Back to Journals » International Journal of Nanomedicine » Volume 20
Novel Modifications and Delivery Modes of Cyclic Dinucleotides for STING Activation in Cancer Treatment
Authors Lu Y, Li Z, Zhu X, Zeng Q , Liu S, Guan W
Received 30 October 2024
Accepted for publication 28 December 2024
Published 6 January 2025 Volume 2025:20 Pages 181—197
DOI https://doi.org/10.2147/IJN.S503780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. RDK Misra
Yanjun Lu,1,* Zhiyan Li,2,* Xudong Zhu,1 Qingwei Zeng,1 Song Liu,1 Wenxian Guan1
1Division of Gastric Surgery, Department of General Surgery, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, People’s Republic of China; 2Division of Thoracic Surgery, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Song Liu; Wenxian Guan, Email [email protected]; [email protected]
Abstract: The microenvironment tends to be immunosuppressive during tumor growth and proliferation. Immunotherapy has attracted much attention because of its ability to activate tumor-specific immune responses for tumor killing. The cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway is an innate immune pathway that activates antitumor immunity by producing type I interferons. Cyclic dinucleotides (CDNs), produced by cGAS sensing cytoplasmic abnormal DNA, are major intermediate activating molecules in the STING pathway. Nowadays, CDNs and their derivatives have widely worked as powerful STING agonists in tumor immunotherapy. However, their clinical translation is hindered by the negative electrical properties, sensitivity to hydrolytic enzymes, and systemic toxicity. Recently, various CDN delivery systems have made significant progress in addressing these issues, either through monotherapy or in combination with other treatment modalities. This review details recent advances in CDNs-based pharmaceutical development or delivery strategies for enriching CDNs at tumor sites and activating the STING pathway.
Keywords: cyclic dinucleotides, stimulator of interferon genes pathway, immunotherapy
Introduction
As one of the biggest dangers threatening human life and health safety, tumors cause nearly 10 million deaths each year.1 The treatment of tumors has always been faced with poor prognosis and unsatisfactory eradication.2,3 In recent years, the strength of immunotherapy has brought new hope for tumor treatment. This emerging treatment has unique advantages in that it precisely attacks tumor cells by the personal immune system, increasing patient tolerance and producing long-lasting anti-tumor effects.4 More than that, combining immunotherapy with other anti-tumor tactics may not only enhance therapeutic efficacy but also reduce tumor recurrence.5 These remarkable superiorities make immunotherapy one of the important development directions of cancer therapy.
The cyclic GMP-AMP synthase(cGAS)-stimulator of interferon genes (STING) pathway is an evolutionarily conserved immune pathway widespread in living organisms.6 After interacting with double-stranded DNA (dsDNA), cGAS catalyzes the formation of 2′, 3′-cyclic guanosine monophosphate (GMP) - adenosine monophosphate (AMP) (2′, 3′-cGAMP).7–9 The generated cGAMP subsequently activates the stimulator of interferon genes (STING) on the endoplasmic reticulum (ER).9,10 Activated STING induces downstream production of the type I interferon and strongly upregulates interferon-driven anti-tumor effects.11 Given that the STING pathway activation is an important link in the anti-tumor responses of multiple immune cell types, many studies have been devoted to exploiting the anti-tumor potential of the STING pathway.12,13
Cyclic dinucleotides (CDNs), as natural STING agonists, have thus entered the public eye.14,15 CDNs are cyclic structures consisting of two nucleotides linked by phosphodiester bonds, including cyclic diadenosine monophosphate (c-di-AMP, CDA), cyclic diguanosine monophosphate (c-di-GMP, CDG), and cyclic GMP-AMP (cGAMP).16,17 Given the natural STING agonistic function of CDNs, many STING agonists based on them come into being, which activate the STING pathway by mimicking the structures and functions of CDNs.18,19
Though showing distinct potency in vitro experiments, CDNs have certain limitations in activating the STING pathway in the clinic.20 First, CDNs suffer from degradation by nucleotide hydrolases because of their high sensitivity to phosphodiesterases (PDEs).21 Second, anionic phosphate groups enriched on CDNs make it difficult for them to penetrate through the negatively charged cell membrane surface to the cytoplasm. This prevents CDNs from effectively activating STING in the cytosol and exerting downstream anti-tumor immune functions.16 Besides, owing to their hydrophilicity, CDNs are highly prone to systemic dissemination and trigger extensive inflammatory cytokine responses, resulting in uncontrollable inflammatory damage.22 Consequently, exogenously dosed CDNs showed poor efficacy as well as serious toxic side effects in clinical trials.23,24 To resolve these issues, emerging drugs and delivery materials have been constructed to increase the accumulation of CDNs at tumor sites and enhance their STING activation abilities.
Here, we summarize the new progress in drug discovery or nano delivery policies centered on CDNs. The new opportunities for its combination with other treatment modalities are also described. With the gradual understanding of the mechanisms of CDNs in STING activation and cell-cell signal transduction,25 many novel medications, such as artificially synthesized CDN analogs, have been developed to enhance the STING activation efficacy by enhancing CDN retention in tumor microenvironment (TME).22,26 Meanwhile, with the rapid development of nano-assisted therapy technology, multiple nanomaterial systems have been used to reform the in vivo delivery of exogenous CDNs. Along with improving the delivery efficiency of CDNs, these nanomaterials reduced systemic toxicity by specifically targeting tumor tissues using their surface modifiers.27,28 Furthermore, certain special delivery systems, such as hydrogels, bacterial carriers, and metal materials have been widely explored to actualize CDNs-based STING activation. These novel modes compensate for the shortcomings of traditional direct administration of CDN drugs and inject new vitality into anti-tumor immunotherapy.
Mechanisms of CDNs-Based STING Agonists
Tumor cells are characterized by genomic instability, susceptibility to oxidative stress, and exuberant metabolism,29,30 which result in micronucleus formation and chromatin fragment leakage, leaving dsDNA naked in the cytoplasm.31,32 Moreover, nuclear and mitochondrial DNA damage will be induced by exogenous stimuli, such as chemotherapy or radiotherapy (RT).32,33 DsDNA activates cGAS to synthesize 2 “, 3” -cGAMP, one form of CDNs, which can be transferred and spread between cells. In diversified organisms, CDA and CDG were first found to directly target STING.34 With the in-depth study of the STING pathway, 2′, 3′ -cGAMP was confirmed to be the more effective molecule that targets STING activation in mammals.35 The endoplasmic reticulum (ER) - transmembrane receptor STING protein in tumor or immune cells binds to 2 “, 3” -cGAMP, oligomerizing and transporting to trans-Golgi vesicles.11 In the Golgi apparatus, STING integrates downstream TANK binding kinase 1 (TBK1) through its C-terminal tail (CTT). TBK1 undergoes conformational changes upon binding to STING, triggering its autophosphorylation and the phosphorylation of STING. Phosphorylated STING unites interferon regulatory factor 3 (IRF3), making it close enough to be phosphorylated by TBK1.36 Then phosphorylated IRF3 forms a dimer and translocates to the nucleus and exerts its transcriptional function, expressing immunostimulatory genes (ISGs) and type 1 interferons (IFNs), including IFN-β.37,38 IFN-β promotes the recruitment and activation of tumor-associated inflammatory cells, as well as the activation and maturation of dendritic cells (DCs). Furthermore, it facilitates the cross-activation of CD8+ T cells, infiltrating and killing primary and metastatic tumors, and forming immune memory (Figure 1).39,40
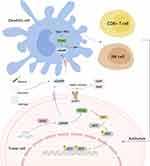 |
Figure 1 Schematic representation of CDNs-mediated STING activation. |
Since being reported, the STING pathway has rapidly become a hotspot for research, followed by the appearance of numerous drugs and material systems for STING activation. Many drugs targeting the accumulated endogenous CDNs have been studied for elevating the 2′, 3′ -cGAMP distribution in TME to decline their hydrolysis by extracellular hydrolases.22,41 Simultaneously, various strategies for CDN delivery are being explored, including polymer particles,42,43 liposomes,44,45 hydrogels,46,47 and engineered bacteria.48,49 Given its inherent characteristics, nanotechnology effectively achieves synergistic treatment of STING pathway activation with other therapies. For example, the combination of immune checkpoint blockade (ICB),50 photothermal therapy (PTT),51 photodynamic therapy (PDT),52 chemodynamic therapy (CDT),53 etc. further enhances its anti-tumor efficacy.
