Back to Journals » Cancer Management and Research » Volume 17
Overall Survival of Patients with Metastatic Renal Cell Carcinoma Following the Introduction of Targeted and Immunotherapies: A Norwegian Retrospective, Real-World Registry Data Study (RECON3)
Authors Puco K , Notland CS , Szulkin R , Jonasson C , Beisland C , Johannesen TB , Solli O , Oldenburg J, Heinrich D
Received 9 August 2024
Accepted for publication 11 December 2024
Published 22 January 2025 Volume 2025:17 Pages 103—112
DOI https://doi.org/10.2147/CMAR.S484947
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Katarina Puco,1 Cathrine S Notland,2 Robert Szulkin,3,4 Christian Jonasson,5 Christian Beisland,6,7 Tom B Johannesen,8 Oddvar Solli,9 Jan Oldenburg,10,11 Daniel Heinrich12
1Department of Oncology, Hematology and Palliative Care, Lovisenberg Diaconal Hospital, Oslo, Norway; 2Department of Medical Affairs, Pfizer AS, Oslo, Norway; 3SDS Life Science, Stockholm, Sweden; 4Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Solna, Sweden; 5Department of Public Health and Nursing, Faculty of Medicine and Health Science, Norwegian University of Science and Technology (NTNU), Trondheim, Norway; 6Department of Urology, Haukeland University Hospital, Bergen, Norway; 7Department of Clinical Medicine, University of Bergen, Bergen, Norway; 8Cancer Registry of Norway, Norwegian Institute of Public Health, Oslo, Norway; 9Health and Value, Pfizer AS, Oslo, Norway; 10Department of Oncology, Akershus University Hospital HF, Lørenskog, Norway; 11Faculty of Medicine, University of Oslo, Oslo, Norway; 12Department of Radiotherapy and Oncology, Innlandet Hospital Trust HF, Division Gjøvik/Lillehammer, Norway
Correspondence: Katarina Puco, Department of Oncology, Hematology and Palliative Care, Lovisenberg Diaconal Hospital, Oslo, Norway, Tel +47 416 86 298, Email [email protected]
Purpose: In Norway, 5-year survival rates of patients with renal cell carcinoma (RCC) are increasing. The objective of this study was to describe the survival of real-world patients with metastatic RCC (mRCC) across Norway and to identify associated factors. The results may provide additional information on the benefits of tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) in clinical practice.
Patients and Methods: We performed a longitudinal, retrospective, non-interventional cohort study using data from four national registries. The study included adults diagnosed with mRCC between 1 January 1995 and 31 December 2018. Primary endpoint was to evaluate overall survival (OS) in all included patients. Secondary endpoints included further analysis of treatment patterns and possible impact on OS. Secondary endpoint analysis was performed in patients diagnosed with mRCC between 1 January 2008 and 31 December 2018, as complete data on systemic therapies were available from 2008 and onwards.
Results: In total, 4078 patients were diagnosed with mRCC in the period from 1995 to 2018. The median OS since initial mRCC diagnosis was 1.17 years. OS appeared to improve over time, 5-year OS was 10% in patients diagnosed in the period 1995– 2001 compared to 25% in 2012– 2015. The secondary analysis included 2338 patients. Fifty-five percent (55%) of the patients received systemic treatment. No differences were observed in the number of treatment lines administered over time or in the number of lines of treatment administered according to tumor histology. Among 343 patients who received ≥ 3 treatment lines, we observed longer OS in patients who received an ICI as a part of their treatment, with a median OS of 4.51 compared to 2.31 years.
Conclusion: Provision of information into registries is mandatory in Norway. This retrospective, registry-based study provides real-world evidence on patient outcomes and treatments of the Norwegian patients with mRCC in the period from 1995 to 2018.
