Back to Journals » Journal of Pain Research » Volume 18
Percutaneous Endoscopic Backhand Holding (EBH) Technique for Extraforaminal Lumbar Disc Herniations: A Modified Operative Technique
Authors Kong M , Gao C, Hao M, Ma X, Zhao J, Luan J, Lin Y, Jin C, Li Q
Received 24 October 2024
Accepted for publication 1 April 2025
Published 16 April 2025 Volume 2025:18 Pages 2069—2080
DOI https://doi.org/10.2147/JPR.S498090
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Meng Kong,1 Changtong Gao,2 Meng Hao,1 Xuexiao Ma,3 Jindong Zhao,1 Jian Luan,1 Yong Lin,1 Canghai Jin,1 Qiang Li1
1Department of Spine Surgery, Qingdao Municipal Hospital, Qingdao, Shandong, People’s Republic of China; 2Department of Oncology, Qingdao Municipal Hospital, Qingdao, Shandong, People’s Republic of China; 3Department of Spine Surgery, Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China
Correspondence: Qiang Li, Department of Spine Surgery, Qingdao Municipal Hospital, No. 5, Middle Dong Hai Road, Qingdao, Shandong Province, 266071, People’s Republic of China, Tel +8617669757975, Email [email protected] Canghai Jin, Department of Spine Surgery, Qingdao Municipal Hospital, No. 5, Middle Dong Hai Road, Qingdao, Shandong Province, 266071, People’s Republic of China, Tel +8618306483878, Email [email protected]
Background: Percutaneous endoscopic lumbar discectomy (PELD) is a minimally invasive surgical (MIS) procedure rapidly improved in the surgical treatment of lumbar disc herniation (LDH). For the treatment of extraforaminal lumbar disc herniations (ELDH), microendoscopic discectomy (MED) or traditional Transforaminal Lumbar Interbody Fusion (MIS-TLIF) are commonly used, but limitations exist due to bony resection. Many classic surgical approaches for PELD have inherent disadvantage when removing migrated ELDH.
Objective: We aimed to present the results of a series of PELD cases that were operated using a modified endoscopic backhand holding (EBH) approach for the treatment of ELDH.
Methods: Seventy-two patients diagnosed with ELDH who underwent PELD via the EBH approach from January 2018 to December 2022 were retrospectively assessed. Pre- and postoperative clinical data, radiographic findings, and surgical techniques were investigated. Neurological recovery examinations were performed preoperatively and at 3 days, 1 month, 3 months, 6 months, 1 year postoperatively.
Results: No major intraoperative complications were noted. Significant improvement in back and leg pain was observed in visual analogue scale (VAS) score, and the mean ODI was decreased from preoperative 78% (range = 60– 98%) to postoperative 30% (20– 40%) (P < 0.001) at 1-month follow-up and obtained further improvement at 1 year (mean ODI = 11%; range = 2– 20%). All patients showed progressive improvement in their initial neurological deficits, with complete recovery of motor weakness. According to the Macnab criteria, overall excellent and good outcomes were obtained in 67 patients (95.7%) – 57 excellent (81.4%), and 10 (14.3%) good – with fair outcomes in three patients (4.3%) at the time of last follow-up.
Conclusion: Percutaneous endoscopic backhand holding is a minimally invasive, safe, valuable, and efficacious surgical procedure for treating ELDH. It is important that surgeons perform PELD using the technique they know best to ensure successful implementation of the surgery.
Keywords: endoscopic backhand holding, PELD, extraforaminal lumbar disc herniations, modified technique, minimally invasive surgical procedure
Introduction
Lumbar disc herniation (LDH), a common cause of sciatica, is divided into several subtypes according to the location of the protrusion relative to the spinal horizontal plane: central, paracentral, foraminal, and extraforaminal herniations. Among the treatments for this disorder, discectomy is considered an effective method to achieve rapid pain relief. With the development of related instruments in recent years, percutaneous endoscopic lumbar discectomy (PELD) and microendoscopic discectomy (MED) have become popular minimally invasive spine surgical (MISS) procedures and have rapidly improved in the treatment of LDH.1 Patients who undergo MISS are more likely to return to normal activities because of smaller surgical scars and shorter hospital stays.
