Back to Journals » International Journal of Nanomedicine » Volume 19
Novel Strategies for Tumor Treatment: Harnessing ROS-Inducing Active Ingredients from Traditional Chinese Medicine Through Multifunctional Nanoformulations
Authors Zhang Z , Li M , Zhang X, Zhou F
Received 21 May 2024
Accepted for publication 28 August 2024
Published 17 September 2024 Volume 2024:19 Pages 9659—9688
DOI https://doi.org/10.2147/IJN.S479212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xing Zhang
Zhengguang Zhang,1,2,* Min Li,3,* Xiaolong Zhang,4,* Fuqiong Zhou1
1Central Laboratory, Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Jiangsu, Nanjing, People’s Republic of China; 2School of Medicine, Nanjing University of Chinese Medicine, Jiangsu, Nanjing, People’s Republic of China; 3Department of Oncology, Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Jiangsu, Nanjing, People’s Republic of China; 4The Affiliated Hospital of Nanjing University of Chinese Medicine, Jiangsu Province Hospital of Chinese Medicine, Jiangsu, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhengguang Zhang; Fuqiong Zhou, Email [email protected]; [email protected]
Abstract: Reactive oxygen species (ROS) encompass a diverse group of chemically reactive molecules or ions distinguished by their substantial oxidative potential. Empirical studies have shown that the targeted administration of high toxic concentrations of ROS can effectively induce tumor cell death in various types. Numerous bioactive ingredients derived from traditional Chinese medicine (TCM), recognized for their ROS-inducing properties, have demonstrated significant anti-tumor activity. Nonetheless, their clinical application has been hindered by challenges such as low solubility, limited bioavailability, and poor selectivity. Multifunctional nanoformulations possess the potential to overcome these challenges and enhance the anticancer efficacy of ROS-inducing active compounds. Through extensive searches of various academic databases and a thorough review and screening of relevant literature, this study aims to systematically summarize and generalize multiple active ingredients in TCM that induce ROS generation, along with their multifunctional nanoformulations, from various perspectives. The objective is to provide new insights and references for fundamental cancer research and clinical treatments. Furthermore, we acknowledge that although numerous active ingredients and their nanoformulations in TCM have demonstrated ROS-inducing and anti-tumor potentials, potentially offering novel strategies for tumor therapy, the underlying mechanisms require further comprehensive investigation.
Keywords: traditional Chinese medicine, nanoformulation, reactive oxygen species, cancer
Introduction
Cancer, recognized globally as a life-threatening disease, has long been a central concern of medical research and treatment.1 Conventional therapeutic modalities, such as surgery, chemotherapy, and radiotherapy, frequently yield suboptimal outcomes and are associated with significant adverse effects, thereby compromising patients’ quality of life.2 Recently, traditional Chinese medicine (TCM) has emerged as a promising strategy in the management of malignant tumors, with its therapeutic efficacy increasingly substantiated by scientific investigations.3,4 Research has indicated that the natural active ingredients found in TCM, which possess various properties that induce ROS generation, exhibit remarkable efficacy in combating malignant tumors.5 Compared to chemotherapeutic agents, these natural ROS-inducing ingredients and different formulations are distinguished by their mild action, ease of acquisition, and reduced side effects on the patient’s body during treatment, thereby enhancing the patient’s well-being.6 It is noteworthy that various ROS-inducing active compounds in TCM exhibit multi-targeting capabilities, enabling concurrent modulation of multiple critical signaling pathways in tumor cells.7 This multipotency not only mitigates the development of drug resistance in tumor cells but also substantially enhances therapeutic efficacy, thereby offering promising prospects for cancer treatment.8
ROS encompass a broad category of oxygen-containing free radicals and peroxides that are prone to free radical formation, which are intricately linked to oxygen metabolism in living organisms. Empirical research has demonstrated that elevated toxic levels of ROS can effectively inhibit the growth and proliferation of tumor cells, thereby exhibiting significant anticancer efficacy.9 The underlying mechanism of this phenomenon is likely attributable to the oxidative damage inflicted by ROS on critical biomolecules, including DNA, proteins, and lipids. This oxidative damage can disrupt the normal physiological functions of tumor cells, consequently inhibiting their growth and proliferation. However, the utilization of ROS is not devoid of risk, as prolonged exposure to elevated concentrations of ROS can also inflict damage on normal cells. Consequently, in practical applications, it is imperative to meticulously regulate the concentration and spatial distribution of ROS to maximize its anticancer efficacy while minimizing adverse effects on healthy tissues.
There remain significant challenges in the effective utilization of ROS-inducing active ingredients derived from TCM for the treatment of malignant tumors. Firstly, these active ingredients exhibit insufficient selectivity towards tumor cells, potentially exerting non-therapeutic effects on normal cells. Secondly, the limited bioavailability and stability of these compounds constrain their anti-tumor efficacy in vivo. Furthermore, the complexity of medicinal dosages and the synergistic relationships among these active ingredients necessitate meticulous selection and combination in clinical applications. To overcome these formidable limitations, multifunctional nanoformulations have emerged as a potentially effective solution.10,11 In recent years, the advancement of multifunctional nanoformulations has yielded four pivotal advancements in the field of cancer treatment:12,13 The first is targeting advantage. By modifying surface functional groups or encapsulating cancer cell membranes, multifunctional nanoformulations can selectively bind to cancer cells, enabling precise targeting therapy and minimizing harm to healthy cells.14 The second aspect pertains to the efficacy of drug delivery. By leveraging the properties of nanoscale materials, multifunctional nanoformulations can circumvent premature drug release and degradation, thereby enhancing drug stability and bioavailability within the body.15 The third benefit is the augmentation of therapeutic efficacy. Multifunctional nanoformulations can transport various drugs or therapeutic agents concurrently, enabling multidimensional treatment and enhancing therapeutic outcomes.16,17 The final advantage is the mitigation of drug resistance. The formulation of multifunctional nanotechnology can disrupt cellular resistance mechanisms to drugs, effectively combating drug resistance in cancer cells and increasing treatment success rates.18 Based on these considerations, multifunctional nanoformulations can facilitate the targeted delivery and release of ROS-inducing active ingredients in TCM specifically at the tumor site. These nanoformulations enhance bioavailability and stability, extend the circulation time of the drug within the body, and minimize damage to normal tissues. Consequently, they significantly improve and enhance the durability of the therapeutic effect.
Currently, there is a notable deficiency in the summarization and generalization of active compounds that stimulate the generation of ROS in TCM within both basic and clinical research domains. Consequently, our research team undertook a comprehensive examination of multiple databases. The outcomes of our inquiry and study are organized into four primary sections: 1. The elucidation of ROS concept, generation, classification, and biological functions; 2. The exploration of the interplay between ROS and tumorigenesis, progression, and therapeutic interventions; 3. The compilation and overview of various ROS-inducing compounds and anticancer mechanisms in TCM; 4. The discussion of advancements in the development of nanoformulations of ROS-inducing compounds in TCM.
The Elucidation of ROS Concept, Generation, Classification, and Biological Functions
ROS constitute a heterogeneous group of oxygen-containing free radicals and peroxides that are integral to oxygen metabolism in living organisms, playing a pivotal role in both physiological and pathological processes in humans.19–21
The generation of ROS can be categorized into endogenous and exogenous sources. Endogenous ROS predominantly originate from redox reactions occurring within intracellular mitochondria and the endoplasmic reticulum, as well as from the enzymatic activities of metabolic systems such as the respiratory chain and cytochrome P450.22,23 Conversely, exogenous ROS generation is induced by environmental factors or exposure to chemical substances, including radiation, pharmaceuticals, and heavy metal ions, which can trigger ROS production.24
Various types of intracellular ROS exist, including superoxide anion radicals (•O2−), hydrogen peroxide (H2O2), hydroxyl radicals (•OH), singlet oxygen (1O2), and Ozone (O3). •O2− is considered the most basic ROS, characterized by an oxygen molecule with an unpaired electron, commonly generated through the mitochondrial respiratory chain and other redox reactions. H2O2 is formed through the interaction of •O2− with peroxidase enzymes, while •OH is generated via the Fenton reaction involving H2O2 or •O2− with transition metals such as iron ions. Additionally, 1O2 represents the excited state of oxygen, typically formed through exposure to light or specific chemical reactions.
ROS possess a broad spectrum of biological functions within organisms.25 Primarily, ROS are instrumental in the eradication of pathogenic microorganisms and are essential to the sterilization process.26 Furthermore, ROS are involved in the modulation of cell growth, proliferation, and various physiological processes through multiple signaling pathways.27 Moreover, optimal levels of ROS can notably enhance apoptosis and aid in the elimination of aberrant cells.28 Additionally, ROS play a crucial role in the regulation of cell differentiation and maturation process.29 As signaling molecules, ROS are pivotal in modulating gene expression, regulating the cellular microenvironment, influencing the cell cycle and apoptotic processes, and promoting cell migration and morphological changes. The synergistic interplay of these roles ensures that cells adhere to a predetermined differentiation program, culminating in the development of mature cells with specialized functions. However, excessive production of ROS can result in oxidative stress, causing damage to intracellular components such as DNA, proteins, and lipids, leading to inflammation, aging, and the onset of various diseases.30,31 Thus, maintaining a proper equilibrium of ROS is crucial for sustaining normal physiological functions. Organisms can mitigate excessive ROS levels through the action of antioxidant enzymes, as well as vitamins A, C, and E, among other antioxidants, to safeguard cells from oxidative damage.32
The Relationship Between ROS and the Genesis, Progression, and Treatment of Malignant Tumors
Initially, scholars held the belief that ROS primarily facilitated tumor progression. Nevertheless, contemporary research has demonstrated that ROS exhibits dual functionality, with its impact contingent upon concentration, duration, and cellular effects.33–35 Elevated ROS levels, which remain beneath the toxic threshold, generally activate growth factor receptors and signaling pathways, thus promoting tumor cell proliferation, metastasis, and malignant transformation via the alteration of tumor-associated gene functionality and the initiation of oncogenesis. Conversely, exceeding the toxicity threshold leads to persistent elevated levels of ROS that intensify oxidative stress damage and instigate the initiation of various ROS-mediated death signaling pathways. This ultimately results in multiple forms of tumor cell death, including ferroptosis, autophagic cell death, apoptosis, pyroptosis, and necroptosis. Notably, levels of ROS are typically higher in tumor tissues relative to normal tissues. Consequently, the utilization of ROS-inducing pharmaceuticals that specifically target tumor tissues or multifunctional materials containing these drugs to stimulate ROS production above the toxic threshold is a promising strategy for treating various forms of cancer. Figure 1 illustrates the primary mechanisms of different types of ROS generation and highlights the distribution of ROS concentration levels in normal and tumor cells.
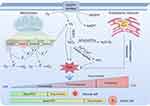 |
Figure 1 The primary mechanisms of different types of ROS generation and distribution of ROS concentration levels in normal and tumor cells (drawn by Figdraw, ID: ASYIW74747). |
It is currently hypothesized that the rational induction of elevated levels of ROS may exhibit superior anticancer effects and broader clinical applicability compared to antioxidant therapies aimed at reducing ROS levels.36 Several ROS inducers and drugs targeting antioxidant enzyme systems, such as cisplatin, oxaliplatin, gemcitabine, ARQ501, and the glutathione inhibitor BSO, have been utilized in clinical settings or are undergoing clinical trials.37,38 In clinical practice, healthcare providers frequently opt to induce high levels of ROS generation as a therapeutic approach for tumor treatment. For instance, oxygen radical therapy functions by administering hyperoxidative compounds like oxygen or hydrogen peroxide solution directly into tumor tissues to elevate the levels of ROS, ultimately leading to tumor cell death. It is important to acknowledge that insufficient ROS levels may trigger pro-cancer signaling pathways like PI3K and HIF, potentially aiding in tumor progression. On the contrary, an elevated concentration of ROS surpassing the toxicity threshold can induce heightened oxidative stress in healthy cells, resulting in detrimental effects on normal tissues and organs, including the heart, liver, and kidneys of the individual. Consequently, it is imperative to carefully manage and maintain ROS levels within the body during cancer therapy to ensure that therapeutic agents selectively target tumor tissues while minimizing harm to healthy tissues.
Summarization of Various ROS-Inducing Active Ingredients in TCM and Their Anti-Cancer Mechanisms
TCM is increasingly recognized as a valuable cultural asset within the field of medicine due to its rich historical background and distinctive therapeutic approaches. In the realm of TCM, a multitude of active ingredients have displayed potential in the treatment of cancer, including various ROS-inducing compounds such as quinones, terpenoids, flavonoids, alkaloids, polyphenols, polysaccharides, sesquiterpene lactones, and others. These ingredients have exhibited inhibitory effects on cell proliferation, as well as the induction of various forms of cell death such as apoptosis, ferroptosis, autophagic cell death, pyroptosis, and necroptosis. We conducted a comprehensive review of representative pieces of literature on various ROS-inducing active ingredients in TCM, elucidated the chemical structural formulae of various types of these compounds (Figure 2), and summarized ROS-induced cell death mechanisms at high concentrations exceeding the toxicity threshold (Figure 3).
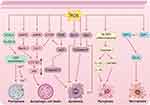 |
Figure 3 ROS-induced cell death mechanisms at high concentrations exceeding the toxicity threshold (drawn by Figdraw, ID: OPRPI96a96). |
Quinones
Quinones, a class of organic compounds containing the structural features of cyclohexadiene dione or cyclohexadiene dimethylene, are commonly found in a variety of TCM plants. This structural characteristic imparts quinones with favorable redox properties. In biological systems, quinones undergo reduction by accepting electrons, leading to the oxidation of oxygen molecules and the generation of ROS such as H2O2 and •O2−. Prominent quinone components renowned for their capacity to stimulate ROS production encompass plumbagin, thymoquinone, shikonin, gambogic acid, emodin, chrysophanol, and numerous other compounds.
Plumbagin
Plumbagin, an active compound derived from the roots of Plumbago zeylanica L., a member of the Plumbaginaceae family, has been shown to elicit a G2/M cell cycle arrest through the generation of ROS and activation of the ATM-p53 signaling pathway. This mechanism leads to genotoxicity and subsequent apoptosis in hepatocellular carcinoma cells.39 Within the realm of lung cancer treatment, plumbagin may also induce apoptosis in lung cancer cells by activating caspase-9 and targeting mitochondria-mediated ROS induction.40 In addressing the prevalent issue of multidrug resistance in oral cancer, plumbagin could serve as a therapeutic agent to counteract this resistance. It achieves this by synergistically initiating the apoptotic pathway via ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction.41
Thymoquinone
Thymoquinone, an active monomer derived from the seeds of Nigella damascena L., a plant in the Ranunculaceae family, has been shown to inhibit the proliferation, migration, and invasiveness of human bladder cancer cells by modulating ROS, autophagic flux, and miR-877-5p.42 Apart from its demonstrated capacity to suppress tumor cell proliferation via ROS-induced apoptosis, thymoquinone has been empirically proven to curtail the stemness of tumor cells and diminish their self-renewal potential.43 Notably, thymoquinone, in exhibiting its independent anti-tumor properties, has been found to augment the cytotoxicity of the pharmaceutical agent 5-fluorouracil when used in conjunction with metformin. This is achieved through the PI3K/mTOR/HIF1α pathway and by amplifying oxidative stress within colon cancer cells.44
Shikonin
Shikonin, a natural compound derived from the dried roots of Arnebia euchroma (Royle) Johnst. and Lithospermum erythrorhizon Sieb. et Zucc. or Arnebia guttata Bunge. in the Boraginaceae family, acts as a potent inhibitor of PKM2. Numerous studies have illustrated the ability of shikonin to impede the proliferation of various cancer cells, including those associated with adult T-cell leukemia/lymphoma, non-small cell lung cancer, colorectal cancer, and osteosarcoma, by inducing forms of cell death linked to ROS, such as apoptosis, ferroptosis, pyroptosis, and autophagy.45–50 These effects are mediated through the modulation of key cellular pathways, including mitochondrial dysfunction, endoplasmic reticulum stress, and the regulation of critical gene expression. Furthermore, our team’s prior research has demonstrated that shikonin can augment the therapeutic efficacy of oxaliplatin in oxaliplatin-resistant colorectal cancer cells by inducing endogenous apoptosis and endoplasmic reticulum stress through ROS.51
Gambogic Acid
Gambogic acid, a bioactive compound sourced from the desiccated resin of the Garcinia hanburyi Hook. f. in the Fabaceae family, has been demonstrated by several researchers to induce endoplasmic reticulum stress via ROS modulation of the JNK pathway, leading to apoptosis and autophagy in prostate cancer cells.52 Additionally, Zhao et al have reported that gambogic acid triggers Noxa-mediated apoptosis in colorectal cancer by activating IRE1α/JNK through ROS signaling.53 In non-small cell lung cancer cells, gambogic acid has been shown to inhibit cell proliferation and induce apoptosis by modulating ROS-mediated endoplasmic reticulum stress.54
Emodin
Emodin is primarily sourced from the desiccated roots and rhizomes of Polygonum palmatum L., Rheum tanguticum Maxim. ex Balf., or Rheum officinale Baill. in the Polygonaceae family. Studies carried out as early as 2005 have demonstrated that emodin instigates apoptosis in human lung adenocarcinoma cells through the ROS-dependent mitochondrial signaling pathway.55 Subsequent research has substantiated emodin’s role as a catalyst for iron death in colorectal cancer cells, accomplished via NCOA4-mediated ferritin autophagy, ROS generation, and the deactivation of the NF-κB pathway.56 Further investigations have illuminated that emodin provokes necrosis in renal cancer cells by amplifying ROS-mediated activation of the JNK signaling pathway, concurrently inhibiting glycolysis via the inactivation of the PI3K/AKT signaling pathway and down-regulation of GLUT1, ultimately facilitating cell death.57
Chrysophanol
Chrysophanol, a natural anthraquinone closely related to emodin, exhibits a diverse array of pharmacological properties, including antimicrobial, antitussive, intestinal motility, and neuroleptic effects, as well as potent antitumor activity. Studies have demonstrated that chrysophanol can impede the proliferation of human tongue squamous carcinoma SAS cells through modulation of mTOR/PPAR-α signaling and accumulation of ROS.58 In oral cancer cell lines, it has been demonstrated that chrysophanol augments the production of ROS while concurrently inhibiting metastasis, invasion, and epithelial-mesenchymal transition.59 Interestingly, when chrysophanol was applied to glioma cells, there was an observed translocation of cytochrome C from the mitochondria to the cytoplasm. This translocation was concomitant with a significant accumulation of ROS and the initiation of apoptosis and cell cycle arrest events.60
Terpenoids
Terpenoids, characterized by the presence of multiple isoprene structural units, typically possess either a carbon-skeleton cycloalkane structure or a linear structure. The mechanism through which these compounds induce ROS generation in organisms involves their interaction with various intracellular oxidoreductases. Terpenoids have the potential to facilitate ROS generation by reducing oxygen molecules through their interaction with intracellular oxidative enzymes. Notable terpenoids capable of inducing ROS generation include costunolide, β-elemene, oridonin, celastrol, oleanolic acid, artesunate, and many other compounds.
