Back to Journals » Drug Design, Development and Therapy » Volume 19
Opioid-Free Anesthesia with Esketamine Combined with Iliac Fascia Block in Elderly Patients Undergoing Hip Surgery
Authors Luo LL, Xiao R , Zhang JP, Xi WF, Xu GH, Yuan H
Received 27 November 2024
Accepted for publication 14 April 2025
Published 28 April 2025 Volume 2025:19 Pages 3337—3349
DOI https://doi.org/10.2147/DDDT.S508805
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Georgios Panos
Lai-Lin Luo,1 Rui Xiao,1 Jin-Peng Zhang,1 Wen-Feng Xi,1 Guang-Hong Xu,2 Hao Yuan1
1Department of Anesthesiology, Fuyang Hospital of Anhui Medical University, Fuyang, Anhui, 236000, People’s Republic of China; 2Department of Anesthesiology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China
Correspondence: Guang-Hong Xu, Department of Anesthesiology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China, Tel +86-551-62922344, Fax +86 551 62923704, Email [email protected] Hao Yuan, Department of Anesthesiology, Fuyang Hospital of Anhui Medical University, Fuyang, Anhui, 236000, People’s Republic of China, Tel +86-558-2200702, Fax +86 558 2200639, Email [email protected]
Background: Most patients with hip fractures are elderly people with relatively high risks of cardiovascular and cerebrovascular accidents. Esketamine has little effect on haemodynamics and has an exact analgesic effect, which is beneficial for patients with intolerance to surgery and anaesthesia. Therefore, we conducted this study to compare the efficacy and safety of esketamine with those of opioids in elderly patients who underwent surgery for hip fractures.
Methods: Seventy-two patients were included in the study, but data from only 68 were analysed. Patients were randomly assigned to either the EKT group (esketamine combined with iliac fascia block) or the OP group (opioids combined with iliac fascia block). Esketamine was used for anaesthesia induction and maintenance in the EKT group, and sufentanil and remifentanil were used in the OP group. The primary endpoint was the area under the curve numeric rating scale (AUCNRS).
Results: The AUCNRS in the EKT group was significantly lower than that in the OP group (6.6± 3.6 vs 9.5± 3.0, P =0.001). The postoperative numeric rating scale (NRS) scores for pain and the number of rescue analgesia were significantly lower in the EKT group than in the OP group (all P < 0.05). The mean blood pressure in the EKT group was significantly greater than those in the OP group after anaesthesia induction (all P < 0.05). The incidence of postoperative nausea and vomiting (PONV) in the OP group was significantly higher than that in the EKT group (P =0.033).
Conclusion: Elderly patients receiving esketamine-based opioid-free anaesthesia had more stable hemodynamics, better postoperative analgesia, and reduced PONV incidence compared to those undergoing opioid-balanced anaesthesia.
Clinical Trial Registration: The trial was registered at the Chinese Clinical Trial Registry on September 1, 2023 (identifier: ChiCTR2300075324).
Keywords: opioid-free anaesthesia, esketamine, hip fractures, elderly individuals, postoperative analgesia
Introduction
Hip fracture, known as terminal fracture, remains one of the most significant injuries worldwide.1 Moreover, hip fractures are more common in elderly and debilitated populations with underlying diseases of varying degrees and poor pain thresholds.2 As their incidence increases with age,3 more than 30% of individuals with hip fractures are older than 85 years.4 Therefore, the risk of mortality in patients with hip fractures ranges between 10% and 25% at 30 days and 1 year respectively.1
Owing to advancements in modern medicine including anaesthesia and surgery, almost all patients can be treated with surgery,5 which is performed under spinal anaesthesia or general anaesthesia.6 Age and preoperative frailty are associated with postoperative complications of noncardiac surgery in elderly patients.7 European guidelines emphasize the importance of thorough preoperative assessment in high-risk elderly patients to optimize perioperative outcomes.8 Therefore, suitable anaesthesia methods and effective perioperative anaesthesia management are important. A meta-analysis revealed that the optimal anaesthesia technique (spinal anaesthesia vs general anaesthesia) for hip fracture surgery remains unclear because of little robust evidence advocating one method over others.1 Spinal anaesthesia rarely causes pulmonary complications and may positively influence patient prognosis. However, elderly patients have calcified bones, which increases the risks of difficult punctures and puncture-related complications. As one of three common general anaesthetics, opioids are associated with a series of side effects, such as constipation, respiratory depression, and postoperative nausea and vomiting (PONV), that affect patient prognosis.9 Therefore, opioid-free anaesthesia (OFA) protocols were introduced and have been increasingly used to reduce the short-term and long-term side effects of opioids.10 A meta-analysis revealed that OFA regimens provide a level of postoperative analgesia similar to that of opioid-balanced anaesthesia.11
OFA is multimodal in that it involves nonopioid agents, including N-methyl-d-aspartate antagonists, anti-inflammatory medications, local anaesthetics, and alpha-2 agonists.10 As a part of OFA, esketamine induces sedative, analgesic, and antidepressant effects and relaxes the bronchial muscles.12 Moreover, esketamine stimulates the sympathetic nervous system, making it an ideal drug for inducing anaesthesia in patients with unstable circulation.12 Previous studies revealed that esketamine, as a part of OFA regimens,13 lowers the risk of PONV,13 and reduces the incidence of postoperative mild chronic pain.14 Min et al2 reported that esketamine used as a substitute for opioids in intravenous analgesia pumps can relieve postoperative pain, reduce perioperative stress responses, and encourage postoperative recovery. To our knowledge, there is minimal evidence advocating the intraoperative administration of esketamine as a potential alternative to opioids for analgesia in elderly patients undergoing hip fracture surgery, particularly in rigorous clinical investigations evaluating its standalone efficacy and safety profile.
