Back to Journals » International Journal of Nanomedicine » Volume 20
Photosynthetic Bacteria: Light-Responsive Biomaterials for Anti-Tumor Photodynamic Therapy
Authors Jiang Y
Received 17 October 2024
Accepted for publication 31 December 2024
Published 10 January 2025 Volume 2025:20 Pages 465—482
DOI https://doi.org/10.2147/IJN.S500314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Jie Huang
Yuan Jiang
Department of Rehabilitation Medicine, School of Clinical Medicine and The First Affiliated Hospital of Chengdu Medical College, Chengdu, Sichuan, People’s Republic of China
Correspondence: Yuan Jiang, Email [email protected]
Abstract: Photodynamic therapy (PDT) is a promising noninvasive tumor treatment modality that relies on generating reactive oxygen species (ROS) and requires an adequate oxygen supply to the target tissue. However, hypoxia is a common feature of solid tumors and profoundly restricts the anti-tumor efficacy of PDT. In recent years, scholars have focused on exploring nanomaterial-based strategies for oxygen supplementation and integrating non-oxygen-consuming treatment approaches to overcome the hypoxic limitations of PDT. Some scholars have harnessed the photosynthetic oxygen production of cyanobacteria under light irradiation to overcome tumor hypoxia and engineered them as carriers of photosensitizers instead of inorganic nanomaterials, resulting in photosynthetic bacteria (PSB) attracting significant attention. Recent studies have shown that light-triggered PSB can exhibit additional properties, such as photosynthetic hydrogen production, ROS generation, and photothermal conversion, facilitating their use as promising light-responsive biomaterials for enhancing the anti-tumor efficacy of PDT. Therefore, understanding PSB can provide new insights and ideas for future research. This review mainly introduces the characteristics of PSB and recent research on light-triggered PSB in anti-tumor PDT to enrich our knowledge in this area. Finally, the challenges and prospects of using PSB to enhance the anti-tumor efficacy of PDT were also discussed.
Keywords: photosynthetic bacteria, PDT, hypoxia, cyanobacteria, purple bacteria, PTT
Graphical Abstract:

Introduction
Photodynamic therapy (PDT) is a non-invasive treatment modality based on the cytotoxic effects of reactive oxygen species (ROS). Its mechanism for ROS generation involves transferring energy from light to certain chemicals known as photosensitizers that can undergo photochemical reactions.1 Many scholars believe that PDT is a promising approach for tumor treatment due to its unique advantages. First, the essential components of PDT are photosensitizers, light, and oxygen (O2). These components are not individually toxic unless combined to trigger photochemical reactions that damage the target tissue or cells.2 Second, photosensitizers accumulate in tumor tissues, which helps confine the treatment area and minimizes damage to surrounding healthy tissues. When exposed to localized light, the photocytotoxic effects of photosensitizers directly damage the organelles of tumor cells (eg, mitochondria, lysosomes, endoplasmic reticulum, and Golgi apparatus), resulting in tumor cell death. PDT also destroys vascular structures and blocks blood vessels in tumor tissues, resulting in significant anti-tumor effects. In addition, PDT can activate the immune system to inhibit tumor metastasis and recurrence.3 Third, advanced endoscopic and fiberoptic light delivery techniques allow PDT to treat hard-to-reach hollow surgical structures (eg, the digestive tract, bronchus, and body cavity). PDT can also be employed for deep-seated and large tumors (>1 cm in size) under the guidance of computed tomography (CT) or ultrasound imaging.4 Fourth, PDT can be repeated as necessary, and importantly, tumor cells do not develop drug resistance during repeated PDT. It is well known that the production levels of ROS are crucial for PDT efficacy, which depends on sufficient O2 levels in the target tissues. However, O2 concentration in solid tumor tissues is only 7–28 mmHg (1–4%), compared to 40–60 mmHg (5–8%) in normal tissues.5,6 The primary reasons solid tumors often experience hypoxia are uncontrolled cell proliferation and abnormal blood vessel formation, which lead to low O2 levels and inadequate supply and diffusion of O2. Hypoxia in tumor tissues significantly limits the effectiveness of PDT, and the oxygen-consuming nature of PDT further exacerbates the hypoxic condition, promoting the proliferation and metastasis of residual tumor cells.7 Although PDT has received clinical approval from the USA Food and Drug Administration (FDA), its clinical application in tumor treatment has been sluggish over the past few decades, and its effectiveness has not received full affirmation and acceptance.8,9 Scholars initially attempted to enhance blood oxygen content and improve tumor blood circulation using hyperbaric oxygen therapy (HBOT), anticoagulant drugs, and warm water baths.10–14 However, these methods have achieved only limited success due to incomplete vascular systems in tumor tissues and the presence of hypoxic areas distant from blood vessels.15 As a result, scholars are actively seeking efficient and practical methods to increase O2 levels in tumor tissues or integrate non-oxygen-consuming treatments to overcome the challenges posed by hypoxia in PDT.
Currently, most of the photosensitizers approved for clinical anti-tumor PDT are second-generation compounds, such as Porfimer sodium salt (Photofrin®), 5-aminolevulinic acid (ALA, Levulan®), Methyl aminolevulinate(MAL, Metvix®), and Tetrasulfonic aluminum phthalocyanine (APkS4, Photosens®), etc. These photosensitizers have improved biochemical properties compared to the first-generation (hematoporphyrin derivatives, HpD).16 However, they still face inherent limitations, such as poor water solubility and stability, shallow penetration depth of excitation light, photodamage, and poor tumor targeting. To overcome these limitations, scholars are developing third-generation photosensitizers by combining the second-generation compounds with specific components (eg, antibodies, carbohydrates, peptides, and amino acids) or encapsulating them in carrier systems.17,18 For instance, combining photosensitizers with nanomaterials (NPs) can significantly enhance the tumor-targeting ability. Scholars have realized that NPs can also serve as delivery systems for O2/catalytic agents to effectively enhance O2 levels in hypoxic areas of tumor tissues or integrate non-oxygen-consuming treatments to improve the anti-tumor efficiency of PDT. To address these aims, scholars often develop NP-based carrier systems that involve complex design and modification,19 which can increase research duration and funding requirements. Recently, some researchers have utilized photosynthesis by chlorophyll-containing microorganisms to generate O2 within tumor tissues to overcome tumor hypoxia, suggesting that these photosynthetic microorganisms can serve as light-triggered oxygen-supplied systems.20 Among these, photosynthetic bacteria (PSB), a specific group of prokaryotic autotrophic organisms with an original photo-energy synthesis system, have attracted attention in alleviating tissue hypoxia due to their unique properties in photosynthetic oxygen production. In addition to rescuing ischemic myocardium and chronic wounds, PSB have been employed as a light-triggered oxygen supplier to enhance the efficacy of anti-tumor PDT. Notably, light-triggered PSB also exhibit the properties of hydrogen production, ROS generation, and photothermal conversion, indicating that they can provide additional therapeutic benefits in tumor tissue, such as hydrogen therapy, photosensitizing effect, and photothermal therapy (PTT). PSB can serve as light-responsive biomaterials in anti-tumor PDT for increasing O2 levels in tumor tissues and integrating non-oxygen-consuming treatments. Compared to inorganic NPs, PSB have several advantages, such as easy modification, high biocompatibility, low toxicity, convenient cultivation, and rapid reproduction.21 Therefore, gaining a deeper understanding of PSB characteristics is essential for utilizing them better in anti-tumor PDT in the future. Therefore, gaining a deeper understanding of PSB characteristics is essential for utilizing them better in anti-tumor PDT in the future. This review will mainly introduce the characteristics of PSB and recent research on light-triggered PSB in anti-tumor PDT, and the challenges and prospects of PSB as light-responsive biomaterials for anti-tumor PDT also will be discussed.