Keywords: real-world data, immunotherapy, molecular targeted therapy, Norway, renal cell carcinoma
Introduction
In 2022, more than 400,000 new cases of kidney cancer were reported worldwide.1 In Norway, incidence rates are rising, with 678 men and 280 women diagnosed in 2023. However, 5-year survival rates are increasing (80.2% and 82.5% in 2019–2023 versus 46.8% and 53% in 1994–1998 for males and females, respectively), likely due to improvements in diagnostic techniques, earlier detection and better available treatments.2
In metastatic renal cell carcinoma (mRCC), the introduction of modern systemic therapies (ie, tyrosine kinase inhibitors [TKIs] and immune checkpoint inhibitors [ICIs]) has improved patient outcomes during the last two decades. In the first trial comparing the effect of TKI sunitinib versus interferon alpha, reported in 2007, sunitinib demonstrated improved outcomes in the first-line treatment.3 Similar improvements have later also been observed in trials investigating other TKIs, such as pazopanib, axitinib, and sorafenib, and later cabozantinib, lenvatinib and tivozanib.4–9 Mammalian target of rapamycin (mTOR) inhibitors temsirolimus and everolimus showed superior effects in patients with poor prognosis and in patients previously treated with TKI sunitinib.10,11 However, later trials have shown superior effect of checkpoint inhibitor nivolumab and TKI cabozantinib over mTOR inhibitor everolimus.12,13
The immunomodulatory properties of antiangiogenic agents provide a scientific rationale for a synergistic effect between TKIs and ICIs, and data from five phase 3 trials, combining checkpoint inhibitors pembrolizumab, nivolumab or avelumab with TKIs axitinib, cabozantinib or lenvatinib, have shown superior clinical outcomes with this approach.14–20 Additionally, PD-1 and CTLA-4 immunotherapy combination, nivolumab and ipilimumab, have shown superior effect to TKI monotherapy, thereby positioning ICI-CTLA-4 and TKI-ICI combinations as the new first-line standard of care from 2018 and onwards.21,22
Randomized clinical trials are often criticized for their rigorous inclusion criteria, and the survival benefits seen in TKIs and ICIs trials may not reflect benefits seen in patients treated in daily clinical practice.23 Thus, real-world data may provide relevant insights into outcomes and treatment use in patients with mRCC.24–27
Our study, the Renal Comparison Study in Norway 3 (RECON3) is a retrospective, non-interventional study, based on the data from four Norwegian national registries. The study objectives were to evaluate survival improvements of the Norwegian mRCC patients in the period from 1995 to 2018 and to describe targeted treatment patterns and the influencing factors in the period from 2008 to 2018. This study is an update to the previously reported RECON trial.26 RECON3 study results may provide additional real-world evidence supporting the effect of TKIs and ICIs observed in randomized clinical trials.
Materials and Methods
Study Design and Patients
This study was a longitudinal, retrospective, non-interventional cohort study based on pseudonymized patient-level data extracted from four national patient registries in Norway. The national registries are established by health authorities and provision of the information is mandatory. The registries have estimated 99% coverage of the whole population and contain validated high-quality data.28
The study included adult patients diagnosed with RCC (ICD10 code C64) between 1 January 1995 and 31 December 2018, identified using the Cancer Registry of Norway (CRN), which also includes death records. Patient data from the CRN were linked with longitudinal data from the Norwegian Prescription Database (NorPD), the Norwegian Patient Register (NPR), and the Norwegian Primary Care Register (KUHR). The NorPD documents all drugs dispensed at pharmacies nationwide from 1 January 2004 onwards. The NPR records details of all hospital visits from 1 January 2008 onwards, including inpatient drug administration, and KUHR collects data on patients treated in primary care from 1 January 2006 onwards, including diagnoses and procedures.
A diagnosis of mRCC was defined according to the criteria provided in Box 1. Data collected from the registries included patient demographics and characteristics, gender, date of birth, age at diagnosis and date of death. Comorbidities were recorded, and comorbidity load was determined using the Charlson comorbidity index (CCI). Data on tumor characteristics, and surgical treatment were also collected, including year of diagnosis, histological subtype, and type of primary tumor surgery. Further on, data included complete information on systemic treatments from 2008 and onwards, defined as dispensation of medications with the Anatomical Therapeutic Classification (ATC) code L01XE04 (sunitinib), L01XE05 (sorafenib), L01XE09 (temsirolimus), L01XE10 (everolimus), L01XE11 (pazopanib), L01XE17 (axitinib), L01XE26 (cabozantinib), L01XE29 (lenvatinib), L01XC07 (bevacizumab), L01XC11 (ipilimumab), L01XC17 (nivolumab), and L01XC34 (tivozanib).
 |
BOX 1 Definitions of mRCC Used in This Study* |
All included patients were followed from the date of diagnosis until death or up to 30 June 2019, which allowed a minimum of 6 months follow-up.