In summary, the agents that activate the cGAS-STING pathway have flourished in the field (Table 1).
 |
Table 1 Cyclic Dinucleotide-Mediated STING Activation |
CDNs and Their Analogues
Many studies have demonstrated that cGAMP will directly activate the STING pathway after transferring to immune cells.37 CDNs have turned into universal STING agonists nowadays.91,92 However, the negative charge and high susceptibility to hydrolysis by extracellular nucleases make CDNs not meet the requirements for becoming a drug molecule.93,94 With the intention of creating STING-targeting medications with enhanced pharmacokinetics and efficacy, kinds of cGAMP analogs have emerged.95
Loads of CDN analogues and their modified derivatives have been synthesized, which demonstrate higher drug activity, tolerance to hydrolytic enzymes, and cell penetration rate. Since CDNs are formed by two nucleotides connected by two phosphodiester bonds, the main modification sites of CDNs are located in the base, ribose, and phosphate backbone.91
Some earlier CDNs are now entering clinical trials. Kim et al reported a macrocycle-bridged STING agonist (MBSA),96 which locked the bioactive u-shaped conformation of the CDNs by injecting a trans cyclic macrocyclic bridge between nucleic acid bases. MBSA exhibited extensive pan-genotypic activity in major human STING variants. E7766, possessing this conformation, has entered clinical trials. During this trial, E7766 exhibited antitumor activity and immune memory response in the bacillus Calmette-Guerin (BCG) unresponsive non-muscle-invasive bladder cancer (NMIBC) model (Figure 2).54 Phosphorothioate modification is the most classical one among the phosphoric acid backbone of oligonucleotides,97 which improves the immunoreactive activity and stability against nucleases degrading CDNs.98 More importantly, phosphorothioate analogs can increase the hydrophobicity of CDNs, making them more easily to be taken up.99 Many drugs approved for clinical trials, such as ADU-S100,55 MK-1454,56,100 BI-1387446,59 are modified in this way. In a Phase I dose-escalation trial, Meric-Bernstam et al reported that ADU-S100 caused significant increases in inflammatory cytokine levels and peripheral blood T cell counts in patients with advanced or metastatic solid tumors or lymphomas, suggesting systemic immune activation. Also, 94% of evaluable injected lesions were stable or reduced in size. However, there existed a certain proportion of treatment-related adverse events, such as pyrexia (17%), chills (15%), and injection site pain (15%).55
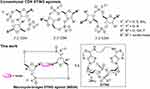 |
Figure 2 Structures of conventional CDN STING agonists and macrocycle-bridged STING agonist (MBSA). Reproduced with permission from Kim DS, Endo A, Fang FG et al. E7766, a Macrocycle-Bridged Stimulator of Interferon Genes (STING) Agonist with Potent Pan-Genotypic Activity. ChemMedChem. 2021;16(11):1741–1744.96 © 2021 Wiley-VCH GmbH. |
Monotherapy with CDNs and their derivatives has mostly been upgraded and updated because of their insignificant clinical efficacy and non-negligible side effects.55,101 In the bargain, combining with other treatment modalities or being the payload agents for novel delivery systems provides new ideas for CDN drugs to activate the STING pathway and related cancer immunotherapy.102,103
Emerging Patterns for CDN Delivery
In recent years, diversified material systems have been explored and utilized to optimize drug delivery efficiency and healing efficacy. Apart from enhancing the targeting potency to reduce medical toxicity, these delivery systems prolonged the half-life of drugs by increasing their clearance resistance.104,105 Besides, these new modes assisted in the combination of CDNs-mediated STING activation with various immunotherapy strategies for tumor killing.106
Polymeric Microparticle
Polymer particles are widely used in nanomedicine synthesis as a classical nano-delivery material.107,108 Polymer nanoparticles (PNPs) are typically constructed through spontaneous complex self-assembly employing biocompatible and biodegradable polymers such as pectin, chitosan, cyclodextrin, etc.109,110 In this process, therapeutic drugs are encapsulated in the core of PNPs. To achieve higher targeting and increase uptake efficiency, PNPs selectively interact with certain cells or tissues via surface functional groups modified by specific proteins, peptides, monoclonal antibodies, etc.111,112 Some PNPs can even achieve lysosomal escape during the process of intracellular acidification maturation, diminishing the destruction of loaded pharmaceuticals.110 Considering the characteristics aforementioned, polymer particles are currently being actively developed for the delivery of CDNs.45
Polymer particles can increase tumor-site retention and fulfill intracellular delivery of unmodified native CDNs, such as CDG and cGAMP.64,67,69 Recently, Xu et al reported an amphiphilic supermolecular drug-drug conjugate (ASDD) (Figure 3). Through hydrogen bonds and hydrophobic interactions, ASDD used nucleotide lipid ligand hydrophobic 3′, 5′ -dioleic acid-deoxycytidine (3′, 5′ -diOA-dC), which was synthesized by oleic acid and deoxycytidine, to assemble with hydrophilic CDG into uniformly stable supramolecular CDG-nanoparticles (CDG-nps).19 CDG-nps were proved to enhance CDG uptake by APC cells at the tumor site, promoting STING activation and immune activation in TME. Their another decomposition product, 3′, 5′ – dioA-dC, was degraded by esterases into oleic acid and deoxycytidine, arising with no negative impact on the organism.
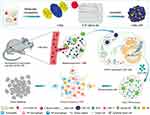 |
Figure 3 Formation of Supramolecular Cyclic Dinucleotide Nanoparticle Delivery System CDG-NPs and Their Application in STING-Mediated Cancer Immunotherapy. Reproduced with permission from Xu L, Deng H, Wu L et al. Supramolecular Cyclic Dinucleotide Nanoparticles for STING-Mediated Cancer Immunotherapy. ACS Nano. 2023;17(11):10,090–10,103.19 Copyright 2023, American Chemical Society. |
Interestingly, CDNs can undergo covalent modifications to alter their pharmacological properties. After polymerizing with other substances and forming polymer particles, their anti-tumor effect may be directly strengthened.65 Dosta et al designed a highly potent poly-drug-conjugated (β-amino ester) (pBAE) nanoparticle formulation (CDN-NP). They employed maleimide-modified ML-317 conjugate with pBAE to form ML-317-linker-pBAE and acrylate-terminated pBAEs polymer mix with arginine oligopeptide (C6-CR3) to form cationic pBAEs. The CDN-NP was born by means of electrostatic complexation between CDNs and C6-CR3, shaping an intravenous STING agonist.66 CDN-NPs internalized by tumor cells were released over time and taken up by immune cells in TME and secondary lymphoid organs. Whereupon CDNs would be cleaved by cathepsins in the cytoplasm and released from CDN-NPs, thereby inducing STING activation for tumor growth inhibition. In multiple syngeneic mouse tumor models, low-dose CDN-NPs combined with immune checkpoint blockade (ICB) treatment induced robust tumor killing and immune memory.
In addition to delivering CDNs agents alone, the polymer system easily achieves the combination of CDNs with other medications.70,74 For example, Wang et al established a combined anti-tumor pattern by integrating CDA and Mn2+ into tannic acid nanostructure (TMA-NPs).73 Magnetic resonance imaging (MRI) displayed that under X-ray irradiation, TMA NPs significantly alleviated hypoxia caused by the oxygenation of Mn2+ in the large tumors, bringing about excessive production of ROS and DNA damage. Accordingly, CDNs released from TMA-NPs, Mn2+ released from phenolic carriers, and DNA fragments released from cell debris worked together to amplify STING activation. The combination of TMA-NPs and RT activated the cascaded STING pathway and fulfilled a remarkable radioimmune therapeutic effect on primary and distant tumors, paving the way for the clinical application of Mn2+-CDN nano-modulators in multimodal tumor treatment.