In 1974, far-lateral (extraforaminal) LDH (F/ELDH) was first defined as a condition where disc protrusion resided beneath or outside the foramen and could bring about clinical syndromes of mechanical compression or subsequent inflammatory stimulation to the exiting nerve root and its dorsal ganglion of responsible segment, and previous studies have reported a varied frequency around 1–12% of this subtype of LDH in various clinical series.2 Usually, more severe and medically resistant pain is identified in these patients compared with other common subtypes due to stimulation of the posterior root ganglion, and surgery should be considered after failure of conservative treatment.
Several techniques have been used for the surgical treatment of ELDH. From the point of view of the operative route, midline incisions and subperiosteal muscle dissection with hemi-laminectomy, partial or complete facetectomy, and paramedian incisions with intermuscular dissections through the intertransverse approach can all be accomplished in MED.3 However, significantly inferior clinical outcomes are often reported because a significant amount of bony resection (especially the zygapophyseal joint) is typically required, which may place the patient at risk of an unstable spine, necessitating spinal fusion surgery in the future. Otherwise, a wide and clear surgical field would not be attained if surgeons were afraid of postoperative segment instability and insufficient bone resection.
PELD through the posterolateral transforaminal approach allows an initial landing as close to the target herniation as possible and has been reported to obtain an effective discectomy without excessive resection of the facet joint.4 The Kambin,5 Yeung,6 Ruetten,7 and modified TESSYS techniques8 have been well described in the literature. However, each of these surgical approaches has limitations in removing extraforaminal disc herniation, especially upward-migrated subtypes. The TESSYS technique demonstrated an outside-in path that required systematic foraminotomy for subsequent resection of intracanal uni- or bilateral disc herniation. Because of its intraspinal tilting 30° camera and circular bevelled working cannula, operating space was always restricted to the inside 0–180° portion of the foramen on the coronal plane. TESSYS technique does not allow the resection of ELDH, as it is often ventrally or further outside, located at the transintervertebral portion of the exiting root. On the other hand, 10% of dysesthesias were reported due to nerve injury induced by extrusion of a working cannula.9
In the current study, we describe a modified operative technique that we named the “Endoscopic Backhand Holding (EBH) approach” and have performed over time, highlighting the surgical skills or tricks in the treatment of ELDH, no matter what kind of transforaminal endoscopic surgical system is applied.
Methods
Between January 2018 and December 2022, 72 patients who were diagnosed with ELDH and underwent PELD performed by the corresponding author via the EBH approach were retrospectively evaluated. This study was designed in accordance with the Declaration of Helsinki and approved by the institutional ethics review board (approval number: 2024-LW-062).
Inclusion/Exclusion Criteria
The inclusion criteria were as follows: 1) symptoms of radicular compression and signs of radiculopathy, as shown by a positive nerve root tension test (straight leg–raising test or femoral tension test) or a corresponding sign of neurological deficit (asymmetrical depressed tendon reflex, impaired sensation in a dermatomal distribution, or weakness in a myotomal distribution); 2) diagnosis of ELDH at the same lumbar segment indicated by computed tomography (CT) and/or magnetic resonance imaging (MRI) scans and no lateral recess stenosis radiologically; 3) failure of systemic conservative treatment and no intraspinal injection with steroids or nonsteroidal drugs within 4 weeks of surgery; and 4) completion of the preoperative and postoperative evaluations and follow-up for more than 12 months. Exclusion criteria were as follows: 1) reoperation at the affected lumbar segment; 2) coexistent lumbar instability, spondylolisthesis, or scoliosis; and 3) comorbidities that may disturb neurological rehabilitation, such as cerebral apoplexy and Parkinson’s disease.
