Back to Journals » Drug Design, Development and Therapy » Volume 19
Network Pharmacology, Molecular Docking, and in vitro Experiments Reveal the Role and Mechanism of Tanshinone IIA in Colorectal Cancer Treatment Through the PI3K/AKT Pathway
Authors Sun J, Qi X , Yang C, Wang S, Jiang J, Wang L, Song J, Yu B , Sun M
Received 11 December 2024
Accepted for publication 9 April 2025
Published 16 April 2025 Volume 2025:19 Pages 2959—2977
DOI https://doi.org/10.2147/DDDT.S492033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Tanshinone IIA in colorectal cancer treatment through PI3K/AKT pathway – Video abstract [492033]
Views: 62
Jinpeng Sun, Xinmeng Qi, Cuiyuan Yang, Shanpeng Wang, Jingwen Jiang, Lijie Wang, Jiacheng Song, Bin Yu, Min Sun
College of Integrative Chinese and Western Medicine, Jining Medical University, Jining, Shandong, 272067, People’s Republic of China
Correspondence: Min Sun, College of Integrative Chinese and Western Medicine, Jining Medical University, Jining, Shandong, 272067, People’s Republic of China, Tel +8615905472137, Email [email protected]
Purpose: To examine the roles and mechanisms of tanshinone IIA (Tan-IIA) in colorectal cancer (CRC) using network pharmacology, molecular docking, and in vitro experiments.
Methods: In network pharmacology studies, Tan-IIA targets for treating CRC were identified using public databases. Employing the protein-protein interaction (PPI) network, gene ontology (GO) enrichment, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses, the core genes and mechanisms of action of Tan-IIA were obtained. Core targets were validated using Gene Expression Profiling Interactive Analysis, the Human Protein Atlas, DriverDBv3, cBioPortal, and the Tumor Immune Estimation Resource database. Molecular docking validates the binding affinity of Tan-IIA to some key targets. Network pharmacology and molecular docking results were validated via in vitro experiments.
Results: Intersecting Tan-IIA and CRC targets led to the identification of 25 potential targets. PPI analysis identified 10 core targets of Tan-IIA for CRC treatment. Database validation revealed that these core targets were expressed at varying levels in both normal and cancer tissues. Their expression could influence patient prognosis and immune cell infiltration levels. GO analysis revealed 170 biological processes, 42 cellular components, and 83 molecular functions. KEGG analysis indicated that Tan-IIA affected CRC through multiple pathways, including the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT), cAMP, and TNF signaling pathways, with the PI3K/AKT pathway being the most enriched. Molecular docking results indicated that Tan-IIA effectively binds to PI3K, AKT, and other partial core targets. In vitro experiments revealed that Tan-IIA suppressed the multiplication and migration of HCT116 and SW480 cells, induced apoptosis, and reduced the PI3K/AKT pathway indicator protein expression, which was reversed by the PI3K/AKT pathway agonist insulin-like growth factor-1.
Conclusion: Network pharmacology, molecular docking, and in vitro validation confirmed that Tan-IIA contributes to CRC treatment through the PI3K/AKT pathway, providing theoretical and experimental foundations for its potential clinical application.
Keywords: tanshinone IIA, colorectal cancer, traditional Chinese medicine, drug-target interactions, PI3K/AKT pathway
Introduction
Colorectal cancer (CRC) is a type of malignant solid tumor with a high incidence in the gastrointestinal tract and ranks among the top causes of morbidity and mortality globally.1 According to data, CRC accounts for 10.9% of global cancer treatment costs, second only to tracheal, bronchial, and lung cancer at 15.4%.2 Modern CRC therapies, including surgery, chemotherapy, radiotherapy, and other modalities, have notable drawbacks, such as high side effects, cost, and the potential for drug resistance. Moreover, these treatments frequently result in a significant decline in the patient’s quality of life.3,4 Therefore, there is a high demand to explore new therapeutic agents or combined adjuvant therapies for CRC.
Traditional Chinese medicine (TCM) is efficacious, has few adverse effects, is relatively inexpensive, and has gained widespread acceptance by doctors and patients as a low-toxicity complementary therapy for patients with cancer.5,6 According to TCM theory, blood stasis is considered a key pathology in tumor development, and activating blood circulation is essential for cancer treatment.7 Danshen (Salvia miltiorrhiza) is a Chinese medicine known to activate blood circulation and remove blood stasis.8 It is commonly used to treat tumors. Tanshinone IIA (Tan-IIA), a diterpene quinone isolated from Danshen, is one of its most pharmacologically active constituents.9 It plays a key role in treating hypertension, coronary heart disease, Alzheimer’s disease, and other conditions. Tan-IIA has impressive antitumor effects against various cancers, including cervical and gastric cancers.10,11 Although limited research suggests that Tan-IIA can treat CRC by modulating reactive oxygen species/c-Jun N-terminal kinase signaling pathways,12 increasing evidence suggests that the therapeutic effects of TCM drugs are exerted through multiple pathways.13 The mechanism of Tan-IIA in CRC treatment is likely multi-targeted and multi-pathway, posing a challenge to the establishment of its precise mode of action and thus requiring further investigation.
