Back to Journals » International Journal of Nanomedicine » Volume 19
Nanoparticles as a Novel Platform for Cardiovascular Disease Diagnosis and Therapy
Authors Tang C, Zhou K, Wu D , Zhu H
Received 21 April 2024
Accepted for publication 18 August 2024
Published 27 August 2024 Volume 2024:19 Pages 8831—8846
DOI https://doi.org/10.2147/IJN.S474888
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Krishna Nune
Chuanyun Tang,1,* Kexun Zhou,2,* Di Wu,1,* Hong Zhu2
1The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, People’s Republic of China; 2Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Zhu, Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, No. 37, GuoXue Xiang, Chengdu, 610041, People’s Republic of China, Tel/Fax +86-28-85423262, Email [email protected]
Abstract: Cardiovascular disease (CVD) is a major global health issue with high mortality and morbidity rates. With the advances in nanotechnology, nanoparticles are receiving increasing attention in diagnosing and treating CVD. Previous studies have explored the use of nanoparticles in noninvasive diagnostic technologies, such as magnetic resonance imaging and computed tomography. Nanoparticles have been extensively studied as drug carriers and prognostic factors, demonstrating synergistic efficacy. This review summarized the current applications of nanoparticles in CVD and discussed their opportunities and challenges for further exploration.
Keywords: nanoparticles, cardiovascular disease, diagnosis, therapy, nanoparticle functionalization
A Letter to the Editor has been published for this article.
Introduction
Modern nanoscience and technology are interdisciplinary fields offering innovative disease prevention, diagnosis and treatment methods.1 With a particle size between 1–100nm, nanoparticles possess attractive properties due to their unique structural characteristics.2 Nanoparticles serve as versatile drug carriers capable of detecting drug aggregation within the body, thereby enabling preliminary assessments of therapeutic efficacy. They also facilitate drug distribution, enhancing drug deposition on specific biological targets while mitigating systemic toxicity and associated side effects.3–5
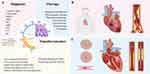
Moreover, the advancement of nanoparticle design is expected to improve therapeutic efficacy and achieve more precise applications. For example, liposome nanoparticles can carry drugs with different properties, including hydrophilic, hydrophobic, and lipophilic compounds. During synthesis, their stability can be tailored by size, surface charge, and lipid composition.6,7 Despite these advantages, their utility is constrained by issues such as low drug loading and suboptimal biological distribution, particularly evident in lipid nanoparticles (LNPs) designed for drug delivery.8,9 Recent developments in polymeric nanoparticles offer potential solutions to these challenges, facilitating the delivery of diverse payloads.10 For instance, polymeric micelles demonstrate versatility in carrying therapeutic agents ranging from small molecules to proteins.11 Previous studies indicated that polymeric micelles can directly intervene in the inflammatory process of coronary artery disease by interacting with inflammatory cells.12 Additionally, inorganic materials such as gold, iron, and silicon dioxide have been used to synthesize nanostructured materials for various drug delivery and imaging applications. For example, magnetic iron oxide nanoparticles, composed of magnetite (Fe3O4) or magnetite (Fe2O3), exhibited superparamagnetic properties at specific sizes and can be used as contrast agents and drug delivery carriers.13 Different nanomaterials and their characteristic features were summarized in Figure 1.
Nanotechnology holds great promise in the field of cardiovascular disease (CVD). CVD is a significant health concern that encompasses diseases of the heart, blood vessels, and blood components. The occurrence and development of CVD are related to a variety of factors, including conventional risk factors (eg, hyperlipidemia, hypertension, diabetes), unhealthy behaviors (eg, smoking, obesity), and immutable factors (family history).14 In 2020, CVD ranked first in terms of morbidity and mortality, outpacing cancer and other diseases.15 The treatment of CVD still presents a challenge due to inadequate drug accumulation at the affected site, requiring high and prolonged dosages that frequently induce severe adverse effects.16 Moreover, atherosclerotic CVD is a polyvascular disorder, complicating the precise localization of unstable or rupture-prone plaques prior to the onset of symptoms, such as stroke or myocardial infarction.17 Therefore, there is an urgent need to investigate biomarkers for predicting risks and monitoring treatments, along with safer and more effective therapeutic strategies.
Based on the occurrence and development mechanisms of CVD, nanoparticles are mainly applied in the following categories: diagnosis, treatment, and drug delivery.18–21 For example, a previous study used polymer comb nanomaterials modified with an anti-C-C motif chemokine receptor 5 (CCR5) peptide and 64Cu for PET-CT nuclear imaging. This modification allowed for long cycle times and optimized biodistribution, which can sensitively detect atherosclerotic (AS) plaque inflammation.22 Meanwhile, nanomaterials can improve the treatment of CVD. Liposomes, biomimics, and inorganic nanomaterials, such as gold and iron oxides, are currently used for CVD treatment.23 Due to the improvement of nanotechnologies, we reviewed the advances related to nanotechnology in CVD. We also discussed the challenges and opportunities of nanoparticles in the new era of CVD (Figure 2).
Application of Nanotechnology in CVD Diagnosis
CVDs encompass a spectrum of conditions affecting the heart and blood vessels, including AS, which involves arterial narrowing and hardening due to lipid and inflammatory plaque buildup. Macrophages, tissue factors, and platelets are implicated in the initiation and progression of AS and are potential targets for noninvasive diagnostic imaging. In the early AS stage, foam cells, generated by macrophages through oxidized low-density lipoprotein (ox-LDL) uptake, play a critical role in plaque formation and inflammation.24 Tissue factors and platelets contribute to local coagulation and thrombosis, exacerbating disease progression. Additionally, platelets influence plaque stability by releasing bioactive molecules that promote inflammation and vascular smooth muscle cells (VSMCs) proliferation.25 Hence, targeting macrophages, tissue factors, and platelets using nanoparticles for improved diagnostic technologies holds promise for early detection and management of CVDs.26
Imaging Diagnosis
Magnetic resonance imaging (MRI) can reveal the accumulation of proinflammatory macrophages. Both superparamagnetic iron oxide nanoparticles (SPIONs) and gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) nanoparticles, used as contrast agents, exhibit macrophage loading. Contrast agents composed of SPIONs are readily phagocytosed by plaque macrophages, leading to signal loss in T2-weighted images and thus enhancing the contrast of arterial wall plaques.27 The surface of SPIONs can be modified by adding targeting fragments, such as dextran and osteobridging protein (OPN), which enables them to bind specifically to corresponding epitopes on the surface of macrophages and increase aggregation of macrophages in vulnerable plaques in the aorta or carotid arteries.28 For example, Kao et al encapsulated MCP-1 motifs on the surface of SPIONs to target monocytes in plaques. Vivo aortic images showed an increase in the degree of darkness in mice chronically fed a high-fat diet, suggesting that MCP-1 motif magnetic nanoparticles have a potential affinity for AS plaques.29 Furthermore, Gd-DTPA improved the contrast of T1-weighted images, and the increased contrast was proportional to the number of macrophages in the plaque. However, the clinical use of Gd-DTPA may be hindered by its potential to cause nephrogenic systemic fibrosis.30
In a study by Wei et al, a magnetic nanoprobe (EGFP-EGF1-SPIONs) was constructed to target tissue factors and specifically detect AS plaques by MRI. The nanoprobe exhibited higher accuracy, better contrast, and better cytocompatibility than typical contrast agents.31 Platelets were closely associated with multiple stages of atherogenesis and development. Wei’s group coated cell membranes from platelets directly onto synthetic nanocarriers, creating platelet membrane-coated nanoparticles (PNPs). These PNPs could bind to different components of AS plaques in vivo. During real-time imaging, an MRI contrast agent to the PNP structure generated sufficient contrast to distinguish plaques.32 Despite limited CVD studies, MRI is advantageous as it is nonionizing, noninvasive, and requires minimal material. Therefore, it could become an alternative imaging method for preclinical development.33
Computed tomography (CT) is an essential diagnostic tool for CVD due to its high spatial resolution and short acquisition time.34 Hyafil found that iodine-containing nanoparticles with high X-ray attenuation coefficients are advantageous for identifying proinflammatory macrophages in vulnerable plaques of the coronary arteries and abdominal aorta. The contrast agent N1177, an iodinated nanoparticle, was rapidly cleared from the bloodstream and accumulated in macrophage-rich tissues. Enhanced signals can be detected 2 hours after intravenous injection of N1177. N1177-enhanced CT does not require radiotracer injection.35 However, the low CT sensitivity requires high doses of nanoradiographs, and their potential toxicity necessitates further study. Moreover, multicolor CT utilizes differences in X-ray absorption and scattering by various tissues and materials to reconstruct the internal structure and composition of an object. This approach can employ gold high-density lipoprotein (HDL) nanoparticles, iodine, and calcium phosphate to identify multiple CVD biomarkers concurrently. Specifically, it leverages macrophage gold HDL accumulation imaging and vascular calcium deposition, synergistically contributing to precise diagnostic capabilities.36
Positron emission tomography (PET) is highly suitable for noninvasive quantitative detection of macrophage-mediated inflammation in CVD due to its high tissue penetration and sensitivity. The lack of specificity of the 18F-FDG radiotracer for macrophages and its susceptibility to uptake by cardiomyocytes, resulting in false-positive signals, can be resolved using nanoparticles. 18F-labeled polyglucose and 89Z-labeled nanoparticles have been widely used to detect proinflammatory macrophages. Moreover, 68Ga-labeled nanoparticle PET specifically targets CVD-associated biomarkers, such as VCAM1, MMR, and LOX1.37 However, PET does not provide anatomical information. Therefore, it is often used combined with MRI or CT. For example, Tu et al used MMP2cNPs as PET-MRI contrast agents to detect MMP-2 in AS plaques and demonstrated the feasibility of this approach.38
Owing to its high spatial resolution and lack of radiation-related risks, noninvasive fluorescence imaging is commonly used to image macrophage-rich vascular lesions. For example, nanoprobes that emit mainly in the near-infrared window allow deep tissue penetration and have been used to detect macrophages in CVD lesions.39 An ongoing trial (NCT04237064) evaluates macrophage-targeted photoacoustic imaging (PAI) to distinguish between plaques with different morphologies. Table 1 summarizes the applications of nanotechnology in the diagnosis of CVD. Table 2 shows the application/localization of nanomaterials depicted in this section. The development of imaging technology will likely lead to more robust and comprehensive techniques to improve CVD diagnosis.