Data Collection
Patient demographic data, symptom duration, operative time, duration of hospital stay, and follow-up duration were routinely recorded. To assess the safety and efficacy, radiographs, and CT/MRI findings were retrospectively reviewed to determine the perioperative complications, prognosis, and reoperation rate. Neurological recovery examinations were performed preoperatively and at 3 days, 1 month, 3 months, 6 months, and 1 year postoperatively. To be specific, preoperative and postoperative visual analogue scale (VAS) scores (0–10) were applied to assess back and leg pain relief (VAS-back and VAS-leg), then Oswestry disability index (ODI, 0–100%) and modified MacNab criteria were measured as the primary outcome to evaluate improvements in neurological function. In particular, four grades were determined in modified MacNab criteria: “Excellent” was defined as no pain, no restriction of mobility, patients received complete relief of symptoms and could return to normal work and activity; “Good” means marked improvement, patients could return to assuasive work but have occasional non-radicular pain, relief of presenting symptoms; “Fair” signifies some improved functional capacity, but handicapped by intermittent pain of sufficient severity to curtail or modify work or leisure activities and the need for pain medications; “Poor” indicates continued objective symptoms of root involvement or worsening, insufficient improvement to enable increase in activities.
Surgical Procedure
We performed all PELD surgeries for patients using the same type of Joimax TESSYS® Isee Spine system (Germany) under general anesthesia (GA) to control for interference factors. The patient was fixed on an operating table in a prone and abdominal-free position with the knee and hip flexed.
Under the guidance of C-arm fluoroscopy, the insertion point on the skin was located superior to the iliac crest, approximately 6–10 cm from the midline, which was designed according to the contour of the body and responsibility segment, and was inner compared to the traditional TESSYS technique. A spinal needle was inserted into the skin along a superolateral to inferomedial direction toward the ventral trunk axis, touching the tip of the superior articular process (SAP) of the inferior vertebra under AP and lateral fluoroscopic control. Depending on the height of the disc in practical segment, the inclination varied from 0° to 20° in the coronal plane of AP view (Line 1, Figure 1) and from 0° to 15° in the sagittal plane of lateral view (Line 2, Figure 1). Briefly, a puncture angle (Line a) more perpendicular to Kambin’s triangle from three-dimensional perspective was achieved (Figure 2). The point of this step is to keep the working sleeve away from the exiting root as much as possible.
The needle was then replaced with a guidewire, the surgical pathway was consecutively expanded to 7 mm using a series of dilators, and a beveled working sleeve was introduced into the dorsal portion of the foramen with an opening facing wrapping around the superior articular process of the inferior vertebra (Figure 1C and D). Then an endoscope was introduced, and the surface of bony anatomical landmarks of SAP was deburred and exposed with a trigger-flex radiofrequency probe. A circular bone saw was used to perform appropriate foraminotomy for visualization if hypertrophy of the facet joint was identified (Figure 3A). A small range of foraminotomy was routinely performed to expose the outer margin of the transforaminal ligamentum flavum (Figure 3B) and, more importantly, to clear the operating space for the following Endoscopic Backhand Holding procedure.
After the disc-flava ligament space was recognized, the beveled working tube was rotated to open facing toward the head direction and was anticlockwise on the left side and clockwise on the right side of the trunk. During this maneuver, the exiting nerve root was located ventrally to the tube, and was often simultaneously sandwiched by the herniated disc fragment (Figure 3C). Hence, appropriate foraminotomy to dilate the space outside the intervertebral foramen10 was necessary to avoid irritation of the nerve. Next, the region of Kambin’s triangle11 was identified in a 90° headward rotating view, and epidural fat here was separated to further trace the anteriorly bordered exiting nerve root (Figure 4). We then continued to revolve the sleeve until its bevel faced outward. In the meanwhile, the 30° lens equipped endoscope should be handled with a consequent backhand shift (Figure 5A). Subsequently, the endoscopic view was switched to the external vertebral canal orientation, in which the entire journey of the exiting nerve root (obliquely downward from Kambin’s triangle and cross the disc to the anterior side) and ganglion should be visualized (Figure 5B and C). Then, via gentle movements of the working cannula, the herniated fragment could be unforcedly identified, pushing the nerve root dorsally upward and removing it using grasping forceps. It should be emphasized that a 180° horizontal reversed endoscopic view was transmitted to the display screen, and which required surgeons to adaptively make an inverse spatial sense in two-dimensional space during anatomical recognition and protrusion excision (Figure 5D). The skin incision was then sutured.
