Back to Journals » International Journal of Nanomedicine » Volume 19
Nanoscale Systems for Local Activation of Hypoxia-Inducible Factor-1 Alpha: A New Approach in Diabetic Wound Management
Authors Saber S, Abdelhady R , Elhemely MA, Elmorsy EA , Hamad RS, Abdel-Reheim MA, El-kott AF , AlShehri MA, Morsy K, Negm S , Kira AY
Received 19 September 2024
Accepted for publication 3 November 2024
Published 21 December 2024 Volume 2024:19 Pages 13735—13762
DOI https://doi.org/10.2147/IJN.S497041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. RDK Misra
Sameh Saber,1 Rasha Abdelhady,2 Mai A Elhemely,3,4 Elsayed A Elmorsy,5 Rabab S Hamad,6 Mustafa Ahmed Abdel-Reheim,4,7 Attalla F El-kott,8 Mohammed A AlShehri,8 Kareem Morsy,8 Sally Negm,9 Ahmed Y Kira10
1Department of Pharmacology, Faculty of Pharmacy, Delta University for Science and Technology, Gamasa, 11152, Egypt; 2Pharmacology and Toxicology Department, Faculty of Pharmacy, Fayoum University, Fayoum, 63514, Egypt; 3School of Medical Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, M20 4BX, UK; 4Department of Pharmacology and Toxicology, Faculty of Pharmacy, Beni-Suef University, Beni Suef, 62521, Egypt; 5Department of Pharmacology and Therapeutics, College of Medicine, Qassim University, Buraidah, 51452, Saudi Arabia; 6Biological Sciences Department, College of Science, King Faisal University, Al Ahsa, 31982, Saudi Arabia; 7Department of Pharmaceutical Sciences, College of Pharmacy, Shaqra University, Shaqra, 11961, Saudi Arabia; 8Department of Biology, College of Science, King Khalid University, Abha, Saudi Arabia; 9Department of Life Sciences, College of Science and Art Mahyel Aseer, King Khalid University, Abha, 62529, Saudi Arabia; 10Department of Pharmaceutics, Faculty of Pharmacy, Delta University for Science and Technology, Gamasa, 11152, Egypt
Correspondence: Mustafa Ahmed Abdel-Reheim; Ahmed Y Kira, Department of Pharmaceutics, Faculty of Pharmacy, Delta University for Science and Technology, Gamasa, 11152, Egypt, Tel +2 1026462867, Fax +2 502770140, Email [email protected]; [email protected]
Abstract: Chronic wounds in diabetic patients experience significant clinical challenges due to compromised healing processes. Hypoxia-inducible factor-1 alpha (HIF-1α) is a critical regulator in the cellular response to hypoxia, enhancing angiogenesis and tissue restoration. Nevertheless, the cellular response to the developed chronic hypoxia within diabetes is impaired, likely due to the destabilization of HIF-1α via degradation by prolyl hydroxylase domain (PHD) enzymes. Researchers have extensively explored HIF-1α activation as a potential pathway for diabetic wound management, focusing mainly on deferoxamine (DFO) as a potent agent to stabilize HIF-1α. This review provides an update of the other recent pharmacological agents managing HIF-1α activation, including novel PHD inhibitors (roxadustat and daprodustat) and Von Hippel‐Lindau protein (VHL) antagonists, which could be potential alternatives for the local treatment of diabetic wounds. Furthermore, it highlights how localized delivery via advanced nanostructures can enhance the efficacy of these novel therapies. Importantly, by addressing these points, the current review can offer a promising area for research. Given that, these novel drugs have minimal applications in diabetic wound healing, particularly in the context of local application through nanomaterials. This gap presents an exciting opportunity for further investigation, as combining these drugs with localized nanotechnology could avoid undesired systemic side effects and sustain drug release within wound site, offering a transformative platform for diabetes wound treatment.
Keywords: nanomedicine, localized delivery, diabetic wound healing, novel prolyl hydroxylase inhibitors
Graphical Abstract:

Introduction
Notably, diabetic wounds, including foot and leg ulcers, affect approximately 20% of diabetic patients with high economic burden. Globally, diabetic wounds represent a primary health concern that attracts special clinical attention primarily due to the reported impaired and delayed healing of such wounds, leading to complications, including severe infections and lower limb amputation.1,2 Of note, hypoxia was reported to show a pivotal role in the hindered healing process of diabetic wounds as decreased oxygen supply to the injured tissues impedes essential cellular repair mechanisms.3
As reported, cellular response to hypoxia is essentially regulated by HIF-1. Activation of HIF-1 stimulates a range of mechanisms that enable cells to compensate for oxygen deficiency, including angiogenesis, consequently improving cell survival and promoting tissue regeneration.4 Therefore, HIF-1 was deemed a crucial factor in initiating and sustaining tissue repair mechanisms vital for wound healing. Nevertheless, HIF-1 function is believed to be compromised in diabetic wounds, thus making it a leading target for the development of novel therapeutic modalities for diabetic wounds.
Prolyl hydroxylase domain (PHD) enzymes are accountable for HIF-1 degradation and loss of function. Therefore, PHD inhibitors were recently developed as wound healing promoters where they demonstrated initial success in stabilizing HIF-1 and restoring its function.5 Meanwhile, the critical obstacle that hinders the clinical application of such recent treatment modality was the systemic severe adverse effects of PHD inhibitors, including excessive erythropoiesis and potential oncogenic risks,6 that prohibited their systemic administration and urged the development of localized delivery for these drugs to their site of action. Notably, clinical trials have been initiated to investigate the effects of these inhibitors. Daprodustat, a PHD inhibitor, has completed Phase I clinical trials for diabetic foot ulcer treatment (ClinicalTrials.gov: NCT01831804), marking a significant advancement in clinical research. Furthermore, a clinical trial is currently examining the effect of localized DFO delivery on diabetic foot ulcer healing (ClinicalTrials.gov: NCT03137966).
Conspicuously, nanotechnology offers an innovative and valid tool for the precise delivery of HIF-1 stabilizers to the wound site, minimizing systemic adverse effects while maximizing their therapeutic efficacy.7 The capacity of various nanotechnological platforms, particularly nanofibers (NFs) and nanohydrogels, in improving the delivery and effectiveness of diabetic wound healing promoters has been previously studied.8–10 Multiple studies have explored strategies to enhance wound healing through the use of nanotechnology. For instance, recent research has demonstrated that nanostructured materials, like nanohydrogels, can support tissue repair by enhancing cell migration and angiogenesis.11 Additionally, immunomodulatory hydrogels have shown efficacy in modulating the diabetic wound environment by addressing key pathological factors such as hyperglycemia-induced inflammation and excessive reactive oxygen species.12,13
The current review provides a concise overview of the HIF-1α role in wound healing and its dysfunction in diabetic wounds. The article then delves into the recent pharmacological agents that target HIF-1α activation in diabetes while examining their potential drawbacks. Moreover, it discusses the role of nanomaterials in overcoming these limitations and enhancing the efficacy of these drugs via local application. Unlike many studies that focus broadly on general wound healing mechanisms, our work distinctly highlights the HIF-1α pathway, a key regulator in tissue repair. By focusing on the stabilization of HIF-1α, we offer a targeted approach to addressing the impaired healing process in diabetic wounds. Furthermore, this review introduces novel pharmacological agents, including roxadustat, daprodustat, and VHL antagonists, which are relatively underexplored in this context, particularly in terms of their localized application through nanomaterials. We anticipate that these insights will advance the development of innovative therapeutic strategies for diabetic wound management, paving the way for more effective treatments by modulating HIF-1α with nanotechnology.
The Intimate Connection Between Wound Healing and Hypoxia
Tissue repair subsequent to injury constitutes a multifaceted cascade encompassing a coordinated interplay of cellular activities and the reorganization of the extracellular matrix (ECM). The triphasic wound healing model involves inflammation, proliferation, and remodeling (Figure 1). A growing body of evidence underscores the pervasive role of hypoxia throughout the various stages of the wound healing cascade. Fluctuations in oxygen tension trigger precisely regulated signaling pathways that respond to hypoxic conditions. While transient hypoxia is a critical signaling mechanism that initiates essential cellular processes for wound repair, such as fibroblast proliferation and angiogenesis, its transition to a chronic state, particularly in diabetic wounds, can significantly hinder the healing cascade. To fully elucidate this interplay, a comprehensive understanding of the individual stages of wound healing and the corresponding impact of hypoxia on each stage is paramount.
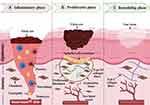 |
Figure 1 Illustration showing the three phases involved in the wound healing process: (A) Inflammatory Phase: characterized by a surge of inflammation and controlled tissue breakdown at the wound site. Platelets, the body’s clotting agents, assemble and form a provisional matrix, a temporary scaffold that acts as the foundation for healing. Meanwhile, neutrophils and monocytes are recruited to the area. Neutrophils act as scavengers, clearing away cellular debris and dead tissue. Monocytes, in turn, transform into macrophages, facilitating the proliferative phase. (B) Proliferative Phase: Characterized by the growth of new tissue. Fibroblasts proliferate, depositing extracellular matrix (ECM) components and collagen fibers. Angiogenesis supplies the new tissue with oxygen and nutrients, while re-epithelialization aids in wound closure. (C) Remodeling Phase: Characterized by the maturation and strengthening of scar tissue. During this phase, the body replaces the less stable type III collagen with the more robust type I collagen, with myofibroblasts aiding in matrix contraction and increased tensile strength, resulting in a more compact and robust collagen matrix. Created in BioRender. Mamdouh, A. (2024) https://BioRender.com/ y77b182. |
Inflammatory Phase
The initial phase of wound healing termed the inflammatory phase, commences upon the disruption of the local vasculature and subsequent extravasation of blood due to tissue injury. This event is a key trigger for platelet aggregation and clot formation, culminating in creating a provisional matrix. This temporary scaffold serves a dual purpose: firstly, it facilitates the migration of essential cells to the wound site, and secondly, it contributes to the formation of granulation tissue. In addition, the coagulation cascade and activated and wounded cells coordinate the release of numerous signaling chemicals. These signaling molecules attract inflammatory cells, such as neutrophils and monocytes, to the wound site. Neutrophils are the initial responders and play a crucial role in debridement, which involves removing cellular debris and facilitating tissue healing. Neutrophils play an active role in fighting against invading germs and aiding in the healing process by releasing substances such as matrix metalloproteinases (MMPs) and reactive oxygen species (ROS).14 These factors facilitate the removal of dead tissue and enhance the transmission of inflammatory signals. Monocytes undergo differentiation and transform into macrophages. At first, macrophages that promote inflammation (M1) emit ROS and cytokines to kill bacteria. Consequently, the growth phase starts when granulation tissue is formed following macrophages’ differentiation into anti-inflammatory macrophages (M2).15
Proliferative Phase
Approximately three days after the injury, the proliferative phase begins, and it’s principally characterized by both macrophage and fibroblast infiltration alongside the formation of granulation tissue in the wound area. Profoundly, macrophages are essential elements of this phase as they release growth factors promoting angiogenesis and fibrous tissue deposition. Moreover, chemotactic attraction of fibroblasts to the site of the wound16 takes place, triggering ECM deposition, and replacing the temporary matrix formed during the inflammatory stage. Notably, a sufficient supply of oxygen and essential nutrients to the newly formed tissue during the proliferative phase is enabled by stimulating angiogenesis in response to pro-angiogenic signals.17 Intriguingly, this phase involves restoration of the outermost skin layer with subsequent wound closure in a step known as re-epithelialization, which is achieved through the growth and movement of keratinocytes along the wound’s edges.18
Remodeling Phase
Eventually, the temporary granulation tissue is replaced by a permanent scar in the so-called remodeling phase, mainly featured in the transformation of the ECM into a stronger and more resilient collagen-based structure. Fibroblasts are crucial in this process as they produce and deposit the collagen fibers.19 Significantly, the initially prevailing type III collagen, often seen in granulation tissue, is progressively substituted by the more durable and resistant type I collagen. The process of collagen remodeling is carefully managed, ensuring a precise equilibrium between the synthesis and breakdown of collagen. MMPs, secreted by various cell types, including macrophages, endothelial cells, and fibroblasts, are responsible for controlled collagen degradation.20 Additionally, under the influence of α-smooth muscle actin and growth factors, some fibroblasts differentiate into myofibroblasts. These specialized cells contribute to matrix contraction and enhance the tensile strength of the developing scar tissue. As the robust collagen matrix establishes itself, the fibroblast-rich granulation tissue is progressively replaced by a relatively acellular, mature scar. The duration of this phase varies with the extent of the injury and can span from days to months, ultimately aiming to restore tissue structure and functionality while enhancing tensile strength.21
Hypoxia Across Wound Healing Phases
The initial inflammatory phase of wound healing is marked by a hypoxic environment. This phenomenon arises from two main factors: the influx of inflammatory cells with high oxygen demands and disruption of the vasculature around the wound, hindering oxygen delivery. Interestingly, these very inflammatory cells preferentially accumulate in hypoxic regions, suggesting a specific role in processes crucial for healing, such as granulation tissue formation and re-epithelialization.22 Moreover, the established hypoxic gradient can influence the function of stromal cells. Studies have shown that acute hypoxia substantially enhances the human dermal fibroblast proliferation.23 Intriguingly, hypoxic conditions significantly enhance the secretory function of fibroblasts, leading to a near tenfold increase in the production of transforming growth factor-β1 (TGF-β1), a well-established regulator of wound healing. Therefore, acute hypoxia appears to induce a transient upsurge in cellular proliferation, thereby contributing to the launch of the wound-healing cascade.24
In a pioneering study, Remensnyder and Majno documented a significant gradient of hypoxia within the wounded cremaster muscle of rats, exhibiting a close correlation with revascularization levels.25 Building on this, Elson et al subsequently corroborated these findings, demonstrating a coordinated and transient induction of HIF-1α and its downstream target genes, including vascular endothelial growth factor (VEGF), during the process of epidermal wound healing.26 These foundational studies highlight the paramount importance of HIF-1 signaling activation and regulation within the context of wound healing. Furthermore, they convincingly demonstrate the critical role played by the adaptation of hypoxic cells, encompassing inflammatory cells, keratinocytes, endothelial cells, and mesenchymal stromal cells, to the hypoxic microenvironment established at the wound site. This adaptation is demonstrably crucial for these cell populations to fulfill their respective functions during the wound-healing process.