Recently emerging network pharmacology offers an appropriate interpretation of the intricate relationships among active ingredients, targets, and diseases in TCM. This approach can clarify the multi-target mechanism of TCM and reveal the possible therapeutic pathways from a holistic and comprehensive perspective.14 Traditional Chinese medicinal monomer Aloin and the traditional TCM formula Zuojin Pills both have substantial antitumor effects.15,16 Molecular docking, a well-established computer-based method, simulates the binding between drug molecules and disease targets, thereby validating novel compounds with therapeutic potential.17
This study aimed to investigate the core targets and mechanisms of Tan-IIA in CRC therapy through network pharmacology and molecular docking, with basic validation using in vitro experiments. This study offers a theoretical and laboratory foundation for the clinical application of drugs that activate blood circulation and treat blood stasis. An experimental overview of this study is shown in Figure 1.
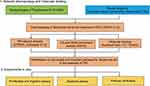 |
Figure 1 Network pharmacological analysis, molecular docking, and in vitro experimental validation of Tan-llA for CRC treatment. |
Materials and Methods
Network Pharmacology and Molecular Docking Analysis
Relevant targets and chemical structures of Tan-IIA were acquired from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (http://tcmspw.com/tcmsp.php). The Universal Protein Resource (UniProt) database (https://www.uniprot.org/) was used to obtain UniProt IDs and gene names of the targets and to screen for human-related genes. GeneCards (https://www.genecards.org/), Pharm GKB (https://www.pharmgkb.org/), and the Therapeutic Target Database (TTD, https://db.idrblab.net/ttd/) were used to screen CRC-related targets. VENNY 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html), a bioinformatic tool for screening cross-targets, was used to identify Tan-IIA for cross-targeting with CRC.
The cross-targets were analyzed using the STRING database (https://string-db.org/), and data with a confidence score ≥0.4 were selected to establish the protein-protein interaction (PPI) network. Target proteins that were independent of the network were removed. The PPI network was imported into Cytoscape 3.7.2 (http://www.cytoscape.org/). We used the MCODE plugin to obtain the clustered subnetwork, and the top 10 core targets were filtered using the CytoHubba plugin. The Database for Annotation, Visualization, and Integrated Discovery (DAVID, https://david.ncifcrf.gov/) was used for enrichment analysis of gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). AutoDock Tools 1.5.7 (https://autodocksuite.scripps.edu/) was used for molecular docking, and the results were visualized and analyzed using PyMOL 3.0 (https://pymol.org/).
Database Validation of Core Targets
The expression of core targets was analyzed using the Gene Expression Profiling Interactive Analysis (GEPIA) online tool (http://gepia.cancer-pku.cn/index.html). Data on the expression of core targets in normal and CRC tissues were obtained from the Human Protein Atlas (HPA) database (https://www.proteinatlas.org/). The association of the 10 core targets with overall survival was evaluated using the DriverDBv3 database (http://driverdb.bioinfomics.org/gene/). Genetic alteration information for core targets was retrieved from the cBioPortal database (https://www.cbioportal.org/). The relationship between core targets and immune cell infiltration, including B cells, CD8+T cells, CD4+T cells, macrophages, neutrophils, and dendritic cells, was explored using the TIMER database (https://cistrome.shinyapps.io/timer/).
Chemical
Tan-IIA was purchased from Shanghai Yuanye Biotechnology Co. (Shanghai, China), verified using high-performance liquid chromatography (≥98%), and dissolved in a 10 g/L stock solution using dimethyl sulfoxide (DMSO; Solarbio, Beijing, China). The solution was aliquoted into sterile centrifuge tubes and stored at −20 °C until needed. Before use, the culture medium was diluted to a series of concentrations (final DMSO concentration ≤ 0.1%). The phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) activator, insulin-like growth factor-1 (IGF-1) (Beyotime, Shanghai, China), was used to verify the modulatory action of Tan-IIA on the PI3K/AKT pathway in CRC cells.18
Cell Culture
The human CRC cell lines HCT116 and SW480 were provided by the American Type Culture Collection and were tested to be free of mycoplasma infection. The cells were cultured in 5% CO2 at 37 °C. The complete medium was a Dulbecco-modified Eagle medium (Pricella, Wuhan, China) containing 10% fetal bovine serum (BIOODIN, Guangzhou, China) and 1% penicillin-streptomycin. Cells were treated with different concentrations of Tan-IIA and 10 μM IGF-1, with 0.1% DMSO as a control.