The modified technique can be divided into two steps based on the operative sequence. The first step was actually similar to the transforaminal approach through a forehand holding of endoscope that obtained 0–180° of intraspinal-oriented view, and then (the second step) gradually shifted to an extraforaminal approach via a backhand holding of the endoscope to make the other half of the extraspinal space visualized. Distinction of the key operating points in several techniques for treating ELDH were listed in Table 1.
 |
Table 1 Studies Involved Different Techniques Treating Extraforaminal Lumbar Disc Herniations (ELDH) |
Patients were routinely administered a small dose of steroids and mannitol 2 days postoperatively, and they were advised to ambulate wearing a lumbar brace the next day and maintain it for 1 month.
Statistical Analysis
Statistical analyses were performed using the SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA). Descriptive statistics (mean, maximum, and minimum) were calculated. The Wilcoxon test was used for pre- and postoperative comparisons of deviation-distributed variables. Statistical significance was defined as P < 0.05.
Results
Preoperative Demographic Characteristics and Outcomes
We performed a median follow-up period of 18.7 months (range = 12–30 months) for 72 patients, including 33 males and 39 females. The mean age at the time of operation was 51.6 years (range = 27–78 years), and the average duration of symptoms was 8.9 weeks (range = 1–16). All patients underwent single-level PELD successfully, and no patient was transferred to other alternative surgical methods or underwent repeated PELD due to incomplete removal of the ruptured particle. The operative segments were located at level L2/3 in 6 (8.3%), L3/4 in 24 (33.3%), L4/5 in 27 (37.5%), and L5/S1 in 15 (20.8%) patients (Table 2).
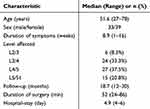 |
Table 2 Demographic Characteristics |
No major complications, such as intraspinal canal infection, dural laceration, cerebrospinal fluid (CSF) leakage, or obvious dural injury were encountered intraoperatively. Only two patients complained of transient paresthesia and intraoperative irritation of the exiting nerve root by radiofrequency bipolar, and these symptoms improved after treatment with gabapentin and mecobalamin within 1 month. The mean operation time was 52.0 minutes (range =, 24–86 minutes) and blood loss was minimal in all cases (not accurately disturbed by flushing water). The mean length of hospital stay was 4.9 days.
Clinical Results
The neural functional recovery of the patients is summarized in Tables 3 and 4. On the day of discharge, significant improvements in back and leg pain were observed, with an average decrease in the VAS score from 3.4 (range = 1–5) and 7.8 (range = 5–10) before surgery to 2.3 (range = 0–3) and 2.1 (range = 0–6), respectively. Alleviation of back and radicular pain was statistically significant (P < 0.001). The mean back pain score further decreased to 1.7 (range = 0–4) at 1 month and 1.2 (range = 1–3) at 3 months postoperative. The VAS of leg pain also remitted to 1.6 (range = 1–3) at 1 month and 1.7 (range = 0–3) at 3 months follow-up. Two patients experienced recurrence of ipsilateral paracentral disc herniation at the primary level (L4/5, L3/4) approximately 4 and 6 months after surgery, respectively, and all required re-PELD after appropriate conservative therapy and were excluded from this study. The remaining 70 cases presented with a mean leg pain score alleviation of 0.5 (range = 0–3) and the same level of back pain (1.2; range 0–3) at 1-year post-operation, which was not significantly different from the statistical value at the final follow-up.
 |
Table 3 VAS and ODI Improvements at Different Time Points |
 |
Table 4 Modified MacNab Criteria to Measure Outcome and Distribution of Patients in This Study |
The mean ODI score decreased from a preoperative 78% (range = 60–98%) to postoperative 30% (range = 20–40%) (P < 0.001) at 1-month follow-up. Further improvement occurred at 1 year (mean ODI = 11%; range = 2–20%) and was maintained at final follow-up at a mean of 10% (range = 2–20%, P = 0.21).