Costunolide
Costunolide, a sesquiterpene lactone isolated from the dried root of the Asteraceae plant, Acacia lappa Decne., possesses a range of pharmacological activities including anti-tumor, anti-inflammatory, anti-allergic, anti-diabetic, and neuroprotective properties. Notably, studies have revealed that costunolide can elicit ROS production in a cell-specific manner, with varying effects observed across different cell lines. For instance, costunolide was found to enhance ROS levels in SK-BR-3 cells while inhibiting ROS elevation in MCF-7 cells.61 In gastric cancer cells, costunolide demonstrated the capacity to modulate ROS accumulation, promote apoptosis, activate autophagy, and induce cell death through the inhibition of the AKT/GSK3β signaling pathway.62 Notably, costunolide was shown to significantly enhance the ROS-inducing properties of cisplatin and its toxic effects on hypopharyngeal SCC FaDu cells when combined with the clinical drug cisplatin.63
β-Elemene
β-elemene, an active monomer derived from Curcuma rcenyujin Y,H. Chenet C. Ling of the Zingiberaceae family, is classified as a national class II non-cytotoxic anti-tumor agent. As a pharmacological agent, β-elemene has demonstrated significant ROS-inducing capabilities, and it can stimulate apoptosis and autophagy in colorectal cancer cells through the regulation of the ROS/AMPK/mTOR pathway.64 Beyond initiating ROS-dependent death in malignant tumors as a standalone treatment,65 β-elemene can also function synergistically with the traditional chemotherapeutic drug, cisplatin, to induce apoptosis in bladder cancer cells via the ROS-AMPK pathway.66
Oridonin
Oridonin, a tetracyclic diterpenoid derived from the whole herb of Rabdosia rubescens in the Labiatae family, has been shown in recent studies to inhibit osteosarcoma cell growth through a dual mechanism involving ferroptosis and apoptosis induction, as well as the promotion of ROS accumulation.67 In a similar vein, oridonin enhances RSL3-induced ferroptosis in breast cancer cells through the activation of the JNK/Nrf2/HO-1 oxidative stress pathway, which results in the accumulation of ROS.68 It is noteworthy that oridonin, apart from promoting ferroptosis, also facilitates cell death by triggering caspase-dependent apoptosis in colorectal cancer cells through the ROS/JNK/c-Jun signaling axis.69
Celastrol
Celastrol, a quinone-methylated triterpenoid compound derived from the dried root of Tripterygium wilfordii Hook. f., a plant in the Celastraceae family, exhibits a diverse range of biological activities, such as anti-inflammatory, anti-rheumatoid, and anticancer properties. Studies have shown that celastrol effectively treats non-small cell lung cancer by inhibiting STAT3 signaling and promoting ROS accumulation.70 Furthermore, Chen et al illustrated that celastrol can induce ROS-mediated apoptosis in gastric cancer cells by specifically targeting peroxiredoxin-2.71 In the realm of drug combinations, it was discovered that the co-administration of erastin, a ferroptosis inducer, and celastrol effectively induced cell death in NSCLC cells at nontoxic concentrations, leading to a marked increase in ROS production, disruption of mitochondrial membrane potential, and stimulation of mitochondrial fission.72
Oleanolic Acid
Oleanolic acid, a triterpenoid compound, is derived from the dried mature fruits of Ligustrum lucidum, a plant in the family Oleaceae Hoffmanns. & Link, and the root bark and stem bark of Aralia chinensis, a plant in the family Araliaceae. Recent studies have shown that oleanolic acid exhibits potential anti-cancer properties by inhibiting the proliferation of cervical cancer Hela cells through the regulation of the ACSL4-related ferroptosis signaling pathway and the promotion of Fe2+ and ROS accumulation.73 Furthermore, oleanolic acid has been shown to trigger autophagic cell death in hepatocellular carcinoma cells via the PI3K/Akt/mTOR and ROS-dependent signaling pathways.74 It is worth noting that the co-administration of oleanolic acid with the FDA-approved drug sorafenib, at sublethal concentrations, presents a promising strategy for enhancing oxidative stress and elevating ROS levels to induce cell death in hepatocellular carcinoma.75
Artesunate
Artesunate, a derivative of Artemisia annua Linn. from the Asteraceae family, is a proven antimalarial drug with additional anti-tumor properties. Recent studies have demonstrated its ability to induce endoplasmic reticulum stress-driven ROS-mediated cell death in hepatocellular carcinoma cells by disrupting unstable iron pools and iron redistribution.76 Moreover, it has been suggested that artesunate instigates ferroptosis in myeloma cells through the inhibition of SREBP2 nuclear translocation and the upregulation of ROS, Fe2+, and lipid peroxidation.77 Additionally, Zhou et al discovered that artesunate induces autophagy-mediated apoptosis in human bladder cancer cells by increasing ROS levels and activating the AMPK-mTOR-ULK1 signaling pathway.78
Flavonoids
Flavonoids, a group of natural compounds characterized by benzene and furan ring structures, have been the subject of recent research indicating their potential dual function as antioxidants and inducers of ROS in various tumor cells and cancer types. This dual role can be attributed to the chemical structure of flavonoids, which feature multiple electrophilic groups and aromatic ring structures, contributing to their antioxidant properties by scavenging free radicals and stabilizing intracellular oxidative conditions. Conversely, flavonoids can interact with cell membrane receptors, leading to the production of ROS and subsequent activation of various cellular signaling pathways, influencing cellular physiological processes. Notable flavonoid components known to induce ROS generation include naringenin,79–81 quercetin,82–84 kaempferol,85–87 luteolin,88–90 wogonin,91,92 apigenin,93–95 hesperetin,96,97 epigallocatechin gallate (EGCG),98–100 hesperidin,101–103 and others. These compounds are commonly present in a variety of fruits, vegetables, teas, and TCM, sharing a common or similar parent core structure that results in significant similarities in physicochemical properties. Research has shown that flavonoids possess the ability to impede tumor growth and metastasis, induce cell cycle arrest, and trigger various forms of cell death by influencing multiple ROS-related signaling pathways, including AMPK, p38, AKT/mTOR, and ASK1/JNK.
Alkaloids
Alkaloids, characterized by the presence of nitrogen atoms and exhibiting base-like properties, are a class of compounds with significant medicinal value. These compounds possess structural features such as nitrogen-containing heterocycles and aromatic rings, which contribute to their diverse biological activities. Among the alkaloid components capable of inducing the generation of ROS are berberine, hydroxycamptothecin, piperlongumine, tetrandrine, vincristine, and numerous other compounds.
Berberine
Berberine, an isoquinoline alkaloid sourced from the medicinal plant Coptis chinensis Franch. in Ranunculaceae family, exhibits a range of pharmacological effects, such as antibacterial, antiviral, hypoglycemic, hypolipidemic, anti-gastric ulcer, and treatment of cardiovascular and cerebral vascular diseases. In recent years, there has been an increasing focus on the potential antitumor effects of berberine. Chen et al have illustrated that berberine can trigger apoptosis in NSCLC cells through the ROS-mediated activation of the ASK1/JNK pathway and the mitochondrial pathway.104 Furthermore, berberine has been shown to impede the progression of renal cancer cells by regulating ROS production and promoting DNA damage.105 In glioblastoma multiforme U87MG cells, berberine has been found to elevate levels of ROS, thiobarbituric acid reactive substances, and protein carbonylation through the induction of oxidative stress.106
7-Ethyl-10-Hydroxycamptothecin
7-ethyl-10-hydroxycamptothecin, also known as SN-38, is an indole alkaloid derived from the seeds or root bark of Camptotheca acuminata Decne. in the Davidia involucrata Baill. This compound is commonly utilized in clinical oncology due to its close association with DNA topoisomerase I, a key enzyme involved in DNA replication and transcription regulation. Research has demonstrated that SN-38 effectively enhances ROS production in LOVO and HCT116 cells, and in combination with chloroquine, it synergistically induces a heightened ROS level. This effect may be attributed to the p53-ROS crosstalk, as well as the activation of lysosomal and mitochondrial apoptotic pathways.107
Piperlongumine
Piperlongumine, a naturally occurring alkaloid present in the fruit or root of the Piperaceae Giseke plant in the Piper longum L. family, exhibits anti-inflammatory, anti-bacterial, anti-tumor, and anti-angiogenic properties. Mitra et al have demonstrated the remarkable therapeutic potential of piperlongumine in combating various types of cancers, particularly breast cancer.108 When combined with piperlongumine, clinical drugs such as doxorubicin and docetaxel have shown synergistic effects that surpass the efficacy of individual agents.109 Moreover, studies have demonstrated that piperlongumine enhances ROS production and diminishes stem cell and epithelial cell mesenchymal transition phenotypes in SOX9-deficient human lung cancer cells, exhibiting heightened anti-metastatic properties.110 Intriguingly, piperlongumine has the potential to act as a potent ROS inducer and, in conjunction with the therapeutic agent bortezomib, it can synergistically instigate cholangiocarcinoma cell death via oxidative stress pathways.111
Tetrandrine
Tetrandrine, a bisbenzylisoquinoline alkaloid derived from the tuberous roots of Stephania tetrandra S. Moore, a plant belonging to the Menispermaceae family, exhibits anti-inflammatory, anti-allergic, platelet aggregation inhibiting, and anticancer properties. Research has indicated that tetrandrine induces ROS production in human lung cancer A549 cells. The inhibition of mitochondrial ATP production using oligomycin and the uncoupling agent FCCP not only amplifies tetrandrine-induced ROS generation but also enhances the cytotoxic effects of tetrandrine.112 Furthermore, certain researchers have shown that tetrandrine-induced ROS also triggers non-liganded Fas-mediated apoptosis through the activation of procaspase-8 and bid cleavage in prostate cancer cells.113 Additionally, Liu et al discovered that tetrandrine suppresses tumor stem cell properties and the epithelial-mesenchymal transition process in triple-negative breast cancer by modulating the SOD1/ROS signaling pathway.114 SOD1, a pivotal antioxidant enzyme prevalent in eukaryotic cells, serves a primary function in catalyzing the dismutation reaction of •O2−, yielding H2O2 and O2. This process is of paramount importance for maintaining intracellular redox homeostasis, since ROS, when present in moderation, act as signaling molecules involved in physiological processes such as cell proliferation and differentiation. Conversely, an overabundance of ROS can lead to oxidative stress, compromising cellular structures and functions, and potentially triggering apoptosis.
Vincristine
Vincristine, an alkaloid derived from Catharanthus roseus (L). G. Don of the Apocynaceae family, is a widely utilized antitumor medication in clinical settings for the management of various cancer types, particularly childhood acute lymphoblastic leukemia. Its primary mode of action involves the inhibition of microtubule protein polymerization and disruption of spindle formation during cell division, thereby impeding the proliferation of cancer cells. Furthermore, vincristine exhibits immunomodulatory and anti-inflammatory properties that have the potential to augment immune function within the body. Research indicates that vincristine initiates a series of axonal pathological events, ultimately leading to mitochondrial dysfunction, increased levels of axonal ROS, and SARM1-mediated axonal degeneration.115 Early studies from 2002 identified the regulatory role of ROS in the initial stages of the mitochondrial signaling pathway responsible for vincristine-induced apoptosis in acute lymphoblastic leukemia cells.116
Polyphenols
Polyphenols are a group of compounds with multiple phenolic hydroxyl groups, distinguished by the presence of multiple hydroxyl groups on various aromatic rings. It is important to note that hydroxyl groups possess the ability to provide hydrogen atoms and engage in antioxidant reactions by directly capturing free radicals, while polyphenolic compounds induce intracellular ROS generation through binding to metal ions or interacting with receptors on cell membranes to modulate redox reactions. Examples of polyphenols capable of inducing ROS generation include curcumin, resveratrol, proanthocyanidin, and various other compounds.
Curcumin
Curcumin, a yellow pigment extracted from the rhizomes of the Zingiberaceae plant Curcuma longa L., is an acidic polyphenol with varied pharmacological properties. A recent scholarly investigation has corroborated that curcumin possesses a distinctive capacity for mitochondrial targeting, which results in mitochondrial dysfunction and fatal mitochondrial phagocytosis. This is accompanied by an increase in SDH activity and an overproduction of ROS, which collectively augment the effectiveness of radioactive iodine in eliminating thyroid cancer cells.117 The ROS-mediated KEAP1/NRF2/miR-34a/b/c cascade signaling pathway is instrumental in curcumin’s anticancer process, demonstrating the potential to impede the metastasis of colorectal cancer cells via a p53-independent mechanism.118 In a similar vein, curcumin has been observed to stimulate cellular pyroptosis and apoptosis, while concurrently inhibiting the proliferation of HepG2 cells through the activation of ROS signaling.119
Resveratrol
Resveratrol, a non-flavonoid polyphenolic organic compound, is commonly present in various fruits and TCM and is recognized for its potent antioxidant properties that safeguard cells against oxidative harm. Interestingly, multiple studies have demonstrated that resveratrol can trigger various cell death pathways reliant on ROS and impede tumor progression. Liu et al discovered that resveratrol can induce ferroptosis in acute myeloid leukemia cells through a ROS-dependent mechanism involving the Hsa-miR-335-5p/NFS1/GPX4 pathway.120 In glioma cells, treatment with resveratrol at a concentration of 15 μM for 48 hours resulted in increased production of ROS, activation of autophagy, induction of endoplasmic reticulum stress and apoptosis, and decreased survival of both 2D and 3D clones.121 Notably, a study conducted by several researchers demonstrated that pairwise combinations of resveratrol, EGCG, and diallyl trisulfide led to enhanced ROS production through the mitochondrial caspase-dependent pathway, ultimately resulting in cell death in the A431 skin cancer cell line.98
Proanthocyanidin
Proanthocyanidin, a polyphenolic polymer, is commonly found in various natural sources such as apples, hawthorns, grapes, strawberries, peanuts, and other plants. It is also recognized as one of the polyphenolic constituents in TCM. Research conducted as early as 2009 demonstrated that proanthocyanidin present in beer can trigger apoptosis, protein carbonylation, and cytoskeleton disorganization in human colorectal adenocarcinoma cells through the generation of ROS.122 Subsequent studies have further revealed that proanthocyanidin derived from cranberry exhibits anti-angiogenic properties and cytotoxic effects on ovarian cancer cells, inducing apoptosis, G2/M phase cell cycle arrest, and ROS generation in chemotherapy-resistant SKOV-3 cells through both endogenous and exogenous pathways.123
Polysaccharides
Polysaccharides, a group of compounds composed of multiple glycosyl units, are prevalent in various organisms such as plants, fungi, and marine species, exhibiting a wide range of biological functions. Several polysaccharide constituents, including lentinan and astragalus polysaccharide, have been identified as capable of inducing the generation of ROS.