 |
Table 1 Nanotechnology in CVD Imaging Diagnosis |
 |
Table 2 The Materials of Imaging Nanoparticles for CVD |
Biomarker Diagnosis
Imaging is the gold standard for diagnosing CVD due to its multiple advantages. However, noninvasive nanoparticle-based diagnostics, such as analyzing clinical samples (eg, blood, saliva, urine), can provide more convenient, rapid, and inexpensive CVD screening and guide imaging follow-up.
Nanoparticles use magnetic and other physicochemical properties to amplify signals and increase sensitivity, providing an advantage in detecting CVD-related molecular and cellular biomarkers. Nanomaterials can overcome the challenge of high background signals in clinical samples and enhance the signal of C-reactive protein (CRP) by adjusting various properties, including electrochemical, optical, and chemiluminescence.40–43 Although CRP is frequently used to predict CVD,44,45 its nonspecific nature as a biomarker of inflammation suggests the need to identify new standalone biomarkers or perform multiplex biomarker assays simultaneously, which leads to the development of nanogold-based microfluidic strategies. This method could integrate the steps of sample processing, reaction, and detection on a single microchip to achieve high-throughput, automation, and rapid detection for myoglobin, d-dimers, and CRP levels simultaneously to confirm the diagnosis of myocardial infarction. Specific immune complexes can be formed independently on adjacent microfluidic tracks, and the 1 mm distance between the tracks allows for the simultaneous detection of up to four different analytes with breakneck speed and high reproducibility.46 An electrochemical immunosensor was designed to detect cardiac troponin I (cTnI) for the early diagnosis of acute myocardial infarction. The sensor was ultrasensitive, enzyme-free, and based on a screen-printed gold electrode (SPGE) modified with graphene quantum dots (GQDs) and gold nanoparticles (AuNPs). The nanoimmunosensor described in the text demonstrated high specificity for cTnI and could measure analytes using four electrochemical techniques, making it a promising tool for diagnosing acute myocardial infarction.47
Plasma has been used to enhance surface Raman scattering for the sensitive measurement of blood auto-antibodies associated with hypertension and urinary microalbumin, which can indicate vascular or endothelial dysfunction.48,49 Electrochemical methods, such as voltammetry, impedance spectroscopy, and galvanometric analysis, have been used to detect cardiac troponin, myoglobin, creatine kinase, and CRP. These methods use quantum dots and metal nanomaterials for direct electron transfer to the sensing analytes.47,50 Electrochemical sensors, which can detect biomarkers by detecting the interaction between biomarkers and sensors, are simple, cost-effective, and accurate. They detect multiple targets with speed and ultrasensitivity, making them a valuable tool for predicting myocardial infarction in patients. There are various electrochemical sensors, such as potentiometric and amperometric sensors. Applying magnetic surface-enhanced Raman scattering (SERS) and biobarcoded particles can facilitate the rapid and sensitive detection of proteins and nucleic acids, which may help identify new patient populations at an early risk of CVD with appropriate biomarkers.51
Application of Nanotechnology in the Treatment of CVD
The development of CVD involves various processes, including myocardial injury and inflammation, valvular and structural problems, and electrical problems. Nanomaterials can enhance the function of other biomaterials to improve their efficiency, efficacy, and durability for use in cardiac patches,52–54 heart valves,55,56 vascular grafts,57,58 stents,59 among others. They can also be used for direct intravenous or intracardiac injections to treat conditions such as ischemia-reperfusion injury.60
Nanostructures That Impact Myocardial Electrophysiology
The myocardium is a functional syncytium composed of cardiomyocytes that can contract and generate force by initiating and propagating electrical signals. After myocardial injury, the heart requires artificial repair, which can be achieved through implantable, injectable, and nanofiber or nanopatterned stent cardiac repair materials.61,62 These materials aid in tissue repair.63,64 An ideal regenerative biomaterial should mimic the multifunctional structure of native tissues rather than being monofunctional, which means it should have appropriate electrical conductivity, growth factor release, cell scaffolding, nonimmunogenicity, mechanical structure, and durability instead of providing mechanical stability. However, existing biomaterials possess only some necessary physicochemical and structural properties.64 Nanomaterials can address the limitations of cardiac regenerative medical technologies. They can provide a sustained release of growth factors for stem cells and other cells to thrive, target the mechanical stability of the natural myocardium, and mimic the conductivity of cardiac syncytia.52–54,63
Gold nanorods formed with albumin electrospun fibers absorb near-infrared light and generate heat to weld functional heart patches securely and safely to the heart, which complements its excellent conductivity, enables efficient cell-to-cell electrical coupling, and avoids myocardial damage caused by traditional cardiac patch suturing processes.65 Furthermore, 3D-printed CVD biomaterials are increasingly sought due to their flexibility and precision in manufacturing. To enhance its performance, nanomaterials can be incorporated into the “ink” of 3D bioprinters to replicate the highly functional tissue of the human heart, possessing contractile, conductive, and thermal properties.66
Targets for Heart Valves
The demand for off-The-shelf implantable tissue-engineered heart valves (TEHVs) has increased due to the rising morbidity and mortality caused by heart valve diseases. Although there has been significant progress in improving the design and performance of TEHV constructs, creating viable, functional human-implantable TEHV constructs remains challenging.67
Nanomaterials provide several strategies to enhance heart valve implants’ safety, mechanical strength, and durability. Nanomaterials can increase valve durability by effectively spatially blocking the interaction of the valve surface with biomolecules while providing sustained-release therapy, thus reducing calcification and immunogenicity.55,56 Erythrocyte membrane-based biomimetic nanomaterials are chemically attached to the valve surface, replacing the traditional glutaraldehyde treatment, which is toxic, immunogenic, and prone to valve degeneration.68 The nanocoating covers the valve surface, and the implant is endothelialized, resulting in improved anticoagulant and anticalcification effects. Jet-spinning technology rapidly fabricates human-sized valves with high biocompatibility and practical functions by combining polymeric gelatin-based nanofibers with an extracellular matrix (ECM).69 Other nanotechnologies have expanded the scope of tissue-engineered valves by developing nano- or microscale porous structures with precise porosity and texture and controlled topography to deliver growth factors or genes to specific cells. In addition, natural heart valves have anisotropic mechanical properties. Therefore, anisotropic nanostructures and microstructures can be fabricated to support the multidimensional biomimetic anisotropy required for proper in vivo function.65,70
Targets for the Vascular System
Serving as the conduit for oxygen, nutrients, and cells to vital organs and tissues, the pivotal role of the vascular system has been underscored. Intravascular injection is the primary method of introducing nanomaterials to treat vascular diseases. The drugs can treat a variety of CVDs, such as lipid dysfunction,71 vascular inflammation,72,73 and endothelial dysfunction,74 either acting on the whole body or through locally targeted delivery using specific nanosurfaces and structures for imaging or treatment.75,76 Nanomaterials can be delivered targeted, allowing them to carry drugs, increase payloads, and mitigate adverse effects. They can also improve drugs that were previously discontinued due to severe side effects or develop new drugs with capability responses. For instance, by combining drugs with nanomaterials, the stability and bioavailability of drugs can be improved, thereby reducing drug dosage and side effects. Nanomaterials improve intravascular and intervascular biomaterials, such as nanoengineered scaffolds, prostheses, and grafts.57–59
Scaffolds and engineered vascular grafts may contain nanomaterials for sustained and local release of drugs and growth factors.59 Vascular grafts can be tissue-engineered from major electrospun polymer nanofibers to mimic the topology, structure, mechanical properties, and biocompatibility of actual blood vessels.57,58,77 Furthermore, integrating 3D printing and nanofibers has created tissue-engineered vascular grafts that exhibit comparable mechanics, elastin, and collagen contents to those of natural blood vessels. This development addresses issues related to thrombosis, immunogenicity, infection, and non-compliance with the mechanical properties of natural tissues.57
