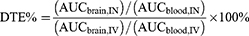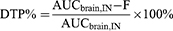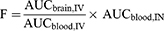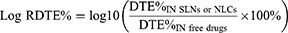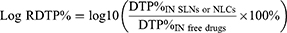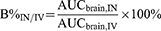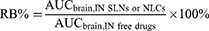Back to Journals » International Journal of Nanomedicine » Volume 19
Nose to Brain: Exploring the Progress of Intranasal Delivery of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
Authors Zheng Y , Cui L, Lu H, Liu Z, Zhai Z, Wang H , Shao L, Lu Z, Song X , Zhang Y
Received 23 September 2024
Accepted for publication 15 November 2024
Published 21 November 2024 Volume 2024:19 Pages 12343—12368
DOI https://doi.org/10.2147/IJN.S497480
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Yang Zheng,1,2,* Limei Cui,1,2,* Haoran Lu,1,2,* Zhen Liu,1,2 Zhaoxue Zhai,2,3 Huikang Wang,1,2 Liting Shao,1,2 Zhaoyang Lu,2,3 Xicheng Song,1,2,4 Yu Zhang1,2,4
1Department of Otorhinolaryngology, Head and Neck Surgery, Yantai Yuhuangding Hospital, Qingdao University, Yantai, People’s Republic of China; 2Shandong Provincial Clinical Research Center for Otorhinolaryngologic Diseases, Yantai, People’s Republic of China; 3Second Clinical Medicine College, Binzhou Medical University, Yantai, People’s Republic of China; 4Shandong Provincial Key Laboratory of Neuroimmune Interaction and Regulation, Yantai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu Zhang, Department of Otolaryngology, Head and Neck Surgery. Yantai Yuhuangding Hospital, Qingdao University, No. 20, East Road, Zhifu District, Yantai, 264000, People’s Republic of China, Tel +86535 6691999, Fax +86535 6240341, Email [email protected] Xicheng Song, Department of Otolaryngology, Head and Neck Surgery. Yantai Yuhuangding Hospital, Qingdao University, Yantai, 264000, People’s Republic of China, Tel +86535 6691999, Fax +86535 6240341, Email [email protected]
Abstract: The intranasal (IN) route of drug delivery can effectively penetrate the blood-brain barrier and deliver drugs directly to the brain for the treatment of central nervous system (CNS) disorders via intra-neuronal or extra-neuronal pathways. This approach has several advantages, including avoidance of first-pass metabolism, high bioavailability, ease of administration, and improved patient compliance. In recent years, an increasing number of studies have been conducted using drugs encapsulated in solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), and delivering them to the brain via the IN pathway. SLNs are the first-generation solid lipid nanocarriers, known for their excellent biocompatibility, high drug-loading capacity, and remarkable stability. NLCs, regarded as the second-generation SLNs, not only retain the advantages of SLNs but also exhibit enhanced stability, effectively preventing drug leakage during storage. In this review, we examined in vivo studies conducted between 2019 and 2024 that used SLNs and NLCs to address CNS disorders via the IN route. By using statistical methods to evaluate pharmacokinetic parameters, we found that IN delivery of SLNs and NLCs markedly enhanced drug accumulation and targeting within the brain. Additionally, pharmacodynamic evaluations indicated that this delivery method substantially improved the therapeutic effectiveness of the drugs in alleviating symptoms in rat models of CNS diseases. In addition, methods for enhancing the efficacy of nose-to-brain delivery of SLNs and NLCs are discussed, as well as advances in clinical trials regarding SLNs and NLCs.
Plain Language Summary: Traditional drug administration routes for the treatment of central nervous system diseases have many limitations due to the existence of the blood-brain barrier (BBB). The intranasal drug administration route crosses the BBB through intra-neuronal pathways as well as extra-neuronal pathways and delivers drugs directly to the brain. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are a type of nanoparticles whose surface is covered by amphoteric surfactants and whose interior is filled with a lipid core. They have the advantages of good biocompatibility, strong drug loading capacity, and strong stability, and can be obtained through a variety of reliable preparation methods. Encapsulating therapeutic drugs into SLNs and NLCs for intranasal delivery can significantly increase drug delivery efficiency and enhance efficacy. In addition, there are various ways to further enhance drug delivery of SLNs and NLCs, such as using gel systems such as chitosan to encapsulate the nanoparticles, piggybacking cell-penetrating peptides onto the surface of the nanoparticles, and modifying the nanoparticles, surface charge of particles, etc.
Keywords: solid lipid nanoparticle, nanostructured lipid carrier, intranasal delivery, central nervous system diseases
Graphical Abstract:

Introduction
In recent years, the number of people suffering from central nervous system (CNS)-related disorders, such as neurodegenerative diseases, depression, and glioblastoma, has increased worldwide.1,2 Traditional drug delivery methods used for treating these CNS ailments, primarily oral and intravenous, encounter a formidable barrier: the blood-brain barrier (BBB) (Figure 1). The presence of the BBB has led to many potential drugs failing to achieve effective therapeutic concentrations in the brain, resulting in their abandonment for clinical development and application.3 Typically, many lipophilic compounds in the peripheral circulation, can passively traverse the BBB in its normal physiological state, with the rate of transport depending on their lipid solubility and molecular size. Essential nutrients such as glucose enter the brain via specific transport proteins, whereas most macromolecules or hydrophilic substances are impeded.4,5 It is worth noting that ATP-binding cassette transporter (ABC transporter) is the main transporter on the BBB that mediates the efflux of lipid-soluble substances. Therefore, indiscriminately increasing a drug’s lipid solubility may paradoxically diminish its ability to penetrate the brain.3,6 This characteristic of BBB serves to protect the CNS from pathogens and toxins while allowing most small-molecule drugs to enter the brain. However, under normal physiological conditions, the entry of nearly all large molecule drugs into the brain is stringently restricted.7 Therefore, there is an urgent need to develop drug delivery routes that can cross the BBB and improve the efficiency of drug transit to the brain.
 |
Figure 1 The blood-brain barrier is a complex structure consisting of vascular endothelial cells and the tight junctions formed between them, pericytes, substrates, and peduncles of astrocytes. |
Numerous invasive strategies such as chemical disruption of the BBB, craniotomy drug delivery, polymer wafers, and microchip technologies; as well as non-invasive approaches including intranasal drug delivery, efflux pump inhibition, and prodrug techniques have been studied to enhance drug transport to the brain.8 Invasive strategies often inflict considerable harm on patients. For example, chemically disrupting the BBB can compromise its selective permeability, leading to uncontrolled diffusion of both low and high molecular weight substances, which may result in elevated cerebrospinal fluid levels and potentially carry risks such as hemiplegia and aphasia.9 Non-invasive strategies, such as inhibiting efflux pumps within the BBB, reduce the expulsion of therapeutic drugs but also diminish the clearance of harmful chemicals, which may increase the risk of brain injury.8 The prodrug approach aims to modify active molecules to adjust their lipophilicity, enhancing permeability and water solubility. Subsequently, it undergoes enzymatic conversion in specific brain regions to yield its active form, thereby achieving the intended therapeutic effect. It is worth noting, however, that due to the action of ABC transporters and the BBB’s restriction on macromolecules, the prodrug strategy also faces significant challenges.10 The intranasal (IN) route is recognized as a highly promising method of administration. After IN formulations enter the nasal cavity, they can be efficiently transported to the brain either directly via the olfactory nerve pathway in the olfactory region or via the trigeminal nerve pathway in the respiratory area. The IN route offers numerous advantages, including avoidance of first-pass metabolism, non-invasive delivery, high bioavailability of drugs, convenience of administration, high safety, and good patient compliance.11 However, IN delivery also faces challenges, such as high mucociliary clearance, susceptibility to enzymatic degradation, and low mucosal penetration of drugs. To overcome these disadvantages, researchers have encapsulated drugs into nanocarriers.12,13
To date, a variety of nanocarriers have been developed for intranasal administration, including lipid-based nanoparticles (LNPs), polymeric nanoparticles, metal-based nanoparticles, stem cells, and exosomes.8,14,15 Among these, solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have garnered increasing interest due to their superior biocompatibility, high drug loading capacity, and high bioavailability and stability when compared with other LNPs.14 Numerous studies have utilized SLNs and NLCs to encapsulate drugs for treating central nervous system (CNS) disorders and delivering them to the brain via the IN route. This article will summarize and analyze recent in vivo studies related to the treatment of CNS diseases by use of drugs encapsulated in SLNs or NLCs.
Intranasal Route of Administration
Nasal Cavity Anatomy
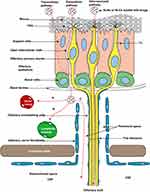
The nose, as a vital organ of the human body, has multiple functions, such as air filtration, humidification, temperature regulation, and olfactory perception.16 The nasal cavity consists of two narrow chambers, each measuring approximately 12 to 14 cm in length, with a wider base tapering to a narrower top, separated by the nasal septum. Each side of the nasal cavity is further divided by the nasal threshold into the nasal vestibule and the proper nasal cavity (Figure 2).7,17
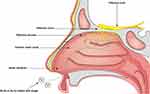 |
Figure 2 Schematic diagram showing the anatomy of the nasal cavity and the entry of SLNs or NLCs into the nasal cavity to reach the olfactory region. The olfactory zone is centrally located at the top of the nasal cavity and covers < 10% of the mucosal surface area; it is primarily responsible for the sense of smell.7 The respiratory mucosa, which constitutes 80–90% of the nasal cavity, is warm and moisturizes inhaled air; it also filters out fine dust and pathogens.7,18 |
The intrinsic nasal mucosa contains two parts: the respiratory zone and olfactory zone. The olfactory zone is an important area for IN delivery. The olfactory epithelium is comprised of supporting cells, basal cells, and olfactory cells, which are closely interconnected through tight junctions (TJ) between the cells. The dynamic replacement of basal cells with neuronal cells can increase the permeability of the mucosa, thereby facilitating the transport of drugs to the brain.17,19 Olfactory nerve cells are a type of bipolar neuron. Their dendrites traverse the olfactory epithelium to reach the mucous layer, while unmyelinated axons on their base combine with axons from other olfactory cells to form olfactory fila. These olfactory fila are enveloped by surrounding olfactory ensheathing cells and olfactory nerve fibroblasts, creating a Schwann cell sheath. This sheath passes through the cribriform plate of the ethmoid bone to enter the cranium, ultimately terminating in the olfactory bulb.20 Olfactory information is then transmitted to brain regions such as the amygdala, piriform cortex, and hypothalamus, providing an anatomical basis for the transport of drugs from the nasal cavity to the brain.8
The respiratory region is innervated by the trigeminal nerve, including its ophthalmic and maxillary branches, which are responsible for sensation in different parts of the nasal cavity.21 The neurons of the trigeminal nerve are in the semilunar ganglion, and the axons reach the brainstem through the pons and have lateral branches that pass through the cribriform plate directly into the olfactory bulb, from which they ultimately affect the caudal and anastomotic sides of the brain, and form a key path for drugs to enter the brain via the trigeminal nerve.8,22 Unlike the olfactory nerve, which is directly exposed to the nasal cavity, trigeminal nerve endings are located under the mucosal epithelium, which is not conducive to drug transport. Thus the mucosa of the olfactory region, which is smaller in size, is more highly valued for nose-to-brain delivery.23 In addition, the mucosa of the respiratory region benefits from its rich blood flow and large surface area, making it also a focal point for intranasal systemic drug delivery.24
Mechanisms of Drug Transport into the Brain by Intranasal Delivery
The mechanisms by which drug molecules enter the brain via IN delivery are diverse, and dominated by the olfactory and trigeminal pathways, which transport drugs to various regions of the brain and distribute them throughout the brain. The olfactory nerve pathway plays a particularly crucial role,23 and the following description will primarily focus on that route.