According to the Macnab criteria, overall excellent and good outcomes were obtained in 67 patients (95.7%) – 57 excellent (81.4%), and 10 (14.3%) good – with fair outcomes in three patients (4.3%) at the time of last follow-up.
Discussion
Due to the limited visual field and operating space, as well as the steep learning curve, dural tears, nerve root injury, incomplete herniation resection, and recurrence are common complications in performing PELD, while ELDH cases usually pose more technical challenges to surgeons in offering PELD than their common median or para-median disc herniation counterparts. The current technique is an expanding application and evolution of the TESSYS technique, which does not require sophisticated and costly instruments, and has proven to be a safer and simpler approach for treating ELDH. The results we share here indicate that its advantages include satisfactory neural decompression, fewer complications, and handlability for multiple types of extraspinal herniations.
As previous studies have described PELD, a limited view and deficient access to herniated disc fragments might cause surgical failure, and it is critical to adopt an available approach to adequately expose extra-canal extruded fragments when treating ELDH,18,19 especially in cases where the protrusion was cranially migrated or located at the shoulder of the exiting nerve root. In the present case series, we introduced a modified method by ingeniously manipulating surgical instruments in a trajectory more horizontal to the disc axial plane and perpendicular to the coronal plane than in the traditional TESSYS technique.9 Thus, an omnibearing flexible view revolving around Kambin’s triangle was obtained without difficulty, which would be clearer than the MED benefit from its water-flushing trait and a certain degree of magnification. Based on this advantage, the nucleus forceps can easily access the disc fragments across the ventral side of the exiting nerve root and simultaneously avoid undetected traction-related injury to the nerve during the rotation of the working sleeve. It must be emphasized that this technique is also suitable for treating the lumbosacral level (at L5/S1 with a higher iliac crest or hypertrophic L5 processus transversus) because of its midline closer puncture point, and its more vertical sleeve would facilitate the identification of the L5 nerve clearly outside of the neuroforamen with minimal irritation on the ganglion. In actual clinical work, deeper serendipity was discovered in the treatment of far-out syndrome by PELD,20 in which the nerve root canal decompression procedure could be achieved under a laterally oriented view by abrasing the hypertrophic bone structure (the transverse process of L5 and the ala of the sacrum) with a high-speed drill and removing impingement of the L5 nerve (Figure 6 and Supplementary Figure 1). Under the evaginable endoscopic view, the head of the drill could easily access the lateral side of the vertebral body and lower end of the transverse process.
In an attempt to trace the exiting nerve root from Kambin’s triangle and follow the surgical steps described previously, anatomical landmarks such as the ventral epidural space of the SAP in the intervertebral foramen usually need to be recognized. The inherent anatomical space from the exiting nerve root and bone of the SAP in Kambin’s triangle cannot be ignored, which could create a sleeve squeezing nerve injury by pushing it against the osseous surface when performing PELD through the foramen.21 In the current study, we observed that the vertical distance from the exiting nerve root to the anterior edge of the SAP was remarkably narrow and thinner than previously reported.10 Obviously, the inherent anatomic space outside the intervertebral foramen was not broad enough to accommodate a 7.5-mm working tube and permit its rotating maneuver. Therefore, appropriate foraminoplasty in PELD is usually necessary to ensure gentle lateral dislocation of the exiting nerve and avoid irritation of the nerve in the foramen, even in the treatment of ELDH.
