Back to Journals » International Journal of Nanomedicine » Volume 20
Photothermal Therapeutic Gold Nanoparticles Loaded with PD-L1 siRNA Enhanced Killing of NSCLC Cells by Immune Cells
Authors Gao L, Yang LH, Jiang FC, Ye WY, Chen X, Liu ZJ, He RQ, Huang WY, Feng ZB, Kong JL, Lai ZF, Chen G
Received 19 January 2025
Accepted for publication 24 June 2025
Published 7 July 2025 Volume 2025:20 Pages 8833—8859
DOI https://doi.org/10.2147/IJN.S518427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Xing Zhang
Li Gao,1,* Li-Hua Yang,2,* Fang-Cheng Jiang,1,3 Wang-Yang Ye,4 Xin Chen,3 Zhao-Ji Liu,3 Rong-Quan He,2 Wan-Ying Huang,1 Zhen-Bo Feng,1 Jin-Liang Kong,5 Ze-Feng Lai,3,6 Gang Chen1
1Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China; 2Department of Medical Oncology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China; 3Guangxi Key Laboratory of Pharmaceutical Precision Detection and Screening, Pharmaceutical College, Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China; 4College of Basic Medicine, Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China; 5Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China; 6State Key Laboratory of Targeting Oncology, Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gang Chen, Email [email protected] Ze-Feng Lai, Email [email protected]
Background: Although immune checkpoint blocking (ICB) therapeutic agents have significantly improved the survival for many cancer patients, the efficacy of ICB therapy for non-small cell lung cancer (NSCLC) patients remains limited. Combining ICB therapy with other strategies that is able to stimulate tumor immunogenicity or increase infiltration of T cells aroused great concern in cancer therapy.
Results: Herein, we constructed a kind of AuNP@NH2-PEG-SH/PD-L1 siRNA nanoparticle complex which showed prominent photothermal therapeutic (PTT) effects triggered by NIR laser and could efficiently deliver PD-L1 siRNA into NSCLC cells (knock-down efficiency were 75.8% and 83% in HCC827 and A549 cells). During the process of photothermal stimulation, discrepancies in heat shock protein expression were observed in NSCLC cells. Knocking down PD-L1 expression in NSCLC cells such as A549 and HCC827 cells activated the co-culturing Jurkat cells and enhanced their tumor-killing effect in vitro (cell inhibition rate was 62.65% for HCC827 cells and 57.03% for A549 cells). The gold nanoparticle complexes exhibited remarkable PTT effect for the engrafted NSCLC cells in zebrafish larvas. Furthermore, the nano complexes could promote the activation of the xenografted human peripheral blood mononuclear cells (PBMCs) and enhance the killing of the NSCLC cells in the larvas.
Conclusion: It is well known that gold nanoparticle is one of the several metal nanoparticles approved for clinical trial by American FDA. This work has demonstrated the outstanding PTT and immunotherapeutic effects of gold nanoparticle complexes on NSCLC, indicating great potential in clinical regiment of lung cancers.
Keywords: non-small cell lung cancer, AuNP, photothermal therapy, antitumor immunity, PD-L1
Background
Immunotherapy has exerted a widespread impact on the survival of cancer patients over the past decade.1 ICB drugs functioned by blocking receptors and ligands involved in the impairment of T cell activation, such as programmed cell death 1 (PD1), cytotoxic T lymphocyte antigen 4 (CTLA4) and its ligand PD-L1, and prevent or reverse the tolerance of acquired peripheral tumor antigens.2 ICB therapy targeting the PD-1/PD-L1 pathway has been established as the standard first-line treatment for non-small cell lung cancer (NSCLC) patients in advanced stage.3,4 However, ICBs yielded limited effect with a response rate of less than 20% in NSCLC patients with wild-type epidermal growth factor receptor (EGFR).5,6 Therefore, the combination of ICB method with other therapeutic methods that could stimulate tumor immunogenicity and increase the infiltration of T cells has become a hot spot in cancer treatment.7 The use of ICB antibodies is confronted with problems such as poor diffusion, low tumor penetration, and immune-related adverse effects.8 Antibody therapy only blocks immune checkpoints on the cell membrane, which are compensated by proteins in the cytoplasm.9,10 To overcome these problems, RNA interference-based PD-1/PD-L1 knockout offers an alternative way to enhance tumor killing mediated by T-cells.11,12
As a widely used RNA interference technique, small interfering RNA (siRNA) has become a promising therapeutic strategy in anticancer research. Double-stranded siRNA can significantly eliminate the expression of a specific gene by specifically targeting the corresponding mRNA through an RNA-induced silencing complex (RISC) that occurs in the cytoplasm.13,14 However, siRNA is usually degraded by relevant enzymes, which remains the biggest obstacle to current siRNA technology. Literature studies have shown that siRNA coupling with gold nanoparticles (AuNP), polyethylenimine (PEI), liposomes, mixed micelles, and other delivery systems can significantly increase its uptake in the cell, thereby improving the efficiency of gene knockout in vitro or in vivo.15–19 So far, a variety of nanomaterials have been developed for transfection of siRNAs into tumor cells, providing valuable experience for siRNA-based drug development.20–22 A targeted exosomal delivery system (designated EGFRApt/Exo/siSurvivin) was engineered by Guo et al through incorporation of survivin-specific siRNA to suppress antiapoptotic pathways, which demonstrated synergistic therapeutic efficacy when administered in combination with cisplatin.23 Through genetic modification of HEK293T cells using tLyp1-Lamp2b fusion plasmids, researchers successfully generated tumor-penetrating exosomes capable of targeted siRNA delivery.24 Yang et al developed a novel EGFRapt-siKRASG12C mutation-targeted three-way junction (3WJ)-based RNA nanocomplex, demonstrating dual targeting specificity for both EGFR-overexpressing NSCLC cells and KRASG12C mutations.25
AuNP consists of gold-bearing inorganic nuclei, usually surrounded by organic monolayers.26 AuNPs are widely regarded as relatively reliable siRNA delivery vectors due to its simplicity, controllable size and shape, surface plasmon resonance (SPR), easy functionalization of the surface, and good biocompatibility.27–30 To prepare non-toxic, surfactant-free and positively charged AuNPs, stabilizers such as L-cysteine, polyethylenimine (PEI) or polyethylene glycol (PEG) was recommended for modification.31–33 It was demonstrated that AuNPs modified with glucose-terminated PEG-block-poly(L-lysine) could deliver PLK1 siRNA to breast cancer cells efficiently,34 and AuNPs functionalized with PEI and folic acid showed high transfection of siRNA into LNCaP prostate cancer cells.35 Multifunctional AuNPs platform could be constructed by multi-modification strategy. For instance, AuNPs modified with both triple helix probe molecular switches and MUC1 aptamer can be used for fluorescence imaging and PTT of tumor.36 However, treatment of NSCLC by combining PTT and ICB with spherical AuNPs carrying PD-L1 siRNA has not been studied comprehensively.
In this study, AuNP@NH2-PEG-SH/PD-L1 siRNA nanoparticle complex was prepared by modifying spherical AuNPs with the positive NH2-PEG-SH and the subsequent electrostatic attachment of negative PD-L1 siRNA. Herein, the PEG coating serves a dual purpose of prolonging the circulation time of the nanoparticles in the bloodstream and shielding the encapsulated siRNA from enzymatic attack.37 The nano complex showed prominent photothermal therapeutic (PTT) effects triggered by 808 nm NIR laser and could efficiently deliver PD-L1 siRNA into the NSCLC cells. Knocking down PD-L1 expression in NSCLC cells such as A549 and HCC827 cells activated the co-culturing Jurkat cells and enhanced their tumor-killing effect in vitro. Besides, the gold nanoparticle complexes exhibited remarkable PTT effect for the engrafted NSCLC cells in zebrafish larvas. Furthermore, the nano complexes could promote the activation of the xenografted human peripheral blood mononuclear cells (PBMCs) and enhance the killing of the NSCLC cells transplanted in the larvae. Although other materials have demonstrated dual-modal capabilities, this study represents the first report of such a NH2-PEG-SH-modified spherical AuNP platform optimized for lung cancer treatment. A dense yet orderly PEGylated monolayer ensured colloidal stability and tumor accumulation via enhanced permeability and retention effect. Moreover, the radially oriented siRNA conjugation through Au-S bonds achieved both sufficient siRNA payloads and preservation of the nanoparticle’s photothermal efficiency. This structural paradigm overcomes the “carrier’s dilemma” observed in prior studies where rod-shaped or hollow gold variants suffered either siRNA loading limitations or severe photothermal decay.38,39 Our approach establishes a streamlined yet effective strategy for concurrent PD-L1 silencing and PTT, offering a more targeted and clinically translatable platform compared to earlier designs that lacked the specific morphological and surface engineering for lung cancer-specific applications (Scheme 1).
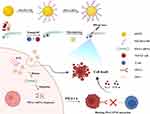 |
Scheme 1 The hypothetical scheme of the construction and application of AuNPs@NH2-PEG-SH/PD-L1 siRNA nanoparticle complex in NSCLC. Abbreviations: NIR, near infrared; PTT, photothermal therapy. |
Methods
Preparation and Characterization of AuNPs@NH2-PEG-SH/PD-L1 siRNA
The Preparation of AuNP
AuNPs were prepared by seeded growth method as previous study.40 Firstly, deionized water of 100mL in a 250mL three-neck flask was heated to 100°C. Chlorauric acid solution (Macklin, C805628-5g, 1%, 1mL) and 1% sodium citrate solution (Aladdin, T433108-500g, 3mL) were then added to the boiled water and heated for obtaining gold seed solution. Subsequently, AuNP solution were prepared in a conical bottle with 8mL gold seed solution, 39.5mL deionized water and 2.5mL 1% chloroauric acid solution. The solution was stirred at a uniform speed. A total of 45mL 0.1% ascorbic acid solution (Aladdin, S431925-250g) was added to the solution at a constant rate (1.5mL/min, 30min) with an infusion pump at the flow rate of 1mLmin-1 within 45min. The AuNP concentrate prepared by the above steps was centrifuged at 2500g for 30min and washed with 0.02% sodium citrate solution by centrifugation at 2500g for 30min. After washing twice, the AuNPs was suspended in 1mL deionized water and fully dispersed by water bath ultrasound.
Characterization of AuNP Solution
The Zeta potential and particle size of AuNP solution were measured by dynamic light scattering nanoparticle at 25°C, and the results were repeated 3 times. The ultraviolet spectrum of AuNP solution was determined by UV-VIS spectrophotometer. The test conditions were set as follows: the scanning range of 200–800nm, the test temperature of 25°C, and deionized water as the dilution solvent. The concentration of AuNP was calculated according to the formula: A = λ*c *l, where A is the absorbance of the solution; λ* is the molar extinction coefficient of the substance at the corresponding absorption wavelength; c is the molar concentration of the solution and l is the absorption path in cm. The molar extinction coefficient of AuNP is 2.33* 1088−1cm−1.41
The Preparation of AuNP@NH2-PEG-SH
The NH2-PEG-SH (Ponsure Biotechnology, PS2-SN-10K) (1mg) is fully dissolved in 1mL 0.1mol/L sodium bicarbonate solution (Aladdin, S112331-500g). Then NH2-PEG-SH solution was added into 1mL AuNP concentrated solution and thoroughly mixed, and the reaction was carried out at 60°C in a constant temperature mixer for 1h. After the reaction, the mixture was centrifuged with deionized water 2500g for 30min and wash three times. The prepared AuNP@NH2-PEG-SH was centrifuged at 2500g in 0.1M sodium bicarbonate solution for 30min and washed with deionized water. Then it was centrifuged at 2500g in sterile 1×PBS containing 0.05% v/v Tween-20 for 30min and washed with deionized water. Finally, it was centrifuged in sterile 1×PBS at 2500g for 30min and washed twice. The prepared AuNP@NH2-PEG-SH was filled to 1mL with deionized water.