NPs and Anti-Tumor PDT
In recent years, scholars have developed various NP-based strategies for oxygen supplementation and have integrated non-oxygen-consuming treatment approaches to overcome the hypoxic limitations of PDT. Hemoglobin (Hb), an O2 carrier, can be encapsulated within NPs for transport to tumor tissues. In addition, NPs made from perfluorocarbons or metal-organic frameworks and other NPs encapsulated within red blood cell membranes can efficiently deliver O2 to tumor tissues.7,22,23 NPs can also carry catalytic agents (eg, catalase, manganese dioxide, and nano-enzymes) to decompose endogenous hydrogen peroxide (H2O2) into O2 and water (H2O) or carry carbon nitride (C3N4) to decompose water to produce O2 and hydrogen (H2), leading to O2 generation within tumor tissues, hydrogen-induced redox stress, and subsequent cell damage.15 Specific photosensitizers (eg, indocyanine green, ICG) and nanomaterials (eg, Gold NPs) can convert light photon energy into heat under near-infrared (NIR) laser irradiation, allowing the combination of PDT and photothermal therapy (PTT) in tumor treatment. PTT does not require O2, while its thermal effect can destroy tumor tissue and improve blood circulation to enhance O2 levels in hypoxic tumor tissues.24,25 As a result, the anti-tumor efficacy of synergistic PDT/PTT is better than that of PDT or PTT alone.26 Currently, various types of NPs have been designed as drug-delivery systems for transporting photosensitizers and other therapeutic agents to tumor tissues. This strategy can reverse hypoxic conditions and enable the combination of PDT with other therapeutic methods, such as PTT, gas therapy, chemotherapy, and immunotherapy. The design and development of NPs are based on the enhanced permeability and retention (EPR) effect in tumor tissues. However, NPs will face the influence of various factors in vivo, such as tumor heterogeneity, poor blood perfusion, abnormal vascular endothelial function, and their physicochemical properties. There is a controversial debate regarding the effectiveness of NPs in improving drug delivery to tumors and enhancing therapeutic efficacy in clinical practice.27–30 To increase tumor accumulation and retention, NPs require more complex design and modification with tumor-targeting ligands,31 which may affect their size, physicochemical properties, synthesis yield, biosafety, biodegradability, immunogenicity, and clinical translation. In contrast, the properties of PSB facilitate the integration of oxygen supplementation and multimodal treatments without the need for complex design and modifications. Therefore, the first step is to understand the characteristics of PSB.
Characteristics of PSB
PSB are Gram-negative facultative anaerobic bacteria that cannot form spores. Along with the general characteristics of facultative anaerobes, one of their most distinctive features is their ability to use light for photosynthesis. PSB exhibits two main types of photosynthesis based on different molecular mechanisms: oxygenic and anoxygenic.32 Cyanobacteria, namely blue-green algae, are oxygenic PSB as they perform oxygenic photosynthesis. The dominant groups of anoxygenic PSB include purple phototrophic bacteria (PPSB, sulfur, and non-sulfur phototrophs) and green sulfur bacteria (GSB) that perform anoxygenic photosynthesis. In addition, PSB possesses other unique properties worth noting.
Hypoxia Chemotaxis
PSB are Gram-negative facultative anaerobes that are sensitive to the O2 content in the surrounding environment and tend to move toward the hypoxic zone to survive in anaerobic conditions.33 Tumor tissues have unique hypoxic microenvironments that attract them to target and move toward the hypoxic regions to colonize.
Phototaxis
PSB have photoreceptors that enable them to perceive specific light stimulation (eg, direction, intensity, and wavelength), allowing them to move towards or away from the light sources. This behavior is known as phototaxis, which helps PSB capture the appropriate light for photosynthesis or escape harmful light sources. Flagellated anoxygenic PSB (eg, PPSB) have flagella, and light signals can drive the flagellar motor to start flagellar rotation and forward the cell.34 PPSB exhibit positive phototaxis in response to far-red light but moves away from visible light.35 In contrast, cyanobacteria lack flagella but have type IV pili. Their IV pili complexes receive signals from photoreceptors to enable particular pili to move towards or flee from light sources.36–38 In addition, a single spherical cyanobacteria cell can act as a microlens to focus incoming light onto the membrane at the back of the cell, facilitating direct and accurate sensing of the light direction.39 Cyanobacteria exhibit positive phototaxis in response to red and green light while escaping from ultraviolet (UV), blue, and high-intensity light.37
Photosynthesis and Photosynthetic Oxygen Production
Photosynthesis involves the conversion of solar energy into chemical energy, which is essential for the growth and metabolic processes of PSB. Although the molecular mechanisms of photosynthesis in oxygenic and anoxygenic PSB differ, the fundamental principles of energy transduction are similar. Cyanobacteria are the only PSB with the ability to produce O2 through photosynthesis by their two types of reaction centers (RC): photosystem I (PSI) and photosystem II (PSII).40,41 Both PSI and PSII are intrinsic protein complexes located in the thylakoid membranes. They consist of a chlorophyll-rich core complex and a chlorophyll-binding peripheral antenna system.42–44 The peripheral antenna system, known as light-harvesting complex I (LHCI) in PSI and light-harvesting complex II (LHCII) in PSII, is responsible for absorbing the external light energy.45 In addition to LHCII, the phycobilisome (PBS) attaches to the surface as an extramembrane light-harvesting antenna. PBS can absorb light between 450 and 650 nm, and even beyond 700 nm in some cases. PBS complements the absorption spectrum of chlorophylls in PSII, leading to more efficient capture of sunlight and energy transfer to the core complex of PSII.46 PSII is a water-oxidizing enzyme that can utilize harvested energy to perform photocatalytic water oxidation to produce O2 in the catalytic core oxygen-evolving complex (OEC), which consists of a tetramanganese-calcium cluster Mn4O5Ca.47 Oxygenic photosynthesis requires synergy between PSI and PSII, which provides the properties of photosynthetic oxygen production under light irradiation in cyanobacteria (see Figure 1).
 |
Figure 1 Schematic representation of photosynthetic oxygen production and hydrogen production in PSB. |
In contrast, anoxygenic PSB have only a single type of RC located within the photosynthetic membrane for conducting anoxygenic photosynthesis. For example, GSB only have type I RC (PSI), while PPSB only have type II RC (PSII-like photosystem).48,49 Anoxygenic PSB also have light-harvesting complexes (LHC), known as LHI and LHII in PPSB or chlorosomes in GSB.50,51 LH1 surrounds the RC to form the core of the photosynthetic complex, while LH2 spreads in the periphery of the RC in most PPSB. In addition to chlorosomes, GSB have another antenna complex called the Fenna-Matthews-Olson (FMO) protein.52 Notably, anoxygenic PSB possess bacteriochlorophylls (BChls) instead of chlorophyll. As the major chromophores of LHC and RC, BChls have light-energy conversion functions. However, the maximum absorbance peak of BChls is located in the near-infrared region,53 which limits the photosynthetic efficiency. Carotenoids act as accessory pigments to absorb the blue-green light and transfer energy to the BChls for expanding the captured light wavelength range, which is beneficial for more light energy transfer from the LHC to the RC.54,55 The RC of anoxygenic PSB can convert sunlight into chemical energy but cannot oxidize water. Therefore, anoxygenic PSB do not have the properties of photosynthetic oxygen production.49
Photosynthetic Hydrogen Production
Most oxygenic and anoxygenic PSB contain nitrogenase or hydrogenase, which enables them to produce H2 through different physiological mechanisms. Cyanobacteria produce H2 through water biophotolysis, which requires the participation of PSI and PSII56 (see Figure 1). In addition to O2, PSII generates hydrogen protons (H+) and electrons through photocatalytic water oxidation during photosynthesis. In non-nitrogen-fixing cyanobacteria, electrons are transferred to bidirectional [Ni, Fe]-hydrogenase to combine with hydrogen protons to produce H2. In contrast, some electrons are transferred to nitrogenases for nitrogen fixation in nitrogen-fixing cyanobacteria. Nitrogenase-obtaining electrons consume the ATP, generated by photophosphorylation, to reduce hydrogen protons to H2.57,58 Anoxygenic PSB produce H2 through photofermentation, mainly involving nitrogenase. Nitrogenase utilizes H+ and electrons derived from organic substrates to produce H2 under hypoxic conditions. LHC capture and transfer light energy to the RC, generating high-energy electrons. Electron transfer establishes a proton gradient, resulting in ATP synthesis through cyclic phosphorylation. Subsequently, nitrogenase consumes ATP and electrons to reduce protons to H2.56,59,60 Another hydrogenase, [Ni Fe]-hydrogenase, can absorb the H2 produced during nitrogen fixation, which may influence the overall hydrogen yields of nitrogenase to a certain extent.61 In short, nitrogenase- and hydrogenase-containing PSB can produce photosynthetic hydrogen when exposed to light. Notably, oxygen can inhibit the activities of both hydrogenases and nitrogenases. Oxygenic photosynthesis in cyanobacteria may limit their H2 production, but this limitation does not apply to anoxygenic PSB.
