Back to Journals » Drug Design, Development and Therapy » Volume 19
Noninferiority of 0.25% versus 0.375% Ropivacaine in Popliteal Sciatic and Saphenous Nerve Blocks for Analgesia After Foot and Ankle Surgery: A Randomized Self-Paired Noninferiority Trial
Authors Wu L, Xi C, Lei G, Li H, Yin Y, Wan M, Wu H, Wang Y, Hu C, Wang G
Received 6 December 2024
Accepted for publication 28 April 2025
Published 19 May 2025 Volume 2025:19 Pages 4093—4104
DOI https://doi.org/10.2147/DDDT.S508528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Lili Wu, Chunhua Xi, Guiyu Lei, He Li, Yue Yin, Meixuan Wan, Haiyao Wu, Yue Wang, Chunhua Hu, Guyan Wang
Department of Anesthesiology, Beijing Tongren Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Guyan Wang, Department of Anesthesiology, Beijing Tongren Hospital, Capital Medical University, 1 Dongjiaominxiang Road, Dongcheng District, Beijing, 100730, People’s Republic of China, Tel +86-10-58268101, Email [email protected]
Purpose: Peripheral nerve blocks are an important part of postoperative analgesia for the extremities. Previously, we reported that a single shot of 0.375% ropivacaine (20 mL) via ultrasound-guided popliteal sciatic and saphenous nerve blocks provided satisfactory analgesia after foot and ankle surgery; however, toe and ankle weakness in the early postoperative period became a concern for patients. Our preliminary data indicate that 0.25% ropivacaine may be effective for postoperative analgesia. Hence, we hypothesized that the analgesic effect of 0.25% ropivacaine would be noninferior to that of 0.375% ropivacaine at the same volume and would reduce the degree of weakness.
Patients and Methods: In this randomized, double-blind, self-paired, noninferiority trial, 31 patients who were scheduled for similar, elective, bilateral foot and ankle surgeries under general anesthesia combined with popliteal sciatic and saphenous nerve blocks were enrolled. Each patient was randomly assigned to receive 0.25% ropivacaine on one side and 0.375% ropivacaine on the other side. The primary outcome was the duration of analgesia, which was defined as the time from the end of the nerve blocks until the first sensation of pain in the surgical area, as indicated by a patient-reported visual analog scale (VAS) score ≥ 1. The secondary outcomes included static VAS scores, motor and sensory block grades, patient satisfaction scores, and the incidence of adverse effects.
Results: The mean duration of analgesia was 31.7 ± 8.3 h for 0.25% ropivacaine, and 31.9 ± 8.5 h for 0.375% ropivacaine (duration difference, − 0.16; 95% CI, − 1.5 to 1.2; P = 0.812). Compared with 0.375% ropivacaine, 0.25% ropivacaine resulted in a lower incidence of motor block at 0, 2, 6 and 12 hours postoperatively (P < 0.05). No differences in static VAS, sensory block or patient satisfaction scores were observed between the two concentrations within 48 hours postoperatively. Furthermore, no nerve block-related adverse events were reported.
Conclusion: The results revealed that 0.25% ropivacaine is not inferior to 0.375% ropivacaine in terms of the analgesic duration of popliteal sciatic and saphenous nerve blocks for bilateral foot and ankle surgery. Moreover, 0.25% ropivacaine reduced the incidence of motor block. Therefore, we recommend 0.25% ropivacaine for postoperative analgesia for foot and ankle surgery.
Keywords: ropivacaine, analgesia, foot and ankle surgery, nerve block, popliteal sciatic nerve, saphenous nerve
Introduction
Foot and ankle surgery often results in severe and sustained pain for 24–48 h postoperatively; this pain can be difficult to control with intravenous or oral medications, and many modalities are needed for postoperative analgesia.1,2 Peripheral nerve blocks (PNBs) are medical procedures that involves the injection of local anesthetics (LAs) into a specific peripheral nerve or a group of nerves, with the goal of blocking the transmission of pain signals to the brain. PNBs have proven to be safe in the control of postoperative pain after limb surgery, as they lead to decreased narcotic use and associated adverse effects, longer analgesia, and greater patient satisfaction, and more referrals.3–6 Ropivacaine is a long-acting amide LA commonly used for PNBs. Previous studies have reported that ropivacaine concentrations of 0.5% to 1% for sciatic and saphenous nerve blocks provide effective analgesia within 24 hours after foot and ankle surgeries.5,7,8 Owing to the increasing demand for patient comfort and advances in surgical methods, popliteal sciatic and saphenous nerve blocks with lower concentrations of LAs combined with general anesthesia are often implemented for elective foot and ankle surgeries.9,10 However, the duration of analgesia with ropivacaine at concentrations less than 0.5% has not been fully evaluated.
