Back to Journals » Journal of Inflammation Research » Volume 18
NLRP3 Inflammasome-Mediated Pyroptosis in Diabetic Nephropathy: Pathogenic Mechanisms and Therapeutic Targets
Authors Chen Y, Chen R , Ji X, Zeng Z, Guan C
Received 20 March 2025
Accepted for publication 15 June 2025
Published 25 June 2025 Volume 2025:18 Pages 8399—8418
DOI https://doi.org/10.2147/JIR.S524246
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Wenjian Li
Yichao Chen,1 Riqiu Chen,2 Xiaozhen Ji,2 Zhifu Zeng,2 Changrong Guan2
1School of Medicine, Hangzhou Normal University, Hangzhou, Zhejiang, 310000, People’s Republic of China; 2Department of Endocrinology, The Lishui Hospital of Wenzhou Medical University, The First Affiliated Hospital of Lishui University, Lishui People’s Hospital, Lishui, Zhejiang, 323000, People’s Republic of China
Correspondence: Changrong Guan, Department of Endocrinology, The Lishui Hospital of Wenzhou Medical University, The First Affiliated Hospital of Lishui University, Lishui People’s Hospital, Lishui, Zhejiang, 323000, People’s Republic of China, Tel +86-18957097205, Email [email protected]
Abstract: Diabetic nephropathy (DN) is a prevalent microangiopathic manifestation of diabetes mellitus (DM) and a pathological sequela of chronic glycemic disorders, characterized by several pathological features including glomerulosclerosis, podocyte loss, tubular epithelial atrophy and abnormal extracellular matrix accumulation. A growing body of research has underscored that chronic inflammatory microenvironments play a central role in the progression of DN. Pyroptosis, a newly defined form of programmed inflammatory necrosis, operates through the following molecular mechanism: inflammasome activation, gasdermin D (GSDMD)-mediated plasma membrane perforation and pro-inflammatory mediator release. Pyroptosis is triggered by the activation of the NOD-like receptor 3 (NLRP3) inflammasome. Classical (caspase-1) or non-classical (caspase-4/5/11) pathways activate pyroptosis by cleaving GSDMD, inducing enzymatic fragmentation of the GSDMD protein. GSDMD-N-terminal domain oligomerizes to form transmembrane pores, which further disrupt cellular osmotic homeostasis as well as membrane integrity. Inflammatory cascades are triggered when IL-1 and IL-18 are released as a result of subsequent cell lysis. This review systematically elucidates the pathobiological interplay between pyroptosis regulatory networks and the pathogenesis of DN and summarizes potential therapeutic compounds that mitigate pyroptosis by inhibiting NLRP3 inflammasome activation or blocking GSDMD pore formation. Preclinical studies suggest that targeting pyroptosis-related signaling molecules including NLRP3, caspase-1 and GSDMD may alleviate renal injury by suppressing inflammation-driven fibrosis and ameliorating glomerular dysfunction. Current studies emphasize that regulating pyroptosis mechanisms could slow DN progression, providing novel insights into the development of nephroprotective strategies.
Keywords: diabetic nephropathy, inflammation, pyroptosis, NLRP3, Caspase-1/4/5/11, GSDMD, IL-1β and IL-18
Introduction
Diabetic nephropathy (DN), also known as diabetic kidney disease (DKD), is one of the leading causes of mortality in people with diabetes mellitus (DM).1 In addition, DN is a significant microvascular sequela of chronic DM and a predominant contributor to end-stage renal disease (ESRD), which is closely linked to increased mortality in diabetic populations.2–4 Epidemiological analyses reveal a rapid escalation in global diabetes prevalence over recent decades.5 There are 463 million people living with diabetes worldwide in 2019 per International Diabetes Federation (IDF) statistics and epidemiological models predict that the number will surge by 51% to 700 million by 2045.6 DM is a global health crisis due to the expanding diabetic epidemic and DN.7 According to the 2023 IDF report, the global incidence of type 2 diabetes (T2DM)-induced nephropathy surged by 74% from 1.4 million (1990s) to 2.4 million (2017s). Growing evidence suggests that DN is the primary single cause of ESRD.8–10 Studies have also reported that diabetes-related ESRD cases increased globally from 22.1% (2000) to 31.3% (2015).11 The above information underscores the critical need for early intervention and comprehensive management of DN in global public health. It is estimated that more than one-fifth of healthcare spending in the United States is primarily for T2DM with most of this focused on complication management for people with T2DM.12 The burden of DN varies across regions of the globe and affects countries with varying income levels. Patients from low- and middle-income countries are often the least able to cope with the burden of DN and the healthcare costs of the disease.13 Patients in low- and middle-income countries are unable to cope with the burden of DN and most healthcare facilities in these countries are unable to adequately meet the specific needs of precision renal care.14
The pathophysiology of DN progression is induced by impaired glucose homeostasis and ROS-mediated oxidative stress (OS) and prolonged inflammation and maladaptive fibrotic remodeling.15 Accumulating evidence has shown that a variety of biological processes including inflammation, fibrosis of the renal interstitial spaces and OS further induce the progression of DN.16–18 Additionally, current studies have demonstrated that cell death is believed to contribute to the progressive renal injury and depletion in DN.19,20 Emerging mechanistic insights suggest that dysregulated programmed cell death pathways, specifically caspase-dependent apoptosis and gasdermin-mediated pyroptotic pathways, exacerbate diabetic microvascular complications, impair renal function during DN.21
Programmed cell death (PCD) is a genetically encoded mechanism that promotes the elimination of cells.22 PCD regulates the shaping of organisms and maintains tissue stability and function.23–25 The disruption of these pathways may result in developmental anomalies or pathologies.26 Unlike cell necrosis, PCD is an efficient mechanism that can clear aging, damaged and malfunctioning cells, thereby maintaining the health of the tissues and organs. However, PCD can be disrupted or imbalanced, resulting in uncontrolled cell death, affecting cell communication and interactions and promoting disease progression.27,28 DN leads to structural and functional damage to glomeruli through excessive apoptosis or impaired autophagy, ultimately resulting in podocyte PCD.29 A variety of PCD forms such as apoptosis, autophagy, ferroptosis, pyroptosis and necroptosis are associated with podocyte damage in DN.30 However, the underlying molecular mechanisms warrant further investigation. Emerging research delineates the specific roles of distinct PCD modalities such as apoptosis, autophagy and pyroptosis, in mediating inflammatory cascades, metabolic disorders and systemic pathologies.31,32 Apoptosis is the most extensively characterized PCD mechanism and contributes to eliminating superfluous or compromised cells.33–35 Both exogenous and endogenous pathways facilitate apoptosis and activate latent cysteine proteases, known as caspases, to systematically break down cellular components through lysis, ultimately leading to apoptosis.36–38 Normal physiological features of cells depend on apoptosis, but dysfunction leads to pathological cell persistence (eg, tumorigenesis) while overactivation leads to tissue atrophy (eg, neurodegenerative diseases, ischemic injury).34,39,40 Autophagy is a lysosome-dependent degradation system and maintains cellular homeostasis through the recycling of organelles and the degradation of proteins.41 Impaired autophagy inversely affects post-mitotic cell populations such as neurons and cardiomyocytes),42 leading to neurodegeneration and cardiac dysfunction.43–45 A similar contradiction exists in tumorigenesis. The catabolic process of autophagy regulates bioenergetic needs by recycling cellular components.46 Tumor cells maintain viability under metabolic stress, which can have adverse effects on the body.47 DN is characterized by abnormal autophagy, which leads to fibrotic remodeling of the kidneys.48 Furthermore, exploring PCD in the context of DN may offer novel strategies to treat DKD.
Pyroptosis is an inflammatory form of caspase-dependent PCD in eukaryotic cells, which is characterized by cell swelling and plasma membrane large bubbles blowing, pore-induced intracellular traps (PITs) forming plasma membrane rupturing and pro-inflammatory intracellular contents releasing such as mature IL-1β and IL-18.49–51 The released intracellular contents attract more immune cells and trigger local inflammation, thereby strengthening the immune defense function of cells.52 This process is initiated by inflammasome activation, which further triggers proteolytic cleavage of gasdermin family proteins (eg, GSDMD) and subsequent plasma membrane pore formation. The features include osmotic disequilibrium, cytosolic content efflux and robust secretion of pro-inflammatory cytokines such as IL-1β and IL-18. Excessive pyroptosis drives uncontrolled and persistent inflammatory responses, thereby serving as a pathogenic driver of inflammatory disorders.53 A growing body of evidence suggests that inflammation plays a contributory role in the pathogenesis of DN.15,54,55 The persistence of pyroptosis can accelerate the progression of renal inflammation by mediating dysregulation of the chronic inflammatory microenvironment. Mechanistically, the activation of pyroptotic signaling pathways exacerbates insulin resistance and metabolic dysfunction in DN.56–59 Consequently, inflammasome activation and pyroptotic cascades critically contribute to the pathogenesis of diabetic complications, particularly DN.60 However, recent papers have reported that pyroptosis is accompanied by a massive expression of pro-inflammatory mediators, leading to the progression of diabetes.52,60,61 Qiu et al suggested that hyperglycemia-induced pyroptosis aggravated myocardial ischemia/reperfusion injury in diabetic rats.62 Che et al have also confirmed that high glucose induces pyroptosis in neuronal cells.63 Few studies have focused on the participation of pyroptosis-dependent signaling pathways in the pathogenesis of DN.
This narrative review systematically examines the molecular mechanisms underlying pyroptotic cell death and elucidates how the interplay between the inflammasome and pyroptosis exacerbates renal pathology in DN. We also summarize current evidence on emerging therapeutic strategies targeting inflammasome activation and pyroptotic signaling pathways, providing new clinical insights into the treatment and management of DN.
Molecular Mechanisms of Pyroptosis
Pyroptosis was initially characterized as an apoptotic-like process in macrophages infected with Salmonella typhimurium64 and Shigella flexneri65 during the 1990s. In 2001, Cookson and Brennan formally differentiated this lytic cell death from apoptosis by coining the term “pyroptosis”66 The pyroptotic cascade comprises four key steps: (1) inflammasome activation, where multiprotein complexes (eg, NLRP3, AIM2) sense pathogen or damage-associated molecular patterns (PAMPs/DAMPs); (2) caspase-mediated signaling, triggering downstream effector pathways; (3) Gasdermin (GSDM) protein cleavage, which liberates the pore-forming N-terminal domain; and (4) plasma membrane permeabilization, driven by GSDM oligomerization. These transmembrane pores disrupt cellular water balance, causing cell enlargement and destruction, release of intracellular components and secretion of inflammation-triggering mediators including IL-1β and IL-18, thereby amplifying systemic inflammation.67,68 Pyroptosis is primarily mediated through canonical (caspase-1-dependent) and non-canonical (caspase-4/5/11-dependent) pathways.
Canonical Pyroptosis
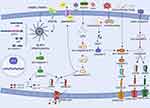
Inflammasome activation is initiated by PAMPs or DAMPs, which further induce the assembly of multiprotein signaling platforms essential for innate immune defense (Figure 1). The inflammasomes consist of intracellular pattern recognition receptors (PRRs), apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), and inflammatory caspases.69 Several PRRs have been identified including the NLR family (NLRP1/3/NLRC4), absent in melanoma 2 (AIM2) and pyrin with the NLRP3 inflammasome serving as the central mediator of canonical pyroptosis.70–72 The NLRP3 protein consists of three distinct functional domains: a nucleotide-binding oligomerization domain (NOD), a leucine-rich repeat (LRR) at the C terminus, which recognizes PAMPs and DAMPs and a pyrin domain (PYD) at the N terminus that recruits ASCs through homotypic interactions.73 Recent studies have found that NIMA-related kinase 7 (NEK7) controls NLRP3 oligomerization, ASC speck formation and caspase-1 activation downstream of potassium efflux, a key upstream signal for inflammasome activation.74 NEK7 mediates the conformational rearrangement of NLRP3 into its active oligomeric state by binding the LRR domain.75 ASC bridges NLRP3 and pro-caspase-1 through its bipartite architecture: its N-terminal PYD binds NLRP3, while the C-terminal caspase activation and recruitment domain (CARD) interacts with pro-caspase-1. Ligand binding triggers NLRP3 oligomerization and ASC speck formation, a process further stabilized by NEK7-mediated scaffolding, thereby facilitating proximity-induced autocleavage of pro-caspase-1 into its active form. Active caspase-1 has a dual function. It processes immature IL-1β/IL-18 into bioactive cytokines while cleaving GSDMD to liberate its N-terminal pore-forming fragment (GSDMD-NT).76 GSDMD-NT subunits released from the cell cause cell swelling and rupture by self-assembling into nanoscale (10–15 nm) transmembrane pores. This process results in pyroptosis, which leads to the proliferation of IL-18 and the initiation of a self-amplifying inflammatory cascade.