Characterization of AuNP@NH2-PEG-SH Solution
The Zeta potential and particle size of AuNP@NH2-PEG-SH were assessed by dynamic light scattering nanoparticle at 25°C, and the results were repeated 3 times. The ultraviolet spectrum of AuNP@NH2-PEG-SH solution was determined by UV-VIS spectrophotometer with the following conditions: the scanning range of 200–800nm, the test temperature of 25°C and deionized water as the dilution solvent. The infrared spectrum of AuNP@NH2-PEG-SH solid material derived through freeze-drying was measured by Fourier infrared spectrometer.
The Serum Stability of AuNP and AuNP@NH2-PEG-SH
AuNP and AuNP@NH2-PEG-SH solution at a volume of 500μL were dissolved in 10mL of aqueous solution containing 10% serum and placed in a water bath at 37°C and 110 RPM. At intervals of 24h, 48h, 72h, 96h, 120h, 144h and 168h, the particle size of each of the two samples was measured by dynamic light scattering at 25°C, and the stability of the two nanoparticles in serum was observed and compared.
The Preparation and Characterization of AuNP@NH2-PEG-SH/siRNA
NC siRNA (GenePharma, sense: UUCUCCGAACGUGUCACGUTT; antisense: UUCUCCGAACGUGUCACGUTT) (5noml) diluted in 250μL DEPC water was added into 5.95mL AuNP@NH2-PEG-SH solution (including AuNP@NH2-PEG-SH 2.5nmol). The solution was mixed uniformly and allowed for reaction under room temperature for precisely 30 minutes. Following the completion of the reaction protocol, the resultant suspension containing composite nanoparticles was subjected to separation using centrifugal filtration technology. Specifically, the solution was transferred to ultrafiltration devices (Millipore Amicon, 30 kDa) and centrifuged at 12,000×g relative centrifugal force for 10 minutes. Subsequent purification involved three sequential washing cycles with PBS (0.01 M, pH 7.4). The residual compounds were removed. Finally, AuNP@NH2-PEG-SH/siRNA was stored in the refrigerator at −20°C.
The morphology of AuNP@NH2-PEG-SH/siRNA was characterized by transmission electron microscopy (TEM) at an accelerated voltage of 80kV. The Zeta potential and ultraviolet spectrum of AuNP@NH2-PEG-SH/siRNA was measured by dynamic light scattering and UV-VIS spectrophotometer according to the methods described above.
The Loading Capacity of AuNP@NH2-PEG-SH for siRNA
A total of 2.5nmol siRNA was dissolved in 125μL DEPC water and the amount of siRNA was fixed at 5pm. The volume of AuNP@NH2-PEG-SH solution required was calculated depending on the concentration ratio of AuNP@NH2-PEG-SH/siRNA (1:6, 1:5, 1:4, 1:3, 1:2, 1:1). AuNP@NH2-PEG-SH/siRNA solution at different concentration ratios were filled to the final volume of 20μL with DEPC water. The control group contained only 5pm of siRNA in 20μL DEPC water. The above solution is mixed with 5×Loading Buffer, and 1% agar-gel electrophoresis is carried out at 100mV for about 20min. TAE solution (0.5X) is used as the electrophoretic buffer. After electrophoresis, the gel imaging system was used for analyzing.
Determination of the Encapsulation Rate of AuNP@NH2-PEG-SH/siRNA
A total of 2.5nmol NC siRNA was dissolved in 125μL DEPC water, and the nucleic acid concentration of the dissolved siRNA was measured by Nanodrop 2000 spectrophotometer. After the test, 1μL of dissolved siRNA sample was added into 23.8μL AuNP@NH2-PEG-SH solution (including 0.0 nmol AuNP@NH2-PEG-SH) for reaction at room temperature for 30min. The prepared nanoparticle composite were centrifuged at 12000rpm for 10min by ultrafiltration tube (30kDa). The nucleic acid concentration of the filtrate was measured again by Nanodrop 2000 spectrophotometer. The encapsulation rate is calculated as follows: encapsulation rate = (total amount of siRNA - amount of siRNA in filtrate)/total amount of siRNA *100%.42
The Effect of Transfection of AuNP@NH2-PEG-SH/PD-L1 siRNA on PD-L1 Expression of NSCLC Cells
The Biocompatibility of AuNP@NH2-PEG-SH and AuNP@NH2-PEG-SH/NC siRNA
HCC827 and A549 cells were purchased from National Collection of Authenticated Cell Cultures (SCSP-538, SCSP-503) and cultured according to the supplier’s manuals. HCC827 cells and A549 cells were seeded into 96-well cell culture plates (1×104 cells/well) and incubated overnight. Different concentrations of AuNP@NH2-PEG-SH (5nM, 10nM, 15nM and 20nM) and AuNP@NH2-PEG-SH/NC siRNA (containing NC siRNA:5nM, 10nM, 15nM, 20nM, 25nM, 30nM and 35nM) diluted in complete medium were added to 96-well plates for co-culture with HCC827 cells or A549 cells. The control group was added with complete culture medium. After incubation for 24h, the liquid in each hole was replaced with 100μL 1640 or F-12K complete medium and 10μLCCK8 reagent, and the plate was incubated away from light. Two hours later, the absorbance of each hole was measured at 450nm. The cell viability was deduced based on the following formula: Cell viability (%) =OD450 (sample)/OD450 (control) ×100%. OD450 (control) was the OD value of the negative control group. OD450 (sample) is the OD value of each experimental group.
The Effect of AuNP@NH2-PEG-SH/PD-L1 siRNA on Viability of NSCLC Cells
Different concentrations of AuNP@NH2-PEG-SH/PD-L1 siRNA (containing PD-L1 siRNA:5nM, 10nM, 15nM, 20nM, 25nM, 30nM and 35nM) diluted in complete medium were added to HCC827 cells and A549 cells in 96-well plates. The following experimental steps were the same as described in the above section. PD-L1 siRNA used in the present study was designed by GenePharma (sense: GCAGUGACCAUCAAGUCCUTT; antisense: AGGACUUGAUGGUCACUGCTT).
Uptake of AuNP@NH2-PEG-SH/FAM-NC siRNA by NSCLC Cells
HCC827 cells or A549 cells were inoculated into confocal laser culture dishes at a concentration of 5×104/ dish and cultured for 24h. After washing with PBS, AuNP@NH2-PEG-SH loaded with FAM labeled NC siRNA (Sangon Biotech, sense: UUCUCCGAACGUGUCACGUTT; antisense: ACGUGACACGUUCGGAGAATT) (15nM) and free FAM NC siRNA solution (15nM) diluted in 2mL base medium were added to the dishes, respectively. After 24 hours of incubation away from light, the cells were washed twice with PBS and fixed with 4% paraformaldehyde at room temperature for 15min. Hoechst 33342 dye solution (Solarbio, C0031) (1mg/mL) was added to each dish and the cells were incubated for 10min at room temperature away from light. After incubation, the dishes were washed twice with PBS, and the uptake of FAM NC siRNA by cells was observed by confocal laser microscope.
For detection of cell uptake with flow cytometry, HCC827 cells or A549 cells were cultured in six-well plates. When the cell density reached about 70%, 2mL AuNP@NH2-PEG-SH/FAM NC siRNA (30nM), free FAM NC siRNA (30nM), lipo2000/FAM NC siRNA (30nM) and basal culture medium were added to cells in different groups. After 24 hours of incubation, cells in each group were cultured with complete culture medium. The next day, the cells were collected in 1.5mL EP tube and washed with PBS for detection of fluorescence intensity by flow cytometry.
Evaluation of PD-L1 Expression in NSCLC Cells Treated with AuNP@NH2-PEG-SH/PD-L1 siRNA
HCC827 cells were cultured in 10cm cell culture dishes. When the cell density reached about 70%, the medium in the dish was discarded, and the cells were treated with AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM), AuNP@NH2-PEG-SH/NC siRNA (30nM), AuNP@NH2-PEG-SH (15nM), lipo2000/PD-L1 siRNA (30nM) and basal culture medium, respectively. For A549 cells cultured in 10cm cell culture dishes, when the cell density reached about 50%, 50ng/mL IFN-γ (meilunbio, MB5954) was added to A549 cells for secretion of PD-L1. After stimulation with IFN-γ for 24h, the cells were treated with AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM), AuNP@NH2-PEG-SH/NC siRNA (30nM), AuNP@NH2-PEG-SH (15nM), lipo2000/PD-L1 siRNA (30nM) and basal culture medium, respectively. Total RNA of NSCLC cells in different treatment groups were extracted using total RNA extraction kit (Servicebio, G3640-50T), and PCR reaction was performed on Bio-Rad system according to the manufacturer’s instructions. The primer sequences used in the present study were: GACCACCACCACCAATTCCAAGAG (forward primer of PD-L1) and TGGAGGATGTGCCAGAGGTAGTTC (reverse primer of PD-L1). The expression data of PD-L1 in different groups were analyzed with 2−ΔΔCt.43 The total protein of NSCLC cells in different groups were extracted using RIPA reagent and detection of PD-L1 protein in different treatment groups was performed through Western blot experiment (PD-L1 antibody: Abcam, ab213524; GAPDH antibody: Cell signaling technology, 5174S). GAPDH was chosen as the internal reference and the protein bands were imaged under the dual color infrared laser imaging system (LI-COR, ODYSSEY Clx). PD-L1 protein expression in different treatment groups was also measured by flow cytometry assay with the use of PE labeled PD-L1 monoclonal antibody (ThermoFisher, 12–5983-41).
The Effect of AuNP@NH2-PEG-SH/PD-L1 siRNA on Biological Function of NSCLC Cells
CCK8 Cell Proliferation Assay
HCC827 cells were cultured in six-well plates and treated with AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM), AuNP@NH2-PEG-SH/NC siRNA (30nM), AuNP@NH2-PEG-SH (15nM) and basal culture medium, respectively. For A549 cells, 50ng/mL IFN-γ was firstly added to A549 cells for secretion of PD-L1. After stimulation with IFN-γ for 24h, the cells were treated with AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM), AuNP@NH2-PEG-SH/NC siRNA (30nM), AuNP@NH2-PEG-SH (15nM) and basal culture medium, respectively. After 24 hours of incubation, the cells in each group were cultured with complete culture medium. The next day, cells were collected and cell suspensions (3×104/mL) were inoculated into 96-well plates for culturing overnight. CCK8 reagent of 10 µL was added to each hole at intervals of 24h, 48h, 72h and 96h. The absorbance of each hole at 450nm was detected.
Scratch Assay
Monolayer HCC827 or A549 cells in different treatment groups was wounded with 200 µL pipette tips. After removal of floating cells by PBS washing, the cells were incubated with serum-free DMEM for 48 h. Cell migration was observed with microscope and three fields were randomly selected and photographed for each well at 0 and 48 h. Percentages of wound healing area was calculated through Image J software.44
The Effect of AuNP@NH2-PEG-SH/PD-L1 siRNA on Cell Cycles of NSCLC Cells Detected by Flow Cytometry
After HCC827 cells or A549 cells of different treatment groups overspread the six-well plate, the medium was discarded and the plates were washed twice with PBS. The cells in each well were collected in a 15mL centrifuge tube and washed with pre-cooled PBS buffer. A total of 500μL pre-cooled 70% ethanol was added to cells followed by gently blowing and mixing. The cells were fixed at 4°C for more than 4 h and centrifuged at 1000g for 5 min. Then, the cells were resuspended with 1 mL pre-cooled PBS buffer and centrifuged again. Propyl iodide staining solution at the volume of 500μL was added to each sample and the cells were gently re-suspended, thoroughly mixed, and incubated at room temperature for 30 min away from light. The cell cycle was measured by flow cytometry and flowjo software was used for analyzing cell cycles.
