Back to Journals » Orthopedic Research and Reviews » Volume 17
Network Pharmacology and Experimental Validation of the Therapeutic Effect of Baji Capsule on LPS-Induced Osteoporosis
Authors Li Q, Li D, Tian C, Liu X, Wang H, Liu H
Received 13 September 2024
Accepted for publication 22 January 2025
Published 11 February 2025 Volume 2025:17 Pages 61—81
DOI https://doi.org/10.2147/ORR.S488478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Clark Hung
Qian Li,1 Dinglin Li,1 Ciqiu Tian,2 Xiangjie Liu,1 Hui Wang,1 Hao Liu1
1Liyuan Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Tongji Medical College of Science and Technology, Wuhan, People’s Republic of China; 2Hubei University of Chinese Medicine, Wuhan, People’s Republic of China
Correspondence: Hao Liu, Liyuan Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology, Tongji Medical College of Science and Technology, Wuhan, People’s Republic of China, Email [email protected]
Purpose: Osteoporosis is a common skeletal disease characterized by impaired bone microarchitecture, decreased bone mineral density and increased bone fragility, leading to a heavy physical and economic burden due to its greatly increased risk of fracture. Baji capsule is a proprietary medicine that can treat menstrual disorders and postmenopausal osteoporosis. However, the efficacy of Baji capsule has not been reported for osteoporosis caused by oxidative stress and inflammation. Therefore, the aim of this study was to evaluate whether Baji capsule has a therapeutic effect on lipopolysaccharide (LPS)-induced inflammatory osteoporosis and to explore the underlying mechanisms through network pharmacology.
Patients and Methods: Osteoporosis model in ICR mice induced with LPS. Mice were treated with vitamin E (100mg/kg), PBS, high-dose Baji capsule (810mg/kg) and low-dose Baji capsule (90mg/kg), respectively. The therapeutic effect of Baji capsule was evaluated by high-resolution micro-computed tomography (Micro-CT) and tissue section staining, serum inflammatory factor levels were assessed by ELISA, serum oxidative stress-related marker levels were determined by kits, and finally the mechanism was explored by network pharmacology and then verified by immunohistochemistry.
Results: Micro-CT results showed that Baji capsule attenuated LPS-induced inflammatory bone loss.Baji capsule also reduced serum inflammatory factor levels and oxygen free radical production. Target screening by network pharmacology yielded a total of 236 active ingredients of Baji capsule, as well as 278 common targets after taking the intersection of Baji capsule active ingredient targets and osteoporosis disease-related targets.
Conclusion: Baji capsule can treat osteoporosis by reducing inflammation and oxidative stress. The therapeutic effects of Baji capsule were shown to be multi-targeted and multi-pathway through network pharmacology. In the future, its anti-inflammatory and antioxidant properties can be utilized to further explore its therapeutic effects on inflammatory diseases, as well as a prospective study for the clinical treatment of osteoporosis.
Keywords: inflammatory, oxidative stress, osteoporosis, traditional Chinese medicine, network pharmacology
Introduction
Osteoporosis is a chronic metabolic disease characterized by impaired bone microarchitecture, decreased bone mineral density and increased bone fragility.1 The prevalence of osteoporosis is high, affecting approximately 75 million people worldwide, including 10 million women.2 Each year, the US healthcare system spends roughly $7 billion on osteoporotic fractures, and by 2040, the annual cost is projected to approach $50 billion. And this economic burden is similar across the globe.3
Currently, the clinically drugs used to treat and prevent osteoporosis are bisphosphonates, parathyroid hormone analogs, and selective estrogen receptor modulators. However, in addition to different degrees of side effects,4–6 these drugs are more effective in osteoporosis caused by estrogen aging, deficiency, and reduced mechanical weight-bearing, and lack specificity for inflammation and oxidative stress-induced osteoporosis. LPS is a component of the outer membrane of Gram-negative bacteria and has been proven to induce inflammation.7,8 LPS induces bone loss by stimulating osteoclast bone resorption and inhibiting osteogenic differentiation of osteoblasts.9 LPS can inhibit osteoblast differentiation by affecting various pathways such as Notch signaling, BMP/Smad signaling and Wnt/β-catenin signaling.10–12 In addition, in vitro experiments demonstrated that LPS can stimulate the intracellular production of excess reactive oxygen species leading to oxidative stress.13 Therefore, we used LPS to create a mouse model of osteoporosis and to explore effective therapies against bacterial-induced bone destruction.
Traditional Chinese medicine (TCM) has been widely used because of its long history, small side effects, most of the raw materials from natural plants and other wonderful characteristics, and now there are a number of Chinese medicines with Chinese medicine extracts have been proved to be able to treat osteoporosis, and has shown good efficacy.14,15 Baji capsule is a proprietary Chinese medicine containing 16 natural herbs: Radix Morindae Officinalis, Polygonum multiflora, Cortex Eucommiae, Herba Cistanches,Dipsaci Radix, Curculiginis Rhizome, Fructus Rosae Laevigatae, Herba Epimedii, Rubus idaeus Linn, Radix Angelicae Sinensis,Codonopsis Radix, Radix Rehmanniae Praeparatae, Lycii Fructus, Radix Glycyrrhizae, Radix Astragali, Rhizoma Cibotii. Among them, the Radix Morindae Officinalis, Cortex Eucommiae, Herba Epimedii,Curculiginis Rhizome, etc. have been confirmed in the literature to be able to reduce the level of serum inflammatory factors and regulate the balance between osteogenesis and osteoblastogenesis thereby treating osteoporosis,16–20 and there are also reports on the therapeutic effect of Baji capsule on the rats model of ovariectomized osteoporosis, but the therapeutic mechanism, active ingredients and molecular targets have not been elucidated.21
Network pharmacology can exhaustively elucidate the molecular mechanisms of TCM in the treatment of various diseases through its key concept of “network target, multi-component” model.22,23 So far, the therapeutic effects of various Chinese medicinal preparations on osteoporosis have been demonstrated by means of network pharmacology, such as Zhuanggu Busui Formula,24 Liuwei Dihuang pill25 and Zhuangguguanjie Formulation.26 Therefore, we also exploited network pharmacology to investigate the route, target, and active ingredient of Baji capsule for its anti-osteoporotic effects.
Materials and Methods
Animals
Animal experiments were supervised and approved by the Animal Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. All the methods were performed in accordance with relevant guidelines and regulations. All the methods were performed in accordance with NC3Rs ARRIVE guidelines. Twenty-five ICR mice (females, 8 weeks) were purchased from Wuhan Hualianke Biotechnology Co. Ltd (Hubei, China) and housed in the Animal Center of Liyuan Hospital, affiliated with Tongji Medical College, Huazhong University of Science and Technology, all of them were maintained in an environment with a 22°C and a 12-hour circadian rhythm.