Lipid Nanoparticles
Nanoliposomes are spherical vesicles composed of one or more phospholipid bilayers. The hydrophilic head of phospholipid molecules is inserted into water, and the hydrophobic tails aggregate with each other to form spherical liposomes with bilayer lipid molecules.113,114 At this point, hydrophobic drugs are encapsulated in lipophilic bilayer shells while hydrophilic drugs are enclosed in the aqueous interior region of liposomes. After administration, liposomes fuse with biofilms by their strong affinity, thereby releasing the loaded active ingredients into the cells.115 Liposomes therefore protect the active drug from degradation in body fluids and overcome barriers to cellular and tissue absorption.116,117 In the bargain, via modifying with different ligands or combining with diverse delivery platforms, lipid nanoparticles upgrade drug targeting and diminish toxic side effects on nontarget organs.118
Cationic nanoliposomes have been widely investigated for CDN delivery.119 Encapsulating CDNs in the aqueous phase of the nanoliposome core is the most classical mode of encapsulation. Koshy et al encapsulated cGAMP in cationic liposomes at different polyethylene glycol (PEG) levels to assess their enzymatic resistance, STING activation, and systemic immune activation ability.75 In both orthotopic melanoma models and invasive lung metastases, pegylated cGAMP liposomes showed definite therapeutic efficacy. By adjusting the surface structure of liposomes, many liposome material models strengthened their stability in body fluids and made for stronger STING activation. For example, Doshi et al prepared a CD103+ DC-targeted cGAMP liposome using a Clec9a-targeting peptide called WH peptide to selectively deliver cGAMP into DCs. Clec9a/DNGR-1 is a type C lectin receptor that highly expressed on CD8+ and CD103+ DCs yet not on any other hematopoietic cells. This resulted in far more encapsulated cGAMP being ingested by DC, lowering fluid clearance and systemic side effects. In MC38 and B16F10 tumor-bearing mouse models, only 0.1 mg/kg intravenous injection of the cGAMP liposomes would induce a strong antitumor immune effect.82
Due to the toxicity issues and insufficient in vivo efficacy of cationic lipids, other forms of lipid nanocarrier systems are being developed vigorously.120,121 For instance, pH-responsive ionizable lipids are neutral at physiological pH, and positively charged only in the acidic environment of the endosome. This feature largely ameliorated the shortcomings of cationic lipids in terms of potency and toxicity.122 Nakamura et al designed a PEGylated CDG ionizable lipid STING-LNPs based on YSK12-C4.44 YSK12-C4 is an ionizable cationic lipid (CLD) with a high affinity for immune cells, which can effectively deliver core drugs into the cytoplasm of cells. In the B16-F10 lung metastasis model, STING-LNPs activated NK cells, transforming the tumor immuno-cold state into the immuno-hot state for innate immune responses. In addition, it has been shown that the shape and property of nanocarriers significantly affect their abilities to be transported in vivo and assimilated by target cells. Non-spherical nanoparticles displayed longer blood circulation half-life and higher cellular internalization efficiency than spherical ones.123 Consequently, Dane et al designed a lipid nanodisc formed by self-assembly of mercapto PEGylated lipids and high melting temperature phospholipids, containing CDN prodrug with a diamine peptide linker (Figure 4a and b).76 They conducted coarse-grained molecular dynamics simulations on the LND model (diameter 40 nm). When passing through a rigid pore (diameter 20 nm) under moderate tension of approximately 330 pN (200 kJ mol−1 nm−1), LND could deform and enter pores smaller than its equilibrium diameter, while traditional liposomes with similar lipid composition and the same diameter could not deform enough to enter the pore. This result indicated that an elliptical flexible disk may diffuse faster than a hard object of the same size. What’s more, it exhibited the same trend in the absence of external forces or airflow interference. In a 24h biodistribution model in MC38 tumor-bearing mice, LND-CDNs showed higher tumor accumulation (7.4%: 1.1%) and lower absorption in other tissues (less than 0.5%) than CDN-encapsulated liposomal formulations (LipoCDN).
 |
Figure 4 Design and characterization of nanoparticles for STING agonist delivery. (a), Chemical structures of the parent CDN STING agonist (1), CDN prodrug (2), diacyl lipid (3) and CDN-PEG-lipid (4). (b), Schematic of LND containing CDN-PEG-lipid. Reproduced with permission from Dane EL, Belessiotis-Richards A, Backlund C et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat Mater. 2022;21(6):710–720.76 © The Author(s) 2022. Creative Commons Attribution 4.0 International License. |
The application of lipid nanocarriers is a canonical pathway for achieving the conjunction of STING activation with other therapeutic approaches. Classical cancer treatment modalities, including chemotherapy or radiotherapy, have been certificated to produce tumor-specific antigens, which are considered the tumor orthotopic vaccine.124,125 Chen et al designed a lipid nanoparticle (LNP) to simultaneously reach cross-presentation of antigens and activation of the STING pathway. Firstly, immune genetic death and tumor-associated antigens (TAAs) release were induced by intratumoral injection of low doses of doxorubicin (DOX). Subsequently, lipid nanoparticles LNP/cGAMP captured TAAs in situ through electrostatic interactions, promoting antigen presentation of APCs. Then, cGAMP released in the cytoplasm activated the STING pathway, which led to type I IFN generation and T cell activation, thereby producing a strong anti-tumor immune response and forming immune memory in the B16F10 tumor model.77
The combination of lipid nano delivery systems with other delivery modalities is also being developed by researchers for CDN delivery. Lipid polymer hybrid nanoparticles (LPHNPs) are advanced core-shell nanostructures composed of liposomes and polymer nano units. Its polymer core region is ordinarily surrounded by a lipid layer. Because of their dual structural characteristics, LPHNPs have the advantages of high stability, high load capacity, high biocompatibility, rate-limiting controlled release, long half-life, and superior therapeutic effect.126 Yang et al reported a hybrid delivery system of lipid polymers. In this system, bacteria-derived CDA was encapsulated into nanoscale coordination polymers (NCP) consisting of a non-toxic zinc phosphate hydrophilic core and PEG-conjugated phospholipids (ZnP). Compared with the half-life of CDA encapsulated liposomes (3.30 hours), ZnCDA-NCP greatly reduced CDA degradation in serum, prolonging its circulation half-life to 12.63 hours in vivo. The increasing half-life naturally led to a significant slowdown in the growth rate of the tumor model.80
Hydrogel
The majority of current STING agonists in clinical trials are administered intratumorally.127 However, drug leakage resulting from intratumoral injection easily reduces curative efficacy and brings about serious toxic damage to surrounding tissues.128 Therefore, controlled-release delivery systems, such as hydrogels formed in situ, become the potential CDN delivery platform.129,130 Hydrogel is a polymer system with excellent biocompatibility and biodegradability.131 Its three-dimensional network structure is composed of one or more hydrophilic polymers, containing abundant water. Besides, there is evidence that hydrogel formulations are mostly prepared under mild conditions, which is more conducive to maintaining the activity of CDNs than that of nano formulations.132
Shortly after cGAMP was proved to have the capacity of STING activation, Lee et al designed a cationic linear polyethyleneimine (LPEI)/hyaluronic acid (HA) hydrogel (LH gel) to load cGAMP which was specifically delivered CDNs to phagocytic macrophages.84 Compared with traditional cationic liposomes, LH/cGAMP gel prompted a prominent 2.5-fold increase in the amount of IFN-β released by immune cells. Recently, Wang et al exploited a supramolecular hydrogel system, which chemically coupled the hydrophilic peptide fragment iRGD with the hydrophobic anticancer medicine camptothecin (CPT) to form a peptide-drug conjugate (diCPT-iRGD).46 In water, self-assembled DiCPT iRGD shaped supramolecular nanotubes (NTs) with positive surface changes, loading negatively charged CDA. After local injection, the CDA-NT solution shaped hydrogel instantly as a repository for internal expansion and discharge of CDA and CPT. In C57BL/6 tumor-bearing mice, the CDA loaded by the hydrogel system represented a sustainable secular liberation, which could still be detected 35 days after in vivo injection.
Considering that hydrogel systems are amenable to local injection, surgery has opened up a unique channel for hydrogel delivery systems. The application of medicine-carried hydrogel to postoperative tumor resection margins has become a reasonable means to induce tumor immunity and prevent local tumor recurrence.86,133 Park et al pioneered the attempt to encapsulate cGAMP by a conventional hyaluronic acid hydrogel 3D scaffold, which was utilized at the tumor resection site after surgery.85 Experiments indicated that hydrogel-encapsulated STING activators had a slower diffusion rate and longer-lasting liberation in vivo, contributing to eliminating residual tumor cells in the immunosuppressive microenvironment. Apart from regulating medicine release through grid size, some stress-responsive hydrogels can even actively respond to changes in light, heat, magnetic field, or pH value to obtain conditional drug release.134 Lately, Fang et al designed a pH-responsive hydrogel system for in situ drug delivery in postoperative tumors.83 The hydrogel system Gel@M/CuO2/DOX/STING was formed by self-assembly of polysulfoxide betaine methacrylate (PSBMA) in saline, encapsulating 2′, 3′ -cGAMP and M/CuO2/DOX nanoparticles (doxorubicin(DOX)-loaded copper peroxide nanoparticles encapsulated by macrophage membrane). After the hydrogel was injected at the tumor site after breast cancer resection, the body fluid pH in TME drove hydrogel backbone ion interaction to degrade, loosing cGAMP and M/CuO2/DOX. Following closely behind were DNA damage induced by DOX, ROS generated by Cu2+-catalyzed Fenton reaction, and activation of the STING pathway, accurately removing residual tumor cells and preventing postoperative tumor recurrence. In addition to the combination with traditional chemotherapy as well as chemodynamic therapy described above, hydrogel delivery systems are also commonly used to combine with other antitumor therapies. For instance, Wang et al combined multiple modalities of antitumor immunotherapy to synthesize a peptide-based supramolecular filament (SF) hydrogel, which was made up of three separated immunomodulators, including α-PD-1 antibody, il-15 cytokine, and CDA (Figure 5a).87 Under the action of matrix metalloproteinase-2 (mmp-2) in tumor tissue, the hydrogel continuously discharged these pharmacological components, which played a synergistic role (Figure 5b).
