Back to Journals » Cancer Management and Research » Volume 17
Optimizing Scalp Cooling: (Ultra)Structural Follicular Characteristic and Restorative Advances
Authors Schaffrin-Nabe D, Josten-Nabe A , Heinze A, Tannapfel A, Schaffrin M, Voigtmann R
Received 1 April 2025
Accepted for publication 20 June 2025
Published 30 June 2025 Volume 2025:17 Pages 1245—1257
DOI https://doi.org/10.2147/CMAR.S526775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Doerthe Schaffrin-Nabe,1 Anke Josten-Nabe,1 Adrian Heinze,2 Andrea Tannapfel,3 Merle Schaffrin,1 Rudolf Voigtmann1
1Praxis für Hämatologie und Onkologie, Bochum, 44799, Germany; 2Noack Statistik GmbH, Bonn, 53119, Germany; 3Ruhr-Universität Bochum, Pathologie, Bochum, 44789, Germany
Correspondence: Doerthe Schaffrin-Nabe, Email [email protected]
Background: Scalp cooling has emerged as a promising intervention for mitigating chemotherapy-induced alopecia, particularly in patients undergoing anthracycline- and taxane-based regimens typically associated with complete hair loss. Despite a visible hair retention rate of 53%, efficacy varies significantly among individuals, influenced by modifiable factors including general health, hair follicle characteristics, and treatment protocols.
Methods: This study evaluated 81 breast cancer patients treated with Epirubicin/Cyclophosphamide followed by weekly Paclitaxel application. Hair preservation was assessed using a cross-section trichometer (Cohen Hair Mass Index [HMI]), light microscopy, and scanning electron microscopy. Key parameters evaluated included pre- and posttherapeutic trichological characteristics such as bulb diameter, shaft diameter, anagen rate, as well as grade of hair shaft damage.
Results: A total of 53% of patients retained visible hair post-treatment (HMI ≥ 50). Trichological factors—particularly bulb diameter, shaft diameter, and anagen rate—strongly predicted hair preservation outcomes. Additionally, hair shaft integrity, specifically surface damage grading, emerged as a critical determinant of clinically meaningful hair retention.
Conclusion: Scalp cooling’s protective effect extends beyond aesthetics, significantly supporting psychological well-being during cancer treatment. Emerging strategies, including cell cycle modulation, antioxidant-based treatments, offer promising avenues to enhance follicular and shaft resilience. While these require further clinical validation, the findings highlight the potential to improve scalp cooling outcomes and raise the standard of supportive oncologic care.
Keywords: scalp cooling, chemotherapy-induced alopecia, hair shaft integrity, trichological parameters, cuticle damage, therapeutic optimization
Introduction
Chemotherapy-induced alopecia is among the most distressing side effects for patients, with studies reporting negative psychological impact in up to 75% of cases, including anxiety, depression, and reduced self-esteem.1,2
Scalp cooling, emerged as a promising intervention to mitigate chemotherapy-induced alopecia with avoidance of visible hair loss in 53% of our patient cohort, is generally well tolerated and current evidence shows the risk scalp metastases is extremely low.3 While this outcome highlights the potential of scalp cooling in preserving hair density, its efficacy remains variable across individuals, necessitating a deeper exploration of contributing factors. Trichological parameters assessed by light- and scanning electron microscopy, particularly those related to the structural and functional characteristics of the hair follicle and shaft, play a pivotal role in determining treatment success. Among these, the integrity of the hair shaft surface emerges as a critical determinant of visible hair preservation. Damage to the cuticle layer, if uncontrolled, predisposes the hair to breakage and visible thinning, underscoring the need for targeted interventions. The potential for restorative strategies aimed at improving hair shaft surface structure, alongside preventive scalp cooling protocols, represents a compelling avenue for enhancing therapeutic outcomes and optimizing patient quality of life during chemotherapy.
Methodology
Study Population
The study population consisted of consecutive 81 patients (79 women and 2 men) with a median age of 56 years, treated between 2021 and 2023 to ensure consistency in chemotherapy protocols and scalp cooling procedures, allowing for controlled analysis of predictive parameters under uniform clinical conditions, undergoing curative treatment for breast carcinoma. The treatment regimen incorporated four cycles of Epirubicin/Cyclophosphamide (EC) (90/600 mg/m² BSA) followed by 12 cycles of weekly Paclitaxel (80 mg/m² BSA) to exert a comparable cell-biologic effect. Patients were excluded from scalp cooling in case of clinically manifested scalp metastases, conditions with generalized hematological involvement (such as leukemia), scalp injuries, or the presence of cryoglobulinemia.
Objectives
The primary analyzed outcomes considered hair preservation, measured by the Hair Mass Index (HMI post treatment), the avoidance of visible hair loss (HMI ≥ 50), and hair loss quantified by the difference in HMI post- HMI pretreatment using a cross-section trichometer. Additional parameters assessed before and after treatment included trichological parameters such as the diameter of the bulb and shaft assessed by light microscopy, and the number of epilated hair follicles in the anagen phase. Particular emphasis was placed on characterizing the degree of hair shaft surface damage via scanning electron microscopy. The study also evaluated potential factors influencing or predicting hair preservation, encompassing general health parameters such as age, internal comorbidities, alopecia-inducing medication (eg, antihypertensives), nicotine use, menopausal status, and hair treatment. Individual hair characteristics (color, length, structure), hair treatment were additionally assessed. Neutrophil nadirs, as indicators of individual hematologic toxicity during cytostatic treatment, were also investigated. Further laboratory testings included evaluation of liver and kidney function, as well as measurements of thyroid and ovarian function.