ROS Generation
During photosynthesis, the conversion of light energy can also lead to the formation of ROS. When the light absorbed by chlorophylls or BChls exceeds the amount required for photosynthesis, the generation of ROS significantly increases. Both PSI and PSII are involved in the generation of ROS.62 PSI is the primary site for ROS production because the electron flow from PSII to PSI can be terminated and accumulated on the stromal side of PSI, leading to O2 reduction to a superoxide radical (O2•−). In contrast, PSII produces ROS under the following conditions: a) when the transfer of light energy from the PSII antenna complex to the RC is limited, chlorophylls or BChls can act as photosynthesizers, absorbing the energy and transitioning from the ground state to the triplet state. The triplet chlorophyll transfers the absorbed energy to surrounding molecular oxygen, forming singlet oxygen generation (1O2). b) when the electron transport chain between the photosystems is inhibited, electrons can leak from the electron acceptor side of PSII to molecular oxygen, forming O2•−. Additionally, incomplete oxidation of water on the electron donor side of PSII can cause the production of hydrogen peroxide (H2O2). O2•− and H2O2 can be further reduced to the harmful hydroxyl radical (HO•) through a series of reactions. However, carotenoids present in PSB can quench excited BChls and 1O2, preventing the photodamage to cells, which can affect the ROS generation.63–65
Photothermal Conversion
Excessive absorbed light energy during photosynthesis can damage the photosystems in PSB. However, PSB have a photoprotective mechanism to dissipate energy as heat through nonphotochemical quenching (NPQ).66 For example, cyanobacterial orange carotenoid protein (OCP) dissipates excess absorbed energy from PSB as heat.67 Carotenoids also play a role by quenching excited BChls and converting the excess energy into heat.64 Zhao et al were the first to demonstrate that PSB could rapidly reach temperatures of 60 °C under NIR light irradiation. Even when inactivated at 65 °C, these bacteria maintained the same photothermal conversion efficiency.68 More recently, scholars have found the photothermal conversion properties of cyanobacterium Synechococcus elongatus (S. elongatus), purple photosynthetic bacterium Rhodobacter johrii (R. johrii), Rhodopseudomonas palustris (R. palustris), and Blastochloris viridis (B. viridis). The photothermal conversion efficiency of these PSB is even higher than that of gold-based particles and metal selenide.69–73 These findings confirmed the photoprotective mechanism endows PSB with photothermal conversion properties.
Application of PSB in Anti-Tumor PDT
In recent years, the application of bacteria in anti-tumor therapy has gained significant attention as a key area of research. Their unique properties and functions offer new perspectives on seeking potential biomaterials in this field. In addition to their inherent properties of PSB, including hypoxia chemotaxis, phototaxis, and light-triggered biological properties, PSB can be modified with special polymers, drugs, and NPs on their surfaces through various physical and chemical methods, which has attracted considerable interest. Some PSB have been employed in research on anti-tumor PDT as hypoxia-targeted carriers for supplying O2 and integrating multimodal treatments.74,75
Cyanobacteria as O2 Suppliers and Photosensitizer Carriers
Most existing research findings are obtained from studies on cyanobacteria (see Table 1). Given their properties of hypoxia chemotaxis and photosynthesis, scholars initially considered using cyanobacteria as O2 suppliers and carriers of photosensitizer to improve the effectiveness of anti-tumor PDT. Shi et al developed a hybrid combining cyanobacteria with the photosensitizer chlorine6 (Ce6) and they named it ceCyan. To form ceCyan, the modified Ce6 with dual-amide-terminated polyethylene glycol (NH2-PEG-NH2) polymers was incubated with cyanobacteria (Synechococcus elongatus PCC 7942, S.7942) (see Figure 2A). The ceCyan can deliver Ce6 to tumor cells while supplying O2 under 660 nm laser light irradiation, which enhances the inhibiting effects of Ce6-induced PDT on tumor cell activity. The mice that received an intravenous injection of ceCyan had only tolerable inflammation, indicating that ceCyan has good biocompatibility. In addition to ceCyan, they also combined protoporphyrin (Ppix) with cyanobacteria to form Ppix-hybridized cyanobacterial cells (Ppix-Cyan), demonstrating that cyanobacteria can be employed to carry other negatively charged chlorin-based photosensitizers, thereby expanding the range of possible combinations between cyanobacteria and photosensitizer.76
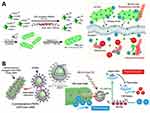 |
Figure 2 (A) Schematic illustration of the synthesis and and mechanism of ceCyan. Reproduced from Huo M, Wang L, Zhang L, Wei C, Chen Y et al. Photosynthetic Tumor Oxygenation by Photosensitizer-Containing Cyanobacteria for Enhanced Photodynamic Therapy. Angew Chem Int Ed Engl. 2020; 59: 1906–1913. © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.76 (B) Schematic illustration of the synthesis and and mechanism of UR-Cyan cells. Reproduced from Huo M, Liu P, Zhang L, Wei C, Wang L et al. Upconversion Nanoparticles Hybridized Cyanobacterial Cells for Near-Infrared Mediated Photosynthesis and Enhanced Photodynamic Therapy. Advanced Functional Materials. 2021; 31: 2,010,196. © 2021 Wiley-VCH GmbH.79 |
 |
Table 1 Literature Examples of Cyanobacteria in Anti-Cancer PDT |
Choosing an ideal photosensitizer for PDT requires considering the wavelength of its excitation light source. Most conventional photosensitizers can often be activated by short-wavelength visible light (400–700 nm) with a low penetration depth within tissues.85 Some scholars chose rare-earth ion-doped upconversion nanoparticles (UCNPs) to address this limitation. Some scholars chose rare-earth ion-doped upconversion nanoparticles (UCNPs) to address this limitation. Shi et al developed a hybrid combining cyanobacteria (S.7942), photosensitizer Rose Bengal (RB), and UCNPs, and it is named UR-Cyan. The negatively charged cyanobacterial cells were incubated with the positively charged RB-loaded URNPs at room temperature (RT) for 1 hour. RB-loaded URNPs were combined with S.7942 to form UR-Cyan cells by electrostatic interactions. The UCNPs consist of a NaYF4: Yb3+, Tm3+, Er3+ @ NaYF4: Yb3+ core-shell structure that absorbs NIR light (980 nm) and converts it into visible light, including green (510–570 nm) and red light (630–680 nm, 680–710 nm). These visible lights are suitable for active S.7942 and RB. As a result, the UR-Cyan cells can provide combined effects of photosynthetic oxygenation and photosensitization effects, eradicating tumor cells in tumor-bearing nude mice when exposed to NIR laser irradiation79(see Figure 2B).