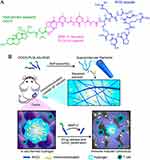 |
Figure 5 Schematic illustration of the designed and studied immunotherapeutic supramolecular filament (SF) hydrogels. (A), Chemical structure of the designed DOCA-PLGLAG-iRGD peptide amphiphile (PA). (B), Schematic illustration of localized immunomodulator delivery using a DOCA-PLGLAG-iRGD supramolecular hydrogel for MMP-2-responsive drug release and tumor microenvironment regulation. Reproduced with permission from Wang F, Su H, Wang Z et al. Supramolecular Filament Hydrogel as a Universal Immunomodulator Carrier for Immunotherapy Combinations. ACS Nano. 2023;17(11):10,651–10,664.87 Copyright 2023, American Chemical Society. |
Other Delivery Methods
Metallic Carriers
Metallic materials, such as gold nanoparticles (AuNPs), have been extensively investigated to exploit their biomedical applications, including pharmaceutical delivery, photoacoustic imaging, and photothermal therapy.135 AuNPs have good biocompatibility and efficient targeting towards tumor tissues in the systemic circulation.136 Surface modification of gold nanoparticles easily assist them in conjugating with other drugs or proteins, making them an ideal carrier for CDNs delivery.137 Zhao et al prepared a polyethyleneimine-modified gold nanorod (GNR-PEI), in which positively charged GNR-PEI can complex with cGAMP through electrostatic interactions, forming a GNR-PEI/cGAMP complex.88 GNR-PEI/cGAMP triggered tumor ablation and in situ TAA generation under near-infrared light irradiation, recruiting antigen-presenting cells at the tumor site. Thereafter, STING activation induced by cGAMP stimulated the proliferation and activation of DCs, sensitizing the anti-tumor immune effect produced by tumor vaccines.
Biomimetic Membranes
The low immunogenicity and tumor-targeting properties of biomimetic membranes make them safe, efficient, precise, and controllable drug delivery platforms.138,139 Rao et al reported a hybrid biofilm nanovesicle (hNV) for the delivery of cGAMP to prevent tumor recurrence and metastasis after surgery.89 The hNV was composed of platelet-derived NVs (P-NVs), M1 macrophage-derived NVs (M1-NVs), and tumor cell-derived NVs overexpressing high-affinity SIRPα variants (SαV-C-NVs) (Figure 6a). Platelet-derived NVs (P-NVs) made hNV effectively accumulated at the surgical wound site by binding to damaged blood vessels and tissues, enriching cGAMP at the postoperative tumor site. Meanwhile, M1 macrophage derived NVs (M1 NVs) repolarized TAM into an M1-like phenotype(Figure 6b). The overexpression of the SIRPα variant on the HNV surface greatly increased its affinity for CD47, competitively blocking the CD47-SIRP signaling axis of tumor cells. Through their synergistic effect, hNV reprogrammed the “cold” tumor to an immunogenic state, prominently inhibiting postoperative tumor recurrence and metastasis.
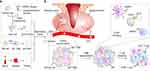 |
Figure 6 Schematic and characterization of hNVs. a, Schematic showing the hNVs consist of engineered SαV-C-NVs, M1-NVs, and P-NVs. b, Schematic showing the hNVs efficiently interact with CTCs in the blood, accumulate in the post-surgical tumor bed, repolarize TAMs towards M1 phenotype, and block the CD47-SIRPα “don’t eat me” pathway, thus promoting macrophage phagocytosis of cancer cells, as well as boosting antitumor Tcell immunity. Reproduced with permission from Rao L, Wu L, Liu Z et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun. 2020;11:4909.89 Copyright © 2020, This is a US government work and not under copyright protection in the US; foreign copyright protection may applyCreative Commons CC BY license. |
Bacteria
Bacteria, as a particular delivery carrier, have attracted great attention because of their unique tumor-targeting properties and active phagocytosis by various immune cells.140,141 Due to the fact that CDNs can be produced by invading bacterial cGAS, engineered bacteria with genetically modified characteristics are ideal carriers for STING agonism.142 Leventhal et al designed an EcN strain SYNB1891 that expressed CDA at the tumor site. SYNB1891 used the hypoxia promoter pfnrs loop with dacA of Listeria monocytogenes to constitute the PfnrS-dacA circuit, initiating CDA expression in the anaerobic environment of tumor tissue.48 SYNB1891 dramatically enhanced the expression of IFN-β1 and decreased tumor growth in B16/F10 tumor-bearing mice model. Apart from bacterial vectors, virus-like nanoparticles, such as cGAMP-VLPs formed from cGAMP coated with HIV-1 structural protein and vesicular stomatitis virus glycoprotein (VSV-G) also exhibited significant STING-based anti-tumor T cell responses.90
Conclusion
The immune reversal capability induced by the STING pathway activation in tumor cells or APCs has made STING agonists a boom in tumor treatment.142 Moreover, conclusive clues demonstrated that anti-tumor immune responses mediated by STING activation play important roles in conventional therapies. Therefore, STING agonists have rapidly evolved today as a potential complementary cure to other treatments.143 Whereas CDNs are the natural upstream STING activating signaling molecules in organisms, an increasing number of novel molecules and delivery systems targeting enriching CDNs at tumor sites are being developed in laboratories and explored in clinical trials.144 Unfortunately, direct systemic administrations of CDNs tended to show severe systemic toxicity. Their therapeutic efficacy failed to meet expectations, which remains to be further explored.145,146
Emerging CDNs-based STING activation strategies have upgraded their targeted efficacy through accurate medicinal action, avoiding harmful side effects. They largely address the shortcomings of direct systemic administration of STING agonists, making STING-mediated anti-tumor immune activation more precise and bringing less impact on other organs.147 Simultaneously, combining with STING activation, RT therapy, chemotherapy, immune checkpoint inhibitors, and metal immunotherapy tend to display superior therapeutic effects.148,149 In this review, we summarize the momentous advances in CDNs-based STING activation, including exploiting new CDN analogues and their derivatives, as well as developing multimodal novel CDN delivery systems.
However, heaps of CDN-targeted STING pathway activation strategies are still in their infancy. Before entering clinical applications, there are still many potential challenges. Firstly, there has been evidence that mouse STING (mSTING) and human STING (hSTING) do not respond consistently to various STING agonists. Regretfully, under the same degree of stimulation, mSTING reacts much more strongly to produce IFN-β or other corresponding cytokines than hSTING. Accordingly, a variety of therapeutic tactics with considerable efficacy in animal experiments may not necessarily be directly extrapolated to clinical patients.150,151 Secondly, it has not been confirmed whether the specific staging of cancer patients (such as whether metastasis occurs) will affect the overall anti-cancer efficacy of STING agonists. Research into this issue may contribute to developing accurate clinical medication guidelines for STING agonists. Additionally, in recent years, many newly developed pharmaceuticals or delivery systems have complex synthesis processes and high heterogeneity, whose cost is extremely huge. It makes it difficult for them to achieve mass production, let alone clinical translation.152
Furthermore, there is much to be investigated regarding the STING pathway. In addition to the anti-tumor immune effects associated with type I IFNs, STING activation has been associated with glucose metabolism,153,154 protein metabolism,155 lipid metabolism,156 and specific apoptosis.157 Nevertheless, a majority of these theories have just been proposed recently and there is still a lack of in-depth understanding of their mechanisms. With the clarification of the framework of STING-related substance metabolism, new metabolic therapeutic agents or regimens will be discovered to optimally combine with STING agonists, promoting anti-tumor activity and minimizing toxicity. It can be foreseen that more pharmaceuticals and delivery patterns will be exploited to facilitate STING activation-dependent tumor treatments in the future.