In addition to satisfactory analgesia, many orthopedic surgeries and foot and ankle surgeries require early recovery of motor function to rule out surgery-induced nerve damage or to achieve early functional recovery and training.11,12 Typically, PNBs with LAs require a threshold volume to reach the target nerve, with the concentration of the LAs being the primary determinant of the efficacy of analgesia and motor block.13,14 As a result, anesthesiologists are interested in the optimal concentrations of LAs to balance analgesia and motor function. Compared with other LAs, ropivacaine has a faster recovery from motor block despite similar sensory properties for regional anesthesia, the management of postoperative analgesia and labor pain.15,16 Previously, we reported that popliteal sciatic and saphenous blocks with 0.375% ropivacaine prior to general anesthesia provided good postoperative analgesia after foot and ankle surgery;17 however, toe and ankle weakness in the early postoperative period became a concern for patients. Reducing the ropivacaine concentration to less than 0.375% with a single shot for postoperative analgesia after foot and ankle surgery has not been studied, and whether it reduces motor block or has an impact on the analgesic effect remains unclear.
Bilateral surgery can be used to address problems in both the feet and ankles simultaneously, so this approach is very popular among patients. However, the risk of systemic and direct neurotoxicity increases as the amount of LAs increases during bilateral surgery.18,19 Thus, the American Society of Regional Anesthesia and Pain Medicine recommends the use of the lowest effective dose of LA (dose = product of volume × concentration) to prevent systemic toxicity.20 Our preliminary data indicate that 0.25% ropivacaine may be effective for postoperative analgesia when administered around the popliteal sciatic and saphenous nerves. Therefore, we hypothesized that the analgesic effect of 0.25% ropivacaine would be noninferior to that of 0.375% ropivacaine in ultrasound-guided popliteal sciatic and saphenous nerve blocks, and would provide better early postoperative functional movement for foot and ankle surgery.
Materials and Methods
Study Design and Ethics
This study was a randomized, double-blinded, paired, noninferiority clinical trial. The trial followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines. The study protocol was presented in Supplemental information 1. Ethical approval was obtained from the Ethics Committee of Beijing Tongren Hospital in February 2021 (TRECKY2021-054), and the study was conducted in accordance with the Declaration of Helsinki. This trial was registered in the Chinese Clinical Trial Registry before patient enrollment (Registration number: ChiCTR2100053929; Date of registration: December 2, 2021; Principal Investigator: Lili Wu). Written informed consent was obtained from all patients prior to randomization.
Participants
The study included patients aged 18–74 years who were scheduled for bilateral similar foot and ankle surgery with general anesthesia combined with popliteal sciatic and saphenous nerve blocks. The inclusion criteria were as follows: American Society of Anesthesiologists (ASA) physical status I‒III, a body mass index (BMI) of 18‒30 kg/m2, and the ability to understand the protocol and provide informed consent. The exclusion criteria were as follows: patients who did not have ASA physical status I‒III, contraindications to PNB (coagulopathy, infection at the puncture site, allergy to general or LAs), anticipated difficult airways, central or peripheral neurological diseases, pregnancy or breastfeeding, chronic opioid use, abnormal liver or kidney function, poorly controlled diabetes (fasting glucose >11 mmol/L), enrollment in other trials, malignant tumors, Charcot osteoarthropathy, and daily use of steroids. Patient recruitment and surgery performance were conducted between December 15, 2021, and February 25, 2022 in Beijing Tongren Hospital, Capital Medical University.
Randomization and Blinding
Patients scheduled for bilateral identical foot and ankle surgeries were assigned a study number (1–33) in order of their enrollment. A computer-generated randomization sequence was prepared by an independent statistician who was not involved in the trial. This sequence contained 33 numbers randomly generated by the computer within 100. These 33 numbers corresponded to the patients’ study numbers in turn. Then, the 33 numbers were sorted from small to large. The patients with the first 16 numbers received plan A, and the patients with the last 17 numbers received plan B. In protocol A, patients received 0.25% ropivacaine on the right side and 0.375% ropivacaine on the left side. In protocol B, patients received 0.25% ropivacaine on the left side and 0.375% ropivacaine on the right side.
The allocation information was kept in sealed, opaque, sequentially numbered envelopes. The envelopes were delivered to a nurse, who was responsible for preparing the trial medication according to the allocation inside the envelopes and was not otherwise involved in the trial. Syringes containing an equivalent volume of indistinguishable local anesthetic were prepared and labelled as either the “right syringe” or the “left syringe” according to the contents. All patients, investigators, anesthesiologists, surgeons, outcome assessors, and data analysts were blinded to the treatment allocation.
