Back to Journals » International Journal of Nanomedicine » Volume 20
Nanoparticle-Based Strategies to Enhance the Efficacy of STING Activators in Cancer Immunotherapy
Authors Qiao Y , Wei L, Su Y, Tan Q , Yang X, Li S
Received 22 January 2025
Accepted for publication 16 April 2025
Published 26 April 2025 Volume 2025:20 Pages 5429—5456
DOI https://doi.org/10.2147/IJN.S515893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Dongwoo Khang
Yi Qiao,1,* Lingyu Wei,2,* Yinjie Su,1 Qinyuan Tan,3 Xuecheng Yang,1 Shengxian Li1
1Department of Urology, The Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 2Department of Gynecologic Oncology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, 510120, People’s Republic of China; 3Department of Urology, The People’s Hospital of Jimo, Qingdao, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuecheng Yang, Department of Urology, Affiliated Hospital of Qingdao University, Qingdao, 266000, People’s Republic of China, Tel +86-18661805062, Email [email protected] Shengxian Li, Department of Urology, Affiliated Hospital of Qingdao University, Qingdao, 266000, People’s Republic of China, +86-18661808767, Email [email protected]
Abstract: The cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway plays a critical role in triggering innate and adaptive immune responses through type I interferon activation and immune cell recruitment, holding significant promise for cancer therapy. While STING activators targeting this pathway have been developed, their clinical application is hindered by challenges such as poor membrane permeability, rapid degradation, suboptimal pharmacokinetics, off-target biodistribution, and toxicity. Nanoparticle-based delivery systems offer a promising solution by enhancing the stability, circulation time, tumor accumulation, and intracellular release of STING activators. Furthermore, combining nanoparticle-delivered STING activators with radiotherapy, chemotherapy, phototherapy, and other immunotherapies enables synergistic antitumor effects through multimodal mechanisms, addressing resistance to monotherapies and reducing risks of recurrence and metastasis. This review outlines the immunomodulatory mechanisms of the cGAS-STING pathway, surveys current STING-targeted activators, and comprehensively discusses recent advances in nanoparticle-mediated delivery strategies for STING activation. Additionally, we explore combinatorial approaches that integrate STING-targeted nanotherapies with conventional and emerging treatments. Finally, we highlight the current status, prospects, and challenges of nanoparticle-based STING activation for cancer immunotherapy.
Keywords: cGAS-STING, nanoparticle, immunotherapy, cancer, drug delivery
Graphical Abstract:

Introduction
Immunotherapy represents a transformative approach to treating malignant tumors, leveraging both the innate and adaptive immune systems to recognize and eliminate cancer cells.1 Among various immune pathways, the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway has garnered increasing attention due to its potent anti-tumor effects. The cGAS-STING pathway is a crucial component of the immune system, capable of detecting cytosolic DNA and triggering the production of type I interferons and other inflammatory cytokines.2–4 The inflammatory cytokines subsequently enhance the recruitment and activation of various immune cells, including dendritic cells (DCs) and cytotoxic T lymphocytes (CTLs), thereby coordinating a robust anti-tumor immune response.5,6 In recent years, numerous STING activators have been identified, demonstrating potential in anti-tumor therapy across cellular, animal, and clinical studies.7 Both the cyclic dinucleotides (CDNs) and non-nucleotide small molecule STING activators face challenges such as poor pharmacokinetics, nonspecific biodistribution, difficulty in crossing the plasma membrane and side effects, limiting their therapeutic application to cancer therapy.8,9
Similar to delivery of cytotoxic drugs required the steps of circulation, accumulation, penetration, internalization and release, STING activators also undergo these barriers in human body.10 In detail, delivery of STING activators into cytoplasm of targeted cells is imperative for successful activation of cGAS-STING pathway owing to the intracellular location of STING protein. Based on it, nanoparticles are ideal delivery systems for targeted delivery of molecules to tumor site, as well as the cytoplasm for STING activation.11 First, the encapsulation of STING activators into nanoparticles enhances their stability and bioavailability during blood circulation and increased the accumulation at tumor site via EPR effect. Second, the shape or the surface modification of nanoparticles facilitate the penetration and uptake of STING activators loaded in the nanoparticles. Finally, the stimuli-response release of STING activators within the cytoplasm of tumor cells not only improves the efficacy but also reduces systemic toxicity.12–14 Leveraging these benefits, nanoparticle-mediated delivery of STING activators provides a novel and effective strategy for cancer immunotherapy.15
Besides successful delivery of STING activator, the tumor heterogeneity and adaptive resistance often limit the effectiveness of single STING therapy.16 This highlights the potential of combination therapies to overcome these challenges. Research indicates that combining surgery, radiotherapy (RT), chemotherapy, phototherapy (PT), including photodynamic therapy (PDT) and photothermal therapy (PTT), tumor vaccines, and other treatments with nanoparticle-mediated STING activators not only enhances tumor treatment outcomes but also mitigates associated side effects.17–22
In this review, we provide a comprehensive summary of the most recent advancements in nanoparticles designed for STING activators delivery, with a particular emphasis on combination with other agents or therapies. First, we investigated nanoparticle designs aimed at prolonging the circulatory persistence of STING activators and enhancing tumor accumulation. Second, we introduced nanoparticles engineered for superior tumor penetration and retention capabilities. Notably, we focus on how the nanoparticles leverage TME-specific triggers or external stimuli to achieve precise activation and release of STING activators, thereby enhancing their therapeutic efficacy and minimizing systemic toxicity. Additionally, the combination therapy based on nanoparticles is also included as an important method for enhancement of STING activators. We also discuss emerging trends and future directions in the development of these innovative systems, which may pave the way for more effective and safe immunotherapeutic strategies.
cGAS-STING Pathway
Cyclic GMP-AMP synthase (cGAS) is located in the cytoplasm, with its N-terminus facilitating nuclear translocation, and its C-terminus hosting the catalytic domain that acts as the active site of cGAS.23,24 STING is a transmembrane dimeric protein embedded in the endoplasmic reticulum (ER), with its C-terminus oriented towards the cytoplasmic space, functioning as a downstream sensor for detecting cytosolic DNA (Figure 1).25 During microbial infections and genomic damage, exogenous or endogenous DNA accumulates in the cytoplasm of mammalian cells, binding to cGAS and activating it (Table 1).26–29 Activated cGAS utilizes adenosine triphosphate (ATP) and guanosine triphosphate (GTP) as substrates to produce cyclic GMP-AMP (cGAMP).30 As a second messenger, cGAMP binds to and activates STING, triggering its oligomerization and translocation via autophagy to the Golgi apparatus, where it initiates downstream signal transduction. Upon activation, STING associates with TANK-binding kinase 1 (TBK1) and undergoes phosphorylation, forming phosphorylated TBK1 (p-TBK1). The p-TBK1 complex then translocate from the ER periphery to the nuclear periphery, where it phosphorylates signal transducer and activator of transcription 6 (STAT6), ultimately inducing the secretion of chemokines such as CCL2 and CCL20.31 Additionally, p-TBK1 catalyzes interferon regulatory factor 3 (IRF3), promoting its translocation to the nucleus and upregulating the expression of type I interferons such as type I interferons-β (IFN-β).31 Subsequently, p-TBK1 activates NF-κB, recruiting it to the nucleus where it activates the transcription of genes encoding pro-inflammatory cytokines.32,33 Following the signaling cascade, STING is internalized into lysosomes. Its accumulation induces lysosomal membrane permeabilization (LMP), releasing hydrolases such as cathepsins into the cytoplasm. These enzymes directly degrade critical proteins (eg, cytoskeletal and mitochondrial components) and activate apoptotic signaling, thereby triggering cell death.34,35
 |
Table 1 Classification of the dsDNA That Activates the cGAS-STING Pathway |
Currently, compelling evidence supports the notion that activation of the STING pathway significantly contributes to anti-tumor therapy. STING pathway activation mediates potent anti-tumor immune responses, including the release of tumor-associated antigens (TAAs), induction of DC maturation, enhancement of antigen presentation, promotion of CTL differentiation and activation, and stimulation of immune cell proliferation.52–54 Within the TME, STING activation is triggered by leaking self-DNA from apoptotic cell nuclei or mitochondria. This activation can initiate antigen-specific immune responses, thereby establishing a positive feedback loop for anti-cancer immunity.
Types of STING Activators
Activators Directly Binding to STING Protein
Cyclic Dinucleotides (CDNs)
Natural CDNs and their analogs are the primary activators of the STING pathway. Natural CDNs primarily include 2′3′-cGAMP and 3′3′-cGAMP. Microbial double-stranded DNA, aberrant genomic DNA, and mitochondrial DNA (mtDNA) in mammals activate cGAS, which in turn releases 2′3′-cGAMP. The isomer 3′3′-cGAMP is naturally produced in bacteria and has been shown to activate the STING pathway and enhance CD8+ T cell function.55–57 CDN homologs, such as c-di-GMP (CDG) and c-di-AMP (CDA), serve as bacterial second messengers regulating various physiological functions and can also activate the STING pathway (Table 2).58–60
 |
Table 2 Current STING Activators and Their Preclinical Applications |
However, CDNs still face many challenges in clinic applications. Firstly, STING protein is located in the ER, and due to the large molecular weight and negative charge of natural CDNs and their homologs, they have difficulty crossing the cell membrane.88,89 Secondly, natural CDNs and their homologs are easily hydrolyzed by phosphodiesterases in the cellular microenvironment, losing their activity.90 To address these issues, researchers have optimized the structure of CDNs to enhance their clinical value. The phosphodiester bond in CDNs is prone to degradation by phosphodiesterases and nucleases, but substituting this bond with a thiophosphodiester bond effectively increases hydrolytic resistance.91,92 Corrales et al synthesized mixed-linkage dithio-CDNs, resulting in R,R- and R,S-diastereomers, specifically ML RR-S2 CDA (ADU-S100).65 Besides ADU-S100, other synthetic CDNs such as IACS-8779, IACS-8803, and MK-1454 have been developed.66,67 Despite the improved stability of these synthetic CDNs, they still achieve STING activation only through intratumoral injection.
Flavonoids
5.6-Dimethylxanthenone-4-acetic acid (DMXAA) was one of the earliest discovered STING activators. Initially applied clinically as an effective vascular-disrupting agent, it demonstrated significant tumor inhibition in mouse models. Subsequent experiments revealed that DMXAA is an activator for mouse STING, showing a high dependency on the STING pathway in mice and requiring CD8+ cells to maximize tumor suppression.69 Flavone-8-acetic acid (FAA) and carbomethoxy-9-acridone (CMA) are small-molecule STING activators structurally similar to DMXAA.93 Unfortunately, all three are specific to mouse STING and ineffective on human STING, likely due to differing residues between human and mouse STING. However, not all flavonoids are mouse-specific; for instance, α-mangostin has been shown to bind and activate human STING.94 Although DMXAA and CMA failed in clinical trials, analysis of their structure and function led to the development of six small-molecule STING activators with acridone backbones, three of which are effective on both human and mouse STING.95 While flavonoids exhibit potential for tumor therapy, successful clinical trials validating their effectiveness in human tumors are lacking.