Abbreviations
cGAS-STING, cyclic GMP-AMP synthase-stimulator of interferon genes; IFNs, interferons; CDNs, Cyclic dinucleotides; 2′, 3′-cGAMP, 2′, 3′-cyclic GMP-AMP; dsDNA, double-stranded DNA; ATP, adenosine triphosphate; GTP, guanosine triphosphate; ER, endoplasmic reticulum; TBK1, TANK binding kinase 1; IRF3, interferon regulatory factor 3; ISGs, immunostimulatory genes; GMP, guanosine monophosphate; AMP, adenosine monophosphate; PDEs, phosphodiesterases; ENPP1, exonucleotide pyrophosphatase/phosphodiesterase 1; EMT, epithelial-mesenchymal transition; QD, quaque die; DSPM, NaGdF4, Nd@NaLuF4@PEG-polyphenol/Mn; MBSA, macrocycle-bridged STING agonist; BCG, bacillus Calmette-Guerin; NMIBC, non-muscle-invasive bladder cancer; PNPs, Polymer nanoparticles; ASDD, amphiphilic supermolecular drug- drug conjugate; 3 ʹ, 5ʹ -diOA-dC, 3 ʹ, 5ʹ -dioleic acid-deoxycytidine; CDG-nps, CDG-nanoparticles; ICB, immune checkpoint blockade; RT, Radiotherapy; PEG, polyethylene glycol; APCs, antigen presenting cells; LPHNPs, Lipid polymer hybrid nanoparticles; CDA, cyclic diadenosine monophosphate (c-di-AMP); CDG, cyclic diguanosine monophosphate (c-di-GMP); NCP, nanoscale coordination polymers; LipoCDNs, CDNs encapsulated liposomal formulations; LipoCDA, CDA encapsulated liposomal formulations; CPT, camptothecin; SF, supramolecular filament; PEI, Polyethylenimine; hNV, hybrid biofilm nanovesicle; Tregs, tumor regulatory T cells; TME, tumor microenvironment; MRI, Magnetic resonance imaging; CLD, cationic lipid; TAAs, tumor-associated antigens; NTs, nanotubes; mmp-2, matrix metalloproteinase-2; AuNPs, gold nanoparticles.
Data Sharing Statement
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.
Funding
This work was supported by the National Natural Science Foundation of China (82172645, 82372805 to W.G.; 82403433 to X.Z.), Key Research and Development Program of Jiangsu Province (BE2022667, BE2022753 to W.G.), Key Project of Nanjing Health Commission (ZKX21013 to W.G.; ZKX24013 to S.L.), Nanjing Drum Tower Hospital Cultivation Program for Outstanding Youth Science Fund of National Natural Science Foundation of China (2023-JCYJ-YP-02 to S.L.).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Debela DT, Muzazu SG, Heraro KD, et al. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med. 2021;9:20503121211034366. doi:10.1177/20503121211034366
3. Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med. 2021;27(1):34–44. doi:10.1038/s41591-020-01195-4
4. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi:10.1038/s41423-020-0488-6
5. Xie Y, Xie F, Zhang L, et al. Targeted anti-tumor immunotherapy using tumor infiltrating cells. Adv Sci. 2021;8(22):e2101672. doi:10.1002/advs.202101672
6. Margolis SR, Wilson SC, Vance RE. Evolutionary origins of cGAS-STING signaling. Trends Immunol. 2017;38(10):733–743. doi:10.1016/j.it.2017.03.004
7. Civril F, Deimling T, de Oliveira Mann CC, et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498(7454):332–337. doi:10.1038/nature12305
8. Ablasser A, Goldeck M, Cavlar T, et al. cGAS produces a 2’-5’-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498(7454):380–384. doi:10.1038/nature12306
9. Sun L, Wu J, Du F, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type-I interferon pathway. Science. 2013;339(6121):1126. doi:10.1126/science.1232458
10. Zhang X, Shi H, Wu J, et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51(2):226–235. doi:10.1016/j.molcel.2013.05.022
11. Liu S, Cai X, Wu J, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347(6227):aaa2630. doi:10.1126/science.aaa2630
12. Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 2020;10(1):26–39. doi:10.1158/2159-8290.CD-19-0761
13. Knelson EH, Ivanova EV, Tarannum M, et al. Activation of tumor-cell STING primes NK-cell therapy. Cancer Immunol Res. 2022;10(8):947–961. doi:10.1158/2326-6066.CIR-22-0017
14. Danilchanka O, Mekalanos JJ. Cyclic dinucleotides and the innate immune response. Cell. 2013;154(5):962–970. doi:10.1016/j.cell.2013.08.014
15. Aline Dias da P, Nathalia Marins de A, Gabriel Guarany de A, et al. The world of cyclic dinucleotides in bacterial behavior. Molecules. 2020;25(10):2462. doi:10.3390/molecules25102462
16. Yan H, Chen W. The promise and challenges of cyclic dinucleotides as molecular adjuvants for vaccine development. Vaccines. 2021;9(8):917. doi:10.3390/vaccines9080917
17. Karaolis DKR, Cheng K, Lipsky M, et al. 3′,5′-cyclic diguanylic acid (c-di-GMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochem Biophys Res Commun. 2005;329(1):40–45. doi:10.1016/j.bbrc.2005.01.093
18. Amouzegar A, Chelvanambi M, Filderman JN, et al. STING agonists as cancer therapeutics. Cancers. 2021;13(11):2695. doi:10.3390/cancers13112695
19. Xu L, Deng H, Wu L, et al. Supramolecular cyclic dinucleotide nanoparticles for STING-mediated cancer immunotherapy. ACS Nano. 2023;17(11):10090–10103. doi:10.1021/acsnano.2c12685
20. Wang Y, Luo J, Alu A, et al. cGAS-STING pathway in cancer biotherapy. Mol Cancer. 2020;19(1):136. doi:10.1186/s12943-020-01247-w
21. Kato K, Nishimasu H, Oikawa D, et al. Structural insights into cGAMP degradation by Ecto-nucleotide pyrophosphatase phosphodiesterase 1. Nat Commun. 2018;9(1):4424. doi:10.1038/s41467-018-06922-7
22. Sun X, Zhang Y, Li J, et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat Nanotechnol. 2021;16(11):1260–1270. doi:10.1038/s41565-021-00962-9
23. Marloye M, Lawler SE, Berger G. Current patent and clinical status of stimulator of interferon genes (STING) agonists for cancer immunotherapy. Pharm Pat Anal. 2019;8(4):87–90. doi:10.4155/ppa-2019-0013
24. Le Naour J, Zitvogel L, Galluzzi L, et al. Trial watch: STING agonists in cancer therapy. Oncoimmunology. 2020;9(1):1777624. doi:10.1080/2162402X.2020.1777624
25. Xie W, Patel DJ. Structure-based mechanisms of 2′3′-cGAMP intercellular transport in the cGAS-STING immune pathway. Trends Immunol. 2023;44(6):450–467. doi:10.1016/j.it.2023.04.006
26. Cho Y, Kang M, Ji SH, et al. Discovery of Orally Bioavailable Phthalazinone Analogues as an ENPP1 Inhibitor for STING-Mediated Cancer Immunotherapy. J Med Chem. 2023;66(22):15141–15170. doi:10.1021/acs.jmedchem.3c01061
27. Fan D, Cao Y, Cao M, et al. Nanomedicine in cancer therapy. Signal Transduct Target Ther. 2023;8(1):293. doi:10.1038/s41392-023-01536-y
28. Riley RS, June CH, Langer R, et al. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196. doi:10.1038/s41573-018-0006-z
29. Parkes EE, Walker SM, Taggart LE, et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst. 2017;109(1):djw199. doi:10.1093/jnci/djw199
30. Crasta K, Ganem NJ, Dagher R, et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 2012;482(7383):53–58. doi:10.1038/nature10802
31. Mackenzie KJ, Carroll P, Martin CA, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548(7668):461–465. doi:10.1038/nature23449
32. Chen YA, Shen YL, Hsia HY, et al. Extrachromosomal telomere repeat DNA is linked to ALT development via cGAS-STING DNA sensing pathway. Nat Struct Mol Biol. 2017;24(12):1124–1131. doi:10.1038/nsmb.3498
33. Riley JS, Quarato G, Cloix C, et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 2018;37(17):e99238. doi:10.15252/embj.201899238
34. Burdette DL, Monroe KM, Sotelo-Troha K, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478(7370):515–518. doi:10.1038/nature10429
35. Kato K, Omura H, Ishitani R, et al. Cyclic GMP-AMP as an endogenous second messenger in innate immune signaling by cytosolic DNA. Annu Rev Biochem. 2017;86:541–566. doi:10.1146/annurev-biochem-061516-044813
