Back to Journals » Cancer Management and Research » Volume 17
Organ Function Preservation in Locally Advanced Low Rectal Cancer Through Total Neoadjuvant Therapy: A Case Report and Literature Review
Authors Liu F, Yu L, Zhao Y, Li G, He X
Received 16 October 2024
Accepted for publication 17 January 2025
Published 24 January 2025 Volume 2025:17 Pages 121—129
DOI https://doi.org/10.2147/CMAR.S499531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Fengyuan Liu, Li Yu, Yuanyuan Zhao, Guoliang Li, Xinjia He
Department of Radiation Oncology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China
Correspondence: Guoliang Li; Xinjia He, Department of Radiation Oncology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Locally advanced rectal cancer (LARC) is a common malignancy that is often managed with neoadjuvant radiotherapy to downstage the tumor and increase the rate of complete response. Recent evidence suggests that total neoadjuvant therapy (TNT) may further improve complete response rates and overall survival compared to conventional treatment methods. This case report describes a 61-year-old male patient with LARC who achieved a clinical complete response following TNT. The treatment regimen followed the CinClare study protocol, which included radiotherapy targeting both the rectum and regional lymph nodes, in combination with chemotherapy consisting of irinotecan and capecitabine. After concurrent chemoradiotherapy, the patient underwent six additional cycles of consolidation chemotherapy, leading to a near-complete clinical response. This case demonstrates the potential effectiveness of a high-intensity, dose-dense regimen involving synchronous radiotherapy followed by a six-cycle consolidation chemotherapy course aimed at optimizing organ preservation. This approach highlights a novel model for enhancing organ preservation in patients with low-grade LARC.
Keywords: rectal cancer, total neoadjuvant therapy, organ preservation, case report
Background
Colorectal cancer (CRC) is the third most common malignancy globally and the second leading cause of cancer-related mortality.1 Rectal cancer accounts for about one-third of all colorectal cancers. It is estimated that there will be more than 46,000 new cases of rectal cancer in the United States in 2024.2 Locally advanced rectal cancer (LARC) is typically defined as stage II/III rectal cancer, characterized by T3/T4 tumors or any lymph node involvement (N+) in TNM staging, without distant metastasis. The use of diffusion-weighted magnetic resonance imaging (DW-MRI) in the evaluation of patients with LARC has gradually increased in recent years due to its ability to reflect the response of rectal cancer to chemoradiotherapy.3–5 The pathological complete response (pCR) rate for LARC patients treated with the conventional approach, including preoperative chemoradiotherapy followed by surgery, is approximately 13%.6 For patients with low rectal cancer where sphincter preservation is challenging, the “Watch & Wait” (W&W) strategy may be adopted if a complete clinical response (cCR) is achieved after chemoradiotherapy, relegating radical surgery to a salvage treatment, thereby enhancing the rate of anal preservation. Recent large clinical trials have explored total neoadjuvant therapy (TNT), which involves shifting radiotherapy and systemic chemotherapy to the preoperative period to improve treatment efficacy.7 The CinClare study suggests that adding irinotecan to the standard regimen of capecitabine combined with long-course radiotherapy may further enhance outcomes for these patients. Here, we present a case of a male patient with LARC, who faced challenges in surgical anal preservation but achieved a near cCR and avoided surgery following a TNT regimen based on the CinClare study protocol. This case underscores the significant clinical impact of this treatment modality for patients with LARC.
Case Presentation
We report the case of a 61-year-old male diagnosed with grade IIIB adenocarcinoma of the rectum, initially considered unresectable The patient presented with altered bowel habits, tenesmus, and episodes of melena alternating with rectal bleeding. There were no associated symptoms such as abdominal pain, distension, fever, or vomiting. His medical history was significant for hypertension, managed for the past four years with nifedipine and valsartan. Additionally, he had a 40-year smoking history, averaging 20 cigarettes per day. On physical examination, the patient appeared in good general condition, with no palpable enlargement of superficial lymph nodes. And there was no family history of malignancy.