Hypoxia and Diabetic Chronic Wounds
After discussing how hypoxia can stimulate beneficial responses in acute settings, it becomes evident that chronic hypoxia presents substantial challenges, particularly in conditions like diabetes, where vascular and metabolic disruptions are prevalent.27 The pathophysiology of diabetic wounds introduces a distinctive set of challenges due to the multifaceted impact of hyperglycemia, peripheral arterial disease (PAD), neuropathy, and related vascular complications (Figure 2). The combination of these variables leads to a consistently low oxygen environment in wounds, significantly hindering crucial healing processes. Recent studies provide mounting evidence that the delayed wound healing observed in diabetic patients might be attributed to worsened hypoxic conditions and defective cellular responses to hypoxia. These characteristics are considered key pathogenic factors.3,28 This section will explore the mechanisms by which hyperglycemia impairs the supply of oxygen and cellular function, how PAD limits blood flow and worsens tissue hypoxia, and how neuropathy impairs sensory perception and vascular regulation, hence sustaining chronic hypoxia in diabetic wounds.
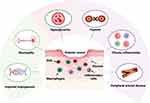 |
Figure 2 Pathophysiology of diabetic wounds: This diagram illustrates the vicious cycle contributing to chronic diabetic wounds. Hyperglycemia increases glucose levels, resulting in oxidative stress and the production of reactive oxygen species (ROS), which damage blood vessels and nerves. Neuropathy impairs sensation, causing unnoticed injuries. Peripheral arterial disease (PAD), resulting from vascular damage, restricts blood flow, impeding healing. Impaired angiogenesis limits the delivery of nutrients and oxygen to the wound site. Chronic inflammation, driven by persistent hyperglycemia, disrupts healing as dysfunctional macrophages fail to resolve inflammation, perpetuating tissue damage. Created in BioRender. Mamdouh, A. (2024) https://BioRender.com/ x89d650. |
Hyperglycemia
Hyperglycemia, a characteristic feature of diabetes, causes a range of biochemical alterations that interfere with the normal wound healing process. One of these alterations is the reduced supply of oxygen to the tissues. Hyperglycemia causes problems with the cells lining the blood vessels and damages the small blood vessels, which reduces the flow of blood to the wound and affects the supply of oxygen.29 Consequently, the wound environment becomes hypoxic, exhibiting reduced quantities of oxygen compared to healthy tissue. Furthermore, elevated glucose levels can potentially modify the characteristics of red blood cells, resulting in reduced flexibility and increased susceptibility to aggregation.30 These modifications decrease the capacity of red blood cells to traverse through microvasculature and effectively transport oxygen to tissues. This impaired oxygen transport exacerbates the hypoxic conditions in diabetic wounds. In addition, high amounts of glucose and the resulting oxidative stress hinder the formation of angiogenic agents, such as VEGF.31 The wound experiences worsened hypoxia due to insufficient blood flow caused by inadequate angiogenesis.
Peripheral Arterial Disease
Diabetic wounds are characterized by vascular insufficiency, a fundamental pathophysiological characteristic. Individuals with diabetes are especially prone to PAD, which is characterized by a gradual constriction and hardening of the arteries that provide blood to the limbs.32 This syndrome causes a considerable reduction in blood flow to the extremities and impairs oxygen delivery to the wound site. Hence, the diminished blood flow resulting from PAD plays a significant role in creating the long-lasting oxygen-deprived conditions seen in diabetic wounds.
Neuropathy
Diabetic neuropathy, a common consequence, is identified as an additional significant element that contributes to the pathophysiology of diabetic wounds. This neuropathy interferes with the function of nerves that control the expansion and contraction of blood vessels. This condition of autonomic neuropathy disrupts the normal control of blood circulation, resulting in an inadequate blood supply to the tissues. Without adequate vascular responses, wounds in diabetic individuals suffer from insufficient oxygenation.33 Neuropathy diminishes the sensation in the extremities, especially the feet, resulting in a heightened susceptibility to undiscovered injury. As a result of ongoing pressure and trauma, these injuries have the potential to progress into chronic wounds.34 Moreover, neuropathy exacerbates the inflammatory response and following stages of wound healing, impeding the body’s ability to maintain the healing process efficiently.35
Hypoxia-Inducible Factor-1 Alpha (HIF-1α)
Understanding the multifaceted impact of hypoxia on diabetic wound pathophysiology underscores the critical need to explore molecular responses and adaptive mechanisms that regulate tissue oxygenation. One mechanism that plays a role in wound repair under ischemia conditions involves HIF-1α, a transcription factor responsible for regulating many processes necessary for this process. Within the diabetic wound milieu, persistent hypoxia alters the normal HIF-1α signaling pathway, leading to a defective cellular response to low oxygen supply. As a result, this malfunction hampers the well-known positive effects of HIF-1α, making the process of wound healing more complicated in individuals with diabetes. This section explores the attributes and regulatory processes that control the function of HIF-1α while clarifying the specific mechanisms via which this pathway becomes compromised in diabetes.
The Role of HIF-1α in the Wound Healing Process
HIF-1 is a transcription factor that plays a critical role in helping cells adapt to low-oxygen settings. It acts as a heterodimer, comprising two subunits: an alpha subunit called HIF-1α and a beta subunit called HIF-1β. In response to hypoxic conditions, HIF-1α is stabilized and moves to the nucleus in response to hypoxic circumstances. At that point, it engages with HIF-1β, creating a functional heterodimer. The dimerization event functions as a molecular mechanism that triggers the activation of transcription for several genes (Table 1) involved in wound healing, such as angiogenesis, metabolic adaption, cell survival, proliferation, and migration.36,37
 |
Table 1 Key HIF-1α Target Genes Associated with Wound Healing |
The crucial function of HIF-1α in wound healing is supported by the hindered angiogenesis and delayed healing found in mice with reduced HIF-1α activity due to either heterozygous knockout or age-related decline.47,48 Likewise, targeted inactivation of HIF-1β in endothelial cells also results in defective wound healing.49 These findings collectively exhibit the multifaceted effects of HIF-1 in promoting tissue repair.
HIF-1α Regulation and Its Dysfunction in Diabetes
Regulation Under Non-Diabetic Conditions
The regulation of the HIF-1α subunit exhibits a tight coupling to oxygen availability, with oxygen levels dictating its fate between degradation or stabilization.50–52 Under normoxic conditions (Figure 3a), specific proline residues (P402 and P564) within HIF-1α are hydroxylated by PHD proteins. Notably, these hydroxylation reactions require co-substrates such as oxygen, 2-oxoglutarate (2-OG), and iron.53 This oxygen-dependent hydroxylation is a molecular recognition tag for the VHLE3 ubiquitin ligase. VHL then targets the hydroxylated HIF-1α for ubiquitination, ultimately resulting in its degradation by the proteasome. The remarkably short half-life of HIF-1α in the presence of oxygen (less than 10 minutes) underscores the remarkable efficiency of this regulatory mechanism.54
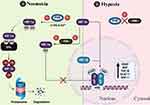 |
Figure 3 Regulation of HIF-1: Panel (A) depicts the normoxic scenario. Under normoxic conditions, prolyl hydroxylase domain (PHD) proteins hydroxylate specific proline residues within HIF-1α. These hydroxylation reactions require oxygen, 2-oxoglutarate (2-OG), and iron (Fe²+) as co-substrates. The presence of these hydroxyl groups on HIF-1α serves as a recognition motif for the von Hippel-Lindau (VHL) E3 ubiquitin ligase. Consequently, VHL targets hydroxylated HIF-1α for ubiquitination, ultimately leading to its proteasomal degradation. An additional layer of regulation is provided by a factor inhibiting HIF-1 (FIH). FIH impedes the recruitment of essential coactivators such as CREB-binding protein (CBP) and p300, thereby dampening the transactivation activity of HIF-1α. Panel (B) illustrates the hypoxic scenario. In response to hypoxic conditions (low oxygen levels), PHD activity becomes significantly reduced due to limited oxygen availability. This abrogation of hydroxylation by PHDs allows HIF-1α to evade recognition and subsequent degradation by the VHL ubiquitin ligase pathway. Therefore, HIF-1α undergoes stabilization and accumulates within the cell. The stabilized HIF-1α translocates to the nucleus, where it heterodimerizes with its partner, HIF-1β. This functional HIF-1α/β complex exhibits a high affinity for hypoxia response elements (HREs) present in the promoter regions of target genes. The binding of the HIF complex to HREs is further facilitated by the recruitment of coactivators, such as CBP and p300. This coordinated assembly on the promoter region culminates in the transcriptional activation of a multitude of genes essential for cellular adaptation to hypoxia. Created in BioRender. Mamdouh, A. (2024) https://BioRender.com/ i10w859. |
Under hypoxic conditions (Figure 3b), the activity of PHD proteins becomes significantly diminished due to the limited availability of oxygen, effectively shutting down the hydroxylation of HIF-1α.55,56 This abrogation of hydroxylation renders HIF-1α invisible to the VHL E3 ubiquitin ligase, thereby thwarting its recognition and subsequent proteasomal degradation. Consequently, HIF-1α undergoes stabilization and translocates to the nucleus, dimerizing with its partner, HIF-1β. This functional HIF-1α/β heterodimer exhibits a high affinity for hypoxia response elements (HREs) present in the target gene promoters. The binding of the HIF complex to HREs is further facilitated by the recruitment of coactivators, such as CREB-binding protein (CBP) and p300. This coordinated assembly on the promoter region culminates in the transcriptional activation of many genes critical for hypoxia adaptation.
Regulation Under Diabetic Conditions
The pathophysiology of diabetic wounds introduces a distinctive set of challenges due to the multifaceted impact of hyperglycemia, neuropathy, and related vascular complications. Combining these variables leads to a consistently low oxygen environment in wounds. One would logically expect an induction of HIF-1α activation to occur as a compensatory response. However, paradoxically, the function of HIF-1α is impaired, exacerbating the delay in healing typically observed in diabetic wounds.
A substantial body of research indicates that hyperglycemia negatively affects HIF-1α activity under hypoxic conditions characteristic of diabetes.57,58 One prominent mechanism involves hyperglycemia-induced promotion of PHD-mediated degradation of HIF-1α. Hyperglycemia enhances the hydroxylation of HIF-1α, resulting in its degradation through PHD- and VHL-dependent pathways, even in hypoxic conditions.59,60
Another contributing mechanism involves the intracellular accumulation of methylglyoxal (MGO), a reactive by-product of glycolysis. Notably, elevated MGO levels, a hallmark of hyperglycemia in diabetes, are associated with increased inflammation and delayed wound healing. Mechanistically, MGO directly modifies HIF-1α, leading to a decrease in its stability and potentially hindering its transcriptional activity.61
Bento et al identified a novel pathway where hyperglycemia-induced MGO modifications enhance HIF-1α binding to molecular chaperones Hsp40 and Hsp70.62 This interaction facilitates the recruitment of chaperone-dependent ubiquitin ligase (CHIP), leading to ubiquitination followed by proteasomal degradation of HIF-1α, independent of PHD and VHL pathways.
Furthermore, MGO appears to directly modify the coactivator protein p300 directly, thereby hindering its interaction with HIF-1α. This impaired interaction between HIF-1α and p300 likely contributes to the diminished transcriptional efficacy of HIF-1α.63
These findings highlight the critical role of HIF-1α dysfunction in the impaired wound healing observed in diabetes. The complex interplay between hyperglycemia-induced oxidative stress, PHD-mediated degradation, and MGO accumulation results in significant impairment of HIF-1α stability and transcriptional activity (Figure 4). Fortunately, these defects have been reported to be partially reversible,4,63 opening avenues for therapeutic interventions aimed at restoring HIF-1α activity and promoting efficient wound healing in diabetic patients.