Animal Models of Osteoporosis
LPS (derived from E. coli 055:B5) was purchased from Sigma-Aldrich, Baji capsule was purchased from Chongqing Xieran Pharmaceutical Co. Ltd, vitamin E,Tween 80 and 4% paraformaldehyde solution were purchased from Wuhan Hualianke Biotechnology Co. Ltd. The powder inside the Baji capsule was removed and dissolved in PBS to make Baji capsule solutions (BJT) of different concentrations. Twenty-five 8-week-old female ICR mice were randomly divided into three treatment groups, namely, the high-concentration BJT group (BJTH), the low-concentration BJT (BJTL) group and the vitamin E group (vitE); as well as the LPS-induced osteoporosis model (LPS) group and the untreated blank control group, with five mice in each group. LPS powder was dissolved in PBS solution and then injected intraperitoneally at a dose of 5 mg/kg into mice in the BJTH, BJTL, vitE and LPS groups on days 0 and 4, whereas the blank control group was injected intraperitoneally with PBS. High-concentration BJT (810 mg/kg), low-concentration BJT (90 mg/kg), and vitamin E (100 mg/kg, dissolved in 0.1% Tween 80) were administered by gavage to the BJTH, BJTL, and vitE groups, respectively, starting on day 0, and continued for 8 days; PBS was gavaged to both LPS and control groups. Gavage was performed at 0.1 mL /10 g body weight. At the end of modeling, all mice in this study were anaesthetised with the inhalational anaesthetic isoflurane, and the mice were anaesthetised and died after ocular blood sampling and autopsy operations.
The collected femurs were fixed in 4% paraformaldehyde for three days and then were scanned and analyzed by micro-CT (SkyScan 1176, Medical sub-Center of Analytical and Testing Center, Huazhong University of Science and Technology, China) to evaluate the bone-trabecular microstructure. Bone volume as a percentage of single bone tissue (BV/TV), trabecular number (Tb.N)., trabecular thickness (Tb.Th)., and trabecular separation (Tb.Sp)., were obtained.
Assay for Serum Chemistry
Serum collected by centrifugation was measured by standard colorimetric methods using commercial kits for alkaline phosphatase (ALP), malondialdehyde (MDA) and catalase (CAT) (Nanjing Jiancheng Bioengineering Institute, China). Serum tumor necrosis factor (TNF-α) and interleukin 6 (IL-6) were measured by enzyme immunoassay using commercial kits (Wuhan Bioswamp Biotechnology Co., Ltd.) according to the manufacturer’s instructions.
Histomorphometric Analysis
Mouse femurs were fixed with 4% paraformaldehyde in for three days at room temperature and decalcified using EDTA decalcification solution (Wuhan servicebio Biotechnology Co., Ltd.) for one month at room temperature, then the tissue samples were embedded in paraffin wax and sectioned in 5μm thick slides. The slides were processed for hematoxylin and eosin (H&E) and tartrate-resistant acid phosphatase (TRAP) staining. Under the microscope, with the femoral head in the centre, select areas in the subchondral region and near the centre point, and the number of bone traps and empty bone traps were counted in each region to obtain the empty bone trap rate. In addition, the number of osteoclasts stained red was counted in each region.
Network Pharmacology
BJT active compounds and targets were screened using the Traditional Chinese Medicine System Pharmacology Database and Analysis Platform (TCMSP, https://tcmspw.com/tcmsp.php). Oral bioavailability (OB ≥ 20%) and drug-likeness (DL ≥ 0.1) were used as the criteria for screening the active compounds. The chemical information repository PubChem (https://pubchem.ncbi.nlm.nih.gov) was used to find the Canonical SMILES of the screened active compounds,27 and then the obtained Canonical SMILES were analyzed using SwissTargetPrediction (STP) (http://www.swisstargetprediction.ch/) to screen the potential target genes of the active compounds.28 Osteoporosis disease targets were obtained by searching the GeneCards (https://www.genecards.org/) and OMIM (https://omim.org/) databases with the keyword “osteoporosis”.29
The STRING database (https://www.string-db.org/) was utilized to screen the active ingredients of Baji capsule and the targets corresponding to osteoporosis diseases according to the criterion of combined score >0.90, and the chosen target proteins were restricted to Homo sapiens. Cytoscape is a platform for integrating molecular interaction network data.30 The intersection of the screened targets was then input into Cytoscape (https://cytoscape.org/) to build a PPI network of drug and disease targets.
DAVID (https://david.ncifcrf.gov/) is a Web-based online database available as a bioinformatics resource for interpreting a large number of gene/protein functions.31 DAVID was used to implement gene ontology (GO) enrichment analysis and KEGG enrichment analysis of intersection targets. GO enrichment analysis was used to explain three biological processes including molecular function (MF), biological process (BP), and cellular component (CC).32 While KEGG enrichment analysis was used to systematically analyze gene functions.33
Molecular Docking
The targets related to inflammation and oxidative stress were found in DAVID, and then the cross-targets with the top 20 CytoNCA scores in the PPI network of drug and disease targets were cross-targeted with the targets related to inflammation and oxidative stress to obtain the key targets, ie, STAT3 and AKT1. Prior to molecular docking, we validated the expression of key targets in bone tissue based on the Human Protein Atlas (HPA, https://www.proteinatlas.org/) to determine whether they were expressed in bone tissue. Next, using PyMoL-2.6.0 and AutoDockTools-1.5.7 software, classical molecular dynamics was used to analyze the binding sites, binding affinities, and interactions between STAT3 and AKT1 and the active ingredients of Baji capsule with CytoNCA scores ranked among the top 35 active ingredients. Crystal structures of key intersecting proteins were obtained from the RCSB Protein Data Bank (PDB, http://www.pdb.org/).34 Mol2 format files of Baji cansule active compounds were downloaded through the TCMSP database. The co-crystallized ligands isolated from the receptors and the active pockets of each key intersecting proteins were probed using AutoDockTools-1.5.6. The molecular docking of active compounds to key targets and the determination of their free binding energies were realized by aotodock. PyMOL and was used for the interaction and binding of active compounds mode visualization and analysis.
Immunohistochemical Analysis
Femurs were fixed, decalcified, wax-packed, and sectioned and the slides were incubated at 37°C for 1 hour with anti-STAT3 and anti-AKT1 (proteintech. Wuhan Three Eagles Biotechnology Co., Ltd). The slides were incubated with HRP-coupled secondary antibody for 30 min and under microscopic observation, ImageJ software was employed to analyze the average optical density (AOD) at 100x magnification.