Study Endpoints
The primary study endpoint was to describe OS in all patients with mRCC diagnosis between 1 January 1995 and 31 December 2018 and according to patient, disease and treatment characteristics and the year of diagnosis.
The secondary endpoints included further analysis of treatment patterns, the use, duration and sequencing of systemic targeted therapies and their potential impact on OS. As complete data on systemic therapies are only available in the registries from 2008 and onwards, analysis related to the use of these were performed on patients diagnosed with mRCC between 1 January 2008 and 31 December 2018.
A standardized window of 3 years before and 3 months after the date of mRCC diagnosis was used to retrieve comorbidity information in NPR. As a result, only patients with an mRCC diagnosis after 1 January 2011 were included in comorbidity analyses.
Statistical Methods
All variables were presented using descriptive statistics, with counts and frequencies provided for categorical variables, and means, standard deviations, median, minimum, maximum, and 1st and 3rd quartiles for continuous variables. Clinical outcomes, including OS and systemic treatment duration, were descriptively assessed using the Kaplan–Meier method together with a Log rank test. OS was defined as the time from the mRCC index date to death or censoring at the end of follow-up (30 June 2019). In the patient cohort where systemic therapies were evaluated (mRCC diagnosed from 2008 and onwards), OS was measured from the start of the first line of therapy. However, a sensitivity analysis, starting follow-up from the third line of therapy, to avoid immortal time bias, was performed. Furthermore, all treatment comparisons of OS were adjusted for several confounders (age, sex, year of diagnosis, histology, metastatic status at primary diagnosis and comorbidities) in a multivariable Cox regression model. Treatment duration was defined as the time from first registered treatment in a treatment line to treatment switch (start of new therapy line) or censoring at death or end of the study period. A multivariable logistic regression model was used to assess which factors (included based on clinical knowledge) were predictive for patients receiving ICI treatment. As this was an observational study, mostly descriptive, with no a priori possibility to dimension the study, no sample size calculation was performed.
Results
Patients
In total, we identified 4159 patients with mRCC diagnosis between 1 January 1995 and 31 December 2018. However, 81 patients were excluded from primary endpoint analysis as they had a death date prior to or on the same date as mRCC diagnosis. Therefore, 4078 patients with mRCC were included in the primary analysis. Secondary endpoint analysis included 2338 patients diagnosed with mRCC from 2008 to 2018, with available complete information on systemic therapy. Patient characteristics are shown in Table 1.
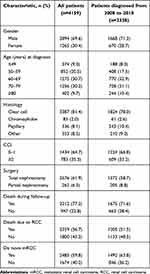 |
Table 1 Characteristics of Patients Diagnosed with mRCC in Norway Between 1995 and 2018 and 2008 to 2018 |
Overall Survival
The median OS from initial mRCC diagnosis was 1.17 years (95% CI 1.11–1.25) (supplementary Figure 1). Probability of survival at 5 years post-mRCC diagnosis was 10% in 1995–2001, 10% in 2002–2005, 16% in 2006–2008, 20% in 2009–2011 and 25% in 2012–2015 (Figure 1). OS improved incrementally over time in patients diagnosed from 1995 to 2018 and the median survival increased from 0.59 years (95% CI 0.5–0.75) in 1995–2001, to 0.75 years (95% CI 0.66–0.91) in 2002–2005, 1.02 years (95% CI 0.87–1.2) in 2006–2008, 1.29 years (95% CI 1.12–1.53) in 2009–2011, 1.46 years (95% CI 1.33–1.7) in 2012–2015, and 1.77 years (95% CI 1.46–2.2) in 2016–2018.