Procedure and Intervention
Patients abstained from food and water for 8 hours before surgery. After arriving at the preoperative preparation room, the patient’s venous access was opened, and routine monitoring of blood pressure (BP), heart rate (HR), and pulse oxygen saturation (SpO2) was conducted.
Oxygen was delivered to the patient at 4 L/min via a face mask. Before nerve block, each patient received 0.1 μg/kg sufentanil and 0.02 mg/kg midazolam intravenously for sedation and analgesia, respectively. Additionally, 10 mg of dexamethasone was administered intravenously to prolong the duration of analgesia as we reported recently.17 All nerve blocks were performed via ultrasound guidance by experienced anesthesiologists with more than 300 PNB procedures (Dr. Wu LL and Dr. Lei GY). We used an ultrasound system with a 4–15 MHz linear probe (Wisonic Navi Sevies, Shenzhen, China) and a 21-gauge, 100-mm insulated nerve blockade needle.
Popliteal sciatic nerve block was performed with the patient in the supine position by resting the foot on an elevated footrest. The transducer was positioned at the level where the tibial nerve and common peroneal nerve start diverging but are still within the common sciatic nerve sheath. The needle was inserted in the plane from the lateral-to-medial direction. The needle tip was finally positioned between the tibial and common peroneal nerves within the paraneural sheath. Ropivacaine was administered in two injections at 6:00 and 12:00 around the bifurcation, with 10 mL per injection, for a total volume of 20 mL.
Saphenous nerve block was performed in the supine position, with the thigh abducted and externally rotated to allow easily access to the medial thigh. The transducer was placed anteromedially, approximately at the junction between the middle and distal thirds of the thigh. The needle was inserted in-plane in a lateral-to-medial orientation and advanced. The saphenous nerve was identified as a hyperechoic, oval or round structure anterolateral to the femoral artery, and 10 mL ropivacaine was injected (Figure 1A and B). We performed aspiration prior to injection to avoid intravascular injection. After administration of the nerve blocks, an independent observer (Dr. Li H or Dr. Hu CH) who was blinded to the treatment, assessed the effectiveness of the block via a pinprick test. This assessment was conducted every 5 minutes for three assessments. Block success was defined as no sensation of cold or pain in the sciatic or saphenous nerve dermatomes 30 min after the final ropivacaine injection.
The patient received general anesthesia when the nerve blocks were determined to be effective. The anesthetic agents used were midazolam 0.02mg/kg, sufentanil 0.2 μg/kg, propofol 1.5–2 mg/kg, and rocuronium 0.6 mg/kg. After induction, a laryngeal mask airway (LMA) was inserted, and intermittent positive pressure ventilation (IPPV) was initiated. The tidal volume was set at 6–8 mL/kg to maintain end-tidal carbon dioxide (EtCO2) levels between 35–40 mmHg. The tourniquet was placed in the middle of the thigh, applied before the start of the procedure and stopped after the procedure. During the operation, the depth of general anesthesia was monitored with the bispectral Index (BIS), and total intravenous anesthesia was performed with a continuous infusion of remifentanil 0.1–0.2 μg/kg/min and propofol 5–8 mg/kg/h to maintain a BIS value between 40–60. The fluctuations in mean arterial pressure (MAP) and heart rate (HR) were maintained within 20% of the preoperative values of the patients. All surgical procedures were conducted by a fixed team of surgeons. Drug infusion was stopped at the end of surgery. Once the patient recovered consciousness, the LMA was removed, and the patient was transferred to the post-anesthesia care unit (PACU) for at least 30 min of observation (Dr. Yue Wang) until they met the discharge criteria.
Postoperative pain management included patient-controlled analgesia (PCA) with sufentanil at 1.5 µg/kg and 24 mg of ondansetron prepared in a 100-mL analgesia device. The device has a continuous background dose of 2 mL per hour, a single additional dose of 2 mL, and a lock-in time of 15 minutes. Patients were allowed to self-administer additional analgesics as needed and were followed by a special team (Dr. Wan MX, Dr. Wu HY or Dr. Yin Y).