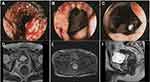
Electronic colonoscopy revealed a circumferential ulcerated neoplasm near the rectum, and a biopsy confirmed the diagnosis of rectal adenocarcinoma (Figure 1A–C). Immunohistochemical analysis at a local hospital showed no evidence of microsatellite instability. Digital rectal examination (DRE) identified a hard, hemorrhagic palpable mass located approximately 3.5 cm from the anal verge. Serum tumor marker analysis revealed a significantly elevated carcinoembryonic antigen (CEA) level of 8.510 ng/mL, while other tumor markers remained within normal ranges. High-resolution magnetic resonance imaging (MRI) of the rectum indicated that the lower margin of the tumor was approximately 37 mm from the anal verge, with the upper and lower tumor margins extending to about 52 mm. More than four abnormal lymph nodes with altered signals were detected in the mesorectal area, the largest having a short diameter of about 8 mm (Figure 1D–F). Both the circumferential resection margin (CRM) and extramural vascular invasion (EMVI) assessments were negative.
Based on these findings, the patient was diagnosed with rectal adenocarcinoma (T3N2M0, stage IIIB) and grade 3 hypertension (high risk). According to the 2017 European Society for Medical Oncology (ESMO) rectal cancer risk stratification, the patient was considered high risk. Given the proximity of the lesion to the anus, surgical preservation of the anus posed a significant challenge, although the patient expressed a strong preference for anus preservation. After a multidisciplinary team (MDT) discussion, neoadjuvant chemoradiotherapy was recommended to achieve tumor regression and enable organ preservation. The patient was treated with the chemoradiotherapy regimen used in the CinClare trial to increase the likelihood of tumor regression and achieving a clinical complete response (cCR). The treatment involved intensity-modulated radiotherapy (IMRT) using 6-MV X-rays at a dose of 5000 cGy over 25 fractions, combined with chemotherapy consisting of irinotecan (80 mg/m² weekly) and capecitabine (625 mg/m² on days 1–5) during the treatment period.
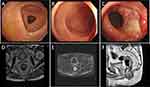
The target area for radiotherapy included the internal iliac lymph node drainage area, occlusive lymph node drainage area, presacral area, and rectal mesenteric lymphatic drainage area, while sparing critical organs such as the bladder, small intestine, and femoral heads (Figure 2A–D). Three irradiation fields with optimal angles were employed, and the gross tumor volume (GTV), clinical target volume (CTV), planning target volume (PTV), and organs at risk were accurately delineated. Dose-volume histogram (DVH) analysis showed that the 5000 cGy dose line encompassed 95.5% of the PTV area. The isodose curve demonstrated that the 95% isodose lines received a higher dose than the prescribed dose. On horizontal, sagittal, and coronal images, critical organs were not encompassed by the 5000 cGy isodose curve, and their irradiation doses remained within tolerable limits. The tumor was also not susceptible to recur in the area out of the 5000 cGy isodose curve. The dose distribution was uniform, with no evidence of hot or cold spots (Figure 3A–E). Following completion of chemoradiotherapy, rectal MRI scans indicated significant reduction of the lesion (Figure 4D–F). Subsequently, the patient received three cycles of chemotherapy with the XELIRI regimen (irinotecan 200 mg/m², capecitabine 1000 mg/m² on days 1–14, every 3 weeks). Electronic colonoscopy revealed a circumferential ulcerative lesion occupying approximately 2/5 of the intestinal lumen, located 4–7 cm from the anal verge (Figure 4A–C). Biopsy pathology indicated moderately active colitis with ulcer formation. The Department of Gastrointestinal Surgery determined that anal preservation remained technically challenging and recommended an additional 2–3 cycles of consolidation chemotherapy.
