Back to Journals » Journal of Pain Research » Volume 18
Nomogram for Predicting Severe Abdominal Pain During Percutaneous Radiofrequency Ablation for Hepatocellular Carcinoma: A Retrospective Study
Authors Tian JM , Zhang J, Liu H, Wang F, Yang QS, Luo R, Yang JJ
Received 25 November 2024
Accepted for publication 11 February 2025
Published 21 February 2025 Volume 2025:18 Pages 837—847
DOI https://doi.org/10.2147/JPR.S506099
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amitabh Gulati
Jin-Ming Tian,* Jianan Zhang,* Hang Liu,* Fuming Wang, Qing-Song Yang, Rong Luo, Ji-Jin Yang
Department of Interventional Radiology, Shanghai Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ji-Jin Yang; Rong Luo, Department of Interventional Radiology, Shanghai Changhai Hospital, Shanghai, People’s Republic of China, Tel +8617717881246 ; +8613003244733, Email [email protected]; [email protected]
Objective: To develop and validate a nomogram for identification of hepatocellular carcinoma (HCC) patients unsuitable for percutaneous radiofrequency ablation (RFA) due to severe abdominal pain.
Methods: In this retrospective study, 530 patients with HBV-HCC underwent RFA between February, 2014, and April, 2024, and treated in the affiliated hospital of Changhai Hospital, Naval Medical University. Patients were divided into a modeling group (373 cases) and a validation group (157 cases) at a 7:3 ratio. Pain severity during the heating process of the radiofrequency ablation system was assessed using the Visual Analog Scale (VAS). Logistic regression was used to determine risk factors for severe pain, based on which a nomogram was developed.
Results: Key predictors of severe abdominal pain included tumor distance to capsule < 1cm, tumor distance to portal vein < 1cm, and history of transarterial chemoembolization (TACE) (P< 0.05). The nomogram showed excellent predictive performance with an AUC-ROC of 0.756 for the modeling group and 0.714 for the validation group.
Conclusion: Tumor distance to capsule, tumor distance to portal vein, and TACE history are independent factors influencing severe abdominal pain during radiofrequency ablation in HBV-HCC patients. The nomogram effectively identifies HCC patients at risk of severe pain during RFA, facilitating doctors to decide on better alternative locoregional therapies.
Keywords: hepatocellular carcinoma, abdominal pain, radiofrequency ablation, nomograms
Introduction
HCC is one of the most prevalent malignancies worldwide and poses a significant burden on global health, particularly in regions with high rates of hepatitis B and C infections.1 As the leading cause of cancer-related mortality, HCC profoundly impacts patients, families, and healthcare systems.2 To address this challenge, various interventional treatments have been developed, including RFA, percutaneous ethanol injection (PEI), microwave ablation (MWA), cryoablation, and TACE.3 Among these, RFA has gained widespread recognition as a minimally invasive and effective locoregional therapy for small to medium-sized HCCs. RFA procedures are typically performed under local anesthesia. While mild to moderate abdominal pain may be tolerable under local anesthesia, but severe pain may be intolerable for patients. In such cases, doctors often face the dilemma of balancing pain management with ensuring adequate thermal energy delivery for effective tumor ablation.4 Adjustments to RFA parameters, such as power, temperature, and duration, to alleviate patient discomfort may inadvertently compromise the extent and efficacy of tumor ablation.5,6 Furthermore, pain experienced during RFA procedures can precipitate complications, including nausea and vomiting. These complications not only add to the patient’s discomfort but also increase the complexity and potential hazards of the procedure.7,8
Despite the clinical significance of intraoperative pain management during RFA procedures for HCC patients, there is a paucity of studies addressing this specific aspect. Therefore, there exists a compelling need for a predictive tool that can anticipate the likelihood of severe abdominal pain during RFA procedures. To address this gap in the field of locoregional therapies, we aim to develop a predictive model that estimates the risk of severe abdominal pain in HCC patients undergoing RFA. By analyzing relevant clinical variables and patient characteristics, we seek to identify predictors of intraoperative pain and establish a tool to assist clinicians in personalizing pain management strategies and optimizing treatment outcomes.