Non-Canonical Pyroptosis
Cytosolic LPS, a structural hallmark of Gram-negative bacteria, directly activates the precursors of caspase-4, −5 and −11.77–81 The activated caspases cleave GSDMD and release GSDMD-NT to disrupt the integrity of plasma membranes. Research has shown that nerve injury-induced protein 1 (NINJ1) is also necessary for inducing plasma membrane rupture during pyroptosis.82 In addition, NINJ1 forms oligomeric clusters at the plasma membrane, which may result in catastrophic disintegration and the release of pro-inflammatory cytokines.83 The caspases-4/5/11 indirectly trigger intracellular IL-18 production by activating the NLRP3 inflammasome, which recruits caspase-1 to mature and produce IL-18.84 Caspase-11 further amplifies this cascade by proteolyzing the pannexin-1 membrane channel, triggering the massive release of ATP.85 Extracellular ATP then binds P2X7 receptors (P2X7R), opening additional K⁺ efflux channels and reinforcing NLRP3 inflammasome assembly.85 This self-sustaining cycle can synergistically amplify pyroptotic cell death and inflammatory cytokine release by linking membrane permeabilization, ion dysregulation and inflammasome activation.
Other Signaling Pathways
Emerging evidence reveals functional crosstalk between apoptotic caspases and pyroptotic pathways.86–88 Caspases-3 can also mediate GSDME-dependent pyroptosis, which has traditionally been considered the primary regulator of apoptosis. Chemotherapeutic drugs and TNF-α trigger caspase-3 to cleave GSDME, releasing its N-terminal pore-forming fragment (GSDME-NT). This fragment translocates to the plasma membrane inducing lytic cell death.89 Caspase-3 activation can occur through apoptotic initiators: caspase-8 (activated by death receptor oligomerization) or caspase-9 (triggered by mitochondrial cytochrome c release via APAF-1).90 In addition to activating caspase-3, caspase-8 can also be activated by Yersinia outer protein-J (Yop-J) and directly induces cleavage of GSDMD to drive pyroptosis.91 Death receptor signaling facilitates caspase-8-mediated proteolysis of GSDMC, resulting in the release of its pore-forming domain (GSDMC-NT).92 Disruption of mitochondrial homeostasis, endoplasmic reticulum stress signaling and redox imbalance (ROS overproduction) further amplifies pyroptosis by activating the caspase-9/3/GSDME axis, thereby linking organelle damage to inflammatory cell death.93 These findings highlight the mechanistic plasticity of caspase in coordinating the apoptosis-pyroptosis transition in different pathological contexts.
Pyroptosis in the Pathogenesis and Progression of DN
DN is a multifactorial inflammation-driven pathology characterized by heterogeneous mechanisms encompassing hemodynamic dysregulation, impaired glucose homeostasis, OS and genetic predisposition.94–96 Evidence indicates that pyroptosis contributes the progression of DN by inducing sustained inflammatory cascades and damaging renal parenchyma.97–99
Pyroptosis in Podocytes
The glomerular filtration barrier, composed of podocytes, endothelial cells and the glomerular basement membrane, is indispensable for renal filtration homeostasis.100,101 DN is caused by the loss of podocytes, terminally differentiated cells at the outermost layer of this barrier.102 Both clinical and experimental studies have consistently demonstrated that podocyte injury is a key player in the progression of DN.103,104 Under hyperglycemic conditions, ROS overgeneration and mitochondrial dysfunction induce oxidative stress, inflammatory activation and cytoskeletal disruption in podocytes, which further promotes apoptosis, detachment and proteinuria.105,106 Ample evidence suggests that diabetic glomerular hyperfiltration imposes mechanical stress on podocytes, exacerbating foot process effacement and accelerating cell loss even in early-stage DN.107–109
Emerging evidence suggests that pyroptosis plays a key role in mediating podocyte demise in DN.110 High glucose upregulates caspase-4/11 and promotes the cleavage of GSDMD in podocytes, whereas inhibition of pyroptosis ameliorates the progression of DN by preserving podocyte integrity and suppressing local inflammation.111 Wu et al further demonstrated that NLRP3 inflammasome blockade mitigates podocyte damage through reducing lipid accumulation in diabetic kidneys.112 The stimulator of interferon genes (STING) pathway has been implicated in regulating NLRP3-dependent pyroptosis.113 STING deficiency attenuates podocyte inflammation, highlighting its therapeutic relevance.114 Mechanistically, the overactivation of the mammalian target of rapamycin (mTOR) in diabetic podocytes enhances NF-κB p65 activity, thereby triggering NLRP3-dependent pyroptosis.115
Multifaceted regulatory mechanisms have been discovered in studies of molecular pathways. LPAR1 regulates lysophosphatidic acid (LPA) signaling at the Egr1 promoter, resulting in increased H3K27 trimethylation.116 This epigenetic alteration elevates Egr1 levels, which in turn activate NLRP3 inflammasomes and pyroptosis in podocytes of streptozotocin (STZ)-induced diabetic mice.116–118 Pharmacological interventions such as dapagliflozin and puerarin exhibit protective effects by targeting the miR-155-5p/HO-1/NLRP3 axis and modulating SIRT1/NLRP3/caspase-1 signaling, respectively.119,120 Additionally, activation of the SIRT6/HIF-1α pathway ameliorates pyroptosis,121 while deficiency of ATP-binding cassette transporter A1 (ABCA1) exacerbates non-canonical pyroptosis-related genes such as caspase-4/11, GSDMD, caspase-1 and IL1β via reduction–oxidation factor 1 (Ref-1)/Apurinic/apyrimidinic endonuclease 1 (APE1) axis.122 Collectively, the findings suggest that pyroptosis is a key driver of podocyte loss, thereby providing an array of molecular targets for therapeutic intervention for DN.
Pyroptosis in Glomerular Endothelial Cells (GECs)
Hyperglycemia-induced damage to GECs occurs in the DN because they are exposed directly to circulating metabolites and inflammatory mediators.123 GEC injury is correlated with podocyte foot process effacement in diabetic mouse models, occurring as early as three weeks after the onset of diabetes.124 Endothelial dysfunction disrupts glomerular permeability and accelerates kidney disease progression, underscoring the importance of GECs in the pathogenesis of DN.125 Hyperglycemia exacerbates metabolic disturbances in GECs, promoting ROS overproduction, mitochondrial dysfunction and subsequent pyroptotic cell death.126 Shen et al demonstrated that notoginsenoside Fc reduces ROS accumulation and alleviates mitochondrial damage by suppressing the expression of HMGCS2, thereby mitigating GEC pyroptosis.127 Tanshinone IIA (Tan-IIA) attenuates NLRP3 inflammasome activation by decreasing the expression of TXNIP, thereby preserving the integrity of GEC in diabetic kidneys.128 The ELABELA (ELA) peptide reversed elevated NLRP3 levels in glomerular endothelial in db/db mice through the inhibition of AMP-activated protein kinase (AMPK), demonstrating the possibility of a novel therapeutic strategy.129
Mechanistic studies further elucidate the involvement of canonical and non-canonical pyroptotic pathways in GEC injury.61,130 Sodium butyrate alleviates hyperglycemia-induced pyroptosis by blocking caspase-1/GSDMD signaling.131 Hirudin targets Irf2 to inhibit GSDMD cleavage and renal damage in STZ-induced DN mice.132 Intriguingly, caspase-11/GSDMD activation drives non-canonical pyroptosis in GECs, as evidenced by reduced renal injury following GSDMD knockout.133 Neutrophil extracellular traps (NETs) also exacerbate GEC pyroptosis in diabetic conditions. Both DNase I treatment and PAD4 gene deletion ameliorate glomerulopathy by degrading NETs and suppressing pyroptosis.21 These findings collectively establish GEC pyroptosis as a critical mediator of diabetic glomerular injury, offering multiple targets for intervention including inflammasome modulation, redox balance restoration and NETs neutralization.
Pyroptosis in Glomerular Mesangial Cells (GMCs)
Glomerular mesangial cells (GMCs) regulate blood flow dynamics, maintain mesangial matrix homeostasis and maintain glomerular capillary architecture.134 Hyperglycemia promotes macrophage-GMC crosstalk and excessive production of inflammatory cytokines and extracellular matrix proteins in diabetic patients. Notably, macrophage-derived exosomes exacerbate diabetic kidney injury by activating NLRP3 inflammasomes in GMCs,135 positioning these cells as central mediators of diabetic nephropathy (DN) progression.
Recent studies have revealed molecular mechanisms governing the pyroptosis of GMCs. Du et al identified circLARP1B as a pro-pyroptotic regulator in high glucose (HG)-treated GMCs. Overexpression of circLARP1B upregulates NLRP3, caspase-1 and IL-1β/IL-18, whereas its silencing suppresses pyroptosis and inflammation via the miR-578/TLR4 axis.136 Similarly, Zhan et al demonstrated that long non-coding RNA -Neat1 (NEAT1) is upregulated in HG-treated rat mesangial cells, influencing pyroptosis and inflammation of DN by modulating the miR-34c/NLRP3 signaling axis.137 These findings highlight that non-coding RNAs are key epigenetic regulators of GMC pyroptotic pathways.
The erythroid 2-related factor 2 (Nrf2)/HO-1 axis represents another therapeutic target for DN. Lu et al discovered that activating Nrf2 signaling attenuated glucose-induced oxidative stress and inflammation in diabetic kidneys.138,139 Specifically, the synthetic Nrf2 activator AB-38b inhibits NLRP3 and IL-1β expression in diabetic GMCs via the Nrf2/HO-1/NLRP3 cascade, demonstrating its potential to counteract pyroptosis-driven ECM accumulation.140 Collectively, these studies indicate that GMC pyroptosis plays a critical role in the progression of glomerulosclerosis and DN.
Pyroptosis in Tubular Epithelial Cells (TECs)
Diabetic tubular injury is driven by hyperglycemia-induced oxidative stress, proteinuria and extracellular matrix (ECM) deposition, which further promote tubular necrosis, apoptosis and epithelial-mesenchymal transition.141,142 TECs death exacerbates renal dysfunction and accelerates DN progression. Several clinical and experimental studies have shown that TEC pyroptosis plays an important role in the pathogenesis of DN.143,144 GSDMD expression is higher in renal biopsies from DN patients and TECs and GSDMD levels are positively correlated with tubular injury severity in DN patients.145 Discoid domain receptor 1 (DDR1) knockdown mitigates HG-induced pyroptosis by suppressing the NF-κB/NLRP3 axis, suggesting a regulatory role for this pathway.146 TLR4/NF-κB signaling is activated in diabetic TECs, resulting in the assembly of NLRP3 inflammasomes and the induction of caspase-1-dependent pyroptosis. The inhibition of TLR4 could reduce the activation of NF-kB, thereby reducing GSDMD cleavage and IL-1β secretion in HG-exposed renal tubular epithelial cells (HK-2), further validating this mechanism.147,148
Non-coding RNAs (lncRNAs) regulate pyroptosis in TECs.149 LncRNA XIST exacerbates pyroptosis by upregulating miR-15b-5p to enhance TLR4/NLRP3 signaling, whereas its silencing alleviates tubular injury.150 The lncRNA MALAT1 suppresses miR-30c to induce NLRP3 inflammasome activation and the inhibition of MALAT1 inhibits caspase-1 and IL-1β release.151,152 The novel lncRNA, PWARSN, is upregulated in proximal TECs of DKD patients and aggravates pyroptosis through activating the TXNIP/NLRP3 pathway.153 HG-induced DNA damage activates AIM2 inflammasomes, leading to pyroptosis in proximal TECs.154 Human umbilical cord mesenchymal stem cells (hUC-MSCs) inhibit NLRP3/caspase-1 signaling and alleviate pyroptosis of renal tubular epithelial cells.155 These findings indicate that suppression of pyroptosis provides a promising therapeutic avenue in the treatment of DN.
Suppressing the Pyroptosis-Related Signaling Pathways for the Therapeutic Regulation of DN
Triptolide
Triptolide is a bioactive diterpenoid derived from Tripterygium wilfordii Hook F with multimodal therapeutic properties. This compound exerts several functions such as anti-inflammatory, antioxidant, hypoglycemic and antifibrotic effects.156–158 Diabetic nephropathy (DN) is characterized by hyperglycemia-induced OS, which induces podocyte injury through the production of reactive oxygen species (ROS) and lipid peroxidation.159 TP suppresses NLRP3 inflammasome activation by activating nuclear factor Nrf2, a master regulator of redox homeostasis.140 Mechanistically, Lv et al demonstrated that TP alleviates podocyte pyroptosis and suppresses the secretion levels of IL-1β and IL-18 via the Nrf2/ROS/NLRP3 axis, thereby attenuating glomerular inflammation and injury.160
Studies have revealed that TP modulates both proliferative and fibrotic pathways in DN.160 Han et al revealed that TP inhibits mesangial cell hyperplasia by suppressing the PDK1/Akt/mTOR cascade, while concurrently ameliorating glomerulosclerosis through miR-137/Notch1 signaling regulation.161,162 The multi-targeted action of TP in combating oxidative stress, inhibiting inflammasomes and modulating epigenetics make it an attractive candidate for the treatment of DN.
