Back to Journals » Patient Related Outcome Measures » Volume 16
Pharmacist Led Telephonic Insulin Titration: A Pilot Study on A1C Control in a Family Medicine Residency
Authors Raghavan P , Chamberlin S , Heidel RE, Wilson GA
Received 15 November 2024
Accepted for publication 4 March 2025
Published 13 March 2025 Volume 2025:16 Pages 79—84
DOI https://doi.org/10.2147/PROM.S502402
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Howland
Priya Raghavan,1 Shaunta Chamberlin,1 Robert Eric Heidel,2 Garland Anthony Wilson1
1Department of Family Medicine, UT Health Science Center Graduate School of Medicine, Knoxville, TN, USA; 2Department of Surgery, UT Health Science Center Graduate School of Medicine, Knoxville, TN, USA
Correspondence: Priya Raghavan, Department of Family Medicine, UT Health Science Center Graduate School of Medicine, 1924 Alcoa Hwy, U-67, Knoxville, TN, 37920, USA, Email [email protected]
Introduction
Diabetes is a major driver of morbidity, mortality, and health care costs within the United States. The Center for Disease Control and Prevention1 in the National Diabetes Statistics Report estimates that nearly 1 in 10 Americans are living with diabetes, or approximately, 11.6% of the US population has diabetes, reporting it as the 8th leading cause of death in 2020. Furthermore, they reported that 3.6 million people 20 years or older, or 12.3%, of all US adults with diagnosed diabetes are started on insulin within a year of diagnosis. The Center for Disease Control and Prevention2 reports that approximately $1 in every $4 health care dollars is spent on caring for people with diabetes. For persons with diabetes, between 48% and 64% of lifetime medical costs are spent on complications related to diabetes. As such, finding more strategies to improve patient access to monitoring and titration of their insulin to better control their diabetes is highly important.
The Covid-19 pandemic exposed a major need in the health care system to improve access to patients with chronic care needs. Diabetic management suffered during the pandemic, Czeisler MÉ, Barrett CE, Siegel KR, et al3 reported both delayed and reduced in-office visits and reduced access to diabetic medications. Additionally, Fragala MS, Kaufman HW, et al4 found decreased diabetic monitoring was observed as evidenced by reduced A1C reporting. It became evident during the Covid-19 pandemic that alternative modalities are needed for diabetes medication management when there is limited access to in-person evaluation.
Sorli C and Heile MK5 identified that many primary care physicians are slow to both initiate and titrate insulin regimens, which is why it is so important to utilize other experts to optimize regimens. Physicians reported in this study that confidence, time and fear are some of the primary drivers for not initiating or utilizing insulin in the treatment of diabetes. Pharmacists are uniquely positioned to assist in the management of diabetes because of their expertise in medication management and frequent interactions with both providers and patients as discussed by Patel D et al.6 Weidman-Evans E, et al7 showed that both frequent telephonic titration and pharmacist titration of insulin have a significant reduction in A1C and glucose compared to insulin titration with in-office visits. Additionally, collaborative medication management between providers and pharmacists not only improves patient outcomes but also decreases overall health expenditures.8 The pandemic revealed a major gap in care for patients with diabetes that needs to be filled. Though the pandemic has passed, there are still patients that have difficulty with consistently coming to in-person appointments, whether it is due to lack of transportation or limited mobility. While there are some studies that look at pharmacist-driven insulin titration in a residency setting, no studies currently exist that have evaluated the use of telephonic insulin titration in a residency practice during a pandemic. Because this study occurred during the pandemic, it was able to show that clinical practices have not significantly changed in their management of diabetes and elucidated the need to utilize virtual modalities and other experts in the field for care.
The goal of this study was to evaluate whether telephonic insulin titration by a clinical pharmacist was as effective at improving A1C compared to in-office titration among patients in a medical residency clinic serving primarily Medicaid and Medicare patients. We hypothesize that telephonic insulin titration by a clinical pharmacist is as effective as in-person insulin titration.
