations have demonstrated effectiveness in the treatment of brain tumors. Abraxane leverages the enhanced permeability and retention (EPR) effect to improve drug delivery to tumor tissues,155 while Doxil’s lipid-based nanoparticles allow for targeted delivery and prolonged circulation time, leading to improved therapeutic outcomes.156 This success indicates that nanoparticle systems can significantly enhance penetration and delivery to the CNS. The absence of clinical studies utilizing nanoparticle-based formulations for treating cognitive impairment underscores the importance of accelerating research to obtain robust evidence. Further testing, including observational studies, multicenter clinical trials and pilot studies, can be strategic steps for advancing this field.
Additionally, other types of nanoparticles, such as nanostructured lipid carriers—the newest generation in lipid-based nanoparticles—can still be explored further for application in this area. In considering the aspect of simplicity, the use of micelle systems can also be explored in future research. The application of basic materials, which typically require surfactants for micelle formation, shows considerable potential in successfully delivering lipophilic drugs into the central nervous system (CNS).157 On the other hand, the need for specific targeting can be further developed through the utilization of PEGylated nanoparticle systems, such as PEGylated liposomes. The presence of PEG allows for the conjugation of targeting ligands, enabling precise delivery. This approach has been successfully demonstrated using BV2 cell membranes to specifically target BV2 cells in the CNS.158 Given the existing evidence, these options remain highly promising for the development of nanoparticle-based drug delivery systems in treating cognitive defects.
Conclusion
The utilization of nanoparticles as drug carriers offers an effective solution to maximize therapy efficacy and optimize drug candidates for cognitive impairment. Findings from this review indicate that nanoparticle-based drug delivery holds promising potential in enhancing the efficacy of various drug candidates for cognitive defects. Modification strategies range from the use of polymeric nanoparticles to metallic nanoparticles and lipid-based nanoparticles. With suitable production methods, these nano-drug delivery systems have demonstrated applicability across various drug candidates that may pose challenges due to their unique physical and chemical properties. Comprehending the achievements attained through these systems offers potential for tackling the diverse challenges encountered in managing cognitive impairments in the future. This, in turn, creates opportunities for researchers to undertake additional clinical trials aimed at securing approval for nanoparticle-based products suitable for broad manufacturing and market distribution.
Acknowledgments
We would like to express our gratitude to the rector of Universitas Padjadjaran for funding the APC, the HIU RKDU Grant from Universitas Padjadjaran Numbers: 1858/UN6.3.1/PT.00/2024 for Gofarana Wilar, and Professor Masanori Shigeno, Ph.D. from Department Biophysical Chemistry Tohoku University for valuable suggestions during revision progress.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gela YY, Fekadu SA, Belsti Y, et al. Cognitive impairment and associated factors among mature and older adults living in the community of Gondar town, Ethiopia, 2020. Sci Rep. 2022;12(1):7545. doi:10.1038/s41598-022-11735-2
2. Pais R, Ruano LP, Carvalho O, Barros H. Global cognitive impairment prevalence and incidence in community dwelling older adults—a systematic review. Geriatrics. 2020;5(4):84. doi:10.3390/geriatrics5040084
3. Dey A, Mukherjee A. Plant-Derived Alkaloids. In: Discovery and Development of Neuroprotective Agents from Natural Products. Elsevier; 2018:237–320. doi:10.1016/B978-0-12-809593-5.00006-9
4. Novak A, Vizjak K, Rakusa M. Cognitive impairment in people with epilepsy. J Clin Med. 2022;11(1):267. doi:10.3390/jcm11010267
5. Pooladgar P, Sakhabakhsh M, Taghva A, Soleiman-Meigooni S. Donepezil beyond Alzheimer’s Disease? A narrative review of therapeutic potentials of donepezil in different diseases. Iran J Pharm Res. 2022;21(1). doi:10.5812/ijpr-128408
6. Zhang X, Lian S, Zhang Y, Zhao Q. Efficacy and safety of donepezil for mild cognitive impairment: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2022;213:107134. doi:10.1016/j.clineuro.2022.107134
7. Shaw KE, Bondi CO, Light SH, et al. Donepezil is ineffective in promoting motor and cognitive benefits after controlled cortical impact injury in male rats. J Neurotrauma. 2013;30(7):557–564. doi:10.1089/neu.2012.2782
8. Mittal KR, Pharasi N, Sarna B, et al. Nanotechnology-based drug delivery for the treatment of CNS disorders. Transl Neurosci. 2022;13(1):527–546. doi:10.1515/tnsci-2022-0258
9. Wu D, Chen Q, Chen X, Han F, Chen Z, Wang Y. The blood–brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther. 2023;8(1):217. doi:10.1038/s41392-023-01481-w
10. Burek M, Förster CY. Blood-Brain Barrier. In: Barichello T editor. Culturing of Rodent Brain Microvascular Endothelial Cells for in vitro Modeling of the Blood-Brain Barrier. Springer; 2019:45–54. doi:10.1007/978-1-4939-8946-1_3.