Although drug-eluting stents have significantly reduced clinical restenosis rates, they also increase the risk of advanced thrombosis.78,79 The characteristics of drug-eluting stents related to their release may be undesirable, hindering the successful use of certain drugs, such as the water-soluble chemotherapeutic drug imatinib.79 Polymeric nanomaterials, such as poly lactic-coglycolic acid (PLGA), can encapsulate sustained scaffold elution. This is because nanomaterials can load efficiently and release more slowly than drug-loaded polymer scaffold coatings. Furthermore, they allow drugs such as imatinib and antiproliferative sirolimus to remain at the scaffold site longer, thus reducing restenosis.79,80
Bionanomaterials, such as exosomes, can promote an anti-inflammatory and pro-angiogenic microenvironment that contributes to re-endothelialization and the reduction of stenosis. Exosomes derived from mesenchymal stromal cells (MSCs) exhibit healing properties in myocardial infarction78,81 and can be eluted from scaffolds to treat ischemia-reperfusion injury.82 Finally, creative strategies can be deployed to maintain the advantages of nanoparticle-coated continuous drug-release scaffolds over the long term. The stent can repeatedly capture magnetic nanomaterials injected throughout the body for multiple local treatments each time the drug is depleted.83
Specific Targets
The pathogenesis of CVD involves specific targets, making nanotechnology tailored to these objectives a potentially universal strategy. Macrophages, pivotal immune cells in AS, can be targeted by nanotechnology to deliver therapeutic agents like drugs or genes to lesion sites, thereby mitigating inflammation and enhancing plaque stability.26 Additionally, T, B, and NK cells contribute significantly to AS progression, suggesting nanotechnology’s potential to modulate immune responses by targeting these cells, thereby theoretically reducing inflammation and promoting plaque stabilization.24 Moreover, miRNAs, small RNA molecules crucial in gene regulation, can be targeted by nanotechnology to modulate gene expression, potentially mitigating inflammation and promoting plaque stability.84
Macrophage
Macrophages play a crucial role in the progression of CVD, including AS. Monocytes can differentiate into macrophages, engulf lipids and cholesterol, and form foam cells, which contribute to the development of AS. Studies have shown that inhibiting monocytes can reduce early AS in mice by reducing foam cell formation, attenuating inflammatory responses, decreasing lipid deposition, inhibiting VSMCs proliferation, and promoting plaque stabilization and repair, highlighting the importance of early detection and treatment targeting macrophages.85 Nanotechnology has emerged as a promising approach to activating or silencing signaling pathways in macrophages, making it a promising topic in CVD therapy.
Circulating monocytes enter AS plaques and differentiate into macrophages. These macrophages are then activated by CD4+ T cells through the CD40-CD40L signaling pathway. To address this issue, Lameijer et al designed recombinant HDL nanoparticles that target tumor necrosis factor receptor-associated factor 6 (CD40-TRAF6). These nanoparticles inhibit the interaction of CD40-TRAF6 with Ly6Chigh monocytes, thus attenuating the proinflammatory effect. Transcriptome analysis revealed that one week of nanoimmunotherapy had a significant anti-inflammatory effect by reducing monocyte migratory capacity.86 Gao et al also developed nanoparticle-mediated mRNAs that selectively delivered mRNA encoding the anti-inflammatory factor interleukin-10 (IL-10) to macrophages within plaques to promote the reduction of inflammation in the plaque microenvironment at advanced AS sites. Furthermore, using nanotherapeutic agents to inhibit the proliferation of proinflammatory macrophages may help stabilize vulnerable plaques.87
Although nanoparticles show great promise in modulating harmful macrophages, their innate immune system activation can cause various disorders, requiring further study. Accurately modulating the local immune response is crucial to minimize potential systemic adverse effects. Furthermore, due to variations in the pathophysiology of AS between animal models and humans, there is no assurance that the nanotherapies used in preclinical studies will be efficacious in humans. Additional assessment of the biosafety, toxicity, immunogenicity, and clearance of the nanotherapies mentioned above and nanoparticle-based visualizers is necessary.
Other Immune Cells
Several experimental studies have shown the involvement of T cells in the development of CVD. For example, antigens such as oxLDL, heat shock proteins (HSPs), and peptides from specific pathogens (HIV and HCV) can activate T cells to release a series of inflammatory factors through the presentation of antigen-presenting cells (APC), promoting inflammatory responses and inducing the development of AS.88,89 Basic experiments have shown that active immunization with the apolipoprotein B-100 (ApoB-100) peptide P210 reduces the development of CVD in animal models. Hyu et al wrapped P210 in peptide amphiphile micelles (PAM) to enhance the efficacy of immunotherapy for CVD to protect the peptide from protease degradation and clearance and increase epitope density. The p210-PAMs were predominantly 15 and 25 nm in size. One week after the injection of P210-PAM, there was a significant increase in CD4+CD25+FoxP3+ regulatory T cells and CD8+CTLA-4+ T cells, a decrease in CD4+ T cell activity, and a significant decrease in total blood cholesterol levels. According to the study, P210-PAMs inhibit the proliferative response of P210-specific CD4+ T cells and the cytotoxic effects of CD8+ T cells, regulate the macrophage phenotype, and significantly inhibit the development of AS.90 Although nanoparticles-based immunotherapy has effectively treated AS, it is not without risks. Immunotherapy can cause immune effector cells to be overactivated and release cytokines in large quantities, leading to a cytokine storm, which can cause symptoms such as capillary leakage and diffuse intravascular coagulation.91 Furthermore, immunotherapy can cause organ-specific toxic reactions, such as myocarditis and hepatitis.92 Therefore, further investigation is necessary for NP-based immunotherapies.
In a preclinical study, Kyaw et al demonstrated that administering apoptotic cells or apoptotic cells mimic liposomes containing phosphatidylserine (PtdSer) can modulate B cell activity and mitigate AS formation in ApoE−/− mice. This modulation stimulates B1a cell proliferation, enhances polyreactive immunoglobulin M (IgM) antibody secretion, and increases anti-inflammatory cytokine production.93 However, specific examples regarding the therapeutic targeting of NK cells, fibroblasts, cardiomyocytes, and endothelial cells in AS treatment are currently lacking. Future research could explore the potential of nanomaterials in addressing AS involving these cell types.
miRNA
MicroRNAs (miRNAs) regulate gene expression and play a key role in CVD. Although miRNAs have become a popular therapeutic target, challenges remain due to their potential off-target effects. Therefore, developing vectors that can be selectively delivered to the injury site is essential. Kheirolomoom et al designed anti-microRNA-712 encapsulated in cationic lipoparticles (CCLs) (anti-miR-712), whose surface was modified by VHPK (a targeted peptide consisting of Valine, Histidine, Proline and Lysine) to target VCAM-1. Optical imaging confirmed the efficient delivery of anti-miR-712 to inflamed mouse aortic endothelial cells, both in vitro and in vivo. The results demonstrated that VHPK-CCL-anti-miR-712 delivery effectively downregulates miR-712 expression induced by d-flow and restored expression of its target genes, tissue inhibitor of metalloproteinase 3 (TIMP3), and the kazal motif of reversion-inducing-cysteine-rich protein (RECK) in endothelial cells. Furthermore, VHPK-CCL anti-miR-712 inhibited atherogenesis at a rate up to 80% lower than nonencapsulated miR-712 and did not affect miR-712 expression in other organs.94 Moreover, Dosta et al developed a novel low-toxicity and high-biocompatible poly beta-amino ester (PBAE) nanoparticle loaded with RNAi, coupled with a VHPK peptide that targets vascular cell adhesion molecule 1 to deliver anti-miR-712. This drug significantly reduced miR-712 expression in mice while preventing the loss of its target gene (TIMP3).95 Therefore, it can potentially treat dysfunctional endothelial cells in vascular diseases. Based on this, endothelial cell therapy can treat dysfunctional vascular diseases.