Other Small Molecule Compounds
The application of high-throughput drug screening technology in modern drug discovery has identified numerous small molecule compounds that interact with specific targets.96,97 Through STING competitive binding assays and cell reporter system screenings, many novel STING activators have been discovered. For example, aminobenzimidazole (ABZI) compounds and their dimeric derivatives (di-ABZIs) have been developed and proven to be effective non-specific STING activators.70 Di-ABZIs exhibit much higher potency than cGAMP. Unlike CDNs, which are restricted to intratumoral injection, intravenous administration of these STING activators in tumor-bearing mice resulted in strong antitumor activity, with 80% of the mice remaining tumor-free by the end of the study. For example, Liu et al discovered two human-specific STING activators, dispiro diketopiperazine (DSDP) and 6-bromo-N-(naphthalen-1-yl)benzo[d][1,3]dioxole-5-carboxamide (BNBC), both of which can activate interferon and cytokine responses and induce an antiviral state.71,72 MSA-2, a non-nucleotide small molecule STING activator, can bind to STING and requires pre-dimerization for binding.73 In the acidic tumor microenvironment, the permeability of MSA-2 increases for preferentially activating STING within tumors. Oral administration of MSA-2 demonstrated good tolerability and antitumor activity in mice. SR-717 also shows significant efficacy after systemic administration, promoting antitumor immunity by activating CD8+ T cells, NK cells, and DC in relevant tissues.74 A new macrocyclic-bridged STING activator, E7766, currently undergoing Phase I clinical evaluation, has shown broad pan-genotypic activity across all major human STING variants and significantly enhanced potency.75 Furthermore, through modification and optimization of a central core template, Compound-53 (C53), was discovered, which bound to a pocket in the transmembrane domain of STING, located between the two subunits of the STING dimer.98,99 This binding induces outward displacement of transmembrane helices in the dimer, mediating the formation of higher-order oligomers.98
Compared to other STING activators, small molecule compounds offer the advantages of high specificity and fewer side effects. However, challenges such as resistance due to tumor gene mutations, balancing multi-target and selectivity, and the accuracy of small molecule drugs before clinical trials continue to hinder their clinical application.
Indirect STING Activators
Chemotherapeutic Drugs
Unlike the aforementioned non-nucleotide small molecule activators, some chemotherapeutic drugs do not directly activate STING but instead induce DNA damage or inhibit DNA repair, causing the accumulation of endogenous DNA in cells, which subsequently activates the cGAS-STING pathway. For example, studies have shown that in bladder and ovarian cancers, cisplatin induces cGAS-STING signaling, promoting T-cell proliferation and enhancing tumor immunogenicity.11,76,100 Doxorubicin-induced DNA damage combined with Mn2+ activates the cGAS-STING pathway. This activation promotes dendritic cell maturation, increases cytotoxic T lymphocyte infiltration, and recruits natural killer cells to the tumor site.19 They used amorphous porous manganese phosphate (APMP) nanoparticles, highly sensitive to the tumor microenvironment, to construct mixed nanoparticles (PL/APMP-DOX NPs) encapsulating doxorubicin (DOX) and phospholipids (PL).101 These nanoparticles remain stable in systemic circulation but can be triggered to release DOX to induce DNA damage and Mn2+ to enhance cGAS-STING activity. Other chemotherapeutic drugs that activate STING through similar mechanisms include camptothecin (CPT), daunorubicin, and other anthracyclines.77,102
Additionally, paclitaxel and 5-fluorouracil activate cGAS-STING through the production of micronuclei.78,79 Targeted therapies can also activate STING. Research has shown that in BRCA1-deficient triple-negative breast cancer and ERCC1-deficient non-small cell lung cancer, poly ADP-ribose polymerase (PARP) inhibitors activate the intrinsic STING pathway in tumor cells by generating micronucleus-associated chromatin fragments.80,103 Furthermore, the CHK1 inhibitor prexasertib accelerates DNA double-strand breaks and STING activation, subsequently enhancing T-cell recruitment and effector cell function in small cell lung cancer (SCLC) mouse models.81
Metal Ion
The significant role of metal ions in the immune system has gained increasing recognition, with metal ion-activated immunotherapy emerging as a promising method for cancer treatment. Most metal ions do not directly activate the cGAS-STING pathway but act as regulators. Ca2+ and Zn2+ are recognized as positive regulators of the cGAS-STING pathway. Firstly, evidence indicates that reducing cytoplasmic Ca2+ flow can inhibit the STING-mediated IFN response, suggesting that STING activation requires an increase in intracellular Ca2+ within the endoplasmic reticulum (ER) and mitochondria.104 And the calcium sensor STIM1 retains STING in the endoplasmic reticulum, preventing aberrant activation of the cGAS-STING pathway, suggesting that calcium homeostasis may stabilize this pathway.105,106 Secondly, calmodulin (CaM) initiates a signaling cascade that phosphorylates calmodulin-dependent protein kinase II (CaMKII) and AMP-activated protein kinase (AMPK), contributing to STING activation.104,107 Additionally, the induction of autophagy is critical for STING activation, as both CaMKII and AMPK target BECN1 within the VPS34 complex, promoting its phosphorylation and inducing autophagy.107 Finally, the increase in cytoplasmic Ca2+ may cause mitochondrial permeability transition (MPT), leading to mtDNA leakage into the cytoplasm, which activates cGAS and subsequently STING.108,109 The zinc finger protein ZCCHC3 enhances the binding of cGAS to DNA, and Zn2+ is essential for the production of cGAMP and the coordination of the interferon response.110–112 Besides, Zn2+ is required for cGAMP folding and liquid-phase separation of cGAMP-DNA complexes.112 Unlike Ca2+ and Zn2+, K+ is considered an inhibitory regulator. Research indicates that intracellular K+ efflux is crucial for inhibiting the cGAS-dependent IFN-β response.113 And as a lack of cytoplasmic K+ results in decreased cGAMP synthesis.106
Among metal ions, Mn2+ is particularly noteworthy. Mn2+ regulates the cGAS-STING pathway by increasing cGAS sensitivity to dsDNA, thereby facilitating cGAMP production and enhancing the activity of CDNs.82,106 Additionally, Mn2+ is considered a direct activator of STING.114,115 Studies have shown that Mn2+ can directly activate cGAS to synthesize noncanonical 2′3′-cGAMP and catalyze the conversion of H2O2 to reactive oxygen species (ROS) for chemodynamic therapy (CDT), leading to the activation of cGAS-STING signaling.21 The unique properties of Mn2+ highlight its immense potential in cancer immunotherapy.
Other Activators
PTT, PDT, radiotherapy, and the ataxia-telangiectasia mutated (ATM) protein activate the STING pathway through the release of mtDNA due to cellular damage or oxidative stress-induced mitochondrial disruption.36,84,116,117 Li et al discovered a pH-sensitive polymer with a tertiary amine seven-membered ring (PCA7).85 Unlike cGAMP, PCA7 binds to a non-competitive site on the surface of STING, distinct from the cGAMP binding pocket, and stimulates prolonged production of pro-inflammatory cytokines. Arginine starvation inhibits gene expression, leading to DNA damage, chromatin leakage, and cGAS-STING activation.86 Sodium-glucose co-transporter 2 (SGLT2) inhibitors can inhibit AKT phosphorylation, thereby upregulating STING expression.87
Strategies for Enhancing the Efficacy of STING Activators by Nanoparticles
Natural CDNs and non-nucleotide small molecule STING activators show promise in cancer immunotherapy but face significant limitations, such as poor stability, low cellular uptake, rapid clearance, systemic toxicity, and off-target effects.118 These challenges hinder their therapeutic potential and clinical translation. Nanoparticle-based delivery systems address these issues by enhancing stability, prolonging circulation time, improving tumor-specific targeting, enabling controlled release, and facilitating intracellular uptake.119,120 Therefore, nanocarrier-mediated delivery strategies play a important role in overcoming the inherent limitations of STING agonists and maximizing their antitumor efficacy.
Nanoparticles with Prolonged Circulation and Enhanced Tumor Accumulation
Lipid Nanoparticles
Lipid nanoparticles (LNPs), which are spherical vesicles encapsulating core substances, are widely used to deliver nucleic acids into cells and effectively neutralize the negative charge of CDNs. Multiple studies have utilized NPs with high fusion characteristics, named YSK05, to deliver c-di-GMP.121,122 These studies demonstrated that these NPs could transfer c-di-GMP into the cell membrane, induce the innate immune system, and promote NK cell-mediated MHC-I non-restricted antitumor immunity, offering a new direction for the immunotherapy of malignant melanoma. However, these studies have limitations, including a lack of comparison between liposomal CDN formulations and free CDNs in terms of antitumor activity, making it difficult to prove the clinical value of liposomal delivery.122 PEGylated liposomal formulations significantly enhance STING pathway activation by improving the pharmacokinetics and tumor-targeting efficiency of cGAMP.90 Compared to free cGAMP, polyethylene glycol (PEG)-modified liposomes (eg, PEG5-cGAMP and PEG10-cGAMP) exhibit prolonged circulation and increased accumulation in tumor-resident APCs, leading to robust immune activation within the tumor microenvironment (Figure 2A and B). This is evidenced by a 200-fold upregulation of IFNB1 and a 1400-fold increase in CXCL9 expression in the lungs of tumor-bearing mice (Figure 2C). Furthermore, PEGylated formulations achieve 50% complete tumor regression and confer 100% survival upon tumor rechallenge, highlighting their superior therapeutic efficacy over non-PEGylated counterparts (Figure 2D and E). These results underscore the critical role of PEGylation in optimizing STING activator delivery and antitumor immunity. Similarly, several studies have demonstrated that conventional intravenous injection of cGAMP-LNPs more effectively activates STING than free Cgamp.123–125 However, only 2–10% of tumor cells or tumor-infiltrating immune cells uptake these LNP-delivered CDNs.124,125 This limited uptake may be due to the restricted diffusion of LNPs within the tumor extracellular matrix (ECM) and potential clearance by the reticuloendothelial system.
 |
Figure 2 Lipid nanoparticles for delivering STING activators in cancer immunotherapy. (A) Schematic of liposomal cGAMP structure and therapeutic strategy. (B) Representative flow cytometry histograms showing the time course of BMDC binding with fluorescein-cGAMP delivered in free or liposomal form. (C) Gene expression analysis of interferon-β (Ifnb1) and chemokine ligand 9 (Cxcl9) in tumor-bearing lung tissues following intravenous injection. (D) Kaplan-Meier curve of overall survival for mice treated with the specified formulation. (E) Kaplan-Meier curve of overall survival for mice previously treated with the specified formulation during the re-challenge period. ****P < 0.0001. (Adapted from Koshy ST, Cheung AS, Gu L, Graveline AR, Mooney DJ. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv Biosyst. 2017;1(1–2):1600013. © 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.90 |
Polymeric Nanoparticles
Polymeric nanoparticles offer numerous advantages in drug and gene delivery due to their versatility, biocompatibility, and tunable properties.126 Polymeric nanoparticles enhance the stability and bioavailability of therapeutic agents by protecting them from degradation during circulation. The ability to design polymers with adjustable molecular weights, charge densities, and degradability further optimizes their performance, making polymer-based systems a powerful platform for delivery of STING activators.126 For instance, a branched polymer-chlorin e6 (Ppa) conjugate (BGSSP) was developed to co-deliver the PARP inhibitor AZD2281 and the photosensitizer chlorin e6 for combined PDT and STING activation (Figure 3A).20 Upon laser irradiation, AZD2281 inhibits DNA repair caused by PDT-induced ROS, promoting cytoplasmic DNA accumulation and subsequent cGAS-STING pathway activation. In a 4T1 tumor model, AZD@BGSSP demonstrated significantly enhanced tumor accumulation compared to BGSSP alone (Figure 3B), achieving 86.3% tumor growth inhibition (Figure 3C). Furthermore, elevated levels of CCL5, CXCL10, and IFN-β in tumor tissues confirmed the potent immune activation mediated by this nanoplatform (Figure 3D). These results highlight the potential of polymer-based nanocarriers for synergistic PDT and STING activation. Similarly, a polymer metal-organic framework (PMOF) nanoparticle with a PEG shell was developed to co-deliver SR-717 and generate singlet oxygen (1O2) upon irradiation.127 The 1O2 not only induced tumor cell apoptosis but also triggered PMOF degradation for rapid SR-717 release, synergistically enhancing anticancer immunity. Beyond synthetic polymers, natural biomacromolecules such as chitosan have also been explored for STING activator delivery. Chitosan facilitates intracellular DNA release, activating the cGAS-STING pathway and promoting the production of IFNs and ISGs, which drive dendritic cell activation and cellular immunity.128 These examples underscore the potential of both synthetic and natural polymer-based systems for optimizing STING-targeted cancer immunotherapy.
