Back to Journals » International Journal of Nanomedicine » Volume 19
Novel Therapeutic Mechanisms and Strategies for Intracerebral Hemorrhage: Focusing on Exosomes
Authors Jiang S, Hu L, Zhou H, Wu J, Zhou J, Yu X, Chen G
Received 13 April 2024
Accepted for publication 20 August 2024
Published 2 September 2024 Volume 2024:19 Pages 8987—9007
DOI https://doi.org/10.2147/IJN.S473611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sachin Mali
Shandong Jiang,1,2 Libin Hu,1,2 Hang Zhou,1,2 Jianan Wu,1,2 Jiayin Zhou,1,2 Xian Yu,1,2 Gao Chen1,2
1Department of Neurosurgery, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310016, People’s Republic of China; 2Key Laboratory of Precise Treatment and Clinical Translational Research of Neurological Diseases, Hangzhou, 310016, People’s Republic of China
Correspondence: Gao Chen, Department of Neurosurgery, Second Affiliated Hospital, School of Medicine, Zhejiang University, Clinical Research Center for Neurological Diseases of Zhejiang Province, Hangzhou, 310009, People’s Republic of China, Email [email protected]
Abstract: Intracerebral hemorrhage (ICH) is a primary, non-traumatic cerebral event associated with substantial mortality and disability. Despite advancements in understanding its etiology and refining diagnostic techniques, a validated treatment to significantly improve ICH prognosis remains elusive. Exosomes, a subtype of extracellular vesicles, encapsulate bioactive components, predominantly microRNAs (miRNAs), facilitating and regulating intercellular communication. Currently, exosomes have garnered considerable interests in clinical transformation for their nanostructure, minimal immunogenicity, low toxicity, inherent stability, and the ability to traverse the blood-brain barrier. A wealth of studies has demonstrated that exosomes can improve the prognosis of ICH through anti-apoptosis, neurogenesis, angiogenesis, anti-inflammation, immunomodulation, and autophagy, primarily via the transportation or overexpression of selected miRNAs. More importantly, exosomes can be easily customized with specific miRNAs or bioactive compounds to establish delivery systems, broadening their potential applications. This review focuses on the therapeutic potential of exosomes in ICH, reviewing the mechanisms of molecular biology mediated by certain miRNAs, discussing the benefits, challenges, and future prospects in ICH treatment. We hope comprehensive understanding of exosomes based on miRNAs will provide new insights into the treatment of ICH and guide the translation of exosome’s research from laboratory to clinical practice.
Keywords: intracerebral hemorrhage, exosomes, extracellular vesicles, microRNAs, stem cells
Graphical Abstract:

Introduction
Spontaneous intracerebral hemorrhage (ICH) represents a catastrophic form of stroke, characterized by blood extravasation within the brain parenchyma. It accounts for 15% of all stroke-related cases.1–3 Despite considerable advancements in the management of ICH and its associated complications, an effective clinical intervention that can significantly ameliorate ICH prognosis remains wanting. In the ICH pathogenesis landscape, hematoma size is a recognized determinant of ICH severity and a robust prognostic indicator, underscoring the potential benefits of early hematoma clearance for prognosis improvement.4 However, recent clinical trials suggest that neither early open nor endoscopic surgical interventions for hematoma evacuation confer significant advantages in functional recovery compared to medical management of ICH.5 This highlights the limited success of mechanical hematoma removal in enhancing ICH outcomes. Consequently, researchers are fervently exploring viable strategies to expedite hematoma resolution and hasten neurological functional recovery via endogenous pathways.
A plethora of potential therapeutic drugs or agents that could promote hematoma degradation have been identified. However, the blood-brain barrier (BBB) restricts their access to the target parenchyma, thus limiting their utility in neurological disease management. This challenge may be circumvented by exosomes, which not only reply on their own biological effects but also given their inherent BBB crossing potential and broad surface-engineering capability, as confirmed by numerous studies and techniques.6–8 The interaction between exosome surface ligands and brain endothelial cell receptors is thought to be the main mechanism.9
Exosomes are lipid bilayer-delimited particles naturally secreted from cells, either through vesicle budding into endosomes or direct vesicle budding from the plasma membrane (PM).10 They are secreted by almost all cell types, including stem cells, macrophages, microglia, astrocytes, and so forth,11 and are present in various body fluids like blood, cerebral spinal fluid (CSF), semen, saliva, plasma, serum, and bronchial fluid.12 Based on their natural targeting capabilities different types of natural exosomes from different donor cells can adhere to various targeting cells. Besides, exosomes can be amended through their parental cells to express a targeting moiety on their surface, or supplemented with desired biological activity.13 Owing to their ability to cross biological barriers, innate stability, and high delivery efficiency,14 exosomes facilitate crucial intercellular communications, including proliferation, differentiation, intercellular signaling, and immune regulation.15
Exosomes contain a myriad of biological molecules, including DNA, RNA, microRNAs, circular RNAs (circRNAs), amino acids, metabolites, and cytosolic proteins, which play pivotal roles in intercellular communication.16 Circular RNAs are a type of noncoding RNA that are particularly abundant in brain tissues, which are produced by the back splicing of long RNA transcripts (including mRNAs and long noncoding RNAs).17 It has demonstrated that exosomal circRNAs are associated with intracranial vascular disease. Jiang X18 first revealed that five plasma brain-derived exosomal circRNAs serve as ideal biomarkers for the diagnosis of acute ischemic stroke and may also be potential targets for therapeutic interventions. Besides, circRNAs have critical influence on critical molecular and cellular processes underpinning pathogenesis of intracranial aneurysms, make circRNAs an enticing avenue for innovative therapeutic approaches, offering as potential diagnostic biomarkers.19 However, among the various components of exosomes, microRNAs (miRNAs) are pivotal to their biological function. They play a significant role in cell proliferation, inflammation, apoptosis, and other biological processes.20–22 As single-stranded non-coding RNAs of 20 to 24 nucleotides, miRNAs are not translated into proteins themselves but regulate protein translation by targeting mRNA, thereby controlling gene expression at the epigenetic level.20 One or both strands of mature miRNA bind to argonaute 2 (Ago2), forming part of the RNA-induced silencing complex (RISC), which targets mRNA for cleavage or translational repression.21,22 While new miRNAs frequently emerge, they are seldom lost.23 However, miRNAs are inherently unstable and prone to degradation, especially in the extracellular environment. This leads to their uneven distribution in various extracellular fluids, impacting the stability and efficiency of signal transmission.24 Without a carrier, it is challenging for miRNAs to be secreted into extracellular fluids or to be specifically recognized and absorbed by recipient cells.25 However, when transported in the form of exosomes, miRNAs can be absorbed by proximate or distant cells, influencing their gene expression and biological function. This is considered the third cellular communication pathway, following cell contact-dependent signaling and soluble molecule-mediated signaling.26 The bilayer lipid membrane structure of exosomes protects the encapsulated miRNA from degradation by ribonuclease, thereby maintaining miRNA stability. Furthermore, miRNAs can be efficiently packaged into exosomes, facilitating their precise delivery to recipient cells and enabling their participation in intercellular communication.27 As research progresses, evidence has emerged showing that exosomes promote recovery after ICH by suppressing cell apoptosis,28 promoting angiogenesis,29 reducing inflammation,30 modulating cell phenotype,31 and autophagy.32 These effects are mediated by the various miRNAs contained within exosomes. Thus, due to their nanoscale size, low expression of membrane-bound proteins, and remarkable therapeutic effects, exosomes are emerging as an excellent candidate for ICH treatment.
Despite the promise of exosomes in the treatment of central nervous system diseases, research has primarily focused on ischemic stroke and Alzheimer’s disease, with ICH receiving less attention. This review, therefore, concentrates on the therapeutic roles and mechanisms of exosomes in ICH, summarizing the therapeutic mechanisms of miRNAs and other exosomal contents in improving ICH prognosis and complications. With the advancement of material technology, exosome-based targeted drug transport systems have also been widely studied, which broaden the clinical application of exosomes in the treatment of ICH. We also discussed the advantages, challenges, and prospects of exosomes in the treatment of ICH. This review aims to support and guide the translation of exosome research from laboratory to clinical practice.
Composition and Biogenesis of Exosomes
Exosomes, membrane-bound carriers with a spheroid shape of diameters ranging from 40 to 160 nm, reflect the nature and physiological state of their donor cell (Figure 1).33 During membrane fusion, exosomes incorporate various surface proteins and markers from parental cell. The main membrane-bound proteins included in exosomes belong to the tetraspanin family (CD9, CD63, CD81, and CD82),34 endosomal sorting complexes required for transport (ESCRTs) proteins, heat shock proteins (HSPs), actin, and adhesion molecules like integrin lymphocyte function-associated antigen 1 (LFA-1).35,36 In addition, proteins such as MHC Class I and II are specific to the donor cell type.37 Exosomes also possess several endosome membrane proteins like flotillin and ALIX, also known as PDC61-interacting proteins.
