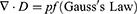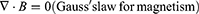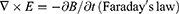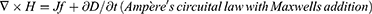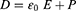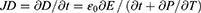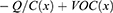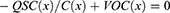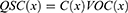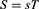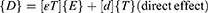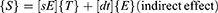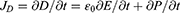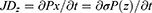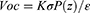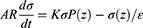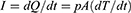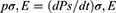Back to Journals » International Journal of Nanomedicine » Volume 19
Nanoscale Generators for Tissue Healing: A Perspective
Authors Swain S, Misra R, Rautray TR
Received 3 August 2024
Accepted for publication 24 October 2024
Published 14 November 2024 Volume 2024:19 Pages 11859—11882
DOI https://doi.org/10.2147/IJN.S480938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Subhasmita Swain,1 RDK Misra,2 Tapash R Rautray1
1Biomaterials and Tissue Regeneration Laboratory, Centre of Excellence, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India; 2Metallurgical, Materials and Biomedical Engineering Department, The University of Texas at El Paso, El Paso, Texas, 79968, USA
Correspondence: Tapash R Rautray; RDK Misra, Email [email protected]; [email protected]
Abstract: Electroactive components can promote tissue healing and control neuronal activity with the support of the tissue environment and offer electrical impulses and biocompatible material habitats. Due to the increasing growth of portable electronics, it is imperative to generate tiny, lightweight power supply appliances with outstanding performance and sustainable energy conversion ability. In order to deal with the energy deficiency of electronic devices, self-powered systems based nanogenerators are committed to capturing ambient energy for electronic device consumption. Nanogenerator assemblies provide a range of benefits, including adjustable shape, flexibility, affordability, and transportability. As such, they represent a novel and intriguing area for biomedical investigation. In living organisms, bioelectrical mechanisms play an integral part in regulating the functions of cells and tissues. An essential component of electroactive assemblies includes self-powered nanogenerators. In conjunction with nanogenerators, biomedicine has contributed to the invention of medical devices based on self-powered system. Currently, one of the most significant energy-based technologies to guarantee the long-term functioning of implanted biomedical devices is the accumulation of biomechanical energy in vivo. This review covers the development of nanogenerators for biomedical applications. Piezoelectric and triboelectric materials, which could foster the evolution of potential applications in the field of bone regeneration and tissue engineering, are the primary focus of this review. These materials are electrically self-sustaining generators that encourage tissue repair involving osteogenic proliferation, differentiation, and microbial sterilization. Eventually, the discussion highlights the potential future scope and challenges related to the nanogenerators.
Keywords: nanogenerator, piezoelectric, triboelectric, tissue engineering
Background
The development of an entirely novel generation of flexible, self-sustaining smart electronics that can supply low-power micro/nanoelectronic devices with electricity instead of hazardous chemical batteries is greatly desired. These devices would not just be self-powered sensors.1 To improve tissue regeneration,2–4 electrical stimulation has emerged as a commonly used method to compensate for the disturbed electrical signalling occurring in injured tissue.5–9 External electric fields can affect the growth and division of several types of cells (such as osteoblasts, nerve cells, and cardiac cells, etc), enhance physiological efficacy, and drive cell-oriented growth.10 Yet, invasive microelectrodes, a source of external power, and electric connections are needed for conventional electrical stimulators.5
Current research on nanogenerators (NGs) demonstrated remarkable developments in the field of non-invasive, self-sustaining electrical stimulation. As an ideal power source, NGs convert electrical signals for physiological and pathological indicator detection, nerve stimulation, tissue repair and cardiac pacing. It also harvests biomechanical energy from physiological processes including, respiration, muscle contraction, gastric peristalsis, and the rate of heartbeat. The usage of wearable and implantable electronics has driven up due to the ongoing research and implementation of proficient electronic devices. The devices need to be compact and possess great mechanical strength, flexibility, and sensitivity for ease of applications.7 A stable, efficient, and reliable energy source is essential for the use of wearable and implantable devices.1 NGs are suitable for self-powering biomedical devices since they gather human thermal, micro- and nano-mechanical energy and transform them into electrical energy. Self-powered autonomous biomedical device development has been facilitated by the conglomeration of biomedicine with NGs.6
In the rapidly expanding domain of the Internet of Things (IoT), NGs are emerging technologies that enable self-sustaining devices, sensors, as well as flexible and portable electronic systems. Due to their lightweight, readily constructed, sustainable, and low upkeep needs, NGs represent a significant advancement in ambient energy-generating technologies. Scalability of manufacturing procedures is also necessary to achieve large-scale nanogenerators.1
The primary focus of this study is on the essential function that NGs serve in tissue engineering. They enable triggers in cellular level by both mechanical and electrical stimulations, and they have a wide range of applications in the disciplines of cardiovascular, neurological, bone, and muscle tissue engineering. Their contribution to the advancement of self-powered systems could be advantageous for enhanced tissue structure and organization, optimized proliferation and differentiation of cells, regulated release of biomolecules and growth factors, interactions among cells, and real-time monitoring of tissue development.8–10
Origin of NGs
The idea of an NG has been proposed based on Maxwell’s displacement current theory. The Maxwell’s fundamental equations of electromagnetism are as follows:11
Where
E = Electric field, H = magnetizing field, B = the magnetic field ρf = the free electric charge density, D = displacement field, Jf= the free electric current density
Where P = polarization field and ε0 permittivity in vacuum
As for an isotropic media, D = εE, where ɛ is the permittivity of the dielectrics.
The Maxwell’s displacement current, designated as the second component in Equation (4),
The output electric current of the NG directly correlates with the second component ∂, P/∂t, in Maxwell’s displacement current. This suggests that NGs are Maxwell’s displacement current applications in the sensor and energy domains.11
Classification of NGs
An NG is a device that accumulates and transforms thermal, nano-mechanical, and micro-energies into electrical energy. Mainly, three categories of NGs may be classified by the power-generating approach used (a) piezoelectric nanogenerators (PENGs), (b) triboelectric nanogenerators (TENGs), and (c) pyroelectric nanogenerators (PyENGs). Various specific applications of TENG and PENG have shown promising results in the field of healthcare and biomedical.12 Mechanical energy is converted into electrical energy by TENGs and PENGs, while thermal energy produced by variations in temperature, which is converted into electrical energy using PyENGs, PENGs and TENGs.
In 2006, the first PENG in history was developed based on a ZnO nanowire array.13 PENGs use piezoelectric polarization to produce an electric field that accelerates electron motion and transforms mechanical energy into electrical energy. Medical implants, wearable appliances, sensors, energy harvesters, etc., rely on PENGs. Polyvinylidene fluoride (PVDF),6 zinc oxide (ZnO),14 barium titanate (BT)15,16 and lead zirconate titanate (PZT) were commonly used as first-generation piezoelectric materials. Then, organic piezoelectric biomaterials were produced, including nanostructured piezoelectric peptides, membranes of M13 phage and protein nanofiber membranes. TENG was designed using the concepts of electrostatic induction and triboelectricity.12 The electrostatic charge established at the interface of two different types of materials generates an electric field that propels electrons in this device. TENG provides a broader range of material options and features greater output power and energy conversion capacity. Since friction is pervasive in daily life, a wide range of applications are made possible by the ability of TENGs to harvest energy from surroundings, including water droplets, human activity, tides, ocean waves, and airflow. Currently, implanted medical devices, wearable electronics, electronic skins, human-computer interfaces, and utilization of blue energy are significant applications of TENG. TENG has the potential to identify and heal ailments by accumulating biomechanical energy from human breathing, heartbeat, and muscle activity.12 The human body produces enough thermal and mechanical energy to power an NG for biomedical device applications.17
The following section of the review discusses thermal and electromagnetic generators and their hybridization with tribo-, piezo-, and pyro-electric NGs.
Teng
TENGs serve as a highly effective, cutting-edge kinetic energy harvesting method that uses electrostatic induction and triboelectrification to convert mechanical energy into electrical energy. One vital aspect of all living things is bioelectricity, which is essential to the medical and physiological sciences. The ability of living cells to produce electrical impulses and react to electrical stimulation is recognized as a crucial characteristic that controls cellular activities and interactions with their surroundings.18–21 Though TENGs are efficient and compact, they are widely used to deliver electrical stimulation to cells that they can control on their own for purposes like functional modification or target selection, which has resulted in the development of novel techniques in biology and medicine.
Working Mechanism of Teng
The method of extracting renewable electrical energy from mechanical energy is known as energy harvesting. Its ability to generate electricity without finite resources, such as gas, oil, or coal, sets it apart from traditional electric generators. Triboelectrification, often known as contact electrification, is the foundation of a TENG.
Electrostatic induction (EI) and contact electrification (CE) are paired to provide the basic operating concept of TENGs. EI is the primary means of converting mechanical energy into electrical energy, whereas contact electrification produces static polarized charges. Yet owing to the intrinsic capacitance, TENG generally has a high internal impedance of numerous mega-ohms.22 Hence, TENG typically has a relatively low current (in the μA range) and a high voltage (several hundred volts). Directly employing TENG as the power supply source will result in inferior energy transfer due to the comparatively low impedances of electronic devices and energy retention units.23 Consequently, the TENG output has pulse-patterned waveforms with arbitrary frequency and amplitude. Hence, traditional power regulation techniques are ineffective due to the highly unpredictable mechanical energy from the environment. TENG must expeditiously implement an effective power regulation system to supply electricity for electrical appliances and power storage units. Furthermore, generating sustainable electricity from TENG without energy storage would be less feasible. Energy storage systems and TENG must be integrated harmoniously to provide renewable energy sources.24 TENGs have significant superiority over conventional energy sources in terms of adaptability and flexibility. The advancement of manufacturing that adopts TENG as a power source continues to be hindered due to the requirement to use large capacitors or conventional inflexible battery packs. In a few investigations, a novel method of integrating TENG with an energy storage unit to create a self-charging power unit (SCPU) has been proposed.25,26
Since a capacitor is the most fundamental electrostatic device, TENG inherently demonstrates capacitive behavior.27 The intrinsic capacitive behaviour of a random TENG is revealed through analysis. Tribo-pairs, or a pair of materials facing each other, are necessary for every triboelectric generator. Under the influence of mechanical stress, the distance (x) separating these two triboelectric surfaces can change. Contact electrification causes the two triboelectric surfaces to accumulate opposing static charges (tribo-charges) on their contact surfaces once they are forced into contact. In addition to the tribo-pairs, the TENG device contains two meticulously insulated electrodes with the intent that charges can only move across them via external circuits. If the amount of transferred charge between two electrodes is Q, then the transferred charge carried by one electrode will be -Q, while the other electrode will possess +Q charge. The electrical potential differential between the two electrodes of TENG is associated mainly with two primary components. The first component comes from the triboelectric charges (produced by polarization), and VOC(x), as a function of the distance (x), represents their contribution towards the voltage. In addition, a variation in electric potential will also be caused by the charge Q that has previously been transferred. In this circumstance, there is the presence of no triboelectric charges as well as the structure is entirely made up of a conventional capacitor, and the contribution of these previously transferred charges between the two electrodes is -Q/C(x) (C = capacitance between the two electrodes).28
The generation of the electrical potential difference between two electrodes will be produced by the separation of the polarized tribo charges. As a result of this electrical potential, electrons will migrate from one electrode to another if an external circuit is present between the two electrodes. By transferring these electrons to the other electrode, they may further screen the electrical potential. These transferred charges (QSC) effectively filter the electrical potential generated by polarized triboelectric charges under short-circuit conditions. Thus, the subsequent equation can be effortlessly derived in short-circuit conditions for TENGs.28 Consequently, the V-Q-X relationship (the principle of electrical potential superposition) can potentially be used to determine the overall voltage differential between the two electrodes.