Hair density evaluation was performed in the anterior vertex region both pre- and post-therapy using the Cross Section Trichometer (Cohen Hair Mass Index Trichometer). This device provides a standardized and reproducible method for quantitatively assessing scalp hair mass from a designated 4 cm² section. Measurements were conducted on hair with a minimum length of 2.5 cm, with results expressed as the Hair Mass Index (HMI). The HMI represents the cross-sectional area of hair (in mm²) per unit scalp area (in cm²), multiplied by 100. Normal HMI values range between 75 and 100, with visible hair thinning (alopecia) starting at HMI values below 50 and complete baldness denoted by HMI values below 20.4
To prepare samples for analysis, approximately 50 hair follicles were extracted from the marked 4 cm² region and mounted between two microscope slides, with the root ends oriented for examination.
Light microscopy was employed to capture images of native hairs at 10x and 40x magnifications using a Leica DM 3000 microscope. Measurements of bulb diameter (normal range: 150–200 µm) and shaft diameter (normal range for the frontal vertex: 75–85 µm) were obtained.5 The anagen phase follicles were identified by the presence of an onion-shaped bulb, while catagen follicles exhibited reduced bulb diameters, and telogen phase follicles featured club-shaped ends. Dystrophic hairs were identified based on abnormalities such as shaft irregularities, breakage, and distortions.
Scanning electron microscopy (SEM) was used to evaluate hair shaft surface integrity before and after therapy. A Zeiss Gemini 982 FESEM was utilized at magnifications ranging from 200x to 2000x. Native hair samples were sputter-coated with gold to enhance imaging resolution prior to analysis.
Representative examples of patients from our study cohort, demonstrating varying patterns and severity grades of hair shaft surface damage, are illustrated in Figure 1. Hair shaft samples from multiple sections were collected and analyzed from a total of 52 patients to ensure consistency in the observed changes. The analysis focused on assessing the extent of damage to the hair shaft surface, according to Kim’s classification of extrinsic hair shaft damage, categorized on a standardized scale ranging from grade 0 to 4, as influenced by the therapeutic intervention (see Box 1).6
 |
Box 1 Definition of Hair Structure Damages via Kim Scale |
Results
Objectives and Descriptive Aspects
Hair Retention (HMI Post) and Avoidance of Visible Hair Loss (HMI Post > 50)
The study evaluated 81 patients with a median pre-treatment Hair Mass Index (HMI pre) score of 75 (IQR 58;92), which decreased to a median of 51 (IQR 17;58) post-treatment. Following therapy, 53% of patients retained an HMI score of 50 or higher, indicating no visible hair loss.
Hair Loss During Therapy (HMI Difference: Post Minus Pre)
The median reduction in HMI score from pre- to post-treatment was −27 (IQR −45; −10). For patients with an initial HMI score below 50, the median change was −20.
Factors potentially influencing outcomes comprised general health conditions such as age, comorbidity, alopecia-inducing medications, smoking status, and menopausal condition. Individual hair characteristics such as color, structure, and length along with hair treatments were taken into account. Additional contributing variables included neutrophil nadirs and specific trichological parameters encompassing bulb and shaft diameter, anagen rate, and shaft surface condition.
In the category of general health factors and comorbidities tend to be associated with poorer hair retention (HMI post (ß =−0,55, p = 0.060)). While elevated neutrophil count is correlated with better hair preservation (ß =+0,24, p=0.054) (Table 1) this trend is also observed in the prevention of visible alopecia, influenced by comorbidity (OR = 0.27, 95% Cl [0.07; 0.98]; p = 0.053) and higher neutrophil nadir count referring to a less pronounced decrease in neutrophil counts (OR 1,97, 95% Cl [1.07; 4.31]; p = 0.055) (Table 2).
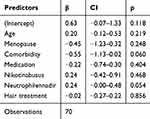 |
Table 1 Factors Influencing HMI Post |
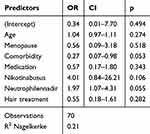 |
Table 2 Predictive Factors for Prevention of Visible Hair Loss HMI Post ≥ 50 |
The use of medications known to induce alopecia predictably leads to greater hair loss during therapy (ß =−0,54; p=0.033). Other previously mentioned potential influencing factors, including hair treatments, do not appear to affect hair loss over time (Table 3)
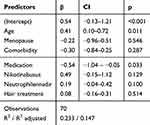 |
Table 3 Parameters Influencing Hair Loss During Treatment (HMI Post – Pre) |
Characteristics such as colour, length or texture do not exhibit a statistically significant correlation with hair loss and simultaneous scalp cooling.