Statistical Analysis
All data were analyzed using SPSS software (version 23.0), and the results are expressed as mean ± standard deviation. We used t-tests to analyze differences between two groups, and ANOVA to analyze differences between multiple groups. P-value <0.05 was considered statistically significant.
Results
Effect of Baji Capsule on Morphology and Parameters of Bone Tissue in Mice
Figure 1A and B show typical 3D images of the femur Micro-CT scans of rats in each experimental group. It can be seen that mice in the LPS group had a large number of cavities in the femoral diaphysis and poor continuity of trabecular. Compared with the mice in the control group, the mice in the LPS group had lower relative bone volume, BV/TV and the Tb.N decreased. In additional, the Tb.Th decreased, Tb.Sp increased. The aqueous solution of Baji capsule improve the bone microstructure changes of bone trabecular in osteoporotic mice and reversed BV/TV, Tb.N, Tb.Th and Tb.Sp. But the trends of Tb.Th and Tb.Sp was not significant and not statistically significant (Figure 1C-F, p<0.05; p<0.05; p>0.05; p>0.05). The BJTH group even had a more significant intervention effect than the vitE group, which was the standard medication group. The BJTH group showed a more significant improvement in bone volume, increasing the area covered by trabecular and the Tb.N.
Chemical Indicators for Serum Testing
Serum levels of the inflammatory factors TNF-α and IL-6 were significantly increased in the LPS group of mice compared with the control group (both p<0.001; Figure 2A–B). In contrast, BJTH, BJTL and vitE treatments all significantly inhibited the LPS-induced increase in serum TNF-α and IL-6 levels (both p<0.001; Figure 2A–B). When the endogenous system fails to provide sufficient antioxidant capacity, oxygen free radicals will lead to lipid peroxidation and MDA accumulation.35 Therefore, MDA level can reflect the level of oxidation in the body.36 Figure 2C shows that MDA levels in serum were higher in the LPS group than in the control group (p<0.01), whereas BJTH treatment significantly reduced MDA levels (p<0.05).Catalase (CAT) is an antioxidant enzyme whose activity reflects the body’s antioxidant capacity.37 LPS intraperitoneal injection decreased the level of CAT activity in serum (Figure 2D, p<0.001).BJTH, BJTL and vitE treatment all significantly increased the level of CAT activity in serum (Figure 2D, p<0.001, p<0.01, p<0.01). ALP is an incipient marker of osteoblast differentiation, and intraperitoneal injection of LPS significantly reduced serum ALP levels in mice (Figure 2E, p<0.01), while BJTH and BJTL treatments significantly reversed serum ALP levels (p<0.05, p<0.05, Figure 2E). Curiously, mouse serum ALP levels were not significantly different between the vitE-treated group and the LPS modeling group.
Morphologic Changes in Bone Tissue and Immunohistochemical Verification of Key Genes Expression in Bone Tissue
Histologic evidence of H&E staining demonstrated intact trabecular structure and morphology, clearly visible osteocytes within the trabecular, and fewer bone traps in the control group.In the LPS group, the number of trabecular in the femur was reduced and structurally disorganized, with a significant increase in empty bone trabecular. In the BJTH group, the pathologic changes in the trabecular structure were reduced, most osteocytes were normal, and there were not many empty bone sockets. The BJTL and vitE groups also reduced the pathologic changes in the bone tissue to different degrees, but to a lesser extent than the BJTH group (Figure 3A). Moreover, quantitative analysis of TRAP staining showed a significant increase in the number of osteoclasts in the LPS group compared to the control group (p<0.05) (Figure 3B). Then, we used immunohistochemical staining to verify the expression of STAT3 and AKT1. The staining sites of STAT3 and AKT1 (Figure 3C–D) were concentrated in the femoral neck and diaphysis. Immunohistochemical stained areas were quantified by comparing optical density values. As shown in Figure 3E and F, LPS intraperitoneal injection significantly inhibited the expression of AKT1 and STAT3 in mice, whereas vitE, BJTH, and BJTL all improved the inhibitory effect of LPS on the expression of AKT1 and STAT3 to varying degrees, of which the improvement effect of BJTH treatment was the most pronounced (p<0.05). However, BJTL treatment did not seem to prevent the inhibitory effect of LPS intraperitoneal injection on AKT1 expression. Treatment with BJTH significantly reduced the number of osteoclasts (p<0.05) (Figure 3 G).
Screening, Collecting Active Compounds and Building Compound-Disease-Target Networks
The obtained interpretation relationships and classifications of 16 herbal medicines, 145 active ingredients and 278 cross-targeting annotations were loaded into Cytoscape software, and the active ingredient target network of herbal medicines of Baji capsule for the treatment of osteoporosis was mapped (Figure 4). The targets corresponding to Baji capsule and osteoporosis respectively were obtained by STRING by setting the interaction score to the highest confidence level (0.9). Figure 4 shows the targets corresponding to the 145 active ingredients of Baji capsule and Figure 5 shows the targets corresponding to osteoporosis disease, and the Venn diagram was constructed by taking the intersection of the two quantities after counting them (Figure 6A). Then, the intersection targets were transferred into Cytoscape software to draw the PPI interaction diagram (Figure 6B). Finally, two PPI diagrams were plotted in order to clearly show the relationship between drug composition, active ingredients and target interactions. Figure 7 represents the active ingredients corresponding to the 16 constituent drugs of Baji capsule. The correlation between the 145 active ingredients and the protein targets is shown in Figure 8.
GO Enrichment Analysis and KEGG Pathway Analysis of Baji Capsule With Osteoporosis Intersection Targets
This partial intersection of targets with targets related to inflammation and oxidative stress was first taken one more time to obtain the core targets. We then performed GO enrichment analysis (p<0.05) on the core targets using the DAVID database and its extension package, and visualized the first 8 items enriched for BP, CC and MF (Figure 9A-C). The results indicate that BP is mainly related to the response to chemicals, organic substrates and oxygen-containing compound, etc. CC mainly includes the plasma membrane, extracellular space and cell surface, etc. MF mainly includes enzyme binding, signaling receptor binding, identical protein binding, and molecular transducer activity, etc.
To further understand the potential pathways of Baji capsule for osteoporosis, we performed KEGG pathway enrichment analysis (p<0.05). And the top 8 pathways with the most statistically significant were visualized, including the pathway in cancer, proteoglycans in cancer, PI3K-Akt signaling pathway, and MAPK signaling pathway (Figure 9D). The connectivity between the core targets and the active ingredients of Baji capsule was calculated and analyzed using cytoNCA in the Cytoscape software, and the two most highly correlated targets, STAT3 and AKT1, were identified.