Lentinan
Lentinan, a polysaccharide compound derived from shiitake mushrooms, is characterized by polysaccharide chains composed of β-(1→3)-D-glucan and β-(1→6)-D-glucan arranged in alternating patterns. Treatment of lung cancer A549 cells with lentinan resulted in elevated levels of ROS and decreased activity of GPX4. The heightened ROS levels triggered the activation of caspase-3, leading to DNA double-strand breaks and inhibition of cell proliferation.124 Further investigations have revealed that the concurrent administration of paclitaxel and lentinan exhibits a synergistic apoptotic impact on A549 cells by promoting ROS production, triggering the activation of the NLRP3 inflammasome, and stimulating the ASK1/p38 MAPK signaling pathway.125
Astragalus Polysaccharide
Astragalus polysaccharide, a polysaccharide compound derived from the roots of the Leguminosae plant Astragalus membranaceus var. mongholicus (Bunge) P.K.Hsiao, primarily consists of polysaccharide chains made up of α-glucose and galactose chains. Recent scholarly investigations have elucidated that an innovative cold-water-soluble polysaccharide, derived from Astragalus membranaceus, possesses the capability to trigger mitochondria-dependent apoptosis in gastric cancer MGC-803 cells. This induction leads to the accumulation of intracellular ROS, the disruption of mitochondrial membrane potential, an augmentation in the Bax/Bcl-2 ratio, and the subsequent activation of caspase-9/-3 expression.126
Sesquiterpene Lactones
Sesquiterpene lactones, a diverse class of secondary metabolites primarily sourced from plants within the Compositae family, exhibit a broad spectrum of biological properties, such as anti-tumorigenic, anti-inflammatory, and antibacterial effects.
Parthenolide
Parthenolide, a naturally occurring compound extracted from the flower buds of the Compositae plant Tanacetum Parthenium, is characterized by its structural composition, which includes an α-methyl-γ-lactone ring and a terpene framework. Recent research has demonstrated the potential of parthenolide to inhibit the progression of various malignant tumors, including lymphoid malignancies, osteosarcoma, breast cancer, and multiple myeloma, through the induction of ROS-mediated apoptosis and autophagic cell death.127–130
Development of Nanoformulations of ROS-Inducing Active Ingredients Derived from TCM
As previously noted, active ingredients sourced from TCM offer distinct advantages over traditional clinical chemotherapeutic agents, including mild effects, multi-targeting capabilities, synergistic properties, and ease of accessibility. However, these active ingredients also present limitations such as poor water solubility, low bioavailability, complex dosage requirements, short circulation periods in the bloodstream, and limited accumulation at tumor sites.
To address these challenges, the utilization of multifunctional nanoformulations emerges as a promising solution. Through the application of advanced nanotechnology, the precise delivery of active compounds capable of inducing ROS generation from TCM can be realized, leading to targeted release of nanomedicines, improved bioavailability, decreased toxicity, and optimal synergistic effects of various drug types. Utilizing nanocarriers facilitates the more precise accumulation of drugs at the tumor site, thereby minimizing harm to normal tissues and greatly enhancing therapeutic efficacy. Furthermore, nanoformulations can extend drug circulation, increase stability, prevent rapid metabolism, and enhance the sustained therapeutic impact.
Through a comprehensive analysis of existing literature, we have compiled a summary of prevalent nanoformulations containing ROS-inducing active compounds in TCM and their potential multifunctional efficacy benefits, as depicted in Figure 4.
 |
Figure 4 An overview of prevalent nanoformulations containing ROS-inducing active ingredients in TCM and their potential multifunctional efficacy benefits (drawn by Figdraw, ID:TRPWO2c2ee). |
Plumbagin-Loaded Nanoformulations
A novel lipid-polymer hybrid nanoparticle formulation loaded with plumbagin and transferrin, developed by Sakpakdeejaroen et al, effectively targets tumors by harnessing the high expression of transferrin receptors on cancer cells.131 Further research has delved into the potential of plumbagin-loaded silver nanoparticles in targeting tumor angiogenesis, inducing ROS generation, and eliciting tumor cell apoptosis.132,133 The immunosuppressive tumor microenvironment (TME) poses a significant challenge in the treatment of hepatocellular carcinoma. To address this, Han et al have formulated a biomimetic nanostructure containing low-dose plumbagin and dihydrotanshinone I, encapsulated within an erythrocyte membrane, which successfully reverses the chemo-immunotherapeutic constraints imposed by the immunosuppressive TME.134
Thymoquinone-Loaded Nanoformulations
The study conducted by Gulbay et al introduced a thymoquinone-loaded nanoparticle that exhibited enhanced cellular uptake and stability of thymoquinone, ultimately resulting in improved therapeutic outcomes for endometrial cancer cells.135 In the context of breast cancer treatment, thymoquinone loaded cubic phase nanoparticles, prepared using the emulsification homogenization method, exhibited favorable subcellular localization and pro-apoptotic effects.136 Furthermore, thymoquinone loaded mesoporous silica nanoparticles were found to inhibit cell invasion and enhance in vitro cytotoxicity through ROS-mediated apoptosis.137 Additionally, Ibrahim et al developed thymoquinone-loaded PLGA nanoparticles for the treatment of melanoma by leveraging sustained release and tailored size to enhance permeability and retention effects.138
Shikonin-Loaded Nanoformulations
Recent research indicates that the utilization of shikonin-loaded nanomedicines has shown promise as an effective approach to cancer treatment. Various nanodrug delivery platforms, including liposomes, polymeric micelles, nanoparticles, nanogels, and nanoemulsions, have been employed to facilitate the targeted delivery of shikonin, thereby enhancing its anti-tumor efficacy.139 For instance, the use of functional liposomes encapsulating shikonin has demonstrated potential for cancer therapy through the induction of ROS-boosting necroptosis.140 Furthermore, Chen et al have devised a delivery nanosystem incorporating shikonin and hyaluronic acid-modified hollow Fe-MOF, serving as an effective glycolysis-meditated agent to enhance the effectiveness of microwave thermotherapy.141 In terms of immunomodulation, Long et al have developed shikonin-loaded and hyaluronic acid-modified MPDA nanoparticles for immunotherapy purposes, which can induce immune-metabolic reprogramming and regulate glycolysis, EMT, and anticancer immunity by inhibiting PKM2. This targeted drug delivery approach shows great promise for the treatment of colorectal cancer.142
Gambogic Acid-Loaded Nanoformulations
In contemporary research, the utilization of gambogic acid nano-delivery has emerged as a promising strategy for tumor therapy.143 A gambogic acid nanoparticle with low toxicity and high tumor-targeting capabilities has been adeptly fabricated through a two-step emulsification process by Huang et al. His nanoparticles, coated with CT26 colon cancer cell membrane on a poly (lactic-co-glycolic acid)/GA foundation, demonstrate the ability to stimulate dendritic cells and effectively immunize against cancer.144 Furthermore, a thermosensitive injectable hydrogel with a rapid gelation time and excellent biocompatibility has been successfully formulated by Zhang et al. This hydrogel has shown notable enhancement in therapeutic efficacy against gastric cancer when encapsulating gambogic acid nanoparticles and the tumor-penetrating peptide iRGD.145
Emodin-Loaded Nanoformulations
A method for emodin-conjugated PEGylation of Fe3O4 nanoparticles has been developed by Ren et al, facilitating the application of these nanoparticles in FI/MRI dual-modal imaging as well as targeted therapy in mice with pancreatic tumor xenografts.146 Additionally, other researchers synthesized ROS/pH dual-sensitive polymer micelles for the simultaneous delivery of emodin and chlorambucil. This approach enhances oxidative stress levels by inducing ROS production and GSH depletion, while also reducing chlorambucil exocytosis. These effects collectively enhance drug chemotoxicity and improve the efficacy of tumor therapy.147
Chrysophanol-Loaded Nanoformulations
A gold-chrysophanol-loaded poly (DL-lactide-co-glycolide) nanoparticle, synthesized by Lu et al, has demonstrated their capability to modulate the p53/ROS crosstalk. These nanoparticles suppress histone deacetylase and AKT signaling pathways by regulating cell cycle proteins, ultimately leading to cell cycle arrest at the sub-G phase. This arrest consequently inhibits prostate cancer cell proliferation and triggers apoptosis. Furthermore, these nanoparticles improve the bioavailability of chrysophanol to a certain extent.148
Terpenoid-Loaded Nanoformulations
Costunolide-Loaded Nanoformulations
Several academics have developed optimized bilosome-based nanoparticles incorporating costunolide, which have demonstrated efficacy in inhibiting colorectal cancer LS174T cells in the sub-G1 phase, inducing apoptosis through up-regulation of caspase-3, TP53, and BAX, and down-regulation of BCL2 mRNA levels, as well as compromising cell membrane integrity. These nanoparticles also enhance cytochrome c release and ROS generation to further promote apoptosis and reduce cell membrane integrity, thereby exerting potent anti-tumor effects. Importantly, these nanoparticles exhibited no cytotoxic effects on normal human colonic epithelial cells, indicating a favorable safety profile and relative selectivity.149
β-Elemene-Loaded Nanoformulations
A novel nanodelivery system, incorporating two-dimensional stanene-based nanosheets and β-elemene, has been developed by Chen et al to counteract the immunosuppressive effects caused by tumor-associated macrophages in solid tumors. This formulation effectively mitigates TAM-induced immunosuppression, thereby augmenting the effectiveness of chemo-immunotherapy.150 Additionally, other researchers utilized a liquid-phase exfoliation method to produce the stanene-based nanosheets essential for creating the β-elemene delivery system. This nanoformulation boasts a remarkable photothermal conversion efficiency and can harness ultrasound for ROS generation, positioning it as a viable option for near-infrared-mediated photothermal therapy and precise delivery of anticancer drugs.151
Oridonin-Loaded Nanoformulations
Recently, a multifunctional therapeutic nanoplatform has been developed for the precise diagnosis and efficient treatment of pancreatic cancer. This nanoformulation utilizes gold nanocages that have been modified with hyaluronic acid and conjugated with anti-glypican-1 antibody, oridonin, gadolinium, and Cy7 dye. The nanoplatform exhibits long-term stability, as well as fluorescent and MRI properties, enabling multimodal imaging and targeted therapy in pancreatic tumor xenografted mice.152 Additionally, Cai et al synthetized a cell-penetrating peptide-modified metal-organic framework nanoplatform for targeted co-delivery of oridonin and survivin siRNA in vivo.153 Furthermore, lipid-layered cisplatin has been co-encapsulated with oridonin in nanoparticles utilizing drug conjugation techniques for the combined treatment of lung cancer.154 Moreover, GE11 peptide-conjugated selenium nanoparticles have been synthesized, designed to specifically target cancer cells overexpressing EGFR. This approach aims to enhance anticancer efficacy while minimizing toxicity to normal cells.155
Celastrol-Loaded Nanoformulations
Niu et al successfully synthesized two nanoconjugates by conjugating celastrol onto PEGylated EpCAM aptamer or antibody dendrimers. Their study demonstrated, for the first time, that these nanoformulations can enhance the specificity of tumor accumulation and penetration in cancer animal models. Additionally, the nanoconjugates were found to effectively deliver celastrol with a favorable biosafety profile.156 Furthermore, Niu et al developed a tumor-targeted, ROS-responsive celastrol-loaded smart nanoparticle that increased endogenous ROS levels through celastrol delivery, thereby inducing tumor cell apoptosis.157 Moreover, other researchers created novel polymeric nanomicelles loaded with celastrol to enhance the inhibition of retinoblastoma growth and angiogenesis by addressing the low water solubility of celastrol.158
Oleanolic Acid-Loaded Nanoformulations
Several researchers have successfully developed a novel EGFR-targeted, oleanolic acid-loaded albumin nanoparticle with promising haemocompatibility and lung safety profiles, demonstrating the potential for targeted delivery of oleanolic acid in lung cancer treatment.159 Furthermore, Kumbham et al utilized oleanolic acid-coupled human serum albumin nanoparticles encapsulated with adriamycin to achieve synergistic chemotherapy effects, effectively inhibiting oropharyngeal carcinoma and melanoma progression.160 Notably, Zhang et al have determined that the co-delivery of cisplatin and oleanolic acid through silicon nanoparticles resulted in a significant enhancement of apoptosis and reversal of multidrug resistance in lung cancer cells.161 Similarly, Bao et al have shown that paclitaxel-loaded oleanolic acid nanoparticles offer the advantage of synergistic chemotherapy and hold promise for the treatment of breast cancer and brain metastasis originating from breast cancer.162
Artesunate-Loaded Nanoformulations
To address the challenges posed by the water solubility and bioavailability of artesunate, Xia et al developed solid lipid nanoparticles loaded with artesunate. This nanoformulation was found to increase Fe2+ levels, inhibit AKT/mTOR signaling, downregulate GPX4 expression, and induce ferroptosis in esophageal squamous cell carcinoma cells.163 Other researchers have employed a nano-chemotherapeutic strategy utilizing PEGylated graphene oxide loaded with superparamagnetic iron oxide nanoparticles and artesunate. This approach effectively induces oxidative stress through the activation of the Fenton effect, leading to the release of Fe2+ and subsequent destruction of cancer cells.164 Recent literature has also highlighted the efficacy of multifunctional artesunate-loaded nanoformulations derived from various materials in the treatment of tumors through chemo-photothermal, chemo-catalytic, and chemo-dynamic mechanisms, all of which have demonstrated significant effectiveness.165–167
Flavonoid-Loaded Nanoformulations
Naringenin-Loaded Nanoformulations
Several researchers’ group developed a chitosan-coated naringenin nanoparticle utilizing chitosan as a base material, demonstrating its ability to impede the proliferation of breast cancer cells and induce apoptosis through the efficient elevation of oxidative stress, facilitation of ROS release, and activation of caspase-3 activity.168 To enhance the therapeutic potential of naringenin’s anticancer properties, researchers have utilized macrophage-encapsulated bionic proteolipid vesicles to deliver the compound. This approach has demonstrated improved biocompatibility of the nanoformulation and has effectively suppressed the proliferation and metastasis of lung cancer cells by modulating the apoptosis signaling pathway.169 Furthermore, naringenin-loaded liquid crystalline nanoparticles have exhibited promising anti-inflammatory and anticancer effects in human airway epithelial-derived basal cells and human lung epithelial cancer cells, respectively.170
Quercetin-Loaded Nanoformulations
Various nanoformulations of quercetin have been studied for their preclinical anticancer activity and mechanisms.171,172 A recent study by Das et al demonstrated the efficacy of a quercetin-encapsulated chitosan nanoparticle in inducing mitochondrial dysfunction, enhancing ROS production, inducing apoptosis, inhibiting colony formation and migration, promoting chromatin condensation in oral cancer CAL33 cells, and inhibiting tumor progression.173 It is worth noting that solid lipid nanoparticles co-coated with a clinically targeted drug erlotinib and quercetin, can achieve synergistic effects by inhibiting the expression of nuclear EGFR and targeting lung cancer through the PI3K/AKT signaling pathway.174
Kaempferol-Loaded Nanoformulations
In 2012, researchers developed kaempferol-containing nonionic polymeric nanoparticles that exhibit potent anti-tumor activity while demonstrating minimal toxicity toward healthy cells.175 Subsequent investigations have revealed that kaempferol, a promising anticancer agent, can be integrated into gold nanoclusters to form nanoparticles. These composite nanoclusters exhibit low cytotoxicity towards normal human cells and high cytotoxicity towards lung cancer A549 cells, enabling targeted delivery of anticancer drugs and facilitating bioimaging through the destruction of cancer cell nuclei.176 Furthermore, Mollaei et al developed a novel nanoformulation of kaempferol, utilizing human serum albumin as an adjuvant and folic acid-linked chitosan polymer to enhance cellular uptake, water solubility, and anti-tumor efficacy of kaempferol.177
Luteolin-Loaded Nanoformulations
To improve the specificity of luteolin in targeting tumor cells, researchers developed a novel luteolin-coated gold nanoparticle capable of inducing cytotoxic effects on HeLa cells by arresting the cell cycle at the subG1 phase and triggering apoptosis in tumor cells via the activation of caspase-3, caspase-8, and caspase-9.178 Furthermore, Fu et al conducted a study in which they developed highly efficient luteolin-loaded nanoformulations utilizing poly(ethylene glycol). Their findings demonstrated that these nanoformulations effectively suppressed the proliferation, migration, and invasion of tumor cells, and exhibited superior efficacy in tumor tissues compared to free luteolin.179 To enhance the treatment of gliomas, Wu et al developed folic acid-modified poly(ethylene glycol)-poly(ε-caprolactone) nano-micelles containing luteolin, which demonstrated inhibition of tumor cell growth, increased cell invasion compared to free luteolin, and induced greater apoptosis. Additionally, no significant adverse effects were observed in mice following treatment.180
Wogonin-Loaded Nanoformulations