Besides the wavelength of the excitation light source, a reasonable lighting control strategy is another important consideration. Zhang et al developed an injectable red blood cell membrane membrane-doped hydrogel system containing NaYF4: Yb/Er UCNPs, RB, and S.7942, namely UCNPs/S7942/RB-RHY (see Figure 3A). They used a 980 nm laser to irradiate UCNPs/S7942/RB-RHY to produce visible light for RB-induced PDT in the tumor-bearing mice. Before this step, the mice were treated with 640 nm laser irradiation three times to alleviate hypoxia in tumor tissue through the photosynthetic oxygenation provided by UCNPs/S7942/RB-RHY. The results indicated that this lighting control strategy led to a more efficient PDT treatment. Interestingly, the generated ROS also contributed to the simultaneous destruction of cyanobacteria within the tumor tissue after PDT.80 Similarly, Sun et al developed a sodium alginate gel containing photosensitizer ICG loading mesoporous silica nanoparticles (MSN-ICG) and cyanobacteria (Synechococcus elongatus UTEX 2973, S. 2973), namely ALG-MI-S2973. After an intratumoral injection of ALG-MI-S2973, the tumor-bearing mice were treated with 640 nm laser irradiation for 3 days and then treated with 808 nm laser on the 4th day. They suggested that this lighting control strategy enhances O2 generation in cyanobacteria under 640 nm laser irradiation, and tumor cells and cyanobacteria can be eliminated by PDT-induced ROS under 808 nm laser irradiation, ensuring therapeutic effect and biosafety83(see Figure 3B). Chang et al reported a novel lighting control strategy based on persistent luminescence material. They developed a CaAl2O4:Eu, Nd blue persistent luminescence material (CAO PLM) that acted as a light source for activating cyanobacteria/photosensitizer hybrids in vivo. Briefly, they covalently linked the photosensitizer verteporfin (Vp) with the -NH2 groups of the outer membrane of S.7942 to form cyanobacteria-verteporfin (Cb-Vp). Subsequently, Cb-Vp was mixed with PEGylated CAP PLM (CaAl2O4:Eu, Nd@PEG) to assemble an exogenous irradiation-free PSB-based platform (CAP + Cb-Vp). They found that CAP + Cb-Vp generated blue persistent luminescence to stimulate photosynthetic oxygenation and photosensitization effects of Cb-Vp, leading to synergistic tumor killing in vivo after pre-excitation by UV light in vitro. Notably, after administering CAP + Cb-Vp, intermittent supplementation with S.7942 and re-irradiation with white light from an LED lamp in subcutaneous tumor tissues can increase blue persistent emission and enhance O2 levels, thereby improving PDT therapeutic outcomes.81
 |
Figure 3 (A) Schematic illustration of the synthesis and mechanism of UCNPs/S7942/RB-RHY. Reprinted from Colloids Surf B Biointerfaces. Volume 201, Zhang X, Zhang Y, Zhang C, Yang C, Tian R et al. An injectable hydrogel co-loading with cyanobacteria and upconversion nanoparticles for enhanced photodynamic tumor therapy. 111640, Copyright 2021, with permission from Elsevier.80 (B) Schematic illustration of the synthesis and animal experiments of ALG-MI-S2973, and tumor growth curves of tumor-bearing mice with different treatments (**P < 0.01 compared with untreated group). Reproduced from Sun T, Zhang Y, Zhang C et al. Cyanobacteria-Based Bio-Oxygen Pump Promoting Hypoxia-Resistant Photodynamic Therapy. Front Bioeng Biotechnol. 2020; 8: 237. Creative Commons.83 |
Cyanobacteria Integrate Multimodal Treatments
Some scholars have also explored the synergistic anti-tumor effects of cyanobacteria/photosensitizer hybrid-induced PDT combined with other therapeutic methods, such as PTT. Yin et al incorporated the photothermal agent Au NPs into the combination of S. 7942 and Ce6 to form a cyanobacteria/photosensitizer/photothermal agent hybrid, and they named Bac@Au-Ce6. They observed that S. 7942 facilitated the accumulation of Au-Ce6 in tumor tissues due to its tumor-targeting ability. Under 660 nm laser irradiation, Bac@Au-Ce6 continuously generated O2 and ROS. Subsequently, Bac@Au-Ce6 generated heat under 808 nm laser irradiation. They found necrosis and apoptosis in the treated tumor tissue, indicating that Bac@Au-Ce6 can simultaneously trigger photosynthetic oxygenation, PDT, and PTT in tumor tissues using different laser irradiation82(see Figure 4A). Qi et al developed a cyanobacteria hybridized with inorganic two-dimensional black phosphorus nanosheets (BPNSs) using amide chemistry-enabled bioconjugation methodology, namely Cyan@BPNSs. Under irradiation with a 660 nm laser, the BPNSs generated singlet oxygen via photosensitization, while the cyanobacteria produced O2 in the tumor tissue77(see Figure 4B). Notably, BPNSs can convert NIR light into heat, leading to PTT.86 Therefore, the hybridization of cyanobacteria and BPNSs can further provide a synergistic strategy for anti-tumor PDT and PTT.
 |
Figure 4 (A) Schematic illustration of the synthesis and mechanism of Bac@Au-Ce6, and transmission electron microscope (TEM) images of Au NPs, Au-Ce6, and Bac@Au-Ce6. Reproduced Yin C, Wang Z, Dai C et al. Light-triggered photosynthetic engineered bacteria for enhanced-photodynamic therapy by relieving tumor hypoxic microenvironment. Theranostics. 2023; 13:1632–1648. Creative Commons.82 (B) Schematic illustration of the synthesis and mechanism of Cyan@BPNSs. Reproduced from Qi F, Ji P, Chen Z, Wang L, Yao H et al. Photosynthetic Cyanobacteria-Hybridized Black Phosphorus Nanosheets for Enhanced Tumor Photodynamic Therapy. Small. 2021; 17: e2102113. © 2021 Wiley-VCH GmbH.77 (C) Schematic illustration of the synthesis and mechanism of SpiD. Reproduced with permission from An X, Zhong D, Wu W, Wang R, Yang L et al. Doxorubicin-Loaded Microalgal Delivery System for Combined Chemotherapy and Enhanced Photodynamic Therapy of Osteosarcoma. ACS Appl Mater Interfaces. 2024; 16: 6868–6878. Copyright © 2024 American Chemical Society.84 |
Cyanobacteria as Therapeutic Agents
Previous studies employed some cyanobacteria extracts as photosensitizers, such as tolyporphin (TP),88 phycocyanin (PC),89 and hydrophilic chlorine derivatives.90 Scholars have further investigated the photosensitizing performance of cyanobacteria to explore novel, safe, and effective natural photosensitizers for anti-tumor PDT. To date, S. platensis has been employed as an unconventional photosensitizer in PDT to kill tongue, oral, and hypopharyngeal cancer cells, while they have no cytotoxic effect on normal cells.87,91 Recently, An et al used an S. platensis-based drug delivery system (SpiD) to transport and release doxorubicin (DOX) into osteosarcoma cells for chemotherapy. S. platensis can provide photosensitization effects under 650 nm laser irradiation, resulting in a synergistic anti-tumor strategy of PDT and chemotherapy84(see Figure 4C). A study found that S. 7942 exhibited photodynamic effects at the tumor site under 660 nm laser irradiation,78 indicating that they may be natural photosensitizers like S. platensis. Another study found that S. 7942 can be used as a photothermal source to generate heat, eliminating tumors under excessive 660 nm light irradiation. Moreover, S. 7942 can release a series of pathogen-associated molecular patterns and act as adjuvants for immune stimulation, leading to an anti-tumor immune memory effect to prevent tumor recurrence.73 In short, whether cyanobacteria act as photosensitizers, photothermal agents, adjuvants, or combined with other drugs, that represents a flexible strategy for enhancing the efficacy of anti-tumor of PDT.
PPSB and Anti-Tumor of PDT
It is also crucial to pay attention to the research findings on light-triggered anaerobic PSB in tumor treatment, such as focusing on PPSB (see Table 2). Unlike cyanobacteria, anaerobic PSB do not produce O2 during photosynthesis. However, scholars have recently harnessed some of their properties to eliminate tumor cells under light irradiation. Zheng and Li’s research team first documented that R. johrii moves from the injection site to the hypoxic tumor regions of tumor-bearing mice due to its property of hypoxic chemotaxis. Subsequent NIR light irradiation further facilitated the localization of R. johrii in tumor tissue due to its NIR phototaxis. Simultaneously, R. johrii converted NIR light into heat in tumor tissue, showing photothermal conversion properties. These findings suggested that R. johrii can act as a photothermal agent providing hypoxia-targeted PTT and act as carriers of other anti-tumor agents (eg, photosensitizers, chemotherapeutics, and prodrugs) providing a combination of multiple strategies.71 Subsequently, they utilized R. palustris to develop an engineered PSB-based tumor vaccine to enhance anti-tumor immunity after PTT. They modified the surface of R. palustris with maleimide to form PSB-MAL through membrane insertion of DSPE-PEG-MAL. Under NIR light irradiation, PSB-MAL-induced PTT leads to tumor cell death and the subsequent release of antigens. PSB-MAL captures the released antigens through a Michael addition reaction. After 1-day and 2-day intervals, NIR light was applied at the tumor periphery to drive PSB-MAL to the tumor margin. At last, PSB-MAL transports tumor antigens to normally functioning antigen-presenting cells (APCs) and promotes dendritic cell (DCs) activation, leading to systemic immune responses and immune memory effects in a mouse tumor model.92 Niu et al combined Rhodobacter sphaeroides (R. sphaeroides) with immunoadjuvant imiquimod (R837)-loaded thermosensitive liposomes (R837@TSL) to form nanoimmunoadjuvant-armed bacteria (R.S-R837@TSL). They found that R.S-R837@TSL effectively targeted hypoxic tumor tissue and converted NIR light into heat for PTT under 808 nm light irradiation. High-temperature-melted R837@TSL released R837 in situ, activating DC-mediated immune responses. Thus, R.S-R837@TSL enables the simultaneous application of PTT and immunoadjuvant-enhanced immunotherapy.93 In addition to its photothermal properties, Yang et al found that R. palustris can generate ROS, leading to effective cytotoxicity in tumor tissues under NIR light irradiation.94 Previous studies have shown that extracts of R. palustris and R. sphaeroides containing bacteriochlorophyll-a can induce photocytotoxicity in promyelocytic leukemia cells under 400 nm-800 nm light irradiation,95 indicating that certain PPSB can act as photosensitizers. However, other scholars have reported that R. johrii produces more H2 after exposure to xenon lamps than after exposure to 808 lasers and red LED light. Elevated levels of H2 can disrupt mitochondrial function, leading to oxidative stress and increased ROS levels.96 In short, light-triggered PPSB can provide benefits for tumor treatment. However, the light-triggered biological properties of PPSB may be affected by the choice of light sources and bacterial strains. Therefore, further experimental research is needed to enrich our understanding of this area.
 |
Table 2 Literature Examples of Light-Triggered PPSB in Tumor Treatment |
Challenges of PSB in Anti-Tumor PDT
The above research showed that light-triggered PSB can integrate O2 supplementation and multimodal treatments without complex design and modifications. These findings will encourage scholars to seek new insights and achievements in anti-tumor PDT. However, we also realize that the studies of PSB as biomaterials for anti-tumor PDT are still in their early stages. There are still many challenges in the preparation and application of PSB.