 |
Figure 3 Polymeric nanoparticles for delivering STING activators in cancer immunotherapy. (A) Schematic diagram of the structure of redox/enzyme-activatable nanomedicines containing GFLG peptides and disulfide bonds after encapsulating the PARP inhibitor AZD2281. (B) Measurement of tumor site accumulation of BGSSP and AZD@BGSSP using IVIS. (C) Tumor growth inhibition (TGI) in different groups. (D) Expression of CCL5, CXCL10, and IFN-β in tumor tissues after different treatments. (G1: Control (−irradiation), G2: BGSSP (−irradiation), G3: AZD@BGSSP (−irradiation), G4: Control (+irradiation), G5: BGSSP (+irradiation), G6: AZD@BGSSP (+irradiation). **P < 0.01. (Adapted from Luo Q, Duan Z, Li X, et al. Branched polymer-based redox/enzyme-activatable photodynamic nanoagent to trigger STING-dependent immune responses for enhanced therapeutic effect. Adv Funct Mater. 2022;32(13). copyright 2021 Wiley‐VCH GmbH).20 |
Biological Membrane-Derived Nanoparticles
Biological membrane-derived nanoparticles offer unique advantages as drug and gene delivery vehicles due to their natural biocompatibility and biological origin.129 These carriers possess inherent properties such as low immunogenicity and excellent bioavailability, allowing them to evade immune clearance and achieve prolonged circulation in the body. Their ability to mimic the surface markers of parent cells enables highly specific targeting to diseased tissues or cells.130 Additionally, exosomes and cell membranes can encapsulate a wide range of therapeutic agents, including small molecules, proteins, and nucleic acids, while protecting them from degradation in the extracellular environment. The natural communication pathways of exosomes further enhance their capability to transfer genetic material or drugs across cellular barriers efficiently.131 These features make cell membrane- and exosome-based delivery systems a powerful and versatile platform for advanced therapeutics. Based on these advantages, plant-derived and cell-derived nanovesicles have been increasingly integrated into multifunctional therapeutic platforms in recent years. Plant-derived nanovesicles (PDNVs) from Artemisia annua contain plant-derived mitochondrial DNA (mtDNA), which not only serve as drug delivery carriers but also activate the cGAS-STING signaling pathway, thereby reprogramming tumor-associated macrophages (TAMs) into an antitumor phenotype.132 Meanwhile, cell membrane-coated composite nanoparticles engineered and functionalized with metallic nanomaterials have been designed to target the tumor microenvironment. By combining near-infrared-II (NIR-II) photothermal therapy with Mn²⁺-mediated STING activation, these nanoparticles enhance dendritic cell maturation and antigen presentation.21 In addition, programmable hybrid cell-derived nanovesicles (hNVs), which integrate components from different cellular sources, have demonstrated the capability to effectively recognize circulating tumor cells and preferentially accumulate at surgical sites. When loaded with cGAMP, these nanovesicles effectively inhibited postoperative tumor recurrence and metastasis in preclinical models of malignant melanoma and triple-negative breast cancer.17 Collectively, these strategies underscore the potential of membrane-based nanovesicle platforms as targeted and sustained therapeutic delivery systems. They highlight the broad applications of these multifunctional and robust systems in cancer immunotherapy and drug delivery.
Inorganic Nanoparticles
Compared to LNPs and polymeric NPs, inorganic nanoparticles exhibit unique physicochemical properties in optics, electricity, magnetism, and catalysis, garnering increasing attention in biomedical applications.133 Inorganic nanoparticles include both non-metallic and metallic types. Non-metallic nanoparticles, such as cationic SiO2 nanoparticles complexed with c-di-GMP have demonstrated extended retention in the tumor microenvironment and effective activation of tumor-infiltrating antigen-presenting cells.134 Although conventional mesoporous silica nanoparticles are generally biocompatible, their small pore sizes, slow biodegradation, and prolonged tissue retention limit their performance.135–137 Recently, silica nanoparticles engineered with a lower-density Si–O–Si matrix and enlarged pore sizes (5–10 nm) have enabled more efficient loading and delivery of biomolecules, effectively inducing robust innate and adaptive immune responses in melanoma models upon CDA delivery.138 For metallic nanoparticles, a biomimetic nano-platform (CMM-DiR) was developed by encapsulating manganese dioxide nanoparticles (MnO2 NPs) and the photothermal agent DiR within a cancer cell membrane (Figure 4A).139 In the TME, MnO2 rapidly degrades, releasing Mn²⁺ to activate STING and generating O₂ to alleviate hypoxia and increase pH, thereby promoting T lymphocyte infiltration. Simultaneously, laser irradiation triggers DiR-mediated photothermal therapy, releasing tumor-associated antigens and transforming the primary tumor into an in situ vaccine. CMM-DiR demonstrated excellent tumor-targeting and long-circulation properties, with DiR fluorescence persisting in tumor cells for up to 36 hours (Figure 4B). In a melanoma model, CMM-DiR/laser treatment significantly inhibited tumor growth and prolonged survival (Figure 4C), while flow cytometry confirmed enhanced intratumoral infiltration of CD8⁺ and CD4⁺ T cells, underscoring the synergistic role of antigen release and STING activation in vaccine efficacy (Figure 4D). Additionally, a composite nanostructure combining gold nanoparticles complexed with single-stranded DNA and DOX assembled onto Mn₃O₄ has achieved synergistic immunotherapeutic and chemotherapeutic effects.140 Collectively, these strategies underscore the potential of inorganic nanoparticle-based systems for multifaceted and efficient cancer treatment.
 |
Figure 4 Inorganic nanoparticles for delivering STING activators in cancer immunotherapy. (A) Schematic illustration of the In Situ STING Activation Vaccination (ISSAV) strategy achieved by the biomimetic nanoplatform (CMM-DiR). (B) Immunofluorescence staining of tumor tissues. Scale bar = 50 μm. (C) Survival percentages of mice under different treatments. (D) Flow cytometry histograms of intratumoral infiltration of CD8+ T cells and CD4+ T cells. **P < 0.01; ***P < 0.001. (Adapted from Nano Today. Yang X, Yang Y, Bian J, et al. Converting primary tumor towards an in situ STING-activating vaccine via a biomimetic nanoplatform against recurrent and metastatic tumors. 38. Copyright 2021, with permission from Elsevier.139 |
Nanoparticles with Enhanced Tumor Penetration and Retention
Tumor penetration is crucial for the effective delivery of drugs as well as STING activators, as their therapeutic efficacy largely depends on reaching not only the tumor periphery but also deeply embedded tumor cells and the immunosuppressive microenvironment.141 Efficient penetration ensures that the STING activators activate tumor-resident APCs, such as dendritic cells and macrophages, promoting robust production of type I interferons and other pro-inflammatory cytokines.46 This activation is essential for priming adaptive immune responses and converting “cold” tumors into “hot” ones, thereby overcoming immune resistance.142 Delivery strategies that enhance tumor penetration, such as nanoparticle carriers with shape-switching properties or surface modifications, significantly improve the therapeutic outcomes of STING activators-based immunotherapies.143
Non-Spherical Structures
In addition to the various carrier materials previously mentioned, nanotube structures have shown distinct advantages in extending the penetration and retention time of NPs in vivo.144 Non-spherical nanoparticles possess a larger surface area for contact with target cells, enhancing adhesion and promoting more effective internalization.145 This increased surface area also improves drug loading efficiency, ensuring efficacy at lower drug concentrations.146 A lipid nanodisc (LND) structure was developed by conjugating CDNs with PEGylated lipids via a cleavable linker, significantly enhancing tumor penetration compared to conventional PEG-lipids (Figures 5A and B).147 In vivo studies demonstrated that LND-CDN effectively stimulated T cell responses in tumor-draining lymph nodes (TDLNs), as confirmed by ELISPOT analysis of spleen cells co-cultured with irradiated tumor cells (Figure 5C). Moreover, LND-CDN treatment achieved complete tumor regression in all treated mice, highlighting its superior therapeutic efficacy (Figure 5D). Similarly, nanotubes self-assembled from an amyloid fibril-forming peptide were employed to load and deliver c-di-GMP, leading to enhanced expression of cytokines such as IFN-β, TNF-α, IL-6, and IL-1β in a melanoma model, thereby inhibiting distal tumor growth with lower biotoxicity.148 These studies collectively demonstrate the potential of nanotube-based and other non-spherical nanoparticles as versatile and efficient platforms for targeted drug delivery and cancer therapy.
 |
Figure 5 Nanoparticles with non-spherical shapes enhance tumor penetration and retention. (A) Schematic of LnD containing CDn-PEG-lipid. (B) Coarse-grained simulation snapshots of LnD (left) and PEGylated liposomes (right). (C) IFN-γ ELISPOT assay of MC38 tumor-bearing mice treated with LnD-CDn or liposome-CDn. (D) The survival curves of MC38 tumor-bearing mice treated via intratumoral injection with 5 nmol LnD-CDN or liposome-CDN. Adapted from Dane EL, Belessiotis-Richards A, Backlund C, et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat Mater. 2022;21(6):710–720. Creative Commons.147 |
Modification with Tumor-Permeable Ligands-
Tumor-permeable ligands are critical for enhancing the efficiency and specificity of drug delivery systems in cancer therapy. These ligands are designed to bind selectively to receptors or biomarkers overexpressed on the surface of tumor cells or within the tumor microenvironment, such as integrins, folate receptors, or transferrin receptors. By facilitating active targeting, tumor-permeable ligands improve the accumulation of therapeutic agents at the tumor site, while minimizing off-target effects on healthy tissues. Human heavy-chain ferritin nanoparticles (HFn NPs) have been utilized to deliver SR717, leveraging their ability to bind transferrin receptor 1 (TfR1) for blood-brain barrier penetration (Figure 6A).149 Furthermore, modification of HFn NPs with the tumor-penetrating peptide RGE (RGERPPR) enhances their tumor tissue distribution. This modification significantly increases the accumulation of RGE-HFn NPs in subcutaneous tumors compared to unmodified HFn NPs (Figures 6B and C). In an orthotopic glioma model, RGE-HFn NPs exhibited stronger fluorescence signals in brain tissues, confirming improved targeting efficiency (Figure 6D). Additionally, SR717-loaded RGE-HFn NPs significantly upregulated the mRNA levels of immune-related genes, including Ifnb1, Cxcl10, Cxcl9, and TNF-α in RAW and THP-1 cells, compared to free SR717, demonstrating potent immune activation (Figure 6E). These findings highlight the potential of tumor-permeable ligands in enhancing the therapeutic efficacy and specificity of drug delivery systems.
 |
Figure 6 Tumor- permeable ligand nanoparticles enhances the targeted effect of STING activators. (A) Structural diagram of SR717@RGE-HFn NPs. (B) Quantitative analysis of the penetration depth of Dox@HFn and Dox@RGE-HFn NPs in GL261 glioblastoma spheroids. (C) Representative ex vivo images of subcutaneous GL261 xenograft tumors and organs treated with PBS, Cy5.5-labeled HFn, or RGE-HFn NPs. (D) Representative IVIS images of brain tissues from mice bearing orthotopic GL261 gliomas. (E) qRT-PCR analysis of mRNA expression levels following treatment with SR717@RGE-HFn NPs, free SR717, or PBS as a control. *P < 0.05; **P < 0.01; ***P < 0.001. Adapted from Wang B, Tang M, Yuan Z, et al. Targeted delivery of a STING agonist to brain tumors using bioengineered protein nanoparticles for enhanced immunotherapy. Bioact Mater. 2022;16:232–248. Creative Commons.149 |
In Suit-Forming Hydrogel for Enhanced Tumor Retention
Recent advances have led to the development of in situ forming hydrogels that substantially enhance the retention of STING activators within tumors, thereby amplifying antitumor immunity. For example, one strategy uses a silk protein‐based injectable hydrogel that enable self-assembly into nanotubes in aqueous solution.150 Negatively charged CDA were electrostatically complexed onto the positively charged NT surface, forming a spherical hydrogel post-injection (Figure 7A). Compared to free CDA, which showed rapid fluorescence decline, CDA-NT maintained high fluorescence intensity in tumors for up to 15 days, indicating sustained drug retention (Figure 7B). In a GL-261 glioblastoma model, CDA-NT hydrogel treatment achieved significant tumor regression and 100% survival, outperforming both free CDA and locally delivered CDA (Figure 7C). Furthermore, CDA-NT treatment enhanced intratumoral infiltration of CD8⁺ T cells and increased the CD8⁺/CD4⁺Foxp3⁺ regulatory T cell ratio, demonstrating potent immune activation (Figure 7D and E). Together, these innovative in situ forming hydrogels offer a promising strategy to maintain high local concentrations of STING activators, ensuring robust and sustained immune responses against tumors.