Consequently, the essential correlation between VOC, C, and QSC is expressed as follows:
The electric double layer (EDL) develops in the CE between solid and liquid (Figure 1). The Wang model of EDL29 suggests the two-stage development of an EDL. The first step is the transmission of electrons between the solid and liquid surfaces; the second stage is the interaction of different ions inside the liquid. As a result, by including the first stage that converts the atoms present on the surface of a solid into ions, the conventional model might be changed. The coexistence of electron exchange and ion adsorption in the solid–liquid interface has been confirmed by recent investigations conducted in practice. Such a modification could impact certain relevant knowledge about electrochemistry and surface chemistry throughout cellular interactions.30
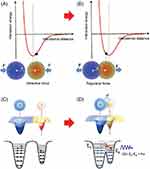 |
Figure 1 The suggested model of overlapping electron cloud explains charge transfer and CE in a generic scenario involving two atoms. When two atoms are subjected to an attractive and repulsive force, their corresponding interatomic interaction potentials are (A) and (B). (C) and (D) demonstrate the electron cloud model and potential energy well model of two atoms of materials (A and B), respectively, nearby and distanced. CE occurs when an external force lowers the potential barrier, allowing the electron to move between atoms (A and B). Reprinted from Wang ZL. Triboelectric nanogenerator (TENG)—sparking an energy and sensor revolution. Adv Energy Mater. 2020;10(17):2000137. © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.29 |
The intricate operation of TENG is demonstrated in Figure 2, which shows a contact-separation format of TENG. There is neither generation nor induction of charge in the beginning state (Figure 2I). The Triboelectric charges are produced on the two interacting surfaces when two distinct materials come into contact (Figure 2II). A potential difference is likely to be generated when these interacting surfaces are separated. This leads to an immediate flow of electrons from the one end of the electrode to the uppermost electrode (Figure 2III), which eventually reaches equilibrium when both surfaces are completely separated out (Figure 2IV). The external load will experience a return flow of electrostatically produced charges to balance the difference in electric potential when both surfaces interact again (Figure 2V).30
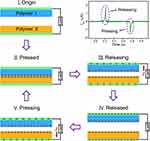 |
Figure 2 Diagrams demonstrating the TENG’s contact-separation mode operation. Reprinted from Luo J, Wang ZL. Recent progress of triboelectric nanogenerators: from fundamental theory to practical applications. EcoMat. 2020;2(4):e12059. Creative Commons.30 |
Four Operational Modes of Tengs
Following the initial release of TENG in 2012, an efficiency of nearly 70% energy (instantaneous) conversion was observed that could provide about 500 W/m2 output power density.31 There are four fundamental modes of operation have been proposed for the TENG, as illustrated in Figure 3, based on the orientation of the electrode arrangement and polarization shift. These are categorized as (A) vertical contact-separation (CS), (B) lateral sliding (LS), (C) single-electrode (SE), and (D) freestanding triboelectric-layer (FT) modes of operation.32 The vertical CS structure, the most fundamental mode, uses the relative orientation that lies perpendicular to the surface. The potential across the two electrodes fluctuates throughout the CS procedure, and an external source of electricity is subsequently generated to balance the potential drop. The lateral sliding process in the external circuit creates an electric current. This process uses the relative displacement that is parallel to the contact surface and the potential difference generated between the two electrodes. This approach can also be accomplished through rotation-influenced sliding. Taking the ground functioning as the standard electrode, the single electrode mode is designed to harvest mechanical energy generated by the mobile object, eliminating the need for a conduction connection. The single-electrode mode served as the model for the freestanding triboelectric-layer mode, which uses two pairs of uniform electrodes instead. The unequal distribution of charges will culminate in electrical output when the site of a mobile object alters. The theoretical approaches associated with these four crucial operating modes have been widely investigated.33 It should be emphasized that in actual implementation, TENGs cannot be confined to a single mode; instead, many modes are frequently blended to maximize their benefits.
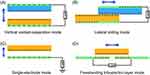 |
Figure 3 The four primary ways of operation of TENGs: (A) vertical contact-separation mode, (B) Lateral sliding mode, (C) single electrode mode, (D) Freestanding Triboelectric-layer mode. Reprinted from Luo J, Wang ZL. Recent progress of triboelectric nanogenerators: from fundamental theory to practical applications. EcoMat. 2020;2(4):e12059. Creative Commons.30 |
Peng
Materials that possess piezoelectric properties are advantageous because they exhibit the potential to transform mechanical energy into electrical energy.34–40 The development of a piezoelectric potential, also known as a piezo-potential, in piezoelectric substances is fundamentally dependent on the breakdown of central symmetry within the crystal structure by an external force.41 In their undisturbed condition, the charge centers of anions and cations correspond to one another. When an outside force is applied, the structural framework deforms.42,43 An electric dipole is formed as a consequence of the separation of the charge centers of the cations and anions, which results in the formation of a piezo-potential. In order to partially obscure the piezo-potential and achieve a new equilibrium state for a distorted crystal that is coupled to external stress, free electrons are stimulated to propagate through the external circuit, thereby activating the energy transformation process.
Thus, if the piezo-potential is continually varied through a high-powered straining phenomenon, the flow of pulsed current streaming via the external circuit is observed. There are two categories of piezoelectric materials: those that include lead (Pb-containing) and those that are devoid of lead (Pb-free). PbxZr1-xTiO3, or lead zirconate titanate, and its numerous doped modified forms are the principal lead-containing piezoelectric substances. These materials have exceptional piezoelectric capabilities. Pb-based materials are likely to have very limited future applications due to the detrimental impacts of Pb on environment and human health. Investigating superior performance based on environment friendly materials is therefore indispensable. The majority lead-free piezoelectric materials have recently been studied including zinc oxide (ZnO), PVDF, and barium titanate (BaTiO3). They exhibit a number of advantages, such as simple structural arrangement, excellent piezoelectricity, ease of manufacturing, inexpensive production, and suitability for large-scale manufacturing and implementation. As a result, lead-containing piezoelectric materials are gradually being replaced by lead-free alternatives. With its potential ability to convert arbitrary mechanical energy into electricity using nanometer-ranged piezoelectric substances, PENG represents a rapidly emerging technology. They are eco-friendly and more capable of producing sustainable electrical energy as compared to chemical batteries. Hence, they are seen as great opportunities for projects involving sustainable energy.44 PENGs represent a highly promising technology that may be able to accomplish the amazing feat of powering these wireless electronics. There are multiple possibilities for generating electricity through ambient energy gathering for useful applications owing to recent advancements in piezoelectric NGs. One technique that has seen a sharp rise in application for power production includes the utilization of PENGs to take advantage of the vibrations near the device. The crystalline structures of the active components in PENGs enable them to efficiently convert mechanical strain into electrical energy. The potential of these active materials is to collect even the smallest vibrations from their environment (typically ambient vibration) and convert them into electrical impulses that can power and operate other devices.45
Flexible electrodes and substrates that can retain their initial electrical and mechanical characteristics even after being bent or stretched are crucial parts of PENGs. Composite material-based NGs, which are typically composed of piezoelectric nanomaterials that are dispersed throughout an elastomeric matrix, may prove beneficial for energy-harvesting applications that require high flexibility and scale. These NGs have a number of advantages, including easy manufacturing, low cost, and mechanical durability.41,46 The integration of multiple nanowires in a parallel arrangement on a single flexible substrate is essential for practical applications for enhancing the output power of TENG. Therefore, a flexible NG based on multiple lateral nanowire arrays was created by merging 700 rows, each of which included around 20,000 lateral ZnO nanowires. The greatest voltage and optimum current that could be obtained, while the system was periodically distorted were 1.26 V and 28.8 nA, respectively.47
There was a surge of interest in PENG-based devices as potential autonomous medical bio-devices for use in clinical settings and the field of biomedicine as a whole.
ZnO-based PENGs: One of the most prevalent applications of ZnO in NGs for tissue engineering is its high piezoelectric characteristics. Significant features of ZnO nanowires include their capacity to produce rectifying piezoelectric currents and their combined piezoelectric and semiconducting properties. Multiple kinds of productive coatings of copolymer are suitable for their fabrication.48
BTO-based PENGs: BTO is an inorganic substance that exhibits a high level of biocompatibility and cytocompatibility. The material is devoid of lead and is often used in composites to enhance the performance of PENG.49
PZT-based PENGs: PZT has significant piezoelectric properties and is widely employed in several applications in medicine, including piezoelectric resonators and ultrasonic transducers. Nevertheless, the cytotoxicity of lead makes it unsuitable for use in implanted devices and wound care.
PVDF-based PENGs: PVDF and its copolymers, like poly(vinylidene fluoride-trifluoro ethylene) (P(VDF-TrFE)), find extensive applications in implantable self-powered devices, tissue engineering, and biomedicine, due to their exceptional flexibility and lack of cytotoxicity. Additionally, they exhibit exceptional physical and chemical durability.50
Two-dimensional PENGs: Wu et al presented the first two-dimensional (2D) PENG consisting of a single atomic layer of MoS2. Since atomic units are very thin, composites made of 2D PENGs are suitable for implanting into the body and may be used in wearable electronic devices. In order to create flexible PENG devices, it is necessary to use electrodes that have a high level of flexibility for both nanowire attachment and energy output. However, metal, indium tin oxide (ITO), and graphene are potential options for materials used in flexible electrodes.
Working Mechanism of Peng
Mechanical distortion is accompanied by the production of electricity in PENG. The PENG typically operates on two different types of operating principles: direct and indirect impacts. When stress is imparted to the piezoelectric substance, the direct effect produces an electrical impulse; in contrast, the indirect effect requires external electrical stimulation in order to cause the material to mechanically respond (Figure 4).51–54 Hooke’s law is used to explain the electrical behavior, which is theoretically related to the piezoelectric phenomenon.55 The explanation for the linear electrical response of a piezoelectric substance is described below.
 |
Figure 4 (a) Direct and indirect response of piezoelectricity, (b) Three-coordination frameworks for the explanation of the tensor orientations for piezoelectric response. Reprinted from Sriphan S, Vittayakorn N. Hybrid piezoelectric-triboelectric nanogenerators for flexible electronics: recent advances and perspectives. J Sci Adv Mater Devices. 2022;7(3):100461. Creative Commons.51 |
The dielectric permittivity, electric displacement, and electric field strength are represented by D and E, respectively. The linear elastic substance is defined by Hooke’s law as
Here, S stands for the strain, T for stress, and s for compliance of the device.