Materials and Methods
Patients
This study protocol was approved by the Medical Research Ethics Committee of Shanghai Changhai Hospital. Although the nature of the study was retrospective, all patients had signed informed consent forms at the time of admission. We retrieved data from the electronic medical record database of our hospital on 748 patients diagnosed with HBV-related primary liver cancer according to the American Association for the Study of Liver Diseases (AASLD) guidelines.9 The inclusion criteria were as follows: (1) patients diagnosed with HBV-related HCC according to the AASLD guidelines; (2) patients classified as BCLC stage 0 or A; (3) patients aged between 30 and 80; and (4) patients with or without a history of TACE treatment. All patients underwent RFA treatments in the interventional radiology department of our hospital from February 2014 to April 2024. Exclusion criteria were as follows: (1) use of more than one ablation needle during the procedure; (2) ablation of more than one tumor focus; (3) maximum diameter of tumor >3 cm; (4) extrahepatic metastasis before surgery with an estimated survival of <6 months; (5) Eastern Cooperative Oncology Group (ECOG) score ≥2; (6) use of non-standard potent opioid analgesics during surgery; (7) severe abdominal pain before surgery or a history of long-term (>1 month) use of analgesics; (8) concurrent other malignancies; (9) hepatic encephalopathy, restlessness, or other conditions preventing cooperation with VAS scoring; (10) lack of complete clinical data. Based on these criteria, 218 patients were excluded from the study.
We ultimately included 530 patients who received RFA treatment, consisting of 463 males (average age 61.91±11.66 years; range 33–85 years) and 67 females (average age 62.21±16.13 years; range 31–85 years). All patients in this study were diagnosed with HBV-related HCC. The inclusion process for the study patients is shown in Figure 1.
 |
Figure 1 Patient Screening Flowchart. |
Ablation Procedure and Anesthesia
All RFA procedures in our department are performed under CT guidance (Philips Brilliance iCT 256 slice CT) by 2 senior interventional radiologists who had over 8 years of experience. In RFA, we use expandable, star-shaped electrodes (RITA StarBurst XL (L=15cm) ELECTROSURGICAL DEVICES; AngioDynamics, Inc, Marlborough, MA, USA) and a 200W RITA radiofrequency ablation system (RITA System Generator 1500X, AngioDynamics, Inc., USA). All patients are awake during the procedures and receive local anesthesia with 50mg (IM) of pethidine hydrochloride and then subcutaneous injection of 10 mL of 2% lidocaine along the planned needle path. If a patient experiences unbearable pain during ablation, an additional 50mg (IM) of pethidine is administered, and respiratory and circulatory systems are monitored. This approach adheres to humane care and ethical standards. However, cases involving additional pethidine are excluded from this study to prevent any influence on the physicians’ pain scoring and the uniformity of the research. Figure 2.
 |
Figure 2 Radiofrequency ablation under CT guidance. |
Intraoperative Pain Assessment
Two interventional radiologists a surgical nurse with six years of experience assessed the severity of abdominal pain during RFA procedures using the Visual Analog Scale (VAS). The assessment tool was an Emoji-Based scale, with emojis above markings from 0–10; the scale starts at 0 for “no pain” and ends at 10 for “most severe pain”. The pain severity assessment is as follows: 0 for no pain; 1–3 for mild pain; 4–6 for moderate pain; and 7–10 for severe pain.10 The patients of all 530 were divided into a mild (VAS 1–3) to moderate (VAS 4–6) abdominal pain group and severe abdominal pain (VAS 7–10) group.
Clinical Data Collection
Based on the requirement that the sample size should be at least ten times the number of variables in a risk factor study, this research included nine variables, based on previous studies and clinical practice experience.11 These variables are gender, age (<60, ≥60), distribution of tumor (left lobe, right anterior lobe, right posterior lobe), tumor distance to capsule (<1cm, ≥1cm), tumor distance to portal vein (<1cm, ≥1cm), maximum diameter of tumor (<2cm, ≥2cm), maximum diameter of ablation lesion (<3cm, ≥3cm), maximum ablation temperature (<95°C, ≥95°C), surgical treatment history (no, yes), and TACE treatment history (no, yes). All data was obtained through the hospital’s electronic medical record system. Data entry was conducted by two doctors simultaneously to ensure the accuracy of the information.