11. Gampe N, Dávid DN, Takács-Novák K, Backlund A, Béni S. In vitro and in silico evaluation of Ononis isoflavonoids as molecules targeting the central nervous system. Deli MA, ed. PLoS One. 2022;17(3):e0265639. doi:10.1371/journal.pone.0265639
12. Bellettato CM, Scarpa M. Possible strategies to cross the blood–brain barrier. Ital J Pediatr. 2018;44(S2):131. doi:10.1186/s13052-018-0563-0
13. Beig A, Agbaria R, Dahan A. Oral delivery of lipophilic drugs: the tradeoff between solubility increase and permeability decrease when using cyclodextrin-based formulations. Tajmir-Riahi HA, ed. PLoS One. 2013;8(7):e68237. doi:10.1371/journal.pone.0068237
14. Lee D, Kim N, Jeon SH, et al. Hesperidin improves memory function by enhancing neurogenesis in a mouse model of Alzheimer’s disease. Nutrients. 2022;14(15):3125. doi:10.3390/nu14153125
15. Tu W, Song M, Fan X. Does resveratrol improve cognition in humans? A scientometric study to an in‐depth review. CNS Neurosci Ther. 2023;29(9):2413–2429. doi:10.1111/cns.14276
16. Paczkowska-Walendowska M, Miklaszewski A, Cielecka-Piontek J. Improving solubility and permeability of hesperidin through electrospun orange-peel-extract-loaded nanofibers. Int J Mol Sci. 2023;24(9):7963. doi:10.3390/ijms24097963
17. Shekh B, Gupta RA. Formulation and optimization of liquisolid compact for enhancing dissolution properties of polyphenol stilbenoid- resveratrol. J Drug Delivery Ther. 2022;12(6–S):65–72. doi:10.22270/jddt.v12i6-S.5707
18. Yao Y, Zhou Y, Liu L, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193. doi:10.3389/fmolb.2020.00193
19. Sadaquat H, Akhtar M, Nazir M, Ahmad R, Alvi Z, Akhtar N. Biodegradable and biocompatible polymeric nanoparticles for enhanced solubility and safe oral delivery of docetaxel: in vivo toxicity evaluation. Int J Pharm. 2021;598:120363. doi:10.1016/j.ijpharm.2021.120363
20. Altammar KA. A review on nanoparticles: characteristics, synthesis, applications, and challenges. Front Microbiol. 2023;14:1155622. doi:10.3389/fmicb.2023.1155622
21. Joudeh N, Linke D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnology. 2022;20(1):262. doi:10.1186/s12951-022-01477-8
22. Paviolo C, Stoddart P. Gold nanoparticles for modulating neuronal behavior. Nanomaterials. 2017;7(4):92. doi:10.3390/nano7040092
23. Ilić T, Đoković JB, Nikolić I, et al. Parenteral lipid-based nanoparticles for CNS disorders: integrating various facets of preclinical evaluation towards more effective clinical translation. Pharmaceutics. 2023;15(2):443. doi:10.3390/pharmaceutics15020443
24. Zhu TT, Wang H, Wen GH, et al. Melanin-like polydopamine nanoparticles mediating anti-inflammatory and rescuing synaptic loss for inflammatory depression therapy. J Nanobiotechnology. 2023;21(1):52. doi:10.1186/s12951-023-01807-4
25. Ding S, Khan AI, Cai X, et al. Overcoming blood–brain barrier transport: advances in nanoparticle-based drug delivery strategies. Mater Today. 2020;37:112–125. doi:10.1016/j.mattod.2020.02.001
26. Pandit R, Chen L, Götz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 2020;165-166:1–14. doi:10.1016/j.addr.2019.11.009
27. Banks WA. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15(4):275–292. doi:10.1038/nrd.2015.21
28. Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi:10.1016/j.nbd.2009.07.030
29. Endicott JA, Ling V. THE biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58(1):137–171. doi:10.1146/annurev.bi.58.070189.001033
30. Fromm MF. Importance of P-glycoprotein at blood–tissue barriers. Trends Pharmacol Sci. 2004;25(8):423–429. doi:10.1016/j.tips.2004.06.002
31. Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev. 2012;64(7):640–665. doi:10.1016/j.addr.2011.11.010
32. Choudhari M, Hejmady S, Narayan Saha R, et al. Evolving new-age strategies to transport therapeutics across the blood-brain-barrier. Int J Pharm. 2021;599:120351. doi:10.1016/j.ijpharm.2021.120351
33. Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis. 2010;37(1):48–57. doi:10.1016/j.nbd.2009.07.028
34. Bron AJ, Argüeso P, Irkec M, Bright FV. Clinical staining of the ocular surface: mechanisms and interpretations. Prog Retin Eye Res. 2015;44:36–61. doi:10.1016/j.preteyeres.2014.10.001
35. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
36. Cockerill I, Oliver JA, Xu H, Fu BM, Zhu D. Blood-brain barrier integrity and clearance of amyloid-β from the BBB. Adv Exp Med Biol. 2018;1097:261–278. doi:10.1007/978-3-319-96445-4_14
37. Wang H, Zhang Z, Guan J, Lu W, Zhan C. Unraveling GLUT-mediated transcytosis pathway of glycosylated nanodisks. Asian J Pharm Sci. 2021;16(1):120–128. doi:10.1016/j.ajps.2020.07.001
38. Teixeira MI, Lopes CM, Amaral MH, Costa PC. Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): a current overview of active targeting in brain diseases. Colloids Surf B Biointerfaces. 2023;221:112999. doi:10.1016/j.colsurfb.2022.112999
39. Abdul Razzak R, Florence GJ, Gunn-Moore FJ. Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int J Mol Sci. 2019;20(12):3108. doi:10.3390/ijms20123108
40. Zhang W, Liu QY, Haqqani AS, et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS. 2020;17(1):47. doi:10.1186/s12987-020-00209-0
41. Baghirov H. Receptor-mediated transcytosis of macromolecules across the blood-brain barrier. Expert Opin Drug Deliv. 2023;20(12):1699–1711. doi:10.1080/17425247.2023.2255138
42. Simonneau C, Duschmalé M, Gavrilov A, et al. Investigating receptor-mediated antibody transcytosis using blood–brain barrier organoid arrays. Fluids Barriers CNS. 2021;18(1):43. doi:10.1186/s12987-021-00276-x
43. Pulgar VM, Berlin S, Olszakier S, Pahari SK, Kahn I. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front Neurosci. 2019;13:12. doi:10.3389/fnins.2018.01019
44. Lu W. Adsorptive-mediated brain delivery systems. Curr Pharm Biotechnol. 2012;13(12):2340–2348. doi:10.2174/138920112803341851
45. Jafernik K, Ładniak A, Blicharska E, et al. Chitosan-based nanoparticles as effective drug delivery systems—A review. Molecules. 2023;28(4):1963. doi:10.3390/molecules28041963
46. Sampath M, Pichaimani A, Kumpati P, Sengottuvelan B. The remarkable role of emulsifier and chitosan, dextran and PEG as capping agents in the enhanced delivery of curcumin by nanoparticles in breast cancer cells. Int J Biol Macromol. 2020;162:748–761. doi:10.1016/j.ijbiomac.2020.06.188
47. Cao S, Deng Y, Zhang L, Aleahmad M. Chitosan nanoparticles, as biological macromolecule-based drug delivery systems to improve the healing potential of artificial neural guidance channels: a review. Int J Biol Macromol. 2022;201:569–579. doi:10.1016/j.ijbiomac.2022.01.017
48. Zhang Q, Hao S, Li L, et al. M cells of mouse and human Peyer’s patches mediate the lymphatic absorption of an Astragalus hyperbranched heteroglycan. Carbohydr Polym. 2022;296:119952. doi:10.1016/j.carbpol.2022.119952
49. Azarmi M, Maleki H, Nikkam N, Malekinejad H. Transcellular brain drug delivery: a review on recent advancements. Int J Pharm. 2020;586:119582. doi:10.1016/j.ijpharm.2020.119582
50. Shamsher E, Khan RS, Davis BM, et al. Nanoparticles enhance solubility and neuroprotective effects of resveratrol in demyelinating disease. Neurotherapeutics. 2023;20(4):1138–1153. doi:10.1007/s13311-023-01378-0
51. Loo YS, Madheswaran T, Rajendran R, Bose RJC. Encapsulation of berberine into liquid crystalline nanoparticles to enhance its solubility and anticancer activity in MCF7 human breast cancer cells. J Drug Deliv Sci Technol. 2020;57:101756. doi:10.1016/j.jddst.2020.101756
52. Wathoni N, Rusdin A, Motoyama K, Joni IM, Lesmana R, Muchtaridi M. Nanoparticle drug delivery systems for α-mangostin. Nanotechnol Sci Appl. 2020;13:23–36. doi:10.2147/NSA.S243017
53. Wang Y, Yan Q, Lan C, et al. Nanoparticle carriers enhance RNA stability and uptake efficiency and prolong the protection against Rhizoctonia solani. Phytopathol Res. 2023;5(1):2. doi:10.1186/s42483-023-00157-1
54. Ai C, Zhao C, Xiang C, et al. Gum arabic as a sole wall material for constructing nanoparticle to enhance the stability and bioavailability of curcumin. Food Chem X. 2023;18:100724. doi:10.1016/j.fochx.2023.100724
55. Ries M, Moulari B, Shetab Boushehri MA, et al. Adalimumab decorated nanoparticles enhance antibody stability and therapeutic outcome in epithelial colitis targeting. Pharmaceutics. 2022;14(2):352. doi:10.3390/pharmaceutics14020352