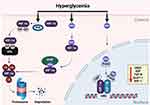 |
Figure 4 Hyperglycemia-induced dysfunction of HIF-1 signaling in diabetes. This figure illustrates the multifaceted mechanisms by which hyperglycemia disrupts HIF-1α stability and function in diabetes. Elevated blood glucose levels promote the degradation of HIF-1α through two main pathways; VHL-dependent pathway: Hyperglycemia stimulates the activity of prolyl hydroxylase domain (PHD) proteins. Hydroxylated HIF-1α becomes a target for the von Hippel-Lindau (VHL) E3 ubiquitin ligase, leading to its ubiquitination and subsequent degradation by the proteasome. MGO-dependent pathway: Hyperglycemia also contributes to the intracellular accumulation of methylglyoxal (MGO), a reactive by-product of glycolysis. MGO directly modifies HIF-1α, decreasing its stability. This modification recruits chaperone-dependent ubiquitin ligase (CHIP), which tags HIF-1α for ubiquitination and proteasomal degradation, independent of the PHD and VHL pathways. Furthermore, MGO disrupts HIF-1α’s transcriptional activity by altering the coactivator protein p300. This modification weakens the interaction between HIF-1α and p300, thereby diminishing HIF-1α’s ability to regulate the expression of target genes. Created in BioRender. Mamdouh, A. (2024) https://BioRender.com/ w30u996. |
Pharmacological Agents for HIF-1α Stabilization
Recent advancements in understanding the mechanisms responsible for impaired HIF-1α function in diabetes offer a compelling rationale for presenting HIF-1α as a promising therapeutic target for developing novel strategies to promote efficient wound healing in diabetic patients. As previously discussed, the PHD enzyme requires the co-substrates 2-OG and iron to hydroxylate specific proline residues on HIF-1α, marking it for degradation. Thus, decreasing or inhibiting these co-substrates presents a potential mechanism for PHD inactivation, thereby stabilizing HIF-1α.
As illustrated in Figure 5, various HIF-1α stabilizing agents, including cobalt chloride, DFO, and 2-OG inhibitors such as roxadustat, and daprodustat, inhibit the PHD degradation pathway by interfering with cofactors. The VHL antagonist VH298 also prevents HIF-1α degradation by inhibiting its interaction with VHL, directly stabilizing HIF-1α.
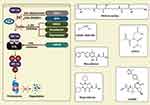 |
Figure 5 Molecular Structures and Mechanisms of HIF-1 Stabilizing Agents: The diagram illustrates the activation of HIF-1 through various mechanisms. Cobalt chloride (CoCl2) and deferoxamine (DFO) replace iron and chelate iron, respectively, reducing iron availability, a cofactor for prolyl hydroxylase domain (PHD) enzymes, thereby inhibiting the PHD degradation pathway. Additionally, 2-oxoglutarate inhibitors, including dimethyloxalylglycine (DMOG), roxadustat, and daprodustat, inhibit the 2-OG cofactor for PHD, resulting in further inhibition of HIF-1α degradation. The VHL protein antagonist VH298 is also shown, which stabilizes HIF-1α by inhibiting the interaction between VHL and hydroxylated HIF-1α. Unlike PHD inhibitors, VHL antagonists directly target the downstream degradation pathway of HIF-1α. Created in BioRender. Mamdouh, A. (2024) https://BioRender.com/ c11t096. |
Therapeutic Agents Targeting Iron
Iron (Fe²+) is a critical cofactor for PHD enzyme activity. It binds reversibly to the active site of this metalloenzyme, facilitating the hydroxylation of HIF-1α. Consequently, strategies to reduce iron availability present a promising therapeutic approach. Iron chelation therapy, such as DFO, can directly sequester iron, limiting its accessibility to PHDs. Alternatively, competitive substitution with non-catalytic metal ions, such as cobalt (Co²+), can displace iron from the active site, effectively inhibiting PHD activity.
Cobalt chloride (CoCl2) can mimic hypoxic conditions by inhibiting PHD enzymes via the competition with iron, leading to HIF-1α stabilization.64 This hypoxia mimetic approach triggers a cellular response similar to that observed under actual hypoxic conditions, promoting wound healing processes. Salts of cobalt have been found to inhibit hydroxylase activity by antagonizing iron.65,66 Huang et al investigated the potential of CoCl2 to enhance fracture healing in Sprague-Dawley rats.67 Their study employed intraperitoneal injection (IP) of CoCl2 at a dose of 15 mg/kg and observed a significant acceleration of bone formation and remodeling during the repair process. Mechanistically, the authors attributed these effects to CoCl2-mediated activation of the HIF-1α pathway.
DFO stands out as one of the pioneering discoveries in HIF activation. Notably, it was among the first identified organic compounds capable of inducing HIF activity.68 DFO exerts its effect by binding to iron, which is a necessary co-factor for the activity of PHD enzymes. By chelating iron, DFO prevents the degradation of HIF-1α, thereby stabilizing it.69 In diabetic mice, DFO has been shown to counteract the transcription-suppressing effects of high glucose, thereby restoring neovascularization and promoting efficient wound healing.70 Beyond its established role in iron chelation, DFO exhibits intriguing antioxidant properties. These properties may extend beyond its ability to bind iron, potentially offering an additional mechanism for promoting wound healing. In particular, research suggests that DFO might prevent MGO-mediated modifications of the coactivator protein p300, thereby enhancing its interaction with HIF-1α.71
Therapeutic Agents Targeting 2-OG
2-OG analogs are pivotal in the stabilization and activation of HIF-1; they competitively inhibit PHD enzymes by occupying the 2-OG binding site and preventing the hydroxylation of HIF-1α.72,73 2-OG analogs can be broadly categorized into classical inhibitors and novel pharmacological agents.
Classical inhibitors, such as dimethyloxalylglycine (DMOG) and N-oxalylglycine (NOG), structurally mimic 2-OG, thereby competitively inhibiting PHD enzymes. Preclinical research has investigated DMOG and NOG extensively, demonstrating their efficacy in stabilizing HIF-1α.74 Several studies have consistently shown that DMOG effectively stabilizes HIF-1α across different cell types, including keratinocytes,75 endothelial cells,76 and fibroblasts.77 In a groundbreaking study by Duscher et al, the first to directly compare DMOG and DFO, researchers investigated their effects on wound healing in diabetic mice.78
Beyond traditional inhibitors like NOG and DMOG, novel pharmacological agents such as roxadustat and daprodustat have emerged.79,80 These drugs offer distinct advantages over their predecessors. While mimicking the 2-OG binding site similar to classical inhibitors, roxadustat and daprodustat boast optimized pharmacokinetic and pharmacodynamic profiles, making them more clinically translatable For instance, daprodustat is undergoing early-stage evaluation in a phase I clinical trial, focusing on evaluating its safety and tolerability when applied topically to both healthy subjects and diabetic wound patients.81 Roxadustat, however, has gone a preclinical investigation, demonstrating its capacity to enhance the healing process of wounds in diabetic rats via HIF-1α activation.82
Von Hippel‐Lindau Protein Antagonist
Markedly, a different approach for activation of HIF-1α was mediated by VHL antagonists suppressing the interaction between VHL and hydroxylated HIF-1α,60 with subsequent angiogenesis stimulation and alleviation of diabetic wounds hypoxia.83 VHL antagonists directly target HIF-1α degradation mechanisms, offering more consistent stabilization of HIF-1α, compared to PHD inhibitors that target upstream PHD enzymes.84
As an example of a VHL antagonist, VH298 could be a promising therapeutic agent accelerating wound healing via promoting angiogenesis and tissue regeneration.60 Qiu et al recently studied the localized delivery of VH298 in a diabetic rat wound-healing model.85 Compared to control groups, wounds treated with VH298 displayed a demonstrably superior healing pattern via HIF-1α activation. The authors emphasize the need for future research to develop extended-release formulations of VH298 to ensure sustained efficacy within the dynamic wound healing microenvironment.
Side Effects and Consideration
HIF-1α stabilizers, though promising for enhancing wound healing, present several significant challenges after systemic exposure. Most current research on novel agents focuses on their effects via systemic routes, which often results in substantial off-target effects. Tampering with the PHD-HIF pathway raises significant concerns due to potential off-target effects. These concerns stem from the involvement of HIF-1α in various pathological processes.86 Although VEGF activation via HIF-1α benefits angiogenesis and wound healing, it can cause undesirable effects. Elevated VEGF levels can promote abnormal angiogenesis, increasing the risk of tumor growth.87,88 Furthermore, systemic VEGF upregulation can lead to vascular leak syndrome, resulting in edema and other complications.89
In addition to oncological concerns, continuous activation of HIF-1α signaling has been implicated in worsening pulmonary hypertension and potentially leading to heart failure, especially in patients with pre-existing conditions.90,91 Also, pharmacological agents that target iron for HIF-1α activation can affect iron metabolism, potentially leading to iron deficiency, which manifests as symptoms in bones.92,93 Notably, patients who are already iron-deficient may experience exacerbated symptoms.
Localized Delivery of HIF-1α Stabilizer via Nanotechnology
After discussing the numerous negative consequences of systemic exposure to HIF-1α stabilizers, localized delivery via nanomaterials advances a promising solution. The main advantage of this strategy is the achievement of both controlled and localized drug delivery of HIF-1α stabilizers within the injured tissues, minimizing any potential systemic adverse effects. This modality could establish a platform for developing novel and efficient therapies for diabetic wounds by targeting HIF-1α. Moreover, the benefits of nanotechnology extend beyond this application; various nanotechnology-based materials offer numerous advantages, making them a viable strategy to address the current research gaps related to the limited application of novel HIF-1α stabilizers for diabetic wound healing. This section will shed more light on the different types, applications, and theoretical potential benefits of nanotechnology-based platforms for targeted delivery of HIF-1α stabilizers.
Advantages of Combining Nanotechnology with HIF-1α Stabilizers
Nanotechnology-based techniques present significant advantages for the targeted delivery of HIF-1α stabilizers in diabetic wound treatment. One of the main advantages is the ability to deliver the drug directly to the site of the wound.94 In sharp contrast to the systemic route of administration where the whole body is likely to be exposed to the drug, the local delivery by nanocarriers ensures enough drug concentration at the precise site where it is most needed while lowering the chances of systemic drug adverse effects. Such targeted treatment is vital, considering that diabetic patients are highly susceptible to drug side effects.
Also noteworthy is that nanotechnology-based drug delivery systems have more potential for controlled release.95 The application of NPs enables the release of HIF-1α stabilizers at a controlled rate, maintaining optimal therapeutic levels over an extended period. Such a release pattern is of great importance in chronic wound management, especially in patients with diabetes, where wound healing is impaired and continuous drug delivery is required. The controlled release further reduces the frequency of drug administration, thus decreasing the persisting need for drug dressing and increasing compliance in patients. Furthermore, this method helps continue a consistent therapeutic effect, thereby enhancing the overall efficacy of the treatment.
Moreover, certain NPs are also well known for their specific properties, such as antibacterial (silver NPs), anti-inflammatory (chitosan NPs), and antioxidant (selenium NPs) ones, that could work on improving the wound healing system by collaborating with HIF-1α stabilizers, targeting many levels of the healing process at the same time.96–98 Even if the NPs do not have these characteristics, they can still serve as a flexible platform for combining several therapeutic agents in one delivery vehicle. HIF-1α stabilizers are not the only molecules NPs can be designed to carry; other bioactive agents such as anti-inflammatory drugs, growth factors, antibiotics, etc. This gives multiple functions simultaneously, making managing diabetic wounds more effective and faster, thus enhancing the patient’s quality of life.
Additionally, a significant advantage attributed to the application of nanoscale materials in wound treatment is that they can act as a surrogate ECM.99 Specifically, these are types of nanomaterials that are synthesized from polymers or proteins, hence the name biocompatible, that tend to have some similarity with the structure and functioning of the ECM, a characteristic crucial for the attachment, growth, and movement of cells.100 These NPs promote tissue regeneration while they hasten the healing process because they give specific structural support, which imitates that of normal skin cells.101 This form of biological support is essential for the management of diabetic ulcers since the normal process of healing is often heavily compromised.
Nanotechnology, in general, has brought new therapeutic strategies that will help to minimize or even overcome such barriers in diabetic wound healing. The next section details the different applications of various nanoparticles, nanohydrogels, NFs, and many other nanotechnologically-based platforms in diabetic wound healing.
Types of Nanomaterials for Diabetic Wound Healing
NPs have a globally debated use and are very efficient in medical therapies, with wound healing being one case in point. NPs typically range in size from 1 to 100 nm and can be synthesized employing a wide range of materials, different structural characteristics, and advantageous properties. The prevalent categories of NPs employed for wound healing include organic NPs, metallic NPs, NFs, and nanohydrogels. Each type has a distinct degree of effectiveness in wound healing. Utilizing these characteristics enables the design of NPs to address specific healing issues, establishing them as innovative agents in wound therapeutics.
Metallic NPs
Silver, gold, and copper NPs have come into focus as wound-healing metallic NPs due to their unique properties, including antimicrobial activity, antioxidant properties, and enhanced cell proliferation ability.102 These types of NPs can be designed to facilitate tissue regeneration and accelerate the healing process by addressing infection and inflammation, two of the most common problems in diabetic wounds.