Therefore, in this study, we compared the effects of esketamine on analgesia, circulation, respiratory function, and prognoses in elderly patients who underwent surgery for hip fractures with those of opioids with the aim of determining whether intraoperative esketamine would benefit patient prognoses more than opioids would.
Methods
This randomized controlled trial (RCT) was approved by the clinical medical research ethics committee of Fuyang Hospital of Anhui Medical University (KY2023055) and registered with the Chinese Clinical Trials Registry on September 1, 2023 (identifier: ChiCTR2300075324). Patients were informed about the study during the preoperative screening visit and signed informed consent form once enrolled. Patients were recruited from September 2023 to September 2024.
Inclusion and Exclusion Criteria
Elderly patients who were aged 65 years or older and were classified as having an American Society of Anaesthesiologists (ASA) physical status II-III were recruited and underwent surgery for hip fractures the following day. All patients were able to communicate and learn normally. The exclusion criteria were as follows: allergy to any of the medicines used in the research or contraindications for peripheral nerve block; history of central nervous system injury or mental disorders (ie, cerebral infarction associated with sequelae or dementia); severe hepatic and renal insufficiency and coagulation dysfunction; myocardial infarction, congestive heart failure, or malignant arrhythmias affecting haemodynamic stability within the past six months; history of chronic analgesic drug use or alcohol abuse; diseases associated with glaucoma or elevated intracranial pressure; diabetes and diabetes-related complications (ie, diabetic ketoacidosis, hyperosmolar coma, and diabetic nephropathy); preoperative delirium or severe cognitive impairment (more than 8 errors on the Simple Mental State Questionnaire, SPMSQ); respiratory failure (arterial partial oxygen pressure less than 60 mmHg) or pulse oxygen saturation (SpO2) less than 90% before surgery; and revision surgery for prosthesis fracture or hip fracture with severe trauma elsewhere. Participants whose iliac fascial block was ineffective, who violated the protocol, experienced massive perioperative bleeding (>800 mL), underwent emergency surgery postoperatively, or had incomplete data were removed from the study.
Randomization and Blinding
Patients were randomly assigned to one of the two treatment groups using a computer-generated randomization sequence. Random sequences are generated by specific researchers, numbered sequentially, and placed in opaque and sealed envelopes.
Before anaesthesia induction, a specific nurse opened one of the consecutively numbered envelopes and prepared the drug solution aseptically (2.5 µg/mL of sufentanil or 2.5 mg/mL esketamine for anaesthesia induction, and 50 µg/mL remifentanil or 2.5 mg/mL esketamine for anaesthesia maintenance). The anaesthesiologists, surgeons, and other nurses were unaware of the grouping, and the patients and the researchers who assessed postoperative outcomes were unaware of that.
In the OP group, sufentanil (which was diluted to 2.5 µg/mL) was used for anaesthesia induction and remifentanil (which was diluted to 50 µg/mL) was used for anaesthesia maintenance.
In the EKT group, esketamine (which was diluted to 2.5 mg/mL) was used for anaesthesia induction and maintenance.
Anesthesia Protocol
After the patients were enrolled and signed the informed consent form, their general information was recorded, and the SPMSQ score and the Frail Screening Scale score were calculated. Patient preparation involved fasting for 8 hours and clear fluids prohibition for 2 hours before the operation.
Once in the operating room, the patients’ electrocardiogram (ECG), pulse, SpO2, and invasive blood pressure were routinely monitored. Peripheral venous access was subsequently established for the delivery of 5 mL/kg/h sodium lactate Ringer’s solution, and basal arterial blood gas analysis was performed before the operation. A bispectral index (BIS) monitor was also used to monitor the depth of anaesthesia.