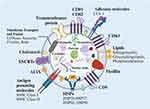 |
Figure 1 The structure and biological composition of exosomes. Exosomes are composed of various proteins: tetraspanins, antigen presenting molecules, adhesion molecules, heat shock proteins (HSPs), ESCRT components, membrane transport, and fusion molecules; proteins in endosome membranes such as flotillin and ALIX. Components in the exosome lumen include proteins, polypeptides, nucleic acids (DNA, RNA, mRNA, noncoding RNA), cytokines, and metabolites. Exosomes also constitute multiple lipids such as cholesterol, sphingomyelin, glycosphingolipids, and phosphatidylserine. Nucleic acids among DNA, mRNAs, non-coding RNAs, etc. The figure was created with https://app.biorender.com/. Abbreviations: HSPs, Heat shock proteins; MHC, Major histocompatibility complex; 1=LFA-1, Lymphocyte function-associated antigen; ESCRTs, Endosomal sorting complexes required for transports. |
Exosomes originate either through the invagination of the plasma membrane (PM) or direct budding from the PM (Figure 2).38 Specifically, exosomes are generated through a process involving the invagination of the plasma membrane via endocytosis, which forms early endosomes. This leads to the creation of intracellular multivesicular bodies (MVBs) or late endosomes containing intraluminal vesicles (ILVs), a process mediated by Rab27a.39 ILVs sequester specific proteins, lipids, and cytosolic components, a process first discovered through the study of vesicular secretion of the transferrin receptor (TFR) by mature reticulocytes.40 Following the transportation of MVBs to the PM via the cytoskeletal and microtubule network, and subsequent exocytosis post fusion to the PM, ILVs are ultimately secreted into the extracellular environment as exosomes.34 In addition to fusing with the PM, MVBs can also merge with lysosomes and autophagosomes for degradation35. Furthermore, MVBs intersect with other intracellular vesicles and organelles, contributing to the diversity in the constituents of exosomes.34 Notably, exosomes can also be generated by direct vesicle budding from the plasma membrane through exocytosis. This complex process demonstrates the intricate nature of exosome formation and release, highlighting their potential as precise delivery systems for intercellular communication.
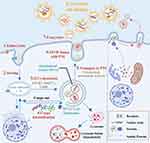 |
Figure 2 Biogenesis of exosomes. In the process of biogenesis, Within the endosomal system, soluble proteins were internalized by endocytosis1 to sort into early endosomes,2 which then mature into late endosomes or MVBs.3 Late endosomes/MVBs are specialized endosomal compartments rich in ILVs, which sequester proteins, lipids, cytosolic compartments, and potential exosome cargoes.4 MVBs containing exosome cargoes get transported to the PM via cytoskeletal and microtubule network,5 fuse with the cell surface5 and the ILVs then get secreted as exosomes.6 The figure was created with https://app.biorender.com/. Abbreviations: ILVs, intraluminal vesicles; MVBs, Multivesicular Bodies; PM, Plasma membrane. |
Through complex biogenesis process, exosomes contain a diverse array of biological molecules, including proteins, nucleic acids (DNA, RNA, noncoding RNA), cytokines, and metabolites, reflecting their varied cellular origins.16 Among these, miRNAs are one of the most abundant RNA species in exosomes. They play significant roles in multiple biological processes, including exocytosis, neurogenesis, and angiogenesis, and participate in exosome-mediated cellular communications.41 All cells, both prokaryotes and eukaryotes, release extracellular vesicles (EVs), particularly exosomes, as part of intercellular communication and disease development.27 Exosomes can transfer a range of these biological macromolecules into target cells, inducing specific signaling cascades that favor cell bioactivity.42 These biological macromolecules are encased within a lipid bilayer, often enriched in cholesterol, sphingomyelin, glycosphingolipids, and phosphatidylserine.43 The lipid content not only protects the macromolecules from degradation but also enhances bioavailability and minimizes adverse reactions. Furthermore, the lipid composition influences cargo sorting, exosome secretion, structure, and signaling, making exosomes an efficient and precise delivery system for intercellular communication.43 As research progresses, the potential of exosomes in the treatment of conditions such as intracerebral hemorrhage is becoming increasingly apparent.
Pathophysiologic Connection Between ICH and Exosomes
Understanding the pathophysiology of ICH is vital for devising effective treatment and management strategies. The relationship between the mechanism of ICH and exosomes is depicted in Figure 3.44 ICH-related pathological brain injury involves both primary and secondary injuries. The primary brain injury occurs when blood vessels rupture to form a hematoma, resulting in direct mechanical damage to the brain tissue due to compression. Post-ICH, the surrounding brain tissue will be faced with ischemic and hypoxic, leading to a shift in cellular metabolism from aerobic phosphorylation to anaerobic glycolysis.45 Exosomes can transmit miR-126,46 miR-125a, miRNA-181b-5p/TRPM7 axis, and others to promote angiogenesis, which is beneficial to improve prognosis by compensating for brain ischemia. Continuous bleeding or hematoma expansion exacerbates mechanical compression injury and ischemic changes in the peripheral brain tissue, accelerating neurological deficits due to increased neuron and glial cell death. Exosomes can transport miR-124,47 miR-17-92,48 and other miRNAs to promote functional recovery via facilitating neural plasticity, myelination, and neurite remodeling. When cell mitochondria or cytomembranes are damaged, excessive release of excitatory neurotransmitters and dysfunction of sodium, potassium, and calcium ions occur, leading to nerve cell death and apoptosis, creating a vicious cycle of brain damage. Reducing apoptosis levels is beneficial for reducing neuron death and functional deficits. Exosomes, loaded with miR‐134, could reduce oligodendrocyte apoptosis by upregulating caspase-8, which suppresses the extrinsic apoptosis signaling cascade.49
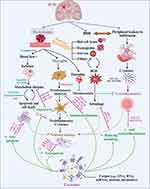 |
Figure 3 The Connection between pathological injury after ICH and exosomes with miRNAs. Hematoma is the key to pathological injury after ICH, which induces mechanical compression and activates bioactive factors. Exosomes can alleviate parenchymal pathological damage and improve prognosis via angiogenesis, neurogenesis, anti-apoptosis, anti-neuroinflammation, immunomodulation, and regulating autophagy, which is mediated by overexpressing or transporting various miRNAs. The figure was created with https://app.biorender.com/. |
Secondary brain injury is caused by the physiological response to the hematoma and the toxic components originating from the hematoma.50 After ICH, the bleeding site and surrounding brain tissue induce inflammation primarily through peripheral leukocyte diapedesis and activation of astrocytes and microglia, excessively releasing proinflammatory factors, including Interleukin (IL), tumor necrosis factor (TNF), nuclear factor (NF), matrix metalloproteinases (MMPs), nitric oxide (NO), reactive oxygen species (ROS), and others.51 Exosomes can alleviate neuroinflammation induced by hemorrhage by transferring miR-183-5p,52 angiotensin-converting enzyme-2,53 and others, thereby reducing IL, NF, TNF, and other proinflammatory factors. Microglia, the brain’s macrophages, play a key role in ICH-induced brain inflammation.54 Under physiological conditions, a balance between the anti-inflammatory and pro-inflammatory phenotypes of microglia helps maintain tissue homeostasis.55 However, under pathological conditions of ICH, overactivation of microglia may trigger and accelerate neuronal damage. Excessive activation of microglia will lead to nervous system inflammation and neuronal damage, correspondingly inflammatory factors secreted by damaged or dead neurons can further promote the activation of microglia.56 Exosomes from various sources can adjust the polarization of microglia from proinflammatory to anti-inflammatory phenotype through complex molecular mechanisms and miRNA-126-5p,57 miRNA-21-3p,57 miR-146a-5P,58 etc. Local increase of thrombin also participates in enhancing neuroinflammation via inhibition of an anti-inflammatory factor--thrombomodulin, which is mediated by C3 and C5 of complement system.59,60 Exosomes derived from thrombin-activated platelet, containing miRNA-223, may also play an important role in regulating neuroinflammation.61
The main approach to hematoma absorption is dependent on hemoglobin breakdown; however, degradation products, including red cell lysate, iron ions, and biliverdin, can lead to secondary damage. High concentrations of iron ions can increase ROS levels and direct brain injury mediated by oxidative stress.62 Free radical generation could contribute to the iron imbalance-induced brain injury,63 which consists of a vicious circle. Exosomes can break this vicious circle by reducing the levels of TNF-α and ROS.64 The process of autophagy after ICH is a complex pathological process, which initiates an increase in ROS, Ca2+ ions, and excitatory neurotransmitters. Accumulation of red blood cell breakdown products and leukocyte infiltration also facilitate the process.36 Modest autophagy is beneficial to protect cells against stressful circumstances and facilitate cell survival. Exosomes can reduce the level of apoptosis via transporting miR-21 or miR-25-3p into target cells.65 Furthermore, the disruption of the BBB caused by physical compression of the hematoma or hemoglobin breakdown products leads to increased BBB permeability in the hemorrhagic area, resulting in edema and peripheral leukocyte infiltration.66
Exosome are also related with stroke through regulating intercellular communication in brain-derived cells. A recent study show that microglia are the main source in brain under physiological conditions for extracellular vesicles (sEVs), including exosomes. This situation changed at 24h after induced stroke-reperfusion, where a significant increase of astrocytic sEVs was observed.67 Furthermore, the result further show that EVs isolated from murine brain and brain-derived cell lines are enriched in prion protein (PrP) and PrP fragment(PrP-C1), with consequences in the regulation of vesicle uptake by recipient cells. Accordingly, when sEVs lack PrP, their uptake by neurons is increased, and conspicuously, they are also more readily taken up by microglia and astrocytes. Moreover, PrP-KO-sEVs were found to colocalize with lysosomes much faster than WT-sEVs. Therefore, increased release of sEVs and exosomes by astrocytes together with elevated levels of PrP in sEVs may play a role in intercellular communication at early stages after stroke, which may be a novel therapeutic method for stroke and ICH.67
In summary, following blood vessel rupture, hemoglobin enters the brain, forming a hematoma which induces pathological changes such as toxicity, edema, inflammation, oxidative stress, and apoptosis, leading to neurological deficits. The mechanism of brain injury after ICH is complex, but we can still improve the therapeutic effect of ICH by affecting one or more pathological pathways. Exosomes alleviate brain injury via angiogenesis, neurogenesis, anti-apoptosis, anti-inflammatory, immunomodulation, autophagy, and other ways, promoting the recovery of nerve function and reducing the mortality and disability rate of ICH. This potential has attracted increasing attention for its application prospects and potential conversion value.