MTT Assay
Furthermore, 96-well plates were spread at a density of 5000 cells/well. After cell attachment, the plates were treated with a medium containing DMSO and varying concentrations of Tan-IIA (2.5, 5, 10, 20, 40, and 80 μM) for 24 and 48 h. Subsequently, 10 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Beyotime) solution was added to each well and incubated for 4 h. The supernatant was carefully discarded; 150 μL DMSO was spiked to each well, and the absorbance was detected at 490 nm after stunning.
Clonogenic Assay
Here, 6-well plates were spread at a density of 2000 cells per well and cultured overnight, followed by treatment with a medium containing various concentrations of Tan-IIA. After 24 h, the cells were grown in a medium without DMSO or Tan-IIA. The medium was replaced every 3 days. After the cells became visible to the naked eye, they were rinsed two times with PBS, fixed in 4% paraformaldehyde (Biosharp, Anhui, China) for 30 min, and stained with crystalline violet (Beyotime) for up to 15 min. They were then rinsed with distilled water, dried, photographed in a well-lit area, and analyzed.
Wound Healing Assay
For the wound healing assay, 6-well plates were spread at a density of 2×106 cells/well and cultured to 80–90% confluence. Wounds were scratched on the cells using a 10 μL sterile pipette tip, rinsed three times with PBS (BIOODIN), and handled with a Tan-IIA-containing medium. The extent of the scratch healing was observed and photographed at 0 and 24 h.
Flow Cytometry for Cell Apoptosis
Regarding flow cytometry for cell apoptosis, 6-well plates were spread at a density of 5×105 cells per well and grown overnight. Various levels of Tan-IIA were added after the wall attachment. Cells were gathered 24 h later and rinsed two times with pre-cooled PBS. Apoptosis was detected using Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining solutions according to the instructions of the Annexin V-FITC/PI Apoptosis Detection Kit (Vazyme, Nanjing, China). After 10 min of incubation in the dark, the sample was mixed with binding buffer, and apoptosis was analyzed using flow cytometry.
Western Blotting
For Western blotting, 6-well plates were spread at a density of 1×106 cells/well and grown overnight, followed by a 24-h intervention with various levels of Tan-IIA. The Western blotting was conducted in accordance with the established protocols outlined.19 Primary antibodies used included p-PI3K, p-AKT (Cell Signaling Technology, Danvers, MA, USA), PI3K, AKT, Bax, Bcl-2, and β-actin (Proteintech, Wuhan, China); bands were treated with a horseradish peroxidase-conjugated secondary antibody (Beyotime). The strips were exposed using ECL chemiluminescence (Biosharp). Protein band intensities were quantified with ImageJ.
Statistical Analysis
Data were analyzed using the t-test (for two groups) or one-way analysis of variance (for more than two groups) using GraphPad Prism 8 and SPSS 22.0. Statistical significance was set at P < 0.05.
Results
Network Pharmacological Analysis and Molecular Docking Validation of Tan-IIA for Treating CRC
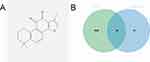
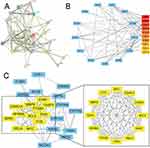
In our study, Tan-IIA was identified in the TCMSP database using oral bioavailability ≥ 30% and drug-likeness ≥ 0.18 as screening criteria. Overall, 39 related genes were identified; the Tan-IIA structures are shown in Figure 2A. Subsequently, we screened 2727 CRC target genes from the GeneCards, Pharm GKB, and TTD databases, with duplicates removed. The identified genes were uploaded to VENNY 2.1.0, yielding 25 cross-targets that were considered potential targets of Tan-IIA in CRC treatment (Figure 2B). The 25 crossover genes were analyzed using the STRING database to create a PPI network. Homo sapiens was selected as the species with a confidence level of ≥ 0.4, which produced a PPI network containing 25 nodes and 113 edges (Figure 3A). The PPI network was then uploaded to Cytoscape 3.7.2 for visual analysis. Using the CytoHubba plugin, we identified the top 10 core targets, namely, TP53, CASP3, BCL2, MMP9, PTGS2, FOS, NFKBIA, MYC, RELA, and CDKN1A (listed in descending order) (Figure 3B). Tan-IIA may have a crucial role in treating CRC by targeting these core targets. The MCODE analysis revealed two clustering subnetworks. The highest-scoring subnetwork included 12 nodes and 59 edges (NPM1, MYC, EDN1, RELA, BCL2, CDKN1A, MMP9, FOS, TP53, NFKBIA, CASP3, and PTGS2) and had a score of 10.727 (Figure 3C).