Light Microscopy Results
Descriptive Analysis (Table 4)
Miniaturized hair bulbs (with a median diameter less than 50 µm) exhibited thinner hair shafts (Mdn = 48 µm, IQR [45;53], 6% patients). In contrast, larger follicles (exceeding 50 µm) demonstated a hair shaft thickness of Mdn =65 µm (IQR [59;76], 94% of patients).
 |
Table 4 Comparison of Critical Conditions of Bulb and Shaft Diameter as Well as Anagen Rate Before and After Treatment Related to Hair Density (HMI Pre- and Post-Therapeutically) |
This trend was confirmed post-treatment: bulb diameter less than 50 µm resulted in a median hair shaft diameter 51 µm (IQR [42; 60] 9.8% patients), whereas normal bulbs produced hair shafts with a median of 59 µm (IQR [53;75] 90.2% patients).
In the patient cohort, 4% initially exhibited miniaturized follicles, which increased to 6% post-therapy, representing the minor subset.
The subsequent Table 4 delineates the relationship between critical conditions of bulb and shaft diameter as well as anagen rate before and after treatment in association with hair preservation.
Comorbidity and related medications likely weakened hair follicles, amplifying chemotherapy’s adverse effects, as supported by the observed reduction in bulb diameter at 111 µm (IQR [101;158] 51% patients) compared to the median of 141 µm (IQR [92;126] 49% patients) without underlying conditions or medication use (see Table 5).
 |
Table 5 Correlation of Internal Comorbidities and Regular Medication Intake with Follicular Baseline Parameters and Hair Density |
This trend is also observed in relation to the anagen rate.
Correlational analysis revealed significant association between hair retention and both pre- and post-treatment factors, with post treatment variables exerting a particularly pronounced effect. Notably, the post-treatment shaft diameter (r=0.62, p < 0.001) showed a robust association with hair retention, followed by the post-treatment anagen rate (r=0.59, p < 0.0001) and bulb diameter (r=0.40, p = 0.016), underscoring their substantial role. Additionally, maintaining stability in shaft diameter (R=0.41, p = 0.008) and anagen rate (r=0.41, p = 0.007) was strongly correlated with improved hair retention outcomes (Table 6).
 |
Table 6 Correlation of Light Microscopy Parameters with HMI Post |
Results SEM
Ultrastructural assessment of hair shaft surface damage before and after therapy, classified according to Kim’s scale.
Descriptive analysis revealed that pre-treatment evaluations exhibited a low median shaft surface damage grade of 1. Post-treatment, the median shaft surface damage increased to grade 2 analogue to Kim’s scale.
At baseline, 81% of patients demonstrated a median intact hair shaft with minor cuticle irregularities of whom 31% retained an unaltered hair shaft post treatment showing no additional structural deviations. In patients with cuticle damage up to grade 2, without cortex involvement, the corresponding Hair Mass Index remains ≥50, indicating no visible hair loss (Table 7).
 |
Table 7 Grade of Shaft Surface Damage and Corresponding Hair Density Pre and Post Treatment |
In the bivariate analysis, the pre-treatment damage grade of the hair shaft significantly influenced hair preservation (ρ=−0.35, p = 0.012); though this effect was surpassed by the stronger impact of the post-therapeutic damage grade (ρ =−0.50, p < 0.001).
Specifically, the greater the degree of damage to the shaft surface post-treatment, the lower the probability of HMI post 50 (ρ =−0.41, p = 0.007).
Surrogate Parameters of Hair Preservation as BEST of BEST
From all the types of parameters previously tested with each HMI outcome variable, we included every significant one in the overall regression analyses. Linear regression was used to predict HMI post.
When predicting HMI Post (Table 8), a lesser post-therapeutic damage degree of the shaft surface led to better hair preservation (ß=−0,30, p=0.034). By trend, having no comorbidities (ß=−0,28, p=0.073) and an elevated nadir neutrophil count (ß=0,21, p=0.083) were associated with better hair preservation. This model explained 33% of the variance (adj.R² = 0.33) and included 51 patients with complete data.
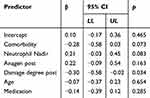 |
Table 8 Hair Retention Post Treatment and Influencing Factors as Every Significant One from All Categories in Overall Regression Analysis as BEST of BEST |
Pragmatic Approach to Evaluate the Efficacy of Scalp Cooling
For prediction purposes, we conducted logistic regression with HMI greater than 50 as the outcome variable. Bulb and shaft diameter, as well as anagen rate, alongside comorbidity and medication use, were calculated as predictors (Table 9). The model fit was moderately strong (Nagelkerke R² = 0.297). The analysis found that in the absence of comorbidity and alopecia-inducing medication, without miniaturized hair follicles and no thin hair shafts (greater than 50 µm) and a minimum anagen rate greater than 50% critical for hair growth, the probability of avoiding visible hair loss was higher by a factor of 3.828 compared to when any of these parameters did not meet these minimal favorable criteria. (OR= 3,828,95% CI (1.25;11.70)).
 |
Table 9 Logistic Regression Predicting HMI > 50 Using Bulb Diameter, Shaft Diameter, Anagen Rate, Comorbidity, and Medication Use as Predictors |
Discussion
To critically evaluate the results of scalp cooling, it is important to scrutinize both the methodology and the interpretations of the outcomes. Eighty-one patients underwent the same cytotoxic drugs to achieve equal cell-biologic effects on hair follicles, which typically induce complete alopecia. Concomitantly receiving scalp cooling, visible hair loss was avoided in a promising 53% of cases, which is consistent with prior studies demonstrating 50–65% efficacy of scalp cooling in anthracycline- and taxane-based regimens, though variations exist due to differences in cooling techniques, patient selection and therapeutic regime.7
The reduction in the median Hair Mass Index from 74 before treatment to 51 post-treatment is significant. This finding implies that while scalp cooling demonstrates efficacy, it may not uniformly benefit all patients. This notable average reduction calls into question the variability in treatment effectiveness. In this regard, factors such as the type, dosage and application modus of cytostatic agents, alongside the scalp cooling protocol, play a critical role in optimizing outcomes.