In the treatment of LDH, traditional open lumbar discectomy through a paraspinal Wiltse approach is considered the gold standard but is associated with the incidence of postoperative low back pain.22 Several minimally invasive spinal surgical procedures, such as endoscopic, microscopic, and tubular technologies, have been widely adopted because of the reduced incidence of tissue damage. Studies have also confirmed that these techniques can achieve good clinical outcomes and improve the quality of life of patients.23 In practice, the optimum operational choice largely depends on the type of disc herniation, and this issue might be less concerning for intraspinal disc herniations. However, this seemed inappropriate for the extraforaminal type of LDH because certain limitations exist in the posterior approach assisted by the tube. When treating ELDH using the posterior approach, the surgical tube is usually oriented to the junction of the articular process, and the transverse process and resection of the articular process are routinely required,24,25 which can easily lead to instability of the corresponding segment. Otherwise, the limited visual field results in unsatisfactory surgical effects due to less facetectomy.26
Regarding optimal anesthetic methods during PELD, controversy often surrounds the feasibility, efficacy, and safety of local (LA) and general anesthesia (GA). Although LA is mostly recommended to reduce the risk of nerve injury,27 pain experienced by patients can represent unpredictable challenges for the successful completion of the operation. Thus, PELD under GA was reported to be an alternative choice that is more psychologically comfortable for patients but may lead to an increased risk of neurologic injury since no subjective feedback of patients was recognized by surgeons during surgery. It was reported that PELD under GA exhibited more postoperative dysesthesia, varying degrees of muscular paralysis, and poor McNab scores at the first postoperative follow-up, possibly due to intense nerve root traction with a lack of patient feedback.28 However, it is important to emphasize that patients with ELDH usually experience severe radiculalgia because of the direct compression and strain to the exiting nerve root by extruded fragments. In most cases, they had to adopt a peculiar compulsive position to relieve symptoms before surgery and could not consciously cooperate with a prone and abdomen-free position while simultaneously flexing the knee and hip. In addition, the exiting nerve root would likely be irritated when rotating the sleeve and tracking the protrusion during the operation. In fact, GA promotes favorable conditions for surgeons to explore residual disc material and achieve optimal decompression by adequately mobilizing nerve roots rather than being distracted by the patient’s discomfort under LA. In addition, effective intraoperative neuromonitoring (IONM) has proven to be of great benefit in performing challenging surgeries safely and successfully, which could bring prompt and accurate changes in signals to be recognized by the surgeon, anesthesiologist, and monitoring personnel when overstimulated manipulation occurred.29 Sometimes, the waking state of patients might help surgeons determine the best time to abort surgery because patients are able to convey their subjective feelings after effective discectomy. To reduce the possibility of disc protrusion residue under GA, we highlight the advantages of the current operative technique for treating ELDH. Under the omnibearing flexible view, the spatial position and entire epispinal journey of the exiting nerve root could be adequately traced, and full nerve decompression could be subsequently performed. Nerve fluctuations accompanied by water pressure changes are an important indirect indication for sufficient endoscopic disc resection. In addition, careful reading of preoperative scans (MRI/CT) has been recommended to measure the distance from not only the herniation but also the exiting root to the facet at different levels of the disc.30 In this retrospective study, no residual disc fragments were observed according to immediate postoperative MRI scan reexamination, which was superior to a previously reported 2.8–15% of patients who underwent PELD.31 As a result, the early reoperation rate then decreased, which was mostly associated with the presence of symptomatic residual disc tissue.32 Thus, the best curative effect would be achieved with maximal safety when treating ELDH with PELD under GA.
Limitations
Our study has some limitations. Firstly, the retrospective design and the limited number of cases are the main inherent limitations of this study, and they may have led to biases. In addition, all cases were operated by EBH technique that the corresponding author was familiar with, and results are not comparable to other techniques. The comparation about operative time, symptom relief, and complications with a control group would make the conclusion more reliable, so finding more evidence-based medical data should be significant in our next work to facilitate the promotion of EBH technique in treating ELDH. Therefore, relatively long-term follow-up duration is also required to corroborate the favorable effect of this technique.
Conclusion
EBH technique represents a series of modified operative skills in PELD that might be a valuable and effective choice for surgeons interested in single-channel endoscopic spinal surgery when curing ELDH. Our study does not emphasize the superiority of EBH technique in PELD over conventional MED or MIS-TLIF in currently established methods but hoping this methodology will imbue surgeons with greater confidence and decrease the anxiety associated with nerve injury and disc residue.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This work was supported by grants from the Key Technology Research and Industrialization Demonstration Project of Qingdao (23-1-4-xxgg-11-nsh) and Medical Health Science and Technology Project of Shandong Province (202304070263).
Disclosure
The authors declare no conflicts of interest related to this work.