Statistical Analysis
Clinical data were analyzed using R software (version 4.3.3). Count data were presented as numbers of cases or ratios. All clinical data were categorical variables, and chi-square tests or the Fisher’s exact test were used to determine any statistical differences between the modeling and validation groups. Univariate analyses were performed using chi-square tests, and variables with statistical significance were included in the multivariate analysis. Subsequently, a multivariate logistic regression analysis was conducted to develop a predictive model for severe abdominal pain during RFA, and a nomogram was constructed. The performance of the model was quantified using the Receiver Operating Characteristic (ROC) curve, and the Area Under the Curve (AUC) was calculated. The clinical utility of the nomogram was evaluated through calibration curves and Decision Curve Analysis (DCA). P<0.05 was considered statistically significant.
Results
Comparison of Clinical Data Between the Modeling Group and the Validation Group
The modeling group and the validation group showed no significant differences in terms of gender, age, distribution of tumor, tumor distance to capsule, tumor distance to portal vein, maximum diameter of tumor, maximum diameter of ablation lesion, maximum ablation temperature, surgical treatment history, and TACE treatment history (all P>0.05, Table 1).
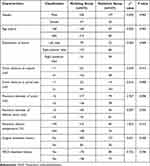 |
Table 1 Baseline Demographic and Clinical Characteristics in Modeling Group and Validation Group |
Univariate and Multivariate Analysis of Factors Causing Severe Abdominal Pain During RFA
Data from the modeling group indicate that severe abdominal pain during radiofrequency ablation is significantly associated with tumor distance to the capsule, tumor distance to portal vein, and TACE treatment history (P < 0.05, Table 2). It shows no significant correlation with gender, age, tumor distribution, maximum diameter of tumor, maximum diameter of the ablation lesion, maximum ablation temperature, or surgical treatment history (P > 0.05, Table 2). Based on the variables that were statistically significant in the univariate analysis, a multivariate logistic regression analysis was conducted, the final results showed that: tumor distance to the capsule, tumor distance to portal vein, and TACE treatment history were all independent factors influencing severe abdominal pain during RFA in HBV-HCC patients (all P <0.05, Table 3).
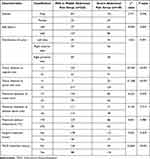 |
Table 2 Univariate Analysis of Severe Abdominal Pain During RFA in Modeling Group |
 |
Table 3 Multivariate Logistic Regression Analysis of the Factors for Severe Abdominal Pain During RFA |
Prediction Model Construction and Validation
Employing the β coefficient from logistic regression analysis, we devised a risk assessment equation: P =1/1+e−Y, where P denotes the probability of experiencing severe abdominal pain during RFA. In this formula, “e” is the natural logarithm, and Y = −1.838+1.345×Tumor distance to capsule+1.301×Tumor distance to portal vein +1.322×TACE treatment history. The ROC analysis results showed that the model’s predictive accuracy in the modeling group was reflected by an AUC of 0.756 (95% CI 0.709–0.803), with a corresponding optimal cutoff value of 0.256 (sensitivity 0.442, specificity 0.940) (Figure 3A). Verification from the validation group supported these results, showing an AUC of 0.714 (95% CI 0.633–0.796) and corresponding optimal is 0.691 (sensitivity 0.935, specificity 0.391), demonstrating the model’s strong predictive ability (Figure 3B). The calibration curve demonstrated general alignment between the predicted and observed outcomes, diagonal dotted line symbolizes the optimal scenario for flawless predictions, while a solid line illustrates the actual performance of the model in this study, the proximity of the model’s curve to the ideal line suggests that the model’s clinical predictive capability is outstanding (Figure 4). Furthermore, the decision curve analysis (DCA) curve indicated a significant net benefit associated with the model (Figure 5).
 |
Figure 3 (A) Receiver operating characteristic (ROC) curves of the modeling group. (B) Receiver operating characteristic (ROC) curves of the validation group. |
 |
Figure 4 Calibration curve of the model. |
 |
Figure 5 Decision curve analysis (DCA) curve of the model. |
Construction and Application Example of a Nomogram for the Prediction of Severe Pain During RFA
To simplify the presentation of these results, we used a nomogram to depict the model visually (Figure 6). For instance, consider a primary HCC patient with a tumor located 0.9cm from the liver capsule, 0.5cm from the portal vein, and no prior TACE treatment. Based on these characteristics, scores are obtained from the point axis, totaling 197.5. A vertical line is then drawn from the position corresponding to 197.5 on the total point axis, intersecting with the risk of severe pain axis. This intersection indicates that the probability of severe pain during RFA is approximately 70%.