Sabra et al utilized self-assembly techniques to create a nanomicellar formulation containing amphiphilic zeaxolysin-lactoferrin encapsulated with rapamycin and wogonin. This nanoformulation demonstrated enhanced cytotoxicity against MCF-7 breast cancer cells and ascites tumor animal models, as well as improved tumor targeting, resulting in increased anti-tumor efficacy and reduced side effects.181 Furthermore, Huang et al developed a nanoformulation consisting of cadmium-telluride quantum dots with wogonin. This formulation was found to effectively address multidrug resistance in cells and induce apoptosis by facilitating the interaction between wogonin and leukemia KA cells.182 To regulate the release rate of wogonin, researchers encapsulated the compound in solid lipids within the nanoformulation, resulting in a sustained and controlled release of the drug over a period of 72 hours, leading to increased cytotoxicity.183
Apigenin-Loaded Nanoformulations
Recently, researchers have incorporated apigenin into chitosan to enhance its hydrophobicity and subsequently coated it with albumin-folic acid to improve stability and bioavailability, resulting in successful targeting of HepG2 cancer cells.184 Additionally, other researchers have developed lactose-tailored PLGA nanoformulations loaded with apigenin, demonstrating increased cytotoxicity and apoptotic potential compared to free apigenin.185
Hesperetin-Loaded Nanoformulations
To address the challenge of poor water solubility in hesperetin, researchers have developed a chitosan folate hesperetin nanoformulation through the covalent conjugation of folic acid with chitosan molecules. This nanoformulation leverages the targeting capabilities of folic acid and the permeability of nanoparticles to enhance the delivery of hesperetin in colorectal cancer and elicit anti-tumor effects.186 Furthermore, it is worth mentioning that additional researchers have developed chitosan nanoparticles incorporating DCLK1-functionalized folic acid conjugated hesperetin, with a specific focus on targeting colon cancer stem cells, using a similar approach.187 Moreover, Wang et al have devised a targeted drug delivery system utilizing hyaluronic acid-modified liposomes loaded with cisplatin and hesperetin to enhance the synergistic anticancer effects of clinical drugs, thereby reducing systemic toxicity and improving the efficacy against triple-negative breast cancer.188
EGCG-Loaded Nanoformulations
Recent research has demonstrated the development of EGCG-loaded flower-shaped Au nanoclusters, gold nanoparticles, and PLGA-encapsulated nanoformulations for enhanced delivery of EGCG to cancer cells.189–191 Han et al employed microfluidics technology to create a fluorinated assembly of EGCG-ligands-siTOX nanoparticles for immunotherapy, with the goal of synergistically modulating tumor cells and exhausted T cells to improve the therapeutic response rate in cold tumors.192 In the realm of liver cancer treatment, He et al developed thermosensitive nanospheres containing fluorinated-modified EGCG, which effectively induce immunogenic cell death and damage-associated molecular patterns.193
Hesperidin-Loaded Nanoformulations
Research has demonstrated that gold nanoparticles loaded with hesperidin exhibit high biocompatibility and serve as effective drug delivery systems, showing promise as novel agents for combating cancer and inflammation, as well as inducing phagocytosis.194 Furthermore, several studies have validated the use of magnetic casein-CaFe2O4 nanohybrid carrier conjugated with progesterone to enhance the cytotoxicity of hesperidin against breast and ovarian cancer.195 Additionally, the nanoformulation of imatinib and hesperidin has been demonstrated to target MDR-1 gene expression, Bax/Bcl-2, caspase-3, and Ki-67 associated with drug resistance and apoptosis, thereby significantly improving the effectiveness of the anticancer treatment compared to using either agent alone.196
Alkaloid-Loaded Nanoformulations
Berberine-Loaded Nanoformulations
Singh et al developed a hydrophilic nanogel comprising chitosan and sodium alginate through the ion gelation technique to deliver berberine hydrochloride, enhancing its uptake and promoting apoptotic cell death mediated by oxidative stress in HepG2 cells.197 Furthermore, researchers have utilized CD44-labelled hyaluronic acid-chitosan liposome carriers to encapsulate berberine and doxorubicin, allowing for sustained release of both compounds in diverse physiological conditions characterized by high permeability and increased cytotoxicity. This approach has shown promising outcomes in the treatment of lung cancer.198 Additionally, berberine-loaded Janus gold mesoporous silica nanocarriers and glucose-coated berberine nanodrugs have been developed for multidimensional tumor therapy.199
SN38-Loaded Nanoformulations
Recent advancements in nanodrug delivery systems for SN38 in the field of antitumor research have shown rapid development.200,201 Liu et al have developed a polymeric, ROS-responsive nanoformulation for in vivo drug delivery, which has shown promising results in enhancing DNA damage and inducing DC cell maturation for cancer immunotherapy through ultrasound-triggered sonodynamic therapy.202 Wu et al developed a biocompatible superparamagnetic chitosan-based nanoplatform for targeted delivery of SN38 in a mouse xenograft colorectal cancer model, resulting in a tumor inhibition rate of 81%.203 Furthermore, various formulations such as SN38-conjugated gold nanoparticles, CD133-targeted self-assembled PEGylated carboxymethylcellulose-SN38 nanoparticles, and novel polymer micelles containing SN38 have shown promising results in preclinical studies across multiple cancer types.204–206
Piperlongumine-Loaded Nanoformulations
A PLGA-based nanomedicine containing piperlongumine was developed by Singh et al, which demonstrated the ability to disrupt glycolytic metabolism in triple-negative breast cancer stem cells through the regulation of GAPDH and FBP1.207 Additionally, this nanomedicine was found to inhibit cancer stem-like cells by modulating STAT3 in a triple-negative breast cancer mammosphere model.208 In the realm of drug conjugation for clinical applications, Liu et al employed PLGA and D-α-tocopheryl polyethylene glycol succinate as carriers for the simultaneous delivery of paclitaxel and piperlongumine. Their nanoformulations exhibited significant antitumor efficacy and minimal toxicity towards non-target tissues in a HepG2 xenograft tumor model when compared to the free drug.209
Tetrandrine-Loaded Nanoformulations
A new bionic platelet membrane-encapsulated delivery system for tetrandrine has been developed using polycaprolactone-b-poly-b-polycaprolactone nanoparticles by Jiang et al. This system demonstrated enhanced evasion of macrophage phagocytosis and improved antitumor efficacy against non-small cell lung cancer.210 Additionally, several research groups have synthesized self-assembled reduction-sensitive paclitaxel dimer prodrug and tetrandrine nanoformulations to enhance the synergistic therapeutic effects and drug release.211 Notably, Li et al developed a novel supramolecular synergistic drug delivery nanoformulation utilizing advanced “carrier-free” self-assembled nanofibers for the simultaneous delivery of paclitaxel and tetrandrine. This formulation was found to enhance the clinical efficacy of paclitaxel in gastric cancer by promoting apoptosis in mitochondria, while also reducing associated side effects.212
Vincristine-Loaded Nanoformulations
Numerous studies have investigated the use of vincristine-based nanoformulations in both preclinical and clinical settings.213 Researchers have utilized smart polymer magnetic nanocarriers with dextran shells, superparamagnetic iron oxide cores, and folic acid to deliver vincristine to testicular tumor cells, resulting in a significant increase in apoptosis levels.214 Wang et al developed an anti-CD133 antibody-targeted therapeutic immunomagnetic albumin microbeads loaded with vincristine, which demonstrated efficacy in suppressing tumor cell migration, invasion, and brain-targeted diagnosis and therapy from an immunotherapeutic standpoint.215 Furthermore, a variety of smart nanoparticles containing vincristine have been engineered for the effective management of diverse tumor types.216,217
Polyphenol-Loaded Nanoformulations
Curcumin-Loaded Nanoformulations
Recent research has demonstrated the widespread utilization of curcumin-loaded nanoformulations for combination therapy involving drug-drug interactions and gene disruption-drug interactions. Vahedi et al developed a dual drug delivery system utilizing a polyamide-amine dendrimer to encapsulate curcumin and form a complex with linc-RoR siRNA at an optimal N/P ratio. This nanoformulation was effectively internalized in MCF-7 breast cancer cells, leading to synergistic cytotoxic effects through the dual delivery of linc-RoR siRNA and curcumin. This resulted in cell cycle arrest in the G1 phase and induction of apoptosis.218 In the realm of breast cancer treatment, other researchers have co-encapsulated gemcitabine with curcumin in folate-modified PLGA nanoparticles, resulting in significant accumulation in MDA-MB-231 cells. This nanoformulation demonstrated enhanced efficacy in inducing cytotoxicity, apoptosis, and cell cycle arrest, as well as overcoming drug resistance in breast cancer compared to individual agent formulations.219 Furthermore, Amandi et al pioneered the construction of artemisinin- and curcumin-loaded liposomal nanoparticles for the co-delivery of these active ingredients in TCM, resulting in the promotion of apoptosis, induction of cell cycle blockade, and significant anti-cancer effects on human colorectal cancer cells.220
Resveratrol-Loaded Nanoformulations
The issue of low bioavailability of resveratrol has been addressed by Wang et al, who developed D-α-Tocopheryl polyethylene glycol 1000 succinate-resveratrol-solid lipid nanoparticles to enhance its therapeutic effectiveness, particularly in combating multidrug resistance in breast cancer.221 Additionally, Liu et al developed gold nanoparticles functionalized with Au-Se-bonded peptides for triple-negative breast cancer therapy. These nanoparticles, loaded with resveratrol, functioned as gatekeepers to prevent glutathione interference in the bloodstream, resulting in prolonged release of resveratrol at the tumor site and enhanced therapeutic efficacy.222 Notably, significant progress has been made by several researchers who have successfully developed biocompatible resveratrol-conjugated gold nanoparticles using an innovative green nanotechnology method. These nanoformulations have exhibited remarkable in vitro stability in various biological environments and have proven effective in human breast (MDAMB-231), pancreatic (PANC-1), and prostate (PC-3) cancer cell lines.223
Proanthocyanidin-Loaded Nanoformulations
Research has progressed in the development of deformable nanoparticles responsive to the TME, with Qian et al introducing a novel nanoformulation. This nanoformulation incorporated a tumor acid-responsive peptide checkpoint inhibitor polymer (PEG-DMA-DPA-1), combined with a mixture of proanthocyanidin and mitoxantrone, aimed at enhancing immunogenic cell death. These nanoparticles exhibited self-assembly within the acidic microenvironment of tumors, ultimately leading to local reprogramming of the TME in CT26 tumor-bearing mice. This innovative approach demonstrated improved efficacy in immunotherapy for colorectal cancer while mitigating associated toxicity.224 Furthermore, ZhuGe et al synthesized silk fibroin nanoparticles containing indocyanine green, followed by the creation of a durable nanoformulation through cross-linking with proanthocyanidin. This formulation effectively targets and eliminates residual glioma cells in the surgical cavity when exposed to near-infrared irradiation.225
Polysaccharides-Loaded Nanoformulations
Lentinan-Loaded Nanoformulations
In response to the issue of selenium nanoparticles aggregating into non-bioactive forms, lentinan-selenium nanoparticles were developed utilizing lentinan as a template, as exemplified by Gao et al. This nanoformulation demonstrates high stability at 4°C for a minimum of 8 weeks, induces cell cycle arrest at the G0/G1 phase in HCT-116 cells, stimulates apoptosis through activation of the mitochondria-mediated apoptotic pathway, and exhibits negligible toxicity towards normal cells.226 Furthermore, certain researchers have engineered selenium nanoparticles functionalized with lentinan. These nanoparticles are capable of targeting the mitochondria of tumor cells through the TLR4/TRAF3/MFN1 signaling pathway, leading to alterations in mitochondrial membrane potential and the generation of ROS, thereby promoting apoptosis in the tumor cells.227
Astragalus Polysaccharide-Loaded Nanoformulations
To overcome the limitations of anti-tumor immunity, focused ultrasound has been utilized to construct a multifunctional nanoformulation, as exemplified by Xiong et al. This nanoformulation consists of poly(ethylene glycol) platinum nanoparticles encapsulating astragalus polysaccharide and gold nanorods, synthesized via the double emulsion method. This novel approach enhances the efficacy of focused ultrasound-induced immunity for sustained and systemic anti-tumor effects, as well as providing imaging and thermal enhancement.228 In the context of hepatocellular carcinoma treatment, a study conducted by Jiao et al prepared nanocomposites by modifying selenium nanoparticles with astragalus polysaccharide, serving as a stabilizer and dispersant. This intervention significantly impeded the proliferation of HepG2 cells in a dose-dependent manner, leading to alterations in cell morphology, cell cycle arrest in the S-phase, and ultimately inducing apoptosis of HepG2 cells via the mitochondria.229 Similarly, selenium nanoparticles combined with astragalus polysaccharides, synthesized by Duan et al, exhibited cytotoxic effects on MCF-7 cells through the inhibition of cellular autophagy and apoptosis mediated by the mitochondrial pathway.230 Furthermore, Wang et al developed a self-assembled delivery system targeting ERα-positive breast tumors using a double-targeted approach involving quercetin-3’3-dithiodipropionic acid, astragalus polysaccharides, and folic acid, demonstrating effective suppression of multidrug resistance.231
Sesquiterpene Lactone-Loaded Nanoformulations
Parthenolide-Loaded Nanoformulations
Researchers have developed a novel polydopamine-coated PLGA nanoformulation containing parthenolide, which demonstrates selective cytotoxicity and induces apoptosis in gastric cancer cells.232 Additionally, Gao et al have created nano-magnetic liposomes encapsulating parthenolide and glucose oxidase. This nanoformulated anticancer strategy combines chemotherapy, chemodynamic therapy, starvation therapy, and magnetically targeted therapy, exhibiting significant synergistic anticancer efficacy in vivo and in vitro with minimal biological toxicity.233 To enhance the treatment of advanced hepatocellular carcinoma, several scholars developed a novel parthenolide nanocrystal drug delivery system aimed at addressing the limited water solubility of parthenolide and enhancing its therapeutic synergy with sorafenib. The nanoformulation demonstrated superior therapeutic efficacy compared to individual administration of parthenolide and sorafenib, as evidenced by increased intracellular uptake, inhibition of cell proliferation and migration, and significant tumor suppression in animal models, all while exhibiting minimal toxicity.234
Discussion
As scientific research advances, experts and scholars in the field of medicine have progressively recognized that ROS play a pivotal role in the occurrence, progression, and treatment of malignant tumors.20 Initially, ROS were predominantly considered detrimental, promoting tumor development, as evidenced by their significantly elevated concentrations in tumor cells compared to normal cells. However, in recent years, mounting evidence suggests that the role of ROS in the development of malignant tumors is dynamic, exhibiting dichotomous effects contingent upon their concentration and duration of exposure.21 Specifically, low-toxic high concentrations of ROS can activate a series of growth factor receptors and signaling pathways, thereby promoting the proliferation, metastasis, and malignant transformation of tumor cells through the modulation of tumor-related genes and the induction of oncogenic mutations, among other mechanisms. Conversely, when the concentration of ROS surpasses the toxicity threshold, it can exacerbate oxidative stress damage and activate multiple ROS-mediated cell death signaling pathways, leading to various forms of cell death in tumor cells and thereby significantly inhibiting tumor growth. Given the elevated levels of ROS in tumor cells, the development of pharmacological agents that induce ROS generation and specifically target tumor tissues to elevate ROS concentrations beyond the toxicity threshold within the tumor has emerged as a promising therapeutic strategy for cancer treatment.
In recent years, a diverse array of active ingredients derived from TCM that induce ROS generation have exhibited distinct advantages as a novel approach to cancer therapy, attributable to their pronounced anti-tumor effects.5 Primarily, these active ingredients are sourced from natural plants or animals, which are abundant and exhibit high biocompatibility with the human body, resulting in relatively low toxicity. This characteristic renders them frequently perceived as safer and more reliable alternatives in the realm of cancer treatment. Secondly, these active constituents in TCM frequently exhibit multi-targeted properties, facilitating synergistic anti-tumor effects among various components, thereby mitigating the risk of drug resistance in tumor cells. Furthermore, guided by the holistic principles inherent in Chinese medicine, these active constituents emphasize the balance of yin and yang as well as the harmonization of qi and blood. These characteristics are instrumental in enhancing overall patient health and bolstering the body’s anti-tumor capabilities. Nonetheless, the ROS-inducing active ingredients derived from TCM encounter several challenges, including poor water solubility and bioavailability, non-specific distribution, and rapid metabolic degradation. To address these limitations, the development of multifunctional nanoformulations represents a promising strategy, potentially enhancing the anticancer efficacy of these ROS-inducing TCM compounds.