The Effects and Mechanism of Exosomes in the Treatment of ICH
ICH treatment initially focuses on hematoma removal via endogenous pathways and protecting surrounding brain parenchyma. Subsequent stages concentrate on repairing damaged neural cells, primarily through reducing inflammation, promoting angiogenesis, and neurogenesis.68 Exosomes can improve ICH prognosis by promoting angiogenesis, neurogenesis, anti-apoptosis, anti-inflammatory, and immunomodulation, aligning with ICH’s pathological mechanisms. Understanding the association between exosomes and these mechanisms could elucidate their therapeutic effect. In the following sections, we will discuss these mechanisms in detail.
Association Between Exosomes and Angiogenesis After ICH
Following an ICH event, vascular integrity is compromised, leading to ischemic stress in adjacent tissues and blood vessels. This stress further exacerbates brain damage. Angiogenesis, the formation of new vascular buds from pre-existing blood vessels, is a complex process that can enhance blood perfusion in the ischemic area.69 This increased blood supply helps maintain neuronal energy metabolism, thereby preventing neurons from entering a harmful cycle of apoptosis and death. As such, promoting cerebral angiogenesis under ischemic or hemorrhagic conditions may be a viable strategy to mitigate brain functional damage after ICH.
Interestingly, research has shown that exosomes from various cell sources, including mesenchymal stem cells (MSCs), cardiac progenitor cells, endometriotic stromal cells, and human induced pluripotent stem cells (iPSCs), can stimulate angiogenesis.70,71 Angiogenesis is a multistep process involving cell proliferation, migration, invasion, vessel formation, and the maturation and stabilization of new sprouts. This process is mediated by activated endogenous progenitor cells, exogenous stem cells, and therapeutic agents like pro-angiogenic miRNAs.72,73 Certain miRNAs, such as miR210, miR126, miR132, and miR21, found in exosomes, can promote angiogenesis by inhibiting gene expression post-transcriptionally.29 For example, exosomes secreted by bone marrow mesenchymal stem cells (BMMSCs) transplanted into mice with ischemic stroke have been shown to enhance neurological and motor function recovery. This improvement is potentially mediated by miR-126, an endothelial cell-specific miRNA known to play a critical role in vascular stabilization.46,74 Exosomes derived from miR-126 overexpressing BMMSCs have been shown to promote the proliferation, migration, and angiogenesis of human umbilical vein vessel endothelial cells (HUVECs) by regulating the PI3K/Akt signaling pathway.75 Other studies have found that the effect of miR-126 is responsive to angiogenic growth factors, including VEGF or fibroblast growth factor.75,76 Exosomes from human adipose-derived mesenchymal stem cells (ADMSCs) have been shown to increase the length, number, and branches of microvessels by transferring miR-125a and the miRNA-181b-5p/TRPM7 axis. Overexpression of TRPM7 reverses the effects of miRNA-181b-5p-exosomes on migration and cerebral capillary microvessel formation. Furthermore, it has been reported that exosomes originating from mouse brain endothelial cells can significantly enhance endothelial capillary formation, leading to higher blood vessel density, larger arterial diameter, and improved neurological cognitive function after ICH.46 A summary of the current knowledge of exosomes in the treatment of ICH via related angiogenesis mechanisms is provided in Table 1.
 |
Table 1 The Source, Component, and Mechanism of Exosomes in the Treatment of ICH Through Angiogenesis |
Association Between Exosomes and Neurogenesis After ICH
Neurogenesis, a process that includes the proliferation of neural stem cells (NSCs), migration of immature neurons and neuroblasts, differentiation into adult neurons, and extension of neurites, culminates in the formation and stabilization of synapses.78 Notably, neurogenesis after a stroke is closely associated with angiogenesis.79,80 Angiogenesis serves as the foundation for neurogenesis, and in turn, neurogenesis is a prerequisite for functional recovery.81 The inhibition of cerebral angiogenesis can negatively impact neurogenesis in both hemorrhagic and ischemic brains. Therefore, encouraging neurogenesis under ischemic or hemorrhagic conditions could be an effective strategy to reduce brain functional damage following ICH.
As previously discussed, neurogenesis and angiogenesis often occur concurrently. Exosomes derived from mouse brain endothelial cells have been linked to improved synaptic remodeling and enhanced neurological cognitive function, attributed to the formation of endothelial capillaries and increased blood vessel density.46 Exosomes carrying miR-126 also foster neuronal formation and axonal growth, thereby aiding the recovery of neurological and motor functions74. Preclinical studies indicate that the systemic administration of exosomes can support the endogenous rewiring of neuronal circuitry, white matter remodeling, angiogenesis, and neurogenesis in the injured brain.82 Exosomes derived from MSCs have been shown to effectively enhance neurological recovery, particularly in spatial learning and motor functions after ICH. This could be attributed to their role in promoting endogenous neurogenesis, including the formation of neuroblasts and mature neurons in the subventricular zone, and myelination in the striatum.83 In line with these findings, exosomes derived from miRNA 133b-overexpressing MSCs have demonstrated the ability to enhance neural plasticity and increase myelination, thereby facilitating functional recovery and neurite remodeling plasticity.84 A summary of the current understanding of exosomes in the treatment of ICH via the related mechanism of neurogenesis is provided in Table 2.
 |
Table 2 The Source, Component, and Mechanism of Exosomes in the Treatment of ICH Through Neurogenesis |
Association Between Exosomes and Anti-Apoptosis After ICH
Apoptosis, or programmed cell death, is a gene-controlled process that maintains internal environmental stability. In the context of ICH, apoptosis and cell death, which contribute to neuronal cell loss, serve as secondary injuries and are significant factors in high mortality and morbidity rates. As such, anti-apoptotic strategies could play a crucial role in mitigating and reversing the detrimental effects of ICH.
A study conducted in vitro has demonstrated that exosomes secreted by MSCs, loaded with miR-134, can reduce oligodendrocyte apoptosis following oxygen-glucose deprivation (OGD). This is achieved through the upregulation of Caspase 8, which suppresses the extrinsic apoptosis signaling cascade.49 Interestingly, the introduction of miR-134 inhibitors to exosomes reduces the expression of Caspase 8 but increases procaspase-8 and caspase-8 cleaved product proteins, which promotes OGD-induced apoptosis.49 Another study revealed that exosomes released by astrocytes can be absorbed by neurons, resulting in a decrease in apoptosis levels and an increase in mitochondrial performance, thereby aiding the functional recovery of damaged neurons.85 Similarly, microglia-derived exosomes can also reduce neuronal apoptosis when taken up by neurons.11 In vivo experiments further substantiate the anti-apoptotic properties of exosomes. A study by Heyu found that injecting MSCs transfected with miR-21 into ICH rats could mitigate neuronal apoptosis and improve neural function.28 This treatment also significantly reduced hematoma and edema sizes. Further research indicated that exosomes derived from miR-21 overexpressing MSCs, when absorbed by neurons, can lessen post-ICH damage by reducing the activation of the NF-κB pathway. This suggests that exosomes secreted by miR-21-modified MSCs can alleviate neuronal apoptosis through miR-21, thereby protecting neurological function after ICH.28 Mice treated with exosomes derived from miR-133b modified MSCs exhibited improved cognitive and motor function compared to the control group. This improvement is attributed to the resistance to neuronal apoptosis by inhibiting RhoA expression and activating the ERK1/2/CREB pathway in hemorrhagic brain tissue.86 Moreover, exosomes overexpressing miR-146a-5p from MSCs can enhance neural function after ICH by reducing neuronal apoptosis and inhibiting the proinflammatory phenotype polarization of microglia.87 A summary of the current understanding of exosomes in the treatment of ICH via the related mechanism of anti-apoptosis is provided in Table 3.
 |
Table 3 The Source, Component, and Mechanism of Exosomes in Treatment of ICH Through Anti- Apoptosis |
Association Between Exosomes and Anti-Inflammation After ICH
Neuroinflammation plays a significant role in the pathogenesis of ICH. In the early stages of ICH, microglia activation, cell death, and the breakdown of products initiate an inflammatory cascade that results in increased neuronal death. Microglia are the initial inflammatory cells that respond to the injury caused by ICH. The subsequent infiltration of blood-derived cells, including monocytes that transform into macrophages within the lesion, further intensifies the neuroinflammatory cascade.88 Within 12 hours post-ICH, inflammatory macrophages and dendritic cells largely outnumber microglia in the hemorrhagic area.54 The extensive neuroinflammatory response induced by ICH appears to exacerbate brain damage.81 As such, reducing neuroinflammation is considered a potential therapeutic target.