The assessment of factors influencing or even predicting hair preservation includes parameters related to overall health, hair condition, and morphological follicular characteristics at the (ultra)structural level.
For instance, the median hair density, measured in individuals with fine hair (defined as exhibiting a shaft diameter of less than 50 μm), is 54 pre-therapies, compared to a density of 82 in individuals with normally dense hair shafts (Table 4). Post-therapy, visible hair loss (HMI < 50) was observed in patients with thin hair shafts after treatment (Mdn HMI = 34), in contrast to those with dense hair shafts. These differences are also reflected in the bulb diameter and the anagen-to-telogen ratio, being easily determined by light microscopy (Table 4).
These findings reaffirm the biological characteristics of the hair follicle as fundamental determinants of hair density.
The duration of the anagen phase and the number of hair follicles in this growth phase determine the quantity of hairs visible on the scalp.
The bulb, as the site of cell proliferation and hair production, correlates with follicular activity and dermal papilla nourishment, where a larger diameter biologically signifies enhanced matrix cell proliferation and the generation of thicker, denser hair. Pathophysiological reduction in follicle size, as seen in miniaturization, reduces hair density (Table 4).
The hair shaft diameter is influenced by the proliferation and differentiation of matrix cells, as well as structural proteins (keratin) in the cortex. With hair thinning due to aging or hormonal influences, hair density visibly decreases even if the number of hair follicles remains constant.8
The degree of shaft surface damage not only affects the optical quality of hair but, more importantly in this context, also influences the susceptibility to hair loss through breakage or split ends.
Even under alopecia-inducing chemotherapy and scalp cooling, these factors statistically represent key levers for avoiding visible alopecia. Regression analysis of all light-microscopically evaluated parameters shows that pretherapeutically evaluated anagen ratio, shaft, and bulb diameter significantly influence hair retention. For post-therapeutically determined parameters a strong association was observed (Table 6).
After this statistical impact of these light-microscopic parameters has been demonstrated, a brief discussion of general trichological parameters is warranted. Hair color or length have not demonstrated statistical significance in this analysis. The texture of hair does not, in principle, play a statistically significant role in hair loss. Patients with curly hair may have a slightly higher chance of avoiding alopecia, but this finding, based on a small subset, is likely biased and not conclusive. Hair treatment does not show a significant correlation with either physical stress, such as blow-drying, or chemical stress, like dyeing or coloring.9–12
Given the aforementioned clinical importance of optimal hair follicle characteristics, such as bulb and shaft diameter, as well as anagen ratio, it is imperative to address modifiable factors.
Statistically significant factors from univariate and multivariate analyses include internal comorbidities, suggesting a strong influence of baseline health on the efficacy of scalp cooling. The negative correlation between internal comorbidities and hair preservation may stem from conditions such as metabolic syndrome, which induce hair loss patterns resembling androgenetic alopecia.13–16 However, this association does not imply direct causation but rather shared pathophysiological mechanisms like endocrine imbalances and genetic predispositions. Comorbidities and related medications likely exacerbate the weakening of hair follicles, thereby intensifying the deleterious effect of chemotherapy. This is supported by the observed relative reduction in hair bulb diameter after treatment, which was markedly diminished in patients with underlying conditions or medication use when compared to those without such factors.
Conversely, it's important to note that the frontoparietal scalp is densely populated with hair follicles in the anagen phase being particularly susceptible to the toxic effects of cytostatic agents.17,18
The anagen rate diminishes in the presence of comorbidities and with the regular administration of pharmacological agents such as antihypertensives, antidepressants, NSAIDs irrespective of chemotherapy involvement. Baseline data indicate that only 60% of hair follicles are in the anagen phase in patients with comorbidities and medication, compared to 70% in patients without such factors, accounting for 47% of the study cohort. Moreover, the study’s older demographic, with a median age of 56 years, exacerbates vulnerabilities due to pharmacokinetic and pharmacodynamic alterations, including diminished renal clearance of agents such as doxorubicin and weekly taxanes.19,20
Medication-induced alopecia remains pathogenetically elusive. Statins have been hypothesized to modulate the hair cycle, while ACE inhibitors are linked to intracellular zinc deficiency, affecting metalloenzymes and hedgehog signalling crucial to hair follicle development. Evidence points to these therapies influencing hair follicle morphogenesis, further implicating medications in weakening follicular resilience and reducing scalp cooling efficacy.19,21,22
Consequently, the significant negative impact of regular medication on hair preservation appears plausible.
