Back to Journals » International Journal of Nanomedicine » Volume 20
Overcoming Biological Barriers in Cancer Therapy: Cell Membrane-Based Nanocarrier Strategies for Precision Delivery
Authors Li Y, Sun H, Cao D, Guo Y, Wu D, Yang M, Wang H, Shao X, Li Y, Liang Y
Received 23 September 2024
Accepted for publication 4 February 2025
Published 13 March 2025 Volume 2025:20 Pages 3113—3145
DOI https://doi.org/10.2147/IJN.S497510
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Sachin Mali
Yuping Li,1,2 Hongfang Sun,1 Dianchao Cao,1 Yang Guo,1 Dongyang Wu,1 Menghao Yang,1 Hongming Wang,2 Xiaowei Shao,2 Youjie Li,1 Yan Liang1
1Department of Biochemistry and Molecular Biology, School of Basic Medicine, Binzhou Medical University, YanTai, ShanDong, 264003, People’s Republic of China; 2Binzhou Inspection and Testing Center, Binzhou, ShanDong, 256600, People’s Republic of China
Correspondence: Yan Liang; Youjie Li, Department of Biochemistry and Molecular Biology, School of Basic Medicine, Binzhou Medical University, YanTai, ShanDong, 264003, People’s Republic of China, Tel/Fax +86 535 6913335; +86 535 6913211, Email [email protected]; [email protected]
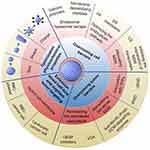
Abstract: Given the unique capabilities of natural cell membranes, such as prolonged blood circulation and homotypic targeting, extensive research has been devoted to developing cell membrane-inspired nanocarriers for cancer therapy, while most focused on overcoming one or a few biological barriers. In fact, the journey of nanosystems from systemic circulation to tumor cells involves intricate processes, encompassing blood circulation, tissue accumulation, cancer cell targeting, endocytosis, endosomal escape, intracellular trafficking to target sites, and therapeutic action, all of which pose limitations to their clinical translation. This underscores the necessity of meticulously considering these biological barriers in the design of cell membrane-mimetic nanocarriers. In this review, we delineate the functions and applications of diverse types of cell membranes in nanocarrier systems. We elaborate on the biological hurdles encountered at each stage of the biomimetic nanoparticle’s odyssey to the target, and comprehensively discuss the obstacles imposed by the tumor microenvironment for precise delivery. Subsequently, we systematically review contemporary cell membrane-based strategies aimed at overcoming these multi-level biological barriers, encompassing hybrid cell membrane (HCM) camouflage, tumor microenvironment remodeling, endosomal/lysosomal escape, multidrug resistance (MDR) reversal, optimization of nanoparticle physicochemical properties, and so on. Finally, we outline potential strategies to accelerate the development of cell membrane-inspired precision nanocarriers and discuss the challenges that must be addressed to enhance their clinical applicability. This review serves as a guide for refining the study of cell membrane-mimetic nanosystems in surmounting in vivo delivery barriers, thereby significantly contributing to advancing the development and application of cell membrane-based nanoparticles in cancer delivery.
Keywords: cell membrane-mimetic, biological barriers, nanodelivery system, delivery efficiency, anti-tumor
Graphical Abstract:

Introduction
As one of the three leading causes of mortality worldwide, the incidence and mortality rates of cancer are increasing globally. Recent studies have indicated that there were 20 million new cancer cases and 9.7 million cancer-related deaths over the past two years.1 The lifetime risk of developing cancer is approximately 20%, with one in nine males and one in twelve females succumbing to the disease. Considering the limitations of surgical interventions and the adverse effects associated with radiotherapy, chemotherapy continues to play an indispensable role in clinical oncology. Over the past few decades, nanovectors have garnered significant attention due to the enhanced permeation and retention (EPR) effect at tumor sites. However, relying solely on passive targeting, the delivery efficiency of conventional nanovectors is a mere 0.6%, with ligand-modified nanovectors achieving only 0.9% delivery to solid tumors via the bloodstream. Conventional nanocarriers, often identified as exogenous substances, are sequestered by the mononuclear phagocyte system (MPS) and subsequently cleared from the bloodstream, leading to suboptimal delivery efficiency. Although several PEGylated nanodelivery systems with enhanced long-term circulation have entered clinical trials, repeated administration of these agents may induce the development of anti-PEG antibodies, thereby hindering their penetration into tumor tissue.2
To improve delivery efficiency, endogenous cell membrane-based nanocarriers have been investigated, demonstrating advantages such as prolonged blood circulation, biocompatibility, biodegradability, and site-specific binding. Although nanocarriers can be encapsulated or directly attached to cells, several limitations must be considered, such as the potential for lysosomal degradation of internalized nanodrugs, immunomodulatory stimulation of surface-bound agents, and uncontrolled release.3 Natural cell membranes are not merely lipid bilayers; glycoproteins and glycolipids on the cell surface play a pivotal role in responding to the intercellular environment, releasing signals, transporting proteins, and stimulating immune reactions.4–7 Otherwise, the biocompatibility and biodegradability of the cell membrane result in minimal adverse reactions within the body. Given the intrinsic advantages of cell membranes, cell membrane-mimetic nanodelivery systems, which consist of a nano-core coated by cell membrane, are being enthusiastically explored. This cell membrane coated nanovectors retain the inherent biological functions of cell membrane and the physicochemical properties of nanoparticles, thereby achieving high drug delivery efficiency.8 The development of the advanced delivery system is inspired by the understanding that living cell membranes engage in intricate cascade responses, and their biological functions are challenging to fully replicate with artificial materials. By encapsulating artificially synthesized nanoparticles within a red cell-membrane layer, these nanoparticles are recognized by the immune system as endogenous components. This evasion of early immune clearance results in extended half-life compared to conventional PEGylated nanomedicines.9 Furthermore, to augment protection against immune phagocytosis, the integration of nanoparticles with immune cell membranes has been a focal point of ongoing research. Notably, the protective shell provided by the immune cell membrane not only endows the nano-core with the capability to evade clearance by the MPS but also plays a crucial role in inhibiting tumor metastasis.10,11 Significantly, the cell membrane derived from tumor development processes exhibits homotypic targeting properties, presenting substantial potential for site-specific drug delivery in cancer therapy.12–14
Despite extensive fundamental research on cell membrane-mimetic nanodelivery systems, the clinical application of cell membrane-based nanovectors remains limited. For cell membrane-mimetic nanocarriers to exhibit high delivery efficiency, they must evade immune detection, penetrate tumor interiors, endosomal/lysosomal escape and prevent pump efflux to effectively eradicate tumor cells. However, achieving the desired therapeutic effects in clinical practice often necessitates increasing the dosage, which consequently leads to heightened side effects and the emergence of MDR. Several obstacles determine the fate of cell membrane-mimetic nanovectors via intravenous administration. These include opsonization and sequestration by the mononuclear phagocyte system, limitations imposed by hemorheology and blood vessel fluid dynamics, intra-tumoral pressure and nanoparticle extravasation, traversal of cellular membranes followed by endosomal compartmentalization, and MDR mediated by drug efflux pumps.15 Despite the potential for overcoming a few obstacles, such as prolonging the half-life of drugs and enhancing their accumulation at tumor sites, current platforms still encounter a complex array of biological barriers that significantly limit site-specific bioavailability, thereby impeding the attainment of optimal delivery efficiency.16 An ideal drug delivery system must efficiently navigate through multi-level biological barriers, including the blood barrier, tumor microenvironment, and cellular barriers, to ensure the targeted release of encapsulated nano-cores at tumor sites.
In this review, we demonstrate that cell membrane-mimicking nanoparticles present a promising avenue for precise anti-tumor drug delivery, attributed to their biocompatibility, extended half-life, and tumor-targeting capabilities. Next, we delineate the framework of the biological challenges encountered at various stages during the systemic transit of nanoparticles to tumor cells. Subsequently, we provided a systematic summary of the current cell membrane-mimetic strategies developed to overcome these multi-level barriers. Importantly, we demonstrate that the failure to address the majority, or even all, biological barriers in the design of cell membrane-mimetic nanocarriers will preclude the realization of the proposed clinical outcomes. Additionally, we outline potential strategies to expedite the development of precision delivery systems and discuss the challenges that must be overcome to enhance their clinical applicability. Despite numerous studies having been conducted on cell membrane-mimetic nanovectors, the journey from the laboratory to the clinical stage remains a formidable challenge, as only one or a few barriers have been merely solved. The review summarizes the barriers faced by anti-tumor nanodrugs and corresponding strategies, providing guidance for the design of novel cell membrane-mimetic nanocarriers in the future.
Functions and Applications of Cell Membranes in Nanodelivery Systems
The nanovectors coated with cell membranes not only retain the EPR property of nanoparticles, but also exhibit the biological functions of cells, such as prolonged circulation time, immune evasion, and targeted delivery to tumors. Furthermore, nanocarriers mimicking cell membranes demonstrate enhanced tissue barrier penetration compared with bare nano-cores, enabling active ingredients to reach the interior of tumors. Hence, a variety of nanovectors camouflaged by endogenous cell membranes have been recognized as an advanced approach for drug delivery in cancer therapy and endogenous cells used to prepare cell membrane-based nanovectors are depicted in Figure 1. In this section, we present the characteristics and fundamental applications ranging from circulating cells to tumor cells. The advantages of cell membrane-based nanodelivery systems endowed by various cell membranes are summarized in Table 1.
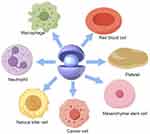 |
Figure 1 Various endogenous cells used to prepare cell membrane-based nanovectors. |
 |
Table 1 The Advantages of Cell Membrane-Based Nanodelivery Systems Endowed by Various Cell Membrane |
Red Blood Cell Membrane
Red blood cells (RBCs) are the most abundant cells in the human body and produced in the bone marrow with a lifespan of 100~120 days. They function as carriers, transporting nutrients and growth factors.32 The transmembrane protein of CD47 expressed on the surface of RBC membrane releases a “don’t eat me” signal via selectively binding to SIRP generated by macrophages, which weakens the phagocytosis of immune system and extends circulation time.17 Gao et al demonstrated that the macrophage clearance rate of gold nanoparticles enclosed by RBC membrane was one-quarter of that of unclosed nano-gold-core (3.2 vs 13.5 ng/1000 macrophage cells), thus indicating that the coating membrane conferred immunosuppressive capacity to gold nanoparticles, enabling the encapsulated gold nanoparticles to evade macrophage uptake.33 Furthermore, the encapsulation of RBC membrane prolongs the circulation time of nanocarriers in the bloodstream leading to increased accumulation at the tumor site via EPR effect.18 Anticancer drug doxorubicin (DOX) loaded poly (lactic-co-glycolic acid) (PLGA) nanoparticles coated by RBC membrane exhibited enhanced inhibition of tumor growth and improved immune compatibility compared to unencapsulated cores, highlighting RBC membrane strengthened the passive targeting of encapsulated therapeutic ingredients via extending circulation lifetime.34
Platelet Membrane
Platelets, as nuclear fragments derived from megakaryocytes, are recruited to areas of injury, inflammation, and wound healing, where they play essential roles in hemostasis and immune defense.35 Similar to RBC membrane, immunomodulatory proteins such as CD47, CD55 and CD59 were discovered on the surface of platelet membrane that prevent the macrophage adsorption and prolong the lifetime in bloodstream.19 In addition to extending blood circulation time, platelets actively target tumors based on the specific interactions between their surface proteins and tumor receptors, for example, P-selectin specifically binds to CD44 receptors.20 Furthermore, the hemostatic properties of platelets contribute to tumor metastasis by providing a protective shield of activated platelets and fibrin deposition that shields circulating tumor cells (CTCs) from immune system eradication.36 Therefore, platelets not only exhibit an affinity for primary tumor cells, but also demonstrate the capability to specifically capture CTCs.
Leveraging the adhesion of activated platelets to CTCs, the approach of utilizing platelet-mimetic nanoplatforms was investigated for CTC-targeted drug delivery systems. Li et al encapsulated biocompatible silica (Si) particles with activated platelet membrane to deliver the major tumor-killing cytokine, tumor necrosis factor-related apoptosis inducing ligand (TRAIL).11 Since the overexpression of TRAIL on the surface of cytotoxic T cells, cancer-killing natural killer cells and activated neutrophils, the platelet membrane-coating TRAIL combined with CTCs and activated the extrinsic apoptosis signaling pathway.37 Additionally, the platelet membrane-mimetic delivery system suppresses phagocytic uptake in comparison to uncoated nanoparticles, relying on the protection provided by differential IgG opsonization and CD47. The utilization of TRAIL coated with platelet membrane effectively circumvents adverse effects, eliminated CTCs, and suppressed tumor metastasis by enhancing the immune response.
Leukocyte Membrane
Given that a significant portion of tumors stem from chronic inflammation, there has been considerable focus on leukocytes. Leukocytes, such as macrophages, neutrophils, and natural killer (NK) cells, which are crucial in the transition from chronic inflammation to malignancy.38 During the progression of inflammation-induced cancers, tumor cells release a variety of chemokines and cytokines, which attract leukocytes to the affected areas and initiate a series of immune responses.39 Hence, the development of synthetic nanoparticles camouflaged with leukocyte membranes, which possess innate cell antigenic properties, presents significant potential in tumor-targeted delivery systems.
Macrophages, as the predominant among tumor-associated leukocytes, phagocytose and degrade “foreign substances”.40 Tumor-associated macrophages release cytokines to modulate the cancer development and metastasis.41 Moreover, MDR has been proven to be associated with the density of tumor-associated macrophages in mouse models.42 Considering the correlation between macrophages and cancers, the combination of nanoparticles and macrophage membrane has been the focus of leukocyte membrane-mimetic nanodelivery systems. The nanoparticles coated with macrophage membrane exhibit prolonged circulation time due to their resistance to phagocytosis. Furthermore, the specific receptors and functional molecules on the macrophage membrane confer the enclosed nanoparticles on the ability to traverse endothelial barriers, attributed to the interaction between ICAM-1 and macrophage adhesion ligand-1 (Mac-1; ITGAM).21 Macrophage membrane was initially used to cloak silicon nanoparticles, which displayed prolong circulation time and preferentially penetrated through inflammatory endothelium.22 The glycans (sialic acid and N-acetylglucosamine) present on the macrophage membrane serve to shield the nanoparticles from serum protein adhesion, thereby preventing subsequent clearance by the mononuclear phagocyte system. The protective layer endowed the nano-core with the capability to reduce surface adsorption of IgG and albumin, resulting in decreased clearance in human THP-1 phagocytic cells (~50% decrease) and murine J774 macrophages (~75% decrease). DOX loaded silicon nanoparticles wrapped by macrophage membrane demonstrate enhanced penetration of tumor endothelium and accumulation at tumor sites, leading to significantly improved therapeutic efficacies.
Neutrophils possess the ability to undergo deformable migration towards tumor-associated inflammatory sites, based on the adhesive molecules such as LFA-1 and VLA-4.23 Leveraging this tumor-homing characteristic, the neutrophil membrane has been utilized as a camouflage layer in delivery systems for targeted therapy. Activated neutrophils not only engulf tumor cells, but also facilitate tumor metastasis. Inflammatory neutrophils bind to CTCs and promote tumor extravasation through the interaction of functional molecules and the formation of neutrophil extracellular traps.43 Inspired by the CTC-targeting property of neutrophils, PLGA nanoparticles covered by neutrophil membrane were developed.24 Chen utilized a non-invasive method to transfer membrane-associated protein cocktails onto the surface nanoparticles in order to inherit the bio-binding capabilities of neutrophils. The encapsulation of the neutrophil membrane significantly increased the efficiency of capturing CTCs and enhanced tropism towards the pre-metastatic niche in vivo, attributed to three key interactions: LFA-1 binding with ICAM-1, CD44 binding with L-selectin, and β1 integrin binding with VCAM-1. After being loaded with carfilzomib, the neutrophil membrane-mimicking nanodelivery system has been demonstrated to effectively prevent early metastasis and potentially inhibit the growth of established metastases with minimal immune-related adverse effects.