The surface of the nasal epithelium is covered with a layer of mucus. Upon entering the nasal cavity, drug molecules first reach this mucus layer and are progressively moved towards the rear of the nasal cavity and cleared by the movement of cilia. Drug molecules must penetrate this barrier to be absorbed by the nasal epithelium.25 Therefore, extending the residence time of drug molecules in the nasal cavity is one of the strategies for enhancing the efficiency of IN delivery, and will be discussed in detail in the subsequent sections. It is worth mentioning that ciliary activity occurs only in the epithelium of the respiratory region, and not the olfactory region, which confers an anatomical advantage to the olfactory region in IN delivery.8 Subsequently, drug molecules are primarily transported via intra- or extra-neural cellular transport mechanisms (Figure 3).
Mechanisms of the Intra-Neuronal Pathway
The intra-neural cellular transport mechanism for drugs delivered via the intranasal (IN) route primarily relies on the internalization of olfactory neurons (or trigeminal neurons), which may involve nonspecific processes or receptor-mediated endocytosis. Studies suggest that receptor-mediated neuronal internalization is uncommon, and phagocytosis is more likely the predominant mode of neuronal internalization.24 After entering the olfactory neuron, drug molecules are encapsulated in vesicles and transported to the olfactory bulb via the Golgi network and axons. Similarly, in trigeminal neurons, these vesicles are then transported to the cerebral bridges.23,26 After the drug-containing vesicles are transported along the axons to the nerve terminals, the drugs are released into the synaptic cleft and bind to postsynaptic cells in the olfactory bulb (including mitral cells and tufted cells).27,28 Ultimately, the drugs are transmitted to various regions of the brain through those cells. An in vivo study in mice found that the transport time of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) within olfactory neurons to the olfactory bulb ranged from approximately 0.74 to 2.67 hours, while in the trigeminal pathway, the process required approximately 3.69 to 13.33 hours.29 This difference may be related to the respective lengths of the olfactory and trigeminal nerves.23 This indicates that the intraneuronal transport of drugs is relatively slow, suggesting that direct brain delivery via the IN route may also depend on additional pathways.
Mechanisms of the Extra-Neuronal Pathway
The extra-neuronal pathway is another important mechanism of IN delivery, and mainly involves paracellular and transcellular transport pathways. In a paracellular transport pathway, drug molecules traverse the nasal epithelium into the lamina propria through gaps between cells of the olfactory or respiratory epithelium. Although open gaps exist between some cells that can be freely traversed by molecules, the majority of nasal epithelial cells are tightly connected by tight junctions (TJs), which tightly regulate the permeability of the nasal epithelium to molecules.24 Notably, TJs do not influence the permeability of lipophilic molecules, whereas hydrophilic molecules must navigate this barrier.30 Within the olfactory epithelium, olfactory neurons are renewed approximately every 30–60 days,31 and neuronal cells undergo apoptosis, leaving large gaps between the surrounding supporting cells until new neuronal cells regrow in the gaps. During this process, the TJs between cells are opened, and allow drugs to enter the lamina propria.24 The transcellular transport pathway, predominantly occurring in the supporting cells of the nasal epithelium, typically involves mechanisms such as receptor-mediated endocytosis, passive diffusion, or fluid-phase endocytosis, which enable drug transport into the lamina propria.32
After entering the lamina propria, drug molecules are transported to various brain regions via multiple pathways. The axons of olfactory sensory neurons are wrapped by olfactory sheath cells and olfactory fibroblasts (ONFs), between which there is a gap known as the perineural space (PNS).24 ONFs form a continuous layer extending to the meninges, allowing the PNS direct access to the subarachnoid space, and thereby allowing drugs to enter the cerebrospinal fluid directly from the lamina propria.33–35 A similar mechanism applies to the trigeminal nerve, and enables rapid drug entry into the brain via the PNS. Studies indicate that drug transport within the PNS is bidirectional,36 and occurs more rapidly than intra-neuronal transport.7 This may be associated with the perivascular space and “perivascular pump” mechanisms.37,38
The lamina propria of the nasal epithelium, and particularly the mucosa of the respiratory zone, is rich in capillaries that are more permeable than the BBB, and allows drug molecules to pass through these vessels into the peripheral circulation and ultimately to the brain.16,39 Additionally, drugs can be absorbed through lymphatic vessels in the lamina propria, and then enter the peripheral circulation via deep cervical lymph nodes.23,38 Walter et al40 conducted rat studies which confirmed that cerebrospinal fluid could drain from the subarachnoid space along the olfactory nerve to the nasal lymphatics. They injected fluorescein isothiocyanate-labeled keyhole limpet hemocyanin (KLH-FITC) into the subarachnoid space of rats and found fluorescence in the nasal mucosa as well as the cervical lymph nodes, demonstrating the pathway of cerebrospinal fluid drainage. Moreover, kinetic evidence suggests that extra-neuronal transport mechanisms appear to play a major role.24
Key Features of SLNs and NLCs for in Delivery
To address the challenges of short nasal residence time and susceptibility to enzymatic degradation in the nasal cavity associated with intranasal (IN) drug delivery, nanocarrier systems have been developed, such as lipid-based nanoparticles (LNPs), polymeric nano-systems, metal-based nanoparticles, stem cells, and exosomes.8,14,15 LNPs can be categorized into four types: 1) Solid Lipid Nanoparticles (SLN), 2) Nanostructured Lipid Carriers (NLC), 3) Nano-emulsions (NE), and 4) Liposomes.41 These carriers demonstrate considerable potential for IN delivery due to 1) lower industrial production costs, 2) excellent biostability and biocompatibility, 3) effective penetration capabilities through biological membranes, 4) enhanced drug loading efficiency, and 5) superior drug targeting abilities.11,41 Among these, SLNs and NLCs are particularly noted for their superior drug protection ability and encapsulation efficiencies,42,43 and have thus been extensively studied in recent years. This article will focus on these two types of nanoparticles.
Solid Lipid Nanoparticles
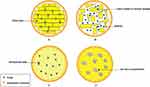
Solid lipid nanoparticles (SLNs) have an interior filled with a solid lipid core covered by an amphiphilic surfactant layer that separates the lipid core from the external aqueous environment. The surfactant layer plays a role in maintaining the stability of the lipid core (Figure 4).14 Notably, the internal solid lipid core of SLNs is not dense. All-atom MD simulations and small-angle scattering (SANS) experiments on SLNs have shown that different non-trident lipid conformations crystallize in the core to form crystals with defects, and drug molecules entering the lipid core are most likely encapsulated in those defects.44 The unique structure of SLNs protects the drug molecules carried within the particles from degradation by various enzymes in the nasal cavity, and also prolongs the release time of the drug.45,46 SLNs also have other advantages, such as the use of temperature-controlled lipid materials in their preparation process. These materials are solid at room temperature, and the therapeutic drug molecules are encapsulated in the defects in the solid lipid matrix.47,48 When the nanoparticles enter the human body, the ambient temperature changes to body temperature, and the solid lipid core of the SLNs transforms into a liquid form to release the encapsulated drug.49 Moreover, the study by Saini et al50 demonstrated that SLNs possess excellent mucoadhesive properties, and compared to free ferulic acid (FA), the FA encapsulated in SLNs exhibits significantly enhanced penetration through the nasal mucosa. However, SLNs also have some drawbacks, such as the possibility of lipid coagulation and crystallization occurring inside the SLNs. These events lead to a decrease in the drug-carrying capacity of SNLs and their structural instability during storage, which in turn leads to premature release of the encapsulated drug.45,51 To date, various reliable techniques for preparing SLNs have been reported, including the use of microfluidic technology,52 the double emulsion method,53 the microemulsion technique,54 the high-pressure homogenization (HPH) method,54 the hot melt-emulsification ultrasonication method,55 high shear homogenization (HSH) technology,56 and the hot melt extrusion (HME) technique.57
Nanostructured Lipid Carriers
Nanostructured lipid carriers (NLCs) are second-generation nano lipid particles developed to improve upon the limitations of SLNs (Figure 4). NLCs contain not only solid lipids but also liquid lipid components in their interior.58 NLCs maintain a solid lipid matrix structure at both room temperature and body temperature.59 Depending on the proportions of lipid and oily components inside the particles and the preparation method, NLCs can be categorized into three types: 1) Imperfect type, 2) Amorphous type, and 3 Multiple oil-solid fat-water (O/F/W) type.59 NLCs share the same advantages as SLNs, such as strong nasal adhesion.60 Besides, NLCs have a higher drug-carrying capacity due to the introduction of liquid lipids.61,62 In addition, the incorporation of liquid lipids enhances the stability of NLCs and prevents the recrystallization of solid lipids, which helps to reduce the early release of a drug, and thus enhances drug-loading efficiency.63 Although NLCs surpass SLNs in terms of stability and drug-loading efficiency, regrettably, there is still insufficient in vivo experimental evidence to prove that the drug delivery efficiency and therapeutic effects of NLCs are superior to those of SLNs.