Transwell Invasion Assay
The Matrigel gel was mixed with serum-free 1640 or F-12K medium at the ratio of 1: 5, and 80μL diluted Matrigel was added to the Transwell chamber. The 24-well Transwell plate was placed at 37°C for solidification. Then, 200 μL serum-free suspension of HCC827 cells or A549 cells of different treatment groups (5 × 105/mL) was added to the upper chamber. Complete medium containing 10% FBS was added to the lower chamber of each well (600μL). The 24-well plates were cultured for 48 h in a 37°C incubator. Subsequently, the Transwell chamber was washed twice with PBS, the cells on the upper surface were wiped off. Then the cells were fixed with anhydrous methanol for 30 minutes, and stained with 0.1% crystal violet for 24 hours. After washing with PBS and air-drying, photos of invaded cells were captured under a fluorescent inverted microscope at a magnification of ×200.45
The in vitro Therapeutic Effect of PTT Combined with PD-L1 siRNA Transfection by AuNP@NH2-PEG-SH on NSCLC Cells
Measurement of the Photothermal Effect of AuNP@NH2-PEG-SH
AuNP@NH2-PEG-SH solution of 15nM was irradiated with a near-infrared laser of 808 nm at the power of 2, 1.5, 1.0 and 0.5W/cm2 for 15min, respectively. Meanwhile, 1×PBS was used as the blank control group. The temperature was recorded every 15s, and the photothermal curve was drawn.
The Effect of PD-L1 siRNA Combined with PTT on Viability of NSCLC Cells
HCC827 cells and A549 cells treated with 50ng/mL IFN-γ were seeded into 96-well cell culture plates (5×103 cells/well) and incubated overnight. AuNP@NH2-PEG-SH (15nM) and AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM) diluted in basical culture medium were added to 96-well plates. After 24 hours of incubation, the cells in the group requiring light treatment were irradiated with 808nm infrared laser for 5min (2W/cm2). After irradiation, the plates were washed twice with PBS and continued for culturing with complete culture media. Cell viability was measured with CCK8 reagent according to the methods described above.
The Effect of AuNP@NH2-PEG-SH/PD-L1 siRNA Combined with PTT on Apoptosis of NSCLC Cells
HCC827 and A549 cells treated with 50ng/mL IFN-γ were cultured in six-well plates and treated with AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM), AuNP@NH2-PEG-SH (15nM) or basical culture medium, respectively. After 24 hours of incubation, the cells requiring laser irradiation were irradiated with 808nm infrared laser for 5min (2W/cm2). After irradiation, the liquid in all holes were replaced with complete medium and incubation of plates continued.
After the cells confluenced in the six-well plate, the cells were collected with trypsin digestion solution without EDTA and centrifuged with pre-cooled PBS twice. The 5×binding buffer was diluted to 1× working solution with double steaming water, and the cells were resuspended with 500μL 1×binding buffer. A total of 5μL Annexin V-APC and 10μL 7-AAD were added to each tube for 5 minutes of incubation away from light. Then, all samples were ready for flow cytometry.
Expression of Hsp70 and Hsp90 in NSCLC Cells Treated with PTT
Total RNA and proteins were harvested from HCC827 or A549 cells treated with AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM), AuNP@NH2-PEG-SH (15nM) and basical culture medium with or without laser irradiation. PCR and Western blot experiments were conducted for comparing Hsp70 and Hsp90 expression in different treatment groups according to the standard protocols (Hsp70 antibody: Abcam, EPR16892; Hsp90 antibody: Abcam, EPR16621-67). GAPDH served as the internal reference for PCR and Western blot experiments. Primer sequences for genes coding Hsp70 and Hsp 90 were: GTGCTGACCAAGATGAAGGAGATC (HSPA1A, forward), GCTGCGAGTCGTTGAAGTAGG (HSPA1A, reverse), ATCCACCACTCTACTCTGTCTCTG (HSP90AA1, forward), CTCAACCTCCTCCTCCTCCATC (HSP90AA1, reverse). GAPDH primers were purchased from Sangon Biotech (B661104-0001).
The Effect of Transfection of AuNP@NH2-PEG-SH/PD-L1 siRNA Into NSCLC Cells on Immune Activity of Jurkat T Cells
Jurkat cells were purchased from National Collection of Authenticated Cell Cultures (SCSP-513) and cultured according to the supplier’s manuals. CD3/CD28 T cell activator (Immunocult, 10971) (25μL/mL) was used for inducing Jurkat cell activation.46 HCC827 or A549 cells stimulated by IFN-γ in six-well plates were treated with complete medium, AuNP@NH2-PEG-SH (15nM), AuNP@NH2-PEG-SH/NC siRNA (30nM), AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM), respectively. The next day, the medium of each well of the six-well plate was discarded, and the plates were washed twice with PBS. CD3/CD28 co-stimulated Jurkat cells were directly added into the six-well plate. NSCLC cells and Jurkat cells were co-cultured in 2mL 1640 complete medium with the ratio of HCC827 (A549)/Jurkcat=1:10, and the same number of activated Jurkat cells were cultured separately in the control group.
The next day, Jurkat cells in all groups were collected by centrifugation. Flow cytometry was performed on Jurkat cells of different co-culture groups or non-co-culture group for examination of cell proliferation (CellTrace™ CFSE Cell Proliferation Detection Kit: ThermoFisher, C34554), CD69 expression (PE labeled human CD69 monoclonal antibody: ThermoFisher, 12–0699-42) and apoptosis. Meanwhile, the supernatants of each group were collected and Elisa was used for detecting the secretion of IL-2 and TNF-ɑ by Jurkat cells according to the kit instructions (Human TNF - α Elisa Kit: Servicebio, GEH0004-96T; Human IL-2 Elisa Kit: Servicebio, GEH0038-96T). The OD values of each hole were acquired under the detection wavelength of 450 nm and the correction wavelength of 630 nm. The concentrations of IL-2 and TNF-ɑ in samples of each group were calculated according to the standard curve equation.
The Effect of Transfection with AuNP@NH2-PEG-SH/PD-L1 siRNA Combined with PTT on the Viability and Apoptosis of NSCLC Cells in Co-Culture System
HCC827 or A549 cells were inoculated in a 12-well plate at a density of 1×105 cells/well and cultured overnight. After cell adhesion, 50ng/mL IFN-γ was added to each group of cells for simulation of PD-L1. At the same time, CD3/CD28 T cell activator (25μL/mL) was used for inducing Jurkat cell activation. After 24 hours, the media of each hole of the six-well plate was discarded, and the cells were treated with complete medium, AuNP@NH2-PEG-SH (15nM), AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM), respectively. The next day, the 808nm laser was used for irradiating all cells in the PTT group with 2.0W/cm2 power for 5min. After irradiation, the plates were cleaned twice with PBS. CD3/CD28 co-stimulated Jurkat cells were directly added into the six-well plate. NSCLC cells and Jurkat cells were co-cultured in 2mL 1640 complete medium with the ratio of HCC827 (A549)/Jurkcat=1:10.
After 24 hours of co-culture, NSCLC cells of each group were collected in a 15mL centrifuge tube and inoculated into 96-well plates at a density of 1×105/ well for culture in complete medium. The next day, 10μL CCK8 reagent was added to each well and mixed with the medium. After two hours of incubation away from light, the absorbance of each hole at 450nm was measured. For flow cytometry experiments of cell apoptosis, NSCLC cells were collected with trypsin digestion solution without EDTA and centrifuged with pre-cooled PBS twice after 24 hours of co-culture. All samples were prepared for detection of cell apoptosis by flow cytometry according to the methods described above.
The Therapeutic Effect of AuNP@NH2-PEG-SH/PD-L1 siRNA in vivo
The Establishment of Zebrafish NSCLC Xenograft Model
Human HCC827 cells were stained with Dil reagent and transplanted into the yolk sac of zebrafish larvas (Ezerinka Biotechnology, 2dpf wild AB and Tg (fli1a: EGFP)) by microinjection (Nanoject III, Drummond, USA). Approximately 500–900 cells were injected into each yolk sac of the larvas for establishment of NSCLC xenograft model. After microinjection, the zebrafish larvas were placed in a juvenile incubator at 28°C for 1h, and then placed in a three-gas incubator for further culture. After 24h, the yolk sac of zebrafish was observed and photographed by stereo-fluorescence microscope. Approval of all in vivo experiments was guaranteed by the Ethical Committee of First Affiliated Hospital of Guangxi Medical University (2024-E788-01).
The Effect of AuNP@NH2-PEG-SH/PD-L1 siRNA Combined with PTT on Tumor Growth of NSCLC Xenografts
Zebrafish larvas transplanted with HCC827 cells were divided into four groups for treatment of AuNP@NH2-PEG-SH/PD-L1 siRNA (10nL, 100nM) with or without PTT and 1×PBS (10nL) with or without laser irradiation (808nm). After the injection of AuNP@NH2-PEG-SH/PD-L1 siRNA or PBS, zebrafish larvas were placed in juvenile incubators at 28°C for 1h and then placed in three-gas incubators for further culture. After 24 hours, all zebrafish larvas were transferred into 96-well plates, larvas requiring laser irradiation were irradiated with 808nm laser for 5min at the power of 1.5W/cm2. After irradiation, the zebrafish larvas were placed in a juvenile incubator at 28°C for 1h, and then placed in a three-gas incubator for further culture. Twenty-four hours later, the fluorescence of NSCLC xenografts in zebrafish yolk sacs were observed and photographed by stereo-fluorescence microscopy. Image J software was used for analyzing and calculating the fluorescence area and fluorescence intensity of NSCLC xenografts.
The Effect of AuNP@NH2-PEG-SH/PD-L1 siRNA Combined with PTT on Tumor Growth of NSCLC Xenografts After Construction of Immune Microenvironment
Peripheral blood lymphocytes were extracted from healthy adult volunteers according to kit instructions. The next day post establishment of NSCLC xenografts, the collected human peripheral blood lymphocytes were transplanted into the yolk sac of zebrafish larvas by microinjection (500~900 cells each tail) for constructing the immune microenvironment of zebrafish larvas. After injection, the zebrafish larvas was placed in a juvenile incubator at 28°C for 1 hour, and then placed in a three-gas incubator for 12 hours.
Zebrafish larvas transplanted with HCC827 cells and human peripheral blood lymphocytes were divided into four groups for treatment of AuNP@NH2-PEG-SH/PD-L1 siRNA (10nL, 100nM) with or without PTT and 1×PBS (10nL) with or without laser irradiation (808nm). After the injection of AuNP@NH2-PEG-SH/PD-L1 siRNA or PBS, zebrafish larvas were placed in juvenile incubators at 28°C for 1h and then placed in three-gas incubators for further culture. After 12 hours, all zebrafish larvas were transferred into 96-well plates, larvas requiring laser irradiation were irradiated with 808nm laser for 5min at the power of 1.5W/cm2. After irradiation, the zebrafish larvas were placed in a juvenile incubator at 28°C for 1h, and then placed in a three-gas incubator for further culture. Twenty-four hours later, the fluorescence of NSCLC xenografts in zebrafish yolk sacs were observed and photographed using the methods described above.
Evaluation of the Systemic Toxicity of AuNP@NH2-PEG-SH/PD-L1 siRNA in Zebrafish
Zebrafish larvas (2dpf wild AB and Tg (fli1a: EGFP)) were injected with 10nL AuNP@NH2-PEG-SH/PD-L1 siRNA (100nM) or 10nL 1×PBS. After injection, zebrafish larvas were placed in a juvenile incubator at 28°C for recovering and then placed in a three-gas incubator for further culture. After 48h, the whole fish was fixed at room temperature in 4% paraformaldehyde for 24h. HE stained tissue sections were made from whole fish according to standard protocols. The morphological changes were observed under microscopy (200x).