Safety
PSB are commonly found in various natural environments, including soils, paddy fields, swamps, lakes, rivers, and oceans. Scholars believe that PSB hold significant potential for research and applications in fields, such as food, nutrition, cosmetics, and medicine. However, similar to other bacteria-based tumor therapies, safety is the primary concern and challenge in the preparation and application of PSB. Yang et al demonstrated that dead R. palustris lost their unique light-triggered biological properties and formed visible aggregations after autoclaving. The solution containing dead bacteria is not suitable for intravenous injection into mice due to the risk of lethal pulmonary embolus,93 suggesting that live PSB remain the preferred option. Notably, PSB contain pathogen-associated molecular patterns (PAMPs) similar to those found in other pathogenic bacteria, such as lipopolysaccharide, peptidoglycan, and flagellin, which can activate the systemic immune response. Previous animal studies have demonstrated that PSB are not pathogenic to major organs, except for causing tolerable inflammation. Cohen et al discovered that only a small amount of PSB remained in the interstitium of Wistar rats 24 hours after the direct intramyocardial injection of S. elongatus. There was no evidence of abscess formation or residual S. elongatus present four weeks after injection. In addition, the rats receiving intravenous injections of S. elongatus had no signs of infection, and blood cultures remained persistently negative for bacterial growth throughout a one-week study period.97 However, the growth and development of live PSB in tumor tissues make their pharmacokinetics differ from those of traditional drugs. Tumor-bearing rodent models cannot fully replicate clinical scenarios in humans,98 meaning that the dosage, administration method, and treatment course of PSB established in existing animal experiments may not be appropriate for human applications. There is also a lack of relevant experimental observations on the clearance process of PSB in human organs, and the potential harm of residual PSB and their biofilms remains unknown. Moreover, certain cyanobacteria have been reported to produce highly toxic secondary metabolites known as cyanotoxins. Prolonged exposure to these cyanotoxins, even at relatively low doses, may lead to liver and kidney damage, cytotoxicity, neurotoxicity, skin toxicity, gastrointestinal disturbances, and other problems.99 However, some scholars have suggested that the immunogenicity of PSB could offer potential benefits for tumor immunotherapy. Large numbers of PSB disappear after PDT/PTT treatment and eradication of tumor lesions, thereby avoiding the need for antibiotics. Many scholars have realized that bacteria are double-edged swords in tumor therapy.100,101 Therefore, ensuring the safety of PSB in the human body while enhancing their effectiveness is crucial. Scholars need to conduct strict clinical trial supervision and long-term follow-up observations in the future.
Bacterial Size and Distribution
Tumor-targeting bacteria can passively enter tumor tissue through leakage from the tumor vascular system and penetrate the tumor interior through intercellular translocation.102 However, the approximately 2 μm length of PSB may hinder their ability to infiltrate solid tumors owing to their sizable dimensions.60 The heterogeneity of tumors and the immune conditions of the tumor microenvironment (TME) can also affect the distribution of PSB, resulting in uneven bacterial colonization in the tumor tissue. These factors are detrimental to PSB-based therapy in tumor treatment.103 In recent years, nanosized outer membrane vesicles (OMVs) derived from bacteria have been used as natural drug nanocarriers for tumor treatment and have gained increasing attention. Similar to other gram-negative bacteria, PSB also produces OMVs. A recent study confirmed that PSB-OMVs can target and accumulate in tumor tissues, inhibit tumor growth, and improve immunosuppressive effects in tumor areas without obvious toxicity and side effects.60 Notably, these nanosized vesicles contain a small amount of chlorophyll or BChls, indicating that they may possess some light-triggered biological properties of the parent PSB, such as ROS production. Hence, PSB-OMVs could replace their parent bacteria and play a more favorable role in anti-tumor PDT. However, similar to the challenges of OMVs in PDT discussed in the previous review,17 scholars may face challenges, such as low yield and heterogeneity.
Preparation of Engineered PSB
Researchers can combine PSB with drugs, nanomaterials, and functional groups using various physical or chemical methods such as covalent conjugation, electrostatic interactions, and streptavidin-biotin binding. The modifiability of PSB allows researchers to achieve tumor-targeted drug delivery and multimodal anti-tumor therapies. However, engineering methods affect the drug loading and release rate of PSB. Lu et al summarized and compared the advantages and disadvantages of different engineering methods in their published review.91 Thus far, scholars have often chosen engineering methods based on their research purposes and experimental conditions, which may lead to different experimental results. Some engineering methods may affect the size, charge potential, membrane rigidity, integrity, and activity of PSB, resulting in undesirable alterations in properties, cell motility, immunogenicity, blood clearance, and body metabolic status. It is necessary to avoid external contamination during separation, purification, and engineering processes due to live PSB cannot be sterilized by filtration or heating. Furthermore, there is no unified standard for the storage parameters of engineered PSB, such as temperature, time, and solvents, which can affect their stability.
Conclusion and Outlook
PDT has considerable therapeutic effects and clinical application prospects in superficial tumors and precancerous lesions. However, the efficacy of PDT in deep tumors is affected by factors such as low light penetration depth, non-targeting photosensitizers, and hypoxia in tumor tissues.104 Tumor hypoxia remains an unavoidable challenge in the clinical application of photodynamic therapy (PDT). In the present review, we introduced the NP-based carrier system for supplementing oxygen and integrating non-oxygen-consuming treatments to enhance the anti-tumor efficacy of PDT. In contrast to NPs, PSB have unique advantages as biomaterials that deserve attention. First, hypoxia chemotaxis gifts PSB a natural tumor-targeting ability, while phototaxis facilitates PSB to achieve light-guided movement to the tumor, which does not require extra modification with tumor-targeting ligands. Second, PSB can carry photosensitizers using a simple coincubation process, while light-triggered PSB can supply O2 by photosynthesis to enhance the photodynamic effects of photosensitizers. Third, light-triggered PSB can be eliminated during PDT, avoiding bacterial residue after treatment. Lastly and most importantly, light-triggered PSB exhibited good performance in photothermal conversion, ROS-induced photocytotoxicity, and H2 production, indicating that PSB can serve as potential photothermal agents, photosensitizers, or H2 suppliers, not just carriers and O2 suppliers of photosensitizers. These advantages make light-triggered PSB supply O2 and integrate various treatment modalities such as PTT, PDT, and gas therapy to reverse the hypoxic limitation of PDT in tumor tissues. Some scholars have identified other light-triggered biological properties of PSB, such as photoacoustic (PA) imaging and second near-infrared (NIR-II, 1000–1700 nm) thermal imaging, which may enable them to act as active PA contrast agents and NIR-II probes for visualizing anti-tumor PDT.95 Notably, engineered PSB possess superior tumor targeting and drug loading capacity, which opens up opportunities for collaboration with additional anti-tumor treatments, such as immunotherapy,92 gene therapy,105 sonodynamic therapy (SDT),106 and chemotherapy.90 Combining with superparamagnetic magnetite endows the engineered PSB external magnetic actuation and magnetic resonance imaging properties.107 The development of an all-in-one diagnostic and therapeutic platform based on engineered PSB could significantly enhance the efficacy of PDT in treating solid tumors and advance the developments in this field. However, the application of PSB in anti-tumor PDT is still in its infancy and faces several challenges. In addition to the already mentioned challenges, we also need to understand the physiological behavior of PSB in the body, including their viability/proliferation manner, OMV biogenesis, and the interaction with quorum sensing (QS) systems quorum sensing (QS) systems. In particular, understanding how QS systems regulate PSB aggregation in tumor tissues and monitoring their numbers have positive implications for the clinical application of PSB. In summary, PSB represents a promising new area of research in anti-tumor PDT and has the potential for various applications. Therefore, we are confident that deepening our understanding of PSB and encouraging the progress of advanced engineering technology will contribute to the clinical transformation of PSB as a light-activatable biomaterial in anti-tumor PDT.