Summarizing the aforementioned trichological data and general health parameters, the following clinical relevance can be derived: In the absence of comorbidities and medications that induce alopecia, and with minimal favourable light-microscopically determined parameters such as the absence of miniaturized hair bulbs greater than 50 µm in diameter, thick hair shafts with a diameter greater than 50 µm, and a critical threshold for hair growth characterized by an anagen rate greater than 50%, the probability to avoid visible alopecia increases by a factor of 3.828 compared to when any of these parameters do not meet these criteria.
Following the statistical analysis of the pathophysiological significance of hair follicle integrity—specifically, the anagen phase, bulb, and shaft diameter in relation to healthy hair retention—focus should now shift to the surface structure of the hair shaft. This is underscored by the notably high correlation between excellent hair preservation in multivariate regression analysis and grade of posttreatment hairshaft surface damage, as among the most significant factors across all categories (Best of Best).
At baseline, the majority of patients exhibited predominantly intact hair shafts with only minor cuticular irregularities. Among this cohort, a subset retained structurally unaltered hair shafts following treatment, displaying no progression in cuticular deviation. In contrast, patients presenting with cuticular damage up to moderate levels, without evidence of cortical disruption, demonstrated preserved hair density parameters, reflected in a Hair Mass Index indicative of the absence of clinically visible alopecia.
The integrity of the cortex appears to be of paramount importance in determining hair density. To further enhance the efficacy of scalp cooling, the integration of real-time shaft monitoring and potentially restorative interventions may prove beneficial for improving hair preservation outcomes.
Building upon the insights gained from our examination of hair follicle integrity and the efficacy of scalp cooling, it is crucial to contextualize these findings within the broader framework of external and internal stressors impacting hair morphology focused on hair shaft surface characteristics. These stressors, encompassing a range of environmental, physiological, and pharmacological factors, play a pivotal role in shaping hair structure and health. As we transition to exploring these multifaceted stressors, we aim to elucidate their complex interactions with hair biology, ultimately influencing outcomes in hair preservation.
External stressors, such as physical and topical chemical exposures, distinctly affect hair morphology. Physical stressors, notably the thermal impact of procedures like blow-drying, typically cause damage restricted to the cuticle layer, sparing the deeper cortical structures.23 In contrast, chemical stressors, such as those from permanent dyes, not only induce swelling of the cuticular overlapping scales but also penetrate deeper into the cortex. This penetration can lead to significant structural damage, including exposure of the cortex due to extensive cuticular detachment up to complete disappearance of the cuticle.24
Chemical and physical stressors primarily impact the visible portions of the hair shaft,25 while chemotherapy affects all rapidly proliferating cells from the bulb upward, alongside other effects on hair follicles.
SEM samples are strategically selected from representative sections as close as possible to the hair bulb. This methodological approach allows for the evaluation of chemotherapy-induced damage as unaffected as possible by external stressors-induced shaft damage if adequate shaft sections are available. In contrast, topical chemical and physical stress are more likely to manifest in the distal, visible parts of the shaft, and thus may generally have a lesser influence on measured shaft effects and the Hair Mass Index measured at a hair length of 2,5 cm. This may potentially explain the non-significant influence of hair shaft surface damage from external chemical and physical stressors.
After the post-therapeutic degree of shaft damage emerged as a significant biomarker associated with alopecic severity, the question of therapeutic implications arises. It is essential to emphasize that damage to the shaft surface was consistently associated with visible alopecia once the cortex structure was exposed, typically following extensive destruction of the cuticle. Among these patients, visible alopecia was observed.
In contrast, in patients with cuticle damage up to grade 2, without cortex involvement, the corresponding Hair Mass Index remains ≥50, indicating no visible hair loss.
This damage assessment focuses on the most proximal shaft sections, reflecting predominantly intrinsic damage. Given that the bulb represents the zone of cellular proliferation, where matrix keratinocytes are actively engaged in forming the hair shaft, treatment of hair damage caused by residual exposure to cytostatics – affecting both the bulb and the hair shaft surface – should be approached on both preventive and regenerative levels. This includes localized strategies, such as follicular targeting, as well as systemic interventions.