In the context of the piezoelectric strain-charge theory, equations (10) and (11) are integrated to give a set of coupled mathematical equations as follows.55
The direct piezoelectric effect matrix is denoted by [d], whereas the matrix describing the indirect piezoelectric effect is represented by [dt]. Electric field and constant or zero stress from the network are denoted by the superscripts E and T, correspondingly. Further, t specifies the transposition of the matrix.
Displacement vectors and electric field vectors, dielectric and elastic variables, including stress and strain tensors, are all coupled up in piezoelectricity. As a consequence of the anisotropic nature of the piezoelectric material, energy extraction and detection depend on the orientation of imparted electrical or mechanical stimuli to correlate the response to the stimulus. The rectangular coordinate system axes, X, Y, and Z, conform to analogous three-axis coordinate systems (i, j, and k) that are configured to determine the potential stimulus orientations provided to the piezoelectric specimen.56 There are two alternative orientations for the stress delivered to the PENG: horizontal and vertical. Therefore, as seen in Figure 4, the markings 1, 2, and 3 denote the directions along the X, Y, and Z axes, respectively, for the purpose of convenience. The numbers 4, 5, and 6 demonstrate the shear forces that are exerted on the piezoelectric substance along the X, Y, and Z axes, correspondingly. For instance, the initial index “3” in the case of the piezoelectric coefficient d33, which was frequently used to determine the piezoelectric characteristics for the PENG system, regulates the electrodes associated with the poling orientation along axis 3, and the second index “3” suggests the orientation of imparted stress along axis 3.
As a result, assuming coordinate systems are taken into account, the piezoelectric linear functional correlations in the strain-charge version are.57
where the permittivity of the dielectric tensor at constant stress is represented by ƐTij and the elastic compliance vector at the constant electric field is represented by sEij (as shown in Figure 5b, the values of the I and J indices range from 1 to 6, although they can also be 1 to 3). Furthermore, both D and E correspond to the three distinct coordinate axes, X, Y, and Z, and represent 3 × 1 tensors. The 6 × 1 tensors S and T represent shear strain or stress (4–6) and vertical strain or stress (1–3). The working mechanism of PENG is exclusively involved in the direct piezoelectric behavior. The piezoelectric material generates piezoelectric polarization charges (σP) at both terminals when it is exposed to an external force. This is accompanied by induced charges (σ(t)), which are transmitted across two metal electrodes by an external load Figure 5ai.
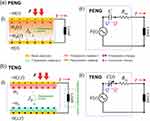 |
Figure 5 The theoretical model elucidating the (a) PENG and (b) TENGs’ analogous circuits and charge-generating process. Reprinted from Sriphan S, Vittayakorn N. Hybrid piezoelectric-triboelectric nanogenerators for flexible electronics: recent advances and perspectives. J Sci Adv Mater Devices. 2022;7(3):100461. Creative Commons.51 |
According to Maxwell’s displacement current JD,58
Here, P represents polarization field.
The PENG is not subjected to an external field of electricity. The displacement current density, having polarization in the z-axis orientation (JDz), is represented as
Similarly, the open-circuit voltage (Voc) for PENG can be defined as:
Here, k is the width of the piezoelectric media.
When the electrical load (Figure 5a.i) is connected, the current transportation expression can be given by,
Where A is the area of the electrode, and R represents the resistance to external loads.
The current signals and output voltage, as well as the transport context, are strongly influenced by the polarization charge magnitude, which is determined by the piezoelectric coefficient of each individual piezoelectric material, as demonstrated by equations 17, 18, and 19. Since piezoelectric medium width is constant, Figure 5a.ii shows the analogous circuit of PENG. Regarding the excitation time V(t), the piezoelectric substance, in this instance, can be regarded to be an AC voltage supplier. Because of the media-electrode junction, parasitic resistance is represented by Rin, and the electrical capacitance C is related to the quantity of electrical charges Q deposited on the metallic electrodes as an outcome of piezoelectric polarization. When force is imparted, piezo-potential is produced, which propels current across the circuit.51
When piezoelectric insulating substances are implemented for producing NGs, the way these materials behave during power production is primarily dictated by their ability to generate alternating current (AC). Carrier movement through the metallic electrodes of NGs through these insulating active substances is prevented by the insulting characteristics of these materials. Therefore, the NG produces electricity via AC. When the active substance frequently undergoes stretching and release, or when it encounters strain and is frequently unfastened, the associated both negative and positive voltage, as well as the current output peaks, can potentially be recorded.59
PyENG
The prospective uses of pyroelectric energy-capturing devices as power generators have garnered significant interest in recent times. PyENGs are a kind of power source that can be used by forthcoming sensor technology that needs extremely minimal power. The pyroelectric effect, in particular, is used as an IR (infrared) sensor since it transforms thermal energy into electric current. Nonetheless, a paradigm transition in pyroelectric systems from sensors to power sources has started due to the improved output efficiency of PyENGs as well as the declining power consumption of the Internet of Things (IoT). Furthermore, to enhance the output performance, PyENGs may be readily hybridized with various energy-capturing systems, such as the TENGs and PENGs. The concepts of PENGs and TENGs include the exchange of outside mechanical energy into electrical energy through the utilization of the piezoelectric and triboelectric effects. Consequently, the pyroelectric phenomenon can efficiently transform thermal energy produced by mechanical friction, devices, and the environment into electric energy, which can then be used as a novel form of power source for IoT devices.60
WORKING MECHANISM of PyENG
Pyroelectric materials exhibit intrinsic spontaneous polarization (Ps) when there is no electric field. The pyroelectric phenomenon refers to the transitory alteration in Ps caused by a shift in the temperature of the pyroelectric materials over time.61 The decline in surface charges attached to the surface arises from decreased Ps due to thermal vibration with the rise in temperature.62 A potential difference is produced across the pyroelectric substance when it is in an open-circuit mode.61 Electricity flows across an external circuit if the pyroelectric substance is in a state of short circuit condition.
Equation (20) defines theoretical short circuit current output as follows;
Where A denotes the surface area of the pyroelectric substance, t represents the duration, Q represents the pyroelectric charge, and i is the produced current.
Equation (21) represents the pyroelectric coefficient of an unclamped object under a uniform electric field and stress.
Where σ denotes constant stress and E represents the electric field. Despite being a vector quantity, the observed pyroelectric coefficient is usually interpreted as a scalar quantity.
Applications of NGs
In order to reflect health information, NGs, which are autonomous sensors, transform biomechanical and thermal energies into electrical impulses, as previously stated. Numerous investigations have been conducted to identify additional biomedical uses for self-powered technology, aside from sensors, as shown in Figure 6. Examples of these include the stimulation of biological tissues with electrical energy produced by natural gas generators or the powering of medical devices. However, as compared to PyENG, PENG and TENG have been more frequently utilized in biological tissues because of the presence of body fluids that affect piezoelectric or triboelectric materials, changing the output of NGs.63
 |
Figure 6 Various applications of NGs in healthcare devices and biomedical engineering. Reprinted from Wang W, Pang J, Su J, et al. Applications of nanogenerators for biomedical engineering and healthcare systems. InfoMat. 2022;4(2):e12262. Creative Commons.63 |
Application of TENG in Various Fields
Global health concerns have sparked intense interest in using a variety of wearable bioelectronics to acquire different physiological information about the human body. However, as the number of elderly people rises, it is more difficult than ever to develop workable healthcare alternatives. In connection to acute diseases, which can be effectively managed within a relatively brief period of time through intensive treatment, chronic diseases demand numerous clinical treatments. These include the necessity for continuous analysis of health data, ongoing monitoring of health conditions, and even on-demand treatment to ensure personalized health care.64 As a result, biomedical devices that are worn or implanted are essential for contemporary clinical treatments, which involve documenting, assessing, and monitoring a wide range of bioactivities, as well as carrying out the therapeutic tasks of the devices, as shown in Figure 7. As a result, the world enters the IoT age, and specialists encourage human well-being through the use of biomedical devices, which are extensively used on the human body to customize, gather, and analyze the health data.
 |
Figure 7 An outline of TENG’s applications in various fields of bioengineering. Reprinted from Matter, volume 4(3), Zhang S, Bick M, Xiao X, Chen G, Nashalian A, Chen J. Leveraging triboelectric nanogenerators for bioengineering. 845–887, copyright 2021, Creative Commons.64 |
TENG in Drug Delivery
Drug delivery systems that are implantable and on-demand possess significant promise for treating particular sites, owing to their exceptional efficiency and regulated, prolonged release. Many ailments, including diabetes, ophthalmic disorders, cancer, etc., can be treated using implanted devices. One major drawback of these devices is their need for a power supply, which is often a lithium (Li) ion battery. After a certain period of time, the battery must be replaced and removed surgically. The expense of having surgery to replace a battery is high, and the patient experiences discomfort.63 The TENG, which transforms mechanical energy into electrical energy, can serve as a power source for the implanted device. A microneedle-integrated TENG for medication delivery was proposed by Bok et al in 2018.65 Salmon deoxyribonucleic acid (sDNA) was utilized to create dissolving microneedles and form an active layer in TENG. Using P.I. and PTFE, the triboelectric property of sDNA was investigated. The drug was delivered by using the output of TENG as a pulse for the electrophoresis action. Furthermore, the researchers examined at how drug deposition in sDNA film affected the TENG’s output performance. To work with the TENG, an sDNA microneedle device with rhodamine intercalation was created. The skin of a pig corpse was used to assess the insertion capabilities of a microneedle.66 The triboelectric device (for the electrophoresis effect) and the drug-intercalated sDNA microneedle patches were the two main components of the device. As a pharmacological model, rhodamine B dye was employed. By absorbing into the skin, the sDNA microneedle could dissolve the drug and penetrate the stratum corneum. Evaluation was given to the practical use of determining whether or not the electric field was transmitted to the inner membrane of the skin. Ouyang et al in his study showed a non-invasive and on-demand drug administration method across the skin using TENG.67 This drug delivery system consisted of three parts: (a) PMU (power management unit), (b) iontophoresis and drug and patch electrodes, and (c) spinning TENG. The drug patch composed of a screen-printed electrode (SPE) coated with polypyrrole (Ppy) thin films that had been loaded with the drug. The skin and SPE were separated by a sponge dipped in phosphate buffer saline (PBS). Dexamethasone sodium phosphate (DEX-P), an anti-inflammatory medication, was utilized by the researchers to illustrate the regulated drug delivery. Iontophoresis therapy was applied to TDD using the patch electrode (C). Drug molecules travel across the stratum corneum as a result of the electro-osmosis and electrophoresis effects of the voltage provided through electrodes A and C. The voltage that was delivered to the drug electrode determined how much drug was released along with varying TENG charging times affect drug release.65
TENG in Tissue Engineering
TENG shows promising results for converting biomechanical signals to electrical ones. Due to their simple architecture, affordability, and dependable performance, TENG, an emerging platform for the generation of electricity, has attracted research efforts from a variety of angles, including property enhancement, mechanism investigation, device fabrication, and on-body applications. By integrating smart 3D structured porous scaffolds and a desirable multi-convex TENG-based sensor, Yue et al (2023)68 developed a tissue battery enabling integrated cartilage treatment using nanotechnology. The suggested cartilage tissue battery is extremely sensitive, self-supportable, repair-induced, implantable, interference-free, and smartly degradable. Within the pressure range of 0–1.8 MPa joint movement, the contact separation TENG mechanism-rooted sensor has an excellent sensitivity of 52.5 V MPa–1, allowing the tissue battery to in situ monitor the real-time status of cartilage healing in the black box. Furthermore, studies conducted both in vivo and in vitro revealed that the soft tissue battery accelerates the cartilage-healing process by converting mechanical energy into electrical current that stimulates chondrocyte growth in the scaffold.
