Back to Journals » Infection and Drug Resistance » Volume 18
Necrotizing Bronchitis with Airway Obstruction Due to Macrolide-Resistant Mycoplasma pneumoniae in a Child: A Case Report
Received 2 October 2024
Accepted for publication 1 January 2025
Published 6 January 2025 Volume 2025:18 Pages 77—82
DOI https://doi.org/10.2147/IDR.S498411
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zhongjie Li,* Yao Xu,* Yujian Xiang*
Department of Pediatrics, Zhejiang University School of Medicine Second Affiliated Hospital Jiashan Branch, the First People’s Hospital of Jiashan, Jiashan, Zhejiang, 314100, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhongjie Li, Department of Pediatrics, Zhejiang University school of medicine second Affiliated Hospital Jiashan Branch, The first People’s Hospital of Jiashan, No. 1218 Tayo South Road, Jiashan, Zhejiang, 314100, People’s Republic of China, Email [email protected]
Abstract: Although infection with Mycoplasma pneumoniae is generally self-limited, it may cause refractory or life-threatening pneumonia with pulmonary or extrapulmonary complications. Necrotizing bronchitis is a rare condition with a high mortality rate. The present report describes a patient with mycoplasma pneumonia and necrotizing bronchitis that caused airway obstruction. A 7-year-old girl presented with fever and cough and was hospitalized. Her symptoms did not improve, and mild hypoxemia was observed. Fiberoptic bronchoscopy revealed diffuse bronchitis with necrotic and hemorrhagic material obstructing the bronchus. The necrotic tissue was removed directly, unblocking the airways. This case underscores the importance of fiberoptic bronchoscopy for early diagnosis and treatment of severe respiratory complications associated with Mycoplasma pneumoniae infection, such as necrotizing bronchitis. Early recognition and timely intervention are critical to improving patient outcomes in such cases.
Keywords: Mycoplasma pneumoniae, necrotizing bronchitis, bronchial fiberscope, airway obstruction
Introduction
Mycoplasma pneumoniae (MP) is a frequent cause of respiratory system infections in children. Mild cases may be self-limiting, with no particular treatment being required.1 Severe pulmonary complications, including obliterative bronchitis, bronchiectasis, pleural effusion, acute respiratory distress syndrome (ARDS),2 necrotizing pneumonitis,3 and necrotizing bronchitis have been reported to be rare manifestations of M. pneumoniae infection. The present report describes a pediatric patient with necrotizing bronchitis.
Case Presentation
A previously healthy 7-year -old girl was admitted to the Department of Pediatrics in August 2023 with a 4-day history of fever and dry cough. She had a continuous high fever and paroxysmal cough, but no phlegm or shortness of breath. There was no family history of cardiovascular disease, coagulation disorder or any other systemic immune disorder. On examination, the patient weighed 25.0 kg and had a body temperature of 39.0°C. Her respiratory rate was 24 breaths/min, her heart rate was 120 beats/minute; and her oxygen saturation was 96% during lung auscultation, respiratory sound of Rhonchi was noted. Her capillary refill time was less than 2 sec, her white blood cell count was 6.7×109/L, and her platelet count was 156 ×109/L. Her hemoglobin, C-reactive protein (CR), procalcitonin, and interleukin (IL)-6 concentrations were 124 g/L, 30.58 mg/dl, 0.09 ng/mL, and 19.6 pg./mL, respectively. Liver and renal function tests and electrolyte concentrations were normal. Nasopharyngeal swabs were negative for adenovirus antigen, respiratory syncytial virus antigen, influenza A virus antigen, and influenza B virus antigen. Her erythrocyte sedimentation rate was 92 mm/h, and her D-dimer and serum ferritin concentrations were 740 pu/L and 157.96 ng/mL, respectively. Chest radiography revealed a high-density patchy shadow in the lower left lung and a blunt left costophrenic angle (Figure 1).
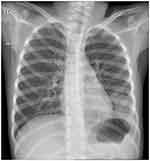 |
Figure 1 Chest-X-ray of the present patient, using a blunt left costophrenic angle, showing a high-density patchy area in the lower left lung. |
Following treatment with intravenous azithromycin (10 mg/kg) once daily, the patient had a recurrent fever, an increasing cough, and an oxygen saturation of 92%. Chest computed tomography (CT) on the fifth day showed a schistose density in the lower lobe of the left lung with partial consolidation (Figure 2). Fiberoptic bronchoscopy, performed because there was no improvement in symptoms, showed diffuse bronchitis with necrotic mucosal and hemorrhagic materials partially obstructing the Bronchus and both bronchi (Figure 3). Pathological examination revealed exfoliated epithelial cells and tissue cells in the inflammatory exudative necrotic tissue (Figure 4), with the latter treated with an additional injection of 80 mg/kg ceftriaxone.