Natural killer cells, as a significant constituent of the innate immune system, have the ability to defend against tumors through cytokine production. Unlike other lymphocytes, surface receptors guide NK cells aggregation to the tumor site in the absence of antigen pre-sensitization.44 NK cells tend to recognize cells with down-regulated major histocompatibility complex (MHC) class I, then, the inhibitory receptors such as Natural Killer Group 2 A (NKG2A) and killer cell immunoglobulin-like receptors (KIRs) would destroy MHC class negative tumor cells as foreign substances.25 Besides, tumor cells could be cytolyzed through the NK-mediated exocytosis pathway involving perforin (PFN) and granzyme B (GrB). Tumor necrosis factor (TNF) superfamily such as FASL (Fas cell surface death receptor ligand) and TRAIL induce tumor necrosis via binding FAS (or CD95) and TRAIL-R1/R2 (DR4/5) on tumor cells.26 The biocompatibility and tumor-targeting properties of NK cell membrane make it a desirable coating layer for delivery systems in tumor immunotherapy. Liposomal nanoparticles encapsulated by NK cell membrane have been developed for targeted tumor treatment under the immune surveillance of diseased/stress cells.27 The combinational delivery system of “NKsome” enhanced tumor-targeted retention relying on the NK surface proteins (CD56 bright receptors and the activated receptors like NKG2-D, NKp30, and NKp44 on). It was demonstrated that NKsome conferred on the nanoparticles a higher affinity towards tumors than normal cells in an in vitro flow-passage assay. Following doxorubicin loading, the NK membrane-mimetic nanodelivery system exhibited promising anti-tumor efficacy against MCF-7-induced tumor models in vivo.
Mesenchymal Stem Cell Membrane
Mesenchymal stem cells (MSCs) are a type of multipotent progenitor cells capable of self-renewal and differentiation into multiple cell types.45 MSCs consist mainly of bone marrow mesenchymal stem cells (BMSCs), adipose mesenchymal stem cells (AMSCs), and umbilical cord mesenchymal stem cells (UMSCs), which are found in various tissues including bone marrow, umbilical cord, adipose tissue, amniotic fluid, placenta, and others.46 MSCs not only possess the capacity for relatively easy isolation and expansion in vivo, but also have the tropism of migrating towards and engrafting into tumor sites, relying significantly on adhesion molecules such as b1- and b2-integrins and L-selectin.28 The homing of MSCs follows the four steps: (i) mobilization and migration of MSCs, (ii) binding and rolling of MSCs on vascular endothelium, (iii) adhesion of MSCs to vascular endothelium, (iv) passing through endothelial cells to target damaged tissues.47
Compared with the tumor-targeted delivery systems solely based on the ligand-receptor interaction, the tumor-homing mechanism of MSCs involves more factors, such as chemokine receptors and endothelial adhesion molecules, hence, MSC-mimetic nanoscale vectors exhibit higher tumor targeting efficiency.48 Inspired by the tumor-homing properties of MSCs, PLGA nanoparticles functionalized with MSC membranes were synthesized for tumor-targeted chemotherapy.29 Under the encapsulation of MSC membrane, PLGA nanoparticles efficiently migrated and passed through the tumor stroma, and are eventually internalized by tumor cells. When loaded with doxorubicin, the MSC membrane-mimetic nanodelivery system displayed remarkable tumor suppression and obvious cellular apoptosis with minimal side effects.
Cancer Cell Membrane
Taking advantage of unlimited proliferation, immune evasion and homologous targeting, cancer cell membrane (CCM) was confirmed as the excellent coating layer to wrap nanoparticles in oncological applications.49 Unlike circulating or immune cells isolated from patient autologous plasma, cancer cells are robust and easy to massively culture in vitro for membrane collection on account of the unrestrictive proliferative capacity.50 The aggregation of homotypic cancer cells has been reported to promote the development of distant organ lesions during metastasis, and this homotypic affinity is mediated by the interaction between galectin-3 on the surface of cancer cells and carcinoembryonic antigen.30 Therefore, CCM with the property of homotypic targeting confers the encapsulated nanoparticles on the capability to specifically target both primary tumors and metastatic lesions.51
The CCM characteristics of homologous adhesion and immune escape have sparked extensive research on CCM membrane-mimetic nanovectors. DOX has been extensively utilized in clinical settings for the treatment of numerous cancers, including breast cancer, ovarian cancer, leukemias, and various lymphomas. It functions by intercalating into DNA to induce topoisomerase II-mediated DNA damage, ultimately leading to cell death.52 Several studies have shown that DOX functionalized by the shell of CCMs exhibits improved therapeutic efficacy and reduced side effects compared to unmodified DOX. The nanocarrier of PLGA-DOX cloaked with HepG2 cell membrane (HepM) was developed for targeted chemotherapy of hepatocellular carcinoma.31 The packing layer of carcinoma cell membrane enhanced the immune compatibility of the nanocarrier by inhibiting uptake by murine macrophage cells. The results of Western blot analysis revealed the enrichment of galectin-1, galectin-3, and CD47 on the surface of HepM-PLGA, which facilitate homotypic targeting. The HepM coated delivery system effectively transported chemotherapy ingredients to tumor lesions in nude mouse models and reduced tumor volume by approximately 90% due to its inheritance of homologous aggregation of HepG2 cells. This HepM-mimicking delivery system demonstrated effective anticancer therapeutics with minimal toxicities, attributed to its immune compatibility and homologous aggregation.
Coating Technology
Currently, the laboratory has mainly employed two methods to synthesize cell membrane-mimetic nanocarriers, physical co-extrusion and ultrasonication. As the common nanotechnology, physical co-extrusion is conducted by repeatedly extruding isolated cell membranes and synthetic nano-cores together back and forth. During the progress, cell membrane is temporarily disrupted into nanofragments, which could surround the nanosubstrates in a stable core-shell structure, generating cell membrane-mimetic nanovehicles that largely match with surface proteins found on the source cells.19 It’s reported that the coated membrane typically possess a right-side-out orientation, which might be facilitated through the encapsulated nanocore and its interaction with the inherently asymmetric charge profile between the inner and outer membrane leaflets, guaranteeing that the surface protein markers retain biological functions.53 However, issues related to low yield and tedious processes limit the applicability of co-extrusion. Similar to co-extrusion, ultrasonication, as an alternative approach, utilizes ultrasonic energy to disrupt the cell membrane, and the fragments randomly encapsulate nanocores.33 In comparison with co-extrusion, this method is simple to operate, demands less equipment, and consumes fewer materials, while the force produced by ultrasonication is greater than that generated from co-extrusion, resulting in structural damage to self-assembled nanoparticles or other nanovectors with lower mechanical strength. As a result, ultrasonication is more suitable for nanoparticles characterized by high mechanical strength, such as PLGA nanoparticles or metal nanoparticles. Conversely, the co-extrusion method is employed for the samples unable to tolerate excessive disruption.
Biological Barriers Encountered by Nanodelivery Systems
Despite decades of development in cell membrane-based nanocarriers, current delivery systems fail to effectively cluster active ingredients at tumor sites, and only a limited number of cell membrane-mimetic delivery systems have been successfully applied in clinical settings. Nanovectors encapsulated by cell membranes exhibit prolonged half-time, biocompatibility and partial tumor targetability, while it’s almost impossible to overcome the complicated series of biological barriers from bloodstream to tumor cells based only on the biological functionalities of cell membrane-coated layers. Unless cell membrane-based delivery systems address multi-level biological barriers, the transition from test bench to clinical arena could not be achieved. Upon intravenous administration, nanoparticles are initially opsonized by plasma proteins and taken up by MPS, leading to non-specific distribution to normal organs such as the liver. Subsequently, high intratumoral fluid pressure significantly impedes the extravasation of nanoparticles into the tumor interior, and nanoparticles internalized by clathrin-mediated endocytosis undergo lysosomal degradation. Finally, upon entering the cell, drug efflux pumps that provide resistance to therapy actively remove chemotherapeutic agents from the cell. In the following section, we elaborate on the biological barriers that need to be addressed in designing advanced drug delivery systems. The framework of biological barriers encountered by nanovectors during intravenous delivery is displayed in Figure 2.
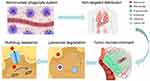 |
Figure 2 Biological barriers encountered by nanovectors during intravenous delivery. Abbreviation: IFP, interstitial fluid pressure. |
Blood Barriers
Blood serves as the first barrier for nanodelivery systems administered by intravenous injection. The development of a protein corona around circulating nanoparticles occurs through the absorption of plasma proteins, including serum albumin, apolipoproteins, complement components, and immunoglobulins.54 Besides, the formation of protein corona depends on the physicochemical properties of nanoparticles, including surface charge, shape, size, and hydrophobicity.55 The protein corona on the surface of nanoparticles facilitates their recognition and binding to specific receptors on phagocytic cells, followed by internalization, transportation to phagosomes, and fusion with lysosomes.56 Therefore, the formation of protein corona reduces the circulation and time-dependent EPR effect of nanovectors. The protein corona not only weakens passive targeting, but also diminishes the active-targeting characteristic of nanovectors by covering targeting ligands with absorbed proteins, resulting in reduced targeting efficiency. The investigation revealed that the protein corona effectively prevented transferrin-combined nanoparticles from binding to their targeted receptors on A549 cells, as well as soluble transferrin receptors, resulting in a loss of targeting specificity.57 Whether delivery systems are based on passive or active targeting properties, the process of protein opsonization in the bloodstream significantly impedes the accumulation of active ingredients at specific sites. This necessitates further investigation into methods for suppressing protein aggregation on the nanovector surface.
Tumor Barriers
The widespread application of nanoparticles is impetus by EPR effect at tumor sites, which is ascribed to the presence of endothelial dysfunction and blood vessel fenestration. According to published data, the nanodelivery efficiency only retains 0.76% of the injected dose (%ID), as analyzed physiologically using pharmacokinetic (PBPK) models.58 Nanosystems relying on passive targeting are unable to achieve satisfactory delivery efficiency, since the unique tumor microenvironment impedes the site-specific accumulation of nanoparticles. The interstitial fluid pressure (IFP) in tumor is a crucial obstacle to hinder the nanoparticle penetration into tumor interiors. The precise mechanism behind the increased IFP has not been clearly demonstrated, but inadequate blood and lymphatic drainage, along with a dense extracellular matrix (ECM), are crucial factors in the elevation of interstitial fluid pressure.59
Disrupted Vasculature
Due to the infinite replicative property of cancer cells, tumor growth attracts blood vessels through PDGF, TGF-β and angiogenic factors released by the infiltrated macrophages and other immune cells. The abnormal secretion of cytokines breaks the balance of normal angiogenetic differentiations, resulting in disrupted vasculature characterized by irregular, saccular and convoluted vessels unable to provide sufficient blood flow. Furthermore, the secretion of vascular endothelial cell growth factor (VEGF) by tumor cells leads to an elevation in vascular permeability, contributing to increased macromolecule leakage from tumor vessels and subsequently raising interstitial colloid osmotic pressure.60 In addition, the lymphatic vessels compressed by growing cancer cells are shown low drainage of fluid and proteins from the tumor interstitium, resulting in a concomitant IFP rise.61
Dense Extracellular Matrix
ECM as the major component in the tumor microenvironment comprises around 300 proteins, such as collagens and glycoproteins, which is modulated by enzyme degradation to retain tumor homeostasis. Meanwhile, the dysregulated ECM components undergo cross-linking to form a dense framework to support tumor proliferation and metastasis, while the dense network plays a critical role in resistance to anti-cancer therapies via dysfunctioning the tumor vascular.62 Various collagen fibrils with 20–42 nm or 75–130 nm interfibrillar space are composed of collagens, as the basic structure of ECM, they hamper the extravasation of macromolecules to distant regions in tumors.63 Besides, the ECM viscosity was increased by glycoproteins consisting of proteins cores linked with carbohydrate chains, resulting in high interstitial pressure and the reduced transportation efficiency of nanovectors in ECM.64 Furthermore, the elevated interstitial fluid pressure is linked to the contractile characteristics of tumor stroma resulting from fibroblast differentiation into smooth muscle cells.65 In normal conditions, loose connective tissues alleviate the IFP increase via expanding the volume of tissues, such as oedema. The volume of tumor is constrained by a denser tumour stroma composed of connective-tissue molecules, resulting in the persistent IFP elevation. Therefore, the dense ECM severely impedes the diffusion of therapeutic agents in solid tumors via increasing interstitial transport resistance and IFP elevation.
Cell Barriers
Although nanodelivery systems can penetrate and disperse within tumors by overcoming tissue barriers, it is crucial for them to be internalized by tumor cells and to release active ingredients to selectively target organelles. However, the process of lysosomal degradation and efflux caused by MDR severely impedes the drugs from exerting their cytotoxic effects on specific sites.
Lysosomal Degradation
It is commonly understood, small hydrophobic molecules can passively diffuse through cell membranes, whereas nanoparticles require active uptake by cells. The classic uptake mechanism for nanoparticles is clathrin-mediated endocytosis, which includes membrane invagination, intracellular vesicle formation, and fusion with lysosomes.66 The uptaken nanoparticles would be degraded by various enzymes in lysosomes and eliminated through cellular efflux.67 Internalization of nanoplatforms via the clathrin-pathway leads to a decrease in intracellular concentration of effective drugs due to lysosomal degradation, posing a challenge for improving delivery efficiency.
MDR
In order to maintain cell homeostasis and prevent the damage from chemotherapeutic agents, carcinoma cells develop resistance to intracellular drugs, leading to reduced therapeutic efficiencies. After long-term exposure to a chemotherapeutic drug, tumor cells not only resist the agent, but also exhibit cross-resistance toward other compounds with different structures and functions, which is known as MDR. Despite the fact that the mechanism of MDR is intricate, involving the activated system of detoxification and dysfunction of apoptosis, the elevated effluxion of intracellular drugs mediated by ATP-binding cassette (ABC) transporters is the primary factor causing MDR.68 Besides, MDR in cancers is associated with the overexpression of ABC transporters, such as P-glycoprotein (P-gp).69 P-gp with the property of substrate promiscuity is capable of effluxing various anticancer compounds, such as taxanes, anthracyclines and vinca alkaloids, leading to reduced intracellular dosage and diminished therapeutic effects.70 Consequently, the need for continuously increasing administration dosages can result in severe patient morbidity, ultimately leading to non-responsive recurrence and failure of chemotherapeutic regimens.
