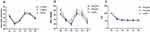Back to Journals » Drug Design, Development and Therapy » Volume 19
Optimal Effect-Site Concentration of Propofol for Hemodynamic Stability During Intubation with Dexmedetomidine: A Randomized Controlled Study
Authors Gao H, Wu J, Chen Y, Wang C, Yao M, Yang Y, Miao C, Liang C
Received 11 December 2024
Accepted for publication 14 April 2025
Published 24 April 2025 Volume 2025:19 Pages 3129—3138
DOI https://doi.org/10.2147/DDDT.S508736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Georgios Panos
Huayuan Gao,1,* Junmei Wu,1,* Youwen Chen MD,1,* Chengyu Wang,2 Minmin Yao,2 Yan Yang,2 Changhong Miao,2,* Chao Liang2,*
1Department of Anesthesiology, Zhongshan Hospital (Xiamen), Fudan University, Xiamen, Fujian, People’s Republic of China; 2Department of Anesthesiology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Liang, Department of Anesthesiology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China, Email [email protected] Changhong Miao, Department of Anesthesiology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China, Email [email protected]
Background: This study aimed to determine the 95% effective concentration (EC95) of propofol via target-controlled infusion (TCI) for endotracheal intubation at three different doses of dexmedetomidine.
Methods: One hundred and eighty patients aged 18– 60 and classified as American Society of Anesthesiologists (ASA) class I–II were enrolled to undergo general anesthesia. Patients were randomly assigned to one of the three groups (A, B, or C), receiving three different doses of dexmedetomidine (0.6, 0.8, or 1 μg/kg) infused over 10 min. Anesthesia was then induced with propofol TCI, followed by rocuronium. The biased coin design method was used to calculate the EC95 of propofol for successful intubation. The primary outcome endpoint was the EC95 of propofol for successful endotracheal intubation at each dexmedetomidine dose.
Results: Sixty patients in each group completed the trial. The time from propofol administration to intubation in group C (132.5 ± 10.7 s) was significantly shorter compared to group A (140.2 ± 14.4 s, P< 0.0001) and group B (142.6 ± 13.2 s, P=0.0037). Both the EC95 and the average total dose of propofol in group B [14.6 (10.8, 14.8) μg/mL and 3.6 ± 1.1 mg/kg] and C [12.7 (11.5, 12.8) μg/mL and 2.8 ± 1.0 mg/kg] were lower than those in group A [14.9 (4.5, 15.0) μg/mL and 3.8 ± 0.9 mg/kg] (P< 0.001). The incidence of hypotension and bradycardia during induction was low in each group.
Conclusion: The EC95 of propofol for endotracheal intubation across three different background doses of dexmedetomidine was determined. We suggest administering 1.0 μg/kg dexmedetomidine and then the EC95 of propofol for successful endotracheal intubation was 12.7 μg/mL.
Registration: Chinese Clinical Trial Registry; Registration number: ChiCTR2400089952, URL:https://www.chictr.org.cn/showproj.html?proj=221236.