56. Dilliard SA, Siegwart DJ. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat Rev Mater. 2023;8(4):282–300. doi:10.1038/s41578-022-00529-7
57. Nkune NW, Abrahamse H. Nanoparticle-based drug delivery systems for photodynamic therapy of metastatic melanoma: a review. Int J Mol Sci. 2021;22(22):12549. doi:10.3390/ijms222212549
58. Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–1198. doi:10.1111/jphp.13098
59. Kim J, Park E, An M. The cognitive impact of chronic diseases on functional capacity in community-dwelling adults. J Nurs Res. 2019;27(1):e3. doi:10.1097/jnr.0000000000000272
60. Bai X, Smith ZL, Wang Y, Butterworth S, Tirella A. Sustained drug release from smart nanoparticles in cancer therapy: a comprehensive review. Micromachines. 2022;13(10). undefined-undefined. doi:10.3390/mi13101623
61. Bai X, Tang S, Butterworth S, Tirella A. Design of PLGA nanoparticles for sustained release of hydroxyl-FK866 by microfluidics. Biomater Adv. 2023;154:213649. doi:10.1016/j.bioadv.2023.213649
62. Nallamuthu I, Devi A, Khanum F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J Pharm Sci. 2015;10(3):203–211. doi:10.1016/j.ajps.2014.09.005
63. Dong N, Zhu C, Jiang J, et al. Development of composite PLGA microspheres containing exenatide-encapsulated lecithin nanoparticles for sustained drug release. Asian J Pharm Sci. 2020;15(3):347–355. doi:10.1016/j.ajps.2019.01.002
64. Mahmood MA, Madni A, Rehman M, Rahim MA, Jabar A. Ionically cross-linked chitosan nanoparticles for sustained delivery of docetaxel: fabrication, post-formulation and acute oral toxicity evaluation. Int J Nanomed. 2019;Volume 14:10035–10046. doi:10.2147/IJN.S232350
65. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi:10.1186/s12951-018-0392-8
66. Silva P, Bonifácio B, Ramos M, Negri K, Maria Bauab T, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomed. 2013;9:1–15. doi:10.2147/IJN.S52634
67. Wang W, Huang Z, Li Y, et al. Impact of particle size and pH on protein Corona formation of solid lipid nanoparticles: a proof-of-concept study. Acta Pharm Sin B. 2021;11(4):1030–1046. doi:10.1016/j.apsb.2020.10.023
68. Nowak M, Brown TD, Graham A, Helgeson ME, Mitragotri S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng Transl Med. 2020;5(2):e10153. doi:10.1002/btm2.10153
69. Mao Z, Campbell CT. Predicting a key catalyst-performance descriptor for supported metal nanoparticles: metal chemical potential. ACS Catal. 2021;11(13):8284–8291. doi:10.1021/acscatal.1c01870
70. Danaei M, Dehghankhold M, Ataei S, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. doi:10.3390/pharmaceutics10020057
71. Carneiro S, Kreutz T, Limberger R, Teixeira H, Veiga Júnior V D, Koester L. Piper aduncum essential oil rich in dillapiole: development of hydrogel-thickened nanoemulsion and nanostructured lipid carrier intended for skin delivery. Pharmaceutics. 2022;14(11):2525. doi:10.3390/pharmaceutics14112525
72. Dourado D, Batista FPR, Philadelpho BO, et al. Resveratrol-loaded attalea funifera oil organogel nanoparticles: a potential nanocarrier against A375 human melanoma cells. Int J Mol Sci. 2023;24(15):12112. doi:10.3390/ijms241512112
73. Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. doi:10.1016/j.arabjc.2017.05.011
74. Topal GR, Mészáros M, Porkoláb G, et al. ApoE-targeting increases the transfer of solid lipid nanoparticles with donepezil cargo across a culture model of the blood–brain barrier. Pharmaceutics. 2020;13(1):38. doi:10.3390/pharmaceutics13010038
75. Slepička P, Slepičková Kasálková N, Siegel J, Kolská Z, Švorčík V. Methods of gold and silver nanoparticles preparation. Materials. 2019;13(1):1. doi:10.3390/ma13010001
76. Németh Z, Csóka I, Semnani Jazani R, et al. Quality by design-driven zeta potential optimisation study of liposomes with charge imparting membrane additives. Pharmaceutics. 2022;14(9):1798. doi:10.3390/pharmaceutics14091798
77. Clogston JD, Patri AK. Zeta Potential Measurement. In: McNeil SE editor. Characterization of Nanoparticles Intended for Drug Delivery. Vol 697. Methods in Molecular Biology. Humana Press; 2011:63–70. doi:10.1007/978-1-60327-198-1_6.