Sanziguben Polysaccharides
Sanziguben polysaccharides (SZP) are a primary component of Sanzigube, which show promising renoprotective effects in DN by inhibiting gut-renal axis modulation and inflammasome.163 Experimental studies demonstrate that SZP treatment reverses hyperglycemia-induced TLR4/NF-κB/NLRP3 pathway activation in DN mice, downregulating ASC, caspase-1, and pro-inflammatory cytokines.164 Notably, diabetic renal injury is exacerbated by gut dysbiosis characterized by reduced microbial diversity and overgrowth of LPS-producing Gram-negative bacteria.165,166 SZP ameliorates this pathological crosstalk by restoring intestinal microbiota homeostasis, thereby reducing LPS translocation through compromised intestinal barriers and subsequent TLR4-mediated renal inflammation.167 Furthermore, Zhou et al revealed that SZP inhibits NF-κB nuclear translocation in glomerular cells, attenuating cytokine-driven mesangial expansion and tubular injury.163 Therefore, SZP can be positioned as a natural medicine for the multifaceted treatment of DN as a dual therapeutic modality that simultaneously targets gut-derived endotoxemia and intrarenal inflammation.
Hirudin
Hirudin is a natural component derived from leech salivary glands, which exerts thrombolytic and blood anticoagulant activities. Studies have suggested that hirudin can accelerate blood circulation and exert renoprotective effects in DN through multimodal mechanisms.168 Previous studies have also demonstrated that hirudin inhibits the migration and pathological angiogenesis of endothelial cells beyond its well-characterized anticoagulant activity.169 The interferon regulatory factor 2 (Irf2) is essential for triggering caspase-4 activation and subsequent cleavage of the GSDMD, which is another hallmark of non-canonical pyroptosis.170 Han et al demonstrated that hirudin ameliorates glomerular injury in streptozotocin-induced DN mice by targeting Irf2-dependent GSDMD transcriptional activation and proteolytic processing.171 The genetic ablation of GSDMD conferred resistance to diabetic renal damage, validating that this pathway plays an important role in the development of DN. Therefore, hirudin concurrently addressed inflammasome-driven pyroptosis in diabetic kidneys, as well as coagulation abnormalities.
Syringaresinol
Syringaresinol (SYR), a naturally occurring polyphenolic lignan, exerts dual renoprotective mechanisms in DN through redox balance restoration and inflammasome suppression.172 Zhang et al revealed that SYR suppresses macrophage pyroptosis by directly targeting the caspase-1/NLRP3 signaling axis, thereby attenuating inflammatory cytokine production.173 Concurrently, Li et al revealed that SYR enhances nuclear translocation and transcriptional activity of Nrf2 by inhibiting KEAP1/Nrf2 degradation.174 Inflammasome assembly is countered by this mechanism, which eliminates hyperglycemia-induced ROS accumulation. Therefore, SYR mitigates the progression of OS-driven renal injury during DNP by scavenging ROS and inhibiting pyroptosis.
AB-38b
AB-38b, a synthetic α, β-unsaturated carbonyl compound derived from biphenyl diester precursors, possesses significant renoprotection in DN through dual modulation of antioxidant and inflammasome pathways.175–177 Several preclinical studies have demonstrated the efficacy of this agent in reducing serum uremic toxins (BUN, Cr) and LDL-C levels while ameliorating glomerular structural abnormalities such as effacement of the foot process and accumulation of extracellular matrix.140,178 Mechanistically, AB-38b activates the Nrf2/ARE signaling axis by suppressing Keap1-mediated degradation, thereby restoring NQO-1 and HO-1 antioxidant enzyme expression in diabetic renal cortices.178 A pioneering study has revealed that A B-38b inhibits ROS overproduction to disrupt TXNIP/NLRP3 formation and lower caspase-1 activation and IL-1β secretion.140 The coordinated actions on OS and pyroptotic signaling highlight the therapeutic potential of AB-38b, although clinical validation should be evaluated in the future.
Punicalagin
Punicalagin (PU) is the primary active component of pomegranate polyphenols in pomegranate peel and mitigates the progression of DN through antioxidative and pyroptosis-suppressive mechanisms.179,180 Growing evidence suggests that PU can enhance superoxide dismutase (SOD) activity, which may attenuate the production of superoxide and nitric oxide (NO) during hyperglycemia.181–186 The redox-balancing effect of this compound is synergistic with its direct inhibition of TXNIP and NLRP3. The kidneys of diabetic mice showed elevated levels of NLRP3, IL-1β, GSDMD and caspase-1. PU treatment inhibited the reversible increase of the TXNIP/NLRP3 axis expression.179 Previous studies have revealed that PU attenuates both LPS-induced macrophage inflammation and hyperglycemia-induced podocyte pyroptosis.181 The compound may be effective in treating DN and other OS-related nephropathy due to its multimodal effects that include free radical scavenging, suppression of inflammatory mediators, and disassembly of inflammasome complexes.
Carnosine
Carnosine, a water-soluble dipeptide comprising β-alanine and L-histidine, exhibits multifaceted biological properties including anti-inflammatory, antioxidant, anti-glycation and carbonyl scavenging activities.187 Research has demonstrated that carnosine exerts potential therapeutic effects in the treatment of DN by modulating pyroptotic pathways.188 Moreover, carnosine attenuated LPS-induced NLRP3 inflammasome activation and pyroptosis in aged rats with cognitive dysfunction.189 The authors demonstrated that carnosine mitigates HG-induced podocyte injury by inhibiting NLRP3 inflammasome assembly and its downstream effectors. Zhu et al reported that carnosine treatment in STZ-induced diabetic mice reduced renal levels of NLRP3, ASC, pro-IL-1β, mature IL-1β and IL-18.190 The immunofluorescence analysis indicated that caspase-1 and GSDMD colocalized with synaptopodin and carnosine effectively inhibited pyroptotic activity.188 Studies have revealed that carnosine attenuates OS and mitigates inflammation in renal cells under high glucose conditions.191,192 Therefore, carnosine can be considered a potential therapeutic agent for DN via targeting inflammasome-driven pyroptosis and maintaining glomerular integrity.
Biochanin A
Biochanin A (BCA), a naturally occurring methoxylated isoflavonoid, exhibits broad-spectrum pharmacological activities with therapeutic potential across multiple disease models.193–199 BCA exhibits a wide range of beneficial effects in addition to its well-described neuroprotective, chemopreventive and hepatoprotective properties, positioning it as a versatile therapeutic option for the treatment of metabolic disorders.193,196 In addition, BCA has been shown to be effective in reducing inflammation in both in vitro and in vivo studies.200–202 Treatment with BCA alleviated renal inflammation in mice with unilateral ureteral obstruction by suppressing NLRP3 inflammasome activation and lowering levels of active caspase-1, IL-1β and IL-18.201 Further studies revealed that BCA mitigates mitochondrial dysfunction and attenuates GSDMD-mediated pyroptosis in diabetic kidneys through enhancing antioxidant activity and modulation of the NF-κB/NLRP3 axis in renal tubular epithelial cells.203 Based upon these findings, BCA can inhibit the activation of apoptotic and pyroptotic cell death pathways, providing a multi-target approach for the treatment of DN.
Notoginsenoside Fc
Panax notoginseng is a widely recognized medicinal herb in traditional Chinese medicine, which exhibits a wide range of pharmacological properties including hemostatic, antithrombotic, anti-atherosclerotic and antitumor effects.204–206 More than 150 saponins have been isolated from the roots and leaves of this medicinal herb.207 Notoginsenoside Fc (Fc), a bioactive component, has shown significant therapeutic potential in the treatment of DN.208 Previous studies have shown that Fc enhances the proliferation and migration of endothelial cells in diabetic vascular tissues under HG conditions, thereby promoting vascular repair.209 A pioneering research has revealed that Fc further attenuates HG-induced endothelial dysfunction by suppressing pro-inflammatory cytokine secretion (eg, TNF-α, IL-1β, IL-6, ICAM-1) and regulating apoptotic-proliferative pathways.210 Wei et al reported that Fc alleviates mitochondrial dysfunction and tubular injury in acute kidney injury models through activation of the SIRT3/SOD2 antioxidant axis.211 Mechanistic studies have shown that Fc mitigates pyroptosis in glomerular endothelial cells (GECs) by downregulating key mediators such as TXNIP, NLRP3, cleaved caspase-1 and GSDMD-NT.211 It has been demonstrated that HMGCS2 modulation promotes antipyroptotic effects in HG-induced GECs, as evidenced by reduced mitochondrial damage and pore formation following knockdown of HMGCS2 in HG-stimulated cells.127 Multifaceted mechanisms including anti-inflammatory, autophagy-enhancing and pyroptosis-inhibitory functions, make Fc a promising multi-target agent for the treatment of DN.
Pyrroloquinoline Quinone
Pyrroloquinoline quinone (PQQ), a naturally occurring redox-active quinone compound, exhibits multifaceted biological effects including antioxidant, anti-aging and immunomodulatory activities.212–216 In addition, PQQ possesses promising therapeutic efficacy against ischemia, inflammatory disorders and metabolic dysregulation and produces remarkable neuroprotective effects.212 A study by Wang et al demonstrated that PQQ protects HK-2 against OS-induced apoptosis through activation of the PI3K/AKT/FOXO3a pathway and SIRT3-mediated mitochondrial homeostasis.217 Furthermore, previous studies have shown that PQQ suppresses HG-induced inflammation and cellular senescence in HK-2 cells by inhibiting ROS overproduction and activating the KEAP1/NRF2 antioxidant axis.218 Qu et al further established that PQQ attenuates renal fibrosis and improves glomerular function in diabetic murine models via targeting the ROS/NF-κB/NLRP3 inflammasome signaling cascade.219 These preclinical studies suggest that PQQ may be renoprotective in the treatment of DN by modulating OS, inflammation and pyroptotic responses.
Astragaloside IV
The herb Astragalus membranaceus is an effective renoprotective herb and has been extensively used in traditional Chinese medicine to treat kidney disease.220 Astragaloside IV (AS-IV), a bioactive saponin derived from Astragalus, exhibits multi-target therapeutic efficacy in the treatment of DN.216,221 AS-IV combats OS, suppresses inflammatory responses and inhibits epithelial-mesenchymal transition (EMT) via selective inhibition of Wnt/β-catenin signaling pathway, thereby attenuating HG-induced renal injury.222 Recently, Gao et al reported that AS-IV promotes the phosphorylation of p62, which competitively disrupts KEAP1-NRF2 binding, promoting NRF2 nuclear translocation to alleviate ROS accumulation and renal fibrosis.223 Previous studies have revealed that AS-IV alleviates podocyte pyroptosis and mitochondrial ultrastructural damage by upregulating SIRT6 expression and downregulating HIF-1α, NLRP3, GSDMD and caspase-1.121 Shen et al revealed that AS-IV restores mitochondrial function and counteracts OS-induced podocyte apoptosis in diabetic kidneys by activating the NRF2-ARE/TFAM pathway.224 He et al also reported that AS-IV elevates endogenous Klotho levels, a nephroprotective protein that suppresses NLRP3 inflammasome-mediated pyroptosis, offering a mechanism to preserve glomerular integrity.225 Therefore, AS-IV presents a promising multi-target agent for the treatment of DN by targeting inflammatory and metabolic dysregulations in diabetic renal pathology.
Ginsenosides
Ginseng, a cornerstone of traditional medicine in East Asian cultures for thousands of years, has been extensively used to treat a wide range of pathologies.226 Preclinical and clinical evidence have reported that ginseng exerts remarkable anti-inflammatory properties and provides potential therapeutic effects in the treatment of DN.226 Ginsenosides are the primary bioactive constituents of ginseng, which exhibit a wide range of therapeutic properties including anti-fibrotic, antioxidant and anti-inflammatory actions.227,228 In addition, ginsenosides alleviate apoptosis and pyroptosis-dependent cell death in diabetic kidneys.115,229 Previous studies have found that ginsenosides mitigate renal dysfunction and downregulate pro-inflammatory cytokine levels (eg, IL-1, IL-6, TNF-α) in diabetic models, confirming their anti-inflammatory efficacy.230 A stereoisomer of 20(S)-ginsenoside Rg3 improves insulin sensitivity, lipid metabolism and OS in STZ-induced DN murine models by modulating MAPK and NF-kB signaling.231,232 Ginsenoside Rg5, a key component of black ginseng, inhibits NLRP3 inflammasome activation (ASC, caspase-1) and suppresses IL-1β/IL-18 secretion, leading to the alleviation of DN.233,234 Studies have shown that Rg5 inhibits the nuclear translocation of NF-B and the phosphorylation of p38 MAPK, which further mitigates the inflammatory response.235 In conclusion, these findings suggest that ginsenosides and their derivatives could potentially be used as multi-target drugs for DN intervention, confirming their clinical translation for the treatment of diabetes-associated renal failure.