Strategies Aimed at Achieving Precise Delivery
Considerable research efforts have focused on integrating innovative functionalities and moieties into biomimetic nanocarriers constructs to effectively overcome biological barriers, leading to the development of multifunctional nanovectors. While these modifications displayed the remarkable versatility and preclinical potential of biomimetic nanocarriers, few biomimetic nanocarriers that simply address one or a few biological barriers have progressed to clinical trials. Therefore, in order to facilitate the transition from laboratory research to clinical application, cell membrane-mimetic nanovectors must effectively overcome a majority, if not all, of the biological barriers. In the following section, we systematically delineate various cell membrane-based strategies aimed at overcoming multi-level biological barriers, as depicted in Figure 3. Furthermore, the cell membrane-mimetic nanocarriers discussed for enhancing delivery efficiency are summarized in Table 2.
 |
Table 2 Cell Membrane-Mimetic Nanocarriers Discussed for Enhancing Delivery Efficiency |
Extension Blood Half-Time and Augment Tumor Targeting
The initial approach to prevent protein adsorption and extend circulation time involved the use of PEG-functionalized nanoparticles. Numerous studies have confirmed that PEG, as an amphiphilic polymer, interacts with water molecules on the nanoparticle surface, forming a protective hydrated shell that effectively inhibits protein aggregation around the nanoparticles.89 Representatively, PEGylated liposomes exhibited an 8-fold increase in circulation time than bare liposomes, as the hydrating shell effectively shielded liposomes from protein destruction.90 Despite the widespread use of PEGylation to improve drug delivery efficiency, the gradual revelation of drawbacks such as the “accelerated blood clearance (ABC) phenomenon upon reuse of PEGylated nanoparticles is becoming apparent”.91 For the sake of avoiding immune responses caused by foreign substances, protein CD47, serving as a “marker of self” was grafted to the surface of nanoparticles to delay phagocytic recognition and clearance.92 Synthetic peptides of CD47 linked with nanobeads exhibited longer circulation and more accumulation at the A549 tumor site, compared to conventional formulations, in the model of immunodeficient IL2rγ null mice in combination with non-obese diabetic (NOD) mice.
Inspired by the inhibition of macrophage clearance through the combination between protein CD47 and signal-regulatory protein α, nanocarriers cloaked by cell membranes carrying protein CD47, such as erythrocyte membrane and platelet membrane, have been widely applied in extension blood circulation.17 The top-down cloaking approach was first employed by polymeric nanoparticles camouflaged with erythrocyte membrane, wherein the coating membrane bestowed the nanodelivery system with the ability to bypass macrophage clearance and extended the circulation time for 72 h.9 As aforementioned, the coating layers of cell membranes endow the wrapped nano-core with biofunctions of long blood circulation, immune escape, and tumor-homing. However, owing to RBC membranes lacking the capacity of tumor-homing, tumor-targeting ligands were inserted on the surface of RBC membrane-mimetic nanoparticles to achieve receptor-specific targeting, which provoked the exploration of hybrid cell membrane.93 HCM exhibits synergistic effects in extension of circulation time and augmentation of tumor targeting. In the following part, we introduce the HCM application in nanodelivery systems.
RBC-Platelet HCM
The RBC-Platelet fusing membrane not only combines the advantages of RBC and platelet, including escape immune recognition and target inflammatory regions, but also exhibits the longer blood half-time than monotypic membrane of RBC membrane or platelet membrane. Diana and coworkers synthesised PLGA nanoparticles coated by different membranes and indicated that the blood half-time of nanocarriers encapsulated by the fusing membrane extended to 51.8 h; however, nanoparticles wrapped by RBC membrane circulated in the bloodstream for 42.4 h and platelet membrane coating nanoparticles only lasted for 38.3 h.94 Driven by inflammatory factors and microthrombosis in the tumor area, nano-polypyrrole wrapped by RBC-Platelet hybrid membrane possessed a better tumor-homing characteristic, which significantly improved therapeutic outcomes compared with bare nano-polypyrrole or monotypic membrane coating nanoparticles.71 The strategy of RBC-Platelet membrane coating layer opened a promising avenue for the development of multi-functional drug delivery systems in cancer treatment.
RBC-Cancer Cell HCM
Based on the tumor-homing characteristic, cancer cell membrane has been widely applied in the cell membrane-based nanodelivery system.95 However, the limitations of CCM are becoming increasingly apparent, such as its relatively short circulation time due to the inability to maintain membrane integrity during the preparation process and completely evade immune surveillance.96 Therefore, the hybrid membrane composed of erythrocytes and cancer cells could achieve long-term circulation via the protective protein of CD47 on RBC surface, which has been demonstrated as an excellent coating layer in cell membrane-mimetic nanodelivery systems.72
The fusing membrane strategy typically combines homogeneous cancer cell membrane to realize homotypic targeting in personalized therapeutics. Dox-loaded CuS nanoparticles cloaked by the bio-fusing membrane of red blood cells and melanoma cells (B16-F10 cells) were fabricated to treat melanoma.72 The DCuS@[RBC-B16] nanoplatform exhibited a longer blood half-time compared to bare CuS nanovectors (20.0% ID/g versus 14.5% ID/g in the blood retention of mouse models after 24-hour injection) and possessed stronger homotypic targeting capabilities in contrast to uncoated CuS nanoparticles or monotypic membrane coating formulations, achieving a 100% inhibitory effect on melanoma tumor growth. Recently, Rezaei and coworkers decorated DOX-loaded reduction-sensitive nano-chitosan with the hybrid membrane of red blood cell and 4T1 cancer cell to transport more DOX to tumor lesions via avoiding immune clearance and depending on self-recognition among homogenous cells.73 Moreover, it was demonstrated that the homotypic targeting property could be optimized by adjusting the ratio of RBC membrane and 4T1 cancer cell membrane.
Leukocyte-Cancer Cell HCM
The interaction with leukocyte membrane also helps to overcome the short blood circulation issue caused by the inability to maintain the integrity of cancer cell membrane, which is attributed to the immune-escape property of leukocytes. Furthermore, nanodelivery systems encapsulated by leukocyte-cancer cell HCM exhibit high inhibitory rate of tumor growth based on the synergistic effect on tumor targeting. Yang and coworkers designed a novel cell membrane-mimetic nanocarrier, leutusome, composed of PTX-loaded liposomal nanoparticles and leukocyte-cancer cell HCM inheriting the leukocyte-specific immune escape and tumor-specific homotypic targeting properties, which significantly extended the plasma half-time from 4.0 h to 8.1 h and enhanced the tumor-accumulating efficiency of encapsulated nano-cores to 80% (Figure 4).74 The elevated tumor-targeting performance effectively suppresses tumor growth without producing systemic adverse effects, suggesting leukocyte-cancer cell HCM could be used as a candidate packing biomaterial for personalized cancer treatment. Leukocyte-cancer cell HCM has also been applied in cancer detection relying on the property of homotypic tumor-targeting and the capability of capturing CTCs. The streptavidin-nanoplatform functionalized by leukocyte-cancer cell HCM exhibited high sensitive identification and effective capture of CTCs, and the capture efficiency reached 97.63% playing a critical role in monitoring tumor metastasis.97
 |
Figure 4 Schematic diagram and advantageous study of leutusome integrating plasma membrane components of leukocytes and tumor cells in enhanced solid tumor homing. (A) Schematic presentation of composite LTM-PTXL. (B) TEM images of PTX-loaded liposomal nanoparticles. (C) In vitro cytotoxicity of PTX-loaded liposomal nanoparticles at various concentrations on HN12 cells after 24 h incubation. **indicates p<0.01 (n=6). (D) Leukocyte membrane helps reduce the uptake of nanoparticles by monocytes and neutrophils in the blood. * Indicates p<0.05 and **indicates p<0.01 (n=4). (E) Relative tumor volume growth was monitored over the treatments. (F) Tumor tissue apoptosis was recorded after the various treatments. NS, not significant; **indicates p<0.01, and ***indicates p<0.001 (n=6). Adapted from He H, Guo C, Wang J et al. Leutusome: a biomimetic nanoplatform integrating plasma membrane components of leukocytes and tumor cells for remarkably enhanced solid tumor homing. Nano Lett. 2018;18(10):6164–6174. Copyright 2018 American Chemical Society.74 Abbreviations: PTX, paclitaxel; LM-PTXL, leukocyte membrane-camouflaged PTX-loaded liposome; TM-PTXL, tumor cell membrane-camouflaged PTX-loaded liposome; LTM-PTXL, hybrid membrane of leukocyte and tumor cell-camouflaged PTX-loaded liposome. |
In summary, the HCM strategy is capable of alleviating the limitations of monotype camouflage, such as the weak tumor-targeting of red cell membrane and the short blood circulation of tumor cell membrane, which facilitates explore into integrating multifunctional cell membrane-mimetic coating layers. Furthermore, the procedure of fusing two cell membranes is straightforward and does not necessitate any chemical modification in contrast to traditional engineered technologies, enabling efficient large-scale production.98 Hybrid cell membranes are obtained by either fusing two cells for membrane extraction or extracting the membrane from each cell and then fusing them.96 The initial method involves the induction of a fused cell through stimulation with polyethylene glycol or electrofusion, followed by extraction of the hybrid cytomembrane using conventional methods. Another approach of cell membrane fusion is to extract each kind of cell membrane, and then to stir the mixture of two membranes at 37 °C or ice bath for 10 min. Moreover, hybrid membrane can also be obtained by sonication of the membrane mixture. These preparation processes are highly applicable, not only for the fusion between eukaryotic cell membranes but also for the hybridization of eukaryotic cell membranes with prokaryotic cell membranes. In addition to the straightforward fabrication process of cell membrane-mimetic nanoparticles, the biomimetic nanovectors offer numerous unique advantages compared to conventional artificial materials, such as low immunogenicity, specific targeting and an abundant functional protein library. In general, decorating nanoplatforms with HCM has been considered as an effective strategy to design multi-functional cell membrane-mimetic nanocarriers for cancer treatment and diagnosis.
Overcoming Tumor Barriers
The disordered vasculature and dense extracellular matrix indeed prevent nanoparticles from penetrating to the interior of solid tumors and reduce the delivery efficiency of chemotherapeutics, which prompts researchers to explore the valid solutions of enhancing transvascular transport of therapeutics in tumors. In order to allow nanoparticles to penetrate to the center of tumors rather than merely accumulate around the tumor periphery, the mitigation of disordered vasculature and dense extracellular matrix is highly desired. In addition to normalizing the tumor microenvironment, modifying the nanoparticles with penetrating peptides has been applied in enhancing the tumor permeability of nanodelivery systems.
Remodeling the Disordered Vasculature
The high IFP caused by the disordered vasculature as a pivotal factor hinders nanoparticles penetrating the solid tumors. The most direct avenue to reduce IFP is to normalize the disordered vasculature.99 Moreover, vascular disruption is capable of tumor lethality via dramatically reducing the blood flow to the center of tumors.100
Vascular normalization has been attempted to alleviate the resistance of nanovectors to penetrating from vasculature to the interior of tumors. The application of antiangiogenic agents can adjust the imbalanced phenomenon during the formation of new vessels to normalize the architecture of the tumor-associated vascular network.101,102 Inhibiting the VEGF secreted by tumor cells can effectively improve the drainage of blood vessels. Moreover, the treatment with exogenous VEGF expanded the diameter of maximum pore and enhanced the transvascular delivery of larger molecules (size range, from 100 nm~300 nm) to internal regions of tumors.103 Currently, dozens of VEGF inhibitors, such as bevacizumab, cinnabar, and apatinib, have entered the clinical stage, which obviously suppress the tumor growth co-administrated with anti-tumor agents via completely combining with VEGFR-2ATP binding sites. Recently, Chen et al designed a cell membrane-mimetic multifunctional nanoplatform-ZrO2@CuO/Cu-MOF-Apatinib-PCM coated by RBC membrane, based on the production of reactive oxygen species (ROS) by nano-Cu under external microwave stimulations.75 The nanodelivery system leveraged RBC membrane to avoid immune clearance and achieved prolonged circulation, resulting in preferentially accumulate at tumor sites. Besides, loaded apatinib as the inhibitor of VEGF can be transported to the tumor microenvironment and significantly increased the drainage of tumor vasculature, which promote Cu nanoparticles penetrate to the deep regions of solid tumors. Thus, the cell membrane-based strategy of codelivery with antiangiogenic drugs encapsulated by cell membranes significantly suppressed the tumor growth and the tumor inhibition rate reached 96.79%, exhibiting as an effective avenue to enhance the tumor permeability of nanovectors.
The strategy of tumor necrosis based on vascular disruption and decreased blood supply has been confirmed by several vascular disrupting agents (VDA) such as combretastatin A4 phosphate (CA4P) and 5.6-dimethylxanthenone-4-acetic acid (DMXAA). Excitingly, it was demonstrated that CA4P and DMXAA possessed the character of tumor-targeting and displayed minimal effects on the vasculature of normal tissues via Phase I and II clinical trials.104 CA4 released from CA4P combined with tubulin, suppressed tubulin polymerization and destabilized the cytoskeleton resulting in changing the endothelial cell morphology, which subsequently induced narrowing vessel lumen, increasing convective resistance and dramatically reducing the blood supply for tumor growth.105 Chen et al developed the matrix metalloproteinase 9 (MMP9)-activated doxorubicin prodrug (MMP9-DOX-NPs), conjugated with CA4, to augment the anti-tumor therapeutic effect and reduce systemic toxicity. The expression of MMP9 was increased by 5.6-fold under the condition of CA4-mediated exacerbated hypoxia, which enhanced the tumor-targeting release of DOX.106 After treatment with VDA, platelets are recruited to the regions of vascular disruption to promote coagulation.107 Inspired by the biological functions of platelets, tirapazamine, as a typically hypoxia-activated prodrug and DMXAA co-loaded mesoporous silica nanoparticles (MSNs) encapsulated by platelet membrane was designed to produce cascade amplification of hypoxia-activated treatment.76 The platelet shell not only protected the nanodelivery system from immune recognition, but also enhanced the tumor-targeting property. DMXAA can selectively destroy the tumor vasculature through inducing apoptosis of vascular endothelial cells aggravating the hypoxia microenvironment, which facilitate the transformation of tirapazamine into radical formation by the reduction of single electron. Considering platelet-mimicking biotaxis and cascade hypoxia amplification, the cell membrane-mimetic hypoxia-sensitive nanovectors loaded with VDA are deemed as the paradigm of effectively inhibiting tumor growth.
Remodeling the Dense Extracellular Matrix
Dense extracellular matrix increases IFP and solid stress, which indeed impede anti-tumor nanocarriers penetration into solid tumors resulting in poor prognosis. Cancer-associated fibroblasts (CAFs), as critical mediators of extracellular matrix, play a vital role in the restricting the infiltration of chemotherapeutics into tumors. Anti-fibrotic drugs, such as losartan and quercetin, are capable of weakening the CAF activity and inducing CAFs damage responses.108,109 The investigation by Ben et al found that losartan, as an angiotensin II receptor antagonist, prevented tumor-related fibroblasts from producing collagen I and also reduced the stromal collagen density in tumors, thereby improving the delivery efficiency and intratumor distribution of PEG-liposome-Doxil.110
In addition to inhibiting the activity of CAFs, the depletion of existing ECM by ECM-degrading enzymes is an effective approach to improve the tumor-penetration efficiency. The major components of ECM, such as elastin and type 1 collagen, are the substrates of neutrophil elastase (NE).111 Li and coworkers firstly synthesized the biomimetic liposomes linked with NE extracted from tumor cell membranes, which selectively degraded tumor ECM and displayed favorable biocompatibility.112 The biomimetic liposomes with chimeric cell membrane proteins (LMP) combined with chemotherapeutics dramatically improved the penetrated efficiency of anti-tumor drugs and suppressed the tumor growth. Besides, employing NE-LMP with the treatment of PD-1 checkpoint blockade allowed massive cytotoxic T lymphocytes (CTLs) accumulate in tumor centers and efficiently kill tumor cells. Therefore, the chemo-immunotherapy based on NE-LMP possessed the potential to improve the survival of the suffering triple-negative breast tumor via depleting the ECM. Besides, collagenase as an effective ECM-degrading enzyme, can destroy the dense extracellular matrix to promote the infiltration of anti-tumor nanodelivery systems. To avoid damaging the normal tissues, Yang et al developed the cell membrane-based DOX loaded Au nanoparticles encapsulated by PDAC tumor cell membrane conjugated with collagenases (Figure 5).77 The coating layer of tumor cell membrane bestowed the delivery system with homologous tumor-targeting and immune escape. Moreover, collagenase was easily attached to the encapsulating membrane through lipid insertion, which enhanced the permeation of DOX loaded Au nanoparticles to deep lesions of tumor tissues. Under the near-infrared (NIR) irradiation at 808 nm, Au nanoparticles were excited and transferred the photon energy to molecular oxygen to produce cytotoxic reactive oxygen species (ROS) for photodynamic therapy (PDT). The cell membrane-mimetic combination treatment of chemo-PDT linked with collagenases exerted a significant role in addressing tumor barriers, which provided a new perspective for enhancing anti-tumor efficacy.