References
1. Chen Z, Zhang L, Dong J, et al. Percutaneous transforaminal endoscopic discectomy compared with microendoscopic discectomy for lumbar disc herniation: 1-year results of an ongoing randomized controlled trial. J Neurosurg Spine. 2018;28(3):300–310. doi:10.3171/2017.7.SPINE161434
2. Abdullah AF, Ditto EW, Byrd EB, Williams R. Extreme-lateral lumbar disc herniations. Clinical syndrome and special problems of diagnosis. J Neurosurg. 1974;41(2):229–234. doi:10.3171/jns.1974.41.2.0229
3. Smith N, Masters J, Jensen C, Khan A, Sprowson A. Systematic review of microendoscopic discectomy for lumbar disc herniation. European Spine J. 2013;22(11):2458–2465. doi:10.1007/s00586-013-2848-8
4. Yoshikane K, Kikuchi K, Okazaki K. Posterolateral transforaminal full-endoscopic lumbar discectomy for foraminal or extraforaminal lumbar disc herniations. World Neurosurg. 2021;146:e1278–e1286. doi:10.1016/j.wneu.2020.11.141
5. Kambin P, Gellman H. Percutaneous lateral discectomy of the lumbar spine a preliminary report. Clin Orthopaedics Related Res. 1983;174(174):127–132. doi:10.1097/00003086-198304000-00017
6. Yeung AT. Minimally invasive disc surgery with the Yeung endoscopic spine system (YESS). Surgical Technol Int. 1999;8:267–277.
7. Ruetten S, Komp M, Godolias G. An extreme lateral access for the surgery of lumbar disc herniations inside the spinal canal using the full-endoscopic uniportal transforaminal approach-technique and prospective results of 463 patients. Spine. 2005;30(22):2570–2578. doi:10.1097/01.brs.0000186327.21435.cc
8. He S, Sun Z, Wang Y, Ma D, Tan W, Lai J. Combining YESS and TESSYS techniques during percutaneous transforaminal endoscopic discectomy for multilevel lumbar disc herniation. Medicine. 2018;97(28):e11240. doi:10.1097/MD.0000000000011240
9. Xin G, Shi-Sheng H, Hai-Long Z. Morphometric analysis of the YESS and TESSYS techniques of percutaneous transforaminal endoscopic lumbar discectomy. Clin Anatomy. 2013;26(6):728–734. doi:10.1002/ca.22286
10. Zhang L, Yang J, Hai Y, et al. Relationship of the exiting nerve root and superior articular process in kambin’s triangle: assessment of lumbar anatomy using cadavers and computed tomography imaging. World Neurosurg. 2020;137:e336–e342. doi:10.1016/j.wneu.2020.01.195
11. Yeung AT, Tsou PM Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine (Phila Pa 1976). 2002;27(7):722–731.
12. Lew SM, Mehalic TF, Fagone KL Transforaminal percutaneous endoscopic discectomy in the treatment of far-lateral and foraminal lumbar disc herniations. J Neurosurg. 2001;94(2 Suppl).
13. Ruetten S, Komp M, Godolias G An extreme lateral access for the surgery of lumbar disc herniations inside the spinal canal using the full-endoscopic uniportal transforaminal approach-technique and prospective results of 463 patients. Spine (Phila Pa 1976). 2005;30(22):2570–2578.
14. Choi G, Lee SH, Bhanot A, Raiturker PP, Chae YS Percutaneous endoscopic discectomy for extraforaminal lumbar disc herniations: extraforaminal targeted fragmentectomy technique using working channel endoscope. 2007;32(2). Spine (Phila Pa 1976).
15. Lübbers T, Abuamona R, Elsharkawy AE Percutaneous endoscopic treatment of foraminal and extraforaminal disc herniation at the L5-S1 level. Acta Neurochir (Wien). 2012;154(10):1789–1795.
16. Yoshikane K, Kikuchi K, Okazaki K Posterolateral Transforaminal Full-Endoscopic Lumbar Discectomy for Foraminal or Extraforaminal Lumbar Disc Herniations. World Neurosurg. 2021;146:e1278–e1286.