Statistical Analysis
All statistical analyses were conducted using SPSS v.22.0 software. Shapiro–Wilk test was performed for evaluation of normal distribution. Two way analysis of variance (ANOVA) was used for comparing the differences between groups in CCK8 cell proliferation assay. ANOVA method was used for analyzing the differences between groups in other experiments, and LSD or Tamhane methods were used for pairwise comparisons. All experiments were repeated at least three times. Two-tailed p values less than 0.05 indicated statistical significance.
Results
Preparation and Characterization of AuNPs@NH2-PEG-SH/PD-L1 siRNA
The average particle size of the gold nanoparticles is 77.07nm (Figure 1A), and the average potential is −36.7mV (Figure 1B). The ultraviolet spectrum results showed that the maximum absorption peak of the AuNP is 548nm (Figure 1C), which explained the reddish-brown appearance. The concentration of AuNP Concentrated solution was calculated to be 419.74nM.
The dynamic light scattering data showed that the mean particle size of AuNP@NH2-PEG-SH is 80.39nm (Figure 1A) and the mean potential is 35.63mV (Figure 1B). It was speculated that NH2-PEG-SH binding with AuNP through gold-sulfur bond increased the particle size of AuNP, and the amino group of NH2-PEG-SH increased the electropositivity of AuNP. The ultraviolet spectrum results showed that AuNP@NH2-PEG-SH displayed a maximum absorption peak at 549nm (Figure 1C), which is consistent with the maximum ultraviolet absorption wavelength of AuNP, indicating that the introduction of NH2-PEG-SH did not change the ultraviolet absorption characteristics of AuNP. The characteristic peaks of AuNP@NH2-PEG-SH were observed at 3432.37, 2921.61, 1640.25, 1384.25 and 1080.18cm, respectively (Figure 1G). Particularly, the hydroxyl peak at 3432.78 cm is considered to be the characteristic peak of gold nanoparticles. The stretching vibration peak of C-H is at 2921.61 cm, and the vibration absorption peak of C-O-C in PEG is at 1080.18cm (Figure 1G), indicating that NH2-PEG-SH is successfully coupled with AuNP.
The particle size of AuNP without surface modification changed little within 72h at 37°C, however; the particle size of AuNP increased from 77.89 nm to 100.06nm after 72h (Figure 1F). On the fifth and sixth days, the size of AuNP without surface modification further increased from 100.06nm to 103.83nm. In contrast, AuNP@NH2-PEG-SH particles did not cluster in the first six days, and the particle size of AuNP@NH2-PEG-SH increased from 83.16nm to 105.63nm on the seventh day (Figure 1F). The increased stability of AuNP might be attributed to the modification of NH2-PEG-SH.
Observation under transmission electron microscope showed that the AuNP@NH2-PEG-SH/siRNA nanoparticle complex is spherical with uniform particle size and good dispersion in aqueous solution (Figure 1D). The average particle size of AuNP@NH2-PEG-SH/siRNA is 81.04nm (Figure 1E), and its particle size is normally distributed. The spherical particles are coated with a thin shell, presumably formed by coupled NH2-PEG-SH and siRNA. Dynamic light scattering data showed that the average potential of AuNP@NH2-PEG-SH/siRNA is −8.72mV (Figure 1B), the negative potential indicated that siRNA has been attached to the surface of AuNP@NH2-PEG-SH by electrostatic adsorption. The UV spectrum results showed that AuNP@NH2-PEG-SH/siRNA exhibited a maximum absorption peak at 543nm (Figure 1C), which was basically consistent with the maximum UV absorption wavelength of AuNP@NH2-PEG-SH and AuNP, indicating that the loading of siRNA had no effect on the UV absorption characteristics of AuNP@NH2-PEG-SH.
As shown in Supplementary Figure 1, when the concentration ratio of AuNP@NH2-PEG-SH to NC siRNA was 1:2 and 1:1, no bright bands appeared on the gel, which confirmed the capacity of AuNP@NH2-PEG-SH for loading NC siRNA. Before combining with AuNP@NH2-PEG-SH, the total amount of siRNA used was 41.2ng. After mixed reaction and ultrafiltration, the amount of free NC siRNA was 4ng. The encapsulation rate of AuNP@NH2-PEG-SH for NC siRNA was 90.3%.
Uptake of AuNP@NH2-PEG-SH/FAM-NC siRNA by NSCLC Cells
As shown in Figure 2A–D, after incubation with AuNP@NH2-PEG-SH solution and AuNP@NH2-PEG-SH/NC siRNA solution at different concentrations, the viability of HCC827 cells and A549 cells was similar to that of the blank control group, reaching more than 90%. The results showed that AuNP@NH2-PEG-SH solution in the range of 0–20 nM and AuNP@NH2-PEG-SH/NC siRNA solution in the range of 0–35 nM had no toxicity on NSCLC cells. The AuNP@NH2-PEG-SH/PD-L1 siRNA exerted inhibiting effect on the viability of HCC827 and A549 cells in a concentration-dependent manner. The maximum inhibitory effect of AuNP@NH2-PEG-SH/PD-L1 siRNA on the viability of HCC827 and A549 cells was achieved at the concentration of 30nM. The maximum inhibition rates of cell viability by AuNP@NH2-PEG-SH/PD-L1 siRNA (30nM) were 33.5% and 27.6% for HCC827 and A549 cells, respectively (p<0.05, Figure 2E and F).
To detect whether AuNP@NH2-PEG-SH/siRNA could be delivered into A549 and HCC827 cells, we loaded FAM labeled NC siRNA onto the surface of AuNP@NH2-PEG-SH. HCC827 cells and A549 cells were incubated with the basal medium containing AuNP@NH2-PEG-SH/FAM-NC siRNA for 24h. The observation results under confocal laser microscopy showed that compared with the free FAM-NC siRNA control group, cells transfected with AuNP@NH2-PEG-SH/FAM-NC siRNA showed obvious green fluorescence signals, and most of the green fluorescence signals were distributed in the cytoplasm, which verified that HCC827 and A549 cells could effectively take up AuNP@NH2-PEG-SH/FAM-NC siRNA (Figure 2G). Flow cytometry experiments further demonstrated the high uptake efficiency of AuNP@NH2-PEG-SH/FAM-NC siRNA in HCC827 cells and A549 cells (Figure 2H–K). The green FAM fluorescence signal in NSCLC cells transfected with AuNP@NH2-PEG-SH/FAM-NC siRNA was significantly higher than that in the blank control group and the Free FAM-NC siRNA group (Figure 2H and I). The uptake efficiency of AuNP@NH2-PEG-SH/FAM-NC siRNA in both HCC827 cells and A549 cells was more than 80%, near to that of the positive control (Figure 2J and K).
Transfection with AuNP@NH2-PEG-SH/PD-L1 siRNA Successfully Knocked Down PD-L1 Expression in NSCLC Cells
To verify whether AuNP@NH2-PEG-SH/PD-L1 siRNA could effectively knock down the expression of PD-L1 in HCC827 cells and A549 cells, we performed RT-qPCR, Western blot and flow cytometry experiments. Because the background expression of PD-L1 was low in A549 cells; A549 cells were stimulated with IFN-γ for inducing high expression of PD-L1 before transfection with AuNP@NH2-PEG-SH/PD-L1 siRNA. PCR results showed that AuNP@NH2-PEG-SH/PD-L1 siRNA could significantly down-regulate the expression of PD-L1 mRNA in HCC827 cells compared with the other control groups, and the knock-down efficiency was 75.8%, near to the positive control lipo2000/PD-L siRNA (p<0.05, Figure 3A). Western blot assay and flow cytometry experiments further verified the ability of AuNP@NH2-PEG-SH/PD-L1 siRNA to significantly reduce PD-L1 protein in HCC827 cells (Figures 3A, 4A and C). The expression level of PD-L1 protein in HCC827 cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA and lipo2000/PD-L1 siRNA was significantly lower than that of the other three groups (p<0.05, Figures 3A, 4A and C). The mRNA and protein expressions of PD-L1 in A549 cells treated with IFN-γ were significantly higher than those in the blank control group (p<0.05, Figures 3B, 4B and D). Similar to the expression change of PD-L1 in HCC827 cells, The mRNA and protein expression of PD-L1 in IFN-γ-treated A549 cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA were remarkably reduced compared with IFN-γ-treated A549 cells transfected with AuNP@NH2-PEG-SH/NC siRNA, AuNP@NH2-PEG-SH or negative control (p<0.05, Figures 3B, 4B and D), and the knockdown efficiency for PD-L1 at mRNA level was 83%, similar to the knock-down effect of positive control lipo2000/PD-L siRNA (Figure 3B).
After successful knocking down of PD-L1 expression, we further investigated the effect of transfection with AuNP@NH2-PEG-SH/PD-L1 siRNA on biological functions of NSCLC cells. Results from CCK8 assay showed that the proliferation capacity of HCC827 cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA at 48h, 72h and 96h was significantly lower than that of the other three control groups (p<0.05, Figure 3C). Results from scratch assay showed that the scratch healing percentage of HCC827 cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA was significantly lower than that of the blank control group (p<0.05, Figure 3E and F). These results indicated that transfection of AuNP@NH2-PEG-SH/PD-L1 siRNA inhibited the migration of HCC827 cells by knocking down PD-L1 expression. After transfection with AuNP@NH2-PEG-SH/PD-L1 siRNA, the invasion ability of HCC827 cells was also significantly inhibited, and the number of cells invaded to the lower chamber 48h later was significantly lower than that of the other three controls (p<0.05, Supplementary Figure 2A and C). Analysis results from cell cycle flow cytometry showed that transfection with AuNP@NH2-PEG-SH/PD-L1 siRNA significantly reduced the proportion of HCC827 cells at G2/M stage compared with the other three controls (p<0.05, Supplementary Figure 3A and C). IFN-γ treatment had no obvious effect on biological functions such as proliferation and invasion of A549 cells. The proliferation capacity of A549 cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA after IFN-γ treatment was significantly lower at 48h, 72h and 96h than that of the other groups (p < 0.05, Figure 3D). In the scratch assay, the healing percentage of scratch area in A549 cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA after IFN-γ treatment was significantly lower than that in the blank control group and the AuNP@NH2-PEG-SH/NC siRNA control group (Figure 3G and H). For transwell invasion assay, the number of A549 cells invaded to the lower compartment in group of transfection with AuNP@NH2-PEG-SH/PD-L1 siRNA after IFN-γ treatment was also significantly lower than that in other groups at 48h (p<0.05, Supplementary Figure 2B and D). Flow cytometry experiments of cell cycle showed that the proportion of IFN-γ-treated A549 cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA in S phase significantly increased by 15.1% compared with that in IFN-γ control group (p<0.05, Supplementary Figure 3B and D), which indicated that transfection with AuNP@NH2-PEG-SH/PD-L1 siRNA might arrest the cell cycle of A549 cells in S phase.
Combined Treatment of PTT and Transfection of AuNP@NH2-PEG-SH/PD-L1 siRNA Boosted Therapeutic Effect in vitro
As shown in Figure 5, under the irradiation of 808nm laser at the power of 2.0W/cm2, the temperature of 15nM AuNP@NH2-PEG-SH solution increased from 22.7°C to 60°C within 12 minutes and remained at about 60°C (Figure 5A and B). The temperature of PBS solution in the control group only increased by 3.4°C after 15min irradiation by 808nm laser (Figure 5B), indicating that the significant increase in the temperature of AuNP@NH2-PEG-SH solution was contributed to the photothermal conversion ability of AuNP@NH2-PEG-SH. Further observation showed that the photothermal conversion capacity of AuNP@NH2-PEG-SH was power-dependent. With the increase of the irradiation power, the rate of temperature increase of AuNP@NH2-PEG-SH solution speed up (Figure 5C). The temperature rise of AuNP@NH2-PEG-SH solution is the fastest under the irradiation of 808nm laser at the power of 2.0W/cm2 (Figure 5C).