Tanshinone IIA
Tanshinone IIA (Tan-IIA) is the main lipophilic bioactive constituent of Salvia miltiorrhiza, a traditional Chinese herb renowned for promoting blood circulation and resolving blood stasis.236 Tan-IIA has traditionally been used to treat cardiovascular diseases.237 Several lines of studies have demonstrated that Tan-IIA has therapeutic potential in DN due to its immune modulatory, anti-inflammatory and antioxidant properties.128,237,238 Studies have revealed that Tan-IIA suppresses pro-inflammatory cytokine production and ameliorates metabolic dysfunction in rodent models with T2DM by inhibiting NF-B-mediated inflammatory cascades and enhancing AMPK activity.239 Chen et al demonstrated that Tan-IIA attenuates proteinuria, podocyte foot process effacement and renal fibrosis in diabetic rats by inhibiting extracellular matrix (ECM) deposition and fibroblast activation.240 Wu et al demonstrated that Tan-IIA inhibits hyperglycemia-induced pyroptosis by targeting the TXNIP/TRX1/NLRP3 inflammasome axis in glomerular endothelial cells.128 Therefore, Tan-IIA could be a potential therapeutic drug candidate for the treatment of DN.
GLP-1 Receptor Agonists
The GLP-1 receptor agonist (GLP-1 RA) was initially developed for its glucose-lowering properties through the mimicry of incretin hormones.241 GLP-1 RA suppresses systemic and renal inflammatory responses, thereby mitigating diabetic complications beyond glycemic control.242 In addition, GLP-1 RA reduces urinary albumin excretion, glomerular filtration rate (eGFR) decline and the risk of macroalbuminuria, thus demonstrating their renoprotective properties.243 Mechanistic studies demonstrate that liraglutide, a GLP-1 receptor agonist, inhibits the activation of the NLRP3 inflammasome in diabetic kidneys, downregulates the levels of caspase-1, GSDMD-NT and IL-1, which alleviates podocyte pyroptosis and ameliorates the progression of DN to end-stage renal disease.244 Li et al further demonstrated that hyperglycemia-induced podocyte pyroptosis is driven by NF-κB/NLRP3 pathway activation. GLP-1 RAs such as liraglutide and semaglutide suppress this pathway and downregulate the expression of genes involved in pyroptosis and inflammation.245 GLP-1RAs have demonstrated therapeutic effects, which provide a greater insight into the diagnosis and treatment of DN.
Dapagliflozin
Dapagliflozin, a pioneering sodium-glucose cotransporter 2 (SGLT2) inhibitor approved for T2DM, exerts glycemic control by promoting urinary glucose excretion.246 Oraby et al found that dapagliflozin also exhibits nephroprotective effects in DN by inhibiting renal inflammation, OS and fibrosis and alleviating glucotoxicity-induced apoptosis.247 Recent research has demonstrated that dapagliflozin alleviates podocyte pyroptosis in DN.248 Zhang et al revealed that dapagliflozin inhibits the miR-155-5p/HO-1/NLRP3 axis in diabetic models, whereas palmitic acid (PA)-induced NLRP3 upregulation in podocytes (MPC5 cells) activates caspase-1 and elevates IL-1β/IL-18 levels (Table 1).249 Dapagliflozin reverses this cascade by restoring HO-1 expression and downregulating NLRP3 inflammasome components, thereby attenuating pyroptosis.249 Further mechanistic studies have shown that dapagliflozin inhibits both the NLRP3/caspase-1 pyroptotic pathway and the NF-kB/AMPK/NLRP3 inflammatory-metabolic pathway, suggesting a link between its renoprotective and antipyroptotic properties.250,251 Therefore, dapagliflozin could be considered an effective drug for suppressing renal pyroptosis, which can address both the metabolic dysfunction of DN and pyroptosis-driven renal dysfunction.
 |
Table 1 Compounds/Agents Targeting Pyroptosis-Related Signaling Pathways for the Therapeutic Regulation of DN |
Conclusions, Current Challenges and Future Perspectives
Pyroptosis, a lytic inflammatory cell death mechanism mediated by canonical caspase-1-dependent and non-canonical caspase-4/5/11-dependent pathways, plays a key role in driving renal cellular damage and the progression of DN. Central to this process is the NLRP3-caspase-1-GSDMD signaling axis, which orchestrates inflammasome activation, membrane permeabilization and the release of pro-inflammatory cytokines, linking hyperglycemia-induced cellular stress to clinical manifestations such as proteinuria and glomerular filtration decline.
Pyroptosis is thought to drive the pathogenesis of DN. Hyperglycemia triggers ROS/mTOR/NF-κB signaling in podocytes, leading to the activation of NLRP3 inflammasomes and inducing irreversible damage to the glomerular barrier. Microvascular injury is exacerbated by the activation of caspase-11/GSDMD and NETs-NLRP3 pathways in GECs. TLR4/NF-B triggers AIM2 inflammasome responses in TECs, which lead to pyroptosis. This review establishes pyroptosis as a unifying pathogenic contributor in DN. Current evidence suggests that lncRNAs regulate transcriptional and post-transcriptional networks in DN, thereby promoting renal inflammation.
The findings of this review redefine the pathophysiology of DN. Pyroptosis is emerging as the predominant type of inflammatory cell death, whereas apoptosis and autophagy have traditionally been the focus of research. The relevance of these findings is further enhanced when contextualized within existing research. Pyroptosis is a result of chronic inflammation related to DN. Previous studies have demonstrated that inhibiting inflammasome components may alleviate renal fibrosis and inflammation. This review provides an overview of specific molecular pathways involved in pyroptotic cell death including the NLRP3 inflammasome and GSDMD-mediated pores. This mechanistic explanation confirms existing studies and also provides a more detailed understanding of how pyroptosis contributes to the progression of DN, involving both metabolic dysregulation and chronic renal inflammation. This conversion provides an early intervention target for the treatment of DN.
The quality of life for patients with DN can be significantly improved through timely prevention and treatment. Targeting and regulating different pathways of pyroptosis can effectively mitigate the pathophysiology of metabolic imbalances, thereby restoring glomerular filtration and tubular reabsorption and alleviating DN. The current therapeutic protocols for DN primarily focus on controlling glycemic control, while neglecting the damage caused by inflammation. Natural compounds and traditional medicines have demonstrated efficacy in alleviating pyroptosis by targeting the NLRP3/GSDMD signaling axis, thereby preserving renal microstructure and slowing functional decline, which suggests a potential complement to metabolic-focused treatments. The validation of pyroptosis biomarkers in human diabetic kidney biopsies will establish the clinical relevance of these markers. Therefore, inhibiting pyroptosis may reduce the incidence of ESRD by alleviating the progression of DN at pre-fibrotic stages. Bioavailability challenges prevent natural compounds from offering advantages across a wide range of targets. Several synthetic agents such as AB-38b, have shown promise in preclinical models and require validation in clinical trials. The development of future drugs should be based on optimizing pharmacokinetics and validating the biomarkers for the early diagnosis of pyroptosis in humans.
The limitations of the existing studies must be acknowledged. Mechanistic insights obtained from animal models may not thoroughly explore the multifactorial pathology of DN in humans. The translation of preclinical findings into clinical practice faces significant challenges. The evaluation of further human cohorts, the optimization of drug delivery methods and addressing the bioavailability of certain natural compounds need to be addressed. The complex interaction between pyroptosis and other cell death pathways such as apoptosis and necroptosis also requires a more comprehensive understanding in order to avoid unintended therapeutic effects.
In summary, suppression of pyroptosis-regulated cell death offers a promising therapeutic avenue for the treatment of DN. However, rigorous clinical trials should be conducted to evaluate the safety and effectiveness of these therapeutic interventions, establish benchmarks against established first-line therapies and address translational challenges. Therefore, this effort will facilitate the development of precision medicine approaches for alleviating the global burden of DN-associated kidney failure.
Funding
This work was supported by the Project from Zhejiang Provincial Medical and Health Science and Technology Program (2022ZH022), China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li X, Lu L, Hou W, et al. Epigenetics in the pathogenesis of diabetic nephropathy. Acta Biochim Biophys Sin. 2022;54(2):163–172. doi:10.3724/abbs.2021016
2. Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. doi:10.1155/2021/1497449
3. Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–1335. doi:10.2522/ptj.20080008
4. Jha R, Lopez-Trevino S, Kankanamalage HR, Jha JC. Diabetes and renal complications: an overview on pathophysiology, biomarkers and therapeutic interventions. Biomedicines. 2024;12(5):1098.
5. Hossain MJ, Al-Mamun M, Islam MR. Diabetes mellitus, the fastest growing global public health concern: early detection should be focused. Health Sci Rep. 2024;7(3):e2004. doi:10.1002/hsr2.2004
6. Ma Q, Li Y, Li P, et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed Pharmacother. 2019;117:109138. doi:10.1016/j.biopha.2019.109138
7. Al-Lawati JA. Diabetes mellitus: a local and global public health emergency! Oman Med J. 2017;32(3):177–179. doi:10.5001/omj.2017.34
8. Yuan CM, Nee R, Ceckowski KA, Knight KR, Abbott KC. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin Kidney J. 2017;10(2):257–262. doi:10.1093/ckj/sfw112
9. Thomas S, Karalliedde J. Diabetic nephropathy. Medicine. 2019;47(2):86–91. doi:10.1016/j.mpmed.2018.11.010
10. Dattani R, McAdoo S. Secondary glomerular disease. Medicine. 2019;47(10):644–648. doi:10.1016/j.mpmed.2019.07.005
11. Cheng H-T, Xu X, Lim PS, Hung K-Y. Worldwide epidemiology of diabetes-related end-stage renal disease, 2000–2015. Diabetes Care. 2021;44(1):89–97. doi:10.2337/dc20-1913
12. Ma X, Liu R, Xi X, Zhuo H, Gu Y. Global burden of chronic kidney disease due to diabetes mellitus, 1990-2021, and projections to 2050. Front Endocrinol. 2025;16:1513008. doi:10.3389/fendo.2025.1513008
13. Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12(2):73–81. doi:10.1038/nrneph.2015.173
14. Cockwell P, Fisher LA. The global burden of chronic kidney disease. Lancet. 2020;395(10225):662–664. doi:10.1016/s0140-6736(19)32977-0
15. Rayego-Mateos S, Morgado-Pascual JL, Opazo-Ríos L, et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int J Mol Sci. 2020;21(11). doi:10.3390/ijms21113798
16. Jin Q, Liu T, Qiao Y, et al. Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols. Front Immunol. 2023;14:1185317. doi:10.3389/fimmu.2023.1185317
17. Tervaert TW, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–563. doi:10.1681/asn.2010010010
18. Pour-Reza-Gholi F, Assadiasl S. Immunological approaches in the treatment of diabetic nephropathy. Curr Diabetes Rev. 2024;21(1):e061123223172. doi:10.2174/0115733998267893231016062205
19. Noh MR, Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of accidental and programmed cell death. Am J Physiol Renal Physiol. 2024;327(1):F4–f20. doi:10.1152/ajprenal.00275.2023
20. Priante G, Gianesello L, Ceol M, Del Prete D, Anglani F. Cell death in the kidney. Int J Mol Sci. 2019;20(14). doi:10.3390/ijms20143598
21. Zheng F, Ma L, Li X, et al. Neutrophil extracellular traps induce glomerular endothelial cell dysfunction and pyroptosis in diabetic kidney disease. Diabetes. 2022;71(12):2739–2750. doi:10.2337/db22-0153
22. Cao Z, Huang D, Tang C, et al. Pyroptosis in diabetes and diabetic nephropathy. Int J Clin Chem. 2022;531:188–196. doi:10.1016/j.cca.2022.04.011
23. Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(4):742–758. doi:10.1016/j.cell.2011.10.033
24. Haider KH, Khan M, Sen CK. Chapter 15 - MicroRNAs With mega functions in cardiac remodeling and repair: the micromanagement of matters of the heart. In: Sen CK, editor. MicroRNA in Regenerative Medicine.