Wang et al69 presented a biomedical TENG that utilized electrostatic induction and tribo-electrification between ethyl cellulose (EC) sheet and a biocompatible medical 317L stainless steel (317L SS) plate. Inductively coupled plasma etching and lithography techniques were used to design the surfaces of EC and 317L SS. After examining efficient sliding friction at the tribo-interface, the mechanism of TENG, and its power output, it was observed that the output power of the TENG rose significantly when the surface pattern density raised, which was commensurate with a boost in the efficiency of sliding friction. The power output of the TENG attained its maximum under ideal conditions, with a generated density of 4 × 104 mm−2 (for 317L SS) along with an etching power of 275 W (for EC). The open-circuit voltage and short-circuit current reached 245 V and 50 μA, respectively, correspondingly sufficient to power approximately 18 commercial LEDs in parallel connection. During a month-long immersion in simulated bodily fluid for biological experiments, open-circuit voltage and short-circuit current remained consistent at 150 V and 24.5 μA, respectively. Biocompatible TENG thereby offers a wide range of possible applications in the biological domain.
Zhang et al discovered a triboelectric NG that is sandwich-shaped and multiplies the frequency of electric energy from lower-frequency mechanical energy.70 Between two PDMS (polydimethylsiloxane) membranes, an Al sheet was placed to increase the frequency by two times, using contact electrification during a single cycle of external force. To improve the functionality of the device, meticulously constructed nano/micro dual-scale patterns (such as V-shaped grooves and pyramids) were built on the apex of the PDMS surface. From the observations, it was demonstrated that 465 V was the output maximum voltage, 13.4 μA/cm2 was the output current density, and 53.4 mW/cm3 was the output energy volume density. Five commercial LEDs connected in parallel have been shown to have a consistently large output, and the first instance of an NG being used to control a biomedical microsystem has been achieved by lighting an implantable three-dimensional microelectrode network for neural prosthesis devoid of the need for extra components. Particularly in the biomedical domain, this sandwich-shaped TENG has intriguing prospective applications in nano/micro devices (Figure 8).
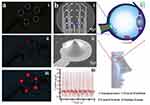 |
Figure 8 The sandwich-shaped TENG’s implementation in electrical devices and biological systems are illustrated: (a) Without the need for a rectification circuit and an energy storage system, five commercial LEDs were parallelly illuminated straight away, (b) (i) and (ii) 3D implantable microelectrode network,(iii) 88 μA of current was obtained when the neural prosthesis was directly powered. Reprinted with permission from Zhang X-S, Han M-D, Wang R-X, et al. Frequency-multiplication high-output triboelectric nanogenerator for sustainably powering biomedical microsystems. Nano Lett. 2013;13(3):1168–1172. Copyright © 2013 American Chemical Society.70 |
In order to monitor physiological processes, Fu et al71 designed a fibrous stretchable TENG-based (FS-TENG) featuring a core-sheath layout. The FS-TENG sensor could provide a short-circuit current and an output open-circuit voltage of over 0.6 μA and 10V, respectively. The FS-TENG sensor had high resilience due to its flexible substrates and stretchy electrodes. Its output voltage remained steady even under a 60% strain extension, indicating the durability of sensors at massive distortion. A remarkable responsiveness towards outside forces and ultralow identification limit were demonstrated by the FS-TENG sensor, which could reach 26.75 V N−1 in reduced pressure and identify ultralow pressure as low as 0.02 N. Presumably, the FS-TENG sensor could sense throughout a wide strain range when the output voltage was employed as the detection signal because it displayed a steady output voltage when strain extension lied at 60%. Leveraging these exceptional characteristics, the FS-TENG sensor was utilized for accurate physiological detection, encompassing large-scale human motions such as bending and stepping as well as subtle human indicators such as phonation, expression, and pulse. Additionally, to measure the spatial dissemination of pressure, FS-TENG sensors weave a tactile sensor network comprising 3 × 3 pixels, demonstrating the possible use of the sensor in the monitoring of outside forces.
An extremely flexible, self-powered, completely translucent contact-separation mode TENG was proposed by Zhao et al72 to serve as a tactile sensor. A double-network ionogel exhibiting stretchability, conductivity, and transparency served as the material for electrode and first friction layer in the TENG, while patterned PDMS (polydimethylsiloxane) served as the second friction layer. When sensing impacting forces within the range of 0.1–1 N, the manufactured sensor has an optimal sensitivity of 1.76 V/N. A strong linearity with impacting forces was maintained by the triboelectric impulse signals at various tensile percentages due to the excellent flexibility of the sensor. An external impulsive force could provide a steady triboelectric current or voltage for detection by inducing contact-separation movement across the layers of highly conductive ionogel and patterned PDMS. At varying tensile percentages (0%~80%), this sensor exhibits excellent stretchability (121%), a high degree of transparency (83%), and a favourable sensitivity to pressure (0.39~1.46 V/N) between 0.1 and 1 N. The self-powered sensor was used to detect various touching pressures, human respiration, finger bending, and heartbeats to illustrate its biomedical uses. Future implementations of wearable sensing devices might greatly benefit from the self-powered skin-like sensor, as it is a blend of stretchability, transparency, and sensitivity.
A super hydrophobic driving platform (SDP) with paralleled operating electrodes and a TENG driving droplet (TNDDS) system with periodic frictional Kapton film and Al foils were the two components of the system which Lin et al73 established to facilitate the directional transportation of bacterial droplets that needed to be eradicated. In order to create an electric field that propels the directed transportation of charged droplets, the current produced by the TENG was transferred to the paralleled operating electrodes. The driving range of droplets was correlated with the number of electrodes, and the critical value corresponding to the propelled droplet volume on SDP was directly connected to the width and distance of the distributed electrodes. The most important feature is that TNDDS could actively move charged droplets of produced triangular Ag NPs (silver nanoparticles) in both directions so as to eliminate and combine with a small bacterial droplet in a tiny half-enclosed network or on an open SDP. Ag+ release provides the potential to eradicate bacteria, while TNDDS’s ability to regulate motion direction successfully eliminates them. With all aspects considered, this strategy presented a viable use for eliminating germs from surfaces of materials powered by TENG and created a fresh path for the anti-adherence of bacterial cells.
For bone regeneration to occur, proliferation and differentiation are essential. Low-level laser treatment promotes cell viability and quickens tissue regeneration, which helps in faster healing. An infrared (IR) laser unit and a flexible TENG make up the self-powered laser curing system (SPLC). Combining a flexible intertwined electrode with a TENG for self-powered flexibility, an implanted self-powered low-level laser cure (SPLC) device was designed by Tang et al.74 Further, it has the ability to stimulate the differentiation and proliferation of embryonic osteoblast cells in mice. The SPLC comprises a versatile TENG and an in vitro laser module. The frictional layer of the TENG is constructed using a PDMS film and ITO. It is shaped into an arch to allow for the bending movement of the knee joint.66 The TENG was surgically inserted between the diaphragm and the liver of the mouse. Upon the movement of the diaphragm, the TENG produced an open-circuit voltage and a short-circuit current of 0.2 V and 0.06 nA, respectively. The electricity was subsequently gathered and utilized to power the laser apparatus. The TENG laser therapy group exhibited a 16.9% increase in alkaline phosphatase (ALP) levels compared to the control group. This increase led to the formation of bone matrix and the maturation of the extracellular matrix in MC3T3-E1 cells. The laser irradiation from the TENG treatment system enhanced mineral deposition in MC3T3-E1 cells, suggesting that this system can stimulate the proliferation, differentiation, and production of osteoblasts. Similarly, in another study, Tian et al75 presented an implanted electrical stimulator. It is explained that following the application of electrical stimulation, the amount of intracellular Ca2+ ions was elevated, indicating that this autonomous electric stimulant greatly enhanced osteoblast adhesion, differentiation, and proliferation. It boosted the overall spreading surface by 78.37% following stimulation for 1h and improved the adhered number of MC3T3-E1 cells (up to 72.76%) after 3h. Additionally, following 3 days of treatment, MC3T3-E1 proliferated by 23.82%, and following 12 days of stimulation, the differentiation level of the cells was enhanced by 28.2%. Furthermore, it was shown that the self-powered electrical stimulator was fueled by the everyday motion of a rat. This suggests that TENG could be used in a practical way to serve as an electroactive medical implant to trigger bone remodeling and osteoblast differentiation, as it was able to efficiently transform the mechanical energy (produced from the daily motion of the rat) into electric energy. The autonomous electrical stimulator mentioned above may be able to affect bone homeostasis and reduce fractures associated with osteoporosis. These findings open up possibilities for the future in situ activation of osteoblasts along with electrically reactive cells (muscle and neuron cells, etc)., the therapeutic treatment of bone damage, as well as remodeling of bones following bone transplantation by implementing TENG.
TENG in Wound Healing
The durability and biodegradability of implantable TENGs are determined by whether they are designed for long-term or short-term usage. TENGs have the advantage of being self-powered, and their utilization as efficient energy harvesters has been the subject of research for the past decade. Wearable TENGs have limited applications; however, implantable TENGs possess an advantage in terms of in vivo self-powering and physiological data sensing.76 However, an enormous portion of the global population is impacted by non-healing sores such as diabetic foot and venous-associated ulcers.58 As a result, they demand high healthcare costs. One of the most appealing wound healing techniques that mimic the natural healing process of the body is ES. The TENG is an approachable option for producing electric pulses because of its affordability, wearability, ease of fabrication, flexibility, etc. Jiang et al developed the bioresorbable TENG (BN-TENG) made from natural materials to regulate the pulse of cardiomyocytes.77 Cell regulation and tissue healing may be aided by the ES. In the future, self-powered cardiomyocyte stimulation may be used to treat some cardiac conditions by healing abnormal cardiomyocytes. Additionally, there is a chance that the stimulation will help the heart tissue regenerate.