Compared with blank control and AuNP@NH2-PEG-SH control group, the cell viability of HCC827 cells and A549 cells after AuNP@NH2-PEG-SH/PD-L1 siRNA transfection with or without PTT was significantly decreased (p<0.05) (Figure 6). The effect of AuNP@NH2-PEG-SH/PD-L1 siRNA transfection combined with PTT on the viability of NSCLC cells was greater than that of AuNP@NH2-PEG-SH-PD-L1 siRNA or PTT treatment alone (p<0.001). Laser irradiation had little effect on viability of HCC827 cells (Figure 6A). The viability of IFN-γ-stimulated A549 cells treated by laser irradiation decreased by 16.78% compared with the blank control group, which was lower than that of the other experimental groups (Figure 6B).
With regard to cell apoptosis, the apoptosis and necrosis rates of HCC827 cells and A549 cells were significantly increased after AuNP@NH2-PEG-SH/PD-L1 siRNA transfection with or without PTT (p<0.05) (Figure 6C–F). The apoptosis and necrosis rates of NSCLC cells treated by AuNP@NH2-PEG-SH/PD-L1 siRNA transfection combined with PTT were higher than those treated by AuNP@NH2-PEG-SH-PD-L1 siRNA transfection or PTT alone (Figure 6C–F) (p<0.01). After treatment of AuNP@NH2-PEG-SH/PD-L1 siRNA transfection or PTT, the necrosis rate of NSCLC cells was higher than early or late apoptosis rates, and the apoptosis of HCC827 cells in all treatment groups was mainly late apoptosis (Figure 6C and D). While the early apoptosis rate of A549 cells in combined treatment group was higher than the late apoptosis rate (Figure 6E and F). Laser irradiation alone caused slight apoptosis and necrosis for HCC827 cells and A549 cells (Figure 6C–F) (p<0.05).
We also investigated changes of Hsp70 and Hsp90 expression in NSCLC cells after PTT treatment. While the mRNA expression of HSPA1A gene in HCC827 cells were significantly decreased in PTT group and the combined treatment group compared with other groups, the mRNA expression of HSP90AA1 gene was significantly increased compared with the blank control and AuNP@NH2-PEG-SH control group (p<0.05) (Supplementary Figure 4A and B). There were no significant differences in the protein expression levels of Hsp70 and Hsp90 in HCC827 cells among all groups (Figure 6G, I and J). The expression of HSPA1A gene in IFN-γ-stimulated A549 cells of PTT group or combined treatment group was significantly lower than that in blank control and AuNP@NH2-PEG-SH/PD-L1 siRNA treatment group (Supplementary Figure 4C) (p<0.05). The expression of HSP90AA1 gene in IFN-γ-stimulated A549 cells was significantly higher in PTT group than in blank control group (p<0.05) (Supplementary Figure 4D). However, the expression of Hsp70 protein in IFN-γ-stimulated A549 cells of PTT group and combined treatment group was significantly higher than that in other control groups. The expression of Hsp90 protein in IFN-γ-stimulated A549 cells of PTT group and combined treatment group was higher than that of blank control and AuNP@NH2-PEG-SH control group (p<0.05) (Figure 6H, K and L).
Transfection of AuNP@NH2-PEG-SH/PD-L1 siRNA Led to Immune Activation
The fluorescence intensity of the proliferation peak of Jurkat cells in the non-co-culture group was similar to that of co-culture group without IFN-γ stimulation, indicating that HCC827 or A549 cells without IFN-γ stimulation did not affect the proliferation ability of Jurkat cells in co-culture system (Supplementary Figure 5A, B, G and H). The fluorescence intensity of the proliferation peak of Jurkat cells co-cultured with IFN-γ-stimulated HCC827 or A549 cells with or without the treatment of AuNP@NH2-PEG-SH and AuNP@NH2-PEG-SH/NC siRNA was significantly up-regulated (p<0.05) (Supplementary Figure 5C-E and I-K). When NSCLC cells stimulated by IFN-γ were treated with AuNP@NH2-PEG-SH/PD-L1 siRNA, the fluorescence intensity of the proliferation peak of co-cultured Jurkat cells significantly diminished compared with other IFN-γ stimulation control group (p<0.05) (Figure 7A and D, Supplementary Figure 5F and L).
Analysis results of flow cytometry experiments showed that the apoptosis rate of Jurkat cells significantly increased after co-culture with IFN-γ-stimulated HCC827 or A549 cells with or without the treatment of AuNP@NH2-PEG-SH and AuNP@NH2-PEG-SH/NC siRNA (p<0.05), (Supplementary Figure 6A–E and G–K). The apoptosis of Jurkat cells was mainly early apoptosis, and the apoptosis rate of Jurkat cells co-cultured with HCC827 cells was higher than that of Jurkat cells co-cultured with A549 cells under the same conditions. When NSCLC cells stimulated with IFN-γ were treated with AuNP@NH2-PEG-SH/PD-L1 siRNA, the apoptosis rate of Jurkat cells was significantly decreased compared with other IFN-γ stimulation control group (p<0.05) (Figure 7B and E, Supplementary Figure 6F and L).
Because CD69 is the earliest marker on the cell surface of activated T cells, we examined the expression rate of CD69 in Jurkat cells of co-culture systems. After co-culture with HCC827 cells or A549 cells without IFN-γ stimulation, the expression rate of CD69 on Jurkat cells decreased slightly (Supplementary Figure 7A–C and H–J). The expression rate of CD69 on Jurkat cells co-cultured with IFN-γ-stimulated HCC827 or A549 cells with or without the treatment of AuNP@NH2-PEG-SH and AuNP@NH2-PEG-SH/NC siRNA significantly decreased compared with that in the non-co-culture group and blank control co-culture group (p<0.05) (Supplementary Figure 7D–F and 7K–M). When NSCLC cells stimulated by IFN-γ were treated with AuNP@NH2-PEG-SH/PD-L1 siRNA, the expression rate of CD69 on Jurkat cells significantly increased compared with other IFN-γ stimulation control group (p<0.05) (Figure 7C and F, Supplementary Figure 7G and N).
Results of ELISA experiments showed that the level of IL-2 secreted by Jurkat cells co-cultured with IFN-γ-stimulated HCC827 or A549 cells with or without the treatment of AuNP@NH2-PEG-SH or AuNP@NH2-PEG-SH/NC siRNA was significantly decreased compared with the blank control group (p<0.05) (Figure 7G and H). After co-culture with IFN-γ-stimulated HCC827 or A549 cells treated by AuNP@NH2-PEG-SH/PD-L1 siRNA, the level of IL-2 secreted by Jurkat cells remarkably increased compared with other IFN-γ stimulation control group (p<0.05) (Figure 7G and H). Similarly, when NSCLC cells stimulated by IFN-γ were treated with AuNP@NH2-PEG-SH/PD-L1 siRNA, the level of TNF-α secreted by Jurkat cells increased significantly compared with other IFN-γ stimulation control group (Figure 7I and J). These results indicated that transfection of PD-L1 siRNA mediated by AuNP@NH2-PEG-SH reduced the binding of PD-L1 and PD-1 by suppressing IFN-γ-induced PD-L1 expression, thus boosting the immune activation of Jurkat cells.
We also evaluated the immunotoxic effects combined with PTT and transfection of PD-L1 siRNA on NSCLC cells. The cell viability of NSCLC cells in the co-culture system was further significantly decreased after being transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA or PTT treatment (p<0.05) (Supplementary Figure 8). The viability of NSCLC cells in the co-culture system treated by AuNP@NH2-PEG-SH/PD-L1 siRNA transfection combined with PTT was the lowest, in which case the inhibition rate was 62.65% for HCC827 cells and 57.03% for A549 cells (Supplementary Figure 8). These results indicated that the toxic effect of Jurkat cells induced by AuNP@NH2-PEG-SH/PD-L1 siRNA transfection on NSCLC cells combined with PTT had enhanced anti-tumor effect on NSCLC cells.
When NSCLC cells in the co culture system were treated by AuNP@NH2-PEG-SH /PD-L1 siRNA transfection or PTT treatment, the apoptosis and necrosis rates of NSCLC cells increased significantly than other control groups (p<0.05) (Figure 8A–G and J–P). Particularly, the apoptosis and necrosis rates of co-cultured NSCLC cells in PTT group were higher than that of co-cultured NSCLC cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA (Figure 8F, G, O and P). The apoptosis and necrosis rates of NSCLC cells in the co-culture system treated by AuNP@NH2-PEG-SH/PD-L1 siRNA combined with PTT were the highest, in which case the apoptosis of NSCLC cells mainly belonged to late apoptosis (Figure 8H, I, Q and R). These results indicated that the decrease of PD-L1 expression in NSCLC cells induced by AuNP@NH2-PEG-SH/PD-L1 siRNA and the toxic effect of Jurkat cells on NSCLC cells combined with PTT could increase the apoptosis and necrosis rate of NSCLC cells.
Therapeutic Effect of AuNP@NH2-PEG-SH/PD-L1 siRNA Combined with PTT in vivo
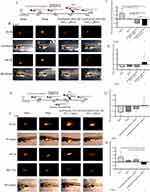
The design of in vivo experiments on zebrafish larvas was shown in Figure 9A and E. The growth rate of fluorescence intensity of NSCLC xenograft in zebrafish larvas treated with AuNP@NH2-PEG-SH/PD-L1 siRNA with or without PTT was significantly lower than that in PBS control group and laser irradiation control group (Figure 9B and C) (p<0.05). Specifically, the growth rate of fluorescence intensity of NSCLC xenograft in zebrafish treated with AuNP@NH2-PEG-SH/PD-L1 siRNA and PTT was further decreased compared with that in zebrafish treated with AuNP@NH2-PEG-SH/PD-L1 siRNA alone (p<0.05) (Figure 9B and C). There was no significant difference in growth rates of fluorescence area among all groups (Figure 9D). According to the analysis results of fluorescence intensity and fluorescence area, the tumor growth of NSCLC xenograft in the combined treatment group was significantly inhibited.
The representative photos of the fluorescence of NSCLC xenografts before and after treatment as well as the fluorescence of transplanted human peripheral blood lymphocytes were shown in Figure 9F. The growth rate of fluorescence intensity of NSCLC xenograft in zebrafish treated with AuNP@NH2-PEG-SH/PD-L1 siRNA and PTT was significantly lower than that in PBS control group and laser irradiation control group (p<0.05) (Figure 9F and G). There was no significant difference in growth rates of fluorescence area among all groups (Figure 9H). According to the analysis results of fluorescence intensity and fluorescence area, transfection of AuNP@NH2-PEG-SH/PD-L1 siRNA combined with PTT could significantly suppress the tumor growth of NSCLC xenografts in zebrafish after construction of immune microenvironment.
As shown in Supplementary Figure 9, there was no morphological abnormality in organs at developmental stages of zebrafish larvas injected with AuNP@NH2-PEG-SH/PD-L1 siRNA and PBS, which proved the biosafety of AuNP@NH2-PEG-SH/PD-L1 siRNA.
Discussion
Multiple nanoparticles have been developed for delivery of PD-L1 siRNA into NSCLC to achieve therapeutic effect.47–49 These breakthroughs in nanomedicine delivery systems demonstrate remarkable potential for creating lung cancer interventions with enhanced therapeutic specificity. Our investigation of PD-L1 siRNA-encapsulated gold nanoparticles aligns with this trend in targeted oncotherapy. Notably, previous researchers have successfully utilized AuNPs to deliver PD-L1 siRNA, demonstrating its potential in lung cancer therapy. Liu et al developed a gold nanoprism (GNP)-based nanoplatform with human PD-L1 siRNA loaded via sequential coating of poly(4-styrenesulfonate) (PSS) and poly(diallyldimethylammonium chloride) (PDADMAC), which possesses properties of siRNA delivery for hPD-L1 gene down-regulation, photoacoustic imaging, and photothermal therapy.50 In the present study, we explored for the first time the ability of NH2-PEG-SH modified spherical gold nanoparticles to deliver PD-L1 siRNA into NSCLC cells and silence PD-L1 expression levels, as well as their therapeutic potential.