Outcomes and Definitions
The primary outcome was the duration of analgesia, which was defined as the time from the end of the nerve blocks until the first sensation of pain at the surgical site as measured by a patient-reported visual analog scale (VAS) score ≥ 1 (on a scale ranging from 0–10). The secondary outcomes included static VAS scores for pain in the PACU and at 2, 6, 12, 24, 36 and 48 h postoperatively; motor block; sensory block; patient satisfaction scores (on a scale ranging from 0–10); and adverse events such as persistent numbness, paranesthesia, motor deficits, vascular injury, local anesthetic toxicity and nerve injury. Motor block was assessed for ankle and toe movements and was graded on a 3-point scale: 0 = no block; 1 = paresis (slight or partial paralysis); and 2 = complete paralysis. The sensory block was graded on a 3-point scale using a cold test: 0 = no block; 1 = analgesia (patient can feel touch, no cold), and 2 = anesthesia (patient cannot feel touch) as described in a previous study.14
Sample Size
This study was a randomized, double-blinded, paired, noninferiority trial. The primary outcome (duration of analgesia) was used to determine the appropriate sample size. We hypothesized that the analgesic duration of 0.25% ropivacaine would be noninferior to that of 0.375% ropivacaine. In a preliminary study with seven patients, the mean duration difference was 0.16 hours, with a standard deviation (SD) of 3.78. PASS 15.0 software (NCSS, LLC; Kaysville, UT, USA) was used for sample size calculation, and a noninferiority margin of 2 hours was considered clinically relevant,21 with a one-sided alpha of 0.025, a beta of 0.2 and a power of 80%. The minimum sample size was calculated to be 27 for each group. Under the assumption of a potential dropout rate of 20%, we planned to recruit 33 patients.
Statistical Analysis
Statistical analysis was performed via IBM SPSS Statistics for Windows, version 24.0 (IBM Corp, Armonk, NY, USA). The Shapiro–Wilk test was used to test whether the data were normally distributed. Normally distributed variables are expressed as the means ± SDs and were analyzed with paired Student’s t test. Nonnormally distributed variables are expressed as medians [IQRs] and were analyzed with the paired Wilcoxon signed-rank test. Categorical variables are presented as n (%). A noninferiority study design was used to assess the primary outcome. Kaplan‒Meier curves were used to analyze time-to-event (the event was defined as a patient reported VAS score ≥ 1) data, and the Log rank test was used to compare the survival distributions between the two concentrations. Sensory and motor block scores were compared via the Mantel‒Haenszel linear‒by‒linear χ2 test. We also used a Bonferroni–Holm correction to test a threshold for the statistical significance of multiple outcomes.22 All tests were two-sided, and P < 0.05 indicated statistical significance.
Results
Patient Characteristics
In total, 33 patients scheduled for bilateral foot and ankle surgeries were recruited between 15 December 2021 and 25 February 2022. One patient each from Protocol A and Protocol B declined to participate, leaving 31 patients who received the allocated intervention. All 31 patients completed the trial and were analyzed for the primary outcome, as presented in the CONSORT flow diagram (Figure 2). The demographic data, patient characteristics, and clinical parameters are listed in Table 1. All nerve blocks were successful and uneventful.
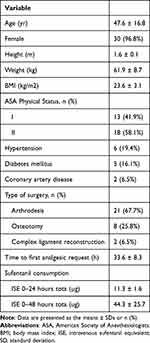 |
Table 1 Demographics and Baseline Values |
 |
Figure 2 CONSORT flowchart diagram. |
Primary and Secondary Outcomes
The mean duration of analgesia was 31.7 ± 8.3 h in the 0.25% ropivacaine group and 31.9 ± 8.5 h in the 0.375% ropivacaine group (duration difference, −0.16; 95% CI, −1.5 to 1.2; P = 0.812), as shown in Table 2. The lower bound of the 95% confidence interval (−1.5) was greater than the noninferiority margin (−2). The Kaplan–Meier curves representing the complete pain relief time revealed that there was no difference between 0.25% ropivacaine and 0.375% ropivacaine (P = 0.900) (Figure 3).
 |
Table 2 Primary and Secondary Outcomes and Clinical Parameters |
 |
Figure 3 Kaplan–Meier survival curve representing the complete pain relief time in the two groups. (P = 0.900). |
There were no significant differences in static VAS scores between the two groups in the PACU or at 2, 6, 12, 24, 36, and 48 h postoperatively. Similarly, the patient satisfaction scores at 12, 24, and 48 h after surgery were not significantly different between the two groups. Moreover, the durations of surgery and tourniquet application were similar between the two groups. Notably, there were no ropivacaine-related adverse events or nerve block complications in either group (Table 2).
Patients with 0.25% ropivacaine had significantly less motor block at PACU, at 2, 6 and 12 h; no other statistically significant differences in block density were observed at 24 h or later (Table 3). There was no significant difference in the sensory block between the two groups at any assessment time point (Table 4). All patients recovered from complete motor block and sensory block at 72 h postoperatively. No nerve block-related complications were reported postoperatively.
 |
Table 3 Motor Block Within 48 hours Postoperatively |
 |
Table 4 Sensory Block Within 48 hours Postoperatively |
Discussion
In this randomized, self-paired study, we found that the duration of analgesia with 0.25% ropivacaine was noninferior to that with 0.375% ropivacaine in ultrasound-guided popliteal sciatic and saphenous nerve blocks for foot and ankle surgeries. Additionally, motor block with 0.25% ropivacaine was milder within 24 hours postoperatively than motor block with 0.375% ropivacaine was.