Potential Targets for Future Applications
Besides the targets mentioned above, new therapeutic strategies for CVD are being identified. For example, AS plaques contain autoantibodies, which connect them to autoimmune diseases.96 However, the initiation of autoantibodies and their response mechanisms during the pathology of AS remains poorly understood. Lorenzo et al performed single-cell sequencing and proteomic analyses and found that free mitochondrial aldehyde dehydrogenase four family member A1 (ALDH4A1) levels were significantly elevated in patients with AS. This finding suggested that ALDH4A1 could be a potential diagnostic marker for AS. The ALDH4A1 antibody has been found to effectively slow the development of AS in mice, making it a potential therapeutic option for CVD.96 Pi et al also identified the P2RY12 receptor as a novel target for AS treatment. This study indicated that administering a P2RY12 receptor inhibitor (CDL) combined with atorvastatin (ATV) effectively mitigated the AS process. Furthermore, it ameliorated lipid accumulation and prevented VSMCs-derived foam cell formation in advanced AS. The mechanism was explored using molecular biology, phosphorylation proteomics, and other methods. There are three main aspects: In vitro experiments showed that the P2RY12 receptor inhibits autophagy, reducing cholesterol efflux and increasing foam cell formation. Moreover, phosphorylated proteomics revealed that the P2RY12 receptor can regulate the autophagy signaling pathway through the PI3K-AKT-mTOR pathway. In addition, in diseased mice, the inhibitor of the P2RY12 receptor (CDL) can promote autophagy of VSMC through the PI3K-AKT-mTOR pathway to treat AS.97
Stent implantation is a highly effective treatment for coronary artery disease; however, it presents several challenges. One challenge is the potential for endothelial hyperplasia, which can lead to restenosis.98 Therefore, it is crucial to clarify the mechanism of restenosis and identify therapeutic targets to counteract endothelial hyperplasia, which could improve the effectiveness of stent-based coronary interventions. Yang et al investigated the mechanism of endothelial hyperplasia after vascular injury and identified a novel therapeutic target, the noncoding small RNA miR-22. They found that miR-22 can reduce pathological intimal hyperplasia after vascular injury by regulating MECP-2 and EVI-1, thus improving vascular remodeling and reducing stenosis.99 This study suggested that miR-22 could be used as a drug coating, providing new possibilities for developing a novel generation of drug-coated scaffolds. We summarized the relevant targets in Table 3.
 |
Table 3 Therapeutic Targets for Nanotechnology in CVD |
Nanotechnology-Based Drug Delivery Systems for CVD
Intravenous Administration
Intravenous administration targets specific cells or receptors by circulating the drug in the bloodstream. For example, Leuschner et al treated AS with optimized lipid nanoparticles and small interfering RNAs that silenced CCR2 to target monocytes. In vivo, when intravenously administered, the substance was quickly cleared from the bloodstream and accumulated mainly in the spleen and bone marrow, targeting monocytes.100 This treatment showed great potential to reduce the number of AS plaques and the size of myocardial infarctions after coronary artery occlusion. Also, the effects of intravenous injection of CeO2 nanoparticles on cardiac function and myocardial remodeling after cardiomyopathy were evaluated. The study indicated this administration effectively suppressed progressive left ventricular dysfunction and enlargement. Furthermore, the degree of mononuclear macrophage aggregation in cardiomyocytes, tumor necrosis factor-alpha, and the levels of IL-1β and IL-6 were significantly reduced.101 Kim et al developed a nanoencapsulated drug (CSNP) with a core composed of methyl-β-cyclodextrin (cyclodextrin) and simvastatin. When administered intravenously, CSNPs interacted with cholesterol to release simvastatin, which removed cholesterol and ultimately reduced AS plaques.75
Intravenous administration involves the rapid entry of nanomedicines into the venous bloodstream, allowing them to target specific cells or receptors as they circulate and reach atheromatous plaques. However, the immune system quickly removes nanoparticles from the circulation, which can reduce their efficacy or duration of effect. Furthermore, it is crucial to target specific nanoparticles to prevent inadvertent injection of nanomedicines into healthy tissues or cells.102
Localized Administration
Although intravenous administration possesses several advantages, it may not be suitable for treating certain diseases. For example, it may be ineffective when the drug cannot be localized sufficiently or when a constant concentration is required for an extended period.102 In such cases, localized nanodelivery may be advantageous, particularly when local cannulation is necessary to prevent restenosis and eliminate blood clots. Vong et al developed a controlled NO-releasing redox injectable hydrogel (NO-RIG) that scavenged overproduced ROS and modulated local NO expression levels when intracardially administered. NO-RIG was transformed into a gel in myocardial tissues, evenly distributed, and lasted over ten days. NO-RIG therapy significantly reduced infarct size and improved cardiac function after myocardial infarction. Furthermore, it improved angiogenesis, possessing great potential in the prevention and treatment of CVD.103
Localized injection of proteins into infarcted lesions is a viable option to initiate cardiac regeneration after myocardial infarction. Qi et al investigated the efficacy of liraglutide loaded onto PLGA-PEG nanoparticles after localized intramyocardial injection. NP-liraglutide persisted for up to 4 weeks in a rat model of myocardial infarction, and it was highly influential in enhancing cardiac function, decreasing infarct size, maintaining vessel wall thickness, stimulating angiogenesis, and inhibiting cardiomyocyte apoptosis.104 Therefore, intramyocardial administration of NP-liraglutide can potentially treat myocardial infarction. Somasuntharam investigated the role of localized deoxyribonuclease metal nanoparticles (AuNPs) injection in treating myocardial infarction by silencing tumor necrosis factor-α (TNF-α). The study found that this administration resulted in a 50% TNF-α knockdown efficiency, leading to significant anti-inflammatory effects and improved cardiac function after myocardial infarction.105 These effects were partly due to the mode of delivery and gene regulation.
Despite its rapid onset of action and long duration of maintenance, localized administration has certain limitations. Further studies are required to investigate the chronic effects of localized administration on cardiomyocyte remodeling and cardiac function. Besides, localized administration may cause an excessive short-term increase in intracardiac drug concentration, which may damage cardiomyocytes. Thus, it is necessary to conduct more fundamental experiments to enhance the efficacy and safety of localized administration.
Nanodelivery Systems for Encapsulation
Therapeutic agents can be encapsulated within nanocarriers without chemical modifications, meeting diverse clinical requirements. For instance, statins, the preferred lipid-lowering drugs, competitively inhibit HMG CoA reductase to reduce intracellular cholesterol synthesis. Nonetheless, many statins exhibit low bioavailability due to rapid metabolism and poor water solubility.106 Nanocarriers can effectively enhance their therapeutic efficacy and minimize adverse effects. Katsuki et al demonstrated that intravenous injection of pitavastatin-incorporated nanoparticles in ApoE (−/−) mice suppressed AS plaques’ instability and rupture propensity. This effect was attributed to the inhibition of macrophage-secreted MCP-1, matrix metalloproteinase-9 and MCP-1-induced monocyte chemotaxis by the pitavastatin-incorporated nanoparticles.107 Additionally, Hossaini et al developed a novel hyaluronic acid-atorvastatin (HA-ATV) conjugate, where the hydrophobic ATV formed the core of nanoparticles (HA-ATV-NPs). HA on the surface of this nanomedicine selectively bound to CD44 expressed on atherosclerotic plaques, leading to targeted and efficient anti-inflammatory effects.108 These findings were corroborated by other studies showing that statin nanomaterials could reduce plaque area in AS animal models.109 However, variations in therapeutic dosage and administration frequency pose challenges in evaluating and comparing the therapeutic efficacy of different nanocarriers.