In this study, the variation range of particle size of AuNP modified with NH2-PEG-SH within seven days was significantly smaller than that of AuNP without modification, indicating that modification by NH2-PEG-SH could increase its stability and reduce agglomeration between particles, which was consistent with literatures. After preparation of AuNP@NH2-PEG-SH/siRNA, we verified that AuNP@NH2-PEG-SH/siRNA could be absorbed by NSCLC cells and the silencing effect of AuNP@NH2-PEG-SH/siRNA for PD-L1 gene was as significant as lipo2000.
It was further noted in in vitro experiments that HCC827 and A549 cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA showed decreased proliferation, while AuNP@NH2-PEG-SH and IFN-γ or NC siRNA alone had no effect on the proliferation of NSCLC cells. These findings indicated that knockdown of PD-L1 expression by AuNP@NH2-PEG-SH significantly inhibited the proliferation of NSCLC cells. Previous studies have shown that PD-L1 could regulate the growth and proliferation of cancer cells and inhibit cell apoptosis without the involvement of PD-1.51–59 Lotfinejad et al demonstrated that knocking down the expression of PD-L1 could significantly reduce the proliferation rate of triple-negative breast cancer cells and induce apoptosis through intrinsic and extrinsic apoptotic pathways.53 In mouse sarcoma models, blocking PD-L1 expression of tumor cells significantly inhibited tumor progression and cellular glycolysis by inhibiting mTOR signaling and reducing the expression of some glycolytic enzymes.54 During cell division, PD-L1 is a subunit of the adhesion complex. PD-L1 could compensate for the loss of cell cycle related protein 5 and compete with WAPL to bind to PDS5B, thus ensuring proper cohesion and separation of sisters chromatids; in immunodeficient NSG mice, consumption of PD-L1 was reported to lead to the production of multinucleated cells and inhibit in vitro cell proliferation and in vivo tumor growth.57 These studies provided possible molecular mechanism of the growth inhibition in NSCLC cells with reduced PD-L1 expression. Further analysis of flow cytometry results indicated that decrease in PD-L1 expression caused by transfection of AuNP@NH2-PEG-SH/siRNA in HCC827 and A549 cells might result in arrest of the G0/G1 and S phase of cell cycle, respectively, thus leading to a decrease in cell proliferation ability.
We also observed the suppressing effect of knocking down PD-L1 expression by AuNP@NH2-PEG-SH/PD-L1 siRNA on the migration and invasion ability of NSCLC cells. Epithelial mesenchymal transformation (EMT) is a phenomenon closely associated with tumor cell migration and invasion.60 Previous studies have shown that PD-L1 plays a crucial role in promoting EMT and maintaining stem cell dryness in cancer stem cells. Chen et al observed that PD-1 fusion protein-mediated stimulation of the cytoplasmic domain of PD-L1 and PD-L1 played a key role in promoting the EMT phenotype of esophageal cancer Eca-109 cells.61 Tieche and Kim et al found that the overexpression of PD-L1 was significantly correlated with EMT of NSCLC cells.62,63 In NSCLC, TGF-β1 could up-regulate the expression of PD-L1 through phosphorylation of Smad2, while the treatment of NSCLC with M7824, a bi-functional drug that can target PD-L1 and TGF-β1, can reduce TGF-β1-mediated EMT.64 Based on the above results, it is assumed that the attenuated migration and invasion ability of NSCLC cells transfected with AuNP@NH2-PEG-SH/PD-L1 siRNA in this study may be related to the involvement of PD-L1 in promoting the EMT phenotype of tumor cells.
Considering the photothermal effects of AuNP, we also investigated the PTT potential of AuNP@NH2-PEG-SH/PD-L1 siRNA system in NSCLC. Multiple nanomaterials have been proven to be suitable for PTT,65–67 among which AuNP is the most studied platform for PTT, which has multiple advantages.68 Under the irradiation of 808nm NIR at the power of 2W/cm2, the AuNP@NH2-PEG-SH prepared in this study showed efficient photothermal conversion, which indicated that it had the therapeutic potential of PTT.
During hyperthermia, when cells and tissues are exposed to elevated temperatures, the supply of oxygen and nutrients cannot meet the needs of the growing tumor mass due to the disorder of blood vessel structure, leading to the development of a hypoxic, acidic and malnourished microenvironment, and cancer cells are generally more sensitive to high temperatures than normal cells.69–71 Literature studies have shown that protein denaturation is a key event in disruption of cell homeostasis.72 The starting temperature of protein denaturation varies for different types of cells. When temperature increases above 41°C, protein denaturation occurs in various organelles, including microsomal membranes, mitochondria, cytosol, nucleus, and nuclear matrix.72–74 Protein denaturation directly leads to enzyme inactivation and reduced membrane receptor binding capacity.75 The above mechanisms may provide a possible explanation for the decreased cell viability and increased mortality of NSCLC cells treated by PTT in the present study. Further in vitro experiments of the present study proved that the inhibition of the intrinsic biological function of tumor cells by PD-L1 reduction combined with PTT could achieve better anti-tumor effects.
Although PTT has the advantages of low cost, minimal side effects, and non-invasive treatment, its efficacy is sometimes compromised by non-lethal heat doses of tumor-area induced by heat shock proteins (HSPs).76,77 In this study, WB results showed increased trend of Hsp70 or Hsp90 protein expression in A549 cells of PTT group and combination treatment group, indicating that A549 cells may initiate self-defense mechanism to resist PTT by up-regulating Hsp70 and Hsp90 expression after heat stress. Hsps protein inhibitors can be introduced in future studies to increase the sensitivity of NSCLC cells to PTT, and further increase the therapeutic effect of AuNP@NH2-PEG-SH-mediated PTT in this study. We noted that Hsp70/Hsp90 protein levels differ between HCC827 and A549 cells post-PTT, which might arise from intrinsic differences in stress response pathways and genetic backgrounds. The induction of HSPs is a complex process regulated by multiple signaling pathways.78,79 In response to heat stress, the heat shock factor 1 (HSF1) is activated, which then binds to the heat shock element (HSE) in the promoter region of HSP genes, leading to their transcription.80 Differences in the activation of HSF1 or the integrity of the HSE in these two cell lines might explain the differential expression of HSPs. We conjectured that KRAS-mutant A549 cells may have a more robust or sensitive HSF1 - HSE axis, allowing for a greater upregulation of HSPs upon heat stress. In contrast, EGFR-mutant HCC827 cells might have a compromised or less responsive HSF1 - HSE pathway, resulting in no observable change in HSP expression. We will further validate these mechanisms using HSF1 or HSE inhibitors in follow-up experiments.
Due to the biological significance of PD-L1 as an immune checkpoint, we also investigated the effect of AuNP@NH2-PEG-SH-mediated transfection of PD-L1 siRNA on the immune activity of Jurkat T cells in co-culture system. ICB therapy targeting PD-1 or PD-L1 has been reported to resulted in adaptive immune resistance in some NSCLC patients,81 and the number of patients who can effectively respond to ICB therapy for a long time is still limited. It is also difficult to find suitable biomarkers to predict the long-term treatment response of NSCLC patients to ICB therapy.82 Therefore, we hypothesized that siRNA therapy targeting PD-L1 may be an effective strategy to assist in enhancing the anti-tumor immune activities of ICB therapy. In this study, we found that Jurkat cells co-cultured with NSCLC cells treated with AuNP@NH2-PEG-SH/PD-L1 siRNA exhibited increased cell proliferation, expression rate of CD69 and secretion of IL-2 and TNF-alpha, which indicated that the blocking of the binding between PD-L1 and PD-1 mediated by transfection of AuNP@NH2-PEG-SH/PD-L1 siRNA increased the immune activity of Jurkat cells.
In addition to directly killing tumor cells through hyperthermia, PTT could also induce ICD in tumor cells, release damage-associated molecular patterns (DAMP) and tumor-associated antigens to trigger adaptive immune responses and activate tumor-specific immune responses.83 PTT could also stimulate anti-cancer vaccine action and produce long-term anti-tumor efficacy in vivo.84 These properties of PTT make it an ideal adjunctive therapy for combined immunotherapy, resulting in the new concept of photothermal immunotherapy.84 PTT-induced ICDs have been combined with immunotherapies such as ICIs, immunoadjuvants, and CAR T-cell therapy for treating a variety of cancers and inducing sustained anti-tumor immune effects.84,85 In the co-culture experiment of this study, we also observed that the immunotoxic effect of Jurkat cells on NSCLC cells combined with PTT could produce increased anti-tumor effect than other groups. Therefore, it was conjectured that the AuNP@NH2-PEG-SH/PD-L1 siRNA prepared in this study beared potential for photothermal immunotherapy.
After in vitro validation, in vivo experiments of the present study further demonstrated the biosafety of AuNP@NH2-PEG-SH/PD-L1 siRNA. In the present work, we used the globally accepted zebrafish animal model. Zebrafish is one of the most important vertebrate model organisms in biomedical research, and the rapid extrauterine development of their fertilized eggs allows for direct visualization and manipulation during embryogenesis and larval development.86 Moreover, the optically transparent tissue of zebrafish during these developmental stages facilitates cellular and subcellular imaging, allowing for real-time monitoring of tumors and their microenvironment.87 Since the zebrafish larvas used in this study only have innate immune system,88 we innovatively isolated the peripheral blood lymphocytes from healthy human volunteers and transplanted them into zebrafish larvas to compensate for the missing adaptive immune system in zebrafish larvas and simulate the in vivo tumor microenvironment of NSCLC patients. The fluorescence intensity of NSCLC xenografts in transparent zebrafish larvas with or without reconstructed immune microenvironment intuitively reflected the therapeutic effects of different treatment groups, which further proved the therapeutic effect of AuNP@NH2-PEG-SH/PD-L1 siRNA for NSCLC in vivo. In the present study, we observed dissociation between tumor cell fluorescence intensity and tumor mass area.
We conjectured that dead cell clearance by phagocytes might be slower due to limited immune cell infiltration in zebrafish xenograft models compared to mammalian systems, which leaded to a lag between cell death and structural regression.
While we prepared AuNP@NH2-PEG-SH/PD-L1 siRNA nanoparticle complex and used zebrafish xenograft models to assess the therapeutic effect, certain limitations should be acknowledged. First, potential off-target effects of siRNA, a common challenge in gene therapy, were not fully evaluated in this work; future studies should employ high-throughput sequencing or functional assays to systematically assess siRNA specificity. Second, although the nanomaterial exhibited short-term stability in vitro, long-term stability during storage and in vivo circulation remains uncharacterized, necessitating further investigations into the durability of the nanoparticle coatings and siRNA payload under physiological conditions. Although zebrafish models offer advantages in visualizing dynamic processes and high-throughput screening, they lack the physiological complexity of mammalian tumor microenvironments, particularly in terms of immune interactions, stromal crosstalk, and organ-specific metastatic niches. It is necessary to establish the mouse tumor-bearing model experiment to verify the efficacy of AuNP@NH2-PEG-SH/PD-L1 siRNA. Future studies could incorporate syngeneic or patient-derived xenograft (PDX) murine models to evaluate the stability, dose-dependent responses, systemic toxicity, and long-term therapeutic outcomes of AuNP@NH2-PEG-SH/PD-L1 siRNA nanoparticle complex under conditions that better recapitulate human pathophysiology. These efforts would strengthen the preclinical relevance of our findings and bridge the gap between zebrafish-based discovery and mammalian translational research.