Molecular Docking Validation
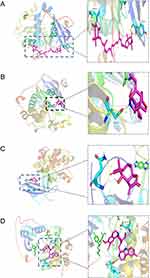
Two key targets (STAT3 and AKT1) screened in the PPI network analysis were molecularly docked with the top 35 active ingredients in the cytoNCA “degree” of the active ingredient target network.In order to analyse whether the key targets could play a role in osteoporosis, we first examined the expression of the key targets recorded in the HPA database before molecular docking and presented them as histograms. The results showed that STAT3 and AKT1 were moderately expressed in bone marrow (Figure 10A and B). The binding energy of ≤-5 kcal/mol was a criterion that an active ingredient could be docked to key targets.38 Therefore, molecular docking was performed several times to screen out the active ingredients that could be docked to key targets. We describe the names and binding energies of the active ingredients screened for their alignment to key targets uniformly in Table 1. Finally, we visualized the seven pairs of active compounds that could bind to the key targets by PyMOL software. Among them, Chrysanthemaxanthin was docked to residues ASN-204, TRY-175 of AKT1 through hydrogen bonding; 11-Hydroxyrankinidine was docked to residue GLU-341 of AKT1 through hydrogen bonding; Helenalin was docked to residue GLN-43 of AKT1 through hydrogen bonding; Marckine was docked to residue ASP-323 and GLU-355 of AKT1 through hydrogen bonding (Figure 11A–D). In addition, Friedelin was docked to residue ASN-647 of STAT3 via hydrogen bonding; Frutinone A was docked to residue ASP-369, LYS-370 and THY-440 of STAT3 via hydrogen bonding; 2-Hydroxyanthraquinone was docked to residue TYR-640 of STAT3 via hydrogen bonding. (Figure 12A–C).
 |
Table 1 Pairing of Molecular Docking Active Ingredients With Key Proteins |
Discussion
LPS-induced inflammation and oxidative stress promote bone resorption and lead to bone loss in animals.39,40 Effective treatment for bacterial-induced bone destruction is limited to the use of antibiotics and surgery[18], but the resulting antibiotic resistance and surgical intolerance as well as the poor efficacy of traditional anti-osteoporotic medications have prompted the search for safer and more effective treatment modalities. In our experiments, we inferred that LPS-induced osteoporosis was associated with increased serum levels of pro-inflammatory factors and oxidative stress; we also demonstrated that Baji capsule counteracted LPS-induced bone loss by promoting STAT3 and AKT1 expression.
Bone tissue structure is an instrumental criterion for assessing bone health.41 In this research, bone micro-CT and bone histopathologic techniques were applied to assess bone structure. Our experimental results showed that both vitE and BJTH treatments improved the bone microstructure changes of bone trabecular in osteoporotic mice and reversed the alterations in parameters such as BV/TV, Tb.N. The results imply that Baji capsule improved LPS-induced bone microstructural damage and was effective in restoring bone morphologic parameters, but it must be instituted with a BJTH treatment, and BJTL treatment cannot achieve the desired results.
Oxidative stress and inflammation are drivers of bone loss.42 Many studies have illustrated that inflammatory cytokines such as TNF-α and IL-6 promote osteoclastogenesis and activation while inhibiting osteoblast proliferation.43–46 Excessive accumulation of reactive oxygen species (ROS) hinders osteoblast proliferation and differentiation; enhances osteoclast differentiation and ultimately leads to more bone resorption.47,48 Moreover, osteoblast death is induced in the presence of dramatically increased ROS levels, leading to structural bone damage and decreased bone density.49 In this study, we found that compared to the LPS group, CAT and ALP activity levels were up-regulated in Baji capsule aqueous solution administration groups; MDA, IL-6 and TNF-α activity levels were decreased. This suggests that Baji capsule can inhibit inflammation and oxidative stress induced by intraperitoneal injection of LPS. Curiously, mouse serum ALP levels were not significantly different between the vitE-treated group and the LPS modeling group, which may be caused by the toxicity of Tween 80, the organic solvent used to dissolve vitE. Furthermore, Intraperitoneal injection of LPS significantly increased the number of osteoclasts in bone tissue, whereas BJTH treatment inhibited the pro-osteoclastogenic effect of LPS. It suggests that at high concentrations of Baji capsule may treat osteoporosis by inhibiting osteoclast formation and promoting osteoclastogenesis.
The study demonstrated the anti-inflammatory, anti-oxidative stress and thus anti-osteoporotic effects of Baji capsule and explored the underlying mechanisms through network pharmacology. The active compounds that can interface with the key targets include: Friedelin, Frutinone A, 2-Hydroxyanthraquinone, Chrysanthemaxanthin, 11-Hydroxyrankinidine, Helenalin and Marckine. Friedelin, a triterpenoid derived from Codonopsis Radix, has demonstrated promising free radical scavenging, anti-inflammatory, and antioxidant properties in animal models.50 In addition, friedelin induces the expression of collagen type X (COL-10), a homotrimeric collagen associated with chondrocyte hypertrophy that promotes cartilage calcification, in mesenchymal stromal stem cell (MSC)[51]. Chondrogenic hypertrophy is a process by which chondrocytes in the central areas increase in size and become hypervascularized during calcification, a process that is accompanied by the differentiation of bone progenitor cells into osteoblasts and the replacement of cartilage by mineralized bone tissue.51 This means that friedelin has the ability to indirectly promote osteogenesis by promoting chondrocyte calcification. Frutinone A is a coumarin derived from Codonopsis Radix. Studies have demonstrated that coumarins exhibit many pharmacological characteristics such as antioxidant, anti-inflammatory and anti-tumor properties.52 Furthermore, some studies have shown that coumarin has an inhibitory effect on osteoclast activity53 and also promotes osteogenic differentiation of stem cells.54 2-hydroxyanthraquinone is an anthraquinone compound derived from Radix Morindae Officinalis. Although 2-hydroxyanthraquinone has not been successfully isolated from Radix Morindae Officinalis, anthraquinones are thought to have anti-inflammatory, anti-osteoporotic and other biological activities.55 Chrysanthemaxanthin and 11-Hydroxyrankinidineis are also active constituents of Codonopsis Radix. Although its pharmacological properties have not been reported, Codonopsis Radix extracts have been demonstrated to have anti-inflammatory, antioxidant, and anti-osteoporotic effects in various