In addition, nucleic acid-based therapies, such as antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), have emerged as potential treatments for CVD. However, these therapies still have drawbacks, such as susceptibility to degradation by RNA enzymes and rapid renal clearance.110,111 Many nanomaterials have been used to deliver RNA, and lipid-based nanoparticles stood out.112 Kheirolomoom et al encapsulated anti-miR-712 in cationic lipid particles modified with a VCAM-1-binding peptide for targeting. The cationic lipid structure improved miRNA transfection efficiency. Experiments in an ApoE-/- mouse model showed a significant reduction in the area of the AS lesion of approximately 43–45% compared to non-targeted nanomaterials and nanomaterials containing mismatched miRNAs, and no side effects were observed.94 Also, ligating phosphorothioate (PS)-modified microRNA-146a to SPION-coated PEG could improve its efficacy by protecting it from nuclease degradation. Vivo experiments showed a reduction in the expression of genes involved in the NF-kB pathway in the aorta of mice in the treatment group compared to the control group. Furthermore, the treatment group showed a 30% reduction in the area of AS lesions.113 These studies indicated that nanomediated delivery systems could address the significant drawbacks of RNA therapies and play a crucial role in suppressing critical factors in the development of AS, which could be achieved by prolonging the circulation time of RNA in vivo through PEGylation of nanoparticles, adding site-specific ligands to improve specificity, and ensuring that the drug is released at the intended site to minimize off-target effects.
Furthermore, Jiang et al prepared trimethyl chitosan nanoparticles modified with atorvastatin and galactose (GTANPs) to encapsulate Baf60a siRNA (siBaf60a) and anti-miR-33 pDNA (pAnti-miR-33) for effective synergistic delivery of statins and nucleic acids. In AS mice lacking ApoE, intravenous administration of GTANPs/siBaf60a resulted in a synergistic reduction in plasma cholesterol and AS plaque area. Furthermore, oral administration of GTANPs/pAnti-miR-33 led to a synergistic increase in plasma high-density lipoprotein cholesterol (HDL-C) and anti-inflammatory cytokine levels, resulting in effective anti-AS outcomes. This study proposed a promising strategy for the synergistic delivery of statins and nucleic acids to treat AS.114
Nanoparticles as Prognostic Factors
Ridker et al conducted a collaborative analysis of patients with or at high risk for AS disease in a multinational CLEAR-Outcomes trial. The trial randomly assigned 13,970 patients intolerant to statins to a group receiving 180 mg of benzoic acid orally daily and a matched placebo group. Patients were followed for myocardial infarction, stroke, risk of a four-component composite event of coronary revascularization or cardiovascular death, and all-cause mortality. Compared to the placebo group, the bempedoic acid trial group exhibited a 21.6% reduction in the median levels of high-sensitivity C-reactive protein (hsCRP) and a 21.1% reduction in mean levels of low-density lipoprotein cholesterol (LDL-C) after six months. The baseline hs-CRP level was significantly associated with major cardiovascular events, cardiovascular mortality, and all-cause mortality. The highest hsCRP quartile had a hazard ratio (HR) of 1.43 for the primary composite endpoint and HRs of 2.00 and 2.21 for cardiovascular and all-cause mortality, respectively. Patients with elevated levels of hsCRP are at a high risk of adverse cardiovascular events, regardless of their levels of LDL-C. Bempedoic acid demonstrated similar efficacy in reducing the risk of cardiovascular events at all levels of hsCRP and LDL-C. The results indicated that increasing quartiles of hsCRP and baseline LDL-C levels were inferior predictors.115 Nanomolecules, combined with inflammation-associated cells such as monocytes/macrophages, can be a powerful prognosticator of AS by quantifying or localizing residual inflammatory regions or cholesterol.
Outlooks and Conclusions
In recent decades, advancements in understanding the pathophysiological mechanisms underlying CVDs have been substantial. The rapid progress in nanotechnology has dramatically accelerated research into novel nanomaterials for diagnosing and treating CVDs, highlighting their increasing importance in clinical practice. Beyond drug delivery, nanoparticles exhibit therapeutic potential in immune modulation, gene regulation, and tissue regeneration, promising to alleviate the burden of AS and enhance cardiovascular prognosis. Additionally, exploring miRNA and other molecular targets has unveiled new avenues for targeted therapies in CVDs.
Despite the substantial promise of nanotechnology, there remains a need for further enhancement in the efficacy of nanomaterials. For instance, comprehensive studies on the pharmacokinetics and pharmacodynamics of these materials are essential for selecting optimal compositions and nanostructural designs. Additionally, while current clinical use indicates acceptable safety profiles for nanoparticles, concerns persist regarding their aggregation and metabolic pathways within the body. The metabolism and excretion of nanoparticles in vivo are intricate processes, while some particles are metabolized by enzymes and eliminated by the immune system, others may accumulate and provoke toxic responses. Several factors, including size, shape, surface chemistry, concentration, and duration of exposure, influence nanoparticle toxicity.116 For instance, silica nanoparticles of varying sizes can selectively induce cytotoxic T cells or promote Th1 cell differentiation.117,118 Furthermore, nanoparticle shape affects cellular interactions through variable contact patterns, influencing adhesion to VSMCs and potentially exacerbating AS plaque calcification.119,120 The electrostatic solid attraction between nanoparticle surface cations and negatively charged cell membranes facilitates their penetration. However, this affinity may also cause adverse effects such as membrane disruption, cell lysis, and degranulation of granulocytes.121 Surface modifications of nanoparticles alter their interactions with atherosclerosis-related cells. For example, polyethylene glycol chains can adsorb onto membrane surfaces, activating integrins and downstream inflammatory pathways in macrophages.122
Strategies to mitigate nanoparticle-induced atherosclerotic effects include optimizing physicochemical and surface properties such as shape, size, surface charge neutrality or slight negativity. Techniques like surface modification and cell membrane coating can also help minimize adverse effects.123 However, current nanoparticle metabolism and toxicity research remains predominantly animal-based, with a notable absence of clinical case analyses. Addressing nanoparticle toxicity effectively demands comprehensive research from molecular mechanisms to clinical applications.
In conclusion, this review underscores diverse diagnostic and therapeutic strategies employing nanomaterials tailored to various aspects of CVDs. Nanotechnology is reshaping the landscape of CVDs diagnosis and treatment, from imaging contrast agents to precise drug delivery systems, as evidenced by numerous foundational studies and successful clinical trials. Looking ahead, continuous advancements in nanotechnology and progress in artificial intelligence and precision medicine hold promise for more accurate disease progression prediction and personalized treatment strategies in CVDs.