The present review systematically examined and analyzed ROS-inducing ingredients derived from TCM. It was found that these active compounds encompass a diverse array of chemical types, including quinones, terpenoids, flavonoids, alkaloids, polyphenols, polysaccharides, and sesquiterpene lactones. Furthermore, the review investigated the diversity of these compounds concerning their anticancer mechanisms, revealing their extensive involvement in the inhibition of cell proliferation, promotion of apoptosis, ferroptosis, autophagic cell death, pyroptosis, and necroptosis, among other processes. Moreover, this paper provides a comprehensive summary of nanoformulations incorporating ROS-inducing compounds, highlighting two principal strategies for their preparation. The first strategy involves the direct nanoformulation and processing of these compounds into nanoparticles. The second strategy employs nano-carrier systems to prepare these compounds, either in isolation or in combination with other drugs, into a diverse array of nanodrugs, including but not limited to liposomes, polymer micelles, gold nanoparticles, metal-organic frameworks, iron oxides, inorganic nanoparticles, and hydrogels. Through the application of advanced nanotechnology and the integration of active ingredients in TCM capable of inducing ROS generation, the development of effective nanosized drug preparations can be realized. This approach not only enhances targeted drug release, increases bioavailability, and reduces toxicity, but also optimizes the efficacy of drug combinations. Nanocarriers facilitate the targeted aggregation of pharmaceuticals within tumor tissues, thereby minimizing collateral damage to healthy tissues, significantly enhancing therapeutic efficacy, and reducing adverse side effects. Besides, nanomedicines can extend the systemic circulation time of drugs, improve their stability, and prevent rapid metabolism, thereby sustaining the therapeutic effect over a prolonged period.
In this manuscript, we present the inaugural systematic induction and comprehensive summary of TCM-derived ROS-inducing components and their nanoformulations, to provide a significant reference for the clinical application of TCM drugs in tumor treatment. This study holds substantial implications for the future exploration of TCM within the realm of modern medicine and is anticipated to introduce novel concepts and methodologies to the field of clinical oncology. Nevertheless, despite our rigorous efforts to systematically organize and synthesize the available data, certain limitations persist in this review. Firstly, considering the extensive range of ROS-inducing compounds present in TCM, our study is limited to examining only some of the more prevalent compounds, thereby not providing a comprehensive analysis of all ROS-inducing compounds and their nanoformulations pertinent to tumor therapy. Secondly, regarding the investigation of the pharmacological mechanisms of action and the clinical efficacy evaluation of these compounds and nanoformulations, our content requires further refinement and in-depth exploration. Lastly, constrained by the scope and depth of the current study, we were unable to comprehensively address the potential side effects and interactions of these compositions. These areas represent critical directions for future research and warrant further detailed examination.
Currently, the development of multifunctional nanomedicine formulations is progressively advancing. Through innovative strategies, multiple research teams have successfully formulated a variety of nanoformulations with broad application prospects. These formulations exhibit significant potential not only in the field of anti-tumor therapy but also in other therapeutic domains, such as the treatment of anaerobic bacterial infections.235–237 Notably, recent advancements in nanomedical research underscore the importance of constructing supramolecular hosts with specific recognition sites and drug-loaded guest molecules based on the host-guest molecular recognition mechanism, employing techniques like self-assembly. This approach aims to achieve precise targeting and controlled release of drugs within the TME. Such supramolecular systems, endowed with specific functions and properties, demonstrate extensive application value in various forms of malignant tumor therapy. For instance, they can enhance the efficacy of chemotherapy and phototherapy,238 facilitate mRNA vaccine delivery,239 modulate the TME to synergize with tumor immunotherapy,240 and enable in situ self-enhanced cancer photochemotherapy.241 These regulatory strategies offer novel insights and methodologies for tumor treatment. It is reasonable to believe that with the continuous advancements in supramolecular chemistry, nanotechnology, and biotechnology, the active ingredients in TCM, particularly those with ROS-inducing properties, will also be able to effectively combine with various supramolecular materials (such as cyclodextrins and pillararenes) based on their inherent attributes. This will facilitate the development of supramolecular nanomedicine formulations derived from TCM. This research domain is anticipated to yield further breakthrough achievements, bringing enhanced hope and well-being to personalized treatment for cancer patients.
Conclusions
Overall, although our study provides new perspectives on the ROS-inducing components of TCM and its nano-formulations for the treatment of tumors, more basic and clinical experiments are needed to verify whether they can widely serve clinical oncology patients in the future.
Data Sharing Statement
The current study does not have any datasets generated or analyzed for data sharing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the National Natural Science Foundation of China (82004118, 82004298), Natural Science Youth Foundation of Nanjing University of Chinese Medicine (NZY82004118), Medical Scientific Research Project of Jiangsu Provincial Health Commission (Z2020015), Outstanding Youth Fund Project of Nanjing Health Science and Technology Development (JQX21010), Traditional Chinese Medicine Youth Talent Training Program of Nanjing (NJSZYYQNRC‐2020‐ZFQ), Construction Project of Nanjing Key Laboratory of Chinese Medicine Preparation and Clinical Pharmacy (NJSYXZDSYS-2023), and Nanjing Medical Science and Technique Development Foundation (QRX17187, YKK21199).
Disclosure
The authors declare no competing interests in this work.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2024;74(3):229–263.
2. Cao M, Li H, Sun D, et al. Current cancer burden in China: epidemiology, etiology, and prevention. Can Biol Med. 2022;19(8):1121–1138. doi:10.20892/j.issn.2095-3941.2022.0231
3. Zhang X, Qiu H, Li C, et al. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15(5):283–298. doi:10.5582/bst.2021.01318
4. Wang S, Fu J, Hao H, et al. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol Res. 2021;170:105728. doi:10.1016/j.phrs.2021.105728
5. Qian Q, Chen W, Cao Y, et al. Targeting reactive oxygen species in cancer via Chinese Herbal Medicine. Oxid Med Cell Longev. 2019;2019:9240426. doi:10.1155/2019/9240426
6. Wang X, Li J, Chen R, et al. Active ingredients from Chinese medicine for combination cancer therapy. Int J Bio Sci. 2023;19(11):3499–3525. doi:10.7150/ijbs.77720
7. Wang Y, Chen M, Yu H, et al. The role and mechanisms of action of natural compounds in the prevention and treatment of cancer and cancer metastasis. Front Biosci. 2022;27(6):192. doi:10.31083/j.fbl2706192
8. Garg P, Garg R, Horne D, et al. Prognostic significance of natural products against multidrug tumor resistance. Cancer Lett. 2023;557:216079. doi:10.1016/j.canlet.2023.216079
9. Perillo B, Di Donato M, Pezone A, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52(2):192–203. doi:10.1038/s12276-020-0384-2
10. Huang J, Zhu Y, Xiao H, et al. Formation of a traditional Chinese medicine self-assembly nanostrategy and its application in cancer: a promising treatment. Chin Med. 2023;18(1):66. doi:10.1186/s13020-023-00764-2
11. Li Q, Lianghao Y, Shijie G, et al. Self-assembled nanodrug delivery systems for anti-cancer drugs from traditional Chinese medicine. Biomater Sci. 2024;12(7):1662–1692. doi:10.1039/D3BM01451G
12. Gao Y, Wang K, Zhang J, et al. Multifunctional nanoparticle for cancer therapy. Medcomm. 2023;4(1):e187. doi:10.1002/mco2.187
13. Azizi M, Jahanban-Esfahlan R, Samadian H, et al. Multifunctional nanostructures: intelligent design to overcome biological barriers. Mater Today Bio. 2023;20:100672. doi:10.1016/j.mtbio.2023.100672
14. He Z, Zhang Y, Feng N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review. Mater Sci Eng C. 2020;106:110298. doi:10.1016/j.msec.2019.110298
15. Malik JA, Ansari JA, Ahmed S, et al. Nano-drug delivery system: a promising approach against breast cancer. Therap Deliv. 2023;14(5):357–381. doi:10.4155/tde-2023-0020
16. Alshammari MK, Alghazwni MK, Alharbi AS, et al. Nanoplatform for the delivery of topotecan in the cancer milieu: an appraisal of its therapeutic efficacy. Cancers. 2023;15(1):65. doi:10.3390/cancers15010065
17. Fu X, Shi Y, Qi T, et al. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal Transduct Target Ther. 2020;5(1):262. doi:10.1038/s41392-020-00342-0
18. Kalave S, Hegde N, Juvale K. Applications of nanotechnology-based approaches to overcome multi-drug resistance in cancer. Curr Pharm Des. 2022;28(38):3140. doi:10.2174/1381612828666220401142300
19. Checa J, Aran JM. Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res. 2020;13:1057–1073. doi:10.2147/JIR.S275595
20. Zuo J, Zhang Z, Luo M, et al. Redox signaling at the crossroads of human health and disease. Medcomm. 2022;3(2):e127. doi:10.1002/mco2.127
21. Yu Y, Liu S, Yang L, et al. Roles of reactive oxygen species in inflammation and cancer. Medcomm. 2024;5(4). doi:10.1002/mco2.519
22. Nohl H, Gille L, Staniek K. Intracellular generation of reactive oxygen species by mitochondria. Biochem. Pharmacol. 2005;69(5):719–723. doi:10.1016/j.bcp.2004.12.002
23. Görlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Signaling. 2006;8(9–10):1391. doi:10.1089/ars.2006.8.1391
24. Liu P, Wu X, Gong B, et al. Review of the mechanisms by which transcription factors and exogenous substances regulate ROS metabolism under abiotic stress. Antioxidants. 2022;11(11):1.
25. Milkovic L, Cipak Gasparovic A, Cindric M, et al. Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells. 2019;8(8):793. doi:10.3390/cells8080793
26. Vaishampayan A, Grohmann E. Antimicrobials functioning through ROS-mediated mechanisms: current insights. Microorganisms. 2022;10(1):61. doi:10.3390/microorganisms10010061
27. Dunnill C, Patton T, Brennan J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14(1):89–96. doi:10.1111/iwj.12557
28. Wang B, Wang Y, Zhang J, et al. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol. 2023;97(6):1439–1451. doi:10.1007/s00204-023-03476-6
29. Chen S, Su Y, Wang J. ROS-mediated platelet generation: a microenvironment-dependent manner for megakaryocyte proliferation, differentiation, and maturation. Cell Death Dis. 2013;4(7):e722. doi:10.1038/cddis.2013.253
30. Xu T, Ding W, Ji X, et al. Oxidative stress in cell death and cardiovascular diseases. Oxid Med Cell Longev. 2019;2019:9030511–9030563. doi:10.1155/2019/9030563
31. Kirkinezos IG, Moraes CT. Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol. 2001;12(6):449–457. doi:10.1006/scdb.2001.0282
32. Didier AJ, Stiene J, Fang L, et al. Antioxidant and anti-tumor effects of dietary vitamins A, C, and E. Antioxidants. 2023;12(3):632. doi:10.3390/antiox12030632
33. Kohan R, Collin A, Guizzardi S, et al. Reactive oxygen species in cancer: a paradox between pro- and anti-tumour activities. Cancer Chemother Pharmacol. 2020;86(1):1–13. doi:10.1007/s00280-020-04103-2
34. Wang Y, Qi H, Liu Y, et al. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11(10):4839–4857. doi:10.7150/thno.56747
35. Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer. 2022;22(5):280–297. doi:10.1038/s41568-021-00435-0
36. Yang Y, Zhu Y, Xi X. Anti-inflammatory and antitumor action of hydrogen via reactive oxygen species. Oncol Lett. 2018;16(3):2771–2776. doi:10.3892/ol.2018.9023
37. Hong H, Cao W, Wang Q, et al. Synergistic antitumor effect of Andrographolide and cisplatin through ROS-mediated ER stress and STAT3 inhibition in colon cancer. Med Oncol. 2022;39(8):101. doi:10.1007/s12032-022-01691-2
38. Li D, Kou Y, Gao Y, et al. Oxaliplatin induces the PARP1-mediated parthanatos in oral squamous cell carcinoma by increasing production of ROS. Aging. 2021;13(3):4242–4257. doi:10.18632/aging.202386
39. Liu H, Zhang W, Jin L, et al. Plumbagin exhibits genotoxicity and induces G2/M Cell Cycle Arrest via ROS-mediated oxidative stress and activation of ATM-p53 signaling pathway in hepatocellular cells. Int J Mol Sci. 2023;24(7):6279. doi:10.3390/ijms24076279
40. Tripathi SK, Rengasamy KRR, Biswal BK. Plumbagin engenders apoptosis in lung cancer cells via caspase-9 activation and targeting mitochondrial-mediated ROS induction. Arch Pharmacal Res. 2020;43(2):242–256. doi:10.1007/s12272-020-01221-6
41. Lin C, Yu C, Lee T, et al. Plumbagin induces the apoptosis of drug-resistant oral cancer in vitro and in vivo through ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction. Phytomedicine. 2023;111:154655. doi:10.1016/j.phymed.2023.154655
42. Zhou X, Wang F, Wu H, et al. Thymoquinone suppresses the proliferation, migration and invasiveness through regulating ROS, autophagic flux and miR-877-5p in human bladder carcinoma cells. Int J Bio Sci. 2021;17(13):3456–3475. doi:10.7150/ijbs.60401
43. Liou YF, Chen PN, Chu SC, et al. Thymoquinone suppresses the proliferation of renal cell carcinoma cells via reactive oxygen species‐induced apoptosis and reduces cell stemness. Environ Toxicol. 2019;34(11):1208–1220. doi:10.1002/tox.22822
44. Farrash WF, Aslam A, Almaimani R, et al. Metformin and thymoquinone co‐treatment enhance 5‐fluorouracil cytotoxicity by suppressing the PI3K/mTOR/HIF1α pathway and increasing oxidative stress in colon cancer cells. Biofactors. 2023;49(4):831–848. doi:10.1002/biof.1947
45. Qian X, Zhu L, Xu M, et al. Shikonin suppresses small cell lung cancer growth via inducing ATF3-mediated ferroptosis to promote ROS accumulation. Chem Biol Interact. 2023;382:110588. doi:10.1016/j.cbi.2023.110588
46. Boonnate P, Kariya R, Okada S. Shikonin Induces ROS-Dependent Apoptosis via mitochondria depolarization and ER Stress in adult T cell leukemia/lymphoma. Antioxidants. 2023;12(4):864. doi:10.3390/antiox12040864
47. Qi H, Zhang X, Liu H, et al. Shikonin induced apoptosis mediated by endoplasmic reticulum stress in colorectal cancer cells. J Cancer. 2022;13(1):243–252. doi:10.7150/jca.65297
48. Tsai M, Chen S, Ong A, et al. Shikonin induced program cell death through generation of reactive oxygen species in renal cancer cells. Antioxidants. 2021;10(11):1831. doi:10.3390/antiox10111831
49. Ju X, Zhang H, Wang J, et al. Shikonin triggers GSDME-mediated pyroptosis in tumours by regulating autophagy via the ROS-MAPK14/p38α axis. Phytomedicine. 2023;109:154596. doi:10.1016/j.phymed.2022.154596
50. Chen Z, Wu F, Li J, et al. Investigating the synergy of Shikonin and Valproic acid in inducing apoptosis of osteosarcoma cells via ROS-mediated EGR1 expression. Phytomedicine. 2024;126:155459. doi:10.1016/j.phymed.2024.155459
51. Zhang Z, Shen C, Zhou F, et al. Shikonin potentiates therapeutic efficacy of oxaliplatin through reactive oxygen species‐mediated intrinsic apoptosis and endoplasmic reticulum stress in oxaliplatin‐resistant colorectal cancer cells. Drug Dev Res. 2023;84(3):537–550. doi:10.1002/ddr.22044
52. Wu J, Wang D, Zhou J, et al. Gambogenic acid induces apoptosis and autophagy through ROS ‐mediated endoplasmic reticulum stress via JNK pathway in prostate cancer cells. Phytother Res. 2023;37(1):310–328. doi:10.1002/ptr.7614
53. Zhao Q, Zhong J, Bi Y, et al. Gambogenic acid induces Noxa-mediated apoptosis in colorectal cancer through ROS-dependent activation of IRE1α/JNK. Phytomedicine. 2020;78:153306. doi:10.1016/j.phymed.2020.153306
54. Zhu M, Jiang Y, Wu H, et al. Gambogic acid shows anti-proliferative effects on non-small cell lung cancer (NSCLC) cells by activating reactive oxygen Species (ROS)-induced endoplasmic reticulum (ER) stress-mediated apoptosis. Med Sci Monit. 2019;25:3983–3988. doi:10.12659/MSM.916835
55. Su Y, Chang H, Shyue S, et al. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem. Pharmacol. 2005;70(2):229–241. doi:10.1016/j.bcp.2005.04.026
56. Shen Z, Zhao L, Yoo S, et al. Emodin induces ferroptosis in colorectal cancer through NCOA4-mediated ferritinophagy and NF-κb pathway inactivation. Apoptosis. 2024. doi:10.1007/s10495-024-01973-2
57. Wang K, Meng X, Chen J, et al. Emodin induced necroptosis and inhibited glycolysis in the renal cancer cells by enhancing ROS. Oxid Med Cell Longev. 2021;2021:1–17.