The ES systems consist of a bridge rectifier, BN-TENG, and PDMS-packed IDT electrodes. Furthermore, wound infection is a significant problem when there is inadequate wound healing. In 2021, Du et al developed a TENG patch loaded with drug and incorporated a surface-engineered electrode coated with Mg-Al layered double hydroxides (LDH).78 The TENG loaded drug exhibited an arch-shaped patch that contained a minocycline container and an electrode consisted of electropositive Mg–Al LDH@Al film and electronegative PTFE. An external force caused these two fabric materials to come into touch with one another and separate, inducing an alternating current. It was discovered that electrical stimulation aided in the healing of wounds and encouraged the release of loaded minocycline by Mg-Al LDH@Al upon electrode with serum fluid contact. In another in vitro investigation, 24 hours after applying this surface-engineered TENG patch, nearly 100% of S. aureus and E. coli were eliminated. Additionally, increased fibroblast migration and proliferation were observed. Mice infected with S. aureus and with full-thickness skin wounds were used in the in vivo study. Using the surface-engineered TENG device, an AC current of 5–40 nA and a voltage of 0.5–4.5 V were obtained from the mouse movements. The result revealed that there were antibacterial effectiveness with 96.7% bacterial cell inhibition and quick wound-healing process while compared with the untreated control group. The development of drug-containing surface-engineered TENG devices has opened up new biomedical application opportunities.78 An innovative form of wound dressing that combines non-invasive monitoring with the advantages of ES has been demonstrated by Divya et al.79 An IDT electrode and a layer of conductive hydrogel known as polydopamine-crosslinked carboxymethyl chitosan comprised the dressing. The conductive hydrogel aids in the transmission of bioelectrical impulses to the wound, activating cells and quickening the healing process. To help the wound heal, the TENG generated a constant output of 30 V and 2 μA. The wound monitoring system operated on its own power supply because of the TENG. By detecting the fluctuating electrical resistance or current across the wound, the electrode enables real-time wound healing monitoring. This surveillance system may be seen using a personal smartphone and was linked to a WiFi network. The system’s data may be electronically shared with distant physicians, enabling dynamic intervention as necessary. The specified recovery index Figure 9 may be used to identify an infected wound from one that healed without infection.79 The formation of a conductive biofilm lowers the electrical resistance of the infected wound with increasing the TENG current and lowering the recovery index value. TENG supplied enough power to run the LED array efficiently, and the light’s intensity dropped as the wound healed. The wound fully healed on day 14 Figure 9 and the LED’s brightness has entirely decreased.79 This cutting-edge wound monitoring device might follow the healing process and provide early alerts of any infections, optimizing the patient clinical outcomes and minimizing the need for visits to the patient.
Jeong et al80 developed a TENG patch that can be fully extended and is based on hydrogel assembly. This patch was intended specifically for promoting skin wound healing. The concept is that ES can cause charged ions to pass through epidermal ion channels and disrupt the transepithelial electrical potential, resulting in the development of an endogenous electric field. The dermal cells, including keratinocytes, fibroblasts, and endothelial cells, located at the periphery of the wound, were directed to migrate into the centre of the wound in order to facilitate the restoration of the damaged skin. The TENG therapy group was able to effectively and safely heal wound surface, because their normal human skin fibroblasts migrated at a rate that was approximately 3.5 times greater than that of the control group, while the wound healed at a rate that was approximately 3 times faster. Similarly, Yao et al created a device for promoting the healing of fractures that may be implanted in the body. This device was made of a biodegradable material and capable of generating electrical stimulation. It comprised of a TENG and a set of dressing electrodes. This device demonstrated excellent adaptability by being able to connect and treat even on irregular tissue surfaces. Additionally, it has the capability to produce consistent biphasic electrical pulses that induce the proliferation and bone healing of MC3T3-E1 cells. ES facilitated fracture rehabilitation in a time frame of 6 weeks. The stimulation group exhibited a 27% increase in bone mineral density and an 83% improvement in flexural strength compared to the control group. The device underwent degradation within 18 weeks through fast autocatalytic hydrolysis during the in vitro experiment. Further, the observation of deterioration occurred after 14 weeks after implantation in vivo. However, the pace of degradation was significantly influenced by the dynamic internal conditions of the animal.
Long et al81 produced a wound-healing electronic bandage using TENG technology. The TENG mechanism generates electrical pulses that cause cells surrounding the wound to migrate, proliferate, and differentiate. The experimental apparatus transforms the mouse’s kinetic energy from breathing into a distinct alternating voltage. This voltage was then applied to the wound, creating an electric field that enhanced skin regeneration at the site of the lesion. The study showed that the experimental group, treated with the electronic bandage, experienced a significantly shorter healing period of just 3 days compared to the untreated group, which took 12 days to recover. Additionally, the low level and safe current developed by the electronic bandage largely alleviated the pain and discomfort experienced by the patients.
Application of PENG in Various Fields
PENG in Tissue Engineering
As reported by Wang et al,82 piezoelectric NFM (nanofiber membrane) of aligned P(VDF-TrFE) scaffold was used with an NG to develop an autonomous, programmable electrically stimulated system for promoting the growth of pre-osteoblasts. The investigation assessed how electrospinning and various post-treatments, such as poling and annealing, affected the ferroelectric characteristics, piezoelectric β phase, wettability of the surface, and sensory detection abilities of NFMs. Comparing the P(VDF-TrFE) NFM with a piezo-coefficient (d31) of 0.03 pC/N, the polarised P(VDF-TrFE) NFM demonstrated superior sensing ability and provided an improved piezo-coefficient (d31) of 22.88 pC/N. The piezoelectric nanofiber NG with P(VDF-TrFE) technology showed −1.7 V and 41.5 nA. Immobilizing the NGs on the flexible culture dish bottom allowed for the real-time acquisition of an appropriate electrical response under dynamic mechanical stimuli, replicating the in vitro electrical stimulation scenario. Additionally, using an identical circuit model, the association between the cells and the NG was simulated. Various P(VDF-TrFE) NG outputs were examined for their influence on in vitro cell proliferation in order to confirm the viability of P(VDF-TrFE) NGs with precise electrical stimulation, and the investigation revealed that pre-osteoblast proliferation had significantly increased. These findings revealed that P(VDF-TrFE) nanofiber NG has large potential applicability for tissue healing and regeneration and highlighted its adaptability for self-generating electrical stimulation systems.
With the objective of facilitating tissue regeneration for diverse tissue engineering applications, in vivo stimulation using electricity has demonstrated considerable potential. The fact that the present postoperative practices involving transcutaneous leads include a high risk for infections and require additional surgery to eliminate the linked electrical interface posed a serious barrier to the prolonged electrical stimulation approach. Wu et al83 investigated an ultrasound-regulated in vivo ES method for peripheral nerve injuries that relies on a biodegradable PENG and does not involve transcutaneous leads. Ultrasound (US) was chosen as an external wireless source of energy to power implanted NGs comprised dissolvable piezoelectric films owing to its advantages in the field of biomedical engineering, including deep tissue penetration and comprehensive clinical safety. After the surgery, the implanted PENG continued to supply electrical stimulation to the biodegradable conducting pathways of peripheral nerves through remotely initiated ultrasound pulses that were programmed. Additionally, they monitored muscle electrophysiological response, allowing for real-time monitoring of the nerve-repairing process upon in situ ES of the regenerated nerves through the implanted NG. A thorough examination of neurologic function restoration investigation, histological evaluation, and analysis of microstructure using a sciatic nerve damage model validated the significant improvement in nerve regeneration provided by the US-driven in vivo ES. For applications in tissue engineering, this study offers a unique method for delivering in vivo ES through a biodegradable, US-responsive PENG.
Webster and Kumarakuru introduced a semi-invasive method that utilizes an implanted PENG device to deliver a DC electric signal within the body, with the aim of stimulating bone development and facilitating the healing of fractures.84 The system consisted of both internal and external devices. A piezoelectric nanowire was inserted between the lower substrates and an upper protective layer within the internally implanted device. The implanted PENG device was a self-sustaining NG, detecting mechanical force exerted either externally on the body or through stimuli from an external source (Figure 9). The electric signal produced by the PENG was transmitted via electrical leads to electrodes positioned at the desired location for bone formation.85–89
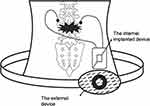 |
Figure 9 System composed of both internal and external device. Reprinted from Kao F-C, Chiu P-Y, Tsai -T-T, Lin Z-H. The application of nanogenerators and piezoelectricity in osteogenesis. Sci Technol Adv Mater. 2019;20(1):1103–1117. Creative Commons.89 |
Electrical stimulation in vivo has demonstrated significant potential in facilitating tissue regeneration for a range of tissue engineering functions. Nevertheless, a notable drawback of the present long-term ES approach is the high susceptibility to infection and the requirement for a further surgical procedure to eliminate the tethered electrical interface associated with the existing postoperative procedures utilizing transcutaneous leads. Hence, biodegradable PENG is used for in vivo ES of peripheral nerve damage using ultrasound as the driving force. Notably, this technique does not require any transcutaneous leads.90
The piezoelectric NG consists of biodegradable materials with piezoelectric properties, including poly(L-lactic acid) (PLLA), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and potassium sodium niobate (KNN) nanowires. It also includes biodegradable encapsulation layers made of poly-ɛ-caprolactone (PCL) films or poly(lactic acid) (PLA) as well as molybdenum (Mo) wires and biodegradable magnesium (Mg) electrodes. Therefore, the advantageous characteristics of the US in the field of biomedical engineering, like its ability to penetrate deep tissues and its high level of clinical protection, the US was chosen as an external wireless energy source to power the implantable NGs. These NGs were constructed using dissolvable piezoelectric films.91–94 The implanted piezoelectric NGs can provide adjustable electrical stimulation to the biodegradable conductive conduits of peripheral nerves beyond the perioperative period. This stimulation was achieved through mechanical excitation that was remotely actuated by programmable US pulses.83,95
PENG in Wound Healing
Electrical stimulation, which mimics the natural electric field of the human body and speeds up the healing process of wounds, is a relatively new and promising therapeutic technique that has come up because of advancements in biomedicine and medical technology. The significance of PENGs in wound treatment is highlighted by their small size and effective electrical conversion. In order to facilitate the process of wound healing, PENG materials must possess biocompatibility, which enables them to effectively sustain the ongoing development and proliferation of cells.96–99
The NG is affixed to the wound, and an electric field produced by the two electrodes permeates the dermis to mimic the body’s natural electric field and hasten the healing process. Deng et al recently showed that the piezoelectric nano-fibrous scaffold PENG is effective in promoting wound healing both in laboratory experiments and in living organisms.100
Wang et al provided evidence of the effectiveness of a PENG in promoting wound healing both in vivo and in vitro. A PENG formed of polarized nanofibers of PVDF-TrFE was fabricated. The PENG was then divided into a film measuring 20 mm × 20 mm and surgically implanted in the subcutaneous thigh area of Sprague Dawley rats known for their high activity levels.101
Bhang et al revealed that ZnO nanorods containing piezoelectric skin patches, oriented in a PDMS (polydimethylsiloxane) matrix, may enhance the migration of cells and re-epithelialization.102
Although PDMS is frequently utilized in wearable devices due to its flexibility and biocompatibility, the significant disparity in modulus between skin and PDMS, as well as its poor adhesion, necessitates the use of additional adherence mechanisms (such as strong tape).103–105 However, this may have negative effects on device wearability and wound healing. Furthermore, the highly interconnected network and the hydrophobic properties of PDMS lead to low permeability, which hinders the advancement of PDMS-based PENGs in clinical applications. Thus, it remains crucial to create PENG patches that possess both efficient electric field generation capabilities and appropriate physical characteristics for the purpose of facilitating skin wound healing in wearable applications. Because of these characteristics, hydrogels derived from mussels are a great choice for use as a matrix in wearable PENGs for wound care.106 Du et al107 developed a bioinspired hybrid patch with piezoelectric and self-adhesive characteristics (HPSP) to expedite the healing of skin wounds. They used electrospun PVDF nanofibers to provide a catechol chemical hydrogel and piezoelectric stimulation to mimic the outcomes of natural wound electric Field (EF) and mussel self-adhesion, respectively.107–110 The HPSP was created by combining PVDF nanofiber-based PENGs with a hydrogel matrix made of polydopamine polyacrylamide (PDA-PAAm). The piezoelectric performance of PENGs was significantly enhanced by the alignment of PVDF nanofibers. Additionally, the self-adhesion and barrier functionalities of the patch were provided by the mussel-inspired PDA-PAAm hydrogel. Experimental findings in vivo and in vitro demonstrated that HPSP promotes the rapid growth and movement of cells and also increases the production of proteins associated with tissue regeneration, such as TGF-β1, VEGF-A, and CD31 near the location of the wound. The combination of PDA-PAAm hydrogels matrix and PENG, along with HPSP, improved collagen deposition, new blood vessel formation, partial regeneration of hair follicles and re-epithelialization. This enables faster and more effective wound healing.111,112
This strongly suggests that the proposed PENG had the most effective impact on encouraging skin regeneration and wound healing. Furthermore, many additional piezoelectric materials, including P(VDF-TrFE)/BaTiO3 nanocomposites and electrospun PVDF/polyurethane membrane, have also demonstrated their ability to effectively make the use of piezoelectric electric fields to enhance the process of bone regeneration and wound healing. Liu and his team investigated that subjecting the piezoelectric PLLA nanofiber scaffold to force or joint stress effectively stimulates chondrogenesis and cartilage regeneration. This is because the scaffold can generate regulated piezoelectric charge. This method demonstrated great potential for wound healing and tissue regeneration by using a biodegradable piezoelectric scaffold in conjunction with controlled mechanical activation.113
PENG in Drug Delivery
Due to the significant clinical need for targeted therapeutics, drug delivery systems have emerged as a prominent area of study in the field of biomedicine. Piezoelectric devices have demonstrated their efficacy in drug delivery in recent years. They primarily operate in two modes: one mode utilizes the inverse piezoelectric effect to precisely control the deformation of the devices through peripheral circuits, enabling the accurate release of drugs in quantifiable amounts.114 Nevertheless, this particular paradigm necessitates intricate peripheral circuits and is mostly employed for transdermal drug administration, making it challenging to implement in living organisms. The second technique relies on the direct piezoelectric effect, which facilitates drug release through electrical stimulation. Given its compact size and user-friendly interface, this method is highly suitable for in vivo applications, particularly for precise drug delivery facilitated by a magnetic field. In a recent investigation, Chen et al invented a successful hybrid core-shell composite nanowire made of FeGa and P(VDF-TrFE).