GO enrichment analysis of the 25 cross-targets was conducted using the DAVID database. Entries with P<0.05 were considered significantly different, and 170 biological process-related, 42 cellular component-related, and 83 molecular function-related entries were screened. These results suggest that Tan-IIA influences the malignant biological behaviors of CRC cells, such as growth and reproduction, by modulating the response to hypoxia and amino acids, translation factor complexes, chromatin proteases, and binding interactions, thereby exerting therapeutic effects on CRC. Based on these analyses, the top 10 biological processes, cellular components, and molecular functions were selected for visualization (Figure 4A). Subsequently, 77 pathways were identified via KEGG analysis (P<0.05). The top 15 signaling pathways were ranked by count values from highest to lowest to create the KEGG signaling pathway enrichment bubble map (Figure 4B). The results suggested that Tan-IIA treats CRC through PI3K-AKT, cAMP, and TNF signaling pathways. We designed a drug target-pathway network map using Cytoscape 3.7.2 (Figure 4C), where most genes were associated with the PI3K/AKT pathway, indicating its potentially significant role in CRC treatment with Tan-IIA. The PI3K/AKT pathway is crucial for tumor development.20 We simulated the binding affinity of Tan-IIA to PI3K, AKT, and the four core targets using molecular docking (Figures 5 and 6). Strong interactions were indicated by low binding energy <5.0 kcal/mol, suggesting good binding ability, and values below −7.0 kcal/mol, indicating strong binding. The binding energies of Tan-IIA for each molecule are listed in Table 1. These findings further confirmed that the core targets predicted by network pharmacology and the PI3K/AKT pathway are crucial for treating CRC with Tan-IIA.
 |
Table 1 The Binding Energies of Tanshinone IIA and Each Molecule |
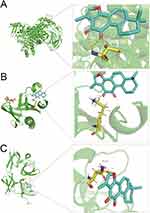 |
Figure 5 Molecular docking results. (A) PI3K, (B) AKT, and (C) TP53. |
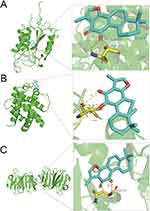 |
Figure 6 Molecular docking results. (A) CASP3, (B) Bcl-2, and (C) MMP9. |
Database Validation of Core Targets of Tan-IIA for CRC Treatment
The top 10 core targets of Tan-IIA for CRC were identified using a previously described methodology. The expression of these targets in normal colon and CRC specimens was analyzed using GEPIA and HPA databases. The outcomes revealed that mRNA expressions of TP53, CASP3, MMP9, and MYC were increased in CRC specimens compared with the levels in normal tissues (P<0.05), as shown in Figure 7. Additionally, Figure 8 illustrates that the expression levels of all 10 core targets were at higher levels in CRC tissues than in normal tissues. The association between core target expression and overall survival was accessed with the DriverDBv3 database, with results presented in Figure 9. Genetic alterations in these 10 core targets were identified in 1515 (65%) of the 2335 patients using the cBioPortal database (Figure 10).
 |
Figure 7 Core gene expression levels obtained from the GEPIA database. Red and gray represent colorectal cancer and normal colorectal tissues, respectively. |
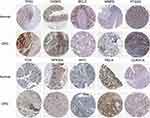 |
Figure 8 Immunohistochemical images of core genes corresponding to normal colorectal tissue versus colorectal cancer tissues retrieved from the HPA. |
 |
Figure 9 Relationship between core gene expression and overall survival in the DriverDBv3 database. Black and red lines represent low and high expressions, respectively. |
 |
Figure 10 Genetic information on core targets revealed by the cBioPortal database. The data showed that 1515 of the 2335 patients (65%) had alterations in these genes. |
We further explored the relationship between core target expression and immune cell infiltration by the TIMER database. As shown in Figure 11, TP53 expression had a positive correlation with tumor purity, B cells, and CD8+T cells and a negative correlation with CD4+T cells, macrophages, neutrophils, and dendritic cells. CASP3, BCL2, MMP9, PTGS2, NFKBIA, RELA, and RELA expression were adversely correlated with tumor purity. FOS expression was negatively correlated with tumor purity and macrophages but positively correlated with other immune cells; MYC expression was positively correlated with tumor purity, CD4+ T cells, macrophages, neutrophils, and dendritic cells but negatively correlated with B and CD8+ T cells; CDKN1A expression was negatively correlated with purity and macrophages but positively correlated with other cells.
Figure 11 Continued.
Tan-IIA Inhibits the Proliferation and Migration of CRC Cells
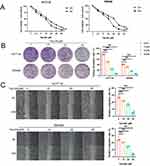
To examine the effect of Tan-IIA on the proliferation of CRC cells, HCT116 and SW480 cells were treated with Tan-IIA for 24 and 48 h, respectively, and cell viability was measured using the MTT assay. These findings suggest that Tan-IIA decreased the viability of HCT116 and SW480 cells in a dose-dependent manner (Figure 12A). The IC50 values for HCT116 and SW480 cells were 15.92 μM and 12.33 μM at 24 h and 8.42 μM and 7.98 μM at 48 h, respectively. Based on these results, we selected concentrations of 0, 10, 20, and 40 μM for subsequent experiments, with a treatment duration of 24 h. The suppressive action of Tan-IIA on CRC cell proliferation was further verified using a clonogenic assay, which demonstrated that the colony formation ability of cells was suppressed significantly with an increase in drug concentration (Figure 12B). The effect of Tan-IIA on the migratory capacity of CRC cells was evaluated using a wound-healing assay. The findings indicated that the migratory abilities of CRC cells were reduced at higher Tan-IIA concentrations (Figure 12C). These findings suggest that Tan-IIA effectively inhibits the proliferation and migration of CRC cells.