All patients received mask oxygen, and then ultrasound-guided iliac fascia block was performed over the inguinal ligament (0.3% ropivacaine 30 mL). The high-frequency probe was placed vertically at the middle third of the inguinal ligament towards the navel. The ultrasound image showed a bow-tie pattern of sartorius and abdominal muscles above the iliac muscle and a pulsating deep iliac circumflex artery below the abdominal muscle. The nerve stimulation needle was inserted from the tail side to the head, and 3 mL of normal saline was given after the iliac fascia was punctured and no blood was withdrawn. We observed that the normal saline had diffused cephalically under the deep iliac circumflex artery. At this time, the syringe was withdrawn again, there was no blood or gas, and 30 mL of 0.3% ropivacaine was given. After confirming that no complications (such as local anaesthetic poisoning or nerve injury) has occurred, the degree of pain in the anterior and lateral thigh skin before block were compared with that after block to evaluate the effectiveness of the block.
Before the induction of anaesthesia, all patients received mask preoxygenation for 5 minutes. For anaesthesia induction in the OP group, 1.5–2.0 mg/kg of propofol, 0.3–0.5 µg/kg of sufentanil, and 0.15–0.2 of mg/kg cis-atracurium were injected, and then a laryngeal mask airway was inserted for mechanical ventilation. In the EKT group, 0.3–0.5 mg/kg of esketamine was used instead of sufentanil for general anaesthesia induction. Mechanical ventilation parameters are set to maintain the end-tidal carbon dioxide (PETCO2) pressure at 35–45 mmHg and SpO2 at 99–100%. Propofol (3–5 mg/kg/h) combined with remifentanil (6–10 µg/kg/h) was infused intravenously for anaesthesia maintenance in the OP group, whereas propofol (3–5 mg/kg/h) combined with esketamine (0.3–0.5 mg/kg/h) was infused intravenously for anaesthesia maintenance in the EKT group to maintain the BIS value within a reasonable range. According to the multimodal analgesia protocol, 50 mg of flurbiprofen was administered intravenously for preventive analgesia during incision closure.
Intraoperative hypertension was defined as an increase in systolic blood pressure (SBP) >20% from the preoperative value and/or an SBP>180 mmHg, and intraoperative hypotension was defined as a decrease in SBP>20% from the preoperative value and/or an SBP<90 mmHg. In patients with intraoperative hypertension, the dosages of propofol and analgesics should be adjusted to improve analgesia and balance the depth of anaesthesia, and 5–10 mg urapidil should be injected intravenously if necessary. Patients with intraoperative hypotension were treated with rapid fluid infusion, followed by an infusion of ephedrine or phenylephrine. When the patient’s heart rate (HR) was <50 bpm, 0.2–0.5 mg of atropine was given by intravenous bolus and repeated if necessary.
Surgical Procedure
Total or half hip replacement and closed reduction internal fixation are surgical methods used to treat hip fractures. All surgeries were performed by the same surgeons using the same surgical procedure. Low-molecular-weight heparin was injected subcutaneously after admission to prevent thrombosis of the lower extremities and then stopped 12 hours before surgery.
Analgesia Scheme
The postoperative multimodal analgesia regimen included an intravenous infusion of flurbiprofen at the time of incision closure, iliac fascia block over the inguinal ligament before surgery, and intravenous patient-controlled analgesia (IPCA). The formula for IPCA was as follows: 100 µg of sufentanil + 8 mg of ondansetron + normal saline =200 mL. The analgesic pump parameters were set as follows: a continuous infusion volume of 2 mL per hour, a self-controlled dose of 4 mL per press, and a self-controlled interval of 10 minutes. In the postanaesthesia care unit (PACU) and ward, the IPCA pump was pressed to provide rescue analgesia when the numeric rating scale (NRS) pain score was > 3, with the highest score being 10. The nurse in the PACU provided the patient and family members with instructions on how to evaluate pain and use the IPCA pump. Nurses in the ward assess the intensity of pain every 6 hours.
Primary and Secondary Outcomes
The primary outcome was the area under the curve numeric rating scale (AUCNRS). Intraoperative systolic/diastolic blood pressure (mean blood pressure) (SBP/DBP (MAP)), heart rate (HR) and haemodynamic parameters at T1 (before the induction of anaesthesia), T2 (after the induction of anaesthesia), T3 (5 minutes after incision), and T4 (1 minute before the end of surgery); changes in arterial oxygen partial pressure (PaO2) and arterial oxygen saturation (SaO2); NRS pain score and the number of remedial analgesia at T5 (in the PACU), T6 (6 hours after surgery), T7 (12 hours after surgery), T8 (24 hours after surgery), and T9 (48 hours after surgery); perioperative adverse events; Ramsay sedation score after surgery; the time to extubation; the length of stay in the PACU; and the length of hospital stay (LOHS) were the secondary outcomes.
Statistical Analysis
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS) version 25.0. The Shapiro‒Wilk test was used for normality analysis of all quantitative data. Levene’s test was used for homogeneity of variances. Quantitative data are expressed as the mean ± standard deviation (SD) or median (interquartile range, IQR) according to their distribution. Normally distributed data were assessed using t-test and repeated-measures ANOVA, whereas skewed quantitative data were analysed using the Mann–Whitney U-test. Qualitative data were analysed via Chi-square (χ2) tests. P <0.05 indicated a statistically significant difference. Multiple comparisons via repeated-measures ANOVA were corrected using Bonferroni method.