Methods
This pilot study occurred in a Family Medicine resident clinic at the University of Tennessee. Providers practicing in clinic include 25 medical residents (9 post-graduate year one, 8 post-graduate year two, and 8 post-graduate year 3), 12 faculty physicians, and one nurse practitioner. The applicability of this study is limited to other primary care practices with access to clinical pharmacy staff. The EMR software utilized in this clinic is GE Centricity Practice Solution. The residency clinic mainly serves the Medicare and Medicaid populations and works closely with the hospital’s pharmacy residency program. The Family Medicine faculty included a full-time, residency-trained clinical pharmacist associated with the pharmacy residency program. The faculty pharmacist and pharmacy residents had already established telephonic diabetes medication management, including insulin titration, visits under a collaborative practice agreement. The faculty clinical pharmacist has several years of experience working with and training both pharmacy and family medicine residents in the management of diabetes. She tracked the patients with diabetes being followed by pharmacy and was available for all questions or concerns from the pharmacy and physician residents. Patient referral for telephonic insulin titration was provider dependent; there was no minimum or maximum A1C required for the clinical pharmacists to work with the patients. The clinical pharmacist calls to the patients were typically once a week or every other week. The regular calls to patients and the active insulin dosing were documented in the chart as a phone note, and this was used to identify patient adherence to titration protocols. There was no specific script used to invite patients to participate in pharmacy-driven titration or during the phone calls.
This is a retrospective cohort study utilizing data previously collected by a clinical pharmacist for patient monitoring and from the EMR. This study was approved by and determined to be exempt by the University of Tennessee IRB, approval number 4755.
The primary outcome of this study assessed the change in A1C between the control and experimental group from the index date to three months. Three months was used as the time-frame as that is the standard time-frame used in the evaluation and treatment of diabetes. A change in A1C of 0.5% was considered significant. For the experimental group, this period was defined as the A1C at initiation with telephonic titration by a clinical pharmacist (± 30 days) and after three months (± 30 days). For the control group, this was defined as the A1C first documented during the intervention period (± 30 days) and then again after three months (± 30 days). There were n = 48 individuals identified in the experimental group and n = 45 individuals in the control group.
The inclusion criteria for this study were patients 18–70 years old with insulin-dependent type 1 or 2 diabetes. The patients had to have been referred to the pharmacist for insulin titration between the dates of April 2020 to April 2021. Patients excluded were those who followed endocrinology for their diabetic management and those who had bariatric surgery during the intervention period. Additionally, patients that did not have an A1C documented in the 3-month follow-up period were excluded from the study.
The control and experimental groups were matched by their time on insulin (new, <five years, >five years), newness to the clinic, insulin regimen (basal, bolus or both), insurance type, and index date. Index date was defined as the first A1C documented at initiation with pharmacist management during the intervention period for the experimental group, and the first A1C documented during the intervention period for the control group. The groups were defined at three months intervals: Group one (April–June), Group two (July–September), Group three (Oct–Dec), and Group four (Jan–April).
Socioeconomic status was indirectly controlled for using insurance type. Time on insulin was used to control for possible response to insulin regimens. Confounding factors not addressed include medication adherence, diabetic education and technical education on using insulin pens or syringes.
A power calculation was performed to adequately power the study. The researchers hypothesized that a small to moderate effect size (f = 0.17) associated with the treatment. Using the aforementioned effect size, an alpha value of 0.05, 80% power, two (2) independent groups, two (2) measurements across time (baseline and post-intervention), a correlation amongst repeated measures of 0.5, a nonsphericity correction of 1.0, and a hypothesized attrition rate of 20%, a total of n = 88 participants (n = 44 in each treatment arm) would be needed for adequate statistical power in the study. The sample size calculation was performed G*Power 3.1.
Data missingness was assessed using frequency and descriptive statistics. In the event of missing data points for the primary outcome, as per MCAR (missing completely at random) analysis, multiple imputation was used. Chi-square analysis was used to compare the intervention arms on insulin regiment, time on insulin, new to clinic, insurance type, and index date so that baseline differences in exposure could be assessed. Frequencies and percentages were reported for the chi-square analyses. Mixed-effects ANOVA was then performed to compare the rate of change in A1C between the two treatment arms. Between-subjects, within-subjects, and interactions effects were reported and interpreted in Table 1. Statistical assumptions (normality, homogeneity of variance, and homogeneity of covariance) were checked before the model interpretation. Marginal means (M) with 95% confidence intervals (95% CI), mean difference with 95% CI, effect sizes (partial eta-squared), and post hoc power were reported and interpreted for the mixed-effects analysis. A sensitivity analysis was performed (analysis of covariance; ANCOVA) for the primary outcome to adjust for baseline differences in A1C. Statistical significance was assumed at an alpha value of 0.05, and all analyses were performed using SPSS Version 29 (Armonk, NY: IBM Corp).
