Back to Journals » Journal of Inflammation Research » Volume 18
Novel Composite Scoring System for Predicting Prognosis in Stage IV Gastric Cancer Patients Treated with Immune Checkpoint Inhibitors
Authors Yang L , Li H , Xia M , Pu X
Received 17 February 2025
Accepted for publication 25 April 2025
Published 22 May 2025 Volume 2025:18 Pages 6491—6504
DOI https://doi.org/10.2147/JIR.S519724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Lingbing Yang,1,* Hongwei Li,2,* Mingyu Xia,3 Xiaomeng Pu4
1Department of Breast Surgery, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 2Department of Gastrointestinal Surgery, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 3Department of Colorectal Surgery, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 4School of Stomatology, Gansu Health Vocational College, Lanzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaomeng Pu, Email [email protected]
Background: Gastric cancer (GC) with distant metastases has a poor prognosis, and immune checkpoint inhibitors (ICIs) effectively improve the survival time of patients with this disease. This study aimed to identify effective prognostic markers that can predict the treatment effect of ICIs in patients with stage IV GC.
Methods: This study included 256 patients with GC with distant metastases who had received treatment with ICIs. A receiver operating characteristic (ROC) curve was used to analyze the predictive ability and optimal cutoff values of immune-inflammatory markers. Kaplan‒Meier survival curves were used to analyze the differences in progression-free survival (PFS) and overall survival (OS) among patients. Cox proportional hazard regression analysis was used to identify independent prognostic factors for PFS and OS.
Results: By comparing the area under the ROC curve (AUC) of immune-inflammatory markers, we selected the preoperative platelet count/(lymphocyte count × prealbumin count) ratio and fibrinogen/albumin ratio to form a combined score (PLPR–FAR score). The ROC curve revealed that when the PLPR–FAR score was used to predict patient PFS and OS, the AUC were 0.614 and 0.672, respectively. The Kaplan‒Meier survival curve revealed that patients with higher PLPR–FAR scores had significantly shorter PFS and OS than those with lower PLPR–FAR scores. Cox proportional hazard regression analysis revealed that the PLPR–FAR score was an independent risk factor for PFS and OS in stage IV GC patients.
Conclusion: The PLPR-FAR score may help identify which patients are more likely to benefit from ICIs treatment, and could serve as a novel and promising prognostic biomarker.
Keywords: gastric cancer, immune checkpoint inhibitors, prognosis, immune-inflammatory markers
Introduction
Gastric cancer (GC) is a type of cancer type with one of the highest incidence and mortality rates worldwide, with over 1 million new cases globally in 2020 and approximately 769,000 deaths attributed to GC.1 The East Asian region has the highest incidence rate of GC.2 Owing to the large number of patients with GC being diagnosed at an advanced stage of cancer, the disease prognosis in these patients has remained poor.3,4 Patients with distant metastases of GC have a median survival time of less than half a year.5 However, the emergence of immune checkpoint inhibitors (ICIs) has brought new hope to patients with metastatic GC, proving to be effective in extending the survival time of patients with GC.6,7
ICIs mainly target programmed death 1 (PD-1) or its ligand (PD-L1). Therefore, the expression levels of PD-L1 on tumor cells or tumor-associated immune cells can reflect the therapeutic effect of ICIs to some extent.8 Studies have also shown that microsatellite instability plays an important role in guiding treatment decisions. Patients with GC with high microsatellite instability benefit less from perioperative and adjuvant chemotherapy but are more sensitive to immunotherapy.9,10 In addition, tumor mutation burden levels have been shown to have similar effects.11,12 However, according to previous reports, these markers are expressed at low levels or not at all in the majority of patients.13,14 Notably, some studies have shown that cancer patients in whom PD-L1 is not expressed may still achieve certain survival benefits from immunotherapy.15 Hence, finding more accessible biomarkers that can effectively predict the outcomes of immunotherapy for patients with stage IV GC is highly important.
Inflammation is an important component of the tumor immune microenvironment. Inflammation has been reported to play a significant role in the occurrence and development of various cancers.16 Moreover, some inflammatory markers have also been found to predict the response of cancer patients to immunotherapy.17 For example, one study indicated that the neutrophil-to-lymphocyte ratio can effectively predict the treatment outcome of stage III non-small cell lung cancer patients receiving immunotherapy.18 In the field of GC, many similar studies have been conducted, and inflammatory indices such as the gastric immune prognostic index and neutrophil-to-lymphocyte ratio are considered effective in predicting the prognosis of advanced GC in patients receiving ICI treatment.19,20 In addition to inflammatory responses, the nutritional status of patients is also an important factor reflecting the treatment tolerance and tumor progression of cancer patients.21 Especially for GC patients, due to reduced food intake and a higher risk of cachexia, malnutrition is very common among them.22,23 Researchers have reported that various nutritional assessment systems, such as the prognostic nutritional index and body mass index, are related to the immunotherapy outcome of patients with GC.24,25 However, it is necessary to consider that GC is a highly heterogeneous disease, especially in patients with distant metastases, and a single hematological indicator has difficulty in effectively predicting the prognosis of GC in patients receiving ICIs treatment.26 In comparison, the combination of multiple indicators is more likely to help identify potential populations that may benefit from immunotherapy.27
The primary aim of this study was to identify inflammatory markers that can reliably predict the treatment outcomes of patients with stage IV GC receiving ICIs therapy. We selected two inflammatory markers with the highest predictive accuracy to develop a combined scoring system. Furthermore, a nomogram was constructed on the basis of this combined score to predict both progression-free survival (PFS) and overall survival (OS) in these patients. This model aims to provide more precise prognostic information, supporting clinicians in making informed treatment decisions and facilitating the development of individualized treatment plans.
Materials and Methods
Patients
This study included 256 GC patients who were treated with immunotherapy at Harbin Medical University Cancer Hospital from September 2018 to August 2022. The types of ICIs received by these patients were camrelizumab (N=135), toripalimab (N=47), sintilimab (N=49), pembrolizumab (N=14) and nivolumab (N=11). The diagnosis of patients was based on tissue samples obtained during gastroscopy, and the pathological tissue was examined by a pathologist to confirm the diagnosis.
The inclusion criteria were as follows: (1) patients with GC which was diagnosed by pathological biopsy; (2) patients with GC with distant metastasis; (3) patients who received more than one cycle of ICIs therapy. The exclusion criteria were as follows: (1) patients who had received neoadjuvant therapy or conversion therapy before the operation; (2) patients with gastric stump cancer; (3) patients with other primary malignant tumors in addition to GC; (4) patients who had received chemotherapy at other institutions before receiving chemotherapy at our institution; (5) patients with autoimmune diseases.
Data Extraction
This study collected 33 clinical data items from 256 patients with GC. Specifically, these data included sex, age, smoking status, drinking status, curative gastrectomy status, tumor location, number of cycles of ICIs therapy received, number of distant metastasis sites, body mass index, 20 hematological indicators, and 6 immune-inflammatory markers. Patients’ blood samples were collected within 7 days prior to starting immunotherapy. The calculation methods for the immune-inflammatory markers are as follows: MLR = monocyte count/lymphocyte count; DIR = direct bilirubin level/indirect bilirubin level; PLPR = platelet count/(lymphocyte count × prealbumin count); NLR = neutrophil count/lymphocyte count; FAR = fibrinogen level/albumin level; APR = alkaline phosphatase level/prealbumin level.
Study Outcome and Follow-up
The primary outcome of this study was the PFS, and the secondary endpoint was the OS of the patients. PFS was defined as the period from the date of the first ICIs treatment to the date of cancer progression or death of the patient. OS was defined as the period from the date of the first ICIs treatment to the date of death of the patient from any cause. The cutoff date for follow-up was set at one year; if some patients experienced cancer progression or death within a year, these patients were recorded as having an outcome event. Patients were followed up by telephone every three months until death or completion of 12 months of follow-up. The last follow-up date was September 1, 2023. During treatment with ICIs, all patients underwent tumor marker or radiological examinations (ultrasound, CT, and gastroscopy) every 3–6 months. Additionally, PET/CT scans were performed as needed and evaluated by radiologists and clinicians to assess tumor progression.
Statistical Analysis
In this study, categorical variables were described as frequencies and percentages; continuous variables were represented by means and standard deviations or medians and interquartile ranges. The predictive performance of immune-inflammatory markers was compared using the receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC). The optimal cutoff value for immune-inflammatory markers was determined on the basis of Youden’s index. The Kaplan‒Meier survival curve was used to evaluate the differences in OS or PFS between patients. The Cox regression model was used to analyze independent risk factors associated with PFS or OS. The hazard ratio (HR) and 95% CI were estimated for each factor. All the statistical analyses were performed using R software version 4.2.3. P < 0.05 was considered statistically significant.
Results
Patient Characteristics
The clinical and pathological characteristics of the patients are presented in Table 1. According to the inclusion and exclusion criteria, a total of 256 patients with stage IV GC who received ICIs treatment were included in this study. The median age of these patients was 60 years. Among them, 175 (68.4%) were male, and 81 (31.6%) were female. Cancer was located in the distal part of the stomach in 119 (46.5%) patients. Moreover, the median number of distant metastasis sites was 2, and the median number of cycles of immunotherapy was 3.
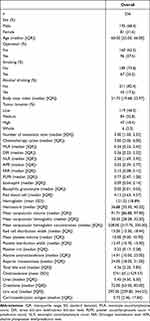 |
Table 1 Clinicopathological Features of All Patients |
Inflammation Index and Combined Score
To more accurately predict the treatment outcome and prognosis of GC in patients receiving ICIs treatment, we performed ROC analysis for each inflammation index. The two immune-inflammatory markers with the highest AUC were selected to form a combined scoring system. The results revealed that when these immune-inflammatory markers were used to predict patient PFS, the optimal cutoff values for the MLR, DIR, PLPR, NLR, FAR, and APR were 0.2192, 0.2126, 1.1836, 2.4757, 0.0989, and 0.6027, respectively. Moreover, their AUC were 0.541 (95% CI: 0.478–0.604), 0.528 (95% CI: 0.510–0.634), 0.605 (95% CI: 0.542–0.665), 0.542 (95% CI: 0.478–0.604), 0.573 (95% CI: 0.478–0.604), and 0.553 (95% CI: 0.490–0.615) (Figure 1A). Moreover, when these immune-inflammatory markers were used to predict patient OS, the optimal cutoff values for the MLR, DIR, PLPR, NLR, FAR, and APR were 0.3285, 0.2126, 0.9199, 2.4757, 0.0965, and 0.6027, respectively. The AUC were 0.561 (95% CI: 0.498–0.623), 0.529 (95% CI: 0.466–0.592), 0.643 (95% CI: 0.581–0.702), 0.572 (95% CI: 0.509–0.634), 0.633 (95% CI: 0.571–0.693), and 0.605 (95% CI: 0.542–0.665) (Figure 1B). Therefore, we constructed the PLPR–FAR scoring system on the basis of the optimal cutoff values. In this scoring system, patients with both a PLPR and FAR below the optimal cutoff values were scored as 0, those with both indicators above the optimal cutoff values were scored as 2, and patients with only one indicator above the optimal cutoff value were scored as 1. ROC analysis indicated that when the PLPR-FAR scoring system was used to predict patients’ PFS, the AUC was 0.614 (95% CI: 0.550–0.678) (Figure 1C). When the PLPR-FAR scoring system was used to predict patients’ OS, the AUC was 0.672 (95% CI: 0.609–0.734) (Figure 1D). In addition, we also performed a 10-fold cross-validation ROC analysis. The results revealed that when the PLPR–FAR scoring system was used to predict patient PFS, the average AUC was 0.600, and when it was used to predict patient OS, the average AUC was 0.630 (Figure S1).
PLPR-FAR and PFS
Chi-square tests revealed that the PLPR–FAR score was significantly associated with clinical characteristics, including the number of metastatic sites and cycles of immunotherapy. Specifically, patients with higher PLPR–FAR scores tended to have more metastatic sites (P=0.011) and fewer cycles of ICIs treatment (P=0.004) (Table S1). Moreover, the mean PFS times for patients with PLPR–FAR scores of 0, 1, and 2 were 10.330 months (95% CI: 9.832–10.828), 8.679 months (95% CI: 7.889–9.469), and 7.659 months (95% CI: 6.553–8.765), respectively. The 1-year PFS rates were 65.2%, 47.7%, and 39.6%, respectively (P<0.001) (Figure 2).
 |
Figure 2 Kaplan-Meier survival curve analysis of PLPR-FAR Score for assessing tumor progression. |
Univariate Cox proportional hazards regression analysis indicated that age (P=0.022), BMI (P=0.009), tumor location (P=0.020), number of cycles of immunotherapy (P=0.002), PLPR–FAR score (P<0.001), platelet distribution width (P=0.030), cholinesterase levels (P=0.005), and uric acid levels (P=0.029) were significantly associated with PFS in patients receiving ICIs treatment for GC. However, multivariate analysis revealed that only age (P=0.015), tumor location (P=0.034), number of cycles of immunotherapy (P=0.019), PLPR–FAR score (P=0.003), and uric acid levels (P=0.009) were independent prognostic factors related to tumor progression (Table 2).
 |
Table 2 Univariate and Multivariate Cox Proportional Hazards Regression Analysis with PFS or OS as the Outcome |
We combined the independent prognostic factors related to tumor progression to construct a nomogram for predicting tumor progression (Figure 3A). On the basis of the nomogram results, we calculated each patient’s risk score and conducted ROC analysis. The results revealed that when the nomogram was used to predict patient tumor progression, the AUC was 0.705 (95% CI: 0.640–0.769) (Figure 3B). The results of the 10-fold cross-validation ROC analysis revealed that the average AUC of the nomogram was 0.700 (Figure S2A). On the basis of the maximum Youden index, we subsequently determined the optimal cutoff value for the risk score, dividing the patients into high-risk and low-risk groups. Kaplan‒Meier survival analysis revealed that the PFS of patients in the high-risk group was significantly shorter than that of patients in the low-risk group (P<0.001) (Figure 3C). Moreover, decision curve analysis revealed that the use of the nomogram to predict patient tumor progression had a good net benefit (Figure 3D).
PLPR-FAR and OS
Chi-square tests revealed that the PLPR–FAR score was significantly associated with the number of metastatic sites (P=0.03) and cycles of immunotherapy (P=0.002) in patients with stage IV GC receiving ICIs treatment (Table S1). Furthermore, Kaplan‒Meier survival analysis indicated that patients with higher PLPR–FAR scores generally had shorter survival times. The mean OS times for patients with PLPR–FAR scores of 0, 1, and 2 were 11.151 months (95% CI: 10.738–11.563), 9.420 months (95% CI: 8.739–10.100), and 8.135 months (95% CI: 7.209–9.062), respectively. The 1-year survival rates were 79.8%, 52.2%, and 43.1%, respectively (P<0.001) (Figure 4).
 |
Figure 4 Kaplan-Meier survival curve analysis of PLPR-FAR score for evaluating patient survival time. |
Univariate Cox proportional hazards regression analysis revealed that characteristics related to OS included body mass index (P=0.003), tumor location (P=0.006), number of metastatic sites (P=0.035), number of cycles of immunotherapy (P<0.001), PLPR–FAR score (P<0.001), PLPR-FAR (P<0.001), red blood cells (P=0.006), hemoglobin (P=0.019), mean corpuscular volume (P=0.033), red cell distribution width (P=0.023), platelet distribution width (P=0.048), cholinesterase levels (P<0.001), and uric acid levels (P=0.033). Multivariate analysis revealed that tumor location (P=0.013), number of cycles of immunotherapy (P=0.005), PLPR–FAR score (P<0.001), and uric acid levels (P=0.011) were independent prognostic factors for OS (Table 2).
On the basis of the results of multivariate analysis, researchers used tumor location, number of cycles of immunotherapy, PLPR-FAR score, and uric acid levels to construct a nomogram for predicting the 1-year mortality rate of patients (Figure 5A). Similarly, we calculated each patient’s risk score and performed ROC analysis. When the nomogram was used to predict the 1-year mortality rate of patients with stage IV GC receiving ICIs treatment, the AUC was 0.754 (95% CI: 0.692–0.815) (Figure 5B). The results of the 10-fold cross-validation ROC analysis revealed that the average AUC of the nomogram was 0.750 (Figure S2B). On the basis of the ROC analysis, we subsequently calculated the optimal cutoff value for the risk score and divided all patients into high-risk and low-risk groups. Kaplan‒Meier survival analysis revealed that the OS of patients in the high-risk group was significantly shorter than that of patients in the low-risk group (P<0.001) (Figure 5C). Decision curve analysis revealed that the nomogram based on the PLPR–FAR score had a good ability to predict patient OS (Figure 5D).
Discussion
In recent years, the application of ICIs in the field of GC has been increasing globally.28 The efficacy of ICIs has been verified by many large clinical trials, showing that it can reduce tumor size and significantly prolong the survival time of some patients.29,30 Biomarkers such as PD-L1 and tumor mutation burden are considered rational markers for assessing the effectiveness of ICIs.11,31 However, the application of these biomarkers in clinical practice has always faced some challenges and limitations. First, the detection process for these tumor-derived markers is often invasive;32 second, studies have indicated that the expression levels of PD-L1 can vary within different areas of the same tumor;33 third, the expression levels of PD-L1 may fluctuate during treatment.34 Fortunately, previous reports have indicated that some cancer patients in whom PD-L1 is not expressed can also gain significant survival benefits from immunotherapy.15 These observations suggest that it is necessary to find new predictive factors to assess which patients with GC are more likely to benefit from the application of ICIs.
Previous studies have revealed a correlation between immune infiltration in the tumor immune microenvironment and the response of patients with GC to immunotherapy.35,36 Some inflammation indices derived from peripheral blood can effectively predict the survival time of patients with advanced GC receiving ICIs treatment.27 Through univariate and multivariate Cox analysis, we found that the PLPR-FAR score is an independent risk factor for both PFS and OS of GC patients receiving ICIs treatment. Characteristics with the same effect also include the extent of GC and the cycles of immunotherapy. Furthermore, through chi-square tests, we found that GC patients with higher PLPR-FAR score tend to have a greater number of metastatic sites and fewer cycles of ICIs treatment. Reports by Chen et al indicated that the number of metastatic sites significantly affects the survival time of patients with advanced GC.37 These findings further confirm that the PLPR–FAR scoring system is a potential new biomarker for determining the effectiveness of immunotherapy, suggesting that patients with GC with a PLPR–FAR score of 0 might be suitable candidates for ICIs treatment.
In previous studies, both the PLPR and FAR have been proven to be significantly associated with the prognosis of GC patients.38,39 However, no studies have explored their potential to predict immunotherapy outcomes in patients with GC. The PLPR consists of platelets, lymphocytes, and prealbumin, whereas the FAR consists of fibrinogen and albumin. The relationship between these hematological markers and cancer has drawn considerable attention. It has been reported that cancer activates the IL-6 synthesis pathway, increasing fibrinogen release and leading to elevated levels of fibrinogen in the blood.40 Fibrinogen is transformed into fibrin by thrombin in the coagulation process, which is vital for tumor progression and metastasis.41,42 The accumulation of fibrin/fibrinogen triggers fibrinolysis, causing degradation of the extracellular matrix, creating a supportive environment for tumor cell attachment, and stimulating mitos.43–45 Tumor cells induce platelet aggregation and thrombin formation, promoting fibrinogen to aggregate around tumor cells, forming a dense fibrin(ogen) layer, which helps tumor cells evade NK cell-mediated killing.46 As fundamental components of various immune-inflammatory markers, lymphocytes are highly important to cancer patients and are capable of inhibiting the migration of circulating tumor cells by secreting interferon-γ.47 Research indicates that lymphocytes play crucial roles in killing tumor cells by promoting cytotoxic cell death and mediating immune responses through cytokine production.48 Albumin and prealbumin, both synthesized by the liver, are blood indicators that can reflect the nutritional status of patients. Patients with lower levels of albumin or prealbumin tend to have shorter survival times.49,50 Additionally, cancer patients with reduced albumin levels have a higher risk of developing venous thromboembolism.51 This study combines these classic hematological indicators to construct the PLPR–FAR scoring system. Survival analysis revealed that patients with lower PLPR–FAR scores tended to have significantly longer PFS and OS (P<0.001). This combined scoring system not only reflects the tumor’s biological characteristics and the patient’s systemic inflammation status but also takes into account nutritional status and coagulation function, providing a multidimensional perspective for assessing the prognosis of stage IV GC.
Recently, nomogram have been widely used to predict the prognosis of patients with various cancers.52,53 In this study, we combined the results of multivariate Cox proportional hazards regression analysis to construct nomogram for PFS and OS of stage IV GC patients receiving ICIs treatment. ROC analysis results show that the AUC for predicting patients’ PFS and OS using nomogram were 0.705 (95% CI: 0.640–0.769) and 0.754 (95% CI: 0.692–0.815), respectively. Additionally, nomogram also have the functionality of visualizing the risk of each feature. For example, according to Figures 3A and 5A, we can clearly understand that the most relevant risk factor for patients’ OS is PLPR-FAR=2; the most relevant risk factor for patients’ PFS is total GC, followed by PLPR-FAR=2. Therefore, Total gastric cancer that combine multiple indicators are convenient and effective for assessing the immunotherapy outcome of GC patients and are worthy of clinical application.
Notably, this study has several limitations. First, this was a retrospective study, and all the subjects were from the same center, which inevitably increased the risk of selection bias. Second, our sample size was small, and although we found that the PLPR–FAR score might be a potential biomarker for predicting the immunotherapy outcome of patients with GC, large prospective randomized controlled studies are needed to address these limitations and verify whether our research results are accurate.
Conclusion
We developed a novel and effective prognostic score called the PLPR-FAR score, which is an independent risk factor for both PFS and OS of patients with stage IV GC receiving ICIs treatment and may help identify which patients are more likely to benefit from ICIs treatment. Furthermore, nomogram developed based on the PLPR-FAR score and traditional clinical features can assist clinicians in planning more suitable treatment schemes for GC patients.
Data Sharing Statement
The data underlying this article cannot be shared publicly due to privacy concerns regarding the individuals involved in the study. The data will be shared on reasonable request to the corresponding author.
Ethical Approval
This study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital and Heilongjiang Provincial Hospital (Ethics Approval No. 2019-185), and the research process was in accordance with the 1964 helsinki Declaration. All patients included in this study have signed written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
2. Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet. 2020;396:635–648. doi:10.1016/S0140-6736(20)31288-5
3. Xu J, Jiang H, Pan Y, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA. 2023;330:2064–2074. doi:10.1001/jama.2023.19918
4. Li Y, Wang JS, Guo Y, et al. Use of the alkaline phosphatase to prealbumin ratio as an independent predictive factor for the prognosis of gastric cancer. World J Gastroenterol. 2020;26:6963–6978. doi:10.3748/wjg.v26.i44.6963
5. Yang D, Hendifar A, Lenz C, et al. Survival of metastatic gastric cancer: significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011;2:77–84. doi:10.3978/j.issn.2078-6891.2010.025
6. Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS One. 2017;12:e0182692. doi:10.1371/journal.pone.0182692
7. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;390:2461–2471. doi:10.1016/S0140-6736(17)31827-5
8. Ren D, Hua Y, Yu B, et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer. 2020;19:19. doi:10.1186/s12943-020-1144-6
9. Pietrantonio F, Miceli R, Raimondi A, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37:3392–3400. doi:10.1200/JCO.19.01124
10. Lucarini A, Arrivi G, Liotta E, et al. Leptomeningeal carcinomatosis in early gastric cancer: a case report and literature review. Healthcare. 2024;12:1184. doi:10.3390/healthcare12121184
11. Bai R, Lv Z, Xu D, et al. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8:34. doi:10.1186/s40364-020-00209-0
12. Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–1458. doi:10.1038/s41591-018-0101-z
13. Shao C, Li G, Huang L, et al. Prevalence of high tumor mutational burden and association with survival in patients with less common solid tumors. JAMA Network Open. 2020;3:e2025109. doi:10.1001/jamanetworkopen.2020.25109
14. Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017. doi:10.1200/PO.17.00073
15. Moehler M, Dvorkin M, Boku N, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN Gastric 100. J Clin Oncol. 2021;39:966–977. doi:10.1200/JCO.20.00892
16. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi:10.1038/nature01322
17. Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33:e22964. doi:10.1002/jcla.22964
18. Bryant AK, Sankar K, Strohbehn GW, et al. Prognostic and predictive value of neutrophil-to-lymphocyte ratio with adjuvant immunotherapy in stage III non-small-cell lung cancer. Lung Cancer. 2022;163:35–41. doi:10.1016/j.lungcan.2021.11.021
19. Chen L, Zhao R, Sun H, et al. The prognostic value of gastric immune prognostic index in gastric cancer patients treated with PD-1/PD-L1 inhibitors. Front Pharmacol. 2022;13:833584. doi:10.3389/fphar.2022.833584
20. Wan M, Ding Y, Mao C, et al. Association of inflammatory markers with survival in patients with advanced gastric cancer treated with immune checkpoint inhibitors combined with chemotherapy as first line treatment. Front Oncol. 2022;12:1029960. doi:10.3389/fonc.2022.1029960
21. Mantzorou M, Koutelidakis A, Theocharis S, et al. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis? Nutr Cancer. 2017;69:1151–1176. doi:10.1080/01635581.2017.1367947
22. Loman BR, Luo M, Baggs GE, et al. Specialized High-Protein Oral Nutrition Supplement Improves Home Nutrient Intake of Malnourished Older Adults Without Decreasing Usual Food Intake. J Parenter Enteral Nutr. 2019;43:794–802. doi:10.1002/jpen.1467
23. Freire PP, Fernandez GJ, Cury SS, et al. The pathway to cancer cachexia: microRNA-regulated networks in muscle wasting based on integrative meta-analysis. Int J Mol Sci. 2019;20:1962. doi:10.3390/ijms20081962
24. Zhang L, Ma W, Qiu Z, et al. Prognostic nutritional index as a prognostic biomarker for gastrointestinal cancer patients treated with immune checkpoint inhibitors. Front Immunol. 2023;14:1219929. doi:10.3389/fimmu.2023.1219929
25. Nie RC, Chen GM, Wang Y, et al. Association between body mass index and survival outcomes in patients treated with immune checkpoint inhibitors: meta-analyses of individual patient data. J Immunother. 2021;44:371–375. doi:10.1097/CJI.0000000000000389
26. Zhang Q, Gong X, Sun L, et al. The predictive value of pretreatment lactate dehydrogenase and derived neutrophil-to-lymphocyte ratio in advanced non-small cell lung cancer patients treated with PD-1/PD-L1 inhibitors: a meta-analysis. Front Oncol. 2022;12:791496. doi:10.3389/fonc.2022.791496
27. Pan Y, Si H, Deng G, et al. A composite biomarker of derived neutrophil-lymphocyte ratio and platelet-lymphocyte ratio correlates with outcomes in advanced gastric cancer patients treated with anti-PD-1 antibodies. Front Oncol. 2021;11:798415. doi:10.3389/fonc.2021.798415
28. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi:10.1016/S0140-6736(21)00797-2
29. Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30:507–519. doi:10.1038/s41422-020-0337-2
30. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–668. doi:10.1038/s41577-020-0306-5
31. Tray N, Weber JS, Adams S. Predictive biomarkers for checkpoint immunotherapy: current status and challenges for clinical application. Cancer Immunol Res. 2018;6:1122–1128. doi:10.1158/2326-6066.CIR-18-0214
32. An HJ, Chon HJ, Kim C. Peripheral blood-based biomarkers for immune checkpoint inhibitors. Int J Mol Sci. 2021;22:9414. doi:10.3390/ijms22179414
33. McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2:46–54. doi:10.1001/jamaoncol.2015.3638
34. Sheng J, Fang W, Yu J, et al. Erratum: expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep. 2016;6:23850. doi:10.1038/srep23850
35. Liu C, Wang Y, Li S, et al. Early change in peripheral CD4(+) T cells associated with clinical outcomes of immunotherapy in gastrointestinal cancer. Immunotherapy. 2021;13:55–66. doi:10.2217/imt-2020-0068
36. Yuan J, Zhao X, Li Y, et al. The association between blood indexes and immune cell concentrations in the primary tumor microenvironment predicting survival of immunotherapy in gastric cancer. Cancers. 2022;14:3608. doi:10.3390/cancers14153608
37. Chen K, Deng X, Yang Z, et al. Sites of distant metastases and the cancer-specific survival of metastatic Siewert type II esophagogastric junction adenocarcinoma: a population-based study. Expert Rev Gastroenterol Hepatol. 2020;14:491–497. doi:10.1080/17474124.2020.1760839
38. Liu Y, Yang Y, Tai G, et al. Correlation between Preoperative Platelet Count/(Lymphocyte Count × Prealbumin Count) ratio and the prognosis of patients with gastric cancer undergoing radical operation. Gastroenterol Res Pract. 2023;2023:8401579. doi:10.1155/2023/8401579
39. Zhang L, Wang Z, Xiao J, et al. Prognostic value of fibrinogen-to-albumin ratio in patients with gastric cancer receiving first-line chemotherapy. Oncol Lett. 2020;20:10. doi:10.3892/ol.2020.11871
40. Yamaguchi T, Yamamoto Y, Yokota S, et al. Involvement of interleukin-6 in the elevation of plasma fibrinogen levels in lung cancer patients. Jpn J Clin Oncol. 1998;28:740–744. doi:10.1093/jjco/28.12.740
41. Zacharski LR, Meehan KR, Algarra SM, et al. Clinical trials with anticoagulant and antiplatelet therapies. Cancer Metastasis Rev. 1992;11:421–431. doi:10.1007/BF01307191
42. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi:10.1038/nature07205
43. Gerner C, Steinkellner W, Holzmann K, et al. Elevated plasma levels of crosslinked fibrinogen gamma-chain dimer indicate cancer-related fibrin deposition and fibrinolysis. Thromb Haemost. 2001;85:494–501. doi:10.1055/s-0037-1615611
44. Pöllänen J, Stephens RW, Vaheri A. Directed plasminogen activation at the surface of normal and malignant cells. Adv Cancer Res. 1991;57:273–328. doi:10.1016/s0065-230x(08)61002-7
45. Hatzfeld JA, Hatzfeld A, Maigne J. Fibrinogen and its fragment D stimulate proliferation of human hemopoietic cells in vitro. Proc Natl Acad Sci U S A. 1982;79:6280–6284. doi:10.1073/pnas.79.20.6280
46. Zheng S, Shen J, Jiao Y, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100:859–865. doi:10.1111/j.1349-7006.2009.01115.x
47. Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28:4045–4051. doi:10.1200/JCO.2010.27.9992
48. Zhang X, Zhao W, Chen X, et al. Combining the Fibrinogen-to-Pre-Albumin Ratio and Prognostic Nutritional Index (FPR-PNI) predicts the survival in elderly gastric cancer patients after gastrectomy. Onco Targets Ther. 2020;13:8845–8859. doi:10.2147/OTT.S264199
49. Yamashita K, Ushiku H, Katada N, et al. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol. 2015;41:1324–1332. doi:10.1016/j.ejso.2015.05.021
50. Zu H, Wang H, Li C, et al. Preoperative prealbumin levels on admission as an independent predictive factor in patients with gastric cancer. Medicine. 2020;99:e19196. doi:10.1097/MD.0000000000019196
51. Königsbrügge O, Posch F, Riedl J, et al. Association between decreased serum albumin with risk of venous thromboembolism and mortality in cancer patients. Oncologist. 2016;21:252–257. doi:10.1634/theoncologist.2015-0284
52. Ma Y, Shi L, Lu P, et al. Creation of a novel nomogram based on the direct bilirubin-to-indirect bilirubin ratio and lactate dehydrogenase levels in resectable colorectal cancer. Front Mol Biosci. 2021;8:751506. doi:10.3389/fmolb.2021.751506
53. Song J, Di Y, Kang X, et al. Development and validation of a nomogram to predict cancer-specific survival with unresected cholangiocarcinoma undergoing external radiotherapy. Front Public Health. 2023;11:1012069. doi:10.3389/fpubh.2023.1012069
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Development and Validation of an Age-Related Gastric Cancer-Specific Immune Index
Wang H, Yin X, Fang T, Lou S, Han B, Gao J, Wang Y, Zhang D, Wang X, Lu Z, Wu J, Zhang J, Wang Y, Zhang Y, Xue Y
Journal of Inflammation Research 2022, 15:6393-6407
Published Date: 23 November 2022
A Novel Neutrophil Extracellular Traps Signature for Overall Survival Prediction and Tumor Microenvironment Identification in Gastric Cancer
Qu Z, Han Y, Zhu Q, Ding W, Wang Y, Zhang Y, Wei W, Lei Y, Li M, Jiao Y, Gu K, Zhang Y
Journal of Inflammation Research 2023, 16:3419-3436
Published Date: 14 August 2023
Identification of Hub Genes Associated with Gastric Cancer via Bioinformatics Analysis and Validation Studies
Zhao T, Chen Z, Liu W, Ju H, Li F
International Journal of General Medicine 2023, 16:4835-4848
Published Date: 26 October 2023
Inhibition Effect of Pancreatic Exocrine Insufficiency on Immune Checkpoint Inhibitor Treatment in Pancreatic Cancer: A Retrospective Study
Luo Q, Dong Y, Liu P, He C, Chen L, Zhang K, Pan C, Gao Y, Qin T
ImmunoTargets and Therapy 2024, 13:45-54
Published Date: 1 February 2024
TCHH as a Novel Prognostic Biomarker for Patients with Gastric Cancer by Bioinformatics Analysis
Yu F, Zhao LX, Chu S
Clinical and Experimental Gastroenterology 2024, 17:61-74
Published Date: 26 February 2024