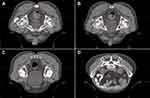 |
Figure 2 Schematic of Target Delineation (A–D) Gross Tumor Volume Schematic Red outlining: Gross Tumor Volume Yellow outlining: Organ at risk. |
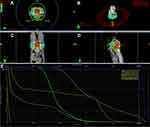 |
Figure 3 Schematic of Radiotherapy Planning: (A–D) Isodose curve schematic. (E) Dose-volume histogram (DVH) schematic. |
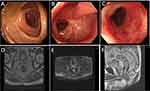
After the 4th to 6th cycles of chemotherapy, MRI scans showed no significant thickening or abnormal signal in the rectal wall (Figure 5D–F), and electronic colonoscopy revealed that the ulceration was replaced by inflammatory granulation tissue, with no residual tumor lesions (Figure 5A–C). Multidisciplinary team (MDT) consultation classified the treatment response as a near clinical complete response (near-cCR) according to the RECIST 1.1 criteria. A “Watch and Wait” (W&W) strategy was suggested to optimize the chances of organ preservation. To date, after six months of follow-up, the patient remains free of disease recurrence, and all serum tumor markers were within normal ranges as of June 2024. No evidence of recurrence or metastasis has been detected during the follow-up period, and the rectal lesion remains in near-cCR.
Discussion and Conclusions
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy worldwide, with adenocarcinoma arising from the mucosa and its glandular structures as the predominant histologic subtype. The adenoma-carcinoma sequence, involving mutations in APC, activating mutations in KRAS, and inactivating mutations in p53, accounts for over 70% of CRC cases.8
The treatment of rectal cancer is primarily guided by clinical staging and involves a multidisciplinary and comprehensive approach. For stage I rectal cancer, satisfactory long-term survival rates can be achieved with surgery alone, without the need for additional treatment. If the stage I tumor is located near the anal verge, local tumor excision or combined postoperative radiotherapy can yield comparable outcomes to radical surgery while preserving anal function.9 For operable stage II–III rectal cancer (T3-4/N+), several randomized studies have demonstrated that preoperative radiotherapy, adjuvant chemoradiotherapy, or postoperative chemoradiotherapy reduce locoregional recurrence rates and significantly improve long-term survival compared to surgery alone. Evidence from multiple randomized trials, including the CAO/ARO-094 trial, indicates that preoperative concurrent chemoradiotherapy improves local control and reduces toxicity compared to postoperative treatment. The results showed a significant reduction in local recurrence rates with preoperative chemoradiotherapy (6% vs 13%, p = 0.006), although there was no significant difference in overall survival (76% vs 74%) and disease-free survival (68% vs 65%). The rates of anal preservation were 39% and 19% in the two groups, respectively (p = 0.004), and both acute and long-term toxic side effects were significantly lower in the preoperative chemoradiotherapy group. Thus, preoperative concurrent chemoradiotherapy improves local control, reduces toxic side effects, and allows for higher rates of anal sphincter preservation.
With advancements in clinical outcomes, the “Watch & Wait” (W&W) strategy has gained international attention for improving quality of life. For patients with low rectal cancer in whom anal sphincter preservation is challenging, those who achieve a complete clinical response (cCR) after chemoradiotherapy can follow the W&W strategy, with radical surgery relegated to salvage treatment, thereby increasing the anal preservation rate. In 2004, Habr-Gama et al first reported that 71 of 265 patients (26.8%) with rectal cancer who received preoperative chemoradiotherapy achieved cCR and found that those without local tumor residue achieved long-term survival under the W&W strategy.10 Although numerous clinical studies and meta-analyses on the W&W strategy have been reported, most are retrospective, and results vary due to geographic differences and small sample sizes. In February 2014, to address these inconsistencies and provide more robust evidence for clinical practice, senior clinical experts from multiple countries established the International Watchful Waiting Database (IWWD, http://www.iwwd.org).11 In July 2018, long-term survival data from 880 cCR patients in the IWWD were published, showing a 2-year local recurrence rate of 25.2%, a 3-year distant metastasis rate of 8.1%, and a 5-year overall survival rate of 84.7%. Tumor recurrence primarily occurred within the first 2 years after treatment, predominantly as intestinal wall recurrence, and was mostly salvageable.12 An update in 2021 showed that the risk of local recurrence in patients maintaining cCR for 3 years was 5% or less, and the risk of systemic recurrence was less than 2% thereafter.13 These findings suggest that as the duration of W&W increases, the probability of local recurrence decreases, with patients eventually reaching a “cured” state, supporting the safety and feasibility of the W&W strategy.