 |
Figure 1 Overall survival of patients with mRCC, stratified by year of diagnosis. Abbreviation: mRCC, metastatic renal cell carcinoma. |
There was a significant difference in OS according to histological subtype and advancing age (p < 0.0001), but no apparent difference according to sex (supplementary Figures 2–4). Median OS was 1.21, 2.21, 1.32 and 0.62 years among patients with clear cell, chromophobe, papillary and other histological subtypes, respectively. More lines of systemic therapy were associated with a better OS; median OS was 0.87, 1.42, 2.62 and 4.09 years for patients who received 1, 2, 3 or ≥4 lines of therapy, respectively (supplementary Figure 5).
Treatment Patterns and Impact on Survival
Of all 2338 patients diagnosed with mRCC between 2008 and 2018, 55% (n = 1281) received systemic treatment. Patients with low comorbidity score were more likely to receive systemic treatment, and 68% of patients with comorbidity CCI score 0–1 received treatment compared with 30% of patients with CCI score of ≥2. Between 2008 and 2018, TKIs were the most frequently prescribed treatment, representing 97.5%, 49.5%, 61.0% and 73.4% of first-, second-, third- and fourth-line therapy received, respectively. Second- and third-line use of mTOR inhibitors decreased over time (40–48% in 2008–2009 versus 3–5% in 2018), whereas use of ICI therapy increased during the same period (from 5% to 30–45%) (supplementary Table 1). Cytokine therapy, with interferon alpha, was used in only one patient after 2008.
As expected, the proportion of patients eligible to receive each subsequent line of therapy decreased (Table 2), but there were no apparent differences in the overall number of lines of treatment received in patients diagnosed between 2008 and 2018, or the number of lines of treatment received according to histology (supplementary Table 2).
 |
Table 2 Distribution of Lines of Treatment According to Year of mRCC Diagnosis (2008 to 2018) |
Furthermore, we descriptively assessed the duration of treatment in 343 patients who received at least 3 lines of therapy. Median duration of first-, second- and third-line treatment was 231 and 155 and 155 days, respectively (Table 3). No apparent differences in treatment duration were detected, regardless of the treatment sequence used. We also performed OS analysis on the various therapy sequences. Patients who received an ICI had a significantly (p < 0.0001) improved OS compared with those who did not; median 4.51 versus 2.31 years (supplementary Figure 6). Furthermore, these differences persisted in a multivariable model adjusting for age, sex, year of diagnosis, histology, metastatic status at primary diagnosis and comorbidities (HR = 0.27, p < 0.0001 in favor of patients treated with ICI) (supplementary Table 3). A sensitivity analysis (to avoid immortal time bias) with OS measured from the start of the third treatment line confirmed these results (HR = 0.45, p < 0.0001) (supplementary Table 3).
 |
Table 3 Median Duration of Treatment Lines Among Patients Who Received ≥ 3 Lines of Treatment |
The most frequently prescribed subsequent treatment after ICI, within the study follow-up period, was cabozantinib (67.6%) followed by axitinib and sunitinib (both <10%).
According to a multivariable prediction model (supplementary Table 4), patients with chromophobe histology were significantly (p = 0.022) more likely to receive an ICI compared with those with clear cell histology, as were patients with a more recent diagnosis of metastatic disease (ie, 2014 onwards; p < 0.001). Conversely, patients with primary mRCC (p = 0.014), males (p = 0.048), and patients with advanced age (70–79 years; p = 0.009 and ≥80 years; p = 0.001) compared to the youngest (≤49 years) were significantly less likely to receive an ICI. Comorbidity load had no significant impact on treatment selection (CCI 0–1 versus ≥2; p = 0.125).
Discussion
This study represents a retrospective analysis of data collected from four Norwegian national registries. As the provision of information into registries is mandatory, these findings are based on a complete national population and provide real-world evidence on the survival of mRCC patients over 23 years, before and after the introduction of modern targeted therapies, and the complete and comprehensive data on the use of systemic therapies in Norway from 1 January 2008 and onwards.