Undesired weakness of the toes and ankles is a common occurrence after popliteal sciatic nerve block. Decreasing the concentration of LAs is often used to minimize motor block, however, it remains unknown whether the analgesic effect is affected and the results are contradictory. A previous study examined the concentration of LAs and reported no difference in the analgesic duration of the interscalene block between 10 mL of 0.75% ropivacaine and 20 mL of 0.375% ropivacaine at a fixed dose.23 However, 20 mL of 0.5% ropivacaine yielded longer postoperative analgesia than did 20 mL of 0.375% ropivacaine after a costoclavicular brachial plexus block.14 A recent systematic review and meta-analysis indicated that the duration of sensory blockade with 0.75% ropivacaine was similar to that with 0.5% ropivacaine, and that 0.5%–1% ropivacaine may provide a similar sensory block duration but longer motor block duration than 0.375% ropivacaine.24 In addition, although low-concentration ropivacaine for continuous sciatic nerve block may provide analgesia for up to several days, catheterization of continuous blocks may not be necessary because of rapid discharge from the hospital after foot and ankle surgery. In this study, two different concentrations of ropivacaine with the same volume of nerve blocks were used in patients who underwent bilateral foot and ankle surgery, with a concentration of 0.375% on one side and 0.25% on the other side. We found that the analgesic duration of 0.25% ropivacaine was approximately 32 hours, and was similar to that of 0.375% ropivacaine. Therefore, we believe that a concentration of 0.25% ropivacaine is sufficient for postoperative analgesic purposes alone and can reduce the total amount of LAs used in bilateral surgery.
The concentration, volume, total dose of LAs, and anatomical structures surrounding the target nerve may influence the analgesic duration of PNBs.23,25 Typically, the volume of LAs is considered paramount in determining analgesic duration, as a greater volume of LAs is more likely to completely surround the target nerve. The ED95 minimal effective anesthetic volume (MEAV) was 16 mL for ultrasound-guided popliteal sciatic nerve block with 0.5% ropivacaine, and 1.9 mL for saphenous nerve block with 2% mepivacaine.26,27 Our previous study revealed that 20 mL for sciatic nerve block and 10 mL for the saphenous nerve block provided analgesia for over 24 hours after foot and ankle surgery.17 Therefore, we applied the same volume of LA in this study to rule out the effect of volume on analgesic efficacy. We deduce that the most important factor for the analgesic duration in our study is the anatomical characteristics of the sciatic nerve at the popliteal fossa, where there are paraneural sheaths covering the tibial nerve (TN) and common peroneal nerve (CPN) above and below their divergence. These anatomical features provide excellent conditions for lower concentrations of the LA to infiltrate the TN and CPN, which are slimmer and more likely to be blocked than the sciatic nerve trunk above the divergence.28 Therefore, 20 mL of 0.25% ropivacaine with subparaneural injection can effectively block both the TN and CPN simultaneously, resulting in long-acting analgesia and sensory block. Our results support the findings of Christiansen et al, who reported that ropivacaine concentrations ranging from 0.04% to 0.5% had similar sensory block durations on the common peroneal nerve in healthy volunteers.29 In addition, the analgesic duration in this study was significantly longer than that reported in previous studies (approximately 24 hours).5,8 We believe that this may be related to our use of a two-point injection method, as it has been reported in the literature that two-point administration may achieve a longer analgesic effect by encircling the longer sciatic nerve.30 Given that the effectiveness of PNBs is dependent on the skill of the operator, further multicenter studies could be performed to confirm our results.
In our study, severe motor block was significantly lower in the 0.25% ropivacaine group than in the 0.375% ropivacaine group within 24 hours postoperatively. This finding is particularly important for facilitating early discharge and rehabilitation after surgery because prolonged dense motor blockade is another limitation of subparaneural popliteal sciatic nerve block that concerns the surgeon, and patients also find it unpleasant. The prolonged postoperative motor blockade may be related to the slow washout (elimination) of the LAs from the relatively avascular subparaneural compartment. Previous studies have shown that both high concentrations and large volumes of local anesthetics can affect motor block, and LA dilution results in reduced motor block duration.29,31 Typically, higher concentrations of ropivacaine, such as 0.5% to 1%, are used in regional anesthesia, primarily because of their rapid onset and effectiveness as intraoperative anesthesia. In our study, general anesthesia was used to ensure patient comfort and to mitigate the effects of thigh tourniquets. The benefits of high-concentration LAs were not detectable, whereas the disadvantages of motor blocks were evident in this situation. Thus, we suggest that a lower concentration of local anesthetics, such as 0.25% ropivacaine, may optimize postoperative analgesia, minimize motor block, and increase patient comfort.