Safety of SLNs and NLCs
The safety of SLNs and NLCs has been one of the central concerns in the field of IN delivery. Its evaluation is multifactorial, involving the formulation components, particle size, surface charge, and other physicochemical properties of SLNs and NLCs.64 Moreover, studies have shown that the surface of many SLNs or NLCs used for delivering chemotherapeutic drugs may contain cationic components and linkers that bind to specific ligands, which could potentially trigger immune responses.65
An in vitro experiment demonstrated that most cells can tolerate doses of SLNs and NLCs up to 1 mg/mL, without showing signs of cytotoxicity.66 However, certain SLN/NLC formulations can be safely added to cells at even higher doses, and the safety of the formulation appears to be closely related to the type of surfactant selected.67 Veider et al68 and Sadegh et al69 assessed the cytotoxicity of SLNs and NLCs using Caco-2 cells and mouse fetal fibroblast cells, respectively, with both demonstrating low cytotoxicity.
Interactions between positively charged nanoparticles and negatively charged cell membranes can lead to cytotoxicity, whereas negatively charged nanoparticles are repelled due to having the same negative charge as the cell membrane, and are not suitable for mucosal delivery.8,70,71 Nevertheless, positively charged surfactants are still needed because they enhance the efficiency and drug-loading capacity of nanoparticles. Furthermore, a study has indicated that SLNs prepared using the cationic surfactant cetyltrimethylammonium bromide (CTAB) can still exhibit low toxicity at concentrations exceeding 1 mg/mL.72 It is important to note that CTAB may increase calcium ion release from neutrophils, leading to cellular damage, whereas other surfactants such as polysorbate 80 and poloxamer 188 demonstrate good biocompatibility and low cytotoxicity.73,74 Moreover, both SLNs and NLCs have demonstrated excellent biocompatibility in terms of blood compatibility, genotoxicity, and also in in vivo experiments.67,75 In the in vitro hemolysis assay by Lakkadwala et al,76 SLNs exhibited only slight hemolytic activity at a concentration of 1 mg/mL. Similarly, in another study, NLCs also demonstrated low hemolytic effects.68
Most in vivo studies on SLNs and NLCs currently focus on their drug delivery efficiency and therapeutic efficacy, with few addressing in vivo safety. Only a limited number of studies conducted in experimental animals have assessed the in vivo safety of SLNs and NLCs, and these findings indicate that SLNs and NLCs are generally safe in mice, rats, Drosophila melanogaster and chickens.66 Overall, SLNs and NLCs have shown good safety profiles for use as nanocarriers. Further work is needed to investigate the in vivo safety of SLNs and NLCs and to explore more suitable formulations to minimize their potential side effects.
Strategies for Enhancing the in Delivery of SLNs and NLCs
Apart from safety, enhancing the efficiency of SLNs and NLCs in delivering drugs to the brain via the IN route is another prominent focus in this field. One approach is to overcome the clearance mechanism of the nasal cilia, in which nanoparticles move backward with the cilia oscillations and are cleared, needs to be overcome.25 It follows that prolonging the residence time of nanoparticles in the nasal cavity should help to enhance drug absorption. Chitosan and its gel system have been developed to extend the mucosal residence time of nanoparticles due to its excellent mucosal adhesion properties. Chitosan, a natural polysaccharide derived from the deacetylation of chitin,77 has exceptional biocompatibility, low toxicity, is non-immunogenic, and biodegradable. It also enhances mucosal adhesion, promotes permeation, and delays drug release.78,79 Substantial evidence indicates that chitosan-coated SLNs and NLCs enhance nose-to-brain transport. For instance, chitosan-coated BPE-CS-NLCs prepared by Noorulla et al68 exhibited longer Tmax,brain times than the free drug (IN), indicating their sustained-release properties.80 Similarly, in two other studies, chitosan-coated SLNs or NLC formulations showed higher Cmax,brain times and brain targeting efficiency when compared to formulations without a chitosan coating.50,81 In addition, thermosensitive in situ gels composed of methylcellulose or poloxamer 407 (Pluronic F127) have also been used to enhance IN delivery. Research conducted by Uppuluri et al82 showed that SLNs wrapped with thermosensitive gels had higher values of Cmax,brain and drug brain targeting efficiency.
Cell-penetrating peptides (CPPs) can selectively interact with the cell membranes of certain cell types, and facilitate the transport of bioactive substances coupled to them across the cell membrane into the cell via different mechanisms.83,84 Research indicates that CPPs play a crucial role in enhancing intra-neuronal transport in IN delivery.85 CPPs are characterized by their low cytotoxicity and can ultimately be degraded into amino acids, and thus exhibit excellent biosafety.86 Rassu et al87 developed BACE1 siRNA-loaded SLNs modified with CPPs (BACE1-CPP-SLNs) for optimal nose-to-brain transport, and evaluated them by using Caco-2 cells as an epithelial-like phenotype model. The results showed that CPPs enhanced epithelial cellular absorption as well as the intra-neuronal cellular transport of BACE1 siRNA. In another study, R9SA-NLCs modified with stearoyl-nonanyl-L-arginine (R9SA) as a CPP were evaluated using a Caco-2 epithelial-like model, and the results showed a 15.6-fold increase in cellular uptake of R9SA-NLCs when compared to blank NLCs.88 Despite some limitations of CPPs, such as non-specific transport and a short blood half-life,86 targeting can be enhanced by loading CPPs onto specifically modified SLNs or NLCs. For instance, SLNs or NLCs can be modified with non-toxic components of neurotoxic substances to enhance targeting of the nervous system.89
After considering the impact of the nanoparticle surface charge on cellular uptake, ie, nanoparticles with a negative surface charge are more likely to traverse the mucus barrier, and nanoparticles with a positive surface charge are more likely to be taken up by cells.90–92 Veider et al68 optimized the preparation of charge-converted SLNs and NLCs by using cetyltrimethylammonium chloride (CTAC) and polyphosphate Graham’s salt. Initially, the nanoparticles carried a negative surface charge after being coated with phosphate groups. However, once the phosphate groups were cleaved by intestinal alkaline phosphatase, the presence of CTAC shifted the surface charge to positive. The results indicated that the optimized formulations of SLNs and NLCs not only significantly increased cellular uptake but also reduced cytotoxicity.
In vivo Study and Evaluation of SLNs and NLCs in CNS Diseases
Due to the presence of the BBB, intravenous drug therapies for CNS disorders face significant challenges. IN-delivered drug-carrying SLNs and NLCs for treatment of CNS-related diseases have garnered increasing attention for their superior ability to cross the BBB. We searched the PubMed, Scopus, Web of Science, and ScienceDirect databases using the following keywords: (SLNs or NLCs) and (Alzheimer’s disease or Parkinson’s disease or multiple sclerosis or depression or migraine or brain tumor or attention deficit hyperactivity disorder or vascular dementia or epilepsy or anxiety or schizophrenia or insomnia or meningitis or cerebrovascular disease). The most recent search was conducted on January 9, 2024. After removing duplicates, we selected 94 research articles published from 2019 to 2024 on the treatment of CNS diseases with SLNs and NLCs. Further selection was based on the following inclusion criteria: 1) protocol included an in vivo study; 2) employed the IN route for delivery; 3) included evaluations of PK, bioavailability, or PD. Ultimately, 38 articles were selected for further analysis. Data describing the PK, bioavailability, PD, nanoparticle size, zeta potential, and drug encapsulation rate in the 38 articles were analyzed to evaluate the use of SLNs and NLCs in CNS diseases.
Evaluation Indicators and Calculation Methods for Pharmacokinetics and Bioavailability
This section will discuss several different parameters in pharmacokinetic and bioavailability studies conducted to evaluate various formulations of SLNs and NLCs for IN delivery. Cmax,brain and Cmax,blood refer to the maximum concentration of a drug that can be achieved in the brain or blood, respectively, after administration. Tmax,brain and Tmax,blood denote the time required to reach the maximum drug concentration. Drug targeting efficiency (DTE) is an important index for evaluating the brain targeting efficiency of IN-delivered drugs, and is calculated from the area under the curve of the brain (AUCbrain) and the area under the curve of the blood (AUCblood).5 AUCbrain and AUCblood indicate the total amount of drug that enters the brain or blood, respectively, after a certain period of time, and is calculated from the area under the curve plotted against the concentration of drug in the brain or blood measured at different time points. The larger the AUC value is, the higher the bioavailability is, and vice versa. DTE% is calculated by the following method:
AUC is determined by the length of the study (AUC0-t or AUC0-∞), IN denotes the intranasal route of delivery, IV denotes the intravenous route of delivery, and DTE% can be expressed as the relative propensity of a drug to accumulate in the brain via IN delivery as compared to IV administration. The value of DTE% ranges from 0 to +∞, and a DTE% value between 100% and +∞ suggests that the IN route has a better brain targeting ability than the IV route. The opposite is true when DTE% is between 0 and 100%. Log DTE% values are sometimes used for standardizing drug distribution.5,93
It is worth noting that the percentages of drug that enter the brain via the olfactory/trigeminal nerve pathway and via the peripheral circulatory route cannot be distinguished merely by calculating the DTE%. Therefore, drug transit percentage (DTP) was introduced, and is calculated as follows:
The F value represents the amount of drug that enters the brain via the peripheral blood route after intranasal administration, and is calculated as follows:
DTP% is calculated by subtracting the amount of drug that enters the brain via the peripheral circulatory route, and represents the proportion of a drug delivered IN that enters the brain via the olfactory/trigeminal nerve pathway. The value of DTP% ranges from -∞ to 100. When drug molecules are not transported via the olfactory/trigeminal pathway, AUCbrain, IN = F; at which time, DTP% = 0; when 0<DTP%<100. This indicates a greater advantage of transport through the olfactory/trigeminal nerve pathway, and the larger the DTP%, the higher the proportion; when -∞<DTP%<0, the opposite is true. Theoretically, a DTP% value of 100 indicates that drugs in peripheral circulation are completely unable to cross the BBB (AUCbrain, IV = 0, F = 0).5,93
Additionally, to compare the brain targeting efficiencies of drug-loaded SLNs or NLCs delivered IN with against free drugs administered via the same route, the values for relative DTE% (Log RDTE%) and relative DTP% (Log RDTP%) were also calculated as follows:
Only when the values of Log RDTE% or Log RDTP% exceed 2 can it be demonstrated that the brain targeting efficiency of drug-loaded SLNs or NLCs delivered intranasally (IN) is superior to that of free drugs.5,93
A special case exists when AUCbrain, IN is very low, and at the same time the value of AUCblood, IN is also very low, so that DTE% and DTP% may be very high. Therefore, it is necessary to introduce a new parameter, B%IN/IV, which is different from DTE% and DTP% for evaluating the brain targeting efficiency of IN delivery. B%IN/IV compares the accumulation of drugs in the brain after delivery via the IN pathway with that of the IV pathway. It can be used to evaluate the accumulation of drugs in the brain after delivery via the IN pathway, after excluding the effect of the IV pathway, and is relevant to the evaluation of bioavailability. The calculation method is as follows:
A B%IN/IV value > 100 indicates that the IN pathway is more conducive to drug accumulation in the brain than the IV pathway, and a Log B% IN/IV value is sometimes used to standardize the distribution.5,93
RB%, also known as relative bioavailability, can also be used to evaluate brain targeting efficiency, which is the ratio of IN-delivered drug-loaded SLNs or NLCs to IN-delivered free drug accumulation in the brain. It is calculated as:
An RB% value > 100 proves that IN-delivered drug-loaded SLNs or NLCs are more advantageous than IN-delivered free drugs in terms of accumulation in the brain. Moreover, RB% values are sometimes used to standardize the distribution of Log RB% values.5,93
Literature reports do not always mention parameters such as DTE% or DTP%; therefore, we recalculated those parameters using AUC0-t (time from 0 to the last measurement) to obtain the drug concentration values they provided.