36. Zhang C, Shang G, Gui X, et al. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567(7748):394–398. doi:10.1038/s41586-019-1000-2
37. Marcus A, Mao AJ, Lensink-Vasan M, et al. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity. 2018;49(4):754–763.e4. doi:10.1016/j.immuni.2018.09.016
38. Zheng J, Mo J, Zhu T, et al. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer. 2020;19(1):133. doi:10.1186/s12943-020-01250-1
39. Dunn GP, Bruce AT, Sheehan KCF, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6(7):722–729. doi:10.1038/ni1213
40. González-Navajas JM, Lee J, David M, et al. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12(2):125–135. doi:10.1038/nri3133
41. Wei X, Zhang L, Yang Y, et al. LL-37 transports immunoreactive cGAMP to activate STING signaling and enhance interferon-mediated host antiviral immunity. Cell Rep. 2022;39(9):110880. doi:10.1016/j.celrep.2022.110880
42. Zhou Q, Zhou Y, Li T, et al. Nanoparticle-mediated STING agonist delivery for enhanced cancer immunotherapy. Macromol Biosci. 2021;21(8):e2100133. doi:10.1002/mabi.202100133
43. Watkins-Schulz R, Tiet P, Gallovic MD, et al. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell- and CD8+ T cell-mediated anti-tumor immunity. Biomaterials. 2019;205:94–105. doi:10.1016/j.biomaterials.2019.03.011
44. Nakamura T, Sato T, Endo R, et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J Immunother Cancer. 2021;9(7):e002852. doi:10.1136/jitc-2021-002852
45. Li Y, Li X, Yi J, et al. Nanoparticle-mediated STING activation for cancer immunotherapy. Adv Healthc Mater. 2023;12(19):e2300260. doi:10.1002/adhm.202300260
46. Wang F, Su H, Xu D, et al. Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat Biomed Eng. 2020;4(11):1090–1101. doi:10.1038/s41551-020-0597-7
47. Mathiyalagan R, Kariyarath Valappil A, Yang DC, et al. gene regulations upon hydrogel-mediated drug delivery systems in skin cancers-an overview. Gels. 2022;8(9):560. doi:10.3390/gels8090560
48. Leventhal DS, Sokolovska A, Li N, et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat Commun. 2020;11(1):2739. doi:10.1038/s41467-020-16602-0
49. Jiang Y, Li X, Qian F, et al. Fine-tuning bacterial cyclic di-AMP production for durable antitumor effects through the activation of the STING pathway. Research. 2023;6:0102. doi:10.34133/research.0102
50. Yi M, Zheng X, Niu M, et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. doi:10.1186/s12943-021-01489-2
51. Song X, Wang M, Liu S, et al. A sequential scheme including PTT and 2′3′-cGAMP/CQ-LP reveals the antitumor immune function of PTT through the type I interferon pathway. Pharmacol Res. 2023;196:106939. doi:10.1016/j.phrs.2023.106939
52. Ding F, Liu J, Ai K, et al. Simultaneous activation of pyroptosis and cGAS-STING pathway with epigenetic/ photodynamic nanotheranostic for enhanced tumor photoimmunotherapy. Adv Mater. 2024;36(7):e2306419. doi:10.1002/adma.202306419
53. Zhao Y, Pan Y, Zou K, et al. Biomimetic manganese-based theranostic nanoplatform for cancer multimodal imaging and twofold immunotherapy. Bioact Mater. 2023;19:237–250. doi:10.1016/j.bioactmat.2022.04.011
54. Huang KC, Chandra D, McGrath S, et al. Correction: pharmacologic activation of STING in the bladder induces potent antitumor immunity in non-muscle invasive murine bladder cancer. Mol Cancer Ther. 2023;22(4):551. doi:10.1158/1535-7163.MCT-23-0121
55. Meric-Bernstam F, Sweis RF, Hodi FS, et al. Phase I dose-escalation trial of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin Cancer Res. 2022;28(4):677–688. doi:10.1158/1078-0432.CCR-21-1963
56. Chang W, Altman MD, Lesburg CA, et al. Discovery of MK-1454: a potent cyclic dinucleotide stimulator of interferon genes agonist for the treatment of cancer. J Med Chem. 2022;65(7):5675–5689. doi:10.1021/acs.jmedchem.1c02197
57. Lyons TW, Thaisrivongs DA, Kuhl N, et al. The first GMP synthesis of MK-2118, a small molecule agonist for stimulator of interferon genes. Org Process Res Dev. 2024;28(6):2309–2316. doi:10.1021/acs.oprd.4c00102
58. Uslu U, Sun L, Castelli S, et al. The STING agonist IMSA101 enhances chimeric antigen receptor T cell function by inducing IL-18 secretion. Nat Commun. 2024;15(1):3933. doi:10.1038/s41467-024-47692-9
59. Kuttruff CA, Fleck M, Carotta S, et al. Discovery of BI 7446: a potent cyclic dinucleotide STING agonist with broad-spectrum variant activity for the treatment of cancer. J Med Chem. 2023;66(14):9376–9400. doi:10.1021/acs.jmedchem.3c00510
60. Carideo Cunniff E, Sato Y, Mai D, et al. TAK-676: a novel stimulator of interferon genes (STING) agonist promoting durable IFN-dependent antitumor immunity in preclinical studies. Canc Res Commun. 2022;2(6):489–502. doi:10.1158/2767-9764.CRC-21-0161
61. Abstract B96: pharmacodynamic studies of SB 11285, a systemically bioavailable STING agonist in orthotopic tumor models | cancer immunology research | American association for cancer research. Availabe from: https://aacrjournals.org/cancerimmunolres/article/8/4_Supplement/B96/469867/Abstract-B96-Pharmacodynamic-studies-of-SB-11285-a.
62. Prabagar M, Bommireddy V, Siegel R, et al. Abstract 524: the novel STING agonist VB-85247 induces robust durable antitumor immune responses by intravesical administration in a non-muscle invasive bladder cancer model. Cancer Res. 2021;81(13_Supplement):524. doi:10.1158/1538-7445.AM2021-524
63. Sibal PA, Matsumura S, Ichinose T, et al. STING activator 2′3′-cGAMP enhanced HSV-1-based oncolytic viral therapy. Mol Oncol. 2024;18(5):1259–1277. doi:10.1002/1878-0261.13603
64. Chen YP, Xu L, Tang TW, et al. STING activator c-di-GMP-loaded mesoporous silica nanoparticles enhance immunotherapy against breast cancer. ACS Appl Mater Interfaces. 2020;12(51):56741–56752. doi:10.1021/acsami.0c16728
65. Wu Y, Li Q, Shim G, Oh YK. Melanin-loaded CpG DNA hydrogel for modulation of tumor immune microenvironment. J Control Release. 2021;330:540–553. doi:10.1016/j.jconrel.2020.12.040
66. Dosta P, Cryer AM, Dion MZ, et al. Investigation of the enhanced antitumour potency of STING agonist after conjugation to polymer nanoparticles. Nat Nanotechnol. 2023;18(11):1351–1363. doi:10.1038/s41565-023-01447-7
67. Li S, Luo M, Wang Z, et al. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat Biomed Eng. 2021;5(5):455–466. doi:10.1038/s41551-020-00675-9
68. Wehbe M, Wang-Bishop L, Becker KW, et al. Nanoparticle delivery improves the pharmacokinetic properties of cyclic dinucleotide STING agonists to open a therapeutic window for intravenous administration. J Control Release. 2021;330:1118–1129. doi:10.1016/j.jconrel.2020.11.017
69. Shae D, Becker KW, Christov P, et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;14(3):269–278. doi:10.1038/s41565-018-0342-5
70. Lu ZD, Chen YF, Shen S, et al. Co-delivery of phagocytosis checkpoint silencer and stimulator of interferon genes agonist for synergetic cancer immunotherapy. ACS Appl Mater Interfaces. 2021;13(25):29424–29438. doi:10.1021/acsami.1c08329
71. Chen C, Tong Y, Zheng Y, et al. Cytosolic delivery of thiolated Mn-cGAMP nanovaccine to enhance the antitumor immune responses. Small. 2021;17(19):e2102241. doi:10.1002/smll.202102241
72. Sun X, Huang X, Park KS, et al. Self-assembled STING-activating coordination nanoparticles for cancer immunotherapy and vaccine applications. ACS Nano. 2024;18(15):10439–10453. doi:10.1021/acsnano.3c11374
73. Wang D, Nie T, Huang C, et al. Metal-cyclic dinucleotide nanomodulator-stimulated STING signaling for strengthened radioimmunotherapy of large tumor. Small. 2022;18(41):2203227. doi:10.1002/smll.202203227