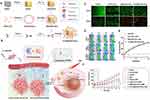 |
Figure 5 Schematic diagram and advantageous study of Col-M@AuNCs/Dox for enhancing tumor penetration and synergistic therapy in pancreatic cancer. (A) Preparation process of Col-M@AuNCs/Dox. (B) Schematic representation of Col-M@AuNCs/Dox for improved intratumoral penetration, complete tumor destruction, and combined treatment and monitoring through ECM degradation. (C) Live/dead staining images of BxPC3 cells after treatment with the different preparations. Live cells were stained with calcein-AM (green), and dead cells were stained with propidium iodide (red.) (scale bar: 100 µm.) (D) In vivo fluorescence images of BxPC3 tumor-bearing, mice after intravenous injection of Cy5.5-labeled AuNCs/Dox, M@AuNCs/Dox and Col-M@AuNCs/Dox. (E) Temperature curves under NIR irradiation. (F) Tumor growth curves during different treatments (n=6) in BxPC3 tumor-bearing mice. Adapted from Yang XY, Zhang JG, Zhou QM et al. Extracellular matrix modulating enzyme functionalized biomimetic Au nanoplatform-mediated enhanced tumor penetration and synergistic antitumor therapy for pancreatic cancer. J Nanobiotechnology. 2022;20(1):524. Creative Commons. http://creativecommons.org/licenses/by/4.0/.77 Abbreviations: AgNCs, Ag nanocages; Dox, doxorubicin; PDAC, pancreatic ductal adenocarcinoma; Col-M@AuNCs/Dox, collagenase-functionalized PDAC tumor cell membrane-coated Dox-loaded Au nanocages; ECM, excessive extracellular matrix; NIR, near-infrared; PTT, photothermal therapy; PDT, photodynamic therapy; ROS, reactive oxygen species. |
Decorating the Surface of Nanodelivery Systems With Tumor-Penetrating Peptides (TPPs)
Apart from remodeling the components and architecture of tumor microenvironment, nanocarriers decorated with TPPs are capable of enhancing the tumor-penetrating efficiency of therapeutic agents. TPPs, such as iRGD, cyclic Lyp-1, and linear Lin TT1, contain similar motifs that respond to R/KXXR/K stimuli proteases on the cell surface, bind with neuropilin-1 receptors and activate the trans-tissue channel to promote the infiltration of nanovectors through the interstitial matrix.113 Hypoxia-activated prodrug tirapazamine (TPZp) and photosensitizer chlorine6 (Ce6) co-loaded nanocarriers camouflaged by RBC membrane and iRGD peptide were developed to facilitate TPZp and Ce6 across solid tumors and overcome hypoxia-mediated metastasis and resistance.78 The protective shell of RBC membrane endowed the cell membrane-mimetic nanoplatform escaped immune clearance and exhibited long circulation time. iRGD as a tumor-penetrating peptide, selectively binds to αV integrins on tumor endothelium and neuropilin-1 receptors, leading to enhanced infiltration into the center of tumors.114 Due to the production of Ce6-mediated ROS for PDT, the hypoxia condition inside the tumor was exacerbated under light irradiation, which triggered the transformation of hypoxia-activated prodrug-TPZp to cytotoxic TPZ, resulting in a synergistic anti-tumor effect of PDT and chemotherapy. Similarly, H Guo et al constructed glucose oxidase (GOX) and TPZ co-loaded into photothermal conversion agent polydopamine (PDA) nanoreactors encapsulated by tumor cell membrane modified with cRGD peptides to dramatically maximum the anti-tumor therapeutic outcoming relying on the combination of starvation therapy and hypoxia-activated chemotherapy.79 Therefore, cell membranes linked with TPPs render the wrapped core of nanovectors with the ability of avoiding immune elimination as well as improving the tumor-penetrating efficiency, which promote massive anti-tumor active ingredients overcome the tissue barriers, and accumulate inside the solid tumors.
Overcoming Cell Barriers
Clathrin-mediated endocytosis, as the classic internalization pathway, is responsible for transportation of extracellular substances, including antigens, receptors, pathogens, growth factors, nutrients as well as anti-tumor nanodelivery systems.66 Once nanovectors are internalized by clathrin-mediated endocytosis, therapeutic agents are typically enzymatically decomposed via the fusion of late endosomes with lysosomes to lose the anti-tumor curative effects. Moreover, chemotherapy, as one of the most effective approaches to treat cancers, could induce tumor cells to develop resistance against chemotherapeutic drugs via the effluxion mediated by P-gp, ultimately resulting in suboptimal treatment outcomes and undesirable side effects. Therefore, nanovehicles functionalized with the function of the ability to enable endosomal escape and inhibit efflux are urgently needed to improve therapeutic outcomes.
Endosomal or Lysosomal Escape
Considering the outer negatively charged endosomal membrane, decoration nanocarriers with cationic polymers is regarded as an effective and convenient strategy to achieve endosomal escape based on membrane flipping and instability. Representatively, cationic polymers, such as poly(l-lysine) (PLL) and poly(ethylene imine) (PEI), are capable of pairing with anionic groups on the surface of endosomes and destabilize the membrane, leading to effective release of therapeutic ingredients from endosomes.115 Furthermore, the incorporation of secondary or tertiary amine groups, such as PEI and histidine, in nanocarrier design possesses the ability to cause the swelling from the influx of water into compartments and eventually rupture, relying on the protonatable property of amine groups.116 Zhu and coworkers developed a 5-fluorouracil loaded nanogels decorated by PEI shell to achieve caspase-dependent apoptosis via lysosomal/mitochondrial pathway.117 After internalization by lysosomes, the protonated PEI promoted release of 5-fluorouracil from nanogels to inhibit the synthesis of nucleic acid and destroyed the lysosomal membrane to discharge Cat B, which could be translocated in mitochondria increasing the permeability of mitochondrial membrane. Subsequently, Cyt C was released from mitochondria and activated caspase-9 inducing a series of apoptotic cascade responses. Similarly, the mechanism of “proton sponge effect” has also been applied in cell membrane-mimetic nanodelivery systems to avoid lysosomal degradation. Small interfering RNA (siRNA) loaded metal-organic framework (MOF) nanocarriers coated by platelet membrane was designed to silence tumor-associated genes.80 The protective layer of platelet membrane endowed the nanoplatforms with the capabilities of immune escape and tumor-targeting. MOF nanoparticles prepared with zinc nitrate hexahydrate and 2-methylimidazole dissociated within the acidic endosomal compartment, which promoted endosomal disruption and siRNA release into the cytosol resulting in knocking down targeting genes. The experimental results demonstrated that the formulation of siRNA loaded MOF nanoparticles encapsulated by cell membrane could greatly optimize the treatment of nucleic acid-induced diseases paving a new avenue for gene therapy.
In addition, the application of membrane-destabilizing peptides in nanocarriers has been deemed as an effective strategy to break endosomal membrane and protect anti-tumor components from enzymatic degradation. Endosomal or lysosomal membrane disruption can be induced by membrane lytic peptides rich in histidine based on protonated His-residues within late endosomes or lysosomes.118 Moreover, the appearance of hemagglutinin (HA) on influenza virus surface assists the genetic materials to escape endosomes and avoid lysosomal degradation.119 Mature HA possesses two subunits, the HA1 subunit facilitates virus adhere to the plasma membrane of target cells to promote endocytosis, while the conformation of HA2 subunit changes, triggered by acidic conditions to facilitate the membrane fusion of virus and endosomes.120 Inspired by the principle of virus escape from endosomes, mRNA loaded nanoparticles encapsulated by cell membrane engineered with HA were synthesized to achieve virus-mimetic endosomal escape and improve the cytosolic delivery efficiency (Figure 6).81 The investigation demonstrated that the combination of mRNA-nanoparticles coated by HA engineered-cell membrane not only represented a compelling paradigm for delivering nucleic acids to cytosolic, but also improved the practicality of cell membrane encapsulated nanoparticles.
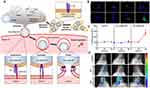 |
Figure 6 Schematic diagram and advantageous study of virus-mimicking cell membrane-coated nanoparticles in cytosolic delivery of mRNA. (A) Illustration of genetically engineered cell membrane-coated nanoparticles for the cytosolic delivery of mRNA. (B) Endosomal escape was observed through fluorescent visualization of B16-WT cells following incubation with WT-DiO-NP and HA-DiO-NP for 1, 4, 8, and 24 hours. Nuclei (blue), endosomes (red), nanoparticles (green); scale bar=20 mm. (C) mRNA transfection was conducted via bioluminescence monitoring over time in the serum of mice intravenously administered with WT-mRNA-NP and HA-mRNA-NP loaded with CLuc mRNA (n=5; mean SD). *p<0.05, **p<0.01 (compared to 0 h); Student’s t-test. (D) Visualization of bioluminescent signal from mice intranasally administered with WT-mRNA-NP and HA-mRNA-NP loaded with CLuc mRNA; high signal (H), low signal (L). Adapted from Park JH, Mohapatra A, Zhou J, et al. Virus-mimicking cell membrane-coated nanoparticles for cytosolic delivery of mRNA. Angew Chem Int Ed Engl. 2022;61(2):e202113671.© 2021 Wiley-VCH GmbH.81 Abbreviations: HA, hemagglutinin; DiO, 3-octadecyl-2-[3-(3-octadecyl-2-(3H)-benzoxazolylidene)-1-propenyl]-, perchlorate; WT-DiO-NP, B16-WT membrane-coated DiO-loaded PLGA cores; HA-DiO-NP, B16-HA membrane-coated DiO-loaded PLGA cores. |
Optimizing the Endocytosis Pathway
Due to nanoparticles mediated by clathrin undergo enzymatic degradation, another valid strategy to avoid lysosomal catabolism is to enable endocytosis independent of clathrin. Decorating nanoplatforms surface with ligands, such as cholesterol, albumin and folic acid (FA), has been manifested to induce the internalization of nanoparticles by target cells through caveolin-mediated pathway.121,122 For instance, paclitaxel loaded nanoparticles bound with albumin were internalized mediated by caveolae, which was attributed to the combination of albumin and glycoprotein 60 (gp60) - the albumin receptor present in caveolae of endothelial cells.123 Li et al developed DOX, indocyanine green (ICG), L-menthol (LM) co-loaded mesoporous silica nanoparticles (MSNs) linked with folic acid covered by methoxy poly (ethylene glycol)-poly(β-amino ester) (MPEG-PAE) finally enclosed by macrophage membrane to achieve multi-level targeting delivery.82 As macrophage membrane endowed the nano-core with characteristics of evading immune clearance and tumor targeting, the accumulation of nanoplatforms in tumors was increased. MPEG-PAE, as a pH-sensitive cationic polymer, sandwiched between the nano-core and macrophage membrane, was protonated and swelled in response to the acidic tumor microenvironment to peel off the macrophage membrane, facilitating release the wrapped nanovectors. Furthermore, the released MSN-FA can selectively bind to the over-expressed FA receptor on tumor cell surface to improve phagocytosis. Under near-infrared light irradiation, ICG was heated and produced reactive oxygen species to kill tumor cells combined with photothermal therapy (PTT) and PDT, which significantly enhanced the therapeutic effect and reduced side effects.
Besides, cell membrane coating layers optimize the cellular internalization pathway and bestow the wrapped nanoplatforms with the ability of bypassing lysosomes and evading enzymatic degradation. Tasciotti et al developed nanovectors camouflaged by leukocyte membrane and revealed few leukocyte membrane-mimetic nanoparticles were trapped in endosomes through transmission electron microscopy, owing to the internalization mediated by cytoskeleton rearrangement and channel formations around nanoparticles.22 As cell membrane possess the characteristic that facilitate more efficient endosomal bypass, mRNA loaded methoxy-poly (ethylene glycol)-block-polylactic acid (mPEG-bPLA) nanoparticles were fused with mammalian cell membranes to transport cargos directly to cytoplasm bypassing endosome/lysosome significantly enhancing delivery efficiency.124 Qiu and coworkers designed siRNA loaded nanoparticles encapsulated by endoplasmic reticulum (ER) membrane derived from cancer cells to effectively deliver siRNA via the endosome-Golgi-ER pathway avoiding lysosomal damage and improving target-gene silencing efficiencies of siRNA.83 Resident proteins on the wrapped layer of ER triggered the directional transport from Golgi apparatus to ER mediated by COPI or COPII vesicles. Hence, the paradigm of nanocarriers encapsulated by cell membrane not only evade lysosomal degradation but also possess the ability to target specific organelles via optimizing intracellular trafficking pathways, providing broad potential for the application of subcell-derived membranes in directional delivery systems.
Alleviating MDR
To overcome the obstacle of MDR in cancer treatment, investigators have devoted massive efforts to combination therapies. Combination therapies could be the co-delivery of chemotherapeutic drugs with MDR inhibitors or chemosensitizers (CSs). Verapamil (VER), as the calcium channel antagonist, can competitively bind with P-glycoprotein, suppress the drug efflux, increase the cell-internalization efficiency of therapeutic drugs to reverse MDR.125 Lee and partners synthesized nanoliposomes co-loaded with DOX and VER, which effectively alleviated the drug resistance mediated by P-glycoprotein and displayed stronger killing efficiency against leukemia, compared with free DOX.126 In addition, curcumin (Cur) derived from the traditional Chinese medicine-Turmeric, as a potential chemotherapeutic sensitizer, exerts synergistic anti-tumor effects in co-administration with chemotherapeutic drugs. Gao et al developed DOX and Cur co-loaded poly(lactic-co-glycolic acid) nanoparticles coated by PEGylated tumor cell membrane to conquer MDR in treatment of DOX-resistant esophageal carcinoma.84 The combination of DOX and Cur was verified to exert a much better anti-tumor effect than Cur or DOX alone through CCK-8 assay, transwell assay, and apoptosis analysis. Besides, the DOX and Cur co-loaded PLGA nanoparticles coated by tumor cell membrane possessed enhanced cytotoxicity towards TE10/DOX cells, while the bare core of PLGA@Cur + DOX displayed no obvious lethality, which was due to the coating layer of cancer cell membrane bestowed the nanovehicles with the ability of homologous recognition and prolonged circulation. The combination of chemo-drugs and chemosensitizers co-delivery system encapsulated by cell membrane provides a new and efficient strategy to surmount MDR in the treatment of cancer.