25. Qian S, Long Y, Tan G, et al. Programmed cell death: molecular mechanisms, biological functions, diseases, and therapeutic targets. MedComm. 2024;5(12):e70024. doi:10.1002/mco2.70024
26. Suqing L, Yurong P, Ting L, et al. The role of regulated programmed cell death in osteoarthritis: from pathogenesis to therapy. J Int J Mol Sci. 2023;24(6):5364.
27. Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Proliferation. 2012;45(6):487–498. doi:10.1111/j.1365-2184.2012.00845.x
28. Guo D, Liu Z, Zhou J, Ke C, Li D. Significance of programmed cell death pathways in neurodegenerative diseases. Int J Mol Sci. 2024;25(18). doi:10.3390/ijms25189947
29. Erekat NS. Programmed cell death in diabetic nephropathy: a review of apoptosis, autophagy, and necroptosis. Med Sci Monit. 2022;28:e937766. doi:10.12659/msm.937766
30. Yang H, Sun J, Sun A, et al. Podocyte programmed cell death in diabetic kidney disease: molecular mechanisms and therapeutic prospects. Biomed Pharmacother. 2024;177:117140. doi:10.1016/j.biopha.2024.117140
31. Zhao S, Guo Y, Yin X. Autophagy, ferroptosis, apoptosis and pyroptosis in metabolic dysfunction-associated steatotic liver disease. Front Biosci. 2024;29(1):30. doi:10.31083/j.fbl2901030
32. Zhao J, Jiang P, Guo S, Schrodi SJ, Apoptosis HD, Autophagy N. Necroptosis, and pyroptosis mediated programmed cell death as targets for innovative therapy in rheumatoid arthritis. Front Immunol. 2021;12:809806. doi:10.3389/fimmu.2021.809806
33. Harada T, Taniguchi F, Izawa M, et al. Apoptosis and endometriosis. Front Biosci. 2007;12:3140–3151. doi:10.2741/2302
34. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi:10.1080/01926230701320337
35. D’Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43(6):582–592. doi:10.1002/cbin.11137
36. Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003;193:10–21. doi:10.1034/j.1600-065x.2003.00048.x
37. Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin. 2005;37(11):719–727. doi:10.1111/j.1745-7270.2005.00108.x
38. Yazdi AS, Guarda G, D’Ombrain MC, Drexler SK. Inflammatory caspases in innate immunity and inflammation. J Innate Immun. 2010;2(3):228–237. doi:10.1159/000283688
39. Lin X, Ouyang S, Zhi C, et al. Focus on ferroptosis, pyroptosis, apoptosis and autophagy of vascular endothelial cells to the strategic targets for the treatment of atherosclerosis. Arch Biochem Biophys. 2022;715:109098. doi:10.1016/j.abb.2021.109098
40. Negoescu A. Apoptosis in cancer: therapeutic implications. Histol Histopathol. 2000;15(1):281–297. doi:10.14670/hh-15.281
41. Chun Y, Kim J. Autophagy: an essential degradation program for cellular homeostasis and life. Cells. 2018;7(12). doi:10.3390/cells7120278
42. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863. doi:10.15252/embj.2021108863
43. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi:10.1038/nature06639
44. Polajnar M, Zerovnik E. Impaired autophagy: a link between neurodegenerative and neuropsychiatric diseases. J Cell & Mol Med. 2014;18(9):1705–1711. doi:10.1111/jcmm.12349
45. Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy. 2014;10(12):2208–2222. doi:10.4161/15548627.2014.981787
46. Ryter SW, Cloonan SM, Choi AM. Autophagy: a critical regulator of cellular metabolism and homeostasis. Mol Cells. 2013;36(1):7–16. doi:10.1007/s10059-013-0140-8
47. Levine B, Kroemer G. Biological Functions of Autophagy Genes: a Disease Perspective. Cell. 2019;176(1–2):11–42. doi:10.1016/j.cell.2018.09.048
48. Cui J, Bai X, Chen X. Autophagy and diabetic nephropathy. Adv Exp Med Biol. 2020;1207:487–494. doi:10.1007/978-981-15-4272-5_36
49. Tan Y, Chen Q, Li X, et al. Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res. 2021;40(1):153. doi:10.1186/s13046-021-01959-x
50. Huston HC, Anderson MJ, Fink SL. Pyroptosis and the cellular consequences of gasdermin pores. Semin Immunopathol. 2023;69:101803. doi:10.1016/j.smim.2023.101803
51. Kovacs SB, Miao EA. Gasdermins: effectors of Pyroptosis. Trends Cell Biol. 2017;27(9):673–684. doi:10.1016/j.tcb.2017.05.005
52. Li S, Feng L, Li G, et al. GSDME-dependent pyroptosis signaling pathway in diabetic nephropathy. Cell Death Discov. 2023;9(1):156. doi:10.1038/s41420-023-01452-8
53. Rao Z, Zhu Y, Yang P, et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12(9):4310–4329. doi:10.7150/thno.71086
54. Rayego-Mateos S, Rodrigues-Diez RR, Fernandez-Fernandez B, et al. Targeting inflammation to treat diabetic kidney disease: the road to 2030. Kidney Int. 2023;103(2):282–296. doi:10.1016/j.kint.2022.10.030
55. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124(3):139–152. doi:10.1042/cs20120198
56. Endocrine D, sAHoWMU MD, City W, et al. Ginsenoside Rb2 improves insulin resistance by inhibiting adipocyte pyroptosis. J Adipocyte. 2020;9(1):302–312.
57. Pengfei Z, Ziqi Y, Lulingxiao N, et al. Hyperglycemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. J Clin Periodontol. 2021;48(10):1379–1392.
58. Yibo Z, Wenjing C, Yongsen Z, Xiaoyi S, Xiaojing Y. Salmonella infection causes hyperglycemia for decreased GLP-1 content by enteroendocrine L cells pyroptosis in pigs. Int J Mol Sci. 2022;23(3):1272.
59. Jun Z, Shan Y, Xu G, et al. Salidroside protects pancreatic β-cells against pyroptosis by regulating the NLRP3/GSDMD pathway in diabetic conditions. Int Immunopharmacol. 2023;114:109543.
60. Al Mamun A, Ara Mimi A, Wu Y, et al. Pyroptosis in diabetic nephropathy. Int J Clin Chem. 2021;523:131–143. doi:10.1016/j.cca.2021.09.003
61. Al Mamun A, Shao C, Geng P, Wang S, Xiao J. The mechanism of pyroptosis and its application prospect in diabetic wound healing. J Inflamm Res. 2024;17:1481–1501. doi:10.2147/jir.S448693
62. Qiu Z, Lei S, Zhao B, et al. NLRP3 inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev. 2017;2017:9743280. doi:10.1155/2017/9743280
63. Che H, Li H, Li Y, et al. Melatonin exerts neuroprotective effects by inhibiting neuronal pyroptosis and autophagy in STZ-induced diabetic mice. FASEB J. 2020;34(10):14042–14054. doi:10.1096/fj.202001328R
64. Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93(18):9833–9838. doi:10.1073/pnas.93.18.9833
65. Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi:10.1038/358167a0
66. Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends microbiol. 2001;9(3):113–114. doi:10.1016/s0966-842x(00)01936-3
67. Ringel-Scaia VM, McDaniel DK, Allen IC. The goldilocks conundrum: NLR inflammasome modulation of gastrointestinal inflammation during inflammatory bowel disease. Crit Rev Immunol. 2016;36(4):283–314. doi:10.1615/CritRevImmunol.2017019158
68. Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. doi:10.1111/imr.12287
69. Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36. doi:10.1038/s41421-020-0167-x
70. Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol. 2010;30(5):693–702. doi:10.1007/s10875-010-9425-2
71. Xu H, Yang J, Gao W, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513(7517):237–241. doi:10.1038/nature13449
72. Hafner-Bratkovič I, Sušjan P, Lainšček D, et al. NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway. Nat Commun. 2018;9(1):5182. doi:10.1038/s41467-018-07573-4
73. Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227(1):106–128. doi:10.1111/j.1600-065X.2008.00734.x
74. He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–357. doi:10.1038/nature16959
75. He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41(12):1012–1021. doi:10.1016/j.tibs.2016.09.002
76. Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi:10.1038/nature18590
77. Wang K, Sun Q, Zhong X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180(5):941–955.e20. doi:10.1016/j.cell.2020.02.002
78. Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi:10.1038/nature13683
79. Rathinam VAK, Zhao Y, Shao F. Innate immunity to intracellular LPS. Nat Immunol. 2019;20(5):527–533. doi:10.1038/s41590-019-0368-3
80. Yang J, Zhao Y, Shao F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr Opin Immunol. 2015;32:78–83. doi:10.1016/j.coi.2015.01.007
81. Liu J, Kang R, Tang D. Lipopolysaccharide delivery systems in innate immunity. Trends Immunol. 2024;45(4):274–287. doi:10.1016/j.it.2024.02.003
82. Zheng XB, Wang X, Gao SQ, et al. NINJ1-mediated plasma membrane rupture of pyroptotic endothelial cells exacerbates blood-brain barrier destruction caused by neutrophil extracellular traps in traumatic brain injury. Cell Death Discov. 2025;11(1):69. doi:10.1038/s41420-025-02350-x
83. Kayagaki N, Kornfeld OS, Lee BL, et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591(7848):131–136. doi:10.1038/s41586-021-03218-7
84. Rühl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45(10):2927–2936. doi:10.1002/eji.201545772
85. Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43(5):923–932. doi:10.1016/j.immuni.2015.10.009
86. Gullett JM, Tweedell RE, Kanneganti TD. It’s all in the PAN: crosstalk, plasticity, redundancies, switches, and interconnectedness encompassed by PANoptosis underlying the totality of cell death-associated biological effects. Cells. 2022;11(9). doi:10.3390/cells11091495
87. Eskander G, Abdelhamid SG, Wahdan SA, Radwan SM. Insights on the crosstalk among different cell death mechanisms. Cell Death Discov. 2025;11(1):56. doi:10.1038/s41420-025-02328-9
88. Wallace HL, Wang L, Gardner CL, et al. Crosstalk between pyroptosis and apoptosis in hepatitis C virus-induced cell death. Front Immunol. 2022;13:788138. doi:10.3389/fimmu.2022.788138
89. Jiang M, Qi L, Li L, Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:112. doi:10.1038/s41420-020-00349-0
90. Xiao F, Gao W, Wang X, Chen T. Amplification activation loop between caspase-8 and −9 dominates artemisinin-induced apoptosis of ASTC-a-1 cells. Apoptosis. 2012;17(6):600–611. doi:10.1007/s10495-012-0706-5
91. Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi:10.1038/nature22393
92. Hou J, Zhao R, Xia W, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22(10):1264–1275. doi:10.1038/s41556-020-0575-z
93. Hu Y, Liu Y, Zong L, et al. The multifaceted roles of GSDME-mediated pyroptosis in cancer: therapeutic strategies and persisting obstacles. Cell Death Dis. 2023;14(12):836. doi:10.1038/s41419-023-06382-y
94. Ratan Y, Rajput A, Pareek A, Singh G. Comprehending the role of metabolic and hemodynamic factors alongside different signaling pathways in the pathogenesis of diabetic nephropathy. Int J Mol Sci. 2025;26(7). doi:10.3390/ijms26073330
95. Dwivedi S, Sikarwar MS. Diabetic nephropathy: pathogenesis, mechanisms, and therapeutic strategies. Hormone Metab Res. 2025;57(1):7–17. doi:10.1055/a-2435-8264
96. Magee C, Grieve DJ, Watson CJ, Brazil DP. Diabetic nephropathy: a tangled web to unweave. Cardiovasc Drugs Ther. 2017;31(5–6):579–592. doi:10.1007/s10557-017-6755-9