This nanowire was designed for the delivery of drugs to specific targets. In a similar manner, Mushtaq et al suggested the creation of a versatile piezoelectric nano-eel with a tail, taking inspiration from the mechanisms of electric eels. This nano-eel was made up of, polypyrrole (Ppy), P(VDF-TrFE), and nickel rings and was intended to be used for drug delivery purposes. The result of the drug release experiment unequivocally indicated that the quantity of drug release may be efficiently regulated by modifying the magnetic field settings, highlighting significant prospects for the advancement of a proficient and manageable drug delivery system.115–120
Summary
NGs represent a comparatively emerging field that generates energy by scavenging thermal, light, and mechanical energies from the surrounding environment. Tribo-electrification is a readily accessible phenomenon that can be applied to a broad range of materials. Many different types of TENGs have found applications in tissue engineering and the biomedical sector as potential micro and nano energy sources and autonomous sensors.
Due to its numerous applications in biomedical studies, electronic skin, as well as wearable and portable smart devices such as stretchable electric circuits, flexible screens, etc., NGs are gaining a lot of interest. Alternatively, wearable and portable electronics are becoming considerably more compact and tinier than they were a few years ago, allowing them to be powered by the renewable energy sources available in the surroundings. It is, therefore, very important to have a green source of energy that functions well for wearable and portable electronic devices.
The use of several electric stimulation technologies in controlling cells opened up an emerging field for tissue engineering; many of these methods have already shown promise in both laboratory and patient studies. By altering signaling pathways, electrical stimulation may encourage the migration of cells and differentiation. Signaling routes are important for tissue engineering and wound healing. Conventional electrical stimulation devices may be replaced by NGs, which will enable the development of electrical stimulation-based wearable, implantable, self-powered, and portable tissue regeneration systems. The fundamental ideas, components, and designs of NGs are reviewed in this article along with their uses in tissue engineering, drug delivery, cardiology, neurology, and other fields. Due to its capacity to connect to the internet of things, self-driving capabilities, and mobility, NGs are valuable for clinical research. Moreover, there is great potential for NGs-based medical devices to revolutionize wound healing treatment because of their inexpensive price, portability, and real-time effect. The unique properties of PENG and TENG, such as being lightweight, flexible, and elastic, make them ideal materials for self-assisted wound healing devices, which mimic the mechanical motions performed by human beings. The mechanical energy generated from daily activities and the recovery process following trauma or surgery may be utilized as power sources to convert into electrical stimulation. This can be used to accelerate wound healing in medical devices based on PENG and TENG technology. Undoubtedly, the extensive study on self-powered TENG and PENG has motivated scientists to investigate innovative approaches for utilizing these technologies in the field of self-sustainable treatments and biomedicine. This review provides a concise overview of the latest in vivo and in vitro investigations exploring the effectiveness and practicality of using TENG and PENG for wound healing. While the research is still in its first phases and the results show promise, there are still several obstacles that need to be resolved before the research can be applied in clinical settings. Firstly, the design of NGs should be reduced and modified to fit the dimensions of wounds. Additionally, wound dressings based on NGs should securely adhere to the wound, allowing for electrical stimulation while effectively preventing interference from body fluids. Non-toxicity, biocompatibility, elasticity, durability, and flexibility are essential requirements for effectively integrating TENGs and PENGs into mechanical systems to generate electrical stimulation for wound healing purposes. However, discharge from wounds and body fluids could damage the functioning mechanism of PENGs and TENGs. Overall, both PENGs and TENGs show great promise for modern application in the field of wound healing due to their numerous advantages. In addition to providing a power source for medical therapy, PENGs and TENGs might be used for clinical bio-sensing that could allow us to track the progress of wound healing, by converting mechanical energy into electrical.
Acknowledgments
Every attempt was made by the authors to cite relevant references in the review article and any omission is unintentional.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mason WP. Piezoelectricity, its history and applications. J Acoust Soc Am. 1981;70(6):1561–1566. doi:10.1121/1.387221
2. Mohapatra B, Rautray TR. Ceramic coatings for wound healing applications Bijayinee Mohapatra’and Tapash R. Rautray2 ¹PG Department of Physics, Govt. In: 2. Biomaterials and Tissue Regeneration Lab. Centre of Excellence in TM Sciences. Autonomous College, Angul, Odisha, India:. Advanced Ceramic Coatings for Biomedical Applications; 2023: 269.
3. Swain S, Misra R, Rautray TR. Ceragenin-CSA13 loaded high strength coatings of nano-and micro-SrHA implanted N-Carboxymethyl chitosan–Polyetheretherketone by low temperature high speed collision approach: in vitro pro-osteogenicity and bactericidal activity against MRSA. Mater Chem Phys. 2023;309:128367. doi:10.1016/j.matchemphys.2023.128367
4. Swain S, Ong JL, Narayanan R, Rautray TR. Ti‐9Mn β‐type alloy exhibits better osteogenicity than Ti‐15Mn alloy in vitro. J Biomed Mater Res B Appl Biomater. 2021;109(12):2154–2161. doi:10.1002/jbm.b.34863
5. Das S, Swain S, Rautray TR. Incorporation of hydroxyapatite and cerium oxide nanoparticle scaffold as an antibacterial filler matrix for biomedical applications. Int J Artif Organs. 2024;2024:03913988241234548.
6. Swain S, Lenka R, Rautray T. Synthetic strategy for the production of electrically polarized polyvinylidene fluoride‐trifluoroethylene—co‐polymer osseo‐functionalized with hydroxyapatite scaffold. J Biomed Mater Res A. 2024;112:1675–1687. doi:10.1002/jbm.a.37720
7. Swain S, Kumari S, Swain P, Rautray T. Polarised strontium hydroxyapatite–xanthan gum composite exhibits osteogenicity in vitro. Mater Today Proc. 2022;62:6143–6147.
8. Swain S, Mangaraj S, Rautray TR. Assessment of polarized piezoelectric SrBi4Ti4O15 nanoparticles as alternative antibacterial agents. Inorg Chem Commun. 2024;162:111965. doi:10.1016/j.inoche.2023.111965
9. Swain S, Mishra S, Patra A, Praharaj R, Rautray T. Dual action of polarised zinc hydroxyapatite-guar gum composite as a next generation bone filler material. Mater Today Proc. 2022;62:6125–6130.
10. Swain S, Bowen C, Rautray T. Dual response of osteoblast activity and antibacterial properties of polarized strontium substituted hydroxyapatite—Barium strontium titanate composites with controlled strontium substitution. J Biomed Mater Res A. 2021;109(10):2027–2035. doi:10.1002/jbm.a.37195
11. Wang ZL. On Maxwell’s displacement current for energy and sensors: the origin of nanogenerators. Mater Today. 2017;20(2):74–82. doi:10.1016/j.mattod.2016.12.001
12. Sun M, Li Z, Yang C, et al. Nanogenerator-based devices for biomedical applications. Nano Energy. 2021;89:106461. doi:10.1016/j.nanoen.2021.106461
13. Wang ZL, Song J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science. 2006;312(5771):242–246. doi:10.1126/science.1124005
14. Mangaraj S, Priyadarshini I, Swain S, Rautray TR. ZnO doped β-TCP ceramic-based scaffold promotes osteogenic and antibacterial activity. Bioinspir Biomim Nanobiomat. 2024;2024:1–8.
15. Pradhan M, Swain S, Rautray TR. Electrically polarized hydroxyapatite with Sr containing barium titanate incorporated with mangiferin for enhanced osteogenic and antibacterial activity. Ferroelectrics. 2024;618(4):1157–1169. doi:10.1080/00150193.2024.2324630
16. Mishra S, Swain S, Rautray TR. Preparation of Eggshell Derived HA-BT Composite for Biomedical Applications. Integr Ferroelectr. 2024;240(1):163–174. doi:10.1080/10584587.2023.2296316
17. Wang ZL. Triboelectric nanogenerators as new energy technology for self-powered systems and as active mechanical and chemical sensors. ACS nano. 2013;7(11):9533–9557. doi:10.1021/nn404614z
18. Priyadarshini I, Swain S, Koduru JR, Rautray TR. Electrically polarized withaferin a and alginate-incorporated biphasic calcium phosphate microspheres exhibit osteogenicity and antibacterial activity in vitro. Molecules. 2022;28(1):86. doi:10.3390/molecules28010086
19. Asian Conference on Indoor Environmental Quality. Polarized Chitosan with Cu substituted hydroxyapatite composite exhibits enhanced osteogenicity and antibacterial efficacy in vitro.