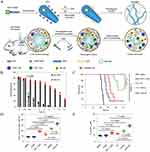 |
Figure 7 In suit-forming hydrogel for delivering STING activators in cancer immunotherapy. (A) Schematics of localized CPT and CDA delivery using a bioresponsive CPT-based nanotube hydrogel for TME regulation and chemoimmunotherapy. (B) Quantitative detection of the intratumoral retention profile of CDA-Cy7. (C) Survival percentages after different treatments. (D) CD8+ T-cell levels were quantified in C57BL/6 mice bearing GL-261 tumors. (E) The ratio of tumor-infiltrating CD8+ T effector (Teff) cells to CD4+Foxp3+ regulatory T (Treg) cells was assessed in C57BL/6 mice bearing GL-261 tumors. Adapted from Wang F, Su H, Xu D, et al. Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat Biomed Eng. 2020;4(11):1090–1101, with permission from SNCSC.150 |
Stimuli-Responsive Nanoparticles for Cytoplasmic Release of STING Activator
pH-Responsive Nanoparticles
pH-responsive delivery systems play a crucial role in tumor therapy, leveraging the acidic characteristics of the tumor microenvironment (pH ~6.5–6.8) and the even lower pH within lysosomes/endosomes (pH~4.5–5.5).151 These systems can trigger the release of drugs or genetic materials in response to the acidic environment either outside or inside tumor cells. For activation of STING protein in cytoplasm, the pH within endosome offers an ideal stimulus for targeted delivery of STING activators. One strategy involves encapsulating oxaliplatin and an immunomodulatory PC7A into nanoparticles that, under acidic conditions, simultaneously release oxaliplatin, which induces DNA damage and activates the STING pathway through the resulting DNA fragments.151 Additionally, the nanoparticles release PC7A monomers that directly bind to STING, triggering a robust immune response and leading to tumor eradication in preclinical models. Another strategy employs pH-responsive nano-prodrugs formulated from amphiphilic diblock copolymers (PEG-b-PDPA) to deliver DMXAA selectively at pH 6.0, thereby activating STING in dendritic cells while preventing premature drug release during circulation; such systems have markedly inhibited tumor progression in melanoma and breast cancer models.152 Furthermore, the nanoSTING-VAX platform was developed using pH-responsive polymer vesicles to co-deliver CDNs, peptide antigens, and adjuvants, enabling tailored cellular immunity against cancer (Figure 8A).153 Fluorescence imaging confirmed enhanced antigen accumulation in inguinal lymph nodes when encapsulated within polymer vesicles (Figure 8B). The vesicles retained pH-responsive degradation capabilities, as evidenced by reduced nanoparticle diameter at endosomal pH (Figure 8C). In vivo, nanoSTING-VAX combined with immunotherapy significantly inhibited tumor growth and prolonged survival (Figure 8D). Additionally, intracellular cytokine staining revealed that nanoSTING-VAX increased the frequency of multifunctional (IFN-γ⁺, TNF-α⁺) antigen-specific CD8⁺ T cells compared to free cGAMP and synthetic long peptides, demonstrating potent immune activation (Figure 8E). Collectively, these systems harness the unique pH conditions of tumor tissues to achieve precise, controlled release and effective STING pathway activation, offering promising strategies for cancer therapy.
 |
Figure 8 pH-responsive nanoparticles enhance nanoparticle delivery. (A) Schematic illustration of the nanoSTING-vax structure. (B) Representative fluorescence images of the draining inguinal lymph nodes (LNs) at the vaccine site 18 hours after subcutaneous administration of nanoSTING-vax. (C) Dynamic light scattering analysis of the number-average particle size distribution of STING-NPs under extracellular and endosomal pH conditions. (D) Tumor survival curves of mice. (E) Percentage of IFN-γ+, TNF-α+, CD8α, + T cells in peripheral blood after ex vivo stimulation with Reps1 and Adpgk epitopes. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Adapted with permission from Shae D, Baljon JJ, Wehbe M, et al. Co-delivery of peptide neoantigens and stimulator of interferon genes agonists enhances response to cancer vaccines. Acs Nano. 2020;14(8):9904–9916, copyright 2020, American Chemical Society.153 |
Reactive Oxygen Species-Responsive Nanoparticles
ROS-responsive nanoparticles have emerged as a promising approach for targeted tumor therapy. Tumor cells often exhibit elevated levels of ROS compared to normal cells, making this a key trigger for drug release. For delivery of STING activators, these systems also enable cell cytoplasm-specific delivery, enhancing activation of STING pathway. For example, a ROS-responsive chitosan hydrogel—synthesized via conjugation of (methylthio) acetic acid to chitosan—enables sustained release of the STING activator DMXAA and indocyanine green (ICG) in the tumor microenvironment, thereby converting an immunosuppressive milieu into an immunogenic one while ensuring excellent biocompatibility and biodegradability.154 Additionally, a ROS-responsive nanoparticle was developed through the self-assembly of a cisplatin-camptothecin prodrug and a ROS-sensitive polymer. This system enables tumor-specific drug release and simultaneously activates both DNA damage and the cGAS-STING pathway (Figure 9A).11 Platinum release was significantly enhanced by H2O2 in a concentration-dependent manner (Figure 9B). In vivo, these nanoparticles demonstrated potent tumor suppression, as indicated by reduced tumor weight (Figure 9C). In vitro analyses revealed elevated IFN-β and IL-6 levels (Figure 9D, left/middle), enhanced dendritic cell maturation, extensive tumor cell apoptosis (Figure 9D, right), and DNA fragmentation (Figure 9E).
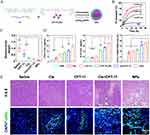 |
Figure 9 ROS-responsive nanoparticles enhance nanoparticle delivery. (A) CPT-Pt(IV) can self-assemble into nanoparticles NPs with the ROS-sensitive polymer (P1) and the lipid polymer mPEG2k-DSPE. (B) Cumulative Pt release from nanoparticles under different conditions at 37°C. (C) Tumor relative tumor weight. (D) Levels of IFN-β and IL-6 in the supernatant of CT26 cells were measured, along with the quantification of the mature BMDCs. (E) H&E and TUNEL staining of tumor tissues from different groups. *P < 0.05; **P < 0.01; ***P < 0.001. Adapted from Cao L, Tian H, Fang M, et al. Activating cGAS-STING pathway with ROS-responsive nanoparticles delivering a hybrid prodrug for enhanced chemo-immunotherapy. Biomaterials. 2022;290:121856. Creative Commons.11 |
Reduction-Responsive Nanoparticles
Reduction-responsive nanoparticles are designed to exploit the reductive environment characteristic within tumor environment, particularly within the intracellular compartments like glutathione (GSH)-rich areas.155 Tumor cells often exhibit elevated levels of reducing agents, such as GSH, compared to normal tissues. Reduction-responsive systems incorporate disulfide bonds or other redox-sensitive linkages that can be cleaved in the presence of high GSH concentrations, triggering the release of therapeutic agents.156 Based on it, these systems allow for precise delivery of STING activators, improving therapeutic outcomes while reducing off-target side effects. In this context, reduction-responsive biodegradable polymers were developed to enhance tumor retention and cytosolic delivery of ADU-S100, promoting robust STING activation in tumor-draining lymph nodes (Figure 10A).157 CPs exhibited glutathione (GSH)-dependent drug release, with only 7.4% CDN released under physiological conditions versus 58.9% in 10 mm GSH (Figure 10B). In a B16F10 melanoma model, combining CPs-CDN with fractionated low-dose X-ray irradiation (3 Gy) achieved superior tumor suppression compared to free CDN or radiation alone. This combination also extended median survival to 42 days, significantly surpassing other groups (Figure 10C). Further analysis revealed that 5 Gy + CPs-CDN treatment increased CD8⁺ T cell infiltration and elevated plasma levels of IFN-β and TNF-α, demonstrating enhanced systemic immune activation (Figure 10D).
 |
Figure 10 Reduction-responsive nanoparticles enhance nanoparticle delivery. (A) Structural diagram of CPs-CDN. (B) In vitro release of Cy3-diAMP from CPs in the presence or absence of 10 mm GSH. (C) Tumor survival rates after treatment with different formulations with or without irradiation. (D) The proportion of CD8+ T cells in the tumor, the expression of CD206 on macrophages in the TME, and the plasma concentrations of IFN-β and TNF-α in mice. *P < 0.05; **P < 0.01; ***P < 0.001. Adapted from Zheng H, Guo B, Qiu X, et al. Polymersome-mediated cytosolic delivery of cyclic dinucleotide STING agonist enhances tumor immunotherapy. Bioact Mater. 2022;16:1–11. Creative Commons.157 |
Photo-Responsive Nanoparticles
Photo-responsive nanoparticles have emerged as a powerful tool for tumor-targeted drug delivery, utilizing light as an external stimulus to achieve precise control over drug release.158 These nanoparticles are typically designed with photosensitive components, such as photo-cleavable linkages, photosensitizers, or photo-thermal agents, which respond to specific wavelengths of light (eg, near-infrared).158 Upon irradiation, these nanoparticles undergo structural changes, generate heat, or produce ROS, triggering the release of therapeutic agents directly at the tumor site. This approach minimizes off-target effects and enhances the therapeutic efficacy of drugs. For example, a multifunctional plasmonic gold-blackbody (AuPB) photo-responsive nano-adjuvant coated with polydopamine (PDA) and loaded with Mn2+ (AuPB@PDA/Mn) was reported.159 Triggered by second NIR-II light, AuPB@PDA/Mn induced local hyperthermia and released Mn2+ ions, activating STING. In a mouse colon cancer model, NIR-II light activation of AuPB@PDA/Mn significantly reduced preoperative tumor burden and minimized tumor recurrence after radical resection.
Nanoparticles-Mediated Combination With Other Therapeutics
Combining STING activator with other therapeutics is critical to maximizing their immunotherapeutic potential, as this approach leverages complementary mechanisms to overcome tumor resistance and enhance anti-tumor efficacy.36 STING activation alone induces innate immune responses and promotes the recruitment and activation of cytotoxic T cells, but combining it with immune checkpoint inhibitors (eg, anti-PD-1/PD-L1) can further amplify adaptive immunity by relieving T-cell exhaustion.160 Similarly, pairing STING activator with chemotherapies, photodynamic/photothermal Therapy (PDT/PTT) or radiotherapies enhances tumor antigen release, creating a more immunogenic environment.161 These synergistic strategies not only improve therapeutic outcomes but also expand the scope of STING activator to treat immunologically “cold” tumors that are typically unresponsive to monotherapies.