In our study, we observed an incremental improvement in OS over time. The factors associated with a better prognosis, regardless of systemic therapy use, were chromophobe histology and younger age. This is in line with previously reported RECON trial from Norway, and similar Danish and Swedish registry-based studies.24–27 However, our study included mRCC patients from a longer time period, 1995 to 2018, which allowed us to analyze survival for a longer follow-up period; before introduction of targeted therapies (1995–2005), cytokine era (2002–2005), during TKI era (2006 and onwards) and after introduction of ICIs (2017 and onwards). Factors likely to have contributed to survival improvements include earlier diagnosis, improved surgical procedures, general improvements in cancer care and more equitable access to health care services.29 In addition, improvements in survival after 2005 may reflect the introduction of modern systemic therapies and increasing number of effective treatments. Thus, the results from our study may provide additional real-world evidence, supporting survival benefits observed in TKI and ICIs randomized trials. Of note, the ICI monotherapy became available from 2016 (reimbursed in Norway from 2017) and the full effect of ICIs on OS is not fully captured by this analysis and is yet to be seen.
As expected, we found that a greater number of lines of systemic therapy was associated with improved OS. However, these results suffer from problems with immortal time bias, ie, only patients that survive long enough will have the possibility to be treated with several treatment lines. Hence, the results should not be interpreted as causal.
The proportion of patients receiving systemic treatment did not change significantly over the study period and was 54.8% in all patients, which reduced to 29.7% among patients with a greater comorbidity load (CCI ≥2). Although there are reasons why some patients would not receive systemic treatment, ie, delayed systemic treatment in oligometastatic disease treated with surgical procedures or radiotherapy, comorbidities, patient preference, we would have expected the proportion of patients receiving treatment to increase over time. However, this was not seen, suggesting that patient outcomes could possibly be improved merely by improving access to treatment.
Further on, we observed significantly improved OS in patients receiving ICI as one of the treatment lines, and multivariable analysis supports this finding. This benefit should be interpreted with caution, as the patient sizes render the robustness of our observations vulnerable to spurious variation, and we cannot rule out there are possible other confounding factors that could have contributed to this difference in survival. However, it is important to note that the number of trials have shown improved outcomes in patients with mRCC treated with ICI. The immunomodulatory properties of antiangiogenic therapies combined with ICIs as well as the enhanced activity of combination therapies with TKI-ICI and ICI-ICI have been demonstrated in six phase 3 studies.14–22,30 Our study was however conducted prior to the introduction of combination therapies, and updated analysis is therefore necessary to investigate this in the future.
With more systemic therapies available in mRCC, optimizing the treatment sequence could possibly improve clinical outcomes. Various clinical trials have demonstrated a lack of cross resistance, thereby permitting sequencing of TKI therapies.5,8,9,12 Efficacy of mTOR inhibitors or ICIs as second-line therapy after a TKI has also been demonstrated and TKIs have shown activity after prior treatment with a TKI and ICI.8,31,32 In our study, we did not observe any differences in treatment duration related to different therapy sequences. Taking that and patient sizes into account, no conclusions could be made on optimal treatment sequence.
There are a number of limitations to our study. First, we lack the necessary clinical information to stratify patients according to the International Metastatic RCC Database Consortium risk groups, as this information was not captured in the source databases. Further on, we lack data on sites of metastatic lesions, as well as data on surgery and radiotherapy of metastasis that may influence prognosis and outcomes. Secondly, medical treatment records were not available for patients with mRCC prior to 2008, therefore the analyses according to treatment received were limited to patients with mRCC diagnosis after 1 January 2008. Finally, as in all retrospective real-world evidence studies, there is a risk of imbalances between studied groups, ie, confounding bias, and hence establishing causal relationships is challenging. All unadjusted analysis should be interpreted descriptively. However, the analysis where treatment differences were tested was adjusted for several important confounders. Nevertheless, residual confounding cannot be ruled out since all prognostic factors and comorbidities were not captured by the registries. In our multivariable analysis, we identified that patients with a more recent diagnosis of metastatic disease (ie, 2014 onwards) were more likely to receive an ICI. This likely reflects the increased access/availability of ICIs over time.