20. Swain S, Misra S, Rautray TR. Osteogenic trace element doped ceramic coating for bioimplant applications. Adv Ceramic Coatings for Biomedical Appli. 2023;2023:293.
21. Swain S, Patra A, Kumari S, Praharaj R, Mishra S, Rautray T. Corona poled gelatin-Magnesium hydroxyapatite composite demonstrates osteogenicity. Mater Today Proc. 2022;62:6131–6135.
22. Niu S, Liu Y, Zhou YS, Wang S, Lin L, Wang ZL. Optimization of triboelectric nanogenerator charging systems for efficient energy harvesting and storage. IEEE Trans Electron Devices. 2014;62(2):641–647.
23. Chen H, Miao L, Su Z, et al. Fingertip-inspired electronic skin based on triboelectric sliding sensing and porous piezoresistive pressure detection. Nano Energy. 2017;40:65–72. doi:10.1016/j.nanoen.2017.08.001
24. Pu X, Li L, Song H, et al. A self‐charging power unit by integration of a textile triboelectric nanogenerator and a flexible lithium‐ion battery for wearable electronics. Adv Mater. 2015;27(15):2472–2478. doi:10.1002/adma.201500311
25. Song Y, Zhang J, Guo H, et al. All-fabric-based wearable self-charging power cloth. Appl Phys Lett. 2017;111(7). doi:10.1063/1.4998426
26. Swain S, Mishra S, Das S, Rautray TR Magnesium-Substituted Hydroxyapatite/Spider Silk fibroin/N-carboxymethyl chitosan based microsphere: a potential template for bone regeneration. 2024.
27. Niu S, Liu Y, Wang S, et al. Theory of sliding‐mode triboelectric nanogenerators. Adv Mater. 2013;25(43):6184–6193. doi:10.1002/adma.201302808
28. Niu S, Liu Y, Wang S, et al. Theoretical investigation and structural optimization of single‐electrode triboelectric nanogenerators. Adv Funct Mater. 2014;24(22):3332–3340. doi:10.1002/adfm.201303799
29. Wang ZL. Triboelectric nanogenerator (TENG)—sparking an energy and sensor revolution. Adv Energy Mater. 2020;10(17):2000137. doi:10.1002/aenm.202000137
30. Luo J, Wang ZL. Recent progress of triboelectric nanogenerators: from fundamental theory to practical applications. EcoMat. 2020;2(4):e12059. doi:10.1002/eom2.12059
31. Tang W, Jiang T, Fan FR, et al. Liquid‐metal electrode for high‐performance triboelectric nanogenerator at an instantaneous energy conversion efficiency of 70.6%. Adv Funct Mater. 2015;25(24):3718–3725. doi:10.1002/adfm.201501331
32. Wang ZL. Triboelectric nanogenerators as new energy technology and self-powered sensors–Principles, problems and perspectives. Farad Disc. 2014;176:447–458. doi:10.1039/C4FD00159A
33. Niu S, Wang ZL. Theoretical systems of triboelectric nanogenerators. Nano Energy. 2015;14:161–192. doi:10.1016/j.nanoen.2014.11.034
34. Swain S, Padhy RN, Rautray TR. Polarized piezoelectric bioceramic composites exhibit antibacterial activity. Mater Chem Phys. 2020;239:122002. doi:10.1016/j.matchemphys.2019.122002
35. Rautray T, Vijayan V, Panigrahi S. Synthesis of hydroxyapatite at low temperature. Ind J Phys. 2007;81:95–98.
36. Swain S, Rautray TR. Estimation of trace elements, antioxidants, and antibacterial agents of regularly consumed Indian medicinal plants. Biol Trace Elem Res. 2021;199(3):1185–1193. doi:10.1007/s12011-020-02228-2
37. Swain S, Kwon TY, Rautray TR. Fabrication of silver doped nano hydroxyapatite-carrageenan hydrogels for articular cartilage applications. BioRxiv. 2021;2020:424664.
38. Swain S, Koduru JR, Rautray TR. Mangiferin-enriched mn–hydroxyapatite coupled with β-TCP scaffolds simultaneously exhibit osteogenicity and anti-bacterial efficacy. Materials. 2023;16(6):2206. doi:10.3390/ma16062206
39. Swain S, Swain P, Parida SK, Rautray TR. Ceramic scaffolds for biomaterials applications. Adv Ceramic Coatings for Biomedical Appli. 2023;2022:223–241.
40. Narayanan R, Kim K, Rautray T. Surface Modification of Titanium for Biomaterial Applications. Hauppauge, NY, USA: Nova Science; 2010:352.
41. Fan FR, Tang W, Wang ZL. Flexible nanogenerators for energy harvesting and self‐powered electronics. Adv Mater. 2016;28(22):4283–4305. doi:10.1002/adma.201504299
42. Swain S, Rautray T. Silver doped hydroxyapatite coatings by sacrificial anode deposition under magnetic field. J Mater Sci Mater Med. 2017;28:1–5. doi:10.1007/s10856-016-5790-6
43. Asian Conference on Indoor Environmental Quality. Osteogenicity and antibacterial property of polarized HA-UHMWPE Composites as orthopedic implant biomaterial.
44. Hu D, Yao M, Fan Y, Ma C, Fan M, Liu M. Strategies to achieve high performance piezoelectric nanogenerators. Nano Energy. 2019;55:288–304. doi:10.1016/j.nanoen.2018.10.053
45. Kumar B, Kim S-W. Recent advances in power generation through piezoelectric nanogenerators. J Mater Chem. 2011;21(47):18946–18958. doi:10.1039/c1jm13066h
46. Dhanabalan SC, Dhanabalan B, Chen X, Ponraj JS, Zhang H. Hybrid carbon nanostructured fibers: stepping stone for intelligent textile-based electronics. Nanoscale. 2019;11(7):3046–3101. doi:10.1039/c8nr07554a
47. Xu S, Qin Y, Xu C, Wei Y, Yang R, Wang Z. Nat virus-enabled silicon anode for lithium-ion batteries. Nanotechnol. 2010;5:366.
48. The 2008 Annual Meeting. Synthesis and characterization of zinc oxide nanoparticles for dye-sensitized solar cells.
49. Dudem B, Kim DH, Bharat LK, Yu JS. Highly-flexible piezoelectric nanogenerators with silver nanowires and barium titanate embedded composite films for mechanical energy harvesting. Appl Energy. 2018;230:865–874. doi:10.1016/j.apenergy.2018.09.009
50. Rajabi AH, Jaffe M, Arinzeh TL. Piezoelectric materials for tissue regeneration: a review. Acta Biomater. 2015;24:12–23. doi:10.1016/j.actbio.2015.07.010
51. Sriphan S, Vittayakorn N. Hybrid piezoelectric-triboelectric nanogenerators for flexible electronics: recent advances and perspectives. J Sci Adv Mater Devices. 2022;7(3):100461.
52. Swain S, Rautray TR, R. N Sr. Mg, and Co substituted hydroxyapatite coating on TiO2 nanotubes formed by electrochemical methods. Adv Sci Lett. 2016;22(2):482–487. doi:10.1166/asl.2016.6888
53. Swain S, Rautray TR. Effect of surface roughness on titanium medical implants. Nanostr MateTheir Appli. 2021;2021:55–80.
54. Mohapatra B, Rautray TR. Strontium-substituted biphasic calcium phosphate scaffold for orthopedic applications. J Korean Ceram Soc. 2020;57:392–400. doi:10.1007/s43207-020-00028-x
55. Covaci C, Gontean A. Piezoelectric energy harvesting solutions: a review. Sensors. 2020;20(12):3512. doi:10.3390/s20123512
56. Mishra S, Unnikrishnan L, Nayak SK, Mohanty S. Advances in piezoelectric polymer composites for energy harvesting applications: a systematic review. Macromol Mater Eng. 2019;304(1):1800463. doi:10.1002/mame.201800463
57. Ramadan KS, Sameoto D, Evoy S. A review of piezoelectric polymers as functional materials for electromechanical transducers. Smart Mater Struct. 2014;23(3):033001. doi:10.1088/0964-1726/23/3/033001
58. Sato T, Murakami Y, Muramoto Y. Estimation of liquid sterilization using critical voltage under high electric field pulses. IEEJ Trans Electr Electron Eng. 2019;14(7):1002–1007. doi:10.1002/tee.22895
59. Chang C, Tran VH, Wang J, Fuh Y-K, Lin L. Direct-write piezoelectric polymeric nanogenerator with high energy conversion efficiency. Nano Lett. 2010;10(2):726–731. doi:10.1021/nl9040719
60. Ryu H, Kim SW. Emerging pyroelectric nanogenerators to convert thermal energy into electrical energy. Small. 2021;17(9):1903469. doi:10.1002/smll.201903469
61. Lang SB. Pyroelectricity: from ancient curiosity to modern imaging tool. Phys Today. 2005;58(8):31–36. doi:10.1063/1.2062916
62. Lingam D, Parikh AR, Huang J, Jain A, Minary-Jolandan M. Nano/microscale pyroelectric energy harvesting: challenges and opportunities. Int J Smart Nano Mater. 2013;4(4):229–245. doi:10.1080/19475411.2013.872207
63. Wang W, Pang J, Su J, et al. Applications of nanogenerators for biomedical engineering and healthcare systems. InfoMat. 2022;4(2):e12262. doi:10.1002/inf2.12262
64. Zhang S, Bick M, Xiao X, Chen G, Nashalian A, Chen J. Leveraging triboelectric nanogenerators for bioengineering. Matter. 2021;4(3):845–887. doi:10.1016/j.matt.2021.01.006
65. Bok M, Lee Y, Park D, et al. Microneedles integrated with a triboelectric nanogenerator: an electrically active drug delivery system. Nanoscale. 2018;10(28):13502–13510. doi:10.1039/C8NR02192A
66. Khandelwal G, Raj NP, Kim SJ. Triboelectric nanogenerator for healthcare and biomedical applications. Nano Today. 2020;33:100882. doi:10.1016/j.nantod.2020.100882
67. Ouyang Q, Feng X, Kuang S, et al. Self-powered, on-demand transdermal drug delivery system driven by triboelectric nanogenerator. Nano Energy. 2019;62:610–619. doi:10.1016/j.nanoen.2019.05.056
68. Yue O, Wang X, Hou M, et al. Smart nanoengineered electronic-scaffolds based on triboelectric nanogenerators as tissue batteries for integrated cartilage therapy. Nano Energy. 2023;107:108158. doi:10.1016/j.nanoen.2022.108158
69. Wang M, Li W, You C, Wang Q, Zeng X, Chen M. Triboelectric nanogenerator based on 317L stainless steel and ethyl cellulose for biomedical applications. RSC Adv. 2017;7(11):6772–6779. doi:10.1039/C6RA28252K
70. Zhang X-S, Han M-D, Wang R-X, et al. Frequency-multiplication high-output triboelectric nanogenerator for sustainably powering biomedical microsystems. Nano Lett. 2013;13(3):1168–1172. doi:10.1021/nl3045684
71. Fu K, Zhou J, Wu H, Su Z. Fibrous self-powered sensor with high stretchability for physiological information monitoring. Nano Energy. 2021;88:106258. doi:10.1016/j.nanoen.2021.106258
72. Zhao G, Zhang Y, Shi N, et al. Transparent and stretchable triboelectric nanogenerator for self-powered tactile sensing. Nano Energy. 2019;59:302–310. doi:10.1016/j.nanoen.2019.02.054
73. Lin J, Li J, Feng S, et al. An active bacterial anti-adhesion strategy based on directional transportation of bacterial droplets driven by triboelectric nanogenerators. Nano Res. 2023;16(1):1052–1063. doi:10.1007/s12274-022-5177-6
74. Tang W, Tian J, Zheng Q, et al. Implantable self-powered low-level laser cure system for mouse embryonic osteoblasts’ proliferation and differentiation. ACS Nano. 2015;9:7867–7873. doi:10.1021/acsnano.5b03567
75. Tian J, Shi R, Liu Z, et al. Self-powered implantable electrical stimulator for osteoblasts’ proliferation and differentiation. Nano Energy. 2019;59:705–714. doi:10.1016/j.nanoen.2019.02.073
76. Peng X, Dong K, Ye C, et al. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci Adv. 2020;6(26):eaba9624. doi:10.1126/sciadv.aba9624
77. Wang ZL, Lin L, Chen J, et al. Triboelectric nanogenerator: vertical contact-separation mode. Triboelectric Nanogenerators. 2016;2016:23–47.