The pathological complete response (pCR) rate after traditional 5-FU-based concurrent chemoradiotherapy is only 10–15%.14 Improving tumor regression, complete remission rates, and long-term survival remains a clinical challenge. Several clinical studies aimed at optimizing treatment modalities, including intensified chemotherapy during concurrent chemoradiotherapy, total neoadjuvant therapy (TNT), and radiotherapy combined with immunotherapy, are currently being conducted.
The question arises whether adding drugs to 5-FU can lead to further tumor regression. Five Phase III clinical studies comparing oxaliplatin plus fluorouracil found that adding oxaliplatin to preoperative chemoradiotherapy did not significantly improve short-term efficacy but increased toxicity.15,16 Over long-term follow-up, there was a mild extension in local control and disease-free survival (DFS), but no improvement in overall survival (OS). This modest benefit is attributed to the neoadjuvant or adjuvant phase, which remains unclear. Therefore, current treatment recommendations do not advocate the use of oxaliplatin in neoadjuvant chemoradiotherapy for rectal cancer. After administration, irinotecan is converted into its active metabolite SN-38, which exerts antitumor effects and is then glucuronidated to inactive SN-38G by UGT1A1 for excretion.17 Mutations in the UGT1A1 gene affect enzyme activity, increasing toxicities such as neutropenia and diarrhea caused by SN-38.
The phase III CinClare trial demonstrated that conventional radiotherapy combined with different doses of irinotecan guided by UGT1A1 genotype (CapIriRT) doubled the pCR rate in patients with locally advanced rectal cancer (LARC) (17% to 33%). Notably, patients with the UGT1A1 *1/*28 genotype received reduced irinotecan doses. However, the addition of irinotecan also resulted in a significant increase in grade 3–4 toxicities (38%), particularly leukopenia, neutropenia, and diarrhea.18,19 This trial suggests that adding irinotecan during radiotherapy increases the likelihood of a complete response but also raises the risk of toxic reactions. With UGT1A1 genotype guidance, toxicities can be managed by adjusting irinotecan dosage. The 2021 Chinese Society of Clinical Oncology (CSCO) guidelines have recommended this regimen for patients with lower rectal cancer requiring anal preservation.
Recent large trials have investigated TNT, which improves overall survival by moving radiation and systemic chemotherapy to the preoperative period to eliminate micrometastatic lesions early. The RAPIDO trial randomized patients with high-risk rectal cancer to standard treatment or an experimental arm. The standard treatment group received preoperative concurrent chemoradiotherapy (capecitabine 825 mg/m² bid, 45–50 Gy/25f), while the experimental group followed a neoadjuvant approach with short-course radiotherapy (5 Gy x 5) plus 6 cycles of neoadjuvant chemotherapy (CAPOX). The study, which included 450 patients in the standard arm and 462 in the experimental arm with a median follow-up of 4.6 years, showed that neoadjuvant chemotherapy had better compliance. Short-course radiotherapy combined with full-dose neoadjuvant chemotherapy significantly reduced tumor recurrence and increased the pCR rate. The 3-year cumulative probability of disease-related treatment failure was 23.7% in the experimental arm versus 30.4% in the standard arm, and the pCR rate in the experimental arm was 27.7% compared to 13.8% in the standard arm.7 TNT can be divided into two modalities: consolidation chemotherapy following long-course concurrent chemoradiotherapy, or induction chemotherapy followed by long-course concurrent chemoradiotherapy. The OPRA and CAO/ARO/AIO-12 studies compared these two approaches, showing similar survival rates for both, but with higher pCR and organ preservation rates in the consolidation chemotherapy group.20,21 Thus, from an organ preservation standpoint, consolidation TNT is more likely to yield better outcomes than induction TNT. Building on the CinClare study, Professor Zhu Ji from Zhejiang Cancer Hospital conducted a randomized controlled trial in which patients received either 6 cycles of XELIRI or 9 cycles of FOLFIRINOX. Interim results showed a cCR rate exceeding 65%.22