Limitations
This study has several limitations. First, we assessed only postoperative static pain, not dynamic pain, because most patients were unable to move their feet and ankles freely within 24 hours because of the motor block. This concern is mitigated by the low static pain scores reported in both groups. Second, it was not possible to evaluate the differences in opioid consumption at different concentrations of ropivacaine since this was a self-controlled experiment. Typically, there are subjective differences in patients’ perceptions and scoring of pain. By comparing the patient’s bilateral feet and ankles, we minimized individual variability in subjective pain experiences, thereby reducing bias in assessing analgesic efficacy. Thirdly, the duration of analgesia in this study was longer than that in previous studies, which may be related to our block skills, and multicenter studies are needed to confirm these results. Finally, most of our patients were diagnosed with first metatarsophalangeal joint (MPJ) arthrodesis and underwent arthrodesis or osteotomy. As previously reported, the number of female patients with this type of disease who need surgical treatment is much higher than that of male patients.32,33 As a result, the majority of our study subjects were women. Further research is needed to determine whether our conclusions can be confirmed in men or other foot and ankle surgeries.
Conclusion
Our results demonstrated that the analgesic duration of 0.25% ropivacaine was noninferior to that of 0.375% ropivacaine for popliteal sciatic and saphenous nerve blocks following bilateral foot and ankle surgery. Moreover, a lower concentration resulted in less severe motor block, thereby promoting early postoperative mobilization. These findings support the use of 0.25% ropivacaine as a suitable choice for achieving effective analgesia with reduced motor block and reducing the total amount of LAs for the purpose of postoperative analgesia after foot and ankle surgery. It may also facilitate early rehabilitation and discharge from the hospital during ambulatory surgery.
Data Sharing Statement
The raw data supporting the conclusions of this article will be available by the authors without undue reservation. After the article is published, readers can contact the corresponding author, Guyan Wang, to obtain data by email.
Acknowledgments
The authors thank all the staffs at the Department of Operation Room of Beijing Tongren Hospital, Capital Medical University, Beijing, China, for their help in this study.
Funding
This work was supported by Beijing Hospitals Authority’s Ascent Plan (No. DFL20220203). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bombardieri AM, Maalouf DB, Kahn RL, et al. A comparison of two different concentrations and infusion rates of ropivacaine in perineural infusion administered at the same total dose for analgesia after foot and ankle surgery: a randomized, double blinded, controlled study. Minerva Anestesiologica. 2019;85(2):139–147. doi:10.23736/S0375-9393.18.12266-8
2. Admiraal M, Smulders PSH, Rutten MVH, et al. The effectiveness of ambulatory continuous popliteal sciatic nerve blockade on patient-reported overall benefit of analgesia in patients undergoing foot or ankle surgery (CAREFREE trial); a randomized, open label, non-inferiority trial. J Clin Anesthesia. 2024;95:111451. doi:10.1016/j.jclinane.2024.111451
3. Memtsoudis SG, Cozowicz C, Bekeris J, et al. Peripheral nerve block anesthesia/analgesia for patients undergoing primary hip and knee arthroplasty: recommendations from the international consensus on anesthesia-related outcomes after surgery (ICAROS) group based on a systematic review and meta-analysis of current literature. Reg Anesth Pain Med. 2021;46(11):971–985. doi:10.1136/rapm-2021-102750
4. Sciard D, Xu K, Soulier A, et al. Postoperative analgesia after surgical repair of distal radius fracture: a randomized comparison between distal peripheral nerve blockade and surgical site infiltration. Minerva anestesiologica. 2023;89(10):876–883. doi:10.23736/S0375-9393.23.16956-2
5. Sort R, Brorson S, Gogenur I, et al. Peripheral nerve block anaesthesia and postoperative pain in acute ankle fracture surgery: the Anankle randomised trial. Br J Anaesth. 2021;126(4):881–888. doi:10.1016/j.bja.2020.12.037