Data Sharing Statement
Data are contained within the article.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
The author gave his consent for publication.
Acknowledgments
The author expresses sincere thanks to Dr. Yaxiong Fang and Ms. Zunzhen Zhou for their assistance.
Funding
This work was supported by grants from the Research Project of the Science and Technology Department of Sichuan Province (2021YJ0217), the Research Project of Sichuan Medical and Health Care Promotion Institute (KY2023SJ0041), and the Medical Research Project of Chengdu (2023203).
Disclosure
The author declares no conflicts of interest in this work.
References
1. Kwiatkowski S, Knap B, Przystupski D, et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi:10.1016/j.biopha.2018.07.049
2. Rkein AM, Ozog DM. Photodynamic therapy. Dermatol Clin. 2014;32:415–425. doi:10.1016/j.det.2014.03.009
3. Kessel D. Critical PDT theory III: events at the molecular and cellular level. Int J Mol Sci. 2022;23:6195. doi:10.3390/ijms23116195
4. Shafirstein G, Battoo A, Harris K, et al. Photodynamic therapy of non-small cell lung cancer. Narrative Rev Future Direct Ann Am Thorac Soc. 2016;13:265–275. doi:10.1513/AnnalsATS.201509-650FR
5. Hamblin MR. Photodynamic therapy for cancer: what’s past is prologue. Photochem Photobiol. 2020;96:506–516. doi:10.1111/php.13190
6. Kessel D, Obaid G, Rizvi I. Critical PDT theory II: current concepts and indications. Photodiagnosis Photodyn Ther. 2022;39:102923. doi:10.1016/j.pdpdt.2022.102923
7. Huang X, Pan J, Xu F, et al. Bacteria-based cancer immunotherapy. Adv Sci. 2021;8:2003572. doi:10.1002/advs.202003572
8. Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92. doi:10.2147/HP.S93413
9. Wan Y, Fu L-H, Li C, Lin J, Huang P. Conquering the hypoxia limitation for photodynamic therapy. Adv Mater. 2021;33:e2103978. doi:10.1002/adma.202103978
10. Maier A, Anegg U, Fell B, et al. Hyperbaric oxygen and photodynamic therapy in the treatment of advanced carcinoma of the cardia and the esophagus. Lasers Surg Med. 2000;26:308–315. doi:10.1002/(SICI)1096-9101(2000)26:3<308::AID-LSM9>3.0.CO;2-B
11. Maier A, Anegg U, Tomaselli F, et al. Does hyperbaric oxygen enhance the effect of photodynamic therapy in patients with advanced esophageal carcinoma? A clinical pilot study. Endoscopy. 2000;32:42–48. doi:10.1055/s-2000-132
12. Tomaselli F, Maier A, Pinter H, Stranzl H, Smolle-Jüttner FM. Photodynamic therapy enhanced by hyperbaric oxygen in acute endoluminal palliation of malignant bronchial stenosis (clinical pilot study in 40 patients). Eur J Cardiothorac Surg. 2001;19:549–554. doi:10.1016/S1010-7940(01)00635-2
13. Yang L, Wei Y, Xing D, Chen Q. Increasing the efficiency of photodynamic therapy by improved light delivery and oxygen supply using an anticoagulant in a solid tumor model. Lasers Surg Med. 2010;42:671–679. doi:10.1002/lsm.20951
14. Hu D, Sheng Z, Gao G, et al. Activatable albumin-photosensitizer nanoassemblies for triple-modal imaging and thermal-modulated photodynamic therapy of cancer. Biomaterials. 2016;93:10–19. doi:10.1016/j.biomaterials.2016.03.037
15. Lai C, Luo B, Shen J, Shao J. Biomedical engineered nanomaterials to alleviate tumor hypoxia for enhanced photodynamic therapy. Pharmacol Res. 2022;186:106551. doi:10.1016/j.phrs.2022.106551
16. Aebisher D, Czech S, Dynarowicz K, et al. Photodynamic therapy: past, current, and future. Int J Mol Sci. 2024;25:11325. doi:10.3390/ijms252011325
17. Jiang Y, Zhou Z, Liu C, Wang L, Li C. Bacterial outer membrane vesicles as drug delivery carrier for photodynamic anticancer therapy. Front Chem. 2023;11:1284292.
18. Aires-Fernandes M, Botelho Costa R, Rochetti Do Amaral S, et al. Development of biotechnological photosensitizers for photodynamic therapy: cancer research and treatment-from benchtop to clinical practice. Molecules. 2022;27:6848. doi:10.3390/molecules27206848
19. Zuo T, Li X, Ma X, et al. Engineering tumor-oxygenated nanomaterials: advancing photodynamic therapy for cancer treatment. Front Bioeng Biotechnol. 2024;12:1383930. doi:10.3389/fbioe.2024.1383930
20. Han D, Zhang X, Ma Y, Yang X, Li Z. The development of live microorganism-based oxygen shuttles for enhanced hypoxic tumor therapy. Mater Today Bio. 2022;18:100517. doi:10.1016/j.mtbio.2022.100517
21. Hamida RS, Ali MA, Redhwan A, Bin-Meferij MM. Cyanobacteria - A promising platform in green nanotechnology: a review on nanoparticles fabrication and their prospective applications. Int J Nanomed. 2020;15:6033–6066. doi:10.2147/IJN.S256134
22. Moloudi K, Abrahamse H, George BP. Nanotechnology-mediated photodynamic therapy: focus on overcoming tumor hypoxia. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024;16:e1937. doi:10.1002/wnan.1937
23. Salim SA, Salaheldin TA, Elmazar MM, Abdel-Aziz AF, Kamoun EA. Smart biomaterials for enhancing cancer therapy by overcoming tumor hypoxia: a review. RSC Adv. 2022;12:33835–33851. doi:10.1039/D2RA06036A
24. Li X, Chen L, Huang M, et al. Innovative strategies for photodynamic therapy against hypoxic tumor. Asian J Pharm Sci. 2023;18:100775. doi:10.1016/j.ajps.2023.100775
25. Zhang C, Hu X, Jin L, et al. Strategic design of conquering hypoxia in tumor for advanced photodynamic therapy. Adv Healthc Mater. 2023;12:e2300530. doi:10.1002/adhm.202300530
26. Bienia A, Wiecheć-Cudak O, Murzyn AA, Krzykawska-Serda M. Photodynamic therapy and hyperthermia in combination treatment-neglected forces in the fight against cancer. Pharmaceutics. 2021;13:1147. doi:10.3390/pharmaceutics13081147
27. Ikeda-Imafuku M, Wang LL, Rodrigues D, Shaha S, Zhao Z, Mitragotri S. Strategies to improve the EPR effect: a mechanistic perspective and clinical translation. J Control Release. 2022;345:512–536. doi:10.1016/j.jconrel.2022.03.043
28. Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921–7924. doi:10.7150/thno.49577
29. Islam W, Niidome T, Sawa T. Enhanced permeability and retention effect as a ubiquitous and epoch-making phenomenon for the selective drug targeting of solid tumors. J Pers Med. 2022;12:1964. doi:10.3390/jpm12121964
30. Gawali P, Saraswat A, Bhide S, Gupta S, Patel K. Human solid tumors and clinical relevance of the enhanced permeation and retention effect: a ‘golden gate’ for nanomedicine in preclinical studies? Nanomedicine. 2023;18:169–190. doi:10.2217/nnm-2022-0257
31. Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med. 2021;11:771. doi:10.3390/jpm11080771
32. Kushkevych I, Procházka J, Gajdács M, Rittmann SKR, Vítězová M. Molecular physiology of anaerobic phototrophic purple and green sulfur bacteria. Int J Mol Sci. 2021;22:6398. doi:10.3390/ijms22126398
33. Fan JY, Huang Y, Li Y, et al. Bacteria in cancer therapy: a new generation of weapons. Cancer Med. 2022;11:4457–4468. doi:10.1002/cam4.4799
34. Wilde A, Mullineaux CW. Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol Rev. 2017;41:900–922. doi:10.1093/femsre/fux045
35. Jiang ZY, Bauer CE. Component of the Rhodospirillum centenum photosensory apparatus with structural and functional similarity to methyl-accepting chemotaxis protein chemoreceptors. J Bacteriol. 2001;183:171–177. doi:10.1128/JB.183.1.171-177.2001
36. Mehdizadeh Allaf M, Peerhossaini H. Cyanobacteria: model Microorganisms and Beyond. Microorganisms. 2022;10:696.
37. Menon SN, Varuni P, Bunbury F, Bhaya D, Menon GI. Phototaxis in cyanobacteria: from mutants to models of collective behavior. mBio. 2021;12:e0239821.