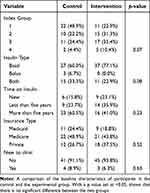 |
Table 1 Demographic Data |
Results
There were no missing observations for the baseline or post-intervention observations of A1c, so no values were imputed. At baseline, there were no significant differences between the in-office and telephonic titration groups for index group, p = 0.07, insulin type, p = 0.08, time on insulin, p = 0.23, insurance type, p = 0.52, or being new to the clinic, p = 0.63. See Table 1 for the frequencies and percentages related to these findings. The primary analysis of A1C yielded significant main and interaction effects. There was a significant between-subjects effect, with the intervention group having a higher mean value (M = 10.02, 95% CI 9.62–10.43) than controls (M = 8.89, 95% CI 8.48–9.31), F(1,91) = 14.87, p < 0.001, partial eta-squared = 0.14, power = 0.97. A significant within-subjects effect was found for A1C from pre-intervention (M = 9.88, 95% CI 0.54–10.23) to post-intervention (M = 9.04, 95% CI 8.70–9.38), F(1,91) = 2046, p < 0.001, partial eta-squared = 0.18, power = 0.99. The interaction effect also yielded significant differences between the treatment arms at baseline (mean difference = 1.55, 95% CI 0.85–2.24, p < 0.001) and at post-intervention (mean difference = 0.72, 95% CI 0.03–1.41, p = 0.04). There intervention group experienced a higher level of decline in A1C across time (mean difference −1.26, 95% CI −0.74 – −1.77; p < 0.001) versus the control group (mean difference −0.43, 95% CI −0.96–0.10; p = 0.11). Finally, the sensitivity analysis found that the intervention group yielded a slightly higher post-intervention A1C finding (M = 9.17, 95% CI 8.70–9.64) versus controls (M = 9.14, 95% CI 8.64–9.64), F(1,89) = 4.39, p = 0.17, partial eta-squared = 0.02, power = 0.28. The marginal means with 95% confidence intervals for these findings are presented in Table 2. The interaction effect is depicted graphically in Figure 1.
 |
Table 2 Results of Mixed-Effects ANOVA and Sensitivity Analysis |
 |
Figure 1 Comparison of Treatment Arms on the Change in A1C. |
Discussion
Finding multi-modal access points for patients to access care is necessary to improve health care outcomes. The Covid-19 pandemic shined a light on how access issues for patients with chronic illness can impact an individual’s health. Specifically, patients with diabetes saw decreased A1C monitoring and insulin titration. This is one of the first studies to look into the impact of the Covid-19 pandemic on patient’s diabetes, and shows the utility of telephonic insulin titration by pharmacy in helping with diabetic control. The preliminary findings of this study show that titration of insulin by a clinical pharmacist is as effective as in-office titration of insulin by a primary care physician. Secondary findings showed that the decrease in A1C at the 3-month marker was significantly greater for the telephonic group than for the in-office group. The major strength of this study is that it shows that during times when patients are unable to get into a clinic, virtual modalities are an effective management tool for achieving glycemic control in patients with diabetes. Although this study was conducted at an Academic Family Medicine Residency clinic, the improvements seen are generally applicable to medically undeserved populations, as the majority of patients seen were Medicaid and Medicare. However, a major limitation is that clinics will need access to a clinical pharmacist to titrate the insulin. A few previous studies have looked at the use of telephonic insulin titration by clinical pharmacists within a residency clinic; however, this is the first study to look at the utility of increased virtual access during a global pandemic where patients were often unable to have in-office visits. On a broader scale, this study confirms the continued need for virtual modalities and use of pharmacists in the management of chronic conditions like diabetes for populations who continue to have limited resources and access to care even following the pandemic. From a policy level, this study shows the impact and need for residency clinics to employ or work closely with pharmacists as it improves access and quality of care, which can also impact a clinic’s re-imbursements. This study provides an alternative to the gold standard of in-person visits, and further studies are needed to determine how best to utilize this resource in a medically underserved population.
Something important to note in this study is that the A1C at initiation was found to be significantly higher in the pharmacy titration group compared to the control group, with a p <0.001, and yet the rate of decrease in A1C was also significantly higher in the intervention group. This might suggest that those being referred for pharmacy titration were sicker than the control group, and yet their drop in A1C was far more rapid than that of the control. This is a significant finding, as it suggests that the frequent telephonic titration might have better A1C improvement; however, this would need to be further evaluated in future studies.