Zeta Potential, Drug Encapsulation Efficiency
Zeta potential is an important parameter for evaluating the stability of SLNs and NLCs. Nanoparticles in solution have an electric charge on their surface. Therefore, they attract ions with the opposite charge and form two ionic layers around the nanoparticles: the Stern layer and the Diffusion layer. The Stern layer is the oppositely charged ion layer adsorbed on the surface of the nanoparticles, and the electrical potential at a certain point of the layer distant from the surface of the nanoparticles is the Stern potential. The interface between the Stern layer and its surrounding portion of the more stable particles and the Diffusion layer with relative movement of the interface is called the sliding surface. The sliding surface away from the surface of the nanoparticle at a point and the nanoparticle surface of the potential difference is zeta potential.94 The larger the absolute value of zeta potential is, the more the solution tends to disperse, and thus the more stable it is; the smaller the absolute value of zeta potential is, the more the solution tends to aggregate, and thus the more unstable it is. Studies have shown that nanoparticles with an absolute zeta potential value > 30 mv have good stability.8 Drug encapsulation efficiency (EE%) is the ratio of the amount of drug encapsulated into the nanoparticles.
Statistical Analysis of Formulation Properties and PK Parameters
We summarized results from 38 papers (Tables 1 and 2) and then used GraphPad Prism and SPSS software to statistically analyze formulation properties and various pharmacokinetic parameters, such as particle size, absolute zeta potential, EE%, Tmax, Cmax, Log DTE%, DTP%, Log RDTE%, Log RDTP%, Log B% IN/IV, and Log RB%. We assessed data distribution normality using the Shapiro–Wilk test; for normally distributed parameters, we compared group means to reference values by using the one-sample T-test and made comparisons between groups using the independent samples T-test. For parameters without a normal data distribution, we used the Wilcoxon signed-rank test to compare median values with reference values and the Mann–Whitney U-test to compare median values between groups. For analyses involving more than two groups, we used one-way analysis of variance (ANOVA) with Tukey’s Honestly Significant Difference (Tukey’s HSD) post-hoc test to compare means across multiple groups.
 |
Table 1 PK Parameters and Main Outcomes of SLN and NLC Formulations |
 |
Table 2 Main Characteristics of SLN and NLC Formulations |
Statistical analyses were conducted on particle size (PS), absolute zeta potential (|ZP|), and encapsulation efficiency (EE%) for all the SLN and NLC formulations mentioned earlier (as shown in Figure 5). Among them, 32 different optimized SLN or NLC formulations had a PS ≤ 200 nm, which is advantageous for nanoparticle transport through the olfactory neuronal pathway for IN delivery as well as for maintaining the stability of nano carriers.129,130 Only 13 optimized formulations exhibited a |ZP| > 30 mv, indicating that those nanoparticles relied on their own zeta potential to maintain their stability, while the stability of the remaining formulations depended more on their surfactant.131 Additionally, the EE% of 35 different formulations exceeded 60%, demonstrating the superior drug encapsulation capabilities of the SLNs and NLCs.
In the realm of PK, DTE% and DTP% serve as crucial indicators for evaluating the brain targeting efficiency of SLNs or NLCs. A comprehensive analysis of both DTE% and DTP% for SLNs, NLCs (IN), and free drugs (IN) revealed a notable correlation (Figure 6). Upon recalculation, only 1 study was found to have a DTE% (94.28) < 100 and a DTP% (−6.07) < 0.118 This discrepancy likely stems from the investigator’s choice to measure drug accumulation in the cerebrospinal fluid rather than in brain tissue, where the transit time to cerebrospinal fluid might be longer.93 In addition, in an in vivo study of an optimized formulation of sumatriptan (ST) piggybacked NLCs,121 we re-calculated the relevant PK parameters and found that its Log RDTE% (1.63) value was < 2 and its Log RDTP% (1.87) value was < 2, which might be related to the NLCs significantly enhancing the transport of ST across the BBB via peripheral circulation pathways, and thus somewhat reducing its direct brain targeting efficiency. In another study of levofloxacin piggybacked SLNs,123 we found that its B%IN/IV value (76.35) was < 100 based on the AUC0-360 in the article, indicating that its brain targeting efficiency via IN delivery was lower than that achieved by IV delivery. Excluding those outliers, the median values of Log DTE%, DTP%, and Log B%IN/IV for drug-loaded SLNs or NLCs delivered via IN were significantly higher than those of free drugs delivered via IN (p < 0.01, p < 0.01, and p < 0.0001, respectively). Additionally, the mean values of Log RB% and Log RDTP% for drug-loaded SLNs or NLCs delivered via IN were significantly higher than the reference values (p < 0.00001 and p < 0.01, respectively), and the median values of their Log RDTE%, Log B%IN/IV, DTP%, and Log DTE% were also significantly higher than the reference values (p < 0.01, p < 0.0001, p < 0.00001, and p < 0.00001, respectively). Yasir et al105 encapsulated haloperidol (HPL) into SLNs (HPL-SLNs) and administered them intranasally for a PK study in a Parkinson’s rat model. We utilized the AUC0-t provided in the article for recalculation, and the results showed that the DTE% of HPL-SLNs was 597.57, indicating that the brain-targeting efficiency of the drug delivered intranasally was higher than that of IV administration. The DTP% of HPL-SLNs was 83.27, further demonstrating the advantage of drug transport into the brain via the olfactory/trigeminal nerve pathways. Additionally, the Log RDTE% and Log RDTP% of HPL-SLNs were 2.64 and 2.49, respectively, providing strong evidence that the brain-targeting efficiency of HPL-SLNs is superior to free HPL during IN delivery. Abo El-Enin et al developed an optimized formulation of berberine-laden (BER) NLCs, encapsulated with chitosan (BER-CS-NLCs), and conducted a PK study in an Alzheimer’s disease (AD) rat model.102 Similarly, we recalculated the B%IN/IV of BER-CS-NLCs to be 626.34, indicating that the IN route is more favorable for drug accumulation in the brain compared to the IV route. Moreover, the RB% of BER-CS-NLCs was 341.94, demonstrating that IN delivery of BER-CS-NLCs has a greater advantage in brain accumulation compared to IN delivery of free BER. These findings suggest that loading drugs onto SLNs or NLCs and administering them intranasally markedly enhances their brain targeting efficiency (Figure 7).
In addition, we found some particularly high Log DTE% values that mostly corresponded with hydrophilic drugs.98,108 Those drugs struggled to penetrate the blood-brain barrier via peripheral circulation pathways, resulting in their lower bioavailability after IV administration, resulting in high Log DTE% values.
Furthermore, the Tmax,brain values for drug-loaded SLNs and NLCs (IN), as well as for drug-loaded SLNs or NLCs delivered via the IV route (IV ALL), and free drug (IN) were statistically analyzed. We used one-way analysis of variance (ANOVA) with Tukey’s Honestly Significant Difference (Tukey’s HSD) post-hoc test to compare the mean values among different groups. No significant differences were found among the groups. This lack of difference might be attributed to some nanoparticles being encapsulated by gel systems,50,80,82,101,119 which would delay drug release and thereby increase Tmax,brain. In an in vivo study on a Parkinson’s rat model, astaxanthin (AST)-loaded NLCs (AST-NLCs) were prepared and encapsulated with chitosan to form AST-CS-NLCs.81 Following IN administration, the AST-NLCs group reached Cmax,brain 4 hours post-administration, while both the AST-CS group and the AST-CS-NLCs group reached their peak concentrations 6 hours after dosing, highlighting the excellent capability of chitosan to prolong drug release. Additionally, the rate of drug transport into the brain is influenced by the size of the nanoparticles and various intrinsic properties of the drug itself. Thus, it seems that SLNs and NLCs do not have a significant effect on the rate of IN delivery.
Due to the varying units of Cmax in presented in different studies, which are difficult to standardize, ratios were directly used for statistical analysis to eliminate that effect. Comparisons between the Cmax,brain values of SLNs or NLCs (IN) and those of ALL IV, and between SLNs or NLCs (IN) and those of free drugs (IN) were made, and a Wilcoxon signed-rank test indicated that the median ratios in each group were significantly higher than the reference values (p < 0.0001, p < 0.001, respectively). Tripathi et al optimized the preparation of cinnarizine (CIN)-loaded NLCs (CIN-NLCs).119 The study revealed that after IN administration, the Cmax,brain of CIN-NLCs was 786.65 μg /mL, approximately 2.03 times higher than that of free CIN administered intranasally. These results demonstrated that SLNs or NLCs (IN) could significantly increase drug accumulation in the brain and also increase the bioavailability of drugs (Figure 8).