6. Gianakos AL, Romanelli F, Rao N, et al. Combination lower extremity nerve blocks and their effect on postoperative pain and opioid consumption: a systematic review. J Foot Ankle Surg. 2021;60(1):121–131. doi:10.1053/j.jfas.2020.08.026
7. Cappelleri G, Ambrosoli AL, Gemma M, et al. Intraneural ultrasound-guided sciatic nerve block: minimum effective volume and electrophysiologic effects. Anesthesiology. 2018;129(2):241–248. doi:10.1097/ALN.0000000000002254
8. Olofsson M, Nguyen A, Rossel J-B, et al. Duration of analgesia after forefoot surgery compared between an ankle and a sciatic nerve block at the popliteal crease: a randomised controlled single-blinded trial. European J Anaesthesiol. 2024;41(1):55–60. doi:10.1097/EJA.0000000000001929
9. Sharrow CM, Elmore B. Anesthesia for the patient undergoing foot and ankle surgery. Clin Sports Med. 2022;41(2):263–280. doi:10.1016/j.csm.2021.11.010
10. Zhang T, Cao Y, Xu R, et al. Spinal anesthesia with peripheral nerve block versus general anesthesia with peripheral nerve block for elective foot and ankle surgeries: a retrospective single-center study. J Foot Ankle Surg. 2022;61(4):706–712. doi:10.1053/j.jfas.2021.11.001
11. Restrepo-Holguin M, Kopp SL, Johnson RL. Motor-sparing peripheral nerve blocks for hip and knee surgery. Curr Opin Anaesthesiol. 2023;36(5):541–546. doi:10.1097/ACO.0000000000001287
12. Lützner J, Gehring R, Beyer F. Slightly better pain relief but more frequently motor blockade with combined nerve block analgesia compared to continuous intraarticular analgesia after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1169–1176. doi:10.1007/s00167-019-05843-2
13. Sotthisopha T, Francisca Elgueta M, Samerchua A, et al. Minimum effective volume of lidocaine for ultrasound-guided costoclavicular block. Reg Anesth Pain Med. 2017;42(5):571–574. doi:10.1097/AAP.0000000000000629
14. Wang S, Fang H, Qin J, et al. Comparison of the efficacy of costoclavicular space brachial plexus blockade with 0.5% versus 0.375% ropivacaine: a randomized, double-blind, single-centre, noninferiority clinical trial. Can J Anaesthesia. 2023;70(1):106–115. doi:10.1007/s12630-022-02327-9
15. Li M, Wan L, Mei W, et al. Update on the clinical utility and practical use of ropivacaine in Chinese patients. Drug Des Devel Ther. 2014;8:1269–1276. doi:10.2147/DDDT.S57258
16. Malhotra R, Johnstone C, Halpern S, et al. Duration of motor block with intrathecal ropivacaine versus bupivacaine for caesarean section: a meta-analysis. Int J Obstetric Anesthesia. 2016;27:9–16. doi:10.1016/j.ijoa.2016.03.004
17. Lei G, Yang S, Wu L, et al. Intravenous injection of dexamethasone is non-inferior to perineural administration for popliteal sciatic nerve and saphenous nerve blocks: a randomized, controlled, triple-blind study. Heliyon. 2024;10(7):e28304. doi:10.1016/j.heliyon.2024.e28304
18. Macfarlane AJR, Gitman M, Bornstein KJ, et al. Updates in our understanding of local anaesthetic systemic toxicity: a narrative review. Anaesthesia. 2021;76(Suppl 1):27–39. doi:10.1111/anae.15282
19. Neal JM. Ultrasound-guided regional anesthesia and patient safety: update of an evidence-based analysis. Reg Anesth Pain Med. 2016;41(2):195–204. doi:10.1097/AAP.0000000000000295
20. Neal JM, Barrington MJ, Fettiplace MR, et al. The third American Society of Regional Anesthesia and Pain Medicine practice advisory on local anesthetic systemic toxicity: executive summary 2017. Reg Anesth Pain Med. 2018;43(2):113–123. doi:10.1097/AAP.0000000000000720
21. McHardy PG, Singer O, Awad IT, et al. Comparison of the effects of perineural or intravenous dexamethasone on low volume interscalene brachial plexus block: a randomised equivalence trial. Br J Anaesth. 2020;124(1):84–91. doi:10.1016/j.bja.2019.08.025
22. Short AJ, Ghosh M, Jin R, et al. Intermittent bolus versus continuous infusion popliteal sciatic nerve block following major foot and ankle surgery: a prospective randomized comparison. Reg Anesth Pain Med. 2019;44(12):1053–1058. doi:10.1136/rapm-2018-100301
23. Lee SC, Jeong JH, Jeong SY, et al. Comparison between two different concentrations of a fixed dose of ropivacaine in interscalene brachial plexus block for pain management after arthroscopic shoulder surgery: a randomized clinical trial. Korean J Anesthesiol. 2021;74(3):226–233. doi:10.4097/kja.20353