Consent for Publication
The authors confirm that the details of any images can be published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gupta AS. Nanomedicine approaches in vascular disease: a review. Nanomedicine. 2011;7(6):763–779. doi:10.1016/j.nano.2011.04.001
2. Diez-Pascual AM, Rahdar A. Functional nanomaterials in biomedicine: current uses and potential applications. ChemMedChem. 2022;17(16):e202200142. doi:10.1002/cmdc.202200142
3. Patra JK, Das G, Fraceto LF, et al. Nano-based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi:10.1186/s12951-018-0392-8
4. Heshmati Aghda N, Dabbaghianamiri M, Tunnell JW, Betancourt T. Design of smart nanomedicines for effective cancer treatment. Int J Pharm. 2022;621:121791. doi:10.1016/j.ijpharm.2022.121791
5. Corma A, Botella P, Rivero-Buceta E. Silica-based stimuli-responsive systems for antitumor drug delivery and controlled release. Pharmaceutics. 2022;14(1):110. doi:10.3390/pharmaceutics14010110
6. Sarfraz M, Afzal A, Yang T, et al. Development of dual drug loaded nanosized liposomal formulation by a reengineered ethanolic injection method and its preclinical pharmacokinetic studies. Pharmaceutics. 2018;10(3):151. doi:10.3390/pharmaceutics10030151
7. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi:10.3389/fphar.2015.00286
8. Leung AK, Tam YY, Chen S, Hafez IM, Cullis PR. Microfluidic mixing: a general method for encapsulating macromolecules in lipid nanoparticle systems. J Phys Chem B. 2015;119(28):8698–8706. doi:10.1021/acs.jpcb.5b02891
9. Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, Langer R. Advances in biomaterials for drug delivery. Adv Mater. 2018;30(29):e1705328. doi:10.1002/adma.201705328
10. Afsharzadeh M, Hashemi M, Mokhtarzadeh A, Abnous K, Ramezani M. Recent advances in co-delivery systems based on polymeric nanoparticle for cancer treatment. Artif Cells Nanomed Biotechnol. 2018;46(6):1095–1110. doi:10.1080/21691401.2017.1376675
11. Liu X, Li C, Lv J, et al. Glucose and H(2)O(2) dual-responsive polymeric micelles for the self-regulated release of insulin. ACS Appl Bio Mater. 2020;3(3):1598–1606. doi:10.1021/acsabm.9b01185
12. Katsuki S, Matoba T, Koga JI, Nakano K, Egashira K. Anti-inflammatory nanomedicine for cardiovascular disease. Front Cardiovasc Med. 2017;4:87. doi:10.3389/fcvm.2017.00087
13. Arias LS, Pessan JP, Vieira APM, Lima TMT, Delbem ACB, Monteiro DR. Iron oxide nanoparticles for biomedical applications: a perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics. 2018;7(2):46. doi:10.3390/antibiotics7020046
14. Goldsborough E, Osuji N, Blaha MJ. Assessment of cardiovascular disease Risk: a 2022 update. Endocrinol Metab Clin North Am. 2022;51(3):483–509. doi:10.1016/j.ecl.2022.02.005
15. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80(25):2361–2371. doi:10.1016/j.jacc.2022.11.005
16. Gershlick AH. Treating atherosclerosis: local drug delivery from laboratory studies to clinical trials. Atherosclerosis. 2002;160(2):259–271. doi:10.1016/S0021-9150(01)00618-9
17. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026
18. Darwitan A, Wong YS, Nguyen LTH, et al. Liposomal nanotherapy for treatment of atherosclerosis. Adv Healthc Mater. 2020;9(14):e2000465. doi:10.1002/adhm.202000465
19. Zhang Q, Jeppesen DK, Higginbotham JN, et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat Cell Biol. 2021;23(12):1240–1254. doi:10.1038/s41556-021-00805-8
20. Kargozar S, Baino F, Hamzehlou S, Hamblin MR, Mozafari M. Nanotechnology for angiogenesis: opportunities and challenges. Chem Soc Rev. 2020;49(14):5008–5057. doi:10.1039/c8cs01021h
21. Li R, Rhee SJ, Bae S, et al. H2O2-responsive antioxidant nanoparticle attenuates whole body ischemia/reperfusion-induced multi-organ damages. J Cardiovasc Pharmacol Ther. 2021;26(3):279–288. doi:10.1177/1074248420969571
22. Luehmann HP, Pressly ED, Detering L, et al. PET/CT imaging of chemokine receptor CCR5 in vascular injury model using targeted nanoparticle. J Nucl Med. 2014;55(4):629–634. doi:10.2967/jnumed.113.132001
23. Hu Q, Fang Z, Ge J, Li H. Nanotechnology for cardiovascular diseases. Innovation. 2022;3(2):100214. doi:10.1016/j.xinn.2022.100214
24. Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630–1645. doi:10.1016/j.cell.2022.04.004
25. Li N. Platelets as an inter-player between hyperlipidaemia and atherosclerosis. J Intern Med. 2024;296(1):39–52. doi:10.1111/joim.13794
26. Nankivell V, Vidanapathirana AK, Hoogendoorn A, et al. Targeting macrophages with multifunctional nanoparticles to detect and prevent atherosclerotic cardiovascular disease. Cardiovasc Res. 2024;120(8):819–838. doi:10.1093/cvr/cvae099
27. Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater. 2014;13(2):125–138. doi:10.1038/nmat3780
28. Chen W, Schilperoort M, Cao Y, Shi J, Tabas I, Tao W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. 2022;19(4):228–249. doi:10.1038/s41569-021-00629-x
29. Kao CW, Wu PT, Liao MY, et al. Magnetic nanoparticles conjugated with peptides derived from monocyte chemoattractant protein-1 as a tool for targeting atherosclerosis. Pharmaceutics. 2018;10(2):62. doi:10.3390/pharmaceutics10020062
30. Tang J, Baxter S, Menon A, et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc Natl Acad Sci U S A. 2016;113(44):E6731–E6740. doi:10.1073/pnas.1609629113
31. Wei Q, Wang J, Shi W, et al. Improved in vivo detection of atherosclerotic plaques with a tissue factor-targeting magnetic nanoprobe. Acta Biomater. 2019;90:324–336. doi:10.1016/j.actbio.2019.04.014
32. Wei X, Ying M, Dehaini D, et al. Nanoparticle functionalization with platelet membrane enables multifactored biological targeting and detection of atherosclerosis. ACS Nano. 2018;12(1):109–116. doi:10.1021/acsnano.7b07720
33. Muslu Y, Utkur M, Demirel OB, Saritas EU. Calibration-free relaxation-based multicolor magnetic particle imaging. IEEE Trans Med Imaging. 2018;37(8):1920–1931. doi:10.1109/TMI.2018.2818261
34. Flohr TG, De Cecco CN, Schmidt B, Wang R, Schoepf UJ, Meinel FG. Computed tomographic assessment of coronary artery disease: state-of-The-art imaging techniques. Radiol Clin North Am. 2015;53(2):271–285. doi:10.1016/j.rcl.2014.11.011
35. Hyafil F, Cornily JC, Feig JE, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13(5):636–641. doi:10.1038/nm1571
36. Cormode DP, Roessl E, Thran A, et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology. 2010;256(3):774–782. doi:10.1148/radiol.10092473
37. Senders ML, Hernot S, Carlucci G, et al. Nanobody-facilitated multiparametric PET/MRI phenotyping of atherosclerosis. JACC Cardiovasc Imaging. 2019;12(10):2015–2026. doi:10.1016/j.jcmg.2018.07.027
38. Tu Y, Ma X, Chen H, et al. Molecular imaging of matrix metalloproteinase-2 in atherosclerosis using a smart multifunctional PET/MRI nanoparticle. Int J Nanomed. 2022;17:6773–6789. doi:10.2147/IJN.S385679
39. Uchida M, Kosuge H, Terashima M, et al. Protein cage nanoparticles bearing the LyP-1 peptide for enhanced imaging of macrophage-rich vascular lesions. ACS Nano. 2011;5(4):2493–2502. doi:10.1021/nn102863y
40. Vilian ATE, Kim W, Park B, et al. Efficient electron-mediated electrochemical biosensor of gold wire for the rapid detection of C-reactive protein: a predictive strategy for heart failure. Biosens Bioelectron. 2019;142:111549. doi:10.1016/j.bios.2019.111549
41. Vashist SK, Schneider EM, Luong JH. Surface plasmon resonance-based immunoassay for human C-reactive protein. Analyst. 2015;140(13):4445–4452. doi:10.1039/C5AN00690B
42. Aray A, Chiavaioli F, Arjmand M, et al. SPR-based plastic optical fibre biosensor for the detection of C-reactive protein in serum. J Biophotonics. 2016;9(10):1077–1084. doi:10.1002/jbio.201500315