Combination with PTT or PDT
PDT and PTT as PTs are extensively studied in anticancer treatment. Unlike radiation therapy and chemotherapy, which typically induce immune suppression, PT has been observed to stimulate immune responses.18,162,163 Photosensitizer molecules absorb light and convert it into ROS or heat to kill tumor cells and induce immune reactions.164 With the rise of tumor immunotherapy in recent years, many researchers have focused on combining PT with other immune stimulants or strategies to enhance tumor immune responses and anticancer treatment outcomes. For example, hollow mesoporous organosilica nanocomposites loaded with platinum nanoparticles (Pt-NPs) and the photosensitizer IR820, exposed to near-infrared irradiation, achieve PDT and PTT treatments.117 Concurrent oxidative stress leads to dual damage of nuclear and mitochondrial DNA, triggering activation of the cGAS/STING signaling pathway. In another strategy, a multifunctional nanoparticle (MDPMH) was developed to integrate PTT, chemotherapy, and immunotherapy.165 Synthesized via ultrasound-assisted self-assembly of DTX, a manganese-modified phthalocyanine sonosensitizer (MnIIIPC), Mn2+, and hyaluronic acid (HA)-PLGA hybrids (Figure 11A), MDPMH synergistically activated the STING pathway by downregulating Bcl-2 and upregulating Bax, thereby promoting tumor cell apoptosis (Figure 11B). In vitro cytotoxicity assays revealed that MDPMH combined with laser irradiation exhibited the strongest antitumor activity, as evidenced by minimal green fluorescence (viable cells) and dominant red fluorescence (dead cells) in Calcein-AM/PI staining (Figure 11C). This multimodal approach highlights the potential of MDPMH for enhancing therapeutic efficacy through coordinated PTT, chemotherapy, and immune activation. Additionally, multifunctional STING-activating nanoparticles containing double-stranded DNA and doxorubicin, which accumulate in tumors via the enhanced permeability and retention effect.140 This study have demonstrated synergistic effects in combining chemotherapy with immunotherapy, leading to effective tumor growth inhibition and prolonged survival.
 |
Figure 11 Combination of STING activator with PTT or PDT. (A) The synthetic route of MDPMH. (B) Relative expression of different proteins Bax, and Bcl-2 after different treatment. (C) A549 cells were stained with Calcein-AM under different conditions, and real-time imaging was performed using a fluorescence microscope. *P < 0.05; **P < 0.01; ***P < 0.001. Adapted from Feng X, Xiong X, Ma S. Docetaxel-loaded novel nano-platform for synergistic therapy of non-small cell lung cancer. Front Pharmacol. 2022;13. Creative Commons.165 |
Combination with Radiotherapy
In addition to surgery and chemotherapy, RT has been extensively used in cancer treatment for decades.166 Combining RT with immunotherapy is a common strategy in oncology. Therefore, numerous researchers have endeavored to synergize nanoparticle-mediated cGAS-STING activators with RT for cancer therapy. For instance, inhalable nanoparticles loaded with cGAMP have demonstrated synergistic effects with fractionated RT in lung tumors, inducing robust antitumor immunity, inhibiting lung metastases, and remodeling the tumor microenvironment.167 Moreover, cGAMP conjugated onto nanoscale metal-organic layers (MOL) not only sensitizes tumors to radiation by enhancing immunogenic cell death but also provides sustained STING activation compared to free cGAMP.18 Additionally biomineralized manganese oxide nanoparticles (Bio-MnO2 NPs), synthesized via enzyme-catalyzed biomineralization, synergize radiotherapy (RT) with cGAS-STING pathway activation. These NPs convert tumor-associated H2O2 into O2 to generate ROS, enhancing radiosensitivity in non-small cell lung cancer (NSCLC) cells. Concurrently, Mn²⁺ released from Bio-MnO2 NPs amplifies cGAS-STING signaling by upregulating phosphorylated STING, TBK1, and IRF3 (Figure 12A and C).168 In a subcutaneous NSCLC model, Bio-MnO₂ NPs combined with fractionated RT (8 Gy × 3) achieved the most pronounced tumor growth inhibition compared to controls (Figure 12B), demonstrating the potential of Mn²⁺-mediated STING activation to enhance therapeutic outcomes.
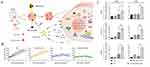 |
Figure 12 Combination of STING activator with radiotherapy. (A) Schematic diagram illustrating the enhanced therapeutic efficacy of STING agonists in tumor treatment through the combination of Bio-MnO2 nanoparticles and radiotherapy. (B) Tumor growth curves of the combination treatment with Bio-MnO2 nanoparticles and radiotherapy. (C) mRNA levels of C-C motif chemokine ligand 5 (CCL5), CXC motif chemokine ligand 10 (CXCL10), and interferon-β (IFN-β) were assessed in A549 and PC9 cells following various treatments. *P < 0.05; **P < 0.01; ***P < 0.001. Adapted from Liu X, Kifle MT, Xie H, et al. Biomineralized manganese oxide nanoparticles synergistically relieve tumor hypoxia and activate immune response with radiotherapy in non-small cell lung cancer. Nanomaterials. 2022;12(18). Creative Commons.168 |
Combination with Immune Checkpoint Therapy
The combination of immune checkpoint inhibitors (ICIs) and STING activators represents a powerful synergy in cancer immunotherapy, addressing complementary aspects of the immune response. Immune checkpoint inhibitors, such as anti-PD-1/PD-L1 antibodies, function by blocking inhibitory signals on T cells, thereby restoring their activity and preventing T-cell exhaustion within the tumor microenvironment.169 However, ICIs alone are often insufficient in “cold” tumors, where there is a lack of immune cell infiltration and tumor antigen presentation. For example, intra-tumoral cyclic dinucleotide therapy combined with systemic extended half-life IL-2 and anti-PD-1 treatment has been shown to halt primary tumor progression and achieve long-term remission in metastatic breast cancer models.170 In addition, a triple-combination nanoplatform (GPS) was engineered by encapsulating the STING activator diABZI within a polymer-conjugated gemcitabine (GEM) prodrug and surface-modifying it with PD-L1 antibodies (αPD-L1) for targeted delivery (Figure 13A).163 The αPD-L1 conjugation significantly enhanced nanoparticle internalization in tumor cells (Figure 13B). In a postoperative recurrence model, GPS therapy completely suppressed metastasis and extended survival, outperforming monotherapy or dual therapy (Figure 13C). Immunoassays further revealed that GPS elevated intratumoral IFN-β and IL-6 levels, confirming enhanced STING pathway activation through efficient activator delivery (Figure 13D). Moreover, a nanoplatform composed of hollow manganese dioxide loaded with MSA-2 and modified with hyaluronic acid to deliver CRISPR-Cas9/sg-PD-L1 plasmids has enhanced tumor microenvironment immunogenicity, effectively suppressing both primary and metastatic cancers.171 Complementarily, engineering CAR-T cells with STING activators such as DMXAA or cGAMP-treated Th/Tc17 cells further improves tumor control.171
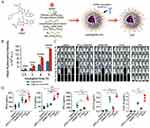 |
Figure 13 Combination of STING activator with immune checkpoint therapy. (A) Schematic diagram of the generation of GEM NP and αPD-L1/GEM NP. (B) PanCO2 cells were treated with DiI@GEM NPs or DiI@αPD-L1/GEM NPs at different time points, and cellular uptake of the two NPs was evaluated using flow cytometry analysis. (C) In vivo bioluminescence imaging of 4T1 recurrence and metastasis in different treatment groups. (D) IFN-β, IL-6, IFN-γ, CXCL9, and CXCL10 levels in the 4T1 TME. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Adapted from Shi X, Shu L, Wang M, et al. Triple-combination immunogenic nanovesicles reshape the tumor microenvironment to potentiate chemo-immunotherapy in preclinical cancer models. Adv Sci. 2023;10(15). © 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.163 |
Conclusions and Outlook
STING plays a crucial role in cancer immunity by activating type I interferons and recruiting immune-related cells, effectively eliciting innate and adaptive immune responses, thereby demonstrating clinical potential in cancer therapy. Currently, an increasing number of STING activators are being discovered or synthesized and validated through experiments. However, these activators face challenges such as difficulty in crossing the cell membrane, susceptibility to degradation, poor pharmacokinetics, nonspecific biodistribution, and toxicity, making it challenging to achieve optimal therapeutic effects through systemic administration via intravenous injection. Intratumoral administration may encounter certain difficulties in drug development and application for different indications.
Nanoparticle-based delivery systems present an effective strategy for improving the clinical efficacy of STING activators. Nanoparticles can be used to facilitate the intravenous administration of STING activators. First, nanoparticles formulated with PEG ot coated with natural biological membranes retain the physicochemical properties of nanomaterials while maintaining the homotypic advantages of cells, thereby facilitating targeted therapy against immune-evasive tumor cells. Additionally, novel nanomaterials have the potential to enable precise targeted therapy, reducing drug dosage and toxicity. By utilizing the microenvironments within tumors, the release of STING activators release can be controlled and directed specifically at tumor sites, limiting harmful effects on normal tissues. Moreover, multifunctional nanoparticle probes designed for both cancer diagnosis and treatment enable simultaneous tumor imaging, precise therapy, and real-time assessment of drug distribution, release, and efficacy.
Combining nanoscale STING activators with novel treatments such as chemotherapy, radiotherapy, phototherapy, and other immunotherapy synergistically targets tumor cells through multiple mechanisms. This approach reduces resistance commonly observed with single therapies and maintains sustained immune pressure on tumor cells, effectively preventing tumor recurrence and metastasis. Radiotherapy and chemotherapy enhance tumor cell immunogenicity and modify the tumor microenvironment, facilitating immune cell infiltration and action. This synergy reduces the requirement for STING activators and induces robust immune responses.36 Phototherapy directly kills tumor cells upon light exposure using photosensitizers to generate reactive oxygen species or heat, triggering tumor antigen release that further augment immune responses initiated by nanoscale STING activators. Notably, integrating nanoparticle-mediated STING activation with immune checkpoint inhibitors further amplifies antitumor immunity by reinvigorating exhausted T cells and converting immunologically “cold” tumors into “hot” ones. Preclinical studies demonstrate that triple-combination strategies (eg, STING activators + PD-L1 blockade + chemotherapy) significantly suppress metastasis and establish long-term immune memory. Cancer vaccines introduce tumor-associated antigens to enhance immune memory and comprehensive immune activation when combined with nanoscale STING activators. These multimodal approaches highlight the transformative potential of STING-targeted nanotherapies in overcoming drug resistance and achieving durable clinical responses.
While NP-mediated STING therapy has shown promise in both preclinical and clinical experiments, several issues still require addressing in future research. Firstly, the cGAS-STING signaling pathway can either recruit immune-supporting cells to suppress malignant transformation or recruit immune-suppressive cells to drive tumor progression, demonstrating a dual effect on tumor development. Highly invasive and unstable tumors can paradoxically exploit STING signaling to stimulate tumor progression. Therefore, further research is needed to elucidate the underlying mechanisms of STING-mediated immune responses in specific tumors, with careful consideration given to the location and dosage of STING release when designing NPs for accurate in vivo delivery. Secondly, multifunctionally modified NPs hold promise for achieving precise STING delivery, but this approach complicates preparation processes, leading to low yields, high costs, batch-to-batch variability, and poor storage stability. Therefore, translating these NPs into clinical practice should involve standardizing preparation processes, optimizing production procedures, and validating safety. To address the dual role of the STING pathway in diverse tumor microenvironments, in-depth investigations into its immunomodulatory mechanisms are warranted. Single-cell sequencing technologies should be leveraged to delineate tumor type-specific STING activation patterns, integrated with stimuli-responsive delivery systems (eg, pH- or enzyme-sensitive nanoparticles, NPs) for spatiotemporally controlled release. Concurrently, optimizing dosage regimens, implementing tumor-targeting modifications, and combining STING activators with immune checkpoint inhibitors or adoptive cell therapies could enhance activation specificity and mitigate protumoral effects. Regarding challenges in multifunctional NP development such as fabrication complexity, high costs, and instable strategies including self-assembly techniques, microfluidic manufacturing, and standardized quality control protocols should be adopted to streamline production, improve batch-to-batch consistency, and enhance storage stability. Lyophilization and cryoprotectant formulations may further extend NP shelf life. Systematic toxicological evaluations and clinical validation are imperative to ensure safety and efficacy, ultimately accelerating the clinical translation of STING-targeted nanomedicines. In conclusion, NP-mediated STING activators present significant potential in combating cancer, but several challenges need addressing in future research to maximize their therapeutic efficacy.