Taking into account the retrospective nature and the limitations of our study, it is difficult to generalize the results. However, our results support the results from a number of randomized trials as well as previously reported real-world evidence studies, suggesting a benefit of systemic targeted therapies.
Conclusion
In conclusion, this retrospective, registry-based, population-wide study of patients with mRCC in Norway, spanning over 23 years, provides evidence of incremental improvements in survival in the period from 1995 to 2018. Our findings may provide additional evidence supporting survival benefits of modern systemic therapies in real-world patients.
Data Sharing Statement
The full dataset consists of de-identified patient-level data obtained and connected from four national health registries in Norway. Sponsor and data owner of the study dataset is Pfizer AS. Access to the full raw patient-level dataset is limited to sponsors for the study, and the vendor contracted to work on the full patient-level dataset for the study, in accordance with Data Privacy and Ethical Approval for the study project. All authors have full access to the complete study report including line listing, study analyses performed, tables and figures. Study protocol including a statistical analysis plan is available.
Ethical Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki, as well as other relevant research practices, legal requirements, and ethical guidelines. The study was approved by the independent regional ethical committee, Regional Committees for Medical Research Ethics South-East Norway on May 29, 2019, reference number 2019/545. Approval for access and use of data was granted by the four national patient registries in Norway: Cancer Registry of Norway (CRN), the Norwegian Prescription Database (NorPD), the Norwegian Patient Register (NPR), and the Norwegian Primary Care Register (KUHR). The informed consent was waived by the ethics committee, considering the potential benefit from the study, and the use of pseudonymized data from a large number of patients, many of whom were presumed to be deceased and hence making it impossible to carry out the project based on obtaining consent.
Funding
Study was fully funded by Medical Oncology, Pfizer AS.
Disclosure
Katarina Puco reports receipt of honoraria for advisory board participation from Astellas, MSD, Bayer, Pfizer; honoraria for speaker services from Astellas, Ipsen, Janssen-Cilag, AstraZeneca, Merck Sharp & Dohme (MSD) and Bayer; reimbursement of travel/meeting expenses from Pfizer and Ipsen; funding to institution for clinical trial participation from Bristol-Myers Squibb (BMS), Debiopharm, Eisai, Incyte, MSD, Novartis, Eli Lilly, Pfizer, Roche. Cathrine S Notland is an employee of Pfizer AS. Robert Szulkin is employed by SDS Life Science and received payment from Pfizer as a statistical consultant in this project. Christian Jonasson was a paid consultant to Pfizer in connection with the development of the study protocol and the data acquisition process. Christian Beisland reports receipt of honoraria for advisory board participation from Merck Sharp & Dohme (MSD) and Bristol-Myers Squibb (BMS) and has received reimbursement of travel/meeting expenses from the Kidney Cancer Association (KCA) during the project period. Tom B Johannesen has no conflicts of interest to disclose. Oddvar Solli is an employee of Pfizer AS. Jan Oldenburg reports receipt of honoraria for advisory board participation for Bristol Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Pfizer, Roche, Astellas, AstraZeneca, Bayer, Ipsen and Janssen-Cilag; and has undertaken institutional research as coordinating project lead for BMS, MSD and Sanofi. Daniel Heinrich reports receipt of honoraria for advisory board participation for Astellas, AstraZeneca, Bayer, EISAI, IPSEN, Janssen-Cilag, Novartis, Organon, Pfizer and Roche; honoraria for speaker services from AAA – a Novartis company, Astellas, Bayer, Bristol-Myers Squibb (BMS), Dagens Medisin, EUSA Pharma, Ferring, Ipsen, Janssen-Cilag, Merck Sharp & Dohme (MSD), Novartis, Norwegian Medicines Agency; funding to institution for clinical trial participation from AstraZeneca, Bayer, BMS, EISAI, Janssen-Cilag, MSD, Pfizer and Roche.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Cancer Registry of Norway. Cancer in Norway 2023 - Cancer incidence, mortality, survival and prevalence in Norway. 2024. Avaliable from: https://www.kreftregisteret.no/globalassets/cancer-in-norway/2023/cin_report-2023.pdf.
3. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi:10.1056/NEJMoa065044
4. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731. doi:10.1056/NEJMoa1303989
5. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi:10.1016/S0140-6736(11)61613-9
6. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi:10.1056/NEJMoa060655
7. Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591–597. doi:10.1200/JCO.2016.70.7398
8. Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95–104. doi:10.1016/S1470-2045(19)30735-1
9. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, Phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–1482. doi:10.1016/S1470-2045(15)00290-9
10. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled Phase III trial. Lancet. 2008;372(9637):449–456. doi:10.1016/S0140-6736(08)61039-9
11. Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi:10.1056/NEJMoa066838
12. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–1823. doi:10.1056/NEJMoa1510016
13. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi:10.1056/NEJMoa1510665
14. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi:10.1056/NEJMoa1816047
15. Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31(8):1030–1039. doi:10.1016/j.annonc.2020.04.010
16. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi:10.1056/NEJMoa1816714
17. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–1573. doi:10.1016/S1470-2045(20)30436-8
18. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi:10.1056/NEJMoa2035716
19. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi:10.1056/NEJMoa2026982
20. Choueiri TK, Powles T, Albiges L, et al. Cabozantinib plus nivolumab and ipilimumab in renal-cell carcinoma. N Engl J Med. 2023;388(19):1767–1778. doi:10.1056/NEJMoa2212851
21. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi:10.1056/NEJMoa1712126
22. Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. doi:10.1136/esmoopen-2020-001079
23. Heng DY, Choueiri TK, Rini BI, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol. 2014;25(1):149–154. doi:10.1093/annonc/mdt492
24. Soerensen AV, Donskov F, Hermann GG, et al. Improved overall survival after implementation of targeted therapy for patients with metastatic renal cell carcinoma: results from the Danish Renal Cancer Group (DARENCA) study-2. Eur J Cancer. 2014;50(3):553–562. doi:10.1016/j.ejca.2013.10.010
25. Wahlgren T, Harmenberg U, Sandström P, et al. Treatment and overall survival in renal cell carcinoma: a Swedish population-based study (2000-2008). Br J Cancer. 2013;108(7):1541–1549. doi:10.1038/bjc.2013.119
26. Beisland C, Johannesen TB, Klepp O, et al. Overall survival in renal cell carcinoma after introduction of targeted therapies: a Norwegian population-based study. Onco Targets Ther. 2017;10:371–385. doi:10.2147/OTT.S123061
27. Lindskog M, Wahlgren T, Sandin R, et al. Overall survival in Swedish patients with renal cell carcinoma treated in the period 2002 to 2012: update of the RENCOMP study with subgroup analysis of the synchronous metastatic and elderly populations. Urol Oncol. 2017;35(9):541.e15–541.e22. doi:10.1016/j.urolonc.2017.05.013
28. Cancer registry of Norway. Available from: https://www.kreftregisteret.no/en/The-Registries/clinical-registries/.
29. Lundberg FE, Andersson TM, Lambe M, et al. Trends in cancer survival in the Nordic countries 1990-2016: the NORDCAN survival studies. Acta Oncol. 2020;59(11):1266–1274. doi:10.1080/0284186X.2020.1822544
30. Rassy E, Flippot R, Albiges L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther Adv Med Oncol. 2020;12:1758835920907504. doi:10.1177/1758835920907504
31. Auvray M, Auclin E, Barthelemy P, et al. Second-line targeted therapies after nivolumab-ipilimumab failure in metastatic renal cell carcinoma. Eur J Cancer. 2019;108:33–40. doi:10.1016/j.ejca.2018.11.031
32. Ornstein MC, Pal SK, Wood LS, et al. Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2019;20(10):1386–1394. doi:10.1016/S1470-2045(19)30513-3
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Adjuvant Therapy for High-Risk Localized Renal Cell Carcinoma: Current Landscape and Future Direction
Buller DM, Antony M, Ristau BT
OncoTargets and Therapy 2023, 16:49-64
Published Date: 24 January 2023