Another prospective approach to reversing MDR is to inhibit gene expression of MDR transporters. Small interfering RNA (siRNA) with the ability of silencing the drug-resistance gene can be co-delivered with chemotherapeutic drugs to improve the anti-tumor effect against MDR cancers.127 Wang and coworkers synthesized Paclitaxel (PTX) and MDR1-siRNA co-delivered lipid/dextran hybrid nanocarriers to deal with PTX-resistant ovarian (OV) cancer.128 The MDR1-siRNA/PTX combination exhibited obvious inhibitory effect on PTX-resistant cancer cells through inhibiting the expression of P-glycoprotein. In addition to suppressing the P-glycoprotein expression through gene interference, it has been reported that NO also exerts an inhibitory effect during the process of P-glycoprotein expression.129 Meanwhile, NO as a valid vasolivator can increase vasodilation and normalize the disordered blood vessels to promote the drug penetration into tumors.130 Based on the advantages of NO in drug delivery systems, Zhang et al developed NO donor, L-Arg loaded Au nanoparticles linked with Gemcitabine (GEM) via disulfide bonds, which was coated by pancreatic ductal adenocarcinoma (PDAC) cell membrane in order to maximum the cytotoxicity toward PDAC cells.85 In the tumor microenvironment, the high level of glutathione (GSH) triggered the release of GEM via cleavaging the disulfide bonds and NO was produced from L-Arg in response to the elevated reactive oxygen species. Besides, the PDAC cell membrane-mimetic nanodelivery system had the advantages of tumor-targeting and enhanced stability. Collectively, the cell membrane-based delivery system combined with NO donors and chemotherapeutic drugs greatly promotes deep-penetration of nanoparticles in tumors, reverses MDR, and achieves stimuli-response release of therapeutic ingredients based on tumor environment, representing the paradigm of treatment of tumors with MDR via inhibiting the expression of MDR related transporters.
Optimization the Physicochemical Property of Nanoparticles
In addition to designing various cell membrane-mimetic strategies that target multi-level biological barriers, the physicochemical properties of nanoparticles, including size, charge and shape, also contribute to the improvement of delivery efficiency. In the subsequent section, we will introduce how to design efficient nanocarriers by optimizing their physicochemical properties.
According to the physiological environment of blood vessels and tumors, the requirements for nanoparticle size vary from the aspect of blood circulation to penetration through solid tumors. In the bloodstream, particles smaller than 5.5 nm could be directly cleared by the filtration of the kidneys.131 It has been reported that the accumulating efficiency at tumor sites elevated with the increase of micelle size, and the tumor accumulation of 100 nm micelles was nearly 10 times that of 35 nm particles after 24 h injection.132 In addition, the size of nanoparticles also affects the penetrating capability in solid tumors.133 Small sized nanoparticles possess stronger tumor penetration, due to experience less resistance during diffusion compared with large-sized nanoparticles.134,135 A series of micellar nanoparticles with different sizes from 30 nm to 100 nm were synthesized to examine the effect of nanoparticle diameter on penetrating abilities in hyperpermeable and hypopermeable tumors, which demonstrated that only 30 nm micellar nanoparticles were capable of penetrating pancreatic tumors with poor permeability.136 Collectively, nanoparticles with large size preferentially accumulate at tumor sites, yet, the penetrating efficiency is poor. On the contrary, small-sized nanoparticles could easily penetrate through solid tumors, but inclined to be directly eliminated by kidneys in blood circulation. Considering the inconsistent requirement of particle size between blood circulation and tumor penetration, optimizing particle size to enhance tumor accumulation and facilitate penetration is extremely significant to improve drug delivery efficiency. With the emergence of stimuli-responsive size-tunable nanoparticles, tremendous efforts have been devoted to design size-reducible nanoparticles triggered by tumor microenvironment such as overexpressed proteases, to balance accumulation and penetration in tumors.137,138 Inspired by the high expression of hyaluronidase (HAase) at tumor sites, Yu et al developed a series of cationized gold nanoclusters (CAuNCs)-PTX@hyaluronic acid (HA) with diameter of 150 nm, 200 nm and 300 nm, respectively, via changing the mass ratio of HA and CAuNCs, and then, cloaked by the RBC membrane to check the effect of nanoparticle diameter on tumor targeting, which showed that 150 nm nanoclusters displayed the best tumor targeting and accumulating efficiency.86 Subsequently, 150 nm CAuNCs-PTX@HA coated by RBC membrane were picked to be loaded with the photosensitizer pheophorbide A (PheoA), ROS-responsive paclitaxel dimer prodrug (PXTK) and anti-PD-L1 peptide (dPPA) to achieve synergistic treatments combined with chemotherapy, PDT and immunotherapy. The outer coating layer of RBC membrane protected the nanocarriers from MPS clearance, extended the circulation time (mean residence time of RBC membrane coated nanoplatforms was 3.23-fold longer comparing with the uncapsulated ones) and facilitated the tumor accumulation. The size of nanovectors accumulating at tumor sites shrinked due to the degradation of HA, which promoted the size-reduced nanoparticles penetrated deep into tumors. Thus, cell membrane-based size-reducible nanocarriers triggered by tumor microenvironment ensure maximum accumulation and penetration at tumor sites, cleverly solving the size conflict.
Besides particle size, surface charge also determines the particle fate in vivo. As well known, neutral or negatively charged particles have been demonstrated to inhibit the formation of protein corona, thereby extending circulation time.139 Nevertheless, nanoparticles with positively charged surface are conducive to improve tumor penetration and cell internalization due to the electrostatic absorption with negatively charged cell membrane.140 Moreover, particles with strong positively-charged surface could break through the compartment membrane via “proton sponge effect” to achieve endosomal escape avoiding the degradation of lysosomes.141 To optimize the physicochemical property of drug carriers, the charge-switching strategy in design of cell membrane-based nanodelivery system could resolve the charge conflict in the process from blood circulation to cellular internalization.142,143 Considering the upregulated level of MMP-9 in numerous malignancies, Zhang and coworkers fused A549 cancer cell membrane with MMP-9-switchable peptide-based charge-reversal liposome membrane (Lipm) to encapsulate lipoic acid-modified polypeptide (LC) nanoparticles mainly composed of cell-penetrating peptide (CPP) co-loaded with decitabine (DTX) and siPGAM1 cloaked by citraconic anhydride-grafted poly-l-lysine (PC) to treat non-small cell lung cancer.87 In the blood circulation, the fused coating layer exhibited negatively charged, resulting in long blood half-time, homogenous targeting and enhanced accumulation at tumor sites. Since the cleavage of MMP-9-sensitive peptides (RRRRRRRRR-PVGLIG-EGGEGGEGG) by the overexpressed MMP-9 at tumor sites, CPP with positive-charge surface was activated to mediate cellular internalization bypassing endosomes. PC in the middle layer was hydrolyzed and transformed the charge from negative to positive in response to acidic tumor microenvironment leading to disruption and collapse of delivery systems, which contributed to efficient release of DTX and siPGAM1. The multistage charge-reversal fused membrane-coated nanocarriers triggered by the tumor environment were proposed as a potential strategy of optimizing the nanovehicle physicochemical property to improve the delivery efficiency.
Apart from size and charge, the nanocarrier shape has an unignorable effect on the accumulation of therapeutics at tumor sites. Numerous reports have indicated that nanovectors with minimal regions of curvature and high aspect ratios exhibit longer blood circulation and more effectively congregate in lesions compared with spherical nanoparticles.144,145 Represently, worm-like nanoparticles composed of siRNA and cationic bovine serum albumin (cBSA) through electrostatic interactions coated with cRGD-RBC membrane was fabricated to silence melanoma-relating genes (Figure 7).88 The decoration of cRGD-RBC membrane bestowed the worm-like nanocarriers with the ability of long blood circulation, deep penetration into tumors and enhanced cellular internalization due to the overexpression of αvβ3 integrin on B16F10 cell surface. The siRNA-cBSA complex exhibited negative-charge surface at neutral pH in bloodstream, while the cationic residues, diisopropylamino ethylamine (DIPEA), linked with bovine serum albumin mediated the disruption of compartment membrane in response to acidic conditions in lysosomes relying on “proton sponge effect”, which promoted the effective release of siRNA into cytoplasm. The worm-like nanoparticles of siRNA and BAS encapsulated by cRGD-inserted RBC membrane performed significant inhibitory effects in orthotropic xenograft mouse models. Altogether, the strategies of overcoming multi-level biological barriers combined with optimizing physiochemical characters should be conducted in designing of an ideal cell membrane-mimetic nanocarrier to extend blood circulation, massively accumulate at tumor sites, efficiently penetrate solid tumors, avoid lysosomal degradation, achieve site-specific release, and inhibit efflux.
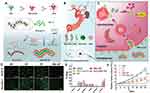 |
Figure 7 Schematic diagram and advantageous study of worm-Like biomimetic nanoerythrocyte carrying siRNA in melanoma gene therapy. Schematic illustration of (A) preparation and charge-reversible profile, (B) B16F10 tumor gene therapy, and (C) cell uptake and siRNA release profile from late endosome/lysosome escape of worm-like biomimetic nanoerythrocytes as targeting charge-reversible siRNA vectors. (D) Fluorescence images of two kinds of cells incubated with each gene vector (NP, RP, PP, and RBC-RP) for 4 h. (E) Biodistribution of cBSA, BSA sphere, RP, RBC-RP, and RGD-RBC-RP in B16F10 tumor-bearing BALB/c mice at 48 h after the injection. Results are expressed as mean ± SD (N = 4). *P < 0.05, **P < 0.01. Scale bars: 100 µm. (F) Relative tumor volume from tumor-bearing BALB/c mice with different treatment. Used with permission from Wang Y, Ji X, Ruan M, et al. Worm-like biomimetic nanoerythrocyte carrying siRNA for melanoma gene therapy. Small. 2018;14(47):e1803002. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.88 Abbreviations: RBC, red blood cell; RP, RBC-reversible polyplex; PP, always-positive polyplex; NP, always-negative polyplex; BSA, bovine serum albumin; cBSA, cationic bovine serum albumin; NC, negative control. |
In summary, cell membrane modifications, such as the conjugation of tumor-penetrating peptides, genetic engineering and membrane fusion, can be employed to improve the biocompatibility and targeting capabilities of bionic nanovectors. Furthermore, the physical properties of nanocores, including charge, size, and shape, should be optimized to meet the requirements of biological barriers at different levels. In addition to therapeutics, associated substances, encompassing functional chemical molecules, enzymes, and genes, could be considered for co-encapsulation to achieve synergistic actions on biological barriers, thereby facilitating site-specific delivery. Enhanced overcoming of the biological barriers confronted by anti-tumor nanoparticles upon intravenous injection, coupled with diverse cell membrane-based tactics (disguising with hybrid cell membranes, remodeling pathological milieus, evading lysosomal degradation, reversing MDR, and refining nanoparticle physicochemical attributes), will further propel the development of cell membrane-mimetic nanoparticles adept at sequentially overcoming these hurdles for precise and targeted delivery.
Perspectives of Cell Membrane-Based Nanoplatforms
With the aid of cell membrane-based nanosystems, anti-tumor ingredients are able to evade clearance by RES, infiltrate tumors, and eradicate tumor cells. In addition to the aforementioned biological barriers, cell membrane-mimetic nanocarriers have also displayed unique advantaged in overcoming blood-brain barriers (BBB). Furthermore, by utilizing the precise release characteristics of cell membrane-mimetic nanocarriers, it is feasible to incorporate contrast agents, antibiotics, analgesic ingredients or surgically isolated cancer cell membrane onto these nanodelivery systems for early diagnosis, anti-intratumor bacterial treatment, pain relief and personalized immunotherapy, thus accelerating the development of precision delivery systems.
Application in Central Nervous System Disorders
The treatment of central nervous system (CNS) disorders, such as glioblastoma, Alzheimer’s disease, brain tumors, Parkinson’s disease and ischemic stroke, continues to encounter significant challenges due to the poor penetration of BBB.146 BBB is composed of brain microvascular endothelial cells (BMECs), pericytes, basement membranes, and the end-feet of astrocytes. BMECs serve as the fundamental component of the BBB and play a crucial role in establishing and maintaining its structural integrity.147 BMECs are located within the inner microvasculature, making their recognition a crucial first step for targeted BBB delivery following systemic administration.148 Distinguished from peripheral microvascular endothelium, BMECs are closely attached and form tight junctions with limited fenestrations and pinocytic vesicles, presenting obstacles for delivery vehicles.149 Hence, brain as a vital part of CNS demonstrates a high level of intricacy, necessitating precise delivery vectors for therapeutics targeting brain lesions.
Cell membrane camouflaged nano-vehicles, characterized by enhanced pharmacokinetic profiles, homotypic-targeting capabilities and excellent biocompatibility, hold significant promise for addressing the challenges associated with treating CNS diseases. Notably, nanoparticles camouflaged by glioblastoma (GBM) cancer cell membranes exhibit excellent permeability across BBB, mediated by the downregulation of tight junction proteins, including Zonula occludens-1 (ZO-1), Claudin-5 and Occludin, ultimately decreasing the tightness of endothelial cells.150 To improve BBB penetration, zou and coworkers developed gboxin loaded PEG-PHB nanoparticles encapsulated by GBM cancer cell-mitochondria hybrid membrane aimed at GBM therapy.151 Gboxin, as the inhibitor of oxidative phosphorylation, suppresses GBM growth through reducing the reactive activity of F0F1ATPase complex V. Moreover, the hybrid membrane of GBM cancer cell and mitochondria bestowed prolonged circulation (4.90 h versus 0.47 h of free Gboxin), biocompatibility, remarkable BBB penetration and dual-tumor cell and mitochondria targeting (5–10 folds lower IC50 than free Gboxin) upon the encapsulated therapeutics, leading to negligible side effects and extended median survival time in U87MG and GBM stem cell X01 orthotopic mouse models. Looking forward, nanocarriers wrapped with cell membrane have opened up new avenues for overcoming the BBB and treating CNS-related diseases.
Tumor Imaging
It is important to choose appropriate diagnostic methods to detect tumors in the early stage, in order to prolong the patient survival and improve recovery rate. However, the conventional diagnostic manners, such as X-ray diffraction, positron emission tomography (PET), X-ray CT, magnetic resonance imaging (MRI) and optical fluorescent light imaging (FLI), do not possess the ability to specially detect early tumors in cancer screening, leading to the misinterpretation and poor prognosis.152,153 Given the shortcomings of traditional imaging techniques under short-wavelength irradiation, including high autofluorescence, poor tissue penetration, and invasive photo-damage to tissues, the application of NIR in optical imaging systems has dramatically improved imaging resolution and minimized side effects.154,155 In addition, rare earth-doped nanoparticles (RENPs) with the advantages of long fluorescence lifetime, narrow emission spectra, low autofluorescence and minimal toxicity have been widely applied in NIR-II (1000–1700 nm) imaging.156 Zhang et al developed RENPs coated by cancer cell membrane for NIR-II imaging to detect tumors and navigate surgery.157 The coating layer of cancer cell membrane protected the RENPs from clearance by the host immune system, promoted massive nanoparticles accumulated at tumor sites and exhibited favorable tumor imaging in the NIR-II window, which holds the potential to achieve precise tumor resection in medical applications.
Apart from enabling early diagnosis and guiding tumor resections in clinical settings, cell membrane-based imaging nanoplatforms have been developed for real-time monitoring and dynamic assessment during photothermal/photodynamic therapy realizing the integration of diagnosis and treatment. Indocyanine green (ICG), as a photosensitizer has been approved by the US Food and Drug Administration for clinical application owing to the merits of low toxicity and favorable optical properties, such as optimum level of tissue penetration, reduced light scattering and minimum auto-fluorescent interference from biological samples.158,159 Besides, ICG can also produce reactive oxygen species or induce hyperthermia effects under NIR laser irradiation to eradicate tumors.160 ICG loaded PLGA nano-cores encapsulated by cancer cell membrane (ICNPs) were applied in PTT combined with fluorescence/photoacoustic (FL/PA) imaging under NIR laser irradiation.161 Benefiting from the homologous-targeting property of coating layer, abundant ICNPs accumulated at tumor sites resulting in FL/PA imaging with high resolution and enhanced cytotoxicity. Recently, hydrogen phosphate ions and ICG co-loaded hollow mesoporous manganese dioxide (HMnO2) modified with poly (allylamine hydrochloride) (PAH), and then, coated by Hella cell membrane (M-HMnO2@ICG) were fabricated for the treatment of cervical cancer through magnetic resonance, photothermal and fluorescence imaging-guided combination treatment.162 The ICG-carrying efficiency and stability were improved through the addition of PAH and hydrogen phosphate ions. Under laser irradiation, M-HMnO2@ICG, stimulated by tumor microenvironment, decomposed to release ICG, produce O2 relying on peroxidase- and catalase (CAT)-like activities to sensitize PDT and facilitate oxidative stress treatment effects (chemodynamic therapy (CDT) and PDT) by consuming GSH. Additionally, PTT mediated by ICG exhibited synergistic effects on PDT. The cell membrane-mimetic phototherapeutic nanosystem was shown to accumulate at tumor sites and significantly suppressed tumor growth in Hela tumor-bearing mice models via fluorescence imaging. Collectively, optical therapies with imaging guidance confer the advantages of minimal drug resistance, non-invasive treatment, and real-time monitoring representing a prospective therapeutic avenue to expand therapeutic outcomes and obviously decrease side effects.