Metallic NPs can accelerate the healing of diabetic wounds in various ways: Silver, copper, and zinc oxide NPs are especially noted for their potent antimicrobial properties, helping to reduce bacterial colonization in chronic wounds.103,104 Gold and titanium NPs have been observed to decrease inflammatory responses, which is a principal factor in controlling the course of diabetic wounds, as chronic inflammation worsens the healing process.105,106
In this context, Li et al developed a hydrogel loaded with DFO and copper NPs as an effective approach to diabetic wound healing.107 The developed approach exhibited excellent antibacterial activity and effectively decreased the persistent inflammatory reactions in diabetic wounds. Additionally, combining DFO and copper NPs synergistically enhanced the expression of HIF-1α and VEGF. In another study, the combination of DFO with silver NPs synergistically enhanced cell migration angiogenesis, reduced inflammation, and demonstrated remarkable antimicrobial activity, resulting in fast wound healing in a rat model of infected diabetic wound.108
Hence, opportunities exist for further investigations of the combining metallic NPs with novel HIF-1α stabilizers. Metallic NPs can stabilize HIF-1α stabilizers, preventing the drug from degrading and ensuring its prolonged activity in hypoxic conditions typical of diabetic wounds. As shown in Figure 6, this combination can synergistically enhance wound healing by the dual action of metallic NPs hindering bacterial colonization and inflammatory responses and HIF-1α stabilizers promoting vascularization in diabetic wounds. Table 2 summarizes the applications of different metallic NPs in wound healing.
 |
Table 2 Overview of Therapeutic Applications of Metallic NPs in Wound Healing |
 |
Figure 6 Loading silver nanoparticles (AgNPs) with HIF-1α stabilizer drugs offers a comprehensive approach to diabetic wound healing. This strategy combines sustained drug release with enhanced angiogenesis, tissue regeneration, and reduced chronic inflammation. AgNPs help address the key challenges of wound healing in hypoxic and diabetic conditions by promoting HIF-1α stability, increasing VEGF production, and providing antibacterial effects, ultimately leading to faster and more effective wound repair. Created in BioRender. Mamdouh, A. (2024) https://BioRender.com/ q68h441. |
Lipid-Based NPs
Lipid-based NPs, including solid lipid NPs and liposomes, are widely explored in biomedical applications due to their biocompatibility, biodegradability, and ability to encapsulate hydrophilic and lipophilic drugs.114 These systems are particularly advantageous for diabetic wound healing as they provide controlled and targeted drug delivery, reducing systemic side effects.
Lipid-based NPs can shield labile agents from degradation in the harsh environment of the wound, thereby maintaining their therapeutic effectiveness.115 In addition, they can be combined with HIF-1α stabilizers and other active substances (antimicrobial peptides, etc)., thereby forming multi-functional systems that cover several aspects of the treatment of diabetic ulcers, from infection prevention to stimulating angiogenesis.
Combining lipid-based NPs and HIF-1α therapies can ensure a controlled and extended release of HIF-1α stabilizers at the site of the wound, enhancing the availability of drugs for a longer time, enabling better angiogenesis and the repair of the tissues. Liposomes can fuse with the cell membranes, allowing drug transport into the target cells.116 Thus, these properties help improve the penetration and action of drugs in wound healing processes. Solid lipid NPs are beneficial in incorporating a solid core polymeric matrix that can protect drugs from degradation while attaining sustained and controlled release features.117 They are particularly advantageous in the administration of drugs that are light-sensitive such as, roxadustat or enzyme-sensitive.
In a recent study, a novel HIF-1α stabilizers, roxadustat, has been incorporated into cubosomes, demonstrating a sustained release of the drug for 48 h and effectively developing a conducive environment for tissue repair by promoting angiogenesis, stabilizing HIF-1α, and collagen synthesis.118 In another study, loading DFO with liposomes significantly enhanced wound healing compared to plain DFO by enhancing drug penetration and sustaining drug release.119 Additionally, DFO-loaded solid lipid NPs have shown a prolonged sustained release with a higher entrapment efficiency, which is significant for such a hydrophilic drug.120 Table 3 summarizes the applications of different metallic NPs in wound healing.
 |
Table 3 Therapeutic Applications of Lipid-Based NPs in Wound Healing |
Ceramic NPs
Ceramic NPs refer to nonorganic nanoparticles made of silica, calcium phosphate, and other compounds. These NPs are stable, biocompatible, and possess structural integrity.126 They have paved their way in the biomedical field, particularly in the application of drug delivery vehicles, because they can encapsulate and protect therapeutic entities and provide controlled release.127
In the diabetic wound healing context, ceramic NPs’ advantages include their ability to assist in tissue engineering, prevent infections, and increase the effectiveness of the agents used in wound healing. Certain ceramic NPs contain calcium phosphate, a natural bone and tissue replacement that enhances angiogenic growth and tissue restoration, essential to the diabetic wound restoration process. In a recent study, local administration of DFO within a triphosphate calcium scaffold has been found to significantly enhance HIF-1 and VEGF expression, promoting bone regeneration in osteoporotic rats.128
HIF-1α stabilizers can be incorporated and released to the wound target by ceramic NPs in a controllable and sustained manner. This increases the local levels and activity duration of HIF-1α, catalyzing faster and improved healing of diabetic wounds. They may also complement the effects of HIF-1α stabilizers, further enhancing the wound microenvironment through promoting neovascularization, decreasing oxidative burden, and expediting tissue repair. The NPs might also play a role in wound healing strategies by interacting with the wound bed and promoting cell proliferation.
In a previous study, DMOG-loaded silica NPs have been shown to exert a synergistic effect on diabetic wound healing.129 In vitro, combining DMOG drugs and Si ions enhanced the expression of attachment, proliferation, migration, and angiogenesis genes. The in vivo study exhibited that they improved the formation of new blood vessels, regeneration of epithelial tissue, and collagen production while reducing inflammation in diabetic wound beds, promoting successful wound healing.
Another interesting ceramic material is bioglass, which contains Ca and Silica ions. Bioglass itself shows high biological activity and can release products that, upon degradation, support osteogenesis, angiogenesis, and wound healing.130 In a previous study, the combination of bioglass and DFO accelerated diabetic wound healing by exhibiting a synergistic effect in endorsing HIF-1α expression and revascularization.131
Protein-Based Nanomaterials
Biomaterials such as collagen, gelatin, silk fibroin, and other protein-based nanomaterials are seeing an increase in biomedicine applications because of their biocompatibility and biodegradability. Because they are derived from nature, they are degraded into non-toxic components, and hence, the possibility of side effects is minimized. Their compatibility with living tissue avoids any immune responses, which are critical in chronic diabetic wound situations.
Collagen helps increase the number of fibroblasts in the wound area, which is essential for ECM synthesis and tissue repair. Due to its hydrophilic properties, collagen facilitates fibroblast penetrating the wound base.132 This particular feature is critical in diabetic wounds as padding of fibroblast infiltrates enriches the ability of tissue repair and remodeling of ECM. Also, Collagen aids in the deposition of its fibers, thereby enhancing the quality structure of the healing tissue.133 Another property of collagen is its ability to enhance cell migration, which is necessary for wound healing and closure. Collagen-based nanomaterials can act as carriers for HIF-1α stabilizers, providing a synergistic benefit in promoting re-epithelialization, angiogenesis, and overall tissue regeneration.134
Gelatin, derived from collagen through partial hydrolysis, is a highly biocompatible and biodegradable material widely used in tissue engineering and wound healing. Since it is quite close in structure to collagen, gelatin has many beneficial characteristics, including easing cell attachment, supporting tissue regeneration, and enhancing wound closure.135 Gelatin’s flexible and stable nature allows it to be easily modified and shaped into various forms, making it an ideal delivery system for HIF-1α stabilizers. In a recent study, DFO-loaded gelatin microspheres enhanced angiogenesis by increasing the expression of HIF-1α and angiogenic growth factors, leading to collagen deposition and fast wound closure in a full-thickness diabetic wound model.136
Silk fibroin belongs to the class of natural proteins sourced from silkworms. It has been primarily used in biomedical applications like wound healing because of its excellent physical strength and biocompatibility. Among other proteins like, for instance, gelatin and collagen, SF has better toughness, mechanical performance, and thermal stability.137 Also, since it has the Arg-Gly-Asp sequence, it helps to expand cell adhesion, proliferation, and migration, critical processes for the successful healing of the skin.138 This protein has been developed into different forms of nanomaterials, which are highly effective as carriers of HIF-1α stabilizers in wound healing.139
Polymeric Nanomaterials
Polymer-based nanomaterials, including hyaluronic acid, chitosan, alginate, and Poly lactic-co-glycolic acid (PLGA), are extensively used in drug delivery due to their tunable characteristics, biocompatibility, and ability for controlled and targeted drug release. These NPs are ideal for diabetic wound healing, as they protect bioactive molecules from degradation, can be engineered to respond to environmental stimuli like pH or temperature, and offer sustained release of therapeutic agents, maintaining a necessary dosage in the wound area for an extended period, reducing the need for dressing changes.
Hyaluronic acid is an abundant glycosaminoglycan present in the ECM of many connective tissues. Its properties of moisture retention, firmness, and supporting cell activity make it critical in diabetic wound healing.140 Its hydrophilic nature maintains a wet environment that is important for cell migration and population while inhibiting dryness and cell death. Furthermore, hyaluronic acid increases fibroblast movement and proliferation of keratinocytes and aids in granulation tissue.141 These properties make it an ideal delivery system for HIF-1α stabilizers in managing chronic diabetic wounds.
Chitosan, a naturally occurring polymer obtained from chitin sourced from exoskeletons of crustaceans, contains many properties that are effective for diabetic wound healing. It is biocompatible, biodegradable, and non-toxic. Chitosan’s antimicrobial properties help inhibit bacterial growth, forming a protective barrier that minimizes infection risks.142 Chitosan modulates the immune response, reducing prolonged inflammation helping wounds progress through various healing phases.97 Its mucoadhesive attribute also assists in localizing the drug at the site of application for a prolonged time.143 It contributes to sustained therapeutic release, which is paramount for enhancing wound healing processes, especially in diabetic ulcers. When combined with HIF-1α stabilizers, these properties accelerate healing by stimulating angiogenesis and tissue regeneration. In a recent study, Zhang et al fabricated a chitosan/hyaluronic acid hydrogel loaded with DFO NPs and investigated its efficacy for wound healing.144 The composite nano hydrogel’s photothermal response facilitated wound healing by efficiently reducing inflammation through controlled DFO release and regulation of the immunological reaction of injured skin tissue.
Alginate-based nanomaterials are frequently used in wound dressings because they maintain a moist wound environment.145 Co-delivery of HIF-1α stabilizers within alginate-based nanomaterials can enhance healing by promoting tissue hydration and angiogenesis. Another critical advantage of alginate is its pH-dependent release characteristics,146,147 which benefit wound healing. Intriguingly, chronic wounds feature alkaline pH, which decreases gradually as the wound healing process progresses. Therefore, utilizing alginate-based nanomaterials that facilitate a higher release of therapeutic agents in an alkaline medium initially, followed by sustained release in an acidic pH, could be highly beneficial. This dual-release mechanism guarantees an initial rapid distribution of the medicine to initiate the healing process, followed by a controlled and continuous release to facilitate ongoing tissue repair and regeneration. The mucoadhesive nature of alginate allows it to stay in place on the wound bed for extended periods.148 This extended retention helps maintain a moist environment, critical for wound healing, particularly in chronic diabetic wounds. Recently, Li et al prepared an alginate-based hydrogel loaded with DFO and copper NPs and explored its efficacy in diabetic wound healing.107 The developed system exhibited a sustained release of DFO for 60 h. The alginate composite shown excellent antibacterial activity and effectively decreased the persistent inflammatory reactions. In vivo, it enhanced angiogenesis and expedited the healing of diabetic wounds while maintaining excellent biocompatibility.
Poly(lactic-co-glycolic acid) (PLGA) is one of the most commonly used copolymers. Its advantages have been demonstrated in various biomedical applications, especially wound healing.149 Given its adjustable mechanical characteristics and regulated degradation rates, PLGA is suitable for promoting tissue regeneration, particularly in diabetic wounds where the healing process is commonly hindered.150 A key feature of PLGA is its ability to provide controlled and sustained drug release. This allows therapeutic agents to be delivered over extended periods, which is crucial for promoting wound closure and tissue repair. PLGA’s surface modification capabilities also improve drug interaction, increasing its efficacy in diabetic wound care. In a recent study, a HIF-1α stabilizer, DFO, was loaded in PLGA NPs, and the NPs were incorporated into a hyaluronic acid hydrogel.151 The PLGA NPs demonstrated a prolonged release of DFO over 12 days and reached 15 days after encapsulating in the hydrogel. Moreover, when evaluated in a mouse model of excisional skin wounds, the composite hydrogel enhanced wound healing and stimulated the development of new blood vessels and granular structures.
Nanohydrogels
Nanohydrogels represent a class of drug delivery systems. They are characterized by precise and targeted drug delivery to specific sites followed by a controlled rate of drug release, merging the properties of hydrogels and NPs.152 As an ideal drug delivery carrier, nanogels exhibit numerous positive characteristics, including exceptional drug loading capacity, enhanced stability, biocompatibility, and remarkable responsiveness to different environmental stimuli.153
Nanohydrogels have a 3D highly swollen network structure, which makes them especially effective in medical applications such as wound healing. These hydrogels provide moist wound care in diabetic patients, which prevents excessive dryness, which is detrimental to wound healing. Their porous structure mimics the ECM, supporting cell adhesion, migration, and proliferation, all vital for wound closure.154 Moreover, Some nanohydrogels are designed to respond to specific stimuli, such as changes in pH or temperature. This responsiveness permits targeted drug delivery and controlled release in response to the wound environment.