Tan-IIA Promotes Apoptosis in CRC Cells
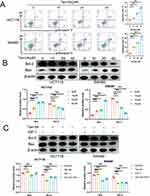
To examine the influence of Tan-IIA on apoptosis of CRC cells, HCT116 and SW480 cells were treated with Tan-IIA for 24 h, and cell apoptosis was measured using flow cytometry. The results suggested that Tan-IIA promoted apoptosis of CRC cells, and the apoptosis rate significantly increased as the drug concentration increased (Figure 13A). Additionally, the effect of Tan-IIA on the expression levels of the apoptotic proteins Bcl-2 and Bax was assessed using Western blotting. Proteins of the Bcl-2 family, comprising Bcl-2 and Bax, are key regulators of the apoptotic pathway in cellular mitochondria.21 The results demonstrated that Tan-IIA decreased Bcl-2 and increased Bax expression in CRC cells (Figure 13B). These results revealed that Tan-IIA may induce apoptosis in CRC cells through the mitochondrial apoptotic pathway, where Bcl-2 and Bax are located, and the changes were reversed by the PI3K/AKT pathway agonist IGF-1 (Figure 13C).
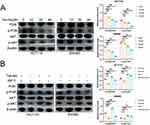
Tan-IIA Inhibits the Activation of the PI3K/AKT Pathway in CRC Cells
The expression levels of PI3K, p-PI3K, Akt, and p-Akt in CRC cells were measured using Western blotting in CRC cells after 24 h of Tan-IIA treatment to evaluate the effects of different concentrations of Tan-IIA on the PI3K/AKT pathway in HCT116 and SW480 cells. The findings showed that Tan-IIA downregulated the expression of p-PI3K and p-AKT in a dose-dependent manner without affecting PI3K and AKT, indicating that Tan-IIA inhibits the activation of the PI3K/AKT pathway in CRC cells (Figure 14A). The optimal dose of Tan-IIA (20 μM) was selected for further experiments to investigate the effects on the PI3K/AKT pathway using IGF-1. We found that IGF-1-induced activation of the PI3K/AKT pathway was reversed by adding Tan-IIA (Figure 14B). These results suggested that the PI3K/AKT pathway plays a significant role in treating CRC with Tan-IIA.
Discussion
In developed countries, the incidence of CRC has consistently remained high, and in China, it is also rising owing to the gradual westernization of lifestyles and dietary habits. Increased intake of animal-based foods, rising obesity rates, and ill-health behaviors, including smoking and alcohol consumption, further escalate the cost of cancer treatment in China.22,23 Given the side effects and limited efficacy of modern medical treatments, identifying and utilizing effective therapeutic agents remains a priority in CRC treatment.
According to the TCM theory, CRC belongs to the category of obstruction in the mass and accumulation, and resolving blood stasis and dispersing lumps is the key to treating tumors.7 Numerous studies have demonstrated the role of blood-activating and stasis-removing drugs in tumor treatment.24,25 In Shennong’s Herbal Classic (Shennong Bencao Jing), Danshen (Salvia miltiorrhiza) is described as the remedy for heart and abdominal ailments as well as resolving accumulations, suggesting its potential therapeutic effect on blood stasis disorders, including cancer.
Tan-IIA, a key pharmacological component of Danshen, has been previously used to treat various circulatory disorders.26 Recent studies have implicated it in inhibiting tumor cell migration and invasion and regulating tumor angiogenesis;27 however, limited studies have focused on CRC. Network pharmacology, which aligns with the Chinese medicine concept of multiple components and targets, offers a clear method for uncovering drug and disease targets. In this study, we investigated the roles and mechanisms of Tan-IIA in CRC using in vitro experiments based on network pharmacology and molecular docking.
We first intersected 39 Tan-IIA-related targets with 2727 CRC-related targets and identified 25 common targets. We constructed a PPI network and imported it into Cytoscape 3.7.2 for visualization and analysis, which revealed two clustered subnetworks using the MCODE algorithm, with the highest cluster score of 10.727. Using the CytoHubba plugin, we screened the top 10 core targets and validated their expression and function using the database. GO enrichment analysis revealed that Tan-IIA regulated CRC cell proliferation, apoptosis, and migration to exert therapeutic effects. Furthermore, KEGG pathway enrichment analysis revealed that the PI3K/AKT, MAPK, cAMP, and TNF pathways are critical signaling pathways in CRC treatment with Tan-IIA, with some roles of the MAPK, cAMP, and TNF pathways dependent on the PI3K/AKT pathway.28–30 The PI3K/AKT pathway is crucial for tumor growth, metastasis, and apoptosis,31 making it a valuable focus for further study. Molecular docking analysis demonstrated that Tan-IIA has a strong binding affinity for PI3K, AKT, and several core targets, suggesting that its therapeutic effect in CRC is mediated through the PI3K/AKT pathway.