Keywords: effective concentration, propofol, dexmedetomidine, opioid-free anesthesia, endotracheal intubation
Introduction
Opioids have long been used for managing stress responses during surgery and for postoperative pain management,1 effectively alleviating nociceptive somatic, visceral, and neuropathic pain. However, during the perioperative period, opioids can trigger respiratory depression, nausea and vomiting, hyperalgesia, pruritus, constipation, urinary retention, and other side effects, which increase patient discomfort, prolonging hospital stay.2 Recently, opioid reduction or avoidance in the perioperative period has gained much interest, with accompanying concepts like opioid-free and opioid-sparing anesthesia (OFA and OSA) being frequently discussed or studied.3–5 The OFA regimen is an innovative approach to pain management that prioritizes patient safety, reduces the opioid-related risks, and seeks to improve recovery outcomes, which have also become integral to the enhanced recovery after surgery (ERAS) regimen.6 Several meta-analyses have shown that OFA reduced postoperative nausea and vomiting (PONV) and other complications during the recovery period compared to opioid-based anesthesia.4,7,8
How to effectively use and combine non-opioids substitutes are the challenges for OFA implementation. Non-opioids mainly include dexmedetomidine, ketamine, magnesium sulfate, nonsteroidal anti-inflammatory drugs, lidocaine, etc.9,10 Different combinations of non-opioid medications have been used for intraoperative anesthesia and postoperative analgesia.10 However, inducing general anesthesia presents unique challenges for OFA. Undesirable hemodynamic fluctuations (tachycardia, hypertension, and arrhythmia) induced by laryngoscopy placement and endotracheal intubation pose a risk to patients.11,12 Traditionally, endotracheal intubation was generally accomplished using sedatives combined with opioids (such as fentanyl, remifentanil, etc.) and muscle relaxants. To avoid opioid use during this period, non-opioid agents have been used in different OFA protocols, including dexmedetomidine, ketamine, lidocaine, and esmolol combined with propofol and muscle relaxants. In some protocols, only sedatives were used. However, the most frequently used combination of OFA induction is dexmedetomidine infusion, with a loading dose of 0.5–1 μg/kg over 10 min, followed by propofol (1–3.5 mg/kg) and a neuromuscular block agent.3 Despite the widespread use of dexmedetomidine and propofol for OFA induction, no studies have systematically investigated or reported the optimal combination doses of these two agents for endotracheal intubation. Additionally, target-controlled infusion (TCI) of propofol has been mostly used in clinical practice for many years, providing stable hemodynamics during induction.13 Therefore, performing an OFA induction with propofol TCI following a background dexmedetomidine infusion seems a reasonable approach. To facilitate the implementation of OFA, it is meaningful to determine the 95% effective concentration (EC95) of propofol via TCI during intubation with dexmedetomidine.
The hypothesis of present study is to determine the EC95 of propofol via TCI to prevent hemodynamic changes during endotracheal intubation at three different dexmedetomidine doses (0.6, 0.8, and 1.0 μg/kg), to identify the optimal combination dose of these two agents for OFA induction. The other collecting outcomes included the hemodynamics and adverse events during induction and intubation.
Methods
Ethical approval for this study (Approval No.: B2023-096R) was provided by the Ethical Committee Zhongshan Hospital (Xiamen branch) Fudan University on 18 February 2024. This study was conducted according to the principles of the Declaration of Helsinki, and written informed consent was obtained from all patients before enrollment. The trial was registered prior to patient enrollment at the Chinese Clinical Trial Registry (www.chictr.org.cn) (ChiCTR2400089952). The study was conducted at Zhongshan Hospital (Xiamen) of Fudan University from October to November 2024. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Participants
This prospective, single-center study included patients scheduled for elective surgery, who had an American Society of Anesthesiologists (ASA) I or II physical status and were aged 18–60 years. Exclusion criteria included a history of difficulty with tracheal intubation, allergy to any study medication, inability to understand the trial procedures, or currently receiving sedative or opioid therapy. Patients were also excluded if they withdrew informed consent, experienced difficulty in completing treatment due to adverse events, or encountered unexpected difficulties with tracheal intubation.
Randomization and Blinding
The enrolled patients were assigned sequentially through a double-blind randomization system in a 1:1:1 ratio, utilizing random number generation software and a block randomization design (block size of 3), to receive one of the three doses of dexmedetomidine (0.6, 0.8, or 1 μg/kg). The allocation sequence was concealed from all members of the study team until the databases were finalized and secured. The anesthesiologist, surgeon, and nurses in the procedure and recovery rooms were blinded to group assignments. Group assignment was obscured behind opaque, sequentially numbered envelopes. Only one research investigator (C.P.) had access to the randomization code for producing study medicines and in case of an emergency during the procedure.
Study Procedures and Interventions
No premedication was administered before induction. After the patient enters the operating room, standard patient monitoring devices to obtain electrocardiograms, non-invasive blood pressure, peripheral pulse oximetry, and bispectral index (BIS) (Covidien) values were applied. The dose of dexmedetomidine, based on the enrollment group A, B, or C, was calculated, and then the respective dexmedetomidine of each group was infused over 10 min.