74. Zheng H, Guo B, Qiu X, et al. Polymersome-mediated cytosolic delivery of cyclic dinucleotide STING agonist enhances tumor immunotherapy. Bioact Mater. 2022;16:1–11. doi:10.1016/j.bioactmat.2022.02.029
75. Koshy ST, Cheung AS, Gu L, et al. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv Biosyst. 2017;1(1–2):1600013. doi:10.1002/adbi.201600013
76. Dane EL, Belessiotis-Richards A, Backlund C, et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat Mater. 2022;21(6):710–720. doi:10.1038/s41563-022-01251-z
77. Chen J, Qiu M, Ye Z, et al. In situ cancer vaccination using lipidoid nanoparticles. Sci Adv. 2021;7(19):eabf1244. doi:10.1126/sciadv.abf1244
78. Baljon JJ, Kwiatkowski AJ, Pagendarm HM, et al. A cancer nanovaccine for co-delivery of peptide neoantigens and optimized combinations of STING and TLR4 agonists. ACS Nano. 2024;18(9):6845–6862. doi:10.1021/acsnano.3c04471
79. Khalifa AM, Nakamura T, Sato Y, et al. Vaccination with a combination of STING agonist-loaded lipid nanoparticles and CpG-ODNs protects against lung metastasis via the induction of CD11bhighCD27low memory-like NK cells. Exp Hematol Oncol. 2024;13(1):36. doi:10.1186/s40164-024-00502-w
80. Yang K, Han W, Jiang X, et al. Zinc cyclic di-AMP nanoparticles target and suppress tumours via endothelial STING activation and tumour-associated macrophage reinvigoration. Nat Nanotechnol. 2022;17(12):1322–1331. doi:10.1038/s41565-022-01225-x
81. Aikins ME, Sun X, Dobson H, et al. STING-activating cyclic dinucleotide-manganese nanoparticles evoke robust immunity against acute myeloid leukemia. J Control Release. 2024;368:768–779. doi:10.1016/j.jconrel.2024.03.022
82. Doshi AS, Cantin S, Prickett LB, et al. Systemic nano-delivery of low-dose STING agonist targeted to CD103+ dendritic cells for cancer immunotherapy. J Control Release. 2022;345:721–733. doi:10.1016/j.jconrel.2022.03.054
83. Fang Y, Huang S, Hu Q, et al. Injectable zwitterionic physical hydrogel with enhanced chemodynamic therapy and tumor microenvironment remodeling properties for synergistic anticancer therapy. ACS Nano. 2023;17(24):24883–24900. doi:10.1021/acsnano.3c05898
84. Lee E, Jang HE, Kang YY, et al. Submicron-sized hydrogels incorporating cyclic dinucleotides for selective delivery and elevated cytokine release in macrophages. Acta Biomater. 2016;29:271–281. doi:10.1016/j.actbio.2015.10.025
85. Park CG, Hartl CA, Schmid D, et al. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci Transl Med. 2018;10(433):eaar1916. doi:10.1126/scitranslmed.aar1916
86. Delitto D, Zabransky DJ, Chen F, et al. Implantation of a neoantigen-targeted hydrogel vaccine prevents recurrence of pancreatic adenocarcinoma after incomplete resection. Oncoimmunology. 2021;10(1):2001159. doi:10.1080/2162402X.2021.2001159
87. Wang F, Su H, Wang Z, et al. Supramolecular filament hydrogel as a universal immunomodulator carrier for immunotherapy combinations. ACS Nano. 2023;17(11):10651–10664. doi:10.1021/acsnano.3c01748
88. Zhao X, Han Y, Sun Y, et al. Combining photothermal ablation-based vaccine with immune checkpoint blockade for synergistic osteosarcoma immunotherapy. Mater Des. 2021;198:109311. doi:10.1016/j.matdes.2020.109311
89. Rao L, Wu L, Liu Z, et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun. 2020;11(1):4909. doi:10.1038/s41467-020-18626-y
90. Jneid B, Bochnakian A, Hoffmann C, et al. Selective STING stimulation in dendritic cells primes antitumor T cell responses. Sci Immun. 2023;8(79):eabn6612. doi:10.1126/sciimmunol.abn6612
91. Lioux T, Mauny MA, Lamoureux A, et al. Design, synthesis, and biological evaluation of novel cyclic adenosine-inosine monophosphate (cAIMP) analogs that activate stimulator of interferon genes (STING). J Med Chem. 2016;59(22):10253–10267. doi:10.1021/acs.jmedchem.6b01300
92. Motedayen Aval L, Pease JE, Sharma R, et al. Challenges and opportunities in the clinical development of STING agonists for cancer immunotherapy. J Clin Med. 2020;9(10):3323. doi:10.3390/jcm9103323
93. Zhang H, You QD, Xu XL. Targeting stimulator of interferon genes (STING): a medicinal chemistry perspective. J Med Chem. 2020;63(8):3785–3816. doi:10.1021/acs.jmedchem.9b01039
94. Shang M, Lu K, Guan W, et al. 2′,3′-cyclic GMP-AMP dinucleotides for STING-mediated immune modulation: principles, immunotherapeutic potential, and synthesis. ChemMedChem. 2022;17(2):e202100671. doi:10.1002/cmdc.202100671
95. Yi M, Niu M, Zhang J, et al. Combine and conquer: manganese synergizing anti-TGF-β/PD-L1 bispecific antibody YM101 to overcome immunotherapy resistance in non-inflamed cancers. J Hematol Oncol. 2021;14(1):146. doi:10.1186/s13045-021-01155-6
96. Kim DS, Endo A, Fang FG, et al. E7766, a macrocycle-bridged stimulator of interferon genes (STING) agonist with potent pan-genotypic activity. ChemMedChem. 2021;16(11):1741–1744. doi:10.1002/cmdc.202100068
97. Flamme M, Hanlon S, Iding H, et al. Towards the enzymatic synthesis of phosphorothioate containing LNA oligonucleotides. Bioorg Med Chem Lett. 2021;48:128242. doi:10.1016/j.bmcl.2021.128242
98. Li L, Yin Q, Kuss P, et al. Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat Chem Biol. 2014;10(12):1043–1048. doi:10.1038/nchembio.1661
99. Novotná B, Vaneková L, Zavřel M, et al. Enzymatic preparation of 2′-5′,3′-5′-cyclic dinucleotides, their binding properties to stimulator of interferon genes adaptor protein, and structure/activity correlations. J Med Chem. 2019;62(23):10676–10690. doi:10.1021/acs.jmedchem.9b01062
100. McIntosh JA, Liu Z, Andresen BM, et al. A kinase-cGAS cascade to synthesize a therapeutic STING activator. Nature. 2022;603(7901):439–444. doi:10.1038/s41586-022-04422-9
101. Harrington KJ, Brody J, Ingham M, et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann Oncol. 2018;29:viii712. doi:10.1093/annonc/mdy424.015
102. Yu X, Yu J, Dai H, et al. Novel formulation of c-di-GMP with cytidinyl/cationic lipid reverses T cell exhaustion and activates stronger anti-tumor immunity. Theranostics. 2022;12(15):6723–6739. doi:10.7150/thno.71010
103. Kong X, Zuo H, Huang HD, et al. STING as an emerging therapeutic target for drug discovery: perspectives from the global patent landscape. J Adv Res. 2023;44:119–133. doi:10.1016/j.jare.2022.05.006
104. Yang Z, Gao D, Zhao J, et al. Thermal immuno-nanomedicine in cancer. Nat Rev Clin Oncol. 2023;20(2):116–134. doi:10.1038/s41571-022-00717-y
105. Huang X, Kong N, Zhang X, et al. The landscape of mRNA nanomedicine. Nat Med. 2022;28(11):2273–2287. doi:10.1038/s41591-022-02061-1
106. Li X, Wang H, Chen Y, et al. Novel emerging nano-assisted anti-cancer strategies based on the STING pathway. Acta Materia Medica. 2023;2:323–341. doi:10.15212/AMM-2023-0023
107. Wang F, Xiao J, Chen S, et al. Polymer vesicles: modular platforms for cancer theranostics. Adv Mater. 2018;30(17):e1705674. doi:10.1002/adma.201705674
108. Vicent MJ, Duncan R. Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol. 2006;24(1):39–47. doi:10.1016/j.tibtech.2005.11.006
109. Luk BT, Fang RH, Zhang L. Lipid- and polymer-based nanostructures for cancer theranostics. Theranostics. 2012;2(12):1117–1126. doi:10.7150/thno.4381
110. Nori A, Kopecek J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv Drug Deliv Rev. 2005;57(4):609–636. doi:10.1016/j.addr.2004.10.006
111. Ma Z, Mao Z, Gao C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surf B Biointerfaces. 2007;60(2):137–157. doi:10.1016/j.colsurfb.2007.06.019
112. Jing X, Zhang Y, Li M, et al. Surface engineering of colloidal nanoparticles. Mater Horiz. 2023;10(4):1185–1209. doi:10.1039/d2mh01512a