 |
Figure 6 A nomogram for predicting severe abdominal pain during Radiofrequency Ablation. |
Discussion
Although RFA has been widely practiced globally for decades, most hospitals and surgeons still rely on experiential judgment to anticipate intraoperative pain and choose ablation methods. Our study successfully developed a predictive nomogram to assess the risk of severe abdominal pain in HCC patients undergoing RFA. To our knowledge, this is the first predictive model for pain during RFA procedures. The model includes key variables—Tumor distance to capsule, tumor distance to portal vein, and TACE treatment history—significant predictors of severe pain. The nomogram demonstrated strong predictive power with an area under the ROC curve of 0.756 for the modeling group and 0.714 for the validation group. These findings highlight the potential of our nomogram as a clinical tool for preoperative risk stratification and personalized pain management around the perioperative period of RFA.
This study, based on multivariate analysis, shows that tumor distance to capsule, tumor distance to portal vein, and TACE treatment history are independent predictors of severe abdominal pain during surgery. The “capsule” refers to the liver’s Glisson’s capsule, a dense fibrous structure enveloping the liver, innervated by branches of the intercostal nerves, which are sensitive to pain and temperature and provide precise localization.12–14 Tumors located near the portal vein may cause severe pain during radiofrequency ablation due to the dense network of autonomic nerves surrounding the portal vein. These nerves, which run alongside the liver’s blood vessels, are highly sensitive to thermal damage. The ablation process can stimulate these nerves, leading to increased pain perception.15,16 TACE blocks the tumor’s blood supply, altering the hemodynamics in the tumor area, which may indirectly reduce the “heat-sink effect” troubling many doctors during ablation, result in a more concentrated thermal effect and hence more severe pain.17 Additionally, the iodized oil accumulated in the lesion post-TACE, being an oily substance with distinct physical and chemical properties, may lead to quicker or more focused heat distribution in the tumor area, increasing pain.
Our results align with Lee’s findings on the proximity of tumors to the peritoneum affecting pain, but our study specifies a more precise distance (<1 cm).11 In Lee’s study, the factor of tumor proximity to the portal vein was also included, but possibly due to the small sample size, it did not show statistical significance. Moreover, the author did not include whether patients had received TACE treatment as a factor.
Our study has limitations. The size of the sample in clinical studies that analyze risk factors and develop predictive models is crucial for the statistical power and broader applicability of the results. In the future, we will gather more patient data to confirm the reliability of our model and plan multi-center studies to establish external validation cohorts to further test the model’s robustness. Additionally, as this was a retrospective study, future efforts should aim to construct prospective, multi-center studies incorporating a broader range of clinical and demographic variables to validate our predictive model, enhance its applicability, and develop a more universally applicable pain prediction tool that might be used for predicting intraoperative pain in patients undergoing other types of ablation like microwave ablation and cryoablation.
Studies on RFA typically focus on survival outcomes, but intraoperative pain is a critical aspect. In most cases, percutaneous RFA is performed under local anesthesia with the patient remaining conscious. This can lead to severe abdominal pain, possibly accompanied by nausea, vomiting, airway obstruction from aspiration, and even intolerable discomfort that may require stopping the procedure. Additionally, doctors often face the dilemma of balancing pain management with ensuring sufficient heat delivery to effectively ablate the tumor. Adjusting RFA parameters to alleviate pain could resulting in insufficient ablation. Thus, our study fills this gap by providing a practical model, which can help doctors design more appropriate anesthesia, pain relief measures, and ablation plans before the procedure.
Conclusion
This study developed and validated a predictive nomogram for severe abdominal pain during RFA in hepatocellular carcinoma patients. Key predictors including tumor proximity to the liver capsule, tumor proximity to the portal vein, and prior TACE treatment. The nomogram demonstrated strong predictive performance, aiding interventional physicians in risk stratification, optimizing pain management strategies, and selecting appropriate alternative options, such as cryoablation or other locoregional therapies.
Key Points
- A nomogram that uses key predictors to accurately forecast severe abdominal pain during RFA for HCC.
- This tool aids doctors in facilitating personalized pain management and potentially guiding alternative treatment choices.