78. Flores D, Almeida CMR, Gomes CR, Balula SS, Granadeiro CM. Tailoring of mesoporous silica-based materials for enhanced water pollutants removal. Molecules. 2023;28(10):4038. doi:10.3390/molecules28104038
79. Kırımlıoğlu GY, Yenilmez E, Başaran E, Yazan Y. Preparation and in vitro characterization of a fluconazole loaded chitosan particulate system. ACTA Pharm Sci. 2019;57(2):203. doi:10.23893/1307-2080.APS.05713
80. Liu Q, Su RC, Yi WJ, Zhao ZG. Biodegradable Poly(Amino Ester) with aromatic backbone as efficient nonviral gene delivery vectors. Molecules. 2017;22(4):566. doi:10.3390/molecules22040566
81. Attia NF, El-Monaem EMA, El-Aqapa HG, et al. Iron oxide nanoparticles and their pharmaceutical applications. Appl Surf Sci Adv. 2022;11:100284. doi:10.1016/j.apsadv.2022.100284
82. Nambara K, Niikura K, Mitomo H, et al. Reverse size dependences of the cellular uptake of triangular and spherical gold nanoparticles. Langmuir. 2016;32(47):12559–12567. doi:10.1021/acs.langmuir.6b02064
83. Xie X, Liao J, Shao X, Li Q, Lin Y. The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles. Sci Rep. 2017;7(1):3827. doi:10.1038/s41598-017-04229-z
84. Dudala T, Yalavarthi P, Mudumala N, et al. A perspective overview on lipospheres as lipid carrier systems. Int J Pharm Investig. 2014;4(4):149. doi:10.4103/2230-973X.143112
85. Opatha SAT, Titapiwatanakun V, Chutoprapat R. Transfersomes: a promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics. 2020;12(9):855. doi:10.3390/pharmaceutics12090855
86. Seo Y, Lim H, Park H, et al. Recent progress of lipid nanoparticles-based lipophilic drug delivery: focus on surface modifications. Pharmaceutics. 2023;15(3):772. doi:10.3390/pharmaceutics15030772
87. Patel D. Nanostructured Lipid Carriers (NLC)-based gel for topical delivery of aceclofenac: preparation, characterization and in vivo evaluation. Sci Pharm. 2012;80(3):749–764. doi:10.3797/scipharm.1202-12
88. Paswan SK, Saini TR. Comparative evaluation of in vitro drug release methods employed for nanoparticle drug release studies. Dissolution Technol. 2021;28(4):30–38. doi:10.14227/DT280421P30
89. Barzegar-Jalali M. Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci. 2008;11(1):167. doi:10.18433/J3D59T
90. Fonar G, Polis B, Meirson T, Maltsev A, Samson AO. Subcutaneous sustained-release of Poly-Arginine Ameliorates Cognitive Impairment in a Transgenic Mouse Model of Alzheimer’s Disease. Adv Alzheimers Dis. 2018;07(04):153–182. doi:10.4236/aad.2018.74011
91.. Rehman M, Madni A, Khan WS, et al. Solid and liquid lipid-based binary solid lipid nanoparticles of diacerein: in vitro evaluation of sustained release, simultaneous loading of gold nanoparticles, and potential thermoresponsive behavior. Int J Nanomed. 2015;2805. doi:10.2147/IJN.S67147.
92. Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as drug delivery systems: a review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers. 2023;15(7):1596. doi:10.3390/polym15071596
93. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
94. Mandal B, Bhattacharjee H, Mittal N, et al. Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine. 2013;9(4):474–491. doi:10.1016/j.nano.2012.11.010
95. Charelli LE, De Mattos GC, De jesus sousa-batista A, Pinto JC, Balbino TA. Polymeric nanoparticles as therapeutic agents against coronavirus disease. J Nanopart Res. 2022;24(1):12. doi:10.1007/s11051-022-05396-5
96. Du H, Liu M, Yang X, Zhai G. The design of pH-sensitive chitosan-based formulations for gastrointestinal delivery. Drug Discov Today. 2015;20(8):1004–1011. doi:10.1016/j.drudis.2015.03.002
97. Zielińska A, Carreiró F, Oliveira AM, et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25(16):3731. doi:10.3390/molecules25163731
98. Khan T, Khan S, Akhtar M, Ali J, Najmi AK. Empagliflozin nanoparticles attenuates type2 diabetes induced cognitive impairment via oxidative stress and inflammatory pathway in high fructose diet induced hyperglycemic mice. Neurochem Int. 2021;150:105158. doi:10.1016/j.neuint.2021.105158
99. Abd-Allah H, Nasr M, Ahmed-Farid OAH, El-Marasy SA, Bakeer RM, Ahmed RF. Biological and pharmacological characterization of ascorbic acid and nicotinamide chitosan nanoparticles against insulin-resistance-induced cognitive defects: a comparative study. ACS Omega. 2021;6(5):3587–3601. doi:10.1021/acsomega.0c05096
100. Siddiqui MA, Akhter J, Bashir DJ, et al. Resveratrol loaded nanoparticles attenuate cognitive impairment and inflammatory markers in PTZ-induced kindled mice. Int Immunopharmacol. 2021;101:108287. doi:10.1016/j.intimp.2021.108287
101. Valenza M, Chen JY, Di Paolo E, et al. Cholesterol‐loaded nanoparticles ameliorate synaptic and cognitive function in
102. Khalin I, Alyautdin R, Wong TW, Gnanou J, Kocherga G, Kreuter J. Brain-derived neurotrophic factor delivered to the brain using poly (lactide-co-glycolide) nanoparticles improves neurological and cognitive outcome in mice with traumatic brain injury. Drug Deliv. 2016;23(9):3520–3528. doi:10.1080/10717544.2016.1199609