Evaluation of Pharmacodynamics in in vivo Studies
In addition to PK evaluations, many in vivo studies have also conducted pharmacodynamic (PD) assessments (Table 1). Islamie et al developed SLNs loaded with asiatic acid (AA-SLNs).100 In a PD study using a rat model of amyloid-beta (Aβ)-induced memory impairment, IN delivery of AA-SLNs played a crucial role in improving spatial and recognition memory deficits caused by Aβ1–42. It significantly reduced Aβ1–42-induced lipid peroxidation, tau hyperphosphorylation, and inflammatory cytokine levels. Additionally, IN administration of AA-SLNs notably decreased Aβ1–42-associated glial activation in the hippocampal CA1 and CA3 subregions. Compared to oral AA at the same dose, IN delivery of AA-SLNs demonstrated a significant advantage in neuroprotection in the AD rat model. In another study, an improved formulation of ferulic acid (FA)-loaded SLNs encapsulated with chitosan (FA-CS-SLNs) was developed.50 In behavioral experiments, IN delivery of FA-CS-SLNs in AD rats resulted in a significant reduction in escape latency (p<0.001 on Day 27) and improvement in cognitive abilities (p<0.001 on Day 27) compared to the free drugs. Additionally, IN FA-CS-SLNs significantly reduced biochemical markers in the brains of AD rats, such as acetylcholinesterase, compared to other groups. This highlights the significant therapeutic effect of IN delivery of FA-CS-SLNs in the treatment of AD. Neha et al developed an optimized formulation of dopamine (DOPA)-loaded NLCs (DOPA-NLCs) for PD studies in a Parkinson’s disease rat model.104 The motor behavior tests in the experimental group of rats administered with IN DOPA-NLCs were similar to those of the control group, while both the rotenone (IN) negative control group and the DOPA (IN) positive control group showed significantly lower performance compared to the control group (p<0.01, p<0.05, respectively). Additionally, in the forced swimming exertion test, the immobility duration in both the rotenone (IN) negative control group and the DOPA (IN) positive control group was significantly higher than that of the control group (p<0.01, p<0.05, respectively), whereas the DOPA-NLCs (IN) group was similar to the control group. In the neurochemical evaluation, the DOPA levels in the DOPA-NLCs (IN) group were comparable to those of the control group, while both the negative and positive control groups had significantly lower DOPA levels than the control group (p<0.01, p<0.05, respectively). This demonstrates that NLCs significantly enhanced the therapeutic efficacy of DOPA in the treatment of Parkinson’s disease. Overall, encapsulating drugs into SLNs or NLCs and delivering them intranasally significantly enhances their therapeutic efficacy in treating CNS diseases.
Limitations and Reflections
Overall, this paper has some inevitable limitations. The formulations of the SLNs and NLCs, as well as the drugs used in the 38 studies we screened varied significantly. In addition, although all studies used rats as in vivo experimental models, the growth environments and feeding conditions of the rats varied considerable, resulting in a high degree of heterogeneity. This made it challenging to maintain small deviations when directly comparing results from different studies, and thereby affected the accuracy of our conclusions. Moreover, in order to avoid inconsistency in calculations, we re-calculated all PK-related parameters. We found that some studies used the value of AUC0-∞ for the relevant calculations, while in this paper, we used the value of AUC0-t for re-calculation, which led to discrepancies between our calculated values and the original values. However, when no AUC0-t values were provided, we had to rely on AUC0-∞ values. Some studies have suggested that the best approach for calculating DTE% and DTP% is to compare SLNs or NLCs (IN) with free drug (IV) to eliminate the effect of the SLNs or NLCs themselves.93 However, not all studies used a free drug (IV) administration protocol, in which case, we had to use the available values from SLNs or NLCs (IN) and compare them to the values for SLNs or NLCs (IV).
Due to economic factors and accessibility, most studies currently use rats as an animal model for IN delivery. However, there are significant anatomical differences between the nasal cavities of rats and humans. The olfactory mucosal area in humans occupies only 10% of the nasal cavity, whereas in rats, it accounts for 50% of the nasal cavity.132,133 In addition, the small size of the anterior nostrils of rats makes it more difficult to administer drugs, and especially viscous agents. In comparison, rabbits, dogs, monkeys, and sheep have larger anterior nostrils, making nasal administration easier and more suitable for PK studies involving IN delivery.134 Furthermore, the proportion of the olfactory region in rabbits, sheep, and monkeys is similar to that in humans (approximately 10%), while in dogs, it is as high as 77%.11 Therefore, considering the economic factors as well as the ethical issues related to animal experimentation, rabbits are the most popular animal models and are often used for PK studies.
While SLNs and NLCs demonstrate significant advantages in treating CNS diseases via IN delivery, they also face numerous challenges. For instance, crystallization of the lipid core in SLNs may reduce their drug-loading capacity, and their structure may become unstable during storage, leading to premature drug release and gelation. The high water content (70–90%) in the formulation may also affect the stability of SLNs.46,135 These drawbacks limit the widespread clinical application of SLNs and NLCs. The majority of ongoing clinical trials using lipid-based nanoformulations are primarily in Phase I or Phase II, and are mostly related to liposomes.136–139 We have meticulously reviewed the current clinical trials involving SLNs and NLCs and summarized them in Table 3. The results show that only two completed clinical trials exist, both of which in Phase I. One of the studies focused on 28 patients with dermatophytosis, evaluating the efficacy of the oxiconazole nitrate SLNs gel system and oxiconazole Nitrate Cream 1% administered topically. The results showed that the oxiconazole nitrate SLNs gel system was superior to the commercially available oxiconazole nitrate cream in terms of clinical improvement, patient satisfaction, and toxic side effects. This indicates that the oxiconazole nitrate SLNs gel system has great potential in the treatment of tinea fungal diseases.140 In another crossover, randomized, placebo-controlled, double-blind study, the clinical efficacy of a xanthan gum-based hydrogel (2%) containing a local anesthetic encapsulated in NLCs was evaluated. Regrettably, the researchers of this study have not disclosed any findings. Another clinical trial aimed to evaluate the safety, reactogenicity, and immunogenicity of an saRNA COVID-19 booster vaccine in participants previously vaccinated or infected with COVID-19. However, this study was terminated before entering Phase II, probably due to safety reasons. However, no studies have focused on IN delivery or the treatment of central nervous system diseases.
 |
Table 3 Clinical Trials on SLNs and NLCs |
Recently, 3D printed models of the human nasal cavity (3D nasal models) have garnered increasing interest among researchers, and are often used to assess the efficacy of IN-delivered agents, as well as intranasal deposition prior to in vivo experiments. This approach helps to reduce the number of experiments conducted on animals and humans, and allows for personalized adjustments based on individual patient anatomies.141–143 Nasal plaster is commonly used to create 3D nasal molds.144 To make these models more closely replicate the actual human nasal cavity environment, artificial mucus or coatings are used to simulate the mucous layer. This enhances the assessment of drug interception in moist nasal environments. Furthermore, techniques such as humidification and the use of flexible nasal sections are employed to further approximate real conditions.141,145 Pina Costa et al146 developed diazepam-loaded NLCs (DZP-NLCs) and evaluated their deposition pattern in a 3D nasal model to successfully obtain the optimal nasal deposition curve for DZP-NLCs. Thus, it is evident that 3D nasal models have substantial potential for use in future research on IN delivery.
Conclusion
Overall, this paper provides a review of recent in vivo studies conducted on SLNs and NLCs delivered via IN administration for the treatment of CNS disorders. Analyses of pharmacokinetic (PK) and bioavailability parameters showed that SLNs and NLCs significantly increased drug accumulation and targeting in the brain when administered intranasally. In addition, PD assessments showed that drug-loaded SLNs or NLCs delivered intranasally significantly improved CNS symptoms in rat models. Thus, despite many challenges, SLNs and NLCs have great potential for use in treatment of CNS diseases via IN delivery. Future efforts should focus on developing SLNs and NLCs formulations with greater stability, aiming to reduce drug release during storage and enhance drug-loading capacity. Additionally, current research on the in vivo safety of SLNs and NLCs is relatively limited; therefore, it is essential to design more animal or clinical trials to further investigate their potential toxic effects. The 3D nasal membrane model shows significant advantages in evaluating intranasal deposition in vitro and could be further developed to better assess the intranasal deposition patterns of SLNs and NLCs. It is expected that more SLNs and NLCs formulations will enter clinical trials and be further used in clinical therapy in the future.