To further enhance treatment efficacy, new antitumor approaches such as immunotherapy have been integrated into neoadjuvant regimens for patients with low-grade rectal cancer. At the 2022 American Society of Clinical Oncology Annual Meeting, Andrea Cercek et al reported on the use of the PD-1 antibody dostarlimab (administered once every 3 weeks for 6 months) in patients with stage II–III rectal cancer with deficient mismatch repair (dMMR). Fourteen patients completed the full 6-month course, with a median follow-up of 12 months. Evaluations using rectal examination, MRI, PET/CT, and colonoscopic biopsy showed that all patients achieved a cCR and entered the W&W phase, with no cases of progression or recurrence reported during the 6–25 month follow-up.23 Thus, immunotherapy is an optimal neoadjuvant strategy for patients with dMMR or high microsatellite instability (MSI-H). However, approximately 95% of rectal cancer patients are microsatellite stable or have normal mismatch repair proteins, for whom immunotherapy alone is ineffective. Studies have shown that radiotherapy can increase tumor cell oxidation levels and pH, regulate cell adhesion molecule expression, and promote extracellular matrix remodeling, thereby enhancing tumor antigen release and immune effector cell recruitment. Immune checkpoint inhibitors (ICIs) can further activate local and systemic immune responses and synergize with chemoradiotherapy for enhanced antitumor effects.24 Several prospective studies on short-course radiotherapy combined with immunotherapy have been conducted worldwide, and initial reports indicate pCR rates ranging from 30% to 60%, suggesting a promising avenue for further exploration.25–27
In conclusion, this paper presents a case of a patient with low-grade locally advanced rectal cancer (LARC) with a challenge of preserving the anal sphincter, who achieved a near-complete clinical response (NEAR-CCR) after undergoing total neoadjuvant therapy (TNT). The patient was treated with simultaneous radiotherapy using a high-intensity, dose-intensity regimen from the CinClare study, followed by a full 6-cycle XELIRI regimen of consolidation chemotherapy. This treatment ultimately resulted in the preservation of anal organ function, proposing a new optimization model to maximize organ preservation in low-grade LARC. Although the findings provide valuable insights, it is important to recognize the limitations of this study, including the lack of UGT1A1 genetic testing, which is crucial for predicting treatment-related toxic reactions. Therefore, genetic testing is recommended to predict a patient’s potential toxic response and guide personalized treatment strategies.
Abbreviations
CRC, Colorectal Cancer; LARC, Locally Advanced Rectal Cancer; TNT, Total Neoadjuvant Therapy; cCR, clinical Complete Response; pCR, pathological Complete Response; W&W, Watch and Wait; IWWD, International Watchful Waiting Database; CRM, circumferential resection margin; EMVI, extramural vascular invasion; CEA, carcinoembryonic antigen; DFS, disease-free survival; OS, overall survival; MDT, multidisciplinary team.
Ethics Approval and Consent to Participate
The studies involving humans were approved by Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The participant provided the written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Consent for Publication
This case study was approved by the Ethics Committee of T Affiliated Hospital of Qingdao University. Written informed consent was obtained from the patient for publication of the case report and the accompanying images.