17. Fanous AA, Tumialán LM, Wang MY. Kambin’s triangle: definition and new classification schema. J Neurosurg Spine. 2019;32(3):390–398. doi:10.3171/2019.8.SPINE181475
18. Lee JS, Woo JY, Jang JS, Jang IT. Combined interlaminar and paraisthmic approach for co-existing intracanal and foraminal lesion. Korean J Spine. 2015;12(4):256–260. doi:10.14245/kjs.2015.12.4.256
19. Sairyo K, Higashino K, Yamashita K, et al. A new concept of transforaminal ventral facetectomy including simultaneous decompression of foraminal and lateral recess stenosis: technical considerations in a fresh cadaver model and a literature review. J Med Investigation. 2017;64(1.2):1–6. doi:10.2152/jmi.64.1
20. Kikuchi K, Abe E, Miyakoshi N, et al. Anterior decompression for far-out syndrome below a transitional vertebra: a report of two cases. Spine J. 2013;13(8):e21–25. doi:10.1016/j.spinee.2013.02.033
21. Wang H, Huang B, Li C, et al. Learning curve for percutaneous endoscopic lumbar discectomy depending on the surgeon’s training level of minimally invasive spine surgery. Clin Neurol Neurosurg. 2013;115(10):1987–1991. doi:10.1016/j.clineuro.2013.06.008
22. Choi KC, Kim JS, Lee DC, Park CK. Percutaneous endoscopic lumbar discectomy: minimally invasive technique for multiple episodes of lumbar disc herniation. BMC Musculoskeletal Disord. 2017;18(1):329. doi:10.1186/s12891-017-1697-8
23. Zhang Y, Chong F, Feng C, Wang Y, Zhou Y, Huang B. Comparison of endoscope-assisted and microscope-assisted tubular surgery for lumbar laminectomies and discectomies: minimum 2-year follow-up results. BioMed Research International. 2019;2019:5321580. doi:10.1155/2019/5321580
24. Tsukamoto A, Oyama A, Yoshimoto M, Iba K, Yamashita T. Extraforaminal stenosis at L2-L3 treated with microendoscopic surgery: report of two cases. J Orthopaedic Case Reports. 2022;12(1):71–74. doi:10.13107/jocr.2022.v12.i01.2624
25. Al-Khawaja DO, Mahasneh T, Li JC. Surgical treatment of far lateral lumbar disc herniation: a safe and simple approach. J Spine Surg. 2016;2(1):21–24. doi:10.21037/jss.2016.01.05
26. Serhan HA, Varnavas G, Dooris AP, Patwadhan A, Tzermiadianos M. Biomechanics of the posterior lumbar articulating elements. Neurosurgical Focus. 2007;22(1):E1. doi:10.3171/foc.2007.22.1.1
27. Sairyo K, Egawa H, Matsuura T, et al. State of the art: transforaminal approach for percutaneous endoscopic lumbar discectomy under local anesthesia. J Med Investigation. 2014;61(3–4):217–225. doi:10.2152/jmi.61.217
28. Chen HT, Tsai CH, Chao SC, et al. Endoscopic discectomy of L5-S1 disc herniation via an interlaminar approach: prospective controlled study under local and general anesthesia. Surg Neurol Int. 2011;2(93). doi:10.4103/2152-7806.82570
29. Hofler RC, Fessler RG. Intraoperative neuromonitoring and lumbar spinal instrumentation: indications and utility. Neurodiagnostic J. 2021;61(1):2–10. doi:10.1080/21646821.2021.1874207
30. Hurday Y, Xu B, Guo L, et al. Radiographic measurement for transforaminal percutaneous endoscopic approach (PELD). European Spine J. 2017;26(3):635–645. doi:10.1007/s00586-016-4454-z
31. Baek J, Yang SH, Kim CH, et al. Postoperative longitudinal outcomes in patients with residual disc fragments after percutaneous endoscopic lumbar discectomy. Pain Physician. 2018;21(4):E457–e466.
32. Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery. 2015;76(4):372–380. discussion 380-371; quiz 381. doi:10.1227/NEU.0000000000000628
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