Wang et al have developed a novel therapeutic approach aiming to enhance diabetic wound healing by utilizing exosomes loaded with the HIF-1α stabilizer, VH298, and integrating these VH298-loaded exosomes (VH-EVs) into a gelatin methacrylate (GelMA) hydrogel. The study aimed to promote angiogenesis and accelerate wound healing through the sustained release and targeted delivery of VH298 to wound sites.155 The GelMA hydrogel, synthesized to mimic extracellular matrix properties, demonstrated suitable mechanical strength, porosity, lower swelling ratio, and slower degradation, resulting in a steady release of VH-EVs for 15 days. In vitro experiments revealed that VH-EVs significantly enhanced the proliferation, tube formation, and migration of human umbilical vein endothelial cells (HUVECs) more effectively than free VH298 or EVs alone. These effects were linked to the upregulation of HIF-1α and VEGF, key proteins involved in angiogenesis. In vivo, VH-EVs incorporated into GelMA hydrogel (Gel-VH-EVs) showed superior wound healing in diabetic mice compared to other treatments. Wounds treated with Gel-VH-EVs exhibited complete closure by day 12, with enhanced granulation tissue formation, collagen deposition, and re-epithelialization.
Deng et al recently conducted a study involving a newly developed hyaluronic acid-based multifunctional hydrogel incorporating silver nanoclusters (AgNCs) and DFO-loaded polydopamine/manganese dioxide (PHMD) NPs aimed at enhancing the healing process of infected diabetic wounds.156 This hydrogel demonstrated several advantageous characteristics: it featured a customizable gelation time ensuring excellent injectability, adjustable mechanical properties with a degradation rate nearing 90% after 3 days at pH 5, pH-responsive behavior facilitating enhanced release of encapsulated AgNCs and DFO in acidic conditions typical of diabetic wounds, superior tissue adherence, and close tissue contact capabilities. The combination of AgNCs and PHMD supplied hydrogel with potent antibacterial activity superior to that of AgNCs alone, demonstrating DFO’s dual role owing to its antibacterial action and HIF-1 activation. Notably, the in vivo study using diabetic rats with S. aureus-infected wounds stated that Hydrogel@AgNCs&PHMD demonstrated a significant ability to accelerate wound closure compared to other treatment groups as evidenced by 80% wound closure by day 7 and almost-complete closure by day 14.
Nanofibers
NFs represent a groundbreaking category of nanomaterials. Electrospun fibers can be created from both natural and synthetic polymers. These fibers are distinguished by a high surface area to volume ratio as well as a remarkable biomimetic structure thus enabling a range of applications, including electronics plus the biomedical field.157 This biomimetic quality facilitates cellular adhesion, proliferation, and migration stimulating tissue repair.
A remarkable further benefit of NFs over other nanomaterial is their inherent porosity. Such characteristic contributes markedly to improved wound healing as it allows efficient nutrients and gas exchange creating an ideal milieu supporting necessary cellular processes vital for tissue regeneration. Additionally, NFs were reported to accelerate the epithelialization process and minimize scarring. This could be attributed to their significant capacity to absorb wound exudates and necrotic tissue (eschar) owing to their swelling ability.158,159 Moreover, NFs could be employed to encapsulate bioactive substances (eg, growth factors, antibiotics, and anti-inflammatory medications), and then achieve a controlled release of these substances. The controlled release achieved by NFs ensures both sustained as well as extended bioactive substances action thus decreasing dosing frequency and improving patient compliance. As per diabetic wound management, incorporating HIF-1 stabilizers into NFs, could specifically improve vascularization and accelerate wound closure. Figure 7 displays the key advantages of NFs in wound healing.
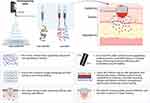 |
Figure 7 Advanced Nanofiber Dressings for Targeted Drug Delivery: NFs resemble the ECM, providing an ideal environment for cell growth and accelerating healing. Their porous structure enables better oxygen exchange, promoting wound recovery. NFs ensure localized and sustained release of drugs directly to the wound site, maximizing efficacy and minimizing systemic side effects. Additionally, NFs absorb wound exudate, maintaining an optimal moisture balance, which supports faster wound healing. Advanced multifunctional NFs, such as core-shell and Janus nanofibers, offer additional benefits by enabling dual drug loading or sequential release, further enhancing their therapeutic potential. Created in BioRender. Mamdouh, A. (2024) https://BioRender.com/ y72h378. |
Recently, Zhu et al established a pioneering design for a wound dressing for diabetic wound healing.160 This dressing is featured with a distinctive NFs bilayer. The outer layer consists of a mixture of a blend of hydrophilic polycaprolactone (PCL) and polyethylene glycol (PEG) NFs loaded with DFO. This layer offers effective exudate management and enhances angiogenesis. Meanwhile, the inner layer is composed of hydrophobic PCL NFs, with a mesh-like structure. The design of this dressing ensured both mechanical strength and biocompatibility. Moreover, the inner mesh structure of PCL NFs enhanced fibroblast attachment and proliferation, crucial for wound closure. In addition, the release kinetics of DFO from the outer layer showed a sustained release profile over time with a release percentage of 86% for 48 h. In a diabetic full-thickness cutaneous wound model, the NFs accelerated wound closure, achieving near complete closure by day 16 compared to standard treatments, underscoring its efficacy in creating a conducive microenvironment that efficiently managed biofluids and stimulated angiogenesis.
Li et al introduced a creative approach to diabetic wound healing using a combination of advanced materials.129 Their strategy centers on aligned, porous poly(L-lactic acid) (PLLA) electrospun fibrous membranes. These membranes incorporate mesoporous silica nanoparticles (DS) specifically designed to deliver DMOG. By incorporating DS into the PLLA nanofibrous scaffold (DS-PL), they established a dual cooperative controllable release system. The study demonstrated that DS-PL effectively released DMOG and Si ions over an extended period of 15 days. This controlled release profile significantly enhanced endothelial cell functions, including proliferation and migration, by stabilizing HIF-1α and upregulating VEGF. Moreover, in vivo studies using a diabetic mouse model revealed accelerated wound closure rates with DS-PL scaffolds, reducing wound size by 95% after 15 days. Furthermore, DS-PL scaffolds stimulated collagen deposition, particularly Collagen I, and promoted re-epithelialization in diabetic wounds, critical for tissue regeneration.
Finally, a growing body of research investigates the application of nanotechnology for the localized delivery of HIF-1α stabilizers in the context of wound healing. These investigations further solidify the promising potential of integrating nanotechnology with the HIF-1α activation pathway. A comprehensive overview of these supplementary research endeavors is presented in Table 4.
 |
Table 4 Advances in Nanotechnology for Targeted Delivery of HIF-1α Stabilizers in Wound Healing |
Future Perspectives
The future of diabetic wound treatment via nanoscale technologies for HIF-1α activation poses several challenges and exciting opportunities. Below are the critical current limitations and potential future research directions.
Clinical Transition
While there are some ongoing clinical applications of HIF-1α stabilizers, their broader clinical use remains limited. For example, a clinical trial is currently investigating the effect of local DFO on the healing process in diabetic foot ulcers (ClinicalTrials.gov: NCT03137966). Similarly, Daprodustat has completed Phase I clinical trials for diabetic foot ulcers treatment (ClinicalTrials.gov: NCT01831804). These trials mark significant steps forward in the clinical translation of HIF-1α stabilizers. However, despite these advancements, the clinical translation of nanomaterials for localized delivery of HIF-1α stabilizers remains a significant challenge. Currently, there are no clinical applications for these nano-based systems. As mentioned, nanomaterials hold the potential to offer several critical advantages that make them particularly suitable for diabetic wound management. However, transitioning these technologies from preclinical studies to clinical practice faces numerous hurdles. First, the scalability of nanomaterial production for large-scale manufacturing remains a significant hurdle, as consistent synthesis methods must be developed. Second, ensuring the long-term safety, biocompatibility, and stability of these materials is crucial, especially for chronic wound treatments. Regulatory approval also poses a challenge due to the novel nature of nanotechnology-based systems, requiring rigorous clinical trials to demonstrate their efficacy and safety.
Bridging the Gap in Preclinical Research
A critical gap in current research is the limited investigation into novel HIF-1α stabilizers beyond DFO and DMOG. To move forward, it is essential to focus on these novel agents, which include PHD inhibitors like roxadustat and daprodustat and VHL antagonists like VH298. These drugs offer a more refined approach to stabilizing HIF-1α but have yet to be fully explored in the context of diabetic wounds. For instance, roxadustat has been investigated in only two studies focusing on its efficacy in wound healing following systemic application via intraperitoneal injection.82,164 While these studies provided foundational insights, systemic application can lead to undesirable side effects, limiting its clinical feasibility. Notably, only one study to date has evaluated the localized application of roxadustat through a cubosomal hydrogel delivery system,118 which successfully avoided systemic side effects, showing a potential direction for future therapeutic interventions. Similarly, daprodustat and VH298 have not been extensively studied in the context of wound healing, representing a significant gap in preclinical exploration.
Moving forward, future research should aim to incorporate these novel agents into advanced nanoscale delivery systems, which can provide localized delivery and minimize systemic exposure. This approach will not only enhance the therapeutic potential of these novel stabilizers but also offer a more controlled and targeted treatment for chronic wounds, particularly in diabetic patients. Additionally, comparative studies evaluating the efficacy of these novel agents are critically needed. Currently, there is only one study comparing the efficacy of DFO and DMOG in wound healing,78 underscoring the necessity for further head-to-head comparisons with newer drugs like roxadustat, daprodustat, and VH298. Such studies would provide valuable insights into the relative strengths and limitations of each stabilizer and could guide the development of more effective therapeutic strategies for diabetic wounds.
Drug Repurposing
Another area for investigation is drug repurposing. It proposes a remarkably attractive approach for accelerating the development of novel therapies for diabetic wounds. A significant advantage of this approach is the acceleration of clinical translation and reducing potential risks associated with new drug development. Significantly, drug repurposing allows leveraging existing FDA-approved drugs with established safety profiles. DFO serves as a prime example of the potential of drug repurposing in wound healing. Originally approved for the treatment of iron overload disorders, DFO has emerged as a potent activator of HIF-1α, a key regulator in the cellular response to hypoxia, which is critical for wound healing. The safety and pharmacokinetics of DFO are already well understood, making it an ideal candidate for repurposing in diabetic wound management.
Looking ahead, future research should focus on identifying and repurposing additional FDA-approved HIF-1α stabilizers or drugs with angiogenic or anti-inflammatory properties that have been previously approved for unrelated conditions By utilizing the drug repurposing strategy, these compounds could provide new therapeutic avenues with reduced development time and improved safety profiles. Moreover, the integration of drug repurposing with advanced nanotechnology platforms offers a powerful synergy. This strategy not only enhances drug efficacy but also minimizes systemic side effects, a significant concern when repurposing drugs for new therapeutic applications. For instance, incorporating DFO into nanocarrier systems for localized delivery has shown promise in preclinical models, and similar strategies should be extended to other repurposed drugs.
Multifunctional Nanomaterials
The advancement of nanotechnology offers transformative potential for diabetic wound healing by enabling the design of multifunctional nanomaterials capable of addressing multiple aspects of the wound healing process. Among these, core-shell and Janus systems represent cutting-edge innovations in nanoscience that have garnered attention due to their ability to integrate multiple therapeutic modalities within a single platform.165,166 These complex nanostructures can be engineered to tackle critical challenges in diabetic wounds, such as hypoxia, infection, inflammation, and impaired angiogenesis, simultaneously.
In the future, research should focus on combining HIF-1α stabilizers with these advanced multifunctional nanomaterials to leverage their synergistic benefits. This could involve developing nanomaterial-based systems with dual functions enabling the co-delivery of HIF-1α stabilizers alongside other therapeutic agents, such as anti-inflammatory drugs or growth factors, offering a comprehensive treatment solution for diabetic wound management.
Conclusion
Impaired wound healing in diabetic patients is a significant medical and public health issue globally, associated with poorer prognosis and higher mortality rates. Hypoxia plays a crucial role in normal wound healing processes via HIF-1α activation, which regulates many essential processes for wound repair under ischemic conditions. However, in diabetic wounds, chronic hypoxia disrupts normal HIF-1α signaling, resulting in a dysfunctional hypoxic response. Consequently, the beneficial effects of HIF-1α are diminished, exacerbating wound healing challenges.
Many studies have emphasized the importance of HIF-1α signaling regulation in diabetic wound healing and have explored pharmacological approaches to stabilize and activate HIF-1α. These approaches include iron substitution, iron chelation, 2-OG inhibition, and the novel strategy of using VHL antagonists. Regardless of the promise of HIF-1α stabilizing agents in enhancing wound healing, systemic exposure to these agents presents significant challenges, including abnormal angiogenesis, increased tumor growth risk, and disrupted iron metabolism. These limitations require a localized drug delivery system.
Nanotechnology-based platforms offer a solution by enabling localized, sustained drug release, enhancing drug efficacy at lower doses, and allowing the co-delivery of multiple therapeutic agents such as HIF-1α stabilizers, growth factors, and antibiotics in a single formulation. This approach simultaneously addresses multiple aspects of wound healing, promoting faster and more effective tissue repair, particularly beneficial in diabetic wound treatment. Additionally, the inherent properties of these nanomaterials, such as mimicking the ECM, degradability, biocompatibility, porosity, and swellability, provide further advantages. Recent studies have demonstrated the promising outcomes of nanotechnology-based delivery systems incorporating HIF-1α stabilizers, in experimental animal models, as evidenced by improved vascularization, accelerated wound closure, and enhanced tissue regeneration. This synergistic strategy represents profound progress in developing practical therapeutic approaches for diabetic wounds, leveraging the key biological pathways regulated by HIF-1.