The PI3K/AKT signaling pathway is a transduction network involved in various life activities in eukaryotic cells, regulating multiple pathways to promote cell growth and proliferation while responding to external stimuli.32,33 It has become a major focus in malignancy-related research. PI3K is a phosphatidylinositol kinase that comprises a heterodimer of regulatory (p85) and catalytic (p110) subunits and functions found in cells. Moreover, AKT is a serine/threonine kinase that is a downstream target of PI3K. Growth factor receptor signaling targets multiple cells by stimulating PI3K and produces phosphatidylinositol-3,4,5-triphosphate (PIP3) to target multiple cells.34 PIP3 can activate AKT and downstream pathways, regulating cell proliferation, migration, and apoptosis.35 Research has shown that many tumors typically carry at least one mutation in the PI3K gene and that PI3K mutations can lead to the emergence and progression of various cancers, including breast, stomach, and ovarian cancers.36,37 In CRC, PI3K mutations are present in approximately 25% of patients, and these mutations may reduce survival time in some cases.38 Therapeutic strategies that inhibit the PI3K/AKT pathway have demonstrated efficacy in CRC.39,40
We conducted an experimental validation based on our network pharmacology, molecular docking, and theoretical inferences. MTT assay and cloning experiments showed that Tan-IIA significantly inhibited CRC cell proliferation in a concentration-dependent manner. Tan-IIA also inhibited cell migration and promoted apoptosis, as observed in the wound healing and Annexin V-PI double staining assays. Additionally, we measured the expression of Bcl-2 and Bax. Aberrant activation of the PI3K/AKT pathway is known to cause abnormal Bcl-2 family expression,41 which aligns with the PPI analysis results. The Bcl-2 family is critical for apoptosis and is closely related to the mitochondrial apoptotic pathway. Tumor cells suppress pro-apoptotic factors by upregulating the anti-apoptotic factors of the Bcl-2 family, thereby preventing apoptosis.42 In our experiments, Tan-IIA substantially lowered the expression of Bcl-2 and enhanced the expression of Bax, indicating that Tan-IIA could promote apoptosis in CRC cells by affecting the mitochondrial apoptotic pathway. Furthermore, Tan-IIA treatment did not alter the total PI3K and AKT levels in CRC cells but markedly diminished p-PI3K and p-AKT levels in a concentration-dependent manner. The suppressive role of Tan-IIA in the PI3K/AKT pathway was reversed by IGF-1, indicating that the PI3K/AKT pathway is crucial for the therapeutic effects of Tan-IIA. However, this study had some limitations, such as the lack of further validation of the effects of Tan-IIA in in vivo experiments, which will be addressed in future studies.
Conclusion
This study investigated the roles and mechanisms of Tan-IIA in treating CRC using network pharmacological prediction and molecular docking. Additionally, relevant in vitro experiments were conducted to validate these findings. Tan-IIA suppressed CRC cell proliferation and migration and induced apoptosis via the PI3K/AKT pathway (Figure 15). This study offers theoretical support and experimental evidence for using Tan-IIA in clinical treatment and highlights its potential as a therapeutic agent for CRC.
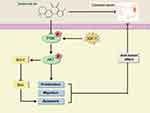 |
Figure 15 A model of Tan-IIA inhibition of CRC progression is described in this study. |
Abbreviations
AKT, protein kinase B; AMPK, AMP-activated protein kinase; CRC, colorectal cancer; DAVID, Database for Annotation, Visualization, and Integrated Discovery; DMSO, dimethyl sulfoxide; FITC, fluorescein isothiocyanate; GEPIA, Gene Expression Profiling Interactive Analysis; GO, gene ontology; HPA, Human Protein Atlas; IGF, insulin-like growth factor-1; KEGG, Kyoto Encyclopedia of Genes and Genomes; MTT, methylthiazolyldiphenyl-tetrazolium bromide; PI, propidium iodide; PI3K, phosphoinositide 3-kinase; PIP3, phosphatidylinositol-3,4,5-triphosphate; PPI, protein-protein interaction; Tan-IIA, tanshinone IIA; TCM, traditional Chinese medicine; TCMSP, Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform; TTD, Therapeutic Target Database; UniProt, Universal Protein Resource.
Data Sharing Statement
The data used to substantiate the results of this experiment may be available upon request from the corresponding author.
Ethical Approval and Informed Consent
This study was approved by the Institutional Ethics Committee of Jining Medical University (JNMC-YX-2025-055).
Consent for Publication
All authors have consented to the publication of this manuscript.