Anesthesia and Biased Coin Design Method
Subsequently, anesthesia induction was initiated using propofol administered via a TCI pump (BRAUN SPACE) with the Schneider pharmacokinetic model. The initial effect site concentration of propofol was set at 3 μg/mL. Rocuronium 0.6 mg/kg was administered after the patient lost consciousness, and endotracheal intubation was performed after the plasma concentration of propofol equaled the effect site concentration.
The biased coin design (BCD) method was employed to ascertain the appropriate dose level of propofol at the 95th quantile (Γ = 0.95) for various doses of dexmedetomidine in individuals. Here, Γ represents the magnitude of the drug effect, as defined by the study’s objectives. Given that EC95 is the objective, Γ = 0.95 was designated to elicit a response in 100 × Γ = 95% of the target population.14 The selection of K-sequential dose levels was predicated on: 1) our clinical experience and 2) our assumption that the dose–response relationship for the anticipated impact is steep with a high slope value. The dosing intervals were selected according to the guidelines of Dixon and Massey, which recommend intervals between 0.5 and 2 times the expected standard deviation (SD).15 Given the anticipated steep slope of the dose–response relationship of propofol in the planned trial, we employed a dosing interval equivalent to the estimated SD, allowing the dose for subsequent patients to be determined by the response of the previous patient. If a positive response was noted in the preceding patient, the subsequent patient in the same designated group was administered the next higher dose of propofol in a preset increment from the prior dose. Upon observing a favorable response, the subsequent patient in the same cohort was randomly administered either the same dose with a 95% probability (P=0.95) or a reduced dose with a decrement from the prior dose at P=0.05. In this study, positive responses were defined as an increase in either maximum mean arterial pressure (MAPmax) or maximum heart rate (HRmax) by ≥20% from baseline, measured from the beginning of tracheal intubation and within 2 min post-intubation. Negative responses (successful endotracheal intubation) were defined as a change in MAPmax or HRmax during intubation of <20% from baseline during intubation. The inability to achieve sedation with the target dose of propofol was regarded as a positive response, prompting additional propofol administration to enhance anesthesia, after which the patient was removed from the trial.
The primary outcome endpoint was the EC95 of propofol required for successful endotracheal intubation at each dexmedetomidine dose. The second outcome endpoints are the HR, MAP, SpO2, and BIS values at the following time points: before administering dexmedetomidine (T0), before anesthesia induction (T1), before tracheal intubation (T2), during tracheal intubation (T3), after tracheal intubation (T4), and 3 min after tracheal intubation (T5). Hypotension (MAP < 60 mm Hg) was treated with 10 mg IV ephedrine, and bradycardia (heart rate <45 bpm) was treated with 0.5–1 mg IV atropine.
Statistical Analysis
Sample Size Estimation
In BCD studies, it is not possible to calculate the sample size in advance due to the dependence of dose allocation on prior responses and the unknown distribution of outcomes (ie, the next patient’s dose depends on the previous patient’s tracheal intubation response). Among approximately 20 subjects, the parameter estimates of all target doses reached equilibrium and tended to stabilize or almost stabilize in 40 subjects.16 To ensure the smooth completion of the experiment, 60 patients were initially included in each group.
Statistics and Analysis
The normality of the distribution was tested using the Shapiro–Wilk test. A descriptive analysis of the sociodemographic variables was performed. Data is presented as mean (standard deviation) when the variables were quantitative and presented normal distribution; otherwise, data is presented as median (interquartile range). For the nominal variables, frequencies were obtained and are presented in counts (percentages). Data analysis was performed using R version 2.4.1, and EC95 was calculated using isotonic regression. Pace and Stylianou described the use of the pool adjacent violators algorithm (PAVA) for monotonic regression by collecting adjacent increasing and decreasing pairs and recalculating the response rate. The 95% confidence interval (CI) of EC95 was calculated using the derived bias-corrected percentile method, with a resampling size of 45, a repetition rate of 2000, and a target gamma of 0.95. For normally distributed data, results are presented as mean ± SD, and an independent sample t-test is used to compare the two groups. If the normality requirement is not met, results are presented as median (M) and interquartile range (IQR), with comparisons between the two groups performed using the non-parametric two-independent sample Mann–Whitney U-test. Two-factor analysis of variance (ANOVA) was used for repeated measures data, and the Bonferroni method was used for post-hoc intergroup comparisons. Count data are expressed as absolute numbers (percentages), and intergroup comparisons are performed using a four-grid table chi-square test. If the four-grid table chi-square test condition is not met, Fisher’s exact probability method is used for comparison. A P-value of <0.05 is considered statistically significant.