Several studies have shown that exosomes can carry miRNAs, proteins, or other contents, which can confer anti-inflammatory effects.31 For instance, exosomes derived from MSCs can mitigate the inflammatory response and improve prognosis following ICH by suppressing IL-1β levels.30 In a study involving diabetic rats with ICH, exosomes derived from BMSCs alleviated neuroinflammation induced by hemorrhage via the transfer of miR-183-5p.52 Moreover, the administration of exosomes secreted from angiotensin-converting enzyme 2-overexpressed endothelial progenitor cells could provide a therapeutic effect on acute ICH. This is achieved by alleviating functional neurological deficits and post-stroke inflammation through the transfer of ACE2 into the hemorrhage area, leading to down-regulated TNF-α and NF-kB, and upregulated IrBα levels.53 The result was further verified by using DX600--an ACE2 inhibitor.89 Exosomes derived from thrombin-activated platelets also play a crucial role in neuroinflammation. Upon the occurrence of ICH, thrombin is released, activating platelets that secrete exosomes containing miRNA-223. This triggers the activation of the NF-KB and MAPK signaling pathways. Through a series of signaling cascade pathways, the expression of miR-223 leads to the down-regulation of adhesion molecules, including ICAM-1.61 As several studies have shown that adhesion molecules exacerbate inflammatory conditions during clot formation inside blood vessels, inhibiting the generation of these adhesion molecules via exosomes could potentially improve inflammatory damage following a stroke.90,91
Furthermore, exosomes can influence neuroinflammation through systemic effects within and between organ systems. Evidence suggests that exosomes from peripheral cells affect the interaction between peripheral and brain inflammation.92 Peripheral inflammation triggers the choroid plexus epithelium to release exosomes into the CSF, which then cross the blood–cerebrospinal fluid barrier to transfer their pro-inflammatory cargo into astrocytes and microglia, leading to brain inflammation.82 Inhibiting exosome secretion to block this exosome-mediated communication can attenuate brain inflammation.92 Additionally, the brain-gut axis can regulate brain parenchyma inflammation under physiological conditions through extracellular vesicles secreted by bacteria. For instance, the K12-derived E. coli strain W3110 can reduce inflammation levels by inhibiting NLRP3 inflammasome activation, which prevents mitochondrial damage and iron death.93 This demonstrates how the exosome system can affect organisms at a systemic level. A summary of the current understanding of exosomes in the treatment of ICH via the related mechanism of anti-neuroinflammation is provided in Table 4.
 |
Table 4 The Source, Component, and Mechanism of Exosomes in Treating ICH Through Anti-Neuroinflammation |
Association Between Exosomes and Immunomodulation After ICH
Microglia display two distinct functional phenotypes: the proinflammatory and anti-inflammatory phenotypes. During the initial stages of ICH, there is a marked increase in the expression of TNF-α in the brain parenchyma surrounding the hematoma, a characteristic marker gene of the proinflammatory phenotype.94 This prompts microglia to secrete a multitude of pro-inflammatory cytokines such as nitric oxide synthase (NOS), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemotactic protein 1 (MCP1), thereby stimulating a nervous system inflammatory response.95 Conversely, microglia with an anti-inflammatory phenotype secrete interleukin-10 (IL-10), interleukin-1R (IL-1R), and transforming growth factor-β (TGF-β), which contribute to systemic protection and repair.96,97 Research has demonstrated that immunomodulation, including the inhibition of proinflammatory phenotypic polarization and acceleration of anti-inflammatory phenotype polarization in microglia, plays a neuroprotective role and could be a potentially important strategy in the treatment of ICH.98 Additionally, some studies have shown that the activation and polarization of microglia can be controlled, further substantiating the potential for recovery through immunomodulation.99
Exosomes secreted by microglia contain a large number of miRNAs, such as miR-126-5p, miR-21-3p, miR-212, among others, which are implicated in the polarization of microglia from a proinflammatory phenotype to an anti-inflammatory phenotype.57 Exosomes derived from MSCs have been shown to inhibit microgliosis and astrogliosis, and promote immunomodulation.100 Further research has indicated that these exosomes facilitate the shift in microglia phenotype from proinflammatory to anti-inflammatory by regulating the CysLT2R-ERK1/2 pathway.101
Similarly, exosomes secreted by BMSCs possess immunomodulatory and anti-inflammatory capabilities, which can be therapeutically beneficial in ICH. This is achieved through the regulation of miR-146a-5P, which may further target IRAKI and NFAT5 genes, inhibiting the activation of inflammatory signaling pathways.58 Another study confirmed that exosomes from BMSCs exert immunomodulation to protect brain parenchyma through the modulation of exosomal H19.102 Moreover, exosomes from BMSCs also regulate the polarization of microglia towards the anti-inflammatory phenotype by downregulating the expression of IL-1b, CD16, CD11b, and NOS, while upregulating CD206.103 A summary of the current understanding of exosomes in the treatment of ICH via the immunomodulation of microglia is provided in Table 5.
 |
Table 5 The Source, Component, and Mechanism of Exosomes in Treating ICH Through Immunomodulation |
Association Between Exosomes and Autophagy After ICH
Autophagy is a process where proteins or organelles are engulfed, endocytosed into vesicles, merged with lysosomes to form autophagic lysosomes, and then the contents within them are degraded. This process fulfills the metabolic requirements of the cell and facilitates the renewal of certain organelles. Numerous studies have indicated that both insufficient and excessive levels of autophagy can exacerbate cell death, whereas moderate autophagy can protect cells against stressful conditions and promote cell survival.104 Autophagy is considered an early-stage mechanism in the pathophysiology of stroke and inflammatory diseases, regulating immune cell homeostasis.105 Conversely, the production of certain inflammatory biomolecules, such as TNF-α, IL-1α, and IFN, can influence the progression of autophagy. Following the onset of ICH, inflammation, oxidative stress, and the accumulation of free iron can trigger apoptosis and autophagy.36 Autophagy may initially exacerbate brain injury, but later stages may be neuroprotective due to the removal of cellular debris.106 In vitro experiments have shown that inhibiting autophagy significantly reduces the inflammatory response induced by OGD. Similarly, in vivo studies have reported a significant reduction in the cerebral infarction area through the acceleration of the autophagic response at target sites.107 Both in vivo and in vitro experiments have confirmed that exosomes derived from astrocytes can mitigate ischemia-induced neuronal damage by inhibiting OGD-induced neuronal autophagy.108 Another study suggested that exosomes secreted by ADMSCs could reduce autophagy in a stroke mouse model by delivering miR-25-3p into target cells.65 Therefore, maintaining a balanced state of autophagy and inflammation may be a potential treatment strategy for ICH. A summary of the current understanding of exosomes in the treatment of ICH via autophagy-related mechanisms is provided in Table 6.
 |
Table 6 The Source, Component, and Mechanism of Exosomes in the Treatment of ICH Through Reducing Autophagy |
The Therapeutic Effect of Exosomes in Complications After ICH
ICH, a severe type of stroke, occurs when blood leaks into the brain parenchyma, contributing to 15% of total stroke-related cases.1–3 While most survivors of ICH experience some degree of spontaneous functional recovery, the improvement is often modest and insufficient for independent daily living, with only 25% of survivors managing to live independently within a year following ICH.109 Complications following ICH include visual impairment, ischemic cerebral infarction, hematoma expansion, cerebral hernia, seizures, hydrocephalus, subarachnoid hemorrhage, and venous thrombotic events.110
Research has demonstrated that exosomes can enhance the recovery from ischemic infarction. For instance, in mice with focal cerebral ischemia, intravenous administration of RVG exosomes loaded with miR-124 reached the peri-ischemic area and improved blood perfusion by promoting angiogenesis and neurogenesis.111 Another study revealed that MSC-derived exosomes could directly influence axonal growth in the ischemic region by inhibiting Argonaut 2 via miR-17-92, leading to axonal growth through the activation of the PTEN/mTOR signaling pathway.48 Additionally, components of exosomes derived from HUMSCs have been shown to possess potential therapeutic roles in treating epilepsy,112 reducing tissue damage, and enhancing tissue repair.113
When blood infiltrates the brain tissue and enters the subarachnoid space, secondary subarachnoid hemorrhage (SAH) occurs. Studies have shown that exosomes also have significant therapeutic effects on SAH through anti-apoptotic pathways. One study found that exosomes secreted by human umbilical cord MSCs with miR-206 knockdown mediated the TrkB/CREB signaling pathway by targeting BDNF, significantly improving neurological deficits and inhibiting apoptosis to prevent early brain damage caused by SAH.114 Another study observed that miR-26b-5p-modified exosomes inhibited apoptosis and the expression of inflammatory mediators during SAH through the MAT2A-mediated p38 MAPK/STAT3 signaling pathway in early brain injury.115 Recently, a study reported that exosomes from BMSCs reduced neuroinflammation and exerted a neuroprotective effect in brain tissues post-SAH by inhibiting the NF-kB and activating AMPK pathways.103 A summary of the current understanding of exosomes in the treatment of complications after ICH is provided in Table 7.
 |
Table 7 The Source, Component, and Mechanism of Exosomes in the Treatment of Complications After ICH |
Application of Exosomes-Based Delivery System in Treating ICH
In the above article, we summarize the inherited properties of exosomes are plausible and beneficial in ameliorating ICH and its complications through kinds of miRNAs by molecular biological mechanisms. Due to the transmembrane capacity of exosomes, they can also be effective carriers to directional transport of the active substances or drugs cross BBB into parenchymal hemorrhage area. BBB is a physiological barrier primarily composed of non-fenestrated endothelial cells that prevent potentially harmful substances from entering the brain environment. Due to tight junctions and selective behavior of BBB, it prevents the penetration of most chemotherapeutic agents or biotech drugs. Therefore, despite the availability of a wide variety of disease ameliorating agents, only 2% of drugs can traverse the BBB.116 Exosome-based delivery systems, a novel drug delivery system, may provide a solution to the transmembrane capacity issue of exosomes. There are several routes for exosomes interact with BBB, including trafficking through macropinocytosis, lipid raft or nonspecific exosome-endothelium interaction, and receptor-mediated transcytosis (Figure 4).117
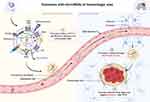 |
Figure 4 Exosomes-based delivery system with miRNAs or bioactive substances to hemorrhage area. Exosomes deliver miRNAs from extracellular fluid into capillaries, such as miR-126, miR-125, miR-124, miR-17-92 and etc,1 targeted cross the BBB,2 then enter the cerebral hemorrhage area to promote nerve function recovery and improve prognosis by various biological effects.3 The figure was created with https://app.biorender.com/. |
How to load bioactive cargo and establish an effective targeted transport system? The origin, payload type, and loading technique are crucial for ensuring the efficient therapeutic application of exosomes. Despite the low uploading efficacy, it is feasible to co-incubate exosomes with a hydrophobic drug in a suitable medium, which allows the drug to diffuse through the exosomal membrane. Typical successful examples of the pattern are curcumin, which was successfully loaded after a 5-min incubation at room temperature,118 or the catalase enzyme, which was successfully loaded after 18 h of incubation in PBS at room temperature.119 Transfecting or infecting parental cells with DNA encoding therapeutically active compounds is also an effective approach. When parental cells released exosomes, therapeutically active compounds will be warped by exosomes.13 Mechanistic studies showed that exosomes secreted from genetically-modified parental cells contained the encoded therapeutic protein, as well as its gene-synthetic material including DNA, mRNA and transcription factor involved in the encoded gene expression.120
Recently, the therapeutic effects of cargo-loaded exosomes have been reported by numbers of studies. A recent study showed that iron oxide and other magnetic nanoparticles packaged in exosomes significantly accumulated at stroke-affected brain tissue,121 showing a 5.1-fold higher accumulation at the injury site. Exosomes loaded with an anti-inflammatory small molecule compound, curcumin, were shown to protect mice from brain inflammation.122 Additionally, exosomes isolated from BMSCs were also loaded with curcumin, which strongly suppressed inflammatory lesions and apoptosis in the ischemic brain after ICH.123 Another therapeutic avenue involves the use of exosomes to transport exogenous siRNA or miRNA. For example, exosomes were used to transport siRNA targeted to miR-150, due to its promotional effect on VEGF.124 It was demonstrated that exosomes loading siRNA attenuated angiogenesis in mice, mediating by the neutralization of miR-150 down-regulated VEGF levels in mice.124 Mice treated with exosomes derived from miR-133b modified MSCs exhibited improved cognitive and motor function compared to the control group.86 Moreover, exosomes overexpressing miR-146a-5p from MSCs can enhance neural function after ICH by reducing neuronal apoptosis and inhibiting the proinflammatory phenotype polarization of microglia.87 The therapeutic effects and mechanisms of exosomes-based therapy on ICH will be elaborated in the following article. A summary of the current understanding of exosomes-based delivery system in the treatment of ICH is provided in Table 8.
 |
Table 8 The Mechanism and Effects of Exosomes Based Delivery System in the Treatment of ICH |
Advantages and Challenges of Exosomes in Treating ICH
Compared to other treatments like stem cell therapy, exogenous systemic injection of exosomes offers several advantages: I: They have a stronger biological effect as they can cross biological barriers and directly fuse with target cells.125 II: They are more convenient to store and can be kept at −80°C for extended periods due to the protective lipid bilayer.125 III: They have a higher transport capacity and can be modified to carry specific cargoes like drugs, cytokines, or miRNAs.126 IV: They offer higher biosafety due to their low immunogenicity as cell-free therapy.127 V: They can be administered in various ways, including intravenously, intramuscularly, transnasally, and subcutaneously, making it easy to control the concentration, dosage, transport route, and administration time. VI: They have excellent editability/modifiability. Exosomes can be used as carrier particles for certain miRNAs, drugs, and existing or newly created compositions to target cross BBB, maximizing clinical benefit and reducing drug concentration. VII: They have a stronger ability to pass the BBB. Exosomes have natural BBB crossing potential through the interaction between exosome surface ligands and brain endothelial cell receptors.128 They can serve as a good delivery vehicle for both hydrophobic and hydrophilic drugs through specific proteins in the exosome membranes and natural homing ability.
Despite these unique features and advantages in treating CNS diseases, there are fewer implications and research in the treatment of ICH compared to ischemic stroke. This may be due to several challenges in translating lab findings to clinical practice: I: Complex interactions. Current studies are mostly limited to a certain substance in exosomes from a single source. However, exosomes contain various bioactive substances, and whether there are synergistic effects of multiple substances in exosomes for the treatment of ICH needs further exploration because the interactions between nerve cells are intricate.129 II: Potential biosafety. Although exosomes have low immunogenicity in terms of structure and chemical components, more experimental studies and repeated verifications are necessary to demonstrate their biosafety and efficacy. III: Lack of standardization. There is no uniform standard technology for the production of exosomes. Different techniques for production and isolation can result in significant heterogeneity and purity differences, hindering the reproducibility and reliability of experimental results and posing a major challenge for their entry into clinical applications.130 IV: Ambiguous mechanism. Questions remain about how exosomes achieve cell-to-cell communication, how they cross the BBB and enter the target neurocyte, and how they transport cargo to the central nervous system. V: Unbalanced distribution. In an exosomal biodistribution study, after the intravenous administration of natural exosomes into the body, they were rapidly removed from the target site and accumulated in organs of the reticuloendothelial system such as the lung, liver, and spleen.131,132 The uneven distribution in vivo limits the biological effects of exosomes. The major advantages and challenges of exosomes in clinical translation are summarized in Figure 5.
 |
Figure 5 Major advantages and challenges of exosomes in the treatment of ICH. Exosomes have the advantages of high biological effect, targeting crossing BBB and convenient transformation, so they have broad application prospects in ICH. However, there are still certain challenges in clinical translation, including ambiguous mechanisms, potential biosafety, unbalanced distribution, and lack of standardization. The figure was created with https://app.biorender.com/. |
Discussion and Prospect
As reviewed above, exosomes derived from various sources, loaded with specific miRNAs and acting through different mechanisms, have shown potential therapeutic effects in improving prognosis after ICH (Tables 1–8). The evidence suggests that exosomes derived from different cells, capable of transporting specific components across the BBB under ischemic or hemorrhagic conditions, offer a promising strategy for ICH treatment. Despite exosomes-based therapy owns unique advantages in nervous system diseases, researchers primary focus on brain tumors, Alzheimer, and other neurological disorders like Parkinson. The research and clinical transformation of ICH is relatively insufficient, which may attribute to sever symptoms, high disability, and mortality of ICH, which limit the development and clinical translation of drugs. With the recent advances in nanomedicines and drug delivery, promoting hemostasis, neuroprotection, and nerve regeneration through exosome may provide insight into hemorrhagic stroke treatment.
While exosome treatment for ICH has shown excellent results in animal experiments, indicating substantial therapeutic potential and application prospects, several questions and challenges remain. In this review, we mainly focused on the therapeutic effects of exosomes through exogenous administration. However, the concept of promoting endogenous exosome secretion as a treatment strategy remains largely unexplored. In certain experimental models, such as an asthma mouse model, heat stimulation promoted exosome secretion from mastocytes, leading to significant reduction in asthma symptoms, which was mediated by promoting the expression of Rab3a and FcεRI, the key factors related to exosome secretion.133 Similarly, in tumor cells, exosomes produced by cells growing on a hard extracellular matrix effectively promoted tumor growth.134 At present, it has not been reported that promoting exosome secretion by a certain kind of cells can improve the recovery of neural defects and improve the healing after ICH. However, there are shreds of evidence that exosomes from peripheral cells can affect the interaction between peripheral and brain inflammation through promoting the activation of astrocytes and microglia, leading to brain inflammation.82,92 Whether a similar strategy could be employed to promote exosome secretion to improve recovery from neural defects and healing post-ICH is an area ripe for exploration.
Furthermore, while some mechanisms of exosome targeting into brain tissue have been discussed, exosomes tend to accumulate in the lung, liver, and spleen. Enhancing the targeted abilities of exosomes to the brain and avoiding these organs is a challenge. Engineered exosomes designed to enhance targeting across the BBB through unique receptors may provide a solution. Surface engineering of various membrane proteins and components, such as CD9,135 CD63,136 PTGFRN,137 LAMP2B,138 and phosphatidylserine,43 could facilitate receptor-mediated transcytosis. Additionally, neurotropic virus-derived peptides like RVG or peptides that bind to specific membrane proteins could be used for exosome modification. Currently, various technologies to efficiently incorporate drugs and active pharmaceutical ingredients into exosomes are being actively developed.139,140 In preclinical studies, engineered exosomes possessing targetability to the brain have shown promising results for CNS delivery. However, for the successful development of clinically approved exosome therapeutics for CNS diseases, there need to be more evidence and studies to establish imaging methods for quantitative/qualitative monitoring of exosomal delivery to the brain parenchyma in vivo as well as uncover the mechanisms underlying the BBB crossing of surface-modified exosomes.25
Many preliminary works have proven that exosomes were sufficient to promote neurovascular remodelling and functional recovery in ischemic and hemorrhagic stroke. However, the clinical investigations of exosome-based therapy in stroke have just begun to explore, and well-designed clinical trials are warranted to promote exosome translation to clinic.141 Clinical trial NCT03384433 was designed to explore the therapeutic effect of exosomes on stroke. Researchers are aiming to evaluate the administration of exosomes derived from allogenic MSC enriched with miR-124 on improvement of disability in patients with acute ischemic stroke which is still under recruiting. Similarly, NCT05035134 aims to explore application of circulating exosomes in early diagnosis and prognosis evaluation after ICH. The purpose of this trial is to observe the relationship between the changes of circulating exosomes and the development and outcome of the disease in patients with ICH, then to search for early serum markers and potential intervention targets for disease monitoring in patients with ICH.
Conclusion
In conclusion, exosomes not only offer potential treatment for multiple pathogenic mechanisms post-ICH and improve prognosis, but also show promise in addressing long-term complications of ICH including SAH, epilepsy, and etc. Although there are many challenges to be overcome before clinical application of exosomes, their potential in ICH treatment is promising and novel. Understanding the mechanisms of exosomes and miRNAs in the process of ICH is critical for guiding their clinical applications. We hope that exosomes-based cell-free therapy will provide a new avenue for ICH treatment in the future.
Acknowledgment
We thank Lingzhi Yu for her comments on the manuscript. Shandong Jiang, Libin Hu, and Hang Zhou are co-first authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was financially supported by the National Natural Science Foundation of China (No.82371299, 82171273, 82171275) and the Key R&D Program of Zhejiang (2022C03133, WKJ-ZJ-2004).
Disclosure
All authors have declared no conflicts of interest in this work.
References
1. Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53–63. doi:10.1016/S1474-4422(05)70283-0
2. Adnan I, Qureshi S, Tuhrim J, Broderick P. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;2001:1.
3. Krishnamurthi RV, Moran AE, Forouzanfar MH, et al. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Global Heart. 2014;9(1):101–106. doi:10.1016/j.gheart.2014.01.003
4. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987. doi:10.1161/01.STR.24.7.987
5. Hanley DF, Thompson RE, Rosenblum M. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint Phase 3 trial. Lancet. 2019;393(10175):1021–1032. doi:10.1016/S0140-6736(19)30195-3
6. Otero-Ortega L, Laso-García F, Gómez-de Frutos M, et al. White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Entific Rep. 2017;7:44433.
7. Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neu Regen Res. 2017;12(1):19. doi:10.4103/1673-5374.198966
8. Yuan D, Zhao Y, Banks WA. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain - ScienceDirect. Biomaterials. 2017;142:1–12. doi:10.1016/j.biomaterials.2017.07.011
9. Zheng M, Huang M, Ma X, Chen H, Gao X. Harnessing exosomes for the development of brain drug delivery systems. Bioconjug Chem. 2019;30(4):994–1005. doi:10.1021/acs.bioconjchem.9b00085
10. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88(1):487–514. doi:10.1146/annurev-biochem-013118-111902
11. Song Y, Li Z, He T, et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics. 2019;9(10):2910–2923. doi:10.7150/thno.30879
12. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi:10.3390/cells8070727
13. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi:10.1016/j.jconrel.2015.07.030
14. Jin S, Lv Z, Kang L, et al. Next generation of neurological therapeutics: native and bioengineered extracellular vesicles derived from stem cells. Asian J Pharm Sci. 2022;17(6):779–797. doi:10.1016/j.ajps.2022.10.002
15. Aminzadeh MA, Rogers RG, Fournier M, et al. Exosome-mediated benefits of cell therapy in mouse and human models of Duchenne muscular dystrophy. Stem Cell Reports. 2018;10:942–955. doi:10.1016/j.stemcr.2018.01.023
16. Lsser C. Exosomes in diagnostic and therapeutic applications: biomarker, vaccine and RNA interference delivery vehicle. Expert opin biol ther. 2015;15(1):103–117. doi:10.1517/14712598.2015.977250
17. Hanan M, Soreq H, Kadener S. CircRNAs in the brain. RNA Biology. 2017;14(8):1028–1034. doi:10.1080/15476286.2016.1255398
18. Jiang X, Zhang R, Lu G, et al. Brain-derived exosomal CircRNAs in plasma serve as diagnostic biomarkers for acute ischemic stroke. J Neuroimm Pharmacol. 2024;19(1):15. doi:10.1007/s11481-024-10113-1
19. Gareev I, Shumadalova A, Ilyasova T, Beilerli A, Shi H. Circular RNAs in intracranial aneurysms: emerging roles in pathogenesis, diagnosis and therapeutic intervention. Non-Coding RNA Res. 2024;9(1):211–220. doi:10.1016/j.ncrna.2023.11.012
20. Abdul-Muneer PM. MicroRNA in the pathophysiology of CNS injury: implication in neuroregenerative medicine. CNS Neurosci Ther. 2016;22(7):543–545. doi:10.1111/cns.12579
21. Mantel PY, Hjelmqvist D, Walch M, et al. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat Commun. 2016;7:12727. doi:10.1038/ncomms12727
22. Meister G. Argonaute proteins: functional insights and emerging roles. Nat Rev Genet. 2013;14:447–459. doi:10.1038/nrg3462
23. Sempere LF, Cole CN, Mcpeek MA, Peterson KJ. The phylogenetic distribution of metazoan microRNAs: insights into evolutionary complexity and constraint. J Exp Zool B Mol Dev Evol. 2010;306 6.
24. Tran N. Cancer Exosomes as miRNA Factories. Trends Cancer. 2016;2(7):329–331. doi:10.1016/j.trecan.2016.05.008
25. Chao HN, Tseng HF, Lin YH, Kuo CY. Strategies for cancer treatment: anti-angiogenesis, exosomes, and micro-RNAs. Current Topics Nutraceutical Res. 2023;21:123–124. doi:10.37290/ctnr2641-452X.21:123-124
26. Zheng D, Huo M, Li B, et al. The role of exosomes and exosomal microRNA in cardiovascular disease. Front Cell Develop Biol. 2020;8:616161. doi:10.3389/fcell.2020.616161
27. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):640.
28. Zhang H, Wang Y, Lv Q, et al. MicroRNA-21 overexpression promotes the neuroprotective efficacy of mesenchymal stem cells for treatment of intracerebral hemorrhage. Front Neurol. 2018;2018:1.
29. Guo-wen H, Qing L, Xin N, Bin J, Liu S-M. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;2015:1.
30. Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Nat Acad Sci. 2016;2016:1.
31. Elia CA, Losurdo M, Malosio ML, Coco S. Extracellular vesicles from mesenchymal stem cells exert pleiotropic effects on Amyloid‐β, inflammation, and regeneration: a spark of hope for alzheimer’s disease from tiny structures? BioEssays. 2019;41(4). doi:10.1002/bies.201800199
32. Zhang H, Wang Y, Lv Q, Gao J, Hu L, He Z. MicroRNA-21 overexpression promotes the neuroprotective efficacy of mesenchymal stem cells for treatment of intracerebral hemorrhage. Front Neurol. 2018;9:9. doi:10.3389/fneur.2018.00009
33. Bobrie A, Colombo M, Raposo GA, Théry C. Théry C: exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi:10.1111/j.1600-0854.2011.01225.x
34. Raghu K. The biology and function of exosomes in cancer. J Clin Investig. 2016;126(4):1208. doi:10.1172/JCI81135
35. Mincheva‐Nilsson L, Baranov V, Nagaeva O, Dehlin E. Isolation and characterization of exosomes from cultures of tissue explants and cell lines. Curr Protoc Immunol. 2016;2016:115.
36. Lee EC, Ha TW, Lee DH, et al. Utility of exosomes in ischemic and hemorrhagic stroke diagnosis and treatment. Int J Mol Sci. 2022;23(15):1.
37. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1). doi:10.1186/s12943-019-0991-5
38. Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1–11. doi:10.1016/j.pharmthera.2018.02.013
39. Song L, Tang S, Han X, et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun. 2019;10(1):1639. doi:10.1038/s41467-019-09720-x
40. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi:10.1016/0092-8674(83)90040-5
41. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9(1). doi:10.1186/s13578-019-0282-2
42. Lai PK, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. 2012;3. doi:10.3389/fphys.2012.00003
43. Tore S, Hessvik NP, Kirsten S, Alicia L. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2018;2018:
44. Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–731. doi:10.1016/S1474-4422(12)70104-7
45. Ivanovic Z. Hypoxia or in situ normoxia: the stem cell paradigm. John Wiley Sons Ltd. 2009;(2):271.
46. Venkat P, ChengchengChopp M, AlexWang F-W, JulieShen Y. Jieli: miR-126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in type 2 diabetes mellitus mice. Stroke J Cereb Circ. 2019;50(10):2865–2874. doi:10.1161/STROKEAHA.119.025371
47. Qiuhong J, Yuhua J, Jingwen P, et al. Increased Brain-Specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS One. 2016;11(9):e0163645. doi:10.1371/journal.pone.0163645
48. Zhang Y, Chopp M, Liu XS, et al. Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Molec Neurobiol. 2017;54(4):2659–2673. doi:10.1007/s12035-016-9851-0
49. Xiao Y, Geng F, Wang G, et al. Bone marrow–derived mesenchymal stem cells–derived exosomes prevent oligodendrocyte apoptosis through exosomal miR‐134 by targeting caspase‐8. J Cell Biochem. 2019;120(2):2109.
50. Zou Y, Liao L, Dai J, et al. Mesenchymal stem cell-derived extracellular vesicles/exosome: a promising therapeutic strategy for intracerebral hemorrhage. Regen Ther. 2023;22:181–190. doi:10.1016/j.reth.2023.01.006
51. Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1-3 in an animal model of intracerebral hemorrhage. Ann Neurol. 2003;54(5):655–664. doi:10.1002/ana.10750
52. Ding H, Jia Y, Lv H, Chang W, Wang D, Wang D. Extracellular vesicles derived from bone marrow mesenchymal stem cells alleviate neuroinflammation after diabetic intracerebral hemorrhage via the miR-183-5p/PDCD4/NLRP3 pathway. J Endocrinol Invest. 2021;44(5):2685–2698. doi:10.1007/s40618-021-01583-8
53. Zazulia AR, Diringer M, Derdeyn C, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke J Cereb Circ. 1999;30:1167–1173. doi:10.1161/01.STR.30.6.1167
54. Zheng H, Chen C, Zhang J, Hu Z. Mechanism and therapy of brain edema after intracerebral hemorrhage. Cerebrovascular Dis. 2016;42(3–4):155.
55. Yin XY, Wang CC, Du P, et al. Muse cells decrease the neuroinflammatory response by modulating the proportion of M1 and M2 microglia in vitro. Neural Regener Res. 2023;18(1):213–218. doi:10.4103/1673-5374.343885
56. Xu L, He D, Bai Y. Microglia-Mediated Inflammation and Neurodegenerative Disease. Molec Neurobiol. 2015;53(10):6709–6715. doi:10.1007/s12035-015-9593-4
57. Yan Z, Ruyi H, Peng W, Yijie S, Liang Z. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater Sci. 2019;7. doi:10.1039/c8bm01449c
58. Yin C, Han Q, Xu D, Zheng B, Zhao X, Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. OncoImmunology. 2019;8(7):e1601479. doi:10.1080/2162402X.2019.1601479
59. Ali A, Andrew E, Stephen T, Lauzurica-Valdemoros R, Borràs FE. Complement in the homeostatic and ischemic brain. Front Immunol. 2015;6:6. doi:10.3389/fimmu.2015.00006
60. Rezaie AR. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost. 2014;112(11):876–882. doi:10.1160/th14-02-0167
61. Li J, Tan M, Xiang Q, et al. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb Res. 2017;154:96–105.
62. Ducruet AF, Zacharia BE, Hickman ZL, et al. The complement cascade as a therapeutic target in intracerebral hemorrhage. Exp Neurol. 2009;219(2):398–403. doi:10.1016/j.expneurol.2009.07.018
63. Xiong XY, Wang J, Qian ZM, Yang QW. Iron and intracerebral hemorrhage: from mechanism to translation. Translat Stroke Res. 2014;5(4):429–441. doi:10.1007/s12975-013-0317-7
64. Kalani A, Chaturvedi P, Kamat PK, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Biotechnol. 2016;79:360–369. doi:10.1016/j.biocel.2016.09.002
65. Hong SB, Yang H, Manaenko A, Lu J, Hu Q. Potential of exosomes for the treatment of stroke. Cell Transplant. 2018;28(8):096368971881699.
66. Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke. 2001;32(12):2932–2938. doi:10.1161/hs1201.099820
67. Brenna S, Altmeppen HC, Mohammadi B, et al. Characterization of brain-derived extracellular vesicles reveals changes in cellular origin after stroke and enrichment of the prion protein with a potential role in cellular uptake. J Extracell Vesicles. 2020;9(1):1809065. doi:10.1080/20013078.2020.1809065
68. Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline From the American Heart Association/American Stroke Association. Stroke. 2022;53(7):e282–e361. doi:10.1161/STR.0000000000000407
69. Zhou JF, Xiong Y, Kang X, et al. Application of stem cells and exosomes in the treatment of intracerebral hemorrhage: an update. Stem Cell Res Ther. 2022;13(1):281. doi:10.1186/s13287-022-02965-2
70. Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8(1):125. doi:10.1186/s13287-017-0578-2
71. Gray WD, French KM, Ghosh-Choudhary S, et al. Identification of Therapeutic Covariant MicroRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology novelty and significance. Circul Res. 2015;116(2):255–263. doi:10.1161/CIRCRESAHA.116.304360
72. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Trans Med. 2014;6(265):265sr6.
73. Fröhlich L. microRNAs at the Interface between osteogenesis and angiogenesis as targets for bone regeneration. Cells. 2019;8(2):121. doi:10.3390/cells8020121
74. Xu R, Bai Y, Min S, Xu X, Tang T, Ju S. In vivo monitoring and assessment of exogenous mesenchymal stem cell-derived exosomes in mice with ischemic stroke by molecular imaging. Int J Nanomed. 2020;Volume 15:9011–9023. doi:10.2147/IJN.S271519
75. Zhang L, Ouyang P, He G, Wang X, He X. Exosomes from microRNA6 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J Cell Mol Med. 2020;25(4):1.
76. Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity - sciencedirect. Dev Cell. 2008;15(2):272–284. doi:10.1016/j.devcel.2008.07.008
77. Otero-Ortega L, Gómez de Frutos MC, Laso-García F, et al. Exosomes promote restoration after an experimental animal model of intracerebral hamorrhage. J Cereb Blood Flow Metab. 2021;41(7):1794. doi:10.1177/0271678X20976843
78. Rahman AA, Amruta N, Pinteaux E, Bix GJ. Neurogenesis after stroke: a therapeutic perspective. Transl Stroke Res. 2021;12(1):1–14. doi:10.1007/s12975-020-00841-w
79. Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23(6):935–942. doi:10.1016/j.conb.2013.09.004
80. Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. doi:10.1523/JNEUROSCI.4323-06.2006
81. Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke. 2020;22(1):29–46. doi:10.5853/jos.2019.02236
82. Zhang ZG, Buller B, Chopp M. Exosomes — beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15(4):193–203. doi:10.1038/s41582-018-0126-4
83. Han Y, Seyfried D, Meng Y, et al. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J Neurosurg. 2018;131(1):290–300. doi:10.3171/2018.2.JNS171475
84. Xin H, Wang F, Li Y, et al. Secondary Release of Exosomes From Astrocytes Contributes to the Increase in Neural Plasticity and Improvement of Functional Recovery After Stroke in Rats Treated With Exosomes Harvested From MicroRNA 133b-Overexpressing Multipotent Mesenchymal Stromal Cells. Cell Transplant. 2017;26(2):243. doi:10.3727/096368916X693031
85. Xin WQ, Wei W, Pan YL, et al. Modulating poststroke inflammatory mechanisms: novel aspects of mesenchymal stem cells, extracellular vesicles and microglia. World J Stem Cells. 2021;13(8):1030–1048. doi:10.4252/wjsc.v13.i8.1030
86. Shen H, Yao X, Li H, et al. Role of exosomes derived from miR-133b Modified MSCs in an experimental rat model of intracerebral hemorrhage. J Mol Neurosci. 2018;64(3):421–430. doi:10.1007/s12031-018-1041-2
87. Duan S, Wang F, Cao J, Wang C. Exosomes Derived from MicroRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial m1 polarization. Drug Des Devel Ther. 2020;14:3143–3158. doi:10.2147/DDDT.S255828
88. Bai Q, Xue M, Yong VW. Microglia and macrophage phenotypes in intracerebral haemorrhage injury: therapeutic opportunities. Brain. 2020;143(5):1297–1314. doi:10.1093/brain/awz393
89. Wang J, Chen S, Yerrapragada SM, Zhang W, Bihl JC. Therapeutic effects of exosomes from angiotensin-converting enzyme 2 -overexpressed endothelial progenitor cells on intracerebral hemorrhagic stroke. Brain Hemorrhages. 2020;2(5):1.
90. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nature Med. 2011;17(7):796–808. doi:10.1038/nm.2399
91. Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory Disequilibrium in Stroke. Circul Res. 2016;119(1):142. doi:10.1161/CIRCRESAHA.116.308022
92. Balusu S, Van Wonterghem E, De Rycke R, et al. Identification of a novel mechanism of blood–brain communication during peripheral inflammation via choroid plexus‐derived extracellular vesicles. EMBO Mol Med. 2016;8(10):1162–1183. doi:10.15252/emmm.201606271
93. Pan J, Wang Z, Huang X, et al. Bacteria-derived outer-membrane vesicles hitchhike neutrophils to enhance ischemic stroke therapy. Advan Mat. 2023;35(38):e2301779. doi:10.1002/adma.202301779
94. Zhao X, Sun G, Zhang J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator‐activated receptor γ in microglia/macrophages. Ann Neurol. 2007;61(4):352–362. doi:10.1002/ana.21097
95. Madore C, Yin Z, Leibowitz J, Butovsky O. Microglia, lifestyle stress, and neurodegeneration. Immunity. 2020;52(2):222–240. doi:10.1016/j.immuni.2019.12.003
96. Chen S, Yan D, Qiu A. The role of macrophages in pulmonary hypertension: pathogenesis and targeting. Int Immunopharmacol. 2020;88(193):106934. doi:10.1016/j.intimp.2020.106934
97. Liu J, Wu F, Zhou H. Macrophage-derived exosomes in cancers: biogenesis, functions and therapeutic applications. Immunol Lett. 2020;227:102–108. doi:10.1016/j.imlet.2020.08.003
98. Lee JH, Zheng ZC, WenyuanWon S, XiaohuanWinter M, Thomas AW, LingYu SP. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol Dis. 2016;96:248–260. doi:10.1016/j.nbd.2016.09.013
99. Kinoshita S, Koyama R. Pro- and anti-epileptic roles of microglia. Neural Regener Res. 2021;16(7):1369–1371. doi:10.4103/1673-5374.300976
100. Drommelschmidt K, Serdar M, Bendix I. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. 2017;60:220–232. doi:10.1016/j.bbi.2016.11.011
101. Zhao Y, Gan Y, Xu G, Yin G, Liu D. MSCs-derived exosomes attenuate acute brain injury and inhibit microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglia M1 polarization. Neuroch Res. 2020;45(5):1180–1190. doi:10.1007/s11064-020-02998-0
102. Zong L, Huang P, Song Q, Kang Y. Bone marrow mesenchymal stem cells-secreted exosomal H19 modulates lipopolysaccharides-stimulated microglial M1/M2 polarization and alleviates inflammation-mediated neurotoxicity. Am J Transl Res. 2021;13(3):935–951.
103. Han M, Cao Y, Guo X, et al. Mesenchymal stem cell-derived extracellular vesicles promote microglial M2 polarization after subarachnoid hemorrhage in rats and involve the AMPK/NF-κB signaling pathway. Biomed Pharmac. 2021;133:111048. doi:10.1016/j.biopha.2020.111048
104. Kang C, Avery L. To be or not to be, the level of autophagy is the question: dual roles of autophagy in the survival response to starvation. Autophagy. 2008;4(1):82.
105. Qian M, Fang X, Wang X. Autophagy and inflammation. Clin Translat Med. 2017;6(1):24. doi:10.1186/s40169-017-0154-5
106. Zhang Y, Khan S, Liu Y, et al. Modes of brain cell death following intracerebral hemorrhage. Front Cell Neurosci. 2022;16:799753. doi:10.3389/fncel.2022.799753
107. Mei J, Hairong W, Mingming J, et al. Exosomes from MiR-30d-5p-ADSCs Reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47:864–878. doi:10.1159/000490078
108. Pei X, Li Y, Zhu L, Zhou Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp. Cell. Res. 2019;382(2):111474. doi:10.1016/j.yexcr.2019.06.019
109. Asch CJV, Luitse MJ, Rinkel GJ, Tweel IVD, Klijn CJ, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–176. doi:10.1016/S1474-4422(09)70340-0
110. Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11(1):101–118. doi:10.1016/S1474-4422(11)70264-2
111. Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7(C):278–287. doi:10.1016/j.omtn.2017.04.010
112. Huang PY, Shih YH, Tseng YJ, et al. Xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly as a potential therapy for rat pilocarpine-induced epilepsy. Brain Behav Immun. 2016;54:45–58.
113. Muthu S, Bapat A, Jain R, Jeyaraman N, Jeyaraman M. Exosomal therapy-a new frontier in regenerative medicine. AME Publish Comp. 2021;8:1.
114. Zhao H, Li Y, Chen L, et al. HucMSCs-Derived miR-206-knockdown exosomes contribute to neuroprotection in subarachnoid hemorrhage induced early brain injury by Targeting BDNF. Neuroscience. 2019;417:11–23.
115. Liu Z, Wang B, Guo Q. MiR-26b-5p-modified hUB-MSCs derived exosomes attenuate early brain injury during subarachnoid hemorrhage via MAT2A-mediated the p38 MAPK/STAT3 signaling pathway. Brain Res Bull. 2021;175:107.
116. Mitragotri S. Devices for overcoming biological barriers: the use of physical forces to disrupt the barriers. Adv Drug Delivery Rev. 2012;65(1):100–103. doi:10.1016/j.addr.2012.07.016
117. Rehman FU, Liu Y, Zheng M, Shi B. Exosomes based strategies for brain drug delivery. Biomaterials. 2023;293:121949. doi:10.1016/j.biomaterials.2022.121949
118. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
119. Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Controll Rel. 2015;207:18–30. doi:10.1016/j.jconrel.2015.03.033
120. Haney MJ, Zhao Y, Harrison EB, et al. Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PLoS One. 2013;8(4):e61852. doi:10.1371/journal.pone.0061852
121. Kim HY, Kim TJ, Kang L, et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243:119942. doi:10.1016/j.biomaterials.2020.119942
122. Zhuang X, Xiang X, Grizzle W, et al. Corrigendum: treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Molecu ther. 2012;20(1):239. doi:10.1038/mt.2011.222
123. Tian T, Zhang HX, He CP, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi:10.1016/j.biomaterials.2017.10.012
124. Liu Y, Zhao L, Li D, et al. Microvesicle-delivery miR-150 promotes tumorigenesis by up-regulating VEGF, and the neutralization of miR-150 attenuate tumor development. Protein and Cell. 2013;4(12):932–941. doi:10.1007/s13238-013-3092-z
125. El Andaloussi S, I M, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi:10.1038/nrd3978
126. Wang C, Guo S, Gu Q. Exosomes: a promising therapeutic strategy for intervertebral disc degeneration. Exp Gerontology. 2022;163:111806. doi:10.1016/j.exger.2022.111806
127. He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi:10.7150/thno.21945
128. Yang L, Han B, Zhang Z, et al. Extracellular vesicle–mediated delivery of circular RNA SCMH1 promotes functional recovery in rodent and nonhuman primate ischemic stroke models. Circulation. 2020;2020:1.
129. Ji N, Wang F, Wang M, Zhang W, Liu H, Su J. Engineered bacterial extracellular vesicles for central nervous system diseases. J Controll Rel. 2023;364:46–60. doi:10.1016/j.jconrel.2023.10.027
130. Hong J, Dauros-Singorenko P, Whitcombe A, Payne L, Swift S. Analysis of the Escherichia coli extracellular vesicle proteome identifies markers of purity and culture conditions. J Extracell Vesicles. 2019;8(1):1632099. doi:10.1080/20013078.2019.1632099
131. Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–155. doi:10.1016/j.jconrel.2014.12.013
132. Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo JK, Choi C. Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Eng Regen Med. 2021;18(4):499–511. doi:10.1007/s13770-021-00361-0
133. Liao W, Chen G, Song L, et al. Temperature regulates Rab3a and mast cell-derived exosomal FcεRI to inhibit mast cell activation. Allergy. 2023;78(6):1707–1710. doi:10.1111/all.15744
134. Wu B, Liu DA, Guan L, et al. Stiff matrix induces exosome secretion to promote tumour growth. Nat Cell Biol. 2023;25(3):415–424. doi:10.1038/s41556-023-01092-1
135. Yim N, Ryu SW, Choi K, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277. doi:10.1038/ncomms12277
136. Stickney Z, Losacco J, McDevitt S, Zhang Z, Lu B. Development of exosome surface display technology in living human cells. Biochem Biophys Res Commun. 2016;472(1):53–59. doi:10.1016/j.bbrc.2016.02.058
137. Dooley K, McConnell RE, Xu K, et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol Ther. 2021;29(5):1729–1743. doi:10.1016/j.ymthe.2021.01.020
138. Alvarez-Erviti L, Seow Y, Yin HF, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnol. 2011;29(4):341. doi:10.1038/nbt.1807
139. Song Y, Kim Y, Ha S, et al. The emerging role of exosomes as novel therapeutics: biology, technologies, clinical applications, and the next. Am J Reproduct Immunol. 2021;85(2):e13329. doi:10.1111/aji.13329
140. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Publishing Group. 2021;7:1.
141. Li Y, Tang Y, Yang GY. Therapeutic application of exosomes in ischaemic stroke. Stroke Vascul Neurol. 2021;6(3):483–495. doi:10.1136/svn-2020-000419
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