Funding
This study is supported by the Natural Science Foundation of Shandong Province (ZR2023QH276).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
3. Benson AB, Venook AP, Adam M, et al. NCCN Guidelines® Insights: rectal Cancer, Version 3.2024. J Natl Compr Canc Netw. 2024;22(6):366–375. doi:10.6004/jnccn.2024.0041
4. De Felice F, Magnante AL, Musio D, et al. Diffusion-weighted magnetic resonance imaging in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Eur J Surg Oncol. 2017;43(7):1324–1329. doi:10.1016/j.ejso.2017.03.010
5. Musio D, De Felice F, Magnante AL, et al. Diffusion-weighted magnetic resonance application in response prediction before, during, and after neoadjuvant radiochemotherapy in primary rectal cancer carcinoma. Biomed Res Int. 2013;2013:1–5. doi:10.1155/2013/740195
6. Rao S, Guren MG, Khan K, et al. Anal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up(☆). Annals of Oncology. 2021;32(9):1087–1100. doi:10.1016/j.annonc.2021.06.015
7. Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, Phase 3 trial. Lancet Oncol. 2021;22(1):29–42. doi:10.1016/s1470-2045(20)30555-6
8. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi:10.1016/S0140-6736(13)61649-9
9. Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679–687. doi:10.1016/s1470-2045(12)70187-0
10. Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–718. doi:10.1097/01.sla.0000141194.27992.32
11. Beets GL, Figueiredo NL, Habr-Gama A, van de Velde CJ. A new paradigm for rectal cancer: organ preservation: introducing the International Watch & Wait Database (IWWD). Eur J Surg Oncol. 2015;41(12):1562–1564. doi:10.1016/j.ejso.2015.09.008
12. van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537–2545. doi:10.1016/s0140-6736(18)31078-x
13. Fernandez LM, Sao Juliao GP, Figueiredo NL, et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the international watch & wait database: a retrospective, international, multicentre registry study. Lancet Oncol. 2021;22(1):43–50. doi:10.1016/S1470-2045(20)30557-X
14. Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. New Engl J Med. 2006;355(11):1114–1123. doi:10.1056/NEJMoa060829
15. Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J clin oncol. 2011;29(20):2773–2780. doi:10.1200/jco.2010.34.4911
16. Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J clin oncol. 2010;28(10):1638–1644. doi:10.1200/jco.2009.25.8376
17. Ando Y, Saka H, Asai G, Sugiura S, Shimokata K, Kamataki T. UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann Oncol. 1998;9(8):845–847. doi:10.1023/a:1008438109725
18. Wang J, Fan J, Li C, et al. the impact of chemotherapy completion on the efficacy of irinotecan in the preoperative chemoradiotherapy of locally advanced rectal cancer: an expanded analysis of the cinclare phase III trial. Clin Colorectal Cancer. 2020;19(2):e58–e69. doi:10.1016/j.clcc.2020.01.004
19. Zhu J, Liu A, Sun X, et al. Multicenter, randomized, phase III trial of neoadjuvant chemoradiation with capecitabine and irinotecan guided by UGT1A1 status in patients with locally advanced rectal cancer. J clin oncol. 2020;38(36):4231–4239. doi:10.1200/jco.20.01932
20. Goffredo P, Quezada-Diaz FF, Garcia-Aguilar J, Smith JJ. Non-operative management of patients with rectal cancer: lessons learnt from the OPRA trial. Cancers. 2022;14(13):3204. doi:10.3390/cancers14133204
21. Fokas E, Schlenska-Lange A, Polat B, et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol. 2022;8(1):e215445. doi:10.1001/jamaoncol.2021.5445
22. Huang CY, Bai MH, Shen JW, et al. Anus preservation in low rectal adenocarcinoma based on MMR/MSI status (APRAM): a study protocol for a randomised, controlled, open-label, multicentre phase III trial. BMC Cancer. 2024;24(1):57. doi:10.1186/s12885-024-11829-2
23. Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. New Engl J Med. 2022;386(25):2363–2376. doi:10.1056/NEJMoa2201445
24. Miyamoto Y, Ogawa K, Ohuchi M, Tokunaga R, Baba H. Emerging evidence of immunotherapy for colorectal cancer. Ann Gastroenterol Surg. 2023;7(2):216–224. doi:10.1002/ags3.12633
25. Passardi A, Monti M, Donati C, et al. Prospective observational study comparing calcium and sodium levofolinate in combination with 5-Fluorouracil in the FOLFIRI Regimen. Oncologist. 2021;26(8):e1314–e1319. doi:10.1002/onco.13762
26. Shamseddine A, Zeidan YH, El Husseini Z, et al. Efficacy and safety-in analysis of short-course radiation followed by mFOLFOX-6 plus avelumab for locally advanced rectal adenocarcinoma. Radiation Oncology. 2020;15(1):233. doi:10.1186/s13014-020-01673-6
27. Wang Y, Shen L, Wan J, et al. Short-course radiotherapy combined with CAPOX and Toripalimab for the total neoadjuvant therapy of locally advanced rectal cancer: a randomized, prospective, multicentre, double-arm, Phase II trial (TORCH). BMC Cancer. 2022;22(1):274. doi:10.1186/s12885-022-09348-z
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.