To propel the clinical translation of AuNP@NH2-PEG-SH/PD-L1 siRNA photothermal immunotherapy, future research should focus on overcoming existing technical barriers through interdisciplinary innovation. First, combination with emerging therapeutic modalities such as gas therapy or catalytic nanomedicine could amplify immunogenic cell death while reducing thermal resistance in tumors.89 Second, intelligent delivery systems refined with stimuli-responsive platforms such as AuNP self-assembly or pH/H2O2-activated drug release may enhance tumor-specific accumulation and spatiotemporal control of siRNA/PTT activation.90,91 Third, imaging-guided precision could be achieved by integrating multimodal imaging agents to monitor HSP70/ROS dynamics and optimize hyperthermia dosing. Finally, addressing clinical challenges such as improving laser penetration depth via NIR-II excitation and validating biocompatibility of nanoparticles in humanized PDX models is critical for safe clinical application. Preclinical validation in PDX models with humanized immune systems will better predict clinical outcomes.
Conclusions
In conclusion, this study demonstrates the feasibility of AuNP@NH2-PEG-SH/PD-L1 siRNA nanocomplex for NSCLC treatment. Our findings showed that transfection and knocking down efficiency of AuNP@NH2-PEG-SH-based delivery system was comparable to that of Lipo2000. The nanoparticle complex also exhibited tumor-killing effect through dual functions of PTT and immunostimulation. Although direct quantitative comparisons with other advanced nanomedicine platforms were not conducted in this study, the properties of AuNP@NH2-PEG-SH/PD-L1 siRNA nanocomplex, such as favorable biocompatibility, transfection efficiency, photothermal effect and immune-killing functions suggest potential advantages in clinical application. Future research could further explore the comparative performance of AuNP@NH2-PEG-SH/PD-L1 siRNA nanocomplex against other nanoplatforms and optimize the system for enhanced clinical translation.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author.
Ethics Approval and Consent to Participate
Approval of all in vivo experiments was guaranteed by the Ethical Committee of First Affiliated Hospital of Guangxi Medical University (2024-E788-01).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was funded by Guangxi Natural Science Foundation Project (2022GXNSFAA035512); Outstanding Young Doctor Project of the First Affiliated Hospital of Guangxi Medical University (2024); Medical Excellence Award Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University (2024). We sincerely thank for the relevant technical support provided by Guangxi Zhuang Autonomous Region Clinical Medicine Research Center for Molecular Pathology and Intelligent Pathology Precision Diagnosis.
Disclosure
The authors declare that they have no competing interests.
References
1. Sun Q, Hong Z, Zhang C, Wang L, Han Z, Ma D. Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduction and Targeted Therapy. 2023;8(1):320. doi:10.1038/s41392-023-01522-4
2. Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21(7):509–528. doi:10.1038/s41573-021-00345-8
3. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, Phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi:10.1016/S0140-6736(18)32409-7
4. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
5. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi:10.1126/science.aar4060
6. Chen X, Gao A, Zhang F, et al. ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation. Theranostics. 2021;11(7):3392–3416. doi:10.7150/thno.52435
7. Guo Y, Zhang Q, Zhu Q, et al. Copackaging photosensitizer and PD-L1 siRNA in a nucleic acid nanogel for synergistic cancer photoimmunotherapy. Sci Adv. 2022;8(16):eabn2941. doi:10.1126/sciadv.abn2941
8. Chen T, Li Q, Liu Z, Chen Y, Feng F, Sun H. Peptide-based and small synthetic molecule inhibitors on PD-1/PD-L1 pathway: a new choice for immunotherapy? Eur. J. Med. Chem. 2019;161:378–398. doi:10.1016/j.ejmech.2018.10.044
9. Han X, Wang L, Li T, et al. Beyond blocking: engineering RNAi-mediated targeted immune checkpoint nanoblocker enables T-cell-independent cancer treatment. ACS nano. 2020;14(12):17524–17534. doi:10.1021/acsnano.0c08022
10. Yoo B, Jordan VC, Sheedy P, et al. RNAi-mediated PD-L1 inhibition for pancreatic cancer immunotherapy. Sci Rep. 2019;9(1):4712. doi:10.1038/s41598-019-41251-9
11. Teo PY, Yang C, Whilding LM, et al. Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: strategies to enhance T cell killing. Adv. Healthcare Mater. 2015;4(8):1180–1189. doi:10.1002/adhm.201500089
12. Borkner L, Kaiser A, van de Kasteele W, et al. RNA interference targeting programmed death receptor-1 improves immune functions of tumor-specific T cells. Cancer Immunol Immunother. 2010;59(8):1173–1183. doi:10.1007/s00262-010-0842-0
13. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):4948. doi:10.1038/35078107
14. Iwamura K, Kato T, Miyahara Y, et al. siRNA-mediated silencing of PD-1 ligands enhances tumor-specific human T-cell effector functions. Genet Ther. 2012;19(10):959–966. doi:10.1038/gt.2011.185
15. Artiga Á, Serrano-Sevilla I, De Matteis L, Mitchell SG, de la Fuente JM. Current status and future perspectives of gold nanoparticle vectors for siRNA delivery. J Mat Chem B. 2019;7(6):876–896. doi:10.1039/C8TB02484G
16. Sun X, Chen Y, Zhao H, et al. Dual-modified cationic liposomes loaded with paclitaxel and survivin siRNA for targeted imaging and therapy of cancer stem cells in brain glioma. Drug Delivery. 2018;25(1):1718–1727. doi:10.1080/10717544.2018.1494225
17. Conde J, Ambrosone A, Sanz V, et al. Design of multifunctional gold nanoparticles for in vitro and in vivo gene silencing. ACS nano. 2012;6(9):8316–8324. doi:10.1021/nn3030223
18. Jhaveri AM, Torchilin VP. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol. 2014;5:77. doi:10.3389/fphar.2014.00077
19. Son S, Nam J, Kim J, Kim S, Kim WJ. i-motif-driven Au nanomachines in programmed siRNA delivery for gene-silencing and photothermal ablation. ACS nano. 2014;8(6):5574–5584. doi:10.1021/nn5022567
20. Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):1738. doi:10.1038/nature03121
21. Kim HJ, Takemoto H, Yi Y, et al. Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS nano. 2014;8(9):8979–8991. doi:10.1021/nn502125h
22. Tiwade PB, Fung V, VanKeulen-Miller R, Narasipura EA, Ma Y, Fenton OS. Non-viral RNA therapies for non-small cell lung cancer and their corresponding clinical trials. Mol Pharmaceut. 2025;22(4):1752–1774. doi:10.1021/acs.molpharmaceut.4c00871
23. Li Z, Yang L, Wang H, Binzel DW, Williams TM, Guo P. Non-small-cell lung cancer regression by siRNA delivered through exosomes that display EGFR RNA aptamer. Nucleic Acid Therapeutics. 2021;31(5):364–374. doi:10.1089/nat.2021.0002
24. Bai J, Duan J, Liu R, et al. Engineered targeting tLyp-1 exosomes as gene therapy vectors for efficient delivery of siRNA into lung cancer cells. Asian J. Pharm. Sci. 2020;15(4):461–471. doi:10.1016/j.ajps.2019.04.002
25. Yang L, Li Z, Binzel DW, Guo P, Williams TM. Targeting oncogenic KRAS in non-small cell lung cancer with EGFR aptamer-conjugated multifunctional RNA nanoparticles. Mol Ther Nucleic Acids. 2023;33:559–571. doi:10.1016/j.omtn.2023.07.027
26. Darweesh RS, Ayoub NM, Nazzal S. Gold nanoparticles and angiogenesis: molecular mechanisms and biomedical applications. Int J Nanomed. 2019;14:7643–7663. doi:10.2147/IJN.S223941
27. Boca SC, Potara M, Toderas F, Stephan O, Baldeck PL, Astilean S. Uptake and biological effects of chitosan-capped gold nanoparticles on Chinese hamster ovary cells. Mater Sci Eng C. 2011;31(2):184–189. doi:10.1016/j.msec.2010.08.015
28. Zhao E, Zhao Z, Wang J, et al. Surface engineering of gold nanoparticles for in vitro siRNA delivery. Nanoscale. 2012;4(16):5102–5109. doi:10.1039/c2nr31290e
29. Baghani L, Noroozi Heris N, Khonsari F, Dinarvand S, Dinarvand M, Atyabi F. Trimethyl-chitosan coated gold nanoparticles enhance delivery, cellular uptake and gene silencing effect of EGFR-siRNA in breast cancer cells. Front Mol Biosci. 2022;9:871541. doi:10.3389/fmolb.2022.871541
30. Han L, Zhao J, Zhang X, et al. Enhanced siRNA delivery and silencing gold-chitosan nanosystem with surface charge-reversal polymer assembly and good biocompatibility. ACS nano. 2012;6(8):7340–7351. doi:10.1021/nn3024688
31. Nguyen LN, Lamichhane P, Choi EH, Lee GJ. Structural and optical sensing properties of nonthermal atmospheric plasma-synthesized polyethylene glycol-functionalized gold nanoparticles. Nanomaterials. 2021;11(7):1678. doi:10.3390/nano11071678
32. Binnemars-Postma KA, Ten Hoopen HW, Storm G, Prakash J. Differential uptake of nanoparticles by human M1 and M2 polarized macrophages: protein Corona as a critical determinant. Nanomedicine. 2016;11(22):2889–2902. doi:10.2217/nnm-2016-0233
33. Shin H, Kwak M, Lee TG, Lee JY. Quantifying the level of nanoparticle uptake in mammalian cells using flow cytometry. Nanoscale. 2020;12(29):15743–15751. doi:10.1039/D0NR01627F
34. Yi Y, Kim HJ, Zheng M, et al. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J Control Release. 2019;295:268–277. doi:10.1016/j.jconrel.2019.01.006
35. Rahme K, Guo J, Holmes JD. Bioconjugated gold nanoparticles enhance siRNA delivery in prostate cancer cells. Methods Mol Biol. 2019;1974:291–301.