studies.56–58 Nuclear factor-kappa B (NF-κB) is a transcription factor that controls cell proliferation and differentiation, and when NF-κB is activated and translocated to the nucleus, it can up-regulate the transcription of relevant genes involved in inducing osteoclast differentiation.59,60 Activation of nuclear factor erythroid type 2-associated factor (Nrf2) binds to and promotes the expression of promoters of certain antioxidant genes, thereby enhancing the body’s antioxidant defense.61 Helenalin is a natural active ingredient derived from Cortex Eucommiae. Helenalin analogs have been reported to activate the Nrf2 pathway and specifically inhibit NF-κB activation.62,63 Marckine (Tubulosine) is an active component of the natural plant Herba Cistanches, belonging to the isoquinoline alkaloids group.64 We know that revitalisation of the inducible nitric oxide synthase (iNOS) pathway facilitates inflammatory induced osteoporosis through repressing bone formation and contributing to osteoblast apoptosis.65 And isoquinoline alkaloids can suppress iNOS expression by inhibiting NF-κB activation and thus may help to combat inflammation-induced osteoporosis.65
Runt-related transcription factor 2 (Runx2) is a key transcription factor in the differentiation of bone marrow mesenchymal stem cells (BMSCs) into osteoblasts.66 STAT3 is a key signaling protein that integrates a variety of cytokines, growth factors, and oncoproteins.67 STAT3 is activated by phosphorylation and transported to the nucleus where it combines with the Runx2 promoter and augments its transcriptional capacity, thereby stimulating the differentiation of BMSCs into osteoblasts.68 In addition, it has been reported that STAT3 deficiency attributes to skeletal defects including craniofacial deformities, osteoporosis and spontaneous fractures, and inhibits osteoblast differentiation.69 In contrast, high expression promotes osteoblast formation and thus increases bone mass.70–72 Akt is an serine/threonine kinase that is a central transducer of the PI3K-Akt signaling pathway,73 and its isoform, Akt1, is expressed in a variety of tissues.74 Activation of AKT1 by phosphorylation activates a variety of downstream substrates, which inhibits apoptosis and promotes cell survival.75 It has been shown that stimulating AKT1 expression can inhibit glucocorticoid-induced apoptosis of osteoblasts, thereby attenuating glucocorticoid-induced osteoporosis.76 It is well known that macrophages are heavily polarised towards M1 during the early inflammatory phase, whereas macrophage M2 polarisation facilitates inflammatory abatement and matrix maturation.77,78 Previous studies have shown that AKT1 promotes macrophage M2 polarisation.79 This can be deduced that AKT1 has a certain anti-inflammatory capacity by promoting macrophage M2 polarisation, thus inhibiting osteoporosis.
The results of KEGG enrichment analysis indicated that the active ingredients of Baji capsule may exert their curative effects on osteoporosis by modulating multiple pathways. The pathways were predominantly enriched in the pathway in cancer, proteoglycans in cancer, PI3K-Akt signaling pathway, MAPK signaling pathway and AGE-RAGE signaling pathway in diabetic complications, etc. It has been demonstrated that binding of the receptor for nuclear factor kappa-B (RANK) on the surface of osteoclast precursor cells to the receptor activator of nuclear factor kappa-B ligand (RANKL) present in stromal cells stimulates osteoclast differentiation and activation through activation of the downstream P13K/AKT pathway.80–82 Furthermore, activated PI3K/AKT signaling also promotes RANKL expression in osteoblasts and regulates the differentiation of mesenchymal stromal cells to osteoblasts.83,84 RANK binding to RANKL also activates the MAPK pathway, which regulates osteoclast differentiation and osteoclast precursor proliferation and apoptosis.85 Moreover, activation of the MAPK pathway promotes osteogenic differentiation and matrix mineralization.86,87 Advanced glycation endproduct (AGE) and AGE receptors (RAGE) bind to each other to stimulate IL-6 production by osteoblasts, inhibit osteoblast phenotypic expression and bone matrix mineralization, and ultimately increase osteoclast resorption.88,89 Type I collagen promotes the progression of an osteoblast phenotype, but it has been shown that collagen osteoblasts modified by AGE are inhibited in function and bone nodule formation, ultimately affecting osteoblast differentiation.90 Collectively, each of these pathways regulates bone homeostasis by modulating the balance between osteoclasts and osteoblasts, and when these pathways are inhibited or activated, an imbalance in bone homeostasis causes the development of osteoporosis.
As we expected, Baji capsule had a therapeutic effect on LPS-induced osteoporosis in mice and could improve the bone microstructural disorders caused by LPS. We found that Baji capsule declined serum IL-6, TNF-α, and MDA levels, implying that Baji capsule can modulate oxidative stress and inflammation-related pathways. In animal experiments, LPS mice were intraperitoneally injected to establish an osteoporotic mouse model, micro-CT was performed to detect bone microstructural changes in mice, serum inflammation, oxidative stress biomarkers and osteogenesis-related markers were detected, and the number of TRAP-positively stained cells in each mouse femur section was counted. Meanwhile, the underlying mechanism of Baji capsule against osteoporosis was explored by network pharmacology. Finally, the key targets predicted by network pharmacology were validated by immunohistochemistry. However, the therapeutic effects and extraction process of some active ingredients screened by network pharmacology were rarely reported and lacked some background support. There was also no correlation study along with in vitro experiments, which are essential to validate and explore more accurate pharmacological effects and mechanisms of Baji capsule.
Conclusion
Baji capsule had a therapeutic effect on LPS-induced osteoporosis in mice and could improve the bone microstructural disorders caused by LPS. Network pharmacological analysis indicated that Baji capsule exerts its therapeutic effects through multi-targets, multi-components, and multi-pathways.The key targets for osteoporosis treatment with Baji capsule may be STAT3 and AKT1, and the potential mechanism may be linked to cancer pathway the regulation of pathway in cancer, proteoglycans in cancer, PI3K-Akt signaling pathway, MAPK signaling pathway, the AGE-RAGE signaling pathway in diabetic complications, and so on. Our study provides basic evidence for osteoporosis treatment with Baji capsule and provides a new drug candidate for the treatment of osteoporosis.