103. Tiwari SK, Agarwal S, Seth B, et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s Disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8(1):76–103. doi:10.1021/nn405077y
104. Sathya S, Manogari BG, Thamaraiselvi K, Vaidevi S, Ruckmani K, Devi KP. Phytol loaded PLGA nanoparticles ameliorate scopolamine-induced cognitive dysfunction by attenuating cholinesterase activity, oxidative stress and apoptosis in Wistar rat. Nutr Neurosci. 2022;25(3):485–501. doi:10.1080/1028415X.2020.1764290
105. Herrera G, Scimonelli T, Lasaga M, Granero G, Onnainty R. Polysorbate 80 coated chitosan nanoparticles for delivery of α-melanocyte stimulating hormone analog (NDP-MSH) to the brain reverse cognitive impairment related to neuroinflammation produced by a high-fat diet (HFD). Neuropharmacology. 2024;253:109969. doi:10.1016/j.neuropharm.2024.109969
106. Yusuf M. Formulation and cognitive evaluation of self-assembled phosphatidylserine-chitosan nanoparticles of lycopene, an innovative technique to lessen STZ-induced oxidative stress: a vital persuader of major neurological diseases. J Drug Deliv Sci Technol. 2021;63:102534. doi:10.1016/j.jddst.2021.102534
107. Darwish AB, Mohsen AM, ElShebiney S, Elgohary R, Younis MM. Development of chitosan lipid nanoparticles to alleviate the pharmacological activity of piperine in the management of cognitive deficit in diabetic rats. Sci Rep. 2024;14(1):8247. doi:10.1038/s41598-024-58601-x
108. Hoang NH, Le Thanh T, Sangpueak R, et al. Chitosan nanoparticles-based ionic gelation method: a promising candidate for plant disease management. Polymers. 2022;14(4):662. doi:10.3390/polym14040662
109. Ravichandran V, Lee M, Nguyen Cao TG, Shim MS. Polysorbate-based drug formulations for brain-targeted drug delivery and anticancer therapy. Appl Sci. 2021;11(19):9336. doi:10.3390/app11199336
110. Roberts R, Smyth JW, Will J, et al. Development of PLGA nanoparticles for sustained release of a connexin43 mimetic peptide to target glioblastoma cells. Mater Sci Eng C. 2020;108:110191. doi:10.1016/j.msec.2019.110191
111. Zhi K, Raji B, Nookala AR, et al. PLGA nanoparticle-based formulations to cross the blood–brain barrier for drug delivery: from R&D to cGMP. Pharmaceutics. 2021;13(4):500. doi:10.3390/pharmaceutics13040500
112. El-Sawah AA, El-Naggar NEA, Eldegla HE, Soliman HM. Green synthesis of collagen nanoparticles by Streptomyces xinghaiensis NEAA-1, statistical optimization, characterization, and evaluation of their anticancer potential. Sci Rep. 2024;14(1):3283. doi:10.1038/s41598-024-53342-3
113. Arun A, Malrautu P, Laha A, Luo H, Ramakrishna S. Collagen nanoparticles in drug delivery systems and tissue engineering. Appl Sci. 2021;11(23):11369. doi:10.3390/app112311369
114. Lu H, Zhang S, Wang J, Chen Q. A review on polymer and lipid-based nanocarriers and its application to nano-pharmaceutical and food-based systems. Front Nutr. 2021;8:783831. doi:10.3389/fnut.2021.783831
115. Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288. doi:10.4103/1735-5362.235156
116. Correia AC, Monteiro AR, Silva R, Moreira JN, Sousa Lobo JM, Silva AC. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: crossing or circumventing the blood–brain barrier (BBB) to manage neurological disorders. Adv Drug Deliv Rev. 2022;189:114485. doi:10.1016/j.addr.2022.114485
117. Liu L, Li M, Xu M, et al. Actively targeted gold nanoparticle composites improve behavior and cognitive impairment in Parkinson’s disease mice. Mater Sci Eng C. 2020;114:111028. doi:10.1016/j.msec.2020.111028
118. Furmann M, Miri AL, Hosni AP, Kerppers II, Machado-Rodrigues A, Mascarenhas LPG. Comparative study of the efficacy of l-tryptophan nanoparticles on motor and cognitive behavior in an Alzheimer’s experimental model. Braz Arch Biol Technol. 2021;
119. Rishitha N, Muthuraman A. Therapeutic evaluation of solid lipid nanoparticle of quercetin in pentylenetetrazole induced cognitive impairment of zebrafish. Life Sci. 2018;199:80–87. doi:10.1016/j.lfs.2018.03.010
120. Kakkar V, Mishra AK, Chuttani K, Chopra K, Kaur IP. Delivery of sesamol-loaded solid lipid nanoparticles to the brain for menopause-related emotional and cognitive central nervous system derangements. Rejuvenation Res. 2011;14(6):597–604. doi:10.1089/rej.2011.1193
121. Sachdeva AK, Misra S, Pal Kaur I, Chopra K. Neuroprotective potential of sesamol and its loaded solid lipid nanoparticles in ICV-STZ-induced cognitive deficits: behavioral and biochemical evidence. Eur J Pharmacol. 2015;747:132–140. doi:10.1016/j.ejphar.2014.11.014
122. Arab D, Kantzas A, Bryant SL. Nanoparticle stabilized oil in water emulsions: a critical review. J Pet Sci Eng. 2018;163:217–242. doi:10.1016/j.petrol.2017.12.091