36. Jiang Y, Zhao W, Zhou H, Zhang Q, Zhang S. ATP-triggered intracellular in situ aggregation of a gold-nanoparticle-equipped triple-Helix molecular switch for fluorescence imaging and photothermal tumor therapy. Langmuir. 2022;38(12):3755–3764. doi:10.1021/acs.langmuir.1c03331
37. Pędziwiatr-Werbicka E, Gorzkiewicz M, Horodecka K, et al. PEGylation of dendronized gold nanoparticles affects their interaction with thrombin and siRNA. J Phys Chem A. 2021;125(4):1196–1206. doi:10.1021/acs.jpcb.0c10177
38. Gan R, Fan H, Wei Z, Liu H, Lan S, Dai Q. Photothermal response of hollow gold nanorods under femtosecond laser irradiation. Nanomaterials. 2019;9(5):711. doi:10.3390/nano9050711
39. Morgan E, Wupperfeld D, Morales D, Reich N. Shape matters: gold nanoparticle shape impacts the biological activity of siRNA delivery. Bioconjugate Chem. 2019;30(3):853–860. doi:10.1021/acs.bioconjchem.9b00004
40. Wang Y, Wang YF, Li X, et al. Nanoparticle-driven controllable mitochondrial regulation through lysosome-mitochondria interactome. ACS nano. 2022;16(8):12553–12568. doi:10.1021/acsnano.2c04078
41. Zagorovsky K, Fernández-Argüelles MT, Bona D, et al. Gold nanoparticle smartphone platform for diagnosing urinary tract infections. ACS Nanoscience Au. 2022;2(4):324–332. doi:10.1021/acsnanoscienceau.2c00001
42. Cui C, Tang J, Chen J, et al. Lactobacillus acidophilus extracellular vesicles-coated UiO-66-NH(2)@siRNA nanoparticles for ulcerative colitis targeted gene therapy and gut microbiota modulation. J Nanobiotechnol. 2025;23(1):301. doi:10.1186/s12951-025-03376-0
43. Hogarth NC, Al-Kawaaz M, Linder MW. Evaluation of turnaround times and performance of in-house ChromaCode high-definition PCR compared to send-out next-generation sequencing in non-small cell lung cancer. Am. J. Clin. Pathol. 2025. doi:10.1093/ajcp/aqaf038
44. Zhang MJ, Wan X, Shi M, Yu Y, Ou R, Ge RS. Curcuminoids WM03 inhibits ovarian cancer cisplatin-resistant cells proliferation and reverses cisplatin resistance by targeting DYRK2. Phytomedicine. 2025;142:156632. doi:10.1016/j.phymed.2025.156632
45. Chen ZX, Liang L, Huang HQ, et al. LPCAT1 enhances the invasion and migration in gastric cancer: based on computational biology methods and in vitro experiments. Cancer Med. 2023;12(12):13438–13454. doi:10.1002/cam4.5991
46. Hußtegge M, Hoang NA, Rebstock J, et al. PD-1 inhibition in patient derived tissue cultures of human gastric and gastroesophageal adenocarcinoma. Oncoimmunology. 2021;10(1):1960729. doi:10.1080/2162402X.2021.1960729
47. Yang G, Zhou D, Dai Y, et al. Construction of PEI-EGFR-PD-L1-siRNA dual functional nano-vaccine and therapeutic efficacy evaluation for lung cancer. Thoracic Cancer. 2022;13(21):2941–2950. doi:10.1111/1759-7714.14618
48. Wen J, Qiu N, Zhu Z, et al. A size-shrinkable matrix metallopeptidase-2-sensitive delivery nanosystem improves the penetration of human programmed death-ligand 1 siRNA into lung-tumor spheroids. Drug Delivery. 2021;28(1):1055–1066. doi:10.1080/10717544.2021.1931560
49. Man RCH, Qiu Y, Leung SWS, Fruhwirth GO, Lam JKW. Co-delivery of PD-L1- and EGFR-targeting siRNAs by synthetic PEG(12)-KL4 peptide to the lungs as potential strategy against non-small cell lung cancer. Eur. J. Pharm. Biopharm. 2024;195:114177. doi:10.1016/j.ejpb.2024.114177
50. Liu B, Cao W, Qiao G, et al. Effects of gold nanoprism-assisted human PD-L1 siRNA on both gene down-regulation and photothermal therapy on lung cancer. Acta Biomater. 2019;99:307–319. doi:10.1016/j.actbio.2019.08.046
51. Clark CA, Gupta HB, Sareddy G, et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 2016;76(23):6964–6974. doi:10.1158/0008-5472.CAN-16-0258
52. Li J, Chen L, Xiong Y, et al. Knockdown of PD-L1 in human gastric cancer cells inhibits tumor progression and improves the cytotoxic sensitivity to CIK therapy. Cell. Physiol. Biochem. 2017;41(3):907–920. doi:10.1159/000460504
53. Lotfinejad P, Kazemi T, Safaei S, et al. PD-L1 silencing inhibits triple-negative breast cancer development and upregulates T-cell-induced pro-inflammatory cytokines. Biomed. Pharmacother. 2021;138:111436. doi:10.1016/j.biopha.2021.111436
54. Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi:10.1016/j.cell.2015.08.016
55. Fan Y, Che X, Hou K, et al. MiR-940 promotes the proliferation and migration of gastric cancer cells through up-regulation of programmed death ligand-1 expression. Exp. Cell. Res. 2018;373(1–2):180–187. doi:10.1016/j.yexcr.2018.10.011
56. Kong T, Ahn R, Yang K, et al. CD44 promotes PD-L1 expression and its tumor-intrinsic function in breast and lung cancers. Cancer Res. 2020;80(3):444–457. doi:10.1158/0008-5472.CAN-19-1108
57. Yu J, Qin B, Moyer AM, et al. Regulation of sister chromatid cohesion by nuclear PD-L1. Cell Res. 2020;30(7):590–601. doi:10.1038/s41422-020-0315-8
58. Zhang N, Zeng Y, Du W, et al. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int J Oncol. 2016;49(4):1360–1368. doi:10.3892/ijo.2016.3632
59. Yang T, Ren C, Lu C, et al. Phosphorylation of HSF1 by PIM2 induces PD-L1 expression and promotes tumor growth in breast cancer. Cancer Res. 2019;79(20):5233–5244. doi:10.1158/0008-5472.CAN-19-0063
60. Aiello NM, Maddipati R, Norgard RJ, et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev. Cell. 2018;45(6):681–95.e4. doi:10.1016/j.devcel.2018.05.027
61. Chen L, Xiong Y, Li J, et al. PD-L1 expression promotes epithelial to mesenchymal transition in human esophageal cancer. Cell. Physiol. Biochem. 2017;42(6):2267–2280. doi:10.1159/000480000
62. Tièche CC, Gao Y, Bührer ED, et al. Tumor initiation capacity and therapy resistance are differential features of EMT-related subpopulations in the NSCLC cell line A549. Neoplasia. 2019;21(2):185–196. doi:10.1016/j.neo.2018.09.008
63. Kim S, Koh J, Kim MY, et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Human Pathol. 2016;58:7–14. doi:10.1016/j.humpath.2016.07.007
64. David JM, Dominguez C, McCampbell KK, Gulley JL, Schlom J, Palena C. A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology. 2017;6(10):e1349589. doi:10.1080/2162402X.2017.1349589
65. Li M, Teh C, Ang CY, et al. Near‐infrared light‐absorptive stealth liposomes for localized photothermal ablation of tumors combined with chemotherapy. Adv. Funct. Mater. 2015;25(35):5602–5610. doi:10.1002/adfm.201502469
66. Guo L, Yan DD, Yang D, et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS nano. 2014;8(6):5670–5681. doi:10.1021/nn5002112
67. Hashida Y, Tanaka H, Zhou S, et al. Photothermal ablation of tumor cells using a single-walled carbon nanotube-peptide composite. J Control Release. 2014;173:59–66. doi:10.1016/j.jconrel.2013.10.039
68. Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(4). doi:10.1002/wnan.1449
69. Imashiro C, Takeshita H, Morikura T, Miyata S, Takemura K, Komotori J. Development of accurate temperature regulation culture system with metallic culture vessel demonstrates different thermal cytotoxicity in cancer and normal cells. Sci Rep. 2021;11(1):21466. doi:10.1038/s41598-021-00908-0
70. Ghahremani FH, Sazgarnia A, Bahreyni-Toosi MH, Rajabi O, Aledavood A. Efficacy of microwave hyperthermia and chemotherapy in the presence of gold nanoparticles: an in vitro study on osteosarcoma. Int J Hyperthermia. 2011;27(6):625–636. doi:10.3109/02656736.2011.587363
71. Shchors K, Evan G. Tumor angiogenesis: cause or consequence of cancer? Cancer Res. 2007;67(15):7059–7061. doi:10.1158/0008-5472.CAN-07-2053
72. Ahmed K, Zaidi SF, Mati Ur R, Rehman R, Kondo T. Hyperthermia and protein homeostasis: cytoprotection and cell death. Journal of Thermal Biology. 2020;91:102615. doi:10.1016/j.jtherbio.2020.102615
73. Lepock JR. How do cells respond to their thermal environment? Int J Hyperthermia. 2005;21(8):681–687. doi:10.1080/02656730500307298
74. Lepock JR. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int J Hyperthermia. 2003;19(3):252–266. doi:10.1080/0265673031000065042
75. Kaur P, Aliru ML, Chadha AS, Asea A, Krishnan S. Hyperthermia using nanoparticles--promises and pitfalls. Int J Hyperthermia. 2016;32(1):76–88. doi:10.3109/02656736.2015.1120889
76. Madersbacher S, Gröbl M, Kramer G, Dirnhofer S, Steiner GE, Marberger M. Regulation of heat shock protein 27 expression of prostatic cells in response to heat treatment. Prostate. 1998;37(3):174–181. doi:10.1002/(SICI)1097-0045(19981101)37:3<174::AID-PROS6>3.0.CO;2-4
77. Gibbons NB, Watson RW, Coffey RN, Brady HP, Fitzpatrick JM. Heat-shock proteins inhibit induction of prostate cancer cell apoptosis. Prostate. 2000;45(1):58–65.
78. Zuo WF, Pang Q, Zhu X, et al. Heat shock proteins as hallmarks of cancer: insights from molecular mechanisms to therapeutic strategies. J hematol oncol. 2024;17(1):81.
79. Hu C, Yang J, Qi Z, et al. Heat shock proteins: biological functions, pathological roles, and therapeutic opportunities. MedComm. 2022;3(3):e161. doi:10.1002/mco2.161
80. Zhang H, Shao S, Zeng Y, et al. Reversible phase separation of HSF1 is required for an acute transcriptional response during heat shock. Nat Cell Biol. 2022;24(3):340–352. doi:10.1038/s41556-022-00846-7
81. Zhang X, Wang X, Hou L, Xu Z, Liu Y, Wang X. Nanoparticles overcome adaptive immune resistance and enhance immunotherapy via targeting tumor microenvironment in lung cancer. Front Pharmacol. 2023;14:1130937. doi:10.3389/fphar.2023.1130937
82. Martin C, Enrico D. Current and novel therapeutic strategies for optimizing immunotherapy outcomes in advanced non-small cell lung cancer. Front Oncol. 2022;12:962947. doi:10.3389/fonc.2022.962947
83. Wen Y, Chen X, Zhu X, et al. Photothermal-chemotherapy integrated nanoparticles with tumor microenvironment response enhanced the induction of immunogenic cell death for colorectal cancer efficient treatment. ACS Appl. Mater. Interfaces. 2019;11(46):43393–43408. doi:10.1021/acsami.9b17137
84. Zhao Y, Liu X, Liu X, et al. Combination of phototherapy with immune checkpoint blockade: theory and practice in cancer. Front Immunol. 2022;13:955920. doi:10.3389/fimmu.2022.955920
85. Guo R, Wang S, Zhao L, et al. Engineered nanomaterials for synergistic photo-immunotherapy. Biomaterials. 2022;282:121425. doi:10.1016/j.biomaterials.2022.121425
86. Astell KR, Sieger D. Zebrafish in vivo models of cancer and metastasis. Cold Spring Harbor Perspectives in Medicine. 2020;10(8):a037077. doi:10.1101/cshperspect.a037077
87. Brown HK, Schiavone K, Tazzyman S, Heymann D, Chico TJ. Zebrafish xenograft models of cancer and metastasis for drug discovery. Expert Opin Drug Discov. 2017;12(4):379–389. doi:10.1080/17460441.2017.1297416
88. Masud S, Torraca V, Meijer AH. Modeling infectious diseases in the context of a developing immune system. Current Topics in Developmental Biology. 2017;124:277–329.
89. Ma G, Liu Z, Zhu C, et al. H(2) O(2) -responsive NIR-II AIE nanobomb for carbon monoxide boosting low-temperature photothermal therapy. Angew Chem. 2022;61(36):e202207213. doi:10.1002/anie.202207213
90. Chen J, Ma Y, Du W, et al. Furin‐instructed intracellular gold nanoparticle aggregation for tumor photothermal therapy. Adv. Funct. Mater. 2020;30:2001566.
91. Gao L, Zheng F, Fu Z, Wang W. Dual-responsive nanoparticles targeting ACE-II senescence for therapeutic mitigation of acute lung injury. J Nanobiotechnol. 2025;23(1):339. doi:10.1186/s12951-025-03382-2
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.