97. Yan M, Li W, Wei R, et al. Identification of pyroptosis-related genes and potential drugs in diabetic nephropathy. J Transl Med. 2023;21(1):490. doi:10.1186/s12967-023-04350-w
98. Wan J, Liu D, Pan S, Zhou S, Liu Z. NLRP3-mediated pyroptosis in diabetic nephropathy. Front Pharmacol. 2022;13:998574. doi:10.3389/fphar.2022.998574
99. Li W, Sun J, Zhou X, Lu Y, Cui W, Miao LM-R. Mini-Review: GSDME-mediated pyroptosis in diabetic nephropathy. Front Pharmacol. 2021;12:780790. doi:10.3389/fphar.2021.780790
100. Miner JH. Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol. 2011;26(9):1413–1417. doi:10.1007/s00467-011-1785-1
101. Miner JH. The glomerular basement membrane. Exp Cell Res. 2012;318(9):973–978. doi:10.1016/j.yexcr.2012.02.031
102. Li X, Zhang Y, Xing X, et al. Podocyte injury of diabetic nephropathy: novel mechanism discovery and therapeutic prospects. Biomed Pharmacother. 2023;168:115670. doi:10.1016/j.biopha.2023.115670
103. Chen Y, Zhang M, Jia R, et al. Podocyte SIRPα reduction in diabetic nephropathy aggravates podocyte injury by promoting pyruvate kinase M2 nuclear translocation. Redox Biol. 2024;78:103439. doi:10.1016/j.redox.2024.103439
104. Kushwaha K, Kabra U, Dubey R, Gupta J. Diabetic Nephropathy: pathogenesis to Cure. Current Drug Targets. 2022;23(15):1418–1429. doi:10.2174/1389450123666220820110801
105. Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. doi:10.2337/diabetes.55.01.06.db05-0894
106. Liu S, Yuan Y, Xue Y, Xing C, Zhang B. Podocyte injury in diabetic kidney disease: a focus on mitochondrial dysfunction. Front Cell Develop Biol. 2022;10:832887. doi:10.3389/fcell.2022.832887
107. Tonneijck L, Muskiet MHA, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28(4):1023–1039. doi:10.1681/asn.2016060666
108. Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16(6):317–336. doi:10.1038/s41581-020-0256-y
109. Kopp JB, Anders H-J, Susztak K, et al. Podocytopathies. Nat Rev Dis Prim. 2020;6(1):68. doi:10.1038/s41572-020-0196-7
110. Lin JS, Susztak K. Podocytes: the weakest link in diabetic kidney disease? Curr Diab Rep. 2016;16(5):45. doi:10.1007/s11892-016-0735-5
111. Cheng Q, Pan J, Zhou Z-L, et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin. 2021;42(6):954–963. doi:10.1038/s41401-020-00525-z
112. Wu M, Yang Z, Zhang C, et al. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism. 2021;118:154748. doi:10.1016/j.metabol.2021.154748
113. Li W, Shen N, Kong L, et al. STING mediates microglial pyroptosis via interaction with NLRP3 in cerebral ischaemic stroke. Stroke Vasc Neurol. 2024;9(2):153–164. doi:10.1136/svn-2023-002320
114. Yang X, Chen Z, Luo Z, et al. STING deletion alleviates podocyte injury through suppressing inflammation by targeting NLRP3 in diabetic kidney disease. Cell Signalling. 2023;109:110777. doi:10.1016/j.cellsig.2023.110777
115. Wang T, Gao Y, Yue R, et al. Ginsenoside Rg1 alleviates podocyte injury induced by hyperlipidemia via targeting the mTOR/NF-κB/NLRP3 axis. Evid Based Complement Alternat Med. 2020;2020:2735714. doi:10.1155/2020/2735714
116. Kim D, Ban KY, Lee GH, Jun HS. Lysophosphatidic acid induces podocyte pyroptosis in diabetic nephropathy by an increase of Egr1 expression via downregulation of EzH2. Int J Mol Sci. 2023;24(12). doi:10.3390/ijms24129968
117. Li HY, Oh YS, Choi JW, Jung JY, Jun HS. Blocking lysophosphatidic acid receptor 1 signaling inhibits diabetic nephropathy in db/db mice. Kidney Int. 2017;91(6):1362–1373. doi:10.1016/j.kint.2016.11.010
118. Zhang MZ, Wang X, Yang H, et al. Lysophosphatidic acid receptor antagonism protects against diabetic nephropathy in a type 2 diabetic model. J Am Soc Nephrol. 2017;28(11):3300–3311. doi:10.1681/asn.2017010107
119. Zhang ZW, Tang MQ, Liu W, et al. Dapagliflozin prevents kidney podocytes pyroptosis via miR-155-5p/HO-1/NLRP3 axis modulation. Int Immunopharmacol. 2024;131:111785. doi:10.1016/j.intimp.2024.111785
120. Wang L, Xie X, Chen Q, Chen Y, Xu X, Liang T. Puerarin reduces diabetic nephropathy-induced podocyte pyroptosis by modulating the SIRT1/NLRP3/caspase-1 pathway. Mol Cell Endocrinol. 2025;595:112409. doi:10.1016/j.mce.2024.112409
121. Zhang M, Liu W, Liu Y, et al. Astragaloside IV inhibited podocyte pyroptosis in diabetic kidney disease by regulating SIRT6/HIF-1α axis. DNA Cell Biol. 2023;42(10):594–607. doi:10.1089/dna.2023.0102
122. Ito M, Ducasa GM, Molina JD, et al. ABCA1 deficiency contributes to podocyte pyroptosis priming via the APE1/IRF1 axis in diabetic kidney disease. Sci Rep. 2023;13(1):9616. doi:10.1038/s41598-023-35499-5
123. Wu T, Ding L, Andoh V, Zhang J, Chen L. The mechanism of hyperglycemia-induced renal cell injury in diabetic nephropathy disease: an update. Life. 2023;13(2). doi:10.3390/life13020539
124. Qi H, Casalena G, Shi S, et al. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes. 2017;66(3):763–778. doi:10.2337/db16-0695
125. Weil EJ, Lemley KV, Mason CC, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82(9):1010–1017. doi:10.1038/ki.2012.234
126. Rizwan H, Pal S, Sabnam S, Pal A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020;241:117148. doi:10.1016/j.lfs.2019.117148
127. Shen Y, Chen W, Lin K, et al. Notoginsenoside Fc, a novel renoprotective agent, ameliorates glomerular endothelial cells pyroptosis and mitochondrial dysfunction in diabetic nephropathy through regulating HMGCS2 pathway. Phytomedicine. 2024;126:155445. doi:10.1016/j.phymed.2024.155445
128. Wu Q, Guan YB, Zhang KJ, Li L, Zhou Y. Tanshinone IIA mediates protection from diabetes kidney disease by inhibiting oxidative stress induced pyroptosis. J Ethnopharmacol. 2023;316:116667. doi:10.1016/j.jep.2023.116667
129. Chen Z, Wang Z, Hu Y, et al. ELABELA/APJ axis prevents diabetic glomerular endothelial injury by regulating AMPK/NLRP3 pathway. Inflammation. 2023;46(6):2343–2358. doi:10.1007/s10753-023-01882-7
130. Hu X, Chen H, Xu H, et al. Role of pyroptosis in traumatic brain and spinal cord injuries. review. Int J Bio Sci. 2020;16(12):2042–2050. doi:10.7150/ijbs.45467
131. Gu J, Huang W, Zhang W, et al. Sodium butyrate alleviates high-glucose-induced renal glomerular endothelial cells damage via inhibiting pyroptosis. Int Immunopharmacol. 2019;75:105832. doi:10.1016/j.intimp.2019.105832
132. Han J, Zuo Z, Shi X, et al. Hirudin ameliorates diabetic nephropathy by inhibiting Gsdmd-mediated pyroptosis. Cell Biol Toxicol. 2023;39(3):573–589. doi:10.1007/s10565-021-09622-z
133. Shao Y, Deng S, Tang W, et al. Molecular mechanism of GSDMD mediated glomerular endothelial cells pyroptosis: an implying in the progression of diabetic nephropathy. Int Immunopharmacol. 2023;122:110632. doi:10.1016/j.intimp.2023.110632
134. Abboud HE. Mesangial cell biology. Exp Cell Res. 2012;318(9):979–985. doi:10.1016/j.yexcr.2012.02.025
135. Liu Y, Li X, Zhao M, et al. Macrophage-derived exosomes promote activation of NLRP3 inflammasome and autophagy deficiency of mesangial cells in diabetic nephropathy. Life Sci. 2023;330:121991. doi:10.1016/j.lfs.2023.121991
136. Du Y, Feng Y, Cai Y, Tian C. CircLARP1B promotes pyroptosis of high glucose-induced renal mesangial cells by regulating the miR-578/TLR4 axis. Int Urol Nephrol. 2024;56(1):283–293. doi:10.1007/s11255-023-03672-4
137. Zhan JF, Huang HW, Huang C, Hu LL, Xu WW. Long non-coding RNA NEAT1 regulates pyroptosis in diabetic nephropathy via mediating the miR-34c/NLRP3 axis. Kidney Blood Pressure Res. 2020;45(4):589–602. doi:10.1159/000508372
138. Wu CT, Deng JS, Huang WC, Shieh PC, Chung MI, Huang GJ. Salvianolic acid C against acetaminophen-induced acute liver injury by attenuating inflammation, oxidative stress, and apoptosis through inhibition of the Keap1/Nrf2/HO-1 signaling. Oxid Med Cell Longev. 2019;2019:9056845. doi:10.1155/2019/9056845
139. Lu C, Fan G, Wang D. Akebia Saponin D ameliorated kidney injury and exerted anti-inflammatory and anti-apoptotic effects in diabetic nephropathy by activation of NRF2/HO-1 and inhibition of NF-KB pathway. Int Immunopharmacol. 2020;84:106467. doi:10.1016/j.intimp.2020.106467
140. Du L, Wang J, Chen Y, et al. Novel biphenyl diester derivative AB-38b inhibits NLRP3 inflammasome through Nrf2 activation in diabetic nephropathy. Cell Biol Toxicol. 2020;36(3):243–260. doi:10.1007/s10565-019-09501-8
141. Shen S, Ji C, Wei K. Cellular senescence and regulated cell death of tubular epithelial cells in diabetic kidney disease. Front Endocrinol. 2022;13:924299. doi:10.3389/fendo.2022.924299
142. Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol. 2016;311(1):F145–61. doi:10.1152/ajprenal.00164.2016
143. Tomita T. Apoptosis in pancreatic β-islet cells in type 2 diabetes. Bosn J Basic Med Sci. 2016;16(3):162–179. doi:10.17305/bjbms.2016.919
144. Zha C, Qi Y, Xing F, Li J. Astragaloside IV inhibits the pyroptosis in the acute kidney injury through targeting the SIRT1/FOXO3a axis. Chem Pharm Bull. 2024;72(10):923–931. doi:10.1248/cpb.c24-00151
145. Yuan S, Wang Y, Li Z, et al. Gasdermin D is involved in switching from apoptosis to pyroptosis in TLR4-mediated renal tubular epithelial cells injury in diabetic kidney disease. Arch Biochem Biophys. 2022;727:109347. doi:10.1016/j.abb.2022.109347
146. Zhao W, He C, Jiang J, et al. The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy. Korean J Physiol Pharmacol. 2022;26(6):427–438. doi:10.4196/kjpp.2022.26.6.427
147. Wang Y, Zhu X, Yuan S, et al. TLR4/NF-κB signaling induces GSDMD-related pyroptosis in tubular cells in diabetic kidney disease. Front Endocrinol. 2019;10:603. doi:10.3389/fendo.2019.00603
148. Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–222. doi:10.1038/s41581-019-0234-4
149. Deng J, Tan W, Luo Q, Lin L, Zheng L, Yang J. Long non-coding RNA MEG3 promotes renal tubular epithelial cell pyroptosis by regulating the miR-18a-3p/GSDMD pathway in lipopolysaccharide-induced acute kidney injury. Front Physiol. 2021;12:663216. doi:10.3389/fphys.2021.663216
150. Xu J, Wang Q, Song YF, et al. Long noncoding RNA X-inactive specific transcript regulates NLR family pyrin domain containing 3/caspase-1-mediated pyroptosis in diabetic nephropathy. World J Diab. 2022;13(4):358–375. doi:10.4239/wjd.v13.i4.358
151. Li X, Zeng L, Cao C, et al. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res. 2017;350(2):327–335. doi:10.1016/j.yexcr.2016.12.006
152. Liu C, Zhuo H, Ye MY, Huang GX, Fan M, Huang XZ. LncRNA MALAT1 promoted high glucose-induced pyroptosis of renal tubular epithelial cell by sponging miR-30c targeting for NLRP3. Kaohsiung J Med Sci. 2020;36(9):682–691. doi:10.1002/kjm2.12226
153. Song Y, Guo F, Zhao YY, et al. Novel lncRNA-prader willi/angelman region RNA, SNRPN neighbour (PWARSN) aggravates tubular epithelial cell pyroptosis by regulating TXNIP via dual way in diabetic kidney disease. Cell proliferation. 2023;56(2):e13349. doi:10.1111/cpr.13349