123. Duong VA, Nguyen TTL, Maeng HJ. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020;25(20):4781. doi:10.3390/molecules25204781
124. Koroleva M, Portnaya I, Mischenko E, Abutbul-Ionita I, Kolik-Shmuel L, Danino D. Solid lipid nanoparticles and nanoemulsions with solid shell: physical and thermal stability. J Colloid Interface Sci. 2022;610:61–69. doi:10.1016/j.jcis.2021.12.010
125. Yukuyama MN, Kato ETM, Lobenberg R, Bou-Chacra NA. Challenges and future prospects of nanoemulsion as a drug delivery system. Curr Pharm Des. 2017;23(3):495–508. doi:10.2174/1381612822666161027111957
126. Juhairiyah F, De Lange ECM. Understanding drug delivery to the brain using liposome-based strategies: studies that provide mechanistic insights are essential. AAPS J. 2021;23(6):114. doi:10.1208/s12248-021-00648-z
127. Gandek TB, Van Der Koog L, Nagelkerke A. A comparison of cellular uptake mechanisms, delivery efficacy, and intracellular fate between liposomes and extracellular vesicles. Adv Healthc Mater. 2023;12(25):2300319. doi:10.1002/adhm.202300319
128. Parveen S, Gupta P, Kumar S, Banerjee M. Lipid polymer hybrid nanoparticles as potent vehicles for drug delivery in cancer therapeutics. Med Drug Discov. 2023;20:100165. doi:10.1016/j.medidd.2023.100165
129. Gajbhiye KR, Salve R, Narwade M, Sheikh A, Kesharwani P, Gajbhiye V. Lipid polymer hybrid nanoparticles: a custom-tailored next-generation approach for cancer therapeutics. Mol Cancer. 2023;22(1):160. doi:10.1186/s12943-023-01849-0
130. Mody V, Siwale R, Singh A, Mody H. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2(4):282. doi:10.4103/0975-7406.72127
131. Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J Drug Deliv Sci Technol. 2019;53:101174. doi:10.1016/j.jddst.2019.101174
132. Sintov AC, Velasco-Aguirre C, Gallardo-Toledo E, Araya E, Kogan MJ. Metal nanoparticles as targeted carriers circumventing the blood–brain barrier. Int Rev Neurobiol Elsevier. 2016;130:199–227. doi:10.1016/bs.irn.2016.06.007
133. Ullah H, Ullah I, Rehman G, et al. Magnesium and Zinc Oxide nanoparticles from datura alba improve cognitive impairment and blood brain barrier leakage. Molecules. 2022;27(15):4753. doi:10.3390/molecules27154753
134. Qiao L, Chen Y, Song X, Dou X, Xu C. Selenium nanoparticles-enriched lactobacillus casei ATCC 393 prevents cognitive dysfunction in mice through modulating microbiota-gut-brain axis. Int J Nanomed. 2022;Volume 17:4807–4827. doi:10.2147/IJN.S374024
135. Baraka SM, Mowaad NA, Ibrahim S, et al. Green synthesized cerium oxide nanoparticles ameliorate hepatic and cognitive dysfunctions in thioacetamide-induced hepatic encephalopathy in rats: modulation of TLR-4/NF-κB/Caspase-3 signaling pathways. J Drug Deliv Sci Technol. 2023;87:104846. doi:10.1016/j.jddst.2023.104846
136. Danish SM, Gupta A, Khan UA, et al. Intranasal cerium oxide nanoparticles ameliorate cognitive function in rats with Alzheimer’s via anti-oxidative pathway. Pharmaceutics. 2022;14(4):756. doi:10.3390/pharmaceutics14040756
137. Muller AP, Ferreira GK, Pires AJ, et al. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater Sci Eng C. 2017;77:476–483. doi:10.1016/j.msec.2017.03.283
138. Pradhan SP, Tejaswani P, Sa N, Behera A, Sahoo RK, Sahu PK. Mechanistic study of gold nanoparticles of Vildagliptin and Vitamin E in diabetic cognitive impairment. J Drug Deliv Sci Technol. 2023;84:104508. doi:10.1016/j.jddst.2023.104508
139. Pradhan SP, Sahoo S, Behera A, Sahoo R, Sahu PK. Memory amelioration by hesperidin conjugated gold nanoparticles in diabetes induced cognitive impaired rats. J Drug Deliv Sci Technol. 2022;69:103145. doi:10.1016/j.jddst.2022.103145
140. Ninsiima HI, Eze ED, Ssekatawa K, et al. Green tea silver nanoparticles improve physiological motor and cognitive function in BALB/c mice during inflammation. Heliyon. 2023;9(3):e13922. doi:10.1016/j.heliyon.2023.e13922
141. Chamgordani MK, Bardestani A, Ebrahimpour S, Esmaeili A. In diabetic male Wistar rats, quercetin-conjugated superparamagnetic iron oxide nanoparticles have an effect on the SIRT1/p66Shc-mediated pathway related to cognitive impairment. BMC Pharmacol Toxicol. 2023;24(1):81. doi:10.1186/s40360-023-00725-3
142. Wang Y, Jiang J, Fu X, et al. Fe3O4@polydopamine nanoparticle-loaded human umbilical cord mesenchymal stem cells improve the cognitive function in Alzheimer’s disease mice by promoting hippocampal neurogenesis. Nanomedicine. 2022;40:102507. doi:10.1016/j.nano.2021.102507
143. Kumar S, Bhushan P, Bhattacharya S. Fabrication of Nanostructures with Bottom-up Approach and Their Utility in Diagnostics, Therapeutics, and Others. In: Bhattacharya S, Agarwal AK, Chanda N, Pandey A, Sen AK editors. Environmental, Chemical and Medical Sensors. Energy, Environment, and Sustainability. Springer Singapore; 2018:167–198. doi:10.1007/978-981-10-7751-7_8.