Acknowledgments
We want to thank Professor Wenzhi Shen for his guidance on the experimental design and some of the experimental reagents and Professor Rong Wang for financial support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81603509 to Bin Yu); the Health Commission of Shandong Province (No. M20245103 to Min Sun); the Key Discipline of Formulary Science of Shandong Province (No. 20221012 to Rong Wang); the Innovative Team Project of Combined Traditional Chinese and Western Medicines for Treating Spleen and Stomach Diseases of Jining Medical College (No. jy201918 to Rong Wang), and Student Innovation Training Program of Jining Medical University (No. cx2023010z and No. S202410443157 to Min Sun).
Disclosure
The authors declare no conflicts of interest.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clinicians. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Chen S, Cao Z, Prettner K, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023;9(4):465. doi:10.1001/jamaoncol.2022.7826
3. Poturnajova M, Furielova T, Balintova S, Schmidtova S, Kucerova L, Matuskova M. Molecular features and gene expression signature of metastatic colorectal cancer (Review). Oncol Rep. 2021;45(4). doi:10.3892/or.2021.7961
4. Qu R, Ma Y, Zhang Z, Fu W. Increasing burden of colorectal cancer in China. Lancet Gastroenterol Hepatol. 2022;7(8):700. doi:10.1016/s2468-1253(22)00156-x
5. Li Z, Feiyue Z, Gaofeng L. Traditional Chinese medicine and lung cancer——From theory to practice. Biomed Pharmacother. 2021;137. doi:10.1016/j.biopha.2021.111381
6. Liu Y, Yang S, Wang K, et al. Cellular senescence and cancer: focusing on traditional Chinese medicine and natural products. Cell Proliferation. 2020;53(10). doi:10.1111/cpr.12894
7.. Wu S, Sun Z, Guo Z, et al. The effectiveness of blood-activating and stasis-transforming traditional Chinese medicines (BAST) in lung cancer progression-a comprehensive review. J Ethnopharmacol. 2023:314. doi:10.1016/j.jep.2023.116565
8. Meim X-D, Cao Y-F, Che -Y-Y, et al. Danshen: a phytochemical and pharmacological overview. Chin J Nat Med. 2019;17(1):59–80. doi:10.1016/s1875-5364(19)30010-x
9.. Ansari MA, Khan FB, Safdari HA, et al. Prospective therapeutic potential of Tanshinone IIA: an updated overview. Pharmacol Res. 2021:164. doi:10.1016/j.phrs.2020.105364
10. Guan Z, Chen J, Li X, Dong N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci Rep. 2020;40(8). doi:10.1042/bsr20201807
11. Liu Z, Zhu W, Kong X, et al. Tanshinone IIA inhibits glucose metabolism leading to apoptosis in cervical cancer. Oncol Rep. 2019. doi:10.3892/or.2019.7294
12. Huo J, Qian J, Cao Y, et al. Tanshinone IIA alleviates the biological characteristics of colorectal cancer via activating the ROS/JNK signaling pathway. Anti-Cancer Agent Med Chem. 2023;23(2):227–236. doi:10.2174/1871520622666220421093430
13. Li X, Liu Z, Liao J, Chen Q, Lu X, Fan X. Network pharmacology approaches for research of Traditional Chinese medicines. Chin J Nat Med. 2023;21(5):323–332. doi:10.1016/s1875-5364(23)60429-7
14. Zhao L, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023:309. doi:10.1016/j.jep.2023.116306
15. Gao J, Yang S, Xie G, Pan J, Zhu F. Integrating network pharmacology and experimental verification to explore the pharmacological mechanisms of aloin against gastric cancer. Drug Des Devel Ther. 2022;16:1947–1961. doi:10.2147/dddt.S360790
16. Wang K, Miao X, Kong F, et al. Integrating network pharmacology and experimental verification to explore the mechanism of effect of Zuojin pills in pancreatic cancer treatment. Drug Des Devel Ther. 2021;15:3749–3764. doi:10.2147/dddt.S323360
17. Paggi JM, Pandit A, Dror RO. The art and science of molecular docking. Annu Rev Biochem. 2024;93(1):389–410. doi:10.1146/annurev-biochem-030222-120000
18. Rong L, Li Z, Leng X, et al. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed Pharmacother. 2020;122:109726. doi:10.1016/j.biopha.2019.109726
19. Jiang X, Du W, Yang C, et al. TBX21 attenuates colorectal cancer progression via an ARHGAP29/RSK/GSK3β dependent manner. Cell Oncol. 2023;46(5):1269–1283. doi:10.1007/s13402-023-00809-6
20. Leiphrakpam P, Are C. PI3K/Akt/mTOR signaling pathway as a target for colorectal cancer treatment. Int J Mol Sci. 2024;25(6):3178. doi:10.3390/ijms25063178