58. Hsu P, Hsu C, Hsia Y, et al. Chrysophanol suppresses cell growth via mTOR/PPAR-α Regulation and ROS accumulation in cultured human tongue squamous carcinoma SAS cells. Curr Issu Molec Biol. 2022;44(4):1528–1538. doi:10.3390/cimb44040104
59. Hsu P, Cheng C, Hsieh P, et al. Chrysophanol regulates cell death, metastasis, and reactive oxygen species production in oral cancer cell lines. Evid Based Complement Alternat Med. 2020;2020:1–10. doi:10.1155/2020/5867064
60. Gu J, Rauniyar S, Wang Y, et al. Chrysophanol induced glioma cells apoptosis via activation of mitochondrial apoptosis pathway. Bioengineered. 2021;12(1):6855–6868. doi:10.1080/21655979.2021.1972079
61. Choi Y, Choi YK, Ko S, et al. Investigation of molecular mechanisms involved in sensitivity to the anti-cancer activity of costunolide in breast cancer cells. Int J Mol Sci. 2023;24(4):4009. doi:10.3390/ijms24044009
62. Xu C, Huang X, Lei X, et al. Costunolide-induced apoptosis via promoting the reactive oxygen species and inhibiting AKT/GSK3β pathway and activating autophagy in gastric cancer. Front Cell Develop Biol. 2021;9. doi:10.3389/fcell.2021.722734
63. Li J, Duan B, Cheng Z, et al. Costunolide enhances cisplatin-induced cytotoxicity in hypopharyngeal SCC FaDu cells by increasing the production of reactive oxygen species. Pathol Res Pract. 2022;236:153966. doi:10.1016/j.prp.2022.153966
64. WANG G, ZHANG L, GENG Y, et al. β-Elemene induces apoptosis and autophagy in colorectal cancer cells through regulating the ROS/AMPK/mTOR pathway. Chinese J Nat Med. 2022;20(1):9–21. doi:10.1016/S1875-5364(21)60118-8
65. Cai S, Xiong Q, Zhao L, et al. β-Elemene Triggers ROS-dependent apoptosis in glioblastoma cells through suppressing STAT3 signaling pathway. Pathol Oncol Res. 2021;27. doi:10.3389/pore.2021.594299
66. Gan D, He W, Yin H, et al. β-elemene enhances cisplatin-induced apoptosis in bladder cancer cells through the ROS-AMPK signaling pathway. Oncol Lett. 2020;19(1):291–300. doi:10.3892/ol.2019.11103
67. Zhang F, Hao Y, Yang N, et al. Oridonin-induced ferroptosis and apoptosis: a dual approach to suppress the growth of osteosarcoma cells. Bmc Cancer. 2024;24(1):1.
68. Ye S, Hu X, Sun S, et al. Oridonin promotes RSL3-induced ferroptosis in breast cancer cells by regulating the oxidative stress signaling pathway JNK/Nrf2/HO-1. Eur J Pharmacol. 2024;974:176620. doi:10.1016/j.ejphar.2024.176620
69. Zhang D, Zhou Q, Huang D, et al. ROS/JNK/c-Jun axis is involved in oridonin-induced caspase-dependent apoptosis in human colorectal cancer cells. Biochem Biophys Res Commun. 2019;513(3):594–601. doi:10.1016/j.bbrc.2019.04.011
70. Zhao Z, Wang Y, Gong Y, et al. Celastrol elicits antitumor effects by inhibiting the STAT3 pathway through ROS accumulation in non-small cell lung cancer. J Transl Med. 2022;20(1). doi:10.1186/s12967-022-03741-9
71. Chen X, Zhao Y, Luo W, et al. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics. 2020;10(22):10290–10308. doi:10.7150/thno.46728
72. Liu M, Fan Y, Li D, et al. Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol Oncol. 2021;15(8):2084–2105. doi:10.1002/1878-0261.12936
73. Xiaofei J, Mingqing S, Miao S, et al. Oleanolic acid inhibits cervical cancer Hela cell proliferation through modulation of the ACSL4 ferroptosis signaling pathway. Biochem Biophys Res Commun. 2021;545:81–88. doi:10.1016/j.bbrc.2021.01.028
74. Shi Y, Song Q, Hu D, et al. Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway. Korean J Physiol Pharmacol. 2016;20(3):237. doi:10.4196/kjpp.2016.20.3.237
75. Lange M, Abhari BA, Hinrichs TM, et al. Identification of a novel oxidative stress induced cell death by Sorafenib and oleanolic acid in human hepatocellular carcinoma cells. Biochem Pharmacol. 2016;118:9–17. doi:10.1016/j.bcp.2016.08.011
76. Jiang Z, Wang Z, Chen L, et al. Artesunate induces ER-derived-ROS-mediated cell death by disrupting labile iron pool and iron redistribution in hepatocellular carcinoma cells. Am J Cancer Res. 2021;11(3):691–711.
77. Liang L, Liu Y, Wu X, et al. Artesunate induces ferroptosis by inhibiting the nuclear localization of SREBP2 in myeloma cells. Int J Med Sci. 2023;20(12):1535–1550. doi:10.7150/ijms.86409
78. Zhou X, Chen Y, Wang F, et al. Artesunate induces autophagy dependent apoptosis through upregulating ROS and activating AMPK-mTOR-ULK1 axis in human bladder cancer cells. Chem Biol Interact. 2020;331:109273. doi:10.1016/j.cbi.2020.109273
79. Du Y, Lai J, Su J, et al. Naringenin-induced oral cancer cell apoptosis Via ROS-mediated Bid and Bcl-xl Signaling Pathway. Curr Cancer Drug Targets. 2024;24:668–679. doi:10.2174/0115680096267430231023091521
80. Chang T, Chi M, Chiang Y, et al. Promotion of ROS-mediated apoptosis, G2/M arrest, and autophagy by naringenin in non-small cell lung cancer. Int J Bio Sci. 2024;20(3):1093–1109. doi:10.7150/ijbs.85443
81. Zhang M, Lai J, Wu Q, et al. Naringenin Induces HepG2 Cell Apoptosis via ROS-Mediated JAK-2/STAT-3 signaling pathways. Molecules. 2023;28(11):4506. doi:10.3390/molecules28114506
82. Jang E, Kim IY, Kim H, et al. Quercetin and chloroquine synergistically kill glioma cells by inducing organelle stress and disrupting Ca2+ homeostasis. Biochem. Pharmacol. 2020;178:114098. doi:10.1016/j.bcp.2020.114098
83. Zhang X, Huang J, Yu C, et al. Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ROS production. Onco Targets Ther. 2020;13:513–523. doi:10.2147/OTT.S228453
84. Ding L, Dang S, Sun M, et al. Quercetin induces ferroptosis in gastric cancer cells by targeting SLC1A5 and regulating the p-Camk2/p-DRP1 and NRF2/GPX4 Axes. Free Radic Biol Med. 2024;213:150–163. doi:10.1016/j.freeradbiomed.2024.01.002
85. Wang F, Wang L, Qu C, et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. Bmc Cancer. 2021;21(1):1.
86. Lee CF, Yang JS, Tsai FJ, et al. Kaempferol induces ATM/p53-mediated death receptor and mitochondrial apoptosis in human umbilical vein endothelial cells. Int J Oncol. 2016;48(5):2007–2014. doi:10.3892/ijo.2016.3420
87. Seydi E, Salimi A, Rasekh HR, et al. Selective cytotoxicity of luteolin and kaempferol on cancerous hepatocytes obtained from rat model of hepatocellular carcinoma: involvement of ROS-mediated mitochondrial targeting. Nutr Cancer. 2018;70(4):594–604. doi:10.1080/01635581.2018.1460679
88. Wang Q, Wang H, Jia Y, et al. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother Pharmacol. 2017;79(5):1031–1041. doi:10.1007/s00280-017-3299-4
89. Kim JK, Kang KA, Ryu YS, et al. Induction of endoplasmic reticulum stress via reactive oxygen species mediated by luteolin in melanoma cells. Anticancer Res. 2016;36(5):2281–2289.
90. Kittiratphatthana N, Kukongviriyapan V, Prawan A, et al. Luteolin induces cholangiocarcinoma cell apoptosis through the mitochondrial-dependent pathway mediated by reactive oxygen species. J Pharm Pharmacol. 2016;68(9):1184–1192. doi:10.1111/jphp.12586
91. KOH H, SUN H, XING Z, et al. Wogonin influences osteosarcoma stem cell stemness through ROS-dependent signaling. In Vivo. 2020;34(3):1077–1084. doi:10.21873/invivo.11878
92. Li SJ, Sun SJ, Gao J, et al. Wogonin induces Beclin-1/PI3K and reactive oxygen species-mediated autophagy in human pancreatic cancer cells. Oncol Lett. 2016;12(6):5059–5067. doi:10.3892/ol.2016.5367
93. Lee Y, Park K, Nam H, et al. Apigenin causes necroptosis by inducing ROS accumulation, mitochondrial dysfunction, and ATP depletion in malignant mesothelioma cells. Korean J Physiol Pharmacol. 2020;24(6):493–502. doi:10.4196/kjpp.2020.24.6.493
94. Park S, Lim W, Bazer FW, et al. Apigenin induces ROS‐dependent apoptosis and ER stress in human endometriosis cells. J Cell Physiol. 2018;233(4):3055–3065. doi:10.1002/jcp.26054
95. Sun Q, Lu N, Feng L. Apigetrin inhibits gastric cancer progression through inducing apoptosis and regulating ROS-modulated STAT3/JAK2 pathway. Biochem Biophys Res Commun. 2018;498(1):164–170. doi:10.1016/j.bbrc.2018.02.009
96. Samandari-Bahraseman MR, Khorsand B, Zareei S, et al. Various concentrations of hesperetin induce different types of programmed cell death in human breast cancerous and normal cell lines in a ROS-dependent manner. Chem Biol Interact. 2023;382:110642. doi:10.1016/j.cbi.2023.110642
97. Zhang J, Song J, Wu D, et al. Hesperetin induces the apoptosis of hepatocellular carcinoma cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, ATP and calcium. Med Oncol. 2015;32(4). doi:10.1007/s12032-015-0516-z
98. Arumugam G, Alagar Yadav S. Synergistic inhibitory actions of resveratrol, epigallocatechin-3-gallate, and diallyl trisulfide against skin cancer cell line A431 through mitochondrial caspase dependent pathway: a combinational drug approach. Med Oncol. 2024;41(3). doi:10.1007/s12032-023-02292-3
99. Guan M, Wu Q, Li M. Epigallocatechin-3-gallate Induced HepG2 Cells Apoptosis through ROSmediated AKT /JNK and p53 Signaling Pathway. Curr Cancer Drug Targets. 2023;23(6):447–460. doi:10.2174/1568009622666220705101642
100. Fang W, Peng ZL, Dai YJ, et al. (-)-Epigallocatechin-3-gallate encapsulated realgar nanoparticles exhibit enhanced anticancer therapeutic efficacy against acute promyelocytic leukemia. Drug Delivery. 2019;26(1):1058–1067. doi:10.1080/10717544.2019.1672830
101. Pandey P, Sayyed U, Tiwari RK, et al. Hesperidin Induces ROS-mediated apoptosis along with cell cycle arrest at G2/M phase in human gall bladder carcinoma. Nutr Cancer. 2019;71(4):676–687. doi:10.1080/01635581.2018.1508732
102. Ning L, Zhao W, Gao H, et al. Hesperidin induces anticancer effects on human prostate cancer cells via ROS-mediated necrosis like cell death. J Buon. 2020;25(6):2629–2634.