Sensor-controlled scalp cooling effectively prevents chemotherapy-induced alopecia, and follicular targeting emerges as a promising complementary intervention. Chemotherapy induces apoptosis in rapidly proliferating matrix keratinocytes, while also elevated oxidative stress in distal hair shaft cells, is highlighting the need for multifaceted protective strategies.26–28
Unlike living cells, distal hair shaft cells lack advanced antioxidative defenses such as radical scavengers. Reactive oxygen species (ROS) can degrade the outermost lipid layer, particularly the 18-methyleicosanoic acid layer, essential for hair integrity. Without this barrier, cuticles become vulnerable to intrinsic and extrinsic stressors. Chemotherapy-induced ROS can damage cuticle cells, extending to the cortex and causing fibril disorientation. Oxidative processes, including keratin oxidation, disulfide bond cleavage, and amino acid modifications, irreversibly damage cortical cells, leading to brittle, dull, and breakage-prone hair shafts.29,30
Topical melatonin and vitamin C-based nanoparticles can effectively counteract oxidative stress in follicular cells, enhancing shaft integrity and improving hair density in conditions like androgenetic alopecia, offering a therapeutic approach for ROS-induced follicular disorders.31
Within the framework of elevated reactive oxygen species (ROS) as chemotherapy-induced agents of damage to the cellular structures of the follicular pigmentary unit, it is imperative to highlight the principle that “the dose makes the poison”. Excessive ROS levels, indicative of oxidative stress, significantly damage hair follicles. Interestingly, Zhang et al demonstrated that mild oxidative stress can paradoxically protect against chemotherapy-induced alopecia by inducing cell cycle arrest through p53 activation, shielding follicles from cytotoxicity.32 Chen et al corroborated this clinically, observing that scalp cooling decelerates the cell cycle in hair follicles, enhancing resistance to chemotherapeutic damage. These findings highlight the critical role of cell cycle arrest in follicular resilience.33
A promising strategy within this framework involves the use of palbociclib, a cyclin-dependent kinase inhibitor. Preclinical studies on human hair follicle organ cultures show that topically and transiently applied palbociclib induces a temporary G1 phase arrest without cytotoxicity or premature catagen phase transition. This allows matrix cell proliferation to resume and reactivates the anagen phase, preventing follicles from entering the shedding phase while preserving chemotherapy efficacy. This targeted approach effectively mitigates hair loss without compromising oncological treatment outcomes.34
Preclinical studies demonstrate that subcutaneous administration of Kartogenin effectively downregulates the TGF-β2/Smad pathway, potentially extending the anagen phase in murine hair follicles. Since TGF-β activity is upregulated during chemotherapy, leading to follicular apoptosis, inhibiting this pathway could play a critical role in mitigating chemotherapy-induced damage.35–37
However, precise timing and duration of anagen phase modulation are essential, particularly when combined with scalp cooling.Transient G1 phase cell cycle arrest is desirable to allow anagen phase reactivation post-chemotherapy without inducing a catagenic shift. Prolonged anagen extension with ongoing matrix keratinocyte proliferation during chemotherapy-induced apoptosis could negate protective effects, underscoring the need for balanced modulation.
Similarly, the temporal application mode of Palifermin requires careful consideration. Regarding the topical use of growth factors, such as keratinocyte growth factors (KGFs), available data are limited to older studies conducted exclusively at the preclinical level and not in conjunction with follicle cooling.38
Recent discussions have focused on hair follicle damage caused by internal stressors, initially affecting the bulb and subsequently the hair shaft, thus bringing both structures into the scope of preventive and restorative therapeutic considerations.
To protect the hair shaft surface, additionally compromised by external physical and chemical stressors, the topical application of a polyphenol complex shows promise by maintaining cuticle integrity and indirectly safeguarding the cortex, enhancing overall resilience.
A polyphenol-enriched shampoo incorporates tannic acid, which stabilizes molecular networks and provides UV protection, reinforcing surface integrity. Gallic acid enhances elasticity by reducing brittleness, while caffeic acid offers potent antioxidant properties, preventing lipid and protein oxidation and scavenging free radicals. Together, these components improve hair quality and reduce pore size within the hair shaft.39
While a detailed exploration of the nutritional aspects lies beyond the scope of this discussion, it is worth noting the importance of food selection in conjunction with achieving the correct nutritional balance.40
Extensive investigations show no cases of scalp metastases among our patients undergoing scalp cooling since its implementation in 2009 in our practise, consistent with international data confirming its safety. This evidence supports informed decision-making by oncologists and patients regarding the integration of scalp cooling into cancer treatment.3,41–43
This study has certain limitations. As a single-center study, the generalizability of our results may be limited. Additionally, due to the technical complexity of scanning electron microscopy (SEM), pre- and post-intervention SEM data were available for a subset of 52 patients. While no formal power calculation was performed for this subgroup, the consistency of the structural changes observed and the clear effect patterns across multiple endpoints support the adequacy of the sample size for exploratory analysis. Future studies with larger and multicenter designs are warranted to confirm and extend these findings.
Conclusion
Scalp cooling shows a clear potential to reduce chemotherapy-induced alopecia, with visible hair retention achieved in 53% of patients—even under aggressive regimens. Efficacy, however, varies, pointing to the need for individualized approaches. Key predictors of success include pre-treatment trichological factors and post-treatment hair shaft integrity.
Future strategies should focus on enhancing follicular and shaft resilience through targeted interventions, such as transient cell cycle arrest or antioxidant therapies, though clinical validation remains essential. Scalp cooling not only preserves hair but also supports patients’ psychological well-being. Larger, multicenter trials are needed to optimize protocols and improve outcomes.