Statistics and Biometry
No complex statistical methods were necessary for this paper. All authors have sufficient statistical expertise to conduct the study.
Abbreviations
RFA, Radiofrequency Ablation; HCC, Hepatocellular Carcinoma; VAS, Visual Analog Scale; TACE, Transarterial Chemoembolization; HBV, Hepatitis B Virus; ROC, Receiver Operating Characteristic; AUC, Area Under the Curve.
Ethical Statement
This retrospective study was conducted in accordance with the Declaration of Helsinki and received approval from the Ethics Committee of Shanghai Changhai Hospital. Although the nature of the study was retrospective, all patients had signed informed consent forms at the time of admission, authorizing the use of their medical data for research purposes. All patient information was anonymized and confidentiality was maintained throughout the study. Institutional Review Board approval was obtained. The scientific guarantor of this publication is Yi-qi Du.
Informed Consent
Written informed consent was not required for this study as per the Institutional Review Board.
Acknowledgments
The authors thank Changhai Hospital, Naval Medical University, for providing the facilities and resources necessary for conducting this research.
Funding
This study did not receive any external funding.
Disclosure
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. There has been no previous reporting of the study subjects or cohorts in this series.
References
1. Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864–884. doi:10.1038/s41571-023-00825-3
2. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi:10.3322/caac.21834
3. Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293–313. doi:10.1038/s41575-020-00395-0
4. Kim TH, Koh YH, Kim BH, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized Phase III trial. J Hepatol. 2021;74:603–612. doi:10.1016/j.jhep.2020.09.026
5. Shi Z-R, Duan Y-X, Cui F, et al. Integrated proteogenomic characterization reveals an imbalanced hepatocellular carcinoma microenvironment after incomplete radiofrequency ablation. J Exp Clin Cancer Res. 2023;42:133. doi:10.1186/s13046-023-02716-y
6. Zhu Y, Li Q, Wang C, et al. Rational design of biomaterials to potentiate cancer thermal therapy. Chem Rev. 2023;123:7326–7378. doi:10.1021/acs.chemrev.2c00822
7. Maeda M, Saeki I, Sakaida I, et al. Complications after radiofrequency ablation for hepatocellular carcinoma: a multicenter study involving 9,411 Japanese patients. Liver Cancer. 2020;9:50–62. doi:10.1159/000502744
8. Zhou C, Peng Y, Zhou K, et al. Surgical resection plus radiofrequency ablation for the treatment of multifocal hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2019;8:19–28. doi:10.21037/hbsn.2018.11.19
9. Singal AG, Llovet JM, Yarchoan M, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922–1965. doi:10.1097/HEP.0000000000000466
10. He S, Renne A, Argandykov D, et al. Comparison of an emoji-based visual analog scale with a numeric rating scale for pain assessment. JAMA. 2022;328:208–209. doi:10.1001/jama.2022.7489
11. Lee S, Rhim H, Kim Y-S, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas: factors related to intraprocedural and postprocedural pain. AJR Am J Roentgenol. 2009;192:1064–1070. doi:10.2214/AJR.08.1350
12. Sag AA, Qadri YJ. Interventional radiology regional anesthesia approaches for intra- and postprocedural pain control. Semin Intervent Radiol. 2022;39:142–149. doi:10.1055/s-0042-1745799
13. Bioulac-Sage P, Lafon ME, Saric J, et al. Nerves and perisinusoidal cells in human liver. J Hepatol. 1990;10:105–112. doi:10.1016/0168-8278(90)90080-b
14. Kimura C. Pain of the peritoneum. Gastroenterol Jpn. 1966;1:8–9. doi:10.1007/BF02798123
15. Mizuno K, Ueno Y. Autonomic nervous system and the liver. Hepatol Res. 2017;47:160–165. doi:10.1111/hepr.12760
16. He KS, Fernando R, Cabrera T, et al. Hepatic hilar nerve block for hepatic interventions: anatomy, technique, and initial clinical experience in thermal ablation of liver tumors. Radiology. 2021;301:223–228. doi:10.1148/radiol.2021203410
17. Zorbas G, Samaras T. A study of the sink effect by blood vessels in radiofrequency ablation. Comput Biol Med. 2015;57:182–186. doi:10.1016/j.compbiomed.2014.12.014
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