78. Du S, Zhou N, Xie G, et al. Surface-engineered triboelectric nanogenerator patches with drug loading and electrical stimulation capabilities: toward promoting infected wounds healing. Nano Energy. 2021;85:106004. doi:10.1016/j.nanoen.2021.106004
79. Divya S, Hajra S, Panda S, et al. A review on the next generation of healing: exploring the use of triboelectric nanogenerators in wound care. Chem Phys Lett. 2023;826:140648. doi:10.1016/j.cplett.2023.140648
80. Jeong S-H, Lee Y, Lee M-G, Song WJ, Park J-U, Sun J-Y. Accelerated wound healing with an ionic patch assisted by a triboelectric nanogenerator. Nano Energy. 2021;79:105463. doi:10.1016/j.nanoen.2020.105463
81. Long Y, Wei H, Li J, et al. Effective wound healing enabled by discrete alternative electric fields from wearable nanogenerators. ACS Nano. 2018;12(12):12533–12540. doi:10.1021/acsnano.8b07038
82. Wang A, Hu M, Zhou L, Qiang X. Self-powered well-aligned P (VDF-TrFE) piezoelectric nanofiber nanogenerator for modulating an exact electrical stimulation and enhancing the proliferation of preosteoblasts. Nanomaterials. 2019;9(3):349. doi:10.3390/nano9030349
83. Wu P, Chen P, Xu C, et al. Ultrasound-driven in vivo electrical stimulation based on biodegradable piezoelectric nanogenerators for enhancing and monitoring the nerve tissue repair. Nano Energy. 2022;102:107707. doi:10.1016/j.nanoen.2022.107707
84. Webster TJ, Kumarakuru H. Self-powered bone growth stimulator: google patents, 2019.
85. Behera DR, Nayak P, Rautray TR. Phosphatidylethanolamine impregnated Zn-HA coated on titanium for enhanced bone growth with antibacterial properties. J King Saud Univ Sci. 2020;32(1):848–852. doi:10.1016/j.jksus.2019.03.004
86. Mangaraj S, Swain S, Rautray TR. Smart Biomaterials and Their Applications. In: Surface Engineering of Biomaterials. CRC Press; 2024:59–84.
87. Rautray T, Kim K-H. Nanoelectrochemical coatings on titanium for bioimplant applications. Mater Technol. 2010;25(3–4):143–148. doi:10.1179/175355510X12723642365322
88. Lenka R, Swain S, Kwon TY, Rautray TR. Surface Modification: carbide-, Silicide-, Nitride-Based Surface. In: Surface Engineering of Biomaterials. CRC Press; 2024:244–270.
89. Kao F-C, Chiu P-Y, Tsai -T-T, Lin Z-H. The application of nanogenerators and piezoelectricity in osteogenesis. Sci Technol Adv Mater. 2019;20(1):1103–1117. doi:10.1080/14686996.2019.1693880
90. Wang L, Mao L, Qi F, et al. Synergistic effect of highly aligned bacterial cellulose/gelatin membranes and electrical stimulation on directional cell migration for accelerated wound healing. Chem Eng J. 2021;424:130563. doi:10.1016/j.cej.2021.130563
91. Rautray TR, Narayanan R, Kim K-H. Ion implantation of titanium based biomaterials. Prog Mater Sci. 2011;56(8):1137–1177. doi:10.1016/j.pmatsci.2011.03.002
92. Swain S, Rautray TR. Cu and Zn substituted hydroxyapatite coatings on titanium oxide nanotubes formed by electrochemical methods. Innovare J Eng Technol. 2016;4:7–10.
93. Mishra S, Swain P, Swain S, Kennedy VJ, Rautray TR. Smart Biomaterial Surface. In: Surface Engineering of Biomaterials. CRC Press; 2024:284–305.
94. Zhao Y, Liang Y, Ding S, Zhang K, Mao H-Q, Yang Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials. 2020;255:120164. doi:10.1016/j.biomaterials.2020.120164
95. Khoshkalam K, Izadi Z, Mirhaji SS, et al. Investigation of the affinity and interaction of fibrinogen with trehalose as a protein stabilizer. J Mol Liq. 2024;402:124713. doi:10.1016/j.molliq.2024.124713
96. Kloth LC. Electrical stimulation technologies for wound healing. Adv Wound Care. 2014;3(2):81–90. doi:10.1089/wound.2013.0459
97. Praharaj R, Swain S, Rautray TR. Bioceramics for antibacterial and antiviral applications. Adv Ceramic Coatings for Biomedical Appli. 2023;2023:347.
98. Priyadarshini I, Swain S, Rautray TR. Mechanism of ceramic coatings degradation. Adv Ceramic Coatings for Biomedical Appli. 2023;2023:33.
99. Mishra S, Rautray TR. Bioceramics for adhesive applications. Adv Ceramic Coatings for Biomedical Appli. 2023;2023:323.
100. Deng W, Zhou Y, Libanori A, et al. Piezoelectric nanogenerators for personalized healthcare. Chem Soc Rev. 2022;51(9):3380–3435. doi:10.1039/d1cs00858g
101. Wang A, Liu Z, Hu M, et al. Piezoelectric nanofibrous scaffolds as in vivo energy harvesters for modifying fibroblast alignment and proliferation in wound healing. Nano Energy. 2018;43:63–71. doi:10.1016/j.nanoen.2017.11.023
102. Bhang SH, Jang WS, Han J, et al. Zinc oxide nanorod‐based piezoelectric dermal patch for wound healing. Adv Funct Mater. 2017;27(1):1603497. doi:10.1002/adfm.201603497
103. Praharaj R, Mishra S, Misra R, Rautray TR. Biocompatibility and adhesion response of magnesium-hydroxyapatite/strontium-titania (Mg-HAp/Sr-TiO2) bilayer coating on titanium. Mater Technol. 2022;37(4):230–239. doi:10.1080/10667857.2020.1825898
104. Mohapatra B, Rautray TR. Phenomenology of Treatment of Biomaterial Surfaces. In: Surface Engineering of Biomaterials. CRC Press; 2024:483–502.
105. Yu Y, Sun H, Orbay H, et al. Biocompatibility and in vivo operation of implantable mesoporous PVDF-based nanogenerators. Nano Energy. 2016;27:275–281. doi:10.1016/j.nanoen.2016.07.015
106. Yu Y, Yuk H, Parada GA, et al. Multifunctional “hydrogel skins” on diverse polymers with arbitrary shapes. Adv Mater. 2019;31(7):1807101. doi:10.1002/adma.201807101
107. Du S, Zhou N, Gao Y, et al. Bioinspired hybrid patches with self-adhesive hydrogel and piezoelectric nanogenerator for promoting skin wound healing. Nano Res. 2020;13:2525–2533. doi:10.1007/s12274-020-2891-9
108. Mohapatra B, Rautray TR. Facile fabrication of Luffa cylindrica-assisted 3D hydroxyapatite scaffolds. Bioinspir Biomim Nanobiomat. 2021;10(2):37–44. doi:10.1680/jbibn.20.00011
109. Mishra S, Rautray TR. Fabrication of Xanthan gum-assisted hydroxyapatite microspheres for bone regeneration. Mater Technol. 2020;35(6):364–371. doi:10.1080/10667857.2019.1685245
110. Rautray T, Kwon T, Kim K. Synthesis of Mg2 incorporated hydroxyapatite by ion implantation and their cell response. Korean Soc Dental Mat Acad Conf. 2011;2011:212–12.
111. Han L, Yan L, Wang K, et al. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017;9(4):e372–e72. doi:10.1038/am.2017.33
112. Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430. doi:10.1126/science.1147241
113. Liu Y, Dzidotor G, Le TT, et al. Exercise-induced piezoelectric stimulation for cartilage regeneration in rabbits. Science Transl Med. 2022;14(627):eabi7282. doi:10.1126/scitranslmed.abi7282
114. Chen X-Z, Hoop M, Shamsudhin N, et al. Hybrid magnetoelectric nanowires for nanorobotic applications: fabrication, magnetoelectric coupling, and magnetically assisted in vitro targeted drug delivery. Adv Mater. 2017;29(8):1605458. doi:10.1002/adma.201605458
115. Mushtaq F, Torlakcik H, Hoop M, et al. Motile piezoelectric nanoeels for targeted drug delivery. Adv Funct Mater. 2019;29(12):1808135. doi:10.1002/adfm.201808135
116. Swain S, Pradhan M, Bhuyan S, Misra R, Rautray TR. On the ion implantation synthesis of ag-embedded over sr-substituted hydroxyapatite on a nano-topography patterned ti for application in acetabular fracture sites. Int J Nanomed. 2024:2024;4515–4531.
117. Swain S, Rautray TR. Ceramic coatings for dental implant applications. Adv Ceramic Coatings for Biomedical Appli. 2023;2023:249–262.
118. Mishra S, Rautray TR. Silver-incorporated hydroxyapatite–albumin microspheres with bactericidal effects. J Korean Ceram Soc. 2020;57(2):175–183. doi:10.1007/s43207-020-00018-z
119. Praharaj R, Mishra S, Rautray TR. The structural and bioactive behaviour of strontium-doped titanium dioxide nanorods. J Korean Ceram Soc. 2020;57(3):271–280. doi:10.1007/s43207-020-00027-y
120. Wang ZL. On the first principle theory of nanogenerators from Maxwell’s equations. Nano Energy. 2020;68:104272. doi:10.1016/j.nanoen.2019.104272
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.