Ethics Statement
The Ethics committee “Ethik Kommission der Ärztekammer westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms-Universität, Gartenstr 210-214, 48147 Münster” reviewed and approved our above mentioned study (2024-454-f-S) on October 6th, 2024. Informed consent was obtained from all study participants and the guidelines outlined in the Declaration of Helsinki were followed.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Trüeb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg. 2009;28(1):11–14. PMID: 19341937. doi:10.1016/j.sder.2008.12.001
2. Rosman S. Cancer and stigma: experience of patients with chemotherapy-induced alopecia. Patient Educ Couns. 2004;52(3):333–339. PMID: 14998604. doi:10.1016/S0738-3991(03)00040-5
3. Lemieux J, Amireault C, Provencher L, Maunsell E. Incidence of scalp metastases in breast cancer: a retrospective cohort study in women who were offered scalp cooling. Breast Cancer Res Treat. 2009;118(3):547–552. PMID: 19241158. doi:10.1007/s10549-009-0342-0
4. Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Intl J Trichol. 2012;4(4):259–264. PMID: 23766610; PMCID: PMC3681107. doi:10.4103/0974-7753.111221
5. Whiting D. Histology of the Human Hair Follicle. In: Blume-Peytavi U, Tosti A, Trüeb R, editors. Hair Growth and Disorders. Berlin, Heidelberg: Springer; 2008. doi:10.1007/978-3-540-46911-7_7
6. Kim YD, Jeon SY, Ji JH, Lee WS. Development of a classification system for extrinsic hair damage: standard grading of electron microscopic findings of damaged hairs. Am J Dermatopathol. 2010;32(5):432–438. PMID: 20414091. doi:10.1097/dad.0b013e3181c38549
7. Brook TS, Seetsen T, Dercksen MW, et al. Results of the Dutch scalp cooling registry in 7424 patients: analysis of determinants for scalp cooling efficacy. Oncologist. 2024;29(10):e1386–e1395. PMID: 38869252; PMCID: PMC11449096. doi:10.1093/oncolo/oyae116
8. Ball JW, Dains JE, Flynn JA, Solomon BS, Stewart RW. Skin, hair, and nails. In: Ball JW, Dains JE, Flynn JA, Solomon BS, Stewart RW, editors. Seidel's Guide to Physical Examination.
9. Lin X, Zhu L, He J. Morphogenesis, growth cycle and molecular regulation of hair follicles. Front Cell Dev Biol. 2022;10:899095. doi:10.3389/fcell.2022.899095
10. Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893. doi:10.3390/jcm12030893
11. He Y, Cao Y, Nie B, Wang J. Mechanisms of impairment in hair and scalp induced by hair dyeing and perming and potential interventions. Front Med. 2023;10:1139607. PMID: 37275367; PMCID: PMC10232955. doi:10.3389/fmed.2023.1139607
12. Huang WY, Lin ET, Hsu YC, Lin SJ. Anagen hair follicle repair: timely regenerative attempts from plastic extra-bulge epithelial cells. Exp Dermatol. 2019;28(4):406–412. PMID: 30664259. doi:10.1111/exd.13889
13. Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, Buendía-Eisman A, Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women: a comparative study. J Am Acad Dermatol. 2010;63(3):420–429. PMID: 20619491. doi:10.1016/j.jaad.2009.10.018
14. Matilainen V, Laakso M, Hirsso P, Koskela P, Rajala U, Keinänen-Kiukaanniemi S. Hair loss, insulin resistance, and heredity in middle-aged women. A population-based study. J Cardiovasc Risk. 2003;10(3):227–231. PMID: 12775957. doi:10.1097/01.hjr.0000070200.72977.c6
15. Arias-Santiago S, Gutiérrez-Salmerón MT, Buendía-Eisman A, Girón-Prieto MS, Naranjo-Sintes R. Hypertension and aldosterone levels in women with early-onset androgenetic alopecia. Br J Dermatol. 2010;162(4):786–789. PMID: 19906217. doi:10.1111/j.1365-2133.2009.09588.x
16. Qiu Y, Zhou X, Fu S, Luo S, Li Y. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv00645. PMID: 34935992; PMCID: PMC9558341. doi:10.2340/actadv.v101.1012
17. Paus R, Foitzik K. In search of the “hair cycle clock”: a guided tour. Differentiation. 2004;72(9–10):489–511. PMID: 15617561. doi:10.1111/j.1432-0436.2004.07209004.x
18. Kanti V, Nuwayhid R, Lindner J, et al. Analysis of quantitative changes in hair growth during treatment with chemotherapy or tamoxifen in patients with breast cancer: a cohort study. Br J Dermatol. 2014;170(3):643–650. PMID: 24641211. doi:10.1111/bjd.12716
19. Mounessa J, Caravaglio JV, Domozych R, et al. Commonly prescribed medications associated with alopecia. J Am Acad Dermatol. 2023;88(6):1326–1337.e2. PMID: 37268392. doi:10.1016/j.jaad.2017.01.060
20. Hurria A, Lichtman SM. Clinical pharmacology of cancer therapies in older adults. Br J Cancer. 2008;98(3):517–522. PMID: 18256586; PMCID: PMC2243152. doi:10.1038/sj.bjc.6604201