Results
One hundred and eighty patients scheduled for various surgeries were finally enrolled. Of these, three declined to participate in the trial, and two did not meet the inclusion criteria. Ultimately, 60 patients were assigned to each group. All patients completed the trial, as illustrated in the CONSORT flow diagram (Figure 1).
 |
Figure 1 Flowchart of the study. |
Table 1 presents the baseline demographic and intraoperative characteristics. No statistically significant differences were observed between the three groups for the demographic characteristics, including age, gender, body mass index (BMI), ASA classification, and surgery type. However, the time from propofol administration to intubation in group C (132.5 ± 10.7 s) was significantly shorter compared to group A (140.2 ± 14.4 s, P<0.0001) and group B (142.6 ± 13.2 s, P=0.0037).
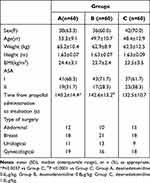 |
Table 1 Patient Characteristics |
The positive or negative responses for each participant at the designated dexmedetomidine dosage are depicted in a conventional graphical representation, with participant sequence on the x-axis and each allocated dosage on the y-axis, respectively (Figure 2A–C). Table 2 shows the observed and PAVA-adjusted response rates with propofol (using the isotonic regression method).
 |
Table 2 Observed and Pool-Adjacent-Violators Algorithm (PAVA)-Adjusted Response Rates with Propofol (Isotonic Regression Method) |
No significant differences in HR (Figure 3A), MAP (Figure 3B), and BIS (Figure 3C) values were observed at T0, T1, T2, T3, T4, and T5 in the three groups. As shown in Table 3, the EC95 and average total dose of propofol in group A were 14.9 (4.5, 15.0) μg/mL and 3.8 ± 0.9 mg/kg, respectively. The EC95 and average total dose of propofol in group B were 14.6 (10.8, 14.8) μg/mL and 3.6 ± 1.1 mg/kg, respectively. The EC95 and average total dose of propofol in group C were 12.7 (11.5, 12.8) μg/mL and 2.8 ± 1.0 mg/kg, respectively. Both the EC95 and average total dose of propofol in groups B and C were significantly lower compared to group A (P<0.001). By quantifying the EC95 of propofol across different doses of dexmedetomidine, these results provide precise, evidence-based guidance for anesthesiologists looking to implement opioid-free induction and intubation.
 |
Table 3 EC95 of Propofol for Smooth Intubation in Different Dexmedetomidine Groups Calculated Using Biased Coin Design Method |
Table S1 presents the number of cases with hypertension, hypotension, tachycardia, and bradycardia during the procedure. No cases of bradycardia occurred in Group A, while three cases were reported in Group B and one case in Group C during the investigation period. Hypotension occurred in four cases each in Groups A and B and in two cases in Group C. However, the differences in these side effects between the groups were not statistically significant.
Discussion
Dexmedetomidine and propofol are the most commonly used combination for OFA induction; however, no consensus on the effective combination of these two agents for endotracheal intubation has been established, especially when propofol is administered via TCI. This study calculated EC95 of propofol via TCI for endotracheal intubation at three different pre-administered doses of dexmedetomidine, providing valuable guidance for OFA implementation.