144. Yang Y, Zheng X, Chen L, et al. Multifunctional Gold Nanoparticles in Cancer Diagnosis and Treatment. Int J Nanomed. 2022;Volume 17:2041–2067. doi:10.2147/IJN.S355142
145. Chiang MC, Yang YP, Nicol CJB, Wang CJ. Gold Nanoparticles in Neurological Diseases: a Review of Neuroprotection. Int J Mol Sci. 2024;25(4):2360. doi:10.3390/ijms25042360
146. Dong J, Carpinone PL, Pyrgiotakis G, Demokritou P, Moudgil BM. Synthesis of precision gold nanoparticles using Turkevich method. KONA Powder Part J. 2020;37:224–232. doi:10.14356/kona.2020011
147. Wahajuddin A. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomed. 2012;3445. doi:10.2147/IJN.S30320
148. Amstad E, Zurcher S, Mashaghi A, Wong JY, Textor M, Reimhult E. Surface functionalization of single superparamagnetic Iron Oxide nanoparticles for targeted magnetic resonance imaging. Small. 2009;5(11):1334–1342. doi:10.1002/smll.200801328
149. Dulińska-Litewka J, Łazarczyk A, Hałubiec P, Szafrański O, Karnas K, Karewicz A. Superparamagnetic Iron Oxide nanoparticles—current and prospective medical applications. Materials. 2019;12(4):617. doi:10.3390/ma12040617
150. Suciu M, Ionescu CM, Ciorita A, et al. Applications of superparamagnetic iron oxide nanoparticles in drug and therapeutic delivery, and biotechnological advancements. Beilstein J Nanotechnol. 2020;11:1092–1109. doi:10.3762/bjnano.11.94
151. Shreffler JW, Pullan JE, Dailey KM, Mallik S, Brooks AE. Overcoming hurdles in nanoparticle clinical translation: the influence of experimental design and surface modification. Int J Mol Sci. 2019;20(23):6056. doi:10.3390/ijms20236056
152. Taheri RA, Goodarzi V, Allahverdi A. Mixing performance of a cost-effective split-and-recombine 3D micromixer fabricated by xurographic method. Micromachines. 2019;10(11):786. doi:10.3390/mi10110786
153. Allan J, Belz S, Hoeveler A, et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul Toxicol Pharmacol. 2021;122:104885. doi:10.1016/j.yrtph.2021.104885
154. Gallo-Orive Á, Moreno-Guzmán M, Sanchez-Paniagua M, Montero-Calle A, Barderas R, Escarpa A. Gold nanoparticle-decorated catalytic micromotor-based aptassay for rapid electrochemical label-free amyloid-β42 oligomer determination in clinical samples from Alzheimer’s patients. Anal Chem. 2024;96(14):5509–5518. doi:10.1021/acs.analchem.3c05665
155. Zhao M, Lei C, Yang Y, et al. Abraxane, the nanoparticle formulation of paclitaxel can induce drug resistance by up-regulation of P-gp. In: leggas M, editor. PLoS One. 2015;Vol. 10(7):e0131429. doi:10.1371/journal.pone.0131429
156. Barenholz Y. (Chezy). Doxil® — the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi:10.1016/j.jconrel.2012.03.020
157. Chen CM, Huang CY, Lai CH, Chen YC, Hwang YT, Lin CY. Neuroprotection effects of kynurenic acid-loaded micelles for the Parkinson’s disease models. J Liposome Res. 2024;1–12. doi:10.1080/08982104.2024.2346986
158. Wang H, Yang W, Xu L, et al. BV2 Membrane-Coated PEGylated-Liposomes Delivered hFGF21 to Cortical and Hippocampal Microglia for Alzheimer’s disease therapy. Adv Healthc Mater. 2024;13(19):2400125. doi:10.1002/adhm.202400125
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