21. Gao Q, Zhou G, Lin SJ, Paus R, Yue Z. How chemotherapy and radiotherapy damage the tissue: comparative biology lessons from feather and hair models. Exp Dermatol. 2019;28(4):413–418. PMID: 30457678. doi:10.1111/exd.13846
22. Alhanshali L, Buontempo M, Shapiro J, Lo Sicco K. Medication-induced hair loss: an update. J Am Acad Dermatol. 2023;89(2S):S20–S28. PMID: 37591561. doi:10.1016/j.jaad.2023.04.022
23. Lee Y, Kim YD, Hyun HJ, Pi LQ, Jin X, Lee WS. Hair shaft damage from heat and drying time of hair dryer. Ann Dermatol. 2011;23(4):455–462. PMID: 22148012; PMCID: PMC3229938. doi:10.5021/ad.2011.23.4.455
24. Dos Santos JD, Edwards HGM, de Oliveira LFC. Raman spectroscopy and electronic microscopy structural studies of Caucasian and Afro human hair. Heliyon. 2019;5(5):e01582. PMID: 31111104; PMCID: PMC6512080. doi:10.1016/j.heliyon.2019.e01582
25. Davis C, Khofar PNA, Karim UKA, et al. Critical assessment on structural analysis of scalp hair using scanning electron microscope (SEM) and compound microscope. Mater Today Proc. 2020;29(Part 1):244–249. doi:10.1016/j.matpr.2020.05.538
26. Trüeb RM. The impact of oxidative stress on hair. Int J Cosmet Sci. 2015;37(Suppl 2):25–30. PMID: 26574302. doi:10.1111/ics.12286
27. Schwartz JR, Henry JP, Kerr KM, Mizoguchi H, Li L. The role of oxidative damage in poor scalp health: ramifications to causality and associated hair growth. Int J Cosmet Sci. 2015;37(Suppl 2):9–15. PMID: 26574300. doi:10.1111/ics.12289
28. Du F, Li J, Zhang S, et al. Oxidative stress in hair follicle development and hair growth: signalling pathways, intervening mechanisms and potential of natural antioxidants. J Cell Mol Med. 2024;28(12):e18486. PMID: 38923380; PMCID: PMC11196958. doi:10.1111/jcmm.18486
29. Hirai T, Ikeda-Imafuku M, Tasaka N, et al. Human hair keratin responds to oxidative stress via reactive sulfur and supersulfides. Adv Redox Res. 2024;10:100091. doi:10.1016/j.arres.2023.100091
30. Robbins CR. Morphological and macromolecular structure. In: Chemical and Physical Behavior of Human Hair. New York, NY: Springer; 2002. doi:10.1007/0-387-21695-2_1
31. Hatem S, Nasr M, Moftah NH, et al. Melatonin vitamin C-based nanovesicles for treatment of androgenic alopecia: design, characterization and clinical appraisal. Eur J Pharm Sci. 2018;122:246–253. doi:10.1016/j.ejps.2018.06.034
32. Zhang Y, Jimenez JJ. Mild oxidative stress protects against chemotherapy-induced hair loss. Front Oncol. 2023;12:1078916. PMID: 36703797; PMCID: PMC9872113. doi:10.3389/fonc.2022.1078916
33. Chen L, Xu Y, Ye X. Low temperature mitigating the paclitaxel-induced damages in mouse cell and hair follicle model. Biochem Biophys Res Commun. 2022;603:94–101. PMID: 35279463. doi:10.1016/j.bbrc.2022.03.031
34. Purba TS, Ng’andu K, Brunken L, et al. CDK4/6 inhibition mitigates stem cell damage in a novel model for taxane-induced alopecia. EMBO Mol Med. 2019;11(10):e11031. PMID: 31512803; PMCID: PMC6783643. doi:10.15252/emmm.201911031
35. Chen Y, Zhou L, Ding Y, et al. Kartogenin regulates hair growth and hair cycling transition. Int J Med Sci. 2022;19(3):537–545. PMID: 35370470; PMCID: PMC8964329. doi:10.7150/ijms.68434
36. Zhu H, Gu X, Xia L, et al. A novel TGFβ trap blocks chemotherapeutics-induced TGFβ1 signaling and enhances their anticancer activity in gynecologic cancers. Clin Cancer Res. 2018;24(12):2780–2793. doi:10.1158/1078-0432.CCR-17-3112
37. Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307(1):1–14. PMID: 11810309. doi:10.1007/s00441-001-0479-6
38. Braun S, Krampert M, Bodó E, et al. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci. 2006;119(23):4841–4849. doi:10.1242/jcs.03259
39. Won HJ, Kim TM, An IS, Bae HJ, Park SY. Protection and restoration of damaged hair via a polyphenol complex by promoting mechanical strength, antistatic, and ultraviolet protection properties. Biomimetics. 2023;8(3):296. PMID: 37504184; PMCID: PMC10807499. doi:10.3390/biomimetics8030296
40. Rajendrasingh RR. Nutritional correction for hair loss, thinning of hair, and achieving new hair regrowth. In: Pathomvanich D, Imagawa K, editors. Practical Aspects of Hair Transplantation in Asians. Tokyo: Springer; 2018. doi:10.1007/978-4-431-56547-5_71
41. Abstracts of the MASCC/ISOO 2016. Annual Meeting. Support Care Cancer. 2016;24(1):1–249. doi:10.1007/s00520-016-3209-z
42. Christodoulou C, Tsakalos G, Galani E, Skarlos DV. Scalp metastases and scalp cooling for chemotherapy-induced alopecia prevention. Ann Oncol. 2006;17(2):350. PMID: 16166175. doi:10.1093/annonc/mdj008
43. Rugo H, Melin S, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163(2):199–205. doi:10.1007/s10549-017-4185-9
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