Data Sharing Statement
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Funding
This project is supported by the National Natural Science Foundation of China (82271147, 82271146); the Key Research and Development Program of Shandong Province (Major Science and Technology Innovation Project) (2020CXGC011302, 2022CXGC020506).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Hong -S-S, Oh KT, Choi H-G, Lim S-J. Liposomal formulations for nose-to-brain delivery: recent advances and future perspectives. Pharmaceutics. 2019;11(10):540. doi:10.3390/pharmaceutics11100540
2. Samaridou E, Alonso MJ. Nose-to-brain peptide delivery – the potential of nanotechnology. Bioorg Med Chem. 2018;26(10):2888–2905. doi:10.1016/j.bmc.2017.11.001
3. Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104(1):29–45. doi:10.1016/j.pharmthera.2004.08.001
4. Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi:10.1016/j.nbd.2009.07.030
5. Nguyen -T-T-L, Maeng H-J. Pharmacokinetics and pharmacodynamics of intranasal solid lipid nanoparticles and nanostructured lipid carriers for nose-to-brain delivery. Pharmaceutics. 2022;14(3). doi:10.3390/pharmaceutics14030572
6. Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Design. 2004;10(12):1295–1312. doi:10.2174/1381612043384844
7. Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Delivery Rev. 2012;64(7):614–628. doi:10.1016/j.addr.2011.11.002
8. Khan AR, Liu M, Khan MW, Zhai G. Progress in brain targeting drug delivery system by nasal route. J Control Release. 2017;268:364–389. doi:10.1016/j.jconrel.2017.09.001
9. Liu J, Chu C, Zhang J, et al. Label-free assessment of mannitol accumulation following osmotic blood–brain barrier opening using chemical exchange saturation transfer magnetic resonance imaging. Pharmaceutics. 2022;14(11):2529. doi:10.3390/pharmaceutics14112529
10. Pavan B, Dalpiaz A. Prodrugs and endogenous transporters: are they suitable tools for drug targeting into the central nervous system? Curr Pharm Design. 2011;17(32):3560–3576. doi:10.2174/138161211798194486
11. Costa CP, Moreira JN, Sousa Lobo JM, Silva AC. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: a current overview of in vivo studies. Acta Pharmaceutica Sinica B. 2021;11(4):925–940. doi:10.1016/j.apsb.2021.02.012
12. Babu SR, Shekara HH, Sahoo AK, Harsha Vardhan PV, Thiruppathi N, Venkatesh MP. Intranasal nanoparticulate delivery systems for neurodegenerative disorders: a review. Therap Del. 2023;14(9):571–594. doi:10.4155/tde-2023-0019
13. Singh S, Shukla R. Nanovesicular-mediated intranasal drug therapy for neurodegenerative disease. AAPS Pharm Sci Tech. 2023;24(7). doi:10.1208/s12249-023-02625-5
14. Zha S, Wong KL, All AH. Intranasal delivery of functionalized polymeric nanomaterials to the brain. Adv Healthcare Mater. 2022;11(11). doi:10.1002/adhm.202102610
15. Li Y, Wu H, Jiang X, Dong Y, Zheng J, Gao J. New idea to promote the clinical applications of stem cells or their extracellular vesicles in central nervous system disorders: combining with intranasal delivery. Acta Pharmaceutica Sinica B. 2022;12(8):3215–3232. doi:10.1016/j.apsb.2022.04.001
16. Keller LA, Merkel O, Popp A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Delivery Transl Res. 2022;12(4):735–757. doi:10.1007/s13346-020-00891-5
17. Gänger S, Schindowski K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics. 2018;10(3):116. doi:10.3390/pharmaceutics10030116
18. Lai SK, Wang -Y-Y, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Delivery Rev. 2009;61(2):158–171. doi:10.1016/j.addr.2008.11.002
19. Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium - a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13(2):339–352. doi:10.1016/0896-6273(94)90351-4
20. Field PM, Li Y, Raisman G. Ensheathment of the olfactory nerves in the adult rat. J Neurocytol. 2003;32(3):317–324. doi:10.1023/B:NEUR.0000010089.37032.48
21. Silver WL, Finger TE. The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann N.Y. Acad Sci. 2009;1170(1):202–205. doi:10.1111/j.1749-6632.2009.03894.x
22. Schaefer ML, Böttger B, Silver WL, Finger TE. Trigeminal collaterals in the nasal epithelium and olfactory bulb: a potential route for direct modulation of olfactory information by trigeminal stimuli. J Comp Neurol. 2002;444(3):221–226. doi:10.1002/cne.10143
23. Crowe TP, Hsu WH. Evaluation of recent intranasal drug delivery systems to the central nervous system. Pharmaceutics. 2022;14(3):629. doi:10.3390/pharmaceutics14030629
24. Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi:10.1016/j.lfs.2017.12.025
25. Pardeshi CV, Belgamwar VS. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: an excellent platform for brain targeting. Expert Opin Drug Delivery. 2013;10(7):957–972. doi:10.1517/17425247.2013.790887
26. Vyas T, Shahiwala A, Marathe S, Misra A. Intranasal drug delivery for brain targeting. Current Drug Delivery. 2005;2(2):165–175. doi:10.2174/1567201053586047
27. Haberly LB, Price JL. The axonal projection patterns of the mitral and tufted cells of the olfactory bulb in the rat. Brain Res. 1977;129(1):152–157. doi:10.1016/0006-8993(77)90978-7
28. Nagayama S. Differential axonal projection of mitral and tufted cells in the mouse main olfactory system. Front Neural Circuits. 2010;4:1662–5110. doi:10.3389/fncir.2010.00120
29. Broadwell RD, Balin BJ. Endocytic and exocytic pathways of the neuronal secretory process and trans synaptic transfer of wheat germ agglutinin‐horseradish peroxidase in vivo. J Comp Neurol. 2004;242(4):632–650. doi:10.1002/cne.902420410
30. Ward PD, Tippin TK, Thakker DR. Enhancing paracellular permeability by modulating epithelial tight junctions. Pharm Sci Technol Today. 2000;3(10):346–358. doi:10.1016/S1461-5347(00)00302-3
31. Cowan CM, Roskams AJ. Apoptosis in the mature and developing olfactory neuroepithelium. Microsc Res Techniq. 2002;58(3):204–215. doi:10.1002/jemt.10150
32. Dhuria SV, Hanson LR, Frey WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharmaceut Sci. 2010;99(4):1654–1673. doi:10.1002/jps.21924
33. Li Y, Field PM, Raisman G. Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia. 2005;52(3):245–251. doi:10.1002/glia.20241
34. Falcone JA, Salameh TS, Yi X, et al. Intranasal administration as a route for drug delivery to the brain: evidence for a unique pathway for albumin. J Pharmacol Exp Ther. 2014;351(1):54–60. doi:10.1124/jpet.114.216705
35. Pang Y, Lin S, Wright C, et al. Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-ohda in rats. Neuroscience. 2016;318(1873–7544):157–165. doi:10.1016/j.neuroscience.2016.01.020
36. Lochhead JJ, Davis TP. Perivascular and perineural pathways involved in brain delivery and distribution of drugs after intranasal administration. Pharmaceutics. 2019;11(11):598. doi:10.3390/pharmaceutics11110598
37. Hadaczek P, Yamashita Y, Mirek H, et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14(1):69–78. doi:10.1016/j.ymthe.2006.02.018
38. Zhang Y-T, He K-J, Zhang J-B, Ma Q-H, Wang F, Liu C-F. Advances in intranasal application of stem cells in the treatment of central nervous system diseases. Stem Cell Res Ther. 2021;12(1): 1–10.
39. Wolburg H, Wolburg-Buchholz K, Sam H, Horvát S, Deli MA, Mack AF. Epithelial and endothelial barriers in the olfactory region of the nasal cavity of the rat. Histochem Cell Biol. 2008;130(1):127–140. doi:10.1007/s00418-008-0410-2
40. Walter BA, Valera VA, Takahashi S, Matsuno K, Ushiki T. Evidence of antibody production in the rat cervical lymph nodes after antigen administration into the cerebrospinal fluid. Arch Histol Cytol. 2006;69(1):37–47. doi:10.1679/aohc.69.37
41. Mishra DK, Shandilya R, Mishra PK. Lipid based nanocarriers: a translational perspective. Nanomed Nanotechnol Biol Med. 2018;14(7):2023–2050. doi:10.1016/j.nano.2018.05.021
42. Qushawy M, Prabahar K, Abd-Alhaseeb M, Swidan S, Nasr A. Preparation and evaluation of carbamazepine solid lipid nanoparticle for alleviating seizure activity in pentylenetetrazole-kindled mice. Molecules. 2019;24(21):3971. doi:10.3390/molecules24213971
43. Joshi MD, Müller RH. Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Biopharm. 2009;71(2):161–172. doi:10.1016/j.ejpb.2008.09.003
44. Pink DL, Loruthai O, Ziolek RM, et al. On the structure of solid lipid nanoparticles. Small. 2019;15(45). doi:10.1002/smll.201903156.
45. Mohammadi-Samani S, Ghasemiyeh P. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288. doi:10.4103/1735-5362.235156
46. Tapeinos C, Battaglini M, Ciofani G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Rel. 2017;264:306–332. doi:10.1016/j.jconrel.2017.08.033
47. McClements DJ, Li Y. Structured emulsion-based delivery systems: controlling the digestion and release of lipophilic food components. Adv Colloid Interface Sci. 2010;159(2):213–228. doi:10.1016/j.cis.2010.06.010
48. Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Delivery Rev. 2002;54(0169–409X):S131–S155. doi:10.1016/S0169-409X(02)00118-7
49. Mehnert W. Solid lipid nanoparticles Production, characterization and applications. Adv Drug Delivery Rev. 2001;47(2–3):165–196. doi:10.1016/S0169-409X(01)00105-3
50. Saini S, Sharma T, Jain A, Kaur H, Katare OP, Singh B. Systematically designed chitosan-coated solid lipid nanoparticles of ferulic acid for effective management of Alzheimer’s disease: a preclinical evidence. Colloids Surf B. 2021;205: 111838.
51. Gordillo-Galeano A, Mora-Huertas CE. Solid lipid nanoparticles and nanostructured lipid carriers: a review emphasizing on particle structure and drug release. Eur J Pharm Biopharm. 2018;133:285–308. doi:10.1016/j.ejpb.2018.10.017
52. Arduino I, Liu Z, Rahikkala A, et al. Preparation of cetyl palmitate-based PEGylated solid lipid nanoparticles by microfluidic technique. Acta Biomater. 2021;121(1878–7568):566–578. doi:10.1016/j.actbio.2020.12.024
53. Subroto E, Andoyo R, Indiarto R, Wulandari E, Wadhiah EFN. Preparation of solid lipid nanoparticle-ferrous sulfate by double emulsion method based on fat rich in monolaurin and stearic acid. Nanomaterials. 2022;12(17):3054. doi:10.3390/nano12173054
54. Khairnar SV, Pagare P, Thakre A, et al. Review on the scale-up methods for the preparation of solid lipid nanoparticles. Pharmaceutics. 2022;14(9):1886. doi:10.3390/pharmaceutics14091886
55. Xu W, Deng Z, Xiang Y, et al. Preparation, characterization and pharmacokinetics of tolfenamic acid-loaded solid lipid nanoparticles. Pharmaceutics. 2022;14(9):1929. doi:10.3390/pharmaceutics14091929
56. Rahmanian-Devin P, Askari VR, Sanei-Far Z, et al. Preparation and characterization of solid lipid nanoparticles encapsulated noscapine and evaluation of its protective effects against imiquimod-induced psoriasis-like skin lesions. Biomed Pharmacother. 2023;1681950–6007:115823. doi:10.1016/j.biopha.2023.115823
57. Bagde A, Patel K, Kutlehria S, Chowdhury N, Singh M. Formulation of topical ibuprofen solid lipid nanoparticle (SLN) gel using hot melt extrusion technique (HME) and determining its anti-inflammatory strength. Drug Delivery Transl Res. 2019;9(4):816–827. doi:10.1007/s13346-019-00632-3
58. Jenning V, Thünemann AF, Gohla SH. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm. 2000;199(2):167–177. doi:10.1016/S0378-5173(00)00378-1
59. Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598–613. doi:10.1016/j.biopha.2018.04.055
60. Devkar TB, Tekade AR, Khandelwal KR. Surface engineered nanostructured lipid carriers for efficient nose to brain delivery of ondansetron HCl using Delonix regia gum as a natural mucoadhesive polymer. Colloids Surf B Biointerfaces. 2014;122:143–150. doi:10.1016/j.colsurfb.2014.06.037
61. Weber S, Zimmer A, Pardeike J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7–22. doi:10.1016/j.ejpb.2013.08.013
62. Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Targeting. 2012;20(10):813–830. doi:10.3109/1061186X.2012.716845
63. Muller R, Petersen R, Hommoss A, Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products☆. Adv Drug Delivery Rev. 2007;59(6):522–530. doi:10.1016/j.addr.2007.04.012
64. Doktorovova S, Kovacevic AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;108(1873–3441):235–252. doi:10.1016/j.ejpb.2016.08.001
65. Haider M, Abdin SM, Kamal L, Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics. 2020;12(3):288. doi:10.3390/pharmaceutics12030288
66. Doktorovova S, Souto EB, Silva AM. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers – a systematic review of in vitro data. Eur J Pharm Biopharm. 2014;87(1):1–18. doi:10.1016/j.ejpb.2014.02.005
67. Doktorovová S, Kovačević AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;108:235–252.
68. Veider F, Akkuş-Dağdeviren ZB, Knoll P, Bernkop-Schnürch A. Design of nanostructured lipid carriers and solid lipid nanoparticles for enhanced cellular uptake. Int J Pharm. 2022;624: 122014.