21. Kandhavelu J, Subramanian K, Naidoo V, et al. A novel EGFR inhibitor, HNPMI, regulates apoptosis and oncogenesis by modulating BCL‐2/BAX and p53 in colon cancer. Br J Pharmacol. 2023;181(1):107–124. doi:10.1111/bph.16141
22. Morgan E, Arnold M, Gini A, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–344. doi:10.1136/gutjnl-2022-327736
23. Zhou M, Yue Y, Wang Y, Yan S. Polysaccharides from Chinese herbs as natural weapons against colorectal cancer. Biosci Rep. 2023;43(5). doi:10.1042/bsr20230041
24. Yang F-R, Li S-Y, Hu X-W, Li X-R, Li H-J. Identifying the antitumor effects of curcumin on lung adenocarcinoma using comprehensive bioinformatics analysis. Drug Des Devel Ther. 2022;16:2365–2382. doi:10.2147/dddt.S371420
25. Zhao H, Han B, Li X, et al. Salvia miltiorrhiza in breast cancer treatment: a review of its phytochemistry, derivatives, nanoparticles, and potential mechanisms. Front Pharmacol. 2022;13. doi:10.3389/fphar.2022.872085
26. Guo R, Li L, Su J, et al. Pharmacological activity and mechanism of Tanshinone IIA in related diseases. Drug Des Devel Ther. 2020;14:4735–4748. doi:10.2147/dddt.S266911
27. Zhang W, Liu C, Li J, et al. Tanshinone IIA: new perspective on the anti-tumor mechanism of A traditional natural medicine. Am J Chin Med. 2022;50(01):209–239. doi:10.1142/s0192415x22500070
28. Zhou Y, Ye Z, Wei W, et al. Macrophages maintain mammary stem cell activity and mammary homeostasis via TNF-α-PI3K-Cdk1/Cyclin B1 axis. NPJ Regen Med. 2023;8(1). doi:10.1038/s41536-023-00296-1
29. Kwon H-W, Shin J-H, Rhee MH, Park C-E, Lee D-H. Anti-thrombotic effects of ginsenoside Rk3 by regulating cAMP and PI3K/MAPK pathway on human platelets. J Ginseng Res. 2023;47(6):706–713. doi:10.1016/j.jgr.2023.04.006
30. Morgos D-T, Stefani C, Miricescu D, et al. Targeting PI3K/AKT/mTOR and MAPK signaling pathways in gastric cancer. Int J Mol Sci. 2024;25(3):1848. doi:10.3390/ijms25031848
31. Glaviano A, Foo ASC, Lam HY, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22(1). doi:10.1186/s12943-023-01827-6
32. Ahmad I, Hoque M, Alam SSM, Zughaibi TA, Tabrez S. Curcumin and plumbagin synergistically target the PI3K/Akt/mTOR pathway: a prospective role in cancer treatment. Int J Mol Sci. 2023;24(7):6651. doi:10.3390/ijms24076651
33. Hoxhaj G, Manning BD. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2019;20(2):74–88. doi:10.1038/s41568-019-0216-7
34. Wang Y, Zhao T, Huang C, et al. Effect and mechanism of Banxia Xiexin decoction in colorectal cancer: a network pharmacology approach. Phytomedicine. 2024;123:155174. doi:10.1016/j.phymed.2023.155174
35. Cheng Y, Bai F, Ren X, et al. Phosphoinositide-binding protein TIPE1 promotes alternative activation of macrophages and tumor progression via PIP3/Akt/TGFβ axis. Cancer Res. 2022;82(8):1603–1616. doi:10.1158/0008-5472.Can-21-0003
36. Li Q, Li Z, Luo T, Shi H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol Biomed. 2022;3(1). doi:10.1186/s43556-022-00110-2
37. Liu R, Chen Y, Liu G, et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020;11(9). doi:10.1038/s41419-020-02998-6
38. Ogino S, Nosho K, Kirkner GJ, et al. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol. 2009;27(9):1477–1484. doi:10.1200/jco.2008.18.6544
39. Belli C, Repetto M, Anand S, Porta C, Subbiah V, Curigliano G. The emerging role of PI3K inhibitors for solid tumour treatment and beyond. Br J Cancer. 2023;128(12):2150–2162. doi:10.1038/s41416-023-02221-1
40. Yu L, Wei J, Liu P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semi Cancer Biol. 2022;85:69–94. doi:10.1016/j.semcancer.2021.06.019
41. Chen S, Bie M, Wang X, et al. PGRN exacerbates the progression of non-small cell lung cancer via PI3K/AKT/Bcl-2 antiapoptotic signaling. Genes Dis. 2022;9(6):1650–1661. doi:10.1016/j.gendis.2021.05.005
42. Lopez A, Reyna DE, Gitego N, et al. Co-targeting of BAX and BCL-XL proteins broadly overcomes resistance to apoptosis in cancer. Nat Commun. 2022;13(1). doi:10.1038/s41467-022-28741-7
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Effects of Salidroside Combined with Paclitaxel on Proliferation, Migration, and Epithelial Mesenchyme of Colorectal Cancer Cells
Hao Y, Li Z, Chang M, Zhang X
Drug Design, Development and Therapy 2022, 16:4079-4089
Published Date: 28 November 2022