Personalized Immunotherapy
Personalized immunotherapy, emerging as a prominent focus in medical research, has the potential to enhance treatment efficacy while minimizing adverse effects on patients through accurate diagnosis and tailored therapeutic regimens.163 Considering solid tumors obtained from resections contain massive tumor-associated antigens (TAAs) as well as neoantigens that can effectively activate anti-tumor immune responses, surgically derived cancer cell membrane (SCM) has been extracted to prepare personalized cancer vaccines for carcinoma immunotherapy.164–166 Imiquimod (R837) was incorporated into thioglycolic-acid-grafted poly(2-methyl-2-oxazoline)-block-poly(2-butyl-2-oxazoline-co-2-butenyl-2-oxazoline) (PMBEOx-COOH) nanoparticles coated with SCM (SCNPs/R837) to mitigate excipient-induced side effects and provide autologous antigens for individualized immunotherapy.167 The findings suggested that SCNPs/R837 were transported to stimulate the resident plasmacytoid dendritic cells (pDCs) in draining lymph nodes (LNs), where activated pDCs released pro-inflammatory cytokines and recruited activated NK cells and CTLs at tumor sites, thereby overcoming the immunosuppressive microenvironment of tumor and inducing tumor cell death.
To enhance tumor-specific cellular immunogenicity, Ren et al developed “ABC” ternary membrane cloaked nanovaccines via a block-copolymer micelle-enabled method. The “ABC” ternary membrane system, consisting of antigen-presenting mature dendritic cell membranes (“A”), bacterial E coli cytoplasmic membranes (“B”) and B16 cancer cell membranes (“C”), accelerates the maturation of dendritic cells (DCs) and promotes cytotoxic T cell multiplication in vivo, effectively inhibiting tumor growth.168 The B16 tumor cell membrane presented a broad spectrum of tumor antigens to avoid immune escape that target a single antigen, E. coli cytoplasmic membranes containing pathogen-associated molecular patterns (PAMPs) triggered innate and adaptive immunity responses similar to infection signals, thereby preventing cytokine storms from cell wall fractions, and dendritic cell membranes promoted cell-to-cell immune communication and enhanced accumulation in lymph nodes. The results revealed that the immunogenicity of ABC was 14- and 304-fold higher, respectively, compared to binary (BC) and single (C) membrane vaccines. Additionally, the cure rate of ABC immunization reached 80% in tumor-bearing mice as a result of the enhanced proliferation of tumor-specific cytotoxic T lymphocytes mediated by the “ABC” ternary membrane system. Furthermore, “ABC” nanovaccines prepared by autologous cancer membranes effectively prevented tumor recurrence and metastasis in a post-surgical recurrence model, demonstrating the new multicellular communication platform of “ABC” ternary membrane nanovaccine. This paves the approach for personalized cancer immunotherapy.
Anti-Intratumoral Bacteria
With the deep understanding of complicated tumor microenvironment, it has been reported that intratumoral bacteria not only facilitate tumor growth and postoperative recurrence but also diminish therapeutic responses of tumor cells, ultimately leading to treatment failure.169,170 Clinically, intravenous antibiotics are commonly utilized in post-surgical cancer treatment to combat bacterial infections.171,172 However, the systemic administration of antibiotics may have several potential drawbacks, bacterial resistance induced by antibiotic overuse, limited effects on tumor-colonizing bacteria and the compromised anti-cancer efficacy, including the elimination of non-pathogenic anticancer bacteria, such as those in the gut microbiome.173,174 Therefore, an alternative strategy to selectively eliminate intratumoral bacteria and enhance anti-tumor efficacy has been highly desirable.
It was found that abundant Fusobacterium nucleatum (F. nucleatum) accumulated within colorectal tumors and adenomas around colon tissues through testing surgically obtained samples from colorectal cancer (CRC) patients,175,176 which significantly exacerbated the immunosuppressive tumor microenvironment via elevating the infiltration of immune suppressive cells,177 reducing the cytotoxic effect of NK cells and T cells,178 and reducing the infiltration of tumor-infiltrating lymphocytes.179 Based on the high affinity between Fap-2 presented on the F nucleatum membrane and D-galactose-β(1-3)-N acetyl-D-galactosamine (Gal-GalNAc) overexpressed on the surface of colorectal tumor cells,180 colistin-loaded liposomes fused with F nucleatum cytoplasmic membrane (FM) was developed to selectively remove tumor-colonized F nucleatum and restore the immunotherapy against colorectal tumors.181 The Colistin-LipoFM, with its tumor-targeting property and retention capacity, exhibited superior antibacterial efficacy compared to free colistin and colistin-loaded liposomes. In addition, the immunosuppressive tumor microenvironment induced by F nucleatum was effectively alleviated, resulting in the restored therapeutic effect of antibodies against cytotoxic T lymphocyte-associated antigen 4 (anti-CTLA4) in F nucleatum infected colorectal cancer models. Consequently, the F nucleatum membrane-mimetic nanoplatform, which targets intratumoral bacteria, exhibited outstanding anti-bacteria efficiency combined with enhanced immunotherapy against F nucleatum-colonized colorectal cancer, thereby highlighting the promising potential of cell membrane-mimetic delivery systems in addressing inflammation within tumors.
Relieve Cancer-Related Pain
Pain, as a prevalent symptom among cancer patients, dramatically impacts their quality of life and accelerates the tumor progression. Reports indicate that approximately 75–90% of cancer patients in metastatic or advanced stages will suffer from cancer-related pain.182,183 Cancer pain is a multifaceted pain condition involving neuropathic, compressive, inflammatory and ischemic mechanisms.184 Within the cancer microenvironment, tumor cells could release algogenic mediators to sensitize and activate nociceptors.185 Pain in certain cancers, such as head and neck cancer, pancreatic cancer, and osteosarcoma, originates from the primary tumor site.186,187 However, in common cancers like breast, kidney, lung cancer and prostate, tumors tend to metastasize to multiple bones throughout the body including the ribs, vertebrae, femur, hip and tibia. The widespread metastasis leads to significant bone pain.188
For breast cancer with bone metastasis, nanoparticles co-loaded with decitabine and JTC801, coated by engineered macrophage membrane (EMM) were constructed to suppress the progression of breast tumors and relieve cancer-related pain.189 The cell membrane-mimetic nanoplatforms, which inherit inflammation-targeting from EMM, effectively addressed the challenge of poor blood supply in bone metastatic tumors and enabled simultaneous targeting of multiple cancer lesions. Subsequently, the combination of JTC801 and decitabine enhanced lytic and inflammatory responses, leading to a synergistic tumoricidal effect, and transformed the tumor into an ideal decoy for EMM, thereby amplifying the tumor-targeting efficiency. Furthermore, JTC801, as μ-opioid receptor (MOR) antagonists, was found to effectively alleviate cancer-associated pain by when evaluated by assessing pain levels in left hindlimb tumor-bearing mice. Comprehensively, the macrophage membrane-mimetic nanoplatform has shown significant inhibitory effects on tumor growth, metastasis, and analgesic activity in mouse models of breast cancer with bone metastasis. Hence, integrating the benefits of cell membrane-mimetic nanovectors into the combination of cancer therapy and pain management has the potential to significantly enhance patient quality of life and caregiver functional status.
Challenges and Future Directions
Despite the considerable advantages of cell membrane-based delivery systems, such as biocompatibility, immune evasion, prolonged blood circulation and tumor targeting properties, most studies regarding fabrication and in vivo or in vitro evaluation still remain at the basic research stage. Hence, to accelerate clinical trails and achieve industrial development, there are some theoretical challenges and technical barriers that need to be overcome (Table 3).
 |
Table 3 The Advantages and Limitations of Cell Membrane-Mimetic Nanovectors |
Cell Sources
In numerous researches, the tumor-targeting capability of cell membrane-mimetic nanovectors is enhanced by utilizing tumor cell membranes derived from donor sources that match host cells. Due to uncontrollable elements and safety concerns, personalized treatment involving extraction of tumor cell membranes from the patient may not be feasible in practical applications. One potential avenue for addressing this concern is to establish several super-cell membranes by pretreating cells to induce the overexpression of intrinsic functional proteins and eliminating unnecessary proteins in accordance with acceptable standard. Otherwise, it is imperative to design a library of biologically derived membranes with distinctive properties and conduct intensive research on the most suitable strategies of cell membrane-mimetic nanocarriers tailored to different tumors, ensuring optimal tumor-targeting properties and improving patient satisfaction.
Potential Immunogenicity
At present, only a few defined proteins on the cell membrane are accountable for tumor-targeting and evading recognition by MPS, while numerous unknown proteins might target other organs and trigger immune responses. Hence, the dynamic mechanism of the cell membrane in traversing biological barriers requires clarification by recreating a 3D microenvironment such as cell–cell and cell-extracellular matrix interactions to determine which are functional proteins and which are the proteins that trigger immune responses. Moreover, most experiments in this review focused on the tumor-targeting property of delivery systems in mouse models; however, the immune response and pharmacokinetics in human body are unpredictable Researchers should put more attention on immune reactions and long-term behaviors, such as absorption, distribution, metabolism and excretion in human systems. Only through repeated confirmation in theory and practice could the potential immunogenicity risks be decreased.
Infancy Technology
The cell membrane-mimicking technology is still in its infancy and its high cost consumption should not be ignored compared with traditional engineering techniques. To achieve industrial development, the following technological barriers must be addressed.
Cell Expansion
To prevent batch-to-batch variations in cell membranes, it is essential to control the purity, quality and phenotype during cell passage. Given this, the bioreactor is highly beneficial for ultra-large-scale cell expansion as it enables better control over the culture environment.190 Moreover, prior to its application, it is crucial to optimize and determine the culture parameters, such as the frequency of medium change, oxygen concentration, and the rotation speed of the impeller, to guarantee the expression level of functional proteins.
Cell Membrane Extraction
The current research usually employs differential centrifugation to extract membranes. However, the method might not completely eliminate all cytoplasmic components and could lead to the loss of membrane fragments, causing nuclear/mitochondrial/cytosol contamination and the reduction of delivery efficiency. Therefore, maintaining the integrity and purity of the cell membrane is an important issue that needs to be taken into account during the extraction process. There is an urgent demand for an established process for the isolation of cell membranes with high yield and purity.
Coating Techniques
There is a risk of immune response for nanoparticles that are not enveloped by cell membranes. Hence, during the assembly process of cell membrane-mimetic nanovectors, it is crucial to control the coating layers encapsulating each nano-core and achieve a homogeneous coating. Compared with conventional methods, such as co-extrusion and ultrasonication, microfluidic electroporation could enable more precise control over the membrane coating process through tunable parameters such as the geometry of the mixing channel, flow rate, voltage, and electric field pulse rate, which significantly enhances the encapsulation efficiency.191
Characterization Tools
The cell membrane-based nanocarriers produced need to be characterized, particularly for the active motifs, to ensure the purity of extracted cell membrane and the integrity of functional proteins. Transmission electron microscopy, stability assays, Western blotting or mass spectrometry protein analysis and binding exclusion assays are all commonly employed approaches for evaluating the uniformity and integrity of the membrane coating, all of which should be optimized to ensure the intended delivery efficiency.
Storage Conditions
Storage conditions, including storage matrix, storage container, time, temperature, etc, exert a decisive influence on the activity of functional proteins after long-term deposit, especially for engineered cell membrane. However, the factors affecting the shelf life of cell membrane-mimetic nanocarriers have not been explored yet. Hence, it is critical to optimize long-term storage conditions to maintain the stability of the coating membrane and improve the shelf life of bionic nanovectors.
Standard Protocols
Standard operating procedures, ranging from cell expansion, cell membrane extraction, to assembly of bionic nanocarriers, should be well-established. Overall, the final product should be manufactured in a sterile environment and in accordance with standard protocols, ensuring cell membrane-mimetic nanomedicines from batch to batch are free of biological contaminants. Only by surmounting the aforementioned challenges can we expedite the process of converting cell membrane-mimetic nanocarriers from the laboratory to clinical practice.
Conclusions
With the development of biomimetic technology, cell membrane-camouflaged nanoplatforms have been widely researched owing to their biocompatibility, long blood circulation, as well as partial tumor-targeting. Nevertheless, the implementation of cell membrane-mimetic nanoparticles has not been satisfactory in clinical trials. After intravenous injection, a series of complicated biological obstacles hinder adequate delivery of therapeutic ingredients to interest sites. Herein, we outlined the biological barriers that determine the final fate of nanocarriers, ranging from blood circulation, tumor microenvironment to cellular internalization, and reviewed cell membrane-based strategies that aim to overcome these impediments and achieve precision delivery at different levels. Moreover, optimizing nanoparticle physicochemical properties based on the requirements of physiological barriers existing in the process of nanoparticles to lesions through systemic routes provides a multifunctional paradigm to improve the delivery efficiency. Additionally, cell membrane-mimetic nanocarriers can be utilized to incorporate contrast agents, antibiotics, analgesic ingredients or patient-self cancer cell membrane for early diagnosis, anti-intratumor bacterial treatment, pain relief and personalized immunotherapy, thus accelerating the development of precision delivery systems.
Despite numerous studies having been conducted on cell membrane-mimetic nanovectors, merely focusing on one or a few biological barriers evidently cannot yield satisfactory delivery outcomes. In the review, we offer a systematic elaboration of biological barriers and corresponding countermeasures, providing guidance for the design of novel cell membrane-mimetic nanocarriers in the future. This advancement not only harbors the potential to translate cell membrane-based nanodelivery systems seamlessly from laboratory to anti-tumor clinical applications, but also underscores the pivotal role of cell membrane-mimetic nanocarriers as a promising therapeutic modality for diverse diseases.