38. Bhaya D. Light matters: phototaxis and signal transduction in unicellular cyanobacteria. Mol Microbiol. 2004;53:745–754. doi:10.1111/j.1365-2958.2004.04160.x
39. Schuergers N, Lenn T, Kampmann R, et al. Cyanobacteria use micro-optics to sense light direction. Elife. 2016;5:e12620. doi:10.7554/eLife.12620
40. Gupta RS. Molecular signatures for the main phyla of photosynthetic bacteria and their subgroups. Photosynth Res. 2010;104:357–372. doi:10.1007/s11120-010-9553-9
41. Pinevich AV. Proposal to consistently apply the international code of nomenclature of prokaryotes (ICNP) to names of the oxygenic photosynthetic bacteria (cyanobacteria), including those validly published under the international code of botanical nomenclature (ICBN)/international code of nomenclature for algae, fungi and plants (ICN), and proposal to change principle 2 of the ICNP. Int J Syst Evol Microbiol. 2015;65:1070–1074. doi:10.1099/ijs.0.000034
42. Pan X, Cao P, Su X, Liu Z, Li M. Structural analysis and comparison of light-harvesting complexes I and II. Biochim Biophys Acta Bioenergy. 2020;1861:148038. doi:10.1016/j.bbabio.2019.06.010
43. Stirbet A, Lazár D, Guo Y, Govindjee G. Photosynthesis: basics, history and modelling. Ann Bot. 2020;126:511–537. doi:10.1093/aob/mcz171
44. Nelson N, Junge W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu Rev Biochem. 2015;84:659–683. doi:10.1146/annurev-biochem-092914-041942
45. Lokstein H, Renger G, Götze JP. Photosynthetic light-harvesting (Antenna) complexes-structures and functions. Molecules. 2021;26:3378. doi:10.3390/molecules26113378
46. Li X, Hou W, Lei J, Chen H, Wang Q. The unique light-harvesting system of the algal phycobilisome: structure, assembly components, and functions. Int J Mol Sci. 2023;24:9733. doi:10.3390/ijms24119733
47. Oliver T, Kim TD, Trinugroho JP, et al. The evolution and evolvability of photosystem II. Annu Rev Plant Biol. 2023;74:225–257. doi:10.1146/annurev-arplant-070522-062509
48. Vasil’ev S, Bruce D. Optimization and evolution of light harvesting in photosynthesis: the role of antenna chlorophyll conserved between photosystem II and photosystem I. Plant Cell. 2004;16:3059–3068. doi:10.1105/tpc.104.024174
49. Hanada S. Anoxygenic photosynthesis -A photochemical reaction that does not contribute to oxygen reproduction. Microbes Environ. 2016;31:1–3. doi:10.1264/jsme2.ME3101rh
50. Hu X, Ritz T, Damjanović A, Autenrieth F, Schulten K. Photosynthetic apparatus of purple bacteria. Q Rev Biophys. 2002;35:1–62. doi:10.1017/S0033583501003754
51. Frigaard NU. Biotechnology of anoxygenic phototrophic bacteria. Adv Biochem Eng Biotechnol. 2016;156:139–154. doi:10.1007/10_2015_5006
52. Kushkevych I, Bosáková V, Vítězová M, Rittmann SKR. Anoxygenic photosynthesis in photolithotrophic sulfur bacteria and their role in detoxication of hydrogen sulfide. Antioxidants. 2021;10:829. doi:10.3390/antiox10060829
53. Gottstein J, Scheer H. Long-wavelength-absorbing forms of bacteriochlorophyll a in solutions of Triton X-100. Proc Natl Acad Sci U S A. 1983;80:2231–2234. doi:10.1073/pnas.80.8.2231
54. Hashimoto H, Uragami C, Cogdell RJ. Carotenoids and photosynthesis. Subcell Biochem. 2016;79:111–139. doi:10.1007/978-3-319-39126-7_4
55. Hashimoto H, Uragami C, Yukihira N, Gardiner AT, Cogdell RJ. Understanding/unravelling carotenoid excited singlet states. J R Soc Interface. 2018;15:20180026. doi:10.1098/rsif.2018.0026
56. Redding KE, Appel J, Boehm M, et al. Advances and challenges in photosynthetic hydrogen production. Trends Biotechnol. 2022;40:1313–1325. doi:10.1016/j.tibtech.2022.04.007
57. Tamagnini P, Axelsson R, Lindberg P, et al. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol Mol Biol Rev. 2002;66:1–20. doi:10.1128/MMBR.66.1.1-20.2002
58. Bothe H, Schmitz O, Yates MG, Newton WE. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol Mol Biol Rev. 2010;74:529–551. doi:10.1128/MMBR.00033-10
59. Ludwig M, Schulz-Friedrich R, Appel J. Occurrence of hydrogenases in cyanobacteria and anoxygenic photosynthetic bacteria: implications for the phylogenetic origin of cyanobacterial and algal hydrogenases. J Mol Evol. 2006;63:758–768. doi:10.1007/s00239-006-0001-6
60. Xiao T, Ma Y, Zhang Z, et al. Tailoring therapeutics via a systematic beneficial elements comparison between photosynthetic bacteria-derived OMVs and extruded nanovesicles. Bioact Mater. 2024;36:48–61. doi:10.1016/j.bioactmat.2024.02.025
61. Srirangan K, Pyne ME, Perry chou C. Biochemical and genetic engineering strategies to enhance hydrogen production in photosynthetic algae and cyanobacteria. Bioresour Technol. 2011;102:8589–8604. doi:10.1016/j.biortech.2011.03.087
62. Pospíšil P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim Biophys Acta. 2012;1817:218–231. doi:10.1016/j.bbabio.2011.05.017
63. Glaeser J, Nuss AM, Berghoff BA, Klug G. Singlet oxygen stress in microorganisms. Adv Microb Physiol. 2011;58:141–173.
64. Cogdell RJ, Howard TD, Bittl R, et al. How carotenoids protect bacterial photosynthesis. Philos Trans R Soc Lond B Biol Sci. 2000;355:1345–1349. doi:10.1098/rstb.2000.0696
65. Maoka T. Carotenoids as natural functional pigments. J Nat Med. 2020;74:1–16. doi:10.1007/s11418-019-01364-x
66. Magdaong NCM, Blankenship RE. Photoprotective, excited-state quenching mechanisms in diverse photosynthetic organisms. J Biol Chem. 2018;293(14):5018–5025. doi:10.1074/jbc.TM117.000233
67. Lu Y, Liu H, Saer R, et al. A molecular mechanism for nonphotochemical quenching in cyanobacteria. Biochemistry. 2017;56:2812–2823. doi:10.1021/acs.biochem.7b00202
68. Zhao E, Liu H, Jia Y, et al. Engineering a photosynthetic bacteria-incorporated hydrogel for infected wound healing. Acta Biomater. 2022;140:302–313. doi:10.1016/j.actbio.2021.12.017
69. Elrad D, Niyogi KK, Grossman AR. A major light-harvesting polypeptide of photosystem II functions in thermal dissipation. Plant Cell. 2002;14:1801–1816. doi:10.1105/tpc.002154
70. Bassi R, Dall’Osto L. Dissipation of light energy absorbed in excess: the molecular mechanisms. Annu Rev Plant Biol. 2021;72:47–76. doi:10.1146/annurev-arplant-071720-015522
71. Zheng P, Fan M, Liu H, et al. Self-propelled and near-infrared-phototaxic photosynthetic bacteria as photothermal agents for hypoxia-targeted cancer therapy. ACS Nano. 2021;15:1100–1110. doi:10.1021/acsnano.0c08068
72. Goto Y, Iwata S, Miyahara M, Miyako E. Discovery of intratumoral oncolytic bacteria toward targeted anticancer theranostics. Adv Sci. 2023;10:e2301679. doi:10.1002/advs.202301679
73. Wang H, Guo Y, Gan S, et al. Photosynthetic microorganisms-based biophotothermal therapy with enhanced immune response. Small. 2021;17:e2007734. doi:10.1002/smll.202007734
74. Liu S, Yang H, Ho MY, Xing B. Recent advances of material-decorated photosynthetic microorganisms and their aspects in biomedical applications. Adv Optical Mater. 2023;11:2203038. doi:10.1002/adom.202203038
75. Lu H, Niu L, Yu L, et al. Cancer phototherapy with nano-bacteria biohybrids. J Control Release. 2023;360:133–148. doi:10.1016/j.jconrel.2023.06.009
76. Huo M, Wang L, Zhang L, et al. Photosynthetic tumor oxygenation by photosensitizer-containing cyanobacteria for enhanced photodynamic therapy. Angew Chem Int Ed Engl. 2020;59:1906–1913.