103. Pandey P, Khan F, Maurya P. Targeting Jab1 using hesperidin (dietary phytocompound) for inducing apoptosis in HeLa cervical cancer cells. J Food Biochem. 2021;45(7). doi:10.1111/jfbc.13800
104. Chen Q, Hou Y, Li D, et al. Berberine induces non-small cell lung cancer apoptosis via the activation of the ROS/ASK1/JNK pathway. Ann Translat Med. 2022;10(8):485. doi:10.21037/atm-22-1298
105. Zhao Y, Lin X, Zeng W, et al. Berberine inhibits the progression of renal cell carcinoma cells by regulating reactive oxygen species generation and inducing DNA damage. Molec Biol Rep. 2023;50(7):5697–5707. doi:10.1007/s11033-023-08381-w
106. Palma TV, Lenz LS, Bottari NB, et al. Berberine induces apoptosis in glioblastoma multiforme U87MG cells via oxidative stress and independent of AMPK activity. Molec Biol Rep. 2020;47(6):4393–4400. doi:10.1007/s11033-020-05500-9
107. Chen P, Luo X, Nie P, et al. CQ synergistically sensitizes human colorectal cancer cells to SN-38/CPT-11 through lysosomal and mitochondrial apoptotic pathway via p53-ROS cross-talk. Free Radic Biol Med. 2017;104:280–297. doi:10.1016/j.freeradbiomed.2017.01.033
108. Mitra S, Biswas P, Bandyopadhyay A, et al. Piperlongumine: the amazing amide alkaloid from Piper in the treatment of breast cancer. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(5):2637–2650. doi:10.1007/s00210-023-02673-5
109. Awasthee N, Shekher A, Rai V, et al. Piperlongumine, a piper alkaloid, enhances the efficacy of doxorubicin in breast cancer: involvement of glucose import, ROS, NF-κB and lncRNAs. Apoptosis. 2022;27(3–4):261–282. doi:10.1007/s10495-022-01711-6
110. Tripathi SK, Sahoo RK, Biswal BK. Exposure of piperlongumine attenuates stemness and epithelial to mesenchymal transition phenotype with more potent anti-metastatic activity in SOX9 deficient human lung cancer cells. Naunyn-Schmiedeberg’s Arch Pharmacol. 2024;397:5631–5647. doi:10.1007/s00210-024-02965-4
111. Naradun N, Talabnin K, Ayuttha KIN, et al. Piperlongumine and bortezomib synergically inhibit cholangiocarcinoma via ER stress-induced cell death. Naunyn-Schmiedeberg’s Arch Pharmacol. 2023;396(1):109–120. doi:10.1007/s00210-022-02305-4
112. Chow LWC, Cheng K, Leong F, et al. Enhancing tetrandrine cytotoxicity in human lung carcinoma A549 cells by suppressing mitochondrial ATP production. Naunyn-Schmiedeberg’s Arch Pharmacol. 2019;392(4):427–436. doi:10.1007/s00210-018-01601-2
113. Chaudhary P, Vishwanatha JK. c-Jun NH2-terminal kinase-induced proteasomal degradation of c-FLIPL/S and Bcl2 sensitize prostate cancer cells to Fas- and mitochondria-mediated apoptosis by tetrandrine. Biochem Pharmacol. 2014;91(4):457–473. doi:10.1016/j.bcp.2014.08.014
114. Liu T, Li K, Zhang Z, et al. Tetrandrine inhibits cancer stem cell characteristics and epithelial to mesenchymal transition in triple-negative breast cancer via SOD1/ROS signaling pathway. Am J Chin Med. 2023;51(02):425–444. doi:10.1142/S0192415X23500222
115. Gomez-Deza J, Slavutsky AL, Nebiyou M, et al. Local production of reactive oxygen species drives vincristine-induced axon degeneration. Cell Death Dis. 2023;14(12):807. doi:10.1038/s41419-023-06227-8
116. Groninger E, Meeuwsen-De BG, De Graaf SS, et al. Vincristine induced apoptosis in acute lymphoblastic leukaemia cells: a mitochondrial controlled pathway regulated by reactive oxygen species? Int J Oncol. 2002;21(6):1339–1345. doi:10.3892/ijo.21.6.1339
117. Zhang L, Qiu L, Xu S, et al. Curcumin induces mitophagy by promoting mitochondrial succinate dehydrogenase activity and sensitizes human papillary thyroid carcinoma BCPAP cells to radioiodine treatment. Toxicol in vitro. 2023;93:105669. doi:10.1016/j.tiv.2023.105669
118. Liu C, Rokavec M, Huang Z, et al. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023;30(7):1771–1785. doi:10.1038/s41418-023-01178-1
119. Manica D, Silva GBD, Silva APD, et al. Curcumin promotes apoptosis of human melanoma cells by caspase 3. Cell Biochem Funct. 2023;41(8):1295–1304. doi:10.1002/cbf.3863
120. Liu J, Gao W, Sheng Y, et al. Resveratrol drives ferroptosis of acute myeloid leukemia cells through Hsa-miR-335-5p/NFS1/ GPX4 pathway in a ROS-dependent manner. Cell Mol Biol. 2023;69(7):131–137. doi:10.14715/cmb/2023.69.7.21
121. Önay Uçar E, Şengelen A, Mertoğlu Kamalı E. Hsp27, Hsp60, Hsp70, or Hsp90 depletion enhances the antitumor effects of resveratrol via oxidative and ER stress response in human glioblastoma cells. Biochem. Pharmacol. 2023;208:115409. doi:10.1016/j.bcp.2022.115409
122. Chung WG, Miranda CL, Stevens JF, et al. Hop proanthocyanidins induce apoptosis, protein carbonylation, and cytoskeleton disorganization in human colorectal adenocarcinoma cells via reactive oxygen species. Food Chem Toxicol. 2009;47(4):827–836. doi:10.1016/j.fct.2009.01.015
123. Kim KK, Singh AP, Singh RK, et al. Anti-angiogenic activity of cranberry proanthocyanidins and cytotoxic properties in ovarian cancer cells. Int J Oncol. 2012;40(1):227–235. doi:10.3892/ijo.2011.1198
124. Li M, Du X, Yuan Z, et al. Lentinan triggers oxidative stress-mediated anti-inflammatory responses in lung cancer cells. Mol Cell Biochem. 2022;477(2):469–477. doi:10.1007/s11010-021-04293-0
125. Liu W, Gu J, Qi J, et al. Lentinan exerts synergistic apoptotic effects with paclitaxel in A549 cells via activating ROS‐TXNIP‐NLRP3 inflammasome. J Cell & Mol Med. 2015;19(8):1949–1955. doi:10.1111/jcmm.12570
126. Yu J, Ji H, Dong X, et al. Apoptosis of human gastric carcinoma MGC-803 cells induced by a novel Astragalus membranaceus polysaccharide via intrinsic mitochondrial pathways. Int J Biol Macromol. 2019;126:811–819. doi:10.1016/j.ijbiomac.2018.12.268
127. Jorge J, Neves J, Alves R, et al. Parthenolide Induces ROS-Mediated Apoptosis in Lymphoid Malignancies. Int J Mol Sci. 2023;24(11):9167. doi:10.3390/ijms24119167
128. Yang C, Yang QO, Kong Q, et al. Parthenolide induces reactive oxygen species-mediated autophagic cell death in human osteosarcoma cells. Cell Physiol Biochem. 2016;40(1–2):146–154. doi:10.1159/000452532
129. D’Anneo A, Carlisi D, Lauricella M, et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013;4(10):e891. doi:10.1038/cddis.2013.415
130. Wang W, Adachi M, Kawamura R, et al. Parthenolide-induced apoptosis in multiple myeloma cells involves reactive oxygen species generation and cell sensitivity depends on catalase activity. Apoptosis. 2006;11(12):2225–2235. doi:10.1007/s10495-006-0287-2
131. Sakpakdeejaroen I, Somani S, Laskar P, et al. Regression of melanoma following intravenous injection of plumbagin entrapped in transferrin-conjugated, lipid-polymer hybrid nanoparticles. Int J Nanomed. 2021;16:2615–2631. doi:10.2147/IJN.S293480
132. Duraipandy N, Dharunya G, Lakra R, et al. Fabrication of plumbagin on silver nanoframework for tunable redox modulation: implications for therapeutic angiogenesis. J Cell Physiol. 2019;234(8):13110–13127. doi:10.1002/jcp.27981
133. Duraipandy N, Lakra R, Kunnavakkam Vinjimur S, et al. Caging of plumbagin on silver nanoparticles imparts selectivity and sensitivity to plumbagin for targeted cancer cell apoptosis. Metallomics. 2014;6(11):2025–2033. doi:10.1039/C4MT00165F
134. Han S, Bi S, Guo T, et al. Nano co-delivery of Plumbagin and Dihydrotanshinone I reverses immunosuppressive TME of liver cancer. J Control Release. 2022;348:250–263. doi:10.1016/j.jconrel.2022.05.057
135. Gulbay G, Secme M, Ilhan H. Exploring the potential of thymoquinone-stabilized selenium nanoparticles: in HEC1B endometrial cancer cells revealing enhanced anticancer efficacy. Acs Omega. 2023;8(42):39822–39829. doi:10.1021/acsomega.3c06028
136. Mehanna MM, Sarieddine R, Alwattar JK, et al. Anticancer activity of thymoquinone cubic phase nanoparticles against human breast cancer: formulation, cytotoxicity and subcellular localization. Int J Nanomed. 2020;15:9557–9570. doi:10.2147/IJN.S263797
137. Goel S, Mishra P. Thymoquinone loaded mesoporous silica nanoparticles retard cell invasion and enhance in vitro cytotoxicity due to ROS mediated apoptosis in HeLa and MCF-7 cell lines. Mater Sci Eng C. 2019;104:109881. doi:10.1016/j.msec.2019.109881
138. Ibrahim WN, Muizzuddin Bin Mohd Rosli L, Doolaanea AA. Formulation, cellular uptake and cytotoxicity of thymoquinone-loaded PLGA Nanoparticles in malignant melanoma cancer cells. Int J Nanomed. 2020;15:8059–8074. doi:10.2147/IJN.S269340
139. Yan C, Li Q, Sun Q, et al. Promising Nanomedicines of Shikonin for Cancer Therapy. Int J Nanomed. 2023;18:1195–1218. doi:10.2147/IJN.S401570
140. Xie W, Li Y, Guo Z, et al. FePd Nanozyme- and SKN-encapsulated functional lipid nanoparticles for cancer nanotherapy via ROS-boosting necroptosis. ACS Appl Mater Interfaces. 2024;16(15):18411–18421. doi:10.1021/acsami.3c18497
141. Chen L, Zhao D, Ren X, et al. Shikonin-Loaded Hollow Fe-MOF Nanoparticles for Enhanced Microwave Thermal Therapy. Acs Appl Sci Engine. 2023;9(9):5405–5417.
142. Long L, Xiong W, Lin F, et al. Regulating lactate-related immunometabolism and EMT reversal for colorectal cancer liver metastases using shikonin targeted delivery. J Exp Clin Cancer Res. 2023;42(1). doi:10.1186/s13046-023-02688-z
143. Fahmy SA, Elghanam R, Rashid G, et al. Emerging tendencies for the nano-delivery of gambogic acid: a promising approach in oncotherapy. Rsc Adv. 2024;14(7):4666–4691. doi:10.1039/D3RA08042K
144. Huang F, Zhang Q, Xiao J, et al. Cancer cell membrane-coated gambogic acid nanoparticles for effective anticancer vaccination by activating dendritic cells. Int J Nanomed. 2023;18:2261–2273. doi:10.2147/IJN.S408521
145. Zhang D, Chu Y, Qian H, et al. Antitumor activity of thermosensitive hydrogels packaging gambogic acid nanoparticles and tumor-penetrating peptide iRGD against gastric cancer. Int J Nanomed. 2020;15:735–747. doi:10.2147/IJN.S231448
146. Ren S, Song L, Tian Y, et al. Emodin-Conjugated PEGylation of Fe(3)O(4) Nanoparticles for FI/MRI dual-modal imaging and therapy in pancreatic cancer. Int J Nanomed. 2021;16:7463–7478. doi:10.2147/IJN.S335588
147. Liang W, Fan Y, Liu Y, et al. ROS/pH dual-sensitive emodin-chlorambucil co-loaded micelles enhance anti-tumor effect through combining oxidative damage and chemotherapy. Int J Pharm. 2023;647:123537. doi:10.1016/j.ijpharm.2023.123537
148. Lu L, Li K, Mao YH, et al. Gold-chrysophanol nanoparticles suppress human prostate cancer progression through inactivating AKT expression and inducing apoptosis and ROS generation in vitro and in vivo. Int J Oncol. 2017;51(4):1089–1103. doi:10.3892/ijo.2017.4095
149. Alamoudi AJ, Badr-Eldin SM, Ahmed AA, et al. Optimized bilosome-based nanoparticles enhance cytotoxic and pro-apoptotic activity of costunolide in LS174T colon cancer cells. Biomed. Pharmacother. 2023;168:115757. doi:10.1016/j.biopha.2023.115757
150. Chen W, Li Y, Liu C, et al. In situ engineering of tumor‐associated macrophages via a nanodrug‐delivering‐drug (β‐Elemene@Stanene) strategy for enhanced cancer chemo‐immunotherapy. Angew Chem Int Ed. 2023;62(41). doi:10.1002/anie.202312436
151. Chen W, Liu C, Ji X, et al. Stanene‐Based Nanosheets for β‐Elemene Delivery and Ultrasound‐Mediated Combination Cancer Therapy. Angew Chem Int Ed. 2021;60(13):7155–7164. doi:10.1002/anie.202016330
152. Qiu W, Chen R, Chen X, et al. Oridonin-loaded and GPC1-targeted gold nanoparticles for multimodal imaging and therapy in pancreatic cancer. Int J Nanomed. 2018;13:6809–6827. doi:10.2147/IJN.S177993
153. Cai M, Yao Y, Yin D, et al. Enhanced lysosomal escape of cell penetrating peptide-functionalized metal-organic frameworks for co-delivery of survivin siRNA and oridonin. J Colloid Interface Sci. 2023;646:370–380. doi:10.1016/j.jcis.2023.04.126
154. Fan X, Wang T, Ji Z, et al. Synergistic combination therapy of lung cancer using lipid-layered cisplatin and oridonin co-encapsulated nanoparticles. Biomed. Pharmacother. 2021;141:111830. doi:10.1016/j.biopha.2021.111830
155. Pi J, Jiang J, Cai H, et al. GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways. Drug Delivery. 2017;24(1):1549–1564. doi:10.1080/10717544.2017.1386729
156. Niu B, Wu Y, Zhou M, et al. Precise delivery of celastrol by PEGylated aptamer dendrimer nanoconjugates for enormous therapeutic effect via superior intratumor penetration over antibody counterparts. Cancer Lett. 2023;579:216461. doi:10.1016/j.canlet.2023.216461
157. Niu W, Wang J, Wang Q, et al. Celastrol Loaded Nanoparticles With ROS-Response and ROS-Inducer for the Treatment of Ovarian Cancer. Front Chem. 2020;8. doi:10.3389/fchem.2020.574614
158. Li Z, Guo Z, Chu D, et al. Effectively suppressed angiogenesis-mediated retinoblastoma growth using celastrol nanomicelles. Drug Delivery. 2020;27(1):358–366. doi:10.1080/10717544.2020.1730522
159. Shukla VN, Vikas Mehata V, Mehata AK, et al. EGFR targeted albumin nanoparticles of oleanolic acid: in silico screening of nanocarrier, cytotoxicity and pharmacokinetics for lung cancer therapy. Int J Biol Macromol. 2023;246:125719. doi:10.1016/j.ijbiomac.2023.125719
160. Kumbham S, Paul M, Itoo A, et al. Oleanolic acid-conjugated human serum albumin nanoparticles encapsulating doxorubicin as synergistic combination chemotherapy in oropharyngeal carcinoma and melanoma. Int J Pharm. 2022;614:121479. doi:10.1016/j.ijpharm.2022.121479
161. Zhang XK, Wang QW, Xu YJ, et al. Co‐delivery of cisplatin and oleanolic acid by silica nanoparticles‐enhanced apoptosis and reverse multidrug resistance in lung cancer. Kaohs J Med Sci. 2021;37(6):505–512. doi:10.1002/kjm2.12365
162. Bao Y, Zhang S, Chen Z, et al. Synergistic chemotherapy for breast cancer and breast cancer brain metastases via paclitaxel-loaded oleanolic acid nanoparticles. Mol Pharmaceut. 2020;17(4):1343–1351. doi:10.1021/acs.molpharmaceut.0c00044
163. Xia Y, Tang Y, Huang Z, et al. Artesunate-loaded solid lipid nanoparticles resist esophageal squamous cell carcinoma by inducing Ferroptosis through inhibiting the AKT/mTOR signaling. Cell Signalling. 2024;117:111108. doi:10.1016/j.cellsig.2024.111108
164. Yadav N, Kannan D, Patil S, et al. Amplified activity of artesunate mediated by iron oxide nanoparticles loaded on a graphene oxide carrier for cancer therapeutics. ACS Appl Bio Mater. 2020;3(10):6722–6736. doi:10.1021/acsabm.0c00632
165. Zhong W, Wong KH, Xu F, et al. NIR-responsive polydopamine-based calcium carbonate hybrid nanoparticles delivering artesunate for cancer chemo-photothermal therapy. Acta Biomater. 2022;145:135–145. doi:10.1016/j.actbio.2022.03.051
166. Xi J, Huang Y, Chen J, et al. Artesunate-loaded poly (lactic-co-glycolic acid)/polydopamine-manganese oxides nanoparticles as an oxidase mimic for tumor chemo-catalytic therapy. Int J Biol Macromol. 2021;181:72–81. doi:10.1016/j.ijbiomac.2021.03.124
167. Wang Y, Zha W, Wang J, et al. Local delivery of artesunate dimer liposomes incorporated injectable hydrogel for H2O2 and pH-independent chemodynamic therapy. Int J Pharm. 2023;636:122822. doi:10.1016/j.ijpharm.2023.122822
168. Ramya Devi KT, Jaganathan MK, Ganesh MR, et al. Chitosan-encapsulated naringenin promotes ROS mediated through the activation of executioner caspase-3. Med Oncol. 2024;41(1):1.
169. Dhanisha SS, Drishya S, Guruvayoorappan C. Encapsulating Naringenin in biomimetic proteolipid vesicles abrogates cancer metastasis by targeting apoptotic signaling axis. Food Chem. 2024;434:137445. doi:10.1016/j.foodchem.2023.137445
170. Wadhwa R, Paudel KR, Chin LH, et al. Anti‐inflammatory and anticancer activities of Naringenin‐loaded liquid crystalline nanoparticles in vitro. J Food Biochem. 2021;45(1). doi:10.1111/jfbc.13572
171. Joshi H, S GD, Kaur G, et al. Nanoformulations of quercetin for controlled delivery: a review of preclinical anticancer studies. Naunyn-Schmiedeberg’s Arch Pharmacol. 2023;396(12):3443–3458. doi:10.1007/s00210-023-02625-z
172. Dogan M. Assessment of mechanism involved in the apoptotic and anti-cancer activity of Quercetin and Quercetin-loaded chitosan nanoparticles. Med Oncol. 2022;39(11). doi:10.1007/s12032-022-01820-x
173. Das P, Ghosh S, Ashashainy V, et al. Augmentation of anti-proliferative efficacy of quercetin encapsulated chitosan nanoparticles by induction of cell death via mitochondrial membrane permeabilization in oral cancer. Int J Biol Macromol. 2023;250:126151. doi:10.1016/j.ijbiomac.2023.126151
174. Ganthala PD, Alavala S, Chella N, et al. Co-encapsulated nanoparticles of Erlotinib and Quercetin for targeting lung cancer through nuclear EGFR and PI3K/AKT inhibition. Colloids Surf. B. 2022;211:112305. doi:10.1016/j.colsurfb.2021.112305
175. Luo H, Jiang B, Li B, et al. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int J Nanomed. 2012;7:3951–3959. doi:10.2147/IJN.S33670
176. Govindaraju S, Roshini A, Lee M, et al. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to A549 lung cancer cells. Int J Nanomed. 2019;14:5147–5157. doi:10.2147/IJN.S209773
177. Mollaei M, Homayouni TM, Es-Haghi A. The folate-linked chitosan-coated Kaempferol/HSA nano-transporters (FCKH-NTs) as the selective apoptotic inducer in human MCF-7 breast cancer cell line. Drug Dev Ind Pharm. 2023;49(10):658–665. doi:10.1080/03639045.2023.2268739
178. Matić IZ, Mraković A, Rakočević Z, et al. Anticancer effect of novel luteolin capped gold nanoparticles selectively cytotoxic towards human cervical adenocarcinoma HeLa cells: an in vitro approach. J Trace Elem Med Biol. 2023;80:127286. doi:10.1016/j.jtemb.2023.127286
179. Fu QT, Zhong XQ, Chen MY, et al. Luteolin-loaded nanoparticles for the treatment of melanoma. Int J Nanomed. 2023;18:2053–2068. doi:10.2147/IJN.S400329
180. Wu C, Xu Q, Chen X, et al. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int J Nanomed. 2019;14:7515–7531. doi:10.2147/IJN.S214585
181. Sabra SA, Elzoghby AO, Sheweita SA, et al. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur J Pharm Biopharm. 2018;128:156–169. doi:10.1016/j.ejpb.2018.04.023
182. Huang B, Liu H, Huang D, et al. Apoptosis induction and imaging of cadmium-telluride quantum dots with wogonin in multidrug-resistant leukemia K562/A02 cell. J Nanosci Nanotechnol. 2016;16(3):2499–2503. doi:10.1166/jnn.2016.10792
183. Baek J, Na Y, Cho C. Sustained cytotoxicity of wogonin on breast cancer cells by encapsulation in solid lipid nanoparticles. Nanomaterials. 2018;8(3):159. doi:10.3390/nano8030159
184. Mabrouk Zayed MM, Sahyon HA, Hanafy NAN, et al. The effect of encapsulated apigenin nanoparticles on HePG-2 Cells through regulation of P53. Pharmaceutics. 2022;14(6):1160. doi:10.3390/pharmaceutics14061160
185. Ganguly S, Dewanjee S, Sen R, et al. Apigenin-loaded galactose tailored PLGA nanoparticles: a possible strategy for liver targeting to treat hepatocellular carcinoma. Colloids Surf. B. 2021;204:111778. doi:10.1016/j.colsurfb.2021.111778
186. Mary Lazer L, Sadhasivam B, Palaniyandi K, et al. Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. Int J Biol Macromol. 2018;107:1988–1998. doi:10.1016/j.ijbiomac.2017.10.064
187. Lazer LM, Kesavan Y, Gor R, et al. Targeting colon cancer stem cells using novel doublecortin like kinase 1 antibody functionalized folic acid conjugated hesperetin encapsulated chitosan nanoparticles. Colloids Surf. B. 2022;217:112612. doi:10.1016/j.colsurfb.2022.112612
188. Wang X, Song Y, Yu L, et al. Co-delivery of hesperetin and cisplatin via hyaluronic acid-modified liposome for targeted inhibition of aggression and metastasis of triple-negative breast cancer. ACS Appl Mater Interfaces. 2023;15(29):34360–34377. doi:10.1021/acsami.3c03233
189. Wu S, Yang X, Luo F, et al. Biosynthesis of flower-shaped Au nanoclusters with EGCG and their application for drug delivery. J Nanobiotechnol. 2018;16(1). doi:10.1186/s12951-018-0417-3
190. Cunha L, Coelho SC, Pereira MDC, et al. Nanocarriers based on gold nanoparticles for epigallocatechin gallate delivery in cancer cells. Pharmaceutics. 2022;14(3):491. doi:10.3390/pharmaceutics14030491
191. Zhang L, Chen W, Tu G, et al. Enhanced Chemotherapeutic Efficacy of PLGA-Encapsulated Epigallocatechin Gallate (EGCG) against human lung cancer. Enhan Chemotherap Eff PLGA-Encapsul Epigalloc Gall. 2020;15:4417–4429.