Dexmedetomidine, an α2 agonist, plays a significant role as a non-opioid agent in OFA. This non-opioid agent acts at both supraspinal and spinal levels, modulating the transmission of nociceptive signals in the central nervous system. Dexmedetomidine also reduces HR, systemic vascular resistance, postoperative pain, and the incidence of PONV due to its opioid‑sparing properties.17,18 Additionally, it enhances hemodynamic stability and reduces anesthetic consumption.19 This makes dexmedetomidine the most used non-opioid for OFA induction, although the use of other opioid substitutes, such as ketamine and esmolol, has also been reported. Some studies have even achieved OFA without any opioid substitute.3 However, these previous studies involving dexmedetomidine for OFA induction have not clearly defined or described the successful induction, intubation, and hemodynamic variables during the induction period. In this study, successful intubation or negative response was defined as an increment in MAPmax and HRmax during intubation of less than 20% from baseline, a common and practical criterion for intubation, as used in a similar study.20
Theoretically, without opioids, anesthesia induction may require relatively large doses of sedatives to suppress cardiovascular responses during tracheal intubation. In a scenario when anesthesia induction and intubation is performed using propofol 3 μg/mL (effect-site concentration) via TCI, co-administered along with remifentanil 0.3 μg · kg−1 · min−1 and rocuronium 0.6 mg/kg, and the propofol (up to 5 μg/mL) was adjusted to BIS values between 40 and 60, however, 55% patients experience hypotension (MAP < 65 mmHg within 2.5 min).21 Furthermore, when an effect site concentration of 4 µg/mL propofol and rocuronium of 0.6 mg/kg was used for induction, the EC50 of remifentanil for blunting the hemodynamic response to tracheal intubation was 2.94 ng/mL.22 Even without muscle relaxants, an effect site concentration of 4 µg/mL propofol combined with remifentanil target concentrations of 4 or 6 ng/mL can provide good or excellent conditions for tracheal intubation and prevent cardiovascular and BIS response during induction.23 However, in the present study, when propofol was combined with the recommended maximum dose (1 µg/kg) of dexmedetomidine, the EC95 of propofol for preventing cardiovascular and BIS response for tracheal intubation was >10 µg/mL. Despite this being a dose-finding study, less than 10% of patients in each group experienced hypotension during the investigation period. These findings, along with previous reports, suggest that while opioids during induction significantly reduce sedative requirements, they also exert stronger cardiovascular suppression compared to dexmedetomidine. In contrast, the combination of dexmedetomidine and propofol for induction and intubation not only eliminates the need for opioids in this period but also provides better hemodynamic stability compared to an opioid-propofol combination.
Another concern of the dexmedetomidine administration is bradycardia. In a multicenter prospective study, the use of balanced OFA with intraoperative continuous dexmedetomidine infusion at rates of 0.4 to 1.4 μg · kg−1 · h−1 was associated with a higher incidence of bradycardia compared to balanced anesthesia with intraoperative continuous remifentanil.24 Additionally, during gastrointestinal endoscopy procedures like endoscopic retrograde cholangiopancreatography (ERCP), a single loading dose of dexmedetomidine combined with propofol was found to reduce propofol consumption, minimize the need for artificial airway intervention, and provide better hemodynamic stability. Importantly, practitioners did not infuse the dexmedetomidine continuously during ERCP since it is associated with hypotension and bradycardia.25–27 Previous OFA studies typically used dexmedetomidine doses ranging from 0.5 to 1.0 μg/kg, infused over 10 min before propofol administration.3 In our study, we selected three doses of dexmedetomidine infuses over 10 min before propofol TCI. Only two cases in the 0.8 μg/kg dexmedetomidine group and one case in the 1.0 μg/kg dexmedetomidine group experienced bradycardia (<45 bpm), while no cases of bradycardia occurred in the 0.6 μg/kg dexmedetomidine group. Therefore, the bolus administration of dexmedetomidine at these doses, in combination with propofol TCI, is considered relatively safe and feasible.
The use of regional analgesia, when possible, is one of the main approaches in OFA and multimodal anesthesia. Since continuous dexmedetomidine infusion is not recommended due to its side effects, OFA induction may be particularly suited for patients undergoing effective regional blockade. With efficient regional anesthesia, such as epidural analgesia for abdominal and thoracic surgeries or paravertebral block for breast surgeries, an effective nociceptive blockade can be achieved, reducing or eliminating the need for opioids.10 Following OFA induction, anesthesia can be maintained using only propofol or inhalational agents with combined non-steroid anti-inflammatory drugs, without the need for opioid substitutes.