43. Xing Y, Gao Q, Zhang Y, et al. The improved sensitive detection of C-reactive protein based on the chemiluminescence immunoassay by employing monodispersed PAA-Au/Fe3O4 nanoparticles and zwitterionic glycerophosphoryl choline. J Mater Chem B. 2017;5(21):3919–3926. doi:10.1039/C7TB00637C
44. Adukauskienė D, Čiginskienė A, Adukauskaitė A, Pentiokinienė D, Šlapikas R, Čeponienė I. Clinical relevance of high sensitivity C-reactive protein in cardiology. Medicina. 2016;52(1):1–10. doi:10.1016/j.medici.2015.12.001
45. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi:10.3389/fimmu.2018.00754
46. Byzova NA, Vengerov YY, Voloshchuk SG, Zherdev AV, Dzantiev ABB. Development of A lateral flow highway: ultra-rapid multitracking immunosensor for cardiac markers. Sensors. 2019;19(24):5494. doi:10.3390/s19245494
47. Mansuriya BD, Altintas Z. Enzyme-free electrochemical nano-immunosensor based on graphene quantum dots and gold nanoparticles for cardiac biomarker determination. Nanomaterials. 2021;11(3):578. doi:10.3390/nano11030578
48. Huang Z, Zhang R, Chen H, et al. Sensitive polydopamine bi-functionalized SERS immunoassay for microalbuminuria detection. Biosens Bioelectron. 2019;142:111542. doi:10.1016/j.bios.2019.111542
49. Li X, Kuznetsova T, Cauwenberghs N, et al. Autoantibody profiling on a plasmonic nano-gold chip for the early detection of hypertensive heart disease. Proc Natl Acad Sci U S A. 2017;114(27):7089–7094. doi:10.1073/pnas.1621457114
50. Tabish TA, Hayat H, Abbas A, Narayan RJ. Graphene quantum dots-based electrochemical biosensing platform for early detection of acute myocardial infarction. Biosensors. 2022;12(2):77. doi:10.3390/bios12020077
51. Jang AS, Praveen Kumar PP, Lim DK. Attomolar sensitive magnetic microparticles and a surface-enhanced raman scattering-based assay for detecting sars-cov-2 nucleic acid targets. ACS Appl Mater Interfaces. 2022;14(1):138–149. doi:10.1021/acsami.1c17028
52. Huang K, Ozpinar EW, Su T, et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci Transl Med. 2020;12(538):eaat9683. doi:10.1126/scitranslmed.aat9683
53. Feiner R, Engel L, Fleischer S, et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat Mater. 2016;15(6):679–685. doi:10.1038/nmat4590
54. Amin DR, Sink E, Narayan SP, Abdel-Hafiz M, Mestroni L, Peña B. Nanomaterials for cardiac tissue engineering. Molecules. 2020;25(21):5189. doi:10.3390/molecules25215189
55. Hu C, Luo R, Wang Y. Heart valves cross-linked with erythrocyte membrane drug-loaded nanoparticles as a biomimetic strategy for anti-coagulation, anti-inflammation, anti-calcification, and endothelialization. ACS Appl Mater Interfaces. 2020;12(37):41113–41126. doi:10.1021/acsami.0c12688
56. Li Y, Zhang Y, Ding JL, et al. Biofunctionalization of decellularized porcine aortic valve with OPG-loaded PCL nanoparticles for anti-calcification. RSC Adv. 2019;9(21):11882–11893. doi:10.1039/C9RA00408D
57. Fukunishi T, Best CA, Sugiura T, et al. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J Thorac Cardiovasc Surg. 2017;153(4):924–932. doi:10.1016/j.jtcvs.2016.10.066
58. Rocco KA, Maxfield MW, Best CA, Dean EW, Breuer CK. In vivo applications of electrospun tissue-engineered vascular grafts: a review. Tissue Eng Part B Rev. 2014;20(6):628–640. doi:10.1089/ten.teb.2014.0123
59. Yin RX, Yang DZ, Wu JZ. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics. 2014;4(2):175–200. doi:10.7150/thno.7210
60. Tang J, Su T, Huang K, et al. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat Biomed Eng. 2018;2(1):17–26. doi:10.1038/s41551-017-0182-x
61. Sadek H, Olson EN. Toward the goal of human heart regeneration. Cell Stem Cell. 2020;26(1):7–16. doi:10.1016/j.stem.2019.12.004
62. Mahmoudi M, Yu M, Serpooshan V, et al. Multiscale technologies for treatment of ischemic cardiomyopathy. Nat Nanotechnol. 2017;12(9):845–855. doi:10.1038/nnano.2017.167
63. Ashtari K, Nazari H, Ko H, et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv Drug Deliv Rev. 2019;144:162–179. doi:10.1016/j.addr.2019.06.001
64. Solazzo M, O’Brien FJ, Nicolosi V, Monaghan MG. The rationale and emergence of electroconductive biomaterial scaffolds in cardiac tissue engineering. APL Bioeng. 2019;3(4):041501. doi:10.1063/1.5116579
65. Malki M, Fleischer S, Shapira A, Dvir T. Gold nanorod-based engineered cardiac patch for suture-free engraftment by near IR. Nano Lett. 2018;18(7):4069–4073. doi:10.1021/acs.nanolett.7b04924
66. Brazhkina O, Davis ME. 3D bioprinting in cardiovascular nanomedicine. Nanomedicine. 2021;16(16):1347–1350. doi:10.2217/nnm-2021-0083
67. Hasan A, Saliba J, Pezeshgi Modarres H, et al. Micro and nanotechnologies in heart valve tissue engineering. Biomaterials. 2016;103:278–292. doi:10.1016/j.biomaterials.2016.07.001
68. Lopez-Moya M, Melgar-Lesmes P, Kolandaivelu K, de la Torre Hernández JM, Edelman ER, Balcells M. Optimizing glutaraldehyde-fixed tissue heart valves with chondroitin sulfate hydrogel for endothelialization and shielding against deterioration. Biomacromolecules. 2018;19(4):1234–1244. doi:10.1021/acs.biomac.8b00077
69. Capulli AK, Emmert MY, Pasqualini FS, et al. JetValve: rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials. 2017;133:229–241. doi:10.1016/j.biomaterials.2017.04.033
70. Vellayappan MV, Balaji A, Subramanian AP, et al. Tangible nanocomposites with diverse properties for heart valve application. Sci Technol Adv Mater. 2015;16(3):033504. doi:10.1088/1468-6996/16/3/033504
71. Tadin-Strapps M, Peterson LB, Cumiskey AM, et al. siRNA-induced liver ApoB knockdown lowers serum LDL-cholesterol in a mouse model with human-like serum lipids. J Lipid Res. 2011;52(6):1084–1097. doi:10.1194/jlr.M012872
72. Beldman TJ, Malinova TS, Desclos E, et al. Nanoparticle-aided characterization of arterial endothelial architecture during atherosclerosis progression and metabolic therapy. ACS Nano. 2019;13(12):13759–13774. doi:10.1021/acsnano.8b08875
73. Kanthi Y, de la Zerda A, Smith BR. Nanotherapeutic shots through the heart of plaque. ACS Nano. 2020;14(2):1236–1242. doi:10.1021/acsnano.0c00245
74. Gasper WJ, Jimenez CA, Walker J, Conte MS, Seward K, Owens CD. Adventitial nab-rapamycin injection reduces porcine femoral artery luminal stenosis induced by balloon angioplasty via inhibition of medial proliferation and adventitial inflammation. Circ Cardiovasc Interv. 2013;6(6):701–709. doi:10.1161/CIRCINTERVENTIONS.113.000195
75. Kim H, Kumar S, Kang DW, Jo H, Park JH. Affinity-driven design of cargo-switching nanoparticles to leverage a cholesterol-rich microenvironment for atherosclerosis therapy. ACS Nano. 2020;14(6):6519–6531. doi:10.1021/acsnano.9b08216
76. Shen M, Li H, Yao S, et al. Shear stress and ROS-responsive biomimetic micelles for atherosclerosis via ROS consumption. Mater Sci Eng C Mater Biol Appl. 2021;126:112164. doi:10.1016/j.msec.2021.112164
77. Tu C, Das S, Baker AB, Zoldan J, Suggs LJ. Nanoscale strategies: treatment for peripheral vascular disease and critical limb ischemia. ACS Nano. 2015;9(4):3436–3452. doi:10.1021/nn507269g
78. Stine SJ, Popowski KD, Su T, Cheng K. Exosome and biomimetic nanoparticle therapies for cardiac regenerative medicine. Curr Stem Cell Res Ther. 2020;15(8):674–684. doi:10.2174/1574888X15666200309143924
79. Masuda S, Nakano K, Funakoshi K, et al. Imatinib mesylate-incorporated nanoparticle-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries. J Atheroscler Thromb. 2011;18(12):1043–1053. doi:10.5551/jat.8730
80. Sane M, Dighe V, Patil R, Hassan PA, Gawali S, Patravale V. Bivalirudin and sirolimus co-eluting coronary stent: potential strategy for the prevention of stent thrombosis and restenosis. Int J Pharm. 2021;600:120403. doi:10.1016/j.ijpharm.2021.120403