 |
Figure 2 Computed tomography (CT) scan of the present patient, showing: a schistose of density in the lower lobe of the left lung with partial consolidation. |
 |
Figure 3 Fiberoptic bronchoscopy of the present patient, showing diffuse tracheobronchitis with necrotic mucosal and hemorrhagic materials partially obstructing the Bronchus and both bronchi. |
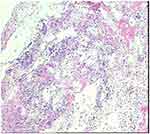 |
Figure 4 Histopathological examination of inflammatory exudative necrotic lung tissue from the present patient, showing exfoliated epithelial cells and tissue cells (original magnification ×100). |
Targeted next-generation sequencing showed M. pneumoniae with an A2063G mutation, conferring resistance to antibiotics, in bronchoalveolar lavage fluid (BALF). Azithromycin was discontinued, and the patient was started on oral clarithromycin, but the family refused treatment with doxycycline.2,4 BALF culture showed bacterial growth without pathogenic bacteria, leading to a cessation of ceftriaxone treatment. The patient’s symptoms improved significantly after bronchoscopy. Chest radiography performed four days after the bronchoscopy, showed a slight increase in fibrous stripes in both lungs, being slightly more prominent in the left lung (Figure 5). The patient continued oral clarithromycin treatment for 10 days. At an outpatient follow-up two months after treatment, she showed no respiratory symptoms.
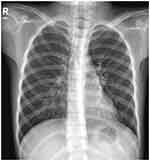 |
Figure 5 X-ray four days after bronchoscopy, showing a slight increase in fibrous stripes in both lungs, with the increase in the left lung being slightly more prominent. |
Discussion
M. pneumoniae is a common pathogen that causes community-acquired pneumonia (CAP) in children, Although M. pneumoniae pneumonia in most patients is a self-limiting illness, some patients may develop serious pneumonia, which can lead to various pulmonary and extrapulmonary complications. Some of these complications can be life threatening, such as necrotizing pneumonia, pulmonary thromboembolism, acute respiratory distress syndrome (ARDS), and necrotizing bronchitis. The present patient was diagnosed with necrotizing bronchitis complicated by M. pneumoniae, a rare condition.
Necrotizing bronchitis is an infrequently encountered necrotic inflammatory process. It can be caused by viral and bacterial infections,5,6 mechanical ventilation,7 rheumatoid arthritis, and ulcerative colitis, as confirmed by histological examination of bronchoscopy samples. Treatment of patients with necrotizing bronchitis is aimed to control the underlying disease, remove necrotic tissue through bronchoalveolar lavage,8 and maintain airway patency, thereby preventing poor outcomes.
The incidence of M. pneumoniae pneumonia refractory to macrolides is increasing. These patients may develop severe or fatal pneumonia. The pathogenic mechanism of M. pneumoniae pneumonia complicated by necrotizing bronchitis may involve the invasion of pathogenic microorganisms and mucosal immune responses in the respiratory tract, destruction of ciliated epithelial cells in the respiratory tract, infiltration of lymphocytes around the bronchi, edema of cell walls and surrounding tissue, and the secretion of cytokines and chemokines, leading to bronchial epithelial hyperplasia and necrosis. Inflammation, necrotic epithelium, and mucus secretion can cause obstruction of the bronchial tubes.9
This report describes a pediatric patient with macrolide-resistant Mycoplasma pneumoniae infection accompanied by necrotizing bronchitis. Timely bronchoscopic lavage and appropriate treatment are crucial. Although macrolide drugs may offer some benefits even in cases of macrolide resistance, the use of alternative antibiotics should be considered based on the severity of the disease,10 some antibiotics are contraindicated in children. For example, fluoroquinolones can negatively influence the growth of bones and articular cartilage, and tetracyclines may cause enamel hypoplasia and exert adverse effects on the bones.
Conclusion
In conclusion, necrotizing bronchitis can be caused by M. pneumoniae without bacterial co-infection. Gradual aggravation of symptoms and hypoxemia in patients with M. pneumoniae pneumonia may result in complicated airway obstruction or necrotizing bronchitis. Bronchoscopy has diagnostic and therapeutic applications in patients with necrotizing bronchitis. It can help clarify the etiology. Bronchoalveolar lavage fluid (BALF) can be analyzed using targeted next-generation sequencing to identify pathogen biology, remove necrotic tissue from blocked air ducts, and maintain airway patency.8,11,12 Critically ill patients with drug-resistant M. pneumoniae infections and those with signs of clinical deterioration may benefit from switching antibiotics or from an appropriate period of corticosteroid treatment.10
The exact mechanism of action underlying the development of necrotizing bronchitis remains unclear. Clinical manifestations are often non-specific, and the diagnosis is based on bronchoscopy. Moreover, The outbreak of drug-resistant M. pneumoniae infections world. In China, resistance rates exceeded 80% in 2023.13 This rise in resistance has led to an increase in patients with severe infections. The number of patients with necrotizing bronchitis caused by M. pneumoniae is expected to grow in the future.