154. Li L, Zhang L, Cai Y, et al. DNA damage-induced AIM2 pyroptosis in high glucose-induced proximal tubular epithelial cell. Front Cell Develop Biol. 2024;12:1457369. doi:10.3389/fcell.2024.1457369
155. Zheng S, Zhang K, Zhang Y, et al. Human umbilical cord mesenchymal stem cells inhibit pyroptosis of renal tubular epithelial cells through miR-342-3p/Caspase1 signaling pathway in diabetic nephropathy. Stem Cells Int. 2023;2023:5584894. doi:10.1155/2023/5584894
156. Fang WY, Tseng YT, Lee TY, et al. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. Br J Pharmacol. 2021;178(15):2998–3016. doi:10.1111/bph.15472
157. Zhang HR, Li YP, Shi ZJ, et al. Triptolide induces PANoptosis in macrophages and causes organ injury in mice. Apoptosis. 2023;28(11–12):1646–1665. doi:10.1007/s10495-023-01886-6
158. Gao J, Zhang Y, Liu X, Wu X, Huang L, Gao W. Triptolide: pharmacological spectrum, biosynthesis, chemical synthesis and derivatives. Theranostics. 2021;11(15):7199–7221. doi:10.7150/thno.57745
159. Jha JC, Ho F, Dan C, Jandeleit-Dahm K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin Sci. 2018;132(16):1811–1836. doi:10.1042/cs20171459
160. Lv C, Cheng T, Zhang B, Sun K, Lu K. Triptolide protects against podocyte injury in diabetic nephropathy by activating the Nrf2/HO-1 pathway and inhibiting the NLRP3 inflammasome pathway. Renal failure. 2023;45(1):2165103. doi:10.1080/0886022x.2023.2165103
161. Han F, Xue M, Chang Y, et al. Triptolide suppresses glomerular mesangial cell proliferation in diabetic nephropathy is associated with inhibition of PDK1/Akt/mTOR pathway. Int J Bio Sci. 2017;13(10):1266–1275. doi:10.7150/ijbs.20485
162. Han F, Wang S, Chang Y, et al. Triptolide prevents extracellular matrix accumulation in experimental diabetic kidney disease by targeting microRNA-137/Notch1 pathway. J Cell Physiol. 2018;233(3):2225–2237. doi:10.1002/jcp.26092
163. Zhou K, Zhang J, Liu C, et al. Sanziguben polysaccharides inhibit diabetic nephropathy through NF-κB-mediated anti-inflammation. Nutr Metab. 2021;18(1):81. doi:10.1186/s12986-021-00601-z
164. Wang F, Liu C, Ren L, et al. Sanziguben polysaccharides improve diabetic nephropathy in mice by regulating gut microbiota to inhibit the TLR4/NF-κB/NLRP3 signalling pathway. Pharm Biol. 2023;61(1):427–436. doi:10.1080/13880209.2023.2174145
165. Sabatino A, Regolisti G, Cosola C, Gesualdo L, Fiaccadori E. Intestinal microbiota in type 2 diabetes and chronic kidney disease. Curr Diab Rep. 2017;17(3):16. doi:10.1007/s11892-017-0841-z
166. Cai TT, Ye XL, Li RR, et al. Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front Pharmacol. 2020;11:1249. doi:10.3389/fphar.2020.01249
167. Fritsche KL. The science of fatty acids and inflammation. Adv Nutr. 2015;6(3):293s–301s. doi:10.3945/an.114.006940
168. Junren C, Xiaofang X, Huiqiong Z, et al. Pharmacological activities and mechanisms of hirudin and its derivatives - a review. Front Pharmacol. 2021;12:660757. doi:10.3389/fphar.2021.660757
169. Han J, Pang X, Zhang Y, Peng Z, Shi X, Xing Y. Hirudin protects against kidney damage in streptozotocin-induced diabetic nephropathy rats by inhibiting inflammation via P38 MAPK/NF-κB pathway. Drug Des Devel Ther. 2020;14:3223–3234. doi:10.2147/dddt.S257613
170. Benaoudia S, Martin A, Puig Gamez M, et al. A genome-wide screen identifies IRF2 as a key regulator of caspase-4 in human cells. EMBO Rep. 2019;20(9):e48235. doi:10.15252/embr.201948235
171. Kayagaki N, Lee BL, Stowe IB, et al. IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci Signaling. 2019;12(582). doi:10.1126/scisignal.aax4917
172. Li G, Yang L, Feng L, et al. Syringaresinol protects against type 1 diabetic cardiomyopathy by alleviating inflammation responses, cardiac fibrosis, and oxidative stress. Mol Nutr Food Res. 2020;64(18):e2000231. doi:10.1002/mnfr.202000231
173. Zhang S, Yang L, Hu D, et al. Syringaresinol alleviates IgG immune complex induced acute lung injury via activating PPARγ and suppressing pyroptosis. Int Immunopharmacol. 2023;124(Pt B):111071. doi:10.1016/j.intimp.2023.111071
174. Li G, Liu C, Yang L, et al. Syringaresinol protects against diabetic nephropathy by inhibiting pyroptosis via NRF2-mediated antioxidant pathway. Cell Biol Toxicol. 2023;39(3):621–639. doi:10.1007/s10565-023-09790-0
175. Du L, Wang J, Chen Y, et al. Correction to: novel biphenyl diester derivative AB-38b inhibits NLRP3 inflammasome through Nrf2 activation in diabetic nephropathy. Cell Biol Toxicol. 2022;38(5):913–914. doi:10.1007/s10565-022-09696-3
176. Kostić K, Brborić J, Delogu G, et al. Antioxidant activity of natural phenols and derived hydroxylated biphenyls. Molecules. 2023;28(6):2646.
177. Chen Y, Wang M, Rosen RT, Ho CT. 2,2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum thunb. J Agric Food Chem. 1999;47(6):2226–2228. doi:10.1021/jf990092f
178. Du L, Wang L, Wang B, et al. A novel compound AB38b attenuates oxidative stress and ECM protein accumulation in kidneys of diabetic mice through modulation of Keap1/Nrf2 signaling. Acta Pharmacol Sin. 2020;41(3):358–372. doi:10.1038/s41401-019-0297-6
179. An X, Zhang Y, Cao Y, Chen J, Qin H, Yang L. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 2020;12(5):1516.
180. Mo Y, Ma J, Gao W, et al. Pomegranate peel as a source of bioactive compounds: a mini review on their physiological functions. Front Nutr. 2022;9:887113. doi:10.3389/fnut.2022.887113
181. Xu X, Li H, Hou X, et al. Punicalagin induces Nrf2/HO-1 expression via upregulation of PI3K/AKT pathway and inhibits LPS-induced oxidative stress in RAW264.7 macrophages. Mediat Inflamm. 2015;2015:380218. doi:10.1155/2015/380218
182. Shabir I, Dar AH, Dash KK, et al. Bioactive potential of punicalagin: a comprehensive review. Appl Food Res. 2024;4(2):100572. doi:10.1016/j.afres.2024.100572
183. Wang Y, Huang M, Yang X, Yang Z, Li L, Mei J. Supplementing punicalagin reduces oxidative stress markers and restores angiogenic balance in a rat model of pregnancy-induced hypertension. Korean J Physiol Pharmacol. 2018;22(4):409–417. doi:10.4196/kjpp.2018.22.4.409
184. Huang L, Lu S, Bian M, et al. Punicalagin attenuates TNF-α-induced oxidative damage and promotes osteogenic differentiation of bone mesenchymal stem cells by activating the Nrf2/HO-1 pathway. Exp Cell Res. 2023;430(1):113717. doi:10.1016/j.yexcr.2023.113717
185. Rao F, Tian H, Li W, Hung H, Sun F. Potential role of punicalagin against oxidative stress induced testicular damage. Asian J Androl. 2016;18(4):627–632. doi:10.4103/1008-682x.168792
186. Zoofeen U, Shah M, Sultan S, et al. Punicalagin improves inflammation and oxidative stress in rat model of pelvic inflammatory disease. Nat Product Res. 2025;39(10):2780–2786. doi:10.1080/14786419.2024.2313183
187. Peters V, Yard B, Schmitt CP. Carnosine and diabetic nephropathy. Curr Med Chem. 2020;27(11):1801–1812. doi:10.2174/0929867326666190326111851
188. Zhu W, Li YY, Zeng HX, et al. Carnosine alleviates podocyte injury in diabetic nephropathy by targeting caspase-1-mediated pyroptosis. Int Immunopharmacol. 2021;101(Pt B):108236. doi:10.1016/j.intimp.2021.108236
189. Shen J, Xu J, Wen Y, Tang Z, Li J, Sun J. Carnosine ameliorates postoperative cognitive dysfunction of aged rats by limiting astrocytes pyroptosis. Neurotherapeutics. 2024;21(4):e00359. doi:10.1016/j.neurot.2024.e00359
190. Liu XQ, Jiang L, Lei L, et al. Carnosine alleviates diabetic nephropathy by targeting GNMT, a key enzyme mediating renal inflammation and fibrosis. Clin Sci. 2020;134(23):3175–3193. doi:10.1042/cs20201207
191. Calabrese V, Scuto M, Salinaro AT, et al. Hydrogen sulfide and carnosine: modulation of oxidative stress and inflammation in kidney and brain axis. Antioxidants. 2020;9(12). doi:10.3390/antiox9121303
192. Caruso G, Fresta CG, Fidilio A, et al. Carnosine decreases PMA-induced oxidative stress and inflammation in murine macrophages. Antioxidants. 2019;8(8). doi:10.3390/antiox8080281
193. Yu C, Zhang P, Lou L, Wang Y. Perspectives regarding the role of biochanin A in humans. Front Pharmacol. 2019;10:793. doi:10.3389/fphar.2019.00793
194. Felix FB, Vago JP, Beltrami VA, et al. Biochanin A as a modulator of the inflammatory response: an updated overview and therapeutic potential. Pharmacol Res. 2022;180:106246. doi:10.1016/j.phrs.2022.106246
195. Sarfraz A, Javeed M, Shah MA, et al. Biochanin A: a novel bioactive multifunctional compound from nature. Sci total environ. 2020;722:137907. doi:10.1016/j.scitotenv.2020.137907
196. Yan J, Qiu P, Zhang X, et al. Biochanin A from Chinese medicine: an isoflavone with diverse pharmacological properties. Am J Chin Med. 2021;49(7):1623–1643. doi:10.1142/s0192415x21500750
197. Anuranjana PV, Beegum F, George KT, et al. Mechanisms behind the pharmacological application of biochanin-A: a review. F1000Res. 2023;12:107. doi:10.12688/f1000research.126059.3
198. Feng ZJ, Lai WF. Chemical and biological properties of biochanin a and its pharmaceutical applications. Pharmaceutics. 2023;15(4). doi:10.3390/pharmaceutics15041105
199. Ramachandran V, Inba KV, Kiran Kumar HR, et al. Biochanin-A: a bioactive natural product with versatile therapeutic perspectives. Curr Drug Res Rev. 2022;14(3):225–238. doi:10.2174/2589977514666220509201804
200. Chen T, Zhou N, Liang Q, et al. Biochanin A: disrupting the inflammatory vicious cycle for dry eye disease. Eur J Pharmacol. 2024;977:176583. doi:10.1016/j.ejphar.2024.176583
201. Ram C, Gairola S, Syed AM, et al. Biochanin A alleviates unilateral ureteral obstruction-induced renal interstitial fibrosis and inflammation by inhibiting the TGF-β1/Smad2/3 and NF-kB/NLRP3 signaling axis in mice. Life Sci. 2022;298:120527. doi:10.1016/j.lfs.2022.120527
202. Suliman FA, Khodeer DM, Ibrahiem A, et al. Renoprotective effect of the isoflavonoid biochanin A against cisplatin induced acute kidney injury in mice: effect on inflammatory burden and p53 apoptosis. Int Immunopharmacol. 2018;61:8–19. doi:10.1016/j.intimp.2018.05.010
203. Ram C, Gairola S, Verma S, et al. Biochanin A ameliorates nephropathy in high-fat diet/streptozotocin-induced diabetic rats: effects on NF-kB/NLRP3 axis, pyroptosis, and fibrosis. Antioxidants. 2023;12(5). doi:10.3390/antiox12051052
204. He X, Deng FJ, Ge JW, Yan XX, Pan AH, Li ZY. Effects of total saponins of Panax notoginseng on immature neuroblasts in the adult olfactory bulb following global cerebral ischemia/reperfusion. Neural Regen Res. 2015;10(9):1450–1456. doi:10.4103/1673-5374.165514
205. Duan H, Zhang Q, Liu J, et al. Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis. Pharmacol Res. 2021;168:105599. doi:10.1016/j.phrs.2021.105599
206. Liu Y, Liu T, Ding K, et al. Phospholipase Cγ2 signaling cascade contribute to the antiplatelet effect of notoginsenoside Fc. Front Pharmacol. 2018;9:1293. doi:10.3389/fphar.2018.01293