192. Han X, Zhang G, Wu X, et al. Microfluidics-enabled fluorinated assembly of EGCG-ligands-siTOX nanoparticles for synergetic tumor cells and exhausted t cells regulation in cancer immunotherapy. J Nanobiotechnol. 2024;22(1). doi:10.1186/s12951-024-02328-4
193. He L, Peng L, Wang L, et al. Investigation of folate-modified EGCG-loaded thermosensitive nanospheres inducing immunogenic cell death and damage-associated molecular patterns in hepatocellular carcinoma. Biochem Biophys Res Commun. 2024;714:149976. doi:10.1016/j.bbrc.2024.149976
194. Sulaiman GM, Waheeb HM, Jabir MS, et al. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci Rep. 2020;10(1). doi:10.1038/s41598-020-66419-6
195. Purushothaman K, P UM, K. M. MSB. Magnetic casein-CaFe2O4 nanohybrid carrier conjugated with progesterone for enhanced cytotoxicity of citrus peel derived hesperidin drug towards breast and ovarian cancer. Int J Biol Macromol. 2020;151:293–304. doi:10.1016/j.ijbiomac.2020.02.172
196. El Sisi AE, Sokkar SS, Ibrahim HA, et al. Targeting MDR‐1 gene expression, BAX/BCL2, caspase‐3, and Ki‐67 by nanoencapsulated imatinib and hesperidin to enhance anticancer activity and ameliorate cardiotoxicity. Fundament Clinic Pharmacol. 2020;34(4):458–475. doi:10.1111/fcp.12549
197. Singh N, Anand SK, Sharma A, et al. Chitosan/alginate nanogel potentiate berberine uptake and enhance oxidative stress mediated apoptotic cell death in HepG2 cells. Int J Biol Macromol. 2024;257:128717. doi:10.1016/j.ijbiomac.2023.128717
198. Uma Maheswari RT, Ajithkumar V, Varalakshmi P, et al. CD44 tagged hyaluronic acid - chitosan liposome carrier for the delivery of berberine and doxorubicin into lung cancer cells. Int J Biol Macromol. 2023;253:126599. doi:10.1016/j.ijbiomac.2023.126599
199. Li X, Wang Z, Wang X, et al. Berberine-loaded Janus gold mesoporous silica nanocarriers for chemo/radio/photothermal therapy of liver cancer and radiation-induced injury inhibition. Int J Nanomed. 2019;14:3967–3982. doi:10.2147/IJN.S206044
200. Qi QR, Tian H, Yue BS, et al. Research Progress of SN38 drug delivery system in cancer treatment. Int J Nanomed. 2024;19:945–964. doi:10.2147/IJN.S435407
201. Chen Y, Wang Z, Wang X, et al. Advances in antitumor nano-drug delivery systems of 10-hydroxycamptothecin. Int J Nanomed. 2022;17:4227–4259. doi:10.2147/IJN.S377149
202. Liu H, Shi Y, Ji G, et al. Ultrasound-triggered with ROS-responsive SN38 nanoparticle for enhanced combination cancer immunotherapy. Front Immunol. 2024;15:1.
203. Wu D, Li Y, Zhu L, et al. A biocompatible superparamagnetic chitosan-based nanoplatform enabling targeted SN-38 delivery for colorectal cancer therapy. Carbohydr Polym. 2021;274:118641. doi:10.1016/j.carbpol.2021.118641
204. Yan Z, Bao H, Zhang Q, et al. Effects of nanoparticle size on antitumor activity of 10-hydroxycamptothecin-conjugated gold nanoparticles: in vitro and in vivo studies. Int J Nanomed. 2016;11(7):929. doi:10.2147/IJN.S96422
205. Alibolandi M, Abnous K, Anvari S, et al. CD133-targeted delivery of self-assembled PEGylated carboxymethylcellulose-SN38 nanoparticles to colorectal cancer. Artif Cells Nanomed Biotechnol. 2018;46(sup1):1159–1169. doi:10.1080/21691401.2018.1446969
206. Guo Y, Gao T, Fang F, et al. A novel polymer micelle as a targeted drug delivery system for 10-hydroxycamptothecin with high drug-loading properties and anti-tumor efficacy. Biophys. Chem. 2021;279:106679. doi:10.1016/j.bpc.2021.106679
207. Singh P, Sen K, Sa P, et al. Piperlongumine based nanomedicine impairs glycolytic metabolism in triple negative breast cancer stem cells through modulation of GAPDH & FBP1. Phytomedicine. 2024;123:155181. doi:10.1016/j.phymed.2023.155181
208. Singh P, Sahoo SK. Piperlongumine loaded PLGA nanoparticles inhibit cancer stem-like cells through modulation of STAT3 in mammosphere model of triple negative breast cancer. Int J Pharm. 2022;616:121526. doi:10.1016/j.ijpharm.2022.121526
209. Liu Q, Zhao D, Zhu X, et al. Coloaded nanoparticles of paclitaxel and piperlongumine for enhancing synergistic antitumor activities and reducing toxicity. J Pharm Sci. 2017;106(10):3066–3075. doi:10.1016/j.xphs.2017.05.027
210. Jiang H, Tang C, Wen Y, et al. Enhanced antitumor efficacy of novel biomimetic platelet membrane-coated tetrandrine nanoparticles in nonsmall cell lung cancer. Mol Pharmaceut. 2023;20(11):5463–5475. doi:10.1021/acs.molpharmaceut.3c00310
211. Jiang M, Zhang R, Wang Y, et al. Reduction-sensitive paclitaxel prodrug self-assembled nanoparticles with tetrandrine effectively promote synergistic therapy against drug-sensitive and multidrug-resistant breast cancer. Mol Pharm. 2017;14(11):3628–3635. doi:10.1021/acs.molpharmaceut.7b00381
212. Li X, Yu N, Li J, et al. Novel “carrier-free” nanofiber codelivery systems with the synergistic antitumor effect of paclitaxel and tetrandrine through the enhancement of mitochondrial apoptosis. ACS Appl Mater Interfaces. 2020;12(9):10096–10106. doi:10.1021/acsami.9b17363
213. Shukla R, Singh A, Singh KK. Vincristine-based nanoformulations: a preclinical and clinical studies overview. Drug Deliv Transl Res. 2024;14(1):1–16. doi:10.1007/s13346-023-01389-6
214. Al-Musawi S, Ibraheem S, Abdul Mahdi S, et al. Smart nanoformulation based on polymeric magnetic nanoparticles and vincristine drug: a novel therapy for apoptotic gene expression in tumors. Life. 2021;11(1):71. doi:10.3390/life11010071
215. Wang X, Lu P, Zhu L, et al. Anti-CD133 antibody-targeted therapeutic immunomagnetic albumin microbeads loaded with vincristine-assisted to enhance anti-glioblastoma treatment. Mol Pharmaceut. 2019;16(11):4582–4593. doi:10.1021/acs.molpharmaceut.9b00704
216. Maia ALC, Ferreira CDA, Barros ALBD, et al. Vincristine-loaded hydroxyapatite nanoparticles as a potential delivery system for bone cancer therapy. J Drug Targeting. 2018;26(7):592–603. doi:10.1080/1061186X.2017.1401078
217. Qiu L, Dong C, Kan X. Lymphoma-targeted treatment using a folic acid-decorated vincristine-loaded drug delivery system. Int J Nanomed. 2018;12:863–872.
218. Vahedi F, Javan B, Sharbatkhari M, et al. Synergistic anticancer effects of co-delivery of linc-RoR siRNA and curcumin using polyamidoamine dendrimers against breast cancer. Biochem Biophys Res Commun. 2024;705:149729. doi:10.1016/j.bbrc.2024.149729
219. Mukhopadhyay R, Sen R, Paul B, et al. Gemcitabine co-encapsulated with curcumin in folate decorated PLGA nanoparticles; a novel approach to treat breast adenocarcinoma. Pharm Res. 2020;37(3). doi:10.1007/s11095-020-2758-5
220. Firouzi Amandi A, Jokar E, Eslami M, et al. Enhanced anti-cancer effect of artemisinin- and curcumin-loaded niosomal nanoparticles against human colon cancer cells. Med Oncol. 2023;40(6). doi:10.1007/s12032-023-02032-7
221. Wang W, Zhou M, Xu Y, et al. Resveratrol-Loaded TPGS-resveratrol-solid lipid nanoparticles for multidrug-resistant therapy of breast cancer: in vivo and in vitro study. Front Bioeng Biotechnol. 2021;9. doi:10.3389/fbioe.2021.762489
222. Liu X, Liu J, Xu S, et al. Gold nanoparticles functionalized with au–se-bonded peptides used as gatekeepers for the off-target release of resveratrol in the treatment of triple-negative breast cancer. ACS Appl Mater Interfaces. 2023;15(2):2529–2537. doi:10.1021/acsami.2c10221
223. Thipe VC, Amiri KP, Bloebaum P, et al. Development of resveratrol-conjugated gold nanoparticles: interrelationship of increased resveratrol Corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int J Nanomed. 2019;14:4413–4428. doi:10.2147/IJN.S204443
224. Qian Y, Mao J, Leng X, et al. Co-delivery of proanthocyanidin and mitoxantrone induces synergistic immunogenic cell death to potentiate cancer immunotherapy. Biomater Sci. 2022;10(16):4549–4560. doi:10.1039/D2BM00611A
225. ZhuGe DL, Wang LF, Chen R, et al. Cross-linked nanoparticles of silk fibroin with proanthocyanidins as a promising vehicle of indocyanine green for photo-thermal therapy of glioma. Artif Cells Nanomed Biotechnol. 2019;47(1):4293–4304. doi:10.1080/21691401.2019.1699819
226. Gao X, Yao Y, Chen X, et al. Lentinan-functionalized selenium nanoparticles induce apoptosis and cell cycle arrest in human colon carcinoma HCT-116 cells. Frontiers in Nutrition. 2022;9. doi:10.3389/fnut.2022.987807
227. Liu H, Qin Y, Zhao Z, et al. Lentinan-functionalized selenium nanoparticles target tumor cell mitochondria via TLR4/TRAF3/MFN1 pathway. Theranostics. 2020;10(20):9083–9099. doi:10.7150/thno.46467
228. Xiong J, Jiang B, Luo Y, et al. Multifunctional nanoparticles encapsulating astragalus polysaccharide and gold nanorods in combination with focused ultrasound for the treatment of breast cancer. Int J Nanomed. 2020;15:4151–4169. doi:10.2147/IJN.S246447
229. Jiao J, Yu J, Ji H, et al. Synthesis of macromolecular Astragalus polysaccharide-nano selenium complex and the inhibitory effects on HepG2 cells. Int J Biol Macromol. 2022;211:481–489. doi:10.1016/j.ijbiomac.2022.05.095
230. Duan Z, Liang M, Yang C, et al. Selenium nanoparticles coupling with Astragalus Polysaccharides exert their cytotoxicities in MCF-7 cells by inhibiting autophagy and promoting apoptosis. J Trace Elem Med Biol. 2022;73:127006. doi:10.1016/j.jtemb.2022.127006
231. Wang B, Guo C, Liu Y, et al. Novel nano-pomegranates based on astragalus polysaccharides for targeting ERα-positive breast cancer and multidrug resistance. Drug Delivery. 2020;27(1):607–621. doi:10.1080/10717544.2020.1754529
232. Karimian Ensaf P, Goodarzi MT, Homayouni Tabrizi M, et al. A novel nanoformulation of parthenolide coated with polydopamine shows selective cytotoxicity and induces apoptosis in gastric cancer cells. Naunyn-Schmiedeberg’s Arch Pharmacol. 2023;397:4435–4445. doi:10.1007/s00210-023-02907-6
233. Gao W, Wei S, Li Z, et al. Nano magnetic liposomes-encapsulated parthenolide and glucose oxidase for ultra-efficient synergistic antitumor therapy. Nanotechnology. 2020;31(35):355104. doi:10.1088/1361-6528/ab92c8
234. Liang P, Wu H, Zhang Z, et al. Preparation and characterization of parthenolide nanocrystals for enhancing therapeutic effects of sorafenib against advanced hepatocellular carcinoma. Int J Pharm. 2020;583:119375. doi:10.1016/j.ijpharm.2020.119375
235. Almatroudi A, Alsahli MA, Alsahli MA, et al. Novel strategies for disrupting cancer-cell functions with mitochondria-targeted antitumor drug-loaded nanoformulations. Int J Nanomed. 2021;16:3907–3936. doi:10.2147/IJN.S303832
236. Zhou S, Shang Q, Wang N, et al. Rational design of a minimalist nanoplatform to maximize immunotherapeutic efficacy: four birds with one stone. J Control Release. 2020;328:617–630. doi:10.1016/j.jconrel.2020.09.035
237. Wu D, Zhang Z, Li X, et al. Cucurbit[10]uril-based supramolecular radicals: powerful arms to kill facultative anaerobic bacteria. J Control Release. 2023;354:626–634. doi:10.1016/j.jconrel.2023.01.040
238. Wu D, Zhang Z, Li X, et al. Dynamically assembled nanomedicine based on host-guest molecular recognition for NIR laser-excited chemotherapy and phototheranostics. Acta Biomater. 2023;168:565–579. doi:10.1016/j.actbio.2023.07.022
239. Qi S, Zhang X, Yu X, et al. Supramolecular lipid nanoparticles based on host-guest recognition: a new generation delivery system of mRNA vaccines for cancer immunotherapy. Adv Mater. 2024;36(23). doi:10.1002/adma.202311574
240. Feng Y, Qi S, Yu X, et al. Supramolecular modulation of tumor microenvironment through pillar[5]arene-based host-guest recognition to synergize cancer immunotherapy. J Am Chem Soc. 2023;145(34):18789–18799. doi:10.1021/jacs.3c03031
241. Wu D, Zhang Z, Li X, et al. Supramolecular theranostic nanomedicine for in situ self-boosting cancer photochemotherapy. Biomacromolecules. 2023;24(2):1022–1031. doi:10.1021/acs.biomac.2c01469
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