77. Qi F, Ji P, Chen Z, et al. Photosynthetic cyanobacteria-hybridized black phosphorus nanosheets for enhanced tumor photodynamic therapy. Small. 2021;17:e2102113. doi:10.1002/smll.202102113
78. Liu L, He H, Luo Z, et al. In situ photocatalyzed oxygen generation with photosynthetic bacteria to enable robust immunogenic photodynamic therapy in triple-negative breast cancer. Adv Funct Mater. 2020;30:1910176. doi:10.1002/adfm.201910176
79. Huo M, Liu P, Zhang L, et al. Upconversion nanoparticles hybridized cyanobacterial cells for near-infrared mediated photosynthesis and enhanced photodynamic therapy. Adv Funct Mater 2021;31:2010196. doi:10.1002/adfm.202010196
80. Zhang X, Zhang Y, Zhang C, et al. An injectable hydrogel co-loading with cyanobacteria and upconversion nanoparticles for enhanced photodynamic tumor therapy. Colloids Surf B Biointerfaces. 2021;201:111640. doi:10.1016/j.colsurfb.2021.111640
81. Chang M, Feng W, Ding L, et al. Persistent luminescence phosphor as in-vivo light source for tumoral cyanobacterial photosynthetic oxygenation and photodynamic therapy. Bioact Mater. 2021;10:131–144. doi:10.1016/j.bioactmat.2021.08.030
82. Yin C, Wang Z, Dai C, et al. Light-triggered photosynthetic engineered bacteria for enhanced-photodynamic therapy by relieving tumor hypoxic microenvironment. Theranostics. 2023;13:1632–1648. doi:10.7150/thno.81718
83. Sun T, Zhang Y, Zhang C, et al. Cyanobacteria-based bio-oxygen pump promoting hypoxia-resistant photodynamic therapy. Front Bioeng Biotechnol. 2020;8:237. doi:10.3389/fbioe.2020.00237
84. An X, Zhong D, Wu W, et al. Doxorubicin-loaded microalgal delivery system for combined chemotherapy and enhanced photodynamic therapy of osteosarcoma. ACS Appl Mater Interfaces. 2024;16:6868–6878. doi:10.1021/acsami.3c16995
85. Wang S, Zhang C, Fang F, et al. Beyond traditional light: NIR-II light-activated photosensitizers for cancer therapy. J Mater Chem B. 2023;11:8315–8326. doi:10.1039/D3TB00668A
86. Dong W, Wang H, Liu H, et al. Potential of black phosphorus in immune-based therapeutic strategies. Bioinorg Chem Appl. 2022;2022:3790097. doi:10.1155/2022/3790097
87. Saberi S, Khoobi M, Alaeddini M, et al. The effect of photodynamic therapy on head and neck squamous cell carcinoma cell lines using spirulina platensis with different laser energy densities. Photodiagnosis Photodyn Ther. 2022;37:102688. doi:10.1016/j.pdpdt.2021.102688
88. Morlière P, Mazière JC, Santus R, et al. Tolyporphin: a natural product from cyanobacteria with potent photosensitizing activity against tumor cells in vitro and in vivo. Cancer Res. 1998;58:3571–3578.
89. Wang CY, Wang X, Wang Y, et al. Photosensitization of phycocyanin extracted from Microcystis in human hepatocellular carcinoma cells: implication of mitochondria-dependent apoptosis. J Photochem Photobiol B. 2012;117:70–79. doi:10.1016/j.jphotobiol.2012.09.001
90. Shen YJ, Cao J, Sun F, et al. Effect of photodynamic therapy with (17R,18R)-2-(1-hexyloxyethyl)-2-devinyl chlorine E6 trisodium salt on pancreatic cancer cells in vitro and in vivo. World J Gastroenterol. 2018;24:5246–5258. doi:10.3748/wjg.v24.i46.5246
91. Saberi S, Modiri-Delshad T, Etemad-Moghadam S, et al. Efficacy of synthesized cubic spirulina platensis photosensitizer in anticancer photodynamic therapy: an in vitro study. Photodiagnosis Photodyn Ther. 2023;42:103511. doi:10.1016/j.pdpdt.2023.103511
92. Han D, Wang F, Ma Y, et al. Redirecting antigens by engineered photosynthetic bacteria and derived outer membrane vesicles for enhanced cancer immunotherapy. ACS Nano. 2023;17:18716–18731. doi:10.1021/acsnano.3c01912
93. Niu MT, Chen QW, Chen Z, et al. Immunoadjuvant-modified Rhodobacter sphaeroides potentiate cancer photothermal immunotherapy. Nano Lett. 2024;24:130–139. doi:10.1021/acs.nanolett.3c03191
94. Yang X, Komatsu S, Reghu S, Miyako E. Optically activatable photosynthetic bacteria-based highly tumor specific immunotheranostics. Nano Today. 2021;37:101100. doi:10.1016/j.nantod.2021.101100
95. Kamal N, Sabaratnam V, Abdullah N, et al. Light-activated cytotoxic compounds from Malaysian microorganisms for photodynamic therapy of cancer. Antonie Van Leeuwenhoek. 2009;95:179–188. doi:10.1007/s10482-008-9301-8
96. Yan H, Fan M, Liu H, et al. Microbial hydrogen ”manufactory” for enhanced gas therapy and self-activated immunotherapy via reduced immune escape. J Nanobiotechnol. 2022;20:280. doi:10.1186/s12951-022-01440-7
97. Cohen JE, Goldstone AB, Paulsen MJ, et al. An innovative biologic system for photon-powered myocardium in the ischemic heart. Sci Adv. 2017;3:e1603078. doi:10.1126/sciadv.1603078
98. Zhou S, Gravekamp C, Bermudes D, Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18:727–743. doi:10.1038/s41568-018-0070-z
99. Zanchett G, Oliveira-Filho EC. Cyanobacteria and cyanotoxins: from impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins. 2013;5:1896–1917. doi:10.3390/toxins5101896
100. Ikryannikova LN, Gorokhovets NV, Belykh DA, Kurbatov LK, Zamyatnin AA Jr. Bacterial therapy of cancer: a way to the dustbin of history or to the medicine of the future? Int J Mol Sci. 2023;24:9726. doi:10.3390/ijms24119726
101. Yarahmadi A, Zare M, Aghayari M, Afkhami H, Jafari GA. Therapeutic bacteria and viruses to combat cancer: double-edged sword in cancer therapy: new insights for future. Cell Commun Signal. 2024;22:239. doi:10.1186/s12964-024-01622-w
102. Suh S, Jo A, Traore MA, et al. Nanoscale bacteria-enabled autonomous drug delivery system (NanoBEADS) enhances intratumoral transport of nanomedicine. Adv Sci. 2018;6:1801309. doi:10.1002/advs.201801309
103. Duong MT, Qin Y, You SH, Min JJ. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 2019;51:1–15. doi:10.1038/s12276-019-0297-0
104. Rodrigues JA, Correia JH. Enhanced photodynamic therapy: a review of combined energy sources. Cells. 2022;11:3995. doi:10.3390/cells11243995
105. Mandhata CP, Sahoo CR, Padhy RN. Biomedical applications of biosynthesized gold nanoparticles from cyanobacteria: an overview. Biol Trace Elem Res. 2022;200:5307–5327. doi:10.1007/s12011-021-03078-2
106. Lu S, Feng W, Dong C, et al. Photosynthetic oxygenation-augmented sonodynamic nanotherapy of hypoxic tumors. Adv Healthc Mater. 2022;11:e2102135. doi:10.1002/adhm.202102135
107. Zhong D, Li W, Qi Y, He J, Zhou M. Photosynthetic biohybrid nanoswimmers system to alleviate tumor hypoxia for FL/PA/MR imaging-guided enhanced radio-photodynamic synergetic therapy. Funct Mater. 2020;30:1910395. doi:10.1002/adfm.201910395
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