Acknowledgments
This work was financially supported by the Natural Science Fund of Shandong Province (ZR2023MH223, ZR2019PH061), the Support Plan for Youth Entrepreneurship and Technology of Colleges and Universities in Shandong (grant no. 2021KJ101).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Asrorov AM, Gu Z, Li F, Liu L, Huang Y. Biomimetic camouflage delivery strategies for cancer therapy. Nanoscale. 2021;13(19):8693–8706. doi:10.1039/d1nr01127h
3. Izci M, Maksoudian C, Manshian BB, Soenen SJ. The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. Chem Rev. 2021;121(3):1746–1803. doi:10.1021/acs.chemrev.0c00779
4. Schroeder A, Heller DA, Winslow MM, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2011;12(1):39–50. doi:10.1038/nrc3180
5. Jing Q, Ruan H, Li J, et al. Keratinocyte membrane-mediated nanodelivery system with dissolving microneedles for targeted therapy of skin diseases. Biomaterials. 2021;278:121142. doi:10.1016/j.biomaterials
6. He Y, Zhang S. She Y, et al.Innovative utilization of cell membrane-coated nanoparticles in precision cancer therapy. Exploration. 2024;20230164. doi:10.1002/EXP.20230164
7. Wu T, Hou X, Li J, et al. Microneedle-mediated biomimetic cyclodextrin metal organic frameworks for active targeting and treatment of hypertrophic scars. ACS Nano. 2021;15(12):20087–20104. doi:10.1021/acsnano.1c07829
8. He Z, Zhang Y, Feng N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review. Mater Sci Eng C Mater Biol Appl. 2020;106:110298. doi:10.1016/j.msec.2019.110298
9. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi:10.1073/pnas.1106634108
10. Zhang Q, Dehaini D, Zhang Y, et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13(12):1182–1190. doi:10.1038/s41565-018-0254-4
11. Li J, Ai Y, Wang L, et al. Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials. 2016;76:52–65. doi:10.1016/j.biomaterials.2015.10.046
12. Chen L, Hong W, Ren W, Xu T, Qian Z, He Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther. 2021;6(1):225. doi:10.1038/s41392-021-00631-2
13. Li Y, Ruan S, Guo J, et al. B16F10 cell membrane-based nanovesicles for melanoma therapy are superior to hyaluronic acid-modified nanocarriers. Mol Pharm. 2022;19(8):2840–2853. doi:10.1021/acs.molpharmaceut.2c00212
14. Yang J, He Y, Zhang M, et al. Programmed initiation and enhancement of cGAS/STING pathway for tumour immunotherapy via tailor-designed ZnFe2O4-based nanosystem. Exploration. 2023;3(6):20230061. doi:10.1002/EXP.20230061
15. Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 2010;28(4):181–188. doi:10.1016/j.tibtech.2009.12.007
16. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi:10.1038/nbt.3330
17. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi:10.1126/science.288.5473.2051
18. Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–135. doi:10.1016/j.addr.2010.03.011
19. Hu C-MJ, Fang RH, Wang K-C, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi:10.1038/nature15373
20. Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A. 2001;98(6):3352–3357. doi:10.1073/pnas.061615598
21. Barreiro O, Yáñez-Mó M, Serrador JM, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157(7):1233–1245. doi:10.1083/jcb.200112126
22. Parodi A, Quattrocchi N, van de Ven AL, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–68. doi:10.1038/nnano.2012.212
23. Chu D, Dong X, Shi X, Zhang C, Wang Z. Neutrophil-based drug delivery systems. Adv Mater. 2018;30(22):e1706245. doi:10.1002/adma.201706245
24. Kang T, Zhu Q, Wei D, et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11(2):1397–1411. doi:10.1021/acsnano.6b06477
25. Borrego F, Kabat J, Kim DK, et al. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38(9):637–660.
26. Dianat-Moghadam H, Mahari A, Heidarifard M, et al. NK cells-directed therapies target circulating tumor cells and metastasis. Cancer Lett. 2021;497:41–53. doi:10.1016/j.canlet.2020.09.021
27. Pitchaimani A, Nguyen TDT, Aryal S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials. 2018;160:124–137. doi:10.1016/j.biomaterials.2018.01.018
28. Hu YL, Fu YH, Tabata Y, Gao JQ. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J Control Release. 2010;147(2):154–162. doi:10.1016/j.jconrel.2010.05.015
29. Yang N, Ding Y, Zhang Y, et al. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy. ACS Appl Mater Interfaces. 2018;10(27):22963–22973. doi:10.1021/acsami.8b05363
30. Fortuna-Costa A, Gomes A, Kozlowski EO, Stelling MP, Pavã£o MSG. Extracellular galectin-3 in tumor progression and metastasis. Front Oncol. 2014;4:138. doi:10.3389/fonc.2014.00138
31. Liu X, Sun Y, Xu S, et al. Homotypic cell membrane-cloaked biomimetic nanocarrier for the targeted chemotherapy of hepatocellular carcinoma. Theranostics. 2019;9(20):5828–5838. doi:10.7150/thno.34837
32. Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Deliv. 2010;7(4):403–427. doi:10.1517/17425241003610633
33. Gao W, Hu CMJ, Fang RH, Luk BT, Su J, Zhang L. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25(26):3549–3553. doi:10.1002/adma.201300638
34. Luk BT, Fang RH, Hu C-MJ, et al. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics. 2016;6(7):1004–1011. doi:10.7150/thno.14471
35. Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2011;34(1):5–30. doi:10.1007/s00281-011-0286-4
36. Hu Q, Qian C, Sun W, et al. Engineered nanoplatelets for enhanced treatment of multiple myeloma and thrombus. Adv Mater. 2016;28(43):9573–9580. doi:10.1002/adma.201603463
37. Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27(48):6207–6215. doi:10.1038/onc.2008.298
38. Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. mol Cancer Res. 2006;4(4):221–233. doi:10.1158/1541-7786
39. Roberts-Thomson IC, Fon J, Uylaki W, Cummins AG, Barry S. Cells, cytokines and inflammatory bowel disease: a clinical perspective. Expert Rev Gastroenterol Hepatol. 2011;5(6):703–716. doi:10.1586/egh.11.74
40. Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 2013;138(2):93–104. doi:10.1111/imm.12023
41. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–1073. doi:10.1189/jlb.0609385
42. Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131(3):463–475. doi:10.1016/j.cell.2007.08.038
43. Park J, Wysocki RW, Amoozgar Z, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8(361):361ra138. doi:10.1126/scitranslmed.aag1711
44. Dahlberg CIM, Sarhan D, Chrobok M, Duru AD, Alici E. Natural killer cell-based therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. 2015;6:605. doi:10.3389/fimmu.2015.00605
45. Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168. doi:10.1016/j.progpolymsci.2015.02.004
46. Compte M, Cuesta ÁM, Sánchez-Martín D, et al. Tumor immunotherapy using gene-modified human mesenchymal stem cells loaded into synthetic extracellular matrix scaffolds. Stem Cells. 2009;27(3):753–760. doi:10.1634/stemcells.2008-0831
47. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. doi:10.1016/j.stem.2009.02.001
48. Kosztowski T, Zaidi HA, Quiñones-Hinojosa A. Applications of neural and mesenchymal stem cells in the treatment of gliomas. Expert Rev Anticancer Ther. 2014;9(5):597–612. doi:10.1586/era.09.22
49. Vijayan V, Uthaman S, Park IK. Cell membrane-camouflaged nanoparticles: a promising biomimetic strategy for cancer theragnostics. Polymers. 2018;10(9):983. doi:10.3390/polym10090983
50. Harris JC, Scully MA, Day ES. Cancer Cell membrane-coated nanoparticles for cancer management. Cancers. 2019;11(12):1836. doi:10.3390/cancers11121836
51. Jin J, Krishnamachary B, Barnett JD, et al. Human cancer cell membrane-coated biomimetic nanoparticles reduce fibroblast-mediated invasion and metastasis and induce T-Cells. ACS Appl Mater Interfaces. 2019;11(8):7850–7861. doi:10.1021/acsami.8b22309
52. Lovitt CJ, Shelper TB, Avery VM. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer. 2018;18(1):41. doi:10.1186/s12885-017-3953-6
53. Hu CM, Fang RH, Luk BT, et al. ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale. 2013;5(7):2664–2668. doi:10.1039/c3nr00015j
54. Tenzer S, Docter D, Kuharev J, et al. Rapid formation of plasma protein Corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8(10):772–781. doi:10.1038/nnano.2013.181
55. Nel AE, Mädler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557. doi:10.1038/nmat2442
56. Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145(3):182–195. doi:10.1016/j.jconrel.2010.01.036
57. Salvati A, Pitek AS, Monopoli MP, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule Corona adsorbs on the surface. Nat Nanotechnol. 2013;8(2):137–143. doi:10.1038/nnano.2012.237
58. Cheng YH, He C, Riviere JE, Monteiro-Riviere NA, Lin Z. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. ACS Nano. 2020;14(3):3075–3095. doi:10.1021/acsnano.9b08142
59. Heldin CH, Rubin K, Pietras K, Östman A. High interstitial fluid pressure-an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–813. doi:10.1038/nrc1456
60. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–1039.
61. Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1(3):219–227. doi:10.1016/s1535-6108(02)00051-x
62. Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi:10.1126/science.1171362
63. Pluen A, Boucher Y, Ramanujan S, et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc Natl Acad Sci U S A. 2001;98(8):4628–4633. doi:10.1073/pnas.081626898
64. Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–592. doi:10.1038/nrc1893
65. Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–503. doi:10.1002/path.1427
66. Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev mol Cell Biol. 2005;6(2):112–126. doi:10.1038/nrm1571
67. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78(1):857–902. doi:10.1146/annurev.biochem.78.081307.110540
68. Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nat Rev mol Cell Biol. 2009;10(3):218–227. doi:10.1038/nrm2646
69. Aller SG, Yu J, Ward A, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323(5922):1718–1722. doi:10.1126/science.1168750
70. Wang J, Seebacher N, Shi H, Kan Q, Duan Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget. 2017;8(48):84559–84571. doi:10.18632/oncotarget.19187
71. Liu Y, Wang X, Ouyang B, et al. Erythrocyte-platelet hybrid membranes coating polypyrrol nanoparticles for enhanced delivery and photothermal therapy. J Mater Chem B. 2018;6(43):7033–7041. doi:10.1039/c8tb02143k
72. Wang D, Dong H, Li M, et al. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12(6):5241–5252. doi:10.1021/acsnano.7b08355
73. Rezaei S, de Araújo Júnior RF, da Silva ILG, Schomann T, Eich C, Cruz LJ. Erythrocyte-cancer hybrid membrane-coated reduction-sensitive nanoparticles for enhancing chemotherapy efficacy in breast cancer. Biomater Adv. 2023;151:213456. doi:10.1016/j.bioadv.2023.213456
74. He H, Guo C, Wang J, et al. Leutusome: a biomimetic nanoplatform integrating plasma membrane components of leukocytes and tumor cells for remarkably enhanced solid tumor homing. Nano Lett. 2018;18(10):6164–6174. doi:10.1021/acs.nanolett.8b01892
75. Chen Z, Wu Q, Guo W, et al. Nanoengineered biomimetic Cu-based nanoparticles for multifunational and efficient tumor treatment. Biomaterials. 2021;276:121016. doi:10.1016/j.biomaterials.2021.121016
76. Zhang M, Ye -J-J, Xia Y, et al. Platelet-mimicking biotaxis targeting vasculature-disrupted tumors for cascade amplification of hypoxia-sensitive therapy. ACS Nano. 2019;13(12):14230–14240. doi:10.1021/acsnano.9b07330
77. Yang X-Y, Zhang J-G, Zhou Q-M, et al. Extracellular matrix modulating enzyme functionalized biomimetic Au nanoplatform-mediated enhanced tumor penetration and synergistic antitumor therapy for pancreatic cancer. J Nanobiotechnology. 2022;20(1):524. doi:10.1186/s12951-022-01738-6
78. Liu H, Jiang W, Wang Q, Hang L, Wang Y, Wang Y. ROS-sensitive biomimetic nanocarriers modulate tumor hypoxia for synergistic photodynamic chemotherapy. Biomater Sci. 2019;7(9):3706–3716. doi:10.1039/c9bm00634f
79. Guo H, Zhang W, Wang L, Shao Z, Huang X. Biomimetic cell membrane-coated glucose/oxygen-exhausting nanoreactor for remodeling tumor microenvironment in targeted hypoxic tumor therapy. Biomaterials. 2022;290:121821. doi:10.1016/j.biomaterials
80. Zhuang J, Gong H, Zhou J, et al. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci Adv. 2020;6(13):eaaz6108. doi:10.1126/sciadv.aaz6108
81. Park JH, Mohapatra A, Zhou J, et al. Virus-mimicking cell membrane-coated nanoparticles for cytosolic delivery of mRNA. Angew Chem Int Ed Engl. 2022;61(2):e202113671. doi:10.1002/anie.202113671
82. Li M, Gao X, Lin C, et al. An intelligent responsive macrophage cell membrane-camouflaged mesoporous silicon nanorod drug delivery system for precise targeted therapy of tumors. J Nanobiotechnology. 2021;19(1):336. doi:10.1186/s12951-021-01082-1
83. Qiu C, Han HH, Sun J, et al. Regulating intracellular fate of siRNA by endoplasmic reticulum membrane-decorated hybrid nanoplexes. Nat Commun. 2019;10(1):2702. doi:10.1038/s41467-019-10562-w
84. Gao Y, Zhu Y, Xu X, et al. Surface PEGylated cancer cell membrane-coated nanoparticles for codelivery of curcumin and doxorubicin for the treatment of multidrug resistant esophageal carcinoma. Front Cell Dev Biol. 2021;9:688070. doi:10.3389/fcell.2021.688070
85. Zhang F, Hu Q, Li B, et al. A biomimetic nanodrug for enhanced chemotherapy of pancreatic tumors. J Control Release. 2023;354:835–850. doi:10.1016/j.jconrel.2023.01.007
86. Yu W, He X, Yang Z, et al. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials. 2019;217:119309. doi:10.1016/j.biomaterials.2019.119309
87. Zhang W, Gong C, Chen Z, Li M, Li Y, Gao J. Tumor microenvironment-activated cancer cell membrane-liposome hybrid nanoparticle-mediated synergistic metabolic therapy and chemotherapy for non-small cell lung cancer. J Nanobiotechnology. 2021;19(1):339. doi:10.1186/s12951-021-01085-y
88. Wang Y, Ji X, Ruan M, et al. Worm-like biomimetic nanoerythrocyte carrying siRNA for melanoma gene therapy. Small. 2018;14(47):e1803002. doi:10.1002/smll.201803002
89. Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. doi:10.1038/nrd1033
90. Harris JM, Martin NE, Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet. 2001;40(7):539–551. doi:10.2165/00003088-200140070-00005
91. Zhao Y, Wang L, Yan M, et al. Repeated injection of PEGylated solid lipid nanoparticles induces accelerated blood clearance in mice and beagles. Int J Nanomed. 2012;7:2891–2900. doi:10.2147/IJN.S30943
92. Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339(6122):971–975. doi:10.1126/science.1229568
93. Fang RH, Hu C-MJ, Chen KNH, et al. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale. 2013;5(19):8884–8888. doi:10.1039/c3nr03064d
94. Dehaini D, Wei X, Fang RH, et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater. 2017;29(16). doi:10.1002/adma.201606209
95. Wang G, Chen X, Liu S, Wong C, Chu S. Mechanical chameleon through dynamic real-time plasmonic tuning. ACS Nano. 2016;10(2):1788–1794. doi:10.1021/acsnano.5b07472
96. Liao Y, Zhang Y, Blum NT, Lin J, Huang P. Biomimetic hybrid membrane-based nanoplatforms: synthesis, properties and biomedical applications. Nanoscale Horiz. 2020;5(9):1293–1302. doi:10.1039/d0nh00267d
97. Ding C, Zhang C, Cheng S, Xian Y. Multivalent aptamer functionalized Ag2S nanodots/hybrid cell membrane-coated magnetic nanobioprobe for the ultrasensitive isolation and detection of circulating tumor cells. Adv Funct Mater. 2020;30(16).