Abbreviations
MP, Mycoplasma pneumoniae; BALF, bronchoalveolar lavage fluid.
Ethical Statement
The authors are responsible for all aspects of the work and ensure that issues related to the accuracy or completeness of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the guidelines of the Declaration of Helsinki (revised 2013). This study was approved by the Ethics Committee of Jiashan First People’s Hospital (NO:2024-070). Acceptance number: LW2024028. Upon review of the institutional policies and ethical guidelines, institutional approval was not deemed necessary for the publication of this case as it adheres to ethical standards, including obtaining written informed consent from the patient’s parents.
Consent for Publication
Written informed consent for publication of her clinical details and clinical images was obtained from the patient’s parents.
Acknowledgments
This is a short text to acknowledge the contributions of specific Guizhi Shi MD, Yiqun Teng MD that aided the efforts of the authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Linde A, Ternhag A, Torner A, Claesson B. Antibiotic prescriptions and laboratory-confirmed cases of Mycoplasma pneumoniae during the epidemic in Sweden in 2011. Euro Surveill. 2012;17(6):20082. doi:10.2807/ese.17.06.20082-en.
2. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17(4):697–728. doi:10.1128/CMR.17.4.697-728.2004.
3. Wang RS, Wang SY, Hsieh KS, et al. Necrotizing pneumonitis caused by Mycoplasma pneumoniae in pediatric patients: report of five cases and review of literature. Pediatr Infect Dis J. 2004;23(6):564–567. doi:10.1097/01.inf.0000130074.56368.4b. PMID 15194841.
4. Tsai TA, Tsai CK, Kuo KC, Yu HR. Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J Microbiol Immunol Infect. 2021;54(4):557–565. doi:10.1016/j.jmii.2020.10.002. PMID 33268306.
5. Arruda BL, Kanefsky RA, Hau S, Janzen GM, Anderson TK, Vincent Baker AL. Mucin 4 is a cellular biomarker of necrotizing bronchiolitis in influenza A virus infection. Microbes Infect. 2023;25(7):105169. doi:10.1016/j.micinf.2023.105169. PMID 37295769.
6. Takahashi S, Nakamura M. Necrotizing tracheobronchitis caused by influenza and Staphylococcus aureus co-infection. Infection. 2018;46(5):737–739. doi:10.1007/s15010-018-1180-y. PMID 30027437.
7. Bua J, Trappan A, Demarini S, Grasso D, Schleef J, Zennaro F. Neonatal necrotizing tracheobronchitis. J Pediatr. 2011;159(4):699–699.e1. doi:10.1016/j.jpeds.2011.04.043. PMID 21683370.
8. Matsui J, Nakahara S, Kikuoka N, et al. Efficacy of bronchial fiberscope in esophageal fistula caused by necrotizing bronchitis. Pediatr Int. 2014;56(1):105–107. doi:10.1111/ped.12174. PMID 24548195.
9. Cimolai N, Taylor GP, Mah D, Morrison BJ. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol Immunol. 1992;36(5):465–478. doi:10.1111/j.1348-0421.1992.tb02045.x. PMID 1513263.
10. Principi N, Esposito S. Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J Antimicrob Chemother. 2013;68(3):506–511. doi:10.1093/jac/dks457. PMID 23169891.
11. Chang J, Kim TO, Yoon JY, et al. Necrotizing tracheobronchitis causing airway obstruction complicated by pandemic 2009 h1N1 influenza: a case report. Medicine. 2020;99(1):e18647. doi:10.1097/MD.0000000000018647. PMID 31895828.
12. Lei W, Fei-Zhou Z, Jing C, Shu-Xian L, Xi-Ling W, Lan-Fang T. Pseudomembranous necrotizing laryngotracheobronchitis due to Mycoplasma pneumoniae: a case report and literature review. BMC Infect Dis. 2022;22(1):183. doi:10.1186/s12879-022-07160-5. PMID 35197010.
13. Meyer Sauteur PM, Beeton ML, Pereyre S. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Mycoplasma and Chlamydia Infections (ESGMAC), and the ESGMAC Mycoplasma pneumoniae Surveillance (MAPS) study group. Pneumonia outbreaks due to re-emergence of Mycoplasma pneumoniae. Lancet Microbe. 2024;5(6):e514. PMID:38342111. doi:10.1016/S2666-5247(23)00406-8
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