Finally, While HIF-1α activation plays a crucial role in promoting angiogenesis, cell proliferation, and tissue regeneration, its use in therapeutic applications must be approached with caution. Excessive or prolonged activation of HIF-1α can lead to adverse effects such as abnormal angiogenesis, edema, and even potential oncogenesis due to its role in promoting blood vessel growth. Beyond the systemic side effects of HIF-1α stabilizers, other challenges in diabetic wound healing, such as chronic infection, excessive inflammation, oxidative stress, and poor immune response, require more than just angiogenesis promotion. This is where multifunctional nanomaterials become especially valuable because they can address these additional factors by delivering multiple therapeutic agents alongside HIF-1α stabilizers.
Multifunctional nanomaterials offer an innovative solution by co-delivering HIF-1α stabilizers alongside other therapeutic agents that target these complications. For instance, chronic infections in diabetic wounds can be controlled through the incorporation of antibacterial agents in nanomaterials, while anti-inflammatory drugs can be delivered to reduce excessive inflammation that hampers healing. Furthermore, multifunctional nanomaterials can carry ROS scavengers to neutralize oxidative stress, protecting cells from damage and promoting tissue regeneration. By targeting these multiple aspects of wound healing simultaneously, multifunctional nanomaterials provide a synergistic approach that enhances the efficacy of HIF-1α stabilizers and addresses the complex nature of diabetic wounds.
Abbreviations
2-OG, 2-oxoglutarate; CHIP, chaperone-dependent ubiquitin ligase; CBP, CREB-binding protein; DFO, deferoxamine; DMOG, dimethyloxalylglycine; ECM, extracellular matrix; HUVECs, human umbilical vein endothelial cells; HREs, hypoxia response elements; HIF-1α, Hypoxia-inducible factor-1 alpha; MMPs, matrix metalloproteinases; MGO, methylglyoxal; NFs, nanofibers; NOG, N-oxalylglycine; PAD, peripheral arterial disease; PHD, prolyl hydroxylase domain; ROS, reactive oxygen species; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor; VHL, Von Hippel‐Lindau protein.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work under grant number RGP2/233/45. This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. KFU242314). The authors would like to thank the Deanship of Scientific Research at Shaqra University for supporting this work. The researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2024-9/1). Special thanks to the International Collaboration Office team at Delta University for Science and Technology for their invaluable support. The graphical abstract was Created in BioRender. Mamdouh, A. (2024) https://BioRender.com/ k86s754.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Chuter V, Schaper N, Hinchliffe R, et al. Performance of non‐invasive bedside vascular testing in the prediction of wound healing or amputation among people with foot ulcers in diabetes: a systematic review. Diabetes/Metab Res Rev. 2024;40(3):e3701. doi:10.1002/dmrr.3701
2. Harding JL, Weber MB, Shaw JE. The global burden of diabetes. Textbook of Diabetes. 2024;28–40.
3. Catrina SB, Zheng X. Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes/Metab Res Rev. 2016;32:179–185. doi:10.1002/dmrr.2742
4. Hong WX, Hu MS, Esquivel M, et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care. 2014;3(5):390–399. doi:10.1089/wound.2013.0520
5. Yeh T-L, Leissing TM, Abboud MI, et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem Sci. 2017;8(11):7651–7668. doi:10.1039/C7SC02103H
6. Hirota K. HIF-α prolyl hydroxylase inhibitors and their implications for biomedicine: a comprehensive review. Biomedicines. 2021;9(5):468. doi:10.3390/biomedicines9050468
7. Tundisi LL, Ataide JA, Costa JSR, et al. Nanotechnology as a tool to overcome macromolecules delivery issues. Colloids Surf B. 2023;222:113043. doi:10.1016/j.colsurfb.2022.113043
8. El-Gizawy SA, Nouh A, Saber S, Kira AY. Deferoxamine-loaded transfersomes accelerates healing of pressure ulcers in streptozotocin-induced diabetic rats. J Drug Delivery Sci Technol. 2020;58:101732. doi:10.1016/j.jddst.2020.101732
9. Jeckson TA, Neo YP, Sisinthy SP, Foo JB, Choudhury H, Gorain B. Formulation and characterisation of deferoxamine nanofiber as potential wound dressing for the treatment of diabetic foot ulcer. J Drug Delivery Sci Technol. 2021;66:102751. doi:10.1016/j.jddst.2021.102751
10. Shen H, Zhang C, Meng Y, et al. Biomimetic hydrogel containing copper sulfide nanoparticles and deferoxamine for photothermal therapy of infected diabetic wounds. Adv Healthcare Mater. 2024;13(8):2303000. doi:10.1002/adhm.202303000
11. Cai E, Qi X, Shi Y, et al. Immunomodulatory melanin@ Pt nanoparticle-reinforced adhesive hydrogels for healing diabetic oral ulcers. Chem Eng J. 2024;488:150372. doi:10.1016/j.cej.2024.150372
12. Qi X, Cai E, Xiang Y, et al. An immunomodulatory hydrogel by hyperthermia‐assisted self‐cascade glucose depletion and ROS scavenging for diabetic foot ulcer wound therapeutics. Adv Mater. 2023;35(48):2306632. doi:10.1002/adma.202306632
13. Qi X, Ge X, Chen X, et al. An immunoregulation hydrogel with controlled hyperthermia‐augmented oxygenation and ROS scavenging for treating diabetic foot ulcers. Adv Funct Mater. 2024;34(33):2400489. doi:10.1002/adfm.202400489
14. Su Y, Richmond A. Chemokine regulation of neutrophil infiltration of skin wounds. Adv Wound Care. 2015;4(11):631–640. doi:10.1089/wound.2014.0559
15. Banerjee K, Madhyastha R, Nakajima Y, Maruyama M, Madhyastha H. Nanoceutical adjuvants as wound healing material: precepts and prospects. Int J Mol Sci. 2021;22(9):4748. doi:10.3390/ijms22094748
16. Shen T, Dai K, Yu Y, Wang J, Liu C. Sulfated chitosan rescues dysfunctional macrophages and accelerates wound healing in diabetic mice. Acta Biomater. 2020;117:192–203. doi:10.1016/j.actbio.2020.09.035
17. Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Delivery Rev. 2019;146:97–125. doi:10.1016/j.addr.2018.09.010
18. Ellis S, Lin E, Tartar D. Immunology of wound healing. Curr Dermatol Rep. 2018;7:350–358. doi:10.1007/s13671-018-0234-9
19. Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4(3):119–136. doi:10.1089/wound.2013.0485
20. Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74(4):589–605. doi:10.1016/j.jaad.2015.08.068
21. Spielman AF, Griffin MF, Parker J, Cotterell AC, Wan DC, Longaker MT. Beyond the scar: a basic science review of wound remodeling. Adv Wound Care. 2023;12(2):57–67. doi:10.1089/wound.2022.0049
22. Bosco MC, Puppo M, Blengio F, et al. Monocytes and dendritic cells in a hypoxic environment: spotlights on chemotaxis and migration. Immunobiology. 2008;213(9–10):733–749. doi:10.1016/j.imbio.2008.07.031
23. Tandara AA, Mustoe TA. Oxygen in wound healing—more than a nutrient. World J Surg. 2004;28:294–300. doi:10.1007/s00268-003-7400-2
24. D’Alessandro S, Magnavacca A, Perego F, et al. Effect of hypoxia on gene expression in cell populations involved in wound healing. Biomed Res Int. 2019;2019(1):2626374. doi:10.1155/2019/2626374
25. Remensnyder J, Majno G. Oxygen gradients in healing wounds. Am J Pathol. 1968;52(2):301.
26. Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1α and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60(21):6189–6195.
27. Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int. 2013;2013(1):385641. doi:10.1155/2013/385641
28. Catrina S-B. Impaired hypoxia-inducible factor (HIF) regulation by hyperglycemia. J Mol Med. 2014;92:1025–1034. doi:10.1007/s00109-014-1166-x
29. Koıtka A, Abraham P, Bouhanick B, Sigaudo-Roussel D, Demiot C, Saumet JL. Impaired pressure-induced vasodilation at the foot in young adults with type 1 diabetes. Diabetes. 2004;53(3):721–725. doi:10.2337/diabetes.53.3.721
30. Szołna-Chodór A, Grzegorzewski B. The effect of glucose and poloxamer 188 on red-blood-cell aggregation. Metabolites. 2021;11(12):886. doi:10.3390/metabo11120886
31. Fadini GP, Albiero M, Bonora BM, Avogaro A. Angiogenic abnormalities in diabetes mellitus: mechanistic and clinical aspects. J Clin Endocrinol Metab. 2019;104(11):5431–5444. doi:10.1210/jc.2019-00980
32. Tresierra-Ayala M, Rojas AG. Association between peripheral arterial disease and diabetic foot ulcers in patients with diabetes mellitus type 2. Med Universitaria. 2017;19(76):123–126. doi:10.1016/j.rmu.2017.07.002
33. Fu-Shin XY, Lee PS, Yang L, et al. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog Retinal Eye Res. 2022;89:101039. doi:10.1016/j.preteyeres.2021.101039
34. Rathur HM, Boulton AJ. The neuropathic diabetic foot. Nat Clin Pract Endocrinol Metab. 2007;3(1):14–25. doi:10.1038/ncpendmet0347
35. Tecilazich F, Veves A. Role of peripheral neuropathy in the development of foot ulceration and impaired wound healing in diabetes mellitus. Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome. 2018;95–104.
36. Cerychova R, Pavlinkova G. HIF-1, metabolism, and diabetes in the embryonic and adult heart. Front Endocrinol. 2018;9:406682. doi:10.3389/fendo.2018.00460
37. Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM. Sustained expression of HIF‐1α in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regener. 2007;15(5):636–645. doi:10.1111/j.1524-475X.2007.00278.x
38. Ahn G-O, Seita J, Hong B-J, et al. Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proc Natl Acad Sci. 2014;111(7):2698–2703. doi:10.1073/pnas.1320243111
39. Komatsu D, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34(4):680–688. doi:10.1016/j.bone.2003.12.024
40. Yu Y, Ma L, Zhang H, et al. EPO could be regulated by HIF-1 and promote osteogenesis and accelerate bone repair. Artif Cells Nanomed Biotechnol. 2020;48(1):206–217. doi:10.1080/21691401.2019.1699827
41. Zhang D, Cui G, Sun C, et al. Hypoxia promotes osteosarcoma cell proliferation and migration through enhancing platelet-derived growth factor-BB/platelet-derived growth factor receptor-β axis. Biochem Biophys Res Commun. 2019;512(2):360–366. doi:10.1016/j.bbrc.2019.03.040
42. Mingyuan X, Qianqian P, Shengquan X, et al. Hypoxia-inducible factor-1α activates transforming growth factor-β1/Smad signaling and increases collagen deposition in dermal fibroblasts. Oncotarget. 2018;9(3):3188. doi:10.18632/oncotarget.23225
43. Sari Y, Sanada H, Minematsu T, et al. Vibration inhibits deterioration in rat deep‐tissue injury through HIF 1–MMP axis. Wound Repair Regener. 2015;23(3):386–393. doi:10.1111/wrr.12286
44. Fulda S, Debatin K-M. HIF-1-regulated glucose metabolism: a key to apoptosis resistance? Cell Cycle. 2007;6(7):790–792. doi:10.4161/cc.6.7.4084
45. Loh SA, Chang EI, Galvez MG, et al. SDF-1α expression during wound healing in the aged is HIF dependent. Plast Reconstr Surg. 2009;123(2S):65S–75S. doi:10.1097/PRS.0b013e318191bdf4
46. Dunn LL, Kong SM, Tumanov S, et al. Hmox1 (heme oxygenase-1) protects against ischemia-mediated injury via stabilization of HIF-1α (hypoxia-inducible factor-1α). Arteriosclerosis Thrombosis Vasc Biol. 2021;41(1):317–330. doi:10.1161/ATVBAHA.120.315393
47. Zhang X, Liu L, Wei X, et al. Impaired angiogenesis and mobilization of circulating angiogenic cells in HIF‐1α heterozygous‐null mice after burn wounding. Wound Repair Regener. 2010;18(2):193–201. doi:10.1111/j.1524-475X.2010.00570.x
48. Zhang X, Sarkar K, Rey S, et al. Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds. J Mol Med. 2011;89:985–995. doi:10.1007/s00109-011-0754-2
49. Han Y, Tao J, Gomer A, Ramirez-Bergeron DL. Loss of endothelial-ARNT in adult mice contributes to dampened circulating proangiogenic cells and delayed wound healing. Vascular Medicine. 2014;19(6):429–441. doi:10.1177/1358863X14559588
50. Koyasu S, Kobayashi M, Goto Y, Hiraoka M, Harada H. Regulatory mechanisms of hypoxia‐inducible factor 1 activity: two decades of knowledge. Cancer Sci. 2018;109(3):560–571. doi:10.1111/cas.13483
51. Saber S, Nasr M, Saad AS, et al. Albendazole-loaded cubosomes interrupt the ERK1/2-HIF-1α-p300/CREB axis in mice intoxicated with diethylnitrosamine: a new paradigm in drug repurposing for the inhibition of hepatocellular carcinoma progression. Biomed Pharmacother. 2021;142:112029. doi:10.1016/j.biopha.2021.112029
52. El-Kashef DH, Youssef ME, Nasr M, et al. Pimitespib, an HSP90 inhibitor, augments nifuroxazide-induced disruption in the IL-6/STAT3/HIF-1α autocrine loop in rats with bleomycin-challenged lungs: evolutionary perspective in managing pulmonary fibrosis. Biomed Pharmacother. 2022;153:113487. doi:10.1016/j.biopha.2022.113487
53. Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular Cell. 2008;30(4):393–402. doi:10.1016/j.molcel.2008.04.009
54. Berra E, Roux D, Richard DE, Pouysségur J. Hypoxia‐inducible factor‐1α (HIF‐1α) escapes O2‐drivenO 2 -driven proteasomal degradation irrespective of its subcellular localization: nucleus or cytoplasm. EMBO Rep. 2001;2(7):615–620. doi:10.1093/embo-reports/kve130
55. Nakayama K, Frew IJ, Hagensen M, et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1α abundance, and modulates physiological responses to hypoxia. Cell. 2004;117(7):941–952. doi:10.1016/j.cell.2004.06.001
56. Choi H, Song BJ, Gong YD, Gwak W, Soh Y. Rapid degradation of hypoxia‐inducible factor‐1α by KRH102053, a new activator of prolyl hydroxylase 2. Br J Pharmacol. 2008;154(1):114–125. doi:10.1038/bjp.2008.70
57. Xiao H, Gu Z, Wang G, Zhao T. The possible mechanisms underlying the impairment of HIF-1α pathway signaling in hyperglycemia and the beneficial effects of certain therapies. Int J Med Sci. 2013;10(10):1412. doi:10.7150/ijms.5630
58. Ramalho AR, Toscano A, Pereira P, Girão H, Gonçalves L, Marques C. Hyperglycemia-induced degradation of HIF-1α contributes to impaired response of cardiomyocytes to hypoxia. Revista Portuguesa de Cardiologia. 2017;36(5):367–373. doi:10.1016/j.repc.2016.09.018
59. Catrina S-B, Zheng X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia. 2021;64:709–716. doi:10.1007/s00125-021-05380-z
60. Li G, Ko C-N, Li D, et al. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat Commun. 2021;12(1):3363. doi:10.1038/s41467-021-23448-7
61. Kim D, Kim K-A, Kim J-H, Kim E-H, Bae O-N. Methylglyoxal-induced dysfunction in brain endothelial cells via the suppression of akt/HIF-1α pathway and activation of mitophagy associated with increased reactive oxygen species. Antioxidants. 2020;9(9):820. doi:10.3390/antiox9090820
62. Bento CF, Fernandes R, Ramalho J, et al. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1α for degradation in the presence of methylglyoxal. PLoS One. 2010;5(11):e15062. doi:10.1371/journal.pone.0015062
63. Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci. 2009;106(32):13505–13510. doi:10.1073/pnas.0906670106
64. Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. doi:10.1016/S0092-8674(01)00507-4
65. Li Q, Chen H, Huang X, Costa M. Effects of 12 metal ions on iron regulatory protein 1 (IRP-1) and hypoxia-inducible factor-1 alpha (HIF-1α) and HIF-regulated genes. Toxicol Appl Pharmacol. 2006;213(3):245–255. doi:10.1016/j.taap.2005.11.006
66. Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55(6):333–339. doi:10.1016/S0753-3322(01)00068-3
67. Huang J, Liu L, Feng M, et al. Effect of CoCl2 on fracture repair in a rat model of bone fracture. Mol Med Rep. 2015;12(4):5951–5956. doi:10.3892/mmr.2015.4122
68. Bellotti D, Remelli M, Deferoxamine B. A natural, excellent and versatile metal chelator. Molecules. 2021;26(11):3255. doi:10.3390/molecules26113255
69. Ikeda Y, Tajima S, Yoshida S, et al. Deferoxamine promotes angiogenesis via the activation of vascular endothelial cell function. Atherosclerosis. 2011;215(2):339–347. doi:10.1016/j.atherosclerosis.2011.01.009
70. Hou Z, Nie C, Si Z, Ma Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1α. Diabetes Res Clin Pract. 2013;101(1):62–71. doi:10.1016/j.diabres.2013.04.012
71. Holden P, Nair LS. Deferoxamine: an angiogenic and antioxidant molecule for tissue regeneration. Tissue Eng B. 2019;25(6):461–470. doi:10.1089/ten.teb.2019.0111
72. Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13(11):852–869. doi:10.1038/nrd4422
73. Smirnova N, Hushpulian D, Speer R, Gaisina I, Ratan R, Gazaryan I. Catalytic mechanism and substrate specificity of HIF prolyl hydroxylases. Biochemistry. 2012;77:1108–1119. doi:10.1134/S0006297912100033
74. Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1α, HIF-2α, and other pathways. J Biol Chem. 2006;281(22):15215–15226. doi:10.1074/jbc.M511408200
75. Kalucka J, Ettinger A, Franke K, et al. Loss of epithelial hypoxia-inducible factor prolyl hydroxylase 2 accelerates skin wound healing in mice. Mol Cell Biol. 2013;33(17):3426–3438. doi:10.1128/MCB.00609-13
76. Weidemann A, Breyer J, Rehm M, et al. HIF-1α activation results in actin cytoskeleton reorganization and modulation of Rac-1 signaling in endothelial cells. Cell Commun Signaling. 2013;11:1–16. doi:10.1186/1478-811X-11-80
77. Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci. 2008;105(49):19426–19431. doi:10.1073/pnas.0805230105
78. Duscher D, Januszyk M, Maan ZN, et al. Comparison of the hydroxylase inhibitor dimethyloxalylglycine and the iron chelator deferoxamine in diabetic and aged wound healing. Plast Reconstr Surg. 2017;139(3):695e–706e. doi:10.1097/PRS.0000000000003072
79. Dhillon S. Roxadustat: first global approval. Drugs. 2019;79:563–572. doi:10.1007/s40265-019-01077-1
80. Dhillon S. Daprodustat: first approval. Drugs. 2020;80(14):1491–1497. doi:10.1007/s40265-020-01384-y
81. Olson E, Mahar KM, Morgan L, Fillmore C, Holland C, Lavery L. Randomized phase I trial to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of topical daprodustat in healthy volunteers and in patients with diabetic foot ulcers. Clin Pharmacol Drug Dev. 2019;8(6):765–778. doi:10.1002/cpdd.654
82. Zhu Y, Wang Y, Jia Y, Xu J, Chai Y. Roxadustat promotes angiogenesis through HIF‐1α/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats. Wound Repair Regener. 2019;27(4):324–334. doi:10.1111/wrr.12708
83. Esakkimuthukumar M, Swaroop AK, Patnaik SK, et al. A novel family of small molecule HIF-1 alpha stabilizers for the treatment of diabetic wounds; an integrated in silico, in vitro, and in vivo strategy. RSC Adv. 2022;12(48):31293–31302. doi:10.1039/D2RA05364K
84. Frost J, Galdeano C, Soares P, et al. Potent and selective chemical probe of hypoxic signalling downstream of HIF-α hydroxylation via VHL inhibition. Nat Commun. 2016;7(1):13312. doi:10.1038/ncomms13312
85. Qiu S, Jia Y, Sun Y, et al. Von Hippel‐Lindau (VHL) protein antagonist VH298 improves wound healing in streptozotocin‐induced hyperglycaemic rats by activating hypoxia‐inducible factor‐(HIF‐) 1 signalling. J Diabetes Investig. 2019;2019(1):1897174. doi:10.1155/2019/1897174
86. Nagel S, Talbot NP, Mecinović J, Smith TG, Buchan AM, Schofield CJ. Therapeutic manipulation of the HIF hydroxylases. Antioxid Redox Signaling. 2010;12(4):481–501. doi:10.1089/ars.2009.2711
87. El-Fattah EE A, Saber S, Youssef ME, et al. AKT-AMPKα-mTOR-dependent HIF-1α activation is a new therapeutic target for cancer treatment: a novel approach to repositioning the antidiabetic drug sitagliptin for the management of hepatocellular carcinoma. Front Pharmacol. 2022;12:720173. doi:10.3389/fphar.2021.720173
88. Saber S, El-Fattah EEA, Abdelhamid AM, et al. Innovative challenge for the inhibition of hepatocellular carcinoma progression by combined targeting of HSP90 and STAT3/HIF-1α signaling. Biomed Pharmacother. 2023;158:114196. doi:10.1016/j.biopha.2022.114196
89. Lim CS, Kiriakidis S, Sandison A, Paleolog EM, Davies AH. Hypoxia-inducible factor pathway and diseases of the vascular wall. J Vascular Surg. 2013;58(1):219–230. doi:10.1016/j.jvs.2013.02.240
90. Cowburn AS, Crosby A, Macias D, et al. HIF2α–arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci. 2016;113(31):8801–8806. doi:10.1073/pnas.1602978113
91. Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1. 5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol. 2008;294(2):H570–H578. doi:10.1152/ajpheart.01324.2007
92. Cheng S, Xing W, Pourteymoor S, Mohan S. Conditional disruption of the prolyl hydroxylase domain‐containing protein 2 (Phd2) gene defines its key role in skeletal development. J Bone Miner Res. 2014;29(10):2276–2286. doi:10.1002/jbmr.2258
93. Haase VH. HIF‐prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodialysis Int. 2017;21:S110–S124. doi:10.1111/hdi.12567
94. Wang W, K-j L, C-h Y, Q-l H, Du Y-Z. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnol. 2019;17(1):82. doi:10.1186/s12951-019-0514-y
95. Simonazzi A, Cid AG, Villegas M, Romero AI, Palma SD, Bermúdez JM. Nanotechnology applications in drug controlled release. Drug Targeting and Stimuli Sensitive Drug Delivery Systems. 2018;81–116.
96. Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. Int J Mol Sci. 2021;22(13):7202. doi:10.3390/ijms22137202
97. Kim S. Competitive biological activities of chitosan and its derivatives: antimicrobial, antioxidant, anticancer, and anti‐inflammatory activities. Int J Polym Sci. 2018;2018(1):1708172. doi:10.1155/2018/1708172
98. Kondaparthi P, Flora S, Naqvi S. Selenium nanoparticles: an insight on its pro-oxidant and antioxidant properties. Front Nanosci Nanotechnol. 2019;6(1):5. doi:10.15761/FNN.1000189
99. Ghomi ER, Lakshminarayanan R, Chellappan V, et al. Electrospun aligned PCL/gelatin scaffolds mimicking the skin ECM for effective antimicrobial wound dressings. Adv Fiber Mater. 2023;5(1):235–251. doi:10.1007/s42765-022-00216-w
100. Wu J, Pan Z, Zhao ZY, et al. Anti‐swelling, robust, and adhesive extracellular matrix‐mimicking hydrogel used as intraoral dressing. Adv Mater. 2022;34(20):2200115. doi:10.1002/adma.202200115
101. Hama R, Reinhardt JW, Ulziibayar A, Watanabe T, Kelly J, Shinoka T. Recent tissue engineering approaches to mimicking the extracellular matrix structure for skin regeneration. Biomimetics. 2023;8(1):130. doi:10.3390/biomimetics8010130
102. Mendes C, Thirupathi A, Corrêa ME, Gu Y, Silveira PC. The use of metallic nanoparticles in wound healing: new perspectives. Int J Mol Sci. 2022;23(23):15376. doi:10.3390/ijms232315376
103. Salvo J, Sandoval C. Role of copper nanoparticles in wound healing for chronic wounds: literature review. Burns Trauma. 2022;10:tkab047. doi:10.1093/burnst/tkab047
104. Haghniaz R, Rabbani A, Vajhadin F, et al. Anti‐bacterial and wound healing‐promoting effects of zinc ferrite nanoparticles. J Nanobiotechnol. 2021;19:1–15. doi:10.1186/s12951-021-00776-w
105. Ovais M, Ahmad I, Khalil AT, et al. Wound healing applications of biogenic colloidal silver and gold nanoparticles: recent trends and future prospects. Appl Microbiol Biotechnol. 2018;102:4305–4318. doi:10.1007/s00253-018-8939-z
106. Javanmardi S, Ghojoghi A, Divband B, Ashrafi J. Titanium dioxide nanoparticle/gelatin: a potential burn wound healing biomaterial. Wounds. 2018;30(12):372–379.
107. Li S, Wang X, Chen J, et al. Calcium ion cross-linked sodium alginate hydrogels containing deferoxamine and copper nanoparticles for diabetic wound healing. Int J Biol Macromol. 2022;202:657–670. doi:10.1016/j.ijbiomac.2022.01.080
108. Hu C, Long L, Cao J, Zhang S, Wang Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem Eng J. 2021;411:128564. doi:10.1016/j.cej.2021.128564
109. Badhwar R, Mangla B, Neupane YR, Khanna K, Popli H. Quercetin loaded silver nanoparticles in hydrogel matrices for diabetic wound healing. Nanotechnology. 2021;32(50):505102. doi:10.1088/1361-6528/ac2536
110. Yu J, Wang X, Li Y, Huang X, Luo X, He X. Synthesis of nerolidol functionalized gold nanoparticles for wound regeneration in people with diabetic foot ulcers in nursing care management. Sci Adv Mater. 2018;10(12):1775–1781. doi:10.1166/sam.2018.3389
111. Ajalli N, Pourmadadi M, Yazdian F, Abdouss M, Rashedi H, Rahdar A. PVA based nanofiber containing GO modified with Cu nanoparticles and loaded curcumin; high antibacterial activity with acceleration wound healing. Curr Drug Deliv. 2023;20(10):1569–1583. doi:10.2174/1567201820666221014090334
112. Saddik MS, Elsayed MM, El-Mokhtar MA, et al. Tailoring of novel azithromycin-loaded zinc oxide nanoparticles for wound healing. Pharmaceutics. 2022;14(1):111. doi:10.3390/pharmaceutics14010111
113. Ahmad MZ, Alasiri AS, Ahmad J, et al. Green synthesis of titanium dioxide nanoparticles using Ocimum sanctum leaf extract: in vitro characterization and its healing efficacy in diabetic wounds. Molecules. 2022;27(22):7712. doi:10.3390/molecules27227712
114. Puri A, Loomis K, Smith B, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2009;26(6):523–580. doi:10.1615/critrevtherdrugcarriersyst.v26.i6.10
115. Samimi S, Maghsoudnia N, Eftekhari RB, Dorkoosh F. Lipid-based nanoparticles for drug delivery systems. Characterization and Biology of Nanomaterials for Drug Delivery. 2019;47–76.