207. Liu H, Lu X, Hu Y, Fan X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol Res. 2020;161:105263. doi:10.1016/j.phrs.2020.105263
208. Gu CZ, Qiao YJ, Wang D, et al. New triterpenoid saponins from the steaming treated roots of Panax notoginseng. Nat Product Res. 2018;32(3):294–301. doi:10.1080/14786419.2017.1356833
209. Liu J, Jiang C, Ma X, Feng L, Wang J. Notoginsenoside Fc accelerates reendothelialization following vascular injury in diabetic rats by promoting endothelial cell autophagy. J Diabet Res. 2019;2019:9696521. doi:10.1155/2019/9696521
210. Liu J, Jiang C, Ma X, Wang J. Notoginsenoside Fc attenuates high glucose-induced vascular endothelial cell injury via upregulation of PPAR-γ in diabetic Sprague-Dawley rats. Vascu Pharmacol. 2018;109:27–35. doi:10.1016/j.vph.2018.05.009
211. Wei M, Gao Y, Cheng D, et al. Notoginsenoside Fc ameliorates renal tubular injury and mitochondrial damage in Acetaminophen-induced acute kidney injury partly by regulating SIRT3/SOD2 pathway. Front Med. 2022;9:1055252. doi:10.3389/fmed.2022.1055252
212. Jonscher KR, Chowanadisai W, Rucker RB. Pyrroloquinoline-quinone is more than an antioxidant: a vitamin-like accessory factor important in health and disease prevention. Biomolecules. 2021;11(10). doi:10.3390/biom11101441
213. Akagawa M, Nakano M, Ikemoto K. Recent progress in studies on the health benefits of pyrroloquinoline quinone. Biosci Biotechnol Biochem. 2016;80(1):13–22. doi:10.1080/09168451.2015.1062715
214. Li J, Zhang J, Xue Q, et al. Pyrroloquinoline quinone alleviates natural aging-related osteoporosis via a novel MCM3-Keap1-Nrf2 axis-mediated stress response and Fbn1 upregulation. Aging Cell. 2023;22(9):e13912. doi:10.1111/acel.13912
215. Harris CB, Chowanadisai W, Mishchuk DO, Satre MA, Slupsky CM, Rucker RB. Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects. J Nutr Biochem. 2013;24(12):2076–2084. doi:10.1016/j.jnutbio.2013.07.008
216. Tamakoshi M, Suzuki T, Nishihara E, Nakamura S, Ikemoto K. Pyrroloquinoline quinone disodium salt improves brain function in both younger and older adults. Food Funct. 2023;14(5):2496–2501. doi:10.1039/d2fo01515c
217. Wang Z, Li Y, Wang Y, Zhao K, Chi Y, Wang B. Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway. Biochem Biophys Res Commun. 2019;508(2):398–404. doi:10.1016/j.bbrc.2018.11.140
218. Wang Z, Han N, Zhao K, Li Y, Chi Y, Wang B. Protective effects of pyrroloquinoline quinine against oxidative stress-induced cellular senescence and inflammation in human renal tubular epithelial cells via Keap1/Nrf2 signaling pathway. Int Immunopharmacol. 2019;72:445–453. doi:10.1016/j.intimp.2019.04.040
219. Qu X, Zhai B, Liu Y, et al. Pyrroloquinoline quinone ameliorates renal fibrosis in diabetic nephropathy by inhibiting the pyroptosis pathway in C57BL/6 mice and human kidney 2 cells. Biomed Pharmacother. 2022;150:112998. doi:10.1016/j.biopha.2022.112998
220. Zhang L, Shergis JL, Yang L, et al. Astragalus membranaceus (Huang Qi) as adjunctive therapy for diabetic kidney disease: an updated systematic review and meta-analysis. J Ethnopharmacol. 2019;239:111921. doi:10.1016/j.jep.2019.111921
221. Gong P, Long H, Yang Q, et al. Astragaloside IV alleviates diabetic nephropathy by modulating the gut-kidney axis and AMPK/PI3K/AKT pathway. Food Bioscience. 2024;62:105448. doi:10.1016/j.fbio.2024.105448
222. Wang E, Wang L, Ding R, et al. Astragaloside IV acts through multi-scale mechanisms to effectively reduce diabetic nephropathy. Pharmacol Res. 2020;157:104831. doi:10.1016/j.phrs.2020.104831
223. Gao P, Du X, Liu L, et al. Astragaloside IV alleviates tacrolimus-induced chronic nephrotoxicity via p62-Keap1-Nrf2 pathway. Front Pharmacol. 2020;11:610102. doi:10.3389/fphar.2020.610102
224. Oh HJ, Nam BY, Wu M, et al. Klotho plays a protective role against glomerular hypertrophy in a cell cycle-dependent manner in diabetic nephropathy. Am J Physiol Renal Physiol. 2018;315(4):F791–f805. doi:10.1152/ajprenal.00462.2017
225. He J, Cui J, Shi Y, et al. Astragaloside IV attenuates high-glucose-induced impairment in diabetic nephropathy by increasing klotho expression via the NF-κB/NLRP3 axis. J Diabet Res. 2023;2023:7423661. doi:10.1155/2023/7423661
226. Ratan ZA, Haidere MF, Hong YH, et al. Pharmacological potential of ginseng and its major component ginsenosides. J Ginseng Res. 2021;45(2):199–210. doi:10.1016/j.jgr.2020.02.004
227. Cho WC, Chung WS, Lee SK, Leung AW, Cheng CH, Yue KK. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;550(1–3):173–179. doi:10.1016/j.ejphar.2006.08.056
228. Zhou P, Xie W, He S, et al. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells. 2019;8(3). doi:10.3390/cells8030204
229. Zhang Y-X, Wan H, Shan G-Y, et al. Ginsenoside Rg5 modulates the TLR4 and BCL-2 pathways by inhibiting NOX1, thereby alleviating inflammation, apoptosis and pyroptosis in hyperuricemia nephropathy. J Ginseng Res. 2025. doi:10.1016/j.jgr.2025.03.009
230. Chen XM, Lin GX, Wang X, et al. Beneficial effects of ginsenosides on diabetic nephropathy: a systematical review and meta-analysis of preclinical evidence. J Ethnopharmacol. 2023;302(Pt A):115860. doi:10.1016/j.jep.2022.115860
231. Kang KS, Yamabe N, Kim HY, Park JH, Yokozawa T. Therapeutic potential of 20(S)-ginsenoside Rg(3) against streptozotocin-induced diabetic renal damage in rats. Eur J Pharmacol. 2008;591(1–3):266–272. doi:10.1016/j.ejphar.2008.06.077
232. Li Y, Hou JG, Liu Z, et al. Alleviative effects of 20(R)-Rg3 on HFD/STZ-induced diabetic nephropathy via MAPK/NF-κB signaling pathways in C57BL/6 mice. J Ethnopharmacol. 2021;267:113500. doi:10.1016/j.jep.2020.113500
233. Zhu Y, Zhu C, Yang H, Deng J, Fan D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol Res. 2020;155:104746. doi:10.1016/j.phrs.2020.104746
234. Shi Y, Wang H, Zheng M, Xu W, Yang Y, Shi F. Ginsenoside Rg3 suppresses the NLRP3 inflammasome activation through inhibition of its assembly. FASEB J. 2020;34(1):208–221. doi:10.1096/fj.201901537R
235. Kim JH, Yi YS, Kim MY, Cho JY. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41(4):435–443. doi:10.1016/j.jgr.2016.08.004
236. Yang C, Mu Y, Li S, Zhang Y, Liu X, Li J. Tanshinone IIA: a Chinese herbal ingredient for the treatment of atherosclerosis. Front Pharmacol. 2023;14:1321880. doi:10.3389/fphar.2023.1321880
237. Wang L, Ma R, Liu C, et al. Salvia miltiorrhiza: a potential red light to the development of cardiovascular diseases. Curr Pharm Des. 2017;23(7):1077–1097. doi:10.2174/1381612822666161010105242
238. Xie F, Zhang B, Dai S, Jin B, Zhang T, Dong F. Efficacy and safety of Salvia miltiorrhiza (Salvia miltiorrhiza Bunge) and ligustrazine injection in the adjuvant treatment of early-stage diabetic kidney disease: a systematic review and meta-analysis. J Ethnopharmacol. 2021;281:114346. doi:10.1016/j.jep.2021.114346
239. Yuan FY, Zhang M, Xu P, et al. Tanshinone IIA improves diabetes mellitus via the NF-κB-induced AMPK signal pathway. Exp Ther Med. 2018;16(5):4225–4231. doi:10.3892/etm.2018.6674
240. Chen X, Wu R, Kong Y, et al. Tanshinone IIA attenuates renal damage in STZ-induced diabetic rats via inhibiting oxidative stress and inflammation. Oncotarget. 2017;8(19):31915–31922. doi:10.18632/oncotarget.16651
241. Zheng Z, Zong Y, Ma Y, et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9(1):234. doi:10.1038/s41392-024-01931-z
242. Yaribeygi H, Maleki M, Sathyapalan T, Jamialahmadi T, Sahebkar A. Anti-inflammatory potentials of incretin-based therapies used in the management of diabetes. Life Sci. 2020;241:117152. doi:10.1016/j.lfs.2019.117152
243. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metabol. 2021;46:101102. doi:10.1016/j.molmet.2020.101102
244. Shi S, Chen X, Yu W, Ke X, Ma T. Protective effect of GLP-1 analog liraglutide on podocytes in mice with diabetic nephropathy. Endocr Connections. 2023;12(10):10.1530/ec–23–0284.
245. Li X, Jiang X, Jiang M, et al. GLP-1RAs inhibit the activation of the NLRP3 inflammasome signaling pathway to regulate mouse renal podocyte pyroptosis. Acta diabetologica. 2024;61(2):225–234. doi:10.1007/s00592-023-02184-y
246. Nazari S, Mirkhani H. Cardiorenal protections of SGLT2 inhibitors in the treatment of type 2 diabetes. Current Diabetes Rev. 2023;19(8):e221222212126. doi:10.2174/1573399819666221222160035
247. Oraby MA, El-Yamany MF, Safar MM, Assaf N, Ghoneim HA. Dapagliflozin attenuates early markers of diabetic nephropathy in fructose-streptozotocin-induced diabetes in rats. Biomed Pharmacother. 2019;109:910–920. doi:10.1016/j.biopha.2018.10.100
248. Chen X, Wang M, Yan Z. Recent advances in understanding the mechanisms by which sodium-glucose co-transporter type 2 inhibitors protect podocytes in diabetic nephropathy. Diabetol Metab Syndr. 2025;17(1):84. doi:10.1186/s13098-025-01655-2
249. Zhang Z, Ni P, Tang M, Song Y, Liu C, Zhao B. Dapagliflozin alleviates renal podocyte pyroptosis via regulation of the HO‑1/NLRP3 axis. Mol Med Rep. 2023;28(5). doi:10.3892/mmr.2023.13087
250. Li H, Zhao Q, Liu D, Zhou B, Gong C, Zhao G. Dapagliflozin attenuates NLRP3/Caspase-1 signaling pathway-mediated pyroptosis of vascular smooth muscle cells by downregulating CTSB. Alt Ther Health Med. 2023;29(6):384–392.
251. El-Rous MA, Saber S, Raafat EM, Ahmed AAE. Dapagliflozin, an SGLT2 inhibitor, ameliorates acetic acid-induced colitis in rats by targeting NFκB/AMPK/NLRP3 axis. Inflammopharmacology. 2021;29(4):1169–1185. doi:10.1007/s10787-021-00818-7
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Pyroptosis: A Novel Intervention Target in the Progression of Osteoarthritis
Chang X, Kang Y, Yang Y, Chen Y, Shen Y, Jiang C, Shen Y
Journal of Inflammation Research 2022, 15:3859-3871
Published Date: 11 July 2022
Extracellular Histones Activate Endothelial NLRP3 Inflammasome and are Associated with a Severe Sepsis Phenotype

Beltrán-García J, Osca-Verdegal R, Pérez-Cremades D, Novella S, Hermenegildo C, Pallardó FV, García-Giménez JL
Journal of Inflammation Research 2022, 15:4217-4238
Published Date: 25 July 2022
Sacubitril/Valsartan Improves Progression of Early Diabetic Nephropathy in Rats Through Inhibition of NLRP3 Inflammasome Pathway
Pan Y, Liu L, Yang H, Chen W, Chen Z, Xu J
Diabetes, Metabolic Syndrome and Obesity 2022, 15:2479-2488
Published Date: 13 August 2022
PANoptosis: A Cell Death Characterized by Pyroptosis, Apoptosis, and Necroptosis
Shi C, Cao P, Wang Y, Zhang Q, Zhang D, Wang Y, Wang L, Gong Z
Journal of Inflammation Research 2023, 16:1523-1532
Published Date: 12 April 2023
The Mechanism of Pyroptosis and Its Application Prospect in Diabetic Wound Healing
Al Mamun A, Shao C, Geng P, Wang S, Xiao J
Journal of Inflammation Research 2024, 17:1481-1501
Published Date: 6 March 2024