69. Sadegh Malvajerd S, Azadi A, Izadi Z, et al. Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: preparation, optimization, and pharmacokinetic evaluation. ACS Chem Neurosci. 2019;10(1):728–739. doi:10.1021/acschemneuro.8b00510
70. Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed. 2012;5577. doi:10.2147/IJN.S36111
71. Liu X, Li H, Jin Q, Ji J. Surface tailoring of nanoparticles via mixed‐charge monolayers and their biomedical applications. Small. 2014;10(21):4230–4242. doi:10.1002/smll.201401440
72. Almeida H, Lobao P, Frigerio C, et al. Preparation, characterization and biocompatibility studies of thermoresponsive eyedrops based on the combination of nanostructured lipid carriers (NLC) and the polymer Pluronic F-127 for controlled delivery of ibuprofen. Pharm Dev Technol. 2017;22(3):336–349. doi:10.3109/10837450.2015.1125922
73. Muller RH, Ruhl D, Runge S, Schulze-Forster K, Mehnert W. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharm Res. 1997;14(4):458–462. doi:10.1023/A:1012043315093
74. Hwang TL, Aljuffali IA, Hung CF, Chen CH, Fang JY. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs). Chem Biol Interact. 2015;235(1872–7786):106–114. doi:10.1016/j.cbi.2015.04.011
75. Vairo C, Collantes M, Quincoces G, et al. Preclinical safety of topically administered nanostructured lipid carriers (NLC) for wound healing application: biodistribution and toxicity studies. Int J Pharm;2019. 569. doi:10.1016/j.ijpharm.2019.05.036
76. Lakkadwala S, Nguyen S, Lawrence J, Nauli SM, Nesamony J. Physico-chemical characterisation, cytotoxic activity, and biocompatibility studies of tamoxifen-loaded solid lipid nanoparticles prepared via a temperature-modulated solidification method. J Microencapsul. 2014;31(6):590–599. doi:10.3109/02652048.2014.898707
77. Cho C-S, Firdous I, Choi Y. Design and application of chitosan microspheres as oral and nasal vaccine carriers: an updated review. Int J Nanomed. 2012;6077. doi:10.2147/IJN.S38330
78. Kumar A, Vimal A, Kumar A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int J Biol Macromol. 2016;91:615–622. doi:10.1016/j.ijbiomac.2016.05.054
79. Shim S, Yoo HS. The application of mucoadhesive chitosan nanoparticles in nasal drug delivery. Mar Drugs. 2020;18(12):605. doi:10.3390/md18120605
80. Noorulla KM, Yasir M, Muzaffar F, et al. Intranasal delivery of chitosan decorated nanostructured lipid carriers of buspirone for brain targeting: formulation development, optimization and in-vivo preclinical evaluation. J Drug Delivery Sci Technol. 2022;67: 102939.
81. Gautam D, Singh S, Maurya P, Singh M, Kushwaha S, Saraf SA. Appraisal of nano-lipidic astaxanthin cum thermoreversible gel and its efficacy in haloperidol induced Parkinsonism. Current Drug Delivery. 2021;18(10):1550–1562. doi:10.2174/1567201818666210510173524
82. Uppuluri CT, Ravi PR, Dalvi AV. Design, optimization and pharmacokinetic evaluation of Piribedil loaded solid lipid nanoparticles dispersed in nasal in situ gelling system for effective management of Parkinson’s disease. Int J Pharm. 2021;606: 120881.
83. Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol Sci. 2017;38(4):406–424. doi:10.1016/j.tips.2017.01.003
84. Rádis-Baptista G. Cell-penetrating peptides derived from animal venoms and toxins. Toxins. 2021;13(2):147. doi:10.3390/toxins13020147
85. Zou -L-L, Ma J-L, Wang T, Yang T-B, Liu C-B. Cell-penetrating peptide-mediated therapeutic molecule delivery into the central nervous system. Curr Neuropharmacol. 2013;11(2):197–208. doi:10.2174/1570159X11311020006
86. Kardani K, Milani A, Shabani H, Bolhassani A. S. Cell penetrating peptides: the potent multi-cargo intracellular carriers. Expert Opin Drug Delivery. 2019;16(11):1227–1258. doi:10.1080/17425247.2019.1676720
87. Rassu G, Soddu E, Posadino AM, et al. Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy. Colloids Surf B. 2017;152:296–301. doi:10.1016/j.colsurfb.2017.01.031
88. Knoll P, Hörmann N, Nguyen Le N-M, Wibel R, Gust R, Bernkop-Schnürch A. Charge converting nanostructured lipid carriers containing a cell-penetrating peptide for enhanced cellular uptake. J Colloid Interface Sci. 2022;628:463–475. doi:10.1016/j.jcis.2022.07.160
89. Soddu E, Rassu G, Giunchedi P, Sarmento B, Gavini E. From naturally-occurring neurotoxic agents to CNS shuttles for drug delivery. Eur J Pharm Sci. 2015;74:63–76. doi:10.1016/j.ejps.2015.04.005
90. Zhi D, Bai Y, Yang J, et al. A review on cationic lipids with different linkers for gene delivery. Adv Colloid Interface Sci. 2018;253:117–140. doi:10.1016/j.cis.2017.12.006
91. Suchaoin W, Pereira de Sousa I, Netsomboon K, Lam HT, Laffleur F, Bernkop-Schnürch A. Development and in vitro evaluation of zeta potential changing self-emulsifying drug delivery systems for enhanced mucus permeation. Int J Pharm. 2016;510(1):255–262. doi:10.1016/j.ijpharm.2016.06.045
92. Liu M, Zhang J, Shan W, Huang Y. Developments of mucus penetrating nanoparticles. Asian J Pharm Sci. 2015;10(4):275–282. doi:10.1016/j.ajps.2014.12.007
93. Pires PC, Santos AO. Nanosystems in nose-to-brain drug delivery: a review of non-clinical brain targeting studies. J Control Release. 2018;270:89–100. doi:10.1016/j.jconrel.2017.11.047
94. Modena MM, Rühle B, Burg TP, Wuttke S. Nanoparticle characterization: what to measure? Adv Mater. 2019;31(32). doi:10.1002/adma.201901556
95. Shehata MK, Ismail AA, Kamel MA. Nose to brain delivery of astaxanthin–loaded nanostructured lipid carriers in rat model of Alzheimer’s disease: preparation, in vitro and in vivo evaluation. Int J Nanomed. 2023;18:1631–1658. doi:10.2147/IJN.S402447
96. Shehata MK, Ismail AA, Kamel MA. Combined donepezil with astaxanthin via nanostructured lipid carriers effective delivery to brain for Alzheimer’s disease in rat model. Int J Nanomed. 2023;18:4193–4227. doi:10.2147/IJN.S417928
97. Abourehab MAS, Khames A, Genedy S, et al. Sesame oil-based nanostructured lipid carriers of nicergoline, intranasal delivery system for brain targeting of synergistic cerebrovascular protection. Pharmaceutics. 2021;13(4):581. doi:10.3390/pharmaceutics13040581
98. Tekade AR, Suryavanshi MR, Shewale AB, Patil VS. Design and development of donepezil hydrochloride loaded nanostructured lipid carriers for efficient management of Alzheimer’s disease. Drug Dev Ind Pharm. 2023;49(9):590–600. doi:10.1080/03639045.2023.2262035
99. Arora D, Bhatt S, Kumar M, et al. QbD-based rivastigmine tartrate-loaded solid lipid nanoparticles for enhanced intranasal delivery to the brain for Alzheimer’s therapeutics. Front Aging Neurosci. 2022;14: 960246.
100. Islamie R, Myint SLL, Rojanaratha T, et al. Neuroprotective effect of nose-to-brain delivery of Asiatic acid in solid lipid nanoparticles and its mechanisms against memory dysfunction induced by Amyloid Beta1-42 in mice. BMC Complement Med Therap. 2023;23(1). doi:10.1186/s12906-023-04125-2.
101. Yasir M, Zafar A, Noorulla KM, et al. Nose to brain delivery of donepezil through surface modified NLCs: formulation development, optimization, and brain targeting study. J Drug Delivery Sci Technol. 2022;75: 103631.
102. El-Enin HA A, Elkomy MH, Naguib IA, et al. Lipid nanocarriers overlaid with chitosan for brain delivery of berberine via the nasal route. Pharmaceuticals. 2022;15(3): 281.
103. Jojo GM, Kuppusamy G, De A, Karri VVSNR. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev Ind Pharm. 2019;45(7):1061–1072. doi:10.1080/03639045.2019.1593439
104. Neha SL, Mishra AK, Rani L, Paroha S, Dewangan HK, Sahoo PK. Design and evaluations of a nanostructured lipid carrier loaded with dopamine hydrochloride for intranasal bypass drug delivery in Parkinson’s disease. J Microencapsul. 2023;40(8):599–612. doi:10.1080/02652048.2023.2264386
105. Yasir M, Chauhan I, Zafar A, et al. Glyceryl behenate-based solid lipid nanoparticles as a carrier of haloperidol for nose to brain delivery: formulation development, in-vitro, and in-vivo evaluation. Braz J Pharm Sci. 2022;58: e20254.
106. Hassan DM, El-Kamel AH, Allam EA, Bakr BA, Ashour AA. Chitosan-coated nanostructured lipid carriers for effective brain delivery of Tanshinone IIA in Parkinson’s disease: interplay between nuclear factor-kappa β and cathepsin B. Drug Delivery Transl Res. 2023;14(2):400–417. doi:10.1007/s13346-023-01407-7
107. Zafar A, Awad Alsaidan O, Alruwaili NK, et al. Formulation of intranasal surface engineered nanostructured lipid carriers of rotigotine: full factorial design optimization, in vitro characterization, and pharmacokinetic evaluation. Int J Pharm. 2022;627: 122232.