Acknowledgments
Yi Qiao and Lingyu Wei contributed equally to this work. This study received financial support from the National Natural Science Foundation of China (Grant 82404089, 82403998); the Natural Science Foundation of Shandong Province (ZR2024QH266); and Youth Research Fund of Affiliated Hospital of Qingdao University (QDFYQN2023117).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Song W, Song SJ, Kuang J, et al. Activating innate immunity by a STING signal amplifier for local and systemic immunotherapy. ACS Nano. 2022;16(10):15977–15993. doi:10.1021/acsnano.2c03509
2. Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. doi:10.1038/nri3581
3. Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24(1):93–103. doi:10.1016/j.immuni.2005.12.003
4. Gao M, He Y, Tang H, Chen X, Liu S, Tao Y. cGAS/STING: novel perspectives of the classic pathway. mol Biomed. 2020;1(1):7. doi:10.1186/s43556-020-00006-z
5. Spadaro F, Lapenta C, Donati S, et al. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119(6):1407–1417. doi:10.1182/blood-2011-06-363564
6. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–353. doi:10.1038/ni.3123
7. Wu JJ, Zhao L, Hu HG, Li WH, Li YM. Agonists and inhibitors of the STING pathway: potential agents for immunotherapy. Med Res Rev. 2020;40(3):1117–1141. doi:10.1002/med.21649
8. Zhao L, Zhuo SH, Wang TY, et al. Cyclic dinucleotide self-assembled nanoparticles as a carrier-free delivery platform for STING-mediated cancer immunotherapy. CCS Chemistry. 2023;6(1):177–195. doi:10.31635/ccschem.023.202302751
9. Guo J, Huang L. Nanodelivery of cGAS-STING activators for tumor immunotherapy. Trends Pharmacol Sci. 2022;43(11):957–972. doi:10.1016/j.tips.2022.08.006
10. Sun Q, Zhou Z, Qiu N, Shen Y. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29(14). doi:10.1002/adma.201606628
11. Cao L, Tian H, Fang M, et al. Activating cGAS-STING pathway with ROS-responsive nanoparticles delivering a hybrid prodrug for enhanced chemo-immunotherapy. Biomaterials. 2022;290:121856. doi:10.1016/j.biomaterials.2022.121856
12. Chen X, Xu Z, Li T, et al. Nanomaterial-encapsulated STING agonists for immune modulation in cancer therapy. Biomark Res. 2024;12(1):2. doi:10.1186/s40364-023-00551-z
13. Zhu L, Wu J, Gao H, et al. Tumor immune microenvironment-modulated nanostrategy for the treatment of lung cancer metastasis. Chin Med J. 2023;136(23):2787–2801. doi:10.1097/CM9.0000000000002525
14. Hao Y, Ji Z, Zhou H, et al. Lipid-based nanoparticles as drug delivery systems for cancer immunotherapy. MedComm. 2023;4(4):e339. doi:10.1002/mco2.339
15. Fan Y, Moon JJ. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines. 2015;3(3):662–685. doi:10.3390/vaccines3030662
16. Ma L, Hernandez MO, Zhao Y, et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell. 2019;36(4):418–430.e6. doi:10.1016/j.ccell.2019.08.007
17. Rao L, Wu L, Liu Z, et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun. 2020;11(1):4909. doi:10.1038/s41467-020-18626-y
18. Luo T, Nash GT, Jiang X, et al. A 2D nanoradiosensitizer enhances radiotherapy and delivers STING agonists to potentiate cancer immunotherapy. Adv Mater. 2022;34(39). doi:10.1002/adma.202110588
19. Zhang X, Wei Z, Ding Z, et al. Boosting doxil-based chemoimmunotherapy via reprogramming tumor-associated macrophages. Chem Eng J. 2023:451. doi:10.1016/j.cej.2022.138971
20. Luo Q, Duan Z, Li X, et al. Branched polymer-based redox/enzyme-activatable photodynamic nanoagent to trigger STING-dependent immune responses for enhanced therapeutic effect. Adv Funct Mater. 2022;32(13). doi:10.1002/adfm.202110408
21. Zhao Y, Pan Y, Zou K, et al. Biomimetic manganese-based theranostic nanoplatform for cancer multimodal imaging and twofold immunotherapy. Bioact Mater. 2023;19:237–250. doi:10.1016/j.bioactmat.2022.04.011
22. Luo M, Wang H, Wang Z, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotech. 2017;12(7):648–+. doi:10.1038/nnano.2017.52
23. Gentili M, Lahaye X, Nadalin F, et al. The N-terminal domain of cGAS determines preferential association with centromeric DNA and innate immune activation in the nucleus. Cell Rep. 2019;26(13):3798. doi:10.1016/j.celrep.2019.03.049
24. Shen R, Liu D, Wang X, et al. DNA damage and activation of cGAS/STING pathway induce tumor microenvironment remodeling. Front Cell Dev Biol. 2022;9:828657. doi:10.3389/fcell.2021.828657
25. Ritchie C, Carozza JA, Li L. Biochemistry, cell biology, and pathophysiology of the innate immune cGAS-cGAMP-STING pathway. Annu Rev Biochem. 2022;91:599–628. doi:10.1146/annurev-biochem-040320-101629
26. Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev mol Cell Biol. 2012;13(12):780–788. doi:10.1038/nrm3479
27. Mackenzie KJ, Carroll P, Martin CA, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548(7668):461–465. doi:10.1038/nature23449
28. Wu J, Li W, Shao Y, et al. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe. 2015;18(3):333–344. doi:10.1016/j.chom.2015.07.015
29. Yin Q, Tian Y, Kabaleeswaran V, et al. Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol Cell. 2012;46(6):735–745. doi:10.1016/j.molcel.2012.05.029
30. Zhang X, Bai XC, Chen ZJ. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity. 2020;53(1):43–53. doi:10.1016/j.immuni.2020.05.013
31. Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5(214):ra20. doi:10.1126/scisignal.2002521
32. Balka KR, Louis C, Saunders TL, et al. TBK1 and IKKε act redundantly to mediate STING-induced NF-κB responses in myeloid cells. Cell Rep. 2020;31(1):107492. doi:10.1016/j.celrep.2020.03.056
33. Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J Virol. 2014;88(10):5328–5341. doi:10.1128/JVI.00037-14
34. Chu TT, Tu X, Yang K, Wu J, Repa JJ, Yan N. Tonic prime-boost of STING signalling mediates Niemann-Pick disease type C. Nature. 2021;596(7873):570–575. doi:10.1038/s41586-021-03762-2
35. Ma Z, Jacobs SR, West JA, et al. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc Natl Acad Sci U S A. 2015;112(31):E4306–4315. doi:10.1073/pnas.1503831112
36. Li Y, Li X, Yi J, et al. Nanoparticle-mediated STING activation for cancer immunotherapy. Adv Funct Mater. 2023. doi:10.1002/adhm.202300260
37. Rongvaux A. Innate immunity and tolerance toward mitochondria. Mitochondrion. 2018;41:14–20. doi:10.1016/j.mito.2017.10.007
38. Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142–1149. doi:10.1038/ni.3558
39. Glück S, Guey B, Gulen MF, et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol. 2017;19(9):1061–1070. doi:10.1038/ncb3586
40. Chen YA, Shen YL, Hsia HY, Tiang YP, Sung TL, Chen LY. Extrachromosomal telomere repeat DNA is linked to ALT development via cGAS-STING DNA sensing pathway. Nat Struct mol Biol. 2017;24(12):1124–1131. doi:10.1038/nsmb.3498
41. Dou Z, Ghosh K, Vizioli MG, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550(7676):402–406. doi:10.1038/nature24050
42. Paijo J, Döring M, Spanier J, et al. cGAS senses human cytomegalovirus and induces type i interferon responses in human monocyte-derived cells. PLoS Pathog. 2016;12(4):e1005546. doi:10.1371/journal.ppat.1005546
43. Lio CWJ, McDonald B, Takahashi M, et al. cGAS-STING signaling regulates initial innate control of cytomegalovirus infection. J Virol. 2016;90(17):7789–7797. doi:10.1128/JVI.01040-16
44. Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341(6152):1390–1394. doi:10.1126/science.1244040
45. Schoggins JW, MacDuff DA, Imanaka N, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505(7485):691–695. doi:10.1038/nature12862
46. Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760–770. doi:10.1038/nri3921
47. Sun B, Sundström KB, Chew JJ, et al. Dengue virus activates cGAS through the release of mitochondrial DNA. Sci Rep. 2017;7(1):3594. doi:10.1038/s41598-017-03932-1
48. Berthelot JM, Drouet L, Lioté F. Kawasaki-like diseases and thrombotic coagulopathy in COVID-19: delayed over-activation of the STING pathway? Emerg Microbes Infect. 2020;9(1):1514–1522. doi:10.1080/22221751.2020.1785336
49. Zhang Y, Yeruva L, Marinov A, et al. The DNA sensor, cyclic GMP-AMP synthase, is essential for induction of IFN-β during Chlamydia trachomatis infection. J Immunol. 2014;193(5):2394–2404. doi:10.4049/jimmunol.1302718
50. Wassermann R, Gulen MF, Sala C, et al. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 2015;17(6):799–810. doi:10.1016/j.chom.2015.05.003
51. Storek KM, Gertsvolf NA, Ohlson MB, Monack DM. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J Immunol. 2015;194(7):3236–3245. doi:10.4049/jimmunol.1402764
52. Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272(5270):1947–1950. doi:10.1126/science.272.5270.1947
53. Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. cGAS-STING pathway in cancer biotherapy. mol Cancer. 2020;19(1):136. doi:10.1186/s12943-020-01247-w
54. Bao T, Liu J, Leng J, Cai L. The cGAS-STING pathway: more than fighting against viruses and cancer. Cell Biosci. 2021;11(1):209. doi:10.1186/s13578-021-00724-z
55. Yoon SH, Waters CM. The ever-expanding world of bacterial cyclic oligonucleotide second messengers. Curr Opin Microbiol. 2021;60:96–103. doi:10.1016/j.mib.2021.01.017
56. Huiting E, Cao X, Ren J, et al. Bacteriophages inhibit and evade cGAS-like immune function in bacteria. Cell. 2023;186(4):864–876.e21. doi:10.1016/j.cell.2022.12.041
57. Gutjahr A, Papagno L, Nicoli F, et al. The STING ligand cGAMP potentiates the efficacy of vaccine-induced CD8+ T cells. JCI Insight. 2019;4(7):e125107. doi:10.1172/jci.insight.125107
58. Burdette DL, Monroe KM, Sotelo-Troha K, et al. STING is a direct innate immune sensor of cyclic-di-GMP. Nature. 2011;478(7370):515–518. doi:10.1038/nature10429
59. Ebensen T, Libanova R, Schulze K, Yevsa T, Morr M, Guzmán CA. Bis-(3’,5’)-cyclic dimeric adenosine monophosphate: strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine. 2011;29(32):5210–5220. doi:10.1016/j.vaccine.2011.05.026
60. Škrnjug I, Rueckert C, Libanova R, Lienenklaus S, Weiss S, Guzmán CA. The mucosal adjuvant cyclic di-AMP exerts immune stimulatory effects on dendritic cells and macrophages. PLoS One. 2014;9(4):e95728. doi:10.1371/journal.pone.0095728
61. Li T, Cheng H, Yuan H, et al. Antitumor activity of cGAMP via stimulation of cGAS-cGAMP-STING-IRF3 mediated innate immune response. Sci Rep. 2016;6:19049. doi:10.1038/srep19049
62. Tang CHA, Zundell JA, Ranatunga S, et al. Agonist-mediated activation of STING induces apoptosis in malignant b cells. Cancer Res. 2016;76(8):2137–2152. doi:10.1158/0008-5472.CAN-15-1885