116. Sato YT, Umezaki K, Sawada S, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6(1):21933. doi:10.1038/srep21933
117. Dolatabadi JEN, Omidi Y. Solid lipid-based nanocarriers as efficient targeted drug and gene delivery systems. TrAC Trends in Analytical Chemistry. 2016;77:100–108. doi:10.1016/j.trac.2015.12.016
118. Nasr M, Kira AY, Saber S. Localized delivery of roxadustat via cubosomes-based thermosensitive in-situ hydrogel enhances diabetic wound healing by stabilizing HIF-1α in rats. J Drug Delivery Sci Technol. 2024;100:106127. doi:10.1016/j.jddst.2024.106127
119. Qayoom A, Aneesha V, Anagha S, Dar JA, Kumar P, Kumar D. Lecithin-based deferoxamine nanoparticles accelerated cutaneous wound healing in diabetic rats. Eur J Pharmacol. 2019;858:172478. doi:10.1016/j.ejphar.2019.172478
120. Karami MA, Makhmalzadeh BS, Rad NM. Preparation and characterization of topical solid lipid nanoparticles containing deferoxamine. Ars Pharmaceutica. 2021;62(3):224–234. doi:10.30827/ars.v62i3.15493
121. Pandey S, Shamim A, Shaif M, Kushwaha P. Development and evaluation of resveratrol-loaded liposomes in hydrogel-based wound dressing for diabetic foot ulcer. Naunyn-Schmiedeberg’s Arch Pharmacol. 2023;396(8):1811–1825. doi:10.1007/s00210-023-02441-5
122. Akbari J, Saeedi M, Morteza-Semnani K, et al. An eco-friendly and hopeful promise platform for delivering hydrophilic wound healing agents in topical administration for wound disorder: diltiazem-loaded niosomes. J Pharm Innovation. 2023;18(3):1111–1127. doi:10.1007/s12247-023-09710-z
123. Arantes VT, Faraco AA, Ferreira FB, et al. Retinoic acid-loaded solid lipid nanoparticles surrounded by chitosan film support diabetic wound healing in in vivo study. Colloids Surf B. 2020;188:110749. doi:10.1016/j.colsurfb.2019.110749
124. Örgül D, Eroğlu H, Tiryaki M, Pınarlı FA, Hekimoglu S. In-vivo evaluation of tissue scaffolds containing simvastatin loaded nanostructured lipid carriers and mesenchymal stem cells in diabetic wound healing. J Drug Delivery Sci Technol. 2021;61:102140. doi:10.1016/j.jddst.2020.102140
125. Haider F, Khan BA, Khan MK. Formulation and evaluation of topical linezolid nanoemulsion for open incision wound in diabetic animal model. AAPS Pharm Sci Tech. 2022;23(5):129. doi:10.1208/s12249-022-02288-8
126. Thomas CS, Kumar Mishra P, Talegaonkar S, Talegaonkar S. Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des. 2015;21(42):6165–6188. doi:10.2174/1381612821666151027153246
127. Treccani L, Klein TY, Meder F, Pardun K, Rezwan K. Functionalized ceramics for biomedical, biotechnological and environmental applications. Acta Biomater. 2013;9(7):7115–7150. doi:10.1016/j.actbio.2013.03.036
128. Wei S, Zhang R-G, Wang Z-Y. Deferoxamine/magnesium modified β-tricalcium phosphate promotes the bone regeneration in osteoporotic rats. J Biomater Appl. 2022;37(5):838–849. doi:10.1177/08853282221121882
129. Ren X, Han Y, Wang J, et al. An aligned porous electrospun fibrous membrane with controlled drug delivery–an efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018;70:140–153. doi:10.1016/j.actbio.2018.02.010
130. Yu H, Peng J, Xu Y, Chang J, Li H. Bioglass activated skin tissue engineering constructs for wound healing. ACS Appl Mater Interfaces. 2016;8(1):703–715. doi:10.1021/acsami.5b09853
131. Kong L, Wu Z, Zhao H, et al. Bioactive injectable hydrogels containing desferrioxamine and bioglass for diabetic wound healing. ACS Appl Mater Interfaces. 2018;10(36):30103–30114. doi:10.1021/acsami.8b09191
132. Chattopadhyay S, Raines RT. Collagen‐based biomaterials for wound healing. Biopolymers. 2014;101(8):821–833. doi:10.1002/bip.22486
133. Brett D. A review of collagen and collagen-based wound dressings. Wounds. 2008;20(12):347–356.
134. Gao W, Sun L, Fu X, et al. Enhanced diabetic wound healing by electrospun core–sheath fibers loaded with dimethyloxalylglycine. J Mat Chem B. 2018;6(2):277–288. doi:10.1039/C7TB02342A
135. Ndlovu SP, Ngece K, Alven S, Aderibigbe BA. Gelatin-based hybrid scaffolds: promising wound dressings. Polymers. 2021;13(17):2959. doi:10.3390/polym13172959
136. Shao Z, Yin T, Jiang J, He Y, Xiang T, Zhou S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact Mater. 2023;20:561–573. doi:10.1016/j.bioactmat.2022.06.018
137. Farokhi M, Mottaghitalab F, Fatahi Y, Khademhosseini A, Kaplan DL. Overview of silk fibroin use in wound dressings. Trends Biotechnol. 2018;36(9):907–922. doi:10.1016/j.tibtech.2018.04.004
138. Patil PP, Reagan MR, Bohara RA. Silk fibroin and silk-based biomaterial derivatives for ideal wound dressings. Int J Biol Macromol. 2020;164:4613–4627. doi:10.1016/j.ijbiomac.2020.08.041
139. Zha S, Utomo YKS, Yang L, Liang G, Liu W. Mechanic-driven biodegradable polyglycolic acid/silk fibroin nanofibrous scaffolds containing deferoxamine accelerate diabetic wound healing. Pharmaceutics. 2022;14(3):601. doi:10.3390/pharmaceutics14030601
140. Graça MF, Miguel SP, Cabral CS, Correia IJ. Hyaluronic acid—Based wound dressings: a review. Carbohydr Polym. 2020;241:116364. doi:10.1016/j.carbpol.2020.116364
141. Della Sala F, Longobardo G, Fabozzi A, Di Gennaro M, Borzacchiello A. Hyaluronic acid-based wound dressing with antimicrobial properties for wound healing application. Appl Sci. 2022;12(6):3091. doi:10.3390/app12063091
142. Matica MA, Aachmann FL, Tøndervik A, Sletta H, Ostafe V. Chitosan as a wound dressing starting material: antimicrobial properties and mode of action. Int J Mol Sci. 2019;20(23):5889. doi:10.3390/ijms20235889
143. Hamedi H, Moradi S, Hudson SM, Tonelli AE, King MW. Chitosan based bioadhesives for biomedical applications: a review. Carbohydr Polym. 2022;282:119100. doi:10.1016/j.carbpol.2022.119100
144. Zhang L, Zhu P, Zhao B, et al. Composite nano hydrogel with dual response and hierarchical drug release for enhanced wound healing. Mater Des. 2024;239:112805. doi:10.1016/j.matdes.2024.112805
145. Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER. Alginate-based composite materials for wound dressing application: a mini review. Carbohydr Polym. 2020;236:116025. doi:10.1016/j.carbpol.2020.116025
146. Lima DS, Tenorio-Neto ET, Lima-Tenório MK, et al. pH-responsive alginate-based hydrogels for protein delivery. J Mol Liq. 2018;262:29–36. doi:10.1016/j.molliq.2018.04.002
147. Zhang Z, Liu H, Yu D-G, Bligh S-WA. Alginate-based electrospun nanofibers and the enabled drug controlled release profiles: a review. Biomolecules. 2024;14(7):789. doi:10.3390/biom14070789
148. Kesavan K, Nath G, Pandit JK. Sodium alginate based mucoadhesive system for gatifloxacin and its in vitro antibacterial activity. Scientia Pharmaceutica. 2010;78(4):941. doi:10.3797/scipharm.1004-24
149. Liu S-J, Kau Y-C, Chou C-Y, Chen J-K, Wu R-C, Yeh W-L. Electrospun PLGA/collagen nanofibrous membrane as early-stage wound dressing. J Membr Sci. 2010;355(1–2):53–59. doi:10.1016/j.memsci.2010.03.012
150. Said SS, Aloufy AK, El-Halfawy OM, Boraei NA, El-Khordagui LK. Antimicrobial PLGA ultrafine fibers: interaction with wound bacteria. Eur J Pharm Biopharm. 2011;79(1):108–118. doi:10.1016/j.ejpb.2011.03.002
151. Li J, Lu X, Weng M, et al. Promoting tissue repair using deferoxamine nanoparticles loaded biomimetic gelatin/HA composite hydrogel. Biomed Mater. 2024;19(4):045009. doi:10.1088/1748-605X/ad46ba
152. Sharma A, Garg T, Aman A, et al. Nanogel—an advanced drug delivery tool: current and future. Artif Cells Nanomed Biotechnol. 2016;44(1):165–177. doi:10.3109/21691401.2014.930745
153. Liu Y, Song S, Liu S, Zhu X, Wang P. Application of nanomaterial in hydrogels related to wound healing. J Nanomater. 2022;2022(1):4656037. doi:10.1155/2022/4656037
154. Ghorbani M, Ghorbani F, Kahrizi S, Naderi-Manesh H, Kahrizi D. The application of nano-hydrogels and hydrogels in wound dressings. Cell Mol Biol. 2023;69(11):125–131. doi:10.14715/cmb/2023.69.11.19
155. Wang Y, Cao Z, Wei Q, et al. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022;147:342–355. doi:10.1016/j.actbio.2022.05.018
156. Deng M, Wu Y, Ren Y, et al. Clickable and smart drug delivery vehicles accelerate the healing of infected diabetic wounds. J Control Release. 2022;350:613–629. doi:10.1016/j.jconrel.2022.08.053
157. Chen K, Hu H, Zeng Y, et al. Recent advances in electrospun nanofibers for wound dressing. Eur Polym J. 2022;178:111490. doi:10.1016/j.eurpolymj.2022.111490
158. Ambekar RS, Kandasubramanian B. Advancements in nanofibers for wound dressing: a review. Eur Polym J. 2019;117:304–336.
159. Wang W, Yu Q, Shao Z, et al. Exudate‐induced gelatinizable nanofiber membrane with high exudate absorption and super bactericidal capacity for bacteria‐infected wound management. Adv Healthcare Mater. 2024;13(9):2303293. doi:10.1002/adhm.202303293
160. Zhu Y, Zhou W, Xiang J, et al. Deferoxamine-loaded Janus electrospun nanofiber dressing with spatially designed structure for diabetic wound healing. Mater Des. 2023;233:112166. doi:10.1016/j.matdes.2023.112166
161. Mandakhbayar N, Ji Y, El-Fiqi A, et al. Double hits with bioactive nanozyme based on cobalt-doped nanoglass for acute and diabetic wound therapies through anti-inflammatory and pro-angiogenic functions. Bioact Mater. 2024;31:298–311. doi:10.1016/j.bioactmat.2023.08.014
162. Nour S, Imani R, Mehrabani M, et al. Biomimetic hybrid scaffold containing niosomal deferoxamine promotes angiogenesis in full-thickness wounds. Mater Today Chem. 2023;27:101314. doi:10.1016/j.mtchem.2022.101314
163. Ding Z, Zhang Y, Guo P, et al. Injectable desferrioxamine-laden silk nanofiber hydrogels for accelerating diabetic wound healing. ACS Biomater Sci Eng. 2021;7(3):1147–1158. doi:10.1021/acsbiomaterials.0c01502
164. Xia R, Tang D, Yang B. Effect of salvia miltiorrhiza combined with roxadustat on wound healing of full-thickness skin defects in diabetic rats and its mechanism. Zhonghua Shao Shang yu Chuang Mian xiu fu za zhi. 2024;40(4):380–388. doi:10.3760/cma.j.cn501225-20231020-00124
165. Ji Y, Zhao H, Liu H, Zhao P, Yu D-G. Electrosprayed stearic-acid-coated ethylcellulose microparticles for an improved sustained release of anticancer drug. Gels. 2023;9(9):700. doi:10.3390/gels9090700
166. Zhang Z, Xia Y, Gong W, Zhou J, D-G Y, Y-f X. Electrospun chitosan//ethylcellulose-vitamin E//ethylcellulose-curcumin tri-chamber eccentric Janus nanofibers for a joint antibacterial and antioxidant performance. Int J Biol Macromol. 2024;281:135753. doi:10.1016/j.ijbiomac.2024.135753
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