98. Chen HY, Deng J, Wang Y, Wu CQ, Li X, Dai HW. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13. doi:10.1016/j.actbio.2020.05.028
99. Chauhan VP, Stylianopoulos T, Martin JD, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383–388. doi:10.1038/nnano.2012.45
100. Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev. 2011;37(1):63–74. doi:10.1016/j.ctrv.2010.05.001
101. Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi:10.1200/JCO.2012.46.3653
102. Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. doi:10.1038/nrclinonc.2010.139
103. Monsky WL, Fukumura D, Gohongi T, et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59(16):4129–4135.
104. Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5(6):423–435. doi:10.1038/nrc1628
105. Song W, Tang Z, Zhang D, Yu H, Chen X. Coadministration of vascular disrupting agents and nanomedicines to eradicate tumors from peripheral and central regions. Small. 2015;11(31):3755–3761. doi:10.1002/smll.201500324
106. Jiang J, Shen N, Ci T, et al. Combretastatin A4 nanodrug-induced MMP9 amplification boosts tumor-selective release of doxorubicin prodrug. Adv Mater. 2019;31(44):e1904278. doi:10.1002/adma.201904278
107. Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7(11):1759–1766. doi:10.1111/j.1538-7836.2009.03586.x
108. Zhao Y, Cao J, Melamed A, et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2019;116(6):2210–2219. doi:10.1073/pnas.1818357116
109. Hu K, Miao L, Goodwin TJ, Li J, Liu Q, Huang L. Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS Nano. 2017;11(5):4916–4925. doi:10.1021/acsnano.7b01522
110. Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108(7):2909–2914. doi:10.1073/pnas.1018892108
111. Chow M-J, Turcotte R, Lin CP, Zhang Y. Arterial extracellular matrix: a mechanobiological study of the contributions and interactions of elastin and collagen. Biophys J. 2014;106(12):2684–2692. doi:10.1016/j.bpj.2014.05.014
112. Li Y-J, Wu J-Y, Hu X-B, Ding T, Tang T, Xiang D-X. Biomimetic Liposome with Surface-Bound Elastase for Enhanced Tumor Penetration and Chemo-Immumotherapy. Adv Healthc Mater. 2021;10(19):e2100794. doi:10.1002/adhm.202100794
113. Xu X, Wu Y, Qian X, et al. Nanomedicine strategies to circumvent intratumor extracellular matrix barriers for cancer therapy. Adv Healthc Mater. 2021;11(1):e2101428. doi:10.1002/adhm.202101428
114. Sugahara KN, Teesalu T, Karmali PP, et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16(6):510–520. doi:10.1016/j.ccr.2009.10.013
115. Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release. 2006;116(2):255–264. doi:10.1016/j.jconrel.2006.06.024
116. Chou LYT, Ming K, Chan WCW. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40(1):233–245. doi:10.1039/c0cs00003e
117. Zhu X, Sun Y, Chen D, et al. Mastocarcinoma therapy synergistically promoted by lysosome dependent apoptosis specifically evoked by 5-Fu@nanogel system with passive targeting and pH activatable dual function. J Control Release. 2017;254:107–118. doi:10.1016/j.jconrel.2017.03.038
118. Liu S, Wang B, Sheng Y, Dong S, Liu G. Rational design of self-assembled mitochondria-targeting lytic peptide conjugates with enhanced tumor selectivity. Chemistry. 2022;28(3):e202103517. doi:10.1002/chem.202103517
119. Russell CJ, Hu M, Okda FA. Influenza hemagglutinin protein stability, activation, and pandemic risk. Trends Microbiol. 2018;26(10):841–853. doi:10.1016/j.tim.2018.03.005
120. Tatulian SA, Tamm LK. Secondary structure, orientation, oligomerization, and lipid interactions of the transmembrane domain of influenza hemagglutinin. Biochemistry. 2000;39(3):496–507. doi:10.1021/bi991594p
121. Bareford L, Swaan P. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59(8):748–758. doi:10.1016/j.addr.2007.06.008
122. Dauty E, Remy JS, Zuber G, Behr JP. Intracellular delivery of nanometric DNA particles via the folate receptor. Bioconjug Chem. 2002;13(4):831–839. doi:10.1021/bc0255182
123. Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7(8):1041–1053. doi:10.1517/14656566.7.8.1041
124. Shin S, Ahn YR, Kim M, Choi J, Kim H, Kim HO. Mammalian cell membrane hybrid polymersomes for mRNA delivery. ACS Appl Mater Interfaces. 2024. doi:10.1021/acsami.4c00843
125. Sharom FJ. The P-glycoprotein efflux pump: how does it transport drugs? J Membr Biol. 1997;160(3):161–175. doi:10.1007/s002329900305
126. Wu J, Lu Y, Lee A, et al. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci. 2007;10(3):350–357.
127. Susa M, Iyer AK, Ryu K, et al. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One. 2010;5(5):e10764. doi:10.1371/journal.pone.0010764
128. Wang C, Guan W, Peng J, Chen Y, Xu G, Dou H. Gene/paclitaxel co-delivering nanocarriers prepared by framework-induced self-assembly for the inhibition of highly drug-resistant tumors. Acta Biomater. 2020;103:247–258. doi:10.1016/j.actbio.2019.12.015
129. Wan MM, Chen H, Da Wang Z, et al. Nitric oxide-driven nanomotor for deep tissue penetration and multidrug resistance reversal in cancer therapy. Adv Sci. 2021;8(3):2002525. doi:10.1002/advs.202002525
130. Vong LB, Nagasaki Y. Nitric oxide nano-delivery systems for cancer therapeutics: advances and challenges. Antioxidants. 2020;9(9):791. doi:10.3390/antiox9090791
131. Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–1170. doi:10.1038/nbt1340
132. Wang J, Mao W, Lock LL, et al. The role of micelle size in tumor accumulation, penetration, and treatment. ACS Nano. 2015;9(7):7195–7206. doi:10.1021/acsnano.5b02017
133. Zhang J, Wang X, Wen J, et al. Size effect of mesoporous organosilica nanoparticles on tumor penetration and accumulation. Biomater Sci. 2019;7(11):4790–4799. doi:10.1039/c9bm01164a
134. Popović Z, Liu W, Chauhan VP, et al. A nanoparticle size series for in vivo fluorescence imaging. Angew Chem Int Ed Engl. 2010;49(46):8649–8652. doi:10.1002/anie.201003142
135. Li HJ, Liu J, Luo YL, et al. Intratumor performance and therapeutic efficacy of PAMAM dendrimers carried by clustered nanoparticles. Nano Lett. 2019;19(12):8947–8955. doi:10.1021/acs.nanolett.9b03913
136. Cabral H, Matsumoto Y, Mizuno K, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6(12):815–823. doi:10.1038/nnano.2011.166
137. Ruan S, Cao X, Cun X, et al. Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials. 2015;60:100–110. doi:10.1016/j.biomaterials.2015.05.006
138. Ruan S, He Q, Gao H. Matrix metalloproteinase triggered size-shrinkable gelatin-gold fabricated nanoparticles for tumor microenvironment sensitive penetration and diagnosis of glioma. Nanoscale. 2015;7(21):9487–9496. doi:10.1039/c5nr01408e
139. Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–515. doi:10.1021/mp800051m
140. Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. Long-circulating poly(ethylene glycol)-poly(D,L-lactide) block copolymer micelles with modulated surface charge. J Control Release. 2001;77(1–2):27–38. doi:10.1016/s0168-3659(01)00451-5
141. Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151(3):220–228. doi:10.1016/j.jconrel.2010.11.004
142. Du JZ, Sun TM, Song WJ, Wu J, Wang J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew Chem Int Ed Engl. 2010;49(21):3621–3626. doi:10.1002/anie.200907210
143. Liu J, Iqbal S, Du XJ, et al. Ultrafast charge-conversional nanocarrier for tumor-acidity-activated targeted drug elivery. Biomater Sci. 2018;6(2):350–355. doi:10.1039/c7bm01025g
144. Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–255. doi:10.1038/nnano.2007.70
145. Park JH, von Maltzahn G, Zhang L, et al. Systematic surface engineering of magnetic nanoworms for in vivo tumor targeting. Small. 2009;5(6):694–700. doi:10.1002/smll.200801789
146. Zengeler KE, Lukens JR. Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders. Nat Rev Immunol. 2021;21(7):454–468. doi:10.1038/s41577-020-00487-7
147. Balkaya M, Prinz V, Custodis F, et al. Stress worsens endothelial function and ischemic stroke via glucocorticoids. Stroke. 2011;42(11):3258–3264. doi:10.1161/STROKEAHA.110.607705
148. Da Silva-Candal A, Brown T, Krishnan V, et al. Shape effect in active targeting of nanoparticles to inflamed cerebral endothelium under static and flow conditions. J Control Release. 2019;309:94–105. doi:10.1016/j.jconrel.2019.07.026
149. Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. doi:10.1126/science.aag2590
150. Zou Y, Wang Y, Xu S, et al. Brain Co-Delivery of Temozolomide and Cisplatin for Combinatorial Glioblastoma Chemotherapy. Adv Mater. 2022;34(33):e2203958. doi:10.1002/adma.202203958
151. Zou Y, Sun Y, Wang Y, et al. Cancer cell-mitochondria hybrid membrane coated Gboxin loaded nanomedicines for glioblastoma treatment. Nat Commun. 2023;14(1):4557. doi:10.1038/s41467-023-40280-3
152. Beaton L, Bandula S, Gaze MN, Sharma RA. How rapid advances in imaging are defining the future of precision radiation oncology. Br J Cancer. 2019. doi:10.1038/s41416-019-0412-y
153. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127–157. doi:10.3322/caac.21552
154. Yang Y, Aw J, Xing B. Nanostructures for NIR light-controlled therapies. Nanoscale. 2017;9(11):3698–3718. doi:10.1039/c6nr09177f
155. Kenry Duan Y, Liu B. Recent advances of optical imaging in the second near-infrared window. Adv Mater. 2018;30(47):e1802394. doi:10.1002/adma.201802394
156. Ren F, Ding L, Liu H, et al. Ultra-small nanocluster mediated synthesis of Nd(3+)-doped core-shell nanocrystals with emission in the second near-infrared window for multimodal imaging of tumor vasculature. Biomaterials. 2018;175:30–43. doi:10.1016/j.biomaterials.2018.05.021
157. Zhang X, Shuqing D, Bingbing Q, et al. Cancer cell membrane-coated rare earth doped nanoparticles for tumor surgery navigation in NIR-II imaging window. Chem Eng J. 2020;385.
158. Jaiswal S, Roy R, Dutta SB, et al. Role of doxorubicin on the loading efficiency of ICG within silk fibroin nanoparticles. ACS Biomater Sci Eng. 2022;8(7):3054–3065. doi:10.1021/acsbiomaterials.1c01616
159. Chaudhary Z, Khan GM, Abeer MM, et al. Efficient photoacoustic imaging using indocyanine green (ICG) loaded functionalized mesoporous silica nanoparticles. Biomater Sci. 2019;7(12):5002–5015. doi:10.1039/c9bm00822e
160. Porcu EP, Salis A, Gavini E, Rassu G, Maestri M, Giunchedi P. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol Adv. 2016;34(5):768–789. doi:10.1016/j.biotechadv.2016.04.001
161. Chen Z, Zhao P, Luo Z, et al. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10(11):10049–10057. doi:10.1021/acsnano.6b04695
162. Wang Y, Xu Y, Song J, et al. Tumor cell-targeting and tumor microenvironment-responsive nanoplatforms for the multimodal imaging-guided photodynamic/photothermal/chemodynamic treatment of cervical cancer. Int J Nanomed. 2024;19:5837–5858. doi:10.2147/IJN.S466042
163. Xu Z, Zhou H, Li T, et al. Application of biomimetic nanovaccines in cancer immunotherapy: a useful strategy to help combat immunotherapy resistance. Drug Resist Updat. 2024;75:101098. doi:10.1016/j.drup.2024.101098
164. van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16(4):219–233. doi:10.1038/nrc.2016.16
165. Lokhov PG, Balashova EE. Cellular cancer vaccines: an update on the development of vaccines generated from cell surface antigens. J Cancer. 2010;1:230–241. doi:10.7150/jca.1.230
166. Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):28. doi:10.1186/s13045-022-01247-x
167. Li S, Dong S, Wu J, et al. Surgically derived cancer cell membrane-coated R837-loaded poly(2-oxazoline) nanoparticles for prostate cancer immunotherapy. ACS Appl Mater Interfaces. 2023;15(6):7878–7886. doi:10.1021/acsami.2c22363
168. Ren H, Li J, Zhang J, et al. Anti-tumor immunity induced by a ternary membrane system derived from cancer cells, Dendritic Cells, and Bacteria. Small. 2023;19(50):e2302756. doi:10.1002/smll.202302756
169. Poore GD, Kopylova E, Zhu Q, et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579(7800):567–574. doi:10.1038/s41586-020-2095-1
170. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi:10.1126/science.aao3290
171. Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 2016;14(5):320–330. doi:10.1038/nrmicro.2016.34
172. Fan Y, Li XD, He PP, et al. A biomimetic peptide recognizes and traps bacteria in vivo as human defensin-6. Sci Adv. 2020;6(19):eaaz4767. doi:10.1126/sciadv.aaz4767
173. Chen S, Zhang L, Li M, et al. Fusobacterium nucleatum reduces METTL3-mediated m(6)A modification and contributes to colorectal cancer metastasis. Nat Commun. 2022;13(1):1248. doi:10.1038/s41467-022-28913-5
174. Sommer MOA, Dantas G, Church GM. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science. 2009;325(5944):1128–1131. doi:10.1126/science.1176950
175. Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology. 2017;152(4):851–866e824. doi:10.1053/j.gastro.2016.11.018
176. Parhi L, Alon-Maimon T, Sol A, et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 2020;11(1):3259. doi:10.1038/s41467-020-16967-2
177. Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. doi:10.1016/j.chom.2013.07.007
178. Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. doi:10.1016/j.immuni.2015.01.010
179. Galeano Niño JL, Wu H, LaCourse KD, et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature. 2022;611(7937):810–817. doi:10.1038/s41586-022-05435-0
180. Casasanta MA, Yoo CC, Udayasuryan B, et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci Signal. 2020;13(641):eaba9157. doi:10.1126/scisignal.aba9157
181. Chen L, Zhao R, Shen J, et al. Antibacterial Fusobacterium nucleatum-mimicking nanomedicine to selectively eliminate tumor-colonized bacteria and enhance immunotherapy against colorectal cancer. Adv Mater. 2023;35(45):e2306281. doi:10.1002/adma.202306281
182. Costantini M, Ripamonti C, Beccaro M, et al. Prevalence, distress, management, and relief of pain during the last 3 months of cancer patients’ life. Results of an Italian mortality follow-back survey. Ann Oncol. 2009;20(4):729–735. doi:10.1093/annonc/mdn700
183. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, Van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi:10.1093/annonc/mdm056
184. Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–577. doi:10.1126/science.aaf8924
185. Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain. 2013;154(Suppl 1):S54–S62. doi:10.1016/j.pain.2013.07.044
186. Dreghorn CR, Newman RJ, Hardy GJ, Dickson RA. Primary tumors of the axial skeleton. Experience of the Leeds Regional Bone Tumor Registry. Spine. 1990;15(2):137–140. doi:10.1097/00007632-199002000-00018
187. Lam DK, Schmidt BL. Orofacial pain onset predicts transition to head and neck cancer. Pain. 2011;152(5):1206–1209. doi:10.1016/j.pain.2011.02.009
188. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi:10.1158/1078-0432.CCR-06-0931
189. Zheng C, Zhang D, Kong Y, et al. Dynamic regulation of drug biodistribution by turning tumors into decoys for biomimetic nanoplatform to enhance the chemotherapeutic efficacy of breast cancer with bone metastasis. Exploration. 2023;3(4):20220124. doi:10.1002/EXP.20220124
190. Hassan MNFB, Yazid MD, Yunus MHM, et al. Large-scale expansion of human mesenchymal stem cells. Stem Cells Int. 2020;2020:9529465. doi:10.1155/2020/9529465
191. Rao L, Cai B, Bu LL, et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11(4):3496–3505. doi:10.1021/acsnano.7b00133
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.