63. Karaolis DKR, Cheng K, Lipsky M, et al. z. Biochem Biophys Res Commun. 2005;329(1):40–45. doi:10.1016/j.bbrc.2005.01.093
64. Um PK, Praharaj M, Lombardo KA, et al. Improved bladder cancer antitumor efficacy with a recombinant BCG that releases a STING agonist. bioRxiv. 2023;2023. doi:10.1101/2023.12.15.571740
65. Corrales L, Glickman LH, McWhirter SM, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11(7):1018–1030. doi:10.1016/j.celrep.2015.04.031
66. Ager CR, Zhang H, Wei Z, Jones P, Curran MA, Di Francesco ME. Discovery of IACS-8803 and IACS-8779, potent agonists of stimulator of interferon genes (STING) with robust systemic antitumor efficacy. Bioorg Med Chem Lett. 2019;29(20):126640. doi:10.1016/j.bmcl.2019.126640
67. Chang W, Altman MD, Lesburg CA, et al. Discovery of MK-1454: a potent cyclic dinucleotide stimulator of interferon genes agonist for the treatment of cancer. J Med Chem. 2022;65(7):5675–5689. doi:10.1021/acs.jmedchem.1c02197
68. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi:10.1146/annurev-immunol-032712-100008
69. Downey CM, Aghaei M, Schwendener RA, Jirik FR. DMXAA causes tumor site-specific vascular disruption in murine non-small cell lung cancer, and like the endogenous non-canonical cyclic dinucleotide STING agonist, 2’3’-cGAMP, induces M2 macrophage repolarization. PLoS One. 2014;9(6):e99988. doi:10.1371/journal.pone.0099988
70. Ramanjulu JM, Pesiridis GS, Yang J, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564(7736):439–443. doi:10.1038/s41586-018-0705-y
71. Liu B, Tang L, Zhang X, et al. A cell-based high throughput screening assay for the discovery of cGAS-STING pathway agonists. Antiviral Res. 2017;147:37–46. doi:10.1016/j.antiviral.2017.10.001
72. Zhang X, Liu B, Tang L, et al. Discovery and mechanistic study of a novel human-stimulator-of-interferon-genes agonist. ACS Infect Dis. 2019;5(7):1139–1149. doi:10.1021/acsinfecdis.9b00010
73. Pan BS, Perera SA, Piesvaux JA, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020;369(6506):eaba6098. doi:10.1126/science.aba6098
74. Chin EN, Yu C, Vartabedian VF, et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science. 2020;369(6506):993–999. doi:10.1126/science.abb4255
75. Kim DS, Endo A, Fang FG, et al. E7766, a macrocycle-bridged stimulator of interferon genes (STING) agonist with potent pan-genotypic activity. ChemMedChem. 2021;16(11):1740–1743. doi:10.1002/cmdc.202100068
76. Grabosch S, Bulatovic M, Zeng F, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019;38(13):2380–2393. doi:10.1038/s41388-018-0581-9
77. Wang X, Huang R, Wu W, et al. Amplifying STING activation by bioinspired nanomedicine for targeted chemo- and immunotherapy of acute myeloid leukemia. Acta Biomaterialia. 2023;157:381–394. doi:10.1016/j.actbio.2022.11.007
78. Hu Y, Manasrah BK, McGregor SM, et al. Paclitaxel induces micronucleation and activates pro-inflammatory cGAS-STING signaling in triple-negative breast cancer. mol Cancer Ther. 2021;20(12):2553–2567. doi:10.1158/1535-7163.MCT-21-0195
79. Tian J, Zhang D, Kurbatov V, et al. 5-Fluorouracil efficacy requires anti-tumor immunity triggered by cancer-cell-intrinsic STING. EMBO J. 2021;40(7):e106065. doi:10.15252/embj.2020106065
80. Pantelidou C, Sonzogni O, De Oliveria Taveira M, et al. PARP inhibitor efficacy depends on CD8+ T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discovery. 2019;9(6):722–737. doi:10.1158/2159-8290.CD-18-1218
81. Sen T, Rodriguez BL, Chen L, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9(5):646–661. doi:10.1158/2159-8290.CD-18-1020
82. Sun X, Zhang Y, Li J, et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat Nanotech. 2021;16(11):1260–+. doi:10.1038/s41565-021-00962-9
83. Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type i interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi:10.1016/j.immuni.2014.10.019
84. Hu M, Zhou M, Bao X, et al. ATM inhibition enhances cancer immunotherapy by promoting mtDNA leakage and cGAS/STING activation. J Clin Invest. 2021;131(3):e139333,139333. doi:10.1172/JCI139333
85. Li S, Luo M, Wang Z, et al. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat Biomed Eng. 2021;5(5):455–466. doi:10.1038/s41551-020-00675-9
86. Hsu SC, Chen CL, Cheng ML, et al. Arginine starvation elicits chromatin leakage and cGAS-STING activation via epigenetic silencing of metabolic and DNA-repair genes. Theranostics. 2021;11(15):7527–7545. doi:10.7150/thno.54695
87. Wu W, Zhang Z, Jing D, et al. SGLT2 inhibitor activates the STING/IRF3/IFN-β pathway and induces immune infiltration in osteosarcoma. Cell Death Dis. 2022;13(6):523. doi:10.1038/s41419-022-04980-w
88. Gonugunta VK, Sakai T, Pokatayev V, et al. Trafficking-mediated STING degradation requires sorting to acidified endolysosomes and can be targeted to enhance anti-tumor response. Cell Rep. 2017;21(11):3234–3242. doi:10.1016/j.celrep.2017.11.061
89. Motedayen Aval L, Pease JE, Sharma R, Pinato DJ. Challenges and opportunities in the clinical development of STING agonists for cancer immunotherapy. J Clin Med. 2020;9(10):3323. doi:10.3390/jcm9103323
90. Koshy ST, Cheung AS, Gu L, Graveline AR, Mooney DJ. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv Biosyst. 2017;1(1–2):1600013. doi:10.1002/adbi.201600013
91. Ding C, Song Z, Shen A, Chen T, Zhang A. Small molecules targeting the innate immune cGAS‒STING‒TBK1 signaling pathway. Acta Pharm Sin B. 2020;10(12):2272–2298. doi:10.1016/j.apsb.2020.03.001
92. Mikkola S, Lönnberg T, Lönnberg H. Phosphodiester models for cleavage of nucleic acids. Beilstein J Org Chem. 2018;14:803–837. doi:10.3762/bjoc.14.68
93. Cavlar T, Deimling T, Ablasser A, Hopfner KP, Hornung V. Species-specific detection of the antiviral small-molecule compound CMA by STING. EMBO J. 2013;32(10):1440–1450. doi:10.1038/emboj.2013.86
94. Zhang Y, Sun Z, Pei J, et al. Identification of α-mangostin as an agonist of human STING. ChemMedChem. 2018;13(19):2057–2064. doi:10.1002/cmdc.201800481
95. Hou S, Lan XJ, Li W, et al. Design, synthesis and biological evaluation of acridone analogues as novel STING receptor agonists. Bioorg Chem. 2020;95:103556. doi:10.1016/j.bioorg.2019.103556
96. Bleicher KH, Böhm HJ, Müller K, Alanine AI. Hit and lead generation: beyond high-throughput screening. Nat Rev Drug Discov. 2003;2(5):369–378. doi:10.1038/nrd1086
97. Macarron R, Banks MN, Bojanic D, et al. Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov. 2011;10(3):188–195. doi:10.1038/nrd3368
98. Lu D, Shang G, Li J, Lu Y, Bai XC, Zhang X. Activation of STING by targeting a pocket in the transmembrane domain. Nature. 2022;604(7906):557–562. doi:10.1038/s41586-022-04559-7
99. Pryde DC, Middya S, Banerjee M, et al. The discovery of potent small molecule activators of human STING. Eur J Med Chem. 2021;209:112869. doi:10.1016/j.ejmech.2020.112869
100. Fu G, Wu Y, Zhao G, et al. Activation of cGAS-STING signal to inhibit the proliferation of bladder cancer: the immune effect of cisplatin. Cells. 2022;11(19):3011. doi:10.3390/cells11193011
101. Hou L, Tian C, Yan Y, Zhang L, Zhang H, Zhang Z. Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate immunity. Acs Nano. 2020;14(4):3927–3940. doi:10.1021/acsnano.9b06111
102. Luthra P, Aguirre S, Yen BC, et al. Topoisomerase II inhibitors induce DNA damage-dependent interferon responses circumventing Ebola virus immune evasion. mBio. 2017;8(2):e00368–17. doi:10.1128/mBio.00368-17
103. Chabanon RM, Muirhead G, Krastev DB, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Invest. 2019;129(3):1211–1228. doi:10.1172/JCI123319
104. Zhang W, Zhou Q, Xu W, et al. DNA-dependent activator of interferon-regulatory factors (DAI) promotes lupus nephritis by activating the calcium pathway. J Biolog Chem. 2013;288(19). doi:10.1074/jbc.M113.457218
105. Srikanth S, Woo JS, Wu B, et al. The Ca2+ sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat Immunol. 2019;20(2):152–162. doi:10.1038/s41590-018-0287-8
106. Wang C, Zhang R, Wei X, Lv M, Jiang Z. Metalloimmunology: the metal ion-controlled immunity. Adv Immunol. 2020;145:187–241. doi:10.1016/bs.ai.2019.11.007
107. Mathavarajah S, Salsman J, Dellaire G. An emerging role for calcium signalling in innate and autoimmunity via the cGAS-STING axis. Cytokine Growth Factor Rev. 2019;50:43–51. doi:10.1016/j.cytogfr.2019.04.003
108. Kanneganti TD, Kundu M, Green DR. Innate immune recognition of mtDNA--an undercover signal? Cell Metabolism. 2015;21(6). doi:10.1016/j.cmet.2015.05.019
109. West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 2017;17(6):363–375. doi:10.1038/nri.2017.21
110. Yang K, Han W, Jiang X, et al. Zinc cyclic di-AMP nanoparticles target and suppress tumours via endothelial STING activation and tumour-associated macrophage reinvigoration. Nat Nanotechnol. 2022;17(12):1322–1331. doi:10.1038/s41565-022-01225-x
111. Lian H, Wei J, Zang R, et al. ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response. Nat Commun. 2018;9(1):3349. doi:10.1038/s41467-018-05559-w
112. Rozenberg JM, Kamynina M, Sorokin M, et al. The role of the metabolism of zinc and manganese ions in human cancerogenesis. Biomedicines. 2022;10(5):1072. doi:10.3390/biomedicines10051072
113. Banerjee I, Behl B, Mendonca M, et al. Gasdermin D restrains type i interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity. 2018;49(3):413–426.e5. doi:10.1016/j.immuni.2018.07.006
114. Lv M, Chen M, Zhang R, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30(11):966–979. doi:10.1038/s41422-020-00395-4
115. Zhao Z, Ma Z, Wang B, Guan Y, Su XD, Jiang Z. Mn2+ directly activates cGAS and structural analysis suggests Mn2+ induces a noncanonical catalytic synthesis of 2’3’-cGAMP. Cell Rep. 2020;32(7):108053. doi:10.1016/j.celrep.2020.108053
116. Pei P, Zhang Y, Jiang Y, et al. Pleiotropic immunomodulatory functions of radioactive inactivated bacterial vectors for enhanced cancer radio-immunotherapy. ACS Nano. 2022;16(7):11325–11337. doi:10.1021/acsnano.2c04982
117. Guo W, Chen Z, Li Z, et al. Improved immunotherapy for gastric cancer by nanocomposites with capability of triggering dual-damage of nuclear/mitochondrial DNA and cGAS/STING-mediated innate immunity. Chem Eng J. 2022;443:136428. doi:10.1016/j.cej.2022.136428
118. Huang C, Tong T, Ren L, Wang H. STING-activating small molecular therapeutics for cancer immunotherapy. Chembiochem. 2024;25(19):e202400255. doi:10.1002/cbic.202400255