24. Wu L, Zhang W, Zhang X, et al. Optimal concentration of ropivacaine for brachial plexus blocks in adult patients undergoing upper limb surgeries: a systematic review and meta-analysis. Front Pharmacol. 2023;14:1288697. doi:10.3389/fphar.2023.1288697
25. Lim JA, Lim H, Lee JH, et al. Local anesthetic volume in ultrasound-guided interscalene block and opioid consumption during shoulder arthroscopic surgery: a retrospective comparative study. Medicine. 2021;100(27):e26527. doi:10.1097/MD.0000000000026527
26. Jeong JS, Shim JC, Jeong MA, et al. Minimum effective anaesthetic volume of 0.5% ropivacaine for ultrasound-guided popliteal sciatic nerve block in patients undergoing foot and ankle surgery: determination of ED50 and ED95. Anaesthesia Intensive Care. 2015;43(1):92–97. doi:10.1177/0310057X1504300114
27. Hoope WT, Hollmann MW, Atchabahian A, et al. Minimum local anesthetic volumes for a selective saphenous nerve block: a dose-finding study. Minerva anestesiologica. 2017;83(2):183–190. doi:10.23736/S0375-9393.16.11496-8
28. Karmakar MK, Reina MA, Sivakumar RK, et al. Ultrasound-guided subparaneural popliteal sciatic nerve block: there is more to it than meets the eyes. Reg Anesth Pain Med. 2021;46(3):268–275. doi:10.1136/rapm-2020-101709
29. Christiansen CB, Madsen MH, Mølleskov E, et al. The effect of ropivacaine concentration on common peroneal nerve block duration using a fixed dose: a randomised, double-blind trial in healthy volunteers. European J Anaesthesiol. 2020;37(4):316–322. doi:10.1097/EJA.0000000000001112
30. Nag K, Ravishankar M, Parthasarathy S, et al. Quantitative assessment of ultrasound-guided sciatic nerve block - A comparison of a single-point versus two-point injection technique: a randomised controlled, double-blinded trial. Indian J Anaesthesia. 2023;67(9):802–808. doi:10.4103/ija.ija_140_23
31. Huang Y, Lu Y, Wang J, et al. Effect of pericapsular nerve group block with different concentrations and volumes of ropivacaine on functional recovery in total hip arthroplasty: a randomized, observer-masked, controlled trial. J Pain Res. 2024;17:677–685. doi:10.2147/JPR.S445000
32. Prat D, Sourugeon Y, Haghverdian BA, et al. “In Situ” joint preparation technique for first metatarsophalangeal arthrodesis: a retrospective comparative review of 388 cases. J Foot Ankle Surg. 2023;62(5):855–861. doi:10.1053/j.jfas.2023.05.004
33. Curran MG, Feeney KM, Murphy EP, et al. Bilateral first metatarsophalangeal joint arthrodesis: an investigation of functional, surgical and radiological outcomes. Foot Ankle Surg. 2024;30(5):411–416. doi:10.1016/j.fas.2024.02.014
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Analgesic Efficacy of an Ultrasound-Guided Transversus Thoracis Plane Block Combined with an Intermediate Cervical Plexus Block on Postoperative Pain Relief After Trans-Areolar Endoscopic Thyroidectomy: A Single Center Prospective Randomized Controlled Study
Zhong R, Zou Y, Bao S, Chen Y, Huang G, Wang L, Chen L, Zhong M, Liang W
Journal of Pain Research 2023, 16:1059-1067
Published Date: 24 March 2023
Sufentanil Improves the Analgesia Effect of Continuous Femoral Nerve Block After Total Knee Arthroplasty

Dong J, Jin Z, Chen H, Bao N, Xia F
Journal of Pain Research 2023, 16:4209-4216
Published Date: 7 December 2023
Analgesic Effects of Different Local Infiltration Anesthesia Techniques Combined with Femoral Nerve Block in Patients Undergoing Total Knee Arthroplasty: A Randomized Controlled Clinical Trial
Gui YK, Xiao R, Luo YR, Liu Y, Da X, Zhu SH, Shi DW, Hu XD, Xu GH
Local and Regional Anesthesia 2023, 16:183-192
Published Date: 22 December 2023
Effect of Pericapsular Nerve Group Block with Different Concentrations and Volumes of Ropivacaine on Functional Recovery in Total Hip Arthroplasty: A Randomized, Observer-Masked, Controlled Trial
Huang Y, Lu Y, Wang J, Lu Q, Bao HF, Liu L, Dong CS
Journal of Pain Research 2024, 17:677-685
Published Date: 14 February 2024
Perineural Dexamethasone is More Efficient Than Perineural Dexmedetomidine in Prolonging Popliteal Sciatic and Saphenous Nerve Blocks: A Single-Center, Prospective, Double-Blinded, Randomized Controlled Trial
Lei G, Wu L, Yin Y, Zhang S, Wang G
Local and Regional Anesthesia 2025, 18:27-38
Published Date: 7 May 2025