Acknowledgments
We sincerely thank the Medical sub-Center of Analytical and Testing Center, Huazhong University of Science and Technology for providing the instrumentation of micro-CT and the training of the experimental operation. All information on human data from publicly available databases was ethically approved by the Ethics Committee of Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li L, Chen B, Zhu R, et al. Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and Sirt6 levels in aging mice. Aging. 2019;11(21):9348–9368. doi:10.18632/aging.102376
2. Schuiling KD, Robinia K, Nye R. Osteoporosis update. J Midwifery Women's Health. 2011;56(6):615–627. doi:10.1111/j.1542-2011.2011.00135.x
3. Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194(2 Suppl):S3–11. doi:10.1016/j.ajog.2005.08.047
4. Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi:10.1001/jama.288.3.321
5. Gyanewali S, Kesharwani P, Sheikh A, et al. Formulation development and in vitro-in vivo assessment of protransfersomal gel of anti-resorptive drug in osteoporosis treatment. Int J Pharm. 2021;608:121060. doi:10.1016/j.ijpharm.2021.121060
6. Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080–1092. doi:10.1016/S0140-6736(21)02646-5
7. Bohannon JK, Hernandez A, Enkhbaatar P, et al. The immunobiology of toll-like receptor 4 agonists: from endotoxin tolerance to immunoadjuvants. Shock. 2013;40(6):451–462. doi:10.1097/SHK.0000000000000042
8. Kassem A, Henning P, Lundberg P, et al. Porphyromonas gingivalis stimulates bone resorption by enhancing RANKL (Receptor Activator of NF-kappaB Ligand) through activation of toll-like receptor 2 in osteoblasts. J Biol Chem. 2015;290(33):20147–20158. doi:10.1074/jbc.M115.655787
9. Bandow K, Maeda A, Kakimoto K, et al. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem Biophys Res Commun. 2010;402(4):755–761. doi:10.1016/j.bbrc.2010.10.103
10. Daigang L, Jining Q, Jinlai L, et al. LPS-stimulated inflammation inhibits BMP-9-induced osteoblastic differentiation through crosstalk between BMP/MAPK and Smad signaling. Exp Cell Res. 2016;341(1):54–60. doi:10.1016/j.yexcr.2016.01.009
11. Xing Q, Ye Q, Fan M, et al. Porphyromonas gingivalis lipopolysaccharide inhibits the osteoblastic differentiation of preosteoblasts by activating Notch1 signaling. J Cell Physiol. 2010;225(1):106–114. doi:10.1002/jcp.22201
12. Tang Y, Zhou X, Gao B, et al. Modulation of Wnt/beta-catenin signaling attenuates periapical bone lesions. J Dent Res. 2014;93(2):175–182. doi:10.1177/0022034513512507
13. Wei L, Chen W, Huang L, et al. Alpinetin ameliorates bone loss in LPS-induced inflammation osteolysis via ROS mediated P38/PI3K signaling pathway. Pharmacol Res. 2022;184:106400. doi:10.1016/j.phrs.2022.106400
14. Lin J, Zhu J, Wang Y, et al. Chinese single herbs and active ingredients for postmenopausal osteoporosis: from preclinical evidence to action mechanism. Biosci Trends. 2017;11(5):496–506. doi:10.5582/bst.2017.01216
15. Jia Y, Sun J, Zhao Y, et al. Chinese patent medicine for osteoporosis: a systematic review and meta-analysis. Bioengineered. 2022;13(3):5581–5597. doi:10.1080/21655979.2022.2038941
16. MengYong Z, CaiJiao W, HuSheng Z, XianWu P, JianMin F, et al. Protective effect of polysaccharides from morinda officinalis on bone loss in ovariectomized rats. Int J Biol Macromol. 2008;43(3):276–278. doi:10.1016/j.ijbiomac.2008.06.008
17. Koh W, Shin J-S, Lee J, et al. Anti-inflammatory effect of cortex eucommiae via modulation of the toll-like receptor 4 pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Ethnopharmacol. 2017;209:255–263. doi:10.1016/j.jep.2017.08.001
18. Liu J, Li D, Sun X, et al. Icariine restores LPS-induced bone loss by downregulating miR-34c Level. Inflammation. 2016;39(5):1764–1770. doi:10.1007/s10753-016-0411-6
19. Zhu FB, Wang Jy, Zhang Yl, et al. Curculigoside regulates proliferation, differentiation, and pro-inflammatory cytokines levels in dexamethasone-induced rat calvarial osteoblasts. Int J Clin Exp Med. 2015;8(8):12337–12346.
20. Zhao L, Liu S, Wang Y, et al. Effects of curculigoside on memory impairment and bone loss via anti-oxidative character in APP/PS1 mutated transgenic mice. PLoS One. 2015;10(7):e0133289. doi:10.1371/journal.pone.0133289
21. Li Y, Lü -S-S, Tang G-Y, et al. Effect of Morinda officinalis capsule on osteoporosis in ovariectomized rats. Chin J Nat Med. 2014;12(3):204–212. doi:10.1016/S1875-5364(14)60034-0
22. Liu ZW, Luo Zh, Meng Qq, et al. Network pharmacology-based investigation on the mechanisms of action of Morinda officinalis how. in the treatment of osteoporosis. Comput Biol Med. 2020;127:104074. doi:10.1016/j.compbiomed.2020.104074
23. Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):110–120. doi:10.3724/SP.J.1009.2013.00110
24. Zhang H, Zhou C, Zhang Z, et al. Integration of network pharmacology and experimental validation to explore the pharmacological mechanisms of zhuanggu busui formula against osteoporosis. Front Endocrinol. 2021;12:841668. doi:10.3389/fendo.2021.841668
25. Lu Z, Huang M, Lin H, et al. Network pharmacology and molecular docking approach to elucidate the mechanisms of Liuwei Dihuang pill in diabetic osteoporosis. J Orthop Surg Res. 2022;17(1):314. doi:10.1186/s13018-022-03194-2
26. Gong W, Chen X, Shi T, et al. Network pharmacology-based strategy for the investigation of the anti-osteoporosis effects and underlying mechanism of zhuangguguanjie formulation. Front Pharmacol. 2021;12:727808. doi:10.3389/fphar.2021.727808
27. Kim S, Chen J, Cheng T, et al. PubChem 2023 update. Nucleic Acids Res. 2023;51(D1):D1373–D1380. doi:10.1093/nar/gkac956
28. Oh KK, Gupta H, Min B-H, et al. Elucidation of prebiotics, probiotics, postbiotics, and target from gut microbiota to alleviate obesity via network pharmacology study. Cells. 2022;11(18):2903. doi:10.3390/cells11182903
29. Yuan M, Zhang Y, Wang L, et al. Study on the mechanism of Tong-Qiao-Huo-Xue decoction regulating apoptosis via ASK1/MKK4/JNK pathway in MCAO/R rats. Phytomedicine. 2022;106:154437. doi:10.1016/j.phymed.2022.154437
30. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.1239303
31. Jiao X, Sherman BT, Huang DW, et al. DAVID-WS: a stateful web service to facilitate gene/protein list analysis. Bioinformatics. 2012;28(13):1805–1806. doi:10.1093/bioinformatics/bts251
32. gene ontology consortium: going forward. Nucleic Acids Res. 2015;43:D1049–56. doi:10.1093/nar/gku1179
33. Kanehisa M, Sato Y, Kawashima M, et al. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457–62. doi:10.1093/nar/gkv1070
34. Berman HM, Westbrook J, Feng Z, et al. The protein data bank. Nucleic Acids Res. 2000;28(1):235–242. doi:10.1093/nar/28.1.235
35. Yilmaz MI, Romano M, Basarali MK, et al. The effect of corrected inflammation, oxidative stress and endothelial dysfunction on fmd levels in patients with selected chronic diseases: a quasi-experimental study. Sci Rep. 2020;10(1):9018. doi:10.1038/s41598-020-65528-6
36. He R, Cui M, Lin H, et al. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018;199:122–130. doi:10.1016/j.lfs.2018.03.020
37. Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F., et al. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother. 2017;87:223–229. doi:10.1016/j.biopha.2016.12.105
38. Li B, Rui J, Ding X, et al. Exploring the multicomponent synergy mechanism of Banxia Xiexin Decoction on irritable bowel syndrome by a systems pharmacology strategy. J Ethnopharmacol. 2019;233:158–168. doi:10.1016/j.jep.2018.12.033
39. Yang J, Su N, Du X, Chen L, et al. Gene expression patterns in bone following lipopolysaccharide stimulation. Cell Mol Biol Lett. 2014;19(4):611–622. doi:10.2478/s11658-014-0216-2
40. Zeng XZ, et al. Artesunate attenuates LPS-induced osteoclastogenesis by suppressing TLR4/TRAF6 and PLCgamma1-Ca(2+)-NFATc1 signaling pathway. Acta Pharmacol Sin. 2020;41(2):229–236. doi:10.1038/s41401-019-0289-6
41. Kalaitzoglou E, Fowlkes JL, Popescu I, et al. Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes Metab Res Rev. 2019;35(2):e3100. doi:10.1002/dmrr.3100
42. Pingali U, Nutalapati C. Shilajit extract reduces oxidative stress, inflammation, and bone loss to dose-dependently preserve bone mineral density in postmenopausal women with osteopenia: a randomized, double-blind, placebo-controlled trial. Phytomedicine. 2022;105:154334. doi:10.1016/j.phymed.2022.154334
43. Damani JJ, De Souza MJ, Strock NC, et al. Associations between inflammatory mediators and bone outcomes in postmenopausal women: a cross-sectional analysis of baseline data from the prune study. J Inflamm Res. 2023;16:639–663. doi:10.2147/JIR.S397837
44. Yu H. Targeting S1PRs as a therapeutic strategy for inflammatory bone loss diseases-beyond regulating S1P Signaling. Int J mol Sci. 2021;22(9).
45. Kwan TS, Marc P, Sandrine T, et al. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15(1):49–60. doi:10.1016/j.cytogfr.2003.10.005
46. Baum R, Gravallese EM. Impact of inflammation on the osteoblast in rheumatic diseases. Curr Osteoporos Rep. 2014;12(1):9–16. doi:10.1007/s11914-013-0183-y
47. Geng Q, Gao H, Yang R, et al. Pyrroloquinoline quinone prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Int J Biol Sci. 2019;15(1):58–68. doi:10.7150/ijbs.25783
48. Lee NK, Choi YG, Baik JY, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106(3):852–859. doi:10.1182/blood-2004-09-3662
49. Qi SS, Shao Ml, Sun Z, et al. Chondroitin sulfate alleviates diabetic osteoporosis and repairs bone microstructure via anti-oxidation, anti-, 2021;12:759843.
50. Antonisamy P, Duraipandiyan V, Aravinthan A, et al., Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms. Eur J Pharmacol, 2015. 750: p. 167–175. 10.1016/j.ejphar.2015.01.015
51. Razwinani M, Motaung KS, The influence of friedelin, resinone, tingenone and betulin of compounds on chondrogenic differentiation of porcine adipose-derived mesenchymal stem cells (pADMSCs). Biochimie, 2022. 196: p. 234–242. 10.1016/j.biochi.2022.01.018
52. Peng XM, Damu GL, Zhou C, Current developments of coumarin compounds in medicinal chemistry. Curr Pharm Des, 2013. 19(21): p. 3884–3930. 10.2174/1381612811319210013
53. Tavares S, Lima V, Bone anti-resorptive effects of coumarins on RANKL downstream cellular signaling: a systematic review of the literature. Fitoterapia, 2021. 150: p. 104842. 10.1016/j.fitote.2021.104842
54. Abdallah BM, Ali EM, 5’-hydroxy Auraptene stimulates osteoblast differentiation of bone marrow-derived mesenchymal stem cells via a BMP-dependent mechanism. J Biomed Sci, 2019. 26(1): p. 51. 10.1186/s12929-019-0544-7
55. Luo H, Wang Y, Qin Q, Wang Y, Xu J, He X, et al., Anti-inflammatory naphthoates and anthraquinones from the roots of Morinda officinalis. Bioorg Chem, 2021. 110: p. 104800. 10.1016/j.bioorg.2021.104800
56. Meng Y, Xu Y, Chang C, et al., Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int J Biol Macromol, 2020. 163: p. 1677–1686. 10.1016/j.ijbiomac.2020.09.117
57. Qin T, Ren Z, Liu X, et al., Study of the selenizing Codonopsis pilosula polysaccharides protects RAW264.7 cells from hydrogen peroxide-induced injury. Int J Biol Macromol, 2019. 125: p. 534–543. 10.1016/j.ijbiomac.2018.12.025
58. Luan F, Ji Y, Peng L, et al., Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: a review. Carbohydr Polym, 2021. 261: p. 117863. 10.1016/j.carbpol.2021.117863
59. Schuman J, Chen Y, Podd A, et al., A critical role of TAK1 in B-cell receptor-mediated nuclear factor kappaB activation. Blood, 2009. 113(19): p. 4566–4574. 10.1182/blood-2008-08-176057
60. Chen J, Song D, Xu Y, et al., Anti-osteoclast effect of exportin-1 inhibitor eltanexor on osteoporosis depends on nuclear accumulation of IkappaBalpha-NF-kappaB p65 complex. Front Pharmacol, 2022. 13: p. 896108. 10.3389/fphar.2022.896108
61. Ma Q, Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol, 2013. 53: p. 401–426. 10.1146/annurev-pharmtox-011112-140320