119. Theivendren P, Kunjiappan S, Pavadai P, et al. Revolutionizing cancer immunotherapy: emerging nanotechnology-driven drug delivery systems for enhanced therapeutic efficacy. ACS Meas Sci Au. 2024. doi:10.1021/acsmeasuresciau.4c00062
120. Zeng YC, Dembele H, Young O, Isinelli G, Ryu JH, Shih W. 764 DNA origami vaccine (Dorivac) for cancer immunotherapy. J Immunother Cancer. 2024;12(Suppl 2). doi:10.1136/jitc-2024-SITC2024.0764
121. Miyabe H, Hyodo M, Nakamura T, Sato Y, Hayakawa Y, Harashima H. A new adjuvant delivery system “cyclic di-GMP/YSK05 liposome” for cancer immunotherapy. J Control Release. 2014;184:20–27. doi:10.1016/j.jconrel.2014.04.004
122. Nakamura T, Miyabe H, Hyodo M, Sato Y, Hayakawa Y, Harashima H. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J Control Release. 2015;216:149–157. doi:10.1016/j.jconrel.2015.08.026
123. Shae D, Becker KW, Christov P, et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;14(3):269–278. doi:10.1038/s41565-018-0342-5
124. Cheng N, Watkins-Schulz R, Junkins RD, et al. A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. JCI Insight. 2018;3(22):e120638,120638. doi:10.1172/jci.insight.120638
125. Wehbe M, Wang-Bishop L, Becker KW, et al. Nanoparticle delivery improves the pharmacokinetic properties of cyclic dinucleotide STING agonists to open a therapeutic window for intravenous administration. J Control Release. 2021;330:1118–1129. doi:10.1016/j.jconrel.2020.11.017
126. Beach MA, Nayanathara U, Gao Y, et al. Polymeric nanoparticles for drug delivery. Chem Rev. 2024;124(9):5505–5616. doi:10.1021/acs.chemrev.3c00705
127. Zhou Q, Dutta D, Cao Y, Ge Z. Oxidation-responsive PolyMOF nanoparticles for combination photodynamic-immunotherapy with enhanced STING activation. ACS Nano. 2023;17(10):9374–9387. doi:10.1021/acsnano.3c01333
128. Carroll EC, Jin L, Mori A, et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016;44(3):597–608. doi:10.1016/j.immuni.2016.02.004
129. Fang RH, Jiang Y, Fang JC, Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83. doi:10.1016/j.biomaterials.2017.02.041
130. Eniola AO, Rodgers SD, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials. 2002;23(10):2167–2177. doi:10.1016/s0142-9612(01)00349-0
131. Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–296. doi:10.1016/j.apsb.2016.02.001
132. Liu J, Xiang J, Jin C, et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnol. 2023;21(1). doi:10.1186/s12951-023-01835-0
133. Wang X, Zhong X, Li J, Liu Z, Cheng L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem Soc Rev. 2021;50(15):8669–8742. doi:10.1039/d0cs00461h
134. An M, Yu C, Xi J, et al. Induction of necrotic cell death and activation of STING in the tumor microenvironment via cationic silica nanoparticles leading to enhanced antitumor immunity. Nanoscale. 2018;10(19):9311–9319. doi:10.1039/c8nr01376d
135. Shen D, Yang J, Li X, et al. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Lett. 2014;14(2):923–932. doi:10.1021/nl404316v
136. Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41(7):2590–2605. doi:10.1039/c1cs15246g
137. Benne N, van Duijn J, Kuiper J, Jiskoot W, Slütter B. Orchestrating immune responses: how size, shape and rigidity affect the immunogenicity of particulate vaccines. J Control Release. 2016;234:124–134. doi:10.1016/j.jconrel.2016.05.033
138. Park KS, Xu C, Sun X, Louttit C, Moon JJ. Improving STING agonist delivery for cancer immunotherapy using biodegradable mesoporous silica nanoparticles. Adv Ther. 2020;3(10). doi:10.1002/adtp.202000130
139. Yang X, Yang Y, Bian J, et al. Converting primary tumor towards an in situ STING-activating vaccine via a biomimetic nanoplatform against recurrent and metastatic tumors. Nano Today. 2021:38. doi:10.1016/j.nantod.2021.101109
140. Zhou M, Wang X, Lin S, et al. Multifunctional STING-activating Mn3 O4 @Au-dsDNA/DOX nanoparticle for antitumor immunotherapy. Adv Healthc Mater. 2020;9(13):e2000064. doi:10.1002/adhm.202000064
141. Khalid A, Persano S, Shen H, et al. Strategies for improving drug delivery: nanocarriers and microenvironmental priming. Expert Opin Drug Deliv. 2017;14(7):865–877. doi:10.1080/17425247.2017.1243527
142. Ying X, Chen Q, Yang Y, et al. Nanomedicines harnessing cGAS-STING pathway: sparking immune revitalization to transform “cold” tumors into “hot” tumors. mol Cancer. 2024;23(1):277. doi:10.1186/s12943-024-02186-6
143. Ding J. Engineered nanomedicines with enhanced tumor penetration. Nano Today. 2019;29:100800. doi:10.1016/j.nantod.2019.100800
144. Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–255. doi:10.1038/nnano.2007.70
145. Larnaudie SC, Sanchis J, Nguyen TH, et al. Cyclic peptide-poly(HPMA) nanotubes as drug delivery vectors: in vitro assessment, pharmacokinetics and biodistribution. Biomaterials. 2018;178:570–582. doi:10.1016/j.biomaterials.2018.03.047
146. Moyer TJ, Kassam HA, Bahnson ESM, et al. Shape-dependent targeting of injured blood vessels by peptide amphiphile supramolecular nanostructures. Small. 2015;11(23):2750–2755. doi:10.1002/smll.201403429
147. Dane EL, Belessiotis-Richards A, Backlund C, et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat Mater. 2022;21(6):710–720. doi:10.1038/s41563-022-01251-z
148. Zhang Z, Liu J, Xiao M, et al. Peptide nanotube loaded with a STING agonist, c-di-GMP, enhance cancer immunotherapy against melanoma. Nano Res. 2023;16(4):5206–5215. doi:10.1007/s12274-022-5102-z
149. Wang B, Tang M, Yuan Z, et al. Targeted delivery of a STING agonist to brain tumors using bioengineered protein nanoparticles for enhanced immunotherapy. Bioact Mater. 2022;16:232–248. doi:10.1016/j.bioactmat.2022.02.026
150. Wang F, Su H, Xu D, et al. Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat Biomed Eng. 2020;4(11):1090–1101. doi:10.1038/s41551-020-0597-7
151. Gao X, Lei G, Wang B, et al. Encapsulation of platinum prodrugs into PC7A polymeric nanoparticles combined with immune checkpoint inhibitors for therapeutically enhanced multimodal chemotherapy and immunotherapy by activation of the STING pathway. Adv Sci. 2023;10(4). doi:10.1002/advs.202205241
152. Zhou L, Hou B, Wang D, et al. Engineering polymeric prodrug nanoplatform for vaccination immunotherapy of cancer. Nano Lett. 2020;20(6):4393–4402. doi:10.1021/acs.nanolett.0c01140
153. Shae D, Baljon JJ, Wehbe M, et al. Co-delivery of peptide neoantigens and stimulator of interferon genes agonists enhances response to cancer vaccines. Acs Nano. 2020;14(8):9904–9916. doi:10.1021/acsnano.0c02765
154. Chen C, Hu M, Cao Y, et al. Combination of a STING agonist and photothermal therapy using chitosan hydrogels for cancer immunotherapy. Biomacromolecules. 2023;24(6):2790–2803. doi:10.1021/acs.biomac.3c00196
155. Song N, Liu W, Tu Q, Liu R, Zhang Y, Wang J. Preparation and in vitro properties of redox-responsive polymeric nanoparticles for paclitaxel delivery. Colloids Surf B Biointerfaces. 2011;87(2):454–463. doi:10.1016/j.colsurfb.2011.06.009
156. Liu KF, Liu YX, Li CX, Wang LY, Liu J, Lei JD. Self-assembled ph and redox dual responsive carboxymethylcellulose-based polymeric nanoparticles for efficient anticancer drug codelivery. ACS Biomater Sci Eng. 2018;4(12):4200–4207. doi:10.1021/acsbiomaterials.8b00920
157. Zheng H, Guo B, Qiu X, et al. Polymersome-mediated cytosolic delivery of cyclic dinucleotide STING agonist enhances tumor immunotherapy. Bioact Mater. 2022;16:1–11. doi:10.1016/j.bioactmat.2022.02.029
158. Pan P, Svirskis D, Rees SWP, Barker D, Waterhouse GIN, Wu Z. Photosensitive drug delivery systems for cancer therapy: mechanisms and applications. J Control Release. 2021;338:446–461. doi:10.1016/j.jconrel.2021.08.053
159. Liang Q, Chen J, Hou S, et al. Activatable Mn2+-Armed nanoagonist augments antitumor immunity in colorectal cancer: a NIR-II Photonic neoadjuvant paradigm. Biomaterials. 2023;300:122206. doi:10.1016/j.biomaterials.2023.122206
160. Zhao L, Chen X, Wu H, He Q, Ding L, Yang B. Strategies to synergize PD-1/PD-L1 targeted cancer immunotherapies to enhance antitumor responses in ovarian cancer. Biochem Pharmacol. 2023;215:115724. doi:10.1016/j.bcp.2023.115724
161. Hao Y, Ma S, Gu Z, et al. Combination of photodynamic therapy and stimulator of interferon genes (STING) agonist inhibits colorectal tumor growth and recurrence. Cancer Commun. 2023;43(4):513–518. doi:10.1002/cac2.12405
162. Sun Y, Zhang Y, Gao Y, et al. Six birds with one stone: versatile nanoporphyrin for single-laser-triggered synergistic phototheranostics and robust immune activation. Adv Mater. 2020;32(48):e2004481. doi:10.1002/adma.202004481
163. Shi X, Shu L, Wang M, et al. Triple-combination immunogenic nanovesicles reshape the tumor microenvironment to potentiate chemo-immunotherapy in preclinical cancer models. Adv Sci. 2023;10(15). doi:10.1002/advs.202204890
164. Yang W, Zhang M, Zhang J. In vivo activated T cell targeting with PD-1/PD-L1 blockade for sequential treatment mediated cancer immunotherapy. Nano Today. 2022;44:101492. doi:10.1016/j.nantod.2022.101492
165. Feng X, Xiong X, Ma S. Docetaxel-loaded novel nano-platform for synergistic therapy of non-small cell lung cancer. Front Pharmacol. 2022;13. doi:10.3389/fphar.2022.832725
166. Allen C, Her S, Jaffray DA. Radiotherapy for cancer: present and future. Adv Drug Deliv Rev. 2017;109:1–2. doi:10.1016/j.addr.2017.01.004
167. Liu Y, Crowe WN, Wang L, et al. An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases. Nat Commun. 2019:10. doi:10.1038/s41467-019-13094-5
168. Liu X, Kifle MT, Xie H, et al. Biomineralized manganese oxide nanoparticles synergistically relieve tumor hypoxia and activate immune response with radiotherapy in non-small cell lung cancer. Nanomaterials. 2022;12(18). doi:10.3390/nano12183138
169. Yong SB, Chung JY, Song Y, Kim J, Ra S, Kim YH. Non-viral nano-immunotherapeutics targeting tumor microenvironmental immune cells. Biomaterials. 2019;219:119401. doi:10.1016/j.biomaterials.2019.119401
170. Milling LE, Garafola D, Agarwal Y, et al. Neoadjuvant STING activation, extended half-life IL2, and checkpoint blockade promote metastasis clearance via sustained NK-cell activation. Cancer Immunol Res. 2022;10(1):26–39. doi:10.1158/2326-6066.CIR-21-0247
171. Xu N, Palmer DC, Robeson AC, et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J Exp Med. 2021;218(2):e20200844. doi:10.1084/jem.20200844
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.