Our study had some limitations. First, we only investigated the combination of dexmedetomidine and propofol during the induction period and did not evaluate the patient’s emergency condition since it was not the primary purpose of this study. Future studies should evaluate the effects of different intraoperative OFA regimens on extubation or postoperative emergency. Second, this study only evaluated the combination of dexmedetomidine and propofol, although ketamine and esmolol have also been used in previous studies.28,29 Further research is needed to determine whether ketamine or esmolol combined with dexmedetomidine and propofol could enhance induction and intubation. Third, our study focused on ASA I–II patients, its applicability to ASA III–IV patients is not sufficiently addressed. Lastly, the dose of 1 µg/kg of dexmedetomidine is possibly acceptable in ASA I-II patients with no cardiovascular morbidity but may lead to an unacceptable rate of hypotension and bradycardia in more severely diseased patients. The conclusion of the present study is therefore limited to fit patients.
Conclusion
In conclusion, our trial is the first reported study to systemically explore the dose combination of dexmedetomidine and propofol via TCI for OFA induction. We determined the EC95 of propofol for endotracheal intubation across three different background doses of dexmedetomidine. Considering both induction time and patient safety, we suggest administering 1.0 μg/kg dexmedetomidine and then the EC95 of propofol for successful endotracheal intubation was 12.7 μg/mL. These findings provide useful guidance for real-world dosing and patient management undergoing opioid-free anesthesia.
Data Sharing Statement
For reasonable data requests, contact the corresponding author by email.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82271215).
Disclosure
The authors disclose no conflicts of interest with respect to this work.
References
1. Shanthanna H, Ladha KS, Kehlet H, Joshi GP. Perioperative Opioid Administration. Anesthesiology. 2021;134(4):645–659. doi:10.1097/ALN.0000000000003572
2. Beloeil H. Opioid-free anesthesia. Best Pract Res Clin Anaesthesiol. 2019;33(3):353–360. doi:10.1016/j.bpa.2019.09.002
3. Olausson A, Svensson CJ, Andrell P, Jildenstal P, Thorn SE, Wolf A. Total opioid-free general anaesthesia can improve postoperative outcomes after surgery, without evidence of adverse effects on patient safety and pain management: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2022;66(2):170–185. doi:10.1111/aas.13994
4. Feenstra ML, Jansen S, Eshuis WJ, van Berge Henegouwen MI, Hollmann MW, Hermanides J. Opioid-free anesthesia: a systematic review and meta-analysis. J Clin Anesth. 2023;90:111215. doi:10.1016/j.jclinane.2023.111215
5. Ao Y, Ma J, Zheng X, Zeng J, Wei K. Opioid-sparing anesthesia versus opioid-free anesthesia for the prevention of postoperative nausea and vomiting after laparoscopic bariatric surgery: a systematic review and network meta-analysis. Anesth Analg. 2024;140:385–396. doi:10.1213/ANE.0000000000006942
6. Echeverria-Villalobos M, Stoicea N, Todeschini AB, et al. Enhanced recovery after surgery (ERAS): a perspective review of postoperative pain management under eras pathways and its role on opioid crisis in the United States. Clin J Pain. 2020;36(3):219–226. doi:10.1097/AJP.0000000000000792
7. Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia. 2019;74(5):651–662. doi:10.1111/anae.14582
8. Salomé A, Harkouk H, Fletcher D, Martinez V. Opioid-free anesthesia benefit-risk balance: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2021;10(10):2069. doi:10.3390/jcm10102069
9. Blum KA, Liew LY, Dutia AR, et al. Opioid-free anesthesia: a practical guide for teaching and implementation. Minerva Anestesiol. 2024;90(4):300–310.10.23736/S0375–9393.23.17824–2.