Sample Size Calculation
In the preliminary trial, eight patients were randomly assigned to each group, and all sixteen patients who were included in the preliminary trial were excluded from the final trial. The sample size was calculated as the difference between two independent means using G*Power 3.1.9.4 software. The mean ± SD of the AUCNRS was 7.4±2.8 in the EKT group and 9.3±2.2 in the OP group. When α was 0.05 and 1-β was 0.8 (two-tails), a sample size of 60 was calculated on the basis of the AUCNRS in the pre-experiment. Considering the 20% drop-out rate, a total of 72 participants were ultimately enrolled.
Results
Of the 96 patients who were initially evaluated, 72 patients were enrolled in the study (Figure 1). Three patients violated the study protocol and one patient was lost to follow-up. Finally, data from 68 patients were analysed.
 |
Figure 1 Flowchart. Abbreviations: EKT, esketamine combined with iliac fascia block; OP, opioids combined with iliac fascia block. |
Demographic Data
The preoperative NRS pain score and preoperative frailty grade were similar between the two groups (Table 1). The average age of the patients was 77.7±6.5 years in the EKT group and 74.3±9.1 years in the OP group (P =0.086). The sex distribution was comparable between the two groups (P =0.787), with a female predominance observed in both groups. The distribution of surgical procedures was similar between the two groups (P =0.715), with the proportion of patients who underwent closed reduction and internal fixation being significantly greater than that of patients who underwent the other two surgical methods. The comparative analysis revealed no statistically significant disparities in ASA classification and body mass index (BMI) between the two groups at baseline (all P >0.05). Moreover, there was no significant difference in the duration of anaesthesia or surgery (all P >0.05).
 |
Table 1 Demographic Characteristics and Operation Details |
Primary Outcome
As shown in Figure 2, the AUCNRS in the EKT group was significantly lower than that in the OP group (6.6±3.6 vs 9.5±3.0, P =0.001).
 |
Figure 2 The area under the curve (AUC) of the NRS score between the two groups (P =0.001). **Indicates a statistical difference between the two groups. |
Secondary Outcomes
Haemodynamic Parameters
As shown in Figure 3, the SBP/DBP (MAP) in both groups decreased after induction of anaesthesia (all P <0.05), and the decrease in SBP in the OP group was significantly greater than that in the EKT group (all P <0.05). At T2, T3, and T4, the MAP in the EKT group was significantly higher than that in the OP group (93.2±17.1 vs 72.7±12.6, P <0.001; 93.8±14.3 vs 80.2±12.0, P <0.001; 102.6±14.4 vs 84.0±11.2, P <0.001) (Table 2). In the EKT group, the HR at T2 was higher than that at T1, but the difference was not significant (P =0.101). Moreover, in the OP group, the HR at T2 was lower than that at T1 (P <0.001). The HR of patients in the EKT group was significantly higher than that of patients in the OP group at T2 and T3 (82.6±12.6 vs 70.9±12.0, P <0.001; 74.9±13.9 vs 66.7±9.5, P =0.006), but the difference in HR at T4 was not significant.
 |
Table 2 The Intraoperative Vital Signs |
As shown in Figure 4, significant differences in the cardiac output and cardiac index were observed between the two groups at T2, T3, and T4 (all P <0.05, Mann–Whitney U-test). The cardiac output and cardiac index in the EKT group were greater than those in the OP group after anaesthesia induction. Peripheral vascular resistance was similar between the two groups at all time points (all P >0.05).
 |
Figure 4 Changes of haemodynamic parameters from T1 to T4 between two groups. |
Changes in Arterial Oxygenation
The postoperative SaO2 were lower than those before surgery in both groups (all P < 0.05). However, both the PaO2 and SaO2 before and after surgery were comparable between the two groups (Table 3).
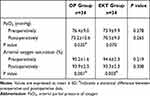 |
Table 3 The Related Details of Respiratory Function |
Postoperative NRS Pain Score and Remedial Analgesia
There were significant differences in postoperative NRS pain scores at T5, T6, T7, T8, and T9 between the two groups (all P < 0.05, Mann–Whitney U-test). Patients in the EKT group had lower NRS pain scores after surgery (Figure 5). As shown in Table 4, there was a significant difference in the number of postoperative rescue analgesia between the two groups (all P < 0.05), and patients in the OP group received more rescue analgesia after surgery than patients in the EKT group.
 |
Table 4 The Details of Anesthesia Recovery, Rescue Analgesia, and Rehabilitation |
 |
Figure 5 Numeric rating scale (NRS) pain scores from T5-T9 in the two groups. |
Ramsay Sedation Score After Surgery
After laryngeal mask removal, four patients in the EKT group and two patients in the OP group experienced drowsiness (Ramsay sedation score of 3), and two patients in the EKT group and two patients in the OP group experienced dysphoria (Ramsay sedation score of 1). At 6 hours after surgery, one patient in each group still had a sedation score of 3.
Adverse Events
As shown in Table 5, patients in the EKT group were more likely to have intraoperative hypertension (20.6% vs 0%, P =0.011), whereas patients in the OP group were more likely to have intraoperative hypotension (94.1% vs 41.2%, P <0.001). Moreover, almost all patients in the OP group developed hypotension, and 4 patients were treated with continuous pumping of vasoactive drugs to maintain circulation.
 |
Table 5 Incidence of Adverse Events |
One patient in each group developed postoperative delirium (POD). Three patients in the OP group developed cutaneous pruritus after surgery, whereas none did in the EKT group (P =0.239). However, the incidence of PONV in the OP group was significantly higher than that in the EKT group: 41.2% in the OP group vs 17.6% in the EKT group (P =0.033).
Details of Recovery
The time to extubation and the length of stay in the PACU after anesthesia were similar between the two groups (all P >0.05) (Table 4). Similarly, there was no significant difference in the LOHS between the two groups after surgery (8.3±3.7 in the OP group vs 7.6±3.1 in the EKT group, P =0.356).
Discussion
The purpose of our study was to investigate the safety and efficacy of opioid-free anaesthesia with esketamine combined with iliac fascia block in patients over 65 years of age who were undergoing hip fracture repair surgery. Our results suggest that, compared with traditional opioids, esketamine can provide more stable intraoperative haemodynamics and better postoperative analgesia in elderly patients without increasing the incidence of postoperative complications. These results are supported by the findings of previous studies that revealed the analgesic effect of esketamine.15–18 However, the intraoperative administration of esketamine alone has not been compared with that of opioids in previous studies. The OFA regimens in previous studies were mostly the combination of dexmedetomidine, esketamine, lidocaine and other drugs,17,18 and our study provided more accurate data for the analgesic effects of esketamine. Second, owing to its established efficacy and safety in elderly patients undergoing hip fracture surgery, esketamine can be considered an additional option for anaesthesia regime.
OFA is multimodal in that a combination of drugs (such as dexmedetomidine, magnesium sulfate, ketamine, lidocaine, and nonsteroidal analgesics) and regional block techniques are used to induce anaesthesia without opioids.19,20 Previous clinical studies involving volunteers and related anatomic studies have confirmed that iliac fascia block on the inguinal ligament can block the sensation of the anterior, medial, and external thighs.21,22 Thus, all patients enrolled in our study underwent iliac fascia block on the inguinal ligament as a part of multimodal analgesia. The results of our study suggest that patients in the OFA group have less severe postoperative pain and are less likely to need remedial analgesia. We speculated that the analgesic effect was better in the EKT group than in the OP group because of the following reasons: a) Opioid-induced hyperalgesia, meaning that patients who are treated with opioids have an increased sensitivity to pain.9 In the OP group, anaesthesia was maintained via remifentanil infusion, which has been strongly correlated with opioid-induced hyperalgesia.18 Moreover, the use of esketamine can prevent hyperalgesia23 and reduce the dose of perioperative opioids.15 b) Rebound pain may occur with iliac fascia block, which is temporary but acute and often occurs after the regression of regional anaesthesia.23 Relevant data show that the incidence of rebound pain after the resolution of peripheral nerve block is approximately 30%-45%.24 A previous triple-blind randomized controlled clinical trial revealed that esketamine can improve pain-sensitive areas and improve the quality of rehabilitation.25 Zeng et al24 suggested that intraoperative small-dose intravenous administration of esketamine can reduce postoperative pain scores, the incidence of rebound pain after a thoracic paravertebral block has stopped, and opioid consumption. c) Existing evidence has shown that perioperative pain, anxiety, and depression are interactional risk factors.2 Pain promotes depression and anxiety, and depression and anxiety enhance subjective pain perception. During the perioperative period, more than 50% of hip fracture patients suffer from depression due to pain, surgery, anesthesia, and restricted activity.3 Singh et al26 reported that 0.2 or 0.4 mg/kg of esketamine had a rapid antidepressant effect. The induction dose of esketamine in our study was 0.3–0.5 mg/kg, which could have an antidepressant effect. Even though patients experienced the same level of pain, the overall NRS pain scores were lower in the EKT group because of the antidepressant effects of esketamine, which relieved depression-related or anxiety-related pain.
In our study, patients who were treated with esketamine rather than opioids during surgery exhibited more stable changes in haemodynamic. This finding is similar to that of a previous study,27 which suggested that esketamine-induced anaesthesia can reduce intraoperative haemodynamic fluctuations. The incidence of hypotension was significantly lower in the EKT group, but the incidence of hypertension was higher. This result may be due to the circulation stimulating effect of esketamine,12 which leads to increases in blood pressure and HR. Propofol combined with esketamine rather than opioids can alleviate circulatory inhibition induced by propofol. Therefore, esketamine is an excellent option for analgesia, sedation, and anaesthesia induction in all patients with impaired haemodynamics.12 However, propofol does not fully mitigate the effect of esketamine on increasing HR. As shown in Figure 3, in the EKT group, the HR at T2 was higher than that before the induction of anaesthesia. However, the difference was not significant, which may be due to the small sample size. The increased HR caused by esketamine leads to an increase in cardiac oxygen consumption,28 which increases the risk of ischemic heart disease. The initial goal of opioids in anesthesia is to control hemodynamic changes,10 which are the increases in blood pressure and HR that result from surgical stress. From this point of view, opioids have an advantage over esketamine. However, intraoperative hypotension is a well-known side effect of anaesthesia that affects patient outcomes. Moreover, it is also a risk factor for postoperative cardiovascular and renal complications and is associated with 30-day mortality.29 A recent meta-analysis highlighted that hypotensive events are a significant concern in elderly patients undergoing hip fracture surgery, particularly under spinal anesthesia.30 It seems that because the patients in the EKT group had better haemodynamics, they had more favourable prognoses. In addition, the cardiac output and cardiac index after anaesthesia induction in the EKT group were greater than those in the OP group. Kamp et al28 reported that esketamine has little effect on haemodynamics and increases cardiac output in a dose-dependent manner, which is similar to our results. None of the patients in the EKT group required conversion to the opioid-balanced regimen, indicating that esketamine can be safely used instead of opioids for general anaesthesia in hip surgery.
In addition, we compared the effects of esketamine with that of traditional opioids on the oxygenation of respiratory function and the time to extubation in elderly patients who underwent hip surgery under general anaesthesia, and no significant differences were observed. Current studies exploring the effects of opioids on the incidence of hypoxemia and the time to extubation have revealed controversial conclusions.31 Some studies revealed that OFA delayed extubation and reduced postoperative oxygen saturation, whereas others revealed that OFA reduced the time to extubation. We believe that opioid use is not solely responsible for decreases in oxygen saturation and delayed extubation. For example, dexmedetomidine, a drug commonly used in OFA, can affect extubation times and oxygen saturation by affecting sedation levels, and the degree of impact varies with the dose.
Notably, despite the prophylactic use of antiemetic drugs during the perioperative period, PONV still occurs in 20%-60% of surgical patients.32 In our study, the incidence of PONV was 41.2% in the OP group, and the incidence of PONV in the EKT group was significantly lower than that in the OP group. We believe that intraoperative exposure to opioids resulted in a higher incidence of PONV in the OP group, which is consistent with previous studies9 revealing that intraoperative opioid administration was a risk factor for PONV and that OFA was associated with a 20% reduction in the incidence of PONV. Esketamine use can reduce the dose of perioperative opioids and the incidence of related postoperative complications.
Massoth et al18 reported that the incidence of postoperative sedation was approximately four times higher in patients who received OFA than in patients who received opioid-balanced anaesthesia, resulting in a significantly longer stay in the PACU. However, this did not occur in our study. We hypothesized that this may be because dexmedetomidine was not used in the OFA regimen of our study. Ketamine use easily causes hallucinations, nightmares, cognitive confusion, and other adverse mental symptoms.2 However, esketamine, as the pure dextrorotatory enantiomer of ketamine, was associated with a significantly reduced incidence of adverse psychiatric symptoms.2 In particular, there were no adverse mental effects in our study, which indicates that the dose of esketamine used in our study is safe.
There are several limitations to this study. First, the observational indicators in the study were limited to short-term results only, and the lack of long-term outcomes limits its clinical relevance. This is a key limitation of this study. In subsequent studies, long-term follow-up evaluations to assess long-term survival, the incidence of chronic pain, and long-term quality of life will improve the clinical relevance and reliability of the findings. Second, patients classified as ASA IV and above were excluded from this study, and this selection bias may indicate that the results may not be applicable to these high-risk patients. In future studies, we will further explore the efficacy of esketamine in patients classified as ASA IV and above. Furthermore, as this was a single-center study, data from multiple centers with a larger sample will be more convincing. Owing to the small sample size, a grouping analysis on the basis of age and comorbidities was not possible. Overall, more and further studies are needed to address these issues.
Conclusions
In conclusion, the OFA protocol integrating esketamine combined with iliac fascia block is feasible and safe for geriatric hip fracture surgery. Esketamine use rather than opioid use could improve haemodynamic stability, reduce the incidence of PONV, and improve postoperative analgesia without increasing the incidence of postoperative complications.
Data Sharing Statement
The datasets generated and/or analysed during the current study are not publicly available due to the privacy policy but are available from the corresponding authors on reasonable requests.
Ethical Adherence
Ethical approval was provided by the clinical medical research ethics committee of Fuyang Hospital of Anhui Medical University, Fuyang, Anhui, China. All patients provided informed consent and all procedures were conducted according to the Declaration of Helsinki.
Acknowledgments
This article is an original work, has not been published before, and is not being considered for publication elsewhere in its final form either in printed or electronic form.
We thank the support of the Specialized Scientific Research of Post-marketing Clinical Research of Innovative Drugs (project number: WKZX2023CX170005). Meanwhile, we thank the nurses involved in the trial for their invaluable support. We also thank the surgeons for their trust in our anesthetics team.
Funding
This work was supported by the Specialized Scientific Research of Post-marketing Clinical Research of Innovative Drugs (project number: WKZX2023CX170005).
Disclosure
None of the authors have any conflicts of interest for this work.
References
1. Kunutsor SK, Hamal PB, Tomassini S, Yeung J, Whitehouse MR, Matharu GS. Clinical effectiveness and safety of spinal anaesthesia compared with general anaesthesia in patients undergoing Hip fracture surgery using a consensus-based core outcome set and patient-and public-informed outcomes: a systematic review and meta-analysis of randomised controlled trials. Br J Anaesth. 2022;129(5):788–800. doi:10.1016/j.bja.2022.07.031
2. Min M, Du C, Chen X, Xin W. Effect of subanesthetic dose of esketamine on postoperative rehabilitation in elderly patients undergoing Hip arthroplasty. J Orthop Surg Res. 2023;18(1):268. doi:10.1186/s13018-023-03728-2
3. Cai J, Chen X, Jin Z, Chi Z, Xiong J. Effects of adjunctive esketamine on depression in elderly patients undergoing Hip fracture surgery: a randomized controlled trial. BMC Anesthesiol. 2024;24(1):340. doi:10.1186/s12871-024-02733-0
4. Guay J, Parker MJ, Gajendragadkar PR, Kopp S. Anaesthesia for Hip fracture surgery in adults. Cochrane Database Syst Rev. 2016;2(2):CD000521. doi:10.1002/14651858.CD000521.pub3
5. Johansen A, Tsang C, Boulton C, Wakeman R, Moppett I. Understanding mortality rates after Hip fracture repair using ASA physical status in the national hip fracture database. Anaesthesia. 2017;72(8):961–966. doi:10.1111/anae.13908
6. Neuman MD, Feng R, Carson JL, et al. Spinal anesthesia or general anesthesia for hip surgery in older adults. N Engl J Med. 2021;385(22):2025–2035. doi:10.1056/NEJMoa2113514
7. Tjeertes EKM, van Fessem JMK, Mattace-Raso FUS, Hoofwijk AGM, Stolker RJ, Hoeks SE. Influence of frailty on outcome in older patients undergoing non-cardiac surgery - a systematic review and meta-analysis. Aging Dis. 2020;11(5):1276–1290. doi:10.14336/AD.2019.1024
8. Lamperti M, Romero CS, Guarracino F, et al. Preoperative assessment of adults undergoing elective noncardiac surgery: updated guidelines from the European society of anaesthesiology and intensive care. Eur J Anaesthesiol. 2025;42(1):1–35. doi:10.1097/EJA.0000000000002069
9. Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia. 2019;74(5):651–662. doi:10.1111/anae.14582
10. Leger M, Perrault T, Pessiot-Royer S, et al. Opioid-free anesthesia protocol on the early quality of recovery after major surgery (SOFA Trial): a randomized clinical trial. Anesthesiology. 2024;140(4):679–689. doi:10.1097/ALN.0000000000004840
11. Feenstra ML, Jansen S, Eshuis WJ, van Berge Henegouwen MI, Hollmann MW, Hermanides J. Opioid-free anesthesia: a systematic review and meta-analysis. J Clin Anesth. 2023;90:111215. doi:10.1016/j.jclinane.2023.111215
12. Trimmel H, Helbok R, Staudinger T, et al. S(+)-ketamine: current trends in emergency and intensive care medicine. Wien Klin Wochenschr. 2018;130(9–10):356–366. doi:10.1007/s00508-017-1299-3
13. Xue Z, Yan C, Liu Y, et al. Opioid-free anesthesia with esketamine-dexmedetomidine versus opioid-based anesthesia with propofol-remifentanil in shoulder arthroscopy: a randomized controlled trial. BMC Surg. 2024;24(1):228. doi:10.1186/s12893-024-02518-9
14. Yan H, Chen W, Chen Y, et al. Opioid-free versus opioid-based anesthesia on postoperative pain after thoracoscopic surgery: the use of intravenous and epidural esketamine. Anesth Analg. 2023;137(2):399–408. doi:10.1213/ANE.0000000000006547
15. Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 2019;393(10180):1558–1568. doi:10.1016/S0140-6736(19)30430-1
16. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117–1127. doi:10.1016/j.bja.2018.02.021
17. Wang D, Sun Y, Zhu YJ, et al. Comparison of opioid‐free and opioid‐inclusive propofol anaesthesia for thyroid and parathyroid surgery: a randomised controlled trial. Anaesthesia. 2024;79(10):1072–1080. doi:10.1111/anae.16382
18. Massoth C, Schwellenbach J, Saadat-Gilani K, et al. Impact of opioid-free anaesthesia on postoperative nausea, vomiting and pain after gynaecological laparoscopy - A randomised controlled trial. J Clin Anesth. 2021;75:110437. doi:10.1016/j.jclinane.2021.110437
19. Hu Y, Zhang Q-Y, Qin G-C, et al. Balanced opioid-free anesthesia with lidocaine and esketamine versus balanced anesthesia with sufentanil for gynecological endoscopic surgery: a randomized controlled trial. Sci Rep. 2024;14(1). doi:10.1038/s41598-024-62824-3
20. Dai J, Li S, Zheng R, Li J. Effect of esketamine on inflammatory factors in opioid-free anesthesia based on quadratus lumborum block: a randomized trial. Medicine. 2023;102(37):e34975. doi:10.1097/MD.0000000000034975
21. Vermeylen K, Desmet M, Leunen I, et al. Supra-inguinal injection for fascia iliaca compartment block results in more consistent spread towards the lumbar plexus than an infra-inguinal injection: a volunteer study. Reg Anesth Pain Med. 2019;44(4):483–491. doi:10.1136/rapm-2018-100092
22. Kantakam P, Maikong N, Sinthubua A, Mahakkanukrauh P, Tran Q, Leurcharusmee P. Cadaveric investigation of the minimum effective volume for ultrasound-guided suprainguinal fascia iliaca block. Reg Anesth Pain Med. 2021;46(9):757–762. doi:10.1136/rapm-2021-102563
23. Zhu Y, Li Q, Liu G, et al. Effects of esketamine on postoperative rebound pain in patients undergoing unilateral total knee arthroplasty: a single-center, randomized, double-blind, placebo-controlled trial protocol. Front Neurol. 2023;14:1179673. doi:10.3389/fneur.2023.1179673
24. Zeng X, Zhang X, Jiang W, Zhou X. Efficacy of intravenous administration of esketamine in preventing and treating rebound pain after thoracic paravertebral nerve block: a prospective randomized, double-blind, placebo-controlled trial. Drug Des Devel Ther. 2024;18:463–473. doi:10.2147/dddt.S448336
25. Helmar Bornemann-cimenti MW, Michaeli K, Edler A, Sandner-Kiesling A, Sandner-Kiesling A. the effects of minimal-dose versus low-dose s-ketamine on opioid consumption, hyperalgesia, and postoperative delirium: a triple-blinded, randomized, active- and placebo-controlled clinical trial. Minerva anestesiologica. 2016;82(10):1069–1072.
26. Singh JB, Fedgchin M, Daly E, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80(6):424–431. doi:10.1016/j.biopsych.2015.10.018
27. Guan Y, Pan H, Cong X, et al. Effect of esketamine on haemodynamic fluctuations in patients undergoing hysteroscopic surgery: a prospective, double-blind randomized clinical trial. Br J Clin Pharmacol. 2024;90(11):2754–2762. doi:10.1111/bcp.16165
28. Kamp J, van Velzen M, Aarts L, Niesters M, Dahan A, Olofsen E. Stereoselective ketamine effect on cardiac output: a population pharmacokinetic/pharmacodynamic modelling study in healthy volunteers. Br J Anaesth. 2021;127(1):23–31. doi:10.1016/j.bja.2021.02.034
29. Simonin M, Delsuc C, Meuret P, et al. Hypobaric unilateral spinal anesthesia versus general anesthesia for hip fracture surgery in the elderly: a randomized controlled trial. Anesth Analg. 2022;135(6):1262–1270. doi:10.1213/ANE.0000000000006208
30. Messina A, La Via L, Milani A, et al. Spinal anesthesia and hypotensive events in Hip fracture surgical repair in elderly patients: a meta-analysis. J Anesth Analg Crit Care. 2022;2(1):19. doi:10.1186/s44158-022-00047-6
31. Beloeil H, Garot M, Lebuffe G, et al. Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery. Anesthesiology. 2021;134(4):541–551. doi:10.1097/ALN.0000000000003725
32. Feng CD, Xu Y, Chen S, et al. Opioid-free anaesthesia reduces postoperative nausea and vomiting after thoracoscopic lung resection: a randomised controlled trial. Br J Anaesth. 2024;132(2):267–276. doi:10.1016/j.bja.2023.11.008
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Opioid-Free Anesthesia for Pain Relief After Laparoscopic Cholecystectomy: A Prospective Randomized Controlled Trial
Yu JM, Tao QY, He Y, Liu D, Niu JY, Zhang Y
Journal of Pain Research 2023, 16:3625-3632
Published Date: 30 October 2023