81. Vandergriff A, Huang K, Shen D, et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8(7):1869–1878. doi:10.7150/thno.20524
82. Hu S, Li Z, Shen D, et al. Exosome-eluting stents for vascular healing after ischaemic injury. Nat Biomed Eng. 2021;5(10):1174–1188. doi:10.1038/s41551-021-00705-0
83. Chorny M, Fishbein I, Yellen BB, et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc Natl Acad Sci U S A. 2010;107(18):8346–8351. doi:10.1073/pnas.0909506107
84. Churov A, Summerhill V, Grechko A, Orekhova V, Orekhov A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int J Mol Sci. 2019;20(22):5547. doi:10.3390/ijms20225547
85. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20(8):589–610. doi:10.1038/s41573-021-00198-1
86. Lameijer M, Binderup T, van Leent MMT, et al. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat Biomed Eng. 2018;2(5):279–292. doi:10.1038/s41551-018-0221-2
87. Gao M, Tang M, Ho W, et al. Modulating Plaque Inflammation via Targeted mRNA Nanoparticles for the Treatment of Atherosclerosis. ACS Nano. 2023;17(18):17721–17739. doi:10.1021/acsnano.3c00958
88. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30(1):1–22. doi:10.1146/annurev-immunol-100311-102839
89. Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
90. Chyu KY, Zhao X, Zhou J, et al. Immunization using ApoB-100 peptide-linked nanoparticles reduces atherosclerosis. JCI Insight. 2022;7(11):e149741. doi:10.1172/jci.insight.149741
91. Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22(2):85–96. doi:10.1038/s41577-021-00547-6
92. O’Leary CL, Pierce N, Patel SP, Naidoo J. Immune-related toxicity in non-small cell lung cancer: current state-of-the-art and emerging clinical challenges. J Thorac Oncol. 2024;19(3):395–408. doi:10.1016/j.jtho.2023.11.018
93. Hosseini H, Li Y, Kanellakis P, et al. Phosphatidylserine liposomes mimic apoptotic cells to attenuate atherosclerosis by expanding polyreactive IgM producing B1a lymphocytes. Cardiovasc Res. 2015;106(3):443–452. doi:10.1093/cvr/cvv037
94. Kheirolomoom A, Kim CW, Seo JW, et al. Multifunctional nanoparticles facilitate molecular targeting and miRNA delivery to inhibit atherosclerosis in ApoE(-/-) mice. ACS Nano. 2015;9(9):8885–8897. doi:10.1021/acsnano.5b02611
95. Dosta P, Tamargo I, Ramos V, et al. Delivery of anti-microRNA-712 to inflamed endothelial cells using poly(β-amino ester) nanoparticles conjugated with VCAM-1 targeting peptide. Adv Healthc Mater. 2021;10(15):e2001894. doi:10.1002/adhm.202001894
96. Lorenzo C, Delgado P, Busse CE, et al. ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature. 2021;589(7841):287–292. doi:10.1038/s41586-020-2993-2
97. Pi S, Mao L, Chen J, et al. The P2RY12 receptor promotes VSMC-derived foam cell formation by inhibiting autophagy in advanced atherosclerosis. Autophagy. 2021;17(4):980–1000. doi:10.1080/15548627.2020.1741202
98. Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J. 2015;36(47):3320–3331. doi:10.1093/eurheartj/ehv511
99. Yang F, Chen Q, He S, et al. miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation. 2018;137(17):1824–1841. doi:10.1161/CIRCULATIONAHA.117.027799
100. Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29(11):1005–1010. doi:10.1038/nbt.1989
101. Wei Y, Zhu M, Li S, et al. Engineered biomimetic nanoplatform protects the Myocardium against ischemia/reperfusion injury by inhibiting pyroptosis. ACS Appl Mater Interfaces. 2021;13(29):33756–33766. doi:10.1021/acsami.1c03421
102. Melero I, Castanon E, Alvarez M, Champiat S, Marabelle A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat Rev Clin Oncol. 2021;18(9):558–576. doi:10.1038/s41571-021-00507-y
103. Vong LB, Bui TQ, Tomita T, Sakamoto H, Hiramatsu Y, Nagasaki Y. Novel angiogenesis therapeutics by redox injectable hydrogel - regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials. 2018;167:143–152. doi:10.1016/j.biomaterials.2018.03.023
104. Qi Q, Lu L, Li H, et al. Spatiotemporal delivery of nanoformulated liraglutide for cardiac regeneration after myocardial infarction. Int J Nanomed. 2017;12:4835–4848. doi:10.2147/IJN.S132064
105. Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15(12):757–769. doi:10.1038/s41569-018-0098-5
106. Somasuntharam I, Yehl K, Carroll SL, et al. Knockdown of TNF-α by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials. 2016;83:12–22. doi:10.1016/j.biomaterials.2015.12.022
107. Katsuki S, Matoba T, Nakashiro S, et al. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation. 2014;129(8):896–906. doi:10.1161/CIRCULATIONAHA.113.002870
108. Hossaini Nasr S, Rashidijahanabad Z, Ramadan S, et al. Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles. Nanoscale. 2020;12(17):9541–9556. doi:10.1039/D0NR00308E
109. Wan J, Yang J, Lei W, et al. Anti-oxidative, anti-apoptotic, and M2 polarized DSPC liposome nanoparticles for selective treatment of atherosclerosis. Int J Nanomed. 2023;18:579–594. doi:10.2147/IJN.S384675
110. Cupido AJ, Kastelein JJP. Inclisiran for the treatment of hypercholesterolaemia: implications and unanswered questions from the ORION trials. Cardiovasc Res. 2020;116(11):e136–e139. doi:10.1093/cvr/cvaa212
111. Fu Q, Hu L, Shen T, Yang R, Jiang L. Recent advances in gene therapy for familial hypercholesterolemia: an update review. J Clin Med. 2022;11(22):6773. doi:10.3390/jcm11226773
112. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi:10.1021/acsnano.1c04996
113. Bai Q, Xiao Y, Hong H, et al. Scavenger receptor-targeted plaque delivery of microRNA-coated nanoparticles for alleviating atherosclerosis. Proc Natl Acad Sci U S A. 2022;119(39):e2201443119. doi:10.1073/pnas.2201443119
114. Jiang T, Xu L, Zhao M, et al. Dual targeted delivery of statins and nucleic acids by chitosan-based nanoparticles for enhanced antiatherosclerotic efficacy. Biomaterials. 2022;280:121324. doi:10.1016/j.biomaterials.2021.121324
115. Ridker PM, Lei L, Louie MJ, et al. Inflammation and Cholesterol as Predictors of Cardiovascular Events Among 13970 Contemporary High-Risk Patients With Statin Intolerance. Circulation. 2024;149(1):28–35. doi:10.1161/CIRCULATIONAHA.123.066213
116. Chen S, Su Y, Zhang M, et al. Insights into the toxicological effects of nanomaterials on atherosclerosis: mechanisms involved and influence factors. J Nanobiotechnology. 2023;21(1):140. doi:10.1186/s12951-023-01899-y
117. Hirai T, Yoshioka Y, Takahashi H, et al. Amorphous silica nanoparticles enhance cross-presentation in murine dendritic cells. Biochem Biophys Res Commun. 2012;427(3):553–556. doi:10.1016/j.bbrc.2012.09.095
118. Vis B, Hewitt RE, Faria N, et al. Non-functionalized ultrasmall silica nanoparticles directly and size-selectively activate T cells. ACS Nano. 2018;12(11):10843–10854. doi:10.1021/acsnano.8b03363
119. Huang LH, Han J, Ouyang JM, Gui BS. Shape-dependent adhesion and endocytosis of hydroxyapatite nanoparticles on A7R5 aortic smooth muscle cells. J Cell Physiol. 2020;235(1):465–479. doi:10.1002/jcp.28987
120. Liu Q, Luo Y, Zhao Y, et al. Nano-hydroxyapatite accelerates vascular calcification via lysosome impairment and autophagy dysfunction in smooth muscle cells. Bioact Mater. 2021;8:478–493. doi:10.1016/j.bioactmat.2021.06.004
121. Hwang TL, Aljuffali IA, Lin CF, Chang YT, Fang JY. Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: lipid nanoparticles versus polymeric nanoparticles. Int J Nanomed. 2015;10:371–385. doi:10.2147/IJN.S73017
122. Luo N, Weber JK, Wang S, et al. PEGylated graphene oxide elicits strong immunological responses despite surface passivation. Nat Commun. 2017;8(1):14537. doi:10.1038/ncomms14537
123. Liu Y, Luo J, Chen X, Liu W, Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nanomicro Lett. 2019;11(1):100. doi:10.1007/s40820-019-0330-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.