10. Shanthanna H, Joshi GP. Opioid-free general anesthesia: considerations, techniques, and limitations. Curr Opin Anaesthesiol. 2024;37(4):384–390. doi:10.1097/ACO.0000000000001385
11. Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987;59(3):295–299. doi:10.1093/bja/59.3.295
12. Edwards ND, Alford AM, Dobson PM, Peacock JE, Reilly CS. Myocardial ischaemia during tracheal intubation and extubation. Br J Anaesth. 1994;73(4):537–539. doi:10.1093/bja/73.4.537
13. Yildirim SA, Dogan L, Sarikaya ZT, Ulugol H, Gucyetmez B, Toraman F. Hypotension after anesthesia induction: target-controlled infusion versus manual anesthesia induction of propofol. J Clin Med. 2023;12(16):5280. doi:10.3390/jcm12165280
14. Pace NL, Stylianou MP, Warltier DC. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107(1):144–152. doi:10.1097/01.anes.0000267514.42592.2a
15. Dixon WJ, Massey FJ Jr. Introduction to Statistical Analysis.4th Ed. Boston, MA, US: McGraw Hill; 2012:426–441.
16. Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometrics. 2002;58(1):171–177. doi:10.1111/j.0006-341x.2002.00171.x
17. Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116(6):1312–1322. doi:10.1097/ALN.0b013e31825681cb
18. Schnabel A, Meyer-Friessem CH, Reichl SU, Zahn PK, Pogatzki-Zahn EM. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain. 2013;154(7):1140–1149. doi:10.1016/j.pain.2013.03.029
19. Thornton C, Lucas MA, Newton DE, Dore CJ, Jones RM. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 2: auditory and somatosensory evoked responses. Br J Anaesth. 1999;83(3):381–386. doi:10.1093/bja/83.3.381
20. Koh GH, Jung KT, So KY, Seo JS, Kim SH. Effect of different doses of intravenous oxycodone and fentanyl on intubation-related hemodynamic responses: a prospective double-blind randomized controlled trial (CONSORT). Medicine (Baltimore). 2019;98(18):e15509. doi:10.1097/md.0000000000015509
21. Sekiguchi R, Kinoshita M, Kawanishi R, Kakuta N, Sakai Y, Tanaka K. Comparison of hemodynamics during induction of general anesthesia with remimazolam and target-controlled propofol in middle-aged and elderly patients: a single-center, randomized, controlled trial. BMC Anesthesiol. 2023;23(1):14. doi:10.1186/s12871-023-01974-9
22. Yon JH, Jo JK, Kwon YS, Park HG, Lee S. Effect-site concentration of remifentanil for blunting hemodynamic responses to tracheal intubation using light wand during target controlled infusion-total intravenous anesthesia. Korean J Anesthesiol. 2011;60(6):398–402. doi:10.4097/kjae.2011.60.6.398
23. Kim SJ, Yoo KY, Park BY, Kim WM, Jeong CW. Comparison of intubating conditions and hemodynamic responses to tracheal intubation with different effect-site concentrations of remifentanil without muscle relaxants during target-controlled infusion of propofol. Korean J Anesthesiol. 2009;57(1):13–19. doi:10.4097/kjae.2009.57.1.13
24. Beloeil H, Garot M, Lebuffe G, et al. Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery. Anesthesiology. 2021;134(4):541–551. doi:10.1097/ALN.0000000000003725
25. Mazanikov M, Udd M, Kylänpää L, et al. Dexmedetomidine impairs success of patient-controlled sedation in alcoholics during ERCP: a randomized, double-blind, placebo-controlled study. Surg Endosc. 2013;27(6):2163–2168. doi:10.1007/s00464-012-2734-1
26. Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. 2019;72(4):323–330. doi:10.4097/kja.19259
27. Chen M, Sun Y, Li X, et al. Effectiveness of single loading dose of dexmedetomidine combined with propofol for deep sedation of endoscopic retrograde cholangiopancreatography (ERCP) in elderly patients: a prospective randomized study. BMC Anesthesiol. 2022;22(1):85. doi:10.1186/s12871-022-01630-8
28. Mansour MA, Mahmoud AA, Geddawy M. Nonopioid versus opioid based general anesthesia technique for bariatric surgery: a randomized double-blind study. Saudi J Anaesth. 2013;7(4):387–391. doi:10.4103/1658-354X.121045
29. Collard V, Mistraletti G, Taqi A, et al. Intraoperative esmolol infusion in the absence of opioids spares postoperative fentanyl in patients undergoing ambulatory laparoscopic cholecystectomy. Anesth Analg. 2007;105(5):1255–1262. doi:10.1213/01.ane.0000282822.07437.02
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.