Major limitations to this study were the duration and size of the study. Due to the lack of duration, it is unclear whether the A1C improvement would have been sustained past 3 months. Additionally, because many patients during that time frame were not able to come into the office for lab draws, there was lack of A1C follow-up data in the chart. Those patient that did not get follow-up A1Cs may have had worse A1C improvement compared to those that did get follow-up labs and could have skewed the data.
Conclusions
While previous studies have looked at the effectiveness of telephonic insulin titration, no previous studies have looked at it during a pandemic where both resources and access are severely limited to patients with diabetes. The goal of this study was to evaluate if telephonic titration is as effective in A1C control as it could have great implications on how we manage diabetes for patients with limited access to the clinic, such as during a pandemic. The findings of this study support both the use of telephonic titration and cooperative management with a clinical pharmacist in the best interest of the patient. In fact, the experimental group showed faster decline in A1C compared to the control even with a higher baseline A1C. The importance of this study in the literature is that it shows that in primarily underserved populations who have limited access to resources even in the absence of a pandemic, telephonic insulin titration can serve as a safe way to manage their diabetes. Additionally, this study shows the importance of a pharmacist in the management of patients with diabetes, and the need for further integration of multi-disciplinary teams not just in residency practices but also in private practice. Lastly, though this studies primary focus was on diabetes, the use of telephonic insulin titration could be expanded and evaluated for other chronic conditions, like asthma control or warfarin monitoring.
In order to address the different baseline A1C characteristics in the two groups, future studies should group people based on A1C severity to identify if the increased rate of decrease in A1C was clinically significant. Further studies should also monitor A1C over a longer period of time to evaluate if the decrease in A1C with the telephonic group is a sustained finding. Additionally, future studies could look at other forms of out-of-office intervention like insulin titration utilizing patient portal messaging, as this has never been reported in the literature as an effective modality for insulin titration. Other considerations for future studies could investigate the role that Continuous Glucose Monitoring might play in helping to collect data for at-home titration compared to the glucometer. Lastly, evaluating family medicine resident passive learning with pharmacy-driven insulin titration would be an important area of study to ensure physician learners are getting a good education in diabetic medication management.
Ethics and Consent Statements
This manuscript was approved by the UTHSC IRB and was granted a consent waiver. Consent waiver was granted by the UTHSC IRB because patients were already receiving the intervention prior to the study and were being tracked by pharmacy. Patient data was kept confidential using a locked excel sheet that was only accessible in the clinic. Additionally, after patient data collection was completed, all patient identifiers were removed.
Acknowledgments
Adam Visconti, Assistant Professor in the Department of Family Medicine, Georgetown University, 4151 Bladensburg Rd, Colmar Manor, MD 20722, [email protected]
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there is no conflict of interest in this work.
References
1. National Diabetes Statistics Report. Center for Disease Control and Prevention. Updated November 29, 2023. https://www.cdc.gov/diabetes/data/statistics-report/index.html.
2. CDC. Cost-Effectiveness of Diabetes Interventions | power of Prevention. https://www.cdc.gov/chronicdisease/programs-impact/pop/diabetes.htm.
3. Czeisler MÉ, Barrett CE, Siegel KR, et al. Health care access and use among adults with diabetes during the COVID-19 pandemic - United States, February-march 2021. MMWR Morb Mortal Wkly Rep. 2021;70(46):1597–1602. doi:10.15585/mmwr.mm7046a2Fragala
4. Fragala MS, Kaufman HW, Meigs JB, Niles JK, McPhaul MJ. Consequences of the COVID-19 pandemic: reduced hemoglobin A1c diabetes monitoring. Popul Health Manag. 2021;24(1):8–9. doi:10.1089/pop.2020.0134
5. Sorli C, Heile MK. Identifying and meeting the challenges of insulin therapy in type 2 diabetes. J Multidiscip Healthc. 2014;7:267–282. doi:10.2147/JMDH.S64084
6. Patel D, Triplitt C, Trujillo J. Appropriate titration of basal insulin in type 2 diabetes and the potential role of the pharmacist. Adv Ther. 2019;36(5):1031–1051. doi:10.1007/s12325-019-00907-8
7. Weidman-Evans E, Evans J, Eastwood R, Fort A. Implementation of a pharmacist-run telephonic insulin titration service. J Am Pharm Assoc. 2012;52(6):e266–e272. doi:10.1331/JAPhA.2012.11225
8. Collaborative Practice Agreements and Pharmacists’ Patient Care Services A Resource for Pharmacists. Center for Disease Control. 2013. https://www.cdc.gov/dhdsp/pubs/docs/translational_tools_pharmacists.pdf.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