62. Widen JC, Kempema AM, Baur JW, et al., Helenalin analogues targeting NF-kappaB p65: thiol reactivity and cellular potency studies of varied electrophiles. ChemMedChem, 2018. 13(4): p. 303–311. 10.1002/cmdc.201700752
63. Li Y, Zeng Y, Huang Q, et al., Helenalin from Centipeda minima ameliorates acute hepatic injury by protecting mitochondria function, activating Nrf2 pathway and inhibiting NF-kappaB activation. Biomed Pharmacother, 2019. 119: p. 109435. 10.1016/j.biopha.2019.109435
64. Tietze LF, Rackelmann N, Muller I, Enantioselective total syntheses of the Ipecacuanha alkaloid emetine, the Alangium alkaloid tubulosine and a novel benzoquinolizidine alkaloid by using a domino process. Chemistry, 2004. 10(11): p. 2722–2731. 10.1002/chem.200306039
65. Chaea HJ, Kim H-R, Kang YJ, et al., Heme oxygenase-1 induction by (S)-enantiomer of YS-51 (YS-51S), a synthetic isoquinoline alkaloid, inhibits nitric oxide production and nuclear factor-kappaB translocation in ROS 17/2.8 cells activated with inflammatory stimulants. Int Immunopharmacol, 2007. 7(12): p. 1559–1568. 10.1016/j.intimp.2007.07.023
66. Komori T, Molecular mechanism of Runx2-dependent bone development. Mol Cells, 2020. 43(2): p. 168–175. 10.14348/molcells.2019.0244
67. Dalagiorgou G, Piperi C, Adamopoulos C, et al., Mechanosensor polycystin-1 potentiates differentiation of human osteoblastic cells by upregulating Runx2 expression via induction of JAK2/STAT3 signaling axis. Cell mol Life Sci, 2017. 74(5): p. 921–936. 10.1007/s00018-016-2394-8
68. Hou X, Tian F, STAT3-mediated osteogenesis and osteoclastogenesis in osteoporosis. Cell Commun Signal, 2022. 20(1): p. 112. 10.1186/s12964-022-00924-1
69. Zhou S, Dai Q, Huang X, et al., STAT3 is critical for skeletal development and bone homeostasis by regulating osteogenesis. Nat Commun, 2021. 12(1): p. 6891. 10.1038/s41467-021-27273-w
70. Chen L, Zhang R-Y, Xie J, et al., STAT3 activation by catalpol promotes osteogenesis-angiogenesis coupling, thus accelerating osteoporotic bone repair. Stem Cell Res Ther, 2021. 12(1): p. 108. 10.1186/s13287-021-02178-z
71. Wang W, Mao J, Chen Y, et al., Naringin promotes osteogenesis and ameliorates osteoporosis development by targeting JAK2/STAT3 signalling. Clin Exp Pharmacol Physiol, 2022. 49(1): p. 113–121. 10.1111/1440-1681.13591
72. Huang X, Zhu Y, Sun S, et al., Exercise maintains bone homeostasis by promoting osteogenesis through STAT3. Int J Biol Sci, 2023. 19(7): p. 2021–2033. 10.7150/ijbs.82744
73. Krzeslak A, [Akt kinase: a key regulator of metabolism and progression of tumors]. Postepy Hig Med Dosw, 2010. 64: p. 490–503.
74. Viglietto G, Amodio N, Malanga D, Scrima M, De Marco C., et al., Contribution of PKB/AKT signaling to thyroid cancer. Front Biosci;2011. 16(4): p. 1461–1487. 10.2741/3799
75. Price J, Zaidi Ak, Bohensky J, et al., Akt-1 mediates survival of chondrocytes from endoplasmic reticulum-induced stress. J Cell Physiol, 2010. 222(3): p. 502–508. 10.1002/jcp.22001
76. Dai WW, Wang L-B, Jin G-Q, et al., Beta-ecdysone protects mouse osteoblasts from glucocorticoid-induced apoptosis in vitro. Planta Med, 2017. 83(11): p. 888–894. 10.1055/s-0043-107808
77. Niu Y, Wang Z, Shi Y, Dong L, Wang C, et al., Modulating macrophage activities to promote endogenous bone regeneration: biological mechanisms and engineering approaches. Bioact Mater, 2021. 6(1): p. 244–261. 10.1016/j.bioactmat.2020.08.012
78. Chen Z, Mao X, Tan L, et al., Osteoimmunomodulatory properties of magnesium scaffolds coated with beta-tricalcium phosphate. Biomaterials, 2014. 35(30): p. 8553–8565. 10.1016/j.biomaterials.2014.06.038
79. Arora S, Dev K, Agarwal B, et al., Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology, 2018. 223(4–5): p. 383–396. 10.1016/j.imbio.2017.11.001
80. Boyle WJ, Simonet WS, Lacey DL, Osteoclast differentiation and activation. Nature, 2003. 423(6937): p. 337–342. 10.1038/nature01658
81. Han J, et al., Gypenoside inhibits RANKL-induced osteoclastogenesis by regulating NF-kappaB, AKT, and MAPK signaling pathways. J Cell Biochem, 2018. 119(9): p. 7310–7318. 10.1002/jcb.27028
82. Sun P, Wang M, Yin GY, Endogenous parathyroid hormone (PTH) signals through osteoblasts via RANKL during fracture healing to affect osteoclasts. Biochem Biophys Res Commun, 2020. 525(4): p. 850–856. 10.1016/j.bbrc.2020.02.177
83. Zhao F, Xu Y, Ouyang Y, et al., Silencing of miR-483-5p alleviates postmenopausal osteoporosis by targeting SATB2 and PI3K/AKT pathway. Aging, 2021. 13(5): p. 6945–6956. 10.18632/aging.202552
84. Ma Y, Ran D, Zhao H, et al., Cadmium exposure triggers osteoporosis in duck via P2X7/PI3K/AKT-mediated osteoblast and osteoclast differentiation. Sci Total Environ, 2021. 750: p. 141638. 10.1016/j.scitotenv.2020.141638
85. Hu XH, Yang X-Y, Lian J, et al., Moringa oleifera leaf attenuate osteoporosis in ovariectomized rats by modulating gut microbiota composition and MAPK signaling pathway. Biomed Pharmacother, 2023. 161: p. 114434. 10.1016/j.biopha.2023.114434
86. Fan S, Gao X, Chen P, et al., Myricetin ameliorates glucocorticoid-induced osteoporosis through the ERK signaling pathway. Life Sci, 2018. 207: p. 205–211. 10.1016/j.lfs.2018.06.006
87. Hsu YL, Chang Jk, Tsai Ch, Chien Tt, Kuo Pl, et al., Myricetin induces human osteoblast differentiation through bone morphogenetic protein-2/p38 mitogen-activated protein kinase pathway. Biochem Pharmacol, 2007. 73(4): p. 504–514. 10.1016/j.bcp.2006.10.020
88. Montagnani A, Gonnelli S, Antidiabetic therapy effects on bone metabolism and fracture risk. Diabetes Obes Metab, 2013. 15(9): p. 784–791. 10.1111/dom.12077
89. Takagi M, Kasayama S, Yamamoto T, et al., Advanced glycation endproducts stimulate interleukin-6 production by human bone-derived cells. J Bone Miner Res, 1997. 12(3): p. 439–446. 10.1359/jbmr.1997.12.3.439
90. Katayama Y, Akatsu T, Yamamoto M, et al., Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J Bone Miner Res, 1996. 11(7): p. 931–937. 10.1002/jbmr.5650110709
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.