108. Nair SC, Vinayan KP, Mangalathillam S. Nose to brain delivery of phenytoin sodium loaded nano lipid carriers: formulation, drug release, permeation and in vivo pharmacokinetic studies. Pharmaceutics. 2021;13(10):1640. doi:10.3390/pharmaceutics13101640
109. Arya RKK, Vijay J, Bisht D, et al. Enhanced brain delivery via intranasal administration of carbamazepine loaded solid lipid nanoparticles: optimization, pharmacokinetic analysis, in-vitro, and in-vivo drug release study. Current Drug Delivery. 2023;20(5):587–600. doi:10.2174/1567201819666220519120837
110. Qizilbash FF, Ashhar MU, Zafar A, et al. Thymoquinone-enriched naringenin-loaded nanostructured lipid carrier for brain delivery via nasal route: in vitro prospect and in vivo therapeutic efficacy for the treatment of depression. Pharmaceutics. 2022;14(3):656. doi:10.3390/pharmaceutics14030656
111. Rai JP, Mohanty PK, Hangargekar SR. Preclinical screening of antidepressant activity of formulated sertraline hydrochloride-loaded solid lipid nanoparticles in rats. J Pharm Res Int. 2021;33:134–138.
112. Silva S, Bicker J, Fonseca C, et al. Encapsulated escitalopram and paroxetine intranasal co-administration: in vitro/in vivo evaluation. Front Pharmacol. 2021;12: 751321.
113. Yasir M, Chauhan I, Zafar A, et al. Buspirone loaded solid lipid nanoparticles for amplification of nose to brain efficacy: formulation development, optimization by Box-Behnken design, in-vitro characterization and in-vivo biological evaluation. J Drug Delivery Sci Technol. 2021;61:102164.
114. Teaima MH, El-Nadi MT, Hamed RR, El-Nabarawi MA, Abdelmonem R. Lyophilized nasal inserts of atomoxetine HCl solid lipid nanoparticles for brain targeting as a treatment of attention-deficit/hyperactivity disorder (ADHD): a pharmacokinetics study on rats. Pharmaceuticals. 2023;16(2):326. doi:10.3390/ph16020326
115. Patel HP, Gandhi PA, Chaudhari PS, et al. Clozapine loaded nanostructured lipid carriers engineered for brain targeting via nose-to-brain delivery: optimization and in vivo pharmacokinetic studies. J Drug Delivery Sci Technol. 2021;64: 102533.
116. Gadhave DG, Tagalpallewar AA, Kokare CR. Agranulocytosis-protective olanzapine-loaded nanostructured lipid carriers engineered for CNS delivery: optimization and hematological toxicity studies. AAPS Pharm Sci Tech. 2019;20(1). doi:10.1208/s12249-018-1213-y
117. Taha E, Nour SA, Mamdouh W, et al. Cod liver oil nano-structured lipid carriers (Cod-NLCs) as a promising platform for nose to brain delivery: preparation, in vitro optimization, ex vivo cytotoxicity & in vivo biodistribution utilizing radioiodinated zopiclone. Int J Pharm X. 2023;5:100160.
118. Bakshi V, Amarachinta PR, Chettupalli AK. Design, development and optimization of solid lipid nanoparticles of rizatriptan for intranasal delivery: invitro & invivo assessment. Mater Today Proc. 2022;66:2342–2357.
119. Tripathi D, Sonar PK, Parashar P, Chaudhary SK, Upadhyay S, Saraf SK. Augmented brain delivery of cinnarizine through nanostructured lipid carriers loaded in situ gel: in vitro and pharmacokinetic evaluation. BioNanoScience. 2021;11(1):159–171. doi:10.1007/s12668-020-00821-2
120. Mathure D, Ranpise H, Awasthi R, Pawar A. Formulation and characterization of nanostructured lipid carriers of rizatriptan benzoate-loaded in situ nasal gel for brain targeting. ASSAY Drug Dev Technol. 2022;20(5):211–224. doi:10.1089/adt.2022.044
121. Masjedi M, Azadi A, Heidari R, Mohammadi-Samani S. Nose-to-brain delivery of sumatriptan-loaded nanostructured lipid carriers: preparation, optimization, characterization and pharmacokinetic evaluation. J Pharm Pharmacol. 2020;72(10):1341–1351. doi:10.1111/jphp.13316
122. Palagati S, Sv S, Kesavan BR. Application of computational tools for the designing of Oleuropein loaded nanostructured lipid carrier for brain targeting through nasal route. DARU J Pharma Sci. 2019;27(2):695–708. doi:10.1007/s40199-019-00304-0
123. Abdel Hady M, Sayed OM, Akl MA. Brain uptake and accumulation of new levofloxacin-doxycycline combination through the use of solid lipid nanoparticles: formulation; optimization and in-vivo evaluation. Colloids Surf B. 2020;193:111076.
124. Mohanty D, Alsaidan OA, Zafar A, et al. Development of atomoxetine-loaded NLC in situ gel for nose-to-brain delivery: optimization, in vitro, and preclinical evaluation. Pharmaceutics. 2023;15(7):1985. doi:10.3390/pharmaceutics15071985
125. Koduru TS, Gupta VN, Veeranna B, Seetharaman S. A dual therapy of nanostructured lipid carrier loaded with teriflunomide—A dihydro-orotate dehydrogenase inhibitor and an miR-155-Antagomir in cuprizone-induced C57BL/6J mouse. Pharmaceutics. 2023;15(4):1254. doi:10.3390/pharmaceutics15041254
126. Gadhave DG, Kokare CR. Nanostructured lipid carriers engineered for intranasal delivery of teriflunomide in multiple sclerosis: optimization and in vivo studies. Drug Dev Ind Pharm. 2019;45(5):839–851. doi:10.1080/03639045.2019.1576724
127. Wang L, Zhao X, Du J, Liu M, Feng J, Hu K. Improved brain delivery of pueraria flavones via intranasal administration of borneol-modified solid lipid nanoparticles. Nanomedicine. 2019;14(16):2105–2119. doi:10.2217/nnm-2018-0417
128. Du W, Li H, Tian B, et al. Development of nose-to-brain delivery of ketoconazole by nanostructured lipid carriers against cryptococcal meningoencephalitis in mice. Colloids Surf B. 2019;183:110446.
129. Cunha S, Costa CP, Loureiro JA, et al. Double optimization of rivastigmine-loaded nanostructured lipid carriers (NLC) for nose-to-brain delivery using the quality by design (QbD) approach: formulation variables and instrumental parameters. Pharmaceutics. 2020;12(7):599. doi:10.3390/pharmaceutics12070599
130. Zhao C, Zhu X, Tan J, Mei C, Cai X, Kong F. Lipid-based nanoparticles to address the limitations of GBM therapy by overcoming the blood-brain barrier, targeting glioblastoma stem cells, and counteracting the immunosuppressive tumor microenvironment. Biomed Pharmacother. 2024;171:116113.
131. Karn-Orachai K, Smith SM, Phunpee S, et al. The effect of surfactant composition on the chemical and structural properties of nanostructured lipid carriers. J Microencapsul. 2014;31(6):609–618. doi:10.3109/02652048.2014.911374
132. Ali J, Ali M, Baboota S, et al. Potential of nanoparticulate drug delivery systems by intranasal administration. Curr Pharm Design. 2010;16(14):1644–1653. doi:10.2174/138161210791164108
133. Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharmaceut Sci. 2005;94(6):1187–1195. doi:10.1002/jps.20318
134. Sabir F, Ismail R, Csoka I. Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: status quo and outlook. Drug Discovery Today. 2020;25(1):185–194. doi:10.1016/j.drudis.2019.10.005
135. Dhiman N, Awasthi R, Sharma B, Kharkwal H, Kulkarni GT. Lipid nanoparticles as carriers for bioactive delivery. Front Chem. 2021;9. doi:10.3389/fchem.2021.580118
136. Almawash S. Solid lipid nanoparticles, an effective carrier for classical antifungal drugs. Saudi Pharm J. 2023;31(7):1167–1180. doi:10.1016/j.jsps.2023.05.011
137. Khan MS, Baskoy SA, Yang C, et al. Lipid-based colloidal nanoparticles for applications in targeted vaccine delivery. Nanoscale Adv. 2023;5(7):1853–1869. doi:10.1039/D2NA00795A
138. Mehrdadi S. Drug delivery of solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) to target brain tumors. Adv Pharm Bull. 2022;13(3):512–520. doi:10.34172/apb.2023.062
139. Mo K, Kim A, Choe S, Shin M, Yoon H. Overview of solid lipid nanoparticles in breast cancer therapy. Pharmaceutics. 2023;15(8):2065. doi:10.3390/pharmaceutics15082065
140. Mahmoud RA, Hussein AK, Nasef GA, Mansour HF. Oxiconazole nitrate solid lipid nanoparticles: formulation, in-vitro characterization and clinical assessment of an analogous loaded carbopol gel. Drug Dev Ind Pharm. 2020;46(5):706–716. doi:10.1080/03639045.2020.1752707
141. Deruyver L, Rigaut C, Lambert P, Haut B, Goole J. The importance of pre-formulation studies and of 3D-printed nasal casts in the success of a pharmaceutical product intended for nose-to-brain delivery. Adv Drug Delivery Rev. 2021;175:113826.
142. Manniello MD, Hosseini S, Alfaifi A, et al. In vitro evaluation of regional nasal drug delivery using multiple anatomical nasal replicas of adult human subjects and two nasal sprays. Int J Pharm. 2021;593:120103.
143. Maaz A, Blagbrough IS, De Bank PA. In vitro evaluation of nasal aerosol depositions: an insight for direct nose to brain drug delivery. Pharmaceutics. 2021;13(7):1079. doi:10.3390/pharmaceutics13071079
144. Williams G, Suman JD. In vitro anatomical models for nasal drug delivery. Pharmaceutics. 2022;14(7):1353. doi:10.3390/pharmaceutics14071353
145. Castile J, Cheng Y-H, Simmons B, Perelman M, Smith A, Watts P. Development of in vitro models to demonstrate the ability of PecSys®, anin situnasal gelling technology, to reduce nasal run-off and drip. Drug Dev Ind Pharm. 2012;39(5):816–824. doi:10.3109/03639045.2012.707210
146. Pina Costa C, Nodilo L N, Silva R, et al. In situ hydrogel containing diazepam-loaded nanostructured lipid carriers (DZP-NLC) for nose-to-brain delivery: development, characterization and deposition studies in a 3D-printed human nasal cavity model. Int J Pharm. 2023;644:123345.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.