113. Lam K, Schreiner P, Leung A, et al. Optimizing lipid nanoparticles for delivery in primates. Adv Mater. 2023;35(26):e2211420. doi:10.1002/adma.202211420
114. El Moukhtari SH, Garbayo E, Amundarain A, et al. Lipid nanoparticles for siRNA delivery in cancer treatment. J Control Release. 2023;361:130–146. doi:10.1016/j.jconrel.2023.07.054
115. Hajj KA, Whitehead KA. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater. 2017;2(10):1–17. doi:10.1038/natrevmats.2017.56
116. Eygeris Y, Gupta M, Kim J, et al. Chemistry of lipid nanoparticles for RNA delivery. Acc Chem Res. 2022;55(1):2–12. doi:10.1021/acs.accounts.1c00544
117. Wang HL, Wang ZG, Liu SL. Lipid nanoparticles for mRNA delivery to enhance cancer immunotherapy. Molecules. 2022;27(17):5607. doi:10.3390/molecules27175607
118. Jeong M, Lee Y, Park J, et al. Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications. Adv Drug Deliv Rev. 2023;200:114990. doi:10.1016/j.addr.2023.114990
119. Hao Y, Ji Z, Zhou H, et al. Lipid-based nanoparticles as drug delivery systems for cancer immunotherapy. MedComm. 2023;4(4):e339. doi:10.1002/mco2.339
120. Guan S, Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(3):133–143. doi:10.1038/gt.2017.5
121. Jörgensen AM, Wibel R, Bernkop-Schnürch A. Biodegradable cationic and ionizable cationic lipids: a roadmap for safer pharmaceutical excipients. Small. 2023;19(17):e2206968. doi:10.1002/smll.202206968
122. Han X, Zhang H, Butowska K, et al. An ionizable lipid toolbox for RNA delivery. Nat Commun. 2021;12(1):7233. doi:10.1038/s41467-021-27493-0
123. Bariwal J, Ma H, Altenberg GA, et al. Nanodiscs: a versatile nanocarrier platform for cancer diagnosis and treatment. Chem Soc Rev. 2022;51(5):1702–1728. doi:10.1039/d1cs01074c
124. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi:10.1038/nm1523
125. Paston SJ, Brentville VA, Symonds P, et al. Cancer vaccines, adjuvants, and delivery systems. Front Immunol. 2021;12:627932. doi:10.3389/fimmu.2021.627932
126. Gajbhiye KR, Salve R, Narwade M, et al. Lipid polymer hybrid nanoparticles: a custom-tailored next-generation approach for cancer therapeutics. Mol Cancer. 2023;22(1):160. doi:10.1186/s12943-023-01849-0
127. Pan BS, Perera SA, Piesvaux JA, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020;369(6506):eaba6098. doi:10.1126/science.aba6098
128. Hong WX, Haebe S, Lee AS, et al. Intratumoral immunotherapy for early-stage solid tumors. Clin Cancer Res. 2020;26(13):3091–3099. doi:10.1158/1078-0432.CCR-19-3642
129. Mikhail AS, Morhard R, Mauda-Havakuk M, et al. Hydrogel drug delivery systems for minimally invasive local immunotherapy of cancer. Adv Drug Deliv Rev. 2023;202:115083. doi:10.1016/j.addr.2023.115083
130. Huang C, Shao N, Huang Y, et al. Overcoming challenges in the delivery of STING agonists for cancer immunotherapy: a comprehensive review of strategies and future perspectives. Mater Today Bio. 2023;23:100839. doi:10.1016/j.mtbio.2023.100839
131. Oliva N, Conde J, Wang K, et al. Designing hydrogels for on-demand therapy. Acc Chem Res. 2017;50(4):669–679. doi:10.1021/acs.accounts.6b00536
132. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6(2):105–121. doi:10.1016/j.jare.2013.07.006
133. Baird JR, Bell RB, Troesch V, et al. Evaluation of explant responses to STING ligands: personalized immunosurgical therapy for head and neck squamous cell carcinoma. Cancer Res. 2018;78(21):6308–6319. doi:10.1158/0008-5472.CAN-18-1652
134. Lei L, Huang D, Gao H, et al. Hydrogel-guided strategies to stimulate an effective immune response for vaccine-based cancer immunotherapy. Sci Adv. 2022;8(47):eadc8738. doi:10.1126/sciadv.adc8738
135. Nejabat M, Samie A, Ramezani M, et al. An overview on gold nanorods as versatile nanoparticles in cancer therapy. J Control Release. 2023;354:221–242. doi:10.1016/j.jconrel.2023.01.009
136. Fan M, Han Y, Gao S, et al. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics. 2020;10(11):4944–4957. doi:10.7150/thno.42471
137. Kesharwani P, Ma R, Sang L, et al. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol Cancer. 2023;22(1):98. doi:10.1186/s12943-023-01798-8
138. Chen R, Yang J, Wu M, et al. M2 macrophage hybrid membrane-camouflaged targeted biomimetic nanosomes to reprogram inflammatory microenvironment for enhanced enzyme-thermo-immunotherapy. Adv Mater. 2023;35(39):e2304123. doi:10.1002/adma.202304123
139. Huang X, Guo H, Wang L, et al. Biomimetic cell membrane-coated nanocarriers for targeted siRNA delivery in cancer therapy. Drug Discov Today. 2023;28(4):103514. doi:10.1016/j.drudis.2023.103514
140. Zhou S, Gravekamp C, Bermudes D, et al. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18(12):727–743. doi:10.1038/s41568-018-0070-z
141. Gurbatri CR, Arpaia N, Danino T. Engineering bacteria as interactive cancer therapies. Science. 2022;378(6622):858–864. doi:10.1126/science.add9667
142. Lam KC, Araya RE, Huang A, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184(21):5338–5356.e21. doi:10.1016/j.cell.2021.09.019
143. Low JT, Brown MC, Reitman ZJ, et al. Understanding and therapeutically exploiting cGAS/STING signaling in glioblastoma. J Clin Invest. 2024;134(2):e163452. doi:10.1172/JCI163452
144. Zaver SA, Woodward JJ. Cyclic dinucleotides at the forefront of innate immunity. Curr Opin Cell Biol. 2020;63:49–56. doi:10.1016/j.ceb.2019.12.004
145. Wang R, Hussain A, Guo Q, et al. cGAS-STING at the crossroads in cancer therapy. Crit Rev Oncol Hematol. 2024;193:104194. doi:10.1016/j.critrevonc.2023.104194
146. Li J, Hubisz MJ, Earlie EM, et al. Non-cell-autonomous cancer progression from chromosomal instability. Nature. 2023;620(7976):1080–1088. doi:10.1038/s41586-023-06464-z
147. Blest HTW, Chauveau L. cGAMP the travelling messenger. Front Immunol. 2023;14:1150705. doi:10.3389/fimmu.2023.1150705
148. Wang Y, Liu Y, Zhang J, et al. Nanomaterial-mediated modulation of the cGAS-STING signaling pathway for enhanced cancer immunotherapy. Acta Biomater. 2024;176:51–76. doi:10.1016/j.actbio.2024.01.008
149. Yang C, Liang Y, Liu N, et al. Role of the cGAS-STING pathway in radiotherapy for non-small cell lung cancer. Radiat Oncol. 2023;18(1):145. doi:10.1186/s13014-023-02335-z
150. Shan B, Hou H, Zhang K, et al. Design, synthesis, and biological evaluation of bipyridazine derivatives as stimulator of interferon genes (STING) receptor agonists. J Med Chem. 2023;66(5):3327–3347. doi:10.1021/acs.jmedchem.2c01714
151. Conlon J, Burdette DL, Sharma S, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190(10):5216–5225. doi:10.4049/jimmunol.1300097
152. Guo J, Huang L. Nanodelivery of cGAS-STING activators for tumor immunotherapy. Trends Pharmacol Sci. 2022;43(11):957–972. doi:10.1016/j.tips.2022.08.006
153. Chen T, Xu ZG, Luo J, et al. NSUN2 is a glucose sensor suppressing cGAS/STING to maintain tumorigenesis and immunotherapy resistance. Cell Metab. 2023;35(10):1782–1798.e8. doi:10.1016/j.cmet.2023.07.009
154. Hu Z, Yu X, Ding R, et al. Glycolysis drives STING signaling to facilitate dendritic cell antitumor function. J Clin Invest. 2023;133(7):e166031. doi:10.1172/JCI166031
155. Fang L, Hao Y, Yu H, et al. Methionine restriction promotes cGAS activation and chromatin untethering through demethylation to enhance antitumor immunity. Cancer Cell. 2023;41(6):1118–1133.e12. doi:10.1016/j.ccell.2023.05.005
156. O’Connor RS. Checkmate: metabolic flexibility with a STING in its tail. Sci Adv. 2023;9(49):eadm6816. doi:10.1126/sciadv.adm6816
157. Zhang R, Kang R, Tang D. The STING1 network regulates autophagy and cell death. Signal Transduct Target Ther. 2021;6(1):208. doi:10.1038/s